Abstract
There has been very little reported on ginsenoside composition and antioxidant activity of hydroponic-cultured ginseng roots (HCR), leaves (HCL), and stems (HCS). We profiled 6 ginsenoside compounds in HCR, HCL, and HCS using high-performance liquid chromatography. Antioxidative activity of HCR, HCL, and HCS were evaluated using total phenolic content (TPC) and total flavonoid content (TFC), 2,2-diphenyl-1-picrylhydrazyl (DPPH) free-radical-scavenging activity assays, and ferric reducing antioxidant power (FRAP) assays. Total ginsenoside contents of HCL and HCS were significantly higher than that of HCR (P<0.05). Rb1 was detected in HCR (23.02 mg/g) but was detected at very low levels in HCL and HCS (2.07~7.30 mg/g). Rg1 was the most abundant ingredient in HCL, followed by Rd; this was different than for HCR and HCS. The TPC and TFC ranged from 52.82~155.31 mg gallic acid equivalent/100 g and 194.71~256.52 mg quercetin equivalent/100 g, respectively, of which HCL contained the highest levels. Moreover, HCL was the most effective in both DPPH and FRAP activities. In this study, we also evaluated the inhibitory effect of HCR, HCL, and HCS on the activities of mushroom tyrosinase through whitening activity test. The inhibitory effect of HCL on tyrosinase activity was higher than that of HCR and HCS. This study provides information about ginsenoside contents and the antioxidative activity of hydroponic-cultured ginseng, and suggests that the whole ginseng plant (including roots, leaves, and stems) may be a beneficial functional vegetables.
Keywords: ginseng parts, antioxidant activity, Panax ginseng, ginsenoside, HPLC
INTRODUCTION
Korean ginseng (Panax ginseng Meyer) is a product of Korea that has been widely used in Asian home remedies and medicine for thousands of years (Kim et al., 2004a). The primary active ingredient in ginseng is saponin ginsenoside. To date, approximately 70 components have been isolated from ginseng (Nah, 1997). Non-saponins, such as polyacetylene, acidic polysaccharides, and polyphenols, have also been identified (Choi et al., 2006). The main bioactive effects of ginseng are anti-cancer activity, cancer cell growth inhibition, hypotensive activity, promotion of cranial nerve cell protection and learning activity, and anti-thrombosis activity. (Van Kampen et al., 2003; Keum et al., 2000; Kim et al., 2003). In addition to saponin, studies have reported antioxidant and antihypertensive effects of polyacetylene and free amino acids. Specifically, various physiological effects, such as antioxidant activities, hypertension inhibition, anti-cancer activities, and whitening activities have been reported in phenolic compounds (Kim et al., 2007; Kong et al., 2009; Nam, 2002; Park et al., 2003).
However, since the generation of ginseng is 4 to 6 years and long-term cultivation is performed, crop protection agents are used to address pest problems. Moreover, the introduction of cheaper ginseng in China and other countries has weakened the competitiveness of domestic ginseng farmers.
Studies have shown that ginseng leaves contain 4- to 5-fold higher contents than ginseng roots (Shi et al., 2007). Ginseng leaves has been identified as excellent new resources. Ginseng leaves contain 30 to 40% of the ginsenosides Rb1, Rb2, Rd, Re, and Rg1 (although amounts vary depending on seasonal variations, geographical differences, and length of cultivation period); specifically, the contents of Re, Rd, Rb1, and F1 ginseng in leaves are relatively higher than that of the roots (Jackson et al., 2003; Xie et al., 2004).
Recently, short-term cultivation of ginseng in hydroponic soil without the use a crop protection agents has attracted attention of consumers under the name ‘sprout ginseng’. Unlike in conventional cultivation, hydroponic cultivation can control the supply of difficult to manage inorganic nutrients necessary for growth. Hydroponic cultivation also has advantages such as allowing easy control of the growth environment (including temperature, light intensity, and water management) and leading to reduction of disease. In particular, hydroponic-cultured ginseng is an excellent functional vegetable since humans can ingest all parts of the plants, including roots, leaves, and stems. Studies on hydroponic-cultured ginseng has shown that root length, diameter, and leaf growth are greater in lower-temperature cultivation than in higher-temperature cultivation. Moreover, hydroponic-cultured ginseng leaves contain Rh1, which is barely detected in multi-year-old ginseng, at levels of 0.15~0.25% (Kim et al., 2010; Lee et al., 2011).
However, hydroponic-cultured ginseng has a low productivity and is difficult to secure market competitiveness compared with conventionally cultivated plants. To increase the added value of hydroponic-cultivated ginseng, it is necessary that it is developed for use in medicines and as a functional food. However, studies on hydroponic-cultured ginseng are lacking. Therefore, we investigated the ginsenoside content and composition of the leaves, stems, and roots of hydroponic-cultured ginseng (HCL, HCS, and HCR, respectively) in the Korea, and confirmed its functional bioactivity (antioxidant and whitening).
MATERIALS AND METHODS
Materials
Nineteen kinds of fresh ginseng cultured using hydroponics were obtained in March, May, and August 2018 at 4 locations in Korea: Gyeonggido (N37°27′08″/E127°57′ 12″), Chungcheongdo (N36°17′51″/E127°57′12″), Jeollado (N35°26′38″/E126°56′44″), and Gyeongsangdo (N35°56′47″/E128°56′54″). Four kinds of 4 to 6 years old ginseng root were obtained at 3 locations in Korea: Pocheon (N37°53′43″/E127°12′03″), Punggi (N36°52′45″/E128°31′26″), and Gunsan (N36°07′56″/E127°29′11″). The cultivation method of hydroponic-cultured ginseng used in this study is as follows. Hydroponic-cultured 1 to 2 year old ginseng (about 25 cm) were harvested about 30 days after planting, except during the winter season. The ginseng were cultivated in artificial soil mixed with granite soil, known locally as ‘Masato’, rice hull, and pearlite. The room temperature was set at 20~23°C in the early stage and 23~25°C after leaves developed, depending on the stage of growth. Illuminance was controlled using a sunscreen. Some farms supplemented plants with nutrients at certain times. Ginseng roots, leaves, and stems were rinsed with tap water, dried using a dryer (Lequip, Seoul, Korea), and stored at −20°C. The standard ginsenosides Rg1, Rb1, Re, Rd, and Rg3 were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). The ginsenoside F1 was purchased from ALB Technology (Kowloon, Hong Kong, China). All chemicals were of reagent grade.
Crude saponin
The crude saponin fraction was prepared by the method of Shin et al. (2001) with some modifications. A 5.0 g powdered dried ginseng sample was extracted with 50 mL of water-saturated butanol for 1 h at 70~80°C under reflux. After cooling, the extracts were washed with 10 mL of water-saturated butanol and 10 mL of water, and was shaken vigorously. The water fraction was then removed. The water-saturated butanol fraction was evaporated, and 50 mL of ethyl ether was added. The ethyl ether fraction was then removed and the butanol fraction was used to quantify the crude saponin fraction.
Analysis of ginsenosides
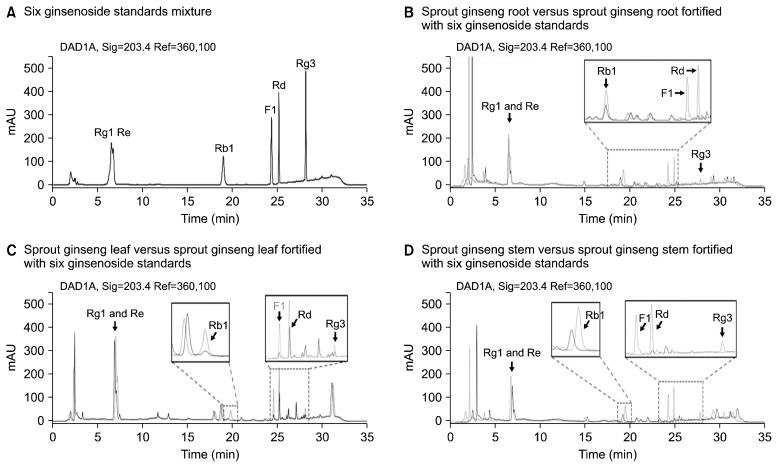
Ginsenosides analysis was performed following the method of Kim et al. (2008) with some modifications. Powdered dried ginseng samples (0.2 g) was accurately weighed, and 2 mL of 70% methanol was added to the samples in a 50 mL conical flask. The flask was then placed in an ultrasonic extractor for 30 min at 50°C. After cooling to room temperature, the methanol extract was centrifuged at 15,000 rpm for 15 min. The solid phase extraction (SPE) cartridge was conditioned with 3.0 mL methanol and 3 mL water before introduction of the sample solution. A 1.0 mL aliquot of the sample solution was introduced into the SPE cartridge at a flow of 0.1 mL/min, and was washed with 10 mL water. Any retained analytes were eluted with 2.0 mL methanol at a flow rate of 0.08 mL/min, and the solution was filtered through a 0.45 μm membrane filter prior to analysis. The high-performance liquid chromatograph used was Agilent 1260 series high-performance liquid chromatography system (Agilent Technologies, Inc., Wilmington, DE, USA) equipped with an ultraviolet (UV) detector. The analytical column used was Halo® RP-Amide (4.6×150 mm, 2.7 μm; Advanced Materials Technology, Wilmington, DE, USA), and samples were detection at 203 nm. The mobile phase was a gradient of acetonitrile (A) and water (B): 27% A (0~6 min), 28% A (6~10 min), 34% A (10~30 min), 80% A (30~33 min), and 27% A (33~35 min). The flow of the mobile phase was 0.8 mL/min and the column temperature was 50°C. An external calibration curve method was used for quantitation of the 6 ginsenosides present in the HCR, HCL, and HCS. The 6 ginsenoside standards were prepared in methanol as a 500 ppm stock solution. Ginsenosides were identified by comparing the retention times of the authentic ginsenoside standards with the peaks observed in the sample aliquots (Fig. 1). Furthermore, each ginsenoside standard was also added to the sample aliquots (sample aliquot+ginsenoside standard is indicated by the red solid line in Fig. 1B~D) to confirm assignment of correct peaks in the ginseng samples. All the calibration curves showed good linearity (r>0.999) across the concentration ranges investigated in this study. The limit of detection (LOD) and limit of quantitation (LOQ) of the 6 ginsenosides were determined using calibration curve as follows:
Fig. 1.
Representative high-performance liquid chromatography (HPLC) chromatograms of a mixture of the 6 ginsenoside standard compounds (A) and hydroponic-cultured ginseng samples (B, C, and D). The black solid line indicates the ginseng sample and the red solid line indicates the same ginseng sample fortified with some ginsenoside standards.
where SD is the standard deviation of a response and S is the slope of the calibration curve. In this study, the LOD ranged from 0.852 μL/mL to 2.268 μL/mL and the LOQ ranged from 0.202 μL/mL to 6.872 μL/mL.
Total phenolic content (TPC) and total flavonoid content (TFC)
Dried ginseng samples (0.2 g) were accurately weighed in extraction tubes; 5.0 mL of 70% methanol was then added and the samples were mixed and heated at 70°C for 10 min. After cooling to room temperature, the extracts was centrifuged at 15,000 rpm for 10 min, and the supernatants were decanted into conical flasks. The extraction step was repeated 3 times. The extracts were pooled, and the volume was adjusted to 10 mL with cold 70% methanol. A 1.0 mL aliquot of the extract was diluted to 5.0 mL with water, and the TPC was calculated by the method of ISO 1450201 (International Organization for Standardization, 2006) with some modifications. Aliquots (1.0 mL) of the diluted sample extracts were transferred to separate tubes containing 5.0 mL of a 1/10 dilution of Folin-Ciocalteu phenol reagents (Sigma-Aldrich Co., St. Louis, MO, USA) in water. Sodium carbonate solution (4.0 mL, 7.5%, w/v) was then added and the tubes were incubated at room temperature for 1 h. The TPC was measured using an UV-visible spectrophotometer (Analytik Jena AG, Jena, Germany) at 725 nm, and the absorbance calculated relative to water. All values were expressed as mg gallic acid equivalent (GAE) per 100 g dry matter of the ginseng samples. Calculation of TFCs carried out following a previously described method with some modifications (Woisky and Salatino, 1998). Aliquots (1.0 mL) of diluted sample extract were added to 4.0 mL of water and incubated 5 min. Sodium nitrate solution (0.3 mL, 5.0%, w/v) was then added, and the aliquots were incubated for a further 6 min. Finally, 2 mL of 1.0 M sodium hydroxide solution and 2.4 mL of water was added. The absorbance was measured at 415 nm. All values were expressed as mg quercetin equivalent (QE) per 100 g dry ginseng sample.
1,1-Diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity
Dried ginseng samples (1.0 g) were added to 10 mL of acetonitrile and 2.0 mL of 0.1 N hydrochloride, and the mixture was extracted using a shaking water bath at 200 rpm for 2 h at room temperature. The crude ginseng extract was filtered through No. 42 Whatman filter paper (GE Healthcare, Maidstone, UK), and the filtrate was concentrated in vacuo at <35°C using a vacuum evaporator (Heidolph Instruments GmbH & Co. KG, Schwabach, Germany). The residue was reconstituted with 5 mL of 80% methanol and was filtered through a 0.45 μm syringe filter. The filtrate was used for in, DPPH (Sigma-Aldrich Co.) radical scavenging activity assay and ferric reducing antioxidant power (FRAP) assay, and then for tyrosinase inhibitory activity assay.
DPPH radical scavenging activity was performed following the method of Blois (1958) with some modifications. A 0.4 mM solution of DPPH was prepared in methanol, and 280 μL of this solution was mixed with a 200 μL aliquot of each ginseng sample. The mixtures were placed in a dark room for 10 min and the absorbance was measured using a spectrophotometer at 517 nm. The DPPH radical scavenging activity was calculated as an inhibition percentage based on the following equation:
where A0 is the absorbance of the control and A1 is the absorbance of the ginseng sample aliquot.
FRAP assay
FRAP assay was performed following the method of Oyaizu (1986) with some modification. A stock solution was prepared by dissolving 10 mM 2,4,6-tris (2-pyridyl)-S-triazine (TPTZ) (Sigma-Aldrich Co.) solution with 20 mM ferric chloride hexahydrate solution in 0.3 M sodium acetate buffer (pH 3.6) and 40 mM hydrochloride. A fresh working solution (25 mL acetate buffer, 2.5 mL TPTZ solution, and 2.5 mL ferric chloride hexahydrate solution) was prepared at 37°C before the experiment. A 20 μL aliquot of the sample extract was mixed with 100 μL of FRAP solution and left in a dark place for 30 min, and the absorbance was measured at 593 nm.
Mushroon tyrosinase activity
In vitro mushroom tyrosinase assays were performed with L-tyrosine and 3,4-dihydroxy-L-phenylalanine (L-DOPA) as substrates for tyrosinase activity. The inhibitory activities of the sample extracts against mushroom tyrosinase catalyzed oxidation of L-tyrosine were determined following the method of Chang et al. (2007) with some modifications. A 10 μL aliquot of the sample extracts were mixed with 180 μL of tyrosinase buffer [1 mM L-tyrosine : 50 mM potassium phosphate buffer (pH 6.5): water=10:10:9]. Mushroom tyrosinase (20 μL, 1,000 units/g) was added to the mixtures and the samples were incubated at 25°C for 30 min. The absorbance was then measured at 490 nm using a microplate reader (Molecular Devices, San Jose, CA, USA). The inhibitory activities on mushroom tyrosinase in L-DOPA oxidation were determined following the method of Masamoto et al. (2003) with some modifications. Sample extract aliquots (20 μL) were mixed with 100 μL of phosphate buffer. Mushroom tyrosinase (20 μL, 1,000 units/g) was then added and mixtures were incubated at 37°C for 5 min. Mixtures were then added to 40 μL L-DOPA (4 mM) in 0.1 M phosphate buffer, incubated at 37°C for 10 min, and the absorbance was measured at 475 nm in a microplate reader. Arbutin was used as a positive control was used for assay. Inhibition of tyrosine and L-DOPA were calculated as follow:
where A0 is the absorbance of the control and A1 is the absorbance of the ginseng sample aliquot.
Statistical analysis
Results are reported as mean±standard deviation (SD). The significance of differences between treatment means were determined using a one-way analysis of variance with SPSS version 12 (SPSS Inc., Chicago, IL, USA) with a significance level of P<0.05.
RESULTS AND DISCUSSION
Total ginsenoside and total crude saponin contents of HCR, HCL, and HCS
The total ginsenoside and total crude saponin contents of HCR, HCL, and HCS were presented in Table 1. The total ginsenoside contents of HCR, HCL, and HCS were 34.01~81.72 mg/g, 76.88~131.60 mg/g, and 55.81~99.23 mg/g, respectively (data not shown). Mean ginsenoside contents were highest in HCL, then HCS and HCR (1.4- and 1.2-fold higher in HCL and HCS than in HCR, respectively). The total ginsenoside content of the older ginseng roots (aged 4 to 6 years) was 80.04 mg/g, 107% of the HCR content, and 90% of the HCS content. Kim et al. (2010) examined the contents of 9 ginsenosides as follows: roots 1.1~1.5%, leaves 13.3~16.1%, and stems 1.2~1.4%. The results differed from this study since a different type of ginsenoside was used for analysis. The content of crude saponin was highest in the order of HCR, HCL, and HCS. Crude saponin contents in HCL were 2.5-fold higher than in HCR; these results were similar to total ginsenoside contents. However, unlike with total ginsenoside contents, crude saponin contents of older ginseng roots were higher than those of HCS. This suggests that the unique ginsenosides reported in this study, such as Rg1 and Rg2 (but not Rg6), were present in ginseng root aged 4 to 6 years old.
Table 1.
Total ginsenoside, crude saponin, total phenlic content (TPC), and total flavonoid content (TFC) in hydroponic-cultured ginseng and ginseng aged four to six years
| Part | Total ginsenosides (mg/g, dry weight base) | Crude saponin (mg/g, dry weight base) | TPC (mg GAE/100 g, dry weight base) | TFC (mg QE/100 g, dry weight base) |
|---|---|---|---|---|
| Hydroponic-cultured ginseng | ||||
| Roots | 74.78±18.27c | 87.26±2.58c | 52.82±38.48c | 194.71±40.91bc |
| Leaves | 107.54±25.78a | 181.52±1.57a | 155.31±45.71a | 256.52±17.27a |
| Stems | 89.20±18.48ac | 91.34±2.25b | 109.82±17.21b | 210.60±25.25b |
| Four to six years old ginseng | ||||
| Roots | 80.04±15.28bc | 99.31±3.27a | 117.21±28.40ab | 222.60±33.21ab |
Results expressed as a mean±SD (n=3).
Values with different letters (a–c) differed statistically by plant part.
GAE, gallic acid equivalent; QE, quercetin equivalent.
Individual ginsenoside compositions of HCR, HCL, and HCS
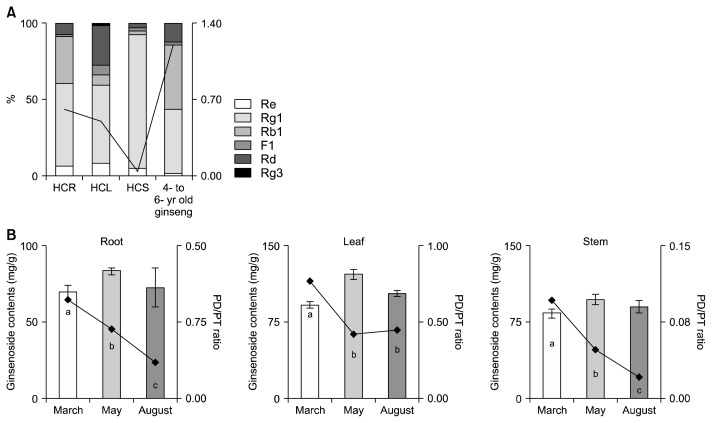
The composition and contents of the individual ginsenosides in the hydroponic-cultured ginseng were further examined (Fig. 2A and Table 2). The composition ratios of HCR were Rg1> Rb1> Rd> Re> F1; the order of these ginsenoside composition ratios were similar to that of the older ginseng root. In HCR and ginseng root aged 4 to 6 years old, Rg1 and Rb1 accounted for 85% and 74% of total ginsenoside, respectively, and Rb1 (HCR, 23.02 mg/g; 4 to 6 years old ginseng root, 33.84 mg/g) was significantly higher than in HCL (7.30 mg/g) and in HCS (2.07 mg/g) (Table 2). Rg1 has been reported to effect phytoestrogen activity, and exert neuroprotective and lung protective effects, and Rb1 has been reported to inhibit the central nervous system, induce sedation and analgesia, impact antipyretic action, and improve liver function and recovery from fatigue (Dong and Kiyama, 2009). The composition ratios of HCL and HCS were Rg1> Rd> Rd> F1> Rg3 and Rg1> Re> Rb1> F1> Rd, respectively. The total ginsenoside content of HCS was similar to that of ginseng root aged 4 to 6 years old, however Rg1 accounted for 88% in HCS, and the composition ratios differed greatly. In HCR and HCS, the ratios of Rd to total ginsenosides were 7.4% and 2.3%, respectively, compared with 26% in HCL (Fig. 2A). Rd has been reported to promote adrenocortical hormone secretion, prevent cancer, and protect the brain against ischemic nerve injury. Unlike HCR and HCS, F1 accounted for 6.5% of total ginsenosides in HCL; F1 has been reported to have whitening effects, such as through inhibiting melanin production and inhibiting UV coagulation (Chang et al., 2008; Ye et al., 2009). Ginseng ginsenosides can be classed as either protopanaxadiol (PD) or protopanaxatriol (PT) according to the structures of the aglycons. The total ginsenoside contents of ginseng is important, but individual ginsenoside composition and PD and PT ratios are also important factors for efficacy (Jin et al., 1999). The PD/PT ratios of HCR, HCL, and HCS were 0.62, 0.51, and 0.05, respectively (Fig. 2B). This is because the sums of Rg1 and Re from PT types were 60.7%, 59.8%, and 93.1%, respectively, compared with only 44.1% in ginseng roots aged 4 to 6 years. Therefore, the PD/PT ratios are lower than those of the older roots because Rg1 and Re are relatively low, and Rb1 is relatively high.
Fig. 2.
Ginsenoside composition of hydroponic-cultured ginseng root (HCR), leaf (HCL), stem (HCS), and 4- to 6-year old ginseng total ginsenosides content (A) and, protopanaxadiol (PD)/protopanaxatriol (PT) ratio of HCR, HCL, and HCS with cultivation month (B). The results are expressed as mean±SD (n=3). Values with different letters (a–c) are statistically different depending on the ginseng part and cultivation month (P <0.05).
Table 2.
Profiling of 6 ginsenosides in hydroponic-cultured ginseng and ginseng aged 4 to 6 years
| (unit: mg/g, dry weight base) | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Part | Ginsenoside | |||||
|
| ||||||
| Re | Rg1 | Rb1 | F1 | Rd | Rg3 | |
| Hydroponic-cultured ginseng | ||||||
| Roots | 5.23±1.23b | 40.19±1.41b | 23.02±1.12a | 0.80±0.15c | 5.54±0.12b | N.D. |
| Leaves | 9.40±2.34a | 54.89±2.23b | 7.30±1.29b | 6.97±1.68a | 27.73±4.69a | 1.25±0.89 |
| Stems | 4.93±1.45b | 78.11±5.57a | 2.07±1.56c | 2.06±0.62b | 2.04±0.69bc | N.D. |
| Four to six years old ginseng | ||||||
| Roots | 1.87±0.01c | 33.45±6.78c | 33.84±6.78a | 1.21±1.12bc | 9.68±1.57b | N.D. |
Results expressed as a mean±SD (n=3).
Values with different letters (a–c) differed statistically by plant part.
N.D., not detected.
The total ginsenoside contents of HCS were similar to that of ginseng roots aged 4 to 6 years (Table 1), but the PD/PT ratio was 20-fold lower than that of the 4 to 6 years old roots (Fig. 2A). Rg1 and Re accounted for 93.1% of total ginsenosides in HCS, but the Rb1 content was low (Fig. 2A). However, in HCR, Rg1 and Re accounted for 61.8% of total ginsenosides, which is a lower proportion than in HCS, and the 6 ginsenosides were distributed evenly, unlike in HCL and HCS. In HCL, Rg1 and Rd accounted for 59.8% of total ginsenosides, which is similar to in HCR.
In conclusion, the ginsenoside composition of HCR is similar to that ginseng roots aged 4 to 6 years, but HCL and HCS have high contents of specific components. Accordingly, the PD/PT ratios for HCR are lower than for ginseng roots aged 4 to 6 years, and the composition ratios are different. Moreover, since the unit weight of HCR is lower than that of 4 to 6 years old ginseng root, the quality and purpose will also differ. However, HCL contained F1, which is virtually undetectable in other parts of the plant, and larger amounts of Rd, which is known to be converted to Rg3 and Rh2, amongst others, at high temperatures. Therefore, Rg3 and Rh2, which have anticancer activities, can be indirectly extracted (Wang et al., 2007).
Ginsenosides of hydroponic-cultured ginseng by cultivation period and area
The total ginsenosides contents of hydroponic-cultured ginseng varied by cultivation period, according to the order of May, August, and March (Fig. 2B). The total ginsenoside content of HCR was 83.71 mg/g in May, which is 1.2-fold higher than in March (70.19 mg/g). Due to the individual ginsenoside content of HCR, Rg1 and F1 contents were 1.6- and 2.0-fold higher in August compared with March, and Rb1 and Rd were 2.1- and 1.2-fold higher in March compared with August, respectively (data not shown).
The total ginsenoside content of HCL was 122.44 mg/g in May, which is 1.3-fold higher than in March (92.14 mg/g), whereas Rg1 and Re were 1.3- and 1.3-fold higher in May than in March, and F1 was 2.0-fold higher in May than in August (data not shown). The total ginsenoside content in HCS was 97.52 mg/g in May, which is 1.2-fold higher than in March (83.74 mg/g). Moreover, in HCS the Re and F1 contents were 2.4- and 4.8-fold higher in August than in March, and Rb1 and Rd were 3.3- and 5.6-fold higher in March than in August, respectively (data not shown). For this reason, PD/PT ratios tended to decrease from March to August. Although contents differed slightly in different parts of the plants, Rg1 and Re (both PT ginsenoside) were generally higher in August, and Rb1 and Rd (both PD ginsenosides) were generally higher in March. Therefore, we concluded that biosynthesis of PD ginsenosides is promoted by relatively low temperatures.
TPC and TFC of HCR, HCL, and HCS
The TPC and TFC in HCR, HCL, and HCS are presented in Table 1. TPC of ginseng was highest in the order of HCL, HCS, and HCR. TPCs in HCL and HCS were 3.8-and 2.1-fold higher, respectively, than in HCR. The TPC of HCR was 45%, which was lower than that of ginseng roots aged 4 to 6 years, and the TPC of HCL was 133%, which is higher than that of ginseng roots aged 4 to 6 years. The TPC of hydroponic-cultured ginseng has been reported to be higher than that of ginseng roots aged 4 to 6 years because the content of phenolic acids in the fruit and root of ginseng increases by 20~30% as the age of the root increases (Chung et al., 2016).
The TFC of ginseng was highest in the order of HCL, HCS, and HCR. The content in HCL was 1.3-fold higher than in HCR. Comparative TFC were relatively similar to the total phenolic content, however the root and stem contents did not significantly differ. From identifying 23 phenolic compounds in ginseng, ginseng leaves were found to have significantly higher phenolic contents than ginseng roots. Therefore, HCL contained higher amounts of phenolic compounds and HCR contained higher amounts of flavonoid compounds. Although the TFCs of hydroponic-cultured ginseng were 87% (roots) and 94% (stems) of that of older ginseng roots, the TFC of the leaves were significantly higher (115%).
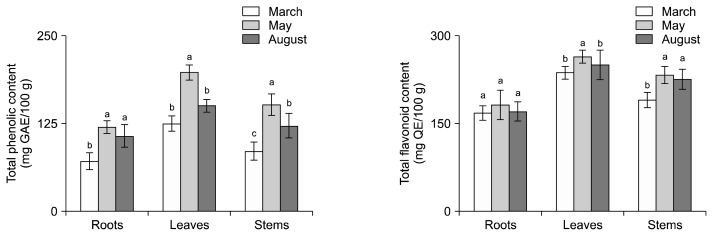
TPC and TFC of HCR, HCL, and HCS by cultivation period
TPC and TFC of hydroponic-cultured ginseng by cultivation period were in the order of May, August, and March for all parts (Fig. 3). The TPC were higher in May than in March (HCR, 1.7-fold; HCL, 1.6-fold; HCS, 1.8-fold). Similarly to the TPC, the TFC was the highest in May. TPC and TFC of hydroponic-cultured ginseng showed the same tendency as the total ginsenoside contents (Fig. 2A), which is related to the cultivation characteristics of ginseng. The 2-years-old ginseng are reported to display the most robust growth at 14~20°C and 60~70% humidity (Lee et al., 2012). The strongest growth periods of hydroponic-cultured ginseng used in this study ranged from 8 to 9 weeks in March, 4 to 5 weeks in May, and 5 to 6 weeks in August. Therefore, the growth of ginseng used in this study was the best in May. In 2018, the mean temperature in May was 12~23°C and the relative humidity was 70%. These conditions were the most suitable for ginseng growth.
Fig. 3.
Total phenolic content (TPC) and total flavonoid content (TFC) of hydroponic-cultured ginseng root, leaf, and stem with cultivation month. The results are expressed as mean±SD (n=3). Values with different letters (a–c) among same parts are statistically different depending on cultivation month (P <0.05). GAE, gallic acid equivalent; QE, quercetin equivalent.
DPPH free radical scavenging activity and FRAP activity in HCR, HCL, and HCS
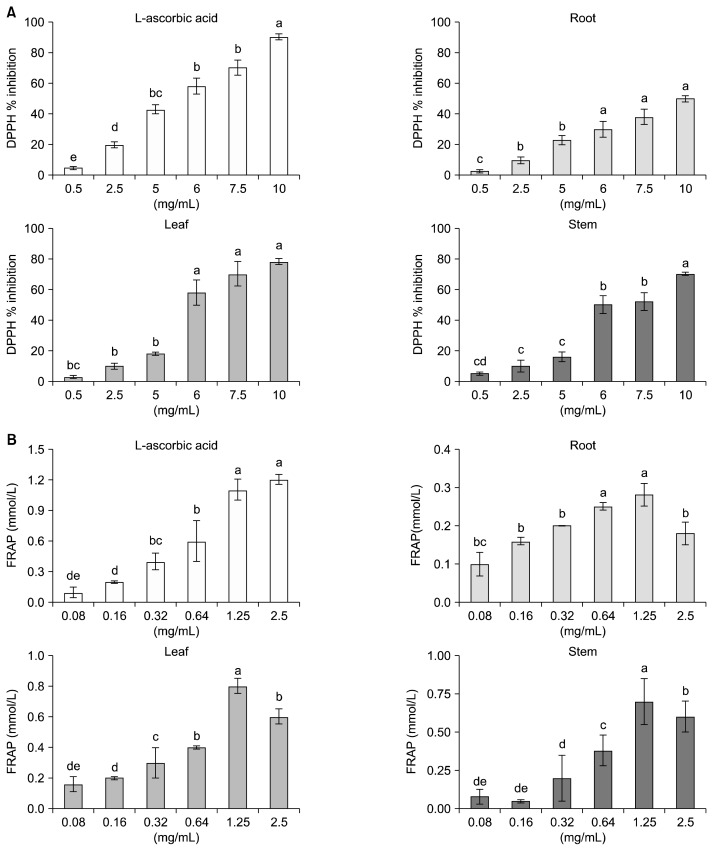
DPPH free radical scavenging activities in HCR, HCL, and HCS are presented in Fig. 4A. DPPH free radical scavenging activity in hydroponic-cultured ginseng correlated with increasing concentrations. DPPH free radical scavenging activity in hydroponic-cultured ginseng was 58.2%, 88.1%, and 78.1% in HCR, HCL, and HCS, respectively; at 10 mg/mL, it was highest in HCL. At 6 mg/mL, DPPH free radical scavenging activity in the HCL and HCS were 60.3% and 50.1%, respectively; however, DPPH free radical scavenging activity in HCR was less than 40%. Compared with the DPPH free radical scavenging activity in L-ascorbic acid (positive control), at a concentration of 10 mg/mL the activity in HCR was 60%, HCL was 90%, and HCS was 80%.
Fig. 4.
2,2-Diphenyl-1-picylhydrazyl (DPPH) free-radical-scavenging activity of hydroponic-cultured ginseng root and leaf (A), and ferric reducing antioxidant power (FRAP) of hydroponic-cultured ginseng root and leaf (B). The results are expressed as mean±SD (n=3). Values with different letters (a–e) are statistically different depending on concentration (P <0.05).
FRAP activity levels in HCR, HCL, and HCS are presented in Fig. 4B. Compared with FRAP activity in L-ascorbic acid, at a concentration of 1.25 mg/mL HCR was 27%, HCL was 73%, and HCS was 68%. These results are in agreement with those obtained for the antioxidant activity determined through DPPH free radical scavenging activities. A strong positive relationship between total phenols and antioxidant activity appears to be the trend in many plant species (Kim et al., 2004b). It is known that the antioxidant potential of phenolics depends on both the quantity and nature of phenolics, such as the molecular weight, the number of aromatic rings, and the nature of hydroxyl group substitution. Therefore, the HCL examined in this study demonstrated good reducing capacity, thereby acting as an efficient reductone.
The correlations between antioxidant activity (DPPH free radical scavenging and FRAP activity) and the TPC of HCR, HCL, and HCS were strong (r=0.9123~0.9987). Further, DPPH activity was most strongly correlated with TPC in HCL (r=0.9987). However, FRAP activity was less strongly correlated with DPPH activity (r=0.9123~0.9523). The correlations between antioxidant activity and TFCs of HCR, HCL, and HCS were similar to those of TPCs. This suggests that DPPH and FRAP are specific for phenolic compounds (Speisky et al., 2012).
Mushroom tyrosinase activity of HCR, HCL, and HCS
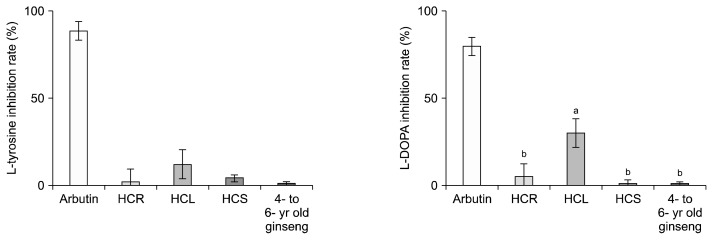
In this study, the highest concentration of the ginsenoside F1 was found in HCL (7.0%). Ginsenoside F1 is reported to have an excellent whitening effect in B16 melanoma cells, including a protective effect against HaCaT cells inhibiting melanin formation, UV-B induced cell damage, and apoptosis (Cho et al., 2010). Therefore, in this study mushroom tyrosinase assays were measured by whitening activity tests. In vitro mushroom tyrosinase assays were performed with L-tyrosine and L-DOPA substrates for tyrosinase activity by whitening activity tests (Fig. 5). Compared with L-tyrosine inhibitory activity of arbutin, which is a known whitening agent, HCL showed higher activity (13.8%) than HCS, HCR, and ginseng roots aged 4 to 6 years, but this did not significantly differ. However, compared with L-DOPA inhibitory activity of arbutin, HCL showed significantly higher activity (30.4%) than HCR, HCS and ginseng root aged 4 to 6 years. These results suggest a weakness in HCL chelates with copper of the tyrosinase active site and inhibits progression of tyrosine to DOPA and from DOPA to quinone. Moreover, the process by which DOPA chrome is generated by autoxidation from DOPA quinone is relatively active.
Fig. 5.
Inhibitory effect on mushroom tyrosinase in hydroponic-cultured ginseng root (HCR), leaf (HCL), stem (HCS), and 4- to 6-year old ginseng. The results are expressed as mean±SD (n=3). Values with different letters (a–c) are statistically different depending on the ginseng part and cultivation month (P <0.05).
In conclusion, this study reports the profile of ginsenoside and the antioxidant activities of HCR, HCL, and HCS. The total ginsenoside contents of HCR, HCL, and HCS were 74.78~107.54 mg/g (dry weight basis) and the ginsenoside contents were found to be significantly higher in HCL and HCS than in HCR. Amongst the 6 ginsenoside studied, the relative composition of HCR was Rg1> Rb1> Rd> Re> F1, and the relative composition order of the ginsenosides was similar to that of ginseng roots aged 4 to 6 years. In HCR and ginseng root aged 4 to 6 years, Rg1 and Rb1 accounted for 85% and 74% of ginsenosides, respectively, and the Rb1 content was significantly higher than in HCL and HCS. The composition ratio of HCL and HCS were in the order of Rg1> Rd> Re> F1> Rg3 and Rg1> Re> Rb1> F1> Rd, respectively. The total ginsenoside content of HCS was similar to that of ginseng root aged 4 to 6 years, but the composition ratio differed greatly. Unlike HCR and HCS, F1 accounted for 6.5% of ginsenosides in HCL. Furthermore, the antioxidant activities in HCL was higher than other parts, and was significantly higher than in ginseng roots aged 4 to 6 years. In addition, DPPH activity was significantly correlated with TPCs of hydroponic-cultured ginseng. This study provides basic information about ginsenoside contents and antioxidant activities of hydroponic-cultured ginseng. This information is potentially useful to hydroponic-cultured ginseng growers and industries involved in the production of high-quality and nutritionally beneficial hydroponic-cultured ginseng products.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- Chang TL, Ding HY, Kao YW. Role of ginsenoside Rd in inhibiting 26S proteasome activity. J Agric Food Chem. 2008;56:12011–12015. doi: 10.1021/jf801427e. [DOI] [PubMed] [Google Scholar]
- Chang TS, Ding HY, Tai SSK, Wu CY. Mushroom tyrosinase inhibitory effects of isoflavones isolated from soygerm koji fermented with Aspergillus oryzae BCRC 32288. Food Chem. 2007;105:1430–1438. doi: 10.1016/j.foodchem.2007.05.019. [DOI] [Google Scholar]
- Cho K, Woo HJ, Lee IS, Lee JW, Cho YC, Lee IN, et al. Optimization of enzymatic pretreatment for the production of fermented ginseng using leaves, stems and roots of ginseng. J Ginseng Res. 2010;34:68–75. doi: 10.5142/JGR.2010.34.1.068. [DOI] [Google Scholar]
- Choi CS, Kim KI, Hong HD, Choi SY, Lee YC, Kim KT, et al. Phenolic acid composition and antioxidative activity of white ginseng (Panax ginseng, C. A. Meyer) J Ginseng Res. 2006;30:22–30. doi: 10.5142/JGR.2006.30.1.022. [DOI] [Google Scholar]
- Chung IM, Lim JJ, Ahn MS, Jeong HN, An TJ, Kim SH. Comparative phenolic compound profiles and antioxidative activity of the fruit, leaves, and roots of Korean ginseng (Panax ginseng Meyer) according to cultivation years. J Ginseng Res. 2016;40:68–75. doi: 10.1016/j.jgr.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong S, Kiyama R. Characterisation of oestrogenic activity of ginsenosides in MCF-7 cells using a customised DNA microarray. Food Chem. 2009;113:672–678. doi: 10.1016/j.foodchem.2008.07.100. [DOI] [Google Scholar]
- International Organization for Standardization. Determination of substances characteristic of green and black tea–Part 1: Content of total polyphenols in tea–colorimetric method using Folin-Ciocalteu reagent. Ref. No. ISO 1450-2-1:2005/Cor. 1:2006(E) 2006 [Google Scholar]
- Jackson CJC, Dini JP, Lavandier C, Faulkner H, Rupasinghe HPV, Proctor JTA. Ginsenoside content of North American ginseng (Panax quinquefolius L. Araliaceae) in relation to plant development and growing locations. J Ginseng Res. 2003;27:135–140. doi: 10.5142/JGR.2003.27.3.135. [DOI] [Google Scholar]
- Jin SH, Park JK, Nam KY, Park SN, Jung NP. Korean red ginseng saponins with low ratios of protopanaxadiol and protopanaxatriol saponin improve scopolamine-induced learning disability and spatial working memory in mice. J Ethnopharmacol. 1999;66:123–129. doi: 10.1016/S0378-8741(98)00190-1. [DOI] [PubMed] [Google Scholar]
- Keum YS, Park KK, Lee JM, Chun KS, Park JH, Lee SK, et al. Antioxidant and anti-tumor promoting activities of the methanol extract of heat-processed ginseng. Cancer Lett. 2000;150:41–48. doi: 10.1016/S0304-3835(99)00369-9. [DOI] [PubMed] [Google Scholar]
- Kim DH, Moon YS, Jung JS, Min SK, Son BK, Suh HW, et al. Effects of ginseng saponin administered intraperitoneally on the hypothalamo-pituitary-adrenal axis in mice. Neurosci Lett. 2003;343:62–66. doi: 10.1016/S0304-3940(03)00300-8. [DOI] [PubMed] [Google Scholar]
- Kim EY, Baik IH, Kim JH, Kim SR, Rhyu MR. Screening of the antioxidant activity of some medicinal plants. Korean J Food Sci Technol. 2004a;36:333–338. [Google Scholar]
- Kim GS, Hyun DY, Kim YO, Lee SE, Kwon H, Cha SW, et al. Investigation of ginsenosides in different parts of Panax ginseng cultured by hydroponics. Korean J Hortic Sci Technol. 2010;28:216–226. [Google Scholar]
- Kim GS, Hyun DY, Kim YO, Lee SW, Kim YC, Lee SE, et al. Extraction and preprocessing methods for ginsenosides analysis of Panax ginseng C.A. Mayer. Korean J Med Crop Sci. 2008;16:446–454. [Google Scholar]
- Kim HS, Lee EH, Ko SR, Choi KJ, Park JH, Im DS. Effects of ginsenosides Rg3 and Rh2 on the proliferation of prostate cancer cells. Arch Pharm Res. 2004b;27:429–435. doi: 10.1007/BF02980085. [DOI] [PubMed] [Google Scholar]
- Kim YC, Hong HD, Rho JH, Cho CW, Rhee YK, Yim JH. Changes of phenolic acid contents and radical scavenging activities of ginseng according to steaming times. J Ginseng Res. 2007;31:230–236. doi: 10.5142/JGR.2007.31.4.230. [DOI] [Google Scholar]
- Kong YH, Rho J, Cho CW, Kim MH, Lee YC, Kim SS, et al. Variation of phenolic ingredient and ginsenoside content in red ginseng extract by acid treatment. J Ginseng Res. 2009;33:194–198. doi: 10.5142/JGR.2009.33.3.194. [DOI] [Google Scholar]
- Lee GA, Chang YK, Park SY, Kim GA, Kim SH, Song BH. Studies on growth responses and yields of Panax ginseng C. A. Meyer grown under hydroponic culture with different temperatures and growth stages. Korean J Med Crop Sci. 2012;20:184–189. doi: 10.7783/KJMCS.2012.20.3.184. [DOI] [Google Scholar]
- Lee SW, Kim GS, Hyun DY, Kim YB, Kim JW, Kang SW, et al. Comparison of growth characteristics and ginsenoside content of ginseng (Panax ginseng C. A. Meyer) cultivated with greenhouse and traditional shade facility. Korean J Med Crop Sci. 2011;19:157–161. doi: 10.7783/KJMCS.2011.19.3.157. [DOI] [Google Scholar]
- Masamoto Y, Ando H, Murata Y, Shimoishi Y, Tada M, Takahata K. Mushroom tyrosinase inhibitory activity of esculetin isolated from seeds of Euphorbia lathyris L. Biosci Biotechnol Biochem. 2003;67:631–634. doi: 10.1271/bbb.67.631. [DOI] [PubMed] [Google Scholar]
- Nah SY. Ginseng; recent advances and trends. J Ginseng Res. 1997;21:1–12. [Google Scholar]
- Nam KY. Clinical applications and efficacy of Korean ginseng. J Ginseng Res. 2002;26:111–131. doi: 10.5142/JGR.2002.26.3.111. [DOI] [Google Scholar]
- Oyaizu M. Studies on products of browning reaction: antioxidative activities of products of browning reaction prepared from glucosamine. Jpn J Nutr Diet. 1986;44:307–315. doi: 10.5264/eiyogakuzashi.44.307. [DOI] [Google Scholar]
- Park CK, Jeon BS, Yang JW. The chemical components of Korean ginseng. Food Industry and Nutrition. 2003;8(2):10–23. [Google Scholar]
- Shi W, Wang Y, Li J, Zhang H, Ding L. Investigation of ginsenosides in different parts and ages of Panax ginseng. Food Chem. 2007;102:664–668. doi: 10.1016/j.foodchem.2006.05.053. [DOI] [Google Scholar]
- Shin JY, Choi EH, Wee JJ. New methods for separation of crude ginseng saponins. Korean J Food Sci Technol. 2001;33:166–172. [Google Scholar]
- Speisky H, López-Alarcón C, Gómez M, Fuentes J, Sandoval-Acuña C. First web-based database on total phenolics and oxygen radical absorbance capacity (ORAC) of fruits produced and consumed within the south Andes region of South America. J Agric Food Chem. 2012;60:8851–8859. doi: 10.1021/jf205167k. [DOI] [PubMed] [Google Scholar]
- Van Kampen J, Robertson H, Hagg T, Drobitch R. Neuroprotective actions of the ginseng extract G115 in two rodent models of Parkinson’s disease. Exp Neurol. 2003;184:521–529. doi: 10.1016/j.expneurol.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Wang CZ, Aung HH, Ni M, Wu JA, Tong R, Wicks S, et al. Red American ginseng: ginsenoside constituents and antiproliferative activities of heat-processed Panax quinquefolius roots. Planta Med. 2007;73:669–674. doi: 10.1055/s-2007-981524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woisky RG, Salatino A. Analysis of propolis: some parameters and procedures for chemical quality control. J Apic Res. 1998;37:99–105. doi: 10.1080/00218839.1998.11100961. [DOI] [Google Scholar]
- Xie JT, Wu JA, Lin E, Wang CZ, Yuan CS. Constituents and effects of ginseng leaf. Orient Pharm Exp Med. 2004;4:1–8. doi: 10.3742/OPEM.2004.4.1.001. [DOI] [Google Scholar]
- Ye R, Li N, Han J, Kong X, Cao R, Rao Z, et al. Neuroprotective effects of ginsenoside Rd against oxygen-glucose deprivation in cultured hippocampal neurons. Neurosci Res. 2009;64:306–310. doi: 10.1016/j.neures.2009.03.016. [DOI] [PubMed] [Google Scholar]