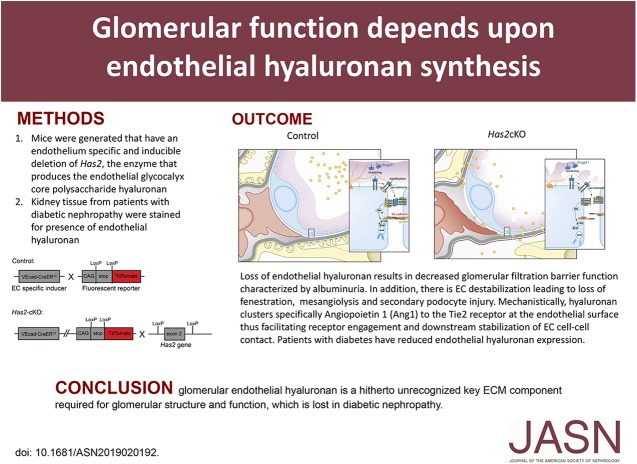
Significance Statement
In previous work, the authors demonstrated that short-term loss of integrity of the luminal part of the glycocalyx envelope that covers endothelial cells resulted in impaired glomerular filtration barrier function. In this study, using knockout mice lacking endothelial hyaluronan synthase 2 (the enzyme that produces hyaluronan, the main structural component of the glycocalyx layer), they found that loss of glomerular endothelial hyaluronan leads to mesangiolysis and glomerulosclerosis. Tissue from patients with diabetic nephropathy showed loss of glomerular endothelial hyaluronan in association with lesion formation. The authors also demonstrated that hyaluronan loss results in disturbed signaling of the extracellular matrix stabilizer angiopoietin 1. These findings suggest that the loss of glomerular endothelial hyaluronan in diabetic nephropathy may be a new therapeutic target to restabilize glomerular lesions.
Keywords: glomerular endothelial cells, glycocalyx, hyaluronan, glomerulosclerosis, angiopoietin-1
Visual Abstract
Abstract
Background
A glycocalyx envelope consisting of proteoglycans and adhering proteins covers endothelial cells, both the luminal and abluminal surface. We previously demonstrated that short-term loss of integrity of the luminal glycocalyx layer resulted in perturbed glomerular filtration barrier function.
Methods
To explore the role of the glycocalyx layer of the endothelial extracellular matrix in renal function, we generated mice with an endothelium-specific and inducible deletion of hyaluronan synthase 2 (Has2), the enzyme that produces hyaluronan, the main structural component of the endothelial glycocalyx layer. We also investigated the presence of endothelial hyaluronan in human kidney tissue from patients with varying degrees of diabetic nephropathy.
Results
Endothelial deletion of Has2 in adult mice led to substantial loss of the glycocalyx structure, and analysis of their kidneys and kidney function showed vascular destabilization, characterized by mesangiolysis, capillary ballooning, and albuminuria. This process develops over time into glomerular capillary rarefaction and glomerulosclerosis, recapitulating the phenotype of progressive human diabetic nephropathy. Using a hyaluronan-specific probe, we found loss of glomerular endothelial hyaluronan in association with lesion formation in tissue from patients with diabetic nephropathy. We also demonstrated that loss of hyaluronan, which harbors a specific binding site for angiopoietin and a key regulator of endothelial quiescence and maintenance of EC barrier function results in disturbed angiopoietin 1 Tie2.
Conclusions
Endothelial loss of hyaluronan results in disturbed glomerular endothelial stabilization. Glomerular endothelial hyaluronan is a previously unrecognized key component of the extracelluar matrix that is required for glomerular structure and function and lost in diabetic nephropathy.
All cells in the body are covered with a glycocalyx layer, a carbohydrate gel composed of the glycosaminoglycans heparan sulfate, hyaluronan (HA), chondroitin sulfate, and associated proteins.1 The glycocalyx is especially critical for the homeostasis of the vasculature. Indeed, the glycocalyx controls vessel perfusion, ensures vessel patency (through its antithrombotic properties), regulates vascular barrier permeability, and prevents vascular inflammation (through precluding leukocyte adhesion to endothelial cells [ECs]).2–4 Despite its importance, the glycocalyx layer is one of the most understudied structures in vascular biology and understanding of its regulation and role in vascular homeostasis is still very limited.5 We previously demonstrated that short-term loss of integrity of the luminal part of the glycocalyx layer resulted in a perturbed glomerular filtration barrier function.6 Given the relevance of this phenomenon for the development of kidney disease, we sought to explore the role of the glycocalyx layer of the endothelial extracellular matrix (ECM) for renal function.
HA, the main structural component of the endothelial glycocalyx layer, is produced by three isoforms of hyaluronan synthase (HAS1, 2, and 3), and these enzymes act on the inner side of the plasma membrane and simultaneously translocate the growing polysaccharide through the plasma membrane into extracellular space. Of these enzymes, HAS2 is the predominant enzyme in HA synthesis during development.4 HAS2 is expressed by most, if not all, cells and is essential for life. Although has1 and has3 null mice develop normally, the has2 null mouse dies at an early embryonic stage when the heart is formed.7 HA is a linear polysaccharide composed of repeating units of glucuronic acid (GlcA) and N-acetyl-glucosamine (GlcNAc) linked together through glycosidic bonds that can be repeated several thousand times. To determine the effects of reduced HA synthesis by ECs in vivo, we inactivated the HA-synthesizing enzyme has2 gene selectively in ECs in adult mice carrying both floxed has2 alleles, and the endothelial-specific cdh5 tamoxifen-inducible Cre-recombinase with a TdTomato reporter to control for recombination efficacy.
In this study we show that (1) loss of HA from the endothelial glycocalyx results in microvascular destabilization, which is most evident in the glomerulus where loss of HA leads to mesangiolysis and glomerulosclerosis, very similarly to what can be observed clinically in diabetes. Loss of HA in the glomerular EC ECM in human diabetic nephropathy was subsequently confirmed. (2) Mechanistically, we demonstrate that HA in the EC ECM serves as a specific scaffold for signaling molecules, where loss of HA results in disturbed signaling of the EC stabilizer angiopoietin 1.
Methods
Mice
Endothelial-specific conditional homozygous has2 knockout (Has2-cKO) mice were generated by breeding B6.Cdh5-Cre-ERT2 with B6.Has2fl/fl.8–12 For reporter analysis, Cdh5-Cre-ERT2.Has2fl/fl and Cdh5-Cre-ERT2 (ctrl) mice were crossed to Rosa-TdTomatofl/fl reporter mice, in which Cre-mediated excision resulted in endothelial Tomato expression, as illustrated in Supplemental Figure 1A and B. Animal experiments were approved by the Animal Care Committee of the Royal Netherlands Academy of Arts and Sciences (permit no. HI 11.2504) or by the Ethical Committee on Animal Care and Experimentation of the Leiden University Medical Center (permit no. 14–033). Two days before tamoxifen treatment and at indicated time intervals (2, 4, 8, and 12 weeks after induction), body weight and BP measurements were performed. One week before the endpoints at 8 and 12 weeks, echocardiography was performed. Additional animals, 4 weeks after tamoxifen, were used for renal cationic ferritin perfusion (n=3 per group) and renal tissue slice collection (n=3 per group), and at 6 weeks after tamoxifen for collection of retinas (n=3–5 per group).
Creation of Neurocan-dsRed Construct
The N terminus rat neurocan construct of the HA-specific neurocan-eGFP (Ncan-eGFP, gift from J. Kappler) construct,13 linked to the BM 40 signal peptide with APLGRGSHHHHHHGGLA and a GSSGA linker at the C terminus, was excised and fused into a pDsRedMonoN1 (ClonTech, Mountain View, CA) plasmid. The modified neurocan-dsRed (Ncan-dsRed) construct was incorporated into a self-inactivating lentiviral vector (pLV-CMV-IE.Ncan-dsRed) and transduced into Hek293 cells for production. Ncan-eGFP and Ncan-dsRed were isolated from supernatant and purified on an Ni-NTA resin column using the incorporated 6xHistidine tag within the protein.
Tissue Slice Imaging
Stained tissue slices, submerged in HBSS, were examined using a Zeiss 710-NLO CLSM microscope (Carl Zeiss, Göttingen, Germany) with a ×20 water dipping objective (W-Plan Apochromat, NA 1.0 DIC M27 70 mm; Carl Zeiss). Sequential 16-bit confocal images (xy dimensions, 0.05×0.05 μm) were recorded using ZEN-2009 Image software (Carl Zeiss) and analyzed with ImageJ, as described earlier.6 For each glomerular image, the amount and location of intensity profiles were quantified by estimating the distance from the luminal half-width of the endothelial TdTomato signal to the half-width of the intraluminal Ncan-eGFP fluorescence along the lines (five per image) of interest as shown in Figure 1, A and B, Supplemental Figure 1, C and D. Total luminal endothelial fluorescence was calculated from fluorescence within this section.
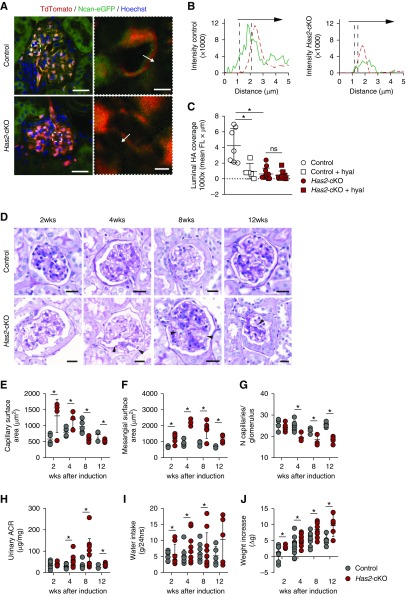
Figure 1.
Loss of endothelial HA and glycocalyx results in progressive glomerulopathy and proteinuria. (A) Left, representative cross-sectional confocal images of glomerular tissue sections, 4 weeks after tamoxifen induction. Sections were stained with the HA-binding probe Ncan-eGFP (green), and successfully recombined ECs were labeled with TdTomato (red). Has2-cKO mice show loss of the endothelial HA layer (scale bar, 30 µm). Right, examples of measurement lines over glomerular free capillary wall (scale bar, 5 µm). (B) Fluorescence intensity plots demonstrate intraluminal (green, left) and subendothelial (green, right) localization of HA in relation to the EC membrane position (red, dotted lines). (C) Luminal HA surface coverage quantification (n=3 per group). (D) Representative images of periodic acid–Schiff–stained glomeruli of control (upper panels) and Has2-cKO (lower panels) mice at 2, 4, 8, and 12 weeks after tamoxifen induction. Arrowheads indicate diffuse widening of capillary loops through mesangiolysis (ballooning; scale bar, 50 µm). (E) Glomerular capillary surface area increased from week 2 onwards. (F) At this early stage, glomerular hypertrophy was the most prominent change in Has2-cKO glomeruli, which was accompanied by induction of matrix expansion. (G) From week 4 onwards capillary rarefaction developed; (E–G) 2 (n=4, 5 per group), 4 (n=6, 4 per group), 8 (n=5 group), and 12 weeks (n=5 group). (H) Albumin-to-creatinine ratio (ACR) in 24-hour urine samples significantly increased in Has2-cKO from week 4. During the 12-week period after tamoxifen induction, (I) Has2-cKO mice drank more water (no change in food intake) and (J) total body weight significantly increased from week 8 onward; 2 (n=8, 12 per group), 4 (n=14 per group), 8 (n=10 per group), and 12 weeks (n=5 per group). Values are given as (C) mean±SEM or (E–J) mean±SD. Difference was assessed by nonpaired two-tailed t test or, when not normally distributed, by two-tailed F-test; *P<0.05. FL, fluorescence intensity.
In Silico Angiopoietin/HA Prediction Model
Angiopoietin 1 (GenBank: AAM92271.1) and angiopoietin 2 (GenBank: AAI43903.1) amino acid sequences were analyzed using online PredictProtein software (www.predictprotein.org/home) and the predicted glycosaminoglycan binding domain within the secondary structure was evaluated against fibrinogen (GenBank: CAA50740.1) and a functional region map was drawn (Figure 3B). 3D structures of Ang1 (target; PDB code 4JYO) and HA (2BVK) from the protein databank (www.rcsb.org/pdb/home) were downloaded and the HA code was transformed to a mol2 format using UCSF Chimera software (www.cgl.ucsf.edu/chimera/) for use as ligand in the online Swissdock software (www.swissdock.ch/docking), to obtain 3D docking models of the Ang1-HA binding domain, as predicted above. Using the UCSF Chimera software, the predicted model with lowest binding energy is shown (Figure 3C).
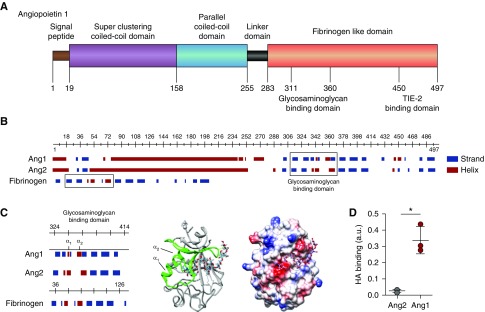
Figure 3.
HA binds angiopoietin 1. (A) Structural characteristics of angiopoietin 1 (Ang1) reveal, besides the superclustering coiled-coil domain (purple) and parallel coiled-coil domain (blue) allowing Ang1 molecules to form clusters, a possible glycosaminoglycan- (GAG) binding domain within the N-terminal fibrinogen-like domain (orange) that neighbors the TIE2-binding site. (B) In contrast to the predicted GAG-binding domain (right box) of angiopoietin 2 (Ang2), the Ang1 GAG-binding domain resembles the HA-binding domain of fibrinogen (left box). (C) Like the predicted HA-binding Link module superfamily,17 the Ang1 GAG-binding domain contains two α-helices separated by an antiparallel β strand (left panel). The 3D ribbon configuration of the fibrinogen-like domain of Ang1 shows the position of the two α-helices in relation to the proposed HA-binding domain (middle panel), and the electrostatic surface projection of the 3D crystallographic structure reveals the positions of negative- (red), neutral- (white), and positive- (blue) charged sites within the HA-binding domain (right panel). (D) Direct binding of recombinant human Ang1 or Ang2 to HA. Values are given as mean±SD and difference was assessed by nonpaired two-tailed t test; *P<0.05.
Angiopoietin/HA Binding Assay
First, 10–20 kDa HA (Lifecore Biomedical LCC, Chaska, MN) was mixed with either Ang1 or Ang2 (R&D Systems) and incubated overnight at rT (control HA alone). Next, bound HA was separated from unbound HA. For HA detection, 20 µg/ml (100 µl) neurocan HA-binding peptide in carbonate buffer (0.2 M, pH 9.4) was incubated overnight at 4°C. Wells were washed, followed by protein block serum-free buffer (Dako) at rT for 1 hour. Next, angiopoietin-bound HA was added and incubated at rT for 2 hours on a shaker, then washed. This was followed by 100 µl HABP-biotin conjugate (DHYALO; R&D Systems) and incubation for 2 hours at rT on a shaker. Wells were washed and 100 µl Substrate Solution (color reagent A and color reagent B; DHYALO; R&D Systems) was added for 30 minutes at rT. Reaction was stopped with Stop Solution (DHYALO; R&D Systems) and absorbance was read at 450 nm within 30 minutes with wavelength correction to 540 or 570 nm. Values are given as bound HA of three separate experiments.
In Vitro Culture Experiments
Primary human glomerular-derived microvascular ECs (hgMVECs) were purchased from Cell Systems (ACBRI-128; Kirkland, WA) and cultured in serum-free EC medium (EC-SFM; Gibco, Thermo-Fisher Scientific, Waltham, MA) supplemented with 1% bovine platelet-poor plasma-derived serum (Biomedical Technologies Inc., Stoughton, MA), 50 µg/ml VEGF-165 (R&D Systems), and 100 µg/ml hFGF (Miltenyi Biotech) (EC-SFM full medium) at 37°C and 5% CO2.
Flow Experiments
Flow experiments were performed using an ibidi flow system (ibidi, Martinsried, Germany) and cells were cultured for 4 days at a constant laminar shear stress of 5 dyne/cm2 in EC-SFM full medium in the presence of 100 ng/ml recombinant human ANG1 (R&D Systems). Control hgMVECs (pLV-CMV-IE) or pLV-CMV-IE.HAS2shRNA silenced cells were seeded into closed perfusion chambers (ibiTreat, 0.4 µ-Slide VI Luer). Medium was refreshed after 1 day, nonadhered cells removed, and cells cultured under laminar flow for another 3 days at 37°C and 5% CO2.
Next, cells were fixed with 4% PFA and 0.2% Triton-X100 in HBSS or 4% PFA in HBSS (for HA staining) for 10 minutes at rT, and stained with primary monoclonal Mouse Anti-Human VE cadherin (CD144, 55–7H1; BD Biosciences), monoclonal Mouse Anti-Human active β-catenin (anti-ABC, 8E7; Merck Millipore), Goat Anti-Human Ang1 (R&D Systems), or Ncan-dsRed then incubated overnight (at 4°C), followed by an appropriate secondary antibody and phalloidin-TRITC (VEcadherin, β-catenin samples) for 1 hour, all in blocking buffer. Cells were counterstained with 10 µg/ml Hoechst 33528 and embedded in Vectashield mounting medium (Vector Laboratories Inc., Burlingame, CA). Cells were examined using a LEICA TCS SP8 X WLL (Leica, Rijswijk, The Netherlands). Quantification is descripted in the Supplemental Material.
Statistical Analyses
Results are presented as mean±SD or mean±SEM; n defines the number of biologic replicates. Differences between groups were assessed by nonpaired two-tailed t test, paired two-tailed t test, or, when not normally distributed, by two-tailed F-test. P values <0.05 were considered statistically significant.
Detailed methods, including information about materials and reagents, immunohistochemistry, electron microscopy, western blotting, and genotyping, are described in the Supplemental Material.
Results
Loss of Endothelial Glycocalyx HA Synthesis In Vivo Leads to Glomerulopathy
To determine the effects of reduced HA expression by ECs in vivo, we inactivated the HA-synthesizing enzyme hyaluronan synthase 2 (has2) gene selectively in ECs in adult mice carrying both floxed has2 alleles, and the endothelial-specific cdh5 tamoxifen-inducible Cre-recombinase with a TdTomato reporter to control for recombination efficacy (Supplemental Figure 1, A and B). Four weeks after tamoxifen, luminal HA was approximately 80% reduced from the endothelial surface in glomeruli of Has2-cKO mice, whereas the specific HA-binding probe Ncan-eGFP still showed the presence of intraluminal HA within the glomerular capillaries of control mice (Figure 1, A–C, Supplemental Figure 1, C and D; hyaluronidase control). Cationic ferritin coverage, which marks glycocalyx coverage, was reduced to 38%±15% in Has2-cKO mice compared with 79%±10% in control mice (Supplemental Figure 1, E and F). This indicates substantial loss of the glycocalyx structure, even though lectin binding of LEA-FITC still demonstrated preservation of other glycocalyx constituents on the endothelial surface (Supplemental Figure 1, G and H). Loss of glycocalyx was predominant on the endothelial surface but could also be noticed in the subendothelial space (Figure 1B, Supplemental Figure 1F).
Analysis of kidneys from Has2-cKO mice revealed that endothelial loss of HA resulted in vascular destabilization, characterized by capillary ballooning, mesangiolysis, and loss of endothelial fenestrations (Figure 1, D–G, Supplemental Figure 2, A–C, Supplemental Table 1). Over the observation period of 12 weeks, this led to capillary rarefaction and severe glomerular injury that included secondary podocyte effacement and parietal epithelial cells that attached to denuded basement membrane where podocytes had disappeared, thus initiating focal segmental glomerulosclerotic lesions (Supplemental Figure 2B). Albuminuria and systemic edema occurred secondary to changes in glomerular capillary structure (Figure 1, H–J). These focal segmental glomerulosclerotic lesions highly resemble the kidney lesions observed in human patients.14,15
Mild Microvascular Disease in the Heart and Retina with Loss of Endothelial HA
Next, we studied the microvasculature in other organs, first focusing on the cardiac microvasculature. With loss of endothelial HA, capillary rarefaction developed in the left ventricle wall (Supplemental Figure 3, A and B). Even though overall myocardial function (ejection fraction) and BP were not altered during the experimental period (data not shown), myocardial contractility, as measured by aortic peak flow, was reduced (Supplemental Figure 3, C and D). Likely to compensate for the impaired contractility, there was also a small increase in ventricular mass (Supplemental Figure 3E) and left ventricle myocyte cross-sectional area from 4 weeks on in Has2-cKO mice, without any myocardial interstitial fibrosis (data not shown). Finally, in the vascular bed in the adult retina, where Tie2 signaling has been shown to regulate blood vessel stabilization and functions,16,17 loss of endothelial HA was associated with typical vaso-regression within the deep retinal capillary plexus, resulting in reduced vascular density and branching (Supplemental Figure 3, H and I).
Diabetic Nephropathy Is Characterized by Loss of Glomerular Endothelial HA
To explore the relevance of these findings for human diabetic microvascular disease, we examined the presence of endothelial HA in kidney tissue obtained both by needle biopsies (n=5) as well as postmortem material (n=5) from patients with varying degrees of diabetic nephropathy. Methenamine silver–periodic acid–Schiff confirmed the various stages of glomerular capillary loss, excessive matrix formation, glomerulosclerosis, and presence of Kimmelstiel–Wilson nodules (Figure 2A, upper panels). Staining for HA using the Ncan-dsRed HA-binding probe demonstrated progressive loss of glomerular capillary endothelial HA with diabetic nephropathic lesion formation (Figure 2A and D, second row and right, respectively) which was accompanied by loss of lectin binding of LEA-FITC (Figure 2A and D, third row and left, respectively), demonstrating loss of other glycocalyx constituents on the endothelial surface as well. With increasing degree of diabetic nephropathy, accumulation of matrix deposition of HA could be observed (Figure 2A, right panels second row) which, however, was no longer related to the endothelium.
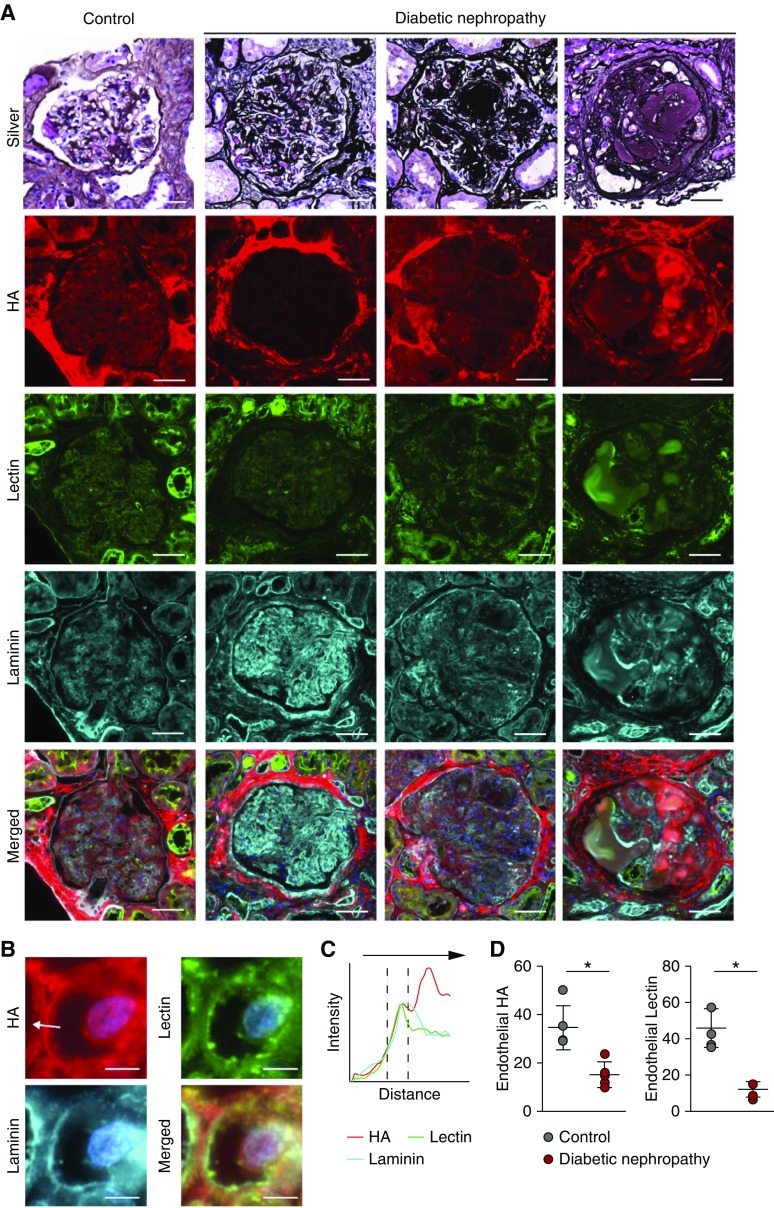
Figure 2.
Early-stage human diabetic nephropathy shows loss of glomerular HA. (A) Representative methenamine silver–periodic acid–Schiff–stained glomeruli of a control glomerulus (left panel) and three consecutive stages of DN in human kidney biopsy specimens. Further, fluorescent images of glomerular tissue sections stained for HA (red, second row), Lycopersicon esculentum lectin (green, third row), and laminin (cyan, fourth row), and merged images. (B) Insets show examples of measurement lines (scale bar, 5 µm). (C) Fluorescence intensity plots demonstrate endothelial localization of HA in relation to the subendothelial basement membrane position (cyan, laminin). (D) Quantification of total endothelial HA (left) or lectin (right) presence (n=5 per group).
Endothelial HA as a Binding Partner for Angiopoietin 1 Signaling
Because (1) Ang1 determines glomerular vascular phenotype,18–20 (2) Ang1 is sequestered to chemically crosslinked HA hydrogels,21 and (3) Ang1 requires ECM binding to signal to its receptor,22 we explored whether HA in the glycocalyx may act as a mediator in confining Ang1 to the cell surface and in inducing endothelial stabilization. To investigate whether a direct link exists between HA and Ang1, we first looked at the secondary structure prediction of the fibrinogen-like domain of Ang1 (Figure 3, A and B). Structural modeling showed potential binding motifs for HA through a lectin-like fold termed the Link module, which has been previously shown to also mediate the binding of fibrinogen to HA.23 This binding motif was different for angiopoietin 2 (Figure 3B). In a 3D docking model, HA was predicted to bind to this domain with a free binding energy ΔG of −12.49 kcal/mol, a value similar to that of the binding of HA to its primary cell surface receptor CD44 (Figure 3, B and C, Supplemental Figure 4, A and B).24 In agreement, we confirmed Ang1, and not Ang2, binding to HA in a direct binding assay (Figure 3D).
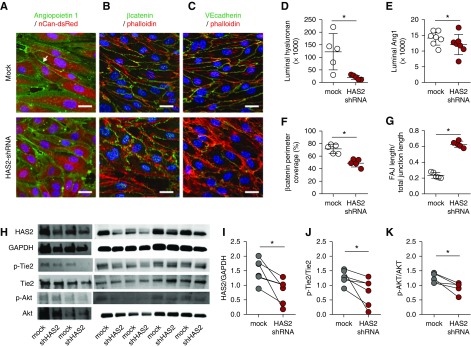
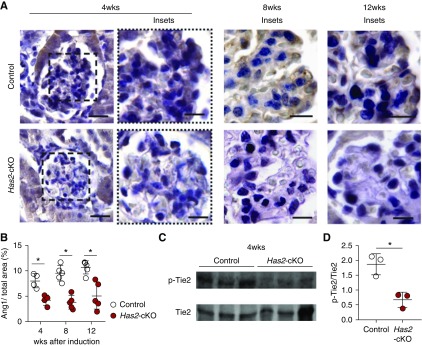
To further corroborate the requirement of endothelial HA as a binding molecule for Ang1 for vascular stabilization, we cultured glomerular ECs exposed to laminar flow, in the presence of Ang1, and silenced HAS2 with a lentiviral shRNA construct, which resulted in approximately 64% reduction of HAS2 protein expression and >95% reduction of surface HA (Figure 4, A, D, H, and I). At the endothelial surface, a significantly reduced presence of Ang1 was observed (Figure 4, A and E), especially at cell-cell contacts (Figure 4A, upper left panel, arrow). This was associated with reduced membrane localization of active β-catenin (Figure 4, B and F), which is required for the linkage between adherens junctions and the actin cytoskeleton. In agreement, more unstable focal adhesion junctions were observed (Figure 4, C and G). Finally, loss of Ang1 binding resulted in reduced activation of its receptor Tie2 and downstream Akt signaling molecules (Figure 4, H, J, and K). We also confirmed in vivo that endothelial loss of has2 resulted in approximately 50% reduction in Ang1 binding to the glomerular endothelium and consequently a decrease of Tie2 signaling in association with glomerular injury and capillary rarefaction (Figure 5, A–D). Loss of myocardial capillaries was also accompanied by reduced Ang1 expression (Supplemental Figure 3, F and G).
Figure 4.
HA angiopoietin 1 clustering stabilizes the endothelial layer. Representative cross-sectional confocal images of primary human glomerular-derived microvascular ECs (hgMVECs), transduced with an empty- (mock) or a HAS2-shRNA–containing lenti-viral construct (HAS2 shRNA) and exposed to a laminar shear stress of 5 dyne/cm2 for 3 days in the presence of 100 ng/ml recombinant human Ang1, stained (A) for HA (red) and Ang1 (green), revealed that especially at cell-cell contact areas (arrow) Ang1 presence is reduced, which is accompanied with (B) reduced membrane-bound active β-catenin (anti-ABC, green) and (C) increased unstable focal adherence junctions (FAJ) as visualized by VE-cadherin (CD144, green) staining. Both in combination with fluorescent-labeled phallotoxin (phalloidin-TRITC, red) to visualize intracellular F-actin and Hoechst 33258 (blue) nuclear stain (scale bar, 20 µm). Silencing of HAS2 results in reduced (D) HA expression, (E) Ang1 binding, (F) membrane-bound β-catenin, and (G) induction of unstable focal adherence junctions (FAJ) (n=5 per group). (H) Western-blot samples stained for HAS2, GAPDH, p-Tie2, Tie2, p-Akt, and Akt. Quantification of (I) HAS2 gene expression, (J) p-Tie2(Y992) over Tie2 expression, and (K) the downstream signaling Akt activation. Values are given as mean±SEM (D–G) or as individual data points (I–K). Differences between control and HAS2-shRNA–treated cells were assessed by paired two-tailed t test; *P<0.05.
Figure 5.
Loss of HA leads to reduction of Ang1/Tie2 signaling in vivo. (A) Representative images of glomerular angiopoietin 1 (Ang1) presence at 4 weeks (left panels) after tamoxifen induction in control (upper panels) and has2-cKO (lower panels) mice (scale bar, 60 µm) with detailed glomerular tufts at 4, 8, and 12 weeks (scale bar, 30 µm). (B) Quantification of glomerular tuft Ang1 (n=5 per group). (C) Western blot samples from kidneys of Has2-cKO and control mice at 4 weeks stained for p-Tie2 and Tie2. (D) Quantification of p-Tie2(Y992) over Tie2 expression. Values are given as mean±SEM or mean±SD and difference was assessed by nonpaired two-tailed t test; *P<0.05.
Discussion
The glycocalyx, together with the proteins that bind to it, constitutes a macromolecular sieve that prevents large molecules from passing through. For macromolecules, such as albumin, that are close in size to that of the spaces between the glycosaminoglycan fibers, steric and hydrodynamic interactions between the macromolecules and the fibers hinder both diffusive and convective transport of the macromolecules through the glycocalyx.25 Indeed, we previously demonstrated that short-term enzymatic removal of HA from the luminal surface of the glomerular vasculature results in albumin sieving.6 Endothelial has2 inactivation also resulted in loss of luminal glomerular glycocalyx dimensions and the occurrence of overt albuminuria. In addition, subendothelial HA was also lost after has2 inactivation and gross structural abnormalities in glomerular capillary structures developed as early as 4 weeks after tamoxifen induction. This was also associated with secondary changes in podocyte structure, making it most likely that disturbed endothelium-podocyte crosstalk was a consequence of loss of HA from the endothelial matrix. Indeed, this study provides evidence that glomerular endothelial glycocalyx HA serves as a critical growth factor signaling platform, where e.g., angiopoietin 1 secretion from podocytes has been shown to be conditional to maintaining the organotypic fenestrated glomerular EC phenotype.20 Indeed, loss of glomerular endothelial fenestration was observed upon loss of glomerular endothelial HA synthesis.
A fundamental characteristic of polysaccharides is their ability to bind to proteins. In particular, diverse sulfation patterns on heparan sulfate are thought to underpin the specificity with which chemokines and signaling proteins bind to heparan sulfate.2 In the case of HA this has been less well established. HA lacks this sulfation patterning and is assumed to be of lesser significance as a scaffold for growth factor binding. However, a few HA-binding matricellular proteins have been identified, including the chemokine chaperone TNF-inducible gene 6 (TSG-6)26,27 and TSG-14 or pentraxin 3 (PTX3), which bind the complement system.28 This study identifies HA as a highly specific and functional binding partner in Ang1-Tie2 receptor signaling. The in vivo study also shows that vascular destabilization with loss of endothelial HA results in organ-specific disease, most notably the kidney, and to a lesser extend the heart and the retina. This further underscores the relevance of the endothelial ECM as an organotypic signaling platform involved in angiocrine signaling and tissue homeostasis.29 It may also point to an increased dependency of these specific vascular beds on Ang1-Tie2 signaling for their function.30 Indeed, glomerular endothelium has a high expression of the Tie2 receptor relative to other vascular beds.17
In this respect, it is of interest to compare these findings with those in mice that have a conditional deletion of Ang1-Tie2 signaling.20 Despite being a prerequisite for normal blood vessel and glomerular development, loss of ang1 activity in adulthood does not immediately result in vascular destabilization. This is in contrast to the spontaneous vascular destabilization in the kidney and to a lesser extend the eye and the heart that we observe with endothelial loss of HA over time. This may point to the fact that the glycocalyx also serves as a molecular scaffold for other growth factor–receptor interactions required for vessel stability, where loss of HA destabilized the glycocalyx as a whole (see Supplemental Figure 1). For example, VEGF receptor activation requires the engagement of specific heparan sulfate domains in the glycocalyx,31 whereas impairment of endothelial Vegf signaling has been associated with glomerular vascular pathology.32 Interestingly, loss of Ang1 signaling was shown to result in enhanced glomerular injury in diabetes,20 further corroborating the potential relevance of the loss of endothelial HA and associated Ang1 binding that we observed in diabetic subjects.
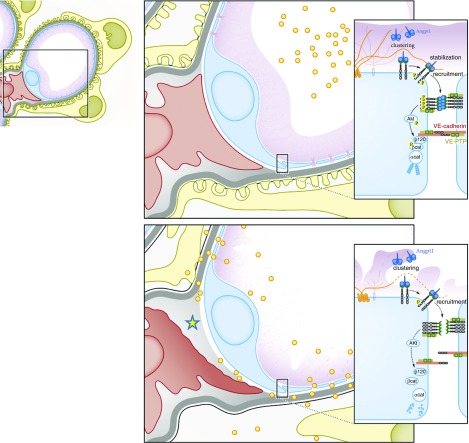
Diabetic microvascular disease is caused by capillary destabilization in susceptible vascular beds such as in the kidney, the retina, and the heart. In the kidney this leads to mesangiolysis, capillary ballooning, and secondary loss of podocytes, resulting in glomerulosclerosis. It is therefore not only of interest that endothelial loss of HA phenocopies this very sequence of events, including the development of microvascular retinal and myocardial disease, but that human diabetic nephropathy is also characterized by progressive endothelial loss of HA, as exemplified in Figure 6. Endothelial HA synthesis and glycocalyx integrity are closely linked to, and dependent upon, cellular glucose metabolism.33,34 It can thus be postulated that disturbances in cellular glycolytic flux, as have recently been associated with the development of diabetic nephropathy,35 may also interfere with endothelial HA synthesis. Changes in endothelial glycolytic flux and glucobiosynthesis have also been shown to be amenable to specific pharmacologic modulation,36 thus constituting endothelial HA synthesis as a new potential therapeutic target.
Figure 6.
Glomerular integrity depends on endothelial surface HA. Schematic overview of loss of endothelial HA results in decreased glomerular filtration barrier function characterized by albuminuria (yellow dots). In addition, there is EC destabilization leading to loss of fenestration, mesangiolysis (yellow asterisk), and secondary podocyte injury. Mechanistically, HA clusters specifically angiopoietin 1 (Ang1) to the TIE2 receptor at the endothelial surface, thus facilitating receptor engagement and downstream stabilization of EC cell-cell contact.
In conclusion, our data provide new fundamental insights into how endothelial function controls glomerular structure and function. Our observations on the loss of glomerular endothelial HA in diabetic nephropathy suggest that this aspect of endothelial function also could serve as a new therapeutic target to restabilize glomerular lesions.
Disclosures
The authors have nothing to declare. Dr. Vink is Chief Scientific Officer of GlycoCheck BV (The Netherlands) and MicroVascular Health Solutions LLC.
Funding
Financial support from the Dutch Kidney Foundation (grants C08.2265 and GLYCOREN consortium C09.03) and the China Scholarship Council grant to Wang (no. 201406170050) are gratefully acknowledged.
Supplementary Material
Acknowledgments
We thank Dr. Yamaguchi (Sanford-Burnham Medical Research Institute, La Jolla, CA) for providing B6.has2flox/flox mice, Iruela-Arispe (Molecular Biology Institute, University of California Los Angeles, Los Angeles, CA) and Dr. Adams (Max Planck Institute for Molecular Biomedicine, University of Münster, Münster, Germany) for providing VE-cadherin(PAC)-CreERT2 mice, and Dr. Kappler (Institut für Biochemie und Molekularbiologie, Universität Bonn, Germany) for providing the neurocan-eGFP construct. We thank Dr. Bajema (Department of Pathology, Leiden University Medical Center, Leiden, The Netherlands) and Dr. Nguyen (Department of Pathology, University Medical Center Utrecht, Utrecht, The Netherlands) for providing postmortem human diabetic nephropathy samples and diabetic nephropathy kidney biopsy samples, respectively. We thank Dr. Koster (Department of Cell and Chemical Biology, Leiden University Medical Center, Leiden, the Netherlands) for facilitating transmission electron microscopy; Dr. Robin and Dr. van Rooij (both Hubrecht Institute, Royal Netherlands Academy of Arts and Sciences, University Medical Center Utrecht, Utrecht, The Netherlands) for facilitating echocardiography equipment use; and Sacha Meldner (Department of Cellular and Molecular Pathology, Germany Cancer Research Institute, Heidelberg, Germany), Jasper van Gemst (Department of Nephrology, Nijmegen Centre for Molecular Life Sciences, Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands), and Angela Koudijs (The Einthoven Laboratory for Vascular Medicine, Department of Internal Medicine, Division of Nephrology, Leiden University Medical Center, Leiden, The Netherlands) for technical assistance.
Dr. van den Berg designed the research study, conducted experiments, acquired data, and wrote the manuscript. Gangqi Wang, Dr. Boels, Cristina Avramut, Erik Jansen, and Wendy Sol conducted experiments, acquired data, and provided helpful comments. Dr. Gröne provided expert knowledge on mouse renal pathology, made experimental and analytic help available, read the manuscript, and provided helpful comments. Dr. Lebrin, Dr. van Zonneveld, Dr. de Koning, and Dr. Vink read the manuscript and provided helpful comments. Dr. Carmeliet read and provided editorial input on the manuscript. Dr. van der Vlag provided reagents, provided helpful comments, and acquired funding. Dr. Rabelink designed the research study, wrote the manuscript, and acquired funding.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019020192/-/DCSupplemental.
Supplemental Figure 1. Generation of conditional endothelial specific hyaluronan synthase 2 knock-out mice.
Supplemental Figure 2. Changes in renal morphology and inflammation markers.
Supplemental Figure 3. Loss of endothelial hyaluronan results in cardiac morphologic- and functional changes and retinal capillary rarefaction.
Supplemental Figure 4. Hyaluronan binds angiopoietin 1.
Supplemental Table 1. Detailed glomerular morphology quantification at 12 weeks after tamoxifen induction.
Supplemental Table 2. Primers used for genotyping.
References
- 1.Dane MJ, van den Berg BM, Lee DH, Boels MG, Tiemeier GL, Avramut MC, et al. : A microscopic view on the renal endothelial glycocalyx. Am J Physiol Renal Physiol 308: F956–F966, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Sarrazin S, Lamanna WC, Esko JD: Heparan sulfate proteoglycans. Cold Spring Harb Perspect Biol 3: 1–33, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bishop JR, Stanford KI, Esko JD: Heparan sulfate proteoglycans and triglyceride-rich lipoprotein metabolism. Curr Opin Lipidol 19: 307–313, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Moretto P, Karousou E, Viola M, Caon I, D’Angelo ML, De Luca G, et al.: Regulation of hyaluronan synthesis in vascular diseases and diabetes. J Diabetes Res 2015: 1–9, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Viola M, Karousou E, D’Angelo ML, Caon I, De Luca G, Passi A, et al.: Regulated hyaluronan synthesis by vascular cells. Int J Cell Biol 2015: 1–8, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dane MJ, van den Berg BM, Avramut MC, Faas FG, van der Vlag J, Rops AL, et al.: Glomerular endothelial surface layer acts as a barrier against albumin filtration. Am J Pathol 182: 1532–1540, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Vigetti D, Viola M, Karousou E, De Luca G, Passi A: Metabolic control of hyaluronan synthases. Matrix biol 35: 8–13, 2014 [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto K, Li Y, Jakuba C, Sugiyama Y, Sayo T, Okuno M, et al.: Conditional inactivation of Has2 reveals a crucial role for hyaluronan in skeletal growth, patterning, chondrocyte maturation and joint formation in the developing limb. Development 136: 2825–2835, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benedito R, Roca C, Sörensen I, Adams S, Gossler A, Fruttiger M, et al.: The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell 137: 1124–1135, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, et al.: A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13: 133–140, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monvoisin A, Alva JA, Hofmann JJ, Zovein AC, Lane TF, Iruela-Arispe ML: VE-cadherin-CreERT2 transgenic mouse: A model for inducible recombination in the endothelium. Dev Dyn 235: 3413–3422, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Nakayama M, Pitulescu ME, Schmidt TS, Bochenek ML, Sakakibara A, et al.: Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature 465: 483–486, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Zhang H, Baader SL, Sixt M, Kappler J, Rauch U: Neurocan-GFP fusion protein: A new approach to detect hyaluronan on tissue sections and living cells. J Histochem Cytochem 52: 915–922, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Nawroth PP, Isermann B: Mechanisms of diabetic nephropathy–old buddies and newcomers part 2. Exp Clin Endocrinol Diabetes 118: 667–672, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Nawroth PP, Isermann B: Mechanisms of diabetic nephropathy--old buddies and newcomers part 1. Exp Clin Endocrinol Diabetes 118: 571–576, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Campochiaro PA, Peters KG: Targeting Tie2 for treatment of diabetic retinopathy and diabetic macular edema. Curr Diab Rep 16: 126, 2016 [DOI] [PubMed] [Google Scholar]
- 17.Wong AL, Haroon ZA, Werner S, Dewhirst MW, Greenberg CS, Peters KG: Tie2 expression and phosphorylation in angiogenic and quiescent adult tissues. Circ Res 81: 567–574, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Augustin HG, Koh GY, Thurston G, Alitalo K: Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol 10: 165–177, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Thurston G, Rudge JS, Ioffe E, Zhou H, Ross L, Croll SD, et al.: Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med 6: 460–463, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Jeansson M, Gawlik A, Anderson G, Li C, Kerjaschki D, Henkelman M, et al.: Angiopoietin-1 is essential in mouse vasculature during development and in response to injury. J Clin Invest 121: 2278–2289, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riley CM, Fuegy PW, Firpo MA, Shu XZ, Prestwich GD, Peattie RA: Stimulation of in vivo angiogenesis using dual growth factor-loaded crosslinked glycosaminoglycan hydrogels. Biomaterials 27: 5935–5943, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu Y, Yu Q: Angiopoietin-1, unlike angiopoietin-2, is incorporated into the extracellular matrix via its linker peptide region. J Biol Chem 276: 34990–34998, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Kohda D, Morton CJ, Parkar AA, Hatanaka H, Inagaki FM, Campbell ID, et al.: Solution structure of the link module: A hyaluronan-binding domain involved in extracellular matrix stability and cell migration. Cell 86: 767–775, 1996 [DOI] [PubMed] [Google Scholar]
- 24.Favreau AJ, Faller CE, Guvench O: CD44 receptor unfolding enhances binding by freeing basic amino acids to contact carbohydrate ligand. Biophys J 105: 1217–1226, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeansson M, Haraldsson B: Glomerular size and charge selectivity in the mouse after exposure to glucosaminoglycan-degrading enzymes. J Am Soc Nephrol 14: 1756–1765, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Higman VA, Briggs DC, Mahoney DJ, Blundell CD, Sattelle BM, Dyer DP, et al.: A refined model for the TSG-6 link module in complex with hyaluronan: Use of defined oligosaccharides to probe structure and function. J Biol Chem 289: 5619–5634, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dyer DP, Thomson JM, Hermant A, Jowitt TA, Handel TM, Proudfoot AE, et al.: TSG-6 inhibits neutrophil migration via direct interaction with the chemokine CXCL8. J Immunol 192: 2177–2185, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wisniewski HG, Vilcek J: Cytokine-induced gene expression at the crossroads of innate immunity, inflammation and fertility: TSG-6 and PTX3/TSG-14. Cytokine Growth Factor Rev 15: 129–146, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Witjas FMR, van den Berg BM, van den Berg CW, Engelse MA, Rabelink TJ: Concise review: The endothelial cell extracellular matrix regulates tissue homeostasis and repair. Stem Cells Transl Med 8: 375–382, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nolan DJ, Ginsberg M, Israely E, Palikuqi B, Poulos MG, James D, et al.: Molecular signatures of tissue-specific microvascular endothelial cell heterogeneity in organ maintenance and regeneration. Dev Cell 26: 204–219, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grünewald FS, Prota AE, Giese A, Ballmer-Hofer K: Structure-function analysis of VEGF receptor activation and the role of coreceptors in angiogenic signaling. Biochim Biophys Acta 1804: 567–580, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J, et al.: VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med 358: 1129–1136, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vigetti D, Deleonibus S, Moretto P, Karousou E, Viola M, Bartolini B, et al.: Role of UDP-N-acetylglucosamine (GlcNAc) and O-GlcNAcylation of hyaluronan synthase 2 in the control of chondroitin sulfate and hyaluronan synthesis. J Biol Chem 287: 35544–35555, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeAngelis PL: Glycosaminoglycan polysaccharide biosynthesis and production: Today and tomorrow. Appl Microbiol Biotechnol 94: 295–305, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Qi W, Keenan HA, Li Q, Ishikado A, Kannt A, Sadowski T, et al.: Pyruvate kinase M2 activation may protect against the progression of diabetic glomerular pathology and mitochondrial dysfunction. Nat Med 23: 753–762, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cantelmo AR, Conradi LC, Brajic A, Goveia J, Kalucka J, Pircher A, et al.: Inhibition of the glycolytic activator PFKFB3 in endothelium induces tumor vessel normalization, impairs metastasis, and improves chemotherapy. Cancer Cell 30: 968–985, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.