INTRODUCTION
The use of genetic testing is growing in medical care, requiring primary care providers (PCPs) to play an expanding role in the consent and disclosure process. Historically many groups have recommended that both pre- and post-test genetic counseling be provided by a genetic trained healthcare provider such as a genetic counselor or medical geneticist, physicians who complete a medical genetics residency. Genetic Counselors are masters-level trained allied health professionals who are certified by the American Board of Genetic Counseling and licensed in 26 states (www.nsgc.org, last accessed 8 Feb 2019). Although the number of Licensed and/or Certified Genetic Counselors (L/CGC) continues to grow, there is only one genetic counselor or medical geneticist for every 132,0001 and 650,0002,3 persons in the US respectively. There is a growing shortage of MD clinical geneticists and 46% of residency positions were unfilled in 2016. As the number of tests increases and clinicians become more familiar as testing moves into routine care, this model limits access to testing, is not scalable, and most importantly this level of engagement with specialists may not be needed by many patients. The risk for psychological sequela with most genetic testing has been shown to be low4. Many patients have an existing relationship with their PCP and access may be increased by not having to visit a different provider.
The CADRe (Consent and Disclosure of Recommendations) workgroup of the NIH funded Clinical Genome Resource (ClinGen) aims to increase access to genetic testing while maintaining effective communication, and the patient-friendly experience historically provided by genetic counselors. To create a transparent process to determining appropriate provider/patient communication about genetic testing, we deliberated about situations where consent and disclosure could be routinely managed by experienced PCPs; studied the issue through focus groups and surveys; and developed a rubric to categorize genetic tests and recommend the level of pre-and-posttest genetic counseling based on test, disorder, and patient characteristics5. The output of CADRe work is used to justify the expanded role for PCPs in the pre- and post-test settings described in this article.
To prepare for the offer and disclosure of genetic testing, it is important that clinicians understand the benefits and limits of genetic testing and understand how to select appropriate tests for specific patients. Further, they must be comfortable with assessing patient understanding and comfort with genetic testing, returning what are sometimes complex results, making a genetic diagnosis, and knowing when to consult with or refer to genetics or other relevant specialties.
With the cost of testing dropping, the ability to test for multiple genes in a single test, and growing online availability, the pre-test workup is less critical to choosing the patient-specific test. But there will always be patients with an unusual presentation and/or unusual medical or family history where consultation with a genetic counselor could benefit the patient. The PCP should consider referring anxious patients or patients with the potential for psychological reactions to a genetic counselor. This article discusses the three levels of consent and disclosure interactions with patients and outlines the roles of the PCP and the genetic counselor.
As new tests enter the market it is important to work with individuals with genetic expertise (L/CGC) to adapt the recommendations in this paper. Patients needing panel tests, exome sequencing, and genome sequencing may continue to benefit from consulting with a L/CGC.
CADRe’s intention in developing the recommendations was that clinicians would start by considering CADRe’s proposed level of communication, and then work with the patient to determine their preferred communication approach after taking into account the clinician’s comfort and experience with the test and condition; the patient’s baseline knowledge of the condition; and any underlying psychosocial concerns. Thus, this is intended to provide a baseline that is adjusted based on clinical judgement and preference.
Defining Levels of Communication
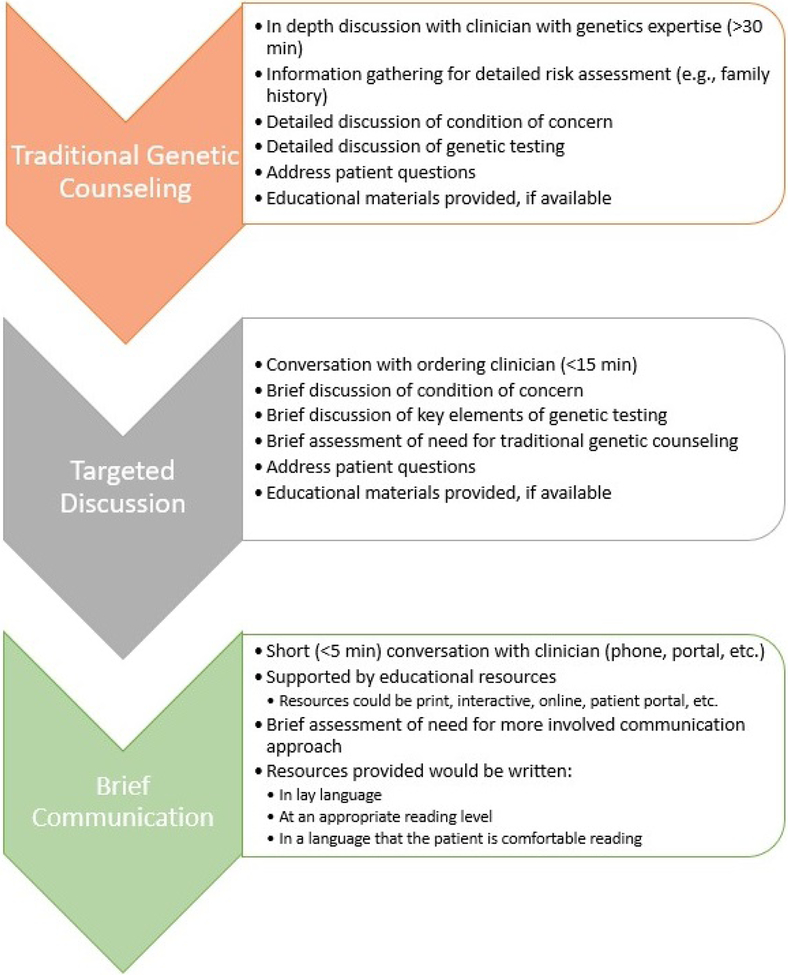
Three levels of patient communication at pre-and post-testing were defined by CADRe and considered in their recommendations: traditional genetic counseling (i.e. detailed discussion by L/CGC); targeted discussion by an experienced and knowledgeable provider; and brief communication with educational and support materials. Those levels are described in Figure 1. These levels were used to evaluate two stages of clinical interaction: pre-test education and consent, and post-test disclosure, education and support.
Figure 1: Communication strategies for genetic testing.
Recommended communication strategies are represented as a schematic. Communication strategies differ regarding depth, and often length, of discussion. Key elements of each communication strategy are denoted as bullet points.
Factors that Impact Complexity ofPre- and Post- Testing Communication
CADRe identified a set of factors that influence clinical complexity, and thus drove recommendations about the appropriate degree of communication required for different patients:
- Test complexity
- Is the choice of test to use obvious?
- Is the interpretation of the test result usually clear-cut?
- Testing situation complexity
- Is the patient symptomatic?
- Is the patient asymptomatic with a concerning family history?
- Is the patient at increased risk to carry a risk allele?
- Is the genetic cause in the family known?
- Implication of the genetic diagnosis to the patient and family
- Is there guidance about management of the genetic condition?
- Is there no significant potential for near-term mortality?
- Evidence of potential for adverse psychological impact
- Based on the clinician’s experience with the patient, the patient’s history, or the severity of the condition(s) in question, is there a low potential that the genetic test result will cause an adverse psychological impact in the patient?
- Clinician knowledge, experience and comfort
- Does the clinician feel comfortable managing pre-test education and post-test results disclosure and support?
- Availability of high-quality and patient-friendly educational materials
- Are there existing materials that can be made available to the patient and support the clinical interaction?
In general, when the answer to all of these questions is “yes,” referral to a genetics specialist (i.e. a L/CGC) for pre- and/or post-test counseling is not always necessary. This model increases patient access and allows the PCP to manage the genetic testing. Additional details about how these test and patient-level variables should be factored into decisions about who should provide education and counseling are described in the sections that follow on pre-test education and consent and post-test disclosure and support. Patient requests for referral to a genetic counselor should be honored and patients with indecision about genetic testing may benefit from referral to a genetic counselor.
PRE-TEST COMMUNICATION
The education and testing consent process is an important step in the genetic testing sequence. Genetic tests can have implications for patients that are different than for many other types of clinical testing and screening, such as prediction of a later onset disorder or exacerbation of presenting symptoms. Results may also be associated with treatment recommendations that may be unexpected for the patient, and results often have implications for others in the family.
Known clinical diagnosis or known familial pathogenic variant
If a patient already has a clinical diagnosis or a known family history of a genetic variant that they have brought to the PCP’s attention, we would assume that a discussion of the condition’s management and prognosis would occur during routine care and outside of the specific discussion of genetic testing. For these indications, we determined that a brief communication about genetic testing with the PCP together with the provision of educational resources is sufficient for consent. The brief communication should ensure that the patient has sufficient knowledge about the condition; understands their risk based on their relationship to the affected person in the family; appreciates what a positive and a negative genetic test result would mean; and wants to proceed with the testing. Supporting patient-friendly educational materials are important to ensure that the patient has additional resources as needed that can be accessed and reviewed as needed.
Clinical symptoms without confirmed diagnosis or unknown familial variant
In situations where a patient has a suggestive but not definitive clinical history, or if a patient is asymptomatic and at risk but with no causative variant identified in affected family members, we recommend a higher degree of communication: a targeted discussion with the ordering clinician about the condition and the potential outcomes of genetic testing. This would include a more detailed discussion about the condition’s natural history and prognosis; the importance of testing affected family members if possible; the testing process; the benefits and limitations of testing; implications for family members; the meaning of a positive, negative, or uncertain result; and anticipatory guidance about how the patient may feel upon receiving all three types of results. The clinician should engage in shared decision making regarding the choice to be tested, including discussion of options if testing is declined.
Complex testing or significant risk for psychological sequela
Outside of these general recommendations, there are other major considerations for the degree of PCP communication and whether to refer to a clinical genetics specialist. Highly-complex testing situations, such as complex testing with multiple possible causative genes; situations where a negative result comes with a high residual risk of the condition; or unusual inheritance patterns should be referred to a genetics specialist. Conditions associated with an increased risk of an adverse psychological impact (such as adult onset progressive neurological conditions and Huntington disease) benefit from referral to a genetics professional for pre-test education and counseling. In addition, if the patient has a personal history or traits that lead the clinician to be concerned about a negative psychological response to the offer of and/or results from genetic testing, a referral to a genetic counselor (L/CGC) is warranted.
Significant risk for near-term mortality or complex management
For conditions with near-term risk of mortality or complex management, we recommend that, at minimum, PCPs undertake a targeted pre-test discussion about the condition in question, the implications of both a positive and a negative test result for the patient and others in the family, and the patient’s psychosocial readiness to receive the test results. Situations where high-quality educational materials are not available to the patient also require clinicians to provide a higher degree of communication about the condition and/or test with the patient.
ALIGNING PRETEST COMMUNICATION WITH RESULTS DISCLOSURE
Depending on the test under discussion and the individual patient, communication of important information may occur in the pre-test communication or the post-test communication. In general, most genetic test results can be returned to patients through a targeted or a brief discussion. Complex testing methods and genetic disorders associated with intricate management recommendations or significant psychological risk can complicate the disclosure process. Pre-test genetic counseling by L/CGC in such cases is paramount. The detailed pre-test discussion should include a plan for disclosure of testing results. As we previously noted, pre-test counseling for most patients will consist of a targeted or a brief discussion. With this model it is likely that a disclosure plan will not be formalized during the relatively limited discussion, although discussion of a results disclosure plan is ideal. In cases where a return of results plan has not been previously discussed, the type of genetic result can guide the PCP on the return of results process.
RESULTS DISCLOSURE
Genetic test results are far from binary. Although a positive (or pathogenic) result can confirm a clinical diagnosis, a pathogenic result may also be associated with a previously unknown disease risk. This can complicate the discussion because risk for disease is different from a diagnosis and can be difficult to understand for both patient and clinician. A crucial component of the results disclosure process ensures patients can understand the personal implications of the result. Whether a patient requires a detailed discussion or traditional genetic counseling about a result can be dependent on a number of factors, which include but are not limited to the implications of the result, the patient’s personal or family history, and the patient’s health and medical literacy. Furthermore, in genetics “positive” and “negative” often have different meanings than other medical tests, a “positive” test can be positive for disease or risk and a “negative” test may mean that an answer was not found but the patient remains at risk. Genetic results (variants) may not always be associated with developing a disease. In order to aid the clinician, genetic variants are interpreted into one of the following categories: pathogenic, likely pathogenic, uncertain significance, likely benign and benign6. In many cases, the category of the variant (i.e. pathogenic or benign) can help guide the return the results process (Table 1).
Table 1:
Method of genetic test result disclosure
| Test Result | Method of Disclosure | |
|---|---|---|
| Pathogenic or likely-pathogenic variant | Targeted discussion (PCP) or L/CGC referral | |
| Adult onset conditions identified in a minor | L/CGC referral | |
| Patient with a history of depression or anxiety | L/CGC referral | |
| Variant of Unknown Significance | L/CGC referral | |
| Benign or likely-benign results | Brief discussion (PCP) | |
| High residual risk due to personal or family history | Targeted discussion (PCP) | |
Pathogenic and likely pathogenic variants
The interpretation of genetic variants is based on the understanding of human biology, functional studies of the gene or protein, and population genetics6. Pathogenic and likely-pathogenic results are highly associated with a disease phenotype. In the past many health care providers referred to these results as “positive”, “abnormal”, or “mutation found” which emphasized the degree of certainty associated with the results. “Positive” result for a risk allele (e.g. BRCA1 pathogenic variant) is not a diagnosis of disease and can require discussion to improve patient understanding.
Pathogenic and likely-pathogenic results often have clear implications for the patient, and a targeted discussion by the patient’s PCP is often appropriate for informed patients. There may be treatment guidelines or familial implications associated with a pathogenic or likely-pathogenic result. The disclosure process could include referral to a L/CGC or a targeted discussion about the result followed by a referral to a sub-specialist for further management or a detailed discussion about the result and follow up recommendations. The disclosure of a pathogenic or likely-pathogenic result is similar to disclosure of most abnormal laboratory or imaging results familiar to the PCP. Discussion of a plan to share results with family members should be included. There are situations that may require a detailed discussion or a referral to a clinician with genetics expertise, which are detailed below.
Pathogenic variants: Adult onset conditions identified in a minor
Decisions about whether to pursue genetic testing for adult-onset conditions in a minor are complex and should be focused on the best interest of the child7,8. As a result, a traditional genetic counseling model is recommended prior to pursing testing9. Occasionally, genetic testing is performed without pre-test counseling or an adult onset condition is an unexpected finding. In such situations, the result should be disclosed to the family or legal guardian10. The result disclosure process is complicated by a number of factors including the need for a formal plan to disseminate the result to the patient at an appropriate age and possible psychosocial implications for the patient. As a result, disclosure of adult onset conditions identified in a minor should include a detailed discussion (traditional genetic counseling) (L/CGC).
Pathogenic variants: Patient’s with a history of depression or anxiety
Receiving a pathogenic genetic result can result in short term depression and anxiety in any patient11. Significant depression or anxiety has been shown to be rare. The risk for depression or anxiety is complex and includes, but is not limited to, the nature of the genetic disorder and the patient’s clinical status. Due to the increased risk of short-term stress, a detailed discussion or a referral to a L/CGC should be considered in patients with an existing history of anxiety or depression.
Variants of unknown significance
Variants of unknown significance (VOUS/VUS) are particularly difficult for the PCP and genetic specialist alike. These results are neither pathogenic nor benign, but a consequence of our current lack of knowledge. This results in a high degree of uncertainty. Not surprisingly, patients have reported feelings of anxiety, worry and uncertainty when receiving VOUS12. Providing counseling may help relieve feelings of anxiety and uncertainty. In a survey of patients who received traditional genetic counseling, the risk perception of individuals with VOUS was similar to those who had a negative test13. Furthermore, the classification of VOUS is not static. Results often will be re-classified in the future, which could impact medical decision-making14. It is important to provide counseling focused on the uncertainty of the result and that the result may be re-classified to either a pathogenic or benign variant in the future. A plan for continual contact between the patient and provider should be established to address the latter. Traditional genetic counseling or a detailed discussion after the PCP consults with a genetics professional is recommended when a VOUS is identified.
Benign and likely benign results
In almost all situations, a benign, likely-benign, or “no variant identified” result should be treated as a negative genetic test result. Historically, many health-care providers may have referred to a benign result as a “polymorphism.” By definition, a polymorphism is a genetic result that occurs at a frequency higher than 1% of the population. Pathogenic variants can be fairly common15, 16 and benign variants may be rare. With current nomenclature, benign indicates that the genetic result is not associated with a disease phenotype. As a result, the majority of benign and likely-benign results can be disclosed to a patient through a brief discussion such as a phone call, an electronic patient portal or through written material.
With “no variant identified” the family and personal medical history can be very important and referral for detailed discussion (genetic counseling) may be indicated. In genetics a “negative” will only place the patient at population risk when a known familial variant is not found. Due to limitations of testing and our knowledge, a patient may remain at high risk after genetic testing when the familial mutation has not been identified (residual risk).
Disclosing genetic results using a brief discussion model has the same caveats as pre-test counseling noted above. The decision to use written communication will be dependent both on the patient and the ability to communicate key components about the benign result. It is important to communicate that both a benign and a negative result indicate a genetic etiology was not identified. This is not equivalent to stating a patient does not have a genetic disease. A genetic etiology may not have been identified due to the limitation of the testing (i.e. incomplete or poor sensitivity testing), other genes not tested, or due to the limited understanding of the disease. In those patients with a clinical diagnosis of a genetic disease or patients in whom there is a high risk due to a personal or family history, a targeted discussion about the benign results is warranted.
Results disclosure and further considerations
Result disclosure is a process. It can begin with the PCP and patients can be referred for genetic counseling or vice-versa. The most important, and difficult, aspect of the results disclosure process is to identify what is in the best interest of the patient. The above serves as general guidelines and will be dependent on the patient, pre-test counseling, and the clinician-patient relationship.
It may be evident to the PCP when a patient will benefit from genetic counseling or a patient may advocate for counseling following a result. It is important to note that patients may desire genetic counseling, but may not request counseling, as a result of anxiety or worry over external judgment related to their need for further counseling17. As a result, all patients should be made aware that genetic counseling is available if needed or desired. The decision to refer a patient for genetic counseling should be patient-centered, although there are situations in which genetic counseling should be strongly considered. In general, these situations mirror the need for genetic counseling prior to testing. Specifically, referral should be considered when a condition has a high risk for adverse psychological reactions or the testing itself is complex. Genetic counseling by a L/CGC may be necessary pre- and post-testing or may only be necessary once during the testing process.
SUMMARY
PCPs are playing an increasing role in the provision of genetic testing by working with genetic counselors to understand the key points necessary for informed consent and the key points for results disclosure. PCPs can leverage their relationship with the patient to determine the level of pre- and post-test communication that will benefit the patient and increase access to genetic testing. There are a limited number of conditions where testing is known to be associated with psychological reactions or choosing the best test can be complicated and in these cases referral to a genetic counselor (L/CGC) is recommended. Working with a genetic counselor, there are many genetic tests where a PCP can provide testing using targeted discussion or a brief discussion accompanied by educational materials and increase patient access to genetic testing.
CASE EXAMPLE
Below are examples to outline the interplay between pre-test and post-test discussion.
BRCA 1 and 2 testing of a woman at increased risk due to known familial mutation. Pre-test consent was brief discussion that did not include management discussion. Patient has high medical literacy, already knows quite a bit about the condition based on family experience, and no history of anxiety. Post-test discussion of a pathogenic variant should be targeted discussion to include familial implications and management discussion. If patient appears anxious, consider genetic counseling with L/CGC.
BRCA 1 and 2 testing of a woman at increased risk due to family history and no family member with history of cancer available to test. Pre-test consent was targeted discussion that included limitations of testing due to lack of known pathogenic variant. Post-test discussion of pathogenic variant could be traditional genetic counseling (L/CGC) if patient needs help with management of familial implications or a targeted discussion.
BRCA 1 and 2 testing of a woman at increased risk due to known familial mutation. Pre-test consent was brief discussion that did not include management discussion. Patient has high medical literacy and no history of anxiety. Post-test discussion of “no mutation found” (negative result) could be brief discussion.
BRCA 1 and 2 testing of a woman at increased risk due to family history and no family member with history of cancer available to test. Pre-test consent was targeted discussion that included limitations of testing due to lack of known pathogenic variant. Post-test discussion of “no mutation found” (negative result) should be traditional genetic counseling or targeted discussion to consider additional testing and management decisions based on family history.
KEY POINTS.
Primary care providers (PCPs) can provide pre-test consent for many genetic tests through a 15–30-minute targeted discussion or by a brief communication (discussion) and educational materials where management is straightforward.
This model of PCPs providing -pre-test consent for straightforward testing situations improves access to genetic testing.
Pre-test and post-test genetic counseling by a licensed/certified genetic counselor (L/CGC) should be considered for conditions with a high risk for adverse psychological impact or high residual risk.
Genetic test result for a pathogenic variant (positive) may appropriately be disclosed by the ordering PCP along with referral to a genetic counselor.
Genetic test result for a benign variant (negative) may often be disclosed by the ordering PCP with a targeted discussion or a brief discussion with educational materials.
SYNOPSIS.
Historically, both pre- and post-test genetic counseling has been standard-of-care for genetic testing. As ordering of genetic test increases in primary care settings, this model should be adapted for primary care providers (PCPs) willing to learn critical information about the test and key concepts that patients need to make an informed testing decision. It is helpful for a PCP to discuss a few initial patients with a genetic counselor to prepare for the key concepts of pre and post-test counseling. Here, the authors provide guidance about the CADRe-recommended level of involvement of the PCP based on the test indication, test complexity, disorder management, and the potential for psychosocial sequela. They provide guidance on consent and results disclosure situations where referral for genetic counseling is needed; where consultation with a genetic counselor is appropriate, followed by a targeted discussion with the patient; and when a brief discussion and educational materials is sufficient.
Acknowledgements:
This work was possible because of the work of members of the ClinGen CADRe workgroup: Kyle Brothers, MD, PhD; Adam H Buchanan, MS, MPH, LCGC; Mildred Cho, PhD; Erin Currey; Miranda LG Hallquist, MSc, LCGC; Laura Hercher, MS, CGC; Louanne Hudgins, MD, FACMG; Seema Jamal, MSc, CGC, CCGC; Dave Kaufman, PhD; Howard Levy, MD, PhD; Nicole Lockhart, PhD; Kelly Ormond, MS, LCGC; Alice Popejoy, PhD; Erin Ramos, MPH, PhD; Myra Roche, MS, CGC; Maureen Smith, MS, CGC; Melissa Stosic, MS, CGC; Wendy Uhlmann, MS, CGC; and Karen Wain, MS, LCGC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE STATEMENT
Disclose any relationship with a commercial company that has a direct financial interest in subject matter or materials discussed in article or with a company making a competing product. If nothing to disclose, please state “The Authors have nothing to disclose.” The authors have nothing to disclose.
Contributor Information
W. Andrew Faucett, Professor, Office of the Chief Scientific Officer, Geisinger, Danville, PA.
Holly Peay, Senior Researcher, Center for Newborn Screening, Ethics, and Disability Studies, RTI International, Research Triangle Park NC.
Curtis R. Coghlin II, Associate Professor, Department of Pediatrics, Section of Genetics, University of Colorado Anschutz Medical Campus.
REFERENCES
- 1.National Society of Genetic Counselors: Genetic Counselor Workforce Initiatives. Available at: http://www.nsgc.org/p/cm/ld/fid=532. Accessed October 5, 2017.
- 2.Summar Marshall L., MS Watson LDTs, Incidental Findings, and the Need for More Geneticists. Medscape. November 2015. Available at http://www.medscape.com/viewarticle/853979 Accessed September 27, 2017.
- 3.Cooksey JA, Forte G, Flanagan PA, Benkendorf J, Blitzer MG. The medical genetics workforce: An analysis of clinical geneticist subgroups. Genet Med. 2006;8(10):603–614. [DOI] [PubMed] [Google Scholar]
- 4.Ringwald J, Wochnowski C, Bosse K, Giel KE, Schäffeler N, Zipfel S, Teufel M. Psychological distress, anxiety, and depression of cancer-affected BRCA 1/2 mutation carriers: A systematic review. J Genet Couns. 2016;25(5):880–91. [DOI] [PubMed] [Google Scholar]
- 5.Ormond KE, Hallquist MLG, Buchanan AH, et al. Developing a conceptual, reproducible, rubric-based approach to consent and result disclosure for genetic testing by clinicians with minimal genetics background. Genet Med. July 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. March 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.COMMITTEE ON BIOETHICS, COMMITTEE ON GENETICS, AND, AMERICAN COLLEGE OF MEDICAL GENETICS AND, GENOMICS SOCIAL, ETHICAL, LEGAL ISSUES COMMITTEE. Ethical and policy issues in genetic testing and screening of children. Pediatrics. 2013;131(3):620–622. [DOI] [PubMed] [Google Scholar]
- 8.Ross LF, Ross LF, Saal HM, et al. Technical report: Ethical and policy issues in genetic testing and screening of children. Genet Med. 2013;15(3):234–245. [DOI] [PubMed] [Google Scholar]
- 9.Botkin JR, Belmont JW, Berg JS, et al. Points to Consider: Ethical, Legal, and Psychosocial Implications of Genetic Testing in Children and Adolescents. Am J Hum Genet. 2015;97(1):6–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green RC, Berg JS, Grody WW, et al. ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. Genet Med. 2013;15(7):565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mella S, Muzzatti B, Dolcetti R, Annunziata MA. Emotional impact on the results of BRCA1 and BRCA2 genetic test: an observational retrospective study. Hered Cancer Clin Pract. 2017;15:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Makhnoon S, Shirts BH, Bowen DJ. Patients’ perspectives of variants of uncertain significance and strategies for uncertainty management. J Genet Couns. January 2019 [DOI] [PubMed] [Google Scholar]
- 13.Richter S, Haroun I, Graham TC, Eisen A, Kiss A, Warner E. Variants of unknown significance in BRCA testing: impact on risk perception, worry, prevention and counseling. Ann Oncol. 2013;24 Suppl 8:viii69-viii74. [DOI] [PubMed] [Google Scholar]
- 14.So M-K, Jeong T-D, Lim W, et al. Reinterpretation of BRCA1 and BRCA2 variants of uncertain significance in patients with hereditary breast/ovarian cancer using the ACMG/AMP 2015 guidelines. Breast Cancer. February 2019. [DOI] [PubMed] [Google Scholar]
- 15.Burnham-Marusich AR, Ezeanolue CO, Obiefune MC, et al. Prevalence of Sickle Cell Trait and Reliability of Self-Reported Status among Expectant Parents in Nigeria: Implications for Targeted Newborn Screening. Public Health Genomics. 2016;19(5):298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grosse SD, Gurrin LC, Bertalli NA, Allen KJ. Clinical penetrance in hereditary hemochromatosis: estimates of the cumulative incidence of severe liver disease among HFE C282Y homozygotes. Genet Med. 2018;20(4):383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vos J, van Asperen CJ, Oosterwijk JC, et al. The counselees’ self-reported request for psychological help in genetic counseling for hereditary breast/ovarian cancer: not only psychopathology matters. Psychooncology. 2013;22(4):902–910. [DOI] [PubMed] [Google Scholar]