Abstract
Cocaine-use disorders are characterized by repeated relapse to drug-seeking and -taking behavior following periods of abstinence. Former drug users display increased activation of the orbitofrontal cortex (OFC) in response to drug-related cues and similar phenomena are also observed in rodent models of drug relapse. The lateral, but not medial, OFC functionally contributes to the maintenance of cue-drug associations; however, less is known about the role of the ventral OFC in this process. To examine the pattern of neuronal activation in OFC subregions in response to drug-associated cues, rats were trained to respond on a lever for a cocaine infusion paired with a complex cue (2-hr sessions, minimum 10 days). Cocaine self-administration was followed by extinction training, in which lever responses resulted in no consequences (2-hr sessions, minimum 7 days). During a 1-hr reinstatement test, drug-seeking behavior (i.e. responses on the drug-paired lever) was examined in the presence or absence of contingent drug-paired cues (Cue TEST vs. Ext TEST, respectively). Rats were overdosed with a ketamine + xylazine cocktail 30 min post session, and transcardially perfused with 4% paraformaldehyde. Cfos protein expression was utilized to measure potential changes in neural activation between the reinstatement test groups. An increase in the number of Cfos-Immunoreactive cells was observed in the ventral and lateral subregions of the OFC in the Cue TEST group. The present findings provide evidence that the ventral and lateral regions of the rat OFC display similar patterns of neuronal activation in response to cocaine-paired cues.
Keywords: reinstatement, drug relapse, rat, cocaine-associated cue, self-administration, Cfos, orbitofrontal cortex
INTRODUCTION
Chronic cocaine-use disorders are defined by alternating cycles of drug use, with increased time spent to obtain or use drug, and repeated attempts to quit or control drug use, throughout which feelings of craving may persist (American Psychiatric Association, 2013; Saunders, 2017). Both environmental and explicit stimuli become associated with drug taking after repeated use, and future exposure to these drug-associated stimuli can precipitate relapse (Ehrman, Robbins, Childress, & O’Brien, 1992; Foltin & Haney, 2000). The rodent self-administration procedure provides a useful model to probe the neural mechanisms underlying relapse. Similar to humans, rats readily self-administer cocaine and subsequently display relapse-like behavior when previous drug-paired cues are presented (Davis & Smith, 1976; Wit & Stewart, 1981). Relapse in the rodent model is defined by reinstatement of drug-seeking behavior (i.e., increased responding on a previously drug-paired lever) when stress, drug-paired cues or drug are presented contingently or non-contingently (Buffalari, Feltenstein, & See, 2013; Erb, Shaham, & Stewart, 1996; See, Grimm, Kruzich, & Rustay, 1999; Venniro, Caprioli, & Shaham, 2016; Wit & Stewart, 1981).
Investigations into the neurobiological underpinnings that contribute to relapse have revealed that when former cocaine users are presented with drug-related stimuli, elements of the mesocorticolimbic circuit are activated (Goldstein & Volkow, 2002). Specifically, increased OFC activation occurs during cocaine intoxication, during self-reported craving and in response to implicit and explicit drug-paired cues (Bonson et al., 2002; Childress et al., 2008; Young et al., 2014). The OFC plays important roles in cognitive functioning, habit formation and reversal learning, and therefore it is hypothesized that dysregulation of this region contributes to the ability of drug-related cues to elicit relapse (Izquierdo, 2017; Kufahl et al., 2005; Lasseter, Xie, Ramirez, & Fuchs, 2010; Schoenbaum, Roesch, Stalnaker, & Takahashi, 2009). When rats receive contingent presentations of previous drug-paired cues, enhanced measures of plasticity (i.e. increased immediate early gene expression) are observed in the OFC. For example, presentation of previous cocaine-paired cues result in increased numbers of Cfos protein-expressing cells (Kufahl et al., 2009; Zavala, Biswas, Harlan, & Neisewander, 2007) and increased expression of Arc and zif268 mRNA in the OFC (Thomas, Arroyo, & Everitt, 2003; Zavala, Osredkar, Joyce, & Neisewander, 2008).
The OFC consists of several heterogeneous subregions spanning the anterior-posterior axis (Izquierdo, 2017); however drug-associated, cue-induced changes in Cfos are often examined in relatively posterior regions (+3.2 to +3.7) of the lateral OFC (lOFC) (Fanous et al., 2012; Kufahl et al., 2009). The lOFC, but not the medial subregion, is functionally important for cue-induced and context-induced reinstatement of cocaine-seeking behavior (Arguello et al., 2017; Fuchs, Evans, Parker, & See, 2004b; Lasseter et al., 2010). While there is evidence that the immediate early gene zif268 is elevated in the ventral OFC (vOFC) during drug reward (Thomas et al., 2003), the involvement of this region in cue-induced drug-seeking behavior is understudied. Therefore, we aimed to determine if the vOFC and lOFC are differentially recruited during contingent presentation of previous drug-paired cues and whether differences might also be observed along the anterior-posterior axis of these regions. To assess neural activation, the number of Cfos-Immunoreactive (IR) cells was quantified throughout the anterior-posterior axis of the vOFC and lOFC.
MATERIALS & METHODS
Animals
Male Sprague Dawley rats (Envigo Inc, Haslett, MI; N=20) were single housed under reversed light-dark conditions (lights off 7am, on 7pm). Humidity and temperature were regulated within the vivarium and rats were fed 20–25g of irradiated rodent chow with water available ad libitum. Rats arrived at 260–275 grams and underwent habituation and handling for 5 days prior to surgery. Protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at Michigan State University (MSU) and followed the National Research Council’s Guide for the Care and Use of Laboratory Rats.
Surgery
Rats were fully anesthetized using a mixture of ketamine + xylazine (80–100 + 5–10 mg/kg, respectively; Henry Schein, Dublin, OH and Akorn Inc, Lake Forest, IL) via intraperitoneal (ip) injection. Intravenous (iv) catheters were constructed in house and consisted of: 22-gauge, 11-mm length guide cannula with a screw type connector (Plastics One, Roanoke, VA), 210/125 μm polypropylene mesh (Sefar Propyltex, Buffalo, NY), 0.64 mm I.D, 1.19 mm O.D. silastic tubing (Dow Corning Corporation, Midland, MI), dental cement and liquid acrylic (Lang Dental Manufacturing, Wheeling, IL) and clear silicone sealant (Henkel Corp, Rocky Hill, CT). Catheters were implanted into the right jugular vein subcutaneously, exiting the rat’s back near the shoulder blade, as previously described (Fuchs, Eaddy, Su, & Bell, 2007; Thomsen & Caine, 2005). Rats were administered pre- and post-surgical oral carprofen analgesia (MediGel Clear H2O, Westbrook, ME). During the 5-day recovery period, catheters were flushed with 0.1 mL cefazolin (100 mg/mL; WG Critical Care, Paramus, NJ) dissolved in heparinized saline (70 U/mL; Fresenius Kabi, Lake Zurich, IL), followed by 0.1 mL heparinized saline (10 U/mL) to prevent infection and maintain catheter patency. Prior to each self-administration session, rats were flushed with 0.1 mL heparinized saline (70 U/mL). After the 2-hr session, rats were flushed with 0.1 mL cefazolin, then 0.1 mL heparinized saline (10 U/mL). During the self-administration phase, rats were periodically given 0.1 mL propofol (10 mg/mL; Hospira Inc, Lake Forest, IL) to test patency of catheters.
Cocaine Self-Administration Training
Self-administration training occurred during a single 2-hr session/day over at least 10 days and was complete after rats met acquisition criteria on at least 10 training days (≥ 10 infusions per session). Cocaine hydrochloride (NIDA Drug Supply System, Research Triangle Park, NC) was dissolved in sterile saline (Hospira Inc, Lake Forest, IL) at a working concentration of 3.0 mg/mL. Rats were placed in operant boxes (29.5 × 24 × 28 cm; Med Associates Inc, St. Albans, NY) with two levers available. Catheters were connected to single-channel swivels (Instech Laboratories Inc, Plymouth Meeting, PA) which connected to a 10.0 mL sterile syringe via tygon tubing. Drug delivery was controlled by an infusion pump (Med Associates Inc, Model PHM-107). The house light remained on for the entirety of the self-administration session. A response on the active lever resulted in a 2-sec infusion (0.15 mg/0.05 mL) overlaid with a 5-sec complex cue presentation and a timeout period of 20 sec. The complex cue consisted of a white light above the active lever and pure tone (80 dB). During the timeout period, active lever responses had no consequences. Inactive lever responses also resulted in no consequences but were still recorded. Lever response and infusion data were recorded using Med-PC IV run on Windows 10.
Extinction Training
After reaching acquisition criteria for self-administration training, daily 2-hr extinction sessions were conducted across a minimum of 7 days in the same operant box. Active and inactive levers were extended during training, and responses were recorded, but resulted in no programmed consequences. Extinction training was complete when responses on the active lever were below criterion for at least two consecutive days (≤ 25 lever responses).
Reinstatement Testing
Rats were split into two groups, with assignment counterbalanced based on previous cocaine intake and lever responses during self-administration and extinction training. Rats in the extinction test group (Ext TEST) received a 1-hr reinstatement test with lever responses resulting in no consequences. The Cue TEST group received a 1-hr reinstatement test in which active lever responses resulted in non-reinforced presentations of the previously drug-paired cue. After the reinstatement test, rats were returned to their transport cage for 30 min before perfusion. Sacrifice occurred 90 min after the start of the test session, which coincides with the timepoint of maximal induction of Cfos protein (Fanous et al., 2012; Hamlin, Clemens, & McNally, 2008; Herrera & Robertson, 1996; Miller & Marshall, 2005; Müller, Curran, Müller, & Guilbert, 1985; Neisewander et al., 2000).
Tissue Sectioning and Preparation
Rats were sacrificed with a lethal dose of ketamine + xylazine (240/15 mg/kg, respectively), transcardially perfused with 0.1 M phosphate buffered saline (PB) for 3 min, followed by 4% paraformaldehyde (PFA) for 15 min (Sigma Aldrich). All solutions were perfused at a rate of 10.0 mL/min (Ismatec IPC ISM931C, Barrington, IL). Brains were extracted and post fixed in 4% PFA for 24 hr at 4°C and subsequently transferred to 30% sucrose + 0.1% sodium azide (Sigma Aldrich) solution for cryoprotection. Five, serial sets of 30-μm coronal brain sections were collected on a freezing sliding microtome (Thermo Fisher Scientific, Microm HM 430) and stored in 0.1 M PB + 0.1% sodium azide at 4°C until they were processed for Cfos detection.
Cfos Immunohistochemistry (IHC)
To stain for Cfos protein, one serial set was slide mounted onto superfrost plus slides (Fisher Scientific). Brain sections containing both vOFC and lOFC were mounted, from bregma +5.12 to +3.72 (Paxinos & Watson, 2014). Slides were coded before the start of IHC and the code was broken after data analysis was complete so as to remain blind to treatment, as previously described (Arguello et al., 2008). Brain sections underwent blocking with 3% normal goat serum (Vector Laboratories Inc, Burlingame CA; Cat# S-1000, Lot# ZD0404) and 0.3% Triton X-100 (Sigma Aldrich) for 1 hr, followed by incubation in rabbit-anti-Cfos primary antibody, in 3% serum + 0.3% Tween-20, overnight at room temperature (Millipore Sigma; Cat# ABE457, Lot# 2987437, 1:1000). Tissues were incubated with biotinylated-goat-anti-rabbit secondary antibody (Vector Laboratories Inc; Cat# BA1000, Lot# ZC0329, 1:200) for 1 hr, followed by quenching of endogenous peroxidases with 0.3% H2O2 for 30 min. IHC was completed using the avidin-biotin diaminobenzidine (ABC-DAB) visualization method (Vector Laboratories Inc; Cat# PK6100), with ABC incubation for 1 hr followed by 20 min of incubation in DAB. Tissue was counter stained using Nuclear Fast Red (RICCA Chemical Company, Arlington TX), dehydrated with increasing concentrations of ethanol (70%, 90%, and 100%) and Citrisolv (Decon Labs, King of Prussia, PA) and cover slipped with DPX (Sigma Aldrich).
Microscopic Analysis and Quantification
Cfos-IR cell number was quantified under brightfield conditions with a Model Eclipse Ni- U microscope (Nikon Instruments Inc, Melville, NY). Images of the left and right vOFC and lOFC were taken at 20X (1920 pixel x 1460 pixel) at 6 bregma points spanning +5.16 to +3.72. A total of 12 images for vOFC and 12 images for lOFC were examined at each bregma point (Nikon NIS Elements Software and Photometrics Dyno Cooled Monochrome Camera). Images were analyzed with NIH Image J version 1.52, thresholded to quantify darkly-stained Cfos-IR cells and three observers separately quantified cell number.
Statistical Analyses
Separate analyses of variance (ANOVAs) or independent t-tests were conducted to examine for possible pre-existing differences in treatment groups for: cocaine intake, lever responses during self-administration training (mean of last 3 days), last day of extinction training and number of days to reach acquisition criteria for self-administration and extinction. Rats that did not acquire self-administration or did not pass the catheter patency test were excluded from analysis. Lever responses during the non-reinforced reinstatement test session were analyzed with mixed factorial ANOVAs with between-subject factors of treatment (Ext vs. Cue TEST) and within-subject factors of test day (Ext Day 7 vs. Test Day) or time bin during reinstatement test (20-min bins). Significant effects, when appropriate, were followed by Tukey’s HSD post-hoc tests, with alpha set at 0.05.
RESULTS
Behavioral History
Rats displayed stable responding on both the active and inactive levers on the last three days of cocaine self-administration (≤ 10% variability in daily cocaine intake). There were no pre-existing differences in the number of days needed to acquire self-administration (t14= 1.47, p=0.17) between groups that would be tested in an Ext TEST vs. Cue TEST. Furthermore, there were no significant pre-existing differences observed in: lever responses during self-administration (active: t14=0.30, p=0.77; inactive: t14=0.57, p=0.58), lever responses during the last day of extinction (active: t14=0.29, p=0.77; inactive: t14= 1.22, p=0.24) or cocaine intake (t14=0.023, p=0.98).
Extinction
Ext TEST and Cue TEST groups did not differ in active or inactive lever responses during extinction training. The 2 × 7 ANOVAs of lever responses revealed a significant extinction day main effect (active: F6,14=39.87, p<0.0001; inactive: F6,14=4.83, p<0.0001), with no treatment main (active: F1,14=0.66, p=0.43; inactive: F1,14=0.034, p=0.86) or extinction day x treatment interaction effects (active: F6,14=0.81, p=0.57; inactive: F6,14=1.53, p=0.18). Therefore, active lever responding decreased by the last day of extinction training for both groups (Ext D1>D7, Tukey’s tests, P<0.01).
Reinstatement Testing
Contingent presentation of the previously cocaine-paired cue resulted in increased cocaine-seeking behavior during the reinstatement test (Fig 1b). The 2 × 2 ANOVA of active lever responses during the last extinction day vs the test day revealed a significant day x treatment interaction effect (F1,14=17.61, p=0.001), day main (F1,14=9.38, p=0.008) and treatment main (F1,14=22.00, p<0.0001) effects. The Cue TEST group exhibited more active lever responses during the test day compared to the last day of extinction (Tukey’s test, p<0.01). Furthermore, the Cue TEST group exhibited greater active responses compared to the Ext TEST group during the 1-hr reinstatement test (Tukey’s test, p<0.01). A time course analysis of active lever responses during the 1-hr reinstatement test revealed significant time main (F2,14=5.55, p=0.009) and treatment main (F1,14=22.15, p<0.001) effects but no time bin x treatment interaction effect (F2,14=2.71, p=0.08) (Fig 1c). Therefore, the Cue TEST group exhibited greater active responses in the first 20-min bin compared to the last 20-min bin of the 1-hr reinstatement test (Tukey’s test, Bin 1>Bin 3, p<0.01).
Figure 1:
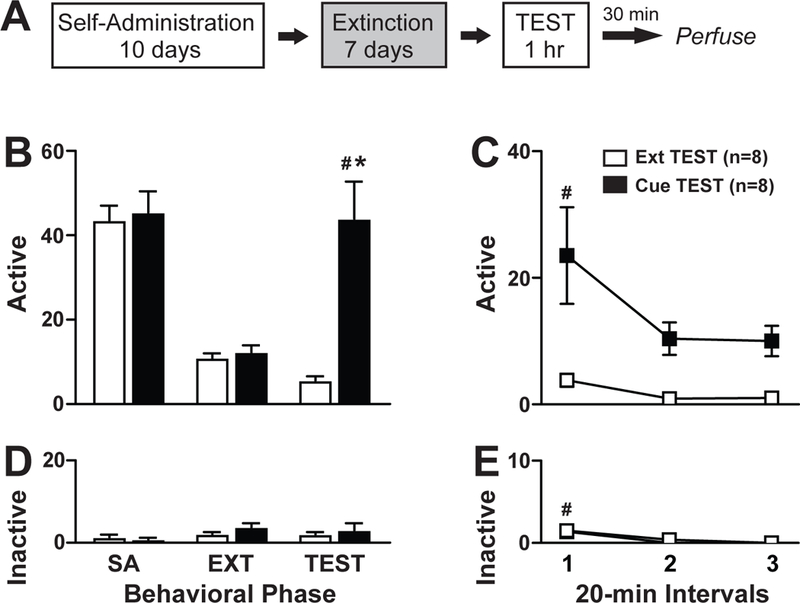
Cue-Induced Reinstatement of Drug-Seeking Behavior. (A) Schematic of the behavioral paradigm consisting of self-administration over at least 10 days, extinction training over at least 7 days and the 1-hr reinstatement test under extinction conditions (Ext TEST) or with contingent presentation of previously drug-paired cues (Cue TEST). (B, D) Active and inactive lever responses during the last 3 days of self-administration training (SA), last day of extinction training (EXT) and during the 1-hr reinstatement test (TEST). (C, E) Active and inactive lever responses during the 20-min intervals of the 1-hr TEST. #Represents significant Tukey’s effect within subject, *represents significant Tukey’s effects between subjects. White bars=Ext TEST Group, n=8; Black bars=Cue TEST group, n=8.
The ANOVA of inactive lever responses during the last day of extinction and the test day revealed no significant day x treatment interaction (F1,14=0.019, p=0.89), day main (F1,14=0.00, p=1.0) or treatment main (F1,14=1.16, p=0.30) effects (Fig 1d). A time course analysis of inactive lever responses during the 1-hr reinstatement test revealed a time main effect (F2,14=12.46, p<0.0001) but no time bin x treatment interaction (F2,14=0.29, p=0.75) or treatment main (F1,14=0.86, p=0.77) effects. Both the Ext TEST and Cue TEST groups exhibited greater inactive lever responses in the first 20-min bin compared to the last 20-min bin of the 1-hr reinstatement test (Fig 1e; Tukey’s test, Bin 1>Bin3, p<0.01).
Cfos-IR Cell Quantification
Cfos protein expression was analyzed throughout the anterior-posterior axis (+5.16 to +3.72) of both the vOFC (magenta) and lOFC (blue) (Fig 2a), with quantification focused on darkly-stained Cfos-IR cells (Fig 2b). The number of Cfos-IR cells in the vOFC was significantly greater in the Cue TEST group compared to the Ext TEST group (Fig 2c; t14=2.86, p=0.013). The 2 × 6 ANOVAs of Cfos-IR cells at the six vOFC bregma points revealed no bregma x treatment interaction (F5,14=0.68, p=0.64) or bregma main (F2,14=2.06, p=0.08) effects. The number of Cfos-IR cells in the lOFC was significantly greater in the Cue TEST group compared to the Ext TEST group (Fig 2e; t14 =2.39, p=0.03). The 2 × 6 ANOVAs of Cfos-IR cells at the six lOFC bregma points revealed no bregma x treatment interaction (F5,14=0.39, p=0.86) or bregma main (F5,14=0.48, p=0.79) effects. Therefore, the number of Cfos-IR cells in both the vOFC and lOFC was increased upon presentation of previous cocaine-paired cues.
Figure 2:
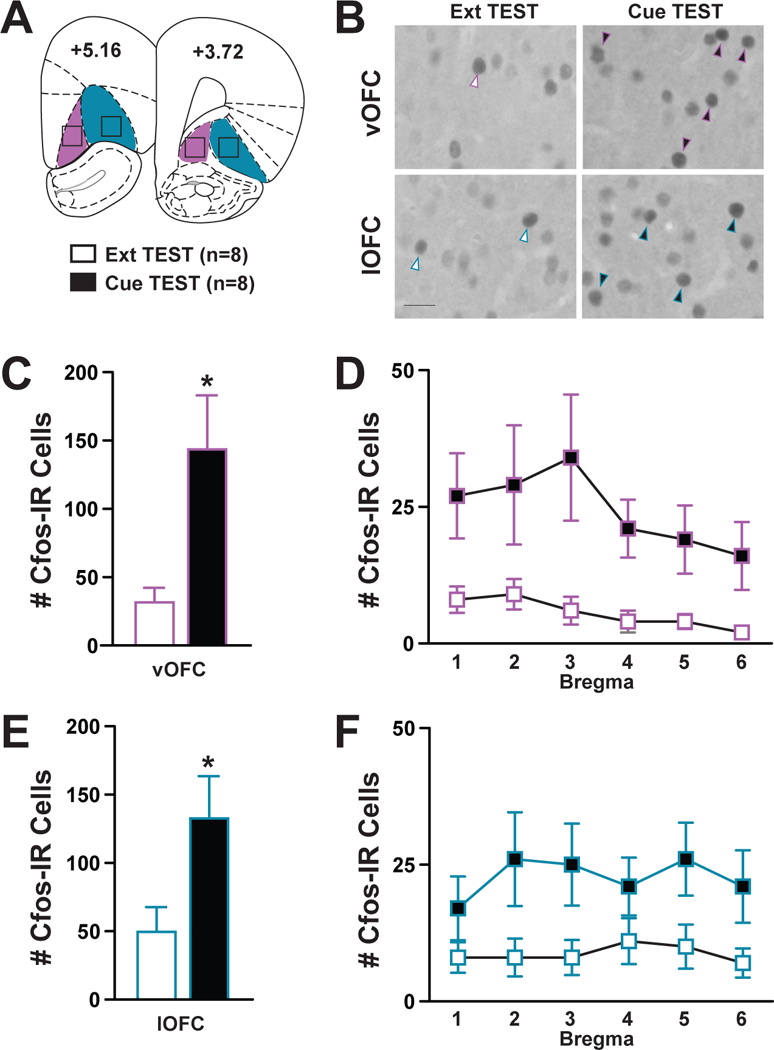
Analysis of Cfos-immunoreactive (IR) Cells following Cue-Induced Reinstatement Behavior. (A) Schematic depicting the representative region of OFC: vOFC (magenta), lOFC (blue) in which Cfos-IR cells were quantified at 6 bregma points from +5.16 to +3.72. (B) Representative images of darkly-stained Cfos-IR cells (arrows) within the vOFC or the lOFC between the Ext and Cue TEST groups; scale bar=100 μm, images taken at 20X magnification. (C, D) Number of Cfos-IR cells in the vOFC separated by bregma. (E, F) Number of Cfos-IR cells in the lOFC separated by bregma. *represents significant effect between subjects. White bars=Ext TEST Group, n=8; Black bars=Cue TEST group, n=8.
DISCUSSION
The present study demonstrates that exposure to previous cocaine-paired cues elicits drug-seeking behavior (Fig 1) and that Cfos expression is increased in both the ventral and lateral regions of the orbitofrontal cortex (vOFC, lOFC) (Fig 2). Specifically, increased cue- induced drug-seeking behavior, defined by increased active lever responses, was observed during the reinstatement test when cocaine-paired cues were presented contingently (Cue TEST) compared to no cue presentation (Ext TEST). Additionally, lever responses were increased on the reinstatement test day compared to the last day of extinction, within the Cue TEST group (Fig 1b, c). Lastly, inactive lever responses were minimal in all groups throughout all phases of the experiment (Fig 1d, e). The cue-induced drug-seeking data is in line with previous reports demonstrating that extinction of lever responses results in robust reinstatement of drug-seeking behavior to an un-extinguished cue (Buffalari et al., 2013), and that the highest level of responding occurs during the first 20-min of the 1-hr test (Arguello et al., 2017; Fuchs, Evans, Parker, & See, 2004a; Fuchs et al., 2004b; Smith, See, & Aston-Jones, 2009).
The current results demonstrating increased neuronal activation in the OFC are in line with previous reports of increased Cfos cell number following a cue-induced reinstatement test. Previous studies utilized a partial reinforcement schedule during self-administration training and continuous cue presentation during the reinstatement test (Kufahl et al., 2009). The current study utilized a continuous reinforcement schedule through all experimental phases, which resulted in lower drug-seeking behavior than previously reported. Nevertheless, we also observed increased Cfos-positive cells in the OFC (Kufahl et al., 2009). Furthermore, we separated analysis of Cfos-IR cells to distinguish between the ventral and lateral portions of the OFC (Izquierdo, 2017). We observed a similar increase in Cfos-IR cells in both the vOFC and lOFC. To our knowledge, this is the first study to report an increase in the number of cells expressing Cfos protein in the vOFC, though there is evidence that levels of zif268 mRNA are also increased in the vOFC (Thomas, Hall, & Everitt, 2002).
To determine whether the increase in Cfos-IR cells occurred throughout OFC, we conducted a bregma analysis. Although we did not observe a bregma x treatment interaction effect, a trend for an effect of bregma was found in the vOFC (Fig 2d). These data suggest that analysis of Cfos at any anterior-posterior level of the vOFC would likely reveal activation-induced changes; whereas, sampling from select levels of the lOFC (Fig 2f; Bregma point 4), may not always reveal increased activation, at least in response to cocaine-related cues. Lastly, an additional novel finding was that the pattern of increase of Cfos-IR cells qualitatively looked similar between anterior and posterior portions of both OFC subregions, which complements previous findings in which immediate early genes were elevated in more posterior levels of the OFC (+3.2 to +3.7) (Fanous et al., 2012; Kufahl et al., 2009; Zavala et al., 2007).
Existing anatomical evidence suggests that vOFC shares similar projection targets with both the medial and lateral portions of the OFC (Hoover & Vertes, 2011). Specifically, the vOFC sends and receives projections from the mOFC, with both regions sending less projections to the lOFC (Hoover & Vertes, 2011; Izquierdo, 2017). In addition, the vOFC sends less projections to limbic areas such as the basolateral amygdala, when compared to the lOFC (Hoover & Vertes, 2011; Price, 2007). Finally, while all three regions of the OFC send projections to the striatum, vOFC and lOFC afferents target ventromedial, whereas mOFC projections target the dorsolateral striatum (Heilbronner et al., 2016). This anatomical information would suggest that the increased neuronal activation within the vOFC and lOFC observed in the present study, corresponds to functional roles of both regions in potentiating cue-related drug-seeking. However, pharmacological and lesion studies have shown a divergent role of the lOFC and the mOFC in that inactivation of the lOFC plays a critical role in cue-induced reinstatement, whereas mOFC does not (Fuchs et al., 2004b). In addition, the role of the vOFC in drug-seeking behavior remains unclear given that pharmacological and lesion manipulations often target both the vOFC and lOFC. Future studies utilizing viral manipulations could overcome this limitation by targeting these distinct OFC subregions to understand their unique contributions to drug-seeking behavior. In conclusion, our results suggest that the vOFC, similar to the lOFC, may play an important role in maintaining cue-drug associations.
Acknowledgements:
This work was supported by NIDA grant R00 DA037271.
Footnotes
The authors have no conflicts of interest to disclose.
REFERENCES
- American Psychiatric Association; (2013). Diagnostic and Statistical Manual of Mental Disorders DMS V Diagnostic and Statistical Manual of Mental Disorders, 5th Edition 10.1176/appi.books.9780890425596.744053 [DOI] [Google Scholar]
- Arguello AA, Harburg GC, Schonborn JR, Mandyam CD, Yamaguchi M, & Eisch AJ (2008). Time course of morphine’s effects on adult hippocampal subgranular zone reveals preferential inhibition of cells in S phase of the cell cycle and a subpopulation of immature neurons. Neuroscience, 157(1), 70–79. 10.1016/j.neuroscience.2008.08.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arguello AA, Richardson BD, Hall JL, Wang R, Hodges MA, Mitchell MP, … Fuchs RA (2017). Role of a Lateral Orbital Frontal Cortex-Basolateral Amygdala Circuit in Cue-Induced Cocaine-Seeking Behavior. Neuropsychopharmacology. 10.1038/npp.2016.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonson KR, S.J G, C.S C, J.M L, J. M, H.L W, … E.D. L (2002). Neural Systems and Cue-Induced Cocaine Craving. Neuropsychopharmacology. 10.1016/S0893-133X(01)00371-2 [DOI] [PubMed] [Google Scholar]
- Buffalari DM, Feltenstein MW, & See RE (2013). The effects of varied extinction procedures on contingent cue-induced reinstatement in Sprague-Dawley rats. Psychopharmacology. 10.1007/s00213-013-3156-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, Ehrman RN, Wang Z, Li Y, Sciortino N, Hakun J, … O’Brien CP (2008). Prelude to passion: Limbic activation by “unseen” drug and sexual cues. PLoS ONE. 10.1371/journal.pone.0001506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis WM, & Smith SG (1976). Role of conditioned reinforcers in the initiation, maintenance and extinction of drug-seeking behavior. The Pavlovian Journal of Biological Science: Official Journal of the Pavlovian. 10.1007/BF03000316 [DOI] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ, Childress AR, & O’Brien CP (1992). Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology, 107(4), 523–529. 10.1007/BF02245266 [DOI] [PubMed] [Google Scholar]
- Erb S, Shaham Y, & Stewart J (1996). Stress reinstates cocaine-seeking behavior after prolonged extinction and a drug-free period. Psychopharmacology. 10.1007/s002130050150 [DOI] [PubMed] [Google Scholar]
- Fanous S, Goldart EM, Theberge FRM, Bossert JM, Shaham Y, & Hope BT (2012). Role of Orbitofrontal Cortex Neuronal Ensembles in the Expression of Incubation of Heroin Craving. Journal of Neuroscience. 10.1523/JNEUR0SCI.1914-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltin RW, & Haney M (2000). Conditioned effects of environmental stimuli paired with smoked cocaine in humans. Psychopharmacology, 149(1), 24–33. 10.1007/s002139900340 [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Eaddy JL, Su ZI, & Bell GH (2007). Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. European Journal of Neuroscience, 26(2), 487–498. 10.1111/j.1460-9568.2007.05674.x [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MC, & See RE (2004a). Differential involvement of the core and shell subregions of the nucleus accumbens in conditioned cue-induced reinstatement of cocaine seeking in rats, 459–465. 10.1007/s00213-004-1895-6 [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MP, & See RE (2004b). Differential Involvement of Orbitofrontal Cortex Subregions in Conditioned Cue-Induced and Cocaine-Primed Reinstatement of Cocaine Seeking in Rats. Journal of Neuroscience, 24(29), 6600–6610. 10.1523/JNEUROSCI.1924-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, & Volkow ND (2002). Drug addiction and its underlying neurobiological basis: neuroimaging evidnce for the involvement of the frontal cortex. American Journal of Psychiatry, 159(10), 1642–1652. 10.1176/appi.ajp.159.10.1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin AS, Clemens KJ, & McNally GP (2008). Renewal of extinguished cocaine- seeking. Neuroscience. 10.1016/j.neuroscience.2007.11.018 [DOI] [PubMed] [Google Scholar]
- Heilbronner SR, Rodriguez-Romaguera J, Quirk GJ, Groenewegen HJ, & Haber SN (2016). Circuit-Based Corticostriatal Homologies Between Rat and Primate. Biological Psychiatry 10.1016/j.biopsych.2016.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera DG, & Robertson HA (1996). Activation of c-fos in the brain. Progress in Neurobiology. 10.1016/S0301-0082(96)00021-4 [DOI] [PubMed] [Google Scholar]
- Hoover WB, & Vertes RP (2011). Projections of the medial orbital and ventral orbital cortex in the rat. Journal of Comparative Neurology, 519(18), 3766–3801. 10.1002/cne.22733 [DOI] [PubMed] [Google Scholar]
- Izquierdo A (2017). Functional Heterogeneity within Rat Orbitofrontal Cortex in Reward Learning and Decision Making. The Journal of Neuroscience, 37(44), 10529–10540. 10.1523/JNEUROSCI.1678-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufahl PR, Li Z, Risinger RC, Rainey CJ, Wu G, Bloom AS, & Li SJ (2005). Neural responses to acute cocaine administration in the human brain detected by fMRI. Neuroimage. 10.1016/j.neuroimage.2005.06.039 [DOI] [PubMed] [Google Scholar]
- Kufahl PR, Zavala AR, Singh A, Thiel KJ, Dickey ED, Joyce JN, & Neisewander JL (2009). c-Fos expression associated with reinstatement of cocaine-seeking behavior by response-contingent conditioned cues. Synapse (New York, N.Y.), 63(10), 823–835. 10.1002/syn.20666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasseter HC, Xie X, Ramirez DR, & Fuchs RA (2010). Prefrontal cortical regulation of drug seeking in animal models of drug relapse. Current Topics in Behavioral Neurosciences, 3(September 2009), 101–117. 10.1007/7854_2009_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, & Marshall JF (2005). Altered Fos expression in neural pathways underlying cue-elicited drug seeking in the rat. European Journal of Neuroscience. 10.1111/j.1460-9568.2005.03974.x [DOI] [PubMed] [Google Scholar]
- Müller R, Curran T, Müller D, & Guilbert L (1985). Induction of c-fos during myelomonocytic differentiation and macrophage proliferation. Nature. 10.1038/314546a0 [DOI] [PubMed] [Google Scholar]
- Neisewander JL, Baker D.a, Fuchs R. a, Tran-Nguyen LT, Palmer a, & Marshall JF (2000). Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 20(2), 798–805. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10632609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, & Watson C (2014). The Rat Brain in Stereotaxic Coordinates Seventh Edition. Elsevier Academic Press. [Google Scholar]
- Price JL (2007). Definition of the orbital cortex in relation to specific connections with limbic and visceral structures and other cortical regions. In Annals of the New York Academy of Sciences (Vol. 1121, pp. 54–71). 10.1196/annals.1401.008 [DOI] [PubMed] [Google Scholar]
- Saunders JB (2017). Substance use and addictive disorders in DSM-5 and ICD 10 and the draft ICD 11. Current Opinion in Psychiatry. 10.1097/YC0.0000000000000332 [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch MR, Stalnaker TA, & Takahashi YK (2009). A new perspective on the role of the orbitofrontal cortex in adaptive behaviour. Nature Reviews Neuroscience. 10.1038/nrn2753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE, Grimm JW, Kruzich PJ, & Rustay N (1999). The importance of a compound stimulus in conditioned drug-seeking behavior following one week of extinction from self-administered cocaine in rats. Drug and Alcohol Dependence, 57(1), 41–49. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10617312 [DOI] [PubMed] [Google Scholar]
- Smith RJ, See RE, & Aston-Jones G (2009). Orexin/hypocretin signaling at the orexin 1 receptor regulates cue-elicited cocaine-seeking. European Journal of Neuroscience. 10.1111/j.1460-9568.2009.06844.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KL, Arroyo M, & Everitt BJ (2003). Induction of the learning and plasticity- associated gene Zif268 following exposure to a discrete cocaine-associated stimulus. European Journal of Neuroscience, 17(9), 1964–1972. 10.1046/j.1460-9568.2003.02617.x [DOI] [PubMed] [Google Scholar]
- Thomas KL, Hall J, & Everitt BJ (2002). Cellular imaging with zif268 expression in the rat nucleus accumbens and frontal cortex further dissociates the neural pathways activated following the retrieval of contextual and cued fear memory. European Journal of Neuroscience, 16(9), 1789–1796. 10.1046/j.1460-9568.2002.02247.x [DOI] [PubMed] [Google Scholar]
- Thomsen M, & Caine SB (2005). Chronic Intravenous Drug Self-Administration in Rats and Mice. In Current Protocols in Neuroscience. 10.1002/0471142301.ns0920s32 [DOI] [PubMed] [Google Scholar]
- Venniro M, Caprioli D, & Shaham Y (2016). Animal models of drug relapse and craving: From drug priming-induced reinstatement to incubation of craving after voluntary abstinence. In Progress in Brain Research (Vol. 224, pp. 25–52). 10.1016/bs.pbr.2015.08.004 [DOI] [PubMed] [Google Scholar]
- Wit H.De, & Stewart J (1981). Psychopharmacology Reinstatement of Cocaine-Reinforced Responding in the Rat, 134–143. [DOI] [PubMed] [Google Scholar]
- Young K.a, Franklin TR, Roberts DCS, Jagannathan, Suh JJ, Wetherill RR, … Childress AR (2014). Nipping cue reactivity in the bud: baclofen prevents limbic activation elicited by subliminal drug cues. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 34(14), 5038–5043. 10.1523/JNEUROSCI.4977-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala AR, Biswas S, Harlan RE, & Neisewander JL (2007). Fos and glutamate AMPA receptor subunit coexpression associated with cue-elicited cocaine-seeking behavior in abstinent rats. Neuroscience. 10.1016/j.neuroscience.2006.12.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala AR, Osredkar T, Joyce JN, & Neisewander JL (2008). Upregulation of Arc mRNA expression in the prefrontal cortex following cue-induced reinstatement of extinguished cocaine-seeking behavior. Synapse, 62(6), 421–431. 10.1002/syn.20502 [DOI] [PMC free article] [PubMed] [Google Scholar]


