Significance Statement
IgA nephropathy (IgAN) is the leading primary GN worldwide. The disease is thought to result from glomerular deposition of circulating immune complexes of IgG bound to galactose-deficient IgA1 (Gd-IgA1). However, routine immunofluorescence microscopy fails to detect IgG in many kidney biopsies from patients with IgAN and the specificity of IgG in immunodeposits has not been tested. The authors show that IgG specific for Gd-IgA1 was extracted from remnant IgAN kidney-biopsy specimens, even when IgG was not detected by routine immunofluorescence. Using confocal microscopy, the authors confirmed that glomerular IgA and IgG colocalize in biopsies, including those negative for IgG by routine immunofluorescence microscopy, suggesting the two form a complex. The results highlight the pivotal role of IgG autoantibodies in IgAN, and bolster the hypothesis that Gd-IgA1–specific IgG autoantibodies are involved in the pathogenesis of the disease.
Keywords: autoantibody, biomarker, immunodeposits, IgA nephropathy
Visual Abstract
Abstract
Background
IgA nephropathy (IgAN) is the leading primary GN worldwide. The disease is thought to result from glomerular deposition of circulating immune complexes of IgG bound to galactose-deficient IgA1 (Gd-IgA1). However, routine immunofluorescence microscopy fails to detect IgG in many kidney biopsies from patients with IgAN and the specificity of IgG in immunodeposits has not been tested.
Methods
We used remnant frozen kidney-biopsy specimens from 34 patients with IgAN; 14 were IgG-positive and 20 were IgG-negative by routine immunofluorescence microscopy. Six patients with primary membranous nephropathy (MN) and eight with lupus nephritis (LN) served as controls. IgG in the kidney tissue was extracted and its amount determined by ELISA. IgG molecular integrity was assessed by SDS-PAGE immunoblotting. Antigenic specificity of extracted IgG was determined by ELISA using phospholipase A2 receptor (PLA2R) or Gd-IgA1 as antigen. In addition, ten other IgAN cases, six IgG-positive and four IgG-negative by routine immunofluorescence, were used for colocalization studies by confocal microscopy.
Results
IgG extracted from MN but not IgAN immunodeposits reacted with PLA2R. Conversely, IgG extracted from IgAN but not MN or LN immunodeposits reacted with Gd-IgA1. Even IgAN kidney-biopsy specimens without IgG by routine immunofluorescence microscopy had IgG specific for Gd-IgA1. Confocal microscopy confirmed the presence of IgG in the IgAN biopsies with colocalization of glomerular IgA and IgG.
Conclusions
These results reveal for the first time that IgAN kidney biopsies, with or without IgG by routine immunofluorescence, contain Gd-IgA1–specific IgG autoantibodies. These findings support the importance of these autoantibodies in the pathogenesis of IgAN.
IgA nephropathy (IgAN), the most common primary GN in the world, is characterized by mesangial IgA as the dominant or codominant Ig by routine immunofluorescence microscopy studies of kidney biopsies.1 A four-hit process has been proposed to explain the pathogenesis of primary IgAN: increased levels of circulatory galactose-deficient IgA1 (Gd-IgA1) (hit 1) lead to development of autoantibodies mostly of the IgG subclass (hit 2). Together, they form pathogenic immune complexes (hit 3) that deposit in glomeruli to incite kidney injury (hit 4).2
Glomerular IgA deposits of IgAN patients are enriched for Gd-IgA1,3,4 but routine immunofluorescence microscopy fails to show IgG in up to 50%–80% of kidney biopsies.5,6 Moreover, the specificity of IgG deposits for the autoantigen has not been defined. In this study, we extracted IgG from glomerular immunodeposits of IgAN remnant kidney-biopsy specimens and tested it for specificity against Gd-IgA1. The presence and specificity of IgG was also ascertained in specimens deemed IgG-negative by routine immunofluorescence. Finally, using confocal microscopy and novel IgG-specific nanobody, we evaluated the glomerular colocalization of IgG and IgA.
Methods
Remnant Kidney-Biopsy Specimens
We used remnant frozen kidney-biopsy specimens from 34 patients with IgAN for extraction of IgG from immunodeposits. The Pathology Department at the University of Alabama at Birmingham (UAB) provided the kidney-biopsy specimens. Fourteen cases had IgG glomerular codeposits (at least 1+ on a 0–4+ scale) by routine immunofluorescence microscopy using an FITC-labeled goat anti-human IgG antibody (MP Biomedicals, Santa Anna, CA). These cases were identified as IgG+RIF. Twenty cases had no IgG detected by routine immunofluorescence microscopy (IgG−RIF). For the confocal microscopy studies, we used remnant frozen kidney-biopsy specimens from ten other IgAN patients; six of those biopsies showed IgG by routine immunofluorescence microscopy and four did not. Remnant frozen kidney-biopsy specimens from six patients with primary membranous nephropathy (MN) served as one group of disease controls, as IgG in these deposits is enriched for antibodies specific for phospholipase A2 receptor (PLA2R).7 Remnant frozen kidney-biopsy specimens from eight patients with lupus nephritis (LN) served as another group of disease controls. Three had diffuse proliferative class 4 lupus nephritis (LN-D) pattern with mesangial IgA deposits and five had membranous class 5 lupus nephritis (LN-M).
The cases were selected after reviewing our local pathology database to identify appropriate groups of tissues: IgG+RIF, IgG−RIF, MN, and LN. Nephropathologists at our institution rendered the pathology readings of all selected biopsy specimens that had been obtained for clinical indications. For this study, a second nephropathologist (H.F.), who was blinded to the original interpretations, reviewed all IgAN cases to confirm the diagnosis and provide scoring for the degree of disease activity and chronicity. The UAB Institutional Review Board approved the study and waived the need for informed consent as remnant tissue specimens were deidentified before conducting the laboratory experiments.
Extraction Procedures
Kidney-biopsy specimens were processed using a published protocol7 with the following modifications: tissue cores were first washed with ice-cold PBS (pH 7.2) supplemented with protease inhibitors (Halt Protease Inhibitor Cocktail; Thermo Fisher Scientific, Waltham, MA) and phosphatase inhibitors (Halt Phosphatase Inhibitor Cocktail; Thermo Fisher Scientific) (PBS-I) to remove freezing medium and blood. The tissues were then cut into small sections and washed twice with PBS-I to remove IgG from interstitial liquid and blood (herein referred to as the one-wash protocol [wash of only the small sections]). Both times, fluids were set aside. The combined fluids from the second wash are referred to as wash. The tissues were then homogenized using a Potter-Elvehjem manual homogenizer and IgG from immunodeposits was extracted twice by acidic buffers supplemented with the protease and phosphatase inhibitors. First extraction used 100 µl of 25 mM citrate buffer, pH 3.2, and the second extraction used 100 µl of 25 mM citrate buffer, pH 2.5. After each extraction, tissue was pelleted by centrifugation (15,000×g, 15 minutes), supernatants were collected, neutralized, and both extracts were combined. These specimens are subsequently referred to as extracts. All steps were performed on ice or at +4°C.
We compared the performance of this optimized protocol with that of a previously published two-washes protocol.8 This protocol included two additional washes with 100 µl PBS-I after homogenization of the biopsy-tissue sections (herein referred to as the two-washes protocol [wash of the small sections as wash 1 and the homogenized sections as wash 2]). IgG from immunodeposits was extracted twice by acidic buffers supplemented with the protease and phosphatase inhibitors and processed as in the one-wash protocol. Using two pools of remnant frozen IgAN kidney-biopsy specimens (IgG+RIF and IgG−RIF), we compared these two protocols based on the results for total IgG and the relative amount of IgG autoantibody in the washes and extracts.
IgAN remnant biopsy specimens had only modest tissue, so we randomly pooled groups of five (or four in one instance) specimens with similar routine immunofluorescence characteristics (IgG+RIF or IgG−RIF). These groups are referred to as pools 1, 2, and 3 for IgG+RIF tissues and pools 4, 5, 6, and 7 for IgG−RIF tissues (Table 1).
Table 1.
Clinical characteristics of patients with IgAN at the time of kidney biopsy
| Patient | Age (yr) | Race | Sex | SCr (mg/dl) | Urinary RBC | UPCR (g/g) | 24 h Urine Protein (g/d) | IgG RIF (0–4+) | IgA RIF (0–4+) | C3 RIF (0–4+) |
|---|---|---|---|---|---|---|---|---|---|---|
| IgG+RIF | ||||||||||
| Pool 1 | ||||||||||
| 1 | 20 | W | F | 2.0 | >25 | 8.00 | 7.71 | 1–2+ | 3–4+ | 2+ |
| 2 | 34 | W | M | 5.3 | 3–10 | 4.26 | 9.97 | 1+ | 2+ | 1–2+ |
| 3 | 43 | B | M | 2.0 | 0–2 | 0.74 | 1.83 | 3+ | 4+ | 4+ |
| 4 | 28 | W | F | 0.8 | >25 | 2.46 | NA | 2–3+ | 3+ | 2–3+ |
| 5 | 72 | O | M | 0.9 | >25 | 0.84 | 1.14 | 3+ | 3+ | 1–2+ |
| Pool 2 | ||||||||||
| 6 | 42 | B | F | 0.7 | NA | NA | 1.56 | 2+ | 3–4+ | 1+ |
| 7 | 38 | W | M | 2.3 | 3–10 | 4.96 | 4.96 | 1+ | 3+ | 3+ |
| 8 | 63 | AS | M | 1.3 | 11–20 | 0.69 | 1.40 | 2+ | 4+ | 1+ |
| 9 | 32 | W | M | 1.1 | NA | 1.30 | 3.80 | 1+ | 2+ | 1+ |
| 10 | 71 | W | F | 1.5 | NA | NA | 6.37 | 2+ | 4+ | Trace |
| Pool 3 | ||||||||||
| 11 | 35 | O | M | 0.9 | 2–3 | 0.65 | 0.82 | 1+ | 3+ | 1+ |
| 12 | 29 | O | F | 0.6 | 0–2 | 0.10 | 0.18 | 2+ | 4+ | 1+ |
| 13 | 30 | O | M | 0.9 | 7–10 | 6.23 | NA | 1+ | 3+ | 3+ |
| 14 | 66 | W | F | 0.6 | NA | 1.20 | 2.50 | 2+ | 3+ | 2+ |
| IgG−RIF | ||||||||||
| Pool 4 | ||||||||||
| 1 | 28 | W | M | 2.3 | 21–50 | 0.54 | NA | 0 | 1+ | 1–2+ |
| 2 | 44 | B | F | 1.4 | 3–10 | NA | NA | 0 | 2–3+ | Trace |
| 3 | 41 | W | M | 1.1 | NA | 0.22 | NA | 0 | 3+ | 2+ |
| 4 | 23 | W | F | 1.2 | NA | 4.55 | 6.09 | 0 | 2+ | Trace |
| 5 | 56 | B | F | 1.3 | NA | 2.63 | 4.17 | 0 | 2+ | 3+ |
| Pool 5 | ||||||||||
| 6 | 75 | W | M | 1.4 | 0 | 0.50 | 1.00 | 0 | 2+ | 1+ |
| 7 | 42 | W | M | 2.3 | 21–25 | 0.30 | 7.40 | 0 | 1+ | 3+ |
| 8 | 77 | W | F | 0.9 | 0–2 | NA | 0.80 | 0 | 3+ | 1+ |
| 9 | 26 | W | M | 0.8 | NA | 0.10 | NA | 0 | 2+ | Trace |
| 10 | 23 | W | F | 0.9 | NA | 0.47 | 0.55 | 0 | 2+ | Trace-1+ |
| Pool 6 | ||||||||||
| 11 | 39 | B | M | 3.6 | NA | NA | 7.17 | 0 | 2+ | 1+ |
| 12 | 38 | B | F | 0.9 | NA | NA | 1.58 | 0 | 1+ | 1+ |
| 13 | 52 | W | M | 0.9 | NA | NA | NA | 0 | 2+ | 0 |
| 14 | 42 | B | M | 1.4 | 0 | 0.15 | 0.30 | 0 | 2+ | Trace |
| 15 | 48 | B | F | 1.2 | 19 | NA | NA | 0 | 2+ | 0 |
| Pool 7 | ||||||||||
| 16 | 61 | W | M | 1.0 | >50 | 1.14 | 2.39 | 0 | 1–2+ | 1+ |
| 17 | 42 | W | M | 1.3 | 2 | 0.71 | 1.70 | 0 | 3+ | Trace |
| 18 | 44 | W | M | 1.5 | 0–2 | 4.37 | NA | 0 | 2+ | Trace |
| 19 | 67 | W | M | 1.3 | NA | 0.15 | 0.23 | 0 | 3+ | 1+ |
| 20 | 45 | W | F | 2.6 | 11–20 | 2.57 | NA | 0 | 3+ | 2+ |
SCr, serum creatinine concentration; RBC, RBC/hpf in resuspended spun urine sediment; UPCR, urine protein-to-creatinine ratio; IgG RIF, IgG staining of renal tissue based on routine immunofluorescence; IgA RIF, IgA staining of renal tissue based on routine immunofluorescence; C3 RIF, Complement component 3 staining of renal tissue based on routine immunofluorescence; W, White; F, female; M, male; B, Black; O, Other; AS, Asian; NA, not applicable.
Three MN biopsy specimens were processed with the two-washes protocol and three with the one-wash protocol. As the MN remnant biopsy specimens had enough tissue with glomeruli, each sample was processed individually (Table 2).
Table 2.
Analysis of IgG total and IgG autoantibody specific for Gd-IgA1 in washes and extracts from remnant frozen kidney-biopsy specimens from patients with IgAN IgG+RIF (pool 1, pool 3) and IgG−RIF (pool 4, pool 7): comparison of the results using the two-washes protocol (pools 1 and 4) and one-wash protocol (pools 3 and 7)
| Preparation | IgG+RIF (Two-Washes Protocol) | IgG+RIF (One-Wash Protocol) | ||
|---|---|---|---|---|
| IgGa | AutoAbb | IgG | AutoAb | |
| W1 | 41.2 | 7.1 | 74.5 | 1.1 |
| W2 | 47.9 | 11.8 | — | — |
| Ext | 10.9 | 81.1 | 25.5 | 98.9 |
| Preparation | IgG−RIF (Two-Washes Protocol) | IgG−RIF (One-Wash Protocol) | ||
| IgG | AutoAb | IgG | AutoAb | |
| W1 | 59.9 | 1.6 | 86.8 | 0.2 |
| W2 | 35.9 | 2.1 | — | — |
| Ext | 4.1 | 96.3 | 13.2 | 99.8 |
AutoAb, Gd-IgA1-specific autoantibody; W1, wash 1; W2, wash 2; Ext, extracts with acidic buffers that were then neutralized.
IgG in ng/ml was determined in washes 1 and 2 and extracts. Values were then expressed in each category as a relative percentage of total isolated IgG.
AutoAb, IgG autoantibody assay was performed for washes 1 and 2 and extracts. One unit (U) of Gd-IgA1–specific IgG was defined as the amount resulting in OD at 490 nm equal to 1.0. The results were normalized per microgram of total IgG. Results in the table were expressed as percentage of total normalized IgG autoantibody.
LN biopsy specimens were processed with the two-washes protocol. These tissues had few glomeruli, so we pooled three LN-D specimens and five LN-M specimens as subgroups of a second disease control (Table 2).
Analysis of Isolated IgG
IgG concentrations in washes and extracts were determined by ELISA.9 IgG molecular integrity was assessed by SDS-PAGE immunoblotting with IgG-specific antibody (Southern Biotech Laboratories, Birmingham, AL). Specificity of IgG autoantibodies was determined by binding to Gd-IgA19 or PLA2R7 in ELISA. IgG autoantibodies specific for Gd-IgA1 were measured on the basis of binding of IgG to Gd-IgA1 coated on ELISA plates (IgG autoantibody assay) or to Gd-IgA1 in solution and detected on the basis of IgG-IgA1 immune-complex formation (IgG-IgA1 immune-complex assay), as described by Suzuki et al.9
PLA2R7 was expressed as a soluble protein using FreeStyle 293-F cells and isolated from serum-free medium by affinity chromatography. One unit (U) of PLA2R-specific IgG autoantibody was defined as the amount resulting in optical density (OD) at 490 nm equal to 1.0. Gd-IgA1 (Ale) was isolated from plasma of a patient with IgA myeloma using ammonium sulfate precipitation, followed by removal of remaining IgG by affinity chromatography and two rounds of size-exclusion chromatography, as previously described.9 Fab fragment of Gd-IgA1 with a complete hinge region with its O-glycans was prepared as we have previously described.9 Gd-IgA1 (Ale) mimics IgA1 in the circulation of patients with IgAN in that it has an elevated content of glycoforms with some hinge-region O-glycans lacking galactose, and in that it is recognized by IgG autoantibodies. These IgG autoantibodies bind to Gd-IgA1 (Ale) and form Gd-IgA1-IgG immune complexes. Thus, this Gd-IgA1 (Ale) served as the antigen in our assays mimicking the IgAN autoantigen. One unit (U) of Gd-IgA1–specific IgG autoantibody was defined as the amount resulting in OD at 490 nm equal to 1.0.
Kidney-Biopsy Specimens Staining and Confocal Microscopy Imaging
Four-micrometer sections of frozen kidney-biopsy-tissue specimens were air-dried at room temperature, fixed in 3.7% paraformaldehyde for 10 minutes, washed in PBS with 0.03% sodium azide, and blocked with blocking buffer (5% BSA in PBS with 0.03 sodium azide). Staining for confocal microscopy used a goat antibody specific for human IgA (Jackson ImmunoResearch Laboratories, West Grove, PA) conjugated to Cy2 (green) and a nanobody specific for the constant heavy-chain domain (CH3) region of human IgG (CaptureSelect IgG-Fc nanobody; Thermo Fisher Scientific) conjugated to biotin that was detected with Streptavidin-Alexa 555 (red). Antibodies were diluted in blocking buffer. Nuclei were stained with DAPI (blue). Endogenous tissue biotin was blocked with avidin/biotin blocking kit (Vector Laboratories, Burlingame, CA). Confocal microscopy imaging was performed using a Nikon fluorescence microscope A1R. Intensity of IgG staining and the IgG-IgA colocalization were measured in an entire glomerular area using equal laser settings for all samples.
Statistical Analyses
Data are expressed as mean±SD or median values. P<0.05 was considered statistically significant. Pearson correlation test was used to determine the correlation between IgG-IgA1 immune-complex assay and IgG autoantibody assay. For image analyses, IgG-IgA colocalization was determined by plotting fluorescence intensities of the respective staining in a single optical layer and analyzing the data using the NIS-Elements software on A1R confocal microscope to calculate Pearson coefficient. Fisher exact test, with two-sided probability,10,11 was used to compare the staining for IgA and C3 by routine immunofluorescence microscopy for the IgG−RIF and IgG+RIF tissues. For the latter analyses, numerical values of 0.25, 0.62, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5, and 4.0 were respectively assigned to specimens that had trace, trace to 1+, 1+, 1+ to 2+, 2+, 2+ to 3+, 3+, 3+ to 4+, and 4+ staining by routine immunofluorescence.
Results
Clinical Data and Kidney-Biopsy Features of Patients with IgAN
The relevant clinical data and renal pathology characteristics of the 34 patients with IgAN are provided in Table 1. By light microscopy, 23 of 34 specimens showed some degree of global glomerulosclerosis, ranging from 3% to 91%. Twenty of 34 specimens showed findings of segmental glomerulosclerosis affecting 3%–72% of sampled glomeruli.
One-Wash Protocol versus Two-Washes Protocol for Extraction of IgG
Using several pools of remnant frozen IgAN kidney-biopsy specimens, we compared the one-wash protocol (IgG+RIF pool 3 and IgG−RIF pool 7) to the two-washes protocol (IgG+RIF pool 1 and IgG−RIF pool 4) on the basis of the results for total IgG and the relative amount of IgG autoantibody in the washes and extracts. For IgG+RIF cases, the acidic extract with the two-washes protocol contained less of the total IgG than with the one-wash protocol (10.9% versus 25.5%) and less of the IgG specific for Gd-IgA1 (autoantibody) (81.1% versus 98.9%, Table 2). Similarly, for IgG−RIF cases, the acidic extract from two-washes protocol contained less of the total IgG (4.1% versus 13.2%), and slightly less of the IgG specific for Gd-IgA1 (autoantibody) (96.3% versus 99.8%, Table 2). This comparative analysis indicated that most of the autoantibody was isolated in the acidic extracts with both protocols. Therefore, we elected to use the one-wash protocol to minimize damage of the proteins by degradation due to extended time of handling, except where stated otherwise.
Antigenic Specificity of Extracted IgG in MN Kidney-Biopsy Tissue
To confirm antigenic specificity of the extracted IgG from the patients with MN, we first developed an in-house version of PLA2R autoantibody assay, modified from the protocol published by Beck et al.7 In this assay, purified soluble portion of recombinant PLA2R was used as an antigen in ELISA and dilutions of serum samples from two patients with primary MN were tested for IgG autoantibody. Sera from two individuals negative for PLA2R antibody served as negative controls (Supplemental Figure 1).
Using this in-house assay, we tested specificity of IgG in washes and extracts from the remnant frozen kidney biopsy specimen of a patient with MN using the one-wash protocol. The IgG in the extracts reacted strongly with PLA2R, whereas IgG in washes had only trace amounts of the autoantibody (Supplemental Table 1). These findings thus validated the procedure for the extraction of IgG from glomerular immunodeposits. Moreover, MN biopsy specimens served as an internal control for the IgAN extraction studies.
Antigenic Specificity of IgG Extracted from IgAN Biopsies Compared with MN Disease Controls using the One-Wash Protocol
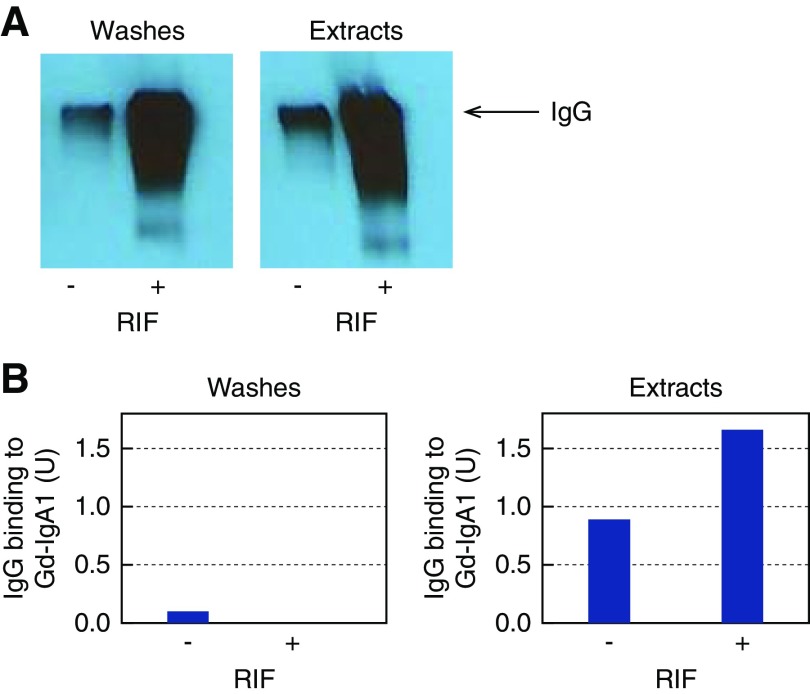
Next, we used this validated extraction procedure with pooled IgG+RIF biopsy specimens from five patients with IgAN (pool 2). For comparison, we pooled IgG−RIF biopsy specimens from five patients with IgAN (pool 5). IgG preparations from washes and extracts were analyzed by SDS-PAGE immunoblotting. The results confirmed the presence and molecular integrity of IgG in all preparations, albeit at lower amounts in IgG−RIF samples compared with IgG+RIF samples (Figure 1A). Next, we tested these IgG preparations for binding to Gd-IgA1 in solution (IgG-IgA1 immune-complex assay). The IgG from extracts of IgG+RIF samples and from extracts of IgG−RIF samples were enriched for Gd-IgA1–specific autoantibodies compared with the IgG from washes (Figure 1B).
Figure 1.
IgG isolated from renal immunodeposits of patients with IgAN is specific for Gd-IgA1. We pooled five biopsy specimens each using remnant frozen tissue that had been IgG-negative (IgG−RIF) (n=5; pool 5) or IgG-positive (IgG+RIF) (n=5; pool 2) by routine immunofluorescence microscopy and used the pools for immunodeposits extraction. (A) SDS-PAGE of washes and extracts under nonreducing conditions followed by immunodetection of IgG by Western blot confirmed the molecular integrity of isolated IgG. Arrow denotes IgG protein band. (B) Analysis of extracts from renal immunodeposits indicated enrichment for IgG autoantibodies specific for Gd-IgA1, as detected by IgG-IgA1 immune-complex assay. One unit (U) was defined as the amount of IgG resulting in optical density (OD) at 490 nm equal to 1.0. RIF, routine immunofluorescence; −, IgG-negative; +, IgG-positive.
To confirm these results for IgG−RIF specimens, we isolated IgG from washes and extracts from another set of five pooled IgG−RIF specimens from patients with IgAN (pool 6). IgG ELISA confirmed IgG in the washes and extracts and the IgG autoantibody assay confirmed that IgG extracted from the immunodeposits was enriched for Gd-IgA1–specific autoantibody (Supplemental Table 2).
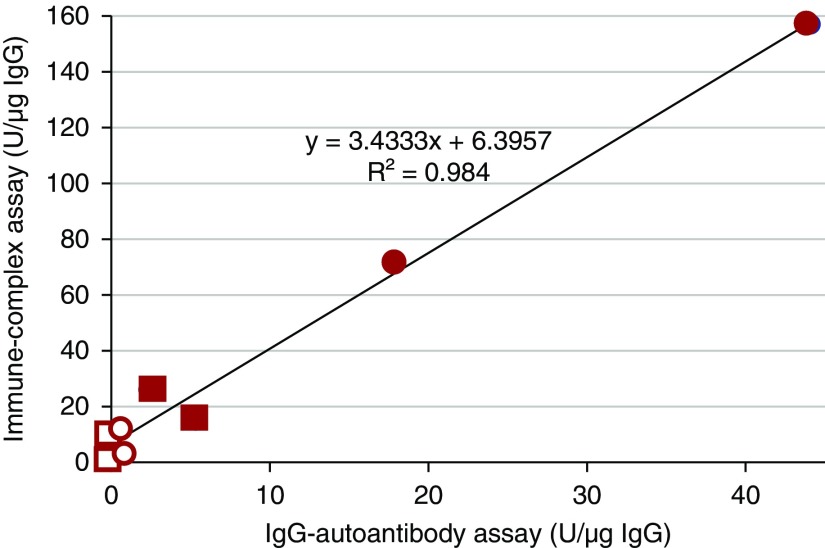
To validate these findings, we used another set of four IgG+RIF specimens (pool 3) and five IgG−RIF specimens (pool 7). Kidney-biopsy specimens from two primary MN patients were processed individually and served as controls. The isolated IgG was quantified in washes and extracts of all specimens. Specificity of IgG preparation for Gd-IgA1 was performed using normalized amount of IgG in the IgG autoantibody assay and in the IgG-IgA1 immune-complex assay (Figure 2, Table 3). Both assays showed that IgG extracted from immunodeposits of the biopsy tissues from patients with IgAN, but not IgG from immunodeposits of the specimens from patients with MN, was enriched for Gd-IgA1–specific autoantibodies (Figure 2, Table 3). To exclude potential binding of IgG autoantibodies to the Fc-fragment N-glycans of Gd-IgA1, we used a purified Fab fragment of Gd-IgA1 that had a complete hinge region with its galactose-deficient O-glycans, similar to our previous studies.9 Using this Fab-Gd-IgA1 in the IgG autoantibody assay, we confirmed that IgG extracted from the immunodeposits of pooled biopsy specimens of IgAN patients is specific for the aberrantly glycosylated hinge region of Gd-IgA1.
Figure 2.
IgG isolated from renal immunodeposits of patients with IgAN but not from renal immunodeposits of patients with MN is specific for Gd-IgA1. Individual IgG samples from two patients with MN, pooled samples for four patients with IgG+RIF IgAN (pool 3), and five patients with IgG-RIF IgAN (pool 7) were assessed in two tests using Gd-IgA1 as the antigen: IgG-IgA1 immune-complex assay and IgG autoantibody assay. One unit (U) was defined as the amount of IgG resulting in optical density (OD) at 490 nm equal to 1.0. Results of the two assays for washes and extracts show excellent correlation (r2=0.98) by Pearson test. Filled circles denote extracts from the two pools of IgAN biopsies; filled squares denote extracts from two MN biopsies. Empty circles denote washes from the same two pools of IgAN biopsies, and empty squares denote washes from the same two MN biopsies.
Table 3.
IgG isolated using the one-wash protocol from renal immunodeposits of pooled remnant frozen kidney-biopsy specimens from patients with IgAN but not from immunodeposits of individual remnant frozen kidney-biopsy specimens of two patients with MN is specific for Gd-IgA1
| Sample | Immune-Complex Assay (U/µg IgG) | IgG Autoantibody Assay (U/µg IgG) |
|---|---|---|
| MN 1 W | 2 | 0.26 |
| MN 2 W | 9 | 0.07 |
| MN 1 Ext | 15 | 5.32 |
| MN 2 Ext | 26 | 2.34 |
| IgAN IgG+RIF W (pool 3) | 11 | 0.17 |
| IgAN IgG−RIF W (pool 7) | 1 | 0.05 |
| IgAN IgG+RIF Ext (pool 3) | 71 | 17.85 |
| IgAN IgG−RIF Ext (pool 7) | 157 | 44.09 |
Gd-IgA1 was used as the antigen in IgG-IgA1 immune-complex assay and IgG autoantibody assay. One unit (U) was defined as the amount of IgG resulting in optical density (OD) at 490 nm equal to 1.0. W, wash; Ext, extract from immunodeposits; IgG+RIF, IgG-positive by routine immunofluorescence (pool 3 of kidney-biopsy specimens); IgG−RIF, IgG-negative by routine immunofluorescence (pool 7 of kidney-biopsy specimens).
Antigenic Specificity of IgG Extracted from IgG−RIF IgAN Specimens Compared with MN and LN Controls using the Two-Washes Protocol
To further confirm the antigenic specificity of the IgG in the extracts from IgG−RIF IgAN specimens, control kidney-biopsy specimens (three from patients with primary MN, three from patients with class 4 LN-D, and five from patients with class 5 LN-M) were used. Analyses of these tissues showed that IgG isolated in the extracts from the biopsies of patients with IgG−RIF IgAN were enriched for autoantibodies specific for Gd-IgA1, whereas those of patients with MN and LN had only small amounts of IgG reacting with Gd-IgA1 (Table 4).
Table 4.
IgG isolated by the two-washes protocol from renal immunodeposits of patients with IgG−RIF IgAN but not from renal immunodeposits of patients with MN and LN is specific for Gd-IgA1
| Kidney Specimens | IgG Autoantibody (U/μg IgG) W1a | IgG Autoantibody (U/μg IgG) W2 | IgG Autoantibody (U/μg IgG) Ext |
|---|---|---|---|
| IgAN (pool 4) | 1 | 3 | 68 |
| MN | 1 | 1 | 5 |
| LN-D | 1 | 1 | 9 |
| LN-M | 0 | 2 | 6 |
Samples were from patients with IgG−RIF IgAN (n=5, pool 4), primary MN (n=3, individually processed, average values shown), LN-D (n=3, pooled), and LN-M (n=5, pooled). W1, wash 1; W2, wash 2; Ext, extracts from immunodeposits.
IgG autoantibody assay was used; one unit (U) of Gd-IgA1–specific IgG was defined as the amount resulting in optical density (OD) at 490 nm equal to 1.0. The results were normalized per microgram of total IgG.
Confocal Microscopy Analyses
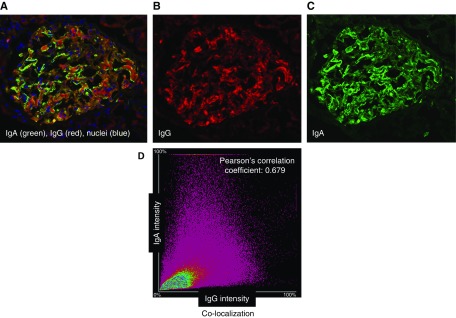
We tested several polyclonal antibodies and a nanobody specific for IgG as alternative reagents for IgG staining. The nanobody, specific for CH3 domain of Fc portion of IgG, provided optimal results. It stained IgG in the biopsy specimens of six additional IgAN kidney biopsies that were IgG-positive and the four IgAN biopsies that were IgG-negative by routine immunofluorescence microscopy; the nanobody also stained IgG in the biopsy specimen of a patient with MN (Supplemental Figure 2). Using this nanobody and an IgA-specific antibody, we stained sections of remnant frozen biopsy tissue from these ten patients with IgAN. Figures 3 and 4 show representative examples of the staining and Supplemental Table 3 summarizes the data for the intensity of IgG staining and the colocalization with IgA. IgG was detected in all specimens, albeit at lower intensity in the four biopsies that showed no IgG by routine immunofluorescence microscopy. Moreover, IgA and IgG exhibited a substantial degree of colocalization (Pearson coefficient r=0.60–0.72 for glomerular areas). Supplemental Figure 3 shows additional analyses of remnant frozen kidney-biopsy tissue from a patient with IgAN without staining for IgG by routine immunofluorescence. Confocal microscopy analysis of the tissue stained with the IgG-specific nanobody and the IgA-specific antibody showed colocalization of IgG and IgA on the basis of a single optical layer image analysis and the line-intensity profiles.
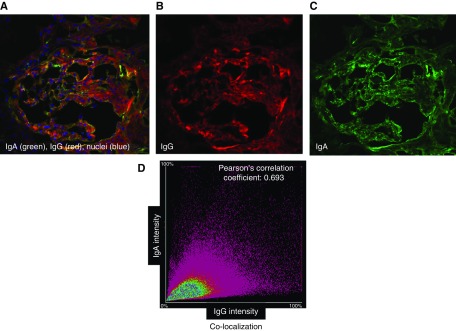
Figure 3.
Confocal microscopic analyses of glomerular Ig deposits of a patient with IgAN with IgG by routine immunofluorescence microscopy show colocalization of IgG and IgA. A remnant frozen kidney-biopsy specimen from a patient with IgAN with IgG staining by routine immunofluorescence microscopy was stained with antibodies against IgA (Cy2, green) and IgG (Alexa 555, red), and a DNA stain (DAPI, blue). (A–C) Single-layer confocal microscopy images with three combined colors (A), red (B), and green (C). (D) Image analysis of entire glomerular area indicated colocalization of individual components, IgA and IgG in glomerular deposits. For this sample, Pearson correlation coefficient (r) is 0.68. Pearson correlation coefficient (r) with value of 1.0 would indicate 100% colocalization.
Figure 4.
Confocal microscopic analyses of glomerular deposits of a patient with IgAN without IgG by routine immunofluorescence microscopy detect IgG and show colocalization of IgG and IgA. A remnant frozen kidney-biopsy specimen from a patient with IgAN without IgG staining by routine immunofluorescence microscopy was stained with antibodies against IgA (Cy2, green) and IgG (Alexa 555, red), and a DNA stain (DAPI, blue). (A–C) Single-layer confocal microscopy images with three combined colors (A), red (B), and green (C). (D) Image analysis of entire glomerular area indicated colocalization of individual components, IgA and IgG, in glomerular deposits. For this sample, Pearson correlation coefficient (r) is 0.69. Pearson correlation coefficient (r) with value of 1.0 would indicate 100% colocalization.
Intensity of Staining for IgA and C3 in IgAN Biopsies
On the basis of the routine immunofluorescence evaluation, the IgG−RIF biopsies had less intense staining for IgA than did the IgG+RIF tissues (P=0.004), but the staining for C3 was comparable (P=0.46). There was no discernable difference on the basis of presence or absence of IgG by routine immunofluorescence in clinical expression of disease at the time of biopsy.
Discussion
Since its initial description in 1968 by Berger and Hinglais,12 much has been learned about IgAN. The disease is currently considered to be an autoimmune disorder in which circulating immune complexes composed of Gd-IgA1 and Gd-IgA1–specific autoantibodies deposit in the kidneys, leading to proliferation of mesangial cells and glomerular injury.2 Central to this hypothesis is the presence of not only the aberrantly glycosylated IgA1 proteins that serve as autoantigens but, equally as important, the development of autoantibodies. There is mounting evidence that IgG (rather than IgA) autoantibodies play the major role in disease pathogenesis13 and prognosis5,14,15 of IgAN. A recent study testing the potential advantage of adding rituximab to standard of care in patients with biopsy-proven IgAN and persistent proteinuria of >1 g/d was completed. The trial failed to show any clinical benefit from rituximab treatment as defined by improvement in eGFR or proteinuria. Importantly, and despite depletion of circulating B cells, the serum Gd-IgA1–specific IgG autoantibody levels did not change during the interventional trial, consistent with the lack of benefit and in line with the critical role of IgG autoantibodies in disease pathogenesis.16
Although several studies have confirmed the presence of Gd-IgA1 in renal immunodeposits,3,4 routine immunofluorescence microscopy fails to reveal IgG in many cases.5,6 Importantly, the specificity of the deposited IgG had never been tested. Our study results reveal that IgG in IgAN renal immunodeposits is enriched for autoantibodies specific for Gd-IgA1. Even IgAN kidney-biopsy specimens without staining for IgG by routine immunofluorescence microscopy contain these autoantibodies (albeit in smaller amounts). Using two different assays, namely the ELISA IgG autoantibody assay and the IgG-IgA1 immune-complex assay in solution, we demonstrated the presence and specificity of the IgG extracted from renal immunodeposits directed at Gd-IgA1. Notably, we used a washing process that removed IgG from the interstitium and vessels at neutral-pH conditions before performing acidic-pH extraction that dissociates immune complexes and releases IgG. We validated this process by comparing the results with those of a more extensive protocol that included extra washing after homogenization of the biopsy tissues. The process to extract IgG maintained the antigenic specificity of the antibody, as demonstrated by the binding of IgG from MN biopsies to PLA2R. Although the IgG extracted from the IgAN biopsies was enriched for Gd-IgA1–specific autoantibody, the IgG from MN and LN biopsies (including those with diffuse proliferative class 4 features with mesangial IgA) had minimal amounts of such IgG. The confocal microscopy studies with IgG-specific nanobody confirmed the presence of IgG in IgAN biopsies without IgG by routine immunofluorescence microscopy and revealed colocalization of IgA and IgG, consistent with the presence of IgA-IgG immune complexes. These findings lend strong support to the currently proposed four-hit hypothesis for the autoimmune mechanism of IgAN pathogenesis.
The more intense glomerular staining for IgA by routine immunofluorescence for the IgG+RIF biopsy specimens than for the IgG−RIF tissues is consistent with deposition of more Gd-IgA1-IgG immune complexes. Furthermore, our confocal microscopy results suggest that the IgG is present even in IgG−RIF, but in amounts below the limit of detection by routine immunofluorescence with the currently used anti-IgG antibody.
Our study has limitations. We did not attempt to test for autoantibody of the IgA subclass that may be present in some patients and may contribute to the pathogenesis of the disease. It would be very difficult to distinguish IgA1 autoantigen from IgA autoantibody. Therefore, we elected to focus on IgG autoantibody, the major autoantibody correlating with serum Gd-IgA1 levels.13 Moreover, we did not use laser microdissection to isolate glomeruli; however, there had been very little IgG staining in the interstitium, tubules, and vessels by routine immunofluorescence microscopy. Finally, because of the small numbers of patients and some missing clinical data, we could not correlate the presence or absence of IgG by routine immunofluorescence with any clinical data at the time of biopsy. Future studies could explore these important questions. Furthermore, comparison of staining of IgG-negative biopsies with various currently used IgG-specific reagent(s) versus IgG Fc-specific nanobody we used in this study can be performed in a larger cohort from different centers.
In conclusion, our study demonstrated the presence of IgG in renal immunodeposits of patients with IgAN, including those without IgG by routine immunofluorescence microscopy. Furthermore, this IgG exhibited specificity for Gd-IgA1, supporting the currently postulated importance of Gd-IgA1–specific IgG autoantibodies in the pathogenesis of the disease.
Disclosures
Dr. Rizk reports grants from NIH, other from IGA Nephropathy Foundation of America, during the conduct of the study; grants from Achillion Pharmaceuticals. Inc., grants from Reata Pharmaceuticals, grants from Calliditas Pharmaceuticals, grants from Retrophin Inc., other from Reliant Glycosciences, LLC., personal fees from Visterra Inc., outside the submitted work; and that none of the companies had any role in the design of the study, interpretation of the results, or drafting of the manuscript. Dr. Saha reports grants from NIH-T32 training grant DK07545, during the conduct of the study. Dr. Lea Novak reports grants from NIH, other from IGA Nephropathy Foundation of America, during the conduct of the study; other from Reliant Glycosciences, LLC, grants from Alexion, grants from Retrophin, outside the submitted work. Dr. Julian reports grants from National Institutes of Health, other from IGA Nephropathy Foundation of America, during the conduct of the study; grants from Calliditas Pharmaceuticals, grants from Retrophin Inc., other from Reliant Glycosciences, LLC, other from Visterra Inc, other from Alexion Pharmaceuticals, outside the submitted work; and that none of the companies had any role in the design of the study, interpretation of the results, or drafting of the manuscript. Dr. Jan Novak reports grants from NIH, other from IGA Nephropathy Foundation of America, during the conduct of the study; other from Reliant Glycosciences, LLC, grants from Alexion, grants from Retrophin, grants from Anthera Pharmaceuticals, personal fees from Visterra, outside the submitted work; In addition, Dr. Jan Novak has a patent “Diagnosing and Treating IgA Nephropathy” US patent application US 14/318,082 filed June 27, 2014. Notice of Allowance 1/19/2017 with royalties paid to University of Alabama at Birmingham. None of the companies had any role in the design of the study, interpretation of results, or drafting the manuscript.
Funding
Research was supported in part by National Institutes of Health grants DK078244 and DK082753 and a gift from IGA Nephropathy Foundation of America. Dr. Saha was supported in part by the National Institutes of Health T32 training grant DK07545. None of the sources of support and companies listed above had any role in the design of the study, interpretation of the results, or drafting of the manuscript.
Supplementary Material
Acknowledgments
Drs. Jan Novak and Julian designed the study. Dr. Saha, Ms. Hall, Dr. Huang, Ms. Brown, Dr. Lea Novak, and Dr. Jan Novak carried out the experiments. Dr. Fatima blindly reviewed all IgAN cases and provided pathology interpretations. Drs. Rizk, Julian, Lea Novak, and Jan Novak analyzed the data. Dr. Rizk drafted all versions of manuscript. Dr. Rizk, Dr. Saha, Dr. Lea Novak, Ms. Hall, Dr. Huang, Dr. Fatima, Dr. Julian, and Dr. Jan Novak revised the manuscript. All authors approved the final version of the manuscript.
The authors thank Drs. David J. Salant and Laurence H. Beck for providing the plasmid for mammalian expression of soluble part of PLA2R, Ms. Cheryl Snyder and Ms. Debbie Debardlabon for retrieving and processing kidney-biopsy remnant specimens, and Drs. Thomas H. Watson, Melanie S. Halvorson, Lautrec W. Radcliff, Paul E. Miller, Charles E. Thomas, and Andreas T. Maddux for retrieval of clinical data. The authors appreciate the use of instrumentation at the University of Alabama at Birmingham high-resolution imaging facility.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018111156/-/DCSupplemental.
Supplemental Table 1. IgG isolated from immunodeposits of a patient with membranous nephropathy is enriched for autoantibodies specific for PLA2R. *One unit (U) of PLA2R-specific IgG was defined as the amount resulting in optical density (OD) at 490 nm equal to 1.0.
Supplemental Table 2. IgG isolated from immunodeposits of five pooled IgAN biopsy specimens IgG-negative by routine immunofluorescence (pool 6) contained IgG autoantibody with specificity for Gd-IgA1. IgG autoantibody assay was used; one unit (U) of Gd-IgA1–specific IgG was defined as the amount resulting in optical density (OD) at 490 nm equal to 1.0.
Supplemental Table 3. Confocal microscopic analyses of intensity of IgG staining and IgG-IgA colocalization in glomerular immunodeposits in remnant frozen kidney-biopsy specimens of IgAN patients. *RIF, routine immunofluorescence; **Pearson coefficient, value of 1 indicates 100% colocalization; #the intensity of IgG staining and the IgG-IgA colocalization were measured in entire glomerular area using equal laser settings for all samples. RIF-positive, six specimens; RIF-negative, three specimens.
Supplemental Figure 1. Detection of IgG autoantibodies specific for PLA2R. Assay for PLAR2-specific IgG autoantibodies from patients with primary MN. Extracellular part of PLA2R was expressed as a soluble protein in FreeStyle 293-F cells and isolated from serum-free medium using affinity chromatography. Purified PLA2R was coated onto the wells of 96-well ELISA as an antigen and dilutions of serum samples from two patients with primary MN were tested for IgG autoantibody. Sera from two controls (negative for PLA2R-specific antibodies) served as negative controls.
Supplemental Figure 2. Confocal microscopy image of glomerular immunoglobulin in membranous nephropathy (MN). (A) A section from a remnant frozen kidney-biopsy specimen from a patient with MN was stained with biotin-labeled nanobody specific for CH3 domain of human IgG (detected with streptavidin-Alexa 555, red) and goat antibody specific for human IgA (detected with Cy2, green). Nuclei were stained with DAPI (blue). Endogenous tissue biotin was blocked with avidin/biotin blocking kit. Staining for IgG is detected. IgA staining is absent. Original magnification, ×200. Inset: enlarged portion of a glomerulus shows granular staining for IgG in glomerular basement membranes. (B) Another section of the specimen was prepared by using only streptavidin-Alexa 555 (red), i.e., the biotin-labeled nanobody specific for CH3 domain of human IgG was omitted. Goat antibody specific for human IgA was also used. Nuclei were stained with DAPI (blue). No staining for IgG or IgA is detected. Original magnification, ×200. This negative control confirms the specificity of the IgG staining with nanobody specific for CH3 of human IgG shown in (A).
Supplemental Figure 3. Analyses of glomerular deposits in a remnant frozen IgAN kidney-biopsy specimen without IgG on routine immunofluorescence show colocalization of IgG and IgA detected by confocal microscopy. (A–C). (A) 4-μm-thick section of the kidney-biopsy specimen was stained with biotin-labeled nanobody specific for CH3 of human IgG (detected with streptavidin-Alexa 555, red) and goat antibody specific for human IgA (detected with Cy2, green). Nuclei were stained with DAPI (blue). Endogenous tissue biotin was blocked with avidin/biotin blocking kit. (A–C) Single-layer confocal microscopy images of a portion of a glomerulus (original magnification, ×600) with three combined colors (A), red (B), and green (C). (D1 and D2) Two line-intensity profiles 1 and 2 respectively, at a single-optical layer, with arrows in (A–C) indicating directions of the two lines. Original magnification, ×600.
References
- 1.Wyatt RJ, Julian BA: IgA nephropathy. N Engl J Med 368: 2402–2414, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Suzuki H, Kiryluk K, Novak J, Moldoveanu Z, Herr AB, Renfrow MB, et al.: The pathophysiology of IgA nephropathy. J Am Soc Nephrol 22: 1795–1803, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hiki Y, Odani H, Takahashi M, Yasuda Y, Nishimoto A, Iwase H, et al.: Mass spectrometry proves under-O-glycosylation of glomerular IgA1 in IgA nephropathy. Kidney Int 59: 1077–1085, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Allen AC, Bailey EM, Brenchley PE, Buck KS, Barratt J, Feehally J: Mesangial IgA1 in IgA nephropathy exhibits aberrant O-glycosylation: Observations in three patients. Kidney Int 60: 969–973, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Wada Y, Ogata H, Takeshige Y, Takeshima A, Yoshida N, Yamamoto M, et al.: Clinical significance of IgG deposition in the glomerular mesangial area in patients with IgA nephropathy. Clin Exp Nephrol 17: 73–82, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellur SS, Troyanov S, Cook HT, Roberts IS; Working Group of International IgA Nephropathy Network and Renal Pathology Society : Immunostaining findings in IgA nephropathy: Correlation with histology and clinical outcome in the Oxford classification patient cohort. Nephrol Dial Transplant 26: 2533–2536, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Beck LH Jr., Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, et al.: M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 361: 11–21, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woodroffe AJ, Wilson CB: An evaluation of elution techniques in the study of immune complex glomerulonephritis. J Immunol 118: 1788–1794, 1977 [PubMed] [Google Scholar]
- 9.Suzuki H, Fan R, Zhang Z, Brown R, Hall S, Julian BA, et al.: Aberrantly glycosylated IgA1 in IgA nephropathy patients is recognized by IgG antibodies with restricted heterogeneity. J Clin Invest 119: 1668–1677, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisher RA: On the interpretation of X2 from contingency tables, and the calculation of P. J R Stat Soc 85: 87–94, 1922 [Google Scholar]
- 11.Larntz K: Small-sample comparisons of exact levels for chi-squared goodness-of-fit statistics. J Am Stat Assoc 73: 253–263, 1978 [Google Scholar]
- 12.Berger J, Hinglais N: [Intercapillary deposits of IgA-IgG]. J Urol Nephrol (Paris) 74: 694–695, 1968 [PubMed] [Google Scholar]
- 13.Placzek WJ, Yanagawa H, Makita Y, Renfrow MB, Julian BA, Rizk DV, et al.: Serum galactose-deficient-IgA1 and IgG autoantibodies correlate in patients with IgA nephropathy. PLoS One 13: e0190967, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nieuwhof C, Kruytzer M, Frederiks P, van Breda Vriesman PJ: Chronicity index and mesangial IgG deposition are risk factors for hypertension and renal failure in early IgA nephropathy. Am J Kidney Dis 31: 962–970, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Shin DH, Lim BJ, Han IM, Han SG, Kwon YE, Park KS, et al.: Glomerular IgG deposition predicts renal outcome in patients with IgA nephropathy. Mod Pathol 29: 743–752, 2016 [DOI] [PubMed] [Google Scholar]
- 16.Lafayette RA, Canetta PA, Rovin BH, Appel GB, Novak J, Nath KA, et al.: A randomized, controlled trial of Rituximab in IgA nephropathy with proteinuria and renal dysfunction. J Am Soc Nephrol 28: 1306–1313, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.