Abstract.
Flea-borne typhus (FBT), although usually perceived as a self-resolving febrile illness, actually encompasses a wide spectrum of disease severity, including fulminant sepsis with multi-organ failure. In endemic Texas and California, the incidence of FBT has more than doubled over the last decade. Clinicians remain unfamiliar with severe septic presentations of FBT when considering the etiologies of acute undifferentiated febrile syndromes. The diagnostic challenges of FBT include the nonspecific and variable nature of both history and physical examination and the lack of diagnostic testing that can provide clinically relevant information early in the course of infection. These barriers perpetuate misdiagnoses in critically ill patients and lead to delay in initiating appropriate antibiotics, which may contribute to preventable morbidity and mortality. This case series describes the clinical and diagnostic trajectories of three patients who developed FBT-associated multi-organ dysfunction. These patients achieved resolution of infection after receiving doxycycline in the context of a high clinical suspicion. Patients residing in FBT-endemic areas presenting with a febrile illness of unknown etiology with a suggestive constellation of hyponatremia, elevated transaminase levels, and thrombocytopenia should be suspected of having FBT. Clinicians should proceed to serologic testing with early doxycycline therapy for potential rickettsiosis. Familiarizing clinicians with the presentation of rickettsiosis-associated septic syndromes and its early and appropriate antibiotic treatment can provide lifesaving care and reduce health-care costs through prevention of the morbidity associated with FBT.
INTRODUCTION
Flea-borne typhus (FBT) is an infection caused by Rickettsia typhi and Rickettsia felis typically presenting as a nonspecific febrile illness without significant complications.1 However, FBT actually encompasses a wide spectrum of disease severity, with its most severe presentation culminating in multi-organ failure requiring intensive care management.2 In the United States, FBT disproportionately affects Texas, California, and Hawaii.1 Texas and California are presently experiencing a resurgence with the reported incidence more than doubling over the last decade, making recognition of this disease increasingly important among clinicians serving these endemic areas.3–5 Still, FBT remains unrecognized and underreported.1,2 We report three cases of FBT (one confirmed and two probable) from South Texas in 2015–2016 manifesting as sepsis with multi-organ failure.
CASE REPORTS
Case 1.
A 53-year-old Hispanic male with a history of alcohol abuse presented with a 1-week history of nausea, vomiting, and diarrhea. Examination revealed a truncal maculopapular rash. He was found to have hyponatremia, elevated transaminase levels, acute kidney injury, and thrombocytopenia (Table 1). The lipase level was within normal limits. An abdominal ultrasound demonstrated no evidence of liver cirrhosis or cholecystitis. A viral hepatitis panel was negative. The patient was started on piperacillin–tazobactam, vancomycin, and doxycycline. On hospital day (HD) 3, he developed melena and hypotension, warranting transfer to the intensive care unit (ICU). The hypotension resolved with 4 L of normal saline. His course was complicated by pancreatitis, a petechial rash, and hemorrhagic conjunctivitis. A repeat ultrasound revealed a bulky pancreas with diffusely decreased parenchymal echogenicity. On HD 5, he developed acute cognitive dysfunction, with facial weakness and dysarthria. A head CT and MRI revealed no acute findings. Neurologic symptoms resolved by HD 8. A biopsy of the rash demonstrated endothelial injury suggestive of rickettsial infection (Figure 1). Blood, urine, and skin biopsy tissue culture were without growth. A rickettsial serologic panel was initially positive for only R. rickettsii IgM at 1:128, suggestive of Rocky Mountain spotted fever (RMSF). With continued doxycycline therapy, the patient improved and was transferred from the ICU to general medicine care on HD 9. Convalescent serum titers 1 month later demonstrated a 4-fold increase in R. rickettsii IgM and IgG titers to 1:1,024, as well as seroconversion to R. typhi, with IgM 1:1,024 and IgG 1:2,048.
Table 1.
Laboratory values during hospitalization for patients 1–3
| Chemistry laboratory values | Reference range | Patient 1 | Patient 2 | Patient 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Admission | Transfer to ICU (HD 3) | Maximum or nadir (when) | Admission | Transfer to ICU (HD 2) | Maximum or nadir (when) | Admission | Transfer to ICU (HD 1) | Maximum or nadir (when) | ||
| Na (mmol/L) | 135–145 | 120 | 120 | 115 (HD 2) | 127 | 138 | 127 (HD 1) | 139 | 142 | 137 (HD 4) |
| HCO3 (mmol/L) | 20–29 | 26 | 17 | 11 (HD 5) | 25 | 21 | 20 (HD 2) | 25 | 18 | 18 (HD 1) |
| Bun (mg/dL) | 7–25 | 28 | 88 | 148 (HD 8) | 18 | 16 | 40 (HD 3) | 11 | 7 | 11 (HD 4) |
| Crt (mg/dL) | 0.6–1.3 | 1.7 | 4.2 | 6.1 (HD 8) | 1.4 | 1.4 | 1.5 (HD 3) | 0.9 | 0.5 | 0.9 (HD 1) |
| Alb (g/dL) | 3.2–5 | 2.5 | 1.7 | 1.5 (HD 11) | 2.4 | 1.7 | 1.6 (HD 6) | 2.9 | 2.2 | 1.8 (HD 4) |
| Alk (U/L) | 45–117 | 125 | 104 | 141 (HD 1) | 164 | 171 | 202 (HD 6) | 255 | 176 | 255 (HD 2) |
| AST (U/L) | < 36 | 215 | 197 | 229 (HD 1) | 156 | 283 | 283 (HD 2) | 137 | 135 | 241 (HD 5) |
| ALT (U/L) | < 46 | 90 | 94 | 101 (HD 1) | 106 | 129 | 129 (HD 2) | 132 | 112 | 169 (HD 5) |
| Tbili (mg/dL) | 0.2–1.2 | 3.7 | 9 | 12.4 (HD 5) | 2.9 | 4.2 | 7.1 (HD 5) | 1.1 | 1.0 | 1.1 (HD 2) |
| LA (mmol/L) | 0.5–2.2 | 3 | 2.5 | 3 (HD 1) | 3.1 | 4.3 | 4.3 (HD 2) | 1 | N/A | 1.1 (HD 3) |
| Arterial pH | 7.35–7.45 | N/A | 7.39 | 7.25 (HD 4) | N/A | 7.29 | 7.29 (HD 2) | N/A | N/A | 7.38 (HD 3) |
| Hematologic laboratory values | Reference Range | Admission | Transfer to ICU (HD 3) | Maximum or nadir (when) | Admission | Transfer to ICU (HD 2) | Maximum or nadir (when) | Admission | Transfer to ICU (HD 1) | Maximum or nadir (when) |
|---|---|---|---|---|---|---|---|---|---|---|
| WBC (K/µL) | 3.4–10.4 | 9.8 | 16.4 | 17.4 (HD 2) | 5.9 | 7.5 | 18.2 (HD 4) | 5.9 | 4.7 | 16.1 (HD 3) |
| Bands (%) | < 10 | N/A | 30 | 30 (HD 3) | 54 | 70 | 70 (HD 2) | 43 | N/A | 43 (HD 1) |
| Hemoglobin (g/dL) | 12.8–17.1 | 15.5 | 11.9 | 8 (HD 12) | 15.2 | 12.9 | 11 (HD 6) | 11.2 | 9 | 8.2 (HD 4) |
| Plt (K/µL) | 140–377 | 37 | < 9 | < 9 (HD 3) | 84 | 46 | 22 (HD 6) | 151 | 118 | 113 (HD 2) |
| Miscellaneous laboratory values | Reference Range | Admission | Transfer to ICU (HD 3) | Maximum or nadir (when) | Admission | Transfer to ICU (HD 2) | Maximum or nadir (when) | Admission | Transfer to ICU (HD 1) | Maximum or nadir (when) |
|---|---|---|---|---|---|---|---|---|---|---|
| BNP (pg/mL) | < 101 | N/A | N/A | N/A | N/A | 282 | 307 (HD 4) | N/A | N/A | 106 (HD 2) |
| Troponin I (ng/mL) | < 0.05 | N/A | 0.23 | 0.23 (HD 3) | N/A | 0.36 | 0.36 (HD 2) | N/A | < 0.03 | < 0.03 (HD 1) |
| LDH (U/L) | 92–240 | N/A | N/A | 427 (HD 1) | N/A | 428 | 428 (HD 2) | N/A | N/A | N/A |
| CK (U/L) | 24–233 | N/A | 151 | 151 (HD 3) | N/A | N/A | 322 (HD 3) | N/A | N/A | N/A |
| INR | 0.8–1.2 | 1.4 | 1.5 | 1.5 (HD 3) | N/A | 1.6 | 1.6 (HD 2) | N/A | N/A | 1.3 (HD 5) |
Alk = alkaline phosphatase; BNP = B-type natriuretic peptide; CK = creatinine kinase; HD = hospital day; INR = international normalized ratio; LA = lactic acid; LDH = lactate dehydrogenase; ICU = intensive care unit.
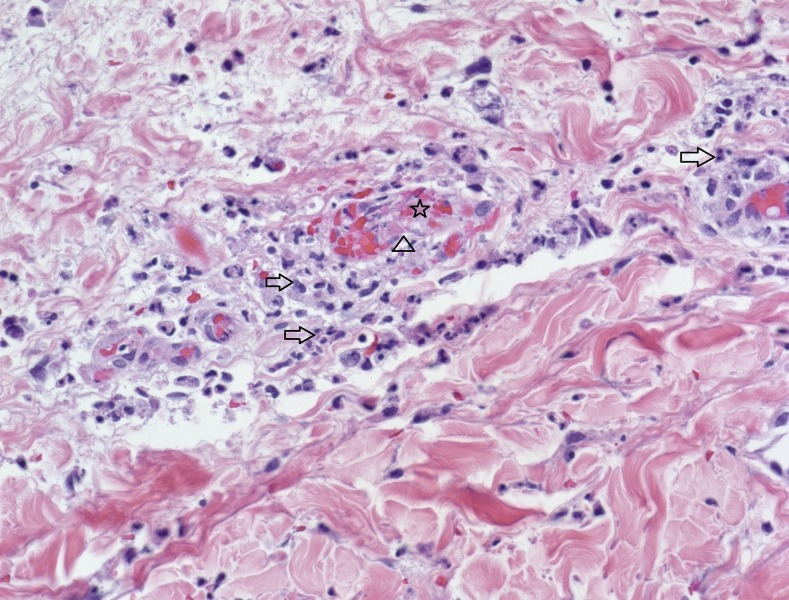
Figure 1.
Case 1: Punch biopsy of skin from the abdomen showing perivascular inflammation (arrows), destruction of vessel wall (triangle), and an intravascular thrombus (star) (hematoxylin and eosin stained section, ×400 magnification). This figure appears in color at www.ajtmh.org.
Case 2.
A 52-year-old Asian male presented with a worsening bitemporal headache and nonproductive cough over the previous 12 days. On arrival in the emergency department, he was afebrile and tachycardic, with a normal blood pressure and an examination notable for bilateral crackles. Laboratory findings revealed hyponatremia, elevated transaminase levels, hypoalbuminemia, acute kidney injury, bandemia, and thrombocytopenia (Table 1). A head CT and a lumbar puncture were unremarkable. Chest X-ray on presentation demonstrated patchy airspace in the right medial lung base. A right upper quadrant ultrasound suggested acute liver injury (Figure 2). Cefepime, vancomycin, and metronidazole were initiated. During the course of his first HD, he became febrile and hypotensive, requiring vasopressor therapy, and he developed respiratory distress, requiring bi-level positive airway pressure ventilation with oxygen supplementation. The patient was transferred to the ICU. Vasopressors were weaned off 12 hours later. Echocardiography revealed right ventricular volume overload (Figure 3A). A chest CT 2 days after presentation demonstrated bilateral pleural effusions, bilateral peribronchovascular opacities with interlobular septal thickening most compatible with pulmonary edema, and an enlarged right atrium (Figure 3B). Azithromycin and doxycycline were added to his antibiotic regimen and a rickettsial serologic panel was ordered. A ventilation/perfusion scan was inconsistent with pulmonary embolism. After aggressive diuretic therapy, he was transitioned to oxygen via high-flow nasal cannula and weaned to room air over the next 4 days. Blood, acid-fast bacilli (AFB) and fungal blood isolators, cerebrospinal fluid, and urine cultures all returned without growth. An HIV-1/2 serologic test and an HIV-RNA polymerase chain reaction (PCR) assay were negative. A nasopharyngeal respiratory pathogen PCR panel (Biofire, Salt Lake City, UT; detects 17 viral and three bacterial targets) was negative. A Legionella urine antigen test was negative. Histoplasma and Coccidioides serologic tests were negative. Serologic tests returned positive for R. typhi, IgM 1:128 and IgG < 1:64. Rocky Mountain spotted fever serologic tests were negative. He continued to clinically improve while narrowing his antibiotic therapy to doxycycline alone and was transferred from the ICU on HD 6.
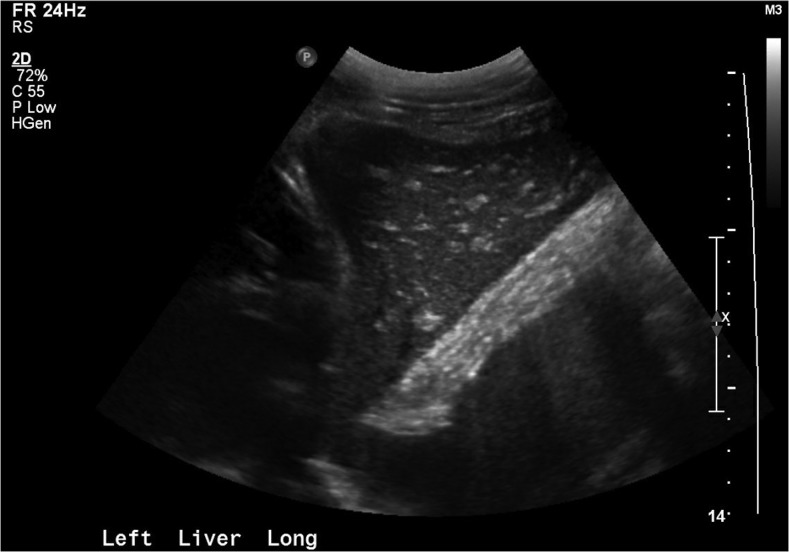
Figure 2.
Case 2: Right upper quadrant ultrasound with “starry sky” appearance, consistent with acute liver injury (echogenic portal triads and venous walls with hypo-echoic, edematous liver parenchyma). This pattern has not only been most commonly reported in viral hepatitis but has also been observed in heart failure, and other infectious, inflammatory, and neoplastic conditions of the liver.20
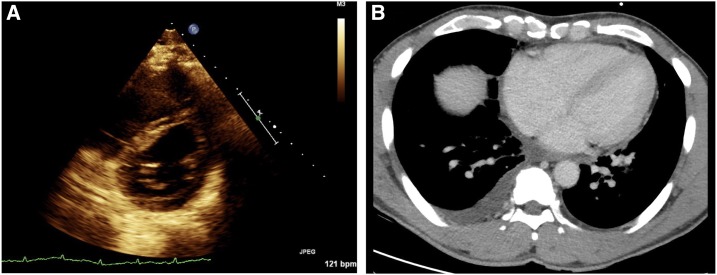
Figure 3.
Case 2: (A) Echocardiogram exhibiting “D sign” of the interventricular septum from right ventricular volume overload. (B) Computerized tomography of the chest demonstrating right atrial enlargement and a right pleural effusion. This figure appears in color at www.ajtmh.org.
Case 3.
A 32-year-old Hispanic female presented with a 1-week history of burning midepigastric pain, headache, subjective fevers, and right flank pain. The flank pain was accompanied by dark urine, dyspnea, chills, and fatigue. She was febrile, tachycardic, and had a mild erythematous rash on her arms and chest. Faint crackles were auscultated at the bilateral lung bases. Initial laboratory results demonstrated elevated transaminases, alkaline phosphatase levels, and bandemia. She developed hemodynamic instability with her blood pressure decreasing to 76/40 mm Hg, which responded to an intravenous fluid bolus. CT of the abdomen was unremarkable. Chest X-rays (Figure 4) revealed a worsening left lower lobe opacity with pleural effusion. A CT angiogram of the chest found no evidence of pulmonary embolism, demonstrating diffuse bilateral interstitial edema and a left lower lobe infiltrate associated with atelectasis and small pleural effusion. She was started on azithromycin and ceftriaxone and admitted to the ICU with a presumptive diagnosis of sepsis secondary to community-acquired pneumonia. On HD 2, the patient experienced hypoxemia with minimal exertion. A chest X-ray showed worsening bilateral pulmonary effusions and pulmonary edema. Furosemide was administered. Nevertheless, she subsequently required mechanical ventilation. Bronchoscopy with bronchoalveolar lavage of the left lower lobe was performed and showed < 10,000 colony-forming units of usual respiratory flora and Candida sp., with AFB stain and culture negative. Blood cultures were negative. Urinalysis and urine culture were inconsistent with a urinary tract infection. An HIV-1/2 serologic test and serum Cryptococcus antigen assay were negative. A nasopharyngeal respiratory pathogen PCR panel (Biofire, as aforementioned) was negative. A Legionella urinary antigen assay was negative. Histoplasma, Coccidioides, and Borrelia burgdorferi serologic tests were negative. Because of increasing leukocytosis and lack of improvement on ceftriaxone and azithromycin, antibiotic coverage was broadened on HD 4 to ceftriaxone, vancomycin, and doxycycline, and a rickettsial serologic panel was sent. The patient improved and was extubated on HD 5. After 7 days of hospitalization, she experienced resolution of all symptoms and was discharged on cefpodoxime and doxycycline. Serologic results returned after discharge and were negative for RMSF and positive for R. typhi IgM 1:128 with IgG < 1:64.
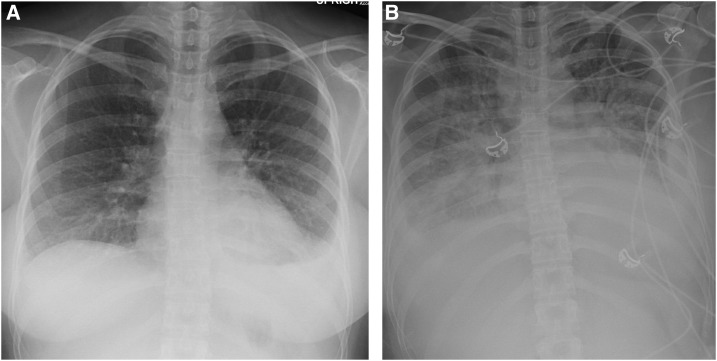
Figure 4.
Case 3: (A) Chest X-ray on admission demonstrating left lower infiltrate with left pleural effusion. (B) Chest X-ray on the second hospital day demonstrating worsening pulmonary edema with bilateral pleural effusion.
DISCUSSION
Flea-borne typhus has often been regarded as a self-limited infection, with a 4% and a 0.6% mortality among untreated and treated adult cases, respectively.6,7 This case series describes the clinical trajectories of three patients with FBT-associated multi-organ failure. In endemic areas, clinicians may not recognize the septic presentations of FBT when considering the potential etiologies of acute undifferentiated febrile illness. Early recognition of FBT before decompensation remains a challenge because of the nonspecific nature of its presentaton.7 Because of its myriad presentations, FBT is often mistaken for other conditions. In one series from Texas, a rickettsiosis was initially suspected in only 11% of the patients.8 The classic clinical triad of fever, headache, and rash for the recognition of FBT is only observed in one-third of patients.1,9,10 Rash may be absent in 52% of cases.10 About 75% of patients do not recall flea exposure.1,10 Risk stratification in FBT is difficult because even patients who are healthy at baseline can suffer fulminant organ dysfunction.1,10
The underlying pathophysiology of R. typhi–induced sepsis is endothelial dysfunction and vasculitis, presenting with distinct diagnostic findings. About 80% of adult patients with FBT present with hypoalbuminemia and elevated levels of transaminases and lactate dehydrogenase; thrombocytopenia, increased alkaline phosphatase levels, and hyponatremia occur in 40–50%.10 Although the diagnosis of FBT by PCR has been described, there are no widely available tests for the diagnosis in the acute phase.11 The indirect fluorescent antibody (IFA) serologic assay remains the gold standard for diagnosis of rickettsioses; however, diagnosis is delayed by the window period of up to 2 weeks of illness before serologic reactivity12 and by the need to send specimens to a reference laboratory. In a compatible clinical case, FBT is confirmed by the IFA method by a 4-fold rise in serum antibody titer to typhus group antigen. A probable diagnosis of FBT is defined as a clinically compatible case with supportive laboratory results (a single IFA titer of 128 or greater while having a lower titer to spotted fever group (R. rickettsii) antigen than to typhus group antigen).8 Only about half of FBT patients tested during the first 6 days of illness will have diagnostic titers, but most patients will have diagnostic titers after 2 weeks of illness.13 The definitive diagnosis of FBT is usually made retrospectively, based on acute and convalescent titers obtained after the initial presentation.1,14 Because convalescent titers are not available for cases 2 and 3, these cases are considered probable FBT.
In this context of initial uncertainty regarding a FBT diagnosis, the evaluation of the septic patient with imaging and microbiologic testing may favor an alternative etiology. Conversely, laboratory studies may disclose findings associated with FBT while ruling out other etiologies. As the course progressed in all three of these cases, evidence of common bacterial causes of sepsis was absent. This series illustrates the necessity for clinicians in FBT-endemic areas to quickly consider a diagnosis of FBT when more common etiologies of multi-organ dysfunction are not supported by diagnostic testing.
Complications occur in 28% of cases of adult FBT.2 Neurologic, ocular, cardiac, pulmonary, gastrointestinal, renal, and hematologic manifestations have been described, although septic shock is rarely reported.8,15–18 In a 2008 series of 33 FBT patients, one-third required ICU admission.4 In another series of 80 patients, three deaths occurred, two of which were precipitated by progressive shock and multi-organ failure.8 We have reported a fatal case of FBT with a course complicated by septic shock.18 Risk factors for increased severity of FBT include older age, male gender, glucose-6-phosphate dehydrogenase deficiency, treatment with sulfa antibiotics, and African origin.1,8,9 Abnormalities associated with increased severity include petechial rash, pulmonary dysfunction, neurologic presentation, hyponatremia, hypokalemia, hypocalcemia, hypoalbuminemia, leukocytosis, thrombocytopenia, and elevated creatinine.8 The patients in this series exhibited abnormalities associated with increased severity. All displayed severe hypoalbuminemia. Patients 1 and 2 showed hyponatremia, lactic acidosis, thrombocytopenia, and coagulopathy. Patient 1 developed renal failure (creatinine 6.1 mg/dL; dialysis not required, recovery after 6 weeks). Uncommon manifestations of FBT observed in this series include pancreatitis,2 gastrointestinal bleeding, hemorrhagic conjunctivitis, and transient ischemic attack in patient 1 and the “starry sky” ultrasound appearance of liver injury and right heart strain in patient 2.2,19 Heart failure may have been due to the myocarditis known to occur in FBT.17 All three patients had prominent leukocytosis and bandemia; the latter is associated with increased mortality.20
The morbidity to individual patients by FBT has costs that are difficult to quantify, including medical care and lost productivity during convalescence. Considering only medical costs, the amount of resources used is related to the severity of illness. The All Patient Refined Diagnosis Related Groups (APR-DRG) that code for the patients’ admissions in this series were obtained. The estimated hospital costs were calculated using the associated relative weight of the respective APR-DRG code.21 This relative weight was multiplied by the state-determined base rate.22 The average estimated cost for inpatient care of these patients was $25,235, ranging from $9,583.40 to $44,601.90. This cost was comparable to the average cost of severe sepsis ($24,638) in U.S. hospitals.23 In another recent study, the median medical cost of a case of FBT was $16,760.24
In this series, each patient required ICU care and organ support, with one requiring vasopressor therapy and two requiring positive pressure ventilation. Sequential organ failure assessment (SOFA) scores provide an assessment of prognosis in the context of sepsis.25 Table 2 provides SOFA scores and the timelines of organ dysfunction for each patient. Increases in SOFA scores ≥ 2 during sepsis are associated with an inhospital mortality > 10%.26 In scrub typhus, SOFA scores correlate with hospital length of stay and mortality rates.27 The SOFA score of patient 1 on admission was 7, reached a maximum of 14 on HD 3 (despite doxycycline on HD 1), and then declined. Patient 2 had a score of 5 on admission, which increased to 11 by HD 2, despite doxycycline on HD 1. Patient 3 had a score of 1 on admission, which increased to 6 by HD 3, but then declined after receiving doxycycline on HD 4. For a summary of the presentation, complications, and severity indicators of these patients, see Table 3. Early initiation of empiric broad-spectrum antibiotics (“escalation”) is an evidence-based intervention for the reversal of the cascade of sepsis and multi-organ dysfunction in critically ill patients.28,29 For the subset of FBT patients that progress to organ failure, a high index of suspicion and targeted anti-rickettsial therapy as soon as FBT is suspected may prevent further decompensation; doxycycline is the preferred drug, with chloramphenicol, azithromycin, and fluoroquinolones as second-line agents.1,30 Thus, we posit that for patients in FBT-endemic areas who present with organ dysfunction due to sepsis of uncertain source, there should be early doxycycline escalation for potential rickettsiosis pending the results of serologic and other diagnostic testing.
Table 2.
SOFA scores and indices of organ system dysfunction during hospitalization
| Patient 1 | Patient 2 | Patient 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Admission | Transfer to ICU | Worst SOFA score | Admission | Transfer to ICU | Worst SOFA score | Admission | Transfer to ICU | Worst SOFA score | |
| Hospital day | 1 | 3 | 5 | 1 | 2 | 2 | 1 | 1 | 3 |
| SOFA score* | 7 | 14 | 14 | 5 | 11 | 11 | 1 | 2 | 6 |
| SOFA parameters | |||||||||
| PaO2 (mm Hg) | N/A | 64 | 73 | N/A | 49 | 49 | N/A | N/A | 56 |
| FiO2 | 0.21 | 0.32 | 0.21 | 0.35 (BI-PAP) | 0.21 (Bi-PAP) | 0.21 (Bi-PAP) | N/A (O2 via nasal cannula) | N/A (O2 via nasal cannula) | 0.5 |
| PaO2/FiO2 ratio | N/A | 200 | 348 | N/A | 233 | 233 | N/A | N/A | 112 |
| Plt (K/µL) | 37 | < 9 | 23 | 84 | 41 | 41 | 151 | 118 | 134 |
| Glasgow Coma Scale | 15 | 14 | 13 | 15 | 15 | 15 | 15 | 15 | 11 |
| TBili (mg/dL) | 3.7 | 9 | 12.4 | 2.9 | 4.2 | 4.2 | 1.1 | 1.0 | 0.7 |
| Level of hypotension | MAP < 70 | MAP < 70 | MAP < 70 | No hypotension | Epinephrine < 0.1 mcg/kg/minutes | Epinephrine < 0.1 mcg/kg/minutes | MAP < 70 | MAP < 70 | No hypotension |
| Crt (mg/dL) | 1.74 | 4.18 | 5.7 | 1.4 | 1.4 | 1.4 | 0.86 | 0.51 | 0.5 |
Plt = platelet count; SOFA = sequential organ failure assessment.
* Reference 25 (specifically efigure 3) provides representative inhospital mortality rates in sepsis for a given SOFA score. Accordingly, SOFA score ≥ 9 is associated with > 20% mortality; SOFA score ≥ 14 is associated with > 40% mortality.
Table 3.
Summary of clinical characteristics of cases 1–3
| Case | Key presenting features | Factors necessitating ICU transfer | Complications | Hospital day when doxycycline was started | Days in ICU | Clinical and laboratory indicators of increased severity* |
|---|---|---|---|---|---|---|
| 1 | Vomiting/diarrhea | Hypotension | Acute kidney injury | 1 | 7 | Bleeding coagulopathy (suspected) |
| Fever | Melena | Coagulopathy | CNS abnormalities | |||
| Rash | Encephalopathy | Hepatic dysfunction | ||||
| Hyponatremia | Hypotension | Hypoalbuminemia | ||||
| Acute kidney injury | Pancreatitis | Hyponatremia | ||||
| Elevated LFTs | Upper gastrointestinal bleed (suspected) | Leukocytosis | ||||
| Thrombocytopenia | Renal dysfunction | |||||
| Coagulopathy | Thrombocytopenia | |||||
| 2 | Headache | Hypotension | Acute kidney injury | 1 | 6 | Hepatic dysfunction |
| Cough | Hypoxic respiratory failure | Hypoxic respiratory failure | Hyponatremia | |||
| Hyponatremia | Septic shock | Leukocytosis | ||||
| Acute kidney injury | Right heart failure | Pulmonary compromise | ||||
| Hypoalbuminemia | Renal dysfunction | |||||
| Elevated LFTs | Thrombocytopenia | |||||
| Bandemia | ||||||
| Thrombocytopenia | ||||||
| Lung infiltrate | ||||||
| 3 | Abdominal | Hypotension | Hypotension | 4 | 6 | Delayed diagnosis |
| Pain | Hypoxic respiratory failure | Hypoxic respiratory failure | Hepatic dysfunction | |||
| Headache | Pneumonia | Hypoalbuminemia | ||||
| Fever | Leukocytosis | |||||
| Dyspnea | Pulmonary compromise | |||||
| Chills | Thrombocytopenia | |||||
| Fatigue | ||||||
| Tachycardia | ||||||
| Rash | ||||||
| Hypoalbuminemia | ||||||
| Elevated LFTs | ||||||
| Bandemia |
ICU = intensive care unit; LFTs = liver function tests (AST/ALT/alkaline phosphatase). All three patients had minimally abnormal potassium and calcium level when corrected for albumin.
* Based on reference 8—disease severity related to older patient age, hyponatremia, hypokalemia, hypocalcemia, renal dysfunction, hypoalbuminemia, leukocytosis, delayed diagnosis, thrombocytopenia, bleeding coagulopathy, cardiac arrhythmias, hepatic dysfunction, pulmonary compromise, CNS abnormalities, previous therapy with sulfa-containing antimicrobial agents, and hemolysis usually associated with glucose-6-phosphate dehydrogenase deficiency.
REFERENCES
- 1.Civen R, Ngo V, 2008. Murine typhus: an unrecognized suburban vectorborne disease. Clin Infect Dis 46: 913–918. [DOI] [PubMed] [Google Scholar]
- 2.Afzal Z, Kallumadanda S, Wang F, Hemmige V, Musher D, 2017. Acute febrile illness and complications due to murine typhus, Texas, USA. Emerg Infect Dis 23: 1268–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray KO, Evert N, Mayes B, Fonken E, Erickson T, Garcia MN, Sidwa T, 2017. Typhus group rickettsiosis, Texas, USA, 2003–2013. Emerg Infect Dis 23: 645–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adjemian J, Parks S, McElroy K, Campbell J, Eremeeva ME, Nicholson WL, McQuiston J, Taylor J, 2010. Murine typhus in Austin, Texas, USA, 2008. Emerg Infect Dis 16: 412–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.California Department of Public Health–Department of Public Health Vector-Borne Disease Section , 2018. Human Flea-Borne Typhus Cases in California. Available at: https://www.cdph.ca.gov/Programs/CID/DCDC/CDPH Document Library/Flea-borneTyphusCaseCounts.pdf. Accessed March 3, 2018. [Google Scholar]
- 6.Eremeeva ME, et al. 2012. Two pathogens and one disease: detection and identification of flea-borne rickettsiae in areas endemic for murine typhus in California. J Med Entomol 49: 1485–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pieracci EG, Evert N, Drexler NA, Mayes B, Vilcins I, Huang P, Campbell J, Behravesh CB, Paddock CD, 2017. Fatal flea-borne typhus in Texas: a retrospective case series, 1985–2015. Am J Trop Med Hyg 96: 1088–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dumler JS, Taylor JP, Walker DH, 1991. Clinical and laboratory features of murine typhus in south Texas, 1980 through 1987. JAMA 266: 365–1370. [PubMed] [Google Scholar]
- 9.Chaliotis G, Kritsotakis EI, Psaroulaki A, Tselentis Y, Gikas A, 2012. International journal of infectious diseases murine typhus in central Greece: epidemiological, clinical, laboratory, and therapeutic-response features of 90 cases. Int J Infect Dis 16: 591–596. [DOI] [PubMed] [Google Scholar]
- 10.Tsioutis C, Zafeiri M, Avramopoulos A, Prousali E, Miligkos M, Karageorgos SA, 2017. Clinical and laboratory characteristics, epidemiology, and outcomes of murine typhus: a systematic review. Acta Trop 166: 16–24. [DOI] [PubMed] [Google Scholar]
- 11.Theunissen C, Cnops L, Van Esbroeck M, Huits R, Bottieau E, 2017. Acute-phase diagnosis of murine and scrub typhus in Belgian travelers by polymerase chain reaction: a case report. BMC Infect Dis 17: 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paris DH, Dumler JS, 2016. State of the art of diagnosis of rickettsial diseases: the use of blood specimens for diagnosis of scrub typhus, spotted fever group rickettsiosis, and murine typhus. Curr Opin Infect Dis 29: 433–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dumler JS, Walker DH, 1994. Diagnostic tests for Rocky Mountain spotted fever and other rickettsial diseases. Dermatol Clin 12: 25–36. [PubMed] [Google Scholar]
- 14.Blanton LS, 2013. Rickettsial infections in the tropics and in the traveler. Curr Opin Infect Dis 26: 435–440. [DOI] [PubMed] [Google Scholar]
- 15.Bernabeu-Wittel M, Villanueva-Marcos JL, de Alarcón-González A, Pachón J, 1998. Septic shock and multiorganic failure in murine typhus. Eur J Clin Microbiol Infect Dis 17: 131–132. [DOI] [PubMed] [Google Scholar]
- 16.Sakamoto N, Nakamura-Uchiyama F, Kobayashi KI, Takasaki T, Ogasawara Y, Ando S, Iwabuchi S, Ohnishi K, 2013. Severe murine typhus with shock and acute respiratory failure in a Japanese traveler after returning from Thailand. J Travel Med 20: 50–53. [DOI] [PubMed] [Google Scholar]
- 17.Walker DH, Parks FM, Betz TG, Taylor JP, Muehlberger JW, 1989. Histopathology and immunohistologic demonstration of the distribution of Rickettsia typhi in fatal murine typhus. Am J Clin Pathol 91: 720–724. [DOI] [PubMed] [Google Scholar]
- 18.Stephens BE, Thi M, Alkhateb R, Agarwal A, Sharkey FE, Dayton C, Anstead GM, 2018. Case report: fulminant murine typhus presenting with status epilepticus and multi-organ failure: an autopsy case and a review of the neurologic presentations of murine typhus. Am J Trop Med Hyg 99: 306–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel NJ, Caserta MP, DiSantis DJ, 2016. The “starry sky” liver. Ultrasound 14: 675–679. [DOI] [PubMed] [Google Scholar]
- 20.Chung JY, Hsu CC, Chen JH, Chen WL, Lin HJ, Guo HR, Huang CC, 2018. Geriatric influenza death (GID) score: a new tool for predicting mortality in older people with influenza in the emergency department. Sci Rep 8: 9312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Texas APR-DRG Grouper , 2018. Available at: https://rad.hhs.texas.gov/sites/rad/files/documents/hospital-svcs/2019/2019-hosp-aprdrg.pdf. Accessed August 14, 2018.
- 22.HHSC Hospital Rate Analysis Department, 2018. All Patient Refined Diagnosis Related Group. Available at: https://rad.hhs.texas.gov/sites/rad/files/documents/hospital-svcs/2018/2018-hosp-urban-sda.pdf. Accessed August 14, 2018.
- 23.Paoli CJ, Reynolds MA, Sinha M, Gitlin M, Crouser E, 2018. Epidemiology and costs of sepsis in the United States-an analysis based on timing of diagnosis and severity level. Crit Care Med 46: 1889–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vohra RF, Walker DH, Blanton LS, 2018. Analysis of health-care charges in murine typhus: need for improved clinical recognition and diagnostics for acute disease. Am J Trop Med Hyg 98: 1594–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singer M, et al. 2016. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315: 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seymour CW, et al. 2016. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315: 762–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balasubramanian P, Sharma N, Biswal M, Bhalla A, Kumar S, Kumar V, 2018. Critical illness scoring systems: sequential organ failure assessment, acute physiology and chronic health evaluation II, and quick sequential organ failure assessment to predict the clinical outcomes in scrub typhus patients with organ dysfunctions. Indian J Crit Care Med 22: 706–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rhodes A, et al. 2017. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock. Intensive Care Med 43: 304–377. [DOI] [PubMed] [Google Scholar]
- 29.Gou Y, Gao W, Yang H, Ma C, Sui S. 2016. De-escalation of empiric antibiotics in patients with severe sepsis or septic shock: a meta-analysis. Heart Lung 45: 454–459. [DOI] [PubMed] [Google Scholar]
- 30.Newton PN, et al. 2019. A prospective, open-label, randomized trial of doxycycline versus azithromycin for the treatment of uncomplicated murine typhus. Clin Infect Dis 68: 738–747. [DOI] [PMC free article] [PubMed] [Google Scholar]