Description of the problem.
Patients with brain cancer are at risk for developing financial toxicity, which is defined as a combination of subjective financial concerns (e.g., anxiety), objective financial consequences of health issues and treatments (e.g., decreased income, medical debt), and patients’ coping behaviors1. The direct costs of care are considerably high in these patients (monthly direct medical cost of approximately $8,4782), and decreased household income is common and reported by at least half of the patients as a result of cancer treatment3. Financial toxicity has been shown to be linked with several clinically relevant patient outcomes, including health-related quality of life (HRQOL)4, symptom burden5, care adherence6, and survival7. Extreme financial distress after cancer diagnosis (manifested by declaring personal bankruptcy) has been reported to be a risk factor for mortality7. Given delay or non-receipt of an indicated imaging tests is a potential outcome of financial toxicity, it is critical for radiologists to understand the financial impact of treatment, which includes surveillance imaging, among patients with this condition.
Providing patients with resources to proactively manage the costs of their cancer care may help to reduce financial toxicity8 and ultimately improve treatment and imaging adherence. Financial navigation can be offered at any stage of patients’ care from surgery to oncology to radiology encounters. However, financial navigation, must be provided in a manner that is acceptable, accessible, less cumbersome, thereby not affecting the flow of clinical care8. In order to better understand how to equip patients and their families with tools that have the potential to reduce financial toxicity, there is an urgent need to study interventions at the patient, clinic, payer, and policy level. In the current study, we aimed to assess the feasibility of providing a financial navigation program alongside treatment in brain cancer patients.
What we did
Procedures for obtaining informed consent and protecting participants were approved and monitored by the Emory Institutional Review Board. The study was Health Insurance Probability and Accountability Act (HIPAA) compliant.
In this prospective single-arm pilot longitudinal observational study, we recruited adult patients with newly diagnosed brain cancer visiting outpatient oncology clinics, identified through prospective review of clinic schedule. Inclusion criteria were age 18 or older, with a new diagnosis of either primary malignant brain cancer (pathology proven) or brain metastasis (diagnosed on brain MRI), and receipt or plan to receive any of surgery, chemotherapy or radiation therapy. New diagnosis was defined as within the first 2 months of diagnosis. Patients who were not able to read or speak English at the time of their appointment were excluded.
Informed consent from eligible patients were obtained in person in the waiting room prior to their oncology clinic visits by a study coordinator. Those who consented were invited to complete a 15-minute paper survey at baseline. Follow-up surveys were completed at 3, 6 and 9 months after enrollment either during follow-up clinic visits (paper survey) or on the phone, if a patient did not have scheduled clinic visit or did not show up to the visit. The surveys included questions to assess financial toxicity, financial coping mechanisms and care-nonadherence due to cost of care. Financial toxicity was measured using the validated Comprehensive Score for financial Toxicity (COST) questionnaire9, which includes 11 questions reflecting five latent dominant themes of affect, coping, family, financial and resources9, and results in a score ranging between 0 to 44. The lower the score, the worse the financial toxicity9. Financial coping mechanisms were measured with questions on decreased spending on food, clothing or leisure10, and financial hardship such as withdrawing money from savings accounts or borrowing money11. Care non-adherence was defined as any self-reported patient-initiated inappropriate cessation of prescribed medication or late- or partial-filling of prescriptions. Caregivers were allowed to help patients answer the survey questions.
Patients who completed the baseline survey were asked to participate in a centralized oncology financial navigation program. The financial navigation program was offered through the Patient Advocate Foundation (PAF)12, a national case-management organization that assists patients with a range of issues, including access to health insurance coverage, debt relief, cost of living and disability applications. Upon consent, a member of PAF initiated the contact with patient and determined patients’ financial or care concerns or needs and attempts were made to secure assistance whenever possible. Subsequently, PAF case managers contacted the participants once per month for 6 months at minimum. In addition, participants could contact their case manager if additional financial issues arose. For each patient, we documented the number of contacts made with the participants (regardless if patient or PAF initiated the contact); all recommendations or interventions made by PAF; and any financial assistance procured through charitable entities on behalf of the patients.
For each patient, mean financial toxicity score (COST) at baseline was reported with standard deviation (SD) and range. Changes in COST from baseline to each time point were compared using paired t-test. Patient characteristics and care non-adherence were summarized with frequencies and percentages or means and SDs, where appropriate. Feasibility for financial navigation program was measured as program participation, type and amount of services provided. For program participation, we calculated proportion of patients who consented and completed at least one contact appointment with a PAF case manager during the 6 months program. We described the type and amount of assistance that were provided to the participants.
Outcomes and Limitations
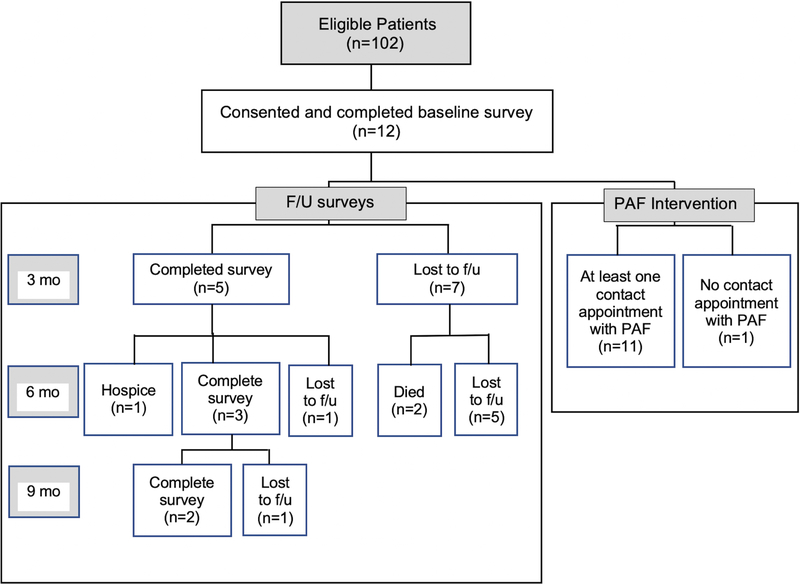
During the study recruitment period (October 2017-December 2018), 102 eligible patients were identified, of whom 12 patients consented to participate in the study (Figure 1). Reasons for nonparticipation were “not interested in this research”, “want to focus on my treatment” and “too overwhelmed with my disease and treatment that do not have time or energy to participate in this research”. Respondents were more likely to be younger on average (45.5 years vs 59.0 years; P=.004) compared with non-respondents. Further, respondents were more likely to be uninsured (16.7% vs. 8.1%) or have Medicaid (16.7% vs. 13.9%) and less likely to have Medicare (16.7% vs. 42.3%) (P=0.04). Gender, race, and marital status did not reach statistical significance at the .05 level between respondents vs. non-respondents.
Figure 1.
Study Flowchart.
Among respondents, 83% (n=10) completed the entire baseline questionnaire; remaining 17% (n=2) completed 95% of the baseline questionnaire. A total of 66% of respondents (n=8) had primary malignant brain cancer; while 34% (n=4) had secondary brain cancer from colon (n=2), lung (n=1) or breast (n=1) primary cancers. Baseline characteristics of the study population is shown in table 1.
Table 1.
Baseline characteristics of study population.
| Mean age, yr (SD) | 45.5 (12.7) |
| Gender, % (n) | |
| Female | 58.3% (7) |
| Male | 41.7% (5) |
| Race, % (n) | |
| White | 58.3% (7) |
| African American | 41.7% (5) |
| Ethnicity, % (n) | |
| Not Hispanic, Spanish or Latino | 90.9% (10) |
| Hispanic, Spanish or Latino | 9.1% (1) |
| Highest level of education, % (n) | |
| High school graduate or less | 27.3% (3) |
| More than high school graduate | 73.7% (8) |
| Marital Status, % (n) | |
| Single/never married | 33.3% (4) |
| Married or living with a partner | 50% (6) |
| Separated, divorced, widowed | 16.7% (2) |
| Employment Status, % (n) | |
| Full-time or part-time | 41.7% (5) |
| Unemployed, disabled, retired | 58.3% (7) |
| Annual household income, % (n) | |
| Less than $60k | 50% (6) |
| $60k or more | 50% (6) |
| Health insurance, % (n) | |
| Medicare | 16.7% (2) |
| Medicaid | 16.7% (2) |
| Private | 33.2% (4) |
| Other types (e.g., marketplace, military) | 16.7% (2) |
| No insurance | 16.7% (2) |
| Mean monthly premium, $ (SD) | 174 (136) |
At baseline, patients’ mean COST score was 9.4 (SD, 9.0), ranging from 0 to 23. This was lower than reported COST scores in the literature for patients with solid tumors (mean of 23)9, indicating brain cancer patients are likely at higher risk of financial toxicity compared to other tumors. All 12 patients reported decreased spending on basic needs (e.g., food and clothing) or leisure activities due to cost of their treatment. A total of 75% (n=9) of patients reported care non-adherence due to cost of treatment. Financial hardship was reported by 58% (n=7) of patients including borrowing money (n=4) or using saving account to pay for treatment (n=5).
Before contact with PAF, 92% (n=11) of patients used some sort of financial support including, using a financial advocate/navigator (n=6), meeting with a social worker (n=10), help with understanding how to pay for care (n=1), and what insurance covers (n=2), free medication samples or help paying for medications (n=2).
A total of 92% (n=11) of consented patients completed at least one contact appointment with a PAF case manager and were considered participants. Participants had an average of 69 contacts [median of 126; interquartile range, 96–142.5] with a PAF case manager over the 6-month study period. The main concerns discussed with PAF staff included debt crisis/cost of living (e.g., inability to afford transportation, food, utility, rent, mortgage), disability (e.g., disability qualification or application assistance), employment (e.g., questions on employment rights such as family and medical leave act [FMLA]), insurance (e.g., general benefit/coverage question, inability to afford care requiring cost-share, inability to afford Marketplace premium), medical decision making (e.g., assistance with clinical trials options), psychosocial support (e.g., counseling for caregivers). Table 2 demonstrates the total number of issues discussed with PAF case managers and percentage of these issues resolved during the 6 months contact with PAF. An issue was considered resolved if the case manager was able to address the specific patient concern/issue, either through education, direct assistance or location of external resource(s). Overall, there were 45 total issues discussed with PAF for 12 patients. Ninety-three percent (n=42) were resolved during the 6 months post enrollment. Total amount of debt relief provided through PAF case management work during the study period was $15,110.
Table 2. Number of issues discussed with PAF case managers and resolved.
Please note that numbers are not at patient-level. A patient may have more than one-time issue in each category.
| # discussed with PAF | # resolved | |
|---|---|---|
| Debt crisis/Cost of living | 11 | 11 |
| Disability issues | 10 | 9 |
| Employment issues | 1 | 1 |
| Insurance issues | 18 | 16 |
| Medical decision-making issues | 1 | 1 |
| Psychosocial support request | 5 | 5 |
An issue was considered resolved if the case manager was able to address the specific patient concern/issue, either through education, direct assistance or location of external resource(s).
There was no significant difference in the COST score of 5 patients who had follow-up scores at 3 months when compared to baseline (mean score of 8.8 at baseline vs. 8.0 at 3 months; p = 0.89), although the sample size is likely too small to detect a significant difference. Supplemental Figure 1 shows a plot of changes in individual patients COST score over time.
We encountered significant limitations. We found that recruitment and follow-up for our pilot study was challenging for several reasons. First, the high rate of mortality13, patients’ rapid cognitive decline14 and high caregiver dependency15 served as a barrier to recruiting patients and following-up during the time. We recruited patients within the first two months of diagnosis, when many patients were coping with physical and psychosocial issues associated with a new cancer diagnosis. Further, many patients had not yet received their hospital bill. Participation in a financial burden study may have been of a less priority for them, with many patients mentioning a preference for focusing on their cancer treatment or participate in a treatment clinical trial. Finally, contact initiation and consistency with PAF was challenging, as some patients did not respond phone calls or messages. Others forgot what PAF was as an organization. Despite these limitations, our findings support the feasibility of centrally provided oncology financial navigation programs once patients express interest in such programs. These results can be used to design future interventions to address whether use of financial navigation program can reduce financial burden resulting from cancer care. For future studies, involvement of all team members (e.g. oncologist, radiologist, social worker, financial counselor) in patient recruitment, addition of patient incentives, use of several methods for recruitment may help improving response rate. Further, routine patient-physician communication about out-of-pocket expenses, may result in better engagement of patients in shared decision-making16 as well as increasing awareness of importance of research studies focusing on financial burden. In addition, we can enhance study participation by helping patients understand that their responses to follow-up surveys are critical in improving clinical outcomes and care adherence.
Challenges of navigating imaging centers for brain cancer patients is variable across institutions and states. Although our pilot study reports the results from one institution, understanding the difficulties these patients face is important. As radiologists are expected to be part of integrated patient care, radiology practices are encouraged to develop processes to identify patients at risk for financial toxicity and imaging non-adherence and refer these patients to existing financial support services within the hospital system. This will help to streamline care and potentially off set many of the missed appointments, care non-adherence and unnecessary costs. Further, our results emphasize the important role of radiologists in recommending evidence-based and cost-effective imaging modality when it comes to cancer diagnosis and follow-up.
Supplementary Material
Supplementary Figure 1. Changes in individual patients COST scores over four time points(baseline, 3, 6, and 9 months) for 12 included patients.
Sources of Support for current project, conflict of Interest and acknowledgement:
This study was coordinated by the ECOG-ACRIN Cancer Research Group (Peter O’Dwyer, MD, and Mitchell D. Schnall, MD, PhD, group co-chairs) and supported by the National Cancer Institute of the National Institutes of Health under award numbers CA189828 and CA180801. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government. This work was supported in part by grant 5UG1CA189828 (Kathleen Gallagher, MPH; Jennifer Obenchain, LPN; Ruth Carlos, MD). The authors would like to acknowledge Bridget Williams and Lisa Kelly from Patient Advocate Foundation, and Debura Coleman from Emory University for all of their hard work and dedication in ensuring the success of this pilot project.
Gelareh Sadigh, MD receives research support from Association of University Radiologists GE Radiology Research Academic Fellowship. Ruth Carlos, MD receives salary support from the Journal of the American College of Radiology and research support from the Harvey L. Neiman Health Policy Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Altice CK, Banegas MP, Tucker-Seeley RD, Yabroff KR. Financial Hardships Experienced by Cancer Survivors: A Systematic Review. J Natl Cancer Inst. 2017;109(2). (https://www.ncbi.nlm.nih.gov/pubmed/27754926) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang S, Long SR, Kutikova L, et al. Estimating the cost of cancer: results on the basis of claims data analyses for cancer patients diagnosed with seven types of cancer during 1999 to 2000. J Clin Oncol. 2004;22(17):3524–3530. (https://www.ncbi.nlm.nih.gov/pubmed/15337801) [DOI] [PubMed] [Google Scholar]
- 3.Johannesen TB, Norum J, Lote K, Scheie D, Hirschberg H. A cost-minimising analysis of standard radiotherapy and two experimental therapies in glioblastoma. Radiother Oncol. 2002;62(2):227–231. (https://www.ncbi.nlm.nih.gov/pubmed/11937250) [DOI] [PubMed] [Google Scholar]
- 4.Zafar SY, McNeil RB, Thomas CM, Lathan CS, Ayanian JZ, Provenzale D. Population-based assessment of cancer survivors’ financial burden and quality of life: a prospective cohort study. J Oncol Pract. 2015;11(2):145–150. PMC4371118 (https://www.ncbi.nlm.nih.gov/pubmed/25515717) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lathan CS, Cronin A, Tucker-Seeley R, Zafar SY, Ayanian JZ, Schrag D. Association of Financial Strain With Symptom Burden and Quality of Life for Patients With Lung or Colorectal Cancer. J Clin Oncol. 2016;34(15):1732–1740. PMC4966336 (https://www.ncbi.nlm.nih.gov/pubmed/26926678) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neugut AI, Subar M, Wilde ET, et al. Association between prescription co-payment amount and compliance with adjuvant hormonal therapy in women with early-stage breast cancer. J Clin Oncol. 2011;29(18):2534–2542. PMC3138633 (https://www.ncbi.nlm.nih.gov/pubmed/21606426) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramsey SD, Bansal A, Fedorenko CR, et al. Financial Insolvency as a Risk Factor for Early Mortality Among Patients With Cancer. J Clin Oncol. 2016;34(9):980–986. PMC4933128 (https://www.ncbi.nlm.nih.gov/pubmed/26811521) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shankaran V, Leahy T, Steelquist J, et al. Pilot Feasibility Study of an Oncology Financial Navigation Program. J Oncol Pract. 2018;14(2):e122–e129. (https://www.ncbi.nlm.nih.gov/pubmed/29272200) [DOI] [PubMed] [Google Scholar]
- 9.de Souza JA, Yap BJ, Wroblewski K, et al. Measuring financial toxicity as a clinically relevant patient-reported outcome: The validation of the COmprehensive Score for financial Toxicity (COST). Cancer. 2017;123(3):476–484. PMC5298039 (https://www.ncbi.nlm.nih.gov/pubmed/27716900) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zafar SY, Peppercorn JM, Schrag D, et al. The financial toxicity of cancer treatment: a pilot study assessing out-of-pocket expenses and the insured cancer patient’s experience. Oncologist. 2013;18(4):381–390. PMC3639525 (https://www.ncbi.nlm.nih.gov/pubmed/23442307) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shankaran V, Jolly S, Blough D, Ramsey SD. Risk factors for financial hardship in patients receiving adjuvant chemotherapy for colon cancer: a population-based exploratory analysis. J Clin Oncol. 2012;30(14):1608–1614. (https://www.ncbi.nlm.nih.gov/pubmed/22412136) [DOI] [PubMed] [Google Scholar]
- 12.Raizer JJ, Fitzner KA, Jacobs DI, et al. Economics of Malignant Gliomas: A Critical Review. J Oncol Pract. 2015;11(1):e59–65. PMC4295423 (https://www.ncbi.nlm.nih.gov/pubmed/25466707) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huse JT, Holland EC. Targeting brain cancer: advances in the molecular pathology of malignant glioma and medulloblastoma. Nat Rev Cancer. 2010;10(5):319–331. (https://www.ncbi.nlm.nih.gov/pubmed/20414201) [DOI] [PubMed] [Google Scholar]
- 14.Munoz C, Juarez G, Munoz ML, et al. The quality of life of patients with malignant gliomas and their caregivers. Soc Work Health Care. 2008;47(4):455–478. (https://www.ncbi.nlm.nih.gov/pubmed/19042496) [DOI] [PubMed] [Google Scholar]
- 15.Sherwood P, Given B, Given C, Schiffman R, Murman D, Lovely M. Caregivers of persons with a brain tumor: a conceptual model. Nurs Inq. 2004;11(1):43–53. (https://www.ncbi.nlm.nih.gov/pubmed/14962346) [DOI] [PubMed] [Google Scholar]
- 16.Sadigh G, Carlos RC, Krupinski EA, Meltzer CC, Duszak R Jr.. Health Care Price Transparency and Communication: Implications for Radiologists and Patients in an Era of Expanding Shared Decision Making. AJR Am J Roentgenol. 2017;209(5):959–964. (https://www.ncbi.nlm.nih.gov/pubmed/28742372) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Changes in individual patients COST scores over four time points(baseline, 3, 6, and 9 months) for 12 included patients.