Abstract
This study assessed the effects of probiotic supplementation on spatial learning and memory, long-term potentiation (LTP), paired-pulse facilitation (PPF) ratios, nitric oxide (NO) concentrations, and lipid profiles in a rat model of amyloid beta (Aβ)(1–42)-induced Alzheimer’s disease (AD). Forty rats were randomly divided into 4 groups. The sham (control and prevention) group received intracerebroventricular (ICV) injections of artificial cerebrospinal fluid, the Alzheimer group received ICV injection of Aβ(1–42), and the probiotic+Alzheimer group received 500 mg probiotics daily (15×109 colony-forming unit) by gavage for 4 weeks before and 2 weeks after injection of Aβ(1–42). The Morris water maze test was performed for evaluation of spatial learning and memory. LTP and PPF ratios were measured to evaluate longterm synaptic plasticity and pre-synaptic mechanisms, respectively. The results showed that probiotic supplementation significantly improved learning, but not memory impairment, and increased PPF ratios compared to those in the Alzheimer group. Both Aβ(1–42) injection and probiotic supplementation alone did not significantly effect plasma level of NO. Probiotic supplementation of rats in the probiotic (6 weeks)+Alzheimer group decreased serum levels of total cholesterol, triglyceride, and very low-density lipoprotein-cholesterol significantly compared to the Alzheimer group. The results of this study suggest that probiotic supplementation may positively impact learning capacity and LTP in rats with AD, most likely via the release of neurotransmitters via presynaptic mechanisms or via a protective effect on serum lipid profiles.
Keywords: probiotics, Alzheimer’s disease, long-term potentiation, biochemical parameters, paired-pulse facilitation
INTRODUCTION
Alzheimer’s disease (AD) is an irreversible neurodegenerative disease that has substantially increased in incidence over the past two decades. In AD, insoluble amyloid beta (Aβ) plaques are deposited throughout the cortex and hippocampus (Lecanu et al., 2010; Salari and Bagheri, 2016). Aβ plaques aggregation is associated with a deficits in spatial learning and memory, and in the synaptic plasticity (Buckner et al., 2005). The hippocampus plays a dominant and important role in spatial learning and memory, and in synaptic plasticity (Bannerman et al., 2014).
Synaptic plasticity is a physiological phenomenon that is involved in neural activity and the consequental changes in synaptic efficacy and neural excitability (Shankar et al., 2008).
In the laboratory, paired-pulse facilitation (PPF) ratios and induction of long-term potentiation (LTP) are fundamental protocols used to examine mechanisms of short-term and long-term synaptic plasticity, respectively (Costa-Mattioli et al., 2009; Zucker and Regehr, 2002). PPF is recorded to probe whether pre-synaptic mechanisms may involve mechanisms of LTP expression since LTP expression may involve increases in the probability of transmitter release from presynaptic terminals (Cazakoff and Howland, 2010; Gerges et al., 2003). Induction of LTP in the cornu ammonis (CA) 1 area of hippocampus by high-frequency stimulation (HFS) reveals an important mechanism of synaptic plasticity and memory, for enhancing the excitatory postsynaptic potentials (fEPSPs) of the CA1 area of the hippocampus (Whitlock et al., 2006). LTP is involved in both post-synaptic and presynaptic mechanisms (Larkman and Jack, 1995). PPF is involved in presynaptic mechanisms through inducing short-lasting increases in synaptic effectiveness (Lauri et al., 2007; Schulz et al., 1995). PPF is used to examine the mechanism of LTP expression by increasing release of neurotransmitters via presynaptic mechanisms (Lauri et al., 2007; Schulz et al., 1995; Schulz et al., 1994).
The possible role of nitric oxide (NO), as an important compensatory signaling molecule, for sustaining synaptic plasticity in neurodegenerative diseases such as AD has been recently examined (Chakroborty et al., 2015; Steinert et al., 2010). NO can either facilitate or suppress plasticity through biphasic effects on mechanisms involved in synaptic plasticity, LTP and consolidation of LTP (Böger, 2007; Chakroborty et al., 2015; Hosseini et al., 2010; Yamada et al., 1999).
There is no cure for AD, but there is a limited number of therapeutic options. Most of the current treatments are palliative and the efficacies of newer therapies remain unproven. Use of acetylcholinesterase (AChE) inhibitors, including rivastigmine, donepezil, and galantamine or memantine (N-methyl-D-aspartate antagonist), is the most common clinically effective approach for treatment of AD (Grover et al., 2012). Herbal plants are traditionally used to treat cognitive disorders and amnesia, however their safety and efficacy needs further investigation (Man et al., 2008). In recent years, the role of probiotic supplements as a therapeutic strategy to improve memory function in a rat model of AD has emerged as a novel and intriguing area for research (Akbari et al., 2016; Jiang et al., 2017). Probiotic supplements are living bacteria of the gut which, when used in adequate amounts, beneficially impact the host human or animal by improving their intestinal microbial balance (Liang et al., 2015). Akbari et al. (2016) showed that a probiotic supplement containing Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifidum, and Lactobacillus fermentum improved impaired spatial learning and memory induced in AD. Romo-Araiza et al. (2018) showed that consumption of probiotics (Enterococcus faecalis) and prebiotics (agave inulin) for 5 weeks improved synaptic plasticity in middle-aged rats. Previous studies have also shown that dietary supplements containing multiple species of live Lactobacillus and Bifidobacteria (L. acidophilus CUL60, L. acidophilus CUL21, B. bifidum CUL20, and Bifidobacterium lactis CUL34) can affect brain functions (O’Hagan et al., 2017). Since probiotic supplementation may be associated with beneficial effects on AD, the aim of the present study was to examine the impact of a long-term dietary supplementation of probiotics containing Lactobacillus and Bifidobacterium strains on learning and memory, electrophysiology (LTP and PPF), NO concentrations, and plasma lipid profiles in a rat model of Aβ(1–42)-induced AD.
MATERIALS AND METHODS
Male Sprague-Dawley rats weighing 220~250 g were housed with free access to standard rat chow diet and tap water for one week in an animal care center (Physiology Research Center, Kashan University of Medical Sciences, Kashan, Iran). Rats were subjected to a 12-h light/dark cycle under controlled temperatures (22±1°C).
Forty male rats were randomly divided into 4 groups: control rats without any surgical or diet intervention; sham-operated rats that received 20 μL artificial cerebrospinal fluid (aCSF) through intracerebroventricular (ICV) bilateral injection for two consecutive days (10 μL/d); Alzheimer group rats that recieved 20 μg/20 μL of Aβ(1–42) injected through ICV for two consecutive days; and probiotic+Alzheimer group rats that received 500 mg probiotics [15×109 colony-forming units (CFU)] for 4 weeks before and for 2 weeks after injection of Aβ(1–42). Spatial learning and memory was evaluated by a Morris water maze (MWM). PPF ratios (fEPSP2/fEPSP1) before induction of HFS were measured before HFS was induced, and was then continuously recorded for 120 min after induction of LTP. At the end of the experiments, blood samples were collected from the right atrium and was stored at −70°C until further analyses. The study protocol was approved by the research ethics committee of Kerman University of Medical Sciences (Ethics committee approval number: IR.KMU.REC.1395.1027).
Aβ(1–42) preparation
Aβ(1–42) was purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Aβ was dissolved in distilled water at 1 μg/1 μL concentration, incubated at 37°C for one week and stored at −20°C.
Probiotic preparation
Odorless and colorless probiotic supplement powder (Iranian Prodigest Capsule) composed of L. acidophilus, B. bifidum, and B. longum was purchased from Gostaresh Milad Pharmed Co. (Teheran, Iran). Five hundred milligrams of the compound (containing 15×109 CFU) was dissolved in 1 mL of drinking water. Due to its special components, the solution was gavaged immediately after its preparation daily.
Surgery procedure
In this study, we established an experimental model of AD similar to that described in previous studies (Liang et al., 2014; Prakash and Kumar, 2014). Briefly, two channels (23-gauge stainless steel) were implanted into ICV (anterior-posterior: −0.8 mm, left-right: ±1.6 mm, and dorsal-ventral: 3.5 mm) using a stereotaxic technique. Animals were then allowed to recover for at least one week. For ICV injection of Aβ and aCSF, a 30-gauge needle connected to a microsyringe (10 μL) with polyethylene-10 tubing was inserted into a preimplanted cannula in each conscious, free moving rat. Aβ and aCSF were then slowly injected (5 μL/15 min) into each lateral ventricular.
Behavioral test
MWM test was used for evaluation of spatial learning and memory. The water maze comprised of a circular tank (140 cm diameter, 60 cm high, and 40 cm deep and filled with 22±1°C water). The MWM test was performed in a room with indirect lighting with an optimal number of surrounding cues. A hidden square black platform or escape platform (10 cm diameter) was submerged 1.5~2.0 cm beneath the water surface in the middle of the target quadrant of the pool. Animal motions were recorded and data were sent to a computer via a camera fixed above the center of the maze. Commercial software (version: 6 XT, Noldus Information Technology, Wageningen, The Netherlands) was used to measure the escape latency, distance travelled, swimming speed, and the number of crossings to the target quadrant during training and probe trials.
Spatial learning phase
Each training trial consisted of 4 trials per day for 4 consecutive days. In each trail, rats were gently released from the start position in the maze North (N), South (S), West (W), and East (E) at the middle of the circular edge of a randomly selected quadrant, with their noses pointed toward the wall. Rats were allowed to swim for a maximum duration of 90 s to find the hidden platform, with an inter-trial interval of 10 min. The mean latency time to find the hidden platform of the water maze task was recorded on each day of testing. If the animal failed to find the platform within 90 s, it was gently guided onto the platform. After completion of training, the animals were returned to their home cages until retention testing (probe trial) 24 h later.
Probe trial test (retention testing)
The probe trial test was performed on day 5, 24 h after the last acquisition day. In the probe test, the platform was removed and the rats was placed in the start position (S in this study) facing the maze wall, as in previous days. Finally, the animals were removed after 60 s circulating the tank searching for the platform. In probe test, the time spent searching, the traveled distance, the swimming speed in the target quadrant and the number of crossings to target quadrant were recorded.
Electrophysiology
fEPSPs were recorded as an important index of neuronal plasticity. The electrophysiological procedure of LTP induction has been previously described (Davari et al., 2013). Briefly, rats were anesthetized with intraperitoneal injections of urethane (1.5 g/kg) and then fixed in a stereotaxic apparatus (Borj Sanat, Tehran, Iran). The recording (3.8 mm posterior to bregma and 2.5 mm lateral to the midline) and stimulating electrodes (4.2 mm posterior to bregma and 3.8 mm lateral to the midline) were inserted into the CA1 stratum radiatum and Schaffer collateral, respectively, according to the atlas of Paxinos and Watson (2005). fEPSPs were recorded following stimulation to the Schaffer collateral pathway. Baseline stimulation was delivered every 30 s. The baseline consisted of 200 μs pulses of a fixed duration with an amplitude varying from 50 to 300 μA. Input-output curves were constructed before induction of HFS by increasing stimulus intensity in 5~10 μA increments. For induction of LTP, stimulus intensity was regulated to evoke a fEPSP about 60% of the maximal response of basal fEPSP. Before and after induction of LTP, we recorded PPF ratios for 5 min. Paired-pulses were delivered at 50 ms-intervals in the Schaffer collateral pathway. The PPF ratio (fEPSP2/fEPSP1) was fEPSP1, the response evoked by the first pulse divided by fEPSP2, the response evoked by the second pulse. After recording PPF, LTP was induced by HFS at 100 Hz. The HFS protocol consisted of 10 bursts of 10 stimuli, of 200 μs duration, and with a pulse cycle of 10,000 μs. fEPSP in response to HFS was then recorded for 120 min. fEPSP were amplified on a preamplifier (Electromadule, Well Services of Iran, Tehran, Iran) and filtered at 1~3,000 Hz.
Measurement of biochemical parameters and plasma NO
Blood samples were pooled from the right atrium of all animals after electrophysiological experiments were completed. Blood samples were centrifuged at 2,500 rpm for 5 min and plasma samples were kept frozen at −80°C until further use. Plasma levels of NO were quantified by the Griess method (Tatsch et al., 2011). Plasma levels of total cholesterol (TC), triglyceride (TG) (Hosseini et al., 2010), and very low-density lipoprotein-cholesterol (VLDL-C) were measured in rats that were pretreated with probiotics for 6 weeks before injection of Aβ(1–42) by using standard laboratory kits purchased from Parsazmun Co. (Karaj, Iran). VLDL-C levels was estimated from data on TG concentrations in plasma according to the following formula: VLDL=TG/5.
Statistical analysis
Results were expressed as mean±standard error of the mean (SEM). Data were analyzed by SPSS 16 (SPSS Inc., Chicago, IL, USA) and Prism 6.0 software (GraphPad, San Diego, CA, USA). Differences between two or more groups were evaluated using the paired Student’s t-test or one-way ANOVA followed by LSD’ post hoc, respectively. Statistical significance was defined as P<0.05.
RESULTS
Effects of probiotic supplement on spatial learning and memory
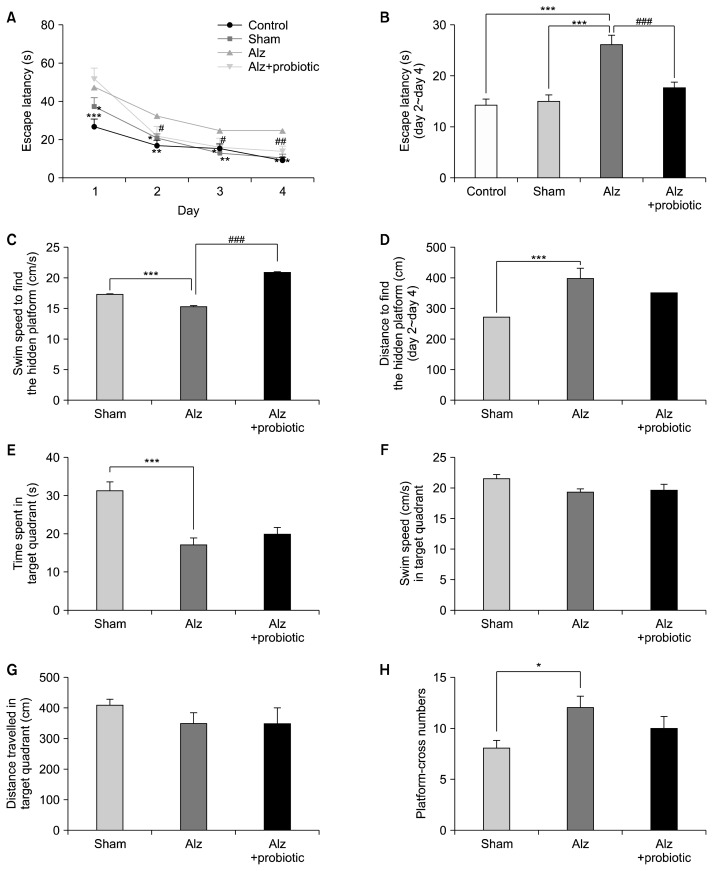
The MWM behavioral test was performed to assess the rats’ spatial learning and memory abilities. The escape latency (the ability of animals to find the platform) during the four consecutive training days are shown in Fig. 1A. Injection of Aβ (ICV) increased escape latency on days of 1, 2, 3, and 4 significantly in comparison with the control and sham groups [(DAY1: F3, 144=11.33; P<0.001), (DAY2: F3, 144=3.63; P<0.014), (DAY3: F3, 144=2.78; P<0.043), and (DAY4: F3, 144=9.09; P<0.001)]. The escape latency and total distance travelled (DAY2~DAY4) to find the hidden platform was significantly increased in the Alzheimer group compared to the control and sham groups (Fig. 1B and 1C: F3, 440=12.17; P<0.001). Treatment with probiotic supplement (500 mg/6 weeks/gavage) decreased escape latency significantly (P<0.001), but did not significantly affect total distance travelled to find the hidden platform compared to sham rats (P<0.228). The mean time spent in the target quadrant was also significantly higher for the Alzheimer group then the sham group (F2, 23=9.49; P<0.001), but no significant differences were observed between the probiotic (6 weeks)+Alzheimer group and the Alzheimer group (Fig. 1D, P<0.96). There were also no significant differences in the swimming speed, the travelled distance in the target quadrant and the number of times the rats crossed the platform area between the all 3 groups (Fig. 1E~1H, P≥0.05).
Fig. 1.
The effects of probiotic supplementation on spatial learning and memory in a rat model of amyloid beta (Aβ)(1–42)-induced Alzheimer’s disease (AD). Rats in Alzheimer (Alz)+probiotic group received 500 mg probiotics [15×109 colony-forming units (CFU)] for 4 weeks before and 2 weeks after injection of Aβ(1–42). (A) Escape latency, (B) total average of escape latency, (C) speed, and (D) distance to find the hidden platform in DAY 2~4 in experimental model of AD in rats was significantly different from control or sham groups. Treatment with probiotics significantly changed delay and speed but not distance to find the hidden platform in compare with Alz group. The time spent in target quadrant (E) and cross number (H), but not speed (F) and distance (G) in probe, in Alz group was significantly different from sham group. Treatment with probiotics did not change any parameters measured in probe day in compare with Alz group. Significant difference with sham group at *P <0.05, **P <0.01, and ***P <0.001, and significant difference between Alz+probiotic group and Alz group at #P <0.05, ##P <0.01, and ###P <0.001.
Effects of probiotic supplementation on LTP in the CA1 area of the hippocampus
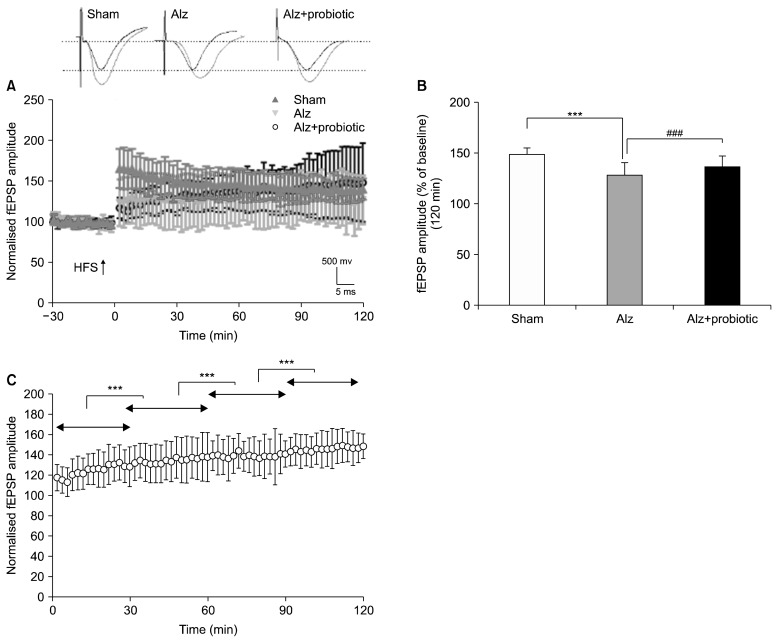
In vivo, LTP measurements were performed to evaluate synaptic plasticity. fEPSPs was recorded 30 min before, continuously during, and for 120 min after the induction of LTP. fEPSPs amplitudes were then calculated as a percent of baseline.
Data showed that the fEPSPs amplitudes differ significantly between groups (Fig. 2A and 2B) (F2, 177=126.39; P<0.001). However, the magnitude of fEPSPs were significantly lower in the Alzheimer group compared to the sham group (P<0.001). The results also showed that treatment with probiotics, significantly reversed the decrease in fEPSPs amplitude observed in the Alzheimer group (Fig. 2B, P<0.001). Moreover, a continuous significant increase in fEPSP amplitude was observed in the probiotic (6 weeks)+Alzheimer group during the 120 min period following LTP induction (P<0.001) (Fig. 2C).
Fig. 2.
The effects of probiotic supplementation on long-term potentiation (LTP) and field excitatory postsynaptic potentials (fEPSPs) in a rat model of Alzheimer’s disease (AD). Rats received 500 mg probiotics [15×109 colony-forming units (CFU)] for 4 weeks before and 2 weeks after injection of amyloid beta (Aβ)(1–42). Probiotic supplementation reversed hippocampal LTP suppression induced by Aβ(1–42) (A and B). Moreover, a continuous significant increase in fEPSP amplitude was observed in mentioned treatment group, during the 120 min period following LTP induction (C). Typical representative traces of fEPSP on top of time course diagram were obtained 30 min before (black traces) and 120 min (red traces) after the induction of LTP. Alz, Alzheimer group; HFS, high-frequency stimulation. Significant difference with sham group at ***P <0.001 and significant difference between Alz+probiotic group and Alz group at ###P <0.001.
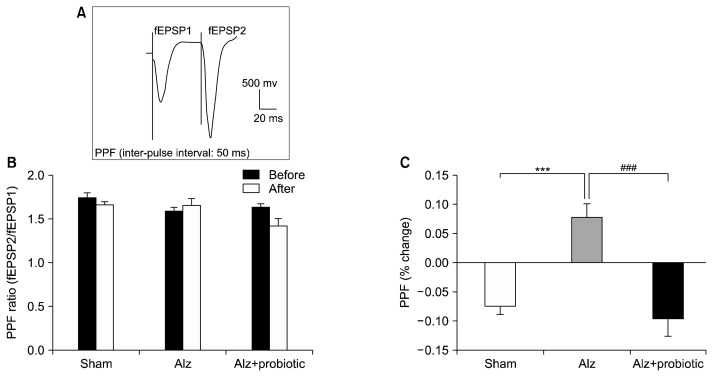
Effects of probiotic supplementation on the PPF ratio
In this study, the PPF ratio (% change) was measured before and after induction of LTP. Results showed that the PPF ratio was significantly increased in the Alzheimer group compared to the sham group (F3, 80=10.75; P<0.001), and that this increase could be reversed by probiotic supplementation (P<0.001; Fig. 3).
Fig. 3.
The effects of probiotic supplementation on paired-pulse facilitation (PPF) change in a rat model of Alzheimer’s disease (AD). Paired-pulse facilitation (PPF) was measured before and 120 min after long-term potentiation (LTP) induction in different groups. The PPF ratio was expressed as the field excitatory postsynaptic potentials (fEPSP) 2/fEPSP1 is the response evoked by the first pulse and fEPSP2 is the response evoked by the second pulse. Representative trace of PPF is shown on the top of the graph (A). There was no significant difference in PPF before and after induction of LTP in each group (B). The % PPF change was measured as % PPF change=(PPF ratio after LTP–PPF ratio before LTP)/(PPF ratio before LTP). Data showed that induction of LTP produced a significant difference between % PPF change between groups (C). Data are presented as mean±SEM. Significant difference between sham group and Alzheimer (Alz) group at ***P <0.001 and between Alz group and Alz+probiotic group at ###P <0.001.
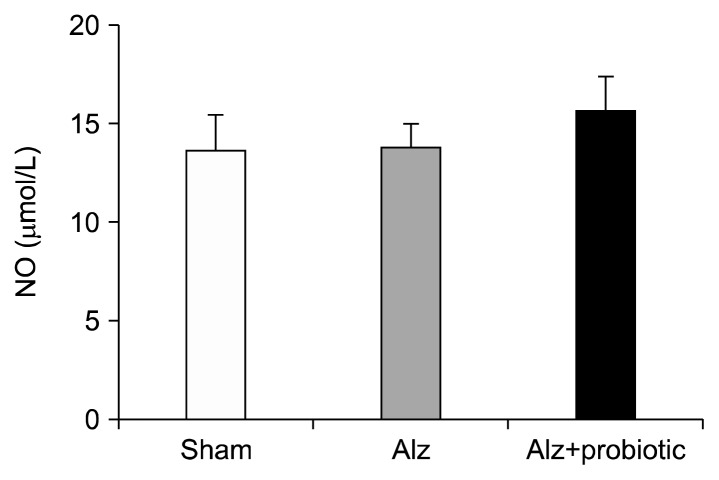
Effects of probiotic supplementation on NO concentrations and biochemical parameters
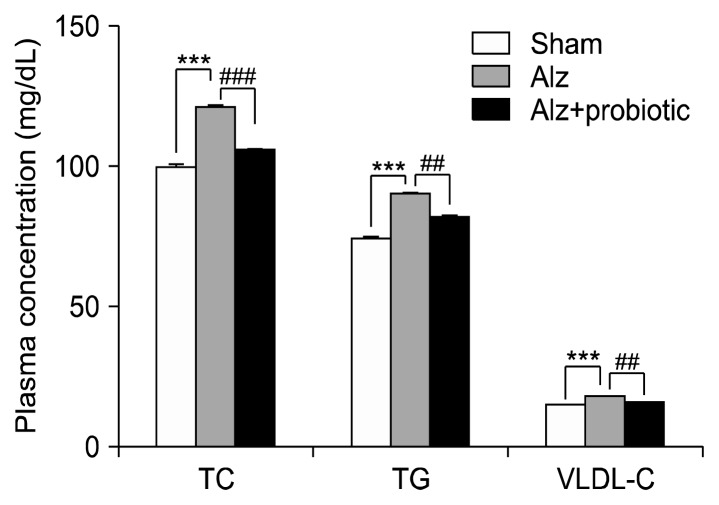
No significant difference in NO concentration were recorded between the 3 groups (Fig. 4; P≥0.05). However, serum concentrations of TC, TG, and VLDL-C were significantly higher in the Alzheimer group compared to the sham group (Fig. 5; P<0.01). The serum levels of these parameters were reduced in the probiotic (6 week)+Alzheimer group compared to the Alzheimer group [TC: (F2, 15=16.29; P<0.001), TG and VLDL-C: (F2, 15= 17.19; P<0.001) (Fig. 5)].
Fig. 4.
The effect of probiotic supplementation on nitric oxide (NO) concentration in a rat model of Alzheimer’s disease (AD). There is no significant difference in NO concentration between groups. Data are presented as mean±SEM.
Fig. 5.
The effects of probiotic supplementation on lipid profile in a rat model of Alzheimer’s disease (AD). Injection of amyloid beta (Aβ)(1–42) (intracerebroventricular) significantly increased the plasma level of total cholesterol (TC), triglycerides (TG), and very low-density lipoprotein-cholesterol (VLDL-C) and treatment with probiotics for 6 weeks before injection of Aβ(1–42) significantly decreased all of these factors in plasma. Data are presented as mean±SEM. Significant difference with sham group at ***P <0.001 and Alzheimer (Alz) group at ##P <0.01 and ###P <0.001.
DISCUSSION
Consistent with previous studies, these results show that Aβ(1–42) injection is associated with learning and memory impairment (Buckner et al., 2005; Pike et al., 2007). Probiotic treatment improved learning impairment, but did not significantly impact spatial memory. Our results are in agreement with previous reports indicating that consumption of probiotics improves cognitive functions in AD patients (Akbari et al., 2016).
Previous studies have also indicated that chronic dietary supplementation with probiotics containing Lactobacillus and Bifidobacterium improves the memory of middle-aged rats (O’Hagan et al., 2017; Romo-Araiza et al., 2018). Emerging evidence has suggested a role of the gut microbiota and probiotic supplementation in regulating cognitive function and metabolic status in AD (Leblhuber et al., 2018; Liang et al., 2015; Minter et al., 2016). There are several possible mechanisms involved in learning and memory deficits caused by injection of Aβ. For example, administration of Aβ into the lateral ventricle is consistent with a major reduction of choline acetyltransferase (Chateauvieux et al., 2010), that may lead to cholinergic synapse dysfunction and cognitive impairment (Nitta et al., 1997). Furthermore, previous studies have shown that Aβ(1–42) toxicity decreases the number of γ-aminobutyric acid (GABA)-ergic and glutaminergic neurons and that it may produce complex imbalances in network activity and lead to impaired spatial learning (Thorajak et al., 2017). Also, deficits in learning and memory in rats treated with Aβ(1–42) may be related to cholinergic and dopaminergic hypoactivity and reduced levels of brain-derived neurotrophic factor (BDNF) proteins in the hippocampus (Itoh et al., 1996; Mozafari et al., 2018).
Our results demonstrated that Aβ(1–42)-induced spatial learning impairment can be improved by pretreatment with probiotics including in L. acidophilus, B. bifidum, and B. longum. In agreement with our findings, other studies have shown that probiotic supplements containing the Gram-positive Lactobacillus and Bifidobacterium, the most commonly used probiotic microorganisms (Sekhon and Jairath, 2010), exert beneficial effects on deficit learning and memory via different mechanisms. Barrett et al. (2012) reported that Lactobacillus brevis and Bifidobacterium dentium can produce GABA by metabolizing glutamate, and this correlates with an increase of GABA in the brain. Whereas Choi et al. (2015) showed that fermentation of Ganoderma lucidum by B. longum increased cognitive enhancing activity by stimulating secretion of memory-related neurotransmitters such as acetylcholine and glutamate, and by inhibiting AChE activity in brain tissues via the cholinergic nervous system in scopolamine-induced memory impaired rats (Choi et al., 2015). Other studies have shown that B. longum up-regulates hippocampal expression of BDNF and plays important roles in improving learning and memory (Bercik et al., 2011; Bercik et al., 2010). The underlying mechanism(s) mediating the beneficial effects of probiotics on spatial learning and memory were not determined in our study; however, some of these aforementioned mechanisms may play an important role in improvement the learning deficit observed in Alzheimer group.
Both LTP and PPF are of interest as a potential cellular substrates in memory (Larkman and Jack, 1995). LTP is a use-dependent form of synaptic plasticity. PPF is defined as an index of presynaptic release of neurotransmitters (Zucker and Regehr, 2002), and is associated with LTP (Schulz et al., 1995). Our results associated probiotic supplementation with an increase in fEPSP amplitudes following LTP induction; this indirectly suggests that the Aβ may impair expression of fEPSPs (1–42), and this may be impacted by probiotics. Also, PPF (% change) after induction of LTP in the Alzheimer group was significantly increased compared to the sham group and was significantly decreased in the probiotic groups; presynaptic neurotransmitter release in the hippocampal CA1 region of rats may therefore be affected by Aβ(1–42) (Boussicault et al., 2016; Soheili et al., 2015) and probiotic treatment may positively impact these changes. Accordingly, our results showed a possible interaction between PPF ratios and LTP. LTP expression in Alzheimer group decreased release of neurotransmitters after induction of LTP, leading to a decrease in fEPSP amplitude. Probiotic treatment presumably caused an increase in release of presynaptic neurotransmitters, resulting in an increase in LTP in the probiotic (6 weeks)+Alzheimer group. In agreement with this study, Romo-Araiza et al. (2018) showed that probiotic and prebiotic supplementation was associated with an improved spatial learning and memory and significant increases in LTP in middle-aged rats. Davari et al. (2013) also reported that probiotic supplementation of streptozotocin-induced diabetic rats improved impaired spatial memory and helped reverse the declines in hippocampal LTP.
Our results did not show any significant differences in NO concentration between the Alzheimer and sham groups. Contrary to our results, increasing evidence has indicated that NO is involved in that pathogenesis of some neurodegenerative diseases including AD, either as a neuroprotective or neurotoxic agent (Chakroborty et al., 2015; Nathan et al., 2005).
Furthermore, our results indicate that Aβ(1–42) injection leads to a significant increase in level of serum TC, TG, and VLDL-C, as compared to sham controls. Pretreatment with probiotics for six weeks before induction of AD by Aβ(1–42) was associated with significant changes in aforementioned biochemical parameters as compared to the sham group. However, Grossi et al. (2018) did not report any significant differences in TC, high-density lipoprotein-cholesterol, or low-density lipoprotein-cholesterol levels between patients with AD and those without; however, in this study TG levels were reported to be lower in AD patients than controls. In addition, Akbari et al. (2016) showed that treatment with probiotics for 12 weeks in patients with AD positively impacts cognitive function, but is not associated with any significant changes in lipid profiles. High level of cholesterol play a dominant role in production of Aβ in AD (Boussicault et al., 2016) and cholesterol-lowering drugs (statins) may decrease the prevalence of AD (Loera-Valencia et al., 2019). Since the effects of supplementation with probiotic on lipid profiles and biochemical parameters is controversial, further animal and human research is needed to determine the effects of acute, subacute and chronic probiotic supplementation (used either as pre-treatment or treatment) on biochemical parameters.
In summary, our results show that Aβ(1–42) induces learning and memory impairment, significantly increases PPF ratios following LTP induction, and decreases in fEPSP amplitudes in Aβ(1–42)-induced Alzheimer group. Furthermore, supplementation probiotics improved learning, but not memory. The probiotics also increased fEPSP amplitudes and decreased PPF ratios, suggestive of an increase in presynaptic neurotransmitter release. Further studies are needed to elucidate the underlying mechanisms of probiotic supplements on learning and memory.
ACKNOWLEDGEMENTS
This work has been supported by a grant from Kerman University of Medical Sciences (No: 95000256).
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- Akbari E, Asemi Z, Daneshvar Kakhaki R, Bahmani F, Kouchaki E, Tamtaji OR, et al. Effect of probiotic supplementation on cognitive function and metabolic status in Alzheimer’s disease: a randomized, double-blind and controlled trial. Front Aging Neurosci. 2016;8:256. doi: 10.3389/fnagi.2016.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman DM, Sprengel R, Sanderson DJ, McHugh SB, Rawlins JN, Monyer H, et al. Hippocampal synaptic plasticity, spatial memory and anxiety. Nat Rev Neurosci. 2014;15:181–192. doi: 10.1038/nrn3677. [DOI] [PubMed] [Google Scholar]
- Barrett E, Ross RP, O’Toole PW, Fitzgerald GF, Stanton C. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J Appl Microbiol. 2012;113:411–417. doi: 10.1111/j.1365-2672.2012.05344.x. [DOI] [PubMed] [Google Scholar]
- Bercik P, Park AJ, Sinclair D, Khoshdel A, Lu J, Huang X, et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol Motil. 2011;23:1132–1139. doi: 10.1111/j.1365-2982.2011.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bercik P, Verdu EF, Foster JA, Macri J, Potter M, Huang X, et al. Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology. 2010;139:2102–2112.e1. doi: 10.1053/j.gastro.2010.06.063. [DOI] [PubMed] [Google Scholar]
- Böger RH. The pharmacodynamics of L-arginine. J Nutr. 2007;137:1650S–1655S. doi: 10.1093/jn/137.6.1650S. [DOI] [PubMed] [Google Scholar]
- Boussicault L, Alves S, Lamazière A, Planques A, Heck N, Moumné L, et al. CYP46A1, the rate-limiting enzyme for cholesterol degradation, is neuroprotective in Huntington’s disease. Brain. 2016;139:953–970. doi: 10.1093/brain/awv384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, et al. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazakoff BN, Howland JG. Acute stress disrupts paired pulse facilitation and long-term potentiation in rat dorsal hippocampus through activation of glucocorticoid receptors. Hippocampus. 2010;20:1327–1331. doi: 10.1002/hipo.20738. [DOI] [PubMed] [Google Scholar]
- Chakroborty S, Kim J, Schneider C, West AR, Stutzmann GE. Nitric oxide signaling is recruited as a compensatory mechanism for sustaining synaptic plasticity in Alzheimer’s disease mice. J Neurosci. 2015;35:6893–6902. doi: 10.1523/JNEUROSCI.4002-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chateauvieux S, Morceau F, Dicato M, Diederich M. Molecular and therapeutic potential and toxicity of valproic acid. J Biomed Biotechnol. 2010;2010 doi: 10.1155/2010/479364. 479364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YJ, Yang HS, Jo JH, Lee SC, Park TY, Choi BS, et al. Anti-amnesic effect of fermented Ganoderma lucidum water extracts by lactic acid bacteria on scopolamine-induced memory impairment in rats. Prev Nutr Food Sci. 2015;20:126. doi: 10.3746/pnf.2015.20.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Mattioli M, Sossin WS, Klann E, Sonenberg N. Translational control of long-lasting synaptic plasticity and memory. Neuron. 2009;61:10–26. doi: 10.1016/j.neuron.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davari S, Talaei SA, Alaei H, Salami M. Probiotics treatment improves diabetes-induced impairment of synaptic activity and cognitive function: behavioral and electrophysiological proofs for microbiome-gut-brain axis. Neuroscience. 2013;240:287–296. doi: 10.1016/j.neuroscience.2013.02.055. [DOI] [PubMed] [Google Scholar]
- Frieden T. Antibiotic resistance threats in the United States, 2013. Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; Atlanta, GA, USA: 2016. pp. 11–28. [Google Scholar]
- Gerges NZ, Aleisa AM, Alkadhi KA. Impaired long-term potentiation in obese zucker rats: possible involvement of presynaptic mechanism. Neuroscience. 2003;120:535–539. doi: 10.1016/S0306-4522(03)00297-5. [DOI] [PubMed] [Google Scholar]
- Grossi MF, Carvalho MDG, Silveira JN, Gonçalves GS, Gomes KB, Bicalho MA, et al. OxLDL plasma levels in patients with Alzheimer’s disease. Arq Neuropsiquiatr. 2018;76:241–246. doi: 10.1590/0004-282x20180012. [DOI] [PubMed] [Google Scholar]
- Grover A, Shandilya A, Agrawal V, Bisaria VS, Sundar D. Computational evidence to inhibition of human acetyl cholinesterase by withanolide a for Alzheimer treatment. J Biomol Struct Dyn. 2012;29:651–662. doi: 10.1080/07391102.2012.10507408. [DOI] [PubMed] [Google Scholar]
- Hosseini M, Dastghaib SS, Rafatpanah H, Hadjzadeh MA, Nahrevanian H, Farrokhi I. Nitric oxide contributes to learning and memory deficits observed in hypothyroid rats during neonatal and juvenile growth. Clinics. 2010;65:1175–1181. doi: 10.1590/S1807-59322010001100021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh A, Nitta A, Nadai M, Nishimura K, Hirose M, Hasegawa T, et al. Dysfunction of cholinergic and dopaminergic neuronal systems in β-amyloid protein-infused rats. J Neurochem. 1996;66:1113–1117. doi: 10.1046/j.1471-4159.1996.66031113.x. [DOI] [PubMed] [Google Scholar]
- Jiang C, Li G, Huang P, Liu Z, Zhao B. The gut microbiota and Alzheimer’s disease. J Alzheimers Dis. 2017;58:1–15. doi: 10.3233/JAD-161141. [DOI] [PubMed] [Google Scholar]
- Larkman AU, Jack JJB. Synaptic plasticity: hippocampal LTP. Curr Opin Neurobiol. 1995;5:324–334. doi: 10.1016/0959-4388(95)80045-X. [DOI] [PubMed] [Google Scholar]
- Lauri SE, Palmer M, Segerstrale M, Vesikansa A, Taira T, Collingridge GL. Presynaptic mechanisms involved in the expression of STP and LTP at CA1 synapses in the hippocampus. Neuropharmacology. 2007;52:1–11. doi: 10.1016/j.neuropharm.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Leblhuber F, Egger M, Schuetz B, Fuchs D. Commentary: effect of probiotic supplementation on cognitive function and metabolic status in Alzheimer’s disease: a randomized, double-blind and controlled trial. Front Aging Neurosci. 2018;10:54. doi: 10.3389/fnagi.2018.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecanu L, Rammouz G, McCourty A, Sidahmed EK, Greeson J, Papadopoulos V. Caprospinol reduces amyloid deposits and improves cognitive function in a rat model of Alzheimer’s disease. Neuroscience. 2010;165:427–435. doi: 10.1016/j.neuroscience.2009.10.033. [DOI] [PubMed] [Google Scholar]
- Liang S, Wang T, Hu X, Luo J, Li W, Wu X, et al. Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience. 2015;310:561–577. doi: 10.1016/j.neuroscience.2015.09.033. [DOI] [PubMed] [Google Scholar]
- Liang SL, Hsu SC, Pan JT. Involvement of dopamine D2 receptor in the diurnal changes of tuberoinfundibular dopaminergic neuron activity and prolactin secretion in female rats. J Biomed Sci. 2014;21:37. doi: 10.1186/1423-0127-21-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loera-Valencia R, Goikolea J, Parrado-Fernandez C, Merino-Serrais P, Maioli S. Alterations in cholesterol metabolism as a risk factor for developing Alzheimer’s disease: potential novel targets for treatment. J Steroid Biochem Mol Biol. 2019;190:104–114. doi: 10.1016/j.jsbmb.2019.03.003. [DOI] [PubMed] [Google Scholar]
- Man SC, Durairajan SS, Kum WF, Lu JH, Huang JD, Cheng CF, et al. Systematic review on the efficacy and safety of herbal medicines for Alzheimer’s disease. J Alzheimers Dis. 2008;14:209–223. doi: 10.3233/JAD-2008-14209. [DOI] [PubMed] [Google Scholar]
- Minter MR, Zhang C, Leone V, Ringus DL, Zhang X, Oyler-Castrillo P, et al. Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer’s disease. Sci Rep. 2016;6:30028. doi: 10.1038/srep30028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozafari N, Shamsizadeh A, Fatemi I, Allahtavakoli M, Moghadam-Ahmadi A, Kaviani E, et al. CX691, as an AMPA receptor positive modulator, improves the learning and memory in a rat model of Alzheimer’s disease. Iran J Basic Med Sci. 2018;21:724–730. doi: 10.22038/IJBMS.2018.28544.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C, Calingasan N, Nezezon J, Ding A, Lucia MS, La Perle K, et al. Protection from Alzheimer’s-like disease in the mouse by genetic ablation of inducible nitric oxide synthase. J Exp Med. 2005;202:1163–1169. doi: 10.1084/jem.20051529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitta A, Fukuta T, Hasegawa T, Nabeshima T. Continuous infusion of β-amyloid protein into the rat cerebral ventricle induces learning impairment and neuronal and morphological degeneration. Jpn J Pharmacol. 1997;73:51–57. doi: 10.1254/jjp.73.51. [DOI] [PubMed] [Google Scholar]
- O’Hagan C, Li JV, Marchesi JR, Plummer S, Garaiova I, Good MA. Long-term multi-species Lactobacillus and Bifidobacterium dietary supplement enhances memory and changes regional brain metabolites in middle-aged rats. Neurobiol Learn Mem. 2017;144:36–47. doi: 10.1016/j.nlm.2017.05.015. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5th ed. Elsevier Academic Press; Cambridge, MA, USA: 2005. pp. 34–38. [Google Scholar]
- Pike KE, Savage G, Villemagne VL, Ng S, Moss SA, Maruff P, et al. β-Amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer’s disease. Brain. 2007;130:2837–2844. doi: 10.1093/brain/awm238. [DOI] [PubMed] [Google Scholar]
- Prakash A, Kumar A. Implicating the role of lycopene in restoration of mitochondrial enzymes and BDNF levels in β-amyloid induced Alzheimer’s disease. Eur J Pharmacol. 2014;741:104–111. doi: 10.1016/j.ejphar.2014.07.036. [DOI] [PubMed] [Google Scholar]
- Romo-Araiza A, Gutiérrez-Salmeán G, Galván EJ, Hernández-Frausto M, Herrera-López G, Romo-Parra H, et al. Probiotics and prebiotics as a therapeutic strategy to improve memory in a model of middle-aged rats. Front Aging Neurosci. 2018;10:416. doi: 10.3389/fnagi.2018.00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salari S, Bagheri M. A review of animal models of Alzheimer’s disease: a brief insight to pharmacologic and genetic models. Physiol Pharmacol. 2016;20:5–11. [Google Scholar]
- Schulz PE, Cook EP, Johnston D. Changes in paired-pulse facilitation suggest presynaptic involvement in long-term potentiation. J Neurosci. 1994;14:5325–5337. doi: 10.1523/JNEUROSCI.14-09-05325.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz PE, Cook EP, Johnston D. Using paired-pulse facilitation to probe the mechanisms for long-term potentiation (LTP) J Physiol Paris. 1995;89:3–9. doi: 10.1016/0928-4257(96)80546-8. [DOI] [PubMed] [Google Scholar]
- Sekhon BS, Jairath S. Prebiotics, probiotics and synbiotics: an overview. J Pharm Educ Res. 2010;1:13–36. [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, et al. Amyloid-β protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soheili M, Tavirani MR, Salami M. Lavandula angustifolia extract improves deteriorated synaptic plasticity in an animal model of Alzheimer’s disease. Iran J Basic Med Sci. 2015;18:1147–1152. [PMC free article] [PubMed] [Google Scholar]
- Steinert JR, Chernova T, Forsythe ID. Nitric oxide signaling in brain function, dysfunction, and dementia. Neuroscientist. 2010;16:435–452. doi: 10.1177/1073858410366481. [DOI] [PubMed] [Google Scholar]
- Tatsch E, Bochi GV, da Silva Pereira R, Kober H, Agertt VA, de Campos MMA, et al. A simple and inexpensive automated technique for measurement of serum nitrite/nitrate. Clin Biochem. 2011;44:348–350. doi: 10.1016/j.clinbiochem.2010.12.011. [DOI] [PubMed] [Google Scholar]
- Thorajak P, Pannangrong W, Welbat JU, Chaijaroonkhanarak W, Sripanidkulchai K, Sripanidkulchai B. Effects of aged garlic extract on cholinergic, glutamatergic and gabaergic systems with regard to cognitive impairment in Aβ-induced rats. Nutrients. 2017;9:E686. doi: 10.3390/nu9070686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- Yamada K, Tanaka T, Zou LB, Senzaki K, Yano K, Osada T, et al. Long-term deprivation of oestrogens by ovariectomy potentiates beta-amyloid-induced working memory deficits in rats. Br J Pharmacol. 1999;128:419–427. doi: 10.1038/sj.bjp.0702811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]