Abstract
Background:
Difficult or failed intubation is a major contributor to morbidity for patients and liability for anesthesiologists. Updated difficult airway management guidelines and incorporation of new airway devices into practice may have affected patient outcomes We therefore compared recent malpractice claims related to difficult tracheal intubation to older claims using the Anesthesia Closed Claims Project database.
Methods:
Claims with difficult tracheal intubation as the primary damaging event occurring in the years 2000–12 (n=102) were compared to difficult tracheal intubation claims from 1993–99 (n=93). Difficult intubation claims from 2000–12 were evaluated for preoperative predictors and appropriateness of airway management.
Results:
Patients in 2000–2012 difficult intubation claims were sicker (78% American Society of Anesthesiologists physical status 3–5, n=78/102) and had more emergency procedures (37%, n=37/102) compared to patients in 1993–99 claims (47% American Society of Anesthesiologists physical status 3–5, n=36/93, p<0.001 and 22% emergency, n=19/93, p=0.025). More difficult tracheal intubation events occurred in non-perioperative locations in 2000–12 than 1993–99 (23%, n=23/102 vs. 10%, n=10/93, p=0.035). Outcomes differed between time periods (p<0.001), with a higher proportion of death in 2000–2012 claims (73%, n=74/102 vs. 42%, n=39/93 in 1993–99 claims, p<0.001 adjusted for multiple testing). In 2000–2012 claims, preoperative predictors of difficult tracheal intubation were present in 76% (78/102). In the 97 claims with sufficient information for assessment, inappropriate airway management occurred in 73% (71/97, kappa=0.44–0.66). A “can’t intubate, can’t oxygenate” emergency occurred in 80 claims with delayed surgical airway in over one-third (39%, n=31/80).
Conclusions:
Outcomes remained poor in recent malpractice claims related to difficult tracheal intubation. Inadequate airway planning and judgment errors were contributors to patient harm. Our results emphasize the need to improve both practitioner skills and systems response when difficult or failed tracheal intubation is encountered.
INTRODUCTION
Difficult or failed airway management in anesthesia is a major contributor to patient morbidity and mortality, including potentially preventable adverse outcomes such as airway trauma, brain damage, or death.1–5 A previous Closed Claims analysis by Peterson et al.1 of malpractice claims associated with adverse difficult intubation events from 1993 to 1999 found a reduction in patient permanent brain damage and death associated with induction of anesthesia, but not other phases of anesthesia.1 In contrast, Peterson et al.1 found that claims prior to the adoption of the first American Society of Anesthesiologists’ Difficult Airway Guideline in 1993,6 showed severe injuries in all phases of anesthesia care.
Since the initial difficult airway guideline was published in 1993, it has been updated twice.7,8 In the nearly 15 years since the Peterson report,1 multiple advanced airway devices, including newer video laryngoscopes and supraglottic airway devices, have been introduced and incorporated into clinical practice. A 2018 study of 421,581 anesthetics in a regional community anesthesia practice found the rates of difficult and failed tracheal intubation decreased fourfold between 2002 and 2015.9 From 2011–2016, the rates of difficult and failed intubation were 1.6 per 1000 and 0.06 per 1000 patients, respectively.9 Brain damage and death are very rare outcomes of difficult airway management. The Fourth National Audit Project of the Royal College of Anaesthetists (NAP4) reported that brain damage or death occurred once for every 180,000 general anesthetics delivered in 2008.5 As malpractice claims are useful to study rare adverse events with severe outcomes, we analyzed claims in the Anesthesia Closed Claims Project database for injuries related to difficult tracheal intubation in the years 2000–2012. We compared patient and case characteristics, adverse outcomes, and timing of difficult airway events in the more recent claims to those from 1993 to 1999 in our previous report. We hypothesized that potentially preventable complications occur with difficult or failed tracheal intubation despite updated practice guidelines and improved airway techniques.
MATERIALS AND METHODS
The Anesthesia Closed Claims Project database is a structured collection of closed anesthesia malpractice claims in the US that has previously been described in detail.10 Procedures have been approved by the University of Washington Human Subjects Committee (IRB application #43939). Data were obtained from a panel of malpractice insurers from throughout the United States and were abstracted from insurance company files by board-certified practicing anesthesiologists. Data were collected from depositions, medical records, autopsy reports, expert witness statements, claims manager summaries, consultant evaluations, and other legal documents. Data collection included the type of surgery, details of the anesthesia care provided, patient demographics, patient outcomes, legal proceedings and any payments made. The on-site anesthesiologist reviewer evaluated the type and severity of injury and the cause of injury (i.e., damaging event). The National Association of Insurance Commissioners’ 10-point scale, which ranges from 0 (no apparent injury) to 9 (death), was used to determine the severity of the injury to the patient in each claim.11 The on-site anesthesiologist reviewer wrote a narrative summary of the claim, including the sequence of events, potential causes of injury and providing additional details relevant to that claim. On-site reviewer assessments were reviewed by the Closed Claims Committee for consistency with study classifications.
For this study, we used the Closed Claims Project database of 11,034 claims collected through 12/31/2016. Inclusion criteria were surgical and procedural anesthesia, obstetric anesthesia, and claims in which the anesthesiologist was called for airway management outside of the operating room (OR), e.g. the postanesthesia recovery room, emergency room, intensive care unit (ICU), or hospital ward. Acute pain management and chronic pain medicine claims were not included in this analysis. Claims associated with difficult intubation of neonates immediately post-delivery were not included; there were no exclusions based on age.
Definition of Variables
Claims in which difficult intubation (defined as multiple attempts at tracheal intubation or failed intubation) was identified as the primary damaging event leading to injury for events that occurred in the years 2000–12 were classified as “difficult tracheal intubation” claims for comparison to difficult tracheal intubation claims previously analyzed by Peterson et al.1 Selection criteria for the claims analyzed by Peterson et al.1 were previously reported and involved reviewer completion of a supplemental questionnaire concerning difficult airway management and tracheal intubation. This supplemental questionnaire was designed to assess the impact of the 1993 difficult airway guidelines on difficult airway management and was discontinued after completion of the Peterson study. For the current study, only claims for events that occurred in 1993–99 (after adoption of guidelines for difficult airway management by the American Society of Anesthesiologists) as analyzed by Peterson et al.1 were included. Claims in the Peterson et al.1 cohort that occurred in the years 1985–1992 were not included.
Permanent brain damage was defined as brain damage with severity of injury in the permanent and disabling range (e.g. 6–8 on the severity of injury scale). Airway injury and other clinical outcomes (other than permanent brain damage and death) were classified exclusive of permanent brain damage or death to yield outcomes in four mutually exclusive categories: death, permanent brain damage, airway injury, and all other injuries. Permanent brain damage or death were defined by the status of the patient at claim resolution. The outcome for a patient suffering permanent brain damage in the time period immediately following a difficult tracheal intubation who died prior to claim closure was classified as death in the database.
The location and phase of care during which difficult tracheal intubation occurred was classified as in Peterson et al.1: pre-induction, induction, intraoperative or intra-procedure, during extubation in the operating room, or during recovery in the post-anesthesia recovery unit. These claims were grouped as “perioperative” claims. Non-operating room anesthetizing (NORA) locations were classified as “perioperative” claims as well, with phase of care classified as described above. Claims that occurred in locations outside of the operating room or recovery area where an anesthesiologist was called to assist (rather than providing procedural anesthesia care) were classified as “outside location.”
For difficult tracheal intubation claims for events that occurred in the years 2000–12, airway management details were abstracted from the claim narratives and classified by three of the authors (KBD, AMJ, MFA). Agreement by two of the authors was required for classification, with disagreements resolved through discussion. Urgency of tracheal intubation was classified as emergency (intubation is required immediately and without delay), urgent (intubation is required, but not immediately), or elective (no urgency, e.g. purely elective case/airway management). The authors identified potential predictors of difficult tracheal intubation including past history of difficult tracheal intubation, acute airway obstruction from any cause, Mallampati grade 3 or 4, limited cervical spine extension, limited mouth opening, secretions or blood in the airway, short neck, thick or bull neck, prior neck irradiation, short thyromental distance, swollen tongue, pre-eclampsia, or prominent teeth if they were noted in the on-site reviewer’s claim narrative. These three authors (KBD, AMJ, MFA) also assessed whether the airway management was appropriate or not, based the 2013 ASA practice guidelines for management of the difficult airway.8 Indications of inappropriate management were classified as inadequate preoperative or airway evaluation, failure to plan for difficult intubation at induction, no backup plan for difficult reintubation after (failed) extubation, failure to use a supraglottic airway as a bridge for oxygenation during difficult intubation, perseveration, and delay or failure to call for a surgical airway in a “can’t intubate, can’t oxygenate” (CICO) emergency. Perseveration was defined as consistent application of any airway management technique or tool more than twice (i.e. greater than or equal to 3 attempts) without deviation or change of technique, or the return to a technique or tool that had previously been unsuccessful.
Statistical Analysis
Inter-rater reliability was determined on a sample of 2000–12 difficult airway claims for the individual indications of inappropriate airway management using kappa scores. Pairwise kappa scores between the three evaluating authors were calculated and the mean of the three pairwise scores reported. Patient and case characteristics, and clinical outcomes for claims occurring in the year 2000–12 were compared to claims that occurred in 1993–99 using chi square test, Fisher’s Exact Test (when cells had expected counts of <5), and independent t-test for equality of means with two-tailed tests and p<0.05 as the criterion for statistical significance. For tables that were larger than 2×2 and expected cell counts of <5, Fisher’s exact test was performed with Monte Carlo significance calculated using 10,000 sampled tables. In order to minimize the incidence of type 1 error, we only tested individual table components if the overall distribution in the table was statistically significant at p<0.05. For tables large than 2×2 where statistically significant distributions were identified, post hoc 2×2 tests on collapsed variables were performed, with both unadjusted and Bonferroni adjusted p-values reported. Multiple testing of factors associated with appropriateness of airway management (location and urgency) were handled in a similar manner, with both unadjusted and Bonferroni adjusted p-values reported. Odds ratios were calculated by logistic regression. The interaction between study cohort (2000–12 claims vs. 1993–99) and phase of care (induction vs. other phases) on outcomes was analyzed by logistic regression. No statistical power calculation was conducted prior to the study. The sample size was based on the available data. All statistical analysis employed IBM SPSS Statistics 25 (International Business Machines Corporation, Armonk, New York).
RESULTS
There were 93 claims related to difficult tracheal intubation for events that occurred in 1993–99 and 102 in 2000–12 (2000–5: n=61, 2006–12, n=41).
Comparison of difficult tracheal intubation claims 2000–12 vs. 1993–99
Patient and case characteristics
Difficult tracheal intubation claims that occurred in 2000–12 had a higher proportion of ASA 3–5 patients undergoing emergency procedures compared to 1993–99 claims (p< 0.001 and p=0.025 respectively, Table 1). The distribution of surgical procedures differed (p=0.045, Table 1), with higher proportions of orthopedic procedures in 1993–99 claims (23%) compared to 2000–12 (9%, odds ratio 3.01 (1.30–7.0), p=0.008, p=0.064 adjusted for multiple testing). More difficult tracheal intubation events occurred in outside locations in 2000–12 than in 1993–99 (p=0.035, Table 1). There were no statistically significant differences in age, sex, or primary anesthetic technique between the two cohorts (Table 1).
Table 1:
Patient and case characteristics
| 1993–99 n (% of 93) |
2000–12 n (% of 102) |
Odds Ratio (95% Confidence Interval) |
p= | |
|---|---|---|---|---|
| Male | 44 (47%) | 62 (61%) | 1.73 (0.98–3.05) | 0.063 |
| ASA 3–5 (n=178) | 36 (47%) | 78 (76%) | 0.277 (0.146–0.53) | <0.001 |
| Emergency (n=187) | 19 (22%) | 37 (37%) | 0.46 (0.241–0.89) | 0.025 |
| Pediatric | 2 (2%) | 2 (2%) | 1.10 (0.152–8.0) | 1.000** |
| Age in years: mean [SD] | 50 [17] | 52 [13] | 2.14 (−2.13 – 6.4) | 0.325 |
| Age >65 years | 17 (18%) | 17 (17%) | 1.12 (0.53–2.34) | 0.851 |
| Obese (n=151) | 44 (62%) | 54 (68%) | 0.79 (0.40–1.53) | 0.499 |
| Primary anesthetic | 0.092** | |||
| General | 83 (89%) | 82 (80%) | 2.02 (0.89–4.6) | |
| Regional | 2 (2%) | 0 (0%) | NA | |
| Monitored anesthesia care | 3 (3%) | 8 (8%) | 0.392 (0.101–1.52) | |
| None | 4 (4%) | 11 (11%) | 0.372 (0.114–1.21) | |
| Combined general + regional | 1 (1%) | 1 (1%) | 1.10 (0.068–17.8) | |
| Procedures | 0.042** | |||
| General surgery | 24 (26%) | 16 (16%) | 1.87 (0.92–3.79) | |
| Orthopedics | 21 (23%) | 9 (9%) | 3.01 (1.30–7.0) | |
| Head, neck, ENT | 20 (22%) | 25 (25%) | 0.84 (0.43–1.65) | |
| Vascular,/cardiothoracic | 10 (11%) | 16 (16%) | 0.65 (0.278–1.51) | |
| Neurologic/spine | 5 (5%) | 10 (10%) | 0.52 (0.172–1.59) | |
| Cesarean delivery | 2 (2%) | 3 (3%) | 0.73 (0.118–4.4) | |
| Gynecology/urology | 6 (6%) | 10 (10%) | 0.63 (0.221–1.82) | |
| Other* | 5 (5%) | 13 (13%) | 0.389 (0.133–1.14) | |
| Location of difficult airway event | 0.035 | |||
| Perioperative | 83 (89%) | 79 (77%) | 2.42 (1.08–5.398) | |
| Outside Location | 10 (11%) | 23 (23%) | 0.41 (0.185–0.92) |
Total N=195 unless otherwise indicated. Claims with missing data excluded. Percentages may sum to < > 100% due to rounding. p-values by chi square or Fisher’s exact test for proportions and t-test for age. Odds ratios based on 1993–99 as the indicator and 2000–12 as the reference category. NA = odds ratio undefined. ASA = American Society of Anesthesiologists Physical Status; SD = standard deviation; ENT = ear, nose, and throat.
Other procedures included ventilator management (n=6), resuscitation (n=8), place/change arterial or central venous catheter (n=2), eye (n=1), and endoscopy (n=1).
p values by Fisher’s exact test with Monte Carlo significance using 10,000 sampled tables.
Phase of care, location, and outcomes of difficult tracheal intubation in perioperative locations
Difficult intubation in perioperative locations were similarly distributed across phases of anesthesia care between the 1993–99 and 2000–12 cohorts (p=0.808, Table 2). Two-thirds of difficult intubation events occurred at induction, 13–14% during the procedure, and 14–16% at extubation in the operating room. Another 4–7% occurred during recovery in the post-anesthesia care unit (Table 2).
Table 2:
Timing of Perioperative Difficult Airway Claims and Outcomes
| Phase | 1993–99 n=83 |
2000–12 n=79 |
||
|---|---|---|---|---|
| # claims (col %) | # BD/D (row %) | # claims (col %) | #BD/D row %) | |
| Preinduction | 1 (1%) | 1 (100%) | 0 | 0 |
| Induction | 52 (63%) | 15 (29%) | 53 (67%) | 40 (75%) |
| Intra-procedure | 12 (14%) | 10 (83%) | 10 (13%) | 7 (70%) |
| Extubation in OR | 12 (14%) | 10 (83%) | 13 (16%) | 12 (92%) |
| Recovery/PACU | 6 (7%) | 4 (67%) | 3 (4%) | 3 (100%) |
Perioperative defined as preinduction through recovery, in the OR or PACU, OR – operating room; PACU = post anesthesia care unit. BD/D = permanent brain damage or death. P=0.808 by Fisher’s exact test for phase by time period. Odds ratio for interaction between phase (excluding pre-induction) and time period on outcome = 5.5 (95% CI=1.07–28.4), p=0.041. CI = confidence interval. Odds ratio by multiple logistic regression.
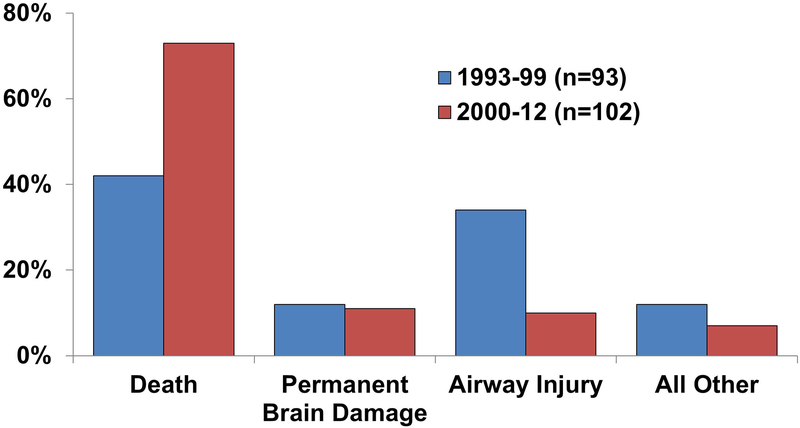
Outcomes differed between time periods (p<0.001, Figure 1). Patients in 2000–12 difficult tracheal intubation claims were more likely to have suffered death than earlier difficult intubation claims (n=74, 73% vs. n=39, 42% for 1993–99 claims, p<0.001, p<0.001 adjusted for multiple testing). Permanent brain damage was similar in the two time periods. Airway injury was more common in the earlier claims (n=32, 34%, p<0.001, p<0001 adjusted for multiple testing). When both phase of care and time period were included in an analysis of perioperative claims, the odds of brain damage or death at induction was 5.5 times greater in 2000–12 compared to 1993–99 (OR 5.5, 95% CI 1.07 – 28.4, p=0.041).
Figure 1:
Clinical outcomes in difficult tracheal intubation claims 1993–99 vs. 2000–12. Airway injury and “all other” outcomes exclude death or permanent brain damage. p<0.001 by chi square test.
For 2000–12 claims, in all locations except the operating room, all claims resulted in brain damage or death. The operating room was the only location in which some difficult tracheal intubation claims did not result in permanent brain damage or death (n=17); brain damage or death was the result for difficult intubation events in all other locations (n= 33 in ICU, 3 PACU, 6 ER, 2 ward, 1 cardiac catheterization lab, 1 radiology). Of the 17 claims that did not result in permanent brain damage or death, most (76%, n=13) occurred during induction of anesthesia (Table 2).
Patient characteristics and airway management techniques in 2000–12 claims
Patient characteristics and urgency of tracheal intubation
Patients were obese in two-thirds (n=54) of the difficult tracheal intubation claims. Most were adults, with only four obstetric patients (all were obese and two were diagnosed with pre-eclampsia; all four had difficult tracheal intubation at induction) and two were pediatric patients (age 24 months undergoing cleft lip/palate with difficult reintubation during phase 1 recovery and age 16 with post-tonsillectomy bleeding with difficult intubation at induction).
Preoperative predictors of a difficult tracheal intubation were present in 76% of difficult tracheal intubation claims (n=78), with nearly half (n=42) possessing two or more predictors of a difficult airway (Table 3). The most common predictors were airway obstruction, past history of difficult intubation, Mallampati grade 3–4, and limited cervical spine extension (Table 3).
Table 3:
Predictors of difficult tracheal intubation and judgment failures in airway management
| Question | # | % |
|---|---|---|
| Indicate any predictors of difficult tracheal intubation (whether known/recognized at the time or not) or factors that contributed to difficult airway management | ||
| Airway obstruction from any cause1 | 31 | 30% |
| Past history of difficult intubation | 21 | 21% |
| Mallampati grade 3–4 | 19 | 19% |
| Limited cervical spine extension | 16 | 16% |
| Limited mouth opening | 13 | 13% |
| Secretions/blood in airway | 12 | 12% |
| Short neck | 10 | 10% |
| Swollen tongue | 6 | 6% |
| Short thyromental distance | 6 | 6% |
| Thick or bull neck | 6 | 6% |
| History of neck irradiation | 5 | 5% |
| Pre-eclampsia | 2 | 2% |
| Prominent teeth | 1 | 1% |
| Number of Predictors | ||
| None | 24 | 24% |
| One | 36 | 35% |
| Two -six | 42 | 41% |
| Inappropriate difficult airway management | ||
| Failure to use supraglottic airway as a bridge (kappa = 0.552) | 27 | 26% |
| Perseveration (kappa = 0.489) | 25 | 25% |
| Failure to plan for difficult tracheal intubation (induction) (kappa = 0.627) | 23 | 23% |
| Delayed calling for, or did not call for, a surgical airway (kappa = 0.436) | 20 | 20% |
| Inadequate preoperative or airway evaluation (kappa = 0.664) | 17 | 17% |
| No backup plan for difficult reintubation (extubation) (kappa= 0.664) | 14 | 14% |
| Number of judgment failures (n=97)2 | ||
| None of the above (appropriate management) | 26 | 27% |
| One | 34 | 35% |
| Two - five | 37 | 38% |
Percentages based on n=102 claims unless otherwise noted.
Causes of airway obstruction: neck hematoma (n=13), allergic reaction (n=4), infection (n=4: 3 neck abscess, 1 acute epiglottis), other upper airway obstruction (n=6: 2 pharyngeal mass, 4 miscellaneous upper airway obstruction), and infraglottic obstruction (n=4: 1 tracheal stenosis, 3 tracheal compression from goiter).
Five claims were excluded from the count of judgment problems because an evaluation of the appropriateness of airway management could not be made.
More than one-third of difficult tracheal intubations involved elective intubations (36%, n=37/102), with almost all occurring in OR/NORA (97%, n=36/102). Urgent tracheal intubation comprised 20% of claims (n=20/102) and all occurred in OR/NORA. Emergency tracheal intubation comprised almost half of the claims (44%, n=45/102) and occurred in a variety of locations (OR [n=20], NORA [n=1], recovery room [n=3], intensive care unit [n=12], emergency room [n=6], and other (ward/non-anesthetizing locations where anesthesia was called to assist with airway management [n=3]).
Airway management techniques:
The most common method used for tracheal intubation during initial attempts was direct laryngoscopy (n=72, 71%). Flexible fiberoptic intubation was the initial technique attempt in 10 cases (10%). Other techniques used for initial attempts included supraglottic airway (n=6), video laryngoscopy (n=2), and blind nasal intubation (n=2). The initial technique in another two cases involved use of a Cook airway exchanger to replace a double lumen endotracheal tube with a single lumen tube or to replace a single lumen tube with a double lumen tube. There was no information regarding use of an assistive device such as direct or video laryngoscopy while exchanging these tubes. In one case the initial technique was jet ventilation. The initial technique could not be determined in 7 claims.
Subsequent techniques used after the initial intubation attempt included direct laryngoscopy in 68 cases (67%), supraglottic airway in 38 (37%), flexible fiberoptic in 20 (20%), and video laryngoscopy in 9 (9%). Additional techniques subsequent to the initial intubation attempt also included blind nasal (n=5), retrograde intubation (n=2), tube changer (n=2), rigid laryngoscopy by the otolaryngology surgeon (n=1), and bronchoscopy (unclear if rigid or flexible, n=1).
Attempts at awake tracheal intubation occurred, but failed, in 11 claims; a flexible fiberoptic scope was used in five claims with direct laryngoscopy used in an additional five (1 unknown technique). Patients were sedated in seven of these claims, and were not sedated in three (1 unknown). Reasons for failed awake intubation, where known, included no or inadequate topical anesthesia (n=3), airway obstruction during topical anesthesia (n=1), and oversedation resulting in apnea, airway obstruction, and inability to ventilate (n=5). In sixteen claims (16%), a supraglottic airway was used as a conduit for intubation. Intubation was successful in three of these claims, and unsuccessful in the remainder.
In claims with sufficient information to evaluate (n=87), a CICO emergency occurred in the majority (92%, n=80). Transtracheal jet ventilation (TTJV) was used in eight cases with five resulting in barotrauma (subcutaneous emphysema with or without pneumothorax). Subsequent attempts at surgical airway proved difficult in four of five of these cases due to presence of subcutaneous emphysema.
Of the 80 claims where a CICO emergency was known to have occurred, obtaining a surgical airway was delayed in over a third of the cases (n=31, 39%). In almost two-thirds of these delays, the delay was due, at least in part, to a delay by the anesthesiologist in calling for a surgical airway (n=20). Other delays in obtaining a surgical airway occurred because the surgeon was not in the hospital (n=4), the surgeon failed to respond to pager (n=1), or the surgeon was reluctant to perform a surgical airway (n=3).
In cases where CICO developed, placement of a supraglottic airway was attempted as a bridge to oxygenation in 36 claims and not attempted in 26. There was insufficient information to evaluate in the remaining CICO claims. In 23 of the claims where placement of a supraglottic airway was attempted, the CICO occurred on induction of anesthesia; oxygenation with a supraglottic airway was unsuccessful in 19 of these cases. Factors contributing to lack of successful supraglottic airway oxygenation included upper airway obstruction or pathology such as neck abscess or prior neck irradiation (n=5), multiple intubation attempts prior to supraglottic airway placement (n=5), and morbid obesity (n=2). In another three of the 23 cases where supraglottic airway was used as a bridge to oxygenation during induction, it was successful but too late to prevent hypoxic injury in two cases and hypoxemia due to negative pressure pulmonary edema occurred in the third case. Other cases where supraglottic airway was attempted as a bridge during CICO (not during induction) included nine cases where difficult tracheal re-intubation occurred at emergence and three cases during the procedure; oxygenation was unsuccessful in all of these cases. In another two cases, supraglottic airway was attempted as a bridge to oxygenation during resuscitation; this was unsuccessful in one case and was successful but delayed in the other.
A surgical airway was performed in 76% of the CICO emergencies (n=61 out of 80 claims). Surgical airways in the OR (n=45) were mostly performed by the case surgeon (general/thoracic/vascular surgeon, ear-nose-throat, or spine surgeons [60%, n=27]). A different surgeon was called to the OR to perform a surgical airway in 29% (n=13). Less commonly, anesthesiologists attempted surgical airways (n=2, 1 successful, 1 not) in the OR. In all 61 claims with attempted surgical airways, the surgical airway was difficult to achieve in 28% of the cases (n=17). Difficulty was attributed to difficult anatomy (n=8), subcutaneous emphysema from jet ventilation (n=4), bleeding and other complications from a surgical airway (n=4), or surgeon inexperience (n=1). Complications occurred in six surgical airways including tracheal transection, esophageal laceration, substantial bleeding, loss of surgical blade in the trachea, or failure to achieve a surgical airway.
One fifth of the claims (n= 21, 21%) had systems issues including lack of assistance and equipment, or lack of systematic communication for airway issues. The more common equipment issues were lack of a difficult airway cart in a suitable location (9 cases with delays to obtain cart) and lack of surgical airway equipment (5 cases where lack of timely availability of emergency airway equipment contributed to delays in accomplishing surgical airways during CICO emergencies). In five cases of difficult reintubation after extubation in the ICU, there had been inadequate communication that intubation in the OR had been difficult.
Appropriateness of airway management:
In the 97 difficult airway claims with sufficient information for assessment, inappropriate difficult airway management occurred in 73% (n= 71, kappa=0.44–0.66, Table 3). Two or more judgment failures occurred in 38% (n=37, Table 3). Only 27% of claims (n=26, Table 3) lacked any judgment failures. The most common failures included failure to use a supraglottic airway as a bridge for oxygenation (n=27, 26% of 102 difficult airway claims, kappa=0.55), perseveration (n=25, 25%, kappa=0.49), and failure to plan for difficult tracheal intubation on induction (n=23, 23%, kappa=0.63, Table 3). Clinical examples of each type of judgment failure are provided in the appendix.
In-hospital location of tracheal intubation was not associated with differences in appropriateness of difficult airway management. In the 97 claims in which an evaluation could be made, perioperative locations (n=16 of 74 appropriate, 22%) was not significantly different compared to airway management in outside locations (n=10 of 23 appropriate, 43%, p=0.039, 0.078 adjusted for multiple testing). Management of difficult tracheal intubation in elective or urgent circumstances was more frequently judged as inappropriate (84%, n=46/55) in contrast to management during emergency intubation (60%, n=25/42, p=0.008, p=0.016 adjusted for multiple testing, odds ratio = 3.48, 95% confidence interval 1.35 to 8.9).
DISCUSSION
Our analysis of difficult tracheal intubation claims in the United States highlights several important findings. Claims for difficult tracheal intubation events in more recent years (2000–2012) more often involved sicker patients (76% ASA 3–5) undergoing emergency procedures compared to claims from 1993–1999. The proportion of difficult tracheal intubation events that occurred in an outside location in 2000–12 claims was also greater than in the earlier claims (23% vs. 11%, p=0.035). Seventy-six percent of patients in 2000–2012 claims had preoperative predictors of difficult tracheal intubation. Almost three-fourths of 2000–12 claims exhibited judgment failures, including lack of a proper airway management plan and, during a CICO emergency, the failure to utilize a supraglottic airway as a bridge to oxygenation and delay in attempting a surgical airway.
Comparison of difficult tracheal intubation events 1993–1999 vs. 2000–2012
Outcomes of difficult tracheal intubation were poor, with a high proportion of death in claims related to difficult intubation in both time periods. However, claims in 2000–2012 exhibited an increased proportion of brain damage or death associated with induction of anesthesia compared to claims in 1993–1999 (Table 2, p<0.001). The increased proportion of brain damage or death associated with more recent difficult tracheal intubation claims may be related to more higher-risk patients, different surgical procedures, and more emergency care. However, the difference may reflect different study methodology in the two time periods with use of a supplemental data collection instrument in the early time period, but not in 2000–2012 claims. Inclusion criteria differed as 1993–1999 cases were not included if the on-site reviewer did not complete the supplemental form. Nonetheless, these findings represent an opportunity to re-evaluate the efficacy of our current algorithms and training for airway management.
Clinical details of difficult tracheal intubation events 2000–2012
Deficiencies in clinical judgment occurred in the majority of recent claims (73%, n=71/97) that could be adequately assessed in our study. Our study is not unique in this finding.5,12–15 In the Danish Anesthesia Database, of emergency surgical airway procedures over a six-year study period, non-technical skills were judged satisfactory in only 37%.12 In the NAP4 study, non-technical skill deficits were identified in 40% of cases with one-quarter considered to be a major contributor to the poor outcome.5,14 Our findings differ from these reports in that ours studied malpractice claims which would be expected to have more judgment errors. However, it is notable that we found judgment errors were more common in elective and urgent procedures compared to emergency tracheal intubation procedures. This result underscores the absence of an airway strategy for elective or urgent cases where the setting may encourage a false sense of security.
A lack of adequate planning for intubation difficulty/failure contributed to the injuries in our study. Published guidelines recommend an airway strategy (coordinated series of airway plans)5 when predictors of difficulty are present.8,16,17 This error was also apparent in claims data from Denmark from 1996–20042, a Norwegian report spanning a 15-year period (2001–2015)4, and the NAP-4 study in UK in 2008–2009.5
Our study again highlights the considerable risk associated with the immediate post-operative period. When difficult tracheal intubation occurred outside the induction phase, brain damage or death was common and there was no improvement in outcomes from 2000–2012 compared to 1993–1999 (Table 2). Only one article regarding extubation is published for every 36 articles published regarding tracheal intubation (OvidTM MedlineTM Search criteria: airway extubation, intratracheal intubation; Wolters Kluver; www.ovid/site/catalog/databases/901.jsp Accessed May 23, 2018). More research and education should be focused on this high-risk stage of care, particularly when difficult tracheal intubation has been encountered, or the nature of the surgery (e.g. procedures around the head and neck, those with fluid shifts, and steep head-down positioning) may make post-extubation airway management more difficult.
Perseveration, defined as the consistent application of any airway management technique/tool ≥3 attempts without deviation or change, or the return to a technique or tool that was previously unsuccessful, was noted in a quarter of 2000–2012 claims (Table 3). Perseveration is a distinct judgment error related to situational awareness and potentially lack of equipment and the skills to use other devices. Moving along a coordinated series of plans in an airway strategy also means not dwelling on techniques already attempted unsuccessfully.
Anesthesiologists appeared reluctant to move down the difficult airway algorithm to placement of a surgical airway. In cases where enough clinical detail was present for review, 80 of 102 claims ultimately degenerated into a CICO emergency. In nearly 4-in-10 CICO claims, obtaining the surgical airway was delayed due to delay in calling for a surgical airway, lack of surgeon availability, and delay in performing a surgical airway. Our results stand in contrast to current guidelines for management of the CICO emergency, which recommend placing a supraglottic device while preparing, in parallel, to perform an immediate emergency “surgical airway” should the supraglottic airway fail to oxygenate the patient.8,16,17
A supraglottic airway was not attempted as a bridge for oxygenation in 26% in our study (Table 3). A supraglottic airway can effectively provide rescue oxygenation in the management of difficult mask oxygenation and tracheal intubation.18,19 The Danish Anaesthesia Database recently reported that placement of a supraglottic airway was attempted in only 12.4% of all difficult airway cases.20 In documented cases of CICO, reported rates of attempted supraglottic airway placement are not much higher, ranging from 18.9–35%.20,21 Cognitive aids and team practice in managing the inevitable CICO emergency may be useful.22
Our study also illustrated that poor outcomes after failed tracheal intubation may occur despite adherence to practice guidelines. Awake intubation wasn’t always effective, insertion of a supraglottic device may not improve oxygenation and waking a patient up after multiple intubation attempts may still yield a poor outcome. Our findings emphasize that supraglottic airway devices cannot be considered fail-safes for the difficult airway in the presence of supra or infraglottic obstruction, multiple preceding intubation attempts, and prior radiation therapy.
We acknowledge the controversy regarding the best technique to establish a surgical airway during a CICO emergency that will achieve both highest first-pass success and least amount of patient harm.23 Traditionally, TTJV has been recommended as a bridge to a definitive surgical airway. However, a recent systematic review found TTJV in CICO was associated with a 32% incidence of barotrauma, 42% incidence of device failure, and, in several cases, subsequent difficulty with open surgical airway attempts due to obliterated anatomy.24 Our study further supports that attempts at TTJV prior to surgical airway need to be weighed against the possibility of making subsequent surgical airway more difficult.
Recommendations to improve management of difficult tracheal intubation
Based on our findings and the literature, we offer some common-sense recommendations related to training and education. Human factors and nontechnical skills (situational awareness, communication, teamwork), or lack thereof, are important drivers of adverse difficult airway management outcomes.21–25 Didactics may be useful to impart knowledge and familiarize practitioners with current guidelines, but are not adequate by themselves. Practitioners must familiarize themselves with locally available airway equipment, which should be placed appropriately within the construct of published difficult airway guidelines.8,16,17 Simple task trainers and/or dedicated manikins should be used to train appropriate handling of this equipment. Correct application of equipment according to recent guidelines when faced with complex and unanticipated difficult airway situations should be rehearsed on a regular basis with the healthcare team focusing not only on technical skills, but to imbue and maintain adequate crew resource management. Incorporation of cognitive aids specific to difficult airway management may cue practitioners to the need to move on to another plan in their airway strategy while ‘in the heat of the moment’. Finally, the education should be malleable so the curricula can swiftly incorporate new evidence as it becomes available.
Limitations
Analysis of closed malpractice claims has well-described limitations: retrospective analysis, lack of randomization, selection, and hindsight bias.10 The database lacks denominators and cannot estimate risk. Cause-effect relationships cannot be established. Claims take 3–7 years between event, claim closure, and incorporation into the database. Hence, new clinical practices and technologies are not fully captured. However, our results are relevant for current practice as Fei et al. reported that the rate of emergency surgical airways was unchanged from 2008–2015 despite increased use of video laryngoscopy for tracheal intubation.26
Data abstraction in 2000–12 claims relied on narratives by reviewers using primary data sources at liability insurers, which may result in missing information. However, reliability of assessments was acceptable (moderate to substantial: 0.436 – 0.664). Kappa values were derived from pairwise assessments; evaluation of each claim by three authors to derive the final assessment improved reliability above the measured kappa values. With an overall failed intubation incidence of 1.3 events/10,000 patient encounters,9 difficulty in conducting prospective randomized trials studying these high-impact low frequency events is self-evident.9 Although risk for injury cannot be determined from closed claims analysis, it identifies patient safety hazards and stimulates research.
Conclusions
In summary, recent difficult intubation claims showed poor outcomes and failures in judgment. Our results emphasize the need to improve both practitioner skills and systems response when difficult or failed tracheal intubation is encountered.
Acknowledgments:
The authors acknowledge the closed claims reviewers from the American Society of Anesthesiologists (ASA) and participation of the following liability insurance companies who have given permission to be acknowledged: Anesthesia Service Medical Group, Inc., San Diego, CA; COPIC Insurance Company, Denver, CO; ISMIE Mutual Insurance Company, Chicago, IL; MAG Mutual Insurance Company, Atlanta, GA; Medical Liability Mutual Insurance Company, New York, NY; Midwest Medical Insurance Company, Minneapolis, MN; NORCAL Mutual Insurance Company, San Francisco, CA; Physicians Insurance A Mutual Company, Seattle, WA; Preferred Physicians Medical Risk Retention Group, Overland Park, KS; Risk Management Foundation, Cambridge, MA; State Volunteer Mutual Insurance Company, Brentwood, TN; The Doctors’ Company, Napa, CA; The University of Texas System, Austin, TX.
Funding Statement: Supported in part by the ASA and the Anesthesia Quality Institute (AQI), Schaumburg, IL. All opinions expressed are those of the authors and do not reflect the policy of the ASA or AQI. REDCap (Research Electronic Data Capture) electronic data capture tools hosted at University of Washington was provided by the Institute of Translational Health Science (ITHS) through UL1 RR025014 from NCRR/NIH. Additional support was provided by institutional funding.
Abbreviations:
- ENT
ear-nose-throat
- OR
operating room
- ICU
intensive care unit
- SpO2
oxygen saturation by pulse oximetry
Appendix: Examples of inappropriate difficult airway management
Inadequate preoperative or airway evaluation (failure to recognize a potentially difficult airway)
Case 1: A morbidly obese 30–40 year old woman with severe pre-eclampsia was scheduled for elective cesarean section. The preoperative assessment was cursory and did not describe pre-eclampsia, an airway exam, other pertinent physical and laboratory findings, or the patient’s past history of a difficult endotracheal intubation. After multiple unsuccessful attempts at a subarachnoid block, general anesthesia was induced via rapid sequence induction using propofol and succinylcholine. The patient’s mouth was difficult to open and bag-mask ventilation difficult. Additional succinylcholine was administered, a laryngoscope inserted, and the larynx was not visualized. A supraglottic airway was placed, but ventilation was unsuccessful. Two-handed bag-mask ventilation was also unsuccessful. A difficult airway cart was called, but it was a floor away. Eventually a surgical airway was performed after the patient arrested. The patient sustained brain death.
Case 2: A 55–65 year old ASA 3 man with metastatic laryngeal cancer treated with neck radiation was scheduled for a gastrostomy tube placement due to dysphagia. While a complete preoperative evaluation had been performed earlier, it was not available at the time of surgery. The anesthesiologist performed a hasty assessment with limited airway evaluation. General anesthesia was induced with propofol and succinylcholine. The patient’s trachea could not be intubated and the patient could not be ventilated with mask or supraglottic airway. Transtracheal jet ventilation was unsuccessful and caused a pneumothorax. A surgical airway was performed after the patient arrested. The patient was resuscitated, but died the next day.
Failure to plan for a difficult tracheal intubation (induction)
Case 3: A 55–65 year old ASA 3E man with a neck abscess was scheduled for incision and drainage by an ENT surgeon. The anesthesiologist decided to do a rapid sequence induction after noting blood and pus in the posterior pharynx. The cords were not visualized on direct laryngoscopy. Bag-mask ventilation was attempted but was unsuccessful. Ventilation was also not successful after a supraglottic airway was placed. The surgeon was called to the room to perform an emergency surgical airway, but there weren’t any instruments available in the room. The patient sustained anoxic brain injury and later died.
Case 4: A 55–65 year old ASA 3E woman with neck swelling, hoarseness, and shortness of breath was brought to the OR for drainage of a neck hematoma post cervical spine fusion. The anesthesiologist performed a rapid sequence induction before the surgeon was present. Multiple attempts at intubation were made using direct laryngoscopy, all without success. Ventilation was difficult and the patient arrested. The surgeon arrived and attempted to perform an emergency surgical airway, at which time the anesthesiologist successfully intubated the patient’s trachea as the hematoma was drained. The patient was resuscitated, but later died of anoxic brain damage.
Case 5: A 20–30 year old ASA 3E woman was scheduled for incision and drainage of a submental salivary gland abscess. The anesthesiologist suggested monitored anesthesia care due to airway concerns, but the surgeon desired general anesthesia. Anesthesia was induced with propofol, fentanyl, and succinylcholine, and the cords were not visualized with direct laryngoscopy. The anesthesiologist called for a video laryngoscope, but the nurses were not able to find it. The patient could not be ventilated and went into cardiac arrest. The anesthesiologist asked the nurses to bring the difficult airway cart, which they also couldn’t find. The anesthesiologist had to leave the room to search for the cart. The anesthesiologist asked the surgeon to perform an emergency cricothyrotomy. However, the surgeon insisted that an electrocautery to be set up first. Nine minutes after cardiac arrest, a surgical airway was secured by the surgeon. The patient was resuscitated but remained in a persistent vegetative state.
No backup plan for difficult reintubation (extubation)
Case 6 (OR): A 40–50 year old ASA 2E man was scheduled for incision and drainage of a submandibular/submental abscess. Anesthesia was induced with a rapid sequence induction with propofol and succinylcholine. Upon laryngoscopy, considerable tongue and paraglottic swelling was noted. The cords could not be visualized, however the anesthesiologist successfully intubated the patient’s trachea using a bougie passed under the epiglottis. At the end of the procedure, an anesthesia team member decided to extubate the patient because the patient was fighting the tube and appeared strong. The SpO2 fell after extubation and an anesthesia team member attempted to open the airway with placement of a nasal and oral airway. Mask ventilation was very difficult. Many intubation attempts were made using a variety of blades and devices. An ENT was called to perform a surgical airway, who suggested a supraglottic airway be inserted instead. After the supraglottic airway was placed, the patient became impossible to ventilate and went into cardiac arrest. The surgical airway was placed with some difficulty. The patient sustained severe hypoxic brain and died.
Case 7 (ICU): A 60–70 year old ASA 3 man with an odontoid fracture/C2 dislocation after an accident in a halo collar was extubated by the ICU team after he met mechanical extubation criteria. The patient’s trachea had been initially intubated at the accident scene. Immediately following extubation, the patient developed upper airway obstruction, bag-mask ventilation was unsuccessful, and the patient arrested. An anesthesiologist was called but could not intubate. A difficult airway cart had to be retrieved from storage. The breast plate was removed and a surgical airway inserted, however the patient had little neurological activity and support was withdrawn.
Failure to use a supraglottic airway as a bridge to oxygenation
Case 8: A 50–55 year old ASA 1 woman underwent bilateral breast augmentation under monitored anesthesia care using propofol, ketamine, and fentanyl in a plastic surgeon’s office. Due to airway obstruction, general anesthesia was induced, succinylcholine was administered, and the anesthesiologist attempted (unsuccessfully) to intubate. SpO2 decreased to 70% for 40 minutes during the many attempts to mask ventilate and intubate the patient’s trachea. The surgeon performed a cricothyrotomy after the patient had marked bradycardia and hypotension. The patient was quickly resuscitated after the cricothyrotomy and required treatment for bilateral pneumothoraces. The patient recovered without neurologic injury, but she complained of difficulty swallowing, a visible scar, an altered voice, and post-traumatic stress disorder.
Perseveration
Case 9: A 60–70 year old ASA 3 obese man underwent general anesthesia using a supraglottic airway for a urologic procedure in the lithotomy position. Due to inadequate ventilation, the anesthesiologist tried to intubate with direct laryngoscopy, but was unable to visualize the vocal cords. Mask ventilation was difficult and the anesthesiologist called for help. Three additional anesthesiologists also attempted direct laryngoscopy multiple times and all failed. An intubating supraglottic airway was attempted without success. The patient arrested. Finally, the patient’s trachea was successfully intubated and he was resuscitated. He sustained severe anoxic brain damage and later died in a long-term care facility.
Case 10: The anesthesiologist was called urgently to the cardiac cath lab to intubate a 60–70 year old morbidly obese ASA 4E man undergoing coronary revascularization after a myocardial infarction. The anesthesiologist gave the patient midazolam prior to performing direct laryngoscopy, but was not able to visualize the vocal cords upon multiple attempts. Bag-mask ventilation became difficult. A second anesthesiologist was called, and was also unable to intubate with multiple direct laryngoscopies and a fiberoptic intubation. SpO2 decreased to 25–30% and the patient arrested. A surgeon was called to place a cricothyrotomy. The patient was resuscitated, but had severe anoxic brain damage and died.
Delay in Surgical Airway during CICO
Case 11: A 1–3 year old child underwent a cleft palate repair under general anesthesia and was extubated at the end of the case. In the recovery room, the patient had substernal retractions with SpO2 of 85–89%. An hour later, the patient required cardiac resuscitation due to heart rate of 30/min and cyanosis. Multiple intubation attempts and supraglottic airway insertion were made for over an hour before a surgical airway was performed. At that time, the patient was asystolic and had a tension pneumothorax. The patient died.
Case 12: A 50–60 year old ASA 3 morbidly obese woman with obstructive sleep apnea presented for elective laparoscopic gastric bypass. The patient’s preoperative airway evaluation did not predict a difficult intubation. After induction with propofol and rocuronium, ventilation was easy. The larynx was not visualized on direct laryngoscopy on two attempts. After the second attempt, bag-mask ventilation became more difficult, SpO2 was in the 80s, and the anesthesiologist called for help. A supraglottic airway was inserted without adequate ventilation and a difficult airway cart brought to the OR. The surgeon was called multiple times but without response as ventilation became impossible. The patient had a hypoxic cardiac arrest. The surgeon arrived 22 min after induction and secured an emergency surgical airway. The patient was resuscitated but sustained hypoxic brain damage requiring assistance with activities of daily living.
Footnotes
Prior Presentations: Preliminary findings were presented at the European Society of Anesthesiology Meeting in Geneva, Switzerland June 5, 2017 (Domino KB et al: Management of the difficult airway: an updated closed claims analysis. Abstract 16AP07–5).
Conflict of interest: None of the authors has a conflict of interest.
Clinical Trial Registry: NA
References
- 1.Peterson GN, Domino KB, Caplan RA, Posner KL, Lee LA, Cheney FW: Management of the difficult airway: A closed claims analysis. Anesthesiology 2005; 103:33–9 [DOI] [PubMed] [Google Scholar]
- 2.Hove LD, Steinmetz J, Christoffersen JK, Møller A, Nielsen J, Schmidt H: Analysis of deaths related to anesthesia in the period 1996–2004 from closed claims registered by the Danish Patient Insurance Association. Anesthesiology 2007; 106:675–80 [DOI] [PubMed] [Google Scholar]
- 3.Cook TM, Scott S, Mihai R: Litigation related to airway and respiratory complications of anaesthesia: An analysis of claims against the NHS in England 1995–2007. Anaesthesia 2010; 65:556–63 [DOI] [PubMed] [Google Scholar]
- 4.Fornebo I, Simonsen KA, Bukholm IRK, Kongsgaard UE: Claims for compensation after injuries related to airway management: A nationwide study covering 15 years. Acta Anaesthesiol Scand 2017; 61:781–9 [DOI] [PubMed] [Google Scholar]
- 5.Cook TM, Woodall N, Frerk C; Fourth National Audit Project: Major complications of airway management in the UK: Results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 1: Anaesthesia. Br J Anaesth 2011; 106:617–31 [DOI] [PubMed] [Google Scholar]
- 6.Caplan RA, Benumof JI, Berry FA, Blitt CD, Bode RH, Cheney FW, Connis RT, Guidry OF, Ovassapian: Guidelines for management of the difficult airway. A report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology 1993; 78:597–602.8457062 [Google Scholar]
- 7.Caplan RA, Benumof JI, Berry FA, Blitt CD, Cheney FW, Connis RT, Guidry OF, NIckinovich DG: Practice guidelines for management of the difficult airway: An updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology 2003; 98:1269–77 [DOI] [PubMed] [Google Scholar]
- 8.Apfelbaum JL, Hagberg CA, Caplan RA, Blitt CD, Connis RT, Nickinovich DG, Hagberg CA; The previous update was developed by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway; Caplan RA, Benumof JL, Berry FA, Blitt CD, Bode RH, Cheney FW, Connis RT, Guidry OF, Nickinovich DG, Ovassapian A: Practice guidelines for management of the difficult airway: An updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology 2013; 118:251–70 [DOI] [PubMed] [Google Scholar]
- 9.Schroeder RA, Pollard R, Dhakal I, Cooter M, Aronson S, Grichnik K, Buhrman W, Kertai MD, Mathew JP, Stafford-Smith M: Temporal trends in difficult and failed tracheal intubation in a regional community anesthetic practice. Anesthesiology 2018; 128:502–10 [DOI] [PubMed] [Google Scholar]
- 10.Cheney FW, Posner K, Caplan RA, Ward RJ: Standard of care and anesthesia liability. JAMA 1989; 261:1599–603 [PubMed] [Google Scholar]
- 11.Sowka M, ed.: Malpractice claims: Final compilation. Brookfield, WI: National Association of Insurance Commissioners; 1980 [Google Scholar]
- 12.Rosenstock C, Hansen E, Kristensen M, Rasmussen L, Skak C, Østergaard D: Qualitative analysis of unanticipated difficult airway management. Acta Anaesthesiol Scand 2006; 50:290–7 [DOI] [PubMed] [Google Scholar]
- 13.Bromiley M: Have you ever made a mistake? Bulletin of the Royal College of Anaesthetists 2008; 48:2442–5 [Google Scholar]
- 14.Cook TM, Woodall N, Harper J, Benger J; Fourth National Audit Project: Major complications of airway management in the UK: Results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 2: Intensive care and emergency departments. Br J Anaesth 2011; 106:632–42 [DOI] [PubMed] [Google Scholar]
- 15.Flin R, Fioratou E, Frerk C, Trotter C, Cook TM: Human factors in the development of complications of airway management: Preliminary evaluation of an interview tool. Anaesthesia 2013; 68:817–25 [DOI] [PubMed] [Google Scholar]
- 16.Law JA, Broemling N, Cooper RM, Drolet P, Duggan LV, Griesdale DE, Hung OR, Jones PM, Kovacs G, Massey S, Morris IR, Mullen T, Murphy MF, Preston R, Naik VN, Scott J, Stacey S, Turkstra TP, Wong DT; Canadian Airway Focus Group: The difficult airway with recommendations for management--part 1--difficult tracheal intubation encountered in an unconscious/induced patient. Can J Anaesth 2013; 60:1089–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frerk C, Mitchell VS, McNarry AF, Mendonca C, Bhagrath R, Patel A, O’Sullivan EP, Woodall NM, AhmadL; Difficult Airway Society intubation guidelines working group: Difficult Airway Society 2015 guidelines for management of unanticipated difficult intubation in adults. Br J Anaesth 2015; 115:827–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parmet JL, Colonna-Romano P, Horrow JC, Miller F,Gonzales J, Rosenberg H: The laryngeal mask airway reliably provides rescue ventilation in cases of unanticipated difficult tracheal intubation along with difficult mask ventilation. Anesth Analg 1998:661–5 [DOI] [PubMed] [Google Scholar]
- 19.Combes X, Le Roux B, Suen P, Dumcrat M, Motamid C, Sauvat S, Duvaldestin P, Dhonneur G: Unanticipated difficult airway in anesthetized patients. Anesthesiology 2004; 100:1146–50 [DOI] [PubMed] [Google Scholar]
- 20.Thompsen JD, Norskov AK, Rosenstock CV: Supraglottic airway devices in difficult airway management: A retrospective cohort study of 658,104 general anaesthetics registered in the Danish Anaesthesia Database. Anaesthesia 2019; 74:151–7 [DOI] [PubMed] [Google Scholar]
- 21.Duggan LV, Lockhart SL, Cook TM, O’Sullivan EP, Dare T, Baker PA: The Airway App: Exploring the role of smartphone technology to capture emergency front-of-neck airway experiences internationally. Anaesthesia 2018; 73:703–10 [DOI] [PubMed] [Google Scholar]
- 22.Duggan LV, Brindley PG, Law JA: Improving communication, teamwork, and action during a “cannot intubate cannot oxygenate (CICO)” emergency: Employing CICO as a cognitive aid mnemonic. Can J Anaesth 2018; 65:1087–92 [DOI] [PubMed] [Google Scholar]
- 23.Baker PA, O’Sullivan EP, Kristensen MS, Lockey D: The great airway debate: Is the scalpel mightier than the cannula? B J Anaesth 2016; 117 Suppl 1:i17–i19 [DOI] [PubMed] [Google Scholar]
- 24.Duggan LV, Ballantyne SB, Law JA, Morris IR, Murphy MF, Griesdale DE: Transtracheal jet ventilation in the ‘can’t intubate can’t oxygenate’ emergency: A systematic review. B J Anaesth 2016; 117 Suppl 1:i28–i38 [DOI] [PubMed] [Google Scholar]
- 25.Cook TM: Strategies for the prevention of airway complications - a narrative review. Anaesthesia 2018; 73:93–111 [DOI] [PubMed] [Google Scholar]
- 26.Fei M, Wanderer JP, Jiang Y, St Jacques PJ: Association between the availability of videolaryngoscopes and the incidence of emergency surgical airway in the perioperative setting of a larger academic medical centre: A retrospective observational study. Brit J Anaesth 2016; 117:824–6 [DOI] [PubMed] [Google Scholar]