Abstract
Objectives
This study aimed to investigate the associations between trimethylamine N-oxide (TMAO) and related metabolites in early pregnancy and the risk of gestational diabetes mellitus (GDM).
Design
A prospective cohort of 22,302 pregnant women from 2010 to 2012 in Tianjin, China, was used to perform a nested case-control study. A total of 243 women with GDM and 243 women without GDM matched by maternal age (±1 year) were used as cases and controls, respectively. Conditional logistic regression and restricted cubic spline were used to examine the full-range risk associations between individual TMAOs metabolites at the first antenatal care visit with GDM. Trimethylamine conversion ratio (TMAR) was defined as trimethylamine (TMA)/its precursors, and trimethylamine N-oxide conversion ratio (TMAOR) was defined as TMAO/TMA. An additive interaction between high TMAR and low TMAOR indicates a state of TMA accumulation, and a mathematical interaction between high TMAR and high TMAOR indicates accumulation of TMAO.
Results
TMA was linearly associated with GDM, whereas TMA precursors and TMAO were inversely associated with GDM with clear threshold effects, i.e., 16 nmol/mL for TMAO, 200 nmol/mL for betaine, 112 nmol/mL for l-carnitine, and 110 and 270 nmol/mL for cholinechloride (a U-shaped relationship). Copresence of TMAR >0.35 and TMAOR ≤0.15 was associated with a markedly higher OR (11.16; 95% CI, 5.45 to 22.8), compared with TMAR >0.35 only (OR = 1.71; 95% CI, 0.42 to 6.95) or TMAOR ≤0.15 only (OR = 2.06; 95% CI, 1.09 to 3.90), with a significant additive interaction. However, the mathematical interaction was nonsignificant.
Conclusions
TMAO metabolites in the early pregnancy were associated with the risk of GDM, whereas TMA was more likely to play a causal role in GDM.
We found that TMAO, TMA, and its precursors were all associated with GDM. A high conversion rate to TMA and a low rate to TMAO had a synergistic effect on GDM.
The prevalence of gestational diabetes mellitus (GDM) has been steadily increasing worldwide (1), especially in low- and middle-income countries. In Tianjin, China, the prevalence of GDM increased from 2.3% in 1999 (2) to 9.3% in 2010 to 2012 (3), quadrupling over a 12-year period. Clinical assessment of GDM typically occurs during the second trimester, and accurate markers in the early pregnancy are still unavailable.
Trimethylamine N-oxide (TMAO) is a small, colorless amine oxide formed from trimethylamine (TMA), which is generated by the metabolism of gut microbiota from dietary precursors (choline, betaine, and l-carnitine) (4, 5). Animal experiments have shown that TMAO can aggravate impaired glucose tolerance and lead to hyperglycemia (6), whereas reduced plasma TMAO helps promote homeostasis of blood glucose and lipids (7). Recently, TMAO has been identified as an independent risk factor for obesity, metabolic syndrome, fatty liver disease, cancer, and cardiovascular disease (CVD) (4, 8, 9). Epidemiological studies have found that consumption of red meat is associated with an elevated risk of type 2 diabetes mellitus (T2DM) (10), and diabetes also associated with higher plasma TMAO levels (11, 12). Although GDM and T2DM share many common risk factors and pathogenesis, only a few studies have explored the role of TMAO in GDM, with inconsistent findings (13–15). It remains uncertain whether TMAO or other related metabolites such as choline, l-carnitine, or TMA play an important role in the etiology and prediction of GDM in early pregnancy.
Furthermore, plasma TMAO levels are influenced by several factors, including diet, age, and flavin monooxygenase 3 (FMO3) (8). Our group has previously reported that decreased concentrations of two secondary bile acids, deoxycholic acid (DCA) and glycoursodeoxycholic acid (GUDCA), in early pregnancy were associated with a markedly elevated risk of GDM (16). Given that both TMAO and bile acids are highly related with gut microbiota, it is of interest to explore whether decreased DCA and GUDCA mediate plausible associations between TMAOs and GDM.
In the current study, we used a case-control design nested within a population-based prospective cohort to investigate associations of individual TMAO metabolites in early pregnancy (i.e., betaine, l-carnitine, cholinechloride, TMA, and TMAO) with the risk of GDM, whether TMAO or TMA is more likely to play a causal role in the etiology of GDM, and whether these associations are mediated via decreased DCA and GUDCA.
Materials and Methods
Study design and participants
The study design was described previously (3, 16). Briefly, a population-based cohort of pregnant women was established from October 2010 to August 2012, based on a universal screening and management system for GDM in the six central urban districts of Tianjin, China, which was initiated in 1999. A total of 22,302 pregnant women were recruited when they attended the primary care hospital for registration of pregnancy and antenatal care. They were followed up longitudinally from their first antenatal care visit to the time of screening for GDM at 24 to 28 weeks’ gestational age and through the postpartum period. Ethics approval was obtained from the Ethics Committee for Clinical Research of the Tianjin Women and Children’s Health Center, Tianjin, China, and all participants provided written informed consent.
A two-step screening procedure was used to identify GDM cases. All pregnant women first underwent a 50-g 1-hour glucose challenge test (GCT) in nonfasting status at 24 to 28 weeks’ gestational age at primary care hospitals. The women with plasma glucose (PG) ≥7.8 mmol/L were referred to the GDM clinic within Tianjin Women and Children’s Health Center, where they underwent a 75-g 2-hour oral glucose tolerance test in the morning after ≥8 hours of overnight fasting. GDM was diagnosed according to the World Health Organization 2013 criteria (17) as fasting PG ≥5.1 mmol/L, 1-hour PG ≥10.0 mmol/L, or 2-hour PG ≥8.5 mmol/L.
Of the 22,302 participants, 2991 pregnant women at an early stage in the universal screening also provided overnight fasting blood samples at their first antennal care visit. We sequentially excluded 227 pregnant women because of a lack of GCT results or lack of oral glucose tolerance test results if their GCT was ≥7.8 mmol/L. Of the remaining 2764 women, 243 who developed GDM were identified as cases and 243 without GDM matched with age (±1 year of the case) were selected as controls for this age-matched nested case-control study (16). The comparison of the 2764 women (which formed the subcohort for identification of cases and controls) with the rest of the cohort was previously published (16).
Data collection procedures
Detailed data collection methods were described previously (16). Briefly, at the first antenatal care visit to the primary care hospital, standardized procedures were used to measure maternal height, weight, and blood pressure by nurses or obstetricians. Weight was measured to an accuracy of 0.1 kg and was remeasured when the GCT was performed. Because weight gain during the first trimester of pregnancy was minimal, we used body weight at the first antenatal care visit as the prepregnancy body weight to estimate prepregnancy body mass index (BMI). BMI groups at the first antenatal care visit were defined based on the criteria recommended for Chinese adults (18). Repeated measurements of body weight were performed, and the difference in body weight between the first antenatal visit and the GCT was calculated and used as the weight gain from the first antenatal care visit to the GCT time. Other data were collected via a series of structured questionnaires completed by care providers or pregnant women at their first antenatal care visit, at the time of the GCT, and at subsequent antenatal care visits, respectively.
Measurement of serum TMAOs
Liquid chromatography–tandem mass spectrometry analysis
After sample pretreatment, an Eksigent Ultra liquid chromatography 100 coupled with an AB 5600 TripleTOF system (AB Sciex) was used to quantify TMAO and related metabolites. A 4.6- × 100-mm XBridge BEH Amide column (Waters) with a 4- × 2.0-mm guard column (Phenomenex) was used to separate components. The separation was achieved under a column temperature of 40°C with a controlled gradient of mobile phase A, which consisted of 0.2% (v/v) formic acid, 5 mM acetic acid amine, and 5% (v/v) acetonitrile in water; and mobile phase B with 0.2% formic acid, 5 mM acetic acid amine, and 5% (v/v) water in acetonitrile, at a flow rate of 0.4 mL/min. The gradient flow was first set at 15% (v/v) A, linearly increased to 80% A during the next 5 minutes, and maintained at this composition for additional 2 minutes, linearly decreased to 15% A during the next 0.5 minutes, and maintained at this composition for additional 2 minutes. The injection volume of each sample was 2 µL. During the analysis of the sample sequence, one quality control sample was run after every 30 injections.
Data processing
The raw data were acquired via PeakView 1.2 software and MultiQuant 2.1 software, based on the z value and the sample retention time.
Statistical analysis
Statistical analysis was performed in SAS version 9.3 (SAS Institute Inc., Cary, NC). Quantitative data were compared between the GDM and non-GDM groups by paired Student t test or the Wilcoxon signed-rank test where the assumption of normal distribution was rejected. Categorical data were compared by McNemar test or Fisher exact test where appropriate.
Conditional binary logistic regression was performed to estimate the ORs and 95% CIs of levels of TMAOs for the risk of GDM in univariate and multivariate analyses. Because it is uncertain whether levels of individual TMAO metabolites (TMAOs) were linearly associated with the risk of GDM (19), we used the restricted cubic spline analysis nested in the conditional logistic regression analysis in univariate and multivariate analyses to examine the full-range associations between TMAO levels and the risk of GDM (20). As suggested, three knots were used in the analysis because the sample was not large (21). We identified threshold points of TMAOs at the points, if any, where GDM risk started to rise steeply, as in the previous investigations (16, 22). We further stratified TMAOs at the selected cutoff points and repeated the conditional logistic regression analysis to show these ORs of TMAOs as categorical variables. Univariate analysis to obtain ORs without adjustment for confounders was first performed and then repeated with adjustment for traditional GDM risk factors. Multivariate model 1 was adjusted for BMI at registration, family history of diabetes in first-degree relatives, systolic and diastolic blood pressure at registration, habitual smoking and drinking before and during pregnancy, gestational age (in weeks) at registration, parity (≥1), educational attainment (>12 years of school), Han nationality, alanine aminotransferase at the first antenatal care visit, and weight gain up to the time of GCT. Multivariate model 2 was adjusted for variables in model 1 and levels of DCA. Multivariate model 3 was adjusted for variables in model 1 and levels of GUDCA. Multivariate model 4 was adjusted for variables in model 1 and levels of DCA and GUDCA.
Based on the TMAO metabolic pathway (19), we created two conversion ratios: TMA conversion ratio (TMAR) and TMAO conversion ratio (TMAOR). TMAR was calculated as TMA divided by the sum of betaine, l-carnitine, and cholinechloride; and TMAOR was calculated as TMAO divided by TMA. We then used the restricted cubic spline curve to identify the threshold points of the two ratios for the risk of GDM. If TMA is the effector for GDM, copresence of a high TMAR and a low TMAOR suggests a state of TMA accumulation and is therefore expected to result in a greatly elevated risk of GDM. An additive interaction between high TMAR and low TMAOR serves the purpose, which is estimated by three measures: relative excess risk due to the interaction (RERI), attributable proportion due to the interaction (AP), and synergy index (SI), with 95% CIs estimated by the delta method (23). Significant RERI >0, AP >0, or SI >1 indicates an additive interaction. In other words, a significant additive interaction suggests that the risk due to exposure to both risk factors is significantly higher than the summation of risks due to exposure to either of them. If TMAO is the effector for GDM, we would expect to find that the overall conversion rate from TMA precursors to TMAO via TMA is significant. The mathematical interaction calculated as TMAR × TMAOR serves the purpose.
In a previous analysis, we found that GUDCA at ≤0.07 nmol/mL and DCA at ≤0.28 nmol/mL had threshold effects, and levels of these two metabolites below the listed cutoff points were strongly associated with an elevated risk of GDM (16). We further set out to determine whether the elevated GDM risk associated with individual TMAOs was mediated by DCA and GUDCA. Then, we performed conditional logistic regression to look for any changes in the ORs of GDM associated with TMAOs, before and after further adjustment for DCA and/or GUDCA, to investigate whether low DCA or low GUCDA was accountable for the effects of individual TMAOs on GDM risk.
Finally, we calculated the areas under the receiver operating characteristic curves (AUCs) to check whether further inclusion of TMAOs would increase the AUCs from a model that included the traditional GDM risk factors only, and a model that included traditional GDM risk factors plus DCA and GUDCA. In all the analyses, a P <0.05 was considered statistically significant.
Results
Characteristics of the study participants
The mean age of participants was 29.2 (SD 3.1) years. These women had a median gestational age of 10.0 (interquartile range 9.0 to 11.0) weeks at their first antenatal care visit. Women with GDM had higher body weight, BMI, systolic and diastolic blood pressure, and alanine aminotransferase at their first antenatal care visit and higher blood glucose at GCT. Women without GDM were less likely to have a family history of diabetes in their first-degree relatives. There were no significant differences in body height, gestational age at registration, proportions of ethnic Han Chinese, educational levels, parity, percentage of habitual smokers and alcohol drinkers, and weight gain from registration to GCT between groups (Table 1). Compared with women without GDM, women with GDM had significantly lower concentrations of betaine, l-carnitine, cholinechloride, and TMAO but had higher concentrations of TMA (Table 1). (Hereafter, the terms betaine,l-carnitine, cholinechloride, TMA, and TMAO will be used to describe their concentrations.)
Table 1.
Clinical and Biochemical Characteristics of the Women Included in the Study
| Characteristics | Non-GDM | GDM | P |
|---|---|---|---|
| No. of subjects | 243 | 243 | |
| Variables at registration | |||
| Age, y | 29.2 ± 3.3 | 29.2 ± 2.7 | 1.0000 |
| Height, cm | 163.2 ± 4.6 | 163.1 ± 5.0 | 0.2796 |
| Weight, kg | 58.2 ± 9.6 | 63.7 ± 10.5 | <0.0001 |
| BMI, kg/m2 | 21.8 ± 3.4 | 23.9 ± 3.6 | <0.0001 |
| BMI in categorya | <0.0001 | ||
| <18.5 kg/m2 | 33 (13.6) | 9 (3.7) | |
| ≥18.5 to <24.0 kg/m2 | 153 (63.0) | 126 (51.9) | |
| ≥24.0 to <28.0 kg/m2 | 45 (18.5) | 77 (31.7) | |
| ≥28.0 kg/m2 | 12 (4.9) | 31 (12.8) | |
| Gestational age, wk | 10.1 ± 2.0 | 10.1 ± 2.1 | 0.9428 |
| Diastolic BP, mm Hg | 67.9 ± 7.7 | 70.6 ± 8.0 | <0.0001 |
| Systolic, mm Hg | 104.0 ± 10.5 | 108.3 ± 10.5 | <0.0001 |
| Han nationality | 234 (96.3) | 238 (97.9) | 0.2850 |
| Family history of diabetes in first-degree relatives | 14 (5.8) | 30 (12.3) | 0.0136 |
| Education >12 y | 132 (54.3) | 135 (55.6) | 0.7797 |
| Parity ≥1 | 12 (4.9) | 14 (5.8) | 0.6831 |
| Habitual smokerb | 13 (5.3) | 15 (6.2) | 0.6949 |
| Alcohol drinker | 57 (23.5) | 72 (29.6) | 0.7415 |
| Alanine aminotransferase, U/L | 16.0 (10.7–21.0) | 19.0 (14.0–26.0) | <0.0001 |
| Betaine, nmol/mL | 290.4 (244.2–378.8) | 229.7 (195.6–279.9) | <0.0001 |
| ≤200 nmol/mL | 19 (7.8) | 71 (29.2) | <0.0001 |
| L-carnitine, nmol/mL | 210.0 (165.8–247.4) | 125.6 (101.7–163.4) | <0.0001 |
| ≤112 nmol/mL | 10 (4.1) | 91 (37.4) | <0.0001 |
| Cholinechloride, nmol/mL | 157.6 (117.8–212.2) | 111.9 (82.9–154.6) | <0.0001 |
| ≤110 nmol/mL | 52 (21.4) | 119 (49.0) | <0.0001 |
| >110 to ≤270 nmol/mL | 174 (71.6) | 103 (42.4) | |
| >270 nmol/mL | 17 (7.0) | 21 (8.6) | |
| TMA, nmol/mL | 114.5 (67.8–199.4) | 209.8 (119.1–358.4) | <0.0001 |
| TMAO, nmol/mL | 18.6 (13.4–27.1) | 10.1 (6.1–17.9) | <0.0001 |
| ≤16 nmol/mL | 90 (37.0) | 169 (69.5) | <0.0001 |
| DCA, nmol/mL | 0.26 (0.15–0.45) | 0.20 (0.10–0.33) | 0.0019 |
| <0.280 nmol/mL | 129 (53.1) | 161 (66.8) | 0.0025 |
| GUDCA, nmol/mL | 0.03 (0.02–0.06) | 0.02 (0.01–0.03) | <0.0001 |
| ≤0.070 nmol/mL | 190 (78.5) | 220 (95.7) | <0.0001 |
| Variables at GCT | |||
| Habitual smoker during pregnancyb | 1 (0.4) | 2 (0.8) | 1.0000 |
| Alcohol drinker during pregnancy | 3 (1.2) | 2 (0.8) | 1.0000 |
| GCT glucose, mmol/L | 6.3 (5.4–7.2) | 9.0 (8.4–10.0) | <0.0001 |
| Weight, kg | 66.7 ± 9.7 | 71.9 ± 10.8 | <0.0001 |
| Weight gain up to GCT, kg | 8.7 ± 3.2 | 8.4 ± 3.6 | 0.1125 |
Data are presented as means ± SD, median (interquartile range), or n (%).
Abbreviation: BP, blood pressure.
BMI groups at the first antenatal care visit were defined based on the criteria recommended for Chinese adults: underweight (<18.5 kg/m2), normal weight (18.5–23.9 kg/m2), overweight (24.0–27.9 kg/m2), and obese (≥28.0 kg/m2).
Habitual smoking before or during pregnancy was defined as continuously smoking one or more cigarettes per day for ≥6 months before pregnancy or smoking one or more cigarettes per day during pregnancy.
Associations between TMAOs and GDM
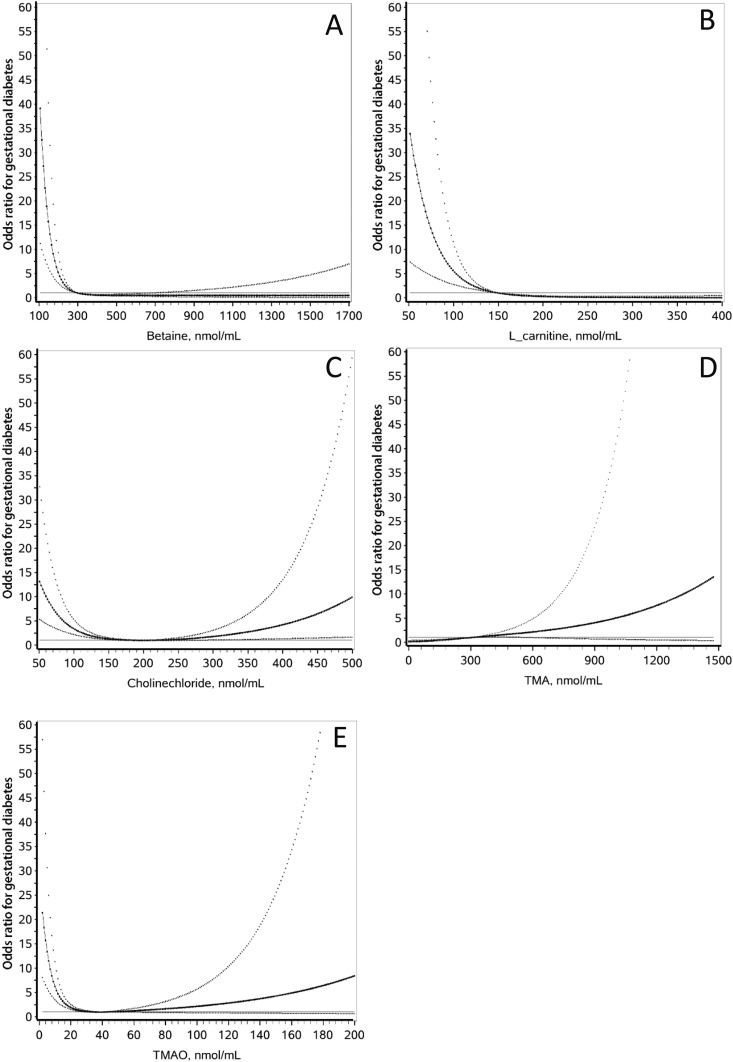
TMA was linearly associated with the risk of GDM (Fig. 1). The increase per SD of TMA was associated with a 2.06-fold increase in the risk of GDM in the univariate analysis (95% CI, 1.60 to 2.65) and a 2.21-fold (95% CI, 1.60 to 3.04) increase in risk in multivariate analysis (Table 2). There was a U-shaped association between cholinechloride and the risk of GDM. Using 110 to 270 nmol/mL as the reference group, both low cholinechloride at ≤110 nmol/mL (OR = 4.87; 95% CI, 2.74 to 8.65) and high cholinechloride at >270 nmol/mL (OR = 3.22; 95% CI, 1.31 to 7.96) were associated with an elevated risk of GDM after adjustment for traditional GDM risk factors. TMAO, betaine, and l-carnitine were inversely associated with elevated risks of GDM, with clear threshold effects, although TMAO tended to be associated with GDM in a U-shaped manner. Decreased TMAO at ≤16 nmol/mL, betaine at ≤200 nmol/mL, and l-carnitine at ≤112 nmol/mL were associated with rapid increases in the risk of GDM. With TMAOs used as categorical variables, TMAO ≤16 nmol/mL was associated with a markedly elevated risk of GDM in the univariate analysis (OR = 3.39; 95% CI, 2.30 to 5.00) and multivariate analysis (OR = 3.21; 95% CI, 2.04 to 5.06) (Table 2). Similarly, betaine at ≤200 nmol/mL was also associated with a markedly elevated risk of GDM in the univariate analysis (OR = 5.00; 95% CI, 2.76 to 9.07) and multivariate analysis (OR = 4.88; 95% CI, 2.51 to 9.50), and l-carnitine at ≤112 nmol/L was associated with an elevated risk of GDM in the univariate analysis (OR = 14.5; 95% CI, 6.34 to 33.2) and multivariate analysis (OR = 14.7; 95% CI, 6.05 to 35.9).
Figure 1.
Associations between individual TMAO metabolites and the risk of GDM in Chinese women. The straight lines are the reference lines at OR = 1. The solid curves and the dotted curves stand for the ORs and their 95% CIs for GDM, respectively. All curves were derived from multivariate analyses. (A) Betaine, (B) l-carnitine, and (E) TMAO were inversely associated with the risk of GDM. (C) The U-shaped association between cholinechloride and the risk of GDM. (D) TMA is linearly associated with the risk of GDM. All metabolites, except for TMA, have a clear threshold effect on GDM.
Table 2.
ORs of GDM by TMAOs
| OR (95% CI) | P | |
|---|---|---|
| Univariate model | ||
| Betaine ≤200 vs >200 nmol/mL | 5.00 (2.76–9.07) | <0.0001 |
| l-carnitine ≤112 vs >112 nmol/mL | 14.5 (6.34–33.2) | <0.0001 |
| Cholinechloride | ||
| ≤110 nmol/mL | 4.27 (2.67–6.81) | <0.0001 |
| >110 ≤ ∼270 nmol/mL | 1.00 | |
| >270 nmol/mL | 2.74 (1.32–5.69) | 0.0070 |
| TMA, per SD | 2.06 (1.60–2.65) | <0.0001 |
| TMAO ≤16 vs >16 nmol/mL | 3.39 (2.30–5.00) | <0.0001 |
| Multivariate model 1 | ||
| Betaine ≤200 vs >200 nmol/mL | 4.88 (2.51–9.50) | <0.0001 |
| l-carnitine ≤112 vs >112 nmol/mL | 14.7 (6.05–35.9) | <0.0001 |
| Cholinechloride | ||
| ≤110 nmol/mL | 4.87 (2.74–8.65) | <0.0001 |
| ≥110 nmol/mL ≤ ∼270 nmol/mL | 1.00 | |
| >270 nmol/mL | 3.22 (1.31–7.96) | 0.0112 |
| TMA, per SD | 2.21 (1.60–3.04) | <0.0001 |
| TMAO ≤16 vs >16 nmol/mL | 3.21 (2.04–5.06) | <0.0001 |
| Multivariate model 2 | ||
| Betaine ≤200 vs >200 nmol/mL | 4.62 (2.35–9.08) | <0.0001 |
| l-carnitine ≤112 vs >112 nmol/mL | 13.51 (5.50–33.2) | <0.0001 |
| Cholinechloride | ||
| ≤110 nmol/mL | 4.57 (2.54–8.22) | <0.0001 |
| ≥110 to ≤∼270 nmol/mL | 1.00 | |
| >270 nmol/mL | 3.11 (1.24–7.80) | 0.0150 |
| TMA, per SD | 2.22 (1.60–3.07) | <0.0001 |
| TMAO ≤ vs >16 nmol/mL | 2.89 (1.82–4.58) | <0.0001 |
| Multivariate model 3 | ||
| Betaine ≤200 vs >200 nmol/mL | 4.44 (2.19–9.00) | <0.0001 |
| l-carnitine ≤112 vs >112 nmol/mL | 12.55 (5.09–30.9) | <0.0001 |
| Cholinechloride, | ||
| ≤110 nmol/mL | 3.75 (2.10–6.72) | <0.0001 |
| ≥110 to ≤∼270 nmol/mL | 1.00 | |
| >270 nmol/mL | 3.54 (1.35–9.24) | 0.0100 |
| TMA, per SD | 2.11 (1.51–2.95) | <0.0001 |
| TMAO ≤16 vs >16 nmol/mL | 3.36 (2.05–5.52) | <0.0001 |
| Multivariate model 4 | ||
| Betaine ≤200 vs >200 nmol/mL | 4.20 (2.06–8.53) | <0.0001 |
| l-carnitine ≤112 vs >112 nmol/mL | 11.3 (4.58–28.1) | <0.0001 |
| Cholinechloride, | ||
| ≤110 nmol/mL | 3.52 (1.94–6.38) | <0.0001 |
| ≥110 to ≤∼270 nmol/mL | 1.00 | |
| >270 nmol/mL | 3.42 (1.29–9.08) | 0.0130 |
| TMA, per SD | 2.10 (1.50–2.93) | <0.0001 |
| TMAO ≤16 vs >16 nmol/mL | 3.05 (1.84–5.05) | <0.0001 |
Multivariate model 1 was adjusted for BMI at registration, family history of diabetes in first-degree relatives, systolic and diastolic blood pressure at registration, habitual smoking and drinking before and during pregnancy, gestational age in weeks at registration, parity (≥1), educational attainment (>12 years of school), Han nationality, alanine aminotransferase at the first antenatal care visit, and weight gain up to the time of GCT.
Multivariate model 2 was adjusted for variables in model 1 and concentrations of DCA.
Multivariate model 3 was adjusted for variables in model 1 and concentrations of GUDCA.
Multivariate model 4 was adjusted for variables in model 1 and concentrations of DCA and GUDCA.
Associations of TMAR and TMAOR with risk of GDM
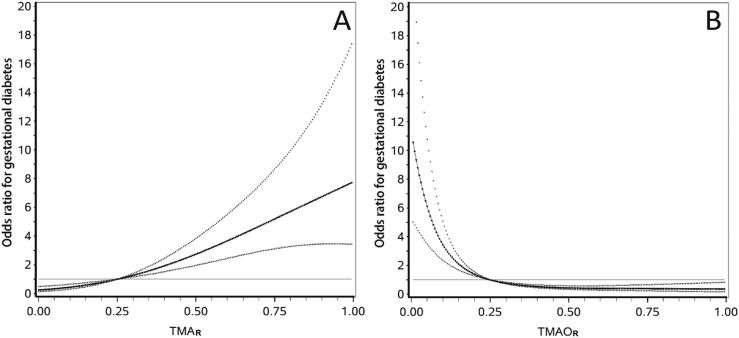
TMAR was positively associated with the risk of GDM (Fig. 2), and the ratio >0.35 was associated with a markedly increased risk of GDM in both univariate analysis (OR = 5.95; 95% CI, 3.71 to 9.55) and multivariate analysis (OR = 6.54; 95% CI, 3.68 to 11.6) (Table 3). On the other hand, TMAOR was inversely associated with the risk of GDM. TMAOR ≤0.15 was associated with a markedly elevated risk of GDM in both univariate analysis (OR = 4.33; 95% CI, 2.78 to 6.75) and multivariate analysis (OR = 4.56; 95% CI, 2.69 to 7.71). Copresence of TMAR >0.35 and TMAOR ≤0.15 greatly increased the ORs of TMAR >0.35 only (OR = 1.71; 95% CI, 0.42 to 6.95) and TMAOR ≤0.15 only (OR = 2.06; 95% CI, 1.09 to 3.90), to an OR of 11.16 (95% CI, 5.45 to 22.8). The additive interaction measures were significant (RERI = 8.38; 95% CI, 0.88 to 15.88; AP = 0.75; 95% CI, 0.49 to 1.02; and SI = 5.72; 95% CI, 1.18 to 27.72) (Table 3). However, the mathematical interaction between TMAR >0.35 and TMAOR >0.15 was not statistically significant.
Figure 2.
(A and B) Associations of TMAR and TMAOR with the risk of GDM in Chinese women. TMAR is defined as TMA/(betaine + l-carnitine + cholinechloride), and TMAOR is defined as TMAO/TMA. The curves are derived from the multivariate analysis. The straight lines are the reference lines at OR = 1. The solid curves and the dotted curves stand for the ORs and their 95% CIs for GDM, respectively. The TMAR curve shows that increase in TMAR is positively associated with the risk of GDM, and the risk of GDM rapidly increases in a linear manner from TMAR = 0.35 upwards. The TMAOR curve shows that TMAOR is inversely associated with the risk of GDM, and the risk of GDM rapidly increases from TMAOR = 0.15 downwards.
Table 3.
ORs of GDM by TMAR and TMAOR
| Cases/Subtotal | OR or Estimate (95% CI) | P | |
|---|---|---|---|
| Univariate model | |||
| TMA ratio >0.35 vs ≤0.35 | 144/189 vs 99/297 | 5.95 (3.71–9.55) | <0.0001 |
| TMAO ratio ≤0.15 vs >0.15 | 190/300 vs 53/186 | 4.33 (2.78–6.75) | <0.0001 |
| Multivariate model 1 | |||
| TMA ratio >0.35 vs ≤0.35 | 144/189 vs 99/297 | 6.54 (3.68–11.6) | <0.0001 |
| TMAO ratio ≤0.15 vs >0.15 | 190/300 vs 53/186 | 4.56 (2.69–7.71) | <0.0001 |
| Univariate additive interaction model | |||
| TMAR >0.35 and TMAOR ≤0.15 | 137/176 | 9.37 (5.25–16.7) | <0.0001 |
| TMAR >0.35 and TMAOR >0.15 | 7/13 | 2.93 (0.85–10.2) | 0.0900 |
| TMAR ≤0.35 and TMAOR ≤0.15 | 53/124 | 2.13 (1.24–3.66) | 0.0065 |
| TMAR ≤0.35 and TMAOR >0.15 | 46/173 | 1.00 | |
| AP | 0.57 (0.14–0.99) | 0.0088 | |
| RERI | 5.32 (–0.31,10.9) | 0.0641 | |
| SI | 2.74 (0.77–9.79) | 0.4470 | |
| Multivariate additive interaction model | |||
| TMAR > 0.35 and TMAOR ≤0.15 | 137/176 | 11.16 (5.45–22.8) | <0.0001 |
| TMAR >0.35 and TMAOR >0.15 | 7/13 | 1.71 (0.42–6.95) | 0.4507 |
| TMAR ≤0.35 and TMAOR ≤0.15 | 53/124 | 2.06 (1.09–3.90) | 0.0261 |
| TMAR ≤0.35 and TMAOR >0.15 | 46/173 | 1.00 | |
| AP | 0.75 (0.49–1.02) | <0.0001 | |
| RERI | 8.38 (0.88–15.9) | 0.0286 | |
| SI | 5.72 (1.18–27.7) | — | |
| Univariate mathematical interaction model | |||
| TMAR ×TMAOR | — | 0.67 (0.17–2.61) | 0.5592 |
| TMAR >0.35 vs ≤0.35 | 144/189 vs 99/297 | 4.41 (2.50–7.76) | <0.0001 |
| TMAOR >0.15 vs ≤0.15 | 190/300 vs 53/186 | 0.47 (0.27–0.81) | 0.0065 |
| Multivariate mathematical interaction model | |||
| TMAR × TMAOR | — | 0.32 (0.07–1.53) | 0.1524 |
| TMAR >0.35 vs ≤0.35 | 144/189 vs 99/297 | 5.41 (2.69–10.9) | <0.0001 |
| TMAOR >0.15 vs ≤0.15 | 190/300 vs 53/186 | 0.48 (0.26–0.92) | 0.0261 |
Significant RERI >0, AP >0, or SI >1 indicates an additive interaction.
Multivariate models were adjusted for BMI at registration, family history of diabetes in first-degree relatives, systolic and diastolic blood pressure at registration, habitual smoking and drinking before and during pregnancy, gestational age in weeks at registration, parity (≥1), educational attainment (>12 years of school), Han nationality, alanine aminotransferase at the first antenatal care visit, and weight gain up to the time of GCT.
Changes in ORs of TMAOs before and after adjustment for bile acids
Adjustment for DCA ≤0.28 nmol/mL (multivariate model 2), GUDCA ≤0.07 nmol/mL (multivariate model 3), or both (multivariate model 4) all slightly attenuated the ORs of betaine ≤200 vs >200 nmol/mL, l-carnitine ≤112 vs >112 nmol/mL, cholinechloride ≤110 nmol/mL or >270 vs ≥110 ≤ ∼270, TMA per SD, and TMAO ≤16 vs >16 nmol/mL, but their statistical significance persisted after the adjustments (Table 2).
Increased predictive values of TMAOs for GDM
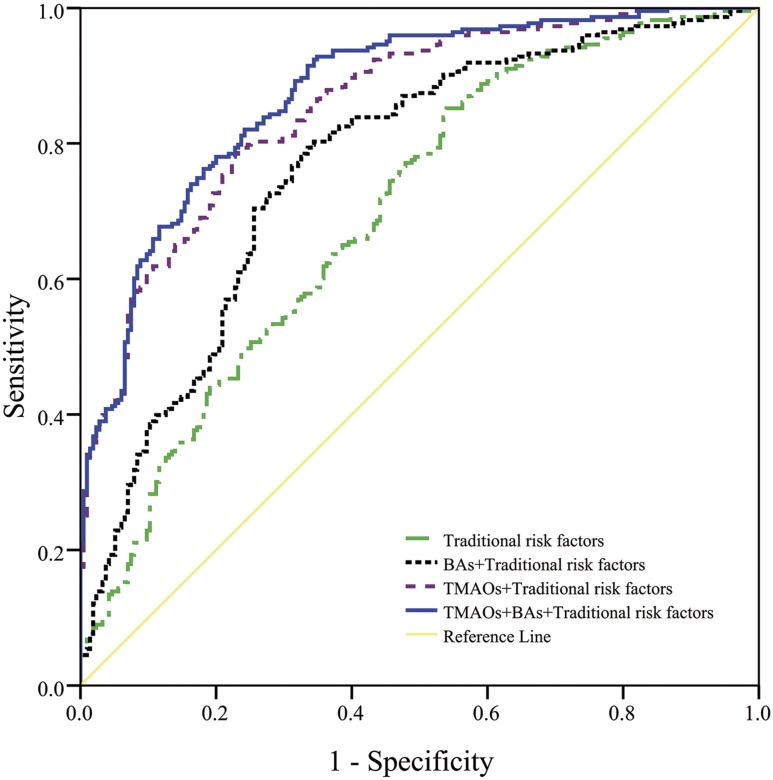
Inclusion of TMAOs in the models markedly increased the AUCs for the model including only traditional risk factors, from 0.70 (95% CI, 0.65 to 0.75), to 0.77 (95% CI, 0.72 to 0.81) for a model including traditional risk factors plus bile acids (DCA and GUDCA). This value was further increased to 0.86 (95% CI, 0.82 to 0.89) for the model including traditional risk factors plus TMAOs (betaine, l-carnitine, cholinechloride, TMA, and TMAO) and to 0.88 (95% CI, 0.84 to 0.91) for the model incorporating traditional risk factors, bile acids, and TMAOs (P < 0.0001 for comparison of any pairs of the three models) (Fig. 3).
Figure 3.
Receiver operating characteristic curves of traditional risk factors, bile acids plus TMAOs for GDM in Chinese women. The green (dash-dot) curve indicates the traditional risk factor model (multivariate model 1 in Table 2), the black (dense dot) curve indicates the traditional risk factor plus bile acids (BAs) (i.e., DCA and GUDCA) model, the purple (sparse dot) curve indicates the traditional risk factor plus TMAOs model (i.e., betaine, l-carnitine, cholinechloride, TMA, and TMAO), and the blue (solid) curve indicates the traditional risk factor plus BAs and TMAOs model.
Discussion
The associations between TMAO and lifestyle-related diseases including T2DM and CVDs are inconsistent and inconclusive, although some studies observed that elevated TMAO was associated with risks of CVD and T2DM (9, 24, 25). For example, a Norwegian study observed an inverse association between plasma concentrations of betaine and an increased risk of T2DM, but the association between TMAO and T2DM was not significant (26). To date, only a few studies have investigated the association between TMAO and GDM risk, and these studies did not produce consistent results. One metabolomics study of pregnant women in the second trimester (n = 54) found that increased urine choline concentrations and decreased concentrations of plasma TMAO and betaine were associated with an increased risk of GDM (13). A Canadian study reported that women with GDM had slightly lower blood concentrations of betaine and TMAO than the control group, although the difference was small and nonsignificant (27). On the other hand, another study of Chinese pregnant women used a nested case-control study design and reported that higher plasma TMAO in the second or third trimester of pregnancy was associated with the increased odds of GDM (15). There are several possible explanations to the different observations regarding the association between TMAO and the risk of GDM. First, the association between TMAO and the risk of GDM seems to be U-shaped, too. Because many factors such as diet are associated with plasma concentrations of TMAO (8), it is worthwhile to investigate whether distributions of TMAO by populations can account for the different associations between TMAO and the risk of GDM in different populations. In this regard, if the distribution of TMAO shifted to the right side, increased TMAO could be associated with an increased risk of GDM. Second, concentrations of TMAO and its metabolites may change during the course of pregnancy. If the distribution of TMAO increased with advancing gestation, TMAO concentrations in later pregnancy could be associated with an increased risk of GDM. Third, the Chinese study did not measure TMA and other TMAO metabolites. Other unadjusted confounders in that Chinese study, such as other TMAO metabolites, may also contribute to the different observations from ours. Our study identified that low TMAO and its metabolites in early pregnancy, including betaine, l-carnitine, and cholinechloride, had threshold effects, and GDM risk sharply increased with decreasing concentrations of these metabolites below certain thresholds.
We found that the copresence of TMAR >0.35 and TMAOR ≤0.15 was associated with a markedly elevated risk of GDM, and the risk due to exposure to both risk factors was significantly greater than the summation of risks due to exposure to either of them alone. It is well known that the conversion from betaine, choline, and l-carnitine to TMA depends on gut microbiota, and the conversion from TMA to TMAO depends on activity of FMOs, especially FMO3 in the liver (4, 5). Presumably, the TMAR is proportional to the rate of gut microbiota transforming these precursors to TMA, whereas TMAOR is proportional to the rate of FMOs transforming TMA to TMAO in the liver. A high TMAR and a low TMAOR were expected to result in a cumulative state of TMA. We demonstrated that this state was associated with a markedly elevated risk of GDM. According to Rothman’s multicausality theory (28), the significant additive interaction suggests that abnormal gut microbiota and decreased activity of FMOs in the liver may be among the component causes of GDM, and copresence of both risk factors constitutes a “sufficient” cause, which results in increased TMA and then the elevated risk of GDM.
How does an increase in serum TMA increase the risk of GDM? Very few studies have investigated TMA in the etiology of GDM, and the biological link between TMA and GDM is still elusive. TMA-induced intracellular calcium mobilization from endoplasmic reticulum (ER) calcium stores contributes toward ER stress (29). Interestingly, ER stress is emerging as an important mechanism involved in many metabolic diseases, such as obesity and diabetes. ER stress can lead to aberrant transcriptional regulation, altered gene expression, ion channel failure, dysmetabolism, impaired signaling, oxidative stress, and inflammation (30, 31). Thus, elevated TMA can result in downstream effects that increase ER stress. There are also epidemiological studies showing that TMA is associated with coronary heart disease. For example, in patients with HIV, TMA (but not TMAO) was associated with atherosclerotic plaque formation (32). Accumulation of TMA can be explained by the following mechanisms. First, TMA is generated by intestinal microbiota in the gut from betaine, l-carnitine, and its metabolites butyrobetaine, choline, and other choline-containing compounds, which are present in the diet (33), and a lack of probiotics can increase the generation of TMA in the gut (8). Second, FMO3 is the main enzyme responsible for conversion of TMA into TMAO. Homozygous or compound heterozygous loss of function mutations in the FMO3 gene result in an excess of odorous TMA. Thus, downregulation or knockout of FMO3 results in trimethylaminuria, which is characterized by fishy odor and could cause inflammation (34). Indeed, it is worthwhile to investigate the plausible mechanism linking TMA to GDM.
We cannot exclude that some TMA precursors may play a role in the etiology of GDM. For example, choline is transformed into TMA by the action of the enzyme choline TMA lyase, whereas betaine is reduced to TMA by betaine reductase in a coupled reduction-oxidation reaction where it acts as an electron acceptor. Alternatively, choline can be transformed into betaine by the sequential action of two enzymes (choline dehydrogenase and betaine aldehyde dehydrogenase) (24, 34). Several lines of evidence suggest a role of choline in regulating insulin resistance and glucose metabolism (35–37). Our study indicated that both betaine and l-carnitine had a clear threshold effect, and decreasing concentrations of betaine at ≤200 nmol/mL and l-carnitine at ≤112 nmol/mL were associated with a marked increase in the risk of GDM, independently of TMA and TMAO. Consistently, dietary intake of choline and betaine does not render people susceptible to developing CVD and may actually prevent CVD by attenuating inflammation (38), possibly via reduced lipid accumulation in liver, heart, and arterial tissues (39).
Both TMAO metabolites and bile acid metabolites are highly associated with gut microbiota. Sun et al. (40) found that metformin improves hyperglycemia partially via a Bacteroides fragilis–GUDCA–intestinal farnesoid X-activated receptor axis. In this regard, our group reported that low GUDCA was associated with an increased risk of GDM (16). However, our observations of very small decreases in the ORs of TMAOs for GDM by adjustment for DCA and GUDCA suggest that the effects of TMAOs were largely independent of DCA and GUDCA.
Our study has several limitations. First, detailed lifestyle factors such as dietary intake were not available for adjustment, which might have potential confounding effects. Second, some women with GDM might have been missed because of the two-step GDM screening procedure. Misclassification of GDM as non-GDM is more likely to lead to underestimation of the effect size. Third, some TMA precursors such as ergothioneine were not available to the analysis, and their impacts on the estimated ORs were unknown. Fourth, our study was a nested case-control study, and the findings can be used only to generate hypotheses. The real predictive values of TMAO metabolites for prediction of GDM need further confirmation in population-based cohort studies.
Our observations have important public health significance and clinical implications. Inclusion of TMAOs in the traditional GDM risk factor model can substantially increase the accuracy of prediction (e.g., 0.86 in the AUC with inclusion of TMAOs and 0.88 with inclusion of TMAOs and bile acids into the model in contrast to 0.836 from the current GDM screening test at 24 to 28 weeks’ gestation) (41). If TMA plays a causal role in GDM, intervention measures aimed at reducing the conversion from TMA precursors to TMA, and at increasing the conversion from TMA to TMAO, are expected to result in a large decrease in the risk of GDM.
In summary, TMAOs in early pregnancy were highly predictive of GDM in Chinese pregnant women. Copresence of TMAR >0.35 and TMAOR ≤0.15 had a large synergistic effect on the risk of GDM. The additive interaction suggests that it is TMA, rather than TMAO, that is more likely to play a role in the etiology of GDM. These effects on GDM risk were largely independent of bile acid metabolites. Future studies are warranted to develop GDM predicting tools that include TMAO metabolites as predictors. Future studies are also warranted to investigate the biological mechanisms underlying the additive interaction between high TMAR and low TMAOR for the development of GDM.
Acknowledgments
The authors thank all doctors, nurses, and research staff at the primary care hospitals, six district-level women and children’s health centers, and Tianjin Women and Children’s Health Center for their participation in this study.
Financial Support: This research was supported by the National Key Research and Development Program of China [grant no. 2018YFC1313900 and 2018YFC1313903 to (X.Y.)] and the National Natural Science Foundation of China [grant no. 81870549 to (X.Y.)]. G.H. was partly supported by the grants from the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK100790) and the National Institute of General Medical Sciences (U54GM104940) of the National Institutes of Health.
Author Contributions: X.Y. and Z.-Z.F. conceived the idea and designed the study; P.S., J. Leng, and W.L. collected the data; Y.-F.C., S.-N.L., and K.Y. conducted the measurement of bile acids; J. Li, X.H., and X.Y. analyzed the data; and X.H., J. Li and X.Y. wrote the first draft. All authors gave critical comments and contributed to the writing of the manuscript and agreed to submit and publish the manuscript. X.Y., X.H, and J. Li take full responsibility for the work as a whole, including the study design, access to the data, and decision to submit.
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Glossary
Abbreviations:
- AP
attributable proportion due to the interaction
- AUC
area under the receiver operating characteristic curve
- BMI
body mass index
- CVD
cardiovascular disease
- DCA
deoxycholic acid
- ER
endoplasmic reticulum
- FMO3
flavin monooxygenase 3
- GCT
glucose challenge test
- GDM
gestational diabetes mellitus
- GUDCA
glycoursodeoxycholic acid
- PG
plasma glucose
- RERI
relative excess risk due to the interaction
- SI
synergy index
- T2DM
type 2 diabetes mellitus
- TMA
trimethylamine
- TMAO
trimethylamine N-oxide
- TMAOR
trimethylamine N-oxide conversion ratio
- TMAR
trimethylamine conversion ratio
References and Notes
- 1. Guariguata L, Linnenkamp U, Beagley J, Whiting DR, Cho NH. Global estimates of the prevalence of hyperglycaemia in pregnancy. Diabetes Res Clin Pract. 2014;103(2):176–185. [DOI] [PubMed] [Google Scholar]
- 2. Yang X, Hsu-Hage B, Zhang H, Yu L, Dong L, Li J, Shao P, Zhang C. Gestational diabetes mellitus in women of single gravidity in Tianjin City, China. Diabetes Care. 2002;25(5):847–851. [DOI] [PubMed] [Google Scholar]
- 3. Leng J, Shao P, Zhang C, Tian H, Zhang F, Zhang S, Dong L, Li L, Yu Z, Chan JC, Hu G, Yang X. Prevalence of gestational diabetes mellitus and its risk factors in Chinese pregnant women: a prospective population-based study in Tianjin, China. PLoS One. 2015;10(3):e0121029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Velasquez MT, Ramezani A, Manal A, Raj DS. Trimethylamine N-oxide: the good, the bad and the unknown. Toxins (Basel). 2016;8(11):326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Canyelles M, Tondo M, Cedó L, Farràs M, Escolà-Gil JC, Blanco-Vaca F. Trimethylamine N-oxide: a link among diet, gut microbiota, gene regulation of liver and intestine cholesterol homeostasis and HDL function. Int J Mol Sci. 2018;19(10):3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gao X, Liu X, Xu J, Xue C, Xue Y, Wang Y. Dietary trimethylamine N-oxide exacerbates impaired glucose tolerance in mice fed a high fat diet. J Biosci Bioeng. 2014;118(4):476–481. [DOI] [PubMed] [Google Scholar]
- 7. Shih DM, Wang Z, Lee R, Meng Y, Che N, Charugundla S, Qi H, Wu J, Pan C, Brown JM, Vallim T, Bennett BJ, Graham M, Hazen SL, Lusis AJ. Flavin containing monooxygenase 3 exerts broad effects on glucose and lipid metabolism and atherosclerosis. J Lipid Res. 2015;56(1):22–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Janeiro MH, Ramírez MJ, Milagro FI, Martínez JA, Solas M. Implication of trimethylamine N-oxide (TMAO) in disease: potential biomarker or new therapeutic target. Nutrients. 2018;10(10):1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368(17):1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim Y, Keogh J, Clifton P. A review of potential metabolic etiologies of the observed association between red meat consumption and development of type 2 diabetes mellitus. Metabolism. 2015;64(7):768–779. [DOI] [PubMed] [Google Scholar]
- 11. Tang WH, Wang Z, Li XS, Fan Y, Li DS, Wu Y, Hazen SL. Increased trimethylamine N-oxide portends high mortality risk independent of glycemic control in patients with type 2 diabetes mellitus. Clin Chem. 2017;63(1):297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Obeid R, Awwad HM, Rabagny Y, Graeber S, Herrmann W, Geisel J. Plasma trimethylamine N-oxide concentration is associated with choline, phospholipids, and methyl metabolism. Am J Clin Nutr. 2016;103(3):703–711. [DOI] [PubMed] [Google Scholar]
- 13. Diaz SO, Pinto J, Graça G, Duarte IF, Barros AS, Galhano E, Pita C, Almeida MC, Goodfellow BJ, Carreira IM, Gil AM. Metabolic biomarkers of prenatal disorders: an exploratory NMR metabonomics study of second trimester maternal urine and blood plasma. J Proteome Res. 2011;10(8):3732–3742. [DOI] [PubMed] [Google Scholar]
- 14. Heianza Y, Sun D, Li X, DiDonato JA, Bray GA, Sacks FM, Qi L. Gut microbiota metabolites, amino acid metabolites and improvements in insulin sensitivity and glucose metabolism: the POUNDS Lost trial. Gut. 2019;68(2):263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li P, Zhong C, Li S, Sun T, Huang H, Chen X, Zhu Y, Hu X, Peng X, Zhang X, Bao W, Shan Z, Cheng J, Hu FB, Yang N, Liu L. Plasma concentration of trimethylamine-N-oxide and risk of gestational diabetes mellitus. Am J Clin Nutr. 2018;108(3):603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li J, Huo X, Cao YF, Li SN, Du Z, Shao P, Leng J, Zhang C, Sun XY, Ma RCW, Fang ZZ, Yang X. Bile acid metabolites in early pregnancy and risk of gestational diabetes in Chinese women: a nested case-control study. EBioMedicine. 2018;35:317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, Dyer AR, Leiva A, Hod M, Kitzmiler JL, Lowe LP, McIntyre HD, Oats JJ, Omori Y, Schmidt MI; International Association of Diabetes and Pregnancy Study Groups Consensus Panel. International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen C, Lu FC; Department of Disease Control Ministry of Health, PR China. The guidelines for prevention and control of overweight and obesity in Chinese adults. Biomed Environ Sci. 2004;17(Suppl):1–36. [PubMed] [Google Scholar]
- 19. Huo X, Li J, Cao Y-F, Li S-N, Shao P, Leng J, Li W, Liu J, Yang K, Ma RCW, Hu G, Fang Z-Z, Yang X. Data from: Trimethylamine N-oxide metabolites in early pregnancy and risk of gestational diabetes: a nested case-control study. ResearchGate 2019. Accessed 2 July 2019. 10.13140/RG.2.2.31888.87049. [DOI] [PMC free article] [PubMed]
- 20. Yang X, So W, Ko GT, Ma RC, Kong AP, Chow CC, Tong PC, Chan JC. Independent associations between low-density lipoprotein cholesterol and cancer among patients with type 2 diabetes mellitus. CMAJ. 2008;179(5):427–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Andersen PK. Regression Modeling Strategies with Applications to Linear Models, Logistic Regression and Survival Analysis. New York, NY: Springer-Verlag; 2001. [Google Scholar]
- 22. Leng J, Zhang C, Wang P, Li N, Li W, Liu H, Zhang S, Hu G, Yu Z, Ma RC, Chan JC, Yang X. Plasma levels of alanine aminotransferase in the first trimester identify high risk Chinese women for gestational diabetes. Sci Rep. 2016;6(1):27291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Andersson T, Alfredsson L, Källberg H, Zdravkovic S, Ahlbom A. Calculating measures of biological interaction. Eur J Epidemiol. 2005;20(7):575–579. [DOI] [PubMed] [Google Scholar]
- 24. Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WH, Bushman FD, Lusis AJ, Hazen SL. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19(5):576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, Peng Y, Zhang D, Jie Z, Wu W, Qin Y, Xue W, Li J, Han L, Lu D, Wu P, Dai Y, Sun X, Li Z, Tang A, Zhong S, Li X, Chen W, Xu R, Wang M, Feng Q, Gong M, Yu J, Zhang Y, Zhang M, Hansen T, Sanchez G, Raes J, Falony G, Okuda S, Almeida M, LeChatelier E, Renault P, Pons N, Batto J-M, Zhang Z, Chen H, Yang R, Zheng W, Li S, Yang H, Wang J, Ehrlich SD, Nielsen R, Pedersen O, Kristiansen K, Wang J. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55–60. [DOI] [PubMed] [Google Scholar]
- 26. Svingen GF, Schartum-Hansen H, Pedersen ER, Ueland PM, Tell GS, Mellgren G, Njølstad PR, Seifert R, Strand E, Karlsson T, Nygård O. Prospective associations of systemic and urinary choline metabolites with incident type 2 diabetes. Clin Chem. 2016;62(5):755–765. [DOI] [PubMed] [Google Scholar]
- 27. Barzilay E, Moon A, Plumptre L, Masih SP, Sohn K-J, Visentin CE, Ly A, Malysheva O, Croxford R, Caudill MA, O’Connor DL, Kim Y-I, Berger H. Fetal one-carbon nutrient concentrations may be affected by gestational diabetes. Nutr Res. 2018;55:57–64. [DOI] [PubMed] [Google Scholar]
- 28. Rothman KJ, Greenland S. Causation and causal inference in epidemiology. Am J Public Health. 2005;95(S1, suppl 1):S144–S150. [DOI] [PubMed] [Google Scholar]
- 29. Schönthal AH. Endoplasmic reticulum stress: its role in disease and novel prospects for therapy. Scientifica (Cairo). 2012;2012:857516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cnop M, Foufelle F, Velloso LA. Endoplasmic reticulum stress, obesity and diabetes. Trends Mol Med. 2012;18(1):59–68. [DOI] [PubMed] [Google Scholar]
- 31. Chhibber-Goel J, Gaur A, Singhal V, Parakh N, Bhargava B, Sharma A. The complex metabolism of trimethylamine in humans: endogenous and exogenous sources. Expert Rev Mol Med. 2016;18:e8. [DOI] [PubMed] [Google Scholar]
- 32. Srinivasa S, Fitch KV, Lo J, Kadar H, Knight R, Wong K, Abbara S, Gauguier D, Capeau J, Boccara F, Grinspoon SK. Plaque burden in HIV-infected patients is associated with serum intestinal microbiota-generated trimethylamine. AIDS. 2015;29(4):443–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zeisel SH, Warrier M. Trimethylamine N-oxide, the microbiome, and heart and kidney disease. Annu Rev Nutr. 2017;37(1):157–181. [DOI] [PubMed] [Google Scholar]
- 34. Fennema D, Phillips IR, Shephard EA. Trimethylamine and trimethylamine N-oxide, a flavin-containing monooxygenase 3 (FMO3)-mediated host-microbiome metabolic axis implicated in health and disease. Drug Metab Dispos. 2016;44(11):1839–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dumas ME, Barton RH, Toye A, Cloarec O, Blancher C, Rothwell A, Fearnside J, Tatoud R, Blanc V, Lindon JC, Mitchell SC, Holmes E, McCarthy MI, Scott J, Gauguier D, Nicholson JK. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc Natl Acad Sci USA. 2006;103(33):12511–12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Raubenheimer PJ, Nyirenda MJ, Walker BR. A choline-deficient diet exacerbates fatty liver but attenuates insulin resistance and glucose intolerance in mice fed a high-fat diet. Diabetes. 2006;55(7):2015–2020. [DOI] [PubMed] [Google Scholar]
- 37. Zhang X, Wang Y, Hao F, Zhou X, Han X, Tang H, Ji L. Human serum metabonomic analysis reveals progression axes for glucose intolerance and insulin resistance statuses. J Proteome Res. 2009;8(11):5188–5195. [DOI] [PubMed] [Google Scholar]
- 38. Rajaie S, Esmaillzadeh A. Dietary choline and betaine intakes and risk of cardiovascular diseases: review of epidemiological evidence. ARYA Atheroscler. 2011;7(2):78–86. [PMC free article] [PubMed] [Google Scholar]
- 39. Salmon WD, Newberne PM. Cardiovascular disease in choline-deficient rats. Effects of choline deficiency, nature and level of dietary lipids and proteins, and duration of feeding on plasma and liver lipid values and cardiovascular lesions. Arch Pathol. 1962;73:190–209. [PubMed] [Google Scholar]
- 40. Sun L, Xie C, Wang G, Wu Y, Wu Q, Wang X, Liu J, Deng Y, Xia J, Chen B, Zhang S, Yun C, Lian G, Zhang X, Zhang H, Bisson WH, Shi J, Gao X, Ge P, Liu C, Krausz KW, Nichols RG, Cai J, Rimal B, Patterson AD, Wang X, Gonzalez FJ, Jiang C. Gut microbiota and intestinal FXR mediate the clinical benefits of metformin. Nat Med. 2018;24(12):1919–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhu WW, Fan L, Yang HX, Kong LY, Su SP, Wang ZL, Hu YL, Zhang MH, Sun LZ, Mi Y, Du XP, Zhang H, Wang YH, Huang YP, Zhong LR, Wu HR, Li N, Wang YF, Kapur A. Fasting plasma glucose at 24–28 weeks to screen for gestational diabetes mellitus: new evidence from China. Diabetes Care. 2013;36(7):2038–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]