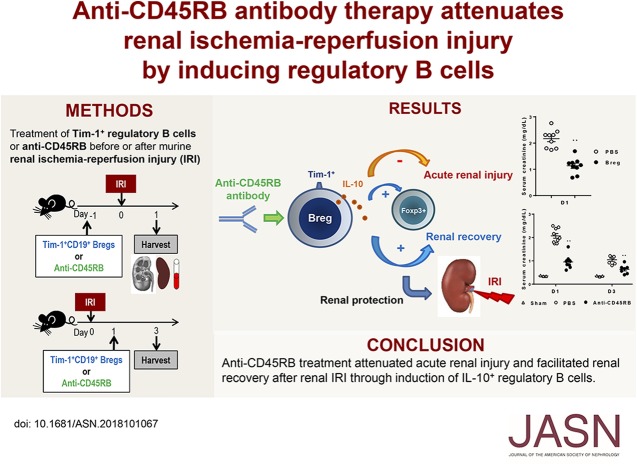
Significance Statement
The role of B cells in renal ischemia-reperfusion injury (IRI) remains controversial, and the role of the recently discovered B cell subset, regulatory B cells, in renal IRI has not yet been studied. The authors demonstrated in mouse models that regulatory B cells attenuated renal IRI. They also found that treatment with anti-CD45RB with or without anti–Tim-1, which induces regulatory B cells and suppresses T cells, attenuated acute renal injury when given before IRI and facilitated renal recovery when given after IRI. The main mechanism underlying the anti-CD45RB–mediated reno-protective effects was induction of IL-10+ regulatory B cells. These findings provide insight into the role of regulatory B cells in renal IRI and suggest that anti-CD45RB may be a potential therapeutic strategy in renal IRI.
Keywords: ischemia-reperfusion injury, regulatory b cell, anti-CD45RB, anti-Tim-1
Visual Abstract
Abstract
Background
Regulatory B cells are a newly discovered B cell subset that suppresses immune responses. Recent studies found that both anti-CD45RB and anti–Tim-1 treatments regulate immune responses by inducing regulatory B cells; however, the role of these cells in renal ischemia-reperfusion injury (IRI) is unknown.
Methods
Using mouse models, including T cell–deficient (RAG1 knockout and TCRα knockout) mice and B cell–deficient (μMT) mice, we investigated the effects of regulatory B cells and anti-CD45RB on IRI and the mechanisms underlying these effects.
Results
Adoptive transfer of regulatory B cells before or after IRI attenuated renal IRI. Anti-CD45RB treatment with or without anti–Tim-1 before IRI increased renal infiltration of CD19+Tim-1+ regulatory B and regulatory T cells. Anti-CD45RB decreased serum creatinine levels, pathologic injury score, tubular apoptosis, and proinflammatory cytokines levels, whereas IL-10 levels increased. Following IRI, anti-CD45RB with or without anti–Tim-1 also induced regulatory B cells, improving renal function and tubular regeneration. In RAG1 knockout mice with B cell transfer, TCRα knockout mice, and wild-type mice with T cell depletion, anti-CD45RB increased regulatory B cells and attenuated IRI. However, anti-CD45RB did not attenuate IRI in RAG1 knockout mice with T cell transfer or μMT mice and induced only mild improvement in wild-type mice with B cell depletion. Furthermore, B cell–deficient mice receiving B cells from IL-10 knockout mice (but not from wild-type mice) did not show renal protection against IRI when treated with anti-CD45RB.
Conclusions
Anti-CD45RB treatment attenuated acute renal injury and facilitated renal recovery after IRI through induction of IL-10+ regulatory B cells, pointing to anti-CD45RB as a potential therapeutic strategy in renal IRI.
Renal ischemia-reperfusion injury (IRI) is an acute inflammatory disease, where both innate and adaptive immune cells are involved.1 Roles of B cells remain controversial in renal IRI and the role of regulatory B cells (Bregs) has not yet been studied. On the one hand, depletion of peritoneal B-1 cells or B cell deficiency in μMT mice attenuated acute renal injury.2,3 μMT mice also showed increased tubular regeneration in the repair phase after IRI compared with wild-type (WT) mice and B cells limit repair processes after IRI.4 On the other hand, the lack of B cells in μMT mice caused more severe injury and worse renal function after IRI than those observed in WT mice.2 In parallel, IL-10–producing B cells have been shown to protect against cerebral IRI.5 These conflicting results suggest that roles of B cells in renal IRI may be variable according to the specific B cell subsets and specific phases of IRI, and there is the possibility of a regulatory role of Bregs in IRI.
Bregs as well as regulatory T cells (Tregs) have recently been proposed to suppress inflammation in autoimmune and alloimmune responses.6–9 Bregs also suppress T helper 1 (Th1), Th17, and cytotoxic CD8+ T cells, along with inducing Tregs via the production of IL-10, TGF-β, and IL-35 or via a contact-dependent mechanism.8,10 They also suppress antigen presentation and proinflammatory cytokine secretion by innate immune cells such as monocytes, dendritic cells, neutrophils, and natural killer cells.8,10 Although multiple subsets of Bregs have been reported, CD5+CD1d+ B10 cells have been regarded as representative of IL-10–producing Bregs.8,10,11 However, CD5+CD1d+ B10 cells account for about 10% of IL-10–secreting B cells and <3% of splenic B cells.12 Instead, recent studies have suggested that Tim-1+ B cells are the major population of IL-10–producing Bregs.9 Tim-1 is a member of the T cell Ig and mucin domain family. Approximately 8% of splenic B cells and 70% of IL-10–producing B cells express Tim-1. Tim-1 signaling in B cells plays an important role in the induction and maintenance of Bregs in response to apoptotic cells.13 In parallel with the expression and role of Tim-1 in Bregs, a low-affinity, inhibitory anti–Tim-1 antibody (RMT1-10) induces Bregs.9
CD45RB is an isoform of CD45, a protein tyrosine phosphatase.14 Anti-CD45RB suppressed cardiac allograft rejection through B cell–dependent mechanisms.15 Furthermore, both anti-CD45RB and anti–Tim-1 treatments induced Bregs and their combination synergistically suppressed islet allograft rejection via Breg-dependent mechanisms.16
However, anti–Tim-1 treatment attenuated experimental autoimmune encephalitis, experimental crescentic GN, and allograft rejection by suppressing Th1 and Th17 effector T cells while inducing Tregs.17–19 Anti-CD45RB also induced Tregs while suppressing Th1 and Th17 effector T cells.20,21 Therefore, it is unclear how anti–Tim-1 or anti-CD45RB treatment suppresses immune responses—in other words, whether T-dependent or B-dependent mechanisms are dominant in their immunomodulatory effects.
Here, we investigated the role of Bregs in renal IRI and determined whether anti-CD45RB treatment, alone or in combination with anti–Tim-1, attenuated IRI. Moreover, we investigated the main mechanisms underlying the beneficial effects of anti-CD45RB treatment on IRI.
Methods
Mice
Male mice, 6–8 weeks old, were used for all experiments. WT (CD45.2) C57BL/6J mice were purchased from Koatech (Pyeongtaek, Korea). CD45.1 C57BL/6J (B6.SJL-PtprcaPep3b/BoyJ), recombination activating gene 1 (RAG1) knockout (KO) (B6.129S7-Rag1tm1Mom/J), μMT (B6.129S2-Ighmtm1Cgn/J), T cell receptor α chain (TCRα) KO (B6.129S2-Tcratm1Mom/J), IL-10 KO (B6.129P2-Il10tm1Cgn/J), and indoleamine-pyrrole 2,3-dioxygenase (IDO) KO mice were purchased from the Jackson Laboratory (Bar Harbor, ME). All experiments were conducted under protocols approved by the Institutional Animal Care and Use Committee of Seoul National University Hospital (IACUC 17-0222-S1A0).
IRI Model and Assessment of Renal Function
Renal IRI was induced by clamping the bilateral renal pedicle for 27 minutes. Serum creatinine and BUN levels were determined using QuantiChrom creatinine and urea assay kits (BioAssay Systems, Hayward, CA), respectively.
Reagents
Anti-CD45RB (MB23G2) and anti–Tim-1 (RMT1-10) antibodies were purchased from BioXCell (West Lebanon, NH). Anti-CD45RB antibody (10 μg) with or without anti–Tim-1 antibody (5 μg) was administrated intraperitoneally 1 day before IRI or 1 day after IRI. In the T cell–depletion experiments, both depleting anti-CD4 (500 μg, GK1.5; BioXCell) and anti-CD8 (500 μg, YTS 169.4; BioXCell) antibodies were intraperitoneally administered 3 days before IRI.22,23 Depleting anti-CD20 antibody (200 μg, 5D2; Genentech, CA) was intraperitoneally administered 7 days before IRI for depletion of B cells.24 Depleting anti-CD25 (300 μg, PC-61.5.3; BioXCell) was intraperitoneally administered a day before and on the day of IRI for the depletion of Tregs. Anti–TGF-β (200 μg, 1D11.16.8; BioXCell) was intraperitoneally administered a day before IRI to neutralize TGF-β.
Adoptive Transfer of B and T Cells
T and B cells were isolated from splenocytes and were purified using MojoSort CD3 T Cell Isolation Kit and MojoSort Pan B Cell Isolation Kit (Biolegend, San Diego, CA), respectively. Purity was >99%. T cells (2×107) or B cells (2×107) were intravenously transferred into RAG1 KO mice 14 days before IRI. FACSAria II (BD Biosciences, San Diego, CA) was used for sorting Tim-1+CD19+ Bregs from B cells that were isolated from CD45.1 mice using the MojoSort Pan B Cell Isolation Kit. Purified Bregs (5×105) were intravenously transferred into CD45.2 mice 1 day before or after IRI.
Flow Cytometric Analysis
Splenocytes and renal leukocytes were prepared as previously described.25 Isolated cells were treated with anti-CD16/32 antibody (BD Biosciences) for blocking Fc receptors before staining with the following anti-mouse antibodies: anti-CD45 (30-F11), anti-CD44 (1M7), 7-aminoactinomycin D, anti-CD19 (1D3), anti–IL-10 (JES5-16E3), rat IgG2b isotype control (BD Biosciences); anti–Gr-1 (RB6-8C5), anti-F4/80 (BM8), anti-CD4 (RM4-5), anti-Foxp3 (FJK-16S), anti-FasL (Fas ligand, MFL3), anti-EBi3 (DNT27), anti-rat IgG2a secondary antibody (Thermo Fisher Scientific, Waltham, MA); anti-CD69 (H1.2F3), anti–Tim-1 (RMT1-4), anti-CD45.1 (A20), anti-IDO (2E2), anti–PD-L1 (programmed death–ligand 1, 10F.9G2), anti-latency associated peptide (TW7-16B4; Biolegend); and anti–IL-12 (p35; R&D System, Minneapolis, MN). Flow cytometric analysis was performed using a FACSCanto II flow cytometer (BD Bioscience) and gating strategies in flow cytometric analysis are shown in Supplemental Figures 1 and 2A. For intracellular cytokine staining, B cells were incubated with LPS (10 μg/ml; Sigma-Aldrich, St. Louis, MO), PMA (50 ng/ml; Sigma-Aldrich), ionomycin (500 ng/ml; Sigma-Aldrich), and GolgiStop (1 μl/ml; BD Biosciences) for 5 hours, as described previously.9
Real-Time PCR
Renal tissue was homogenized using TRIzol reagent (Thermo Fisher Scientific) and RNA was reverse-transcribed into cDNA using the High Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). Each reaction mixture consisted of 2× SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA) and 10 pmol/μl of corresponding primers (Supplemental Table 1). Real-time PCR was performed using a QuantStudio 3 Real-Time PCR Instrument (Thermo Fisher Scientific).
Histologic Analysis
Kidney tissues were fixed in 4% paraformaldehyde and paraffin-embedded sections were used for histologic studies. Tubular injury score was assessed in periodic acid–Schiff–stained kidney sections as previously described.26 Briefly, kidney injury was assessed by two independent researchers in a blinded manner based on the morphologic criteria such as tubular cell necrosis, cast formation, and loss of tubular brush borders. Overall tubular injury was semiquantitatively scored by calculating the percentage of affected tubules (0, unaffected; 1, 1%–25%; 2, 26%–50%; 3, 51%–75%; 4, 76%–100%) in at least three randomly selected, high-power fields in each section. Terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling staining (Roche Diagnostics, Risch-Rotkreuz, Switzerland) and Ki67 staining (Abcam, Cambridge, UK) were used to assess renal tubular apoptosis and regeneration, respectively.
For immunofluorescence staining, kidney cryosections (4 μm) were stained with rat anti-mouse CD19 (Thermo Fisher Scientific) and rabbit anti-mouse Tim-1 (Abcam) for 4 hours, and then incubated with goat anti-rat IgG–Alexa Flour 488 and goat anti-rabbit IgG–Alexa Flour 568 (Thermo Fisher Scientific) for 1 hour at room temperature. Next, they were treated with 4′,6-diamidino-2-phenylindole (Thermo Fisher Scientific) for 2 minutes.
Statistical Analysis
All data are shown as the mean±SEM. Continuous variables were analyzed using two-tailed t test. P values <0.05 were considered statistically significant. All analyses were performed using GraphPad Prism software 7.0 (La Jolla, CA).
Results
Bregs Attenuated Acute Renal Injury after IRI
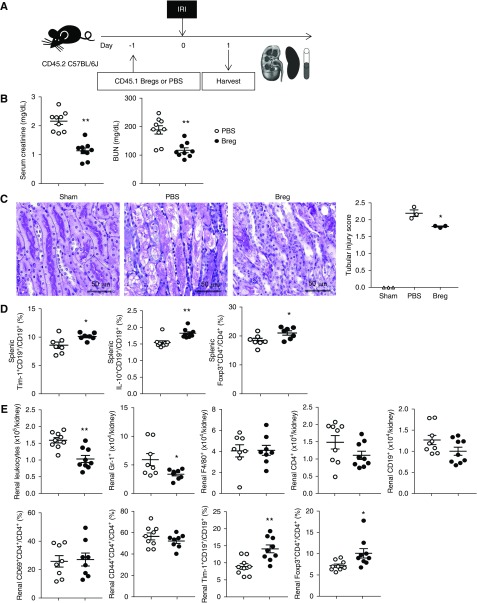
When Tim-1+CD19+ Bregs sorted from CD45.1 mice were transferred to CD45.2 mice a day before IRI, infiltration of CD45.1+ Bregs was found in both the spleen and kidneys (Supplemental Figure 2). Both serum creatinine and BUN levels at 1 day after IRI were significantly lower in the Breg group than those in the PBS group (Figure 1, A and B). Tubular injury was also attenuated by Breg transfer (Figure 1C). In the spleen, proportions of Tim-1+CD19+ cells and of IL-10+CD19+ cells among CD19+ B cells were increased in the Breg group (Figure 1D). Transfer of Bregs increased the proportion of splenic Foxp3+CD4+ Tregs among CD4+ T cells (Figure 1D). Leukocyte infiltration into the kidney was reduced in the Breg group compared with that in the PBS control group (Figure 1E). Transfer of Bregs suppressed the infiltration of Gr-1+ neutrophils into renal tissue. Although the infiltration of activated CD4+ T cells (CD69+CD4+ and CD44+CD4+), as well as of total CD4+ T cells, was not changed, renal Tregs were increased in the Breg group (Figure 1E). When we depleted Tregs after Breg transfer, Treg induction was ameliorated in both the spleen and kidneys (Supplemental Figure 3, A and C). However, either renal functional improvement (Supplemental Figure 3B) or Breg increase (Supplemental Figure 3D) by Breg transfer were not remarkably attenuated. Next, renal B cell infiltration showed a decreasing trend in the Breg group compared with that in the PBS group, and renal Tim-1+ Bregs were significantly increased in the Breg group (Figure 1E). Taken together, Breg transfer before IRI increased the renal infiltration of both Bregs and Tregs and attenuated acute renal injury after IRI.
Figure 1.
Pre-IRI therapy with Bregs attenuated acute renal injury after renal IRI. (A) Tim-1+CD19+ Bregs sorted from CD45.1 mice were transferred to CD45.2 mice 1 day before IRI; mice were harvested 1 day after IRI. (B) Levels of serum creatinine and BUN at 1 day after IRI. (C) Renal tubular injury scores from periodic acid–Schiff staining on day 1. Original magnification, ×200. (D) Flow cytometry analysis of splenic Bregs and Tregs. (E) Flow cytometry analysis of renal leukocytes. Results were expressed as dot plots with the mean±SEM. n=3–9. *P<0.05, **P<0.01, compared with the PBS group. Bregs, regulatory B cells; IRI, ischemia-reperfusion injury; Tregs, regulatory T cells.
Bregs Facilitated Renal Recovery after IRI
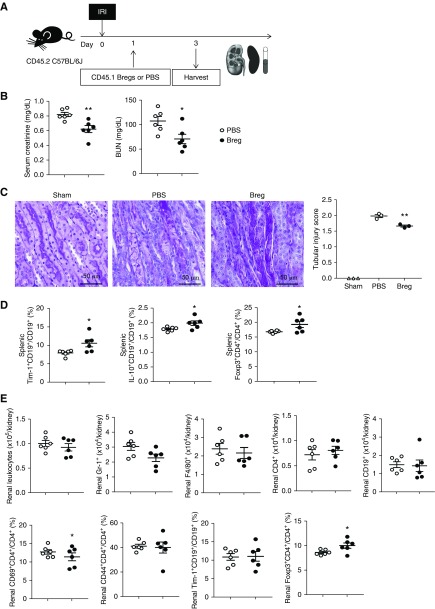
Next, we investigated the therapeutic effects of Breg transfer after IRI. When CD45.1+Tim-1+CD19+ Bregs were transferred to CD45.2 mice 1 day after IRI, both serum creatinine and BUN levels on day 3 were significantly lower in the Breg group than in the PBS control group (Figure 2, A and B). Tubular injury score was also lower in the Breg group (Figure 2C). Both Tim-1+CD19+ B cells and IL-10+CD19+ B cells were increased in the Breg group (Figure 2D). Foxp3+CD4+ Tregs were also increased (Figure 2D). Breg transfer after IRI did not induce significant changes in renal leukocyte infiltration on day 3 after IRI, except for in the cases of Foxp3+CD4+ Tregs (Figure 2E). Overall, Breg transfer after IRI facilitated renal recovery after IRI.
Figure 2.
Post-IRI therapy with Bregs facilitated renal recovery after renal IRI. (A) Tim-1+CD19+ Bregs sorted from CD45.1 mice were injected 1 day after IRI; mice were harvested 3 days after IRI. (B) Levels of serum creatinine and BUN at 1 day after IRI. (C) Renal tubular injury scores from periodic acid–Schiff staining on day 1. Original magnification, ×200. (D) Flow cytometry analysis of splenic Bregs and Tregs. (E) Flow cytometry analysis of renal leukocytes. Results were expressed as dot plots with the mean±SEM. n=3–6. *P<0.05, **P<0.01, compared with the PBS group. Bregs, regulatory B cells; IRI, ischemia-reperfusion injury; Tregs, regulatory T cells.
Treatment with Anti-CD45RB with or without Anti–Tim-1 Antibody before IRI Attenuated Acute Renal Injury after IRI
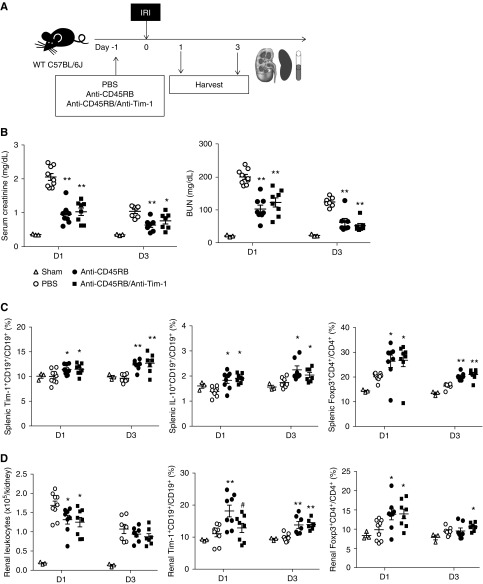
To investigate the effects of anti-CD45RB and anti–Tim-1 on renal IRI, anti-CD45RB with or without anti–Tim-1 antibody was administered to WT mice 1 day before IRI (Figure 3A). Levels of serum creatinine and BUN on days 1 and 3 after IRI were lower in the anti-CD45RB group than in the PBS control group; however, the addition of anti–Tim-1 to anti-CD45RB did not improve the beneficial effects of anti-CD45RB in IRI (Figure 3B). When we compared beneficial effects of anti-CD45RB monotherapy and anti–Tim-1 monotherapy on IRI, anti-CD45RB treatment showed better effects than anti–Tim-1 treatment (Supplemental Figure 4), and we therefore used anti-CD45RB–based therapy in the subsequent experiments. Anti-CD45RB with or without anti–Tim-1 treatment increased splenic Tim-1+CD19+ B cells, IL-10+CD19+ B cells, and Foxp3+CD4+ Tregs on days 1 and 3 after IRI (Figure 3C, Supplemental Figure 5, A and B). Renal leukocyte infiltration on day 1 was suppressed by anti-CD45RB treatment (Figure 3D). Renal infiltration of Tim-1+CD19+ B cells, IL-10+CD19+ B cells, and Foxp3+CD4+ Tregs (Figure 3D, Supplemental Figure 5, A and B) on days 1 and 3 was increased by anti-CD45RB with or without anti–Tim-1 treatment. Tim-1+CD19+ Bregs were mainly found in renal medulla on day 1 after IRI (Supplemental Figure 6).
Figure 3.
Anti-CD45RB with or without anti–Tim-1 treatment before IRI attenuated acute renal injury after renal IRI. (A) Anti-CD45RB, anti-CD45RB with anti–Tim-1 or PBS was administered to WT mice 1 day before IRI; mice were harvested on days 1 and 3 after IRI. (B) Levels of serum creatinine and BUN after IRI. (C) Flow cytometry analysis of splenic Bregs and Tregs after IRI. (D) Flow cytometry analysis of renal leukocytes, Bregs, and Tregs after IRI. Results were expressed as dot plots with the mean±SEM. n=3–9. *P<0.05, **P<0.01, compared with the PBS group. #P<0.05, anti-CD45RB group compared with the anti-CD45RB/anti–Tim-1 group. Bregs, regulatory B cells; IRI, ischemia-reperfusion injury; Tregs, regulatory T cells; WT, wild type.
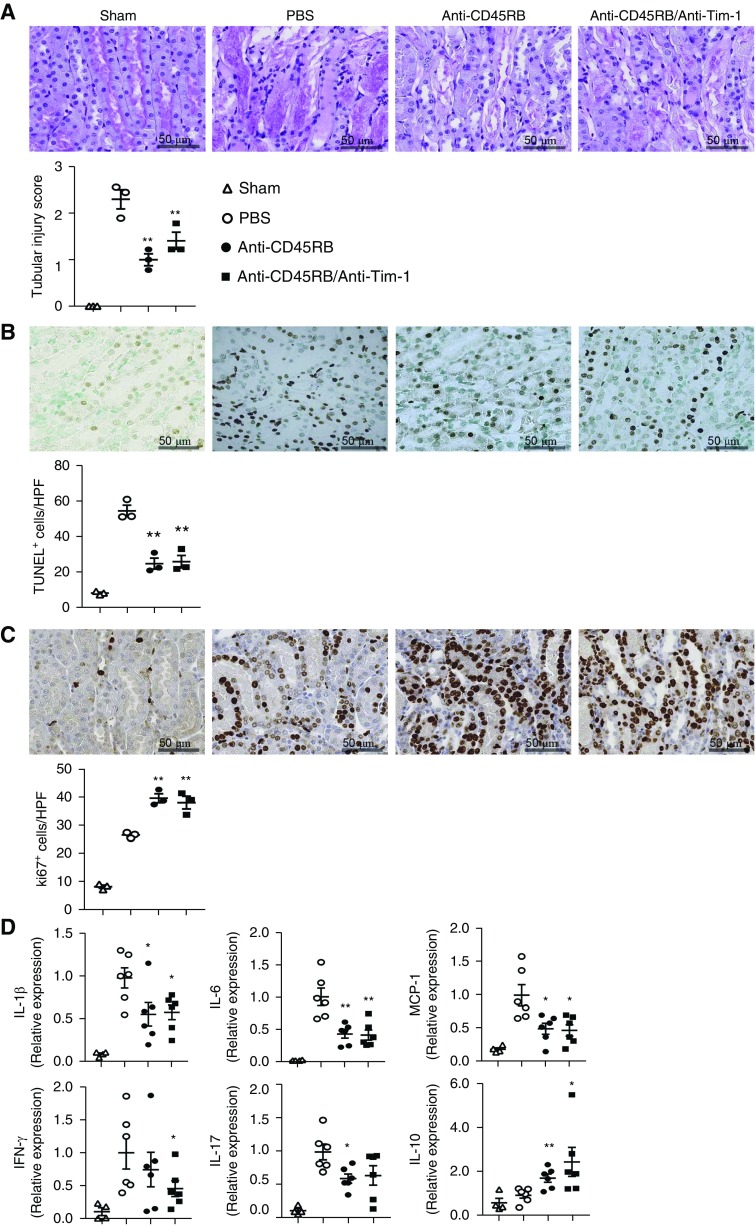
Renal tubular injury on day 1 after IRI was attenuated by anti-CD45RB with or without anti–Tim-1 treatment (Figure 4A). Anti-CD45RB treatment decreased renal tubular apoptosis (positive staining in the terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling assay) on day 1 (Figure 4B) and increased renal tubular regeneration (Ki67+) on day 3 after IRI (Figure 4C). Renal expression of proinflammatory cytokines such as IL-1β, IL-6, monocyte chemoattractant protein-1, IFN-γ, and IL-17, was reduced; whereas renal expression of IL-10 was increased by anti-CD45RB with or without anti–Tim-1 treatment (Figure 4D). Taken together, treatment with anti-CD45RB with or without anti–Tim-1 antibody before IRI increased the renal infiltration of Bregs and Tregs, suppressed the renal expression of proinflammatory cytokines, and attenuated acute renal tissue injury after IRI.
Figure 4.
Pre-IRI treatment with anti-CD45RB and anti-Tim-1 improved renal histologic injury and suppressed renal expression of proinflammatory cytokines. (A) Renal tubular injury scores from periodic acid–Schiff staining on day 1. Original magnification, ×200. (B) Renal tubular apoptosis measured by TUNEL staining on day 1. Original magnification, ×200. (C) Renal tubular regeneration measured by Ki67 staining on day 3. Original magnification, ×200. (D) Renal cytokine expression measured by quantitative real-time PCR on day 1. Results were expressed as dot plots with the mean±SEM. n=3 (A–C) or 6 (D). *P<0.05, **P<0.01, compared with the PBS group. HPF, high-power field; IRI, ischemia-reperfusion injury; MCP-1, monocyte chemoattractant protein-1; PAS, periodic acid–Schiff; TUNEL, terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling.
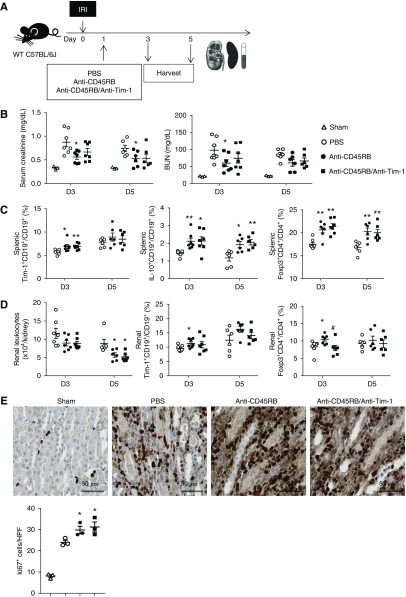
Treatment with Anti-CD45RB with or without Anti–Tim-1 Antibody after IRI Facilitated Renal Recovery after IRI
In parallel with the therapeutic effects of Bregs on IRI, we investigated whether treatment with anti-CD45RB with or without anti–Tim-1 antibody 1 day after IRI facilitated renal recovery after IRI (Figure 5A). Administration of anti-CD45RB on day 1 after IRI significantly reduced levels of serum creatinine and BUN on day 3 after IRI (Figure 5B). Proportions of splenic IL-10+ Bregs and Tregs on days 3 and 5 after IRI were higher both in the anti-CD45RB group and in the anti-CD45RB/anti–Tim-1 group compared with those in the PBS control group (Figure 5C). Anti-CD45RB treatment suppressed renal leukocyte infiltration on day 5, but increased infiltration of Bregs and Tregs on day 3 (Figure 5D). Furthermore, renal tubular regeneration (Ki67+) on day 5 after IRI was increased by anti-CD45RB with or without anti–Tim-1 treatment (Figure 5E). Overall, treatment with anti-CD45RB with or without anti–Tim-1 antibody at 1 day after IRI increased the renal infiltration of Bregs as well as Tregs and facilitated renal recovery after IRI.
Figure 5.
Anti-CD45RB with or without anti–Tim-1 treatment after IRI-facilitated renal recovery after renal IRI. (A) Anti-CD45RB, anti-CD45RB with anti–Tim-1, or PBS was administered to WT mice 1 day after IRI; mice were harvested on days 3 and 5 after IRI. (B) Levels of serum creatinine and BUN after IRI. (C) Flow cytometry analysis of splenic Bregs and Tregs after IRI. (D) Flow cytometry analysis of renal leukocytes, Bregs, and Tregs after IRI. (E) Renal tubular regeneration measured by Ki67 staining on day 5. Original magnification, ×200. Results were expressed as dot plots with the mean±SEM. n=6–7 (A–D) or 3 (E). *P<0.05, **P<0.01, compared with the PBS group. #P<0.05, anti-CD45RB group compared with the anti-CD45RB/anti–Tim-1 group. Bregs, regulatory B cells; HPF, high-power field; IRI, ischemia-reperfusion injury; Tregs, regulatory T cells; WT, wild type.
T Cells Were Dispensable in the Reno-Protective Effects of Anti-CD45RB with or without Anti–Tim-1 Treatment against IRI
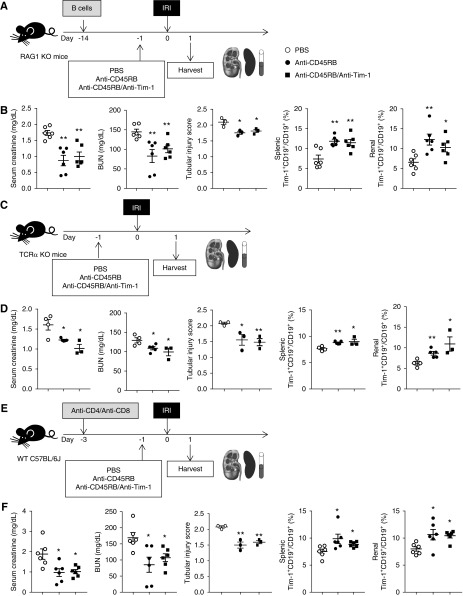
To clarify the mechanism of anti-CD45RB/anti–Tim-1–mediated beneficial effects on renal IRI, we used T cell–deficient mouse models, such as RAG1 KO mice with B cell transfer (Figure 6A), TCRα KO mice (Figure 6C), and WT mice with T cell depletion (Figure 6E, Supplemental Figure 5C). In RAG1 KO mice with B cell transfer, serum creatinine and BUN levels as well as tubular injury score were significantly lower in both the anti-CD45RB group and the anti-CD45RB/anti–Tim-1 group than in the PBS control group (Figure 6B). Anti-CD45RB with or without anti–Tim-1 treatment increased Tim-1+ Bregs in both the spleen and kidney in these mice, similarly as in WT mice (Figure 6B). Anti-CD45RB treatment led to the same results in TCRα KO mice (Figure 6D) and WT mice with T cell depletion (Figure 6F), as were observed in RAG1 KO mice with B cell transfer.
Figure 6.
T cells were dispensable in the reno-protective effects of anti-CD45RB with or without anti–Tim-1 treatment against IRI. (A) B cells from WT mice were transferred to RAG1 KO mice 2 weeks before IRI. Anti-CD45RB, anti-CD45RB with anti–Tim-1, or PBS was administered to RAG1 KO mice with transferred B cells 1 day before IRI; mice were harvested on 1 day after IRI. (B) Levels of serum creatinine and BUN. Flow cytometry analysis of splenic and renal Bregs in the RAG1 KO model. (C) Anti-CD45RB, anti-CD45RB with anti–Tim-1, or PBS was administered to TCRα KO mice 1 day before IRI; mice were harvested 1 day after IRI. (D) Levels of serum creatinine and BUN. Flow cytometry analysis of splenic and renal Bregs in TCRα KO mice. (E) Both anti-CD4 and anti-CD8 were administered to WT mice to deplete T cells 3 days before IRI. Anti-CD45RB, anti-CD45RB with anti–Tim-1, or PBS was administered 1 day before IRI; mice were harvested 1 day after IRI. (F) Levels of serum creatinine and BUN. Flow cytometry analysis of splenic and renal Bregs in WT mice with T cell depletion. Results were expressed as dot plots with the mean±SEM. n=6. *P<0.05, **P<0.01, compared with the PBS group. Bregs, regulatory B cells; IRI, ischemia-reperfusion injury; KO, knockout; RAG1, recombination activating gene 1; WT, wild type.
B Cells Were Indispensable in the Reno-Protective Effects of Anti-CD45RB with or without Anti–Tim-1 Treatment against IRI
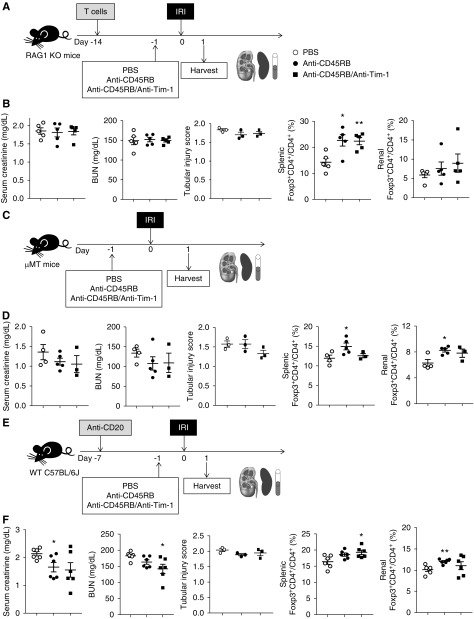
To further elucidate the role of B cells in the anti-CD45RB–mediated beneficial effects on renal IRI, we investigated whether anti-CD45RB with or without anti–Tim-1 treatment showed similar protective effects against IRI in B cell–deficient mouse models. In both RAG1 KO mice with T cell transfer (Figure 7A) and μMT mice (Figure 7C), anti-CD45RB with or without anti–Tim-1 treatment decreased neither serum creatinine nor BUN levels after IRI (Figure 7, B and D), although treatment increased Tregs in the spleens and kidneys (Figure 7, B and D). There was no significant change in tubular injury score upon anti-CD45RB treatment (Figure 7, B, D, and F). In WT mice with B cell depletion (Figure 7E, Supplemental Figure 5D), serum creatinine and BUN levels decreased slightly in the anti-CD45RB and anti-CD45RB/anti–Tim-1 treatment groups (Figure 7F). Tregs were also increased in both the spleens and kidneys (Figure 7F). These results suggested that beneficial effects of anti-CD45RB on renal IRI were mainly mediated by B cells rather than T cells.
Figure 7.
B cells were indispensable in the reno-protective effects of anti-CD45RB with or without anti–Tim-1 treatment against IRI. (A) T cells from WT mice were transferred to RAG1 KO mice 2 weeks before IRI. Anti-CD45RB, anti-CD45RB with anti–Tim-1, or PBS was administered to RAG1 KO mice with transferred T cells 1 day before IRI; mice were harvested on 1 day after IRI. (B) Levels of serum creatinine and BUN. Flow cytometry analysis of splenic and renal Tregs in RAG1 KO mice. (C) Anti-CD45RB, anti-CD45RB with anti–Tim-1, or PBS was administered to μMT mice 1 day before IRI; mice were harvested 1 day after IRI. (D) Levels of serum creatinine and BUN. Flow cytometry analysis of splenic and renal Tregs in μMT mice. (E) Anti-CD20 was administered to WT mice to deplete B cells 1 week before IRI. Anti-CD45RB, anti-CD45RB with anti–Tim-1, or PBS were administered 1 day before IRI; mice were harvested 1 day after IRI. (F) Levels of serum creatinine and BUN. Flow cytometry analysis of splenic and renal Tregs in WT mice with B cell depletion. Results were expressed as dot plots with the mean±SEM. n=6. *P<0.05, **P<0.01, compared with the PBS group. IRI, ischemia-reperfusion injury; KO, knockout; RAG1, recombination activating gene 1; Tregs, regulatory T cells; WT, wild type.
Reno-Protective Effects of Anti-CD45RB Treatment against IRI Were Dependent on IL-10+ Bregs
Finally, B cells isolated from IL-10 KO mice were transferred to RAG1 KO mice to investigate the role of IL-10+ Bregs in the anti-CD45RB–mediated beneficial effects on renal IRI (Figure 8A). Anti-CD45RB treatment did not improve renal function on day 1 after IRI in RAG1 KO mice with IL-10–deficient B cell transfer (Figure 8B), in contrast to the protective effects of anti-CD45RB treatment on renal function in RAG1 KO mice with WT B cell transfer (Figure 6B). Anti-CD45RB treatment resulted in an increasing trend of IL-10–deficient Tim-1+ B cells in both the spleens and kidneys (Figure 8C). Next, LAP (a surrogate surface marker of TGF-β)+ B cells27 were induced by anti-CD45RB treatment after IRI in the kidneys and spleen; however, anti–TGF-β could not ameliorate beneficial effects of anti-CD45RB on IRI in either renal function or Breg induction (Supplemental Figure 7A), suggesting a dispensable role of TGF-β of Bregs in anti-CD45RB–mediated protective effects on IRI. When expression of other immunosuppressive mediators in Bregs was assessed, there was no increased expression of IDO, PD-L1, FasL, or IL-35 (IL-12α or EBI3) in Bregs in response to anti-CD45RB treatment (Supplemental Figure 7, B–E). In parallel, anti-CD45RB treatment attenuated IRI and increased Bregs in the RAG1 KO mice that were transferred by IDO KO B cells, confirming a dispensable role of IDO of Bregs in anti-CD45RB–mediated reno-protective effects (Supplemental Figure 7B).
Figure 8.
Reno-protective effects of anti-CD45RB treatment against IRI were dependent on IL-10+ regulatory B cells. (A) B cells sorted from IL-10 KO mice were transferred to RAG1 KO mice 2 weeks before IRI. Anti-CD45RB or PBS was administered to RAG1 KO mice with transferred IL-10–deficient B cells 1 day before IRI; mice were harvested on 1 day after IRI. (B) Levels of serum creatinine and BUN. (C) Flow cytometry analysis of splenic and renal Bregs. Results were expressed as dot plots with the mean±SEM. n=6. Bregs, regulatory B cells; IRI, ischemia-reperfusion injury; KO, knockout; RAG1, recombination activating gene 1; Tregs, regulatory T cells.
Discussion
This study demonstrated that Bregs attenuate renal IRI. Furthermore, treatment with anti-CD45RB with or without anti–Tim-1 antibody before IRI attenuated acute renal injury, and such treatment after IRI facilitated renal recovery after IRI. A dominant mechanism of the reno-protective effects of anti-CD45RB treatment against IRI was the induction of IL-10+ Bregs.
Roles of B cells in renal IRI have been controversial and the specific role of Bregs, a newly discovered B cell subset, has not previously been studied separately from those of other B cell subsets in IRI. Roles of B cells in IRI seem to vary according to B cell subsets and phases of IRI.2,4 For example, total B cells reduced renal injury in acute phase within 1 day after IRI, but reduced renal regeneration in the recovery phase around day 3.2,4 Moreover, peritoneal B-1 cells contributed to acute renal injury after IRI.2 Interestingly, reduced IL-10 levels in μMT mice, in which acute injury after IRI was more severe than that in WT mice, suggest the role of Bregs in IRI.2 Indeed, Bregs were beneficial in both the acute injury phase and the recovery phase after IRI in this study, similarly to the way in which Tregs ameliorated renal IRI by reducing acute renal injury and facilitating renal recovery.28–30 IL-10–producing Tim-1+ Bregs attenuated renal IRI in parallel with their beneficial effects on ischemic brain injury.5 When we investigated Tim-1+ and IL-10+ Bregs among the various B cell subsets, we found that Bregs exist in renal tissues and that their induction attenuates renal IRI.
Immunosuppressive cell therapy is a promising immune modulating therapy, but cell isolation and culture processes take a long time and carry a risk of contamination. In contrast, induction agents capable of inducing immunosuppressive cells would be much more convenient and demonstrate similar efficacy. Both anti–Tim-1 and anti-CD45RB antibodies induce Bregs under inflammatory conditions.9,15,16 Therefore, we attempted to suppress renal IRI using these Breg-inducing antibodies, rather than through treatment with Bregs themselves. As expected, both pretreatment and post-treatment with anti-CD45RB with or without anti–Tim-1 antibody attenuated renal IRI by decreasing acute injury or increasing renal regeneration, in parallel with the reno-protective effects of Bregs.
We used anti-CD45RB, rather than anti–Tim-1, antibody as the primary agent because anti-CD45RB treatment showed better protective effects against renal IRI and better Breg induction than anti–Tim-1 treatment. Inhibition of Tim-1 on renal epithelial cells may have reduced beneficial effects of Tim-1 inhibition on immune cells in renal IRI, because Tim-1 on renal epithelial cells protects against renal IRI through the phagocytosis of apoptotic cells and subsequent inhibition of NF-κB activation.31
Previous studies have shown that anti–Tim-1 monotherapy attenuates both hepatic and renal IRI; however, these studies focused on the suppression of effector T cells and macrophages without analyzing the roles of B cells.32,33 Therefore, we sought to dissect the roles of B cells and T cells in the anti-CD45RB– and anti–Tim-1–mediated beneficial effects on renal IRI. In RAG1 KO mice with transferred B cells, TCRα KO mice, and T cell–depleted WT mice, the reno-protective effects of anti-CD45RB treatment were intact, indicating that T cells were dispensable in these effects. In contrast, the reno-protective effects of anti-CD45RB treatment were abrogated in RAG1 KO mice with transferred T cells, indicating that B cells were indispensable in these effects. Anti-CD45RB–mediated reno-protective effects were seriously diminished but present in B cell–depleted WT mice, indicating that T cells may partially contribute to these reno-protective effects. Taken together, anti-CD45RB with or without anti–Tim-1 treatment attenuates renal IRI mainly through Breg induction.
In regard to the potential side effects of anti-CD45RB treatment, a previous study reported that anti-CD45RB led to impaired CD8+ T cell response and delayed influenza virus clearance.34 However, influenza virus was eventually eliminated in the CD45RB group, although 3 days later than the control group. Moreover, this study used a much higher dose of anti-CD45RB than our regimen in IRI. Anti-CD45RB increased susceptibility to infection to a much lesser extent than the conventional, broad-spectrum immunosuppressants such as tacrolimus and mycophenolate mofetil which carry a significant infection risk. Therefore, we believe that infection risk of anti-CD45RB treatment is relatively low in renal IRI; however, this potential risk should still be taken into consideration.
We observed the mild expansion of Tregs after IRI in the anti-CD45RB group and the anti-CD45RB/anti–Tim-1 group. Treg expansion might have been attributed to the direct effects of anti-CD45RB and anti–Tim-1 treatment, because of the effects of both antibodies on T cells.17–21 Alternatively, induction of Bregs by both antibodies may induce the expansion of Tregs, in parallel with previous in vitro and in vivo studeis.35,36 When Treg induction by Breg transfer was ameliorated by anti-CD25, Treg depletion did not significantly attenuate renal functional improvement. These results suggest that Treg induction by Breg does not play the dominant role in Breg-mediated reno-protective effects against IRI, although it might partially contribute to the beneficial effects.
IL-10–mediated suppression has been suggested as the main immunosuppressive mechanism of Bregs, especially Tim-1+ Bregs.8,9,16 This study demonstrated that anti-CD45RB treatment–induced Tim-1+ Bregs and IL-10+ Bregs in both the kidney and spleen and increased the renal expression of IL-10 after IRI. Furthermore, adoptive transfer of B cells from IL-10 KO mice did not mediate anti-CD45RB–induced renal protection against IRI, whereas transfer of B cells from WT mice did. In contrast, role of TGF-β, IDO, PD-L1, FasL, or IL-35 in Bregs appears to be limited in anti-CD45RB treatment–induced beneficial effects on renal IRI. Overall, these results suggest that anti-CD45RB–mediated renal protection against IRI is mainly mediated by the induction of IL-10+ Bregs.
Balance between immune effector cells and suppressor cells determines the degree of acute injury and repair processes in IRI.1 As various innate and adaptive immune effectors participate in IRI, previous studies have demonstrated that various immune suppressors, such as Tregs and NKT cells, play a role in controlling IRI.28,30,37 This study provides insight into the role of Bregs in renal IRI; however, the relative importance of Bregs among various immunosuppressive cells in IRI at different time points remains unknown, and further studies are needed to elucidate this issue under various conditions.
To the best of our knowledge, this is the first study to demonstrate the role of Bregs in renal IRI. We success\fully demonstrated not only the beneficial effects of anti-CD45RB on renal IRI but also its underlying mechanisms. This study suggests that anti-CD45RB treatment represents a potential therapeutic strategy for preventing or treating renal IRI in future clinical settings.
In conclusion, treatment of anti-CD45RB with or without anti–Tim-1 before or after renal IRI, attenuated IRI by reducing acute injury and facilitating recovery after IRI, mainly through induction of IL-10+ Bregs.
Disclosures
None.
Funding
This research was supported by Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (2017R1D1A1B03035852, 2018R1A2B3001179).
Supplementary Material
Acknowledgments
Dr. Koo, Dr. Suh, and Dr. Yang designed the study; Dr. Fang, Dr. Koo, Prof. Lee, Mr. Jang, Ms. Xu, Ms. Hwang, Ms. Park, Prof. Yan, Ms. Y-M Ryu and Prof. Kim performed experiments; Dr. Fang, Dr. Koo, Prof. Lee, Dr. J-H Ryu, Dr. Suh and Dr. Yang analyzed the data; Dr. Fang, Dr. Koo, Dr. Suh, and Dr. Yang wrote the manuscript; all authors approved the final version of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018101067/-/DCSupplemental.
Supplemental Table 1. Primer sets used for real-time reverse transcription-PCR.
Supplemental Figure 1. Flow cytometric gating strategies for various leukocyte populations in the spleen and kidneys.
Supplemental Figure 2. Adoptively-transferred regulatory B cells were found in the spleen and kidneys after IRI.
Supplemental Figure 3. Impact of Treg depletion on the renoprotective effects of Breg transfer against IRI.
Supplemental Figure 4. Comparison of the renoprotective effects of anti-CD45RB and anti-Tim-1 treatment against renal IRI.
Supplemental Figure 5. Flow cytometric diagrams for regulatory B cells and depletion of B or T cells.
Supplemental Figure 6. Renal infiltration of regulatory B cells after IRI.
Supplemental Figure 7. Role of immunosuppressive molecules of regulatory B cells in the renoprotective effects of anti-CD45RB treatment against IRI.
References
- 1.Jang HR, Rabb H: Immune cells in experimental acute kidney injury. Nat Rev Nephrol 11: 88–101, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Renner B, Strassheim D, Amura CR, Kulik L, Ljubanovic D, Glogowska MJ, et al.: B cell subsets contribute to renal injury and renal protection after ischemia/reperfusion. J Immunol 185: 4393–4400, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burne-Taney MJ, Ascon DB, Daniels F, Racusen L, Baldwin W, Rabb H: B cell deficiency confers protection from renal ischemia reperfusion injury. J Immunol 171: 3210–3215, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Jang HR, Gandolfo MT, Ko GJ, Satpute SR, Racusen L, Rabb H: B cells limit repair after ischemic acute kidney injury. J Am Soc Nephrol 21: 654–665, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bodhankar S, Chen Y, Vandenbark AA, Murphy SJ, Offner H: Treatment of experimental stroke with IL-10-producing B-cells reduces infarct size and peripheral and CNS inflammation in wild-type B-cell-sufficient mice. Metab Brain Dis 29: 59–73, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarvaria A, Madrigal JA, Saudemont A: B cell regulation in cancer and anti-tumor immunity. Cell Mol Immunol 14: 662–674, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fillatreau S: Regulatory roles of B cells in infectious diseases. Clin Exp Rheumatol 34[Suppl 98]: 1–5, 2016 [PubMed] [Google Scholar]
- 8.Rosser EC, Mauri C: Regulatory B cells: Origin, phenotype, and function. Immunity 42: 607–612, 2015 [DOI] [PubMed] [Google Scholar]
- 9.Ding Q, Yeung M, Camirand G, Zeng Q, Akiba H, Yagita H, et al.: Regulatory B cells are identified by expression of TIM-1 and can be induced through TIM-1 ligation to promote tolerance in mice. J Clin Invest 121: 3645–3656, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peng B, Ming Y, Yang C: Regulatory B cells: The cutting edge of immune tolerance in kidney transplantation. Cell Death Dis 9: 109, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tedder TF: B10 cells: A functionally defined regulatory B cell subset. J Immunol 194: 1395–1401, 2015 [DOI] [PubMed] [Google Scholar]
- 12.Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF: A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity 28: 639–650, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Yeung MY, Ding Q, Brooks CR, Xiao S, Workman CJ, Vignali DA, et al.: TIM-1 signaling is required for maintenance and induction of regulatory B cells. Am J Transplant 15: 942–953, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Penninger JM, Irie-Sasaki J, Sasaki T, Oliveira-dos-Santos AJ: CD45: New jobs for an old acquaintance. Nat Immunol 2: 389–396, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Deng S, Moore DJ, Huang X, Lian MM, Mohiuddin M, Velededeoglu E, et al.: Cutting edge: Transplant tolerance induced by anti-CD45RB requires B lymphocytes. J Immunol 178: 6028–6032, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Lee KM, Kim JI, Stott R, Soohoo J, O’Connor MR, Yeh H, et al.: Anti-CD45RB/anti-TIM-1-induced tolerance requires regulatory B cells. Am J Transplant 12: 2072–2078, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao S, Najafian N, Reddy J, Albin M, Zhu C, Jensen E, et al.: Differential engagement of Tim-1 during activation can positively or negatively costimulate T cell expansion and effector function. J Exp Med 204: 1691–1702, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nozaki Y, Nikolic-Paterson DJ, Snelgrove SL, Akiba H, Yagita H, Holdsworth SR, et al.: Endogenous Tim-1 (Kim-1) promotes T-cell responses and cell-mediated injury in experimental crescentic glomerulonephritis. Kidney Int 81: 844–855, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Ueno T, Habicht A, Clarkson MR, Albin MJ, Yamaura K, Boenisch O, et al.: The emerging role of T cell Ig mucin 1 in alloimmune responses in an experimental mouse transplant model. J Clin Invest 118: 742–751, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Camirand G, Wang Y, Lu Y, Wan YY, Lin Y, Deng S, et al.: CD45 ligation expands Tregs by promoting interactions with DCs. J Clin Invest 124: 4603–4613, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deng CY, Wang XF, Qi H, Li FR: Effects of anti-CD45RB monoclonal antibody for T lymphocyte subsets in mice heart transplantation model. Scand J Immunol 84: 86–94, 2016 [DOI] [PubMed] [Google Scholar]
- 22.Grcević D, Lee SK, Marusić A, Lorenzo JA: Depletion of CD4 and CD8 T lymphocytes in mice in vivo enhances 1,25-dihydroxyvitamin D3-stimulated osteoclast-like cell formation in vitro by a mechanism that is dependent on prostaglandin synthesis. J Immunol 165: 4231–4238, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Ashkenazi E, Baranovski BM, Shahaf G, Lewis EC: Pancreatic islet xenograft survival in mice is extended by a combination of alpha-1-antitrypsin and single-dose anti-CD4/CD8 therapy. PLoS One 8: e63625, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marino J, Paster JT, Trowell A, Maxwell L, Briggs KH, Crosby Bertorini P, et al.: B cell depletion with an anti-CD20 antibody enhances alloreactive memory T cell responses after transplantation. Am J Transplant 16: 672–678, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koo TY, Lee JG, Yan JJ, Jang JY, Ju KD, Han M, et al.: The P2X7 receptor antagonist, oxidized adenosine triphosphate, ameliorates renal ischemia-reperfusion injury by expansion of regulatory T cells. Kidney Int 92: 415–431, 2017 [DOI] [PubMed] [Google Scholar]
- 26.Jo SK, Sung SA, Cho WY, Go KJ, Kim HK: Macrophages contribute to the initiation of ischaemic acute renal failure in rats. Nephrol Dial Transplant 21: 1231–1239, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Lee KM, Yeh H, Zhao G, Wei L, O’Connor M, Stott RT, et al.: B-cell depletion improves islet allograft survival with anti-CD45RB. Cell Transplant 23: 51–58, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kinsey GR, Sharma R, Huang L, Li L, Vergis AL, Ye H, et al.: Regulatory T cells suppress innate immunity in kidney ischemia-reperfusion injury. J Am Soc Nephrol 20: 1744–1753, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim MG, Koo TY, Yan JJ, Lee E, Han KH, Jeong JC, et al.: IL-2/anti-IL-2 complex attenuates renal ischemia-reperfusion injury through expansion of regulatory T cells. J Am Soc Nephrol 24: 1529–1536, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gandolfo MT, Jang HR, Bagnasco SM, Ko GJ, Agreda P, Satpute SR, et al.: Foxp3+ regulatory T cells participate in repair of ischemic acute kidney injury. Kidney Int 76: 717–729, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Yang L, Brooks CR, Xiao S, Sabbisetti V, Yeung MY, Hsiao LL, et al.: KIM-1-mediated phagocytosis reduces acute injury to the kidney. J Clin Invest 125: 1620–1636, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uchida Y, Ke B, Freitas MC, Ji H, Zhao D, Benjamin ER, et al.: The emerging role of T cell immunoglobulin mucin-1 in the mechanism of liver ischemia and reperfusion injury in the mouse. Hepatology 51: 1363–1372, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rong S, Park JK, Kirsch T, Yagita H, Akiba H, Boenisch O, et al.: The TIM-1:TIM-4 pathway enhances renal ischemia-reperfusion injury. J Am Soc Nephrol 22: 484–495, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lim B, Sutherland RM, Zhan Y, Deliyannis G, Brown LE, Lew AM: Targeting CD45RB alters T cell migration and delays viral clearance. Int Immunol 18: 291–300, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Mielle J, Audo R, Hahne M, Macia L, Combe B, Morel J, et al.: IL-10 producing B cells ability to induce regulatory T cells is maintained in rheumatoid arthritis. Front Immunol 9: 961, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu Y, Liu F, Li C, Chen Y, Weng D, Chen J: IL-10-producing B cells suppress effector T cells activation and promote regulatory T cells in crystalline silica-induced inflammatory response in vitro. Mediators Inflamm 2017: 8415094, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang SH, Lee JP, Jang HR, Cha RH, Han SS, Jeon US, et al.: Sulfatide-reactive natural killer T cells abrogate ischemia-reperfusion injury. J Am Soc Nephrol 22: 1305–1314, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.