Abstract
In the United States, multistate Salmonella outbreaks are most commonly linked to a food source; however, contact with live animals can also result in outbreaks of human illness. To characterize Salmonella outbreaks linked to animal contact and examine differences compared to foodborne outbreaks, we analysed data reported to the Centers for Disease Control and Prevention through the National Outbreak Reporting System (NORS) from 2009 to 2014 with a primary mode of transmission listed as “animal contact” or “food.” Four hundred and eighty-four outbreaks with animal contact or foodborne transmission were reported through NORS; of these outbreaks, 99 (20.5%) resulted from Salmonella transmission through animal contact and 385 (79.5%) resulted from foodborne transmission, which resulted in 3,604 (19.8%) and 13,568 (80.2%) illnesses, respectively. A higher proportion of illnesses among children aged <1 year and children aged 1–4 years were linked to animal contact outbreaks compared to foodborne outbreaks (15.2% vs. 1.4%, p < 0.01 and 24.5% vs. 5.6%, p < 0.01, respectively). Illnesses resulting in hospitalizations (OR: 1.81, 95% CI: 1.62, 2.02) were more likely to be associated with animal contact compared to food. Animal contact outbreaks reported to NORS were more likely to be multistate compared to foodborne outbreaks (OR: 5.43, 95% CI: 3.37, 8.76) and had a longer median duration (99.0 days vs. 9.0 days, p < 0.01). Characterizing the differences between outbreaks of illness linked to animal contact and outbreaks linked to food provides useful information to investigators to improve public health response.
Keywords: animal contact, foodborne, outbreak, Salmonella enterica
1 |. INTRODUCTION
Non-typhoidal Salmonella enterica infections cause an estimated 1.2 million gastrointestinal illnesses each year in the United States, resulting in approximately 19,000 hospitalizations and 390 deaths (Scallan et al., 2011). Most human infections result in self-limiting gastrointestinal symptoms that begin 12–72 hr after exposure and last <7 days (CDC, 2015). Most people do not need medical treatment to recover; however, young children (aged <5 years), older adults (aged >65 years), and people with weakened immune systems are at risk of serious infection, which can lead to complications and possibly death (CDC, 2015). Salmonella infections tend to have a seasonal trend, with a higher frequency reported in the warmer months, peaking in the late summer (CDC, 2013). While the majority of Salmonella infections are considered sporadic, many Salmonella infections are identified as part of an outbreak and linked back to a specific source every year (CDC, 2014; Gould et al., 2013).
Salmonellosis is a nationally notifiable infectious disease in the United States, which means reporting of laboratory-confirmed infections to the state or local health department is mandated by legislation and regulation (CDC, 2015). Detection of multistate outbreaks of Salmonella infections is facilitated by PulseNet, the national molecular subtyping laboratory network for enteric disease surveillance, which uses pulsed-field gel electrophoresis and whole genome sequencing to identify clusters of illness that are investigated (Boxrud, Monson, Stiles, & Besser, 2010; Swaminathan, Barrett, Hunter, & Tauxe, 2001). After an outbreak investigation has been completed, data are voluntarily reported by state and local health departments through the Centers for Disease Control and Prevention (CDC) National Outbreak Reporting System (NORS) (Hall et al., 2013).
Although most salmonellosis outbreaks result from foodborne transmission, they can also result from contact with animals and animal environments. Non-typhoidal Salmonella infections transmitted through animal contact have been estimated to cause 11% of all salmonellosis annually (Hale et al., 2012; Hoelzer, Moreno Switt, & Wiedmann, 2011). Large multistate outbreaks have been linked to contact with animals, including live poultry, turtles, other reptiles, and small household pets (Basler et al.,; Hall et al., 2010; Harris, Neil, Behravesh, Sotir, & Angulo, 2010; Loharikar et al., 2012; Marsden-Haug et al., 2013; Mitchell et al., 2013). Differences between outbreaks linked to animal contact and outbreaks linked to food have been reported in the literature; however, no systematic analyses have been conducted to assess these differences using data collected at the national level (Basler et al.,; Hall et al., 2010). The initial questionnaire used to interview patients with Salmonella infections varies from state to state, as do the questions about various food and animal exposures. Identifying and understanding any differences between salmonellosis outbreaks linked to animal contact and food might help investigators solve outbreaks more quickly by focusing hypothesis-generating on more likely sources. This would allow earlier implementation of prevention efforts, perhaps resulting in fewer illnesses. Therefore, we compared characteristics of salmonellosis outbreaks linked to animal contact to those linked to food, with the intent of identifying pertinent differences and helping to guide investigation efforts when the mode of transmission is unknown.
2 |. MATERIAL S AND METHODS
We conducted this analysis using CDC NORS surveillance data and selected outbreaks based on the following inclusion criteria: first reported illness onset date occurred 1 January 2009–31 December 2014; laboratory-confirmed etiology of S. enterica; and a primary mode of transmission indicated as either animal contact or food, as determined by the investigating health agency. Foodborne outbreaks were designated with either a confirmed or probable food vehicle. Only foodborne outbreaks with a confirmed food vehicle were included in this analysis. Animal contact outbreaks did not have a probable vehicle designation in NORS, so all animal contact outbreaks had a confirmed animal vehicle.
2.1 |. Measures and definitions
For purposes of this analysis, a case was defined as illness in a person linked to an outbreak; outbreaks were defined using CDC NORS definitions (CDC, 2017). Outbreak size was calculated using the estimated number of primary illnesses, which included all laboratory-confirmed and probable primary cases. Probable primary cases were defined as cases who were epi-linked to a laboratory-confirmed case or exposure, but do not have laboratory confirmation of infection. Secondary cases for each outbreak were defined as ill people without exposure to the implicated vehicle who became ill after contact with a primary case. Cases were classified by outbreak as confirmed or probable, and as primary or secondary, by the investigating health agency.
2.2 |. Statistical methods
We calculated frequencies for demographic and outcome variables (hospitalizations, deaths, emergency room visits, and non-emergency room outpatient healthcare provider visits). If not reported, case counts for age categories and sex categories were recreated based on reported percentages and estimated primary ill. When age or sex was unknown, the data were considered missing for this analysis. We compared case counts per outbreak and duration of outbreak by outbreak type (animal contact vs. food outbreaks) using Wilcoxon rank-sum tests. We compiled the number of total outbreaks and percentage of total outbreaks for the top 15 most common Salmonella serotypes for animal contact and foodborne outbreaks. We used simple logistic regression to calculate odds ratios (ORs) and 95% confidence intervals (95% CIs) for age, sex, and outcome by outbreak type. All statistical analyses were performed using SAS 9.3.
3 |. RESULTS
From 2009 to 2014, 99 Salmonella illness outbreaks with animal contact as the primary mode of transmission were reported to NORS. These outbreaks resulted in 3,604 estimated primary illnesses, 586 hospitalizations, and 6 deaths. In the same time period, 385 Salmonella illness outbreaks linked to food were reported, resulting in 13,568 illnesses, 1,616 hospitalizations, and 15 deaths (Table 1). Of all illnesses in this analysis, 20.5% resulted from animal contact. The majority of illnesses among young children aged <1 year and aged 1–4 years resulted from animal contact (72.7% and 51.7%, respectively; Figure 1). Patients aged <1 year (OR = 25.0; 95% CI: 20.2, 30.8), patients aged 1–4 years (OR = 10.0; 95% CI: 8.7, 11.6), and patients aged 5–9 years (OR = 5.48; 95% CI: 4.65, 6.46) had greater odds of being linked to animal contact outbreaks compared to those aged 20–49 years. Females were more likely than males to be linked to animal contact outbreaks than to foodborne outbreaks (OR = 1.22; 95% CI: 1.13, 1.32).
TABLE 1.
Number and percentage of outbreak-associated illnesses in animal contact and food outbreaks, by age group, sex, healthcare utilization, and survival—National Outbreak Reporting System, United States, 2009–2014
| Animal contact n = 3,604 (19.8%) |
Foodborne n = 13,568 (80.2%) |
||
|---|---|---|---|
| Characteristic | No. (%) | No. (%) | Odds ratio (95% CI) |
| Age group (years) | |||
| <1 | 387 (15.2) | 145 (1.4) | 24.98 (20.25, 30.83) |
| 1–4 | 623 (24.5) | 583 (5.6) | 10.00 (8.67, 11.55) |
| 5–9 | 312 (12.2) | 533 (5.2) | 5.48 (4.65, 6.46) |
| 10–19 | 248 (9.7) | 1,267 (12.3) | 1.83 (1.56, 2.16) |
| 20–49 | 546 (21.4) | 5,112 (49.5) | 1.00 (reference) |
| 50–74 | 362 (14.2) | 2,184 (21.1) | 1.55 (1.35, 1.79) |
| 75+ | 70 (2.8) | 509 (4.9) | 1.29 (0.99, 1.68) |
| Missing | 1,056 | 3,235 | |
| Sex | |||
| Male | 1,487 (46.5) | 5,601 (51.6) | 1.00 (reference) |
| Female | 1,708 (53.5) | 5,255 (48.4) | 1.22 (1.13, 1.32) |
| Missing | 409 | 2,712 | |
| Healthcare and survivala | |||
| Hospitalization | |||
| Yes | 586 (28.2) | 1,616 (17.8) | 1.81 (1.62, 2.02) |
| No | 1,495 (71.8) | 7,447 (82.2) | |
| Missing | 1,523 | 4,505 | |
| Deathb | Fisher’s Exact Test | ||
| Yes | 6 (0.2) | 15 (0.2) | p = 0.14 |
| No | 2,549 (99.8) | 9,288 (99.8) | |
| Missing | 1,049 | 4,265 | |
| ER visit | |||
| Yes | 52 (26.0) | 941 (18.2) | 1.58 (1.14, 2.18) |
| No | 148 (74.0) | 4,225 (81.8) | |
| Missing | 3,404 | 8,402 | |
| Healthcare visit | |||
| Yes | 176 (64.0) | 2,443 (40.0) | 2.67 (2.07, 3.43) |
| No | 99 (36.0) | 3,666 (60.0) | |
| Missing | 3,329 | 7,459 |
Variables are reported based on information available.
Due to low expected cell frequencies, Fisher’s exact test was used to calculate p value for this outcome.
FIGURE 1.
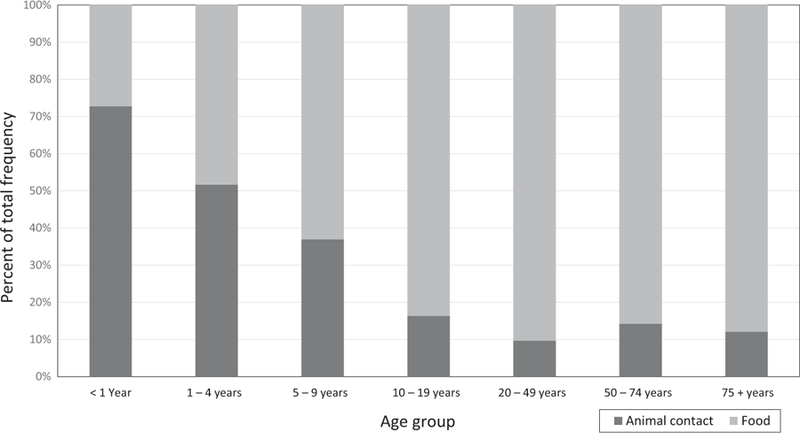
Per cent of total illnesses in each age group by primary mode of transmission—National Outbreak Reporting System, United States, 2009–2014
Patients in animal contact outbreaks had greater odds of hospitalization than patients in foodborne outbreaks (OR = 1.81; 95% CI: 1.62, 2.02). Patients linked to animal contact outbreaks also had higher odds of visiting an emergency room (OR = 1.58; 95% CI: 1.14, 2.18) or visiting an outpatient healthcare provider (OR = 2.67; 95% CI: 2.07, 3.43) than patients in foodborne outbreaks; however, a large percentage of data was missing for both variables among patients in both outbreak types. We did not observe a significant difference in the proportion of deaths comparing animal contact and foodborne outbreaks (0.2% vs. 0.2%, Fisher’s exact test, p = 0.14).
The estimated number of primary illnesses for animal contact outbreaks (median = 10 cases per outbreak) was not significantly different from foodborne outbreaks (median = 14 cases per outbreak; p = 0.24; Table 2). The duration of animal contact outbreaks (median = 99.0 days) was significantly longer than foodborne outbreaks (median = 9.0 days, p < 0.01). Multistate exposure was more common among animal contact outbreaks compared to foodborne outbreaks (OR = 5.43; 95% CI: 3.37–8.76; Table 2). Animal contact outbreaks occurred sporadically, with multiple peaks throughout the year. The highest frequency of outbreaks occurred in the early spring, likely as a result of the number of human salmonellosis outbreaks linked to live poultry. Foodborne outbreaks peaked during the mid-summer months (Figure 2). The most common Salmonella serotypes seen in animal contact outbreaks were Typhimurium, I 4,[5],12:i:-, and Montevideo. The most common Salmonella serotypes seen in foodborne outbreaks were Enteritidis, Typhimurium, and Newport (Table 3).
TABLE 2.
Characteristics of outbreaks linked to animal contact and food—National Outbreak Reporting System, United States, 2009–2014
| Animal contact n = 99 (20.5%) |
Foodborne n = 385 (79.5%) |
||
|---|---|---|---|
| Illnesses per outbreak | Median (IQR) | p-value | |
| Estimated primary ill (No. of illnesses) | 10 (4–42) | 14 (7–30) | 0.236 |
| Total secondary (No. of illnesses) | 0 (0–0) | 0 (0–1) | 0.079 |
| Duration of outbreaka | n = 91 | n = 363 | |
| Days | 99.0 (27.0–217.0) | 9.0 (3.0–27.0) | <0.001 |
| Multistate exposure | No. of outbreaks (%) | Odds ratio | |
| Yes | 51 (51.5) | 63 (16.4) | 5.43 (95% CI: 3.37, 8.76) |
| No | 48 (48.5) | 322 (83.6) |
Outbreak duration was calculated by determining the difference in the first date of illness onset to the last date of illness onset. When last date of illness onset was missing, duration could not be calculated and missing values were excluded.
FIGURE 2.
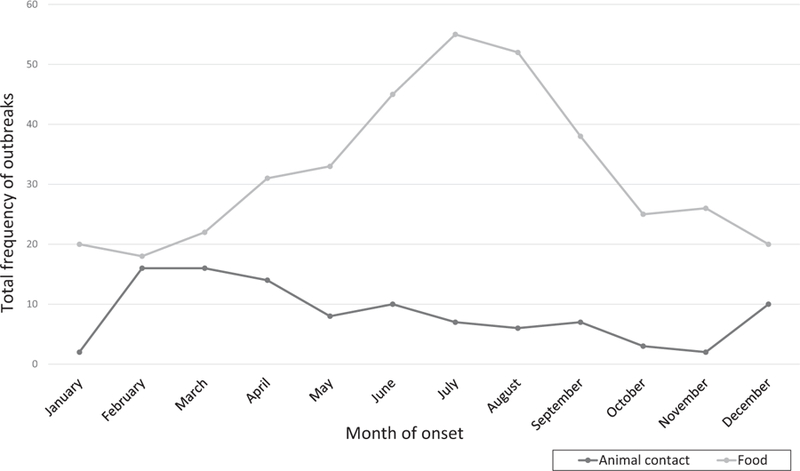
Month of outbreak onset by primary mode of transmission—National Outbreak Reporting System, United States, 2009–2014
TABLE 3.
Top 15 most commonly reported Salmonella enterica serotypes: number and percentage of all outbreaks, by primary mode of transmission—National Outbreak Reporting System, United States, 2009–2014
| Animal contact outbreaks |
Foodborne outbreaks |
||||
|---|---|---|---|---|---|
| Serotype | No. | % | Serotype | No. | % |
| 1. Typhimurium | 26 | 23.0 | 1. Enteritidis | 101 | 24.4 |
| 2. I 4,[5],12:i:- | 10 | 8.8 | 2. Typhimurium | 57 | 13.8 |
| 3. Montevideo | 8 | 7.1 | 3. Newport | 36 | 8.7 |
| 4. Infantis | 7 | 6.2 | 4. Heidelberg | 30 | 7.2 |
| 5. Hadar | 6 | 5.3 | 5. I 4,[5],12:i:- | 19 | 4.6 |
| 6. Sandiego | 6 | 5.3 | 6. Javiana | 19 | 4.6 |
| 7. Enteritidis | 5 | 4.4 | 7. Infantis | 14 | 3.4 |
| 8. Pomona | 5 | 4.4 | 8. Braenderup | 13 | 3.1 |
| 9. Braenderup | 4 | 3.5 | 9. Montevideo | 11 | 2.7 |
| 10. Johannesburg | 4 | 3.5 | 10. Saintpaul | 9 | 2.2 |
| 11. Newport | 4 | 3.5 | 11. Uganda | 8 | 1.9 |
| 12. Paratyphi B | 4 | 3.5 | 12. Mbandaka | 7 | 1.7 |
| 13. Poona | 4 | 3.5 | 13. Muenchen | 7 | 1.7 |
| 14. Muenchen | 3 | 2.6 | 14. Thompson | 7 | 1.7 |
| 15. Thompson | 3 | 2.6 | 15. Typhimurium var Cope | 7 | 1.7 |
4 |. DISCUSSION
This is the first analysis of NORS data collected at the national level to compare characteristics of animal contact and foodborne outbreaks; significant differences between outbreak types were observed in this analysis. Although the overall proportion of illnesses resulting from animal contact was smaller than those resulting from food, a higher proportion of illnesses in children aged <5 years resulted from animal contact. These findings are consistent with previous observations in the literature, and past outbreak reports indicating young children are disproportionately affected by Salmonella illness outbreaks linked to animal contact (Basler et al., 2015; Basler et al., 2014; J. Hall et al., 2010; Harris et al., 2010; Hoelzer et al., 2011; Levy et al., 1999; Loharikar et al., 2012; Whitten, Bender, Smith, Leano, & Scheftel, 2015). Patients linked to animal contact had greater odds of hospitalization compared to foodborne outbreaks. This might indicate that illnesses linked to animal contact have the potential to be more severe or that healthcare utilization differs for illnesses linked to animal contact-related outbreaks.
Animal contact outbreaks in this analysis were more likely to be longer in duration and more likely to occur across multiple states compared to foodborne outbreaks. This is an important distinction as outbreaks of longer duration might require more staff time and resources. The full range of duration for animal contact outbreaks included a minimum of 2.0 days to a maximum of 862.0 days, while the full range of duration for food outbreaks was from 1.0 to 428.0 days. Although animal contact outbreaks can be point source in nature, such as an exposure at a petting zoo or other animal-related event, animal contact outbreaks have also been linked to pets and animals that are widely distributed through commercial channels. In animal contact outbreaks, especially those linked to commercially distributed animals or pets, longer outbreak duration might result from animals shedding Salmonella bacteria intermittently, which can contaminate their environments or expose people long after the animal has been purchased or acquired (Hoelzer et al., 2011). Unless an affected food is shelf-stable, foodborne outbreaks are often shorter in duration because the entirety of the affected food is consumed over a short period of time, or because foodborne outbreaks might be associated with perishable items, such as fresh produce. Additionally, while recalls can be issued for contaminated food products, which can reduce exposure (and presumably overall outbreak) duration, the same cannot be done for animals or pets that people have purchased.
There were limitations to this analysis. First, outbreaks are voluntarily reported to NORS by state and local health departments, and reporting practices vary by state. Thus, underreporting might have influenced the number of total animal contact and food outbreaks, as well as the number of illnesses included in this analysis. Additionally, reporting health agencies can edit or delete reports through the NORS platform at any time, and the current analyses may not reflect future report revision. Second, incomplete reports and missing data for demographic variables were common in the dataset. Data were imputed using known information and categorizing missing data as unknown, which might not accurately represent demographics of illnesses with unknown data. Additionally, although the differences in sex were statistically significant, the effect size was small and might be of little practical significance. Hospitalizations, deaths, emergency room visits, and outpatient healthcare provider visits were analysed based on number of illnesses with information available, which might have led to an overestimated proportion of outcomes. A higher proportion of hospitalization, emergency room visit, and outpatient healthcare provider visit data were missing for animal contact outbreaks, which might contribute to an overestimated proportion of illnesses with each outcome. For emergency room visits, the percentage of missing data for animal contact outbreaks and food outbreaks was 94.4 and 61.9, respectively. Outpatient healthcare provider visit percentages missing were also high, at 92.4% for animal contact outbreaks and 55.0% for food outbreaks. Many variables (e.g. age, gender, and clinical outcomes) were available only at an aggregate outbreak level; therefore, no multivariable analysis could be conducted. Finally, only foodborne outbreaks with a confirmed food vehicle were included in this analysis, so this analysis might not be representative of all foodborne outbreaks reported to NORS. Some foodborne outbreaks in NORS are reported as having a probable vehicle rather than a confirmed vehicle; however, the categorization of animal contact outbreaks as probable is not an option for reporting in NORS at this time. Therefore, this analysis of outbreaks with a confirmed vehicle might be more representative for animal contact outbreaks than for foodborne outbreaks.
Characterizing the differences between outbreaks of illness linked to animal contact and outbreaks linked to food provides useful information to investigators to improve public health response. Although Salmonella illness outbreaks linked to animal contact account for a small proportion of all Salmonella infections annually, they disproportionately affect young children and result in more adverse outcomes compared to foodborne outbreaks. Investigating health agencies should ensure that detailed questions about animal contact are asked of patients in outbreaks of salmonellosis, especially when the demographics and outbreak characteristics are consistent with the findings of this report. It is important to quickly and efficiently identify animal contact illness outbreaks, investigate sources of infections, and implement control measures to reduce the burden of disease and prevent adverse outcomes.
Impacts.
Outbreaks of Salmonella enterica infections from 2009 to 2014 linked to animal contact were significantly more likely to impact young children (aged <5 years) than food outbreaks.
Salmonellosis outbreaks linked to animal contact from 2009 to 2014 resulted in more hospitalizations com- pared to food outbreaks.
Prompt identification, investigation, and implementation of control measures are critical to reducing the number of illnesses and decreasing adverse outcomes during salmonellosis outbreaks linked to animal contact.
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: DISCLAIMER
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of Centers for Disease Control and Prevention.
REFERENCES
- Basler C, Bottichio L, Higa J, Prado B, Wong M, & Bosch S. (2015). Notes from the field: Multistate outbreak of human salmonella Poona infections associated with pet turtle exposure - United States, 2014. MMWR. Morbidity and Mortality Weekly Report, 64(29), 804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basler C, Forshey TM, Machesky K, Erdman MC, Gomez TM, Nguyen TA, & Behravesh CB. (2014). Multistate outbreak of human Salmonella infections linked to live poultry from a mail-order hatchery in Ohio–March-September 2013. MMWR. Morbidity and Mortality Weekly Report, 63(10), 222. [PMC free article] [PubMed] [Google Scholar]
- Boxrud D, Monson T, Stiles T, & Besser J. (2010). The role, challenges, and support of pulsenet laboratories in detecting foodborne disease outbreaks. Public Health Reports, 125(Suppl 2), 57–62. 10.1177/00333549101250s207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (2013). FoodNet 2013 annual report. Retrieved from http://www.cdc.gov/foodnet/reports/annual-reports-2013.html.
- CDC (2014). National Salmonella surveillance annual report, 2012. Atlanta, GA: Retrieved from http://www.cdc.gov/ncezid/dfwed/pdfs/salmonella-annual-report-2012-508c.pdf [Google Scholar]
- CDC (2015). Salmonella homepage. Retrieved from http://www.cdc.gov/salmonella/general/technical.html
- CDC (2017). National outbreak reporting system (NORS) guidance. Retrieved from https://www.cdc.gov/nors/downloads/guidance.pdf
- Gould LH, Walsh KA, Vieira AR, Herman K, Williams IT, Hall AJ, & Cole D. (2013). Surveillance for foodborne disease outbreaks - United States, 1998–2008. MMWR Surveillance Summary, 62(2), 1–34. [PubMed] [Google Scholar]
- Hale CR, Scallan E, Cronquist AB, Dunn J, Smith K, Robinson T, … Clogher P. (2012). Estimates of enteric illness attributable to contact with animals and their environments in the United States. Clinical Infectious Diseases, 54(Suppl 5), S472–479. 10.1093/cid/cis051 [DOI] [PubMed] [Google Scholar]
- Hall AJ, Wikswo ME, Manikonda K, Roberts VA, Yoder JS, & Gould LH. (2013). Acute gastroenteritis surveillance through the National outbreak reporting system, United States. Emerging Infectious Diseases, 19(8), 1305–1309. 10.3201/eid1908.130482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Poulson M, Fawcett L, Cosgrove S, Lujan K, Torres P, … Williams IT. (2010). Multistate outbreak of human Salmonella typhimurium infections associated with aquatic frogs - United States, 2009. MMWR. Morbidity and Mortality Weekly Report, 58(51), 1433–1436. [PubMed] [Google Scholar]
- Harris JR, Neil KP, Behravesh CB, Sotir MJ, & Angulo FJ. (2010). Recent multistate outbreaks of human salmonella infections acquired from turtles: A continuing public health challenge. Clinical Infectious Diseases, 50(4), 554–559. 10.1086/649932 [DOI] [PubMed] [Google Scholar]
- Hoelzer K, Moreno Switt AI, & Wiedmann M. (2011). Animal contact as a source of human non-typhoidal salmonellosis. Veterinary Research, 42, 34 10.1186/1297-9716-42-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy C, Finnerty M, Hansen G, Cory J, McGuill M, Matyas B, … Davis JP. (1999). Reptile-associated salmonellosis–selected states, 1996–1998. MMWR. Morbidity and Mortality Weekly Report, 48(44), 1009–1013. [PubMed] [Google Scholar]
- Loharikar A, Briere E, Schwensohn C, Weninger S, Wagendorf J, Scheftel J, … Behravesh CB. (2012). Four multistate outbreaks of human Salmonella infections associated with live poultry contact, United States, 2009. Zoonoses Public Health, 59(5), 347–354. 10.1111/j.1863-2378.2012.01461.x [DOI] [PubMed] [Google Scholar]
- Marsden-Haug N, Meyer S, Bidol SA, Schmitz J, Culpepper W, Barton Behravesh C, … Anderson TC. (2013). Notes from the field: Multistate outbreak of human Salmonella typhimurium infections linked to contact with pet hedgehogs - United States, 2011–2013. MMWR. Morbidity and Mortality Weekly Report, 62(4), 73. [PMC free article] [PubMed] [Google Scholar]
- Mitchell JR, Forshey TM, Nowicki S, Mohr M, Roney CS, Gomez T, … Barton Behravesh C. (2013). Notes from the field: Multistate outbreak of Salmonella infantis, newport, and lille infections linked to live poultry from a single mail-order hatchery in Ohio–March-September, 2012. MMWR. Morbidity and Mortality Weekly Report, 62(11), 213. [PMC free article] [PubMed] [Google Scholar]
- Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, … Griffin PM. (2011). Foodborne illness acquired in the United States–major pathogens. Emerging Infectious Diseases, 17(1), 7–15. 10.3201/eid1701.091101p1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan B, Barrett TJ, Hunter SB, & Tauxe RV. (2001). PulseNet: The molecular subtyping network for foodborne bacterial disease surveillance, United States. Emerging Infectious Diseases, 7(3), 382–389. 10.3201/eid0703.010303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitten T, Bender JB, Smith K, Leano F, & Scheftel J. (2015). Reptile-associated salmonellosis in Minnesota, 1996–2011. Zoonoses Public Health, 62(3), 199–208. 10.1111/zph.12140 [DOI] [PubMed] [Google Scholar]


