Abstract.
Madariaga virus (MADV), previously known as South American eastern equine encephalitis virus (SA EEEV; family Togaviridae, genus Alphavirus), is a mosquito-borne virus associated mainly with equine disease. In 2010, the first human outbreak by MADV was reported in Central America, but the mosquito vectors and vertebrate hosts involved in the outbreak were not identified. In Argentina, the first epizootic of MADV was in 1930, and since then, several epizootics by MADV have been reported. However, the potential vectors and hosts involved in the transmission cycle remain unknown. In the present study, MADV was detected in Culex (Culex) spp. mosquitoes and the phylogenetic analysis showed that the MADV fragment amplified grouped with the lineage/subtype III of the SA EEEV complex. Our results provide information about the natural infection with MADV in mosquitoes collected in a wild environment of Argentina and its genetic relatedness.
Madariaga virus (MADV) is a mosquito-borne, single-stranded, positive-sense RNA virus (family Togaviridae, genus Alphavirus) belonging to the South American eastern equine encephalitis virus (SA EEEV) complex. Three lineages/subtypes (II, III, and IV), which circulate in Central and South America, have been described.1–4
Up to 2010, MADV infection was associated mainly with equine disease.5 However, in that year, 13 human positive cases with MADV disease were reported in Panama, being the first human outbreak associated with this virus. Nonetheless, the mosquito vectors and vertebrate hosts involved in the outbreak were not identified.6
The first equine epizootic probably associated with MADV ever reported occurred in Buenos Aires Province (Argentina) in 1930, but the virus was not identified until 1953.7 Since then, several epizootics by MADV have been documented, and in some cases, the etiology would appear to be mixed or the virus would show an overlapping distribution with Western equine encephalitis virus.8,9 During an interepidemic period between 1977 and 1980, arbovirus serological surveys performed in wild and domestic birds, wild mammals, and horses from central and northeastern Argentina detected antibodies against MADV in wild birds and horses from Chaco Province, in northeastern Argentina.10 However, in 1981, MADV was the only etiologic agent responsible for the first outbreak in horses from Santiago del Estero Province in the central region of Argentina.11 This epizootic was characterized by low morbidity and high mortality, with absence of antibodies or very low titers against MADV in humans and absence of antibodies in domestic poultry and other animals.11 The last epizootic by MADV recorded in Argentina was in 1988 and occurred in Castelli, Chaco Province. This epizootic was the first and only record for this province.9
Despite the circulation history of MADV in Argentina, the potential vectors and hosts involved in its transmission cycle remain unknown. Therefore, the aim of this work was to look for MADV activity in wild mosquitoes collected in Chaco Province.
Mosquitoes were collected in a wild environment of Chaco Province between 2009 and 2011. This environment is located in the provincial park Pampa del Indio, which belongs to a climatic transition zone between the wet Chaco and dry Chaco eco regions. Adult mosquitoes were collected with CDC light traps supplemented with dry ice. Each mosquito was taxonomically determined under a stereoscopic microscope on a chill table and pooled by species, sex, collection site, and date, with a maximum of 50 adults per pool. Some of the specimens were difficult to identify to the species level because they were damaged. On the other hand, some specimens of the subgenus Culex could not be identified to the species level because of the morphological similarities of some females and the lack of clarity in the key distinctive features. A total of 1,243 mosquitoes belonging to eight genera and 30 species (Table 1) were grouped in 116 pools and analyzed by RT-nested PCR for alphavirus detection (195 bp), targeting the NSP4 gene.12
Table 1.
List of mosquitoes surveyed for molecular detection of alphaviruses in Chaco Province, Argentina, between 2009 and 2011
| Species | Pools | Number of mosquitoes |
|---|---|---|
| Aedeomyia (Aedeomyia) squamipennis | 2 | 29 |
| Aedes (Ochlerotatus) albifasciatus | 1 | 1 |
| Aedes (Ochlerotatus) hastatus | 9 | 182 |
| Aedes (Ochlerotatus) scapularis | 10 | 281 |
| Aedes (Ochlerotatus) spp. | 10 | 258 |
| Aedes (Ochlerotatus) stigmaticus | 5 | 24 |
| Anopheles (Anopheles) neomaculipalpus | 1 | 1 |
| Anopheles (Nissorhynchus) evansae | 1 | 1 |
| Anopheles benarrochi | 1 | 2 |
| Anopheles spp. | 1 | 1 |
| Culex (Culex) amaliae | 1 | 6 |
| Culex (Culex) apicinus | 2 | 2 |
| Culex (Culex) bidens | 5 | 20 |
| Culex (Culex) chidesteri | 2 | 6 |
| Culex (Culex) coronator | 1 | 2 |
| Culex (Culex) eduardoi | 2 | 5 |
| Culex (Culex) maxi | 2 | 3 |
| Culex (Culex) spp. | 7 | 136 |
| Culex (Melanoconion) educator | 1 | 4 |
| Culex (Melanoconion) intrinactus | 4 | 9 |
| Culex (Melanoconion) ocossa | 2 | 3 |
| Culex (Melanoconion) spp. | 2 | 6 |
| Culex (Microculex) imitator | 1 | 1 |
| Haemagogus (Conopostegus) leucocelaenus | 1 | 2 |
| Haemagogus (Haemagogus) spegazzini | 4 | 6 |
| Mansonia (Mansonia) titillans | 7 | 25 |
| Mansonia (Mansonia) spp. | 2 | 4 |
| Psorophora (Grabhamia) paulli | 1 | 2 |
| Psorophora (Grabhamia) varinervis | 2 | 2 |
| Psorophora (Janthinosoma) albigenu | 5 | 83 |
| Psorophora (Janthinosoma) cyanescens | 4 | 20 |
| Psorophora (Janthinosoma) discruscians | 2 | 2 |
| Psorophora (Janthinosoma) ferox | 8 | 45 |
| Psorophora (Janthinosoma) spp. | 4 | 62 |
| Psorophora (Psorophora) ciliata | 1 | 1 |
| Wyeomyia (Phoniomyia) diabolica | 1 | 2 |
| Wyeomyia (Phoniomyia) spp. | 1 | 4 |
| Total | 116 | 1,243 |
One pool of 40 Culex (Cux.) spp. collected in the summer of 2011 was positive, and the nested amplified product was purified and sequenced in both directions at Macrogen Inc., Seoul, Republic of Korea. The amplified fragment (PI74) was edited using the program MEGA X13 and its relatedness with the complete GenBank database was assessed with the Basic Local Alignment Search Tool (http://blast.ncbi.nlm.nih.gov). This analysis showed that PI74 displayed a high similarity score with MADV (99.3% identity, E-value 6e-66). In addition, a maximum likelihood phylogenetic tree was built including all MADV strains available at GenBank and Virus Pathogen Database and Analysis Resource (https://www.viprbrc.org/) databases for the NSP4 partial region. Sequences of EEEV (lineage/subtype I of the North American EEEV complex) were used as outgroup to root the tree. The analysis was carried out with the IQ-Tree software v1.6 (Vienna, Austria),14 using an appropriate substitution model according to the Akaike information criterion, estimated with the jModelTest v2.1 software (A Coruña, Spain).15 Confidence was evaluated with the bootstrap method (1,000 replicates).
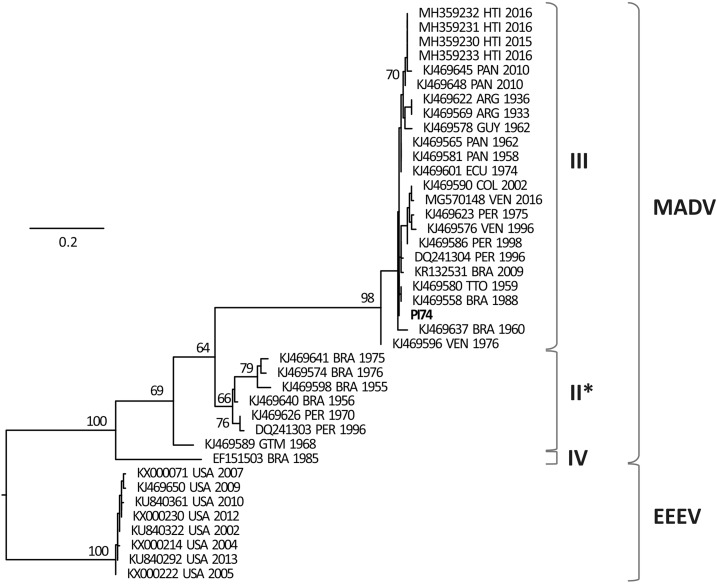
After the extensive period of time since the first detection of MADV in Argentina, this study confirmed the first detection of natural infection with MADV in mosquitoes collected in a wild environment of Argentina. The phylogenetic analysis showed that the MADV (PI74) detected grouped within the largest cluster of the virus, which contains sequences from several Central and South American countries (Figure 1) and belongs to the lineage/subtype III of the SA EEEV complex. This lineage/subtype was previously isolated in Argentina (1936 and 1933). This evidence supported the endemicity of MADV and indicated that the subtropical area of Argentina has the ecological and environmental conditions to maintain the activity of MADV.
Figure 1.
Phylogenetic tree of the partial NSP4 gene of Madariaga virus (MADV). Eastern equine encephalitis virus (EEEV) was used as outgroup to root the tree. Bootstrap values higher than 50% are shown at nodes for relevant groups. The isolate obtained in this work is shown in bold. Subtypes II–IV are detailed. * Non-monophyletic cluster using this region.
Different authors have suggested that MADV is maintained by vertebrate hosts with limited mobility such as rodents4,7,16,17 and that the genetic diversity of MADV lineages could be favored by the greater diversity of mosquito species found in subtropical and tropical areas.7 In different countries of Central and South America, several species of wild mosquitoes, mainly of the genus Culex, subgenus Melanoconion, have been found to be infected with MADV, thus suggesting an important role of these species in the transmission cycle of MADV.5 In the Amazon Basin region of Peru, the repeated isolations and study of susceptibility to infection in Culex (Mel.) pedroi suggest that this species is an enzootic vector in this region. However, in Argentina, in contrast to the wide distribution and great abundance of species of the subgenus Culex in the temperate zone, where most MADV epizootics have occurred, the distribution and richness of the species of the subgenus Melanoconion are significantly reduced.18 In the present study, MADV was detected in Culex (Culex) spp. mosquitoes, which are considered mainly ornithophilic but can also feed on mammals.19 Stein et al.20 found that Culex (Culex) spp. mosquitoes change their host selection from birds to mammals in different environments, selecting mainly birds in urban areas and mammals in wild areas. These authors also found that in wild areas, species of the subgenus Melanoconion were captured mainly in mammal baited traps.20 In Argentina, during the epizootic that occurred in Chaco Province in 1988, different species of mosquitoes were collected in autumn, when the epizootic was still occurring, and Culex (Culex) bidens was one of the most abundant species, being present both on the farm and in the nearby wild environment where the traps were placed.9 The finding of Culex (Culex) spp. naturally infected with MADV suggests that other subgenera, different from Melanoconion, could be involved in the enzootic cycle of MADV in Argentina.
Further studies focused on the biological and molecular characterization of epizootic and enzootic MADV strains isolated in Argentina as well as host and vector competence assays are required to disentangle the maintenance of MADV in subtropical areas of Argentina.
Acknowledgments:
We thank Viviana Ré and Gerardo Deluca for their technical assistance, and Dirección de Fauna y Áreas Naturales Protegidas, Subsecretaria de Recursos Naturales de la Provincia de Chaco, Ministerio de Producción of Argentina for authorization for the collection and transportation of biological material from the Parque Provincial Pampa del Indio Pampa del Indio to the Instituto de Medicina Regional of the Universidad Nacional del Nordeste, Chaco, Argentina. The American Society of Tropical Medicine and Hygiene (ASTMH) assisted with publication expenses.
REFERENCES
- 1.Casals J, 1964. Antigenic variants of eastern equine encephalitis virus. J Exp Med 119: 547–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brault AC, Shope RE, Lopez RN, Weaver SC, Tesh RB, Kang W, Chavez CL, Gutierrez LF, Cachón MF, Powers AM, 1999. Genetic and antigenic diversity among eastern equine encephalitis viruses from North, Central, and South America. Am J Trop Med Hyg 61: 579–586. [DOI] [PubMed] [Google Scholar]
- 3.Van Regenmortel MHV, et al. 2000. Virus Taxonomy: Classification and Nomenclature of Viruses. Seventh Report of the International Committee on Taxonomy of Viruses . Van Regenmortel MHV, Fauquet CM, Bishop DHL, eds. San Diego, CA: Academic Press. [Google Scholar]
- 4.Weaver SC, Powers AM, Brault AC, Barrett AD, 1999. Molecular epidemiological studies of veterinary arboviral encephalitides. Vet J 157: 123–138. [DOI] [PubMed] [Google Scholar]
- 5.Scott TW, Weaver SC, 1989. Eastern equine encephalomyelitis virus: epidemiology and evolution of mosquito transmission. Adv Virus Res 37: 277–328. [DOI] [PubMed] [Google Scholar]
- 6.Carrera J-P, et al. 2013. Eastern equine encephalitis in Latin America. N Engl J Med 369: 732–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arrigo NC, Adams AP, Weaver SC, 2010. Evolutionary patterns of eastern equine encephalitis virus in North versus South America suggest ecological differences and taxonomic revision. J Virol 84: 1014–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sabattini MS, Monath TP, Mitchell CJ, Daffner JF, Bowen GS, Pauli R, Contigiani MS, 1985. Arbovirus investigations in Argentina, 1977–1980. I. Historical aspects and description of study sites. Am J Trop Med Hyg 34: 937–944. [PubMed] [Google Scholar]
- 9.Sabattini MS, Avilés G, Monath TP, 1998. Historical, epidemiological and ecological aspects of arboviruses in Argentina: Togaviridae, Alphavirus. Travassos da Rosa A.P.A, Vasconcelos P.F.C, Travassos da Rosa J.F.S, eds. Overview of Arbovirology in Brazil and Neighbouring Countries. Belém, Brazil: Instituto Evandro Chagas, 135–152. [Google Scholar]
- 10.Monath TP, Sabattini MS, Pauli R, Daffner JF, Mitchell CJ, Bowen GS, 1985. Arbovirus investigations in Argentina, 1977–1980. IV. Serologic surveys and sentinel equine program. Am J Trop Med Hyg 34: 966–975. [PubMed] [Google Scholar]
- 11.Sabattini MS, Daffner JF, Monath TP, Bianchi TI, Cropp CB, Mitchell CJ, Aviles G, 1991. Localized eastern equine encephalitis in Santiago del Estero Province, Argentina, without human infection. Medicina (B Aires) 51: 3–8. [PubMed] [Google Scholar]
- 12.Sánchez-Seco MP, Rosario D, Quiroz E, Guzmán G, Tenorio A, 2001. A generic nested-RT-PCR followed by sequencing for detection and identification of members of the Alphavirus genus. J Virol Methods 95: 153–161. [DOI] [PubMed] [Google Scholar]
- 13.Kumar S, Stecher G, Li M, Knyaz C, Tamura K, 2018. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35: 1547–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen L-T, Schmidt HA, von Haeseler A, Minh BQ, 2015. IQ-tree: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol 32: 268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darriba D, Taboada GL, Doallo R, Posada D, 2012. jModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9: 772–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vittor AY, et al. 2016. Epidemiology of emergent madariaga encephalitis in a region with endemic venezuelan equine encephalitis : initial host studies and human cross-sectional study in Darien, Panama. PLoS Negl Trop Dis 10: e0004554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arrigo NC, Paige Adams A, Watts DM, Newman PC, Weaver SC, 2010. Cotton rats and house sparrows as hosts for North and South American strains of eastern equine encephalitis virus. Emerg Infect Dis 16: 1373–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rossi GC, 2015. Annotated checklist, distribution, and taxonomic bibliography of the mosquitoes (Insecta: Diptera: Culicidae) of Argentina. Check List 11: 1–15. [Google Scholar]
- 19.Molaei G, Huang S, Andreadis TG, 2012. Vector-host interactions of Culex pipiens complex in northeastern and southwestern USA. J Am Mosq Control Assoc 28: 127–136. [DOI] [PubMed] [Google Scholar]
- 20.Stein M, Zalazar L, Willener JA, Almeida FL, Almirón WR, Stein M, Zalazar L, Willener JA, Almeida FL, Almirón WR, 2013. Culicidae (Diptera) selection of humans, chickens and rabbits in three different environments in the province of Chaco, Argentina. Mem Inst Oswaldo Cruz 108: 563–571. [DOI] [PMC free article] [PubMed] [Google Scholar]