Abstract.
Dengue, chikungunya, and Zika viruses, primarily transmitted by Aedes species mosquitoes, have caused large outbreaks in the Americas, leading to travel-associated cases and local mosquito-borne transmission in the United States. We describe the epidemiology of dengue, chikungunya, and noncongenital Zika virus disease cases reported from U.S. states and territories in 2017, including 971 dengue cases, 195 chikungunya cases, and 1,118 Zika virus disease cases. Cases of all three diseases reported from the territories were reported as resulting from local mosquito-borne transmission. Cases reported from the states were primarily among travelers, with only seven locally acquired mosquito-transmitted Zika virus disease cases reported from Texas (n = 5) and Florida (n = 2). In the territories, most dengue cases (n = 508, 98%) were reported from American Samoa, whereas the majority of chikungunya (n = 39, 100%) and Zika virus disease (n = 620, 93%) cases were reported from Puerto Rico. Temporally, the highest number of Zika virus disease cases occurred at the beginning of the year, followed by a sharp decline, mirroring decreasing case numbers across the Americas following large outbreaks in 2015 and 2016. Dengue and chikungunya cases followed a more seasonal pattern, with higher case numbers from July through September. Travelers to the United States and residents of areas with active virus transmission should be informed of both the ongoing risk from dengue, chikungunya, and Zika virus disease and personal protective measures to lower their risk of mosquito bites and to help prevent the spread of these diseases.
INTRODUCTION
Dengue, chikungunya, and Zika viruses are tropical and subtropical arboviruses transmitted to humans by Aedes (Stegomyia) species mosquitoes, such as Aedes aegypti and Aedes albopictus.1–3 Humans are the primary amplifying host for all three viruses. Dengue and Zika viruses belong to the genus Flavivirus; chikungunya virus belongs to the genus Alphavirus. Dengue is caused by any of four antigenically distinct dengue viruses (DENV-1–4). Clinical presentations of dengue, chikungunya, and Zika virus disease are similar, especially during the acute phase.4 Although most DENV infections are asymptomatic, the infection can cause symptoms ranging from a febrile illness with headache, myalgia, arthralgia, and rashes to severe and sometimes fatal manifestations including plasma leakage, hemorrhage, and organ impairment.5 For most individuals, chikungunya is characterized by acute fever and polyarthralgia; some patients have persistent joint pains for months to years following the initial illness.2,6 Most Zika virus infections are asymptomatic or result in mild clinical illness, characterized by acute onset of fever, maculopapular rash, arthralgia, or nonpurulent conjunctivitis.1 Congenital Zika virus infection can result in fetal loss, microcephaly, or other birth defects.1 Rare but serious complications, including Guillain–Barré syndrome and meningoencephalitis, may occur with any of the three arboviral diseases.
Beginning in the second half of the 20th century, DENV transmission expanded across the Americas, with case numbers increasing over time, reaching a peak of 2.4 million cases reported to the Pan American Health Organization in 2015.5,7,8 Dengue transmission is classified as frequent or continuous in Puerto Rico, the U.S. Virgin Islands, and American Samoa, and all three U.S. territories have a history of periodic outbreaks.9 From 1980 to 2015, dengue cases within the U.S. states occurred primarily among travelers to endemic regions, with limited mosquito-borne transmission occurring in Hawaii, Florida, and Texas.3,10–13
Before 2014, chikungunya cases in the United States occasionally occurred in travelers returning from the Africa, Asia, and Oceania regions.4,14 In the Americas, the first identification of local transmission of chikungunya virus occurred in late 2013 and resulted in large outbreaks in the Caribbean, Central America, South America, and Mexico during 2014–2015. These outbreaks led to a sharp increase in travel-associated cases in U.S. states, widespread transmission in Puerto Rico and the U.S. Virgin Islands, and limited local transmission in Florida and Texas.4,15,16 Mirroring the declining incidence of chikungunya in the Americas, the United States experienced a decreased number of chikungunya cases in 2015 and 2016.15–17
Similar to chikungunya, before the identification of local transmission of Zika virus in 2015 in the Americas, small numbers of cases were identified among travelers returning to the United States from the Africa, Asia, and Oceania regions.18 In 2015 and 2016, large outbreaks of Zika virus occurred in the Americas, resulting in an increase in travel-associated cases in U.S. states, widespread transmission in Puerto Rico and the U.S. Virgin Islands, and limited local transmission in Florida and Texas.19–23
This report describes confirmed and probable cases of dengue, chikungunya, and noncongenital Zika virus disease with illness onset during 2017 reported from U.S. states and the District of Columbia, as well as the U.S. territories of Puerto Rico, the U.S. Virgin Islands, and American Samoa.
MATERIALS AND METHODS
In the United States, dengue, chikungunya, and Zika virus disease became nationally notifiable conditions in 2010, 2015, and 2016, respectively.24 Reported cases are classified as confirmed or probable according to national case definitions, which include laboratory testing, clinical, and epidemiologic criteria.25–27 Dengue cases are further classified by clinical syndrome, including dengue, dengue-like illness, and severe dengue. Cases are reported to the CDC through ArboNET, the national arboviral disease surveillance system. We included confirmed and probable cases of dengue, chikungunya, and noncongenital Zika virus disease reported from U.S. states, the District of Columbia, Puerto Rico, the U.S. Virgin Islands, and American Samoa with illness onset during January 1–December 31, 2017. Asymptomatic infections and congenital Zika virus disease cases were excluded.
RESULTS
In 2017, a total of 2,284 cases of dengue (n = 971), chikungunya (n = 195), and Zika virus disease (n = 1,118) were reported in the United States (Table 1). Of those, 1,059 (46%) were reported from U.S. states and 1,225 (54%) from U.S. territories.
Table 1.
Characteristics of confirmed and probable dengue, chikungunya, and Zika virus disease cases reported to ArboNET from U.S. states and territories, 2017
| Characteristic | Dengue | Chikungunya | Zika virus disease | |||
|---|---|---|---|---|---|---|
| States (N = 451), No. (%) | Territories (N = 520), No. (%) | States (N = 156), No. (%) | Territories (N = 39), No. (%) | States (N = 452), No. (%) | Territories (N = 666), No. (%) | |
| Confirmed case | 183 (41) | 509 (98) | 45 (29) | 4 (10) | 383 (85) | 501 (75) |
| Travel associated | 451 (100) | 0 (0) | 156 (100) | 0 (0) | 437 (97) | 1 (< 1) |
| Age-group (years) | ||||||
| < 20 | 64 (14) | 345 (66) | 10 (6) | 22 (56) | 51 (11) | 213 (32) |
| 20‒39 | 176 (39) | 92 (18) | 47 (30) | 6 (16) | 195 (43) | 201 (31) |
| 40‒59 | 147 (33) | 56 (11) | 71 (46) | 6 (15) | 144 (32) | 163 (24) |
| ≥ 60 | 64 (14) | 27 (5) | 28 (18) | 5 (13) | 62 (14) | 89 (13) |
| Female | 218 (48) | 257 (49) | 79 (51) | 16 (41) | 295 (65) | 396 (59) |
| Pregnant | 3 (1) | 6 (1) | 4 (3) | 1 (3) | 71 (16) | 25 (4) |
| Race | ||||||
| Asian | 134 (30) | 11 (2) | 73 (47) | 0 (0) | 8 (2) | 1 (0) |
| White | 131 (29) | 4 (1) | 21 (14) | 0 (0) | 261 (58) | 9 (1) |
| Pacific Islander | 17 (4) | 486 (93) | 5 (3) | 0 (0) | 3 (< 1) | 0 (0) |
| Other* | 40 (8) | 1 (< 1) | 16 (10) | 0 (0) | 57 (13) | 11 (2) |
| Unknown | 129 (29) | 18 (3) | 41 (26) | 39 (100) | 123 (27) | 645 (97) |
| Ethnicity | ||||||
| Hispanic or Latino | 79 (18) | 0 (0) | 11 (7) | 0 (0) | 220 (49) | 7 (1) |
| Not Hispanic or Latino | 243 (54) | 505 (97) | 95 (61) | 0 (0) | 114 (25) | 13 (2) |
| Unknown | 129 (29) | 15 (3) | 50 (32) | 39 (100) | 118 (26) | 646 (97) |
| Onset of illness | ||||||
| January–March | 63 (14) | 27 (5) | 14 (9) | 9 (23) | 136 (30) | 492 (74) |
| April–June | 59 (13) | 63 (12) | 40 (26) | 8 (21) | 83 (18) | 46 (7) |
| July–September | 198 (44) | 176 (34) | 60 (38) | 16 (41) | 170 (38) | 56 (8) |
| October–December | 131 (29) | 254 (49) | 42 (27) | 6 (15) | 63 (14) | 72 (11) |
| Hospitalized | 152 (34) | 228 (44) | 25 (16) | 9 (23) | 9 (2) | 24 (4) |
| Died | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (< 1) |
* Includes race reported as American Indian or Alaska Native, black or African American, other, and multiple. Each of these categories was reported for ≤ 5% of the cases for each virus.
Dengue.
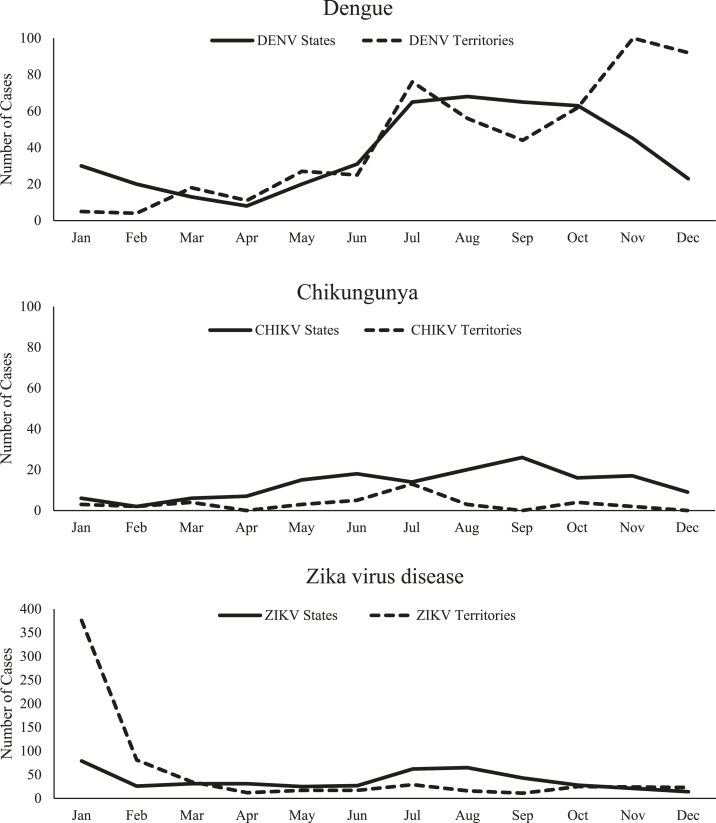
Of the 971 dengue cases reported in 2017, 451 cases (183 confirmed and 268 probable) cases were reported from 26 states (Table 1, Figure 1). All were travel associated; the highest numbers were reported from California (n = 130), Texas (n = 43), and New York (n = 38), New Jersey (n = 25), and Illinois (n = 23), which together accounted for 57% of all cases. The most frequently reported travel locations were Asia (n = 282, 63%), Mexico (n = 53, 12%), and the Caribbean (n = 29, 6%) (Table 2). The median age of case-patients was 38 years (interquartile range [IQR]: 26–51 years), and 387 (86%) were adults aged ≥ 20 years. Although a notable proportion (n = 129; 29%) were missing data for race, the most frequently reported categories were Asian (n = 134; 30%) and white (n = 129; 29%). The highest number of cases (n = 198; 44%) occurred from July through September (Figure 2). Nine (0.9%) severe dengue cases were reported; 152 (35%) case-patients were hospitalized, and there were no deaths.
Figure 1.
Dengue, chikungunya, and Zika virus disease cases reported to ArboNET by state or territory of residence, 2017. Circles represent case counts by area.
Table 2.
Travel locations for confirmed and probable dengue, chikungunya, and Zika virus disease cases reported to ArboNET from U.S. states, 2017
| Region | Dengue (N = 451), No. (%) | Chikungunya (N = 156), No. (%) | Zika virus disease (N = 452), No. (%) |
|---|---|---|---|
| No travel | 0 (0) | 0 (0) | 15 (3) |
| Asia | 282 (63) | 120 (77) | 11 (2) |
| Americas | 110 (24) | 31 (20) | 408 (90) |
| Caribbean | 29 (6) | 7 (4) | 223 (49) |
| North America* | 53 (12) | 5 (3) | 117 (26) |
| Central America | 18 (4) | 6 (4) | 40 (9) |
| South America | 10 (2) | 13 (8) | 28 (6) |
| Africa | 21 (5) | 2 (1) | 2 (< 1) |
| Oceania | 20 (4) | 0 (0) | 5 (1) |
| Europe | 0 (0) | 1 (1) | 9 (0) |
| Multiple | 0 (0) | 0 (0) | 4 (1) |
| Unknown | 18 (4) | 2 (1) | 7 (2) |
* All travel to Mexico.
Figure 2.
Dengue, chikungunya, and Zika virus disease cases reported to ArboNET by month of symptom onset and place of residence—United States, 2017.
A total of 520 dengue cases (509 confirmed and 11 probable) were reported from U.S. territories. Among these, 508 (98%) were reported from American Samoa, and all were due to local mosquito-borne transmission (Table 1, Figure 1). Almost all (n = 509; 98%) cases in the territories were confirmed. The median age of dengue case-patients reported from the territories was 16 years (IQR: 10–26 years), and 228 (44%) were aged < 20 years. Most (n = 486; 93%) dengue cases in the territories were Pacific Islanders reported from American Samoa. Of the 520 cases reported from the territories, the infecting DENV was identified in 120 (23%), all from American Samoa and DENV-2. The number of cases increased over the year, with 254 (49%) reported during October–December (Figure 2). A total of 228 (44%) case-patients were hospitalized, and there were no deaths.
Chikungunya.
Overall, 156 chikungunya cases (45 confirmed and 111 probable) were reported from 28 (56%) states (Table 1, Figure 1). The largest numbers of cases were reported from California (n = 32), New York (n = 19), Texas (n = 15), New Jersey (n = 12), and Massachusetts (n = 11), which together accounted for 57% of all cases. All the cases were associated with travel outside the contiguous United States, including 120 (77%) to Asia and 31 (20%) to other locations in the Americas (Table 2). Race was reported for roughly three-quarters (n = 115; 74%) of cases, among which the most frequently reported was Asian (n = 73; 47%). The median case-patient age was 46 years (IQR: 35–56 years), and 146 (94%) were adults aged ≥ 20 years; 79 (51%) were female. Cases occurred throughout the year, but the highest proportion (n = 60; 38%) had illness onset during July–September (Figure 2). A total of 25 (16%) case-patients were hospitalized, and none died.
In U.S. territories, 39 chikungunya cases (four confirmed and 35 probable) were reported, all from Puerto Rico and due to local mosquito-borne transmission (Table 1, Figure 1). The median case-patient age was 17 years (IQR: 8–42 years), and 22 (56%) were aged < 20 years; 16 (41%) were female. Cases occurred throughout the year, but the highest proportion (n = 16; 41%) had illness onset during July–September (Figure 2). Nine (23%) case-patients were hospitalized, and none died.
Zika virus disease.
A total of 452 Zika virus disease cases (383 confirmed and 69 probable) were reported from 43 U.S. states (Table 1, Figure 1). Over 60% of the cases were reported from four states: Florida (n = 110; 24%), New York (n = 64; 14%), Texas (n = 54; 12%), and California (n = 49; 11%). Case-patients were mostly female (n = 295; 65%) and the median age was 38 years (IQR: 26–52 years); 401 (89%) case-patients were aged ≥ 20 years. Case-patients were primarily white (n = 261; 58%). Roughly a third had no ethnicity reported, and almost half (n = 220; 49%) reported Hispanic or Latino ethnicity. A total of 71 (16%) case-patients were pregnant. Most (n = 437; 97%) cases were travel associated, with travel most frequently to the Caribbean (n = 223; 51%) or Mexico (n = 117; 27%). No travel was reported among 15 Zika virus disease cases in the states. Seven cases attributed to local mosquito-borne transmission were identified in Texas (n = 5) and Florida (n = 2). An additional seven cases were attributed to sexual transmission in five states. One case-patient had a laboratory exposure to Zika virus. January had the highest monthly case count (n = 79), but the third quarter (July–September) had the highest quarterly count (n = 170; 38%) (Figure 2). One case of Guillain–Barré syndrome was identified in a traveler. Nine (2%) Zika virus disease case-patients were hospitalized, and none died.
The territories reported 666 Zika virus disease cases; all but one case in the territories was associated with local mosquito-borne transmission (Table 1). The majority (n = 620; 93%) were reported from Puerto Rico, with the remaining 46 (7%) from the U.S. Virgin Islands (Figure 2). In the territories, cases were mostly confirmed (n = 501; 75%) and female (59%). Four percent of case-patients were pregnant. The median age was 31 years (IQR: 17–50 years), with 32% of cases aged < 20 years. The peak of Zika virus disease case numbers occurred in January (n = 376; 56%), with a steep drop in February and relatively few cases reported during the remainder of the year. One case of Guillain–Barré syndrome was reported. Twenty-four (4%) Zika virus disease cases were hospitalized. One fatal case was reported from Puerto Rico in an adult male aged 50–60 years with a history of hypertension and cardiomyopathy who presented for care following a recent febrile illness and died from cardiac arrest shortly after admission.
DISCUSSION
Although dengue has historically caused periodic outbreaks, and chikungunya and Zika viruses have recently caused widespread outbreaks in the Americas, case numbers for all three diseases were at low levels in the Americas in 2017. Zika virus disease accounted for the highest number of reported cases and most of the cases were associated with the end of the Zika virus epidemic, although U.S. states also had an increase during the warm summer months (July–September). With the exception of dengue in American Samoa, which reported a dengue outbreak during 2016–2018, chikungunya and dengue also generally followed the expected seasonality of mosquito-borne diseases in the northern hemisphere, with an increased number of cases during the warm summer months.28,29
American Samoa and Puerto Rico had the highest number of dengue and Zika virus disease cases, respectively, and almost all cases of the three arboviral diseases in the territories were the result of local mosquito-borne transmission. With the exception of a small number of Zika virus disease cases (n = 15), the remainder of all chikungunya, dengue, and Zika virus disease cases reported by U.S. states occurred among travelers to areas with active virus transmission. This difference likely explains the patterns seen by age, race, and ethnicity by location and virus. Cases in the territories tended to be younger, and cases reported from U.S. states occurred primarily in adult travelers. Travel-associated Zika virus disease cases from the states primarily traveled to the Caribbean and Mexico, whereas in contrast, most travel-associated chikungunya and dengue cases reported travel to Asia. Asian race was most frequently reported among chikungunya and dengue cases, and Zika virus disease case-patients were more frequently white and reported Hispanic ethnicity. These race and ethnicity findings could change in future years depending on the global epidemiology of these three diseases and improved reporting.
A higher proportion of dengue cases were hospitalized (39%) than chikungunya (17%) or Zika virus disease cases (3%). The hospitalization rate for chikungunya was slightly higher than other published reports, which ranged from 0.5% to 8.7%.30–33 The only fatality observed was a Zika virus disease case reported from Puerto Rico; however, the limited available clinical information suggested comorbidities likely contributed.
Approximately equal numbers of dengue and chikungunya cases were identified among males and females; this differs from some previous reports for chikungunya, where up to 66% of reported cases were among females.21,34 A higher proportion of Zika virus disease cases were identified in females, similar to previous reports.21,35–37 Factors associated with increased identification of Zika virus disease cases among females could include increased testing of women of childbearing age, differences in care-seeking behavior or mosquito exposure, increased risk from sexual transmission, or increased susceptibility to development of disease after infection.
The low proportion of dengue (41%) and chikungunya (29%) cases reported from U.S. states that are confirmed suggests that molecular diagnostic methods should be encouraged for patients presenting to health-care providers during the acute phase of illness. In addition, cases that are diagnosed using commercially available serologic methods may benefit from further confirmatory testing.
There are limitations associated with the identification, classification, and passive reporting of arboviral diseases. All three viruses can cause a spectrum of disease presentations, including mild disease, which may have resulted in individuals not visiting a provider, or providers not ordering appropriate tests. Antibody responses to dengue and Zika viruses exhibit significant cross-reactivity, especially with secondary Flavivirus infections.38,39 This can lead to misdiagnosis or cases that meet the case definition for both diseases, often making it difficult to correctly classify and report a case.40 This could result in erroneous duplicate reporting of cases, which would be challenging to identify in the de-identified dataset. Also, serologic tests can also remain positive for extended periods of time in some individuals,41 which, coupled with nonspecific signs and symptoms, make it difficult to determine whether the findings of serologic testing are associated with the patient’s illness. Similarly, diagnostic tests vary in their diagnostic accuracy, which can lead to false-positive or false-negative test results and cases being missed or incorrectly diagnosed and reported. Demographic and other key variables, including race and ethnicity, were frequently missing or incompletely reported, which limits the representativeness of the data. Finally, in areas with ongoing mosquito-borne transmission, determining other modes of transmission is often not possible. In these areas, cases are attributed to mosquito exposure, but it is likely that some Zika virus disease case-patients were infected through other transmission modes, such as sexual contact.
Currently, no vaccines to prevent chikungunya or Zika virus disease are licensed in the United States, and effective, evidence-based methods to control Ae. aegypti are lacking.42,43 A dengue vaccine was approved by the U.S. Food and Drug Association in early 2019; however, its use is limited to individuals aged 9–16 years living in endemic areas who have a laboratory-confirmed previous dengue infection. Modeling data suggest that the combination of population growth and the expansion of areas climatically suitable for Ae. aegypti will increase the number of people at risk for these and other emerging viruses transmitted by this vector.44,45 Continued virus transmission and sporadic outbreaks are likely in areas where mosquito-borne transmission has been established. Areas with Ae. aegypti but without previous virus transmission, including some U.S. states where most of the populations are immunologically naive, remain at risk for outbreaks. Fortunately, the risk of widespread transmission in U.S. states is likely mitigated by factors such as use of air conditioners, window screens, and a more temperate climate.46 Whereas chikungunya and Zika viruses are presumed to provide long-term, possibly life-long, protection from reinfection, infection with any individual DENV does not provide long-term protection against the other three DENVs, and new outbreaks could occur in the Americas in the near future. These findings from 2017 surveillance data indicate ongoing risk from dengue, chikungunya, and Zika viruses among travelers from U.S. states to areas with active virus transmission, as well as among residents of U.S. territories. Persons traveling to or living in risk areas should be educated about ways to avoid mosquito bites (e.g., using insect repellents and installing intact screens) to help mitigate their risk of infection and further spread of these diseases. Pregnant women should refer to current published guidance when they or their sexual partners are considering travel to areas with a risk of Zika. Clinicians should report suspect cases, particularly in areas not known to have active virus circulation, to help target public health interventions.
CONCLUSION
During 2017, U.S. states and territories reported 2,284 confirmed and probable cases of dengue, chikungunya, and Zika virus disease. The territories reported the highest number of dengue and Zika virus disease cases, which were primarily associated with local mosquito-borne transmission; most chikungunya cases occurred among travelers returning to the states. Cases of all three diseases in the states were primarily associated with travel to areas with ongoing virus transmission, although a small number of Zika virus disease cases were the result of infection occurring in the states from mosquitoes or through other transmission routes. Ongoing surveillance for all three diseases in the U.S. states and territories is critical to better understand the global risk of these diseases, detect possible local transmission, provide appropriate clinical care, and identify and protect groups at highest risk for infection and disease.
Acknowledgments:
We thank the state, local, and territorial health departments reporting to ArboNET.
REFERENCES
- 1.Petersen LR, Jamieson DJ, Powers AM, Honein MA, 2016. Zika virus. N Engl J Med 374: 1552–1563. [DOI] [PubMed] [Google Scholar]
- 2.Staples JE, Breiman RF, Powers AM, 2009. Chikungunya fever: an epidemiological review of a re-emerging infectious disease. Clin Infect Dis 49: 942–948. [DOI] [PubMed] [Google Scholar]
- 3.Brunkard JM, López JLR, Ramirez J, Cifuentes E, Rothenberg SJ, Hunsperger EA, Moore CG, Brussolo RM, Villarreal NA, Haddad BM, 2007. Dengue fever seroprevalence and risk factors, Texas–Mexico border, 2004. Emerg Infect Dis 13: 1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yactayo S, Staples JE, Millot V, Cibrelus L, Ramon-Pardo P, 2016. Epidemiology of chikungunya in the Americas. J Infect Dis 214 (Suppl 5): S441–S445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization and the Special Programme for Research and Training in Tropical Diseases (TDR) , 2009. Dengue Guidelines for Diagnosis, Treatment, Prevention and Control, New edition Geneva, Switzerland: World Health Organization. [Google Scholar]
- 6.Feldstein LR, Rowhani-Rahbar A, Staples JE, Weaver MR, Halloran ME, Ellis EM, 2017. Persistent arthralgia associated with chikungunya virus outbreak, US Virgin Islands, December 2014–February 2016. Emerg Infect Dis 23: 673–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.San Martin JL, Brathwaite O, Zambrano B, Solorzano JO, Bouckenooghe A, Dayan GH, Guzman MG, 2010. The epidemiology of dengue in the Americas over the last three decades: a worrisome reality. Am J Trop Med Hyg 82: 128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan American Health Organization , 2017. Dengue Fever in the Americas: Number of Reported Cases By Country or Territory. Available at: http://www.paho.org/data/index.php/en/mnu-topics/indicadores-dengue-en/dengue-nacional-en/252-dengue-pais-ano-en.html?showall=&start=1. Accessed November 1, 2018. [Google Scholar]
- 9.Jentes ES, Lash RR, Johansson MA, Sharp TM, Henry R, Brady OJ, Sotir MJ, Hay SI, Margolis HS, Brunette GW, 2016. Evidence-based risk assessment and communication: a new global dengue-risk map for travellers and clinicians. J Travel Med 23: taw062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention , 2010. Locally acquired dengue-key west, Florida, 2009–2010. MMWR Morb Mortal Wkly Rep 59: 577–581. [PubMed] [Google Scholar]
- 11.Effler PV, Pang L, Kitsutani P, Vorndam V, Nakata M, Ayers T, Elm J, Tom T, Reiter P, Rigau-Perez JG, 2005. Dengue fever, Hawaii, 2001–2002. Emerg Infect Dis 11: 742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rigau-Perez JG, Gubler DJ, Vorndam AV, Clark GG, 1994. Dengue surveillance–United States, 1986–1992. MMWR CDC Surveill Summ 43: 7–19. [PubMed] [Google Scholar]
- 13.Ramos MM, et al. 2008. Epidemic dengue and dengue hemorrhagic fever at the Texas–Mexico border: results of a household-based seroepidemiologic survey, December 2005. Am J Trop Med Hyg 78: 364–369. [PubMed] [Google Scholar]
- 14.Gibney KB, Fischer M, Prince HE, Kramer LD, St George K, Kosoy OL, Laven JJ, Staples JE, 2011. Chikungunya fever in the United States: a fifteen year review of cases. Clin Infect Dis 52: e121–e126. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention , 2018. Chikungunya Virus in the United States. Available at: https://www.cdc.gov/chikungunya/geo/united-states.html. Accessed July 3, 2018. [Google Scholar]
- 16.Lindsey NP, Staples JE, Fischer M, 2018. Chikungunya virus disease among travelers-United States, 2014–2016. Am J Trop Med Hyg 98: 192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan American Health Organization , 2018. Chikungunya. Available at: https://www.paho.org/hq/index.php?option=com_topics&view=article&id=343&Itemid=40931&lang=en. Accessed July 3, 2018. [Google Scholar]
- 18.Hennessey MJ, Fischer M, Panella AJ, Kosoy OI, Laven JJ, Lanciotti RS, Staples JE, 2016. Zika virus disease in travelers returning to the United States, 2010–2014. Am J Trop Med Hyg 95: 212–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walker WL, Lindsey NP, Lehman JA, Krow-Lucal ER, Rabe IB, Hills SL, Martin SW, Fischer M, Staples JE, 2016. Zika virus disease cases–50 states and the District of Columbia, January 1–July 31, 2016. MMWR Morb Mortal Wkly Rep 65: 983–986. [DOI] [PubMed] [Google Scholar]
- 20.Adams L, et al. 2016. Update: ongoing Zika virus transmission–Puerto Rico, November 1, 2015-July 7, 2016. MMWR Morb Mortal Wkly Rep 65: 774–779. [DOI] [PubMed] [Google Scholar]
- 21.Hall V, et al. 2018. Update: noncongenital Zika virus disease cases–50 U.S. States and the District of Columbia, 2016. MMWR Morb Mortal Wkly Rep 67: 265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamer DH, et al. 2017. Travel-associated Zika virus disease acquired in the Americas through February 2016: a GeoSentinel analysis. Ann Intern Med 166: 99–108. [DOI] [PubMed] [Google Scholar]
- 23.Wilder-Smith A, Chang CR, Leong WY, 2018. Zika in travellers 1947–2017: a systematic review. J Travel Med 25: 1–10. [DOI] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention , 2018. Surveillance Case Definitions for Current and Historical Conditions. Available at: https://wwwn.cdc.gov/nndss/conditions/. Accessed July 3, 2018. [Google Scholar]
- 25.Centers for Disease Control and Prevention , 2015. Dengue Virus Infections 2015 Case Definition. Available at: https://wwwn.cdc.gov/nndss/conditions/dengue/case-definition/2015/. Accessed July 3, 2018. [Google Scholar]
- 26.Centers for Disease Control and Prevention, Arboviral Diseases , 2015. Neuroinvasive and Non-neuroinvasive 2015 Case Definition. Available at: https://wwwn.cdc.gov/nndss/conditions/chikungunya-virus-disease/case-definition/2015/. Accessed July 3, 2018. [Google Scholar]
- 27.Centers for Disease Control and Prevention Zika Virus Disease and Zika Virus Infection 2016 Case Definition. Available at: https://wwwn.cdc.gov/nndss/conditions/zika/case-definition/2016/06/. Accessed July 3, 2018. [Google Scholar]
- 28.Cotter CJ, Tufa AJ, Johnson S, Matai’a M, Sciulli R, Ryff KR, Hancock WT, Whelen C, Sharp TM, Anesi MS, 2018. Outbreak of dengue virus type 2—American Samoa, November 2016–October 2018. MMWR Morb Mortal Wkly Rep 67: 1319–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwartz E, Weld LH, Wilder-Smith A, von Sonnenburg F, Keystone JS, Kain KC, Torresi J, Freedman DO, GeoSentinel Surveillance N, 2008. Seasonality, annual trends, and characteristics of dengue among ill returned travelers, 1997–2006. Emerg Infect Dis 14: 1081–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsu CH, et al. 2019. Risk factors for hospitalization of patients with chikungunya virus infection at sentinel hospitals in Puerto Rico. PLoS Negl Trop Dis 13: e0007084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar A, Best C, Benskin G, 2017. Epidemiology, clinical and laboratory features and course of chikungunya among a cohort of children during the first Caribbean epidemic. J Trop Pediatr 63: 43–49. [DOI] [PubMed] [Google Scholar]
- 32.Rolle A, et al. 2016. Severe sepsis and septic shock associated with chikungunya virus infection, Guadeloupe, 2014. Emerg Infect Dis 22: 891–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Genderen FT, Krishnadath I, Sno R, Grunberg MG, Zijlmans W, Adhin MR, 2016. First chikungunya outbreak in Suriname; clinical and epidemiological features. PLoS Negl Trop Dis 10: e0004625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharp TM, Ryff KR, Alvarado L, Shieh WJ, Zaki SR, Margolis HS, Rivera-Garcia B, 2016. Surveillance for chikungunya and dengue during the first year of chikungunya virus circulation in Puerto Rico. J Infect Dis 214 (Suppl 5): S475–S481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lozier MJ, et al. 2018. Differences in prevalence of symptomatic Zika virus infection, by age and sex-Puerto Rico, 2016. J Infect Dis 217: 1678–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lozier M, et al. 2016. Incidence of Zika virus disease by age and sex—Puerto Rico, November 1, 2015–October 20, 2016. MMWR Morb Mortal Wkly Rep 65: 1219–1223. [DOI] [PubMed] [Google Scholar]
- 37.Gordon A, et al. 2019. Prior dengue virus infection and risk of Zika: a pediatric cohort in Nicaragua. PLoS Med 16: e1002726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, Stanfield SM, Duffy MR, 2008. Genetic and serologic properties of Zika virus associated with an epidemic, Yap state, Micronesia, 2007. Emerg Infect Dis 14: 1232–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Calisher CH, Karabatsos N, Dalrymple JM, Shope RE, Porterfield JS, Westaway EG, Brandt WE, 1989. Antigenic relationships between flaviviruses as determined by cross-neutralization tests with polyclonal antisera. J Gen Virol 70: 37–43. [DOI] [PubMed] [Google Scholar]
- 40.Lindsey NP, et al. 2018. Ability to serologically confirm recent Zika virus infection in areas with varying past incidence of dengue virus infection in the United States and U.S. territories in 2016. J Clin Microbiol 56: e01115–e01117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Griffin I, Martin SW, Fischer M, Chambers TV, Kosoy O, Falise A, Ponomareva O, Gillis LD, Blackmore C, Jean R, 2019. Zika virus IgM detection and neutralizing antibody profiles 12–19 months after illness onset. Emerg Infect Dis 25: 299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bowman LR, Donegan S, McCall PJ, 2016. Is dengue vector control deficient in effectiveness or evidence?: Systematic review and meta-analysis. PLoS Negl Trop Dis 10: e0004551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Achee NL, Gould F, Perkins TA, Reiner RC, Jr., Morrison AC, Ritchie SA, Gubler DJ, Teyssou R, Scott TW, 2015. A critical assessment of vector control for dengue prevention. PLoS Negl Trop Dis 9: e0003655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Monaghan AJ, Sampson KM, Steinhoff DF, Ernst KC, Ebi KL, Jones B, Hayden MH, 2018. The potential impacts of 21st century climatic and population changes on human exposure to the virus vector mosquito Aedes aegypti. Clim Change 146: 487–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu-Helmersson J, Stenlund H, Wilder-Smith A, Rocklov J, 2014. Vectorial capacity of Aedes aegypti: effects of temperature and implications for global dengue epidemic potential. PLoS One 9: e89783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reiter P, Lathrop S, Bunning M, Biggerstaff B, Singer D, Tiwari T, Baber L, Amador M, Thirion J, Hayes J, 2003. Texas lifestyle limits transmission of dengue virus. Emerg Infect Dis 9: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]