Abstract.
The “Top End” of Australia is presently experiencing a gonorrhea epidemic. Gonococcal infection is usually limited to mucosal tissues but can lead to disseminated gonococcal infection (DGI), joint destruction, and severe sepsis. This study aimed to explore the epidemiology, presentation, management, and health-care impact of DGI in the Top End of the Northern Territory. Health records of patients diagnosed with proven, probable, or possible DGI between January 2010 and September 2018 were analyzed retrospectively. One hundred six cases of DGI were identified. Ninety-four patients (88.7%) were Indigenous Australian. The incidence of proven and probable DGI in the Indigenous population was 27.1 per 100,000 person-years, compared with 7.1 in the Top End population overall. Of 7,540 laboratory-proven gonococcal notifications, 1.3% (n = 97) were complicated by DGI. The highest incidence was in the 15–19-year age-group. Thirteen cases (12.3%) occurred in patients younger than 15 years. High rates of comorbid alcohol misuse, diabetes, systemic lupus erythematosus, rheumatic fever, and complement deficiency were observed. The “classic triad” of tenosynovitis, dermatitis, and polyarthralgia was rare. Ninety-four patients (88.7%) presented with purulent arthritis. Disseminated gonococcal infection was estimated to cause at least 10.0% of nonpenetrating septic arthritis in the Top End and 1,234 days of hospitalization during the study period. DGI is an important cause of morbidity in the Top End, particularly in the young, remote Indigenous Australian population. Clinical presentation varies from classical teaching. Urgent action in the health and community sector is required, particularly for at-risk populations, to prevent further debilitating and costly complications of gonococcal infection.
INTRODUCTION
The Northern Territory (NT) is burdened by some of the highest rates of gonorrhea infection in Australia.1 The annualized gonorrhea notification rate for the NT in the first quarter of 2018 was higher than in every first quarter since 2013. From 2013 to 2017, the NT rate remained steady, but approximately seven times the national notification rate.2
Gonococcal infection is usually limited to mucosal tissues but can lead to gonococcal bacteremia and disseminated gonococcal infection (DGI). An estimated 0.5% to 3% of persons with gonococcal infection develop DGI.3–5 Two clinical syndromes have been described: a “classic triad” of tenosynovitis, polyarthralgia, and dermatitis and purulent arthritis with or without associated findings.6 These may represent a spectrum of DGI, with the polyarthralgia syndrome progressing to purulent arthritis later in the disease course.6,7 Untreated DGI can cause joint destruction and severe sepsis.8
A 2015 study exploring the epidemiology of gonococcal arthritis in the Indigenous population in Central Australia demonstrated a high burden of DGI, particularly in young women.9 No previous publications have described the epidemiology, clinical features, and management of DGI in the “Top End” of the NT.
Gonococcal infection is not presently considered vaccine preventable. However, a recent retrospective cohort study in New Zealand found a significant reduction in hospitalization caused by gonorrhea after introduction of the meningococcal B vaccine.10 The adjusted vaccine effectiveness was estimated at 24% (95% CI: 1–42%). The possible introduction of a meningococcal B vaccination to the routine immunization schedule in the NT is under consideration. It is, therefore, timely to review the current status of DGI in the Top End.
MATERIALS AND METHODS
Setting.
The Top End is defined in this study as including the SA3 census regions of Daly–Tiwi–West Arnhem, East Arnhem, Katherine, and the SA4 region of Darwin, and 25.5% of the population are Indigenous Australians.11 During the study period, the Top End Health Service included three public hospitals and 26 remote clinics. All patients fulfilling the case definition for DGI were eligible for inclusion if they presented to these sites between 1 January 2010 and 30 September 2018.
Study design and data collection.
Cases of DGI were identified and matched retrospectively using 1) hospital discharge coding systems from all hospitals in the study area and 2) the NT Notifiable Diseases System (NTNDS) database. As the term DGI is not defined in International Classification of Diseases-10 codes, various codes for gonococcal infection and septic arthritis were used to identify hospitalized DGI cases. To estimate the proportion of septic arthritis caused by DGI, cases of purulent arthritis diagnosed between 1 October 2017 and 30 September 2018 were extracted using hospital discharge coding data. NTNDS data were also filtered to select cases in which Neisseria gonorrhoeae was isolated from nonmucosal sites, including blood, synovial fluid, tissue samples, and skin lesions. This identified additional cases that were not hospitalized or were missed through the hospital coding search. Cases were defined as proven, probable, or possible DGI based on previously published case definitions (Table 1).5,6,9
Table 1.
Case definitions for categories of disseminated gonococcal infection
| Proven | Neisseria gonorrhoeae isolated from culture or PCR testing of blood, synovial fluid, skin lesions, or otherwise sterile sources. |
| Probable | Clinical features of disseminated infection and the expected response to therapy, with N. gonorrhoeae isolated from culture or PCR testing of mucosal site(s) (including urogenital, anorectal, or pharyngeal specimens) in the absence of positive blood or other sterile-site specimens. |
| Possible | Clinical features of disseminated infection and the expected response to therapy, in the absence of positive cultures or PCR for N. gonorrhoeae. |
Demographic and clinical data were extracted from electronic and paper medical records. This included patient comorbidities (diabetes, alcohol misuse, pelvic inflammatory disease [PID], rheumatic fever, connective tissue disease, immunodeficiency, intravenous drug use, and pregnancy), clinical presentation (fever, dermatitis, tenosynovitis, arthralgia, arthritis, and joints affected), laboratory data (culture and gonococcal polymerase chain reaction [PCR] results, synovial fluid leukocyte count and crystal analysis, serum complement levels, antinuclear antibody titers, past and concurrent sexually transmitted infections [STIs], and STIs within 12 months after DGI), management (surgery, antibiotics, length of admission, and self-discharge), and mortality within 5 years of DGI. Alcohol misuse was considered to be present if any reference was made to hazardous alcohol consumption or previous alcohol-related injury in the patient’s medical record.
Population data were obtained from the 2011 and 2016 Australian census.11,12 The total number of gonococcal notifications was based on data collected by the NTNDS and published in the NT Disease Control Bulletins.13
Data analysis.
The incidence was determined by dividing the number of incident cases of DGI by the total number of person-years accumulated in the study population. Incidence values and 95% CIs were determined using Microsoft® Excel (version 16.21.1, Microsoft Corp., Redmond, WA). Other statistical analyses were performed using IBM® SPSS Statistics (version 25.0.0.0, IBM, Armonk, NY). Categorical data were compared using the chi-squared test. Parametric data were compared using the t-test or analysis of variance (ANOVA) test and nonparametric data using the Mann–Whitney or Kruskal–Wallis test.
Ethics approval.
Ethics approval was obtained from the Human Research Ethics Committee of the NT Department of Health (HREC 2018-3251).
RESULTS
Incidence and demographics.
As significant population growth occurred in the Top End during the study period, population data from the approximate midpoint of the study (the mean of 2011 and 2016 census data) were used for incidence calculations. The estimated population at this midpoint was 177,440 and the Indigenous population was 40,907 (23.1%). In remote areas, the estimated population was 48,733 and the Indigenous population was 29,408 (60.0%).
Between January 2010 and September 2018, there were 7,540 laboratory-proven gonococcal notifications in the Top End.13 During the same period, 106 cases of proven, probable, or possible DGI were identified, including 97 cases of proven or probable DGI. Table 2 describes their demographics. Of the 106 cases, two have been previously reported in the literature.14 Proven or probable DGI complicated 1.3% of all gonococcal notifications.
Table 2.
Demographics of patients with DGI in the Top End
| Male (n = 51) | Female (n = 55) | All (n = 106) | |
|---|---|---|---|
| Age in years (median, interquartile range) | 36 (25–47) | 27 (18–37) | 32 (20–44) |
| Ethnicity, n (%) | |||
| Indigenous | 39 | 55 | 94 (88.7%) |
| Non-Indigenous | 12 | 0 | 12 (11.3%) |
| DGI, n (%) | |||
| Proven | 40 | 38 | 78 (73.6%) |
| Probable | 4 | 15 | 19 (17.9%) |
| Possible | 7 | 2 | 9 (8.5%) |
| Geographic location at symptom onset (%) | |||
| Remote Top End NT | 33 | 50 | 83 (78.3%) |
| Urban Top End NT (Darwin) | 14 | 1 | 15 (14.2%) |
| Southern NT | 2 | 3 | 5 (4.7%) |
| Interstate/overseas | 2 | 1 | 3 (2.8%) |
| Age-group in years, n (%) | |||
| 5–9 | 1 | 0 | 1 (0.9%) |
| 10–14 | 1 | 11 | 12 (11.3%) |
| 15–19 | 5 | 7 | 12 (11.3%) |
| 20–24 | 5 | 8 | 13 (12.3%) |
| 25–29 | 3 | 4 | 7 (6.6%) |
| 30–34 | 7 | 7 | 14 (13.2%) |
| 35–39 | 6 | 5 | 11 (10.4%) |
| 40–44 | 5 | 5 | 10 (9.4%) |
| 45–49 | 6 | 6 | 12 (11.3%) |
| 50–54 | 4 | 0 | 4 (3.8%) |
| 55–59 | 2 | 2 | 4 (3.8%) |
| 60–64 | 3 | 0 | 3 (2.8%) |
| 65–69 | 2 | 0 | 2 (1.9%) |
| ≥ 70 | 1 | 0 | 1 (0.9%) |
DGI = disseminated gonococcal infection; NT = Northern Territory.
The overall incidence of proven and probable DGI in the Top End was 7.1 (95% CI: 5.72–8.61) per 100,000 person-years. The incidence was significantly higher in the Indigenous than the non-Indigenous population, at 27.1 (95% CI: 21.7–33.5) compared with 1.3 (95% CI: 0.7–2.2) per 100,000 person-years (P < 0.001), and in remote compared with urban areas (Table 3). There was no significant gender difference.
Table 3.
Incidence of proven or probable disseminated gonococcal infection stratified by ethnicity, gender, age, and remoteness
| Population | Incidence per 100,000 person-years | |||
|---|---|---|---|---|
| Top End urban (95% CI) | Top End remote (95% CI) | P-value (urban vs remote) | Top End overall | |
| Ethnicity: | ||||
| All | 1.4 (0.8–2.4) | 22.2 (17.7–27.5) | < 0.001 | 7.1 |
| Indigenous | 6.7 | 36.9 | < 0.001 | 27.1 |
| Non-Indigenous | 0.9 | 1.3 | 0.76 | 1.3 |
| Gender | ||||
| Male | 2.5 | 14.5 | 0.004 | 6.1 |
| Female | 0.2 | 25.7 | < 0.001 | 8.0 |
| Age (years): | ||||
| 5-9 | 0.0 | 2.8 | 0.09 | 1.0 |
| 10–14 | 0.0 | 37.2 | < 0.001 | 12.5 |
| 15–19 | 0.0 | 41.6 | < 0.001 | 13.4 |
| 20–24 | 0.0 | 37.2 | < 0.001 | 10.7 |
| 25–29 | 0.0 | 21.6 | < 0.001 | 5.5 |
| 30–34 | 3.3 | 30.0 | < 0.001 | 9.9 |
| 35–39 | 3.6 | 25.8 | < 0.001 | 9.1 |
| 40–44 | 1.3 | 31.5 | < 0.001 | 8.7 |
| 45–49 | 0.0 | 38.2 | < 0.001 | 10.3 |
| 50–54 | 1.5 | 9.2 | 0.02 | 3.4 |
| 55–59 | 1.8 | 17.2 | < 0.001 | 5.4 |
| 60–64 | 4.4 | 7.3 | 0.38 | 5.0 |
| 65–69 | 6.5 | 11.9 | 0.21 | 5.1 |
| ≥ 70 | 2.5 | 0.0 | 0.11 | 2.1 |
Bold values indicate P < 0.05.
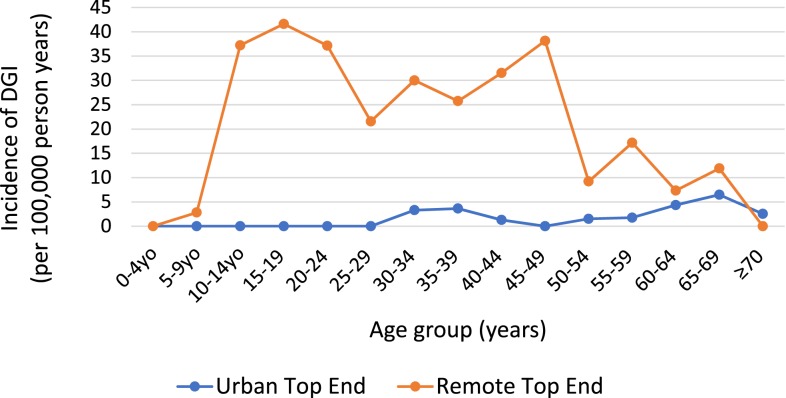
Thirteen cases (12%) of DGI occurred in patients younger than 15 years, with 12 cases in the 10-14-year age-group and one in the 5–9-year age-group. The highest age-stratified incidence of DGI was in 15–19-year-olds, followed by 10–14 and 20–24-year-olds. Incidence was significantly higher in remote than urban areas in all age groups from 10 to 59 years (Figure 1) and across both genders.
Figure 1.
Incidence of DGI per 100,000 person-years in the Top End, stratified by age and remoteness. This figure appears in color at www.ajtmh.org.
There were high rates of comorbid diabetes, alcohol misuse, rheumatic fever, and cirrhosis (Table 4). Thirty-three patients (31.1%) were previously well. The background age-standardized prevalence of lifetime risky alcohol use has been reported at 9.8% in Indigenous Australians and 9.7% in non-Indigenous Australians nationally.16 The background prevalence of diabetes is 17.9% among Indigenous Australians and 5.1% in non-Indigenous Australians.17 Patients with systemic lupus erythematosus (SLE) were also over-represented, with a prevalence of 4.3% in this Indigenous cohort compared with 0.05% among Indigenous Australians in the NT.18,19 One patient had concurrent gonococcal PID and DGI.
Table 4.
Comorbidities of patients with proven, probable, and possible DGI
| Proven DGI (n = 78) | Probable DGI (n = 19) | Possible DGI (n = 9) | Total (n = 106) | |
|---|---|---|---|---|
| Pregnancy | 1 | 0 | 0 | 1 (0.9%) |
| Diabetes | 18 | 3 | 2 | 23 (21.7%) |
| Alcohol misuse | 34 | 4 | 6 | 44 (41.5%) |
| Current IVDU | 0 | 0 | 1 | 1 (0.9%) |
| History of pelvic inflammatory disease | 6 | 2 | 0 | 8 (7.5%) |
| History of acute rheumatic fever | ||||
| Definite15 | 3 | 1 | 2 | 6 (5.7%) |
| Probable or possible15 | 7 | 6 | 0 | 13 (12.3%) |
| Immunodeficiency | ||||
| On immunosuppressant medication | 2 | 1 | 0 | 3 (2.8%) |
| HIV infection | 0 | 0 | 0 | 0 |
| Connective tissue disease | 3 | 2 | 1 | 6 (5.7%) |
| HTLV-I infection | 2 | 0 | 0 | 2 (1.9%) |
| Cirrhosis | 7 | 0 | 0 | 7 (6.6%) |
| Splenectomy | 0 | 1 | 0 | 1 (0.9%) |
DGI = disseminated gonococcal infection; IVDU = intravenous drug use.
Clinical manifestations.
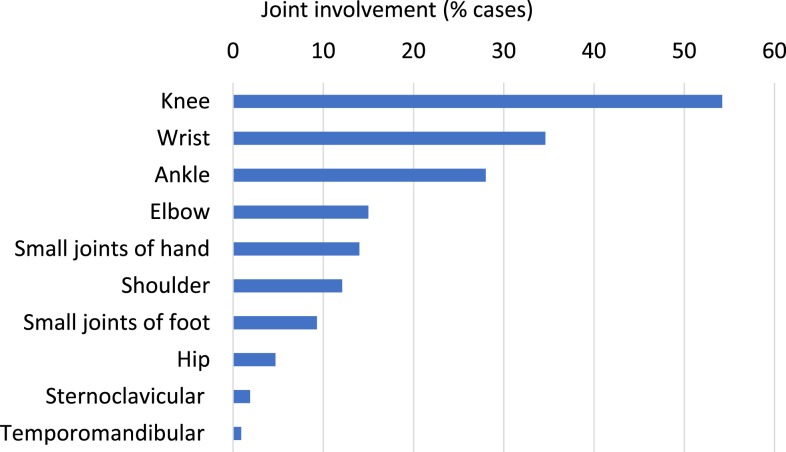
Arthritis was the most common symptom of DGI, seen in 94 patients (88.7%) (Table 5). The knee, wrist, ankle, and elbow were most often affected3,5,7 (Figure 2). Dermatitis occurred in 10 patients (9.4%), including nine with concurrent purulent arthritis. The rash was pustular in most cases (n = 9). The “classic triad” was observed only in one patient. Eighteen patients (17.0%) were initially labeled with undifferentiated sepsis, with DGI later diagnosed on blood cultures. One patient developed gonococcal endocarditis, requiring transfer interstate for mitral valve replacement.
Table 5.
Clinical presentation of disseminated gonococcal infection
| Clinical features n (%) | Proven (n = 78) | Probable (n = 19) | Possible (n = 9) | Total (n = 106) |
|---|---|---|---|---|
| Fever (37.7°C or greater) | 49 | 12 | 5 | 66 (62.3%) |
| Arthritis | ||||
| Yes | 66 | 19 | 9 | 94 (88.7%) |
| No | 6 | 0 | 0 | 6 (5.7%) |
| Unknown | 6 | 0 | 0 | 6 (5.7%) |
| Number of joints affected | ||||
| Monoarthritis | 28 | 6 | 6 | 40 (37.7%) |
| Oligoarthritis (2–4 joints) | 21 | 7 | 1 | 29 (27.3%) |
| Polyarthritis (≥ 5 joints) | 15 | 6 | 2 | 23 (21.7%) |
| Unknown | 8 | 0 | 0 | 8 (7.5%) |
| Type of joints affected | ||||
| Knee | 45 | 8 | 5 | 58 (54.7%) |
| Wrist | 25 | 10 | 2 | 37 (34.9%) |
| Ankle | 19 | 9 | 2 | 30 (28.3%) |
| Elbow | 14 | 2 | 0 | 16 (15.1%) |
| Small joints of hand | 9 | 5 | 1 | 15 (14.2%) |
| Shoulder | 9 | 4 | 0 | 13 (12.3%) |
| Small joints of foot | 4 | 4 | 2 | 10 (9.4%) |
| Hip | 2 | 1 | 2 | 5 (4.7%) |
| Sternoclavicular joint | 1 | 1 | 0 | 2 (1.9%) |
| Temporomandibular joint | 0 | 1 | 0 | 1 (0.9%) |
| Tenosynovitis | 4 | 0 | 0 | 4 (3.8%) |
| Dermatitis | 10 | 0 | 0 | 10 (9.4%) |
| Classic triad (tenosynovitis, dermatitis, and polyarthralgia) | 1 | 0 | 0 | 1 (0.9%) |
Figure 2.
Joint involvement. This figure appears in color at www.ajtmh.org.
Investigation results.
Table 6 describes the laboratory investigations performed during diagnostic workup for DGI. Blood cultures were positive for N. gonorrhoeae in 25 patients (23.6%). Arthrocentesis and synovial fluid culture and/or PCR testing were performed in 70 cases, with a positive result in 58 (54.7%). Of 28 patients with a positive synovial fluid culture, 23 also underwent synovial fluid PCR testing, and all of these PCR results were positive. Synovial fluid from 15 patients tested PCR positive but culture negative, giving synovial fluid culture a sensitivity of 48.3% when compared with PCR in this study. One patient developed gonococcal spontaneous bacterial peritonitis in the context of cirrhosis, without associated PID.
Table 6.
Laboratory investigations in disseminated gonococcal infection
| Male (n = 51) | Female (n = 55) | P-value (M vs F) | Total (n = 106) | |
|---|---|---|---|---|
| Microbiology positive, n (%) | ||||
| Blood culture | 15 | 10 | 0.17 | 25 (23.6%) |
| Synovial fluid PCR | 28 | 25 | 0.61 | 53 (50.0%) |
| Synovial fluid culture | 15 | 13 | 0.50 | 28 (26.4%) |
| Ascitic fluid culture | 1 | 0 | 0.33 | 1 (0.9%) |
| Urine PCR | 14 | 30 | 0.005 | 44 (41.5%) |
| Urine culture | 6 | 0 | 0.009 | 6 (5.7%) |
| Genital/urethral swab PCR | 2 | 10 | 0.02 | 12 (11.3%) |
| Genital/urethral swab culture | 1 | 3 | 0.34 | 4 (3.8%) |
| Pharynx swab PCR | 2 | 0 | 0.13 | 2 (1.9%) |
| Pharynx swab culture | 0 | 0 | – | 0 |
| Eye swab PCR | 1 | 0 | 0.30 | 1 (0.9%) |
| Eye swab culture | 1 | 0 | 0.30 | 1 (0.9%) |
| Skin swab PCR | 2 | 0 | 0.14 | 2 (1.9%) |
| Skin swab culture | 0 | 0 | – | 0 |
| > 1 positive microsite | 16 | 23 | 0.27 | 39 (36.8%) |
| Specimen tested for beta-lactamase | 31 | 27 | 58 (54.7%) | |
| Beta-lactamase negative | 25 | 27 | 0.03 | 52 |
| Arthrocentesis performed | 37 | 33 | 0.17 | 70 (66.0%) |
| Mean leukocyte count × 109/L (±SD) | 184.3 (230.6) | 118.7 (151.5) | 0.16 | 151.5 (196.4) |
| Median leukocyte count × 109/L | 136.0 | 64.0 | – | 92.1 |
| Mean % neutrophils (±SD) | 87.4 (16.8) | 86.6 (15.5) | 0.85 | 87.0 (16.1) |
| Uric acid crystals detected | 6 | 0 | 0.009 | 6 (5.6%) |
| Calcium pyrophosphate crystals detected | 1 | 0 | 0.30 | 1 (0.9%) |
| Immune function | ||||
| Tested for HIV | 41 | 41 | 0.47 | 82 (77.4%) |
| Positive for HIV | 0 | 0 | – | 0 |
| ANA tested (at any time) | 36 | 42 | 0.72 | 78 (73.6%) |
| Positive ANA | 9 | 11 | 0.76 | 19 |
| C3/C4 tested (at any time) | 16 | 20 | 0.59 | 36 (34.0%) |
| Low C3 and/or C4 | 6 | 8 | 0.67 | 14 |
PCR = polymerase chain reaction; ANA = anti-nuclear antibody. Bold values indicate P < 0.05.
Of the 78 patients with proven DGI, 73 were tested for gonococcus at a mucosal site (urine, genital swab, throat, or eye swab). Of these, 34 patients had a positive mucosal result, including 26 on urine and eight on genital swabs. By definition, all patients in the probable DGI group and no patients in the possible group had a positive mucosal result. All patients with positive urine or genital culture also had a positive PCR result from the same site.
Of the 97 cases with a positive gonococcal specimen (proven and probable cases), 58 underwent beta-lactamase testing (59.8%) via culture or PCR. The proportion increased from 2015 with advancement in PCR techniques. Fifty-two (89.7%) of the specimens tested were beta-lactamase negative, indicating penicillin sensitivity.
Among patients with gonococcus isolated in synovial fluid (PCR or culture), the length of hospital stay was significantly longer if uric acid crystals were also detected in the fluid (30 days ± 14, versus 12 days ± 6, P = 0.01).
Serum complement levels were available for 36 patients, with a low level observed in 14 (low C3 and C4 in seven patients; low C3 in 4; and low C4 in 3). Four of the 14 abnormal results occurred during admission with DGI and 10 during other encounters.
Rates of concurrent, past, and future STIs are outlined in Table 7. Twenty-seven patients were retested for gonococcal infection through Territory Pathology within 12 months after treatment of DGI and seven had a positive result.
Table 7.
Other sexually transmitted infections in patients with DGI
| Male (n = 51) | Female (n = 55) | P- value (M vs F) | Total (n = 106) | |
|---|---|---|---|---|
| STI test during admission | ||||
| Chlamydia-positive test (% of those tested) | 4 | 9 | 0.18 | 13 (13%) |
| Not tested | 5 | 3 | 8 | |
| Trichomonas-positive test (% of those tested) | 1 | 15 | < 0.001 | 16 (18%) |
| Not tested | 10 | 9 | 19 | |
| Syphilis-positive test (% of those tested) | 0 | 1 | 0.33 | 1 (2%) |
| Not tested | 21 | 25 | 46 | |
| Previous STI | ||||
| Gonorrhea-positive test (% of those tested) | 3 | 9 | 0.09 | 12 (29%) |
| Not tested | 36 | 28 | 64 | |
| Chlamydia-positive test (% of those tested) | 2 | 4 | 0.46 | 6 (14%) |
| Not tested | 36 | 27 | 63 | |
| Trichomonas-positive test (% of those tested) | 1 | 8 | 0.02 | 9 (33%) |
| Not tested | 39 | 40 | 79 | |
| Syphilis-positive test (% of those tested) | 1 | 5 | 0.11 | 6 (15%) |
| Not tested | 37 | 30 | 67 | |
| STI in 12 months after treatment of DGI | ||||
| Gonorrhea-positive test (% of those tested) | 3 | 4 | 0.77 | 7 (26%) |
| Not tested | 41 | 38 | 79 | |
| Chlamydia-positive test (% of those tested) | 0 | 1 | 0.33 | 1 (4%) |
| Not tested | 41 | 37 | 78 | |
| Trichomonas-positive test (% of those tested) | 0 | 5 | 0.045 | 5 (20%) |
| Not tested | 41 | 40 | 81 | |
| Syphilis-positive test (% of those tested) | 1 | 0 | 0.30 | 1 (6%) |
| Not tested | 43 | 45 | 88 | |
DGI = disseminated gonococcal infection; STI = sexually transmitted infection. Bold values indicate P < 0.05.
Management.
Table 8 outlines the management of DGI in this cohort. One hundred three of 106 patients were hospitalized, with a mean length of stay of 11.6 days (including hospital in the home admissions). The rate of self-discharge from hospital was 30.2% (at least one time). The cumulative length of stay attributable to DGI during the study period was 1,234 days.
Table 8.
Treatment and outcomes of disseminated gonococcal infection
| Proven (n = 78) | Probable (n = 19) | Possible (n = 9) | Total (n = 106) | |
|---|---|---|---|---|
| Surgery | ||||
| Arthroscopy or arthrotomy (1 or more procedures) | 41 | 0 | 0 | 41 (38.7%) |
| Antibiotics | ||||
| Cephalosporin | 45 | 10 | 5 | 60 (56.6%) |
| Penicillin | 2 | 0 | 0 | 2 (1.9%) |
| Both cephalosporin and penicillin | 26 | 8 | 4 | 38 (35.8%) |
| Azithromycin | 40 | 10 | 1 | 51 (48.1%) |
| No antibiotics (self-discharged) | 2 | 0 | 0 | 2 (1.9%) |
| Other antibiotic/unknown | 3 | 0 | 0 | 3 (2.8%) |
| Duration of antibiotic treatment (median, interquartile range) | 10 (7–15) | 7 (7–11) | 7 (6–11) | 10 (7–14) |
| Hospitalization | ||||
| Patients hospitalized | 75 | 19 | 9 | 103 (97.2%) |
| Length of hospital stay in days (median, interquartile range) | 10 (7-15) | 8 (7-12) | 7 (6-13) | 10 (7-14) |
| Cumulative length of hospital stay (days) | 956 | 185 | 93 | 1,234 |
| Self-discharged from hospital | 23 | 5 | 4 | 32 (30.2%) |
| Mortality | ||||
| Within 1 month of diagnosis | 1 | 0 | 0 | 1 (0.9%) |
| Within 1 year | 2 | 0 | 0 | 2 (1.9%) |
| Within 5 years | 4 | 1 | 1 | 6 (5.7%) |
Six patients (5.7%) died within 5 years of DGI, of whom five were Indigenous. The mean age at death was 35 years. In one case, DGI directly contributed to mortality. This was an 18-year-old patient who developed severe DGI sepsis while immunosuppressed on rituximab for autoimmune disease. Causes of death in the other patients included a motor vehicle accident and non-DGI sepsis in the context of rheumatic heart disease.
Microbiology of septic arthritis.
Between 1 October 2017 and 30 September 2018, 80 patients were discharged from hospital in the Top End with a diagnosis of septic arthritis (excluding 33 cases of digital septic arthritis following penetrating injury) (Table 9). Proven DGI accounted for eight of these cases. In 28 cases, no organism was identified, of which 16 underwent arthrocentesis, but gonococcal PCR testing was not performed on the synovial fluid. A further five did not undergo arthrocentesis. Therefore, DGI could not be excluded as the cause of septic arthritis in an additional 21 patients.
Table 9.
Microbiology snapshot of septic arthritis in the Top End (1 October 2017 to 30 September 2018)
| Causative organism | Number of cases |
|---|---|
| Methicillin-susceptible Staphylococcus aureus (MSSA) | 16 |
| Streptococcal species | 8 |
| Neisseria gonorrhoeae | 8 |
| Methicillin-resistant Staphylococcus aureus (MRSA) | 7 |
| Melioidosis | 5 |
| Other Gram-negatives (Escherichia coli, Klebsiella, Kingella, and Pseudomonas) | 5 |
| Other Staphylococcal species | 1 |
| MSSA and Streptococcal species | 1 |
| MRSA and Streptococcal species | 1 |
| Arthrocentesis performed—no organism identified on culture | 23 |
| Gonococcal PCR negative | 7 |
| Gonococcal PCR not performed | 16 |
| Arthrocentesis not performed | 5 |
| Total | 80 |
DISCUSSION
This study describes the burden of DGI in the Top End, identifying the young, remote Indigenous Australian population as being at greatest risk. The rates of DGI between Indigenous and non-Indigenous people in our region differed significantly and those living in remote areas were notably over-represented. A crude incidence of 36.9 per 100,000 person-years was found in the remote Indigenous population, similar to the incidence of 39.9 per 100,000 person-years recorded among Indigenous Australians in Central Australia in 2015.9 These cases represent just the “tip of the iceberg” of the gonococcal epidemic in the NT.
Although previous studies suggest a female predominance of DGI,3,9 in our cohort, there was no significant gender-difference. Only one patient developed DGI during pregnancy despite pregnancy being a known risk factor.3 This low rate may reflect significant efforts directed toward STI screening in early pregnancy in the Top End over recent decades.
Patients with DGI tended to present with purulent arthritis rather than the “classic triad,” as observed in Central Australia previously.9 Possible explanations include characteristics of locally circulating gonococcal strains, genetic immune factors in the Indigenous population, delayed presentation, or predisposing comorbidities. It was not uncommon for DGI to involve atypical joints, such as small joints of the hands and feet, sternoclavicular joints and, temporomandibular joints. A high proportion of patients presented with undifferentiated sepsis. Local health-care staff need to be aware that the distinct clinical phenotype seen in the Top End varies from classical teaching. Concerningly, a significant proportion of patients in our cohort (22.6%) were not tested for HIV. All patients with a newly diagnosed STI require HIV testing20 and DGI may represent the first presentation of HIV infection.21
We found that DGI can occur concurrently with crystal arthropathy, which is associated with increased length of hospital stay. This supports previous literature suggesting more complicated DGI disease in patients with concurrent gout.14 DGI should be considered in the differential diagnosis for polyarthropathy even if crystal arthropathy is present and a sexual history sought. Making the diagnosis of DGI is also complicated by coexisting high rates of rheumatic fever and SLE, which can cause similar symptoms and occur concurrently in the same patient. This again emphasizes the need for routinely taking a sexual history and considering DGI.
The high rate of complement deficiency in our cohort aligns with the established association between hypocomplementemia and Neisseria infections.22,23 Reduction in complement to subnormal levels from DGI itself is reported to be uncommon.5 As there is a high incidence of other conditions associated with hypocomplementemia (SLE, post-streptococcal and, glomerulonephritis) among Indigenous Australians,18,24 this group may be particularly susceptible to gonococcal infection or to dissemination once infected. The high rates of alcohol misuse and diabetes observed in this study suggest that these conditions are also risk factors for DGI in our population.
Our laboratory findings highlight the excellent sensitivity of PCR testing in comparison with culture alone in diagnosis of DGI. If PCR was not available, 25 of the 76 proven DGI cases would have remained unconfirmed. However, it is critical that specimens continue to be sent for culture and PCR to capture ongoing genomic, epidemiologic, and antimicrobial information for current management and future policies and planning.
Gonococcal infection was estimated to cause at least 10.0% of cases of septic arthritis in the Top End (with a further 26.3% culture-negative and gonococcal PCR not performed). This is similar to the rate of 12.0% reported in 1996.25 Local health-care staff should routinely consider PCR testing of synovial fluid and urogenital samples (in addition to cultures) in evaluating septic arthritis. Current national guidelines advise intravenous flucloxacillin as first-line therapy for septic arthritis,26 but this regimen provides inadequate gonococcal arthritis coverage and may require local review.
It is likely that cases of nonhospitalized probable and possible DGI were missed in this study as these would not have been captured by hospital discharge coding or the NTNDS filters. Hospitalized cases may also have been overlooked if no discharge summary was completed (required for coding). Therefore, our incidence data probably underestimates the true burden of DGI. Another limitation of the incidence calculations was the use of midpoint population estimates. Comorbidity results may also be underestimated if medical history was incompletely documented or conditions such as alcohol misuse were unrecognized. Data on past and future STIs were incomplete as community laboratory results were not accessed.
DGI has the potential to cause long-term morbidity through joint destruction and complications of sepsis. In addition, reproductive implications from gonococcal PID are a key consideration not explored here. The association of DGI with other debilitating comorbidities and the impact of self-discharge from hospital in complicating management and outcomes cannot be overstated. High rates of recurrent STIs within 12 months of DGI were also observed, raising concerns about capacity and effectiveness of contact tracing and sexual health and well-being education. Follow-up screening results were only available for a small proportion of patients.
With the high number of positive culture specimens captured in this group, there is potential for further information to be gained regarding gonococcal strains, transmission dynamics, and targeted prevention strategies. Protective strategies from vaccination, as suggested by preliminary studies of meningococcal B vaccination,10 will require understanding of these strains and further research.
From a health system perspective, our results highlight that DGI, a preventable disease, is causing alarming morbidity and consuming significant health-care resources. The extremely high incidence of DGI in the 10–14 and 15–19-year-old remote population demonstrates an urgent need to direct strategies toward 1) health and well-being and sexual health awareness among young people in the remote Top End and 2) knowledge of this disease burden among the health services they access. In liaison with community health services and schools, investment in culturally appropriate STI prevention strategies in this population has the potential to be very cost-effective, in addition to the marked individual health benefits for patients.
Acknowledgments:
We thank all Top End Health Service clinical and laboratory staff involved in the patients’ care.
REFERENCES
- 1.Kirby Institute , 2018. HIV, Viral Hepatitis and Sexually Transmissible Infections in Australia: Annual Surveillance Report 2018. Sydney, Australia: Kirby Institute, UNSW. [Google Scholar]
- 2.Sexual Health & Blood Borne Virus Unit (SHBBVU) , 2018. Surveillance Update for Notifiable Sexually Transmitted Infections and Blood-Borne Viruses in the Northern Territory (NT). Darwin, Australia: Centre for Disease Control, Department of Health, NT Government; 9–11. [Google Scholar]
- 3.Bardin T, 2003. Gonococcal arthritis. Best Pract Res Clin Rheumatol 17: 201–208. [DOI] [PubMed] [Google Scholar]
- 4.Barr J, Danielsson D, 1971. Septic gonococcal dermatitis. Br Med J 1: 482–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holmes K, Sparling P, Stamm W, Piot P, Wasserheit J, Corey L, Cohen M, 2008. Sexually Transmitted Diseases. 4th ed New York, NY: McGraw-Hill. [Google Scholar]
- 6.Klausner JD, 2019. Disseminated Gonococcal Infection. Available at: https://www.uptodate.com/contents/disseminated-gonococcal-infection. Accessed February 13, 2019. [Google Scholar]
- 7.Rice PA, 2005. Gonococcal arthritis (disseminated gonococcal infection). Infect Dis Clin North Am 19: 853–861. [DOI] [PubMed] [Google Scholar]
- 8.Thiery G, Tankovic J, Brun-Buisson C, Blot F, 2001. Gonococcemia associated with fatal septic shock. Clin Infect Dis 32: E92–E93. [DOI] [PubMed] [Google Scholar]
- 9.Tuttle CS, Van Dantzig T, Brady S, Ward J, Maguire G, 2015. The epidemiology of gonococcal arthritis in an Indigenous Australian population. Sex Transm Infect 91: 497–501. [DOI] [PubMed] [Google Scholar]
- 10.Paynter J, Goodyear-Smith F, Morgan J, Saxton P, Black S, Petousis-Harris H, 2019. Effectiveness of a group B outer membrane vesicle Meningococcal vaccine in preventing hospitalization from gonorrhea in New Zealand: a retrospective cohort study. Vaccines (Basel) 7: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Australian Institute of Health and Welfare (AIHW) , 2016. 2016 Census QuickStats. Canberra: AIHW. Available at: http://quickstats.censusdata.abs.gov.au/census_services/getproduct/census/2016/quickstat/036. Accessed March 3, 2019. [Google Scholar]
- 12.AIHW , 2011. 2011 census QuickStats. Canberra: AIHW. Available at: http://quickstats.censusdata.abs.gov.au/census_services/getproduct/census/2011/quickstat/0. Accessed February 12, 2019. [Google Scholar]
- 13.Centre for Disease Control, Northern Territory Government , 2018. Health Library Services ePublications. Available at: https://digitallibrary.health.nt.gov.au/prodjspui/handle/10137/506. Accessed February 14, 2019. [Google Scholar]
- 14.Smith EL, Hodgetts KE, Ralph AP, Anstey NM, 2019. Case report: severe disseminated gonococcal infection with polyarticular gout: two cases in older travelers. Am J Trop Med Hyg 100: 209–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.RHDAustralia (ARF/RHD writing group), National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand , 2012. Australian Guideline for Prevention, Diagnosis and Management of Acute Rheumatic Fever and Rheumatic Heart Disease. 2nd ed Darwin, Australia: Menzies School of Health Research. [Google Scholar]
- 16.Australian Bureau of Statistics , 2013. Australian Aboriginal and Torres Strait Islander Health Survey: First results, Australia, 2012–2013. Cat. No. 4727.0.55.001 Canberra, Australia: ABS. [Google Scholar]
- 17.AIHW , 2017. Aboriginal and Torres Strait Islander Health Performance Framework 2017: Supplementary Online Tables. Cat. no. WEB 170. Canberra: AIHW. Available at: https://www.aihw.gov.au/reports/indigenous-health-welfare/health-performance-framework/contents/tier-1-health-status-and-outcomes/1-09-diabetes. Accessed June 9, 2019. [Google Scholar]
- 18.Vincent FB, Bourke P, Morand EF, Mackay F, Bossingham D, 2013. Focus on systemic lupus erythematosus in Indigenous Australians: towards a better understanding of autoimmune diseases. Intern Med J 43: 227–234. [DOI] [PubMed] [Google Scholar]
- 19.Anstey NM, Bastian I, Dunckley H, Currie BJ, 1993. Systemic lupus erythematosus in Australian aborigines: high prevalence, morbidity and mortality. Aust N Z J Med 23: 646–651. [DOI] [PubMed] [Google Scholar]
- 20.SHBBVU , 2016. NT Guidelines for the Management of Sexually Transmitted Infections in the Primary Health Care Setting. Darwin, Australia: Centre for Disease Control, Department of Health, NT Government. [Google Scholar]
- 21.Amir O, Nguyen VD, Barnett BJ, 2003. Acute human immunodeficiency virus infection presenting as disseminated gonococcal infection. South Med J 96: 284–286. [DOI] [PubMed] [Google Scholar]
- 22.Ross SC, Densen P, 1984. Complement deficiency states and infection: epidemiology, pathogenesis and consequences of neisserial and other infections in an immune deficiency, Medicine (Baltimore) 63: 243–273. [PubMed] [Google Scholar]
- 23.Mitchell SR, Nguyen PQ, Katz P, 1990. Increased risk of neisserial infections in systemic lupus erythematosus. Semin Arthritis Rheum 20: 174–184. [DOI] [PubMed] [Google Scholar]
- 24.Chaturvedi S, Boyd R, Krause V, 2018. Acute post-streptococcal glomerulonephritis in the northern territory of Australia: a review of data from 2009 to 2016 and comparison with the literature. Am J Trop Med Hyg 99: 1643–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morgan DS, Fisher D, Merianos A, Currie B, 1996. An 18 year clinical review of septic arthritis from Tropical Australia. Epidemiol Infect 117: 423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Therapeutic Guidelines , 2014. Bone and Joint Infections. Version 15. West Melbourne, Australia: Therapeutic Guidelines Limited. 365–380. [Google Scholar]