Abstract
Background
Precious corals known as coralliid corals (Anthozoa: Octocorallia) play an important role in increasing the biodiversity of the deep sea. Currently, these corals are highly threatened because of overfishing that has been brought on by an increased demand and elevated prices for them.The deep sea precious corals Pleurocorallium elatius and P. konojoi are distributed in Japanese waters and have distinct morphological features: (1) the terminal branches of the colony form of P. elatius are very fine, while those of P. konojoi are blunt and rounded, (2) the autozooids of P. elatius are arranged in approximately four rows, while those of P. konojoi are clustered in groups. However, previous genetic analysis using mtDNA and nuclear DNA did not indicate monophyly. Therefore, it is important to clarify their species status to allow for their conservation.
Methodology
We collected a total of 87 samples (60 of Corallium japonicum and 27 of P. konojoi) from around the Ryukyu Islands and Shikoku Island, which are geographically separated by approximately 1,300 km. We used a multiplexed inter-simple sequence repeat (ISSR) genotyping by sequencing (MIG-seq) and obtained 223 SNPs with which to perform STRUCTURE analysis and principle coordinate analysis (PCoA). In addition, two relatively polymorphic mtDNA regions were sequenced and compared.
Results
P. elatius and P. konojoi share a same mtDNA haplotype, which has been previously reported. However, MIG-seq analysis clearly distinguished the two species based on PCoA and STRUCTURE analysis, including 5% of species-specific fixed SNPs.
Conclusion
This study indicated that P. elatius and P. konojoi are different species and therefore both species should be conserved separately. Our findings highlight the importance of the conservation of these two species, especially P. elatius, whose population has been dramatically depleted over the last 100 years. The study also demonstrated the effectiveness and robustness of MIG-seq for defining closely related octocoral species that were otherwise indistinguishable using traditional genetic markers (mtDNA and EF).
Keywords: MIG-seq, Incomplete lineage sorting, Species delimitation, Deep sea coral, Octocoral
Introduction
Precious corals belong to family Coralliidae (Anthozoa: Octocorallia) play an important role in increasing the biodiversity of the deep sea by providing a complex habitat structure, suitable for other species to live in Roberts, Wheeler & Freiwald (2006). Their beautiful red and pink axis has drawn great attention and they have been harvested for ornaments, jewelry, and currency since ancient times (Chen, 2012; Clark & Rowden, 2009). Recent studies, however, indicated precious corals are vulnerable to overfishing because of their low fecundity and slow growth rate (Nonaka, Nakamura & Muzik, 2015; Luan et al., 2013; Torrents et al., 2005). Consequently, there is an increasing need for the conservation of overexploited precious corals in order to avoid local extinction (CITES-Netherlands, 2007; CITES-Qatar, 2009).
Species delimitation is important for conserving precious corals due to the fact that the species is the most fundamental unit for preservation (De Queiroz, 2007). However, species identification of the precious corals is sometimes difficult, especially for closely related species. The use of morphological characteristics in determining the species identification of octocorals, including precious corals, is the typical method; however, variations, and high frequency of homoplasy sometimes hinder identification (Figueroa & Baco, 2014). More recently, molecular and phylogenetic approaches using partial mitochondrial DNA (mtDNA) have emerged as useful tools to identify octocoral species (McFadden et al., 2011; Pante et al., 2012; Quattrini et al., 2014). In Coralliidae, the intergenic region 1 (IGR1) in mtDNA has a relatively high intra-species variation and is proposed as a useful marker for distinguishing between precious coral species (Tu, Dai & Jeng, 2015). Although mtDNA is widely used for species delimitation, discordances with the morphological characteristics of species still remain. For example, morphologically distinct species in Hemicorallium cannot be delineated using mtDNA (Ardila, Giribet & Sánchez, 2012; Tu, Dai & Jeng, 2015). Indeed, the slow mtDNA evolution has long been recognized for anthozoans (Hellberg, 2006; France & Hoover, 2002). Octocoral species in particular have a slow mutation rate of mtDNA, due to octocoral-specific DNA repairing enzymes (Bilewitch & Degnan, 2011; France & Hoover, 2002). These facts indicate that a higher resolution genetic marker is required to elucidate the species status of closely related precious coral species.
The advent of high-throughput sequencing technology has rendered a large number of loci of non-model organisms accessible in a short time period. For example, the restriction site associated DNA sequence (RADseq) (Baird et al., 2008) has been applicable in non-model organisms to delimit closely related species of many reference taxa. In deep sea octocorals, only a few studies have applied RADseq for species delimitation. RADseq has helped fine-tune the species status of genus Chrysogorgia (Pante et al., 2014) and revealed robust species boundaries in the genus Paragorgia using the Bayesian model based-method (Herrera & Shank, 2016). Additional studies using the high-throughput technology to delineate closely related precious coral species would be helpful to reveal species boundaries.
Multiplexed inter simple sequence repeat (ISSR) genotyping by sequencing (MIG-seq) is an easy, cost-effective, novel method to obtain a moderate number of single nucleotide polymorphisms (SNPs) of non-model organisms using polymerase chain reaction (PCR) and high-throughput technology. The number of available SNPs from MIG-seq analysis is generally less than those using other techniques such as RAD-seq (Miller et al., 2007). However, MIG-seq has several advantages, namely that putatively neutral loci adjacent to microsatellite regions can be obtained and the method can be performed with small amounts and/or low-quality DNA, and is relatively easy to perform cheaply. The ISSR regions are first amplified by PCR using universal primer sets, indexed by a second PCR, and then sequenced to obtain up to a few thousand SNPs. Closely related species of Heliopora were successfully delineated using MIG-seq (Richards et al., 2018), implying the effectiveness of this method for other closely related octocoral species.
Our target species, deep sea precious corals P. elatius and P. konojoi are distributed in Japanese waters at depths of 115–330 m (Nonaka & Muzik, 2009). P. elatius and P. konojoi can be distinguished by at least two morphological features: (1) terminal branches of the colony form of P. elatius are fine, while those of P. konojoi are blunt and rounded, (2) autozooids of P. elatius are arranged in approximately four rows, while those of P. konojoi are clustered in groups (Nonaka et al., 2012). Nevertheless, a previous molecular analysis suggested the two species shared a major haplotype at eight mtDNA regions and EF-1, indicating that the species status of these two is unclear (Tu, Dai & Jeng, 2015). Because these two species, especially P. elatius, are facing the risk of local extinction due to overfishing (Nonaka & Muzik, 2009), clarifying whether or not they are the same species with gene flow occurring between them is important for devising conservation strategies.
In this study, we applied MIG-seq analysis to clarify the species status of the two closely related precious corals, P. elatius and P. konojoi in order to obtain basic information for the conservation of precious coral species. We also sequenced widely used mtDNA regions for comparison with the MIG-seq results.
Materials and Methods
Sample collection and DNA extraction
In total, 87 samples of P. elatius and P. konojoi (27 P. elatius and 60 P. konojoi) were collected from depths of 100 to 330 m using either a traditional coral net, underwater remotely operated vehicles (ROV), or submarines off Ryukyu, Kyushu, and Shikoku, Japan (Table 1). We collected some samples under the Kochi Prefecture sampling permit number Sa 401, Sa 412, and Sa 423 in Kochi. Samples were preserved in 90% ethanol and genomic DNA was extracted using the hot alkaline solution method (Meeker et al., 2007) followed by ethanol precipitation (Richards et al., 2018).
Table 1. Precious coral samples used in this study.
| Location | Coordinate | MIG-seq | mtDNA | Sampling year | Sampling method | ||
|---|---|---|---|---|---|---|---|
| Pleurocorallium konojoi | Shikoku Island | Kouchi | 32°30′41.87″N 132°49′42.26″E | 47 | 0 | Apr.11-Nov.14 | traditional coral net |
| Ryukyu Islands | Iou Is. | 30°47′31.812″N 132°18′13.5″E | 5 | 4 | Dec.07-Aug.09 | manned submarine | |
| Take Is. | 30°48′36.684″N 130°25′43.356″E | 7 | 7 | Feb.08-May.09 | manned submarine | ||
| Tanega Is. | 30°36′34.38″N 130°58′43.932″E | 1 | 1 | Dec.07-Aug.08 | manned submarine | ||
| 60 | 12 | ||||||
| Pleurocorallium elatius | Shikoku Island | Kouchi | 32°30′41.87″N 132°49′42.26″E | 1 | 0 | Jul.11 | traditional coral net |
| Ryukyu Islands | Iou Is. | 30°47′31.812″N 132°18′13.5″E | 2 | 4 | Jun.05-Apr.09 | manned submarine | |
| Take Is. | 30°48′36.684″N 130°25′43.356″E | 2 | 0 | Sep.07-Oct.08 | manned submarine | ||
| Tanega Is. | 30°36′34.38″N 130°58′43.932″E | 3 | 3 | Feb.06-Dec.07 | manned submarine | ||
| Yaku Is. | 30°20′40.524″N 130°30′45.756″E | 4 | 1 | Aug.07-Aug.09 | manned submarine | ||
| Amami Is. | 28°16′4.224″N 129°21′43.596″E | 1 | 0 | Aug.07 | manned submarine | ||
| Tokuno Is. | 27°47′25.512″N 128°58′0.48″E | 2 | 0 | Oct.07-Aug.09 | manned submarine | ||
| Izena Is. | 26°56′2.976″N 127°56′28.932″E | 1 | 1 | Jan.06 | ROV | ||
| Hateruma Is. | 24°11′40.272″N 123°33′45.54″E | 3 | 0 | Sep.07-Mar.08 | ROV | ||
| Ikema Is. | 24°55′49.836″N 125°14′42.216″E | 1 | 1 | May.09 | ROV | ||
| Tarama Is. | 24°39′19.908″N 124°41′48.12″E | 1 | 1 | Aug.05-Dec.07 | ROV | ||
| Ishigaki Is. | 24°24′23.04″N 124°10′31.584″E | 5 | 1 | Nov.05-Mar.09 | ROV | ||
| Nakanokami Is. | 24°11′40.272″N 123°33′45.54″E | 1 | 1 | Mar.08-Apr.08 | ROV | ||
| 27 | 13 | ||||||
| Total | 87 | 25 |
MtDNA analysis
Two mtDNA regions were sequenced for P. elatius and P. konojoi: IGR1 region using primers (IGR1-Co-F and IGR1-Co-R, Tu, Dai & Jeng, 2015) and putative mitochondrial DNA mismatch repairing gene (mitochondrial mutS-like protein) regions (MSH-Co-F and MSH-Co-R, Tu, Dai & Jeng, 2015) following the original protocols. Two regions were directly sequenced from both directions using Big Dye v 3.1 and the Abi3730 sequencer. All of the sequence data were manually checked using Bioedit ver. 7.0.9.0 (Hall, 1999) and then all the sequences were aligned on GENETIX ver. 12. The two mtDNA region sequences were concatenated and used for subsequent analysis. To assess genetic diversity, the number of haplotypes, number of polymorphic sites, haplotype diversity (h) (Nei, 1987), and nucleotide diversity (Π) were calculated using DNaSP ver. 5.1 (Librado & Rozas, 2009). A median-joining network (Bandelt, Forster & Rohl, 1999) was constructed using NETWORK ver. 5.003, including gap sites, to visualize the evolutionary relationships between haplotypes obtained from P. elatius and P. konojoi. A maximum likelihood tree using MEGA 7.0 (Kumar, Stecher & Tamura, 2016) was constructed using concatenated mtDNA sequences. The best model was estimated using MEGA 7.0 based on the corrected Akaike Information Criterion and the Tamura 3-parameter model (Tamura, 1992) was used to construct a phylogenetic tree. Confidence values for phylogenetic trees were inferred using 1,000 bootstrap replicates. All mtDNA sequences obtained in this study were deposited (DDBJ Accession number: LC464485-LC464516, LC475110-LC475134).
MIG-seq analysis
We performed a MIG-seq analysis to detect genome-wide SNPs following the protocol by Suyama & Matsuki (2015). Briefly, MIG-seq amplifies putatively neutral, anonymous genome-wide ISSR regions (Gupta et al., 1994; Zietkiewicz, Rafalski & Labuda, 1994), including a few hundred to a few thousand SNPs, using 8 pairs of multiplex ISSR primers (MIG-seq primer set 1) for the first PCR. Then the DNA libraries from each sample with a different index were pooled and sequenced using MiSeq (sequencing control software v2.0.12, Illumina) with the MiSeq Reagent v3 150 cycle kit (Illumina). Image analysis and base calling were performed using real-time analysis software v1.17.21 (Illumina). We analyzed a total of 87 individuals with 27 P. elatius and 60 P. konojoi individuals collected from the Ryukyu Islands to Shikoku Island, geographically separated by approximately 1,300 km (Table 1).
To eliminate low-quality reads and primer sequences from the raw data, we used the FASTX-toolkit version 0.0.14 (fastaq_quality_filter) (Gordon & Hannon, 2012; http://hannonlab.cshl.edu/fastx_toolkit/index.html) with a fastq-quality-filter setting of –Q 33 –q 30 –p 40. We removed adapter sequences for the Illmina MiSeq run from both the 5’ end (GTCAGATCGGAAGAGCACACGTCTGAACTCCAGTCAC) and the 3′end (CAGAGATCGGAAGAGCGTCGTGTAGGGAAAGAC) using Cutadapt version 1.13 (Martin, 2011), and then we excluded short reads less than 80 bp. The quality-filtered sequence data were demultiplexed and filtered through the software Stacks v1.46 (Catchen et al., 2011; Catchen et al., 2013). We used Stacks v. 1.4 (Catchen et al., 2013) to stack the reads and extract SNPs. First, we used the U-stacks with the option settings of ‘minimum depth of coverage required to create a stack (m)′ = 3, ‘maximum distance allowed between stacks (M)′ = 1, ‘maximum distance allowed to align secondary reads to primary stacks (N)′ = 1, and the deleveraging (d) and removal (r) algorithms enabled. Secondly, we used the C-stacks with the option ‘number of mismatches allowed between sample loci when build the catalog (n)′ = 4, followed by the S-stacks. To confirm the consistency of the results using different sets of SNPs with different amounts of missing data, we first created three different SNP sets using population software implemented in Stacks v. 1.4 by restricting the data analysis to different criteria: (i) the minimum percentage of individuals required to process a locus across all data (r) was set at 70% and restricting data analysis to a single SNP per locus, (ii) r was set at 50% and a single SNP per locus was used, and (iii) r was set at 50% and all SNPs per locus were used. Confirming that all the results were consistent irrespective of the data sets, we only showed the results using (i) r, 70%, and single SNP per locus. For all of the above analyses , we set the following parameters: the minimum number of populations that a locus must be present in to process a locus (p)′ = 1, the minimum minor allele frequency required to process a nucleotide site at a locus (min_maf) = 0.01, the maximum observed heterozygosity required to process a nucleotide site at a locus (max_obs_het) = 0.9. BayeScan v 2.0 was used to detect possible SNPs under natural selection assuming two morphological species with a default setting.
A Bayesian individual-based assignment approach as implemented in STRUCTURE 2.3.4 was used to examine the genetic boundaries between P. elatius and P. konojoi individuals. Twenty independent runs were performed in STRUCTURE using an admixture model and allele frequency correlated model without any morphological priors. Both the length of the burn-in period and the number of Markov Chain Monte Carlo analyses (MCMC) were 200,000. We estimated lrtri K (Evanno, Regnaut & Goudet, 2005), the most likely number of clusters using STRUCTURE HARVESTER (Earl & vonHoldt, 2012), CLUMPAK (Kopelman et al., 2015), and DISTRUCT (Rosenberg, 2004) to summarize and visualize the STRUCTURE results. In addition, individual-based principle coordinate analysis (PCoA) was performed using GeneAlex ver. 6.5 to visualize the genetic relationship among different individuals in 2 dimensions. Data files were converted to each software using PGDspider ver 2.0.8.3 (Lischer & Excoffier, 2011).
Results
MtDNA analysis
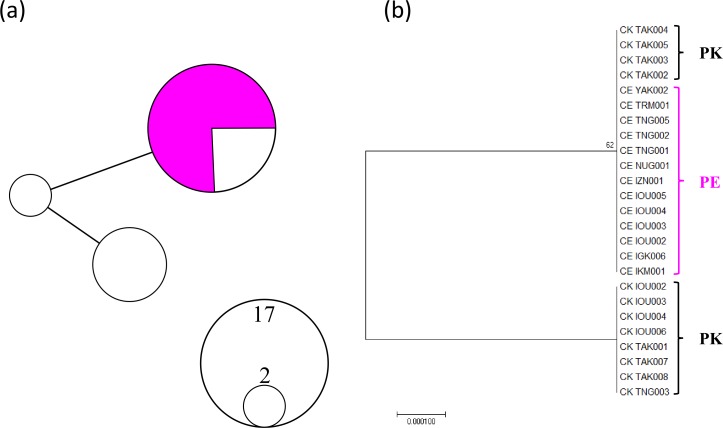
A total of 961 bp (456 bp IGR1 sequences and 505 bp MutS after trimming unreliable sequences) from 25 individuals (13 P. elatius and 12 P. konojoi) were obtained and concatenated to reconstruct the phylogenetic tree (Fig. 1). The genetic diversity of the concatenated sequences for P. elatius and P. konojoi was low, with only three haplotypes with two polymorphic sites, including one gap, uncovered across 25 sequences in the concatenated sequence. The haplotype diversity (h) was 0.453 and nucleotide diversity (π) was 0.00047. The haplotype network analysis revealed P. elatius and P. konojoi shared a major haplotype (Fig. 1A). Four out of 12 P. konojoi individuals shared a major haplotype with P. elatius and all the P. elatius individuals shared the major haplotype with P. konojoi. The reconstructed phylogenetic tree indicated that the two morphological species are not monophyletic (Fig. 1B). P. konojoi included one polymorphic site and one gap site in IGR1 and no polymorphic sites in MutS regions, while All P. elatius shared a single haplotype at both loci.
Figure 1. MtDNA haplotype network considering gap regions (A) and maximum likelihood phylogenetic tree excluding gap regions (B).
P. elatius (PE) is shown in pink and P. konojoi (PK) is shown in black/white. (A) The size of the circle represents the number of haplotypes found in the analysis. (B) Only the nodes with bootstrap values (>50) were indicated.
MIG-seq analysis
In total, 25,444,986 raw reads with an average of 292,471 reads per sample were obtained for 87 individuals by MIG-seq analysis, of which 25,241,178 reads remained after filtering out low quality reads. We obtained 16,011,472 reads with an average of 184,039 reads per individual after two step filtering. The three SNP data sets created using different criteria resulted in a different number of loci: (i) r = 0.7 and only a single SNP per locus resulted in 223 loci, (ii) r = 0.5 and only a single SNP per locus resulted in 762 SNPs, and (iii) r was set at 0.5 and all SNPs per locus resulted in 2251 SNPs. All data sets indicated consistent STRUCTURE and PCoA results, regardless of the different ratio of missing data and possible linkage disequilibrium (Supplemental Information 1).
BayeScan indicated that all the loci were neutral (q-values >0.05). Among the 223 SNPs examined, 12 SNPs indicated species-specific alleles (fixed substitution between the species) that could be observed.
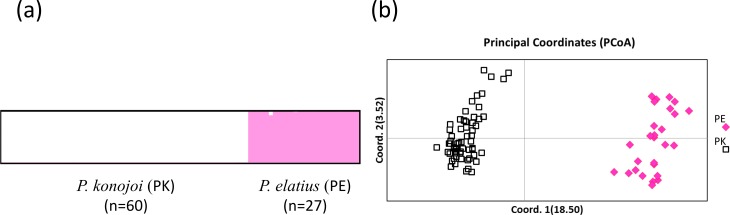
Both STRUCTURE and PCoA indicated the same patterns in all of the SNPs sets; P. elatius and P. konojoi are genetically distinct. Calculation of lrtri K of the STRUCTURE results indicated that K = 2 best explained the data with a mean likelihood = − 5, 666.970 and mean similarity score among 10 independent runs = 0.999. Clear genetic differences corresponded with the morphological differences without any genetically admixed individuals (Fig. 2A). PCoA indicated two morphological species are clearly separated by the x-axis, which explains 18.5% of the data (Fig. 2B).
Figure 2. STRUCTURE (A) and PCoA result (B).
(A) K = 2 with Mean (LnProb) = − 5666.970 and Mean (similarity score) among 10 runs = 0.999 and PCoA results using 223 SNPs data obtained by MIG-seq analysis (B). X axis indicates 18.5% and Y axis indicates 3.52% of the data. PK and PE represent P. konojoi and, P. elatius respectively.
Discussion
Japan has been the main exporter of the precious corals to mainland China. The increased demand of the precious corals in mainland China in recent years resulted in elevated prices for raw corals, and promoted illegal fishing and overexploitation. Precise harvest data for precious corals is currently unavailable due to the inconsistent application of CITES listings, unreported trade as personal or household effects, and illegal and unreported trade. Under these circumstances, having species-specific customs codes for CITES would be necessary to obtain accurate trade data for conservation (Shiraishi, 2018).
Previously, P. elatius and P. konojoi were considered morphologically different; traditional genetic analysis using mtDNA and nuclear elongation factor region (Tu, Dai & Jeng, 2015) did not indicate their monophyly and thus they have been genetically indistinguishable. In the present study, we confirmed that P. elatius and P. konojoi share a same haplotype (Fig. 1), which is consistent with the previous study. MIG-seq analysis, however, successfully discriminated P. elatius from P. konojoi. PCoA and STRUCTURE results indicated that the two species are clearly different with no intermediate genotypes, indicating no current hybridization between the two species. It is possible that extremely slow substitution rates of mtDNA of P. elatius and P. konojoi resulted in non-reciprocal monophyly due to the maintenance of an ancestral shared variation presented at the time of divergence. On the other hand, it is possible that a strong purifying selection could encourage them to split to reciprocal monophyly in nuclear DNA, as the MIG-seq analysis suggested more than 5% of the loci showed fixed substitution between P. elatius and P. konojoi, while BayeScan indicated all MIG-seq loci are all neutral.
This study demonstrated that P. elatius and P. konojoi are indeed different species without any hybridization or on-going gene flow consistent with morphological differences, and therefore require separate conservation management plans since P. elatius populations cannot be replenished by P. konojoi populations. P. elatius made up 20.6% of the total catch of precious corals in Japan from 1904 to 1920. Currently, this has dropped to 2.0% because the biomass of P. elatius has been dramatically depleted due to overfishing (Iwasaki, 2018); thus, there is an urgent need to devise and implement conservation strategies for P. elatius.
This study highlighted the effectiveness of the MIG-seq analysis using the high throughput sequencing technology for delimiting octocoral species. This study, as well as a previous study on shallow water octocoral species, demonstrated that MIG-seq analysis can uncover genetic lineages that are undetectable when using mtDNA or ITS2 (Richards et al., 2018). MIG-seq provides a time-saving (3 days at shortest), simple (two PCR steps), and economical (15 US dollars per sample) approach that is applicable even for small amounts of degraded or valuable samples for SNP genotyping. Data comparison among different species or genera is easy as the MIG-seq always uses the same multiplex primer sets, which would also be suitable for nuclear barcoding among different species. Further application of MIG-seq analysis to a wider range of octocoral taxa would help to clarify the unsolved status of closely related species.
Conclusions
This study indicated that P. elatius and P. konojoi are different species and that both species should be conserved separately. The results highlighted the importance of conservation of these two species, especially P. elatius, which has been dramatically depleted in the past 100 years. Again, it does suggest that separate conservation strategies will be required for the two different morphospecies. This study also demonstrated the effectiveness and robustness of MIG-seq for delimiting closely related octocoral species that were previously indistinguishable when using traditional genetic markers (mtDNA and EF). Further application of MIG-seq analysis for a wider range of octocoral or other taxa would aid in verifying the robustness of MIG-seq analysis on species delimitation.
Supplemental Information
Acknowledgments
We are grateful to Mr. Masao Nakano, Yoshihiko Niiya, Katsuhiro Fujita for collecting samples in Kochi. We thank Mr. Hideaki Yuasa, Ms. Akemi Yoshida for their help in the analysis. Computations were partially performed on the NIG supercomputer at ROIS National Institute of Genetics.
Funding Statement
This study was supported by the Japan Society for the Promotion of Science (JSPS) Grant-in-aid for young scientists (A) (17H04996),16H04722, a Grant-in-Aid for Research Fellows (201921342), and the Sumitomo Foundation Fiscal 2018 Grant for Environmental Research Projects and the Program to Disseminate Tenure Tracking System from the Ministry of Education, Culture, Sports, Science and Technology (MEXT). Additional support was granted by the Japan Society for the Promotion of Science (JSPS) KAKENHI (Grant Number 16H04722). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Kenji Takata performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Hiroki Taninaka analyzed the data, approved the final draft.
Masanori Nonaka and Fumihito Iwase conceived and designed the experiments, contributed reagents/materials/analysis tools, approved the final draft.
Taisei Kikuchi, Yoshihisa Suyama and Satoshi Nagai analyzed the data, contributed reagents/materials/analysis tools, approved the final draft.
Nina Yasuda conceived and designed the experiments, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Field Study Permissions
The following information was supplied relating to field study approvals (i.e., approving body and any reference numbers):
Samples were collected under the Kochi Prefecture sampling permit numbers Sa 401, Sa 412, and Sa 423. Other samples were from sample collections in Okinawa Churaumi Aquarium.
Data Availability
The following information was supplied regarding data availability:
All specimens and the accession numbers are available in the Supplemental files. mtDNA data is available at DDBJ: LC464485-LC464516–LC475110-LC475134. MIG-seq data is available at NCBI: PRJDB8153, BioSample: SAMD00166505–SAMD00166591, and DRA: DRX168319-DRX168404 (Experiment), DRR177792-DRR177878 (RUN): https://ddbj.nig.ac.jp/DRASearch/submission?acc=DRA008354.
References
- Ardila, Giribet & Sánchez (2012).Ardila NE, Giribet G, Sánchez JA. A time-calibrated molecular phylogeny of the precious corals: reconciling discrepancies in the taxonomic classification and insights into their evolutionary history. BMC Evolutionary Biology. 2012;12:246. doi: 10.1186/1471-2148-12-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird et al. (2008).Baird NA, Etter PD, Atwood TS, Currey MC, Shiver AL, Lewis ZA, Selker EU, Cresko WA, Johnson EA. Rapid SNP discovery and genetic mapping using sequenced RAD markers. PLOS ONE. 2008;3:e3376. doi: 10.1371/journal.pone.0003376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandelt, Forster & Rohl (1999).Bandelt HJ, Forster P, Rohl A. Median-joining networks for inferring intraspecific phylogenies. Molecular Biology and Evolution. 1999;16:37–48. doi: 10.1093/oxfordjournals.molbev.a026036. [DOI] [PubMed] [Google Scholar]
- Bilewitch & Degnan (2011).Bilewitch J, Degnan S. A unique horizontal gene transfer event has provided the octocoral mitochondrial genome with an active mismatch repair gene that has potential for an unusual self-contained function. BMC Evolutionary Biology. 2011;11:228. doi: 10.1186/1471-2148-11-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catchen et al. (2011).Catchen JM, Amores A, Hohenlohe P, Cresko W, Postlethwait JH. Stacks: building and genotyping loci de novo from short-read sequences. G3: Genes, Genomes, Genetics. 2011;1:171–182. doi: 10.1534/g3.111.000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catchen et al. (2013).Catchen J, Hohenlohe PA, Bassham S, Amores A, Cresko WA. Stacks: an analysis tool set for population genomics. Molecular Ecology. 2013;22:3124–3140. doi: 10.1111/mec.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen (2012).Chen CS. Management of the precious coral fishery in Taiwan: progress and perspectives. Marine Policy. 2012;36:623–629. doi: 10.1016/j.marpol.2011.10.016. [DOI] [Google Scholar]
- CITES-Netherlands (2007).CITES-Netherlands Inclusion of all species in the genus Corallium in Appendix II of CITES. 2007. [15 January 2019]. This taxon comprises 26 closely related species. Consideration of proposals for Amendment of Appendices I and II. CITES CoP14 Inf. 36 p1. https://www.cites.org/sites/default/files/common/cop/14/inf/E14i-36.pdf .
- CITES-Qatar (2009).CITES-Qatar Consideration of proposals for amendment of appendices I and II. CITES CoP15 Prop. 21, p 1. 2009. https://www.cites.org/sites/default/files/eng/cop/15/prop/E-15-Prop-21.pdf. [15 January 2019]. https://www.cites.org/sites/default/files/eng/cop/15/prop/E-15-Prop-21.pdf
- Clark & Rowden (2009).Clark MR, Rowden AA. Effect of deepwater trawling on the macro-invertebrate assemblages of seamounts on the Chatham Rise, New Zealand. Deep Sea Research Part I: Oceanographic Research Papers. 2009;56:1540–1554. doi: 10.1016/j.dsr.2009.04.015. [DOI] [Google Scholar]
- De Queiroz (2007).De Queiroz K. Species concepts and species delimitation. Systematic Biology. 2007;56:879–886. doi: 10.1080/10635150701701083. [DOI] [PubMed] [Google Scholar]
- Earl & vonHoldt (2012).Earl DA, vonHoldt BM. STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conservation Genetics Resources. 2012;4:359–361. doi: 10.1007/s12686-011-9548-7. [DOI] [Google Scholar]
- Evanno, Regnaut & Goudet (2005).Evanno G, Regnaut S, Goudet J. Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Molecular Ecology. 2005;14:2611–2620. doi: 10.1111/j.1365-294X.2005.02553.x. [DOI] [PubMed] [Google Scholar]
- Figueroa & Baco (2014).Figueroa DF, Baco AR. Octocoral mitochondrial genomes provide insightsinto the phylogenetic history of gene order rearrangements, order reversals, andcnidarian phylogenetics. Genome Biology and Evolution. 2014;7:391–409. doi: 10.1093/gbe/evu286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- France & Hoover (2002).France SC, Hoover IL. DNA sequences of the mitochondrial COI gene have low levels of divergence among deep-sea octocorals (Cnidaria: Anthozoa) Hydrobiogia. 2002;471:149–155. doi: 10.1023/A:1016517724749. [DOI] [Google Scholar]
- Gordon & Hannon (2012).Gordon A, Hannon GJ. FASTX-Toolkit. 2012. FASTQ/A short-reads pre-processing tools (unpublished). http://hannonlab.cshl.edu/fastx_toolkit/
- Gupta et al. (1994).Gupta M, Chyi YS, Romero-Severson J, Owen JL. Amplification of DNA markers from evolutionarily diverse genomes using single primers of simple-sequence repeats. Theoretical and Applied Genetics. 1994;89:998–1006. doi: 10.1007/BF00224530. [DOI] [PubMed] [Google Scholar]
- Hall (1999).Hall T. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series. 1999;41:95–98. [Google Scholar]
- Hellberg (2006).Hellberg ME. No variation and low synonymous substitution rates in coral mtDNA despite high nuclear variation. BMC Evolutionary Biology. 2006;6:24. doi: 10.1186/1471-2148-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera & Shank (2016).Herrera S, Shank TM. RAD sequencing enables unprecedented phylogenetic resolution and objective species delimitation in recalcitrant divergent taxa. bioRxiv. 2016;019745:1–44. doi: 10.1016/j.ympev.2016.03.010. [DOI] [PubMed] [Google Scholar]
- Iwasaki (2018).Iwasaki N. Housekisango no jijokuteki riyou. Seibutsu No Kagaku Iden. 2018;72(3):214–220. [Google Scholar]
- Kopelman et al. (2015).Kopelman NM, Mayzel J, Jakobsson M, Rosenberg NA, Mayrose I. CLUMPAK: a program for identifying clustering modes and packaging population structure inferences across K. Molecular Ecology Resources. 2015;15:1179–1191. doi: 10.1111/1755-0998.12387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, Stecher & Tamura (2016).Kumar S, Stecher G, Tamura K. MEGA 7: molecular evolutionary genetics analysis version 7.0 for BiggerDatasets. Molecular Biology and Evolution. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Librado & Rozas (2009).Librado P, Rozas J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25:1451–1452. doi: 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
- Lischer & Excoffier (2011).Lischer HE, Excoffier L. PGDSpider: an automated data conversion tool for connecting population genetics and genomics programs. Bioinformatics. 2011;28:298–299. doi: 10.1093/bioinformatics/btr642. [DOI] [PubMed] [Google Scholar]
- Luan et al. (2013).Luan NT, Rahman MA, Maki T, Iwasaki N, Hasegawa H. Growth characteristics and growth rate estimation of japanese precious corals. Journal of Experimental Marine Biology and Ecology. 2013;441:117–125. doi: 10.1016/j.jembe.2013.01.012. [DOI] [Google Scholar]
- Martin (2011).Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet Journal. 2011;17:10–12. doi: 10.14806/ej.17.1.200. [DOI] [Google Scholar]
- McFadden et al. (2011).McFadden CS, Benayahu Y, Pante E, Thoma JN, Nevarez PA, France SC. Limitations of mitochondrial gene barcoding in Octocorallia. Molecular Ecology Resources. 2011;11:19–31. doi: 10.1111/j.1755-0998.2010.02875.x. [DOI] [PubMed] [Google Scholar]
- Meeker et al. (2007).Meeker ND, Hutchinson SA, Ho L, Trede NS. Method for isolation of PCR-ready genomic DNA from zebrafish tissues. Biotechniques. 2007;43:610–614. doi: 10.2144/000112619. [DOI] [PubMed] [Google Scholar]
- Miller et al. (2007).Miller MR, Dunham JP, Amores A, Cresko WA, Johnson EA. Rapid and cost-effective polymorphism identification and genotyping using restriction site associated DNA (RAD) markers. Genome Research. 2007;17:240–248. doi: 10.1101/gr.5681207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei (1987).Nei M. Molecular evolutionary genetics. New York: Columbia University Press; 1987. [Google Scholar]
- Nonaka & Muzik (2009).Nonaka M, Muzik K. Recent harvest records of commercially valuable precious corals in the Ryukyu Archipelago. Marine Ecology Progress Series. 2009;397:269–278. doi: 10.1155/2012/519091. [DOI] [Google Scholar]
- Nonaka, Nakamura & Muzik (2015).Nonaka M, Nakamura M, Muzik K. Sexual reproduction in precious corals (Coralliidae) collected in the Ryukyu Archipelago. Pacific Science. 2015;69:15–46. [Google Scholar]
- Nonaka et al. (2012).Nonaka M, Nakamura M, Tsukahara M, Reimer JD. Histological examination of precious corals from the Ryukyu Archipelago. Journal of Marine Biology. 2012;2012 doi: 10.1155/2012/519091. Article 519091. [DOI] [Google Scholar]
- Pante et al. (2014).Pante E, Abdelkrim J, Viricel A, Gey D, France S, Boisselier MC, Samadi S. Use of RAD sequencing for delimitingspecies. Heredity. 2014;114:450–459. doi: 10.1038/hdy.2014.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pante et al. (2012).Pante E, France SC, Couloux A, Cruaud C, McFadden CS, Samadi S, Watling L. Deepsea origin and in-situ diversification of chrysogorgiid octocorals. PLOS ONE. 2012;7:e38357. doi: 10.1371/journal.pone.0038357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quattrini et al. (2014).Quattrini AM, Etnoyer PJ, Doughty C, English L, Falco R, Remon N, Rittinghouse M, Cordes EE. A phylogenetic approach to octocoral community structure in the deep Gulf of Mexico. Deep Sea Research Part II: Topical Studies in Oceanography. 2014;99:92–102. doi: 10.1016/j.dsr2.2013.05.027. [DOI] [Google Scholar]
- Richards et al. (2018).Richards ZT, Yasuda N, Kikuchi T, Foster T, Mitsuyuki C, Stat M, Suyama Y, Wilson NG. Integrated evidence reveals a new species in the ancient blue coral genus Heliopora (Octocorallia) Scientific Reports. 2018;8:15875. doi: 10.1038/s41598-018-32969-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, Wheeler & Freiwald (2006).Roberts JM, Wheeler AJ, Freiwald A. Reefs of the deep: the biology and geology of cold-water coral ecosystems. Science. 2006;312:543–547. doi: 10.1126/science.1119861. [DOI] [PubMed] [Google Scholar]
- Rosenberg (2004).Rosenberg NA. DISTRUCT: a program for the graphical display of population structure. Molecular Ecology Notes. 2004;4:137–138. doi: 10.1046/j.1471-8286.2003.00566.x. [DOI] [Google Scholar]
- Shiraishi (2018).Shiraishi H. Seeing red. Precious coral trade in Asia. https://www.traffic.org/site/assets/files/11127/seeing-red.pdf Traffic report. 2018
- Suyama & Matsuki (2015).Suyama Y, Matsuki Y. MIG-seq: an effective PCR-based method for genome-wide single-nucleotide polymorphism genotyping using the next-generation sequencing platform. Scientific Reports. 2015;5:16963. doi: 10.1038/srep16963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura (1992).Tamura K. Estimation of the number of nucleotide substitutions when there are strong transition-transversion and G+C-content biases. Molecular Biology and Evolution. 1992;9:678–687. doi: 10.1093/oxfordjournals.molbev.a040752. [DOI] [PubMed] [Google Scholar]
- Torrents et al. (2005).Torrents O, Garrabou J, Marschal C, Harmelin JG. Age and size at first reproduction in the commercially exploited red coral Corallium rubrum (L.) in the Marseilles area (France, NW Mediterranean) Biological Conservation. 2005;121:391–397. doi: 10.1016/j.biocon.2004.05.010. [DOI] [Google Scholar]
- Tu, Dai & Jeng (2015).Tu TH, Dai CF, Jeng MS. Phylogeny and systematics of deep-sea precious corals (Anthozoa: Octocorallia: Coralliidae) Molecular Phylogenetics and Evolution. 2015;84:173–184. doi: 10.1016/j.ympev.2014.09.031. [DOI] [PubMed] [Google Scholar]
- Zietkiewicz, Rafalski & Labuda (1994).Zietkiewicz E, Rafalski A, Labuda D. Genome fingerprinting by simple sequence repeat (SSR)-anchored polymerase chain reaction amplification. Genomics. 1994;20:176–183. doi: 10.1006/geno.1994.1151. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
All specimens and the accession numbers are available in the Supplemental files. mtDNA data is available at DDBJ: LC464485-LC464516–LC475110-LC475134. MIG-seq data is available at NCBI: PRJDB8153, BioSample: SAMD00166505–SAMD00166591, and DRA: DRX168319-DRX168404 (Experiment), DRR177792-DRR177878 (RUN): https://ddbj.nig.ac.jp/DRASearch/submission?acc=DRA008354.