Abstract.
In Brazil, the reported number of syphilis cases among pregnant women has markedly increased. We conducted a cross-sectional study to determine the prevalence of Treponema pallidum infection and associated factors in pregnant women in Dourados, Mato Grosso do Sul, Brazil. Participants voluntarily completed a risk-factor questionnaire and provided blood specimens for unlinked anonymous testing for the presence of antibodies against T. pallidum, the causative agent of syphilis. Data of newborns were obtained from medical records. We performed univariate and multivariate regression analyses to assess associations with syphilis. The seroprevalence of syphilis in pregnant women was 4.4% (n = 29/661). Twenty-five newborns were seropositive for T. pallidum, and complications due to syphilis were observed in 28% (n = 7/25). Although 96.5% (n = 28/29) of women with syphilis received antenatal care, Venereal Disease Research Laboratory tests were performed in the first trimester for 47.6% (n = 10/21) of women. Women who received treatment in the third trimester (28.6%; n = 6/21) were considered successfully treated at the time of delivery. The use of illicit drugs during pregnancy (odds ratio [OR]: 13.3, 95% CI: 1.9–91.2) and a history of abortion (OR: 3.7, 95% CI: 1.7–8) were associated with syphilis. Our findings highlight that the poor quality of antenatal care services contributes to the high prevalence of syphilis. In addition, there are social and behavioral risk factors associated with syphilis in pregnant women. Future studies are needed to determine limitations of clinical management and control services available to pregnant women with syphilis.
INTRODUCTION
Syphilis is an infectious disease caused by the spirochete bacterium Treponema pallidum1 and is considered a public health problem, especially in developing countries such as Brazil.2,3 As per the WHO estimation, 11 million people are infected with T. pallidum annually worldwide.4 Specifically, there is a great concern about pregnant women because this pathogen can be transmitted vertically to the fetus and can cause congenital syphilis.5,6 According to the WHO data, approximately 1.5 million pregnant women worldwide are reported to be infected with syphilis annually. Furthermore, about half of untreated pregnant women are presented with adverse clinical outcomes such as congenital syphilis (36%), stillbirth (26.4%), low birth weight (23.4%), premature birth (23.2%), neonatal death (16.2%), and abortion (14.9%).4,7,8 It is important to note that 70% of infected children do not present with symptoms at birth.9
In 2010, the WHO and member states of the Pan American Health Organization (PAHO) approved an action plan to eliminate mother-to-child transmission of syphilis, setting a target for 2015 to reduce the incidence of congenital syphilis to 0.5 cases per 1,000 live births.1,10 Brazil is a signatory to the PAHO and WHO and has launched several strategies, such as an operational plan for reducing the vertical transmission of HIV and syphilis, the Pact for Life, and the Rede Cegonha.11
However, despite various efforts, Brazil is far from achieving the goal of eliminating congenital syphilis. Data from the epidemiological bulletin of the Ministry of Health revealed that the number of reported cases of congenital syphilis has been increasing every year in Brazil. The detection rate increased from 1.9 cases per 1,000 live births in 2005 to 6.5 cases per 1,000 in 2015, representing an increase of 242%. There has also been arise in the number of syphilis cases reported in pregnant women, from 0.5 per 1,000 live births in 2005 to 11.2 per 1,000 in 2015, accounting for an increase of 2,140% over a period of 10 years. In 2015, an alarmingly high detection rate was observed in Mato Grosso do Sul, in the Midwest region of Brazil (21.9 cases per 1,000 live births).12
Although there may be underreported cases of syphilis, Mato Grosso do Sul had the highest rate of syphilis detection among pregnant women in Brazil during 2015.13 Thus, studies on the prevalence and risk factors of syphilis, particularly in pregnant women, are important for developing and implementing preventive measures for this disease.13 To better understand these features and develop future prevention strategies, we conducted a multicenter, cross-sectional study including 661 pregnant women from the Midwest region of Brazil.
MATERIAL AND METHODS
Study setting.
Bordering Paraguay and Bolivia, Mato Grosso do Sul is a state in the Midwest region of Brazil with a population of 2.5 million people, comprising an estimated 53,694 pregnant women (2015).14 We performed a cross-sectional study at the University Hospital (UH), a medium-sized public hospital with 237 beds that provides medium-to-high–complexity care in various specialties, such as gynecology and obstetrics. This is the only reference hospital for the care of pregnant women with risk factors, covering 33 cities (comprising approximately 725,000 inhabitants). The hospital has an average of 1,000 patient visits and 300 births per month.
Study population.
Pregnant women and their newborns were included in this study. To calculate the sample size, we used a 1.6% prevalence of syphilis among pregnant women in Brazil.15 For population size, we used the value of 3,014 births occurring in the UH during 2014. A 95% CI and accuracy of 0.8% were considered in statistical analyses. We added 20% more individuals to account for patients who refused to participate. Pregnant women from other cities, Indigenous women, those younger than 18 years, and women with fetal loss before 22 weeks were excluded from this study. To avoid selection bias, risk factors were determined only for pregnant women from Dourados.
Data collection.
We performed data collection during the postpartum period, in three stages. 1) Each participant was interviewed using a standardized questionnaire. The variables obtained during the interview included age, gender, marital status, educational attainment, drug use, sexual history, sexually transmitted infections (STIs), obstetric, antenatal, diagnosis, and treatment. In addition, the participant’s ethnic group or skin color (white, black, Indigenous, Asian, or mixed) was self-reported. 2) We assessed medical records and antenatal cards of puerperal women. 3) After appropriate antisepsis, a vacuum tube system was used to obtain 10 mL of peripheral venous blood from each woman; the samples were processed to obtain serum, which was stored at −20°C until serological assay. Data of newborns were obtained from the medical records. The interviews, data collection, and blood collection were performed by a team of previously trained health professionals. The team collected data on all days of the week and in all periods until an adequate sample was obtained.
Serological testing.
Rapid treponemal tests (ABON Biopharm, Hangzhou, China) were performed as screening tests in pregnant women and newborns. In addition, serum samples were tested using an ELISA ICE* Syphilis (DiaSorin, Saluggia, Italy) for detection of anti–T. pallidum IgG and IgM. For the ELISA and rapid treponemal tests, reactive samples were serially diluted and titrated to detect anticardiolipin antibodies using the Venereal Disease Research Laboratory (VDRL) test (Abbott Murex, Dartford, United Kingdom). Laboratory tests were carried out according to the manufacturers’ instructions and all tests were run against positive and negative controls. Venereal Disease Research Laboratory titers were used to determine treatment failure or immunological memory in pregnant women.16 Successful treatment was considered as a decrease of two or more titers or presentation of negative titers. Persistence of or increase in titers was indicative of therapeutic failure or reinfection.17 A case of syphilis was confirmed to be positive for any pregnant woman with a reactive treponemal test and a reactive non-treponemal test or reactive with any titers.18 Participants who did not receive adequate treatment during prenatal care were treated during the postpartum period.
Data analysis.
Results of the interviews and serological tests were recorded in the electronic data capture program and analyzed using SAS version 9.2 (SAS Institute, Cary, NC). Variables were evaluated for their distribution pattern, and the choice of statistical method used was based on the distribution pattern of the variables. Variables with asymmetric and normal distributions were analyzed using nonparametric and parametric tests, respectively. Dichotomous or categorical data were analyzed using the chi-square or Fisher’s exact test. For continuous variables, the t-test was used. Univariate analysis was performed to verify the association between the independent and dependent variables. Multivariate analysis (95% CI) using the logistic model was performed for variables that reached a level of significance (P ≤ 0.05), further verifying the joint presence of possible risk factors. Those variables with a significant association of P ≤ 0.05 were retained in the model. The selection of variables for the final model was performed in steps, choosing the best regression equation for the backward stepwise regression model.
Ethical considerations.
This study was conducted with the approval of the research Ethics Committee at the Universidade Federal da Grande Dourados (no. 1,402,529). Following the National Health Council Resolution 466 of 12 December 2012, all eligible participants provided written informed consent to participate in this study. To protect the identity of pregnant women, we only used those variables that were necessary for the present study.
RESULTS
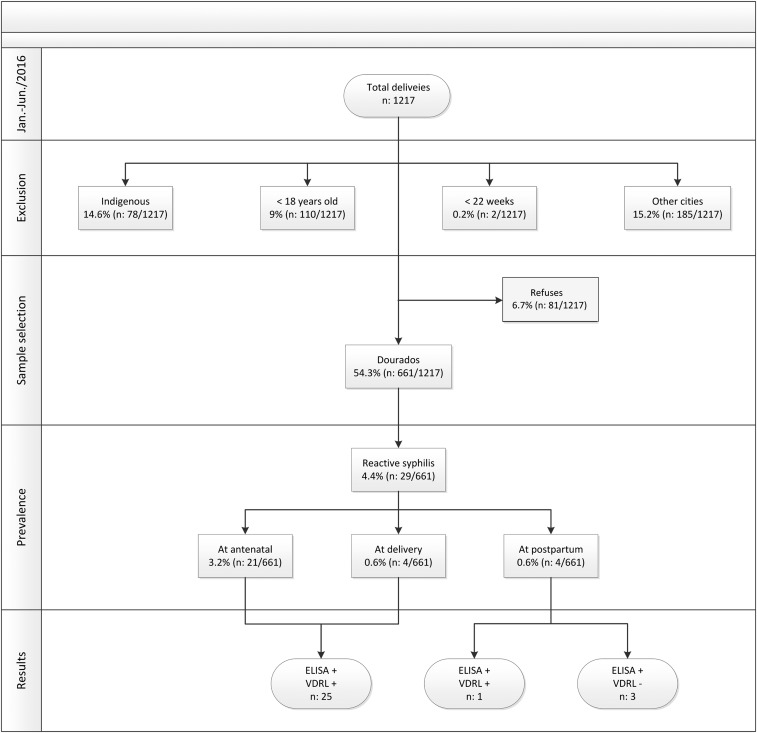
In 2016, there were 5,246 births reported in Dourados, including 3,332 (63.5%) in the UH, 1,829 (34.9%) in private hospitals, and 85 (1.6%) in other settings. From January to June 2016, the UH reported 1,217 births. Of 1,217 pregnant women, 742 (60.97%) from Dourados were invited to participate, and 661 (64%) women agreed to the interview and provided their blood samples (Figure 1). The following women were excluded from this study: 21.9% (185) of women who were from other cities, 14.6% (178) who were Indigenous, 9% (110) who were younger than 18 years, and 0.2% (2) with fetal loss before 22 weeks. The mean age of the 661 included women was 26 years (±5.8 years, range: 18–48 years). Of these, 60.8% of women had completed a high school education (n = 401/660), 51.4% were of mixed ethnicity (n = 340/661), 77% were married (n = 508/660), and 51.7% were homemakers (n = 342/661) (Table 1). Antenatal care was received by 98.2% (n = 649/661) of women, 97.9% (n = 641/655) received public health services, and 66.5% (n = 431/648) received treatment in the first trimester of gestation. During antenatal care, VDRL tests were conducted for 88.3% (n = 573/649) of pregnant women; however, 48.6% (n = 321/661) of women made it to the first trimester and 55.9% (n = 323/649) had no information of undergoing rapid screening tests for syphilis recorded on their antenatal card. HIV testing was performed during antenatal care in 91.2% (n = 592/649) of pregnant women as recommended by the Brazilian Ministry of Health, and 0.8% (n = 5/592) were seropositive (Table 2).
Figure 1.
Flowchart of study design and detection of infection caused by Treponema pallidum in pregnant women.
Table 1.
Socioeconomic, obstetric, and risk behavior characteristics of pregnant women (n = 661) admitted for childbirth at the University Hospital of Dourados, Mato Grosso do Sul, in 2016
| Variables | Number/Percentage |
|---|---|
| Age, years (range) (±SD) | 26 (18–48) (±5.8) |
| Level of education | |
| Completed elementary school | 259/660 (39.2%) |
| High school or higher education | 401/660 (60.8%) |
| Family income | |
| Up to three times minimum wage | 595/648 (91.8%) |
| Four times minimum wage or more | 53/648 (8.2%) |
| Ethnic group/skin color | |
| White | 252/661 (38.1%) |
| Mixed | 340/661 (51.4%) |
| Marital status | |
| Single | 152/660 (23%) |
| Married | 508/660 (77%) |
| Occupation | |
| Homemaker | 342/661 (51.7%) |
| Other occupation | 319/661 (48.3%) |
| Number of children | |
| Up to two children | 446/661 (67.5%) |
| Three children or more | 215/661 (32.5%) |
| Type of birth | |
| Normal | 328/661 (49.6%) |
| Cesarean | 333/661 (50.4%) |
| History of adverse outcomes in previous pregnancies | |
| Spontaneous abortion | 139/661 (21%) |
| Stillborn | 17/661 (2.6%) |
| Premature birth | 87/661 (13.2%) |
| Neonatal death | 17/661 (2.6%) |
| Age of first sexual intercourse (years) | |
| ≤ 17 | 514/650 (79.1%) |
| ≥ 18 | 136/650 (20.9%) |
| Condom use | |
| Always | 111/655 (17%) |
| No or sometimes | 544/655 (83%) |
| Sexual history | |
| Fixed sex partner | 586/661 (88.6%) |
| History of STIs in the last year | 26/661 (3.9%) |
| History of vaginal discharge | 449/661 (67.9%) |
| History of genital lesion | 31/661 (4.7%) |
| History of genital warts | 30/661 (4.5%) |
| History of genital vesicle | 15/661 (2.27%) |
| History of sexual intercourse with (ex) convict | 46/661 (7%) |
| History of sexual partner using non-injectable illicit drugs | 59/661 (8.9%) |
| History of sexual partner using injecting drugs | 3/661 (0.4%) |
| Number of partners in the last 2 years | |
| One | 537/650 (82.6%) |
| Two or more | 113/650 (17.4%) |
| Mean (±SD) | 1.27 (±0.7) |
| Alcohol and drug use | |
| Tattoo | 262/661 (39.6%) |
| Piercing | 214/661 (32.4%) |
| History of blood transfusion | 45/661 (6.8%) |
| History of sharing syringe or needle | 5/661 (0.8%) |
| Smoker | 70/661 (10.6%) |
| History of alcohol use | 456/661 (69%) |
| History of illicit drug use | 27/661 (4.1%) |
| Use of alcohol in the current pregnancy | 136/661 (20.6%) |
| Use of illicit drugs in the current pregnancy | 5/661 (0.8%) |
STI = sexually transmitted infection.
Table 2.
Variables related to the antenatal care of pregnant women (n = 661) admitted for childbirth to the University Hospital of Dourados, MatoGrosso do Sul; data from the antenatal card, 2016
| Characteristics of antenatal care | Number/percentage |
|---|---|
| Received antenatal care | |
| Yes | 649/661 (98.2%) |
| No | 8/661 (1.2%) |
| Yes, but did not bring the antenatal card | 4/661 (0.6%) |
| Type of antenatal service | |
| Public | 641/655 (97.9%) |
| Private | 14/655 (2.1%) |
| Time of starting antenatal care | |
| First trimester | 431/648 (66.5%) |
| Second or third trimester | 217/648 (33.5%) |
| Number of antenatal care visits | |
| One to five | 166/648 (25.6%) |
| Six or more | 482/648 (74.4%) |
| VDRL test performed | |
| Yes | 573/649 (88.3%) |
| No | 4/649 (0.7%) |
| Not reported | 72/649 (11.1%) |
| Achievement quarter of VDRL | |
| First trimester | 321/661 (48.6%) |
| Second trimester | 170/661 (25.7%) |
| Third trimester | 187/661 (28.3%) |
| Not informed | 64/661 (9.7%) |
| VDRL result | |
| Positive | 18/573 (3.1%) |
| Not reported | 5/573 (0.9%) |
| VDRL titer | |
| 1/1 to 1/4 | 9/661 (1.4%) |
| ≥ 1/8 | 9/661 (1.4%) |
| Rapid screening test for syphilis | |
| Yes | 258/649 (39.7%) |
| No | 28/649 (4.3%) |
| Not reported | 323/649 (55.9%) |
| HIV testing | |
| Yes | 592/649 (91.2%) |
| No | 2/649 (0.3%) |
| Not reported | 55/649 (8.5%) |
| HIV test result | |
| Reactive | 5/592 (0.8%) |
| Not reported | 1/592 (0.2%) |
VDRL = Venereal Diseases Research Laboratory.
The seroprevalence of syphilis in pregnant women was 4.4% (n = 29/661) (Figure 1), 72.4% (n = 21/29) of whom were diagnosed with syphilis during antenatal care. Venereal Disease Research Laboratory testing was performed in the first trimester for 47.6% (n = 10/21) of women; however, 28.6% (n = 6/21) underwent treatment in the third trimester and 28.6% (n = 6/21) had no record of syphilis treatment on their antenatal card. There was no information recorded on the antenatal card about rapid screening tests in 46.4% (n = 13/28) of women, only 38% (n = 8/21) received three doses of benzathine penicillin, and 4.8% (n = 1/21) received erythromycin; 95% (n = 20/21) reported that their partners were called in for treatment during antenatal care, of which only 50% (n = 10/20) received treatment (Supplemental Table 1). Six pregnant women (28.6%, n = 6/21) whose partners were registered on their antenatal cards successfully completed treatment at least 30 days before childbirth. Only one pregnant woman (4.8%, n = 1/21) had reduced titers at childbirth, and one (4.8%, n = 1/21) had a history of adequate treatment during a previous pregnancy (i.e., the presence of low titers during antenatal care and at childbirth). Therefore, these two women were considered to have received successful treatment (9.5%, n = 2/21). Five pregnant women (23.8%, n = 5/21) self-reported completion of treatment during pregnancy; however, there was no record of treatment on their antenatal card, making it impossible to evaluate the success of their treatment. Four pregnant women (19%, n = 4/21) did not have a reduction in their titers, suggesting a possibility of reinfection or inadequate treatment.16
At childbirth, 3.8% (n = 25/661) of pregnant women and their newborns tested positive for syphilis, and treatment was prescribed in 60% (n = 15/25) and 88% (n = 22/25), respectively. Complications were observed in 28% (n = 7/25) of newborns with congenital syphilis, requiring hospitalization; among these newborns, 12% had (n = 3/25) neurosyphilis, 8% (n = 2/25) had low birth weight, 4% (n = 1/25) succumbed to neonatal death, and 4% (n = 1/25) were born prematurely. Although there was only one woman who did not receive treatment during prenatal care, 94.5% (n = 20/22) of newborns presented with titers lower than or equal to maternal titers (Supplemental Table 2).
Of the 29 pregnant women with syphilis, 18 (62.1%) had an elementary school education, 96% (n = 28/29) had their first sexual intercourse at or before 17 years of age, 86.2% (n = 25/29) did not use a condom during sexual intercourse, 6.9% (n = 2/29) used illicit drugs during pregnancy, and 48.3% (n = 14/29) reported a history of previous abortion. In the multivariate analysis, use of illicit drugs during pregnancy (OR: 13.3, 95% CI: 1.9–91.2) and a history of abortion (OR: 3.7, 95% CI: 1.7–8) were associated with syphilis during pregnancy (Table 3).
Table 3.
Variables associated with syphilis seropositivity in pregnant women admitted for childbirth to the University Hospital of Dourados, Mato Grosso do Sul; data from univariate and multivariate analyses (P ≤ 0.05), 2016
| Variables | Syphilis test result | Univariate | Multivariate | |||
|---|---|---|---|---|---|---|
| Positive (%) n = 29 | Negative (%) n = 632 | OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Level of education | 0.01 | |||||
| Elementary school | 18/29 (62.1%) | 241/631 (38.2%) | 2.6 (1.2–5.7) | – | – | |
| High school or higher education | 11/29 (37.9%) | 390/631 (61.8%) | 1 | – | – | |
| Alcohol and drug use | ||||||
| Smoker | 7/29 (24.1%) | 63/632 (10%) | 2.9 (1.9–7) | 0.015 | – | – |
| Use of illicit drugs in the current pregnancy | 2/29 (6.9%) | 3/632 (0.5%) | 15.5 (2.5–96.9) | < 0.01 | 13.3 (1.9–91.2) | < 0.01 |
| Risk behaviors | ||||||
| Tattoo | 20/29 (69%) | 242/632 (38.3%) | 3.6 (1.6–8) | < 0.01 | – | – |
| Age at first sexual intercourse (years) | – | – | – | 0.018 | – | – |
| ≤ 17 | 28/29 (96.5%) | 486/621 (78.3%) | – | – | – | |
| ≥ 18 | 1/29 (3.4%) | 135/621 (21.7%) | 7.8 (1–57.7) | – | – | |
| Sexual history | ||||||
| Genital lesion | 5/29 (17.2%) | 26/632 (4.1%) | 4.8 (1.7 –13.7) | < 0.01 | – | – |
| Sexual relationship with (ex) inmate | 6/29 (20.7%) | 40/632 (6.3%) | 3.9 (1.5–10) | < 0.01 | – | – |
| Sexual partner using non-injectable illicit drugs | 7/29 (24.4%) | 52/632 (8.2%) | 3.6 (1.5–8.6) | < 0.01 | – | – |
| History of adverse outcomes | ||||||
| History of abortion | 14/29 (48.3%) | 125/632 (19.8%) | 3.8 (1.8–8) | < 0.01 | 3.7 (1.7–8) | < 0.01 |
| History of neonatal death | 3/29 (10.3%) | 14/632 (2.2%) | 5.1 (1.4–18.9) | < 0.01 | – | – |
| Number of children | – | – | – | < 0.01 | – | – |
| Up to two children | 12/29 (41.4%) | 434/632 (68.7%) | 1 | – | – | |
| Three children or more | 17/29 (58.6%) | 198/632 (31.3%) | 0.3 (0.1–0.7) | – | – | |
OR = odds ratio.
DISCUSSION
The alarming increase in the number of syphilis cases in Brazil has become a public health problem, especially among pregnant women, owing to the serious health complications resulting from the transmission of infection to the fetus. In this study, we determined the prevalence of syphilis infection in a population of pregnant women. According to our results, syphilis infection among pregnant women is higher in Brazil than in some African countries, such as Tanzania (2.3%), Ethiopia (2.9%), and Ghana (2.8%).19–21 Over the past 10 years (2005–2015), the detection rate of syphilis among pregnant women in Brazil has markedly increased.22 This is probably due to the increased coverage of diagnostic testing and regular follow-up of pregnant women.18 Despite an increase in access to antenatal care in Brazil, challenges remain to improving the quality of these services.23
In this study, 47.6% (n = 10/21) of pregnant women were diagnosed with syphilis infection in the first trimester. However, treatment was received in the third trimester by 28.6% (n = 6/21) of women. Only 9.5% (n = 2/21) successfully received treatment 30 days before delivery and had their partners treated concomitantly. A delay in initiating treatment may increase the exposure risk of the fetus to T. pallidum, and the duration of fetal exposure is a critical factor in mother-to-child transmission of infection.24 Thus, the time of treatment initiation is important during pregnancy, and perinatal mortality is reduced by 70% when treatment is provided before the 21st week of gestation.25,26 Increased fetal exposure time to T. pallidum may have been responsible for adverse outcomes in 28% (n = 7/25) in newborns. This result indicates that the antenatal care available at the facilities in the study area is inadequate and does not sufficiently contribute to the control and prevention of adverse outcomes caused by syphilis among pregnant women. Furthermore, 55.9% (n = 323/649) of our participants did not have a record of a rapid screening test for syphilis. In addition, 28.6% (n = 6/21) of women were diagnosed with syphilis infection during antennal care and had no record of treatment. Thus, it is impossible to know whether screening and treatment for syphilis were provided to these patients. This reflects a flaw in the follow-up of pregnant women in preventing syphilis-related problems, which can affect the health of both mother and child. Furthermore, poor record keeping can compromise the organization and planning of antenatal services and quality of care.27,28
According to our data, illicit drug use during pregnancy resulted in a staggering 13-fold increased risk of developing syphilis. Our study showed that 86.2% (n = 25/29) of pregnant women with syphilis reported irregular use of condoms. The strong association between syphilis and illicit drug use may be related to not using condoms. High prevalence of STIs has been reported among drug users.29,30 Nonadherence to safe sexual practices is the main cause of the high prevalence of STIs in this population.30,31 Thus, syphilis infection may be an indicator of continued engagement in behaviors associated with the acquisition and transmission of HIV and other STIs. Comprehensive prevention services of syphilis, including counseling for risk reduction, increased access to condoms, and early treatment for syphilis especially in the first trimester of pregnancy, can help reduce the incidence rate of syphilis and other STIs among pregnant women.
Abortion history was associated with syphilis among the included pregnant women (OR: 3.7, 95% CI: 1.7–8.0). Complications such as abortion were observed in women who received antenatal care but who were not tested or treated for syphilis.7 In addition, early fetal loss as a result of no or inadequate treatment occurs in 14.9% of pregnant women with syphilis.8 Our study showed that 96.5% (n = 28/29) of pregnant women with syphilis received antenatal care; however, only 9.5% (n = 2/21) of those with syphilis were provided appropriate treatment. Thus, the association between abortion history (21%) and syphilis in pregnant women may be a consequence of lacking or inadequate treatment.
Although the results of this cross-sectional study could not detect causality of the transmission source, our findings demonstrate a strong correlation between syphilis and pregnancy, contributing further evidence for increasing efforts to improve the health-care needs of this vulnerable group in Brazil. In our study, incomplete antenatal cards may have led to error bias. It is also possible that some women received diagnostic and treatment services, despite reporting otherwise; however, as required by the Ministry of Health, all care services must be recorded on the antenatal card by health professionals.27 Despite these limitations, in this study, we identified a high prevalence of T. pallidum infection among pregnant women in the study area, highlighting its association with sociodemographic, behavioral, and institutional variables.
Our results show that antenatal coverage in Brazil is insufficient to ensure the control of syphilis. Failure in the delivery of effective antenatal care services occurs owing to delayed treatment and poor information recording on the antenatal card of pregnant women with syphilis. There are challenges that must be overcome to implementing the guidelines of the Ministry of Health and WHO such that health-care systems can provide appropriate treatment to all patients. In addition, syphilis treatment should be prioritized by health managers and professionals, and more effective prevention and control measures should be available to patients. Further studies are needed to identify potential obstacles faced by public service workers who are inefficiently managing and controlling syphilis among pregnant women.
Supplementary Material
Acknowledgments:
We are grateful to the University Hospital of Dourados/MS for their support and to the women participants, without whom this study could not have been performed. We also appreciate the staff of the GPBMM/UFGD study group for their support.
Note: Supplemental tables appear at www.ajtmh.org.
REFERENCES
- 1.World Health Organization , 2007. The Global Elimination of Congenital syphilis: Rationale and Strategy for Action. Geneva, Switzerland: WHO. [Google Scholar]
- 2.Avelleira JCR, Bottino G, 2006. Sífilis: diagnóstico, tratamento e controle. An Bras Dermatol 81: 111–126. [Google Scholar]
- 3.World Health Organization, Dept. of Reproductive Health and Research , 2003. Guidelines for the Management of Sexually Transmitted Infections. Geneva, Switzerland: WHO. [Google Scholar]
- 4.World Health Organization, Centers for Disease Control and Prevention , 2012. Investment Case for Eliminating mother-to-Child transmission of syphilis: Promoting Better Maternal and Child Health and Stronger Health Systems. Geneva, Switzerland: WHO. [Google Scholar]
- 5.World Health Organization , 2014. Global Guidance on Criteria and Processes for Validation: Elimination of Mother-to-Child Transmission (EMTCT) of HIV and Syphilis. Geneva, Switzerland: WHO. [Google Scholar]
- 6.Wolff T, Shelton E, Sessions C, Miller T, 2009. Screening for syphilis infection in pregnant women: evidence for the U.S. preventive services task force reaffirmation recommendation statement. Ann Intern Med 150: 710–716. [DOI] [PubMed] [Google Scholar]
- 7.Newman L, Kamb M, Hawkes S, Gomez G, Say L, Seuc A, Broutet N, 2013. Global estimates of syphilis in pregnancy and associated adverse outcomes: analysis of multinational antenatal surveillance data. PLoS Med 10: e1001396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qin J, Yang T, Xiao S, Tan H, Feng T, Fu H, 2014. Reported estimates of adverse pregnancy outcomes among women with and without syphilis: a systematic review and meta-analysis. PLoS One 9: e102203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Milanez H, 2016. Syphilis in pregnancy and congenital syphilis: why can we not yet face this problem? Rev Bras Ginecol Obstet 38: 425–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.González MA, 2010. Regional initiative for the Elimination of Mother-to-Child Transmission of HIV and Congenital Syphilis in Latin America and the Caribbean: Regional Monitoring Strategy. Washington, DC: Pan American Health Organization. [Google Scholar]
- 11.Ministério da Saúde , 2014. Estratégias para eliminação da Transmissão Vertical do HIV e da Sífilis. Brazil. [Google Scholar]
- 12.Ministério da Saúde , 2016. Boletim Epidemiológico de Sífilis. Saúde Sdve, ed. Brasília, Brasil: Ministério da Saúde. [Google Scholar]
- 13.Lafetá KRG, Martelli Júnior H, Paranaíba LMR, Silveira MF, 2016. Maternal and congenital syphilis, underreported and difficult to control. Rev Bras Epidemiol 19: 63–74. [DOI] [PubMed] [Google Scholar]
- 14.Ministério da Saúde , 2015. DATASUS. Brazil. [Google Scholar]
- 15.Szwarcwald CL, Junior AB, Miranda AE, Paz LC, 2007. Resultados do estudo sentinela-parturiente, 2006: desafios para o controle da sífilis congênita no Brazil. DST J Bras Doenças Sex Transm 19: 128–133. [Google Scholar]
- 16.Ministério da Saúde , 2016. Diretrizes Para Controle da Sífilis Congênita: Manual de olso. Saúde Md, ed. Brasília, Brazil: Secretaria de Vigilância em Saúde, 72. [Google Scholar]
- 17.Kennedy EJ, Creighton ET, 1998. Venereal Disease Research Laboratory (VDRL) Slide Test. Atlanta, GA: CDC, ed. Manual of Syphilis Tests. [Google Scholar]
- 18.Ministério da Saúde , 2015. Boletim Epidemiológico de Sífilis. Brazil: Ministério da Saúde 4. [Google Scholar]
- 19.Lawi JD, Mirambo MM, Magoma M, Mushi MF, Jaka HM, Gumodoka B, Mshana SE, 2015. Sero-conversion rate of Syphilis and HIV among pregnant women attending antenatal clinic in Tanzania: a need for re-screening at delivery. BMC Pregnancy Childbirth 15: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Endris M, Deressa T, Belyhun Y, Moges F, 2015. Seroprevalence of syphilis and human immunodeficiency virus infections among pregnant women who attend the University of Gondar teaching hospital, northwest Ethiopia: a cross sectional study. BMC Infect Dis 15: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Volker F, Cooper P, Bader O, Uy A, Zimmermann O, Lugert R, Gross U, 2017. Prevalence of pregnancy-relevant infections in a rural setting of Ghana. BMC Pregnancy Childbirth 17: 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ministério da Saúde , 2017. Indicadores e Dados Básicos da Sífilis nos Municípios Brasileiros. Departamento de DST, AIDS e Hepatites Virais, Secretaria de Vigilância em Saúde, Brazil. [Google Scholar]
- 23.Viellas EF, Domingues RM, Dias MA, Gama SG, Theme Filha MM, Costa JV, Bastos MH, Leal Mdo C, 2014. Prenatal care in Brazil. Cad Saude Publica 30 (Suppl 1): S1–S15. [DOI] [PubMed] [Google Scholar]
- 24.Berman SM, 2004. Maternal syphilis: pathophysiology and treatment. Bull World Health Organ 82: 433–438. [PMC free article] [PubMed] [Google Scholar]
- 25.Lago EG, 2016. Current perspectives on prevention of mother-to-child transmission of syphilis. Cureus 8: e525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gust DA, Levine WC, St Louis ME, Braxton J, Berman SM, 2002. Mortality associated with congenital syphilis in the United States, 1992–1998. Pediatrics 109: E79–9. [DOI] [PubMed] [Google Scholar]
- 27.Ministério da Saúde , 1998. Assistência Pré-Natal: Normas e Manuais Técnicos. Brasília, Brazil: Ministério da Saúde, 62. [Google Scholar]
- 28.Santos Neto ET, Oliveira AE, Zandonade E, Gama SG, Leal Mdo C, 2012. Prenatal patient cards and quality of prenatal care in public health services in Greater Metropolitan Vitoria, Espirito Santo State, Brazil [article in Portuguese]. Cad Saude Publica 28: 1650–1662. [DOI] [PubMed] [Google Scholar]
- 29.Hwang LY, Ross MW, Zack C, Bull L, Rickman K, Holleman M, 2000. Prevalence of sexually transmitted infections and associated risk factors among populations of drug abusers. Clin Infect Dis 31: 920–926. [DOI] [PubMed] [Google Scholar]
- 30.Khan MR, Berger A, Hemberg J, O'Neill A, Dyer TP, Smyrk K, 2013. Non-injection and injection drug use and STI/HIV risk in the United States: the degree to which sexual risk behaviors versus sex with an STI-infected partner account for infection transmission among drug users. AIDS Behav 17: 1185–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DeHovitz JA, Kelly P, Feldman J, Sierra MF, Clarke L, Bromberg J, Wan JY, Vermund SH, Landesman S, 1994. Sexually transmitted diseases, sexual behavior, and cocaine use in inner-city women. Am J Epidemiol 140: 1125–1134. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.