Abstract.
Schistosomiasis afflicts an estimated 10 million pregnant women in Africa annually. With mounting evidence of adverse impacts to reproductive health resulting from urogenital schistosomiasis, including increased transmission of HIV, further research on prenatal disease epidemiology is warranted, with implications for maternal and fetal health. Between October 2016 and March 2017, we conducted a cross-sectional study examining the prevalence of urogenital schistosomiasis and its association with sexually transmitted infections (STIs) other than HIV among pregnant women visiting antenatal clinics in Kisantu health zone, Democratic Republic of Congo. An extensive sociodemographic and clinical survey was administered to consenting participants, with urine samples and vaginal swabs collected to deduce active schistosomiasis and STIs, respectively. In total, 17.4% of expectant mothers were infected with Schistosoma haematobium, 3.1% with Chlamydia trachomatis (CT), 1.4% with Neisseria gonorrhoeae (NG), and 14.6% with Trichomonas vaginalis (TV). Women infected with urogenital schistosomiasis were at significantly increased odds of harboring a CT, NG, or TV infection (adjusted odds ratio = 3.0, 95% CI: 1.5, 6.0), but reports of clinical symptoms were low, ranging from 17.2% of schistosomiasis to 30.8% of TV cases. Laboratory confirmation of schistosomiasis and STIs provided objective evidence of disease in a cohort with low symptomology where syndromic management may not suffice. Shedding light on local risk factors and associated coinfections of urogenital schistosomiasis can identify unique intervention opportunities for prenatal care in trematode-endemic regions and aid in reducing adverse pregnancy outcomes.
INTRODUCTION
The Democratic Republic of the Congo (DRC) is estimated to hold the third highest schistosomiasis case rate in Africa and remains endemic for three distinct species, including Schistosoma haematobium.1,2 Commonly resulting in morbidities of the kidneys, bladder, and genital tract—collectively designated as urogenital schistosomiasis—S. haematobium infection may also lead to female genital schistosomiasis (FGS), whereby schistosome ova deposited in the genital tract cause granulomatous inflammation and “sandy patch” lesions.3,4 New estimates suggest that FGS may occur in as many as 150 million African girls and women, defining this infection as one of the most common and emergent gynecologic conditions in sub-Saharan Africa.5
Emergence of FGS may be linked in part to its co-endemicity with HIV throughout much of the continent. Recent epidemiologic studies suggest that genital infection with S. haematobium may increase the risk of HIV acquisition and transmission, accelerate disease progression, and lower the effectiveness of antiretroviral therapy.6,7 Local pathology, including increased viral shedding at schistosomal lesions in the cervix or vagina, and systemic immunologic effects, including induction of a Th2 bias, may be at the root of this propagation.8–10 Although the prevalence of HIV in the DRC is relatively low (provincial rates ranging from 1.6% to 8% among screened pregnant women), the prevalence of other sexually transmitted infections (STIs) remains unknown and could potentially be mediated by urogenital schistosomiasis through similar modes of action, as we hypothesize here.11 Indeed, clinical evidence for such a relationship exists, as concordant chlamydia, gonorrhea, mycoplasma, and trichomoniasis have previously been recorded among both men and women harboring S. haematobium infections.12 However, more epidemiologic studies are required to support this link as a dearth of literature describes direct associations between schistosomiasis and non-HIV STIs.
Better understanding the role of S. haematobium infections and their potential modification of STI risk is especially warranted among pregnant women, 10 million of whom are estimated to harbor schistosomiasis each year in Africa alone.13 Morbidities resulting from maternal schistosomiasis infections, including both systemic effects such as anemia and localized inflammation of the urogenital tract, may serve as independent risk factors for adverse pregnancy outcomes.13 Pregnant mice with schistosomiasis showed evidence of increased abortion, maternal and infant death, as well as significantly decreased maternal lifespan and offspring birth weight compared with uninfected controls.14,15 Human studies of prenatal schistosomiasis also suggest serious reproductive health effects, including infertility, ectopic pregnancy, and abortion may result from FGS.3,4,16,17 In addition to deleterious pregnancy consequences, intrauterine exposure to schistosomiasis may cause immunomodulation in the developing fetus with implications for the child’s future susceptibility to a host of infectious diseases, including schistosomiasis.18–20 Finally, by increasing the risk of maternal HIV acquisition and potentially modifying the incidence of other STIs, urogenital schistosomiasis may further exert indirect assaults to healthy pregnancy. Chlamydia, gonorrhea, and trichomoniasis remain highly prevalent among antenatal care (ANC) clinic attendees throughout the developing world and have consistently been associated with preterm delivery and low birth weight in a range of prospective and retrospective studies. Stillbirth, premature membrane rupture, and intrauterine growth restriction have also been linked with maternal infection, and 30–50% of infants born to mothers with prenatal chlamydia or gonorrhea infections develop ophthalmia neonatorum.21,22
Unfortunately, screening for schistosomiasis and STIs is excluded from routine ANC in many resource-constrained settings, including DRC, where access to diagnostic testing remains costly and logistically unfeasible.22–24 Thus, the true burden of urogenital infection during pregnancy remains unknown, creating missed opportunities for proper management of diseases harmful to maternal and infant health. This pilot study provides an assessment of the prevalence of urogenital schistosomiasis, chlamydia, gonorrhea, and trichomoniasis in a group of pregnant women living in an S. haematobium–endemic district of DRC and describes the relationships between them—the first estimates of their kind in the country, to our knowledge. We also provide an assessment of the breadth and frequency of urogenital symptoms reported by this cohort, providing insights into the utility of syndromic diagnostic procedures during pregnancy, the present standard of care in many low- and middle-income countries (LMICs). Local risk factors for prenatal urogenital schistosomiasis are also identified, with implications for regional public health programming.
METHODS
Study site and population.
Between October 2016 and March 2017, pregnant women visiting one of three ANC clinics in Kisantu health zone, Kongo Central Province (formerly, Bas Congo), DRC, were recruited for participation in a prospective cohort study. The efforts described in this article pertain only to cross-sectional examination of baseline data, which were collected over the course of the recruitment period. Each clinic was chosen at random from a stratified list of urban, periurban, and rural ANC facilities in Kisantu provided by the health zone’s chief medical officer, and convenience samples were taken from each clinic site as women arrived for regular prenatal care. In total, 367 women were enrolled over the study baseline period from urban Kintanu Etat clinic (n = 223), periurban Ngeba clinic (n = 63), and rural Lemfu clinic (n = 81) (Figure 1).
Figure 1.
Study sites: Kintanu Etat, Ngeba, and Lemfu antenatal clinics, Kisantu health zone, Kongo Central Province, Democratic Republic of the Congo. Clinic GPS points were collected by study authors. Basemap,25 road and river layers,26 and Kisantu health zone boundary layer27 all were sourced from open access imagery data. The map was generated with ArcMap 10.6 software (Esri, Redlands, CA). This figure appears in color at www.ajtmh.org.
Eligible participants were 18 years of age or older and between 4 and 35 weeks pregnant at the time of enrollment. Those beyond 35 weeks of gestation were excluded from participation in the study to allow for a longitudinal assessment of schistosomiasis-induced birth outcomes in the cohort (described elsewhere). Because of high illiteracy rates in the DRC, women meeting the eligibility criteria were asked to provide oral informed consent, recorded via thumbprint. Ethical approval for the study, including use of oral consent practices, was provided by institutional review boards at both the University of California Los Angeles and the Kinshasa School of Public Health.
Study design and procedures.
Enrolled participants were asked to respond to a questionnaire and provide biological samples for infection assessment over the course of 3 days in the baseline period: at enrollment (questionnaire administration and initial urine and vaginal swab specimen collection) and for the two following days (second and third urine collection). All women took part in the survey and 362 (99%) provided both urine and vaginal swab samples; 353 Chlamydia trachomatis (CT)/Neisseria gonorrhoeae (NG) (98%) and 359 Trichomonas vaginalis (TV) (99%) swabs were available for final laboratory analysis following a few instances of misplaced or contaminated samples. Those testing positive for S. haematobium and/or any STI were notified during their clinic visit or by phone to share test results and provide chemotherapeutics. Survey administration, sample collection, and disease counseling were performed by local clinic staff trained in study procedures.
Questionnaires.
Participants were administered a questionnaire covering basic demographic data, water supply and sanitation, sexual practices, and medical history of schistosomiasis and STIs. Women were also asked about their current symptoms, specifically whether they were experiencing hematuria (bloody urine), dysuria (painful urination), dyspareunia (pain during vaginal intercourse), abnormal vaginal discharge, or a genital lesion at the time of clinic visit. An open-ended symptom question was also included. Questionnaires were completed in a private, one-on-one setting in patient rooms.
Biological specimen collection and laboratory testing.
Over three consecutive days, participants were asked to provide a urine sample to their health-care provider. Schistosoma haematobium exhibits diurnal variation with peak egg excretion around noon daily; as such, attempts were made to collect urine samples from each participant between the hours of 10 am and 3 pm.4 After thorough mixing of fresh urine samples, filtration, and staining with Lugol’s iodine, the specimens were examined by light microscopy at ×10 magnification, with results expressed as the number of S. haematobium eggs per 10 mL of urine. A senior laboratory technician re-examined 5% of the samples for quality control. Because of the inconsistent release of schistosome ova by adult worms into host urine (limiting the sensitivity of urine microscopy), a single positive urine sample was considered sufficient for schistosomiasis positive classification.28 A trained clinician also collected vaginal swab specimens from each participant at baseline to test for STIs of interest (Xpert® CT/NG and TV kits; Cepheid, Sunnyvale, CA). All vaginal swab specimens were analyzed for CT, NG, and TV via DNA assay, also known as nucleic acid amplification test (NAAT), using GeneXpert® machine (Cepheid). Results from HIV tests were also recorded via patient recall or medical records, although serology was not performed as part of this study.
Treatment and follow-up testing.
Women testing positive for S. haematobium were administered a single dose of 40 mg/kg oral praziquantel on site at their clinic visit.29 Women positive for CT, NG, or TV were provided treatment for themselves and their untested sexual partners in accordance with WHO guidelines,30–32 and were followed-up for tests of cure 4–8 weeks later.
Statistical analysis.
Binary variables for urogenital schistosomiasis, CT, NG, CT, or NG (CT/NG), TV, and general STI status (positive versus negative) were created for use in statistical analyses based on laboratory results of urine microscopy and vaginal swab NAAT. Descriptive statistics of study population demographics were calculated, and chi-square tests of proportions were conducted to describe differences between women infected and uninfected with urogenital schistosomiasis. Crude and multivariable logistic regression models were then performed to examine the association between various environmental exposures of interest and S. haematobium infection during pregnancy. Confounders included in the adjusted models were defined a priori via literature review: maternal age, education level, civil status, and clinic site.
Next, proportions of prevalent STI cases were tabulated according to maternal schistosomiasis status. Prevalence odds ratios (OR) were also calculated via logistic regression to describe the relative odds of positive NAAT for CT, NG, CT/NG, TV, or any STI among women infected or uninfected with S. haematobium. Adjusted models could not be run for CT and NG outcomes alone because of sparse data and were instead run according to CT/NG status. This designation is warranted, given the biological (bacterial) and clinical (infections often acute and self-limiting) properties shared between CT and NG which distinguish them from other STIs such as TV.33 In addition to maternal demographic confounders, adjusted regressions controlled for risky sexual behavior, defined as having a primary sex partner who is actively engaged with one or more additional sex partners. HIV-positive women (n = 2) were also excluded from all CT, NG, and TV analyses to control for confounding associated with HIV’s distinct mode of action and severe immunocompromising pathology. Finally, a histogram was created to describe the frequency of patient symptom reports among cases of each tested infection, and the sensitivity and specificity of syndromic testing in our cohort were calculated using laboratory-confirmed diagnostics as our gold standard (urine microscopy and NAAT). Complete case analysis was implemented for all statistical tests. SAS® software, version 9.4 (SAS Institute Inc., Cary, NC) was used to conduct all statistical analyses for this study.
RESULTS
General enrollment information.
In total, 367 women between 4 and 34 weeks of gestational age provided urine samples for S. haematobium diagnosis and completed a standardized questionnaire. A majority of study participants were either married or cohabitating with a steady partner (70.5%) and had experienced at least one previous pregnancy (68.9%); half were in their third trimester (Table 1).
Table 1.
Demographic characteristics of pregnant women in Kisantu health zone, Democratic Republic of the Congo, by Schistosoma haematobium infection status (October 2016–March 2017)
| S. haematobium status | P-value* | ||
|---|---|---|---|
| Positive (%) | Negative (%) | ||
| Age group (years) | |||
| 18–24 | 41 (22.2) | 144 (77.8) | 0.03 |
| 25–34 | 15 (10.9) | 123 (89.1) | |
| 35+ | 8 (18.6) | 35 (81.4) | |
| Education | |||
| Some primary or less | 13 (18.6) | 57 (81.4) | 0.12 |
| Completed primary | 37 (21.0) | 139 (79.0) | |
| Secondary and beyond | 14 (11.8) | 105 (88.2) | |
| Civil status | |||
| Married/cohabitating | 36 (14.0) | 222 (86.0) | < 0.01 |
| Not in a union | 28 (25.9) | 80 (74.1) | |
| Pregnancy history | |||
| Primigravid | 23 (21.3) | 85 (78.7) | 0.08 |
| Multigravid | 33 (13.8) | 206 (86.2) | |
| Current pregnancy† | |||
| First trimester | 9 (28.1) | 23 (71.9) | 0.20 |
| Second trimester | 27 (18.0) | 123 (82.0) | |
| Third trimester | 28 (15.1) | 157 (84.9) | |
| Clinic site | |||
| Kintanu Etat | 54 (24.2) | 169 (75.8) | < 0.01 |
| Ngeba | 3 (4.8) | 60 (95.2) | |
| Lemfu | 7 (8.6) | 74 (91.4) | |
| Total | 64 (17.4) | 303 (82.6) | – |
* P-values calculated using Wald chi-square tests.
† First trimester defined as 0–12 weeks, second trimester 13–27 weeks, and third trimester 28+ weeks.
Risk factors of S. haematobium infection.
Sixty-four women (17.4%) were infected with S. haematobium and displayed a mean infection intensity of 10.8 eggs/10 mL urine; heavy infection (> 50 eggs/10 mL urine) was seen in 6.3% of infected women. No difference in S. haematobium status was noted according to the participants’ trimester of pregnancy; however, age and civil status were significant predictors of infection among our study cohort, with the highest proportion of schistosomiasis cases occurring in 18–24 year olds. Health center location and enrollment period were also associated with disease; higher schistosomiasis prevalence was noted between February and March, coinciding with the end of the rainy season in Southwestern DRC. Women involved in farming were less likely to test positive for S. haematobium than women involved in other occupations (OR = 0.4, 95% CI: 0.2, 0.8), although this relationship was not significant after adjustment for age, education level, civil status, and ANC clinic location (Table 2). Differential water sourcing for general household use, laundry, and bathing did not appear to have a significant or distinguishable impact on schistosomiasis outcomes nor did reports of house flooding events in the year preceding the questionnaire. Women indicating prior schistosome infection appeared to be at increased but statistically insignificant odds of current urogenital schistosomiasis (adjusted odds ratio [aOR] = 2.5, 95% CI: 0.6, 10.7) (Table 2).
Table 2.
Environmental risk factors for Schistosoma haemoatobium infection among pregnant women in Kisantu health zone
| SCH positive | Crude | Adjusted* | ||||
|---|---|---|---|---|---|---|
| n | % | OR | 95% CI | OR | 95% CI | |
| Season/enrollment date† | ||||||
| October–December 2016 | 18 | 9.3 | 1.0 | – | 1.0 | – |
| February–March 2017 | 46 | 26.4 | 3.5 | (2.0, 6.4) | 4.6 | (2.4, 8.9) |
| Occupation | ||||||
| Other | 37 | 24.5 | 1.0 | – | 1.0 | – |
| Fishing | 3 | 15 | 0.5 | (0.2, 1.9) | 0.7 | (0.2, 2.5) |
| Farming | 24 | 12.3 | 0.4 | (0.2, 0.8) | 0.6 | (0.3, 1.1) |
| Main HH water source | ||||||
| Piped | 31 | 21.4 | 1.0 | – | 1.0 | – |
| In-ground (groundwater) | 13 | 25 | 1.2 | (0.6, 2.6) | 1.3 | (0.6, 2.9) |
| Well | 20 | 12.1 | 0.5 | (0.3, 0.9) | 1.3 | (0.5, 3.0) |
| Surface | 0 | – | n/a | (Excluded because of empty cell) | ||
| Laundry water source | ||||||
| Piped | 29 | 21.5 | 1.0 | – | 1.0 | – |
| In-ground (groundwater) | 14 | 20.3 | 0.9 | (0.5, 1.9) | 2.0 | (0.8, 4.6) |
| Well | 13 | 14.1 | 0.6 | (0.3, 1.2) | 1.3 | (0.5, 3.1) |
| Surface | 8 | 11.4 | 0.5 | (0.2, 1.1) | 0.6 | (0.3, 1.7) |
| Bathing water source | ||||||
| Piped | 28 | 20.1 | 1.0 | – | 1.0 | – |
| In-ground (groundwater) | 14 | 19.7 | 1.0 | (0.5, 2.0) | 1.9 | (0.8, 4.5) |
| Well | 13 | 14 | 0.6 | (0.3, 1.3) | 1.4 | (0.6, 3.6) |
| Surface | 9 | 14.3 | 0.7 | (0.3, 1.5) | 1.0 | (0.4, 2.5) |
| Primary HH water collection | ||||||
| Other family member | 7 | 12.3 | 1.0 | – | 1.0 | – |
| Interviewee | 57 | 18.4 | 1.6 | (0.7, 3.7) | 1.5 | (0.6, 3.6) |
| Proximity to main water source | ||||||
| Within 500 m | 47 | 19.3 | 1.0 | – | 1.0 | – |
| Farther than 500 m | 17 | 13.8 | 0.7 | (0.4, 1.2) | 1.3 | (0.6, 2.6) |
| House flooding events, past year | ||||||
| No | 54 | 16.8 | 1.0 | – | 1.0 | – |
| Yes | 10 | 21.7 | 1.4 | (0.6, 2.9) | 1.4 | (0.6, 3.2) |
| Prior SCH infection, past year | ||||||
| No | 60 | 17.2 | 1.0 | – | 1.0 | – |
| Yes | 3 | 21.4 | 1.3 | (0.4, 4.8) | 2.5 | (0.6, 10.7) |
HH = household; OR = odds ratio; SCH = schistosomiasis (Schistosoma haematobium).
* Adjusted for age, education level, civil status, and antenatal care clinic location.
† The rainy season runs from October to March in southwestern Democratic Republic of the Congo.
Sexually transmitted infection prevalence and comorbidities.
In sum, 65 women (18.1%) were found to be positive for at least one STI at the time of interview: 11 women (3.1%) with chlamydia, five (1.4%) with gonorrhea, and 52 (14.6%) with trichomoniasis. Thirty-two percent of STI-positive women had concordant S. haematobium infection; reciprocally, 33.3% of schistosomiasis-positive women presented with STI. Women with a schistosomiasis infection were at substantially increased odds of NG and TV coinfection. After adjusting for age, educational attainment, civil status, and risky sexual behavior, those presenting with any STI had three times the odds of concurrent schistosomiasis (aOR = 3.0, 95% CI: 1.5, 6.0) (Table 3).
Table 3.
Increased odds of STIs among pregnant women with urogenital schistosomiasis
| No. Tested | SCH + (%) | SCH − (%) | OR (95% CI) | aOR (95% CI) | |
|---|---|---|---|---|---|
| CT | 351 | 2/62 (3.2) | 9/289 (3.1) | 1.0 (0.2, 4.9) | – |
| NG | 351 | 4/62 (6.5) | 1/289 (0.3) | 19.9 (2.2, 180.9) | – |
| CT/NG | 351 | 6/ 62 (9.7) | 10/289 (3.5) | 3.0 (1.0, 8.6) | 2.7 (0.9, 8.3) |
| TV | 357 | 15/63 (23.8) | 37/294 (12.6) | 2.2 (1.1, 4.3) | 2.3 (1.1, 4.8) |
| Any STI | 359 | 21/63 (33.3) | 44/296 (14.9) | 2.9 (1.6, 5.3) | 3.0 (1.5, 6.0) |
aOR = adjusted odds ratio; CT = chlamydia (Chlamydia trachomatis); NG = gonorrhea (Neisseria gonorrhoeae); OR = odds ratio; SCH = schistosomiasis (Schistosoma haematobium); TV = trichomoniasis (Trichomonas vaginalis); STI = sexually transmitted infection. Schistosomiasis (Schistosoma haematobium) tested via urine microscopy, STI via nucleic acid amplification testing (NAAT, GeneXpert). All but five women (362/367 = 99%) underwent two vaginal swabs for STI determination. An additional nine CT/NG and three TV swabs were misplaced or contaminated before laboratory analysis, and two HIV+ women were excluded from all STI analyses (see Methods). Only one woman had missing laboratory results for both of her completed swabs. Adjusted odds ratios not calculated individually for CT or NG infections because of sparse data. Adjusted odds ratios adjusted for participant age, educational attainment, civil status, and sharing of primary sexual partner with other concurrent partners.
Syndromic reporting.
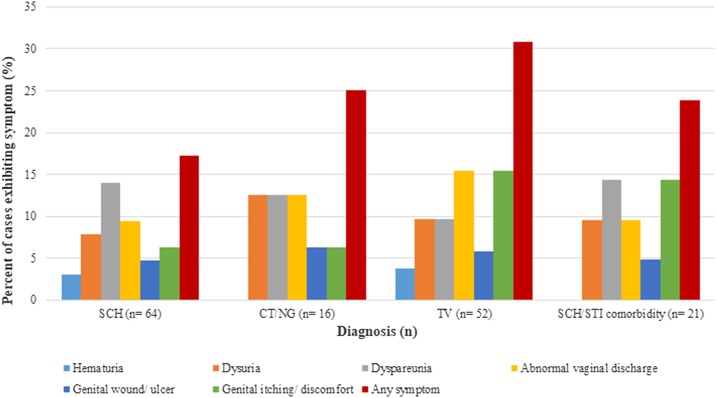
Overall, the presence of reported symptoms was low among the study participants diagnosed with any urogenital disease. Only 17.2% of S. haematobium cases, 25.0% of CT/NG cases, and 30.8% of TV cases reported any symptom at interview; of the 21 women harboring a schistosomiasis/STI coinfection, less than one-quarter (23.8%) displayed clinical signs of infection. Gross hematuria was the least common symptom across all infection types, accounting for less than 4% of schistosomiasis- and TV-positive women; no incidents of bloody urine were disclosed by coinfected patients. Nine schistosomiasis cases presented with dyspareunia (14.1%), and fewer than 10% reported dysuria, abnormal vaginal discharge, or genital lesions or discomfort—common clinical indicators of urogenital infection. Of the 16 participants with CT/NG infection, only two (12.5%) reported dysuria, dyspareunia, or abnormal discharge; among 52 TV cases, the most commonly reported symptoms were of abnormal discharge and genital itching or discomfort (15.4%). Finally, of those harboring a schistosomal coinfection, 14.3% noted dyspareunia or genital itching, 9.5% painful urination or abnormal discharge, and 4.8% genital lesions (Figure 2).
Figure 2.
Symptomology of disease outcomes, by case percentage. CT = chlamydia (Chlamydia trachomatis); NG = gonorrhea (Neisseria gonorrhoeae); SCH = schistosomiasis (Schistosoma haematobium); STI = sexually transmitted infection; TV = trichomoniasis (Trichomonas vaginalis). This figure appears in color at www.ajtmh.org.
When compared with laboratory confirmation methods, syndromic diagnostics showed suboptimal specificity and extremely low sensitivity for all disease types. Pregnant women who were NAAT-positive for CT/NG infection were three times as likely to be categorized as false negatives than to be correctly identified as infected on the basis of reported symptoms alone (sensitivity = 25.0%). Similarly, the sensitivity of symptom-based diagnoses for TV and S. haematobium fell below the probability of correct diagnosis by chance (< 50%) when compared with a gold standard NAAT or urine microscopy test, respectively (Table 4).
Table 4.
Sensitivity and specificity of syndromic diagnoses for STIs and urogenital schistosomiasis
| CT/NG | TV | S. haematobium | |||||||
|---|---|---|---|---|---|---|---|---|---|
| NAAT+ | NAAT− | Total | NAAT+ | NAAT− | Total | Urine+ | Urine− | Total | |
| Symptomatic | 4 | 75 | 79 | 16 | 65 | 81 | 11 | 72 | 83 |
| Asymptomatic | 12 | 260 | 272 | 36 | 240 | 276 | 53 | 231 | 284 |
| Total | 16 | 335 | 351 | 52 | 305 | 357 | 64 | 303 | 367 |
| Sensitivity | (4/16) = 25% | (16/52) = 31% | (11/64) = 17% | ||||||
| Specificity | (260/335) = 78% | (240/305) = 79% | (231/303) = 76% | ||||||
CT/NG = chlamydia or gonorrhea (Chlamydia trachomatis or Neisseria gonorrhoeae); NAAT = nucleic acid amplification test; STI = sexually transmitted infection; TV = trichomoniasis (Trichomonas vaginalis). For each infection of interest, syndromic recognition (defined as the presence of any urogenital symptom in patient reports) was compared against the appropriate gold standard laboratory test: NAAT for STIs and urine microscopy for schistosomiasis.
DISCUSSION
In this study, we estimate the prevalence of S. haematobium, the most commonly implicated causal agent in cases of urogenital schistosomiasis and FGS, and elucidate local risk factors of disease among a pregnant cohort in Kisantu health zone, DRC. We also present previously unreported estimates of CT, NG, and TV prevalence for this area—STIs omitted from routine ANC screenings nationwide and throughout much of the developing world. Our results demonstrate a high prevalence of both STIs and urogenital schistosomiasis, as well as a strong association between the two in this population. Specifically, NG and TV were significantly associated with concurrent S. haematobium infection, supporting our hypothesis that S. haematobium infection likely increases host susceptibility to progressive infection of the female genital tract. This research represents, to the best of our knowledge, only the second study to describe the relationship between laboratory-confirmed urogenital schistosomiasis and sexually transmitted bacterial and protozoan agents,12 as well as the first study to show this phenomenon during the prenatal period. Other reports of S. haematobium and STIs coexisting within the same population have also been undertaken,34,35 however, they do not provide estimates of association between these diseases, as presented here.
Our results reiterate the importance of studying both schistosomiasis and STIs in vulnerable and often overlooked adult populations of trematode-endemic residence and speak to the importance of incorporating additional screening and treatment activities into standard ANC. We find that in a cross-sectional snapshot of the Kisantu health zone, almost one-fifth of participating pregnant women carry active S. haematobium infection. Notably, the Congo Basin is home to two additional trematodes, Schistosoma mansoni and Schistosoma intercalatum, that commonly manifest in humans as intestinal schistosomiasis and likely contribute to a higher total schistosome burden among pregnant Congolese women than is captured here.2,36 The infected women in our study range in gestational age from 6 to 32 weeks, signaling schistosomiasis-induced threats to healthy pregnancy at every stage of in utero development. The lack of association found between urogenital schistosomiasis and trimester of pregnancy further reinforces this point and raises concerns about impacts to maternal physiology during sensitive periods of embryonic and fetal development.
In concert with previous literature on schistosomiasis risk, younger women were more likely to be infected with S. haematobium in this population.12,37 Environmental determinants of schistosomiasis also appeared to play a role, with infection prevalence spiking at the end of the rainy season (26% in February–March compared with 9% in October–December), suggesting disease seasonality. A statistically significant difference in the distribution of schistosomiasis among women visiting different ANC clinics across Kisantu, likely according to location of residence, also indicates potential geographic disparities in environmental trematode reservoirs around the health zone. Unexpectedly, women listing high-risk water contact occupations such as fishing and farming were found to have a lower burden of S. haematobium infection than women holding other positions. This may be a result of low statistical power or could indicate that frequent water contact associated with domestic chores, recreation, and personal hygiene is a more important driver of schistosomiasis than occupational freshwater exposure in this population. Alternatively, myriad jobs with low water contact frequency or duration may be unintentionally captured in our survey as farming,38 thereby diluting or masking the observed effect of agricultural work with high water contact on infection status. Regardless, our results underline the importance of studying local risk factors of disease uniquely tailored to specific populations and geographies, which may be vital to the implementation of effective public health interventions.
Beyond identifying local determinants of schistosomiasis, we found moderate prevalence of poorly characterized STIs among pregnant women of Kisantu and show that these diseases are significantly associated with S. haematobium infection. Indeed, the odds of harboring CT, NG, or TV infection were three times higher among those with microscopically identifiable schistosomiasis in urine. The prevalence of STI in the subgroup of pregnant women with urogenital schistosomiasis (33%) was also consistent with that reported by Leutscher and others12 who found concordant STI in 35% of nonpregnant Madagascan women aged 15–49 years with S. haematobium infection. With evidence of increased coinfection of pregnant women established for all three STIs of interest, these results hold important implications for maternal, fetal, and infant health.
In many low-resource settings, diagnosis of STIs primarily occurs through the syndromic approach, whereby assessment of the clinically symptomatic patient is used to detect markers of disease as a proxy for diagnostic testing.23,39 A lack of user-friendly, affordable diagnostic technologies and limited availability of trained laboratory personnel have led to continued reliance on this approach in DRC, where current national protocol does not include genital CT, NG, or TV testing as a part of routine ANC.24,40 Unfortunately, schistosomiasis, CT, and NG frequently manifest asymptomatically in women.3,40,41 Even when urogenital symptoms do appear, many are shared by both STIs and FGS,34 and some commonly arise during normal pregnancy; this further hinders clinical distinction between infection types and highlights the importance of laboratory diagnosis during gestation.41,42 Myriad cases of urogenital schistosomiasis and STIs thus go undetected or differentially diagnosed, masking the true burden of disease in endemic regions, hindering treatment programs, and perpetuating an unmitigated risk to pregnant women and their children.
Beyond issues of mischaracterization, low recognition of symptoms may limit women’s ability to manage urogenital infections. Notably, almost no schistosome-positive women in our study had bloody or painful urine at the time of positive test, classic indicators of urogenital infection which are frequently substituted for egg-based testing in resource-scarce environments.43 Indeed, if we use common symptom-based measures of diagnosis for urogenital schistosomiasis and STIs alone, this study suggests that we are liable to miss nearly 70% of cases in similar groups. The few symptoms reported in our cohort align well with other investigations of self-reported and clinically verified S. haematobium and STI symptomology in regions of endemic schistosomiasis, which describe low or insignificant rates of abnormal vaginal discharge, lower abdominal pain, dyspareunia, and contact bleeding in women with urogenital infection.17,34,35 Taken together, our results indicate that urogenital schistosomiasis and STIs are likely severely underdiagnosed where syndromic testing is relied upon exclusively, especially in trematode-endemic settings where coinfection may exacerbate STI incidence. Automated laboratory testing, including the use of NAAT systems such as the GeneXpert, offers solutions for improved STI confirmation and control in resource-limited settings. Such diagnostic tools should be considered for broader use in LMICs following cost-effective analyses and assessment of other contextual considerations.
Limitations.
Several limitations of this study should be noted. First, the sample size of our cohort (N = 367) may have been insufficient for capturing rare risk factors of schistosomiasis or STI infection. Second, urinary S. haematobium egg excretion was used as a proxy indicator of general urogenital schistosomiasis. Although most FGS cases are caused by S. haematobium,3 they may result from other schistosome species and can only be officially pathologically diagnosed—often with invasive biopsy or other logistically prohibitive tissue extractions, which were beyond the capabilities of this study. Despite this, urinary excretion of schistosome ova has been significantly associated with genital ova deposition,34 and up to 75% of women displaying urinary S. haematobium ova also exhibit genital egg deposition,44 thereby presenting urine microscopy as a suitable tool for identification and classification of urogenital schistosomiasis for the purposes of this study.
Although urine microscopy remains a standard of urogenital schistosomiasis diagnosis exhibiting high specificity, sensitivity may be hindered by egg excretion misaligning with urine sample collection; thus, we attempted to collect urine samples during peak excretion at midday.4,28 Furthermore, a 5–7 week prepatent period separates actual infection with S. haematobium cercariae and diagnosable manifestation of disease, hindering test sensitivity during early infection.37 Finally, women with schistosomiasis were recently shown to be less likely than men to excrete eggs during active infection.45 Hence, it is likely that nondifferential misclassification (under-detection) of schistosomiasis occurred in our cohort, downwardly biasing estimates of disease prevalence. Selection bias is also of potential concern because women with symptomatic schistosomiasis or STI may be more likely to visit antenatal clinics than uninfected or asymptomatically infected women. However, this is unlikely as the women in our study attended regular prenatal check-ups, and most were symptom-free, suggesting that physical discomfort was not an important driver of care-seeking behaviors.
Finally, we are limited by the cross-sectional nature of this study in determining the temporality of infection among women harboring a schistosomiasis/STI coinfection. Based on the trematode lifecycle, a biological mechanism by which having an STI could increase one’s risk of water contact remains improbable; however, we make no claims about the causality of the associations noted here. Further research to characterize the incidence of urogenital schistosomiasis and STIs in adult and pregnant women—including an assessment of the longitudinal progression of coinfection—is warranted. Investigations performed in larger and more diverse cohorts will help define the generalizability of our findings to pregnant populations in greater DRC and other S. haematobium–endemic settings. Nonetheless, this study is novel in its examination of a panel of urogenital coinfections among pregnant women and lays the groundwork for further work on this topic.
CONCLUSION
In this study, we expand on the very limited evidence of association between urogenital schistosomiasis and bacterial and protozoan STIs, specifically chlamydia, gonorrhea, and trichomoniasis. We demonstrate that these infections are highly correlated in a cohort of pregnant women living in DRC and occur across the entire gestational term. We also show that syndromic diagnoses of schistosomiasis and STIs may be insufficient to recognize or correctly identify most cases of infection during pregnancy. Symptoms of urogenital schistosomiasis may differ substantially for pregnant adults compared with school-aged children, on whom many clinical diagnostic tools for schistosomiasis are designed and implemented.12 We recommend adjusting schistosomiasis management strategies to better account for prevention and control of asymptomatic infection—whether via expansion of mass drug administration campaigns to the communal level, or by targeted praziquantel distribution to patients visiting ANC clinics in the prenatal period. We also argue for the importance of diagnosis and treatment of STIs as a part of routine adult care, especially in pregnant women vulnerable to deleterious downstream effects to mother and fetus.45,46 Investment in expanded prenatal care that includes urogenital schistosomiasis and broad-spectrum STI screening will be essential to curbing the substantive communal and health systems costs caused by these infections during pregnancy.
Acknowledgments:
We would like to thank Cepheid for donating critical diagnostic testing materials for this study, including the GeneXpert NAAT machine and STI test kits. We would also like to recognize the support of Risa Hoffman and Janell Moore at UCLA in developing STI survey questions, Cyrus Sinai at UCLA for mapping our study area, and Maxim Masisa of the FELTP program for his contributions to data collection. Finally, we would like to acknowledge the Kisantu ANC clinic staff members for their immeasurable assistance in survey administration and specimen collection, without which this study would not have been possible.
REFERENCES
- 1.Hotez PJ, Kamath A, 2009. Neglected tropical diseases in sub-Saharan Africa: review of their prevalence, distribution, and disease burden. Plos Negl Trop Dis 3: e412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hürlimann E, et al. 2011. Towards an open-access global database for mapping, control and surveillance of neglected tropical diseases. Plos Negl Trop Dis 5: e1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization , 2015. Female Genital Schistosomiasis: A Pocket Atlas for Clinical Health-Care Professionals. Geneva, Switzerland: WHO, 49. [Google Scholar]
- 4.Gray DJ, Ross AG, Li YS, McManus DP, 2011. Diagnosis and management of schistosomiasis. BMJ 342: d2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hotez PJ, 2013. Female genital schistosomiasis (FGS): sub-Saharan Africa’s secret scourge of girls and women. PLoS Speaking of Medicine. Available at: https://blogs.plos.org/speakingofmedicine/2013/05/06/female-genital-schistosomiasis-fgs-sub-saharan-africas-secret-scourge-of-girls-and-women/. Accessed December 5, 2018. [Google Scholar]
- 6.Walson JL, Herrin BR, John-Stewart G, 2009. Deworming helminth co-infected individuals for delaying HIV disease progression. Cochrane Database Syst Rev 3: Cd006419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gallagher M, Malhotra I, Mungai PL, Wamachi AN, Kioko JM, Ouma JH, Muchiri E, King CL, 2005. The effects of maternal helminth and malaria infections on mother-to-child HIV transmission. AIDS 19: 1849–1855. [DOI] [PubMed] [Google Scholar]
- 8.Kjetland EF, Ndhlovu PD, Gomo E, Mduluza T, Midzi N, Gwanzura L, Mason PR, Sandvik L, Friis H, Gundersen SG, 2006. Association between genital schistosomiasis and HIV in rural Zimbabwean women. AIDS 20: 593–600. [DOI] [PubMed] [Google Scholar]
- 9.Poggensee G, Kiwelu I, Weger V, Göppner D, Diedrich T, Krantz I, Feldmeier H, 2000. Female genital schistosomiasis of the lower genital tract: prevalence and disease-associated morbidity in northern Tanzania. J Infect Dis 181: 1210–1213. [DOI] [PubMed] [Google Scholar]
- 10.Salgame P, Yap GS, Gause WC, 2013. Effect of helminth-induced immunity on infections with microbial pathogens. Nat Immunol 14: 1118–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ministère du Plan et Suivi de la Mise en œuvre de la Révolution de la Modernité–MPSMRM/Congo, Ministère de la Santé Publique–MSP/Congo, and ICF International , 2014. République Démocratique du Congo Enquête Démographique et de Santé (EDS-RDC) 2013–2014. Rockville, MD: MPSMRM, MSP, and ICF International. [Google Scholar]
- 12.Leutscher PDC, Ramarokoto C-E, Hoffmann S, Jensen JS, Ramaniraka V, Randrianasolo B, Raharisolo C, Migliani R, Christensen N, 2008. Coexistence of urogenital schistosomiasis and sexually transmitted infection in women and men living in an area where Schistosoma haematobium is endemic. Clin Infect Dis 47: 775–782. [DOI] [PubMed] [Google Scholar]
- 13.Friedman JF, Mital P, Kanzaria HK, Olds GR, Kurtis JD, 2007. Schistosomiasis and pregnancy. Trends Parasitol 23: 159–164. [DOI] [PubMed] [Google Scholar]
- 14.el-Nahal HM, Kaddah MA, Hassan SI, Abdel Ghany A, Ibrahim AM, Ramzy RM, Mostafa EA, 1998. Effect of Schistosoma mansoni infection on offsprings born from infected mothers. J Egypt Soc Parasitol 28: 523–538. [PubMed] [Google Scholar]
- 15.Amano T, Freeman GL, Jr., Colley DG, 1990. Reduced reproductive efficiency in mice with schistosomiasis mansoni and in uninfected pregnant mice injected with antibodies against Schistosoma mansoni soluble egg antigens. Am J Trop Med Hyg 43: 180–185. [DOI] [PubMed] [Google Scholar]
- 16.Christinet V, Lazdins-Helds JK, Stothard JR, Reinhard-Rupp J, 2016. Female genital schistosomiasis (FGS): from case reports to a call for concerted action against this neglected gynaecological disease. Int J Parasitol 46: 395–404. [DOI] [PubMed] [Google Scholar]
- 17.Randrianasolo BS, et al. 2015. Gynecological manifestations, histopathological findings, and schistosoma-specific polymerase chain reaction results among women with Schistosoma haematobium infection: a cross-sectional study in Madagascar. J Infect Dis 212: 275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nash TE, Cheever AW, Ottesen EA, Cook JA, 1982. Schistosome infections in humans: perspectives and recent findings. NIH conference. Ann Intern Med 97: 740–754. [DOI] [PubMed] [Google Scholar]
- 19.Malhotra I, Mungai P, Wamachi A, Kioko J, Ouma JH, Kazura JW, King CL, 1999. Helminth- and Bacillus Calmette-Guerin-induced immunity in children sensitized in utero to filariasis and schistosomiasis. J Immunol 162: 6843–6848. [PubMed] [Google Scholar]
- 20.Ndibazza J, et al. 2012. Impact of anthelminthic treatment in pregnancy and childhood on immunisations, infections and eczema in childhood: a randomised controlled trial. PLoS One 7: e50325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sutton MY, Sternberg M, Nsuami M, Behets F, Nelson AM, St Louis ME, 1999. Trichomoniasis in pregnant human immunodeficiency virus-infected and human immunodeficiency virus-uninfected congolese women: prevalence, risk factors, and association with low birth weight. Am J Obstet Gynecol 181: 656–662. [DOI] [PubMed] [Google Scholar]
- 22.Mullick S, Watson-Jones D, Beksinska M, Mabey D, 2005. Sexually transmitted infections in pregnancy: prevalence, impact on pregnancy outcomes, and approach to treatment in developing countries. Sex Transm Infect 81: 294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dhana A, Luchters S, Moore L, Lafort Y, Roy A, Scorgie F, Chersich M, 2014. Systematic review of facility-based sexual and reproductive health services for female sex workers in Africa. Glob Health 10: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mayaud P, Mabey D, 2004. Approaches to the control of sexually transmitted infections in developing countries: old problems and modern challenges. Sex Transm Infect 80: 174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Esri , 2009. World Imagery Map [Basemap]. Available at: http://www.arcgis.com/home/item.html?id=10df2279f9684e4a9f6a7f08febac2a9. Accessed April 6, 2018. [Google Scholar]
- 26.World Resources Institute , 2013. Congo Basin Forest Atlases: DRC. Available at: http://www.wri.org/our-work/project/congo-basin-forests/democratic-republic-congo#project-tabs. Accessed April 6, 2018. [Google Scholar]
- 27.American Red Cross , 2019. DRC Health Data. Available at: https://data.humdata.org/dataset/drc-health-data. Accessed January 30, 2019. [Google Scholar]
- 28.Corcoran C, da Silva M, 2014. Diagnosing schistosomiasis: an update. PATHCHAT11. Available at: https://www.ampath.co.za/pdfs/ampathchats/pathchat-11-diagnosing-schistosomiasis-an-update.pdf. Accessed May 15, 2019. [Google Scholar]
- 29.World Health Organization Coordinated , 2006. Use of Anthelminthic Drugs in Control Interventions-A Manual for Health Professionals and Programme Managers. Geneva, Switzerland: WHO. [Google Scholar]
- 30.World Health Organization , 2016. WHO Guidelines for the Treatment of Chlamydia trachomatis. Geneva, Switzerland: WHO. [PubMed] [Google Scholar]
- 31.World Health Organization , 2016. WHO Guidelines for the Treatment of Neisseria Gonorrhoeae. Geneva, Switzerland: WHO. [PubMed] [Google Scholar]
- 32.Walker G, 2004. Interventions for Trichomoniasis in Pregnancy. Geneva, Switzerland: The WHO Reproductive Health Library. [Google Scholar]
- 33.van Liere GAFSV, Dukers-Muijrers NHTM, Wolffs PFG, Hoebe CJPA, 2013. P3. 186. Substantial natural clearance of genital and extragenital Chlamydia trachomatis and Neisseria gonorrhoeae in STD clinic attendees. Sex Transm Infect 89: A206. [Google Scholar]
- 34.Kjetland EF, et al. 2008. Female genital schistosomiasis—a differential diagnosis to sexually transmitted disease: genital itch and vaginal discharge as indicators of genital Schistosoma haematobium morbidity in a cross-sectional study in endemic rural Zimbabwe. Trop Med Int Health 13: 1509–1517. [DOI] [PubMed] [Google Scholar]
- 35.Yirenya-Tawiah D, Annang TN, Apea-Kubi KA, Lomo G, Mensah D, Akyeh L, Bosompem KM, 2014. Chlamydia trachomatis and Neisseria Gonorrhoeae prevalence among women of reproductive age living in urogenital schistosomiasis endemic area in Ghana. BMC Res Notes 7: 349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.World Health Organization , 2018. Fact Sheet: Schistosomiasis. Available from: https://www.who.int/news-room/fact-sheets/detail/schistosomiasis. Accessed December 20, 2018. [Google Scholar]
- 37.Colley DG, Bustinduy AL, Secor WE, King CH, 2014. Human schistosomiasis. Lancet 383: 2253–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.FAO , 2010. Crop Calendar: Democratic Republic of the Congo. Available from: http://www.fao.org/agriculture/seed/cropcalendar/searchbycountry.do. Accessed January 8, 2018. [Google Scholar]
- 39.Behets FM, Matendo R, Vaz LM, Kilese N, Nanlele D, Kokolomami J, Okitolando EW, Van Rie A, 2006. Preventing vertical transmission of HIV in Kinshasa, Democratic Republic of the Congo: a baseline survey of 18 antenatal clinics. Bull World Health Organ 84: 969–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.World Health Organization , 2007. Global Strategy for the Prevention and Control of Sexually Transmitted Infections: 2006–2015. Geneva, Switzerland: WHO. [Google Scholar]
- 41.Romoren M, Sundby J, Velauthapillai M, Rahman M, Klouman E, Hjortdahl P, 2007. Chlamydia and gonorrhoea in pregnant Batswana women: time to discard the syndromic approach? BMC Infect Dis 7: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Romoren M, Velauthapillai M, Rahman M, Sundby J, Klouman E, Hjortdahl P, 2007. Trichomoniasis and bacterial vaginosis in pregnancy: inadequately managed with the syndromic approach. Bull World Health Organ 85: 297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.King CH, Bertsch D, 2013. Meta-analysis of urine heme dipstick diagnosis of Schistosoma haematobium infection, including low-prevalence and previously-treated populations. Plos Negl Trop Dis 7: e2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kjetland EF, Ndhlovu PD, Mduluza T, Gomo E, Gwanzura L, Mason PR, Kurewa EN, Midzi N, Friis H, Gundersen SG, 2005. Simple clinical manifestations of genital Schistosoma haematobium infection in rural Zimbabwean women. Am J Trop Med Hyg 72: 311–319. [PubMed] [Google Scholar]
- 45.Colombe S, Lee MH, Masikini PJ, van Lieshout L, de Dood CJ, Hoekstra PT, Corstjens PLAM, Mngara J, van Dam GJ, Downs JA, 2018. Decreased sensitivity of schistosoma sp. egg microscopy in women and HIV-infected individuals. Am J Trop Med Hyg 98: 1159–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moodley P, Sturm AW, 2000. Sexually transmitted infections, adverse pregnancy outcome and neonatal infection. Semin Neonatol 5: 255–269. [DOI] [PubMed] [Google Scholar]