Significance Statement
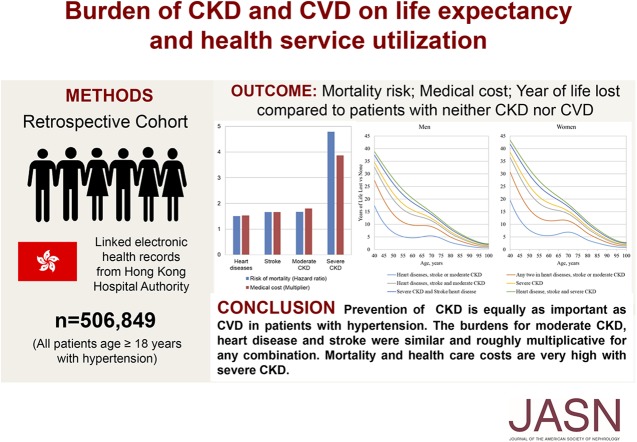
The relative effects of the burdens of CKD and cardiovascular disease on risk of mortality, direct medical costs, and life expectancy in people with hypertension are unknown. In this retrospective cohort study of 506,849 patients with hypertension in Hong Kong, co-occurrence of these conditions was associated with significant incrementally elevated mortality risk, direct medical costs, and reduced life expectancy. The authors found extremely high mortality risk and cost increases for severe CKD, exceeding the combined effects from heart disease and stroke. Moderate CKD, heart disease, and stroke had burdens that were similar individually and roughly multiplicative for any combination. These findings suggest that CKD prevention and intervention to reduce mortality and health care costs in people with hypertension should be given priority equal to that for cardiovascular disease.
Keywords: hypertension, chronic kidney disease, cardiovascular disease, mortality risk, life expectancy, health service utilization
Visual Abstract
Abstract
Background
The relative effects of combinations of CKD, heart disease, and stroke on risk of mortality, direct medical costs, and life expectancy are unknown.
Methods
In a retrospective cohort study of 506,849 Chinese adults in Hong Kong with hypertension, we used Cox regressions to examine associations between all-cause mortality and combinations of moderate CKD (eGFR of 30–59 ml/min per 1.73 m2), severe CKD (eGFR of 15–29 ml/min per 1.73 m2), heart disease (coronary heart disease or heart failure), and stroke, and modeling to estimate annual public direct medical costs and life expectancy.
Results
Over a median follow-up of 5.8 years (2.73 million person-years), 55,666 deaths occurred. Having an increasing number of comorbidities was associated with incremental increases in mortality risk and medical costs and reductions in life expectancy. Compared with patients who had neither CKD nor cardiovascular disease, patients with one, two, or three conditions (heart disease, stroke, and moderate CKD) had relative risk of mortality increased by about 70%, 160%, and 290%, respectively; direct medical costs increased by about 70%, 160%, and 280%, respectively; and life expectancy at age 60 years decreased by about 5, 10, and 15 years, respectively. Burdens were higher with severe CKD.
Conclusions
This study demonstrated extremely high mortality risk and medical cost increases for severe CKD, exceeding the combined effects from heart disease and stroke. Mortality risks and costs for moderate CKD, heart disease, and stroke were similar individually and roughly multiplicative for any combination. These findings suggest that to reduce mortality and health care costs in patients with hypertension, CKD prevention and intervention merits priority equal to that of cardiovascular disease.
Hypertension (HTN) is the most prevalent chronic disease, affecting 1.13 billion people with US$370 billion in direct medical costs spent annually on this condition worldwide.1–4 It is a key risk factor for CKD and cardiovascular diseases (CVD) including heart diseases and stroke.5 Both CKD and CVD are leading causes of mortality globally.6 The prevalences of CKD and CVD are rising rapidly, and the prevalence of coexisting CKD and CVD even more so, increasing 321% from 2000 to 2014 in the United Kingdom.7 As life expectancy increases, the global prevalence of HTN with CVD and/or CKD will continue to increase, resulting in growing burdens in terms of health care costs and premature mortality.
Several studies have examined the risk of mortality or reduction in life expectancy in patients with HTN compared with those with normotension, without specifically examining the effects of CKD or CVD.8–10 Because of the heterogeneity in mortality risks across diverse hypertensive populations, these findings may be too generic with limited clinical application. Some studies have shown that patients with CKD have similar mortality risks and medical costs to those with heart disease or stroke.11–13 However, these studies have evaluated mortality risk associated with CKD, heart diseases, and stroke separately,11–13 ignoring the relative effects of these conditions when combined. Furthermore, there is currently limited evidence on the effect of CKD and/or CVD on medical costs, the economic implications of which are quite important to health system sustainability. This study aimed to address this knowledge gap by evaluating the relative effects of various combinations of CKD, heart disease, and stroke on mortality and direct medical costs. Knowledge of these can help inform health policy planning in terms of resource allocation and treatment priorities for patients with HTN.
Methods
Study Design and Setting
This was a retrospective cohort study. Data were extracted from the electronic Clinical Management System (CMS) of the Hong Kong Hospital Authority, which is the statutory body of all 42 public sector hospitals, 47 specialist outpatient clinics, and 73 primary care clinics in Hong Kong. Hong Kong has a pluralistic health care setting with a public health system which evolved from a tax-funded British National Health Service model operating alongside a largely ambulatory fee-for-service private sector.14 Currently, over 90% of patients with diagnosed chronic disease in Hong Kong are managed by the Hospital Authority,15 where health care costs are highly subsidized by the government or completely waived if household income falls below a certain level. In terms of the ecology of health care in Hong Kong, a previous study demonstrated similar monthly prevalence estimates for hospital-based events including accident and emergency attendances and hospitalizations in Hong Kong as in the United States and United Kingdom, but higher use of ambulatory care (including outpatient care) in Hong Kong.16
Inclusion and Exclusion Criteria
Patients were eligible for this study if they were with initially diagnosed with HTN before or at the first visit, aged ≥18 years, and managed in the outpatient clinics of the Hong Kong Hospital Authority between August 2009 and March 2012. Patients who did not have a record of eGFR, were diagnosed with ESRD at baseline, or had no further visits after baseline were excluded. Patients diagnosed with HTN were defined using the International Classification of Primary Care-2 (ICPC-2) codes of K86 and K87; by the prescription of an antihypertensive drug; systolic BP ≥140 mm Hg or diastolic BP ≥90 mm Hg in the year before baseline, which was defined as the first date of attendance in the outpatient clinic during the inclusion period. Each subject was followed from baseline to the date of all-cause mortality, or last follow-up (as of the censoring date of September 30, 2017), whichever came first. All baseline and outcome measures were extracted from the electronic CMS database of the Hong Kong Hospital Authority. Patient information including demographics and clinical data, such as diagnosis, prescription use, laboratory test results, accident and emergency visits, hospitalization, and outpatient clinic visits, are directly recorded into the CMS by health care professionals at the point of care. A previous study found that the electronic health records achieved a high data completion rate for information related to demographics and socioeconomic factors (100%) and prescription of antihypertensive drugs (99.98%).17 The coding accuracy of the clinical diagnoses entered into the CMS has been validated, and the database has been adopted for several high quality population-based epidemiologic studies.18–21
The study was approved by the Institutional Review Boards in Hong Kong. Informed consent from individual subjects was deemed not needed as all information was extracted anonymously from the computerized administrative system of the Hospital Authority.
Disease Status
Four baseline diseases were included: (1) heart diseases including coronary heart disease and heart failure, (2) stroke, (3) moderate CKD, and (4) severe CKD. The severity of CKD was based on the latest eGFR record before baseline. Moderate and severe CKD were defined by eGFR categories 30–59 and 15–29 ml/min per 1.73 m2, respectively. The eGFR was calculated based on the creatinine level from blood testing according to the abbreviated Modification of Diet in Renal Disease Study formula recalibrated for Chinese (eGFR in ml/min per 1.73 m2=186×[(serum creatinine in μmol/L)×0.011]−1.154×[age]−0.203×[0.742 if female]×1.233, where 1.233 is the adjusted coefficient for Chinese).22 Otherwise, definitions for each condition were based on clinical parameters or diagnostic codes (ICPC-2 or the International Classification of Diseases, Ninth Edition, Clinical Modification) as described in Supplemental Table 1.
Main Outcome Measures
The primary outcome was the incidence of all-cause mortality. Secondary outcomes were annual direct public medical cost and life expectancy. Mortality data were retrieved from the Hong Kong Death Registry, a population-based government official registry recording all of the registered deaths for the citizens in Hong Kong. The costs of each type of service included visits to general and specialist physicians as well as allied health professionals including clinical psychologists, dietitians, occupational therapists, and physiotherapists. Attendance to accident and emergency services and hospital inpatient days of stay are calculated as the product of frequency of attendance and per unit cost. The frequencies of using health services were extracted from the computerized administrative system of the Hong Kong Hospital Authority. Supplemental Table 2 shows the unit costs of relevant health services used by the Government of the Hong Kong Special Administrative Region Gazette and Hospital Authority Ordinance (Chapter 113) in 2013.23 The highly subsidized health policy is for eligible persons (mainly permanent residents; for detailed definitions, see Supplemental Table 2) in Hong Kong. To reflect the original economic burdens from the service provider’s perspective, the unit costs for noneligible persons, which are based on a cost recovery basis, were adopted in this study. The unit costs of the charge include the package costs covering all medical services used during the visits, including consultation, investigations, medications, and other treatments. The annual direct medical costs were the total cost of each type of service utilization.
Baseline Covariates
Baseline covariates consisted of patient sociodemographics, clinical parameters, and treatment modalities. Sociodemographics encompassed gender, age, and smoking status. Clinical parameters included systolic and diastolic BP, lipid profile (LDL cholesterol [LDL-C], total cholesterol/HDL cholesterol ratio [TC/HDL-C ratio] and triglyceride levels), body mass index (BMI), fasting glucose level, and diabetes mellitus identified using the ICPC-2 codes of T89 or T90. Treatment modalities included the prescription of antihypertensive drugs (including angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, β-blockers, calcium channel blockers, diuretics, and other antihypertensive drugs), the prescription of antidiabetic drugs, and lipid-lowering agents. All assays were performed in laboratories accredited by the College of American Pathologists, the Hong Kong Accreditation Service, or the National Association of Testing Authorities, Australia.
Statistical Analyses
All missing baseline covariates including smoking status, BP, lipid profile, BMI, and fasting glucose were handled by multiple imputations to minimize the potential for bias due to missing data.24 Missing data were imputed five times by the chained equation method including all baseline covariates and mortality outcomes, but not costs. Pooled estimates and their corresponding 95% confidence intervals (CIs) were aggregated based on Rubin rules.25 Descriptive statistics for the subjects’ baseline characteristics were summarized. Cumulative incidences and incidence rates for all-cause mortality with 95% CIs were reported. Incidence rates of outcome events were estimated by exact 95% CIs based on a Poisson distribution.26 Multivariable Cox proportional hazards regression models (model 1) were used to estimate the main effects of heart disease, stroke, and moderate and severe CKD on the incidence of all-cause mortality adjusted by age and gender. To observe and control for potential confounding of the effects of heart disease, stroke, and CKD by effects of other potential predictors of mortality, a further three models were developed. For model 2, adjustments were made for age, gender, and smoking status. For model 3, intermediate risk factors (systolic BP, diastolic BP, LDL-C, BMI, TC/HDL-C ratio, triglycerides, and fasting glucose) were also included. For model 4, the diagnosis of diabetes and use of medications (number of HTN drugs used, use of antidiabetic drugs, and use of lipid-lowering drugs) were added. The proportional hazards assumption was also checked by examining plots of the scaled Schoenfeld residuals for covariates against time. The presence of multicollinearity was assessed using the variance inflation factor. Hazard ratios (HRs) with the corresponding 95% CIs and P values for each disease status group (combinations of heart disease, stroke, moderate and severe CKD; defined in the previous section) were reported. Subgroup analyses were conducted to evaluate the associations between different disease status groups and mortality, stratified by gender (male/female). Stratifications were conducted by gender (male, female), age (<70 years, ≥70 years), smoking status (nonsmoker, smoker), BMI (<27.5 kg/m2, ≥27.5 kg/m2), systolic BP (<140 mm Hg, 140–159 mm Hg, ≥160 mm Hg), fasting glucose (<6.1 mmol/L, ≥6.1 mmol/L), LDL-C (<3 mmol/L, ≥3 mmol/L), TC/HDL-C ratio (<4, ≥4), diagnosis of diabetes mellitus (no, yes), number of anti-HTN drugs used (<2, ≥2), use of antidiabetic drugs (no, yes), and use of lipid-lowering drugs (no, yes) at baseline. The interactions of heart disease, stroke, and CKD were evaluated to confirm whether the effects of these diseases were truly multiplicative, as assumed by the primary main effects model.
For secondary outcomes, the annual direct medical cost for each patient in each disease status group was estimated based on the weighted average of the annual costs for health service attendances up to 5-years after baseline. The adjusted ratios of annual costs between disease status groups were evaluated by the generalized linear method with γ family and log link function. This model is commonly applied to estimate the multiplicative effects of complications on medical costs.27–29 Following the approach above, the four models with varying levels of adjustment were refit with the interactions between CVD, stroke, and CKD also included. The losses in life expectancy due to the different numbers of diseases were estimated using a flexible parametric survival model for relative survival.30 This model is widely used to calculate life expectancy,31–33 and allows the evaluation of the loss by extrapolating the estimated linear trend at the end of follow-up without the model assumption of proportional hazards. Age and gender were included as covariates with age treated as time dependent. Age was also modeled continuously and nonlinearly using restricted cubic splines.
All significance tests were two-tailed and those with a P value <0.05 were considered statistically significant. Statistical analyses were performed using Stata version 13.0.
Results
Supplemental Figure 1 shows the flow of patients. A total of 540,480 patients with a diagnosis of HTN were extracted from the Hospital Authority database. After excluding 33,631 patients, 506,849 (93.8%) patients with HTN fulfilled the inclusion criteria and were included in the final analysis. Among those that were excluded, 28,858 (5.7%) had a prior diagnosis of heart disease, 35,095 (6.9%) had a prior diagnosis of stroke, 27,851 (5.5%) had moderate CKD, and 1876 (0.4%) had severe CKD at baseline. The proportions of patients with one, two, and three diagnoses among heart diseases, stroke, and moderate/severe CKD were 71,071 (14.0%), 9908 (2.0%), and 931 (0.2%), respectively. As shown in Supplemental Table 3, the average data completion rates were over 75% for all baseline covariates. The baseline subject characteristics across different disease status groups are summarized in Table 1. Overall, the mean age of subjects was 66 years old (SD 12) and 56% were female. The mean ages for each individual disease subgroup were 8–12 years older than the overall mean.
Table 1.
Baseline covariates of patients by disease status after multiple imputation
| Factor | Total (n=506,849) | Neither CVD nor Moderate/Severe CKD (n=424,939) | Heart Diseases (n=28,858) | Stroke (n=35,095) | Moderate CKD (n=27,851) | Severe CKD (n=1876) |
|---|---|---|---|---|---|---|
| Sociodemographic | ||||||
| Gender | ||||||
| Female (%) | 56 | 57 | 52 | 48 | 60 | 62 |
| Male (%) | 44 | 43 | 48 | 52 | 40 | 38 |
| Age (years) | 66±12 | 64±12 | 75±11 | 74±11 | 78±9 | 78±10 |
| Smoking status | ||||||
| Nonsmoker (%) | 91 | 91 | 93 | 91 | 93 | 93 |
| Smoker (%) | 9 | 9 | 7 | 9 | 7 | 7 |
| Clinical characteristics | ||||||
| Systolic BP (mm Hg) | 137±17 | 137±17 | 137±19 | 137±18 | 139±20 | 141±21 |
| Diastolic BP (mm Hg) | 76±11 | 77±11 | 72±11 | 73±11 | 71±11 | 70±12 |
| LDL-C (mmol/L) | 3.1±0.9 | 3.2±0.9 | 2.7±0.9 | 2.8±0.9 | 3.0±1.0 | 2.9±1.0 |
| TC/HDL-C ratio | 4.1±1.5 | 4.1±1.3 | 3.9±1.3 | 3.9±2.9 | 4.2±1.4 | 4.3±2.0 |
| Triglyceride (mmol/L) | 1.5±1.0 | 1.6±1.0 | 1.5±0.9 | 1.4±0.9 | 1.6±1.2 | 1.7±1.2 |
| BMI (kg/m2) | 26±4 | 26±4 | 25±5 | 25±5 | 25±5 | 25±5 |
| Fasting glucose (mmol/L) | 6.2±2.1 | 6.2±2.1 | 6.2±2.1 | 6.1±1.9 | 6.3±2.5 | 6.4±2.6 |
| Diagnosis of diabetes (%) | 39 | 38 | 42 | 38 | 50 | 59 |
| Number of anti-HTN drugs used | ||||||
| 0 (%) | 8 | 8 | 4 | 5 | 3 | 3 |
| 1 (%) | 49 | 50 | 39 | 45 | 35 | 34 |
| 2 (%) | 34 | 33 | 39 | 36 | 40 | 40 |
| ≥3 (%) | 10 | 9 | 18 | 15 | 22 | 24 |
| Use of antidiabetic drugs (%) | 15 | 31 | 35 | 31 | 44 | 50 |
| Use of lipid-lowering agent (%) | 14 | 11 | 39 | 37 | 18 | 23 |
The values are presented as mean±SD or %, as appropriate. Heart diseases include coronary heart disease and heart failure. Moderate CKD, eGFR 30–59 ml/min per 1.73 m2; severe CKD, eGFR 15–29 ml/min per 1.73 m2. Patients had more than one clinically diagnosis of diseases (e.g., heart and stroke) and thus the sum of the patients in the table is not equal to the total of patients (506,849) in this study. Mean age±SD for (1) heart diseases including coronary heart disease and heart failure was 72±11 (n=27,885); (2) stroke was 73±11 (n=21,394); (3) moderate CKD was 77±10 (n=20,590); (4) severe CKD was 77±10 (n=1202); (5) heart diseases and stroke was 78±10 (n=2904); (6) heart diseases and moderate CKD was 81±8 (n=3299); (7) stroke and moderate CKD was 79±9 (n=3132); (8) heart diseases and severe CKD was 78±10 (n=243); (9) stroke and severe CKD was 81±8 (n=330); (10) heart diseases, stroke, and moderate CKD was 82±8 (n=830); and (11) heart diseases, stroke and severe CKD was 83±7 (n=101). CVD, cardiovascular disease; LDL-C, LDL cholesterol; TC/HDL-C, total cholesterol/HDL cholesterol; BMI, body mass index; HTN, hypertension.
Over a median follow-up period of 5.8 years, 55,666 deaths accrued over 2.7 million person-years at risk. The incidence rate for mortality ranged from 1.38 to 37.32 per 100 person-years among the different disease status groups (Table 2). There was an elevated pattern of mortality risk with increasing cumulative number of conditions among CKD, heart diseases, and stroke. Compared with the reference group (patients without moderate/severe CKD and CVD), the HRs for mortality, adjusted for all baseline covariates, were 1.51 (95% CI, 1.48 to 1.55) in subjects with a history of heart diseases, 1.66 (95% CI, 1.62 to 1.70) in those with stroke, 1.67 (95% CI, 1.63 to 1.71) in those with moderate CKD, and 4.79 (95% CI, 4.52 to 5.08) in those with severe CKD. A similar pattern was observed across all of the subgroups except for subjects aged <70 years, for whom the associations with CKD were stronger (Supplemental Figure 2). The HRs for patients with two or more conditions were generally consistent with multiplicative effects (P>0.05 for deviation from multiplicative effects), with the exception of the HR for those with a history of both stroke and moderate CKD. Supplemental Table 4 displays the adjusted HRs of all-cause mortality by each combination of disease status at baseline. Compared with patients without CKD and CVD, any one, two, and all three conditions (moderate CKD) increased the relative risks of mortality by 52%–72%, 154%–161%, and 285%, respectively. Similar patterns but with higher risk were observed for those with severe CKD.
Table 2.
Mortality rates and adjusted hazard ratios, by disease status at baseline
| Disease Status at Baseline | No. of Participants | No. of Deaths | Person-Years | Incidence Rate (Cases/100 Person-Years) | Hazard Ratio (95% CI) | |||
|---|---|---|---|---|---|---|---|---|
| Age and Sex | Age, Sex, and Smoking | Age, Sex, Smoking, and Intermediate Risk Factorsa | Age, Sex, Smoking, Intermediate Risk Factors, Diagnosis of Diabetes, and Medicationsb | |||||
| No CVD or moderate/severe CKD | 431,840 | 31,084 | 2,338,373 | 1.33 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Heartdiseases | 24,367 | 5167 | 139,982 | 3.69 | 1.45 (1.42,1.49) | 1.46 (1.42,1.49) | 1.46 (1.43,1.50) | 1.51 (1.48,1.55) |
| Stroke | 20,025 | 3928 | 171,649 | 2.29 | 1.64 (1.60,1.68) | 1.63 (1.60,1.67) | 1.60 (1.56,1.63) | 1.66 (1.62,1.70) |
| Moderate CKD | 35,320 | 8368 | 131,498 | 6.36 | 1.69 (1.66,1.73) | 1.69 (1.65,1.73) | 1.70 (1.66,1.74) | 1.67 (1.63,1.71) |
| Severe CKD | 1832 | 956 | 6315 | 15.14 | 5.07 (4.79,5.36) | 5.05 (4.77,5.34) | 4.91 (4.64,5.21) | 4.79 (4.52,5.08) |
Heart diseases include coronary heart disease and heart failure. Moderate CKD, eGFR 30–59 ml/min per 1.73 m2; severe CKD, eGFR 15–29 ml/min per 1.73 m2. The P values for all hazard ratios were <0.001 by multivariable Cox proportional hazard regression. CI, confidence interval; CVD, cardiovascular disease.
Intermediate risk factors include systolic and diastolic BP, LDL cholesterol, body mass index, total cholesterol/HDL ratio, triglycerides, and fasting glucose at baseline.
Diagnosis of diabetes and medications include number of antihypertensive drugs used, use of antidiabetic drugs, and lipid-lowering drugs at baseline.
The annual public direct medical costs ranged from US$1709 to US$9346 among different disease status groups as shown in Table 3. The trend of medical costs with number of diseases was similar to that of mortality. Compared with the reference group, the medical cost multipliers, adjusted for all of the baseline covariates, were 1.53 (95% CI, 1.47 to 1.59) for subjects with a history of heart disease, 1.66 (95% CI, 1.60 to 1.72) for those with stroke, 1.80 (95% CI, 1.73 to 1.87) for those with moderate CKD, and 3.87 (95% CI, 3.34 to 4.47) for those with severe CKD. Except for those with both stroke and moderate CKD for whom costs were modestly lower, the multipliers for subjects with two or more conditions were consistent with multiplicative effects (P>0.05). Supplemental Table 5 displays the adjusted multiplier of geometric mean medical costs for all-cause mortality for each disease combination at baseline. Compared with patients without CKD and CVD, any one, two, and all three conditions (moderate CKD) increased the ratios of mean medical costs by 53%–86%, 146%–184%, and 276%, respectively. Similar patterns with higher medical costs were observed for those with severe CKD.
Table 3.
Estimation of annual public direct medical cost by disease status at baseline
| Disease Status at Baseline | Average public direct medical cost (US$±SD) | Multiplier (95% CI) | |||
|---|---|---|---|---|---|
| Age and Sex | Age, Sex, and Smoking | Age, Sex, Smoking, and Intermediate Risk Factorsa | Age, Sex, Smoking, Intermediate Risk Factors, Diagnosis of Diabetes, and Medicationsb | ||
| No CVD or moderate/severe CKD | 1709±6989 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Heart diseases | 4335±11,119 | 1.55 (1.49,1.61) | 1.55 (1.49,1.62) | 1.49 (1.43,1.54) | 1.53 (1.47,1.59) |
| Stroke | 4146±12,464 | 1.66 (1.60,1.72) | 1.65 (1.60,1.71) | 1.61 (1.55,1.67) | 1.66 (1.60,1.72) |
| Moderate CKD | 5048±12,903 | 1.89 (1.82,1.97) | 1.89 (1.81,1.96) | 1.83 (1.76,1.90) | 1.80 (1.73,1.87) |
| Severe CKD | 9346±16,042 | 4.16 (3.59,4.82) | 4.14 (3.57,4.80) | 3.95 (3.41,4.58) | 3.87 (3.34,4.47) |
Heart diseases include coronary heart disease and heart failure. Moderate CKD, eGFR 30–59 ml/min per 1.73 m2; severe CKD, eGFR 15–29 ml/min per 1.73 m2. The P values for all multiplier were <0.001 by generalized linear model with γ family and log link function. CI, confidence interval; CVD, cardiovascular disease.
Intermediate risk factors include systolic and diastolic BP, LDL cholesterol, body mass index, total cholesterol/HDL ratio, triglycerides, and fasting glucose at baseline.
Diagnosis of diabetes and medications include number of antihypertensive drugs used, use of antidiabetic drugs, and lipid-lowering drugs at baseline.
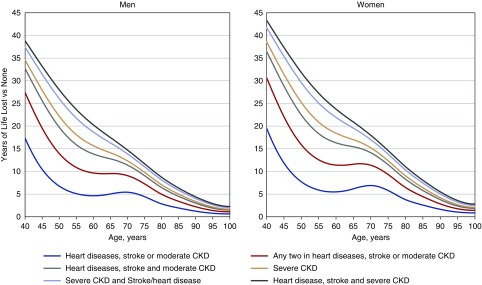
Figure 1 demonstrates the estimated reduction in life expectancy across different disease groups compared with the reference group. At the age of 60, the years of life lost (YLL) for males (females) with one condition (moderate CKD, heart disease, or stroke) was approximately 4.6 years (95% CI, 3.0 to 6.3) (females 5.1 years; 95% CI, 3.0 to 6.3). For those with two conditions, the YLL was approximately 9.7 years (95% CI, 7.3 to 12.0) (females 11.4 years; 95% CI, 8.2 to 14.6). For those with all three conditions, the YLL was approximately 13.9 years (95% CI, 11.7 to 16.0) (females 16.3 years; 95% CI, 13.4 to 19.2). The reduction in life expectancy for males and females with severe CKD, heart disease, and stroke (males 20.3 years; 95% CI, 19.2 to 21.3; females 23.8 years; 95% CI, 22.4 to 25.1), was far greater than in those with moderate CKD, heart disease, and stroke. The YLL for younger patients was much greater than for older patients. For example, in subjects aged 40 years with three conditions (moderate CKD, heart diseases, and stroke) the YLL was 32.7 years (95% CI, 24.9 to 40.5) for males and 36.5 years (95% CI, 26.7 to 46.3) for females.
Figure 1.
Demonstrates greater reductions in life expectancy with increasing number of conditions and worsening severity of CKD as well as greater loss of life in younger patients. Years of life lost by disease status of participants at baseline compared with those with neither moderate/severe CKD nor cardiovascular disease (CVD). Heart diseases includes coronary heart disease and heart failure. Moderate CKD, eGFR 30–59 ml/min per 1.73 m2; severe CKD, eGFR 15–29 ml/min per 1.73 m2.
Discussion
This was a retrospective cohort study of a large data set with almost 56,000 deaths accrued over 2.7 million person-years of risk, providing sufficient power to estimate mortality risk, direct medical costs, and reductions in life expectancy associated with varying combinations of CKD, heart disease, and stroke. Our results demonstrated greater reductions in life expectancy with increasing number of conditions and worsening severity of CKD as well as greater loss of life in younger patients.
The findings highlight the burden from renal complications in patients with HTN, showing that moderate CKD carries a similar burden of mortality risk and annual direct medical costs as having a prior stroke or heart disease, and that the burden of severe kidney disease is even greater than the combined effect from stroke and heart disease. A previous population-based study in Canada also demonstrated that patients with moderate to severe CKD had similar mortality risks compared with those with previous myocardial infarction.11 A review found similar annual direct medical costs of CKD, heart diseases, and stroke in the United States.34 Our findings support the National Kidney Foundation and the American College of Cardiology/American Heart Association in their suggestion that CKD should be regarded as a coronary-heart-disease risk equivalent.35,36 This study found that moderate CKD confers a mortality risk and medical cost increase similar to those with CVD, and extremely high mortality risk and health care cost under the burden of severe CKD. Compared with those without any CKD or CVD, the risk of mortality was increased by approximately 70%, 160%, and 290% in patients with one, two, and three conditions (moderate CKD, heart disease, and stroke). A similar pattern was observed for direct medical costs, which were increased by around 70%, 160%, and 280%, respectively. A similar trend with higher mortality risks and annual direct medical costs was also observed for the combinations of severe CKD, heart diseases, and stroke. Our findings suggest that the associations of CKD, heart diseases, and stroke with mortality and direct medical cost are likely to be multiplicative and virtually nonoverlapping, consistent with previous observations. These results are consistent with previous observations that associations of CKD are largely independent of CVD.13 Our findings therefore support the importance of CVD prevention in patients with CKD, as well as the prevention of CKD in patients with CVD,35,37,38 and suggest that prevention of CKD and CVD may play equally important roles in decreasing the disease burden in patients with HTN. This suggests that prevention of both CKD and CVD may play an equally important role in decreasing the disease burden in patients with HTN. It also provides evidence that both primary and secondary prevention of CVD and CKD may be of equal importance in patients with HTN.
The Framingham Heart Study, involving 3089 participants in the 1950s, estimated an 11-year loss in life expectancy due to myocardial infarction or stroke in patients with HTN aged 60 years, nearly double our estimates.12 Ethnic differences and the historic characteristics of the Framingham Heart Study discourage extrapolation of those findings to this context. Compared with the Framingham Study, our sample size was much larger and more detailed analytical techniques were used. Nevertheless, despite all of the medical advances and interventions currently available to reduce the risks of mortality due to CVD, this study demonstrates that CVD still substantially reduces life expectancy in patients with HTN. Two population-based cohort studies conducted in Taiwan and Canada have demonstrated similar findings. Taiwanese patients aged 60 with moderate and severe loss of kidney function had reductions in life expectancy of around 4 and 13 years; analogous reductions in the Canadian study were 4 and 9 years.13 In our study, the average reductions in life expectancy in patients with severe CKD, heart disease, and stroke were around 20 years at the age of 60, and 40 years at the age of 40. These reductions were even greater than the estimated 10 years of reduced life expectancy for lifelong smoking and 11 years for HIV infection.39–41 However, direct comparisons with the United States, Canada, Taiwan, and Hong Kong may not be completely justified given differing population structures and disease patterns.
Our findings revealed that about one fifth of our patients had one or more complications including CKD, heart disease, and stroke, and the number of patients with CKD was higher than the number with heart disease or stroke. The early stages of CKD are frequently unrecognized as the symptoms associated with uremia do not usually appear until the onset of severe CKD,42 and thus early CKD usually progresses to advanced stages asymptomatically. Previous studies have highlighted under-diagnosis, under-treatment, and low awareness of CKD.43 Recent literature suggests that the early stages of CKD might be reversible by disease-modifying interventions including educational programs, monitoring, and pharmacologic means.44 Although the current trend for most treatment guides is to focus on CVD risk screening and risk-stratified care plans, both CKD and CVD share several common risk factors. Formal systematic screening to detect and manage early CKD may have the potential to help delay or even prevent subsequent progression to advanced stages of CKD. Although most clinicians are aware of the importance of the control and prevention of CVD, CKD has been relatively overshadowed and neglected. Our findings therefore highlight the need for better management of CKD in patients with HTN to prevent or minimize CKD deterioration.
The main strength of this study was the large cohort of patients with HTN managed in primary care clinics. Multiple imputation was used for handling missing data and multiple adjustments with a comprehensive list of confounding variables were included to evaluate and compare the burdens of CKD and CVD. All information was extracted from an administrative database widely used and managed by the Hong Kong Hospital Authority, helping to provide more accurate and reliable data.
Our study has several potential limitations. This study only included patients with HTN in Hong Kong. The findings on mortality risk and health care cost may be subject to temporal changes and modifications in health policy, our results may not be generalizable to general populations and hypertensive populations in other regions or countries. Researchers should thus be cautious when applying our study findings to other settings. Moreover, although several key clinical parameters and different medications were included in the analyses, other potential important confounders such as drug compliance and lifestyle behaviors were not available. Also, costs were not included in the multiple imputation model for missing predictors. Lastly, further studies with longer follow-up durations are needed to reappraise the life expectancy projection.
This territory-wide study showed the extremely high mortality risk and health care cost under the burden of severe CKD. The burdens for moderate CKD, heart diseases, and stroke were similar and roughly multiplicative for combinations. The reductions in life expectancy for those with CKD and CVD were substantial. Our findings highlight the importance of optimizing the management of both CKD and CVD, and the potential benefits of effective prevention of CKD and CVD among patients with HTN.
Disclosures
None.
Funding
This study was funded by the Health Services Research Fund, Food and Health Bureau, Hong Kong Special Administrative Region (reference numbers EPC-HKU-2 and 13142471).
Supplementary Material
Acknowledgments
The authors wish to acknowledge the contributions of the Risk Assessment Management Program (RAMP) team at the Hospital Authority head office, the Chiefs of Service and RAMP program coordinators in each cluster, and the Statistics and Workforce Planning Department at the Hong Kong Hospital Authority.
Dr. Wan, Dr. Yu, and Prof. Lam contributed to the study design and acquisition of data, researched the data, contributed to the statistical analysis and interpretation of the results, and wrote the manuscript. Dr. Chin contributed to the interpretation of the results and wrote the manuscript. Mr. Tang contributed to the statistical analysis. Dr. Fong and Dr. Choi contributed to the interpretation of the results. All authors reviewed and edited the manuscript.
Dr. Wan is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. No funding organization had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or in the preparation of the manuscript.
The data sets generated and/or analyzed during this study are not publicly available as the data are from patient records.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018101037/-/DCSupplemental.
Supplemental Figure 1. Flow chart of patients.
Supplemental Figure 2. Adjusted hazard ratios of patients with any disease compared with patients without diseases associated with all-cause mortality in selected subgroups.
Supplemental Table 1. Definition of the diseases.
Supplemental Table 2. Unit cost of public healthcare services.
Supplemental Table 3. Data completion rates of baseline covariates of patients by disease status.
Supplemental Table 4. Adjusted hazard ratios of all-cause mortality by disease status at baseline.
Supplemental Table 5. Multiplier of annual public direct medical cost by disease status at baseline.
References
- 1.Zhou B, Bentham J, Di Cesare M, Bixby H, Danaei G, Cowan MJ, et al. ; NCD Risk Factor Collaboration (NCD-RisC): Worldwide trends in blood pressure from 1975 to 2015: A pooled analysis of 1479 population-based measurement studies with 19·1 million participants. Lancet 389: 37–55, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J: Global burden of hypertension: Analysis of worldwide data. Lancet 365: 217–223, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Lawes CM, Vander Hoorn S, Rodgers A; International Society of Hypertension: Global burden of blood-pressure-related disease, 2001. Lancet 371: 1513–1518, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Gaziano TA, Bitton A, Anand S, Weinstein MC; International Society of Hypertension: The global cost of nonoptimal blood pressure. J Hypertens 27: 1472–1477, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Forouzanfar MH, Alexander L, Anderson HR, Bachman VF, Biryukov S, Brauer M, et al. ; GBD 2013 Risk Factors Collaborators: Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990-2013: A systematic analysis for the global burden of disease study 2013. Lancet 386: 2287–2323, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang H, Naghavi M, Allen C, Barber R, Carter A, Casey D, et al. ; GBD 2015 Mortality and Causes of Death Collaborators: Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: A systematic analysis for the global burden of disease study 2015 [published correction appears in Lancet 389: e1, 2017]. Lancet 388: 1459–1544, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tran J, Norton R, Conrad N, Rahimian F, Canoy D, Nazarzadeh M, et al. : Patterns and temporal trends of comorbidity among adult patients with incident cardiovascular disease in the UK between 2000 and 2014: A population-based cohort study. PLoS Med 15: e1002513, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turin TC, Murakami Y, Miura K, Rumana N, Kita Y, Hayakawa T, et al. ; NIPPON DATA80/90 Research Group: Hypertension and life expectancy among Japanese: NIPPON DATA80. Hypertens Res 35: 954–958, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Loukine L, Waters C, Choi BC, Ellison J: Health-adjusted life expectancy among Canadian adults with and without hypertension. Cardiol Res Pract 2011: 612968, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kiiskinen U, Vartiainen E, Puska P, Aromaa A: Long-term cost and life-expectancy consequences of hypertension. J Hypertens 16: 1103–1112, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Tonelli M, Muntner P, Lloyd A, Manns BJ, Klarenbach S, Pannu N, et al. ; Alberta Kidney Disease Network: Risk of coronary events in people with chronic kidney disease compared with those with diabetes: A population-level cohort study. Lancet 380: 807–814, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Franco OH, Peeters A, Bonneux L, de Laet C: Blood pressure in adulthood and life expectancy with cardiovascular disease in men and women: Life course analysis. Hypertension 46: 280–286, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJ, Mann JF, et al. : Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. Lancet 382: 339–352, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Leung GM, Tin KY, Chan WS: Hong Kong’s health spending projections through 2033. Health Policy 81: 93–101, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Lau IT: A clinical practice guideline to guide a system approach to diabetes care in Hong Kong. Diabetes Metab J 41: 81–88, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leung GM, Wong IO, Chan WS, Choi S, Lo SV; Health Care Financing Study Group: The ecology of health care in Hong Kong. Soc Sci Med 61: 577–590, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Wong MCS, Jiang JY, Tang JL, Lam A, Fung H, Mercer SW: Health services research in the public healthcare system in Hong Kong: An analysis of over 1 million antihypertensive prescriptions between 2004-2007 as an example of the potential and pitfalls of using routinely collected electronic patient data. BMC Health Serv Res 8: 138, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lau WC, Chan EW, Cheung CL, Sing CW, Man KK, Lip GY, et al. : Association between dabigatran vs warfarin and risk of osteoporotic fractures among patients with nonvalvular atrial fibrillation. JAMA 317: 1151–1158, 2017 [DOI] [PubMed] [Google Scholar]
- 19.Chan EW, Lau WC, Leung WK, Mok MT, He Y, Tong TS, et al. : Prevention of dabigatran-related gastrointestinal bleeding with gastroprotective agents: A population-based study. Gastroenterology 149: 586–595.e3, 2015 [DOI] [PubMed] [Google Scholar]
- 20.Wong AYS, Root A, Douglas IJ, Chui CSL, Chan EW, Ghebremichael-Weldeselassie Y, et al. : Cardiovascular outcomes associated with use of clarithromycin: population based study. BMJ 352: h6926, 2016 [DOI] [PubMed] [Google Scholar]
- 21.Wong AY, Wong IC, Chui CS, Lee EH, Chang WC, Chen EY, et al. : Association between acute neuropsychiatric events and Helicobacter pylori therapy containing clarithromycin. JAMA Intern Med 176: 828–834, 2016 [DOI] [PubMed] [Google Scholar]
- 22.Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y, et al. : Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease [published correction appears in J Am Soc Nephrol 17: 3540, 2006]. J Am Soc Nephrol 17: 2937–2944, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Government of the Hong Kong Special Administrative Region: Hospital authority ordinance (chapter 113) revision to list of charges. 2013. [Google Scholar]
- 24.Royston P: Multiple imputation of missing values. Stata J 4: 227–241, 2004 [Google Scholar]
- 25.Rubin DB: Multiple Imputation for Nonresponse in Surveys, New York, John Wiley & Sons, 2004 [Google Scholar]
- 26.Ulm K: A simple method to calculate the confidence interval of a standardized mortality ratio (SMR). Am J Epidemiol 131: 373–375, 1990 [DOI] [PubMed] [Google Scholar]
- 27.Manning WG, Mullahy J: Estimating log models: To transform or not to transform? J Health Econ 20: 461–494, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Clarke P, Gray A, Legood R, Briggs A, Holman R: The impact of diabetes-related complications on healthcare costs: Results from the United Kingdom Prospective Diabetes Study (UKPDS Study No. 65). Diabet Med 20: 442–450, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Jiao F, Wong CKH, Tang SCW, Fung CSC, Tan KCB, McGhee S, et al. : Annual direct medical costs associated with diabetes-related complications in the event year and in subsequent years in Hong Kong [published correction appears in Diabet Med 36: 655, 2019]. Diabet Med 34: 1276–1283, 2017 [DOI] [PubMed] [Google Scholar]
- 30. Royston P, Lambert PC: Flexible parametric survival analysis using Stata: Beyond the Cox model. College Station, TX, Stata Press, 2011.
- 31.Andersson TM, Dickman PW, Eloranta S, Lambe M, Lambert PC: Estimating the loss in expectation of life due to cancer using flexible parametric survival models. Stat Med 32: 5286–5300, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Nordio M, Limido A, Maggiore U, Nichelatti M, Postorino M, Quintaliani G; Italian Dialysis and Transplantation Registry: Survival in patients treated by long-term dialysis compared with the general population. Am J Kidney Dis 59: 819–828, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Nelson CP, Lambert PC, Squire IB, Jones DR: Relative survival: What can cardiovascular disease learn from cancer? Eur Heart J 29: 941–947, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Muka T, Imo D, Jaspers L, Colpani V, Chaker L, van der Lee SJ, et al. : The global impact of non-communicable diseases on healthcare spending and national income: A systematic review. Eur J Epidemiol 30: 251–277, 2015 [DOI] [PubMed] [Google Scholar]
- 35.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, et al. ; American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention: Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, high blood pressure research, clinical cardiology, and epidemiology and prevention. Circulation 108: 2154–2169, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Weiner DE, Tighiouart H, Stark PC, Amin MG, MacLeod B, Griffith JL, et al. : Kidney disease as a risk factor for recurrent cardiovascular disease and mortality. Am J Kidney Dis 44: 198–206, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Shiba N, Shimokawa H: Chronic kidney disease and heart failure--bidirectional close link and common therapeutic goal. J Cardiol 57: 8–17, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Barrows IR, Raj DS: Janus face of coronary artery disease and chronic kidney disease. J Am Heart Assoc 5: e003596, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doll R, Peto R, Boreham J, Sutherland I: Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ 328: 1519, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.May M, Gompels M, Delpech V, Porter K, Post F, Johnson M, et al. : Impact of late diagnosis and treatment on life expectancy in people with HIV-1: UK Collaborative HIV Cohort (UK CHIC) study. BMJ 343: d6016, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lohse N, Hansen AB, Pedersen G, Kronborg G, Gerstoft J, Sørensen HT, et al. : Survival of persons with and without HIV infection in Denmark, 1995-2005. Ann Intern Med 146: 87–95, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Li PK, Kwan BC, Leung CB, Kwan TH, Wong KM, Lui SL, et al. ; Hong Kong Society of Nephrology: Prevalence of silent kidney disease in Hong Kong: The screening for Hong Kong asymptomatic renal population and evaluation (SHARE) program. Kidney Int Suppl (94):S36–S40, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Wen CP, Cheng TY, Tsai MK, Chang YC, Chan HT, Tsai SP, et al. : All-cause mortality attributable to chronic kidney disease: A prospective cohort study based on 462 293 adults in Taiwan. Lancet 371: 2173–2182, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Morrison AS: Screening in Chronic Disease, New York, Oxford University Press, 1992 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.