Abstract
Responses to inhaled environmental agents are controlled by the coordinated actions by multiple immune cell types, including macrophages, dendritic cells and lymphocytes. Recent evidence indicates that some structural cells can also contribute to the initiation and propagation of immune responses. For example, airway epithelial cells can promote eosinophilic inflammation in response to upon allergen inhalation. Much remains to be learned, however, regarding how each of these cell types interacts with the others, and how these interactions shape immune responses to inhaled agents. Such studies have been hampered by the lack of reliable methods to isolate multiple and distinct populations of cells from the same tissue sample. Consequently, investigators have had to choose between using different protocols with different animals to isolate different populations of cells, or accept that for some populations, cell yields are very low. To overcome these difficulties, we have developed a convenient and practical method to isolate and purify subpopulations of epithelial and endothelial cells from mouse lung. Here, we describe these methods in detail.
Keywords: alveolar epithelial cells, bronchiolar epithelial cells, endothelial cells, epithelial cells, flow cytometry, lymphatic endothelial cells, lung, type 1 alveolar epithelial cells, type 2 alveolar epithelial cells, vascular endothelial cells
1. Introduction
Allergic asthma is a complex disease involving multiple cell types, including leukocytes and structural cells such as epithelial and endothelial cells. Upon their inhalation, allergens are sensed by epithelial cells, which can communicate with leukocytes through both direct and indirect pathways [1]. Alveolar epithelial cells (AECs) are in constant contact with alveolar macrophages, and bronchiolar epithelial cells (BECs) adhere tightly to dendritic cells (DCs) [2,3], suggesting that signals from epithelial cells might affect the context in which allergen-derived peptides are presented to T cells. In addition to this direct contact with leukocytes, epithelial cells also produce cytokines (e.g. IL-25, IL-33 and TSLP) and chemokines (e.g. CCL17 and CCL22) that contribute to allergic responses by recruiting and modifying the actions of type 2 helper T (Th2) cells, type 2 innate lymphoid cells (ILC2) and eosinophils [4,5]. Furthermore, AECs comprise type 1 AECs (AEC1) and type 2 AECs (AEC2), and the later can directly affect T cell responses by presenting antigens to them [6].
Vascular endothelial cells (VECs) and lymphatic endothelial cells (LECs) both play important roles during allergic responses. By capturing tissue-derived chemokines and displaying cell adhesion molecules, VECs promote arrest of circulating leukocytes and their recruitment to underlying tissue [7]. VECs also produce many cytokines that can either promote or suppress allergic inflammation [8]. The lymphatics drain extracellular fluid along with soluble proteins from both endogenous and exogenous sources, and are also the conduit for antigen-bearing DCs migrating from the lung to regional lymph nodes [9,10]. LECs that line these vessels can directly modulate T cell responses through their display of functional molecules, such as programmed cell death ligand 1 (PD-L1) [11].
Although our understanding of how lung stromal cells influence the initiation and elicitation of allergic inflammation continues to improve, much remains to be learned. In particular, the roles of individual stromal cell subpopulations during allergic inflammation are poorly understood. This is partly because distinct cell populations have different susceptibilities and resistance to the enzymes used for tissue digestion, and an optimum balance between cell yield and cell viability in a single isolation protocol has proved elusive. For example, tissue digestion methods optimized to isolate VECs or adherent leukocytes markedly reduce the viability of LECs and AEC1. Therefore, the isolation of different cell populations from the mouse lung has required separate protocols in which different mice are used [12,6,13,8,14]. In this book chapter, we describe a single method we have developed to isolate and purify LECs, VECs, BECs, AEC1, AEC2 in addition to CD45+ leukocytes from the same lung tissue. This method is expected to facilitate studies of how various cell types interact with, and change the activities of, other cell types, thereby leading to and improved understanding of their roles during allergic inflammation.
2. Materials
2.1. Lung digestion
Phosphate buffered salt solution (PBS) (Mg− Ca−) pH 7.4 (Life Technologies)
3-mL syringes with 20-gauge (G) needles
1-mL syringe with 1.5 inch 20-G needle and polyethelene tubing (BD Diagnostic Systems; 0.86 mm inside diameter, 1.27 mm outside diameter)RPMI1640 (PRMI) supplemented with 10 mM HEPES (Life Technologies)
Elastase (Worthington): 150 U/mL (=25.9mg/mL), stored at 4° C
Dispase II: 200 U/mL (=250 mg/mL), stored at −20° C
Liberase TM: 5 mg/mL in PBS, stored at −20° C
DNase I: 20 mg/mL in water, stored at −20° C (Note 1)
Digestion medium #1: RPMI containing 4 U/mL Elastase, 1 U/mL Dispase and 200 μg/mL DNase
Digestion medium #2: RPMI containing 25 μg/mL Liberase and 200 μg/mL DNase
Curved micro-serrefine clamp (Fine Science Tools #18055–06)
35-mm petri dish
60-mm petri dish
Incubator, 5 – 10 % CO2, 37° C
Cell strainer 70-μm
Ammonium-chloride-potassium (ACK) lysis buffer: 8 mg/L NH4Cl, 1 mg/L KHCO3, 3.7 mg/L EDTA·Na2·2H2O
FACS buffer: 0.5% BSA, 0.1% NaN3, and 2 mM EDTA in PBS
2.2. Stromal cell enrichment
-
17.
FACS buffer: 0.5% BSA, 0.1% NaN3, and 2 mM EDTA in PBS (pH 7.2 – 7.4), filter-sterilized and stored at 4° C
-
18.
1 μg/mL Biotinylated anti-CD45 pan leukocyte marker antibody (clone 30-F11)
-
19.
1 μg/mL Biotinylated anti-TER119 mAb
-
20.
EasySep™ Streptavidin RapidSpheres™ (Stem Cell Technologies)
-
21.
EasySep™ magnet (Stem Cell Technologies)
2.3. Cell staining
-
22.
FACS buffer: 0.5% BSA, 0.1% NaN3, and 2 mM EDTA in PBS (pH 7.2 – 7.4), filter-sterilized and stored at 4° C
-
23.
Normal mouse serum
-
24.
Normal rat serum
-
25.
Antibody dilution buffer: 5 % normal mouse serum, 5 % normal rat serum, and 5 μg/mL (1:100) Fc blocker in FACS buffer
-
26.
Antibodies: (Optimal final concentration of Ab is usually 0.5 – 2 μg/mL in antibody dilution buffer)
Fc blocker: anti-mouse CD16/CD32 (clone 2.4G2)
Pan leukocyte marker: CD45 (clone 30-F11)
Pan endothelial cell marker: CD31 (clone 390),
LEC markers: CD90.2 (clone 53–2.1) [15,3], T1a/Podoplanin (Pdpn) (clone 8.1.1) [16]
Pan epithelial cell marker: CD326/EpCAM (clone G8.8) [17]
BEC marker: CD24 (clone 30-F1) [18]
AEC1 marker: T1a/Pdpn (clone 8.1.1) [19,20]
AEC2 marker: MHC class-II (I-Ab clone AF6–120.1 for H-2b mice, e.g C57BL/6, or I-Ad clone AMS-32.1 for H-2d mice, e.g. BALB/c) [6]
-
27.
Streptavidin-BUV395 (BD)
-
28.
7-aminoactinomycin-D (7-AAD)
-
29.
Round bottom 96 well plate
-
30.
Plate rotor for centrifugation
-
31.
5-mL round bottom polystyrene tube (FACS tubes) (with and without 50-μm cell strainer cap)
-
32.
Flow cytometer [e.g. FACS LSR-II (BD)]
2.4. Cell sorting
-
33.
Cell sorter [e.g. FACS ARIA-II (BD)]
-
34.
RPMI + FBS: RPMI 1640 with 10% FBS
-
35.
Fetal bovine serum (FBS)
-
36.
5-mL round bottom polystyrene tube (FACS tubes) (BD, snap cap and 50-μm cell strainer cap)
-
37.
Flow cytometer [e.g. FACS ARIA-II (BD)]
3. Methods
3.1. Tissue digestion
Perfuse lung by injecting PBS (1 – 3 mL PBS, but higher volume of PBS can be injected if necessary) to the right ventricle to remove red blood cells (RBCs).
Remove salivary glands and muscle to expose the upper trachea. Make a small incision on the anterior side of the thickest band of cartilage using sharp forceps. The incision should be just large enough to allow a 20-G needle to pass though.
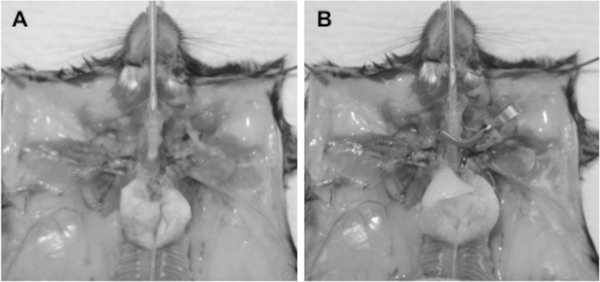
Remove the thymus, heart and diaphragm carefully to keep the lung and trachea intact (Fig. 1A).
Lavage once with 1 mL PBS using a 1.5 inch, 20-G needle connected with polyethylene tubing and 1 mL syringe. Lavage with 1 mL digestion medium #1 containing Elastase, Dispase and DNase (Fig. 1A).
Inflate the lung with 1 mL digestion medium #1, clamp trachea with a micro-serrefine clamp then remove needle from the trachea (Fig. 1B). Confirm no leakage of digestion medium from the lung or trachea.
Remove the trachea and lung with the clamp from mouse then transferred into a well of a 35-mm petri dish containing 2 mL digestion medium #1.
Incubate the dish containing tissues for 45 minutes at 37 °C in a CO2 incubator.
After the incubation, remove trachea gently using 2 sets of forceps, and transfer only the lung to a new 35-mm dish containing 1 mL cold RPMI.
Cut lung tissue to make small pieces (1 mm or smaller) in the same dish using sharp scissors.
Transfer tissue pieces to a 50-mL conical tube using 5-mL pipettes. Rinse the dish with 10 mL cold RPMI, and transfer all tissue pieces to the same tube.
Gently rock the tube on a rotator for 5 minutes at room temperature (RT).
Place a 70-μm cell strainer on a 50-mL new conical tube. Transfer all tissue pieces into the cell strainer. Wash the strainer by applying 10 mL cold RPMI onto the strainer. Do not dissociate tissues mechanically (Note 2). Close lid, and keep the tube on ice until step #15.
Transfer the cell strainer to a 60-mm petri dish, and add 5 mL digestion medium #2 onto the strainer.
Incubate the dish containing tissues for 30 minutes at 37 °C in a CO2 incubator.
Place the cell strainer on a 50-mL conical tube that was kept on ice from step #12. Rinse the dish with 10 mL cold FACS buffer, and transfer the buffer to the tube through the strainer. Do not dissociate tissues mechanically (Note 2). Discard the strainer.
Centrifuge the tube containing cells at 500 g-force for 5 minutes at 4 °C.
If necessary, remaining RBCs can be removed using ammonium-chloride-potassium (ACK) lysis buffer. After RBC lysis, remove aggregates using cell strainers to prepare single cell suspension.
Resuspend cells in FACS buffer and count cells (Note 3).
Figure 1. Inflation of mouse lung for tissue digestion.
(A) After perfusion of the lung, salivary glands and muscle are removed to expose the upper trachea. The thymus, heart and diaphragm are also carefully removed. A polyethylene tubing connected to a 20-G needle and a 1-mL syringe filled with 1 mL PBS is inserted through an incision into the trachea, and the lungs are lavaged with the PBS. (B) After this lavage, the lungs are again lavaged, this time with 1 mL digestion medium #1. The lungs are then inflated with digestion medium #1, the syringe pulled back slightly, and the trachea closed with a micro-serrefine clamp. The polyethylene tubing can now be removed from the trachea. The trachea and lung are then dissected from the animal, together with the clamp. Care must be taken to avoid damage to the lung and trachea and consequent leakage of digestion medium.
3.2. Stromal cell enrichment
If leukocytes are necessary for the study, skip this section, and go to section 3.3. If leukocytes are not desired, the following method is useful to enrich epithelial and endothelial cells.
Resuspend 107 − 108 cells in 1 mL FACS buffer. If you have more than 108 cells, have an additional tube for every 108 cells. Transfer the cell suspension to a 5-mL round bottom tube.
-
Add biotinylated anti-CD45 mAb to the cell suspension for a final concentration of 1 μg/mL. Mix, close lid, and incubate the tube on ice for 30 minutes (Note 4).
If RBCs need to be depleted, anti-TER119 mAb can be added together with anti-CD45 antibody at 1 μg/mL.
Vortex the tube gently for 30 seconds. Add 50 μL Streptavidin RapidSpheres to the tube and mix. Close lid and incubate the tube on ice for 20 minutes.
Add 2.5 mL cold FACS buffer and mix gently. Place the tube without cap into an EasySep magnet, and incubate for 5 minutes at room temperature. Swirl the magnet occasionally (Note 5).
Invert the magnet and tube for pouring the cell suspension into a new 15-mL conical tube. These cells should be CD45-negative stromal cells. CD45-positive cells can be recovered from the 5-mL tube if necessary.
Centrifuge the cells at 500 g-force rpm for 5 minutes, resuspend them in FACS buffer, and count.
-
For cell analysis, proceed to section 3.3 Staining of endothelial and epithelial cells.
For cell sorting, proceed to section 3.4 Sorting of endothelial and epithelial cells.
3.3. Staining of endothelial cells and epithelial cells
-
9.
Place approximately 1×106 (1×105 ~ 2×106) cells in each well of round bottom 96-well plate. Afterwards, use multichannel pipette. Centrifuge the plate at 800 g-force for 3 minutes at 4 °C, and discard supernatant.
-
10.
Add 50 μL of Ab dilution buffer. Incubate the cells on ice for 5 – 10 minutes.
-
11.
Prepare 2x Ab cocktail in Ab dilution buffer. Add 50 μL of 2x Ab solution to the 50 μL of cells and mix well. The Ab composition of the cocktail depends on the goal of the experiment, but an example is shown below (Note 6).
CD24-PE, CD31-APC-Fire750, CD45-BUV395 (or streptavidin-BUV395 after stromal cell enrichment), CD90.2-eFluor 450, CD326-BV510, MHC class II (I-Ab or I-Ad)-FITC, T1a/Pdpn-Alexa Fluor 647 (or APC).
Protect cells from light and incubate on ice for 30 minutes.
-
12.
Wash cells with FACS buffer twice. 1st time, add 100 μL FACS buffer, and 2nd time, resuspend pellet with 200 μL FACS buffer.
-
13.
Suspend cells in 200 μL FACS buffer, and transfer cells into a FACS tube through 50 μm cell strainer cap (BD 5-mL round bottom tube with blue cap).
-
14.
Add 7-AAD at 0.5 μg/mL in final concentration.
3.4. Flow cytometric analysis
Flow cytometer’s setting depends on the instrument and the goal of the experiment. An example is shown in Table 1.
Run standard samples (unstained cells and cells stained with single fluorochrome).
Set voltage of each channel.
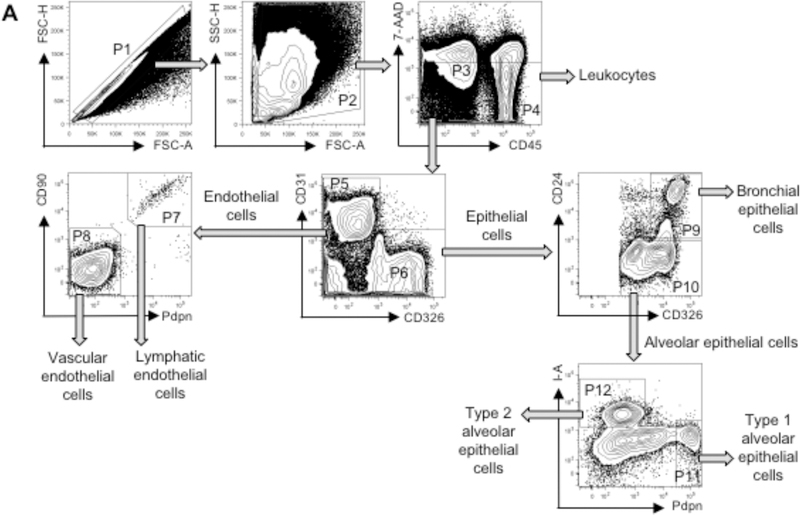
Gate on singlets (P1 in FSC-A vs. FSC-H) and leukocytes and stromal cells (P2 in FSC-A vs. SSC) (Fig. 2).
Adjust compensation manually. We do not recommend using “Auto Comp”, which cannot adjust compensation well for lung stromal cells. Set gates for positive cells (not autofluorescent cells) and negative cells, then adjust the compensation value in each channel. Repeat same procedures for all channels (Note 7).
-
Gate on viable stromal cells (P3: 7-AAD− CD45−) and leukocytes (P4: 7-AAD− CD45+) under P2. Gate endothelial cells (P5: CD31+ CD326−) and epithelial cells (P6: CD31− CD326+) under P3.
Endothelial cells are segregated into LECs (P7: CD90+ Pdpn+) and VECs (P8: CD90lo Pdpnlo).
Epithelial cells are segregated into BECs (P9: CD24+) including ciliated BECs (CD24hi) and other BECs (CD24intermediate), and AECs (P10: CD24lo) including AEC1 (P11: Pdpnhi MHC- IIlo) and AEC2 (P12: MHC-II+ Pdpnlo).
Collect 10,000 cells or more in P5 or P6 dependent on the goal of the experiment.
Absolute cell number is calculated based on total cell count and percentage of each population.
Table 1.
Flow cytometer’s setting
| Target molecules | Fluorochromes | Lasers and channels | Filters | |
|---|---|---|---|---|
| CD45 | BUV395 | Ultra Violet-B | 379/34 BP | - |
| CD90 | eFluor 450 | Violet-C | 450/50 BP | - |
| CD326 | BV510 | Violet-B | 525/50 BP | 505 LP |
| MHC-II I-A | FITC | Blue-B | 525/50 BP | - |
| Dead cells | 7-AAD | Blue-A | 710/50 BP | 695 LP |
| CD24 | PE | Yellow Green-E | 585/15 BP | - |
| T1a/Podoplanin (Pdpn) | Alexa Fluor 647 | Red-B | 660/20 BP | 750 LP |
| CD31 | APC-Fire 750 | Red-A | 780/60 BP | 735 LP |
BP: band pass, LP: long pass
Figure 2. Gating strategy for lung endothelial and epithelial cell analysis by flow cytometry.
Gates shown include single cells (P1), leukocytes and stromal cells (P2), viable stromal cells (P3: 7-AAD− CD45−), viable leukocytes (P4: 7-AAD− CD45+), endothelial cells (P5: CD31+ CD326−), epithelial cells (P6: CD326+ CD31−), LECs (P7: CD90+ Pdpn+), VECs (P8: CD90lo Pdpnlo), BECs (P9: CD24+), AECs (P10: CD24lo), AEC1 (P11: Pdpnhi MHC-IIlo) and AEC2 (P12: MHC- II+ Pdpnlo). BECs can be further segregated into CD24hi ciliated BECs and other CD24intermediate BECs (18). Approximate frequencies of each population (percent of parental population) are as follows: P1, 82%; P2, 68%; P3, 20%; P4, 53%; P5, 27%; P6, 53%; P7, 2%; P8, 98%; P9, 15%; P10, 80%; P11, 13%; and P12, 32%.
3.5. Sorting of Epithelial and Endothelial Cells
Add 4 mL RPMI + FBS to each FACS collection tube to coat tubes. Incubate the tubes at 37 °C.
Place isolated lung cells in 15-mL conical tube (up to 10×108 cells/tube). Fill the tube with sterile FACS buffer. Spin the tube at 500 g-force for 5 minutes, and remove supernatant.
Resuspend cells with 1 mL Ab dilution buffer containing antibodies listed above (section 3.2). Protect cells from light and incubate on ice for 30 minutes.
Wash cells with FACS buffer twice. 1st time, add 14 mL buffer and spin the tube at 500 g-force for 5 min, and remove supernatant. 2nd time, resuspend pellet with 15 mL buffer and spin the tube at 500 g-force for 5 minutes.
Suspend cells in FACS buffer at 3 – 4×106/mL, and transfer cells into a FACS tube through 50 μm cell strainer cap (BD 5-mL round bottom tube with blue cap).
Add 0.5 μg/mL 7-AAD (final concentration).
Remove 3 mL RPMI + FBS from each collection tube, to leave 1 mL of media. Place tubes on ice.
Run cells on a cell sorter (e.g FACS ARIA-II). Lasers, channels, filters, and gating strategies can be the same as those described above (section 3.4) (Table 1).
Sort cell populations of interest. P3: stromal cells, P4: leukocytes, P5: endothelial cells, P6: epithelial cells, P7: LECs, P8: VECs, P9: BECs, P10: AECs, P11: AEC1, P12: AEC2 (Fig. 2).
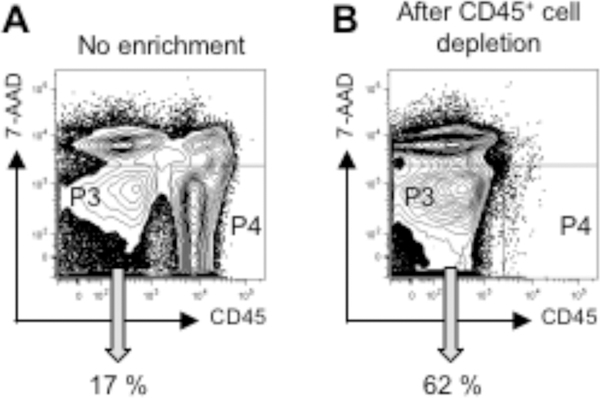
Enrichment of stromal cells by leukocyte depletion prior to the cell sorting is useful to facilitate stromal cell collection (Fig. 3).
If cells need to be cultured after the sorting, wash them thoroughly to remove NaN3 and EDTA.
Figure 3. Enrichment of lung stromal cells by leukocyte depletion.
Lung cells were analyzed by flow cytometry after cell preparation without enrichment (A) or after magnet-base negative selection of CD45+ leukocytes (B). The precentage of live stromal cells (P3, CD45− 7-AAD−) is indicated. Live leukocytes (P4, CD45+ 7-AAD−) are 65% and 0.2% in (A) and (B), respectively.
4. Notes
Use distilled water to dissolve DNase I. Do not use PBS.
Mechanical dissociation reduces cell viability especially for AEC1.
Cell number can vary, but we usually obtain 2 – 2.5×107 cells from each mouse.
1 μg/mL anti-CD45 mAb usually works well to enrich CD45-negative cells, but if depletion is not satisfactory, mAb dose can be increased up to 5 μg/mL.
Magnetic beads and magnet from other manufactures can be used, but do not use a column-based magnetic sorter since cell recovery from these columns is low.
Combinations of Abs and fluorochromes listed above have been verified by us. However, if changes to Ab combinations need to be made, they should be carefully tested to avoid false-positive or -negative signals.
Because different cell populations have different background signals (including autofluorescence), auto-comp cannot adjust the compensation precisely.
Acknowledgements
We thank Miranda Lyons-Cohen, Wan-Chi Lin, Seddon Y. Thomas and Kymberly M. Gowdy for help and advice with lung cell isolation, Carl Bortner and Maria Sifre for help with flow cytometric analysis. This work was supported by the Intramural Research Program of the National Institutes of Health, the National Institute of Environmental Health Sciences (ZIA ES102025-09).
References
- 1.Lambrecht BN, Hammad H (2014) Dendritic cell and epithelial cell interactions at the origin of murine asthma. Ann Am Thorac Soc 11 Suppl 5:S236–243. doi: 10.1513/AnnalsATS.201405-218AW [DOI] [PubMed] [Google Scholar]
- 2.Sung SS, Fu SM, Rose CE Jr., Gaskin F, Ju ST, Beaty SR(2006) A major lung CD103 (alphaE)-beta7 integrin-positive epithelial dendritic cell population expressing Langerin and tight junction proteins. J Immunol 176 (4):2161–2172 [DOI] [PubMed] [Google Scholar]
- 3.Lyons-Cohen MR, Thomas SY, Cook DN, Nakano H (2017) Precision-cut Mouse Lung Slices to Visualize Live Pulmonary Dendritic Cells. J Vis Exp (122). doi: 10.3791/55465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hammad H, Lambrecht BN (2015) Barrier Epithelial Cells and the Control of Type 2 Immunity. Immunity 43 (1):29–40. doi: 10.1016/j.immuni.2015.07.007 [DOI] [PubMed] [Google Scholar]
- 5.Kaplan AP (2001) Chemokines, chemokine receptors and allergy. Int Arch Allergy Immunol 124 (4):423–431. doi:53777 [DOI] [PubMed] [Google Scholar]
- 6.Lo B, Hansen S, Evans K, Heath JK, Wright JR (2008) Alveolar epithelial type II cells induce T cell tolerance to specific antigen. J Immunol 180 (2):881–888 [DOI] [PubMed] [Google Scholar]
- 7.Celie JW, Beelen RH, van den Born J (2009) Heparan sulfate proteoglycans in extravasation: assisting leukocyte guidance. Front Biosci (Landmark Ed) 14:4932–4949 [DOI] [PubMed] [Google Scholar]
- 8.Teijaro JR, Walsh KB, Cahalan S, Fremgen DM, Roberts E, Scott F, Martinborough E, Peach R, Oldstone MB, Rosen H (2011) Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell 146 (6):980–991. doi: 10.1016/j.cell.2011.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson LA, Jackson DG (2008) Cell traffic and the lymphatic endothelium. Ann N Y Acad Sci 1131:119–133. doi: 10.1196/annals.1413.011 [DOI] [PubMed] [Google Scholar]
- 10.Alitalo K (2011) The lymphatic vasculature in disease. Nat Med 17 (11):1371–1380. doi: 10.1038/nm.2545 [DOI] [PubMed] [Google Scholar]
- 11.Cohen JN, Tewalt EF, Rouhani SJ, Buonomo EL, Bruce AN, Xu X, Bekiranov S, Fu YX, Engelhard VH (2014) Tolerogenic properties of lymphatic endothelial cells are controlled by the lymph node microenvironment. PloS one 9 (2):e87740. doi: 10.1371/journal.pone.0087740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamamoto K, Ferrari JD, Cao Y, Ramirez MI, Jones MR, Quinton LJ, Mizgerd JP (2012) Type I alveolar epithelial cells mount innate immune responses during pneumococcal pneumonia. J Immunol 189 (5):2450–2459. doi: 10.4049/jimmunol.1200634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singer BD, Mock JR, D’Alessio FR, Aggarwal NR, Mandke P, Johnston L, Damarla M (2016) Flow-cytometric method for simultaneous analysis of mouse lung epithelial, endothelial, and hematopoietic lineage cells. Am J Physiol Lung Cell Mol Physiol 310 (9):L796–801. doi: 10.1152/ajplung.00334.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakano H, Cook DN (2013) Pulmonary antigen presenting cells: isolation, purification, and culture. Methods Mol Biol 1032:19–29. doi: 10.1007/978-1-62703-496-8_2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jurisic G, Iolyeva M, Proulx ST, Halin C, Detmar M (2010) Thymus cell antigen 1 (Thy1, CD90) is expressed by lymphatic vessels and mediates cell adhesion to lymphatic endothelium. Exp Cell Res 316 (17):2982–2992. doi: 10.1016/j.yexcr.2010.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farr AG, Berry ML, Kim A, Nelson AJ, Welch MP, Aruffo A (1992) Characterization and cloning of a novel glycoprotein expressed by stromal cells in T-dependent areas of peripheral lymphoid tissues. The Journal of experimental medicine 176 (5):1477–1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trzpis M, McLaughlin PM, de Leij LM, Harmsen MC (2007) Epithelial cell adhesion molecule: more than a carcinoma marker and adhesion molecule. Am J Pathol 171 (2):386–395. doi: 10.2353/ajpath.2007.070152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen H, Matsumoto K, Brockway BL, Rackley CR, Liang J, Lee JH, Jiang D, Noble PW, Randell SH, Kim CF, Stripp BR (2012) Airway epithelial progenitors are region specific and show differential responses to bleomycin-induced lung injury. Stem Cells 30 (9):1948–1960. doi: 10.1002/stem.1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dobbs LG, Williams MC, Gonzalez R (1988) Monoclonal antibodies specific to apical surfaces of rat alveolar type I cells bind to surfaces of cultured, but not freshly isolated, type II cells. Biochimica et biophysica acta 970 (2): 146–156 [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez RF, Dobbs LG (1998) Purification and analysis of RTI40, a type I alveolar epithelial cell apical membrane protein. Biochimica et biophysica acta 1429 (1):208–216 [DOI] [PubMed] [Google Scholar]