Abstract
Unintentional body weight loss is common in patients with dementia and is linked to cognitive impairment and poorer disease outcomes. It is proposed that some dementia medications with market approval, while aiming to improve cognitive and functional outcomes of a patient with dementia, are associated with reported body weight or body mass index loss. This review presents evidence in the published literature on body weight loss in dementia, describes selected theories behind body weight loss, evaluates the potential impact of approved dementia pharmacotherapies on body weight, considers the potential role for medical foods, understands the potential influence of treatments for neuropsychiatric symptoms and signs, and finally, summarizes this important area.
1 Introduction
Dementia is a clinical syndrome characterized by progressive deterioration of cognitive abilities stemming from neurodegenerative and/or ischemic pathologies. Alzheimer’s disease (AD), vascular dementia (VaD), dementia with Lewy bodies (LBD), and frontotemporal dementia (FTD) are the most common subtypes [1]. The prevalence of dementia is growing rapidly. It has been estimated that worldwide, 46.8 million people lived with dementia in 2015 [2]. This number is expected to double every 20 years without the development of therapeutic or preventive strategies [2]. The burden of dementia on society and healthcare systems is equally staggering. Total worldwide costs of US$818 billion were estimated for 2015, which are expected to exceed US$1 and US$2 trillion thresholds by 2018 and 2030, respectively [3].
An intense search for effective dementia medications targeting potential etiologies and symptoms throughout the DOI dementia course is ongoing. Available pharmacologic interventions for dementia do not address the underlying etiology, but instead are symptomatic and designed to slow cognitive decline and improve the deteriorating functional status that characterizes dementia. While these treatments can be effective, some may have considerable side effects such as body weight (BW) or body mass index (BMI) loss. In general, epidemiologic studies have described a paradoxical life course association of BMI in association with dementia such that overweight and obesity in mid-life increase the risk for dementia, while a decline in BW or BMI and/or underweight (BMI <20 kg/m2) may precede and/or accompany dementia onset [4, 5], as well as continue to predict dementia course and outcomes after diagnosis [6, 7]. Thus, medications that cause iatrogenic BW or BMI loss may provoke adverse outcomes in a frail elderly patient, such as one with dementia.
Here, we explore the published literature on BW loss in dementia, describe selected theories behind BW loss, evaluate the potential impact of approved dementia pharmacotherapies on BW, consider the potential role for medical foods, understand the potential influence of treatments for neuropsychiatric symptoms and signs, and finally, summarize this important area. To identify relevant studies that describe BW in patients who received drugs that treat dementia symptoms, we searched PubMed and Web of Science for English publications with the terms ‘dementia’, ‘Alzheimer’, ‘donepezil’, ‘galantamine’, ‘ri-vastigmine’, ‘memantine’, ‘body weight’, ‘nutritional status’, ‘body mass index (BMI)’, and ‘weight loss’ with no date restrictions on 31 July, 2016.
2 Body Weight Loss in Dementia
Unintentional BW loss is a prominent clinical feature in some individuals with dementia [7–9]. For example, data indicate that 30–40% of patients with dementia may experience clinically significant weight loss [10–12]. Body weight loss in dementia may be characteristic of reduced nutritional status, which is partially explained by a reduced food intake [11]. It has been shown that 14–45% of community-dwelling patients with mild-to-moderate AD, and up to 68% of patients with severe AD, are at risk of malnutrition [13–15]. Body weight loss is also observed in transgenic mouse models of dementia that recapitulate underlying neurodegenerative pathologies [16–20], albeit inconsistently [21, 22], as this loss may also be explained by model-specific developmental problems [16] or strain-specific genetic effects [23].
Unintentional BW loss is detrimental for the frail elderly because it is associated with higher rates of mortality [24–27], institutionalization [28], adverse health outcomes, decline in functional status, and overall poorer quality of life [29, 30]. In elderly with dementia, unintentional BW loss is associated with syndrome severity [7, 31–33], higher rates of institutionalization [34], a higher incidence of behavioral problems [35], and ultimately mortality [6, 36–38]. In contrast, a BMI equal to or higher than overweight (≥25.0 kg/m2) has been associated with reduced mortality in dementia [39, 40]. Thus, it appears that low or healthy adult BMI can lead to poorer health outcomes in patients with dementia, while a higher BMI may be protective.
As aforementioned, several epidemiologic studies report a paradoxical association of BW or BMI in dementia. Most of these studies use traditional categories of BMI as follows: underweight, <20.00 kg/m ; healthy body size, 20.00–24.99 kg/m2; overweight, 25.00–29.99 kg/m2; and obese ≥30.00 kg/m2. An overweight or obese state in midlife may increase dementia risk in late life [4, 41–43]. Strikingly, a subsequent decline in BW or BMI in late life (e.g., >4% per year [44]) towards the underweight or even healthy BMI range precedes a dementia diagnosis by years, and may signify a prodromal phase, [9, 45–48] and/or accompany dementia [4, 8, 49–51]. In other words, the epidemiology suggests that during mid-life, an increased BMI or central adiposity [52, 53], increases the risk for dementia in old age, while in late life, a decline in body weight to a BMI within the underweight or even healthy range is associated with a higher risk of dementia in the years to come. Supporting evidence of the significance of BW loss during prodromal dementia are observations of unintentional BW loss from mid- to late-life increasing the risk for mild cognitive impairment (MCI) [54]. The elderly with MCI are often malnourished compared with their cognitively intact peers [55]; and patients with MCI that are losing BW show more accelerated progression towards dementia [44, 56, 57]. In summary, it is clear that BW and BMI are intricately linked with dementia before and after diagnosis, and that BW or BMI loss or underweight can lead to poorer health outcomes in dementia.
3 Theories of Body Weight Loss in Dementia
Numerous explanations have been put forth to explain associations observed between malnutrition, BW loss, cognitive decline, and dementia [58, 59]. First, patients with dementia can experience BW loss from reduced energy intake owing to their decreased mental status, which causes them to simply forget or refuse to eat [60]. This may result from impairments in episodic memory and attention that are associated with medial temporal lobe atrophy [11]. Second, changes in behavior, such as repetitive actions and other behavioral disorders may both demand larger amounts of energy and reduce energy intake. Third, sensory changes such as deterioration of the olfactory bulb and/or diminished gustatory perception can contribute to weight loss as a result of cholinergic deficits [58]. Fourth, dysphagia, or difficulties swallowing, may reduce energy intake [61]. Fifth, hypermetabolism, defined as an elevated basal metabolic rate of >10% [in energy (e.g., kJ) per unit time], is suggested to cause BW loss. Studies in patients with AD show increased [62], but also reduced [63] and no change [64, 65] in energy metabolism. However, hypermetabolism and BW loss were observed in a transgenic mouse model of AD using calorimetric cages [19]. Still, it remains unclear whether hypermetabolism is a feature of all or a subset of AD cases.
Sixth, neuroendocrine dysregulation in dementia may also give rise to BW loss. Because the biggest component of BW or BMI loss is loss of adipose tissue [66, 67], discussion of adipose as the largest endocrine organ in the body deserves attention. Adipose tissue secretes hundreds of cytokines, peptides, and hormones, collectively referred to as adipokines, which interact with the brain to control energy metabolism and other brain functions [68–70]. Leptin is secreted by adipocytes [71], expressed in the brain [72, 73], and is present in the blood and cerebrospinal fluid in quantities that are proportional to body fat mass [74–77]. In the brain, leptin regulates food intake and energy expenditure by acting on the hypothalamus through a negative feedback loop [78, 79]. Leptin also supports hippocampal neurogenesis, axonal growth, and learning and memory processes [79–81]. Analogous to the evidence that low adiposity, measured as BMI in the underweight ranges, may be associated with a higher risk for dementia in the elderly [4, 8, 49–51], multiple studies associate low plasma leptin (after adjusting for BW) with an increased risk for AD [82, 83] and cognitive decline [84, 85] later in life. Furthermore, when AD manifests clinically, plasma leptin may be markedly reduced independently of BMI [86, 87] (although stable levels have also been reported [88, 89]). Because in vitro experiments and in vivo animal studies show that leptin is neurotrophic and neuroprotective in both healthy neuronal tissue and AD-stricken brains [90–93], it is plausible that disrupted leptin signaling may contribute to AD pathology. Strikingly, by employing a mouse model that mimics the toxic amyloid-β peptide aggregation pathology of AD, it has been demonstrated that the ensuing morbidly low BW and a low plasma leptin level were associated with inhibited leptin signaling in the brain and aberrant responses of hypothalamic neurons, symptoms that progressively worsened as the amyloid-β burden increased [94]. Thus, these data presented on leptin are an example of the potentially tremendous role of the fat-brain axis in aging-associated changes in both brain and periphery. Owing to the large amount of accumulated adipose tissue in most adults aged 65 years and older who are entering a period of increasing dementia risk, the impact of adipose tissue loss as a result of aging, aging brain, and iatrogenic instigators is meaningful.
Finally, other theories of BW loss not discussed here, are those consequent to dementia, such as the presence of multi-morbidities, adverse effects of other medications unrelated to dementia, loss of autonomy, and changing socio-environmental factors [58, 95].
4 Pharmacotherapies for Dementia: Effects on Body Weight and Body Mass Index
The US Food and Drug Administration (FDA) has currently approved four unique formulations for the treatment of dementia: donepezil, rivastigmine, galantamine, and memantine [96]. Donepezil, rivastigmine, and galantamine are acetylcholinesterase inhibitors (AChEIs) and vary only slightly in pharmacological properties. Acetylcholinesterase inhibitors are at the forefront of symptomatic treatment for AD and LBD [97], diseases that are most often characterized by a loss of cholinergic neurons in the brain, reducing the amount of acetylcholine available for neurotransmission, leading to a deficient cholinergic system [98]. Acetylcholinesterase inhibitors bind to brain cholinesterase enzymes in a reversible manner and inhibit them from breaking down acetylcholine at the synapse level. Thus, AChEIs increase the level and duration of neurotransmitter action in forebrain regions, in an attempt to compensate for the loss of functioning cholinergic neurons [99]. Most systematic reviews and meta-analyses that assess the efficacy of AChEIs report modest improvements in cognitive function and other benefits in patients with mild-to-moderate AD or LBD [100–102], but marginal effects of uncertain significance in VaD [103].
Despite the positive cognitive effects of AChEIs, numerous phase IV clinical trials note an unintentional side effect that may be cause for concern. In a meta-analysis of AChEI treatment in AD, it was shown that there was an almost three-fold higher odds of the adverse event, BW loss, among patient groups taking AChEIs compared with placebo treatment groups. Furthermore, this BW loss was associated with adverse gastrointestinal side effects [100]. Notably, AChEIs induce dose-dependent nausea, vomiting, anorexia, and diarrhea [100, 104–106] caused by cholinergic hyperstimulation of the internal muscarinic receptor in the gastrointestinal tract [106, 107]. Thus, AChEIs may influence nutritional status through adverse effects on the peripheral nervous system leading to BW loss [108].
Since the market approval of AChEIs, the association between AChEI treatment and BW loss has been investigated. One group observed a 23% risk of BW loss in individuals with dementia taking AChEIs [109], while others concluded AChEI treatment is protective against BW loss seen in dementia [110] (however, the latter study lacked a control group). Crucially, in a meta-analysis of 25 clinical AChEI trials and others, an increased risk of BW loss was observed for every AChEI [111, 112]. This provided the first report of pooled estimates of effect addressing whether AChEIs may cause BW loss in dementia. While there are also reports of patients regaining BW after AChEI discontinuation [104, 105], no randomized controlled trials (RCT) assessing discontinuation have been conducted.
Memantine, the fourth compound currently approved by the FDA for the management of dementia, is a partial N-Methyl D-Aspartate receptor (NMDAR) antagonist. In dementia, an excitotoxic mechanism, partially owing to excess glutamate causing over-activation of NMDARs, triggers various neurotoxic events that lead to necrosis or apoptosis. Memantine works through uncompetitive blocking of the glutamatergic NMDARs when they are excessively open, blocking the imminent excitotoxic cascade [113]. Despite a paucity of evidence and high heterogeneity among studies, memantine is associated with clinical benefits on cognitive, functional, and global outcome scales in patients with mild-to-severe AD [114–116], although efficacy in mild AD has been disputed [117]. Minimal benefits have been detected in VaD [114] and FTD [118], but not in LBD [119, 120].
Studies that pooled trial data of adverse events have reported no significant difference between memantine and placebo groups in drop-out rates or the incidence of adverse events [114, 116, 121], and no significant effects were found on BW loss and other adverse events that are associated with AChEIs [114, 116, 121]. There is the caveat with memantine as with all dementia medications, however, that the BW of those prescribed may have already dropped to such low levels that no further influence of the medication is observed on BW. This is however, speculative, as to our knowledge, no data exist on this important point. Overall, memantine displays a better safety and tolerability profile than AChEIs [121].
5 Potential Role for Medical Foods
The pursuit of novel dementia treatments has led to the development of novel nutritional interventions such as medical foods. Medical foods are not dietary supplements owing to legal definitions, and variation exists cross-nationally as to whether a medical prescription is necessary for their use, thus complying with the pharmacotherapy framework of this review [122]. The term medical food is defined as “a food which is formulated to be consumed or administered enterally under the supervision of a physician and which is intended for the specific dietary management of a disease or condition for which distinctive nutritional requirements, based on recognized scientific principles, are established by medical evaluation” [123]. In contrast, dietary supplements are products (e.g., capsules, liquids, or soft gels) that contain a dietary ingredient intended to add to the nutritional value of the diet [122]. Both medical foods and nutritional supplements have been developed to improve dementia outcomes, but for the sake of brevity and the focus of this review on pharmacotherapies, only medical foods are discussed here. (For systematic appraisals on the effectiveness of nutritional supplements in dementia, the reader is directed elsewhere [124–126]).
Treatment rationales for medical foods are based on specific micronutrient needs that arise from underlying disease pathologies, and that may not be met by simply modifying the usual diet. Of high relevance for this review, medical foods may also provide a way to combat BW loss. For AD, three medical foods are being developed that claim to offer symptomatic relief: Axona®, CerefolinNAC®, and Souvenaid®. Axona® provides a ketogenic agent that targets metabolic deficiencies in AD [127]. Results from a multi-center RCT show that APOE-4 (an important AD-susceptibility gene) negative patients with mild-to-moderate AD taking Axona®, improve on the Alzheimer’s Diseases Assessment Scale-Cognitive subscale after 90 days [128]. It should be noted that 23% of patients in the treatment arm discontinued the trial because of adverse events, mostly related to the gas- trointestinal system. CerefolinNAC® addresses the hyperhomocysteinemia and oxidative stress associated with disorders such as MCI and AD. CerefolinNAC® increases the bioavailability of folate and vitamin B12. One prospective case–control study has shown that CerefolinNAC® significantly slows cognitive decline in dementia disorders compared with no treatment [129]. Unfortunately, there are a paucity of published studies on the treatment effects of Axona® and CerefolinNAC® , and these studies have not addressed patient BW or nutritional status.
The third medical food, Souvenaid® , acts as a vehicle for the Fortasyn Connect formulation and was designed to deliver a specific combination of nutritional precursors and cofactors for phospholipid synthesis that are essential for the synthesis of neuronal membranes and synaptic function [130]. Deterioration of neuronal membranes and synaptic function have been shown to underlie cognitive decline in AD [131]. Therefore, this formulation is targeted to improve synaptic formation and function, with the ultimate goal of ameliorating cognitive symptoms in patients with AD [132].
Several industry-sponsored clinical trials assessing the efficacy of Fortasyn Connect at various stages of AD have been completed; however, evidence remains inconclusive. Randomized controlled trials have reported modest positive outcomes for once-daily treatment on the measures of cognitive function in FTD [133] and mild AD [134, 135], while a much larger RCT of add-on treatment reported no effect in mild-to-moderate AD [136]. In addition, these studies noted that Souvenaid® was remarkably well tolerated by patients. In recognition of poor nutritional status often accompanying dementia, a secondary analysis was undertaken to examine the effect of Souvenaid® on BMI and functional abilities compared with an iso-caloric control product in mild AD. Overall, a small increase of marginal significance in BMI was reported in the active vs. control group after 24 weeks [137]. Thus, this study suggests that a medical food can increase BMI in patients with mild AD, although the underlying mechanism by which Souvenaid® contributes to a potential increase in BMI remains unknown.
At this moment, whether Souvenaid® can be an effective (add-on) treatment that may be beneficial to nutritional status in dementia remains an open question. There is no evidence from long-term clinical trials available yet, although a two-year RCT trial known as ‘LipiDiDiet’ has recently been completed in a population with prodromal AD (DR Gustafson and AJ Kiliaan are investigators on the LipiDiDiet team). Preliminary results indicate that Souvenaid® does not significantly benefit nor slow cognitive decline in the treatment group; however, various secondary measures such as medial temporal lobe atrophy and neuropsychological testing scores were significantly improved compared with the control group [138]. While too early to draw definitive conclusions regarding neuroprotective effects of Souvenaid®, the prospect that a medical food intervention can positively affect early disease course, where conventional medication often fails, and alleviate potential side effects of traditional medications, is hopeful. While the LipiDiDiet study has been extended, more long-term clinical trials studying the effects of medical foods as interventions would be worthwhile.
6 Pharmacotherapies for the Treatment of Neuropsychiatric Symptoms of Dementia and their Effect on Body Weight
As aforementioned, AChEIs and NMDAR antagonists are the only medications with marketing approval for the treatment of dementia [96]. However, several other medications are prescribed to treat concomitant neuropsychiatric symptoms. These symptoms are referred to as behavioral and psychological symptoms of dementia (BPSD). These symptoms occur irrespective of dementia subtype and include a wide array of non-cognitive disturbances such as agitation, aggression, hallucinations, and depression [139]. Behavioral and psychological symptoms of dementia are associated with high levels of distress and poor outcomes for the patient and caregiver, as well as increased use of healthcare resources [139]. Behavioral and psychological symptoms of dementia are therefore a meaningful intervention target [140, 141]; and unexpected benefits may arise from BPSD medications. For example, dronabinol, a synthetic cannabinoid used to decrease nausea and improve appetite in patients with human immunodeficiency virus, is used as an off-label treatment for BPSD-related aggression and agitation in the elderly. Interestingly, a placebo-controlled crossover study and a non-randomized retrospective study also suggest that it may alleviate anorexia [142, 143]. However, BW did not change in a more recent RCT [144], hence it is not yet possible to draw conclusions. Herein, antidepressants and antipsychotics are briefly discussed as they are more frequently used as an off-label treatment for BPSD.
6.1 Antidepressants
Increased appetite and BW gain are well-known side effects of antidepressants. Antidepressants are routinely prescribed to treat agitation or depressive symptoms that appear in dementia and use is reported almost twice as high among AD patients compared with controls [145]. However, the orexigenic effect of antidepressants in patients with dementia is rarely addressed. Mirtazapine, a noradrenergic antagonist, has potent orexigenic effects [146]. Of all marketed antidepressants, mirtazapine is associated with maximum BW gain, an effect strongly predicted by the H1-histamine receptor antagonistic action of this drug type [147]. The orexigenic effect of mirtazipine warrants off-label prescriptions to treat anorexia and underweight in various conditions [148, 149], but only a handful of retrospective open-label studies have investigated whether antidepressants such as mirtazapine alleviate BW loss in patients with dementia. One report documented three AD cases with co-morbid depression who regained appetite and BW within weeks after initiating mirtazapine [150]. Another study examined the impact of various antidepressants on nutritional parameters in the institutionalized elderly with and without dementia. All patients, irrespective of dementia status, experienced BW gain and lower risk of malnutrition when taking antidepressants (except for selective serotonin reuptake inhibitors [SSRIs]) [151]. The third and final retrospective open-label study investigated whether mirtazapine alleviated BW loss in AD, and observed a mean BW increase of 4.6% after 6 months [152]. Of note, these studies were not placebo controlled, and sample sizes were underpowered and highly heterogeneous (e.g., many patients also received AChEIs [151]).
Management of BPSD with pharmacological agents is an important but complex component of dementia treatment owing to a lack of treatment options and possible adverse interactions caused by concomitant use of other types of medications. Despite the scarcity and varied quality of clinical practice guidelines for the management of BPSD, there is advancement. Recent systematic comparison and appraisal of BPSD guidelines concluded there was sufficient agreement for the use of antidepressants to manage co-morbid depression in BSPD [153]. However, only SSRIs were considered. Mirtazapine may be preferable in BPSD because SSRIs are associated with a high propensity for drug–drug interactions [154]. Unlike mirtazapine, SSRIs and tricyclic antidepressants are associated with many serotonin-related adverse effects, including nausea, insomnia, headaches, anxiety, and sexual dysfunction [155]. In contrast, mirtazapine is less likely to cause drug–drug interactions and overall has a more favorable side-effect profile than other classes of antidepressant medications [146]. Particularly because of the latter, mirtazapine has been recommended as treatment for depression in dementia over other antidepressants [156]. Of note, studies have suggested that second-generation antidepressants in general appear less effective in elderly populations, and that there is no substantial difference between the efficacy of mirtazapine, sertraline (SSRI), and placebo in the treatment of primary co-morbid depression symptoms in BPSD [157, 158]. However, a follow-up cost-effectiveness study concluded that mirtazapine treatment may carry more benefits over placebo or sertraline in terms of improving quality of life and reducing unpaid care time and cost, potentially caused by improving insomnia and anxiety [159].
6.2 Antipsychotics
Antipsychotics, most often second-generation antipsychotics (SGAs), are another class of medications prescribed for the pharmacological treatment of BPSD. Second-generation antipsychotics are commonly used to manage typical symptoms such as aggression and psychosis, and do so with modest efficacy [160]. In addition, SGAs are associated with an increased risk for BW gain and metabolic side effects related to morbidity in adults, such as diabetes mellitus and hyperlipidemia [161]. Similar to the orexigenic property of antidepressants, H1 receptor affinity of antipsychotic agents was found to be a strong predictor of these effects [162]. The relationship between metabolic side effects and antipsychotic treatment for the elderly with dementia was investigated in a meta-analysis of 27 studies [163]. It was concluded that, owing to a paucity of publications and relatively short trial durations, limited evidence indicates a possible association between BW gain and antipsychotic drug use in patients with mild-to-moderate dementia. This relationship was more convincing for SGAs, and no significant effects on glucose homeostasis or lipid metabolism were found [163].
While BW gain resulting from SGA use might be considered helpful in elderly populations where BW loss is common and detrimental, it can easily be overshadowed by several SGA-associated adverse effects. First, SGA treatment in elderly patients with dementia is linked to a 1.7-fold increased risk for cardiovascular events and mortality compared with placebo [164]. Therefore, the FDA actively warns against off-label prescription of antipsychotics in elderly patients [165] and nearly all clinical BPSD guidelines recommend caution and timely discontinuation [153]. Second, drug–drug interactions need to be considered. For example, if a BPSD patient taking an antipsychotic agent requires an opioid analgesic, there will be an increased risk of sedation, dizziness, and falls [166]. Third, because antipsychotics interact in part with dopaminergic pathways, they cannot be readily applied in every clinical case of dementia. For instance, treatment of psychosis with SGAs in Parkinson’s disease dementia is difficult, considering certain SGAs can exacerbate parkinsonian symptoms [167], which is possibly evidenced by the higher risk of bone fractures associated with SGA prescription in Parkinson’s disease dementia [168].
7 Discussion and Perspectives
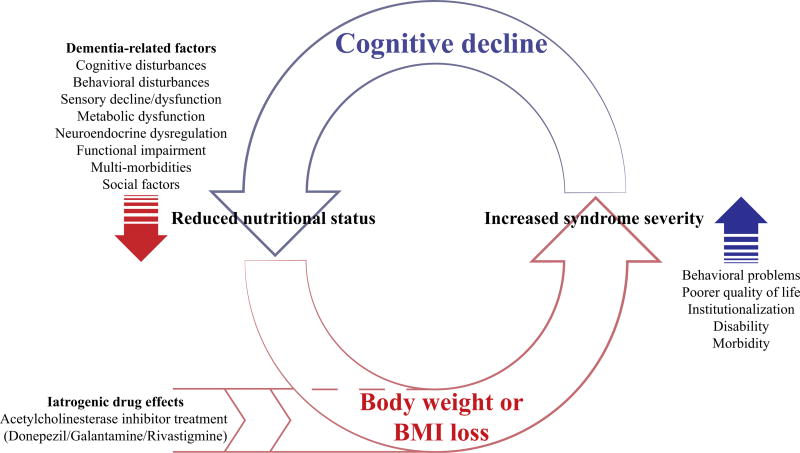
The evidence presented in this review demonstrates that unintentional BW loss in dementia is problematic, and that the association between AChEI treatment and BW loss is a concern. See Fig. 1 for an illustration of factors involved in BW loss in dementia. Importantly, there is increasing evidence that AChEIs, prescribed to relieve cognitive symptoms of dementia, are associated with two or even three times the risk of clinically significant BW loss [111]. In a vulnerable population where BW loss and poor nutritional status are predictive of adverse outcomes, clinicians need to consider these risks when evaluating their choice to prescribe an AChEI. In contrast, memantine has shown no significant relationship with BW loss [114, 116, 121]. Thus, although the cognitive benefits of memantine in AD, FTD, and VaD may be small, the absence of effects on BW may make memantine a more suitable treatment option for elderly patients in whom BW loss is a hazard. However, we acknowledge that memantine may be more likely prescribed among those with more severe dementias, and thus for those who have already lost a substantial amount of BW.
Fig. 1.
Vicious cycle of cognitive impairment and body weight or body mass index (BMI) loss and progression to dementia. Body weight or BMI loss is often an important correlate of dementia. In dementia, BW or BMI loss is associated with adverse health outcomes [6, 7, 31–38], cognitive decline, and cognitive impairment [44, 54, 56, 57], which evokes a deleterious cycle driving further BW or BMI loss. Acetylcholinesterase inhibitors approved for the symptomatic treatment of dementia contribute to BW or BMI loss [111, 112], most likely via side effects including nausea, anorexia, and vomiting, which are likely the result of cholinergic hyperstimulation [106, 107]
Common pharmacotherapies for the treatment of BPSD have demonstrated orexigenic effects in patients with dementia. Mirtazapine, which has a mild side-effect profile that reduces insomnia and increases appetite [146], could be a primary candidate for BPSD treatment when BW loss or malnutrition are concerns. To our knowledge, there is currently no consensus on the use of mirtazapine for treatment of BPSD. Future experimental studies on the orexigenic effect of mirtazapine in the elderly are critical to establish whether mirtazapine is a viable treatment option. Second-generation antipsychotics seem to have a similar effect on BW, but the potential benefit is overshadowed by their more serious adverse effects [164, 165].
Maintaining a healthy BW in individuals with dementia is an integral part of patient care. Decreased nutritional and energy intakes contribute to BW loss [11, 65], which suggests promising avenues for non-pharmacological interventions such as medical foods. Systematic appraisals have indicated that nutritional supplements can improve nutritional status [125, 126], and the same may hold for medical foods [137]. In addition, other lifestyle and social factors may be improved to enhance the quality of the dining experience for the elderly. The introduction of an aquarium in the dining area of residents with long-term dementia increased food intake by 25% and significantly improved BW [169]. There is also interest in other modifiable lifestyle factors, including transformation of usual diets such as adopting the Mediterranean dietary pattern or enhancing essential fatty acid intake [170]. Evidence on single and mixed food-based interventions that improve BW in dementia remain scarce. Future research in this area will benefit both patients and caregivers.
Finally, as a reminder, there is ample evidence that excess BW is associated with serious morbidities in adult populations [171, 172], while among the elderly, BMI in the overweight or obese range appears to positively affect survival [39, 40, 50]. The protective effect of higher BMI on mortality in later life, in contrast to middle age, is frequently termed the ‘obesity paradox’, and has been observed in other conditions, such as coronary artery disease and stroke [173, 174]. However, an obesity paradox is not consistently observed across studies in association with cognition and dementia; and with the global pandemic of obesity and emergence of new medications, it is unclear what associations will be observed in the very near future. Whether low adiposity or BW loss in dementia should be treated with interventions promoting BW gain has no straightforward answer and requires a personalized medicine approach.
8 Conclusion
More research is needed in clinical and pre-clinical areas of BW, BMI, and dementia. First, studies of body composition and metabolic aspects of adipose tissue are needed to clarify what BW and BMI represent in association with brain health. Second, definitions of optimal BW and BMI are needed for healthy brain and peripheral aging. Third, the relative influences of other factors, such as environmental, sensory, and physical function, need to be addressed more comprehensively and quantified for better use in models of aging. Finally, not only do dementia medications require further inquiry, but other medications, in addition to the role of polypharmacy in peripheral (BW loss) and brain health and disorders.
Key Points.
Body weight loss is common in adults with preclinical and clinical dementia, and linked to worsening cognitive impairment.
Some approved dementia medications are associated with loss of body weight.
In the elderly, more attention to polypharmacy targeting both brain and peripheral health and disorders is essential.
Acknowledgments
Funding
Support for this review is as follows: DR Gustafson, State University of New York, Downstate Medical Center institutional research funds and the Swedish Research Council for Health, Working Life and Welfare, AGECAP 2013–2300, 2013–2496; AA Franx, IC Arnoldussen, AJ Kiliaan AJ, EFRO project BriteN (PROJ-00405). The Women’s Interagency HIV Study (WIHS), National Institute of Allergy and Infectious Diseases (UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, and UO1-AI-42590).
Footnotes
Compliance with Ethical Standards
Conflict of interest
Bart A.A. Franx, Ilse A.C. Arnoldussen, Amanda J. Kiliaan, and Deborah R. Gustafson have no conflicts of interest directly relevant to the content of this article. No support was obtained specifically for writing or publishing this review. Dr. Kiliaan and Dr. Gustafson were Investigators in LipiDiDiet, a multicenter translational project funded by the European Commission (http://www.lipididiet.eu). Dr. Kiliaan performed animal experiments using Fortysyn Connect® . Dr. Gustafson analyzed epidemiological data on the association between dementia and adiposity, cardiovascular risk factors, and the APOE genotype. Neither Dr. Kiliaan nor Dr. Gustafson was directly involved in the clinical trial of Souvenaid® in patients with biomarker-based mild cognitive impairment.
References
- 1.World Health Organization. Dementia: a public health priority. Geneva: World Health Organization; 2012. p. 19. [Google Scholar]
- 2.Prince M, Wimo A, Guerchet M, et al. World Alzheimer Report 2015: the global impact of dementia. World Alzheimer’s Report. London: Alzheimer’s Disease International (ADI; 2015. An analysis of prevalence, incidence, costs and trends. [Google Scholar]
- 3.Wimo A, Guerchet M, Ali G-C, et al. The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimer’s Dementia. 2016 doi: 10.1016/j.jalz.2016.07.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Emmerzaal TL, Kiliaan AJ, Gustafson DR. 2003–2013: a decade of body mass index, Alzheimer’s disease, and dementia. J Alzheimers Dis. 2015;43(3):739–55. doi: 10.3233/JAD-141086. [DOI] [PubMed] [Google Scholar]
- 5.Gustafson DR, Backman K, Joas E, et al. 37 years of body mass index and dementia: observations from the prospective population study of women in Gothenburg, Sweden. J Alzheimers Dis. 2012;28(1):163–71. doi: 10.3233/JAD-2011-110917. [DOI] [PubMed] [Google Scholar]
- 6.Faxén-Irving G, Basun H, Cederholm T. Nutritional and cognitive relationships and long-term mortality in patients with various dementia disorders. Age Ageing. 2005;34(2):136–41. doi: 10.1093/ageing/afi023. [DOI] [PubMed] [Google Scholar]
- 7.Albanese E, Taylor C, Siervo M, et al. Dementia severity and weight loss: a comparison across eight cohorts: the 10/66 Study. Alzheimers Dement. 2013;9(6):649–56. doi: 10.1016/j.jalz.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nourhashemi F, Deschamps V, Larrieu S, et al. Body mass index and incidence of dementia: the PAQUID study. Neurology. 2003;60(1):117–9. doi: 10.1212/01.wnl.0000038910.46217.aa. [DOI] [PubMed] [Google Scholar]
- 9.Stewart R, Masaki K, Xue Q, et al. A 32-year prospective study of change in body weight and incident dementia: the Honolulu-Asia Aging Study. Arch Neurol. 2005;62(1):55–60. doi: 10.1001/archneur.62.1.55. [DOI] [PubMed] [Google Scholar]
- 10.White H, Pieper C, Schmader K, et al. Weight change in Alzheimer’s disease. J Am Geriatr Soc. 1996;44(3):265–72. doi: 10.1111/j.1532-5415.1996.tb00912.x. [DOI] [PubMed] [Google Scholar]
- 11.Gillette-Guyonnet S, Nourhashémi F, Andrieu S, et al. Weight loss in Alzheimer disease. Am J Clin Nutr. 2000;71(2):637s–42s. doi: 10.1093/ajcn/71.2.637s. [DOI] [PubMed] [Google Scholar]
- 12.Guerin O, Andrieu S, Schneider SM, et al. Different modes of weight loss in Alzheimer disease: a prospective study of 395 patients. Am J Clin Nutr. 2005;82(2):435–41. doi: 10.1093/ajcn.82.2.435. [DOI] [PubMed] [Google Scholar]
- 13.Droogsma E, Van Asselt DZB, Scholzel-Dorenbos CJM, et al. Nutritional status of community-dwelling elderly with newly diagnosed Alzheimer’s disease: prevalence of malnutrition and the relation of various factors to nutritional status. J Nutr Health Aging. 2013;17(7):606–10. doi: 10.1007/s12603-013-0032-9. [DOI] [PubMed] [Google Scholar]
- 14.Roque M, Salva A, Vellas B. Malnutrition in community-dwelling adults with dementia (NutriAlz Trial) J Nutr Health Aging. 2013;17(4):295–9. doi: 10.1007/s12603-012-0401-9. [DOI] [PubMed] [Google Scholar]
- 15.Gillioz AS, Villars H, Voisin T, et al. Spared and impaired abilities in community-dwelling patients entering the severe stage of Alzheimer’s disease. DementGeriatr Cogn Disord. 2009;28(5):412–7. doi: 10.1159/000255635. [DOI] [PubMed] [Google Scholar]
- 16.Pugh PL, Richardson JC, Bate ST, et al. Non-cognitive behaviours in an APP/PS1 transgenic model of Alzheimer’s disease. Behav Brain Res. 2007;178(1):18–28. doi: 10.1016/j.bbr.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 17.Vloeberghs E, Van Dam D, Franck F, et al. Altered ingestive behavior, weight changes, and intact olfactory sense in an APP overexpression model. Behav Neurosci. 2008;122(3):491–7. doi: 10.1037/0735-7044.122.3.491. [DOI] [PubMed] [Google Scholar]
- 18.Wirths O, Breyhan H, Schäfer S, et al. Deficits in working memory and motor performance in the APP/PS1ki mouse model for Alzheimer’s disease. Neurobiol Aging. 2008;29(6):891–901. doi: 10.1016/j.neurobiolaging.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Knight EM, Verkhratsky A, Luckman SM, et al. Hypermetabolism in a triple-transgenic mouse model of Alzheimer’s disease. Neurobiol Aging. 2012;33(1):187–93. doi: 10.1016/j.neurobiolaging.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Wiesmann M, Zerbi V, Jansen D, et al. A dietary treatment improves cerebral blood flow and brain connectivity in aging apoE4 mice. Neural Plast. 2016;2016:6846721. doi: 10.1155/2016/6846721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rijpma A, Jansen D, Arnoldussen IAC, et al. Sex differences in presynaptic density and neurogenesis in middle-aged ApoE4 and ApoE knockout mice. J Neurodegener Dis. 2013;2013:531326. doi: 10.1155/2013/531326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jansen D, Zerbi V, Janssen CIF, et al. Impact of a multi-nutrient diet on cognition, brain metabolism, hemodynamics, and plasticity in apoE4 carrier and apoE knockout mice. Brain Struct Funct. 2014;219(5):1841–68. doi: 10.1007/s00429-013-0606-7. [DOI] [PubMed] [Google Scholar]
- 23.Reed DR, Bachmanov AA, Tordoff MG. Forty mouse strain survey of body composition. Physiol Behav. 2007;91(5):593–600. doi: 10.1016/j.physbeh.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Z, Klimentidis YC, Bea JW, et al. Body mass index, waist circumference, and mortality in a large multiethnic post-menopausal cohort—results from the Women’s Health Initiative. J Am Geriatr Soc. 2017 doi: 10.1111/jgs.14790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Veronese N, Li Y, Manson JE, et al. Combined associations of body weight and lifestyle factors with all cause and cause specific mortality in men and women: prospective cohort study. BMJ. 2016;355:i5855. doi: 10.1136/bmj.i5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wallace JI, Schwartz RS, LaCroix AZ, et al. Involuntary weight loss in older outpatients: incidence and clinical significance. J Am Geriatr Soc. 1995;43(4):329–37. doi: 10.1111/j.1532-5415.1995.tb05803.x. [DOI] [PubMed] [Google Scholar]
- 27.Winter JE, MacInnis RJ, Wattanapenpaiboon N, et al. BMI and all-cause mortality in older adults: a meta-analysis. Am J Clin Nutr. 2014;99(4):875–90. doi: 10.3945/ajcn.113.068122. [DOI] [PubMed] [Google Scholar]
- 28.Payette H, Coulombe C, Boutier V, et al. Nutrition risk factors for institutionalization in a free-living functionally dependent elderly population. J Clin Epidemiol. 2000;53(6):579–87. doi: 10.1016/s0895-4356(99)00186-9. [DOI] [PubMed] [Google Scholar]
- 29.Keller HH, Østbye T, Goy R. Nutritional risk predicts quality of life in elderly community-living Canadians. J Gerontol Ser A Biol Sci Med Sci. 2004;5(1):M68–74. doi: 10.1093/gerona/59.1.m68. [DOI] [PubMed] [Google Scholar]
- 30.Alibhai SM, Greenwood C, Payette H. An approach to the management of unintentional weight loss in elderly people. Can Med Assoc J. 2005;172(6):773–80. doi: 10.1503/cmaj.1031527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guerin O, Soto M, Brocker P, et al. Nutritional status assessment during Alzheimer’s disease: results after one year (the REAL French Study Group) J Nutr Health Aging. 2005;9(2):81–4. [PubMed] [Google Scholar]
- 32.Vellas B, Lauque S, Gillette-Guyonnet S, et al. Impact of nutritional status on the evolution of Alzheimer’s disease and on response to acetylcholinesterase inhibitor treatment. J Nutr Health Aging. 2005;9(7):75–80. [PubMed] [Google Scholar]
- 33.Soto ME, Secher M, Gillette-Guyonnet S, et al. Weight loss and rapid cognitive decline in community-dwelling patients with Alzheimer’s disease. J Alzheimers Dis. 2012;28(3):647–54. doi: 10.3233/JAD-2011-110713. [DOI] [PubMed] [Google Scholar]
- 34.Andrieu S, Reynish W, Nourhashemi F, et al. Nutritional risk factors for institutional placement in Alzheimer’s disease after one year follow-up. J Nutr Health Aging. 2001;5(2):113–7. [PubMed] [Google Scholar]
- 35.White HK, McConnell ES, Bales CW, et al. A 6-month observational study of the relationship between weight loss and behavioral symptoms in institutionalized Alzheimer’s disease subjects. J Am Med Dir Assoc. 2004;5(2):89–97. doi: 10.1097/01.JAM.0000110646.48753.EF. [DOI] [PubMed] [Google Scholar]
- 36.White H, Pieper C, Schmader K. The association of weight change in Alzheimer’s disease with severity of disease and mortality: a longitudinal analysis. J Am Geriatr Soc. 1998;46(10):1223–7. doi: 10.1111/j.1532-5415.1998.tb04537.x. [DOI] [PubMed] [Google Scholar]
- 37.Gambassi G, Landi F, Lapane K, et al. Predictors of mortality in patients with Alzheimer’s disease living in nursing homes. J Neurol Neurosurg Psychiatry. 1999;67(1):59–65. doi: 10.1136/jnnp.67.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luchsinger JA, Patel B, Tang M-X, et al. Body mass index, dementia, and mortality in the elderly. J Nutr Health Aging. 2008;12(2):127–31. doi: 10.1007/BF02982565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.García-Ptacek S, Kåreholt I, Farahmand B, et al. Body-mass index and mortality in incident dementia: a cohort study on 11,398 patients from SveDem, the Swedish Dementia Registry. J Am Med Dir Assoc. 2014;15(6):447. doi: 10.1016/j.jamda.2014.03.001. e1–7. [DOI] [PubMed] [Google Scholar]
- 40.de Souto Barreto P, Cadroy Y, Kelaiditi E, et al. The prognostic value of body-mass index on mortality in older adults with dementia living in nursing homes. Clin Nutr. 2017;36(2):423–8. doi: 10.1016/j.clnu.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 41.Beydoun MA, Beydoun HA, Wang Y. Obesity and central obesity as risk factors for incident dementia and its subtypes: a systematic review and meta-analysis. Obes Rev. 2008;9(3):204–18. doi: 10.1111/j.1467-789X.2008.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anstey KJ, Cherbuin N, Budge M, et al. Body mass index in midlife and late-life as a risk factor for dementia: a meta-analysis of prospective studies. Obes Rev. 2011;12(5):e426–37. doi: 10.1111/j.1467-789X.2010.00825.x. [DOI] [PubMed] [Google Scholar]
- 43.Tolppanen AM, Ngandu T, Kareholt I, et al. Midlife and late-life body mass index and late-life dementia: results from a prospective population-based cohort. J Alzheimers Dis. 2014;38(1):201–9. doi: 10.3233/JAD-130698. [DOI] [PubMed] [Google Scholar]
- 44.Besser LM, Gill DP, Monsell SE, et al. Body mass index, weight change, and clinical progression in mild cognitive impairment and Alzheimer disease. Alzheimer Dis Assoc Disord. 2014;28(1):36–43. doi: 10.1097/WAD.0000000000000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buchman AS, Wilson RS, Bienias JL, et al. Change in body mass index and risk of incident Alzheimer disease. Neurology. 2005;65(6):892–7. doi: 10.1212/01.wnl.0000176061.33817.90. [DOI] [PubMed] [Google Scholar]
- 46.Johnson DK, Wilkins CH, Morris JC. Accelerated weight loss may precede diagnosis in Alzheimer disease. Arch Neurol. 2006;63(9):1312–7. doi: 10.1001/archneur.63.9.1312. [DOI] [PubMed] [Google Scholar]
- 47.Barrett-Connor E, Edelstein SL, Corey-Bloom J, et al. Weight loss precedes dementia in community-dwelling older adults. J Am Geriatr Soc. 1996;44(10):1147–52. doi: 10.1111/j.1532-5415.1996.tb01362.x. [DOI] [PubMed] [Google Scholar]
- 48.Knopman DS, Edland SD, Cha RH, et al. Incident dementia in women is preceded by weight loss by at least a decade. Neurology. 2007;69(8):739–46. doi: 10.1212/01.wnl.0000267661.65586.33. [DOI] [PubMed] [Google Scholar]
- 49.Atti AR, Palmer K, Volpato S, et al. Late-life body mass index and dementia incidence: nine-year follow-up data from the Kungsholmen Project. J Am Geriatr Soc. 2008;56(1):111–6. doi: 10.1111/j.1532-5415.2007.01458.x. [DOI] [PubMed] [Google Scholar]
- 50.Fitzpatrick AL, Kuller LH, Lopez OL, et al. Midlife and late-life obesity and the risk of dementia: cardiovascular health study. Arch Neurol. 2009;66(3):336–42. doi: 10.1001/archneurol.2008.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Power BD, Alfonso H, Flicker L, et al. Body adiposity in later life and the incidence of dementia: the Health in Men Study. PLoS ONE. 2011;6(3):e17902. doi: 10.1371/journal.pone.0017902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gustafson DR, Backman K, Waern M, et al. Adiposity indicators and dementia over 32 years in Sweden. Neurology. 2009;73(19):1559–66. doi: 10.1212/WNL.0b013e3181c0d4b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Whitmer RA, Gustafson DR, Barrett-Connor E, et al. Central obesity and increased risk of dementia more than three decades later. Neurology. 2008;71(14):1057–64. doi: 10.1212/01.wnl.0000306313.89165.ef. [DOI] [PubMed] [Google Scholar]
- 54.Alhurani RE, Vassilaki M, Aakre JA, et al. Decline in weight and incident mild cognitive impairment: Mayo Clinic Study of Aging. JAMA Neurol. 2016;73(4):439–46. doi: 10.1001/jamaneurol.2015.4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Orsitto G. Different components of nutritional status in older inpatients with cognitive impairment. J Nutr Health Aging. 2012;16(5):468–71. doi: 10.1007/s12603-012-0024-1. [DOI] [PubMed] [Google Scholar]
- 56.Sobow T, Fendler W, Magierski R. Body mass index and mild cognitive impairment-to-dementia progression in 24 months: a prospective study. Eur J Clin Nutr. 2014;68(11):1216–9. doi: 10.1038/ejcn.2014.167. [DOI] [PubMed] [Google Scholar]
- 57.Cova I, Clerici F, Rossi A, et al. Weight loss predicts progression of mild cognitive impairment to Alzheimer’s disease. PLoS ONE. 2016;11(3):e0151710. doi: 10.1371/journal.pone.0151710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aziz NA, van der Marck MA, Pijl H, et al. Weight loss in neurodegenerative disorders. J Neurol. 2008;255(12):1872–80. doi: 10.1007/s00415-009-0062-8. [DOI] [PubMed] [Google Scholar]
- 59.Droogsma E, van Asselt D, De Deyn P. Weight loss and undernutrition in community-dwelling patients with Alzheimer’s dementia. Z Gerontol Geriatr. 2015;48(4):318–24. doi: 10.1007/s00391-015-0891-2. [DOI] [PubMed] [Google Scholar]
- 60.Miyamoto K, Higashino S, Mochizuki K, et al. Evaluation of weight loss in the community-dwelling elderly with dementia as assessed by eating behavior and mental status. Asia Pac J Clin Nutr. 2011;20(1):9–13. [PubMed] [Google Scholar]
- 61.Easterling CS, Robbins E. Dementia and dysphagia. Geriatr Nurs. 2008;29(4):275–85. doi: 10.1016/j.gerinurse.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 62.Wolf-Klein GP, Silverstone FA, Lansey SC, et al. Energy requirements in Alzheimer’s disease patients. Nutrition. 1995;11(3):264–8. [PubMed] [Google Scholar]
- 63.Poehlman E, Toth M, Goran M, et al. Daily energy expenditure in free-living non-institutionalized Alzheimer’s patients A doubly labeled water study. Neurology. 1997;48(4):997–1002. doi: 10.1212/wnl.48.4.997. [DOI] [PubMed] [Google Scholar]
- 64.Prentice AM, Leavesley K, Murgatroyd PR, et al. Is severe wasting in elderly mental patients caused by an excessive energy requirement? Age Ageing. 1989;18(3):158–67. doi: 10.1093/ageing/18.3.158. [DOI] [PubMed] [Google Scholar]
- 65.Donaldson KE, Carpenter WH, Toth MJ, et al. No evidence for a higher resting metabolic rate in noninstitutionalized Alzheimer’s disease patients. J Am Geriatr Soc. 1996;44(10):1232–4. doi: 10.1111/j.1532-5415.1996.tb01375.x. [DOI] [PubMed] [Google Scholar]
- 66.Baumgartner RN, Heymsfield SB, Roche AF. Human body composition and the epidemiology of chronic disease. Obes Res. 1995;3(1):73–95. doi: 10.1002/j.1550-8528.1995.tb00124.x. [DOI] [PubMed] [Google Scholar]
- 67.Heymsfield SB, Gonzalez MC, Shen W, et al. Weight loss composition is one-fourth fat-free mass: a critical review and critique of this widely cited rule. Obes Rev. 2014;15(4):310–21. doi: 10.1111/obr.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Arnoldussen IAC, Kiliaan AJ, Gustafson DR. Obesity and dementia: adipokines interact with the brain. Eur Neuropsychopharmacol. 2014;24(12):1982–99. doi: 10.1016/j.euroneuro.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kiliaan AJ. Arnoldussen IAC, Gustafson DR. Adipokines: a link between obesity and dementia? Lancet Neurol. 2014;13(9):913–23. doi: 10.1016/S1474-4422(14)70085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ahima RS. Adipose tissue as an endocrine organ. Obesity. 2006;14(S8):242S–9S. doi: 10.1038/oby.2006.317. [DOI] [PubMed] [Google Scholar]
- 71.Zhang Y, Proenca R, Maffei M, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 72.Wiesner G, Vaz M, Collier G, et al. Leptin is released from the human brain: influence of adiposity and gender. J Clin Endocrinol Metabol. 1999;84(7):2270–4. doi: 10.1210/jcem.84.7.5854. [DOI] [PubMed] [Google Scholar]
- 73.Wilkinson M, Brown R, Imran SA, et al. Adipokine gene expression in brain and pituitary gland. Neuroendocrinology. 2007;86(3):191–209. doi: 10.1159/000108635. [DOI] [PubMed] [Google Scholar]
- 74.Maffei M, Halaas J, Ravussin E, et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1(11):1155–61. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- 75.Considine RV, Sinha MK, Heiman ML, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334(5):292–5. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 76.Ruhl CE, Harris TB, Ding J, et al. Body mass index and serum leptin concentration independently estimate percentage body fat in older adults. Am J Clin Nutr. 2007;85(4):1121–6. doi: 10.1093/ajcn/85.4.1121. [DOI] [PubMed] [Google Scholar]
- 77.Schwartz MW, Peskind E, Raskind M, et al. Cerebrospinal fluid leptin levels: relationship to plasma levels and to adiposity in humans. Nat Med. 1996;2(5):589–93. doi: 10.1038/nm0596-589. [DOI] [PubMed] [Google Scholar]
- 78.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395(6704):763–70. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 79.Harvey J, Shanley LJ, O’Malley D, et al. Leptin: a potential cognitive enhancer? Biochem Soc Transact. 2005;33(5):1029–32. doi: 10.1042/BST20051029. [DOI] [PubMed] [Google Scholar]
- 80.Garza JC, Guo M, Zhang W, et al. Leptin increases adult hippocampal neurogenesis in vivo and in vitro. J Biol Chem. 2008;283(26):18238–47. doi: 10.1074/jbc.M800053200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Irving AJ, Harvey J. Leptin regulation of hippocampal synaptic function in health and disease. Philos Trans R Soc B Biol Sci. 2014;369(1633):20130155. doi: 10.1098/rstb.2013.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lieb W, Beiser AS, Vasan RS, et al. Association of plasma leptin levels with incident Alzheimer disease and MRI measures of brain aging. JAMA. 2009;302(23):2565–72. doi: 10.1001/jama.2009.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zeki Al Hazzouri A, Stone KL, Haan MN, et al. Leptin, mild cognitive impairment, and dementia among elderly women. J Gerontol Ser A Biol Sci Med Sci. 2013;68(2):175–80. doi: 10.1093/gerona/gls155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Holden KF, Lindquist K, Tylavsky FA, et al. Serum leptin level and cognition in the elderly: findings from the Health ABC Study. Neurobiol Aging. 2009;30(9):1483–9. doi: 10.1016/j.neurobiolaging.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Littlejohns TJ, Kos K, Henley WE, et al. Serum leptin and risk of cognitive decline in elderly italians. J Alzheimers Dis. 2015;44(4):1231–9. doi: 10.3233/JAD-141836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Baranowska-Bik A, Bik W, Styczynska M, et al. Plasma leptin levels and free leptin index in women with Alzheimer’s disease. Neuropeptides. 2015;52:73–8. doi: 10.1016/j.npep.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 87.Khemka VK, Bagchi D, Bandyopadhyay K, et al. Altered serum levels of adipokines and insulin in probable Alzheimer’s disease. J Alzheimers Dis. 2014;41(2):525–33. doi: 10.3233/JAD-140006. [DOI] [PubMed] [Google Scholar]
- 88.Oania R, McEvoy LK. Plasma leptin levels are not predictive of dementia in patients with mild cognitive impairment. Age Ageing. 2015;44(1):53–8. doi: 10.1093/ageing/afu160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Teunissen CE, van der Flier WM, Scheltens P, et al. Serum leptin is not altered nor related to cognitive decline in Alzheimer’s disease. J Alzheimers Dis. 2015;44(3):809–13. doi: 10.3233/JAD-141503. [DOI] [PubMed] [Google Scholar]
- 90.Greco SJ, Hamzelou A, Johnston JM, et al. Leptin boosts cellular metabolism by activating AMPK and the sirtuins to reduce tau phosphorylation and β-amyloid in neurons. Biochem Biophys Res Commun. 2011;414(1):170–4. doi: 10.1016/j.bbrc.2011.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Doherty GH, Beccano-Kelly D, Yan SD, et al. Leptin prevents hippocampal synaptic disruption and neuronal cell death induced by amyloid β. Neurobiol Aging. 2013;34(1):226–37. doi: 10.1016/j.neurobiolaging.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 92.Greco SJ, Bryan KJ, Sarkar S, et al. Leptin reduces pathology and improves memory in a transgenic mouse model of Alzheimer’s disease. J Alzheimers Dis. 2010;19(4):1155–67. doi: 10.3233/JAD-2010-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Perez-Gonzalez R, Alvira-Botero MX, Robayo O, et al. Leptin gene therapy attenuates neuronal damages evoked by amyloid-[beta] and rescues memory deficits in APP/PS1 mice. Gene Ther. 2014;21(3):298–308. doi: 10.1038/gt.2013.85. [DOI] [PubMed] [Google Scholar]
- 94.Ishii M, Wang G, Racchumi G, et al. Transgenic mice overexpressing amyloid precursor protein exhibit early metabolic deficits and a pathologically low leptin state associated with hypothalamic dysfunction in arcuate neuropeptide Y neurons. J Neurosci. 2014;34(27):9096–106. doi: 10.1523/JNEUROSCI.0872-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hansen M-LH, Waldorff FB, Waldemar G. Prognostic factors for weight loss over 1-year period in patients recently diagnosed with mild Alzheimer disease. Alzheimer Dis Assoc Disord. 2011;25(3):269–75. doi: 10.1097/WAD.0b013e3182096624. [DOI] [PubMed] [Google Scholar]
- 96.Qaseem A, Snow V, Cross JT, et al. Current pharmacologic treatment of dementia: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2008;148(5):370–8. doi: 10.7326/0003-4819-148-5-200803040-00008. [DOI] [PubMed] [Google Scholar]
- 97.Lam B, Hollingdrake E, Kennedy JL, et al. Cholinesterase inhibitors in Alzheimer’s disease and Lewy body spectrum disorders: the emerging pharmacogenetic story. Hum Genom. 2009;4(2):1. doi: 10.1186/1479-7364-4-2-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tiraboschi P, Hansen L, Alford M, et al. Cholinergic dysfunction in diseases with Lewy bodies. Neurology. 2000;54(2):407–11. doi: 10.1212/wnl.54.2.407. [DOI] [PubMed] [Google Scholar]
- 99.Stahl SM. The new cholinesterase inhibitors for Alzheimer’s disease, Part 2: illustrating their mechanisms of action. J Clin Psychiatry. 2000;61(11):813–4. doi: 10.4088/jcp.v61n1101. [DOI] [PubMed] [Google Scholar]
- 100.Birks J. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst Rev. 2006;(1):CD005593. doi: 10.1002/14651858.CD005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zemek F, Drtinova L, Nepovimova E, et al. Outcomes of Alzheimer’s disease therapy with acetylcholinesterase inhibitors and memantine. Expert Opin Drug Saf. 2014;13(6):759–74. doi: 10.1517/14740338.2014.914168. [DOI] [PubMed] [Google Scholar]
- 102.Buckley JS, Salpeter SR. A risk-benefit assessment of dementia medications: systematic review of the evidence. Drugs Aging. 2015;32(6):453–67. doi: 10.1007/s40266-015-0266-9. [DOI] [PubMed] [Google Scholar]
- 103.Kavirajan H, Schneider LS. Efficacy and adverse effects of cholinesterase inhibitors and memantine in vascular dementia: a meta-analysis of randomised controlled trials. Lancet Neurol. 2007;6(9):782–92. doi: 10.1016/S1474-4422(07)70195-3. [DOI] [PubMed] [Google Scholar]
- 104.Stewart JT, Gorelik AR. Involuntary weight loss associated with cholinesterase inhibitors in dementia. J Am Geriatr Soc. 2006;54(6):1013–4. doi: 10.1111/j.1532-5415.2006.00756.x. [DOI] [PubMed] [Google Scholar]
- 105.Gallini A, Sommet A, Salandini AM, et al. Weight-loss associated with anti-dementia drugs in a patient with Parkinson’s disease. Move Disord. 2007;22(13):1980–1. doi: 10.1002/mds.21669. [DOI] [PubMed] [Google Scholar]
- 106.Imbimbo BP. Pharmacodynamic-tolerability relationships of cholinesterase inhibitors for Alzheimer’s disease. CNS Drugs. 2001;15(5):375–90. doi: 10.2165/00023210-200115050-00004. [DOI] [PubMed] [Google Scholar]
- 107.Uchiyama T, Chess-Williams R. Muscarinic receptor subtypes of the bladder and gastrointestinal tract. J Smooth Muscle Res. 2004;40(6):237–47. doi: 10.1540/jsmr.40.237. [DOI] [PubMed] [Google Scholar]
- 108.Gauthier S. Cholinergic adverse effects of cholinesterase inhibitors in Alzheimer’s disease. Drugs Aging. 2001;18(11):853–62. doi: 10.2165/00002512-200118110-00006. [DOI] [PubMed] [Google Scholar]
- 109.Sheffrin M, Miao Y, Boscardin WJ, et al. Weight loss associated with cholinesterase inhibitors in individuals with dementia in a national healthcare system. J Am Geriatr Soc. 2015;63(8):1512–8. doi: 10.1111/jgs.13511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Droogsma E, van Asselt DZ, van Steijn JH, et al. Effect of long-term treatment with galantamine on weight of patients with Alzheimer’s dementia. J Nutr Health Aging. 2013;17(5):461–5. doi: 10.1007/s12603-012-0420-6. [DOI] [PubMed] [Google Scholar]
- 111.Soysal P, Isik AT, Stubbs B, et al. Acetylcholinesterase inhibitors are associated with weight loss in older people with dementia: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2016;87(12):1368–74. doi: 10.1136/jnnp-2016-313660. [DOI] [PubMed] [Google Scholar]
- 112.Stewart JT, Lewis DC, George LV, Mattox KM, Stover KT. Involuntary weight loss after switching acetylcholinesterase inhibitors. Ann Long-Term Care Clin Care Aging. 2013;21(3):32–4. [Google Scholar]
- 113.Olivares D, Deshpande VK, Shi Y, et al. N-methyl D-aspartate (NMDA) receptor antagonists and memantine treatment for Alzheimer’s disease, vascular dementia and Parkinson’s disease. Curr Alzheimer Res. 2012;9(6):746–58. doi: 10.2174/156720512801322564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.McShane R, Areosa Sastre A, Minakaran N. Memantine for dementia. Cochrane Database Syst Rev. 2006;(2):CD003154. doi: 10.1002/14651858.CD003154.pub5. [DOI] [PubMed] [Google Scholar]
- 115.Di Santo SG, et al. A meta-analysis of the efficacy of donepezil, rivastigmine, galantamine, and memantine in relation to severity of Alzheimer’s disease. J Alzheimers Dis. 2013;35(2):349–61. doi: 10.3233/JAD-122140. [DOI] [PubMed] [Google Scholar]
- 116.Matsunaga S, Kishi T, Iwata N. Memantine monotherapy for Alzheimer’s disease: a systematic review and meta-analysis. PLoS ONE. 2015;10(4):e0123289. doi: 10.1371/journal.pone.0123289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schneider LS, Dagerman KS, Higgins JT, et al. Lack of evidence for the efficacy of memantine in mild Alzheimer disease. Arch Neurol. 2011;68(8):991–8. doi: 10.1001/archneurol.2011.69. [DOI] [PubMed] [Google Scholar]
- 118.Kishi T, Matsunaga S, Iwata N. Memantine for the treatment of frontotemporal dementia: a metaanalysis. Neuropsychiatr Dis Treat. 2015;11:2883–5. doi: 10.2147/NDT.S94430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Matsunaga S, Kishi T, Iwata N. Memantine for Lewy body disorders: systematic review and meta-analysis. Am J Geriatr Psychiatry. 2015;23(4):373–83. doi: 10.1016/j.jagp.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 120.Stinton C, McKeith I, Taylor JP, et al. Pharmacological management of Lewy body dementia: a systematic review and metaanalysis. Am J Psychiatry. 2015;172(8):731–42. doi: 10.1176/appi.ajp.2015.14121582. [DOI] [PubMed] [Google Scholar]
- 121.Jones RW. A review comparing the safety and tolerability of memantine with the acetylcholinesterase inhibitors. Int J Geriatr Psychiatry. 2010;25(6):547–53. doi: 10.1002/gps.2384. [DOI] [PubMed] [Google Scholar]
- 122.US Food and Drug Administration. [Accessed 3 Mar 2017];What is a dietary supplement? 2015 https://www.fda.gov/AboutFDA/Transparency/Basics/ucm195635.htm.
- 123.US Food and Drug Administration. [Accessed 3 Mar 2017];Medical foods guidance documents and regulatory information. 2016 https://www.fda.gov/food/guidanceregulation/guidancedocumentsregulatoryinformation/medicalfoods/default.htm.
- 124.Hines S, Wilson J, McCrow J, et al. Oral liquid nutritional supplements for people with dementia in residential aged care facilities. Int J Evid Based Healthc. 2010;8(4):248–51. doi: 10.1111/j.1744-1609.2010.00186.x. [DOI] [PubMed] [Google Scholar]
- 125.Allen VJ, Methven L, Gosney MA. Use of nutritional complete supplements in older adults with dementia: systematic review and meta-analysis of clinical outcomes. Clin Nutr. 2013;32(6):950–7. doi: 10.1016/j.clnu.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 126.Liu W, Cheon J, Thomas SA. Interventions on mealtime difficulties in older adults with dementia: a systematic review. Int J Nurs Stud. 2014;51(1):14–27. doi: 10.1016/j.ijnurstu.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 127.Roman MW. Axona® (Accera, Inc): a new medical food therapy for persons with Alzheimer’s disease. Issues Ment Health Nurs. 2010;31(6):435–6. doi: 10.3109/01612841003768231. [DOI] [PubMed] [Google Scholar]
- 128.Henderson ST, Vogel JL, Barr LJ, et al. Study of the ketogenic agent AC-1202 in mild to moderate Alzheimer’s disease: a randomized, double-blind, placebo-controlled, multicenter trial. Nutr Metabol. 2009;6(1):1–25. doi: 10.1186/1743-7075-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hara J, Shankle WR, Barrentine LW, et al. Novel therapy of hyperhomocysteinemia in mild cognitive impairment, Alzheimer’s disease, and other dementing disorders. J Nutr Health Aging. 2016;20(8):825–34. doi: 10.1007/s12603-016-0688-z. [DOI] [PubMed] [Google Scholar]
- 130.Wurtman R. A nutrient combination that can affect synapse formation. Nutrients. 2014;6(4):1701. doi: 10.3390/nu6041701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298(5594):789–91. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 132.van Wijk N, Broersen LM, de Wilde MC, et al. Targeting synaptic dysfunction in Alzheimer’s disease by administering a specific nutrient combination. J Alzheimers Dis. 2014;38(3):459–79. doi: 10.3233/JAD-130998. [DOI] [PubMed] [Google Scholar]
- 133.Pardini M, Serrati C, Guida S, et al. Souvenaid reduces behavioral deficits and improves social cognition skills in frontotemporal dementia: a proof-of-concept study. Neurodegener Dis. 2015;15(1):58–62. doi: 10.1159/000369811. [DOI] [PubMed] [Google Scholar]
- 134.Scheltens P, Kamphuis PJGH, Verhey FRJ, et al. Efficacy of a medical food in mild Alzheimer’s disease: a randomized, controlled trial. Alzheimer’s Dement. 2010;6(1):1–10. doi: 10.1016/j.jalz.2009.10.003. e1. [DOI] [PubMed] [Google Scholar]
- 135.Scheltens P, Twisk JWR, Blesa R, et al. Efficacy of souvenaid in mild Alzheimer’s disease: results from a randomized, controlled trial. J Alzheimers Dis. 2012;31(1):225–36. doi: 10.3233/JAD-2012-121189. [DOI] [PubMed] [Google Scholar]
- 136.Shah RC, Kamphuis PJ, Leurgans S, et al. The S-Connect study: results from a randomized, controlled trial of Souvenaid in mild-to-moderate Alzheimer’s disease. Alzheimers Res Ther. 2013;5(6):59. doi: 10.1186/alzrt224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kamphuis PJ, Verhey FR, Olde Rikkert MG, et al. Effect of a medical food on body mass index and activities of daily living in patients with Alzheimer’s disease: secondary analyses from a randomized, controlled trial. J Nutr Health Aging. 2011;15(8):672–6. doi: 10.1007/s12603-011-0339-3. [DOI] [PubMed] [Google Scholar]
- 138.LipiDiDiet Consortium. [Accessed 15 Apr 2017];Two-year, EU-funded study reveals a medical food in the form of a daily nutritional drink can help to conserve memory, the ability to think and perform everyday tasks, as well as reduce brain shrinkage in people with prodromal Alzheimer’s disease (AD) 2016 http://www.lipididiet.eu/index.php?id=7679&tx_ttnews%5Btt_news%5D=19795&cHash=7e6c6d43aef1446c8c21b054af05a684.
- 139.Cerejeira J, Lagarto L, Mukaetova-Ladinska E. Behavioral and psychological symptoms of dementia. Front Neurol. 2012;3:73. doi: 10.3389/fneur.2012.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA. 2005;293(5):596–608. doi: 10.1001/jama.293.5.596. [DOI] [PubMed] [Google Scholar]
- 141.Wang J, Yu JT, Wang HF, et al. Pharmacological treatment of neuropsychiatric symptoms in Alzheimer’s disease: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2015;86(1):101–9. doi: 10.1136/jnnp-2014-308112. [DOI] [PubMed] [Google Scholar]
- 142.Volicer L, Stelly M, Morris J, et al. Effects of dronabinol on anorexia and disturbed behavior in patients with Alzheimer’s disease. Int J Geriatr Psychiatry. 1997;12(9):913–9. [PubMed] [Google Scholar]
- 143.Woodward MR, Harper DG, Stolyar A, et al. Dronabinol for the treatment of agitation and aggressive behavior in acutely hospitalized severely demented patients with noncognitive behavioral symptoms. Am J Geriatr Psychiatry. 2014;22(4):415–9. doi: 10.1016/j.jagp.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 144.van den Elsen GAH, Ahmed AIA, Verkes R-J, et al. Tetrahydrocannabinol for neuropsychiatric symptoms in dementia: a randomized controlled trial. Neurology. 2015;84(23):2338–46. doi: 10.1212/WNL.0000000000001675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Puranen A, Taipale H, Koponen M, et al. Incidence of antidepressant use in community-dwelling persons with and without Alzheimer’s disease: 13-year follow-up. Int J Geriatr Psychiatry. 2017;32(1):94–101. doi: 10.1002/gps.4450. [DOI] [PubMed] [Google Scholar]
- 146.Anttila SA, Leinonen EV. A review of the pharmacological and clinical profile of mirtazapine. CNS Drug Rev. 2001;7(3):249–64. doi: 10.1111/j.1527-3458.2001.tb00198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Salvi V, Mencacci C, Barone-Adesi F. H1-histamine receptor affinity predicts weight gain with antidepressants. Eur Neuropsychopharmacol. 2016;26(10):1673–7. doi: 10.1016/j.euroneuro.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 148.Davis MP, Khawam E, Pozuelo L, et al. Management of symptons associated with advanced cancer: olanzapine and mirtazapine. Expert Rev Anticancer Ther. 2002;2(4):365–76. doi: 10.1586/14737140.2.4.365. [DOI] [PubMed] [Google Scholar]
- 149.Chinuck RS, Fortnum H, Baldwin DR. Appetite stimulants in cystic fibrosis: a systematic review. J Hum Nutr Diet. 2007;20(6):526–37. doi: 10.1111/j.1365-277X.2007.00824.x. [DOI] [PubMed] [Google Scholar]
- 150.Raji MA, Brady SR. Mirtazapine for treatment of depression and comorbidities in Alzheimer disease. Ann Pharmacother. 2001;35(9):1024–7. doi: 10.1345/aph.10371. [DOI] [PubMed] [Google Scholar]
- 151.Thomas P, Hazif-Thomas C, Clement JP. Influence of antidepressant therapies on weight and appetite in the elderly. J Nutr Health Aging. 2003;7(3):166–70. [PubMed] [Google Scholar]
- 152.Segers K, Surquin M. Can mirtazapine counteract the weight loss associated with Alzheimer disease? A retrospective open-label study. Alzheimer Dis Assoc Disord. 2014;28(3):291–3. doi: 10.1097/WAD.0b013e3182614f52. [DOI] [PubMed] [Google Scholar]
- 153.Azermai M, Petrovic M, Elseviers MM, et al. Systematic appraisal of dementia guidelines for the management of behavioural and psychological symptoms. Ageing Res Rev. 2012;11(1):78–86. doi: 10.1016/j.arr.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 154.Spina E, Santoro V, D’Arrigo C. Clinically relevant pharmacokinetic drug interactions with second-generation antidepressants: an update. Clin Ther. 2008;30(7):1206–27. doi: 10.1016/s0149-2918(08)80047-1. [DOI] [PubMed] [Google Scholar]
- 155.Fawcett J, Barkin RL. Review of the results from clinical studies on the efficacy, safety and tolerability of mirtazapine for the treatment of patients with major depression. J Affect Disord. 1998;51(3):267–85. doi: 10.1016/s0165-0327(98)00224-9. [DOI] [PubMed] [Google Scholar]
- 156.Leong C. Antidepressants for depression in patients with dementia: a review of the literature. Consult Pharm. 2014;29(4):254–63. doi: 10.4140/TCP.n.2014.254. [DOI] [PubMed] [Google Scholar]
- 157.Banerjee S, Hellier J, Dewey M, et al. Sertraline or mirtazapine for depression in dementia (HTASADD): a randomised, multi-centre, double-blind, placebo-controlled trial. Lancet. 2011;378(9789):403–11. doi: 10.1016/S0140-6736(11)60830-1. [DOI] [PubMed] [Google Scholar]
- 158.Tedeschini E, Levkovitz Y, Iovieno N, et al. Efficacy of antidepressants for late-life depression: a meta-analysis and meta-regression of placebo-controlled randomized trials. J Clin Psychiatry. 2011;72(12):1660–8. doi: 10.4088/JCP.10r06531. [DOI] [PubMed] [Google Scholar]
- 159.Romeo R, Knapp M, Hellier J, et al. Cost-effectiveness analyses for mirtazapine and sertraline in dementia: randomised controlled trial. Br J Psychiatry. 2013;202(2):121–8. doi: 10.1192/bjp.bp.112.115212. [DOI] [PubMed] [Google Scholar]
- 160.Maglione M, Maher AR, Hu J, et al. Off-label use of atypical antipsychotics: an update. Comparative effectiveness reviews. Vol. 43. Rockville: Agency for Healthcare Research and Quality; 2011. [PubMed] [Google Scholar]
- 161.Rummel-Kluge C, Komossa K, Schwarz S, et al. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and metaanalysis. Schizophr Res. 2010;123(2–3):225–33. doi: 10.1016/j.schres.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Matsui-Sakata A, Ohtani H, Sawada Y. Receptor occupancy-based analysis of the contributions of various receptors to antipsychotics-induced weight gain and diabetes mellitus. Drug Metabol Pharm. 2005;20(5):368–78. doi: 10.2133/dmpk.20.368. [DOI] [PubMed] [Google Scholar]
- 163.Atti A, Gozzi BF, Zuliani G, et al. A systematic review of metabolic side effects related to the use of antipsychotic drugs in dementia. Int Psychogeratr. 2014;26(1):19–37. doi: 10.1017/S1041610213001658. [DOI] [PubMed] [Google Scholar]
- 164.Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294(15):1934–43. doi: 10.1001/jama.294.15.1934. [DOI] [PubMed] [Google Scholar]
- 165.US Food and Drug Administration. Postmarket drug safety information for patients and providers. [Accessed 15 Apr 2017];Information for healthcare professionals: conventional antipsychotics. 2008 http://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm124830.htm.
- 166.Kennedy WK, Jann MW, Kutscher EC. Clinically significant drug interactions with atypical antipsychotics. CNS Drugs. 2013;27(12):1021–48. doi: 10.1007/s40263-013-0114-6. [DOI] [PubMed] [Google Scholar]
- 167.Fernandez HH, Trieschmann ME, Friedman JH. Treatment of psychosis in Parkinson’s disease. Drug Saf. 2003;26(9):643–59. doi: 10.2165/00002018-200326090-00004. [DOI] [PubMed] [Google Scholar]
- 168.Dore DD, Trivedi AN, Mor V, et al. Atypical antipsychotic use and risk of fracture in persons with Parkinsonism. Move Disord. 2009;24(13):1941–8. doi: 10.1002/mds.22679. [DOI] [PubMed] [Google Scholar]
- 169.Edwards NE, Beck AM. The influence of aquariums on weight in individuals with dementia. Alzheimer Dis Assoc Disord. 2013;27(4):379–83. doi: 10.1097/WAD.0b013e3182769b34. [DOI] [PubMed] [Google Scholar]
- 170.Beydoun MA, Beydoun HA, Gamaldo AA, et al. Epidemiologic studies of modifiable factors associated with cognition and dementia: systematic review and meta-analysis. BMC Public Health. 2014;14(1):1–33. doi: 10.1186/1471-2458-14-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Vazquez G, Duval S, Jacobs DR, et al. Comparison of body mass index, waist circumference, and waist/hip ratio in predicting incident diabetes: a meta-analysis. Epidemiol Rev. 2007;29(1):115–28. doi: 10.1093/epirev/mxm008. [DOI] [PubMed] [Google Scholar]
- 172.de Koning L, Merchant AT, Pogue J, et al. Waist circumference and waist-to-hip ratio as predictors of cardiovascular events: meta-regression analysis of prospective studies. Eur Heart J. 2007;28(7):850–6. doi: 10.1093/eurheartj/ehm026. [DOI] [PubMed] [Google Scholar]
- 173.Romero-Corral A, Montori VM, Somers VK, et al. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet. 2006;368(9536):666–78. doi: 10.1016/S0140-6736(06)69251-9. [DOI] [PubMed] [Google Scholar]
- 174.Vemmos K, Ntaios G, Spengos K, et al. Association between obesity and mortality after acute first-ever stroke: the obesity-stroke paradox. Stroke. 2011;42(1):30–6. doi: 10.1161/STROKEAHA.110.593434. [DOI] [PubMed] [Google Scholar]