Abstract
Artemisia princeps, the Korean mugwort, is an edible plant that has various beneficial effects on health, and which has been used as a part of traditional folk medicine. The current study investigated the possible effects of solvent (H2O, n-BuOH, 85% aq. MeOH, and n-hexane) partitioned fractions of A. princeps crude extract (APE) on adipogenic differentiation of 3T3-L1 mouse pre-adipocytes. Characteristics of the differentiated adipocytes were evaluated by Oil red O staining of intracellular lipid droplets, analyzing mRNA and protein levels of peroxisome proliferator-activated receptor (PPAR) γ, CCAAT/enhancer-binding protein (C/EBP) α, and sterol regulatory element-binding protein (SREBP)-1c, and immunoblotting of phosphorylated mitogen-activated protein kinase (MAPK) pathway proteins such as p38, extracellular-signal-regulated kinase (ERK), and c-Jun N-terminal kinase (JNK). Introduction of APE fractions to differentiating adipocytes resulted in lowered lipid accumulation and downregulation of the PPARγ pathway. APE fractions significantly decreased mRNA and protein expression of PPARγ, C/EBPα, and SREBP-1c. Analysis of MAPK pathway activation showed similar results since treatment with the APE fraction treatment decreased levels of phosphorylated p38, ERK, and JNK. Overall, the n-BuOH and n-hexane fractions were observed to be the most active fractions to suppress adipogenesis-related signaling in 3T3-L1 cells. The promising ability of APE fractions to inhibit adipocyte differentiation of 3T3-L1 cells suggest that A. princeps has potential to be utilized as a source of anti-obesity compounds.
Keywords: 3T3-L1, Adipogenesis, Artemisia princeps, MAPK, PPARγ
INTRODUCTION
Deterioration of energy metabolism regulation results in several health-threatening problems. Obesity is one of the most prevalent complications of energy metabolism, arising from an impaired balance between energy intake and expenditure (Desai et al., 2013). Obesity is reported to be the leading cause of several diseases and metabolic disorders, such as type 2 diabetes, coronary complications, osteoporosis, osteoarthritis, hypertension, and cancer (Grundy, 2004; Balistreri et al., 2010; Cao, 2011). Most of the deleterious effects of obesity can be in adipose tissue. Adipocytes, the building cells of adipose tissue, are important for several metabolisms, such as glucose, appetite, and inflammation via adipokines (Rosen and Spiegelman, 2006). Adipokines are hormones secreted by adipocytes that affect almost all organs and systems of the body (Fantuzzi, 2005). In obese patients, adipocytes undergo abnormal proliferation, which is characterized by excessive fat accumulation. Increased lipid storage is achieved by irregular adipocyte growth, a result of obesity-linked stimulation of adipocyte differentiation. In this context, preventing pre-adipocytes from undergoing adipocyte differentiation may be regarded as a promising therapeutic target to delay or suppress the onset and progression of pathological weight gain (Ghaben and Scherer, 2019). Aside from on the market drugs, natural products are safe and cost-effective alternatives for daily use to regulate adipocyte differentiation and reduce risk of obesity (Sun et al., 2016). Dietary supplementation of bioactive compounds consumed via a diet rich in plants is one way to regulate energy metabolism and lower the risk of developing obesity-related complications.
Several plants have been reported to exhibit inhibitory effects on adipogenesis via their bioactive ingredients, which inhibit adipocyte differentiation and lipid accumulation through different mechanisms. With the aim of developing natural products exhibiting anti-adipogenic effects, we have previously reported that Artemisia scoparia inhibits the differentiation of 3T3-L1 adipocytes via interacting with 5′-adenosine monophosphate (AMP)-activated protein kinase (AMPK)-regulated peroxisome proliferator-activated receptor (PPAR) γ signaling (Oh et al., 2018). Different Artemisia species have been studied for beneficial health properties against various complications such as hyperglycemia (Ahmad and Abdul-Hussian, 2016), fatty liver (Jang et al., 2015), coagulation (Lv et al., 2018), hypertension (Siddiqui et al., 2017), and excessive fat storage (Baek et al., 2015). A. princeps, known as Korean mugwort or ssuk in Korea, is a common plant in Korea and Japan that is used as part of daily diets and is an important ingredient in traditional medicinal recipes against gastric-discomforts and hematological problems (Yun et al., 2016). Studies have reported that A. princeps extracts and its isolated constituents possess promising bioactivities and show anti-diabetic (Dabe and Kefale, 2017), antioxidant (Hirano et al., 2017), and antibacterial effects (Choi et al., 2015). It is known that A. princeps contains high amounts of bioactive compounds, especially flavonoids and terpenoids, and certain essential oils, all of which have been reported to be highly active biocompatible nutraceuticals.
In this regard, the current study aimed to investigate the anti-obesity potential of A. princeps for inhibiting differentiation of 3T3-L1 mouse pre-adipocytes. Solvent-based fractions of A. princeps extracts were tested for their ability to inhibit adipogenesis. Results were suggested to help uncover the mechanism of action of the anti-obesity effect A. princeps and its bioactive molecules.
MATERIALS AND METHODS
Plant material
Whole plants of A. princeps were collected by hand in September 2007 at Onggumdo tidal flat of Ganghwado, Korea. The shade-dried materials of A. princeps was kept in −25°C until chemically investigated.
Chemicals and reagents
Extraction solvents were acquired from Samchun [Seoul, Korea: methylene chloride, D1602; methanol (MeOH), M1450], Junsei Chemical Co., Ltd. [Tokyo, Japan: n-butanol (n-BuOH), 6313050380], and JT Baker (Philipsburg, NJ, USA: n-hexane, 9304-03). Cell culture and assay reagents, Oil red O stain, and staining solvents were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Gene specific primers were from Bioneer (Daejeon, Korea) and the primary antibodies were purchased from Cell Signaling Technology, Inc. [Danvers, MA, USA: PPARγ, #2443; CCAAT/enhancer-binding protein (C/EBP) α, #2295; p-38, #4511; p-c-Jun N-terminal kinase (JNK), #9255; p-extracellular-signal-regulated kinase (ERK), #4370], Abcam [Cambridge, UK: sterol regulatory element-binding protein-1c (SREBP-1c), ab3259], and Santa Cruz Biotechnology (Dallas, TX, USA: β-actin, sc-47778). Sources for other reagents and equipment were given in the designated sections when they are first mentioned.
Extraction and fractionation
A. princeps specimens were washed with water and dried in the shade. Dried samples were cut into small pieces followed by maceration. Powder-like samples were obtained, which were extracted for 24 h at room temperature using methylene chloride as the extraction solvent. The extracts were set aside, and the previous step was repeated to extract the remaining sediment. The remaining sample residue was then extracted again using MeOH using the same conditions. Combined extracts were dried using a rotary evaporator yielding the A. princeps crude extract (APE). Crude extract was subjected to solvent partition using methylene chloride and H2O. The methylene chloride layer was concentrated in vacuo and further partitioned between n-hexane and 85% aqueous (aq.) MeOH. The aqueous layer was also subjected to repartition using n-BuOH and H2O as solvents. The whole procedure yielded four solvent-partitioned fractions of A. princeps crude extract: n-hexane (4.77 g), 85% aq. MeOH (9.63 g), n-BuOH (14.21 g), and H2O (20.28 g).
Cell culture and differentiation
Mouse 3T3-L1 fibroblasts (10092.1; Korean Cell Line Bank, Seoul, Korea) undergoes pre-adipocyte-to-adipocyte differentiation under certain conditions. 3T3-L1 fibroblasts were grown in with Dulbecco’s modified Eagle medium (10-013-CVR; Corning Inc., Corning, NY, USA) containing 10% fetal bovine serum in appropriate flasks or wells, and incubated in at 37°C with a humidified atmosphere containing 5% CO2. Cell differentiation was induced on the second day (day 0) after the cells reached confluence. At day 0, cell culture medium was replaced with differentiation medium [cell culture medium enriched with insulin (5 μg/mL), methylisobutylxanthine (0.5 mM), and dexamethasone (0.25 μM) with or without samples]. Following 48 h of incubation, differentiation medium was changed to feeding medium, cell culture medium containing insulin (5 μg/mL). Feeding medium was replaced with fresh culture medium after 2 days, and was further renewed every 48 h until lipid droplets were observed under microscope in untreated control cells. Different concentrations of APE fractions were added to each cell cultures at day 0 and were included in every medium change until the day the control cells were differentiated into mature adipocytes (day 8).
Cell viability assay
Cytotoxicity of the APE fractions was assessed by colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay based on the conversion of formazan crystals in the mitochondria of living cells as previously described (Kwon et al., 2019). Briefly, mouse 3T3-L1 fibroblast pre-adipocytes were seeded in 96-well plates and treated with various concentrations of APE fractions. The cell culture medium was removed after 2 days of incubation and 100 μL MTT solution (1 mg/mL) was added to each well. The plate was incubated in the dark at 37°C for 4 h, followed by removal of the solution from wells. The cells were then washed with phosphate buffer saline (PBS). Finally, 10 μL of 100% dimethyl sulfoxide was added to each well and the absorbance at 540 nm was measured using GENios FL microplate reader (Tecan Austria GmbH, Grödig, Austria). Viability of the sample-treated cells was calculated as a percentage of the untreated blank group, which were considered to have 100% viability.
Oil red O staining
Levels of stored triglycerides in differentiated mature 3T3-L1 adipocytes were verified by staining intracellular lipid droplets with Oil red O. Mature adipocyte cells treated with or without APE fractions during differentiation (day 0 to 8) were then subjected to staining procedures as described previously (Kwon et al., 2019). Briefly, mature adipocytes differentiated in 6-well plates were washed with PBS and fixed with formaldehyde (3.7%, v/v in distilled water) following the removal of medium from the wells. Fixed cells were then incubated with filtered Oil red O staining solution (0.5%, w/v in a mixture of 60% isopropanol and 40% distilled water) for 1 h at room temperature. Oil red O staining solution was then removed, and the stained lipid droplets were imaged using a light microscope (Nikon, Tokyo, Japan). The stain entrapped by the lipid droplets was then eluted from the cells by addition of 100% isopropanol. The amount of stain was then calculated by the measuring absorbance at 500 nm using a GENios FL microplate reader (Tecan Austria GmbH).
Reverse transcription (RT)-polymerase chain reaction (PCR) assay
Total RNA was isolated from mature 3T3-L1 adipocytes treated with APE fractions (day 0 to day 8) using Trizol reagent (Invitrogen, Carlsbad, CA, USA). cDNA synthesis was performed using 2 μg total RNA and a Moloney murine leukemia virus reverse transcriptase master mix (Invitrogen) containing 140 U reverse transcriptase, 1 mM deoxynucleoside triphosphates, and 500 ng oligo(dT), following manufacturer’s instructions. cDNA amplification was carried out using DNA polymerase containing ready to use master mix (Invitrogen) following the manufacturer’s recommended protocol. PCR conditions were as follows: 30 cycles of 95°C for 45 s for denaturation, 60°C for 1 min for annealing, and 72°C for 45 s for extending. The sequences of gene specific oligonucleotide primers were: PPARγ, forward 5′-TTT TCA AGG GTG CCA GTT TC-3′ and reverse 5′-AAT CCT TGG CCC TCT GAG AT-3′; SREBP-1c, forward 5′-TGT TGG CAT CCT GCT ATC TG-3′ and reverse 5′-AGG GAA AGC TTT GGG GTC TA-3′; C/EBPα, forward 5′-TTA CAA CAG GCC AGG TTT CC-3′ and reverse 5′-GGC TGG CGA CAT ACA GTA CA-3′; β-actin, forward 5′-CCA CAG CTG AGA GGG AAA TC-3′ and reverse 5′-AAG GAA GGC TGG AAA AGA GC-3′. Amplified cDNA was then loaded to 1.5% agarose gels and separated by electrophoresis for 30 min at 100 V. Gels were stained by incubation in 1 mg/mL ethidium bromide solution for 15 min and the gel was imaged using a CAS-400SM Davinch-Chemi imagerTM (Davinch-K, Seoul, Korea).
Western blot analysis
Immunoblotting of adipogenesis specific proteins was carried out using Western blotting as described previously (Kwon et al., 2019). Mature 3T3-L1 adipocytes in 6-well plates treated with or without APE fractions during adipogenesis were lysed with 1 mL of radioimmunoprecipitation assay buffer (Sigma-Aldrich Co.) for 30 min at 4°C, followed by vigorous pipetting. The amounts of protein in cell lysates were analyzed with a bicinchoninic acid protein assay kit (Thermo Fisher Scientific, Rockford, IL, USA), following manufacturer’s protocol. Cell lysates containing equal amounts (20 μg) of protein were loaded onto 12% sodium dodecyl sulfate-polyacrylamide gel and separated by electrophoresis. Separated proteins were then transferred onto membrane (polyvinylidene fluoride, GE Healthcare, Amersham, UK), and incubated for 1 h at room temperature in 5% skimmed milk for blocking. Membranes were washed with 1× Tris buffered saline with Tween 20 (TBST) and incubated with primary antibodies (diluted 1:1,000) targetting PPARγ, SREBP-1c, C/EBPα, phosphorylated (p−) p38, p-ERK, p-JNK, and β-actin in primary antibody dilution buffer containing 1×TBST and 5% bovine serum albumin (overnight at 4°C). After washing with 1× TBST, membranes were incubated with horseradish-peroxidase-conjugated anti mouse (cat. no. 7076; Cell Signaling Technology, Inc.) or anti rabbit (cat. no. 7074; Cell Signaling Technology, Inc.) secondary antibodies (diluted 1:1,000) at room temperature for 1 h. Detection of immunoreactive proteins were detected using a chemiluminescence ECL assay kit (GE Healthcare) according to the manufacturer’s instructions. Images of immunoreactive bands were taken with a CAS-400SM Davinch-Chemi imagerTM (Davinch-K).
Statistical analysis
Results were expressed as the mean±standard deviation (SD) of three separate experiments. Statistical differences between the means of the sample groups were assessed by the analysis of variance (ANOVA) using SAS v9.1 software (SAS Institute, Cary, NC, USA) followed by Duncan’s multiple range test. Statistical significances between the groups were determined at P<0.05 level.
RESULTS AND DISCUSSION
One of the most promising approaches for preventing obesity and related complications is a diet supplemented with functional foods. Recently, plants part of traditional folk medicine have been gaining much interest due to their bioactivities (Fabricant and Farnsworth, 2001; Yeung et al., 2018). These studies have generated promising results against several complications, diseases, and disorders. A. princeps is one such plant with reported bioactivity. Considering its potential, the current study reported the potential of solvent-based fractions from A. princeps extracts against obesity by studying their effects on fat accumulation and adipocyte differentiation.
Effect of APE solvent fractions on viability of 3T3-L1 cells
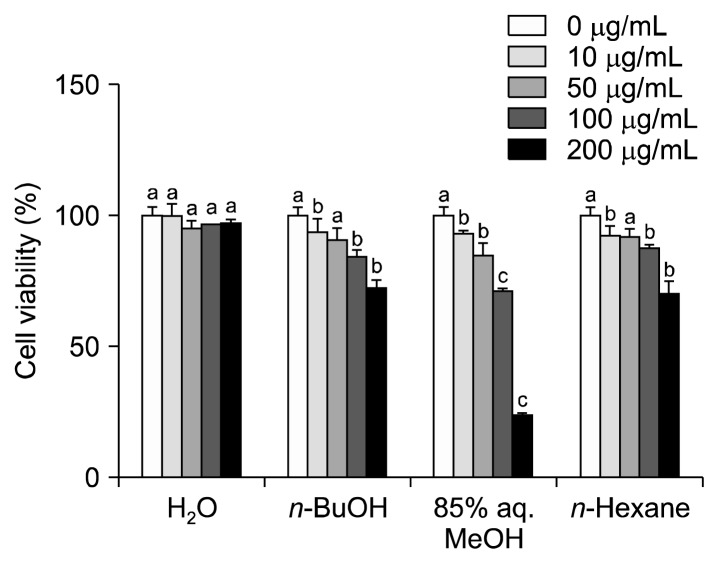
Before conducting obesity-related assays, cytotoxicity of the samples was tested on 3T3-L1 mouse pre-adipocytes using MTT formazan assays. Pre-adipocytes were incubated with different concentrations of APE fractions (10, 50, 100, and 200 μg/mL). None of the treatments below 200 μg/mL showed any significant toxic effect on 3T3-L1 cells after 48 h incubation (Fig. 1). Therefore, the highest concentration that was used in further assays was 100 μg/mL. However, at this concentration (100 μg/mL), treatment with H2O, n-BuOH, 85% aq. MeOH, and n-hexane extract fractions decreased cell viability to the 96.72, 84.23, 71.28, and 87.55% of untreated control cells, respectively. Therefore, for further comparisons and interpretations 85% aq. MeOH fraction was deemed slightly toxic at the concentration of 100 μg/mL. First analyses of the comparative activity of the different fractions were carried out using 50 μg/mL treatment, and further investigation using 100 μg/mL treatment was considered if any fraction other than 85% aq. MeOH was identified as the most active. This non-toxicity of APE fractions showed that any inhibitory presence of the samples during adipogenesis occurred through suppression of cellular pathways rather than by inducing cytotoxicity in differentiating adipocytes.
Fig. 1.
Effect of Artemisia princeps crude extract (APE) and APE fractions (H2O, n-BuOH, 85% aq. MeOH, and n-hexane) on cell viability of 3T3-L1 pre-adipocytes. Different letters (a–c) over bars indicate statistically significant difference among cells treated with the same treatment concentration at P <0.05 level according to Duncan’s multiple range test.
Effect of APE solvent fractions on lipid accumulation of differentiating pre-adipocytes
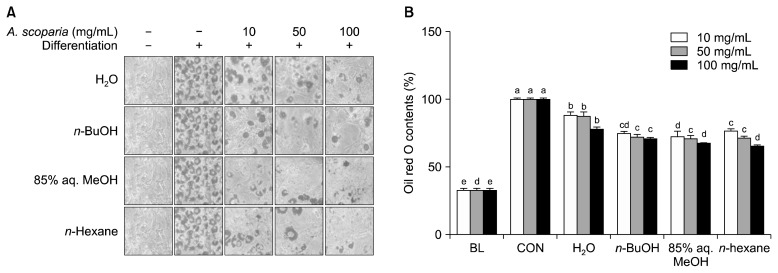
Mouse 3T3-L1 fibroblasts were induced to differentiate into mature adipocytes through incubation with differentiation-inducing medium in the absence and presence of different concentrations of APE solvent fractions. Levels of lipid accumulation in mature adipocytes were confirmed by staining intracellular lipid stores with Oil red O. Adipocyte lipid stores treated with APE fractions were lower compared with those of the untreated control group (Fig. 2A). Levels of inhibition were then quantified by comparing the amount of staining bound to intracellular triglycerides. Oil red O eluded from cells were calculated through measuring absorbance (Fig. 2B). All tested fractions could inhibit lipid accumulation at similar levels at the 50 μg/mL dose, except the H2O fraction. H2O, n-BuOH, 85% aq. MeOH, and n-hexane fractions decreased the intracellular lipids in differentiated adipocytes to 87.51, 72.10, 71.21, and 71.28% of untreated control cells, respectively.
Fig. 2.
Effect of Artemisia princeps crude extract (APE) fractions (H2O, n-BuOH, 85% aq. MeOH, and n-hexane) on lipid accumulation of differentiating 3T3-L1 adipocytes. Cells were treated from initiation of adipocyte differentiation until the adipocyte maturation. (A) Images of 3T3-L1 adipocytes following Oil red O staining of intracellular lipid droplets. (B) Lipid contents of 3T3-L1 cells calculated as the Oil red O stain amount relative to the control group. Different letters (a–e) over bars indicate statistically significant difference among cells treated with the same treatment concentration at P <0.05 level according to Duncan’s multiple range test. BL, untreated non-differentiated pre-adipocytes, CON, untreated differentiated adipocytes.
The most common visible symptom of obesity is abundance of adipose tissue. During obesity progression, adipocyte differentiation occurs at irregularly high levels and, per their characteristics, adipocytes accumulate high amounts of fat as intracellular triglycerides (Desai et al., 2013). Cells undergo adipogenesis to form new adipocytes and store lipids. Therefore, intervening in either adipogenesis signaling or lipid accumulation pathways may prevent cells from storing lipids.
Through decreasing levels of lipids in adipocytes, APE solvent fractions show to possess potential to suppress adipocyte-specific characteristics and/or adipogenesis. The effect of APE fractions on lipid accumulation suggest that APE can help hinder fat tissue expansion observed in obesity.
Effect of APE solvent fractions on the PPARγ pathway
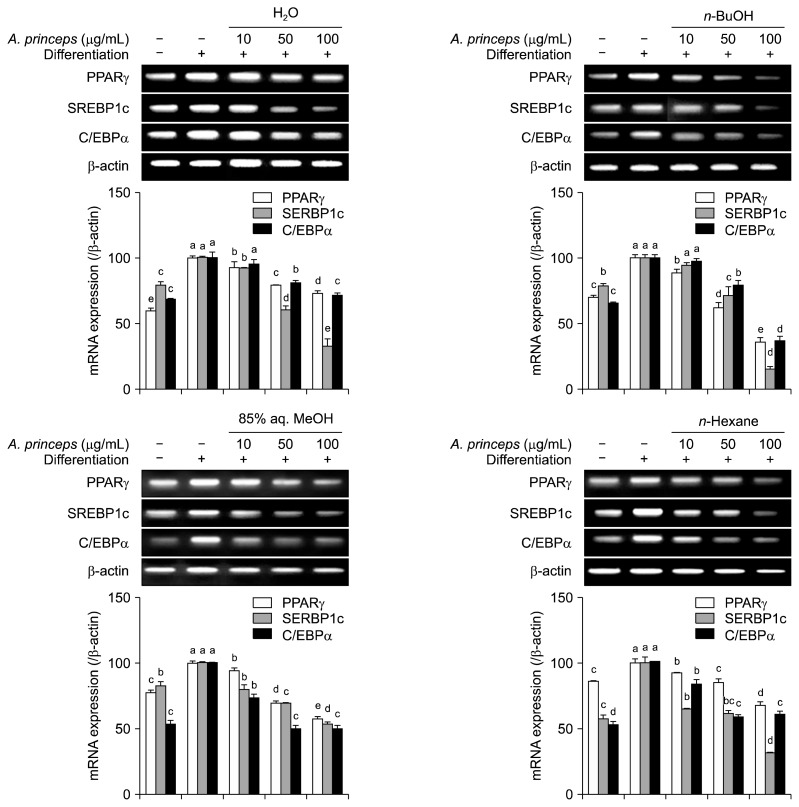
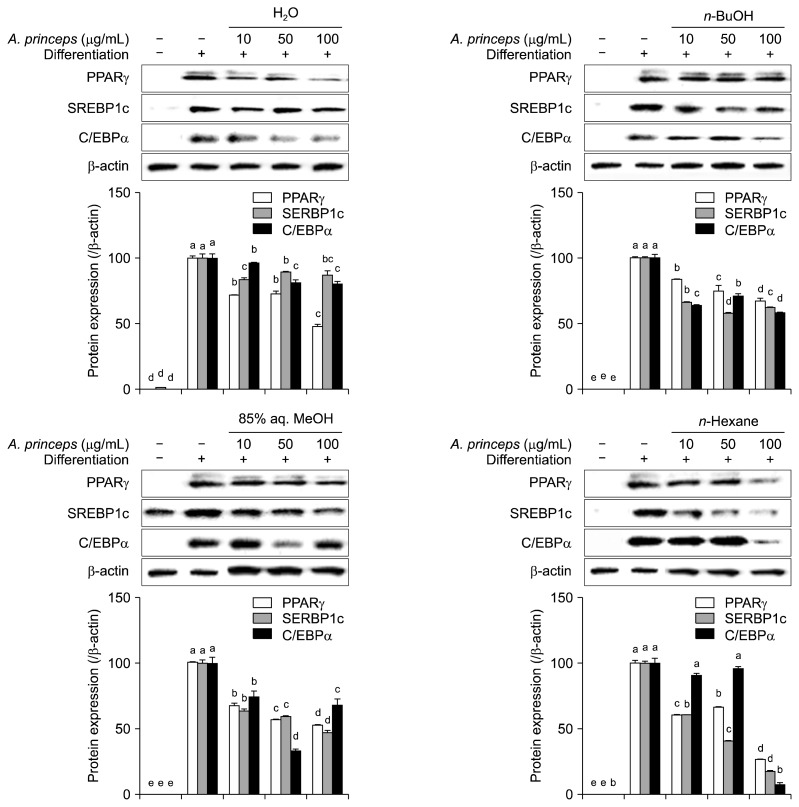
The effect of APE fractions on intracellular adipogenesis signaling pathways was analyzed in 3T3-L1 mature adipocytes treated with or without samples during adipocyte differentiation. Changes in the mRNA and protein expression levels of PPARγ, C/EBPα, and SREBP-1c were examined by RT-PCR and Western blotting, respectively. The PPARγ pathways is the main regulator of the adipocyte differentiation and consists of several transcription factors and proteins such as C/EBPα and SREBP-1c (Payne et al., 2009). Suppression of PPARγ activation and inhibition of its transcription factor expression was shown to hinder adipogenesis, resulting in a lowered adipocyte profile and decreased fat storage in differentiating adipocytes (Christodoulides and Vidal-Puig, 2010). All of the tested APE fractions were able to suppress expression of both mRNA (Fig. 3) and protein (Fig. 4) of PPARγ, C/EBPα, and SREBP-1c in a dose-dependent manner. The n-BuOH fraction showed the highest amount of inhibition by lowering PPARγ, SREBP-1c, and C/EBPα mRNA expression levels to 36.0, 16.3, and 37.3%, respectively of untreated control group at the 100 μg/mL concentrations (Fig. 3). Similar inhibitory patterns were observed for protein levels; n-hexane was the most active fraction for suppressing PPARγ, SREBP-1c, and C/EBPα protein amounts (decreased to 26.2, 17.3, and 7.8%, respectively of untreated control cells at 100 μg/mL concentration) (Fig. 4). However, 85% aq. MeOH fractions only showed strong inhibition at 100 μg/mL concentrations; this was regarded as slightly toxic due to the cell viability assay results and, therefore, was excluded from the comparison. Active fractions (n-BuOH and n-hexane) were also observed as the most active at 50 μg/mL concentrations, as well in comparison with 85% aq. MeOH fractions. Results suggest that APE fractions can decrease lipid accumulation in differentiating cells through intervening in PPARγ pathways. Beside PPARγ, the other PPARγ-dependent proteins in the signaling cascade, SREBP-1c and C/EBPα, were also suppressed at both mRNA and protein levels, further demonstrating inhibition of the PPARγ pathway by APE fractions.
Fig. 3.
Effect of Artemisia princeps crude extract (APE) fractions (H2O, n-BuOH, 85% aq. MeOH, and n-hexane) on mRNA expression levels of peroxisome proliferator-activated receptor-gamma (PPARγ), sterol regulatory element-binding protein-1c (SREBP-1c), and CCAAT/enhancer-binding protein alpha (C/EBPα) analyzed by RT-PCR in differentiating 3T3-L1 adipocytes. β-Actin was used as the internal control. Changes in mRNA levels after quantification of the bands were expressed as a percentage of control cells normalized against the internal control. Different letters (a–e) over bars indicate statistically significant difference among cells treated with the same target gene at P <0.05 level according to Duncan’s multiple range test.
Fig. 4.
Effect of Artemisia princeps crude extract (APE) fractions (H2O, n-BuOH, 85% aq. MeOH, and n-hexane) on protein levels of peroxisome proliferator-activated receptor-gamma (PPARγ), sterol regulatory element-binding protein-1c (SREBP-1c), and CCAAT/enhancer-binding protein alpha (C/EBPα) analyzed by Western blotting in differentiating 3T3-L1 adipocytes. β-Actin was used as the internal control. Changes in protein levels after quantification of the bands are given as percentage of control cells normalized against the internal control. Different letters (a–e) over bars indicate statistically significant difference among cells treated with the same target gene at P <0.05 level according to Duncan’s multiple range test.
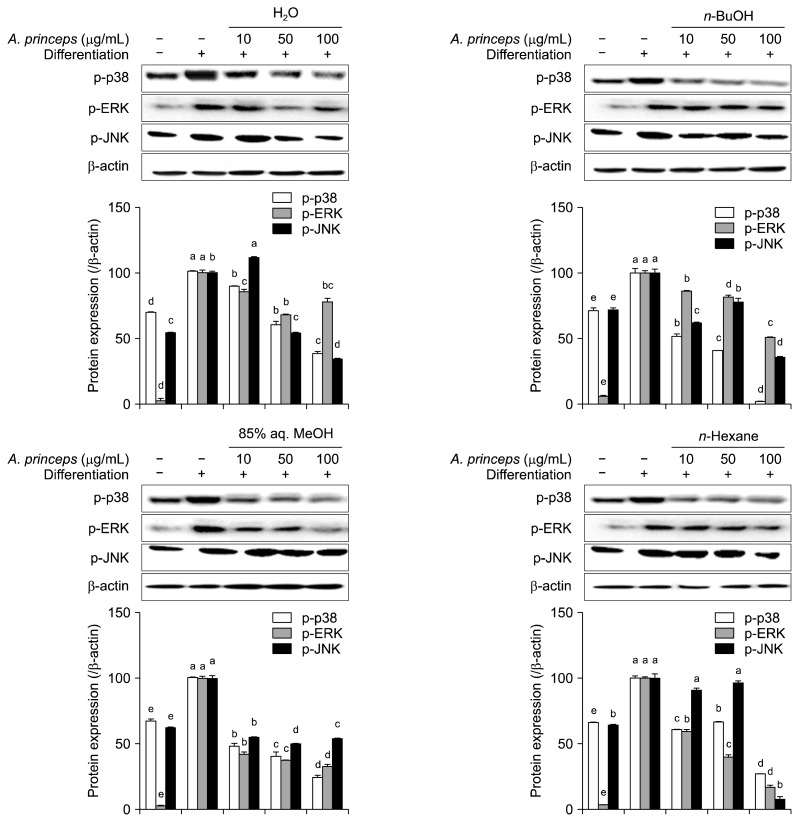
Effect of APE solvent fractions on the mitogen-activated protein kinase (MAPK) pathway
Adipocyte differentiation is mainly regulated by PPARγ signaling, which is closely linked with MAPK signaling. Studies have shown that PPARγ and C/EBP family proteins are phosphorylated by ERK and p38 members of the MAPK signaling (Bost et al., 2005; Qi et al., 2017). Some studies have also indicated a positive role for JNK in preadipocyte to adipocyte differentiation (Bost et al., 2005). Despite some contradictory results that show these MAPK family proteins can also negatively regulate adipogenesis in some stages of adipocyte formation from stem cells (Tsang et al., 2018), arrested fibroblasts with pre-adipocyte characteristics, such as 3T3-L1 cells, undergo adipogenesis where ERK, p38, and JNK are all upregulated during differentiation.
Control cells were also differentiated into mature adipocytes without sample treatment; these cells showed elevated amounts of p-p38, p-ERK, and p-JNK levels analyzed by Western blotting (Fig. 5). Treatment with APE fractions decreased levels of activated p38, ERK, and JNK. All fractions showed a similar dose-dependent trend of suppressing activation of the MAPK pathway. At 100 μg/mL concentrations, n-BuOH decreased p-p38 to 1.1% of untreated control adipocytes, whereas n-hexane decreased amounts of p-ERK and p-JNK to 17.3 and 7.8%, respectively (Fig. 5).
Fig. 5.
Effect of Artemisia princeps crude extract (APE) fractions (H2O, n-BuOH, 85% aq. MeOH, and n-hexane) on protein levels of phosphorylated (p−) p-38, ERK, and JNK, as analyzed by Western blotting in differentiating 3T3-L1 adipocytes. β-Actin was used as the internal control. Changes in protein levels after the quantification of the bands are given as percentage of control cells normalized against the internal control. Different letters (a–e) over bars indicate statistically significant difference cells treated with the among same target gene at P <0.05 level according to Duncan’s multiple range test.
Overall, all APE fractions showed anti-obesity potential by inhibiting adipogenic differentiation of 3T3-L1 cells via suppressing PPARγ and MAPK pathways. However, of all tested samples, treatment with H2O fractions produced fewer significant results compared with n-BuOH, 85% aq. MeOH, and n-hexane. Mechanistic analysis indicated that APE fractions suppress lipid accumulation in adipocytes during adipogenesis mainly due to their intervention of MAPK signaling pathway. Current results suggest that treatment with APE fractions downregulates MAPK pathway protein activation, especially p-38 and ERK, which in turn decrease expression of PPARγ, C/EBPα, and SREBP-1c. This downregulation hinders adipocyte characteristics in differentiating 3T3-L1 pre-adipocytes. Deteriorated adipogenesis resulted in lowered amounts of intracellular lipids. Results also show that the active fractions 85% aq. MeOH, n-hexane, and n-BuOH suppressed adipogenesis at different levels in the different stages. Their effect on lipid accumulation inhibition was similar; however, each fraction most greatly affected different proteins in the signaling cascade. Nevertheless, MAPK pathway analysis suggests that APE fractions show anti-adipogenesis effects largely through downregulation of p-38 and ERK. According to studies by Engelman et al. (1998) and Hu et al. (2003), these proteins are directly linked to activation of C/EBPβ and translocation of glucose transport receptors. These results indicate that anti-adipogenesis treatment with APE fractions inhibits PPARγ pathway activation via downregulation of MAPK signaling. Active anti-adipogenic extracts of different plants with different solvents, such as hexane and butanol, have been shown to yield mainly fatty acids, and their derivatives can inhibit adipocyte differentiation among other phenolic substances (Azain, 2004; Kennedy et al., 2010; Sergent et al., 2012). On the other hand, Artemisia genus plants have yielded flavonoids and similar phenolic compounds with anti-obesity effect (Lim et al., 2013; Hwang et al., 2016). Based on these reports, we suggest that APE fractions, which contain high amounts of fatty acid and flavonoid derivatives, show promising anti-obesity effects. Further studies to analyze the active fractions of A. princeps are suggested to provide better understanding of their mechanism of action; these studies should isolate and characterize the potential bioactive anti-obesity compounds. Overall, the current study shows that A. princeps is a promising plant to study for development of anti-obesity nutraceuticals and functional foods.
ACKNOWLEDGEMENTS
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. NRF-2017R1A2B4009588).
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- Ahmad ZA, Abdul-Hussian BA. Effect of Artemisia herb on induced hyperglycemia in wistar rats. Al-Qadisiyah J Vet Med Sci. 2016;15:63–69. [Google Scholar]
- Azain MJ. Role of fatty acids in adipocyte growth and development. J Anim Sci. 2004;82:916–924. doi: 10.2527/2004.823916x. [DOI] [PubMed] [Google Scholar]
- Baek HK, Shim H, Lim H, Shim M, Kim CK, Park SK, et al. Anti-adipogenic effect of Artemisia annua in diet-induced-obesity mice model. J Vet Sci. 2015;16:389–396. doi: 10.4142/jvs.2015.16.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balistreri CR, Caruso C, Candore G. The role of adipose tissue and adipokines in obesity-related inflammatory diseases. Mediators Inflamm. 2010;2010 doi: 10.1155/2010/802078. 802078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bost F, Aouadi M, Caron L, Binétruy B. The role of MAPKs in adipocyte differentiation and obesity. Biochimie. 2005;87:51–56. doi: 10.1016/j.biochi.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Cao JJ. Effects of obesity on bone metabolism. J Orthop Surg Res. 2011;6:30. doi: 10.1186/1749-799X-6-30. doi: 10.1186/1749-799X-6-30. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi NY, Kang SY, Kim KJ. Artemisia princeps inhibits biofilm formation and virulence-factor expression of antibiotic-resistant bacteria. BioMed Res Int. 2015;2015 doi: 10.1155/2015/239519. 239519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christodoulides C, Vidal-Puig A. PPARs and adipocyte function. Mol Cell Endocrinol. 2010;318:61–68. doi: 10.1016/j.mce.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Dabe NE, Kefale AT. Antidiabetic effects of artemisia species: a systematic review. Anc Sci Life. 2017;36:175–181. doi: 10.4103/asl.ASL_87_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai M, Beall M, Ross MG. Developmental origins of obesity: programmed adipogenesis. Curr Diab Rep. 2013;13:27–33. doi: 10.1007/s11892-012-0344-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman JA, Lisanti MP, Scherer PE. Specific inhibitors of p38 mitogen-activated protein kinase block 3T3-L1 adipogenesis. J Biol Chem. 1998;273:32111–32120. doi: 10.1074/jbc.273.48.32111. [DOI] [PubMed] [Google Scholar]
- Fabricant DS, Farnsworth NR. The value of plants used in traditional medicine for drug discovery. Environ Health Perspect. 2001;109:69–75. doi: 10.1289/ehp.01109s169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. [DOI] [PubMed] [Google Scholar]
- Ghaben AL, Scherer PE. Adipogenesis and metabolic health. Nat Rev Mol Cell Biol. 2019;20:242–258. doi: 10.1038/s41580-018-0093-z. [DOI] [PubMed] [Google Scholar]
- Grundy SM. Obesity, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab. 2004;89:2595–2600. doi: 10.1210/jc.2004-0372. [DOI] [PubMed] [Google Scholar]
- Hirano A, Goto M, Mitsui T, Hashimoto-Hachiya A, Tsuji G, Furue M. Antioxidant Artemisia princeps extract enhances the expression of filaggrin and loricrin via the AHR/OVOL1 pathway. Int J Mol Sci. 2017;18:E1948. doi: 10.3390/ijms18091948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Chan E, Wang SX, Li B. Activation of p38 mitogen-activated protein kinase is required for osteoblast differentiation. Endocrinology. 2003;144:2068–2074. doi: 10.1210/en.2002-220863. [DOI] [PubMed] [Google Scholar]
- Hwang DI, Won KJ, Kim DY, Yoon SW, Park JH, Kim B, et al. Anti-adipocyte differentiation activity and chemical composition of essential oil from Artemisia annua. Nat Prod Commun. 2016;11:539–542. [PubMed] [Google Scholar]
- Jang E, Kim BJ, Lee KT, Inn KS, Lee JH. A survey of therapeutic effects of Artemisia capillaris in liver diseases. Evid Based Complement Alternat Med. 2015;2015 doi: 10.1155/2015/728137. 728137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy A, Martinez K, Schmidt S, Mandrup S, LaPoint K, McIntosh M. Antiobesity mechanisms of action of conjugated linoleic acid. J Nutr Biochem. 2010;21:171–179. doi: 10.1016/j.jnutbio.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon MS, Kim JA, Oh JH, Karadeniz F, Lee JI, Seo Y, et al. Antiadipogenic activity of solvent-partitioned fractions from Limonium tetragonum in 3T3-L1 preadipocytes. J Life Sci. 2019;29:60–68. [Google Scholar]
- Lim DW, Kim YT, Jang YJ, Kim YE, Han D. Anti-obesity effect of Artemisia capillaris extracts in high-fat diet-induced obese rats. Molecules. 2013;18:9241–9252. doi: 10.3390/molecules18089241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv JL, Li ZZ, Zhang LB. Two new flavonoids from Artemisia argyi with their anticoagulation activities. Nat Prod Res. 2018;32:632–639. doi: 10.1080/14786419.2017.1332603. [DOI] [PubMed] [Google Scholar]
- Oh JH, Karadeniz F, Seo Y, Kong CS. Artemisia scoparia inhibits adipogenesis in 3T3-L1 pre-adipocytes by downregulating the MAPK pathway. J Life Sci. 2018;28:999–1006. [Google Scholar]
- Payne VA, Au WS, Lowe CE, Rahman SM, Friedman JE, O’Rahilly S, et al. C/EBP transcription factors regulate SREBP1c gene expression during adipogenesis. Biochem J. 2009;425:215–223. doi: 10.1042/BJ20091112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi R, Liu H, Wang Q, Wang J, Yang F, Long D, et al. Expressions and regulatory effects of P38/ERK/JNK MAPKs in the adipogenic trans-differentiation of C2C12 myoblasts. Cell Physiol Biochem. 2017;44:2467–2475. doi: 10.1159/000486169. [DOI] [PubMed] [Google Scholar]
- Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–853. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergent T, Vanderstraeten J, Winand J, Beguin P, Schneid YJ. Phenolic compounds and plant extracts as potential natural antiobesity substances. Food Chem. 2012;135:68–73. doi: 10.1016/j.foodchem.2012.04.074. [DOI] [Google Scholar]
- Siddiqui MJ, Kamarudin MFB, Mohammed Al-Shami AK, Mat So’ad SZ, Jamshed SQ. Moxibustion (Artemisia plant at acupuncture point) as alternative therapy in hypertension: a promising approach. J Pharm Bioallied Sci. 2017;9:279–281. doi: 10.4103/jpbs.JPBS_24_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun NN, Wu TY, Chau CF. Natural dietary and herbal products in anti-obesity treatment. Molecules. 2016;21:E1351. doi: 10.3390/molecules21101351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang EJ, Wu B, Zuk P. MAPK signaling has stage-dependent osteogenic effects on human adipose-derived stem cells in vitro. Connect Tissue Res. 2018;59:129–146. doi: 10.1080/03008207.2017.1313248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung AWK, Heinrich M, Atanasov AG. Ethnopharmacology–a bibliometric analysis of a field of research meandering between medicine and food science? Front Pharmacol. 2018;9:215. doi: 10.3389/fphar.2018.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun C, Jung Y, Chun W, Yang B, Ryu J, Lim C, et al. Anti-inflammatory effects of Artemisia leaf extract in mice with contact dermatitis in vitro and in vivo. Mediators Inflamm. 2016;2016 doi: 10.1155/2016/8027537. 8027537. [DOI] [PMC free article] [PubMed] [Google Scholar]