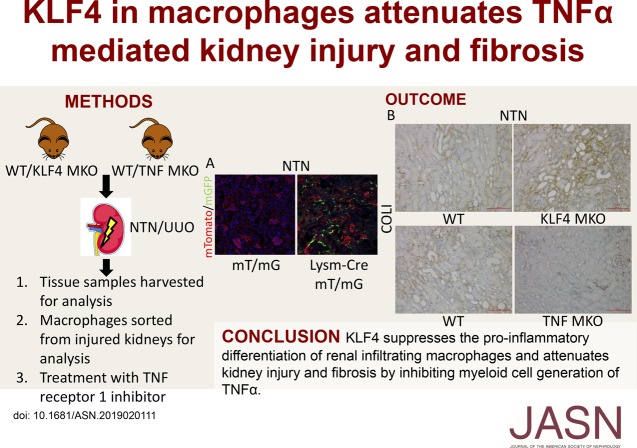
Significance Statement
Proinflammatory M1 macrophages initiate kidney injury, but mechanisms through which persistent M1-dependent kidney damage culminates in fibrosis of the kidney require elucidation. In murine CKD models featuring robust macrophage accumulation, the authors found that macrophage-specific deficiency of Krüppel-like factor 4 (KLF4, a zinc-finger transcription factor that suppresses inflammation) augmented the M1 polarization and expression of TNFα (KLF4’s downstream effector) in macrophages infiltrating the kidney, as well as exacerbated glomerular matrix deposition, tubular damage, and interstitial fibrosis. Mice with macrophage-specific TNF deletion exhibited decreased kidney damage and fibrosis. TNF receptor-1 inhibition in wild-type mice and mice with macrophage-specific KLF4 deficiency reduced susceptibility to kidney damage, fibrosis, and necroptosis, and abrogated differences in these parameters between experimental groups. These findings indicate that macrophage KLF4 ameliorates CKD by mitigating TNF-dependent injury and fibrosis.
Keywords: macrophages, KLF4, necroptosis, renal fibrosis
Visual Abstract
Abstract
Background
Polarized macrophage populations can orchestrate both inflammation of the kidney and tissue repair during CKD. Proinflammatory M1 macrophages initiate kidney injury, but mechanisms through which persistent M1-dependent kidney damage culminates in fibrosis require elucidation. Krüppel-like factor 4 (KLF4), a zinc-finger transcription factor that suppresses inflammatory signals, is an essential regulator of macrophage polarization in adipose tissues, but the effect of myeloid KLF4 on CKD progression is unknown.
Methods
We used conditional mutant mice lacking KLF4 or TNFα (KLF4’s downstream effector) selectively in myeloid cells to investigate macrophage KLF4’s role in modulating CKD progression in two models of CKD that feature robust macrophage accumulation, nephrotoxic serum nephritis, and unilateral ureteral obstruction.
Results
In these murine CKD models, KLF4 deficiency in macrophages infiltrating the kidney augmented their M1 polarization and exacerbated glomerular matrix deposition and tubular epithelial damage. During the induced injury in these models, macrophage-specific KLF4 deletion also exacerbated kidney fibrosis, with increased levels of collagen 1 and α-smooth muscle actin in the injured kidney. CD11b+Ly6Chi myeloid cells isolated from injured kidneys expressed higher levels of TNFα mRNA versus wild-type controls. In turn, mice bearing macrophage-specific deletion of TNFα exhibited decreased glomerular and tubular damage and attenuated kidney fibrosis in the models. Moreover, treatment with the TNF receptor-1 inhibitor R-7050 during nephrotoxic serum nephritis reduced damage, fibrosis, and necroptosis in wild-type mice and mice with KLF4-deficient macrophages, and abrogated the differences between the two groups in these parameters.
Conclusions
These data indicate that macrophage KLF4 ameliorates CKD by mitigating TNF-dependent injury and fibrosis.
Progression of CKD features persistent injury to the renal parenchyma, culminating in extracellular matrix deposition and loss of renal function. Myeloid cells accumulate in the glomerulus and/or tubulointerstitium in CKD of diverse etiologies. Among the inflammatory myeloid cells, the infiltrating macrophages have profound effects on renal damage, repair, and fibrosis depending on their state of polarization.1 In a simplified schema, “M1” macrophages drive parenchymal injury via the generation of proinflammatory cytokines including TNFα and IL1β. In contrast, “M2” macrophages facilitate tissue repair via the secretion of immunosuppressive cytokines. However, the signals through which macrophages in the injured kidney polarize toward an M1 phenotype and the mechanisms through which persistent M1-dependent injury trigger kidney fibrosis warrant investigation.
Krüppel-like factor 4 (KLF4), a DNA-binding transcriptional regulator, is highly expressed in monocytes and regulates a wide range of biologic activities including cell proliferation and differentiation.2 KLF4 is an essential regulator of macrophage polarization in adipose tissues, suppressing bactericidal activity and accelerating wound healing.3 However, the effects of myeloid KLF4 on the progression of CKD have not been interrogated.
In our hands, stimulating renal macrophages with angiotensin II suppressed their generation of proinflammatory cytokines and ameliorated renal fibrosis,4 while inducing macrophage expression of mRNA for KLF4 (Supplemental Figure 1). We therefore posited that myeloid KLF4 could mitigate renal inflammation and fibrogenesis. We tested this hypothesis in mice using two conditional mutant lines that harbor deletion of KLF4 or its downstream effector TNFα selectively from infiltrating myeloid cells. For models that feature both prominent renal macrophage accumulation and activation of the renin-angiotensin system, we subjected our animals to nephrotoxic serum nephritis (NTN) and unilateral ureteral obstruction (UUO). We find that KLF4 deficiency in infiltrating myeloid cells stimulates their production of TNFα with consequent tubular epithelial cell necroptosis and exaggerated renal interstitial fibrosis.
Methods
Animals
For this study, floxed KLF43 or TNF mice5 from the C57BL/6 background were backcrossed for at least six generations to the 129SVE strain and then crossed with 129SVE LysM-Cre mice to generate LysM-Cre+ KLF4flox/flox (KLF4 MKO), LysM-Cre+ TNFflox/flox (TNF MKO) mice, and wild-type (WT) (Cre negative) littermates. Genotyping methods were performed as previously described.4 LysM-Cre recombinase mice were crossed with mT/mG reporter mice (Gt[ROSA]26Sortm4[ACTB-tdTomato,-EGFP]Luo/J; strain 007576; Jackson Labs) to map the distribution of LysM-Cre–positive macrophages in the NTN kidney. Mice were bred and maintained in the Association for Assessment and Accreditation of Laboratory Animal Care–accredited animal facilities at the Durham Veterans Affairs Medical Center according to National Institutes of Health (NIH) guidelines. All of the animal studies were approved by the Durham Veterans Affairs Medical Center Institutional Animal Care and Use Committee and conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Animals had free access to standard rodent chow and water. Eight- to 12-week-old male mice were used for experiments.
NTN Model
Mice received an intraperitoneal injection of sheep IgG (250 μg/20 g) in the 100 μl sterile 1× PBS mix with CFA 100 μl/20 g at day 0. Tail injection of sheep anti-mouse glomerular basement membrane serum (PTX-001S; Probetex, San Antonio, TX) was performed at day 5. For TNF receptor 1 (TNFR1) inhibition, WT and KLF4 MKO animals received intraperitoneal injections of vehicle or R-7050 (12 mg/kg; Cayman Chemical) every other day for the duration of the experiment. At day 14, mice were euthanized and urine and kidney tissues were harvested for further analysis.
UUO Model
UUO model was performed as described previously.6 Mice were anesthetized with isoflurane and then the left ureter was isolated and ligated. Mice were euthanized 7 days later, and the obstructed kidneys were harvested for analysis.
Assessment of Renal Injury
Mouse kidney samples were fixed in 10% formalin (Sigma-Aldrich) overnight and embedded in paraffin. Paraffin sections (5-μm thick) were stained by the Periodic acid–Schiff reagent and the histologic changes were assessed by light microscopy. The glomerular matrix deposition was quantitated as the average matrix percentage for all glomeruli in each individual mouse, and these average values were used for analysis between groups. The tubular injury score was evaluated based on the following morphologic changes including necrotic tubules, loss of brush borders, tubule dilation, cast formation, tubular epithelial swelling, and vacuolar degeneration: 0, no morphologic deformities; 1, <10%; 2, 10%–25%; 3, 25%–50%; 4, 50%–75%; 5, ≥75%. More than ten contiguous fields were examined in each slide under ×200 magnification and the values were averaged. Kidney sections were stained with anti–collagen type 1 and F4/80 to visualize interstitial collagen deposition and macrophage infiltration as described previously.7 BUN levels were measured in individual samples using the Urea Nitrogen Colorimetric Detection Kit (EIABUN; Invitrogen). Urinary concentrations of albumin were measured in individual samples using a murine microalbuminuria ELISA kit (Albuwell M; Exocell). Urinary creatinine concentrations were measured by the Creatinine Companion kit from Exocell.
Terminal Deoxynucleotidyl Transferase–Mediated Digoxigenin-Deoxyuridine Nick-End Labeling Staining
Terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling (TUNEL) staining was performed according the manufacturer’s instructions of the In Situ Cell Death Detection Kit, Fluorescein (Roche, Germany). TUNEL-positive cells in eight high-powered fields of each tissue section were quantified by a blinded observer.
Hydroxyproline Assay
Kidney tissues were weighed and homogenized in distilled water, and hydroxyproline concentrations were measured according to the manufacturer’s instructions of the Hydroxyproline Assay Kit (ab222941; Abcam).
Western Blots
Kidney tissues were homogenized in RIPA Buffer (R0278; Sigma-Aldrich). The concentration of protein was quantitated using the DC Protein Assay Kit (500-0111; Bio-Rad Laboratories). Equal samples went through electrophoresis with 4%–12% Bis-Tris Gels and transferred to polyvinylidene difluoride membranes. The blots were blocked by 5% milk in Tris-buffered saline/Tween 20 and followed by incubation with anti–collagen type 1 antibody (1310-01; SouthernBiotech), anti–α smooth muscle actin (anti-αSMA) antibody (A5228; Sigma-Aldrich), anti–glyceraldehyde-3-phosphate dehydrogenase antibody (2118; Cell Signaling Technology), anti–receptor-interacting protein kinase-3 (anti-RIPK3) antibody (95702; Cell Signaling Technology), anti–mixed-lineage kinase domain-like protein (anti-MLKL) antibody (37705; Cell Signaling Technology), anti–phosphorylated RIPK3 (ab195117; Abcam), and anti–phosphorylated MLKL (ab196436; Abcam) overnight in 4°C. The blots were then washed and incubated with secondary antibodies for 1 hour at room temperature. Bands were detected using an enhanced chemiluminescence detection system and quantified for densitometry by ImageJ.
RNA Isolation and Real-Time PCR Analysis
Total RNA was isolated from tissues by the RNeasy Mini Kit or RNeasy Micro Kit according to the manufacturer’s instructions (Qiagen). cDNA was synthesized using the High-Capacity cDNA Reverse Transcription Kit (4368813; Thermo Fisher). Real-time PCR was performed on an ABI 7900HT system using TaqMan probes for Klf4, Serpine1 (Pai-1), Lcn2 (neutrophil gelatinase–associated lipocalin [Ngal]), Col1a1, Tgfb1, fibronectin, Tnfa, Il1b, IFNg, C-C motif chemokine ligand 2 (Ccl2), Ccl5, Egr2, and resistin-like molecule α (Retnla).
Flow Cytometry and Isolation of Hematopoietic Cells from the Kidney
Mice were deeply anesthetized and a single-cell suspension was prepared as described previously.4 The resulting cell suspensions were treated with Fc block (BioLegend, San Diego, CA) and incubated with the following fluorescent antibodies: CD11b-FITC (clone M1/70), CD45–Brilliant Violet 510 (clone 30-F11), lymphocyte antigen 6 complex (Ly6C) –phycoerythrin-cyanine 7 (clone HK1.4), Ly6G-phycoerythrin-cyanine 5.5 (clone 1A8), CD11c-phycoerythrin (clone N418), MHCII–Brilliant Violet 645 (clone M5/114), CD24-phycoerythrin-cyanine 7 (clone M1/69), CD64-allophycocyanin (clone X54-5/7.1), and 4′,6-diamidino-2-phenylindole–Brilliant Violet 405 for 30 minutes at 4°C. Cells were then washed and fixed (BD Cytofix; BD Biosciences, San Jose, CA). During intracellular flow cytometry, cell suspensions were treated with LPS for 6 hours and then incubated with Fc block and selected surface antibodies. Cells were then fixed (BD Cytofix/Cytoperm; BD Biosciences) and incubated with anti–TNFα-allophycocyanin (clone MP6-XT22). Flow cytometry was performed on a FACSCanto II (BD Biosciences) and data were analyzed by FlowJo 10.2. To isolate CD45+CD11b+Ly6Chi myeloid cells from the kidneys, the sorting strategy was similar and CD45+CD11b+Ly6Chi myeloid cells were harvested for PCR analysis.
Statistical Analysis
All values are expressed as means±SEM. Comparison between groups was assessed using a two-tailed unpaired t test. For comparisons between groups with non-normally distributed variables, we used a Mann–Whitney test.
Results
LysM-Expressing Myeloid Cells Accumulate in Kidney during NTN
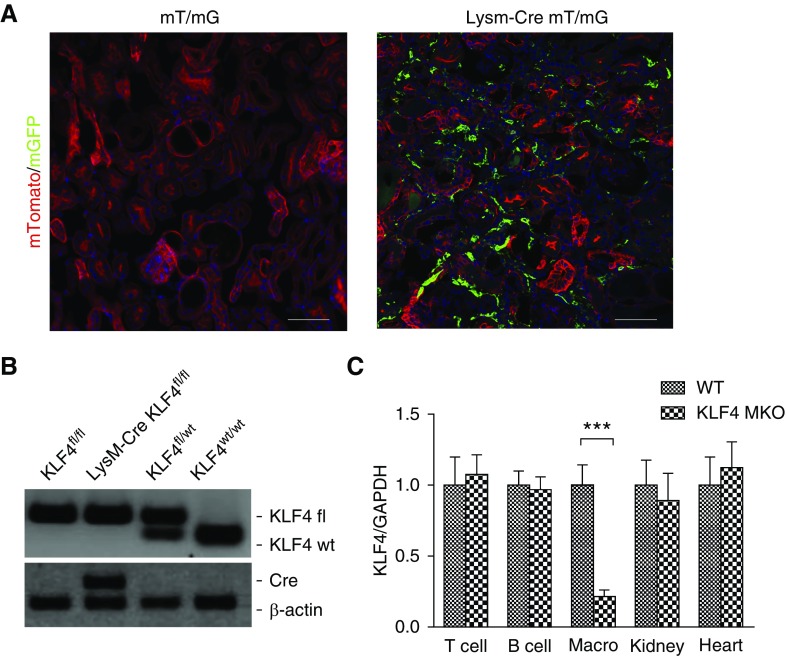
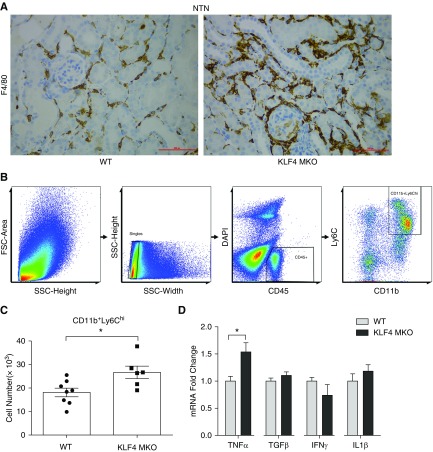
To examine the accumulation of LysM-expressing macrophages in the kidneys during inflammatory CKD, we induced NTN in a LysM-Cre mT/mG reporter mouse line in which LysM-expressing macrophages fluoresce green, but all other cell lineages fluoresce red. By day 14 of NTN, robust enhanced green fluorescent protein–positive macrophages were detected in the renal parenchyma, confirming that LysM-Cre–mediated gene deletion affects macrophages infiltrating the NTN kidney (Figure 1A). To investigate the actions of KLF4 in these infiltrating myeloid cells, we crossed KLF4 floxed mice with LysM-Cre recombinase mice (Figure 1B), thereby generating mice lacking KLF4 expression selectively in myeloid cells (KLF4 MKO) (0.21±0.04 versus 1.00±0.14 au; P=0.0004) (Figure 1C).
Figure 1.
LysM-expressing myeloid cells accumulate in kidney during NTN. (A) Representative images of kidney sections from mT/mG and LysM-Cre+ mT/mG mice at day 14 of NTN. (B) Representative images of KLF4 flox and Lysz-Cre determination. (C) mRNA expression for KLF4 in isolated T cells, B cells, CD11b+ splenic macrophages (Macro), and in whole kidney and heart from WT and KLF4 MKO mice (n≥6 for each group). Scale bars, 100 μm. Data represent the mean±SEM. ***P<0.001.
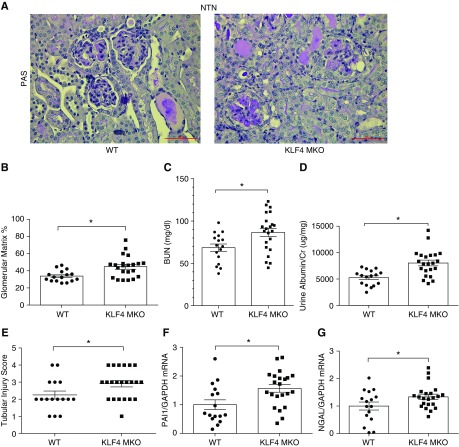
Myeloid KLF4 Attenuates Glomerular and Tubular Injury
We then evaluated whether deletion of KLF4 in infiltrating macrophages affects the severity of glomerular and tubular injury during NTN. KLF4 MKO and WT (LysM-Cre− KLF4flox/flox) sham controls had similar renal architecture, BUN levels, and urinary albumin-to-creatinine ratios (ACRs) (Supplemental Figure 2). At 14 days after NTN induction, kidneys from both groups showed robust damage. However, KLF4 MKO kidneys exhibited more deposition of glomerular matrix at day 14 of NTN compared with WTs (44.8%±2.6% versus 33.9%±1.6%; P<0.05) (Figure 2, A and B). Consistent with this augmented glomerular damage, KLF4 MKO mice compared with WTs had higher BUN levels (87±5 versus 69±4 mg/dl; P<0.05) and urinary ACRs (8030±536 versus 5261±364 μg/mg; P<0.05) at 14 days after NTN (Figure 2, C and D). KLF4 MKO mice also developed more severe tubular injury at day 14 of NTN compared with WTs (2.1±0.2 versus 2.9±0.2 au; P<0.05) (Figure 2E). Plasminogen activator inhibitor 1 (PAI1) has been implicated as a mediator of progression of kidney damage and fibrosis, and NGAL is widely accepted as a marker for renal tubular damage.8,9 The mRNA levels of PAI1 (1.6±0.1 versus 1.0±0.1 au; P<0.05) and NGAL (1.4±0.1 versus 1.0±0.1 au; P<0.05) were significantly upregulated in nephritic kidneys from KLF4 MKO mice compared WTs, confirming that KLF4 deficiency in macrophages augments the glomerular and tubular damage after NTN (Figure 2, F and G).
Figure 2.
Myeloid KLF4 attenuates glomerular and tubular injury. (A and B) Renal pathology in WT and KLF4 MKO mice at day 14 of NTN. (A) Representative images of kidney sections from WT and KLF4 MKO mice with summary data of (B) glomerular matrix deposition (%), (C) BUN, (D) ACR, and (E) tubular injury scores. (F and G) Renal mRNA expression of (F) PAI1 and (G) NGAL (n=16 for WT and n=22 for KLF4 MKO in NTN). Scale bars, 50 μm. Data represent the mean±SEM. *P<0.05.
KLF4 Deficiency in Macrophages Exacerbates Kidney Fibrogenesis
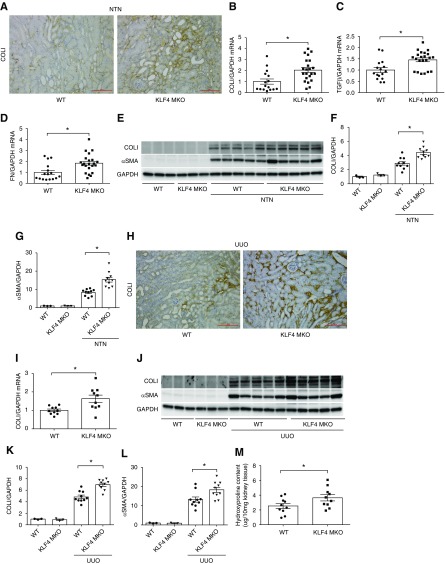
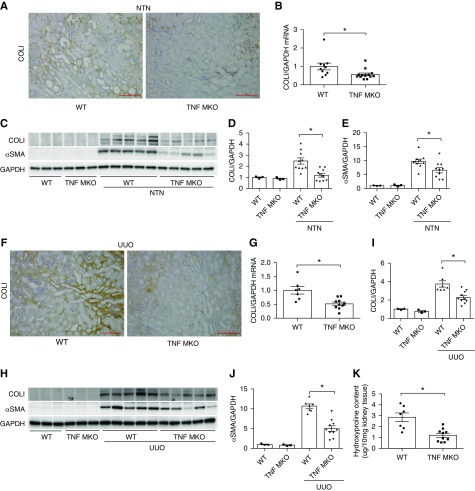
To evaluate whether KLF4 in myeloid cells influences interstitial fibrosis accruing from NTN, we measured mRNA and protein levels for collagen and fibrosis mediators in the NTN kidneys. First, immunohistochemistry staining for collagen I (COLI) of kidney sections after NTN revealed diffuse interstitial collagen deposition in both groups (Figure 3A). At day 14 after NTN, mRNA expressions for COLI, TGFβ, and fibronectin were significantly enhanced in KLF4 MKO kidneys compared with WT mice (Figure 3, B–D). By Western blot analysis, NTN kidneys from KLF4 MKO mice showed markedly higher levels of COLI protein and αSMA than WTs (Figure 3, E–G).
Figure 3.
KLF4 deficiency in macrophages exacerbates kidney fibrogenesis. (A–G) Renal fibrosis in WT and KLF4 MKO mice at day 14 of NTN. (A) Representative images of COLI immunostaining in kidney sections from WT and KLF4 MKO mice at day 14 of NTN. (B–D) mRNA expression of COLI, TGFβ, and fibronectin (FN) in WT and KLF4 MKO kidneys at day 14 of NTN. (E) Western blot for COLI and αSMA in whole kidney at day 14 of NTN. (F and G) Semiquantification of COLI and αSMA protein expression (n=3 for sham control and n=10 for WT/KLF4 MKO in NTN). (H–M) Renal fibrosis in WT and KLF4 MKO mice at day 7 of UUO. (H) Representative images of COLI immunostaining in kidney sections from WT and KLF4 MKO mice at day 7 of UUO. (I) Renal mRNA expression of COLI in WT and KLF4 MKO mice at day 7 of UUO (n=10 for WT and KLF4 MKO). (J) Western blot for COLI and αSMA in obstructed and contralateral control kidney at day 7 of UUO. (K and L) Semiquantification of (K) COLI and (L) αSMA protein expression (n=3 for contralateral control and n=10 for WT/KLF4 MKO in UUO). (M) Hydroxyproline content in obstructed WT and KLF4 MKO kidneys at day 7 of UUO (n=10 for WT and KLF4 MKO). Scale bars, 100 μm. Data represent the mean±SEM. *P<0.05. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
To confirm these effects of myeloid KLF4 in a separate model of macrophage-dependent renal fibrosis, we subjected our experimental animals to the UUO models. Consistent with immunohistochemistry staining for COL1 (Figure 3H), the mRNA levels of COLI were significantly higher in the obstructed KLF4 MKO kidneys compared with WTs (Figure 3I). Similarly, gene expression for the M1 inflammatory markers IFNγ and CCL5 were upregulated in the KLF4 MKO cohort, whereas the M2 anti-inflammatory markers Egr2 and Retnla were downregulated in the obstructed KLF4 MKOs (Supplemental Figure 3, A–D). Compared with WTs, the protein levels of COLI and αSMA were significantly enhanced in obstructed KLF4 MKO kidneys (Figure 3, J–L), which is consistent with the hydroxyproline content (Figure 3M). Thus, myeloid KLF4 mitigates the severity of inflammatory kidney injury and consequent fibrosis.
KLF4 in Infiltrating Ly6Chi Myeloid Cells Suppresses Their Generation of TNFα
As KLF4 regulates the polarization of macrophages in other tissues, we then measured the accumulation and activation of infiltrating macrophages in the NTN kidneys. Immunohistochemistry staining demonstrated increased infiltration of F4/80-positive macrophages in the interstitium of the KLF4 MKO kidneys compared with WTs (Figure 4A). Ly6C marks proinflammatory myeloid cells capable of elaborating cytokines such as TNFα and IL1β that are known to drive both renal injury and fibrosis.10 To examine the Ly6C-expressing macrophage population at day 14 NTN, single-cell suspensions of CD45+ hematopoietic cells were prepared from the kidneys of KLF4 MKO and WT mice and parsed via flow cytometry to isolate CD11b+Ly6Chi macrophages (Figure 4B). Compared with WTs, NTN kidneys from KLF4 MKO mice contained higher numbers of CD11b+Ly6Chi macrophages, suggesting KLF4 deficiency in macrophages mitigates the accumulation of proinflammatory myeloid cells into the kidney after NTN (Figure 4C). We then measured the mRNA expression for cytokines directly in those infiltrating macrophages. Compared with WTs, mRNA levels for KLF4 in renal CD11b+ Ly6Chi macrophages from KLF4 MKO kidneys were significantly lower at day 14 of NTN (Supplemental Figure 4A). By contrast, CD11b+ Ly6Chi macrophages infiltrating the NTN kidneys of KLF4 MKO had upregulated mRNA expression for TNFα, CCL2, and CCL5 (Figure 4D, Supplemental Figure 4, B–D), whereas the expressions for M2 markers TGFβ and Retnla mRNA were similar between groups (Figure 4D, Supplemental Figure 4, E and F). By including additional gating markers (Supplemental Figure 5A), we also detected increased numbers of CD64+ macrophages and CD11b+TNF+ myeloid cells in the KLF4 MKO kidneys (Supplemental Figure 5, B and C), whereas neutrophil and dendritic cell numbers were similar between the WT and KLF4 MKO kidneys during NTN (Supplemental Figure 5, D and E). These data suggest that KLF4 in renal infiltrating macrophages may ameliorate kidney damage and fibrosis by suppressing their production of TNFα.
Figure 4.
KLF4 in infiltrating Ly6Chi myeloid cells suppresses their generation of TNFα. (A) Representative images of F4/80 immunostaining in kidney sections from WT and KLF4 MKO mice at day 14 of NTN. (B) Representative flow cytometry gating strategy for single-cell suspensions from NTN kidneys. (C) Number of CD11b+Ly6Chi macrophages in kidneys from WT and KLF4 MKO mice at day 14 of NTN (n=8 for WT and n=6 for KLF4 MKO). (D) mRNA expression for TNFα, TGFβ, IFNγ, and IL1β in CD11b+Ly6Chi macrophages sorted from NTN kidneys of WT and KLF4 MKO mice (n≥3 for each group). Scale bars, 50 μm. Data represent the mean±SEM. *P<0.05.
TNFα in Infiltrating Myeloid Cells Aggravates Nephrotoxic Nephritis
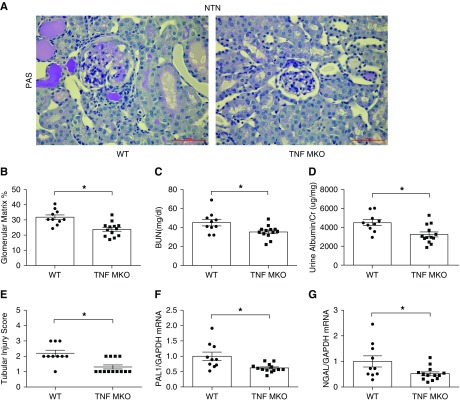
To directly interrogate the role of myeloid TNFα in inflammatory kidney damage, we subjected mice lacking TNFα solely in macrophages (TNF MKO) and WT controls (LysM-Cre− TNFflox/flox) to NTN. TNF MKO animals showed robust and selective deletion of mRNA for TNF in splenic macrophages (Supplemental Figure 6A). At day 14 of NTN, the number of CD11b+Ly6Chi macrophages in TNF MKO kidneys was significantly decreased compared with WTs (Supplemental Figure 6B). mRNA levels for TNF in the renal CD11b+Ly6Chi macrophages from the TNF MKO cohort were markedly reduced (Supplemental Figure 6C). However, TNFα deficiency in macrophages did not affect mRNA levels for CCL2, CCL5, TGFβ, or Retnla in these cells (Supplemental Figure 6, D–G). WT and TNF MKO sham control kidneys had normal architecture and function (Supplemental Figure 7, A–C). By contrast, at day 14 of NTN, TNF MKO mice had less glomerular matrix deposition (23.8%±1.2% versus 31.8%±1.6%; P<0.05) than WT mice (Figure 5, A and B), decreased BUN levels (35.4±1.9 versus 45.2±3.4 mg/dl; P<0.05) (Figure 5C), and lower urinary ACRs (3568±363.1 versus 4664±317.4 μg/mg; P<0.05) (Figure 5D). The TNF MKO kidneys also exhibited less tubular damage (Figure 5E) with markedly attenuated mRNA expressions for PAI1 and NGAL (Figure 5, F and G). Thus, TNFα generation in macrophages augments the severity of glomerular and tubular damage during NTN.
Figure 5.
TNFα in infiltrating myeloid cells aggravates nephrotoxic nephritis. (A and B) Renal pathology in WT and Lysz-Cre+ TNFfl/fl (TNF MKO) mice at day 14 of NTN. (A) Representative images of kidney sections from WT and TNF MKO mice with summary of (B) glomerular matrix deposition (%), (C) BUN, (D) ACR, and (E) renal tubular injury scores in WT and TNF MKO mice. (F and G) Renal mRNA expression of (F) PAI1 and (G) NGAL (n=10 for WT and n=13 for TNF MKO in NTN). Scale bars, 50 μm. Data represent the mean±SEM. *P<0.05. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; PAS, Periodic acid–Schiff.
TNFα Derived from Macrophages Contributes to Renal Fibrogenesis
To examine the severity of renal fibrosis, immunohistochemistry staining for COLI was performed on kidney sections from both experimental groups at day 14 of NTN. TNF MKO kidneys exhibited less interstitial COLI deposition than WTs (Figure 6A). At the mRNA level, expression of COLI was significantly decreased in TNF MKO kidneys versus WTs (Figure 6B). Western blot analysis of the NTN kidneys confirmed that the TNF MKO mice contained less COLI protein and αSMA expression than in WTs, despite similar levels of these proteins in WT and TNF MKO sham control kidneys (Figure 6, C–E). Moreover, after UUO, TNF MKO kidneys had less interstitial staining and downregulated mRNA levels for COLI compared with those in the obstructed WT kidneys (Figure 6, F and G). These findings were consistent with the protein levels of COLI and αSMA and hydroxyproline content (Figure 6, H–K). Thus, macrophage TNFα promotes renal scar formation in two separate models, suggesting that the upregulation of myeloid TNFα accruing from KLF4 deficiency enhances kidney fibrogenesis.
Figure 6.
TNF derived from macrophages contributes to renal fibrogenesis. (A–E) Renal fibrosis in WT and TNF MKO mice at day 14 of NTN. (A) Representative images of COLI immunostaining in kidney sections from WT and TNF MKO mice at day 14 of NTN. (B) Renal mRNA expression for COLI in WT and TNF MKO mice at day 14 of NTN (n=10 for WT and n=13 for TNF MKO). (C) Western blot for COLI and αSMA in whole kidney at day 14 of NTN. (D and E) Semiquantification of COLI and αSMA protein expression (n=3 for sham control and n=10 for WT/TNF MKO in NTN). (F) Representative images of COLI immunostaining in kidney sections from WT and TNF MKO mice at day 7 of UUO. (G) Renal mRNA expression of COLI in WT and TNF MKO mice at day 7 of UUO (n=7 for WT and n=10 for TNF MKO in UUO). (H) Western blot for COLI and αSMA in obstructed and contralateral control kidney at day 7 of UUO. (I and J) Semiquantification of (I) COLI and (J) αSMA protein expression (n=3 for contralateral control, n=7 for WT, and n=10 for TNF MKO in UUO). (K) Hydroxyproline content in obstructed WT and TNF MKO kidneys at day 7 of UUO (n=7 for WT and n=10 for TNF MKO in UUO). Scale bars, 100 μm. Data represent the mean±SEM. *P<0.05. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Augmented Renal Fibrosis and Necroptosis in KLF4 MKO Mice Is Abrogated by TNFα Receptor Inhibition
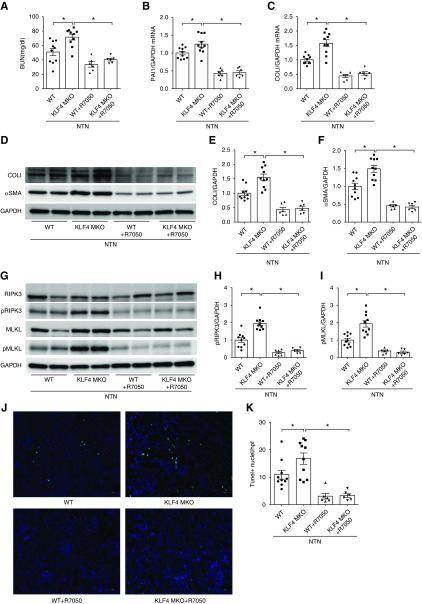
To determine if TNF upregulation permitted by KLF4 deficiency in macrophages contributes to the greater severity of renal damage seen in the KLF4 MKOs, WT and KLF4 MKO animals were treated with the TNFR1 inhibitor R-705011 during NTN. R-7050 treatment significantly reduced BUN levels in WT and KLF4 MKO mice and equalized the BUN levels between the two groups (Figure 7A). At 14 day of NTN, the mRNA levels of PAI1 and COLI in WT and KLF4 MKO kidneys were similarly inhibited by treatment with R-7050 (Figure 7, B and C). TNFR1 inhibition with R-7050 attenuated the protein levels of COLI and αSMA in WT and KLF4 MKO at day 14 of NTN and equalized the levels of these proteins between the two groups (Figure 7, D–F). Because TNFα drives necroptosis in epithelial cells, we examined necroptosis markers in NTN kidneys from KLF4 MKO and WT mice. The total protein levels of RIPK3 and MLKL in sham control WT and KLF4 MKO kidneys were similar, whereas the phosphorylated forms of these proteins could not be detected (Supplemental Figure 8). At day 14 of NTN compared with WTs, KLF4 MKO kidneys exhibited increased levels of phosphorylated RIPK3 and phosphorylated MLKL (Figure 7, G–I). However, R-7050 treatment significantly reduced and equalized the protein levels of phosphorylated RIPK3 and phosphorylated MLKL in the WT and KLF4 MKO mice during NTN (Figure 7, G–I). Finally, numbers of TUNEL-positive cells in WT and KLF4 MKO kidneys at 14 days of NTN were decreased by R-7050 treatment (Figure 7, J and K). These data suggest that KLF4 deficiency in macrophages permits exaggerated kidney damage, fibrosis, and necroptosis during NTN via TNF-dependent actions in kidney epithelial cells.
Figure 7.
Augmented renal fibrosis and necroptosis in KLF4 MKO mice is abrogated by TNFα receptor inhibition during NTN. (A) BUN in WT and KLF4 MKO mice with/without R-7050 treatment during NTN. (B and C) Renal mRNA levels of (B) PAI1 and (C) COLI in WT and KLF4 MKO mice with/without R-7050 treatment. (D) Western blot for COLI and αSMA in whole kidney at day 14 of NTN. (E and F) Semiquantification of (E) COLI and (F) αSMA protein expression. (J) Representative images of TUNEL staining in kidney sections from WT and KLF4 MKO mice at day 14 of NTN. (K) Semiquantification of TUNEL-positive cells per high-powered field (n=10 for WT and KLF4 MKO in NTN alone, n=6 for WT and KLF4 MKO in NTN+R-7050). Scale bars, 50 μm. Data represent the mean±SEM. *P<0.05. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; pMLKL, phosphorylated MLKL; pRIPK3, phosphorylated RIPK3.
Discussion
Myeloid cells infiltrating the kidney can serve both injurious and reparative functions in acute kidney disease and CKD, and the processes governing macrophage polarization in the damaged kidney are complex.12,13 In this study, we demonstrate that KLF4-dependent signals in macrophages mitigate kidney injury, fibrosis, and necroptosis by inhibiting production of macrophage-derived TNFα. First, the severity of kidney injury and fibrosis in KLF4 MKO animals exceeds that seen in WT controls in two models of inflammatory CKD. Second, KLF4 deficiency permits upregulation of TNFα expression within CD11b+Ly6Chi myeloid cells infiltrating the injured kidney, whereas deletion of TNFα selectively from macrophages attenuates kidney injury and fibrosis after NTN or UUO. Third, treatment with the TNFR1 inhibitor R-7050 attenuates renal injury, fibrosis, and necroptosis, and abrogates the differences in these parameters between WT and KLF4 MKO mice during NTN. Thus, these experiments elucidate a mechanism through which KLF4 in myeloid cells protects against inflammatory CKD and provide direct evidence that M1 macrophages trigger TNF-dependent kidney fibrosis and necroptosis during CKD.
KLF4, an important transcription factor of the KLF family, has been implicated in a variety of biologic processes from cellular proliferation, differentiation, and self-renewal.14–16 Whereas KLF4 deficiency inhibits the expression of trafficking molecules and differentiation of bone marrow–derived monocytes, induction of KLF4 in the macrophage favors the transition from the proinflammatory M1 to anti-inflammatory M2 phenotype by a Stat6-dependent mechanism.2,3 In earlier experiments, we reported that activating type 1 angiotensin receptors on macrophages suppressed their transition toward proinflammatory M1 polarization and limited renal epithelial cell damage and kidney fibrosis after obstruction.4 We therefore posited that KLF4 in myeloid cells could play a protective role in inflammatory CKD by suppressing their M1 polarization. Consistent with this hypothesis, we find that KLF4 deletion in infiltrating macrophages augments the expression of M1 cytokines and chemokines TNFα, CCL2, and CCL5, leading to more severe glomerular and tubular damage and augmented kidney fibrosis. Moreover, compared with WTs, obstructed KLF4 MKO kidneys have enhanced expression of M1 markers and downregulated M2 marker expression. These findings are consistent with those of Sharma et al.17 who demonstrated that KLF4 regulates the production of proinflammatory cytokines in myeloid cells infiltrating the vasculature and thereby constrains atherogenesis. Similarly, Yoshida et al.18 demonstrated that Tie2-Cre–mediated KLF4 deletion aggravates inflammation and injury during ischemic AKI, and Tie2 is expressed not only in endothelial cells but also in hematopoietic progenitor cells. Thus, augmenting M1 macrophage differentiation renders multiple organs including the kidney more susceptible to damage, whereas transfer of CD206+ M2 macrophages attenuates glomerular injury during NTN.19
To further investigate the effects of KLF4 on renal macrophage polarization, infiltrating CD11b+Ly6Chi inflammatory macrophages were sorted from kidneys of KLF4 MKO mice and WTs. Among the proinflammatory M1 cytokines, only TNFα in sorted CD11b+Ly6Chi was significantly increased, whereas levels of M2 markers were not altered in KLF4-deficient renal macrophages. Because our sorting analysis focused on Ly6Chi myeloid cells, we acknowledge that our gating strategy could have missed effects of KLF4 deletion on the M2 polarization of mature myeloid cells that downregulated Ly6C after renal infiltration. Nevertheless, based on our sorting results we posited that augmented TNFα expression might account for the exacerbated kidney injury and fibrosis seen with myeloid KLF4 deficiency.
TNFα regulates the progression of both acute kidney disease and CKD. However, TNFα is produced by a wide range of cell lineages including macrophages, Th1 lymphocytes, mesangial cells, and glomerular and tubular epithelial cells such that establishing the source of TNFα driving specific renal pathologies poses a challenge.20–22 Global TNFα deficiency attenuates murine NTN, and TNF blockade ameliorates glomerular inflammation and crescent formation in rat crescentic GN.23–25 On the other hand, TNFα also performs immunoregulatory functions, for example, by promoting exhaustion of T lymphocytes,26 such that some patients treated with TNF blockade have had enhanced risk of developing GN.27 Thus, a better understanding of the cell-specific actions of TNFα in inflammatory kidney disease is necessary to better guide the manipulation of TNFα signals in humans.
In this regard, TNFα produced in the kidney and in myeloid cells alters the severity of renal damage. TNFα generated by renal epithelial cells contributes to the development of cisplatin-induced renal damage.28,29 TNFα generated in the kidneys contributes to angiotensin II–induced BP elevation and glomerular damage.30 Muller et al.31 demonstrated that nephritis was exacerbated in transgenic mice expressing membrane-bound precursor molecule TNF compared with WTs, marked by increased albuminuria and glomerular deposits. On the other hand, macrophage-derived TNFα is also important in the pathogenesis of kidney disease because conditional ablation of TNFα in macrophages significantly reduced albuminuria, histopathologic damage, and kidney macrophage infiltration after 12 weeks of streptozotocin-induced diabetes.32 Whereas Muller and colleagues reported that expression of a membrane-bound TNF precursor on renal parenchymal cells but not in the bone marrow aggravates nephritis at day 5 of NTN, we find that glomerular matrix deposition, tubular injury, and interstitial fibrosis are significantly decreased in TNF MKO mice at day 14 of NTN compared with WTs. Because more infiltrating proinflammatory macrophages accumulate in the kidney during the late phase of NTN, the discrepancies between the two studies may be related to the time point of harvest. Because TNFα is an important mediator of renal fibrogenesis,33 we focused on a later NTN time point to understand if suppression of TNFα by KLF4 affects kidney scar formation. Indeed, we see that myeloid KLF4 deficiency aggravates whereas TNF deficiency mitigates renal fibrosis in both the NTN and UUO models.
Necroptosis is a specific form of programmed cell death that critically depends on RIPK1, RIPK3, and MLKL.34–36 Studies have highlighted the involvement of necroptosis in AKI, and tubular epithelial cells are major targets of necroptosis activators.37–41 Accordingly, RIPK3 blockade or deficiency in tubular epithelial cells improves allograft survival and decreases renal inflammation in allogeneic kidney transplant recipients.42 However, the role of necroptosis in the pathogenesis of chronic inflammation and fibrosis is not clearly established. In nonalcoholic steatohepatitis, RIPK3-dependent Jun-(N)-terminal kinase activation stimulates the generation of proinflammatory cytokines and chemokines, which enhances the recruitment of macrophages and promotes necroptosis.43 TNFα is a key activator that drives parenchymal cell death by stimulating RIPK3-associated necroptosis.31,34 In our study, we find that the augmented TNFα expression in infiltrating CD11b+Ly6Chi subsets from KLF4 MKO kidneys is associated with upregulated renal protein levels of necroptosis markers. Moreover, we find that KLF4 deficiency in infiltrating macrophages exaggerates their polarization toward an M1 phenotype at 14 days of NTN and that treatment with a TNFα receptor inhibitor, R-7050, significantly inhibits and equalizes renal fibrosis and necroptosis in WT and KLF4 MKO animals during NTN. Although these studies suggest that TNF is influencing necroptosis in our injury model, we acknowledge that TNF can have pleiotropic effects on renal injury independent of necroptosis. Moreover, our studies do not discriminate whether renal scar formation accruing from necroptosis depends solely on RIPK3 without involving MLKL because KLF4 deficiency in myeloid cells enhances phosphorylation of both RIPK3 and MLKL in our models.40,44
In summary, KLF4 suppresses the proinflammatory differentiation of renal infiltrating macrophages and attenuates kidney injury and fibrosis by inhibiting myeloid cell generation of TNFα. Our studies further implicate proinflammatory M1 macrophages in promoting the pathogenic accumulation of extracellular matrix in the renal interstitium after chronic epithelial cell injury and highlight the capacity of myeloid cell-derived TNF to exacerbate renal fibrogenesis and necroptosis.
Disclosures
None.
Funding
This work was supported by NIH grants DK087893 and HL128355 and US Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development grant BX000893. Dr. Wen was in receipt of an American Heart Association Predoctoral Fellowship 18PRE34030402.
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019020111/-/DCSupplemental.
Supplemental Figure 1. Angiotensin (Ang) II induces KLF4 mRNA expression in M1-activated macrophages.
Supplemental Figure 2. Normal renal architecture and function in KLF4 MKO sham controls.
Supplemental Figure 3. mRNA expression for M1 and M2 markers in obstructed kidneys from WT and KLF4 MKO mice.
Supplemental Figure 4. KLF4 deficiency in macrophages permits induction of M1 markers in macrophages infiltrating the NTN kidney.
Supplemental Figure 5. Myeloid cell characterization in WT and KLF4 MKO kidneys during NTN.
Supplemental Figure 6. Characterization of macrophages from WT and TNF MKO mice during NTN.
Supplemental Figure 7. Normal renal architecture and function in TNF MKO sham controls.
Supplemental Figure 8. Renal RIPK3 and MLKL protein levels in WT and KLF4 MKO sham controls.
References
- 1.Lee S, Huen S, Nishio H, Nishio S, Lee HK, Choi BS, et al.: Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol 22: 317–326, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alder JK, Georgantas RW 3rd, Hildreth RL, Kaplan IM, Morisot S, Yu X, et al.: Kruppel-like factor 4 is essential for inflammatory monocyte differentiation in vivo. J Immunol 180: 5645–5652, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liao X, Sharma N, Kapadia F, Zhou G, Lu Y, Hong H, et al.: Krüppel-like factor 4 regulates macrophage polarization. J Clin Invest 121: 2736–2749, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang JD, Patel MB, Griffiths R, Dolber PC, Ruiz P, Sparks MA, et al.: Type 1 angiotensin receptors on macrophages ameliorate IL-1 receptor-mediated kidney fibrosis. J Clin Invest 124: 2198–2203, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grivennikov SI, Tumanov AV, Liepinsh DJ, Kruglov AA, Marakusha BI, Shakhov AN, et al.: Distinct and nonredundant in vivo functions of TNF produced by t cells and macrophages/neutrophils: Protective and deleterious effects. Immunity 22: 93–104, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Rudemiller NP, Patel MB, Zhang JD, Jeffs AD, Karlovich NS, Griffiths R, et al.: C-C motif chemokine 5 attenuates angiotensin II-dependent kidney injury by limiting renal macrophage infiltration. Am J Pathol 186: 2846–2856, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang JD, Patel MB, Song YS, Griffiths R, Burchette J, Ruiz P, et al.: A novel role for type 1 angiotensin receptors on T lymphocytes to limit target organ damage in hypertension. Circ Res 110: 1604–1617, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolignano D, Donato V, Coppolino G, Campo S, Buemi A, Lacquaniti A, et al.: Neutrophil gelatinase-associated lipocalin (NGAL) as a marker of kidney damage. Am J Kidney Dis 52: 595–605, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Oda T, Jung YO, Kim HS, Cai X, López-Guisa JM, Ikeda Y, et al.: PAI-1 deficiency attenuates the fibrogenic response to ureteral obstruction. Kidney Int 60: 587–596, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Lin SL, Castaño AP, Nowlin BT, Lupher ML Jr., Duffield JS: Bone marrow Ly6Chigh monocytes are selectively recruited to injured kidney and differentiate into functionally distinct populations. J Immunol 183: 6733–6743, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Mulay SR, Eberhard JN, Desai J, Marschner JA, Kumar SV, Weidenbusch M, et al.: Hyperoxaluria requires TNF receptors to initiate crystal adhesion and kidney stone disease. J Am Soc Nephrol 28: 761–768, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ricardo SD, van Goor H, Eddy AA: Macrophage diversity in renal injury and repair. J Clin Invest 118: 3522–3530, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lech M, Gröbmayr R, Ryu M, Lorenz G, Hartter I, Mulay SR, et al.: Macrophage phenotype controls long-term AKI outcomes--kidney regeneration versus atrophy. J Am Soc Nephrol 25: 292–304, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aziz A, Soucie E, Sarrazin S, Sieweke MH: MafB/c-Maf deficiency enables self-renewal of differentiated functional macrophages. Science 326: 867–871, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Qin S, Liu M, Niu W, Zhang CL: Dysregulation of Kruppel-like factor 4 during brain development leads to hydrocephalus in mice. Proc Natl Acad Sci U S A 108: 21117–21121, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shankman LS, Gomez D, Cherepanova OA, Salmon M, Alencar GF, Haskins RM, et al.: KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med 21: 628–637, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma N, Lu Y, Zhou G, Liao X, Kapil P, Anand P, et al.: Myeloid Krüppel-like factor 4 deficiency augments atherogenesis in ApoE-/- mice--brief report. Arterioscler Thromb Vasc Biol 32: 2836–2838, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshida T, Yamashita M, Iwai M, Hayashi M: Endothelial krüppel-like factor 4 mediates the protective effect of statins against ischemic AKI. J Am Soc Nephrol 27: 1379–1388, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Du Q, Tsuboi N, Shi Y, Ito S, Sugiyama Y, Furuhashi K, et al.: Transfusion of CD206+ M2 macrophages ameliorates antibody-mediated glomerulonephritis in mice. Am J Pathol 186: 3176–3188, 2016 [DOI] [PubMed] [Google Scholar]
- 20.Gómez-Guerrero C, López-Armada MJ, González E, Egido J: Soluble IgA and IgG aggregates are catabolized by cultured rat mesangial cells and induce production of TNF-alpha and IL-6, and proliferation. J Immunol 153: 5247–5255, 1994 [PubMed] [Google Scholar]
- 21.Jevnikar AM, Brennan DC, Singer GG, Heng JE, Maslinski W, Wuthrich RP, et al.: Stimulated kidney tubular epithelial cells express membrane associated and secreted TNF alpha. Kidney Int 40: 203–211, 1991 [DOI] [PubMed] [Google Scholar]
- 22.Neale TJ, Rüger BM, Macaulay H, Dunbar PR, Hasan Q, Bourke A, et al.: Tumor necrosis factor-alpha is expressed by glomerular visceral epithelial cells in human membranous nephropathy. Am J Pathol 146: 1444–1454, 1995 [PMC free article] [PubMed] [Google Scholar]
- 23.Karkar AM, Smith J, Pusey CD: Prevention and treatment of experimental crescentic glomerulonephritis by blocking tumour necrosis factor-alpha. Nephrol Dial Transplant 16: 518–524, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Khan SB, Cook HT, Bhangal G, Smith J, Tam FW, Pusey CD: Antibody blockade of TNF-alpha reduces inflammation and scarring in experimental crescentic glomerulonephritis. Kidney Int 67: 1812–1820, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Timoshanko JR, Sedgwick JD, Holdsworth SR, Tipping PG: Intrinsic renal cells are the major source of tumor necrosis factor contributing to renal injury in murine crescentic glomerulonephritis. J Am Soc Nephrol 14: 1785–1793, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Beyer M, Abdullah Z, Chemnitz JM, Maisel D, Sander J, Lehmann C, et al.: Tumor-necrosis factor impairs CD4(+) T cell-mediated immunological control in chronic viral infection. Nat Immunol 17: 593–603, 2016 [DOI] [PubMed] [Google Scholar]
- 27.Stokes MB, Foster K, Markowitz GS, Ebrahimi F, Hines W, Kaufman D, et al.: Development of glomerulonephritis during anti-TNF-alpha therapy for rheumatoid arthritis. Nephrol Dial Transplant 20: 1400–1406, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Zhang J, Rudemiller NP, Patel MB, Wei Q, Karlovich NS, Jeffs AD, et al.: Competing actions of type 1 angiotensin II receptors expressed on T lymphocytes and kidney epithelium during cisplatin-induced AKI. J Am Soc Nephrol 27: 2257–2264, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramesh G, Reeves WB: TNF-alpha mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J Clin Invest 110: 835–842, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang J, Patel MB, Griffiths R, Mao A, Song YS, Karlovich NS, et al.: Tumor necrosis factor-α produced in the kidney contributes to angiotensin II-dependent hypertension. Hypertension 64: 1275–1281, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Müller MB, Hoppe JM, Bideak A, Lux M, Lindenmeyer MT, Müller S, et al.: Exclusive expression of transmembrane TNF aggravates acute glomerulonephritis despite reduced leukocyte infiltration and inflammation. Kidney Int 95: 75–93, 2019 [DOI] [PubMed] [Google Scholar]
- 32.Awad AS, You H, Gao T, Cooper TK, Nedospasov SA, Vacher J, et al.: Macrophage-derived tumor necrosis factor-α mediates diabetic renal injury. Kidney Int 88: 722–733, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meldrum KK, Misseri R, Metcalfe P, Dinarello CA, Hile KL, Meldrum DR: TNF-alpha neutralization ameliorates obstruction-induced renal fibrosis and dysfunction. Am J Physiol Regul Integr Comp Physiol 292: R1456–R1464, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Günther C, Martini E, Wittkopf N, Amann K, Weigmann B, Neumann H, et al.: Caspase-8 regulates TNF-α-induced epithelial necroptosis and terminal ileitis. Nature 477: 335–339, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rickard JA, O’Donnell JA, Evans JM, Lalaoui N, Poh AR, Rogers T, et al.: RIPK1 regulates RIPK3-MLKL-driven systemic inflammation and emergency hematopoiesis. Cell 157: 1175–1188, 2014 [DOI] [PubMed] [Google Scholar]
- 36.Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, et al.: RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 325: 332–336, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Linkermann A, Bräsen JH, Darding M, Jin MK, Sanz AB, Heller JO, et al.: Two independent pathways of regulated necrosis mediate ischemia-reperfusion injury. Proc Natl Acad Sci U S A 110: 12024–12029, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Linkermann A, Heller JO, Prókai A, Weinberg JM, De Zen F, Himmerkus N, et al.: The RIP1-kinase inhibitor necrostatin-1 prevents osmotic nephrosis and contrast-induced AKI in mice. J Am Soc Nephrol 24: 1545–1557, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mulay SR, Desai J, Kumar SV, Eberhard JN, Thomasova D, Romoli S, et al.: Cytotoxicity of crystals involves RIPK3-MLKL-mediated necroptosis. Nat Commun 7: 10274, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sureshbabu A, Patino E, Ma KC, Laursen K, Finkelsztein EJ, Akchurin O, et al.: RIPK3 promotes sepsis-induced acute kidney injury via mitochondrial dysfunction. JCI Insight 3: e98411, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu Y, Ma H, Shao J, Wu J, Zhou L, Zhang Z, et al.: A role for tubular necroptosis in cisplatin-induced AKI. J Am Soc Nephrol 26: 2647–2658, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lau A, Wang S, Jiang J, Haig A, Pavlosky A, Linkermann A, et al.: RIPK3-mediated necroptosis promotes donor kidney inflammatory injury and reduces allograft survival. Am J Transplant 13: 2805–2818, 2013 [DOI] [PubMed] [Google Scholar]
- 43.Gautheron J, Vucur M, Reisinger F, Cardenas DV, Roderburg C, Koppe C, et al.: A positive feedback loop between RIP3 and JNK controls non-alcoholic steatohepatitis. EMBO Mol Med 6: 1062–1074, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Imamura M, Moon JS, Chung KP, Nakahira K, Muthukumar T, Shingarev R, et al.: RIPK3 promotes kidney fibrosis via AKT-dependent ATP citrate lyase. JCI Insight 3: e94979, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.