ABSTRACT
Metamorphosis is an intricate developmental process in which large-scale remodelling of mRNA and microRNA (miRNA) profiles leads to orchestrated tissue remodelling and organogenesis. Whether, which, and how, ribonucleases (RNases) are involved in the RNA profile remodelling during metamorphosis remain unknown. Human Regnase-1 (also known as MCPIP1 and Zc3h12a) RNase remodels RNA profile by cleaving specific RNAs and is a crucial modulator of immune-inflammatory and cellular defence. Here, we studied Drosophila CG10889, which we named Drosophila Regnase-1, an ortholog of human Regnase-1. The larva-to-adult metamorphosis in Drosophila includes two major transitions, larva-to-pupa and pupa-to-adult. regnase-1 knockout flies developed until the pupa stage but could not complete pupa-to-adult transition, dying in puparium case. Regnase-1 RNase activity is required for completion of pupa-to-adult transition as transgenic expression of wild-type Drosophila Regnase-1, but not the RNase catalytic-dead mutants, rescued the pupa-to-adult transition in regnase-1 knockout. High-throughput RNA sequencing revealed that regnase-1 knockout flies fail to remodel mRNA and miRNA profiles during the larva-to-pupa transition. Thus, we uncovered the roles of Drosophila Regnase-1 in the larva-to-adult metamorphosis and large-scale remodelling of mRNA and miRNA profiles during this metamorphosis process.
KEYWORDS: Regnase-1, RNase, metamorphosis, drosophila, mRNA, miRNA
Introduction
During animal metamorphosis, large-scale remodelling of mRNA and microRNA (miRNA) profiles leads to orchestrated tissue remodelling and organogenesis [1–3]. Modulation of mRNA stability is responsible for ~50% of all changes in gene expression [4]. miRNAs are important post-transcriptional regulators of gene expression that silence target mRNAs using sequence complementarily [5,6]. Ribonuclease (RNase) degradation of mRNA and miRNAs is an important mechanism to control the levels of selected transcripts and their expression in cells. However, whether, which, and how, RNases are involved in the large-scale remodelling of mRNA and miRNA profiles during metamorphosis are not fully understood.
Human Regnase-1, also known as MCPIP1 (Monocyte Chemoattractant Protein-1–Induced Protein 1) and Zc3h12a is an RNase that regulates RNA stability [7]. Regnase-1 possesses a Zc3h12a-like Ribonuclease NYN domain and a CCCH-type zinc finger domain (Figure 1(a)). The Zc3h12a-like Ribonuclease NYN domain active site consists of four conserved aspartate residues that coordinate a single magnesium in the catalytic cleft [8]. The CCCH-type zinc finger domain contributes to recognition of target mRNAs [8]. Regnase-1 orthologues are found from C. elegans and Drosophila to mice and human, sharing the same functional domains, indicating their important biological roles.
Figure 1.

RNase catalytic activity of Regnase-1 is required for the larva-to-adult metamorphosis.
(a) Domain structures of Drosophila Regnase-1 (CG10889) and human Regnase-1. Amino acid sequence alignment of Regnase-1 orthologues around the residues mutated in Figure 1(e) is also shown. Dme, Drosophila melanogaster; Cel, Caenorhabditis elegans; Dre, Danio rerio; Hsa, Homo sapiens. (b) Knockout (KO) allele of Drosophila Regnase-1 created in this study. Nucleotide residues targeted by sgRNA is italicised. The 5-nt residues deleted are shown in green. Amino acid residues after the frameshift mutation in the KO allele are shown in magenta. (c) Life cycle of Drosophila. (d) mRNA expression levels of regnase-1, drosha, dicer-1, and RpL32, in wild-type third instar larvae and early pupae, determined by mRNA-seq. Mean ± SD for three biological replicates. (e) Representative images of third instar larvae, early pupae, dead late pupae, and eclosed adult flies, for indicated genotypes. Successful metamorphosis rates are shown with the numbers of successful metamorphosis event/the numbers of examined pupae shown in parenthesis.
Regnase-1 has been implicated to regulate gene expression in an RNase-dependent manner [9,10]. Human and mice Regnase-1 regulate immune responses by degrading cytokine mRNAs [7,11–16]. Regnase-1 is crucial to suppress the autoimmune response under non-immunogenic conditions. Regnase-1 also exhibits broad-spectrum antiviral effects. Regnase-1 appears to cleave their mRNA targets within specific 3ʹ UTR regions containing an RNA stem loop [7,13,14]. Human Regnase-1 has also been reported to cleave select precursors of microRNAs (pre-miRNAs) in cytoplasm, thereby counteracting Dicer for miRNA generation and inhibiting the maturation of the miRNAs [17,18]. Regnase-1 knockout mice have a spontaneous severe phenotype of autoimmune inflammatory disease along with elevated serum immunoglobulin levels and auto-antibodies [7]. Human and mice Regnase-1 also regulate non-immune related biological processes such as iron homeostasis, differentiation, tumour growth, angiogenesis, epithelial regeneration, and hematopoietic stem and progenitor cell homeostasis [19–24]. C. elegans Regnase-1 (REGE-1) regulates expression of genes involved in lipid metabolism, control of body fat, and innate immunity, by degrading mRNAs including that encoding a transcription factor [10]. Thus, the evolutionary conserved Regnase-1 RNases are involved in remodelling RNA profiles during important biological processes.
However, the biological and molecular functions of Regnase-1 orthologs in other species remain unknown. CG10889, the closest Regnase-1 ortholog in Drosophila, hereafter termed Drosophila Regnase-1, shares 31% identify and 43% similarity in amino acid sequence to human Regnase-1 (Figure 1(a)).
Here, to uncover biological and molecular roles of Drosophila Regnase-1, we generated and analysed Drosophila Regnase-1 knockout and rescue flies. We found that Drosophila Regnase-1 is required for larva-to-adult metamorphosis. Moreover, we found that RNase catalytic activity of Drosophila Regnase-1 is essential for the larva-to-adult metamorphosis. The larva-to-adult metamorphosis in Drosophila includes two transitions: larva-to-pupa and pupa-to-adult (Figure 1(c)). High-throughput RNA sequencing analysis revealed that Drosophila Regnase-1 is crucial for mRNA and miRNA profile remodelling during the larva-to-pupa transition. This study defines Drosophila Regnase-1 as an RNase that has an essential biological role in larva-to-adult metamorphosis and a molecular role in remodelling mRNA and miRNA profiles during the metamorphosis.
Results
Regnase-1 is ubiquitously expressed
Publically available high-throughput RNA sequencing data sets (FlyBase: http://flybase.org/reports/FBgn0038769 and FlyAtlas2: http://flyatlas.gla.ac.uk/FlyAtlas2/index.html?search=gene&gene=Regnase-1&idtype=cgnum#mobileTargetG) show that regnase-1 mRNA is expressed in every tissue and at every developmental stage examined (Supplementary Figure S1). We confirmed that regnase-1 is expressed in third instar larvae and early pupae by high-throughput mRNA sequencing (Figure 1(d)). regnase-1 mRNA expression level (as judged by normalised counts) was similar between in third instar larvae and early pupae, as were mRNAs of drosha and dicer-1, two RNases involved in miRNA biogenesis, and mRNA of a housekeeping gene RpL32 (Figure 1(d)).
Regnase-1 is required for larva-to-adult metamorphosis
In order to determine biological roles of Regnase-1, we generated a knockout allele of regnase-1 (regnase-1KO) by CRISPR-Cas9 genome editing (Figure 1(b)). The regnase-1KO/KO homozygous mutant embryos hatched and became larva, showing that zygotic Regnase-1 is not required for embryogenesis while maternal Regnase-1 contribution cannot be excluded. The regnase-1KO/KO could develop up to the early pupa stage without any apparent phenotypic defects (Figure 1(e)). However, none (0/72) of the regnase-1KO/KO pupae successfully completed the pupa-to-adult transition. Instead, all of the regnase-1KO/KO flies died in puparium case without eclosion while they did not appear to show any organ- or tissue-specific problems (Figure 1(e)) and Supplemental Figure S2). To exclude the possibility that this phenotype was due to secondary mutation(s) including those caused by CRISPR off-targeting, we generated and characterised a trans-heterozygous mutant (regnase-1KO/Df), which has the regnase-1KO allele and the Df(3R)ED6027 chromosomal deficiency allele (regnase-1Df) uncovering the regnase-1 gene. regnase-1KO/Df trans-heterozygous mutant showed the same phenotype as regnase-1KO/KO homozygous mutant. In contrast, the vast majority of wild-type (regnase-1+/+, 162/167) and two heterozygous controls (regnase-1KO/+, 148/148 and regnase-1Df/+, 125/126) completed the pupa-to-adult transition successfully. These results suggested that Regnase-1 is required for the larva-to-adult metamorphosis.
To confirm that Regnase-1 is required for the larva-to-adult metamorphosis, we examined if a Regnase-1 transgene expressed in a regnase-1KO/Df background can rescue the metamorphosis. We used the UAS-Gal4 system with an Actin5C-Gal4 driver to express transgenic Regnase-1 ubiquitously (Act5C-Gal4> Regnase-1). 95% (18/19) of the regnase-1KO/Df pupae expressing wild-type Regnase-1 transgene (Act5c-Gal4> Regnase-1WT; regnase-1KO/Df) completed the pupa-to-adult transition (Figure 1(e)). Thus we conclude that Regnase-1 is required for the larva-to-adult metamorphosis.
Regnase-1 RNase activity is required for larva-to-adult metamorphosis
We next sought to determine whether Regnase-1 RNase catalytic activity is required for larva-to-adult metamorphosis. Human, mice, Drosophila, and C. elegans Regnase-1 have four conserved catalytic Asp residues in the Zc3h12a-like Ribonuclease NYN domain that coordinate a magnesium ion in the catalytic cleft [8]. We created RNase catalytic-deficient Regnase-1 transgenes by mutating one Asp residues in the catalytic cleft, Asp138, to alanine or asparagine (D138A and D138N, respectively) (Figure1(a)). The corresponding mutations in human Regnase-1 abolish its RNase activity [7,25]. In addition, we created another mutant Regnase-1 transgene containing arginine mutation at Cys302, which forms the conserved zinc-finger motif in the CCCH zinc-finger domain (C302R). This cysteine is perfectly conserved among human, mice, Drosophila, and C. elegans Regnase-1. The corresponding mutant of human Regnase-1 retains the RNase activity [7]. None (0/16 and 0/10) of the regnase-1KO/Df pupae expressing the RNase-catalytic dead Regnase-1 transgene (Act5c-Gal4> Regnase-1D138A; regnase-1KO/Df and Act5c-Gal4> Regnase-1D138N; regnase-1KO/Df) could complete the pupa-to-adult transition (Figure 1(e)). In contrast, all (24/24) of the regnase-1KO/Df pupae expressing the Regnase-1 transgene with a mutation in the zinc-finger motif (Act5c-Gal4> Regnase-1C302R; regnase-1KO/Df) could complete the pupa-to-adult transition. Expression levels of transgenic Regnase-1 proteins were similar among wild-type and point mutant as judged by Western blot (Supplementary Figure S3). We concluded that the RNase catalytic activity, but not the zinc-finger motif, of Regnase-1 is required for the larva-to-adult metamorphosis. In the following, we call regnase-1KO/+ as regnase-1-/+ or -/+, and regnase-1KO/Df as regnase-1-/-or -/-.
Regnase-1 knockout pupae exhibit altered miRNA profiles
Since Drosophila Regnase-1 RNase catalytic activity is required for the larva-to-adult metamorphosis (Figure 1) and human Regnase-1 cleaves select mRNAs and pre-miRNAs, we hypothesised that Drosophila Regnase-1 is required to establish normal mRNA profile and/or miRNA profile in the pupal stage.
First, we performed high-throughput sequencing of small RNAs from three biological replicates each of the third instar larvae and early pupae of regnase-1-/- and control (regnase-1+/+ and regnase-1-/+) flies (Supplementary Table S1). None of miRNAs or endo-siRNAs showed significant (p-value <0.05), more than two-fold differential expression in regnase-1-/- third instar larvae compared with the two control third instar larvae (Figure 2(a)). In contrast, 7 miRNAs (miR-1012-3p, miR-92b-3p, miR-1012-5p, miR-5-5p, miR-305-3p, miR-317-3p, miR-306-3p) showed significant, more than two-fold upregulation and 9 miRNAs (miR-193-5p, miR-274-5p, miR-932-5p, miR-210-3p, miR-987-5p, miR-957-3p, let-7-5p, miR-277-3p, miR-956-3p) showed significant, more than two-fold downregulation in regnase-1-/- early pupae compared with the two control early pupae (Figure 2(b)). Expression levels of top five upregulated and downregulated miRNAs are shown in Figure 3.
Figure 2.

miRNA profile is dysregulated in regnase-1 knockout early pupae.
Heatmaps of miRNA and endo-siRNA expression levels determined by high-throughput sequencing of small RNAs. Means of three biological replicates were used. (a-c) show the same data using different normalisation references and in different orders of miRNAs. (a) miRNA and siRNA abundance relative to those in wild-type (+/+) third instar larvae. miRNAs are sorted in descending order of the fold-changes in regnase-1-/- (-/-) third instar larvae. (b) miRNA and siRNA abundance relative to those in wild-type (+/+) early pupae. miRNAs are sorted in descending order of the fold-changes in regnase-1-/- (-/-) early pupae. (c) miRNA and siRNA abundance relative to those in wild-type (+/+) third instar larvae. miRNAs are sorted in descending order of the fold-changes in wild-type early pupae. Only miRNAs whose mean abundance was more than 100 reads per million total reads in either regnase-1+/+, -/+ or -/- samples and three endo-siRNAs (esi-1.1, esi-1.2, and esi-2.1) are shown. Third instar larva is shown as ‘larva’ for simplicity.
Figure 3.

Regnase-1 is required for remodelling miRNA profile during the larva-to-pupa transition.
(a-b) Abundance of top five miRNAs that are significantly (a) upregulated and (b) downregulated in regnase-1-/- early pupae compared with control early pupae, determined by high-throughput sequencing. Mean ± SD for three biological replicates. P-value <0.05 is indicated by *. Third instar larvae are shown as ‘Larvae’ for simplicity.
miRNA profile remodelling occurred during the larva-to-pupa transition in the wild-type (+/+) strain. Thirteen miRNAs showed significant, more than two-fold upregulation and 22 miRNAs and one endo-siRNA (esi-1.1) showed significant, more than two-fold downregulation in wild-type early pupae compared with wild-type third instar larvae (Figure 2(c)).
Most of the miRNA level dysregulation observed in regnase-1-/- early pupae compared with the two control early pupae seemed to result from a failure to remodel miRNA profile during the larva-to-pupa transition (Figure 2(b,c) and 3). This is particularly obvious for the miRNAs that are downregulated in regnase-1-/- early pupae compared with control early pupae. For example, the top five miRNAs downregulated in regnase-1-/- early pupae (miR-193-5p, miR-274-5p, miR-932-5p, miR-210-3p) compared with control early pupae showed significant upregulation during the larva-to-pupa transition in the control strains (Figure 3). These miRNAs showed higher levels in control early pupae compared with control third instar larvae. However, levels of these miRNAs in regnase-1-/- early pupae remained as low as those in third instar larvae, indicating a failure of miRNA level remodelling during the larva-to-pupa transition in regnase-1-/-.
High-throughput sequencing results were confirmed by Northern blots (Supplementary Figure S4). For example, miR-315-5p was upregulated in regnase-1-/- pupae compared with control pupae. In contrast, other miRNAs, such as miR-1, showed no difference between regnase-1-/- and control pupae.
Together, these results revealed that Regnase-1 is required for normal miRNA expression profile in pupae, and more specifically, for regulated miRNA profile remodelling during the larva-to-pupa transition. In contrast, Regnase-1 is not required for normal miRNA expression profile in third instar larvae.
Regnase-1 knockout pupae exhibit altered mRNA profiles
Next, we examined the hypothesis that Regnase-1 is also required for establishing normal mRNA profile by high-throughput sequencing of polyA+ mRNAs of three biological replicates each of the third instar larvae and early pupae of regnase-1-/- and control (regnase-1+/+ and regnase-1-/+) (Table 1, Table 2, and Supplementary Table S2).
Table 1.
Top 30 genes upregulated in regnase-1-/- early pupae compared with control early pupae.
| Gene | Fold-change (-/- vs +/+) | Fold-change (-/- vs -/+) | Adjusted p-value (-/- vs +/+) | Adjusted p-value (-/- vs -/+) |
|---|---|---|---|---|
| CG12057 | 54.6 | 167.0 | 1.1E-25 | 1.4E-24 |
| CG12951 | 18.2 | 85.6 | 4.8E-10 | 1.0E-14 |
| CG31789 | 26.9 | 82.9 | 3.9E-15 | 5.3E-17 |
| CG16727 | 45.4 | 63.6 | 4.9E-24 | 3.8E-22 |
| CG10912 | 9.7 | 60.5 | 7.9E-05 | 4.9E-14 |
| CG7953 | 32.6 | 45.0 | 7.9E-21 | 7.9E-24 |
| Est-P | 17.3 | 39.9 | 3.5E-15 | 2.4E-14 |
| CG7290 | 29.1 | 39.6 | 3.6E-12 | 3.3E-12 |
| CG11865 | 39.8 | 38.9 | 1.8E-06 | 2.1E-06 |
| CG8997 | 15.9 | 38.7 | 6.7E-28 | 7.3E-37 |
| CG7017 | 37.9 | 37.6 | 1.6E-19 | 3.6E-19 |
| CR46031 | 35.4 | 36.1 | 1.2E-05 | 1.2E-05 |
| CG11854 | 15.0 | 34.9 | 2.5E-10 | 3.2E-15 |
| CG31205 | 35.5 | 34.1 | 4.8E-07 | 6.2E-07 |
| CG34282 | 7.1 | 32.7 | 1.2E-06 | 1.2E-11 |
| CG13215 | 25.0 | 30.0 | 3.8E-11 | 2.4E-12 |
| CG32023 | 9.7 | 28.9 | 2.8E-03 | 5.1E-05 |
| CR45371 | 19.3 | 28.0 | 1.5E-04 | 1.7E-05 |
| CG6277 | 27.5 | 26.5 | 6.9E-09 | 1.1E-08 |
| CG14957 | 6.1 | 26.2 | 5.6E-04 | 3.0E-10 |
| CG12115 | 43.2 | 26.2 | 6.4E-10 | 3.1E-09 |
| Jon65Aii | 6.7 | 26.0 | 1.7E-05 | 2.9E-12 |
| CG5770 | 44.5 | 24.1 | 4.3E-19 | 4.3E-18 |
| Sgs5 | 28.4 | 22.9 | 1.6E-10 | 4.4E-10 |
| CG14300 | 30.3 | 22.8 | 6.2E-09 | 1.5E-08 |
| Listericin | 9.1 | 21.7 | 1.6E-07 | 1.7E-10 |
| CG10725 | 22.1 | 21.2 | 1.2E-08 | 1.9E-08 |
| CR43771 | 7.8 | 20.7 | 1.6E-02 | 5.7E-04 |
| Jon66Ci | 8.2 | 20.4 | 7.8E-06 | 3.7E-08 |
| Peritrophin-15a | 11.4 | 20.0 | 2.1E-09 | 1.9E-12 |
Table 2.
Top 30 genes downregulated in regnase-1-/- early pupae compared with control early pupae.
| Gene | Fold-change (-/- vs +/+) | Fold-change (-/- vs -/+) | Adjusted p-value (-/- vs +/+) | Adjusted p-value (-/- vs -/+) |
|---|---|---|---|---|
| CG13231 | 0.00111 | 0.00082 | 1.1E-54 | 8.2E-60 |
| fln | 0.00105 | 0.00086 | 1.4E-73 | 6.0E-78 |
| Cpr30F | 0.00109 | 0.00093 | 2.2E-111 | 7.9E-117 |
| TpnC41C | 0.00097 | 0.00094 | 3.2E-46 | 1.0E-46 |
| CG42323 | 0.00109 | 0.00102 | 3.7E-113 | 2.3E-115 |
| Cpr30B | 0.00130 | 0.00120 | 2.6E-127 | 2.2E-130 |
| CG7031 | 0.00166 | 0.00121 | 6.8E-34 | 2.2E-37 |
| Acp1 | 0.00277 | 0.00152 | 7.8E-19 | 6.9E-23 |
| Crys | 0.00158 | 0.00155 | 4.6E-107 | 8.0E-108 |
| Scp1 | 0.00173 | 0.00161 | 5.1E-81 | 4.7E-83 |
| Osi21 | 0.00200 | 0.00172 | 2.2E-23 | 1.4E-24 |
| Cpr76Ba | 0.00181 | 0.00172 | 9.7E-20 | 4.7E-20 |
| CG14625 | 0.00245 | 0.00176 | 2.5E-29 | 1.1E-32 |
| CG12003 | 0.00229 | 0.00176 | 8.5E-35 | 5.6E-38 |
| Cpr64Aa | 0.00202 | 0.00191 | 1.1E-137 | 2.5E-140 |
| Cpr72Ec | 0.00202 | 0.00192 | 5.5E-61 | 4.8E-62 |
| CG7214 | 0.00218 | 0.00206 | 9.6E-100 | 1.1E-101 |
| Osi11 | 0.00174 | 0.00212 | 6.2E-25 | 1.9E-23 |
| Cpr76Bd | 0.00234 | 0.00213 | 1.3E-88 | 2.3E-91 |
| CG34205 | 0.00213 | 0.00218 | 8.3E-114 | 3.6E-113 |
| CG42449 | 0.00303 | 0.00227 | 6.8E-31 | 4.2E-34 |
| Osi12 | 0.00339 | 0.00236 | 1.0E-46 | 6.0E-53 |
| Cpr49Ae | 0.00295 | 0.00251 | 5.9E-98 | 1.4E-103 |
| Act88F | 0.00382 | 0.00278 | 5.6E-123 | 2.0E-137 |
| trp | 0.00364 | 0.00300 | 9.8E-38 | 1.9E-40 |
| Obp73a | 0.00448 | 0.00304 | 6.8E-18 | 1.4E-20 |
| Cpr92A | 0.00322 | 0.00305 | 2.2E-48 | 2.4E-49 |
| CG15888 | 0.00322 | 0.00316 | 4.1E-31 | 2.2E-31 |
| CG42367 | 0.00340 | 0.00325 | 1.6E-19 | 7.6E-20 |
| Acp65Aa | 0.00359 | 0.00338 | 3.6E-108 | 1.5E-110 |
Sample-to-sample distance analysis and principal component analysis examining the levels of all mRNAs showed that mRNA profiles in regnase-1-/- third instar larvae were similar to those in the two control third instar larvae (Figure 4). They clustered closely together in both of the analyses. mRNA profiles of the two control early pupae were significantly different from those of the two control third instar larvae, showing that there is a large-scale mRNA profile remodelling during the larva-to-pupa transition. However, mRNA profiles of regnase-1-/- early pupae were significantly different from those of control early pupae and instead showed some degree of similarity to mRNA profiles of third instar larvae. These results suggested that Regnase-1 is required for normal mRNA profile in pupae, and more specifically, for mRNA profile remodelling during the larva-to-pupa transition.
Figure 4.
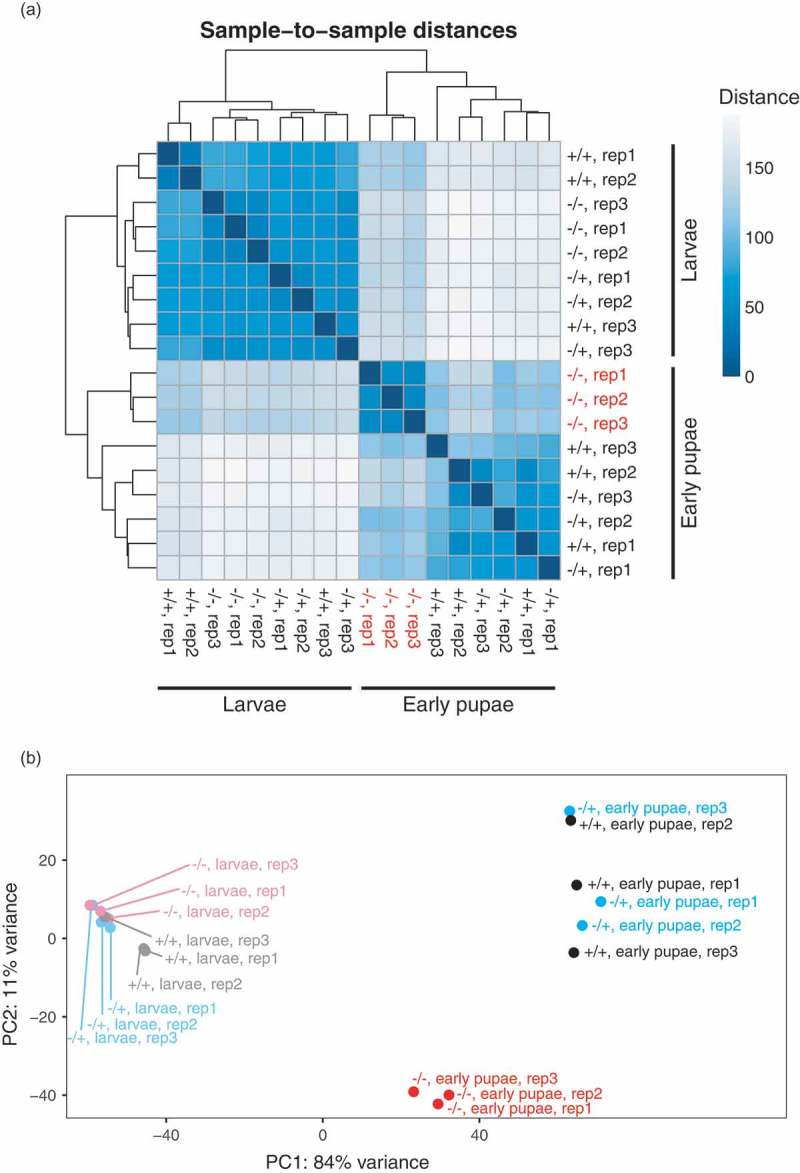
Regnase-1 is required for normal mRNA profile in pupae.
(a) Sample-to-sample distance matrix of mRNA profiles determined by mRNA-seq. (b) Principal component analysis of mRNA profiles. Third instar larvae are shown as ‘larvae’ for simplicity.
To further confirm this, we examined differential expression of individual genes (Figure 5). Only 497 genes were significantly (FDR<0.05) upregulated and 184 genes were downregulated out of ~17,000 examined genes in regnase-1-/- third instar larvae compared with wild-type (+/+) third instar larvae (Figure 5(a)). Only 68 genes were upregulated and 46 genes were downregulated in regnase-1-/- third instar larvae compared with heterozygous mutant control (-/+) third instar larvae. These numbers were similar to those found in the comparison between the two controls (224 upregulated and 24 downregulated). In contrast, much larger numbers of genes showed differential expression in regnase-1-/- early pupae compared with the two control early pupae (Figure 5(b)). 1716 genes were upregulated and 2190 genes were downregulated in regnase-1-/- early pupae compared with wild-type early pupae. 1648 genes were upregulated and 1939 genes were downregulated in regnase-1-/- early pupae compared with -/+ early pupae. Only 68 genes were upregulated and 4 genes were downregulated in -/+ early pupae compared with +/+ early pupae, showing that these two controls have similar mRNA profiles as expected. These results further revealed that Regnase-1 is required for normal mRNA profile in pupae but is not in third instar larvae.
Figure 5.
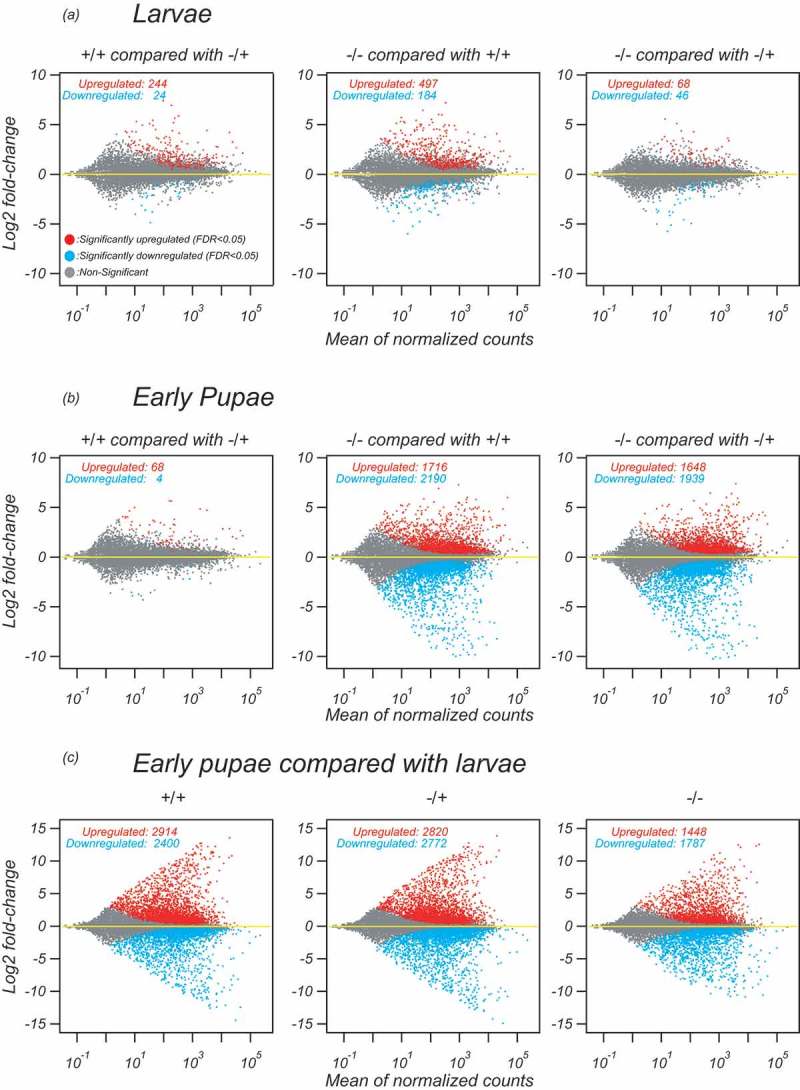
MA plots show that Regnase-1 is required for normal mRNA profile in pupae.
MA plots of mRNA expression. x-axis shows the means of normalised counts among the six biological samples tested (three biological replicates each for two genotypes or developmental stages that are compared in each graph). y-axis shows log2 fold-change in the normalised counts between the two genotypes or developmental stages. (a) MA plots of mRNA profiles in third instar larvae, comparing pair-wisely among regnase-1 +/+, -/+, and -/-. (b) MA plots of mRNA profiles in early pupae, comparing pair-wisely among regnase-1 +/+, -/+, and -/-. (c) MA plots of mRNA profiles comparing between early pupae and third instar larvae of regnase-1+/+, -/+, and -/-. Third instar larvae are shown as ‘Larvae’ for simplicity.
Regnase-1 is required for large-scale remodelling of mRNA profile during larva-to-pupa transition
During the larva-to-pupa transition, large-scale mRNA profile remodelling occurs. In fact, as many as 2914 genes were upregulated and 2400 genes were downregulated in wild-type early pupae compared with wild-type third instar larvae (Figure 5(c)). Similarly, as many as 2820 genes were upregulated and 2772 genes were downregulated in -/+ early pupae compared with -/+ third instar larvae. In contrast, a smaller number of genes exhibited differential expression upon the larva-to-pupa transition in regnase-1-/-. Only 1448 genes were upregulated and 1787 genes were downregulated in regnase-1-/- early pupae compared with regnase-1-/- third instar larvae, showing that regnase-1-/- could not fully remodel mRNA profile during the larva-to-pupa transition. In fact, out of the 2914 genes upregulated from third instar larvae to early pupae in wild-type, 76 (3%) were upregulated and 1647 (57%) were downregulated in regnase-1-/- early pupae compared with wild-type early pupae (Supplementary Figure S5). Out of the 2400 genes downregulated from third instar larvae to early pupae in wild-type, 1061 (44%) were upregulated and 65 (3%) were downregulated in regnase-1-/- early pupae compared with wild-type early pupae. This is consistent with the results of the sample-to-sample distance analysis and principal component analysis that showed that mRNA profiles of regnase-1-/- early pupae are located somewhat in between those of control third instar larvae and those of control early pupae (Figure 4). These results further supported the idea that Regnase-1 is required for mRNA profile remodelling during the larva-to-pupa transition.
To further test this idea, we examined the expression of the top 5 genes that were upregulated and downregulated in regnase-1-/- early pupae compared with control early pupae. All of the top 5 upregulated genes (CG12057, CG12951, CG31789, CG16727, CG10912), whose mRNA levels were significantly increased in regnase-1-/- early pupae than in control early pupae, showed high expression levels in third instar larvae of all three genotypes (+/+, -/+, and -/-) (Figure 6(a)). Their expression levels were significantly reduced in the two control (+/+ and -/+) early pupae compared in third instar larva. In contrast, their expression levels were not fully reduced in regnase-1-/- early pupae. We confirmed these results using an independent method, qRT-PCR (Figure 6(b)).
Figure 6.
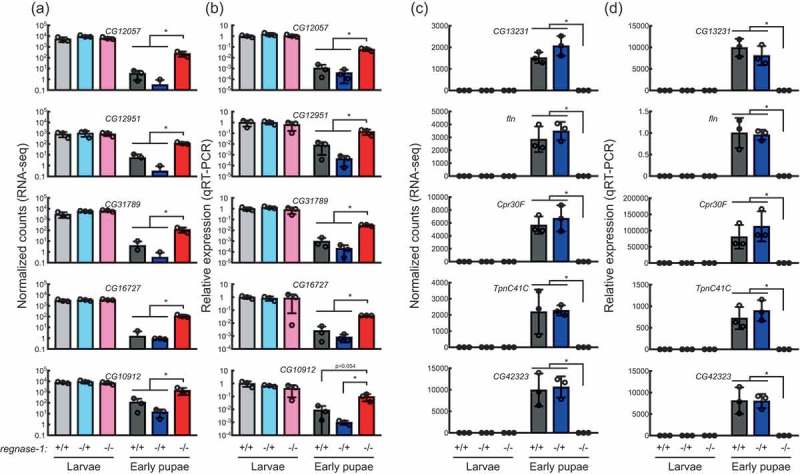
Regnase-1 is required for remodelling mRNA profile during the larva-to-pupa transition.
Abundance of top five mRNAs that are significantly (A, B) upregulated and (C, D) downregulated in regnase-1-/- early pupae compared with control early pupae, determined by high-throughput sequencing. (A,C) Normalised counts in RNA-seq. (B,D) Relative expression normalized by rp49 determined by qRT-PCR. Expression relative to wild-type (regnase-1+/+) third instar larvae are shown except in fln, where expression relative to wild-type (regnase-1+/+) early pupae larvae are shown. Mean ± SD for three biological replicates. P-value <0.05 is indicated by *. Third instar larvae are shown as ‘Larvae’ for simplicity.
A similar observation was obtained when we examined the expression of the top five downregulated genes (CG13231, fln, Cpr30F, TpnC41C, CG42323), whose mRNA levels were significantly decreased in regnase-1-/- early pupae than in control early pupae (Figure 6(c)). They showed low expression levels in third instar larvae of all three genotypes as judged by both RNA-seq and qRT-PCR (Figure 6(c,d)). Their expression levels were significantly increased in the two control early pupae compared in third instar larva. In contrast, their expression levels remained low in regnase-1-/- early pupae. These results further confirmed that Regnase-1 is required for mRNA profile remodelling during the larva-to-pupa transition.
Pathway dysregulation in Regnase-1 mutant pupae
A total of 1146 genes were significantly upregulated and 1679 genes were downregulated in regnase-1-/- early pupae compared with the two control early pupae. Using these differentially expressed genes, we performed GO term/KEGG pathway enrichment analyses to determine the pathways dysregulated in regnase-1-/- early pupae.
GO term analysis of the 1146 upregulated genes revealed high enrichment for GO term biological processes related to cell cycle (ex. mitotic cell cycle process, chromosome segregation, regulation of cell cycle, stem cell differentiation, cell division) and metabolism (ex. amino sugar metabolic process, aminoglycan metabolic process, macromolecule catabolic process) were highly enriched (Table 3). Similarly, GO term cellular components related to cell cycle (ex. chromosomal part, condensed chromosome, spindle) and metabolism (ex. peptidase complex) were highly enriched. Cell cycle and metabolism are finely regulated during larva-to-adult metamorphosis [26,27]. GO term molecular functions of chitin binding, endopeptidase activity, organic acid and anion transmembrane transporter activity, serine hydrolase activity, were enriched. KEGG pathways of proteasome, DNA replication, and endocytosis were enriched. Proteasome activity has been shown to be important for larva-to-pupa transition [28].
Table 3.
Significantly enriched GO terms and KEGG pathways associated with genes upregulated in regnase-1-/- early pupae compared with control early pupae.
| p-Value | |
|---|---|
| GOterm Biological Process | |
| Mitotic cell cycle process | 9.9E-09 |
| Amino sugar metabolic process | 2.0E-06 |
| Aminoglycan metabolic process | 1.7E-05 |
| Chromosome segregation | 1.7E-05 |
| Cellular response to DNA damage stimulus | 2.3E-05 |
| Organelle fission | 2.5E-05 |
| Macromolecule catabolic process | 4.4E-05 |
| Regulation of cell cycle | 5.0E-05 |
| Stem cell differentiation | 7.9E-05 |
| Cell division | 9.8E-05 |
| Organic acid transport | 1.5E-04 |
| Regulation of organelle organisation | 1.7E-04 |
| Peripheral nervous system development | 2.4E-04 |
| Urogenital system development | 2.9E-04 |
| Response to wounding | 3.2E-04 |
| Cell fate commitment | 7.7E-04 |
| Notch signaling pathway | 1.0E-03 |
| Chromosome organisation | 1.1E-03 |
| Cell proliferation | 1.1E-03 |
| Cell death | 1.4E-03 |
| Meiotic cell cycle | 2.1E-03 |
| Anatomical structure regression | 2.1E-03 |
| Cell cycle phase transition | 2.6E-03 |
| Embryonic organ development | 3.4E-03 |
| GOterm Cellular Component | |
| Peptidase complex | 6.1E-09 |
| Chromosomal part | 1.7E-04 |
| Condensed chromosome | 3.9E-04 |
| Spindle | 6.3E-04 |
| Cytoplasmic region | 1.8E-03 |
| Microtubule-associated complex | 2.1E-03 |
| Midbody | 2.5E-03 |
| GOterm Molecular Function | |
| Chitin binding | 7.9E-07 |
| Endopeptidase activity | 9.2E-07 |
| Organic acid transmembrane transporter activity | 6.9E-06 |
| Serine hydrolase activity | 1.2E-05 |
| Anion transmembrane transporter activity | 4.7E-04 |
| KEGG Pathway | |
| Proteasome | 7.8E-11 |
| DNA replication | 4.9E-04 |
| Endocytosis | 9.8E-04 |
GO term analysis of the 1679 downregulated genes revealed high enrichment for GO term biological processes related to metabolism (ex. generation of precursor metabolites and energy, organic acid metabolic process, purine-containing compound metabolic process, cofactor metabolic process) and signaling pathways (ex. synaptic signaling, G-protein coupled receptor signaling pathway, regulation of membrane potential) and mitochondrion organisation, were highly enriched (Table 4). Signalling cascades including that for ecdysone steroid hormone are crucial for larva-to-adult metamorphosis [29]. GO term cellular components of mitochondrial part, ribosome, contractile fibre, extracellular matrix, and transporter complex were highly enriched. GO term molecular functions of structural molecule activity, cation transmembrane transporter activity, calcium ion binding, and ammonium ion binding, were enriched. KEGG pathways of neuroactive ligand–receptor interaction, phototransduction, metabolic pathways, carbon metabolism, and ribosome, were enriched.
Table 4.
Significantly enriched GO terms and KEGG pathways associated with genes downregulated in regnase-1-/- early pupae compared with control early pupae.
| p-Value | |
|---|---|
| GOterm Biological Process | |
| Mitochondrion organisation | 0.0E+00 |
| Generation of precursor metabolites and energy | 1.7E-11 |
| Cation transport | 9.9E-11 |
| Cuticle development | 1.5E-09 |
| Synaptic signaling | 3.1E-09 |
| G-protein coupled receptor signaling pathway | 1.8E-08 |
| Ion transmembrane transport | 3.7E-08 |
| Organic acid metabolic process | 5.2E-08 |
| Purine-containing compound metabolic process | 9.8E-08 |
| Regulation of membrane potential | 2.4E-07 |
| Peptide biosynthetic process | 3.0E-07 |
| Cofactor metabolic process | 8.2E-07 |
| Muscle system process | 1.3E-06 |
| Sensory perception of light stimulus | 2.0E-06 |
| Nucleobase-containing small molecule metabolic process | 2.8E-05 |
| Glycosyl compound metabolic process | 5.0E-05 |
| Sulphur compound metabolic process | 1.7E-04 |
| Response to abiotic stimulus | 2.4E-04 |
| Pigment metabolic process | 2.9E-04 |
| Hydrogen transport | 1.0E-03 |
| Sensory perception of mechanical stimulus | 1.5E-03 |
| Regulation of behaviour | 1.7E-03 |
| Response to toxic substance | 1.7E-03 |
| Mating | 2.4E-03 |
| Small molecule biosynthetic process | 2.4E-03 |
| Synapse organisation | 2.5E-03 |
| Locomotory behaviour | 3.1E-03 |
| GOterm Cellular Component | |
| Mitochondrial part | 0.0E+00 |
| Ribosome | 3.3E-16 |
| Contractile fibre | 1.0E-13 |
| Extracellular matrix | 9.8E-10 |
| Transporter complex | 2.0E-09 |
| Rhabdomere | 1.3E-07 |
| Respiratory chain | 7.5E-07 |
| Oxidoreductase complex | 3.0E-06 |
| Synapse | 4.3E-05 |
| Envelope | 1.1E-04 |
| Cytochrome complex | 5.4E-04 |
| Anchored component of membrane | 8.5E-04 |
| Lipid particle | 2.1E-03 |
| Actin-based cell projection | 8.9E-03 |
| GOterm Molecular Function | |
| Structural molecule activity | 0.0E+00 |
| Cation transmembrane transporter activity | 7.6E-07 |
| Calcium ion binding | 5.5E-06 |
| Ammonium ion binding | 1.8E-05 |
| Electron carrier activity | 3.5E-05 |
| Metallopeptidase activity | 6.9E-05 |
| Passive transmembrane transporter activity | 1.2E-04 |
| Oxidoreductase activity, acting on the aldehyde or oxo group of donors | 1.8E-04 |
| Cofactor binding | 2.0E-04 |
| Exopeptidase activity | 3.0E-04 |
| Oxidoreductase activity, acting on peroxide as acceptor | 1.0E-03 |
| Heme-copper terminal oxidase activity | 1.3E-03 |
| Oxidoreductase activity, acting on a heme group of donors | 1.3E-03 |
| Antioxidant activity | 1.3E-03 |
| Tetrapyrrole binding | 1.4E-03 |
| Monooxygenase activity | 3.9E-03 |
| Transferase activity, transferring sulphur-containing groups | 5.6E-03 |
| Transmembrane receptor activity | 5.7E-03 |
| Transferase activity, transferring alkyl or aryl (other than methyl) groups | 6.4E-03 |
| KEGG Pathway | |
| Neuroactive ligand–receptor interaction | 6.1E-08 |
| Phototransduction | 1.9E-07 |
| Metabolic pathways | 4.7E-07 |
| Carbon metabolism | 4.9E-06 |
| Ribosome | 7.4E-06 |
| Oxidative phosphorylation | 8.3E-06 |
| Citrate cycle (TCA cycle) | 1.6E-05 |
| Porphyrin and chlorophyll metabolism | 1.2E-04 |
| Drug metabolism – cytochrome P450 | 9.3E-04 |
| Glycolysis/Gluconeogenesis | 9.6E-04 |
| Metabolism of xenobiotics by cytochrome P450 | 1.3E-03 |
| Glycine, serine and threonine metabolism | 1.6E-03 |
| Valine, leucine and isoleucine degradation | 2.9E-03 |
Dysregulation of these pathways may underlie the failure to complete the larva-to-adult metamorphosis in regnase-1-/-.
Discussion
Here we describe a previously uncharacterised Drosophila RNase, Regnase-1, involved in the remodelling of mRNA and miRNA profiles during the larva-to-adult metamorphosis. We generated and studied regnase-1 knockout and rescue flies (Figure 1). regnase-1 knockout flies developed up to the pupal stage, but they died in puparium case without eclosing as an adult fly. Therefore, Regnase-1 is essential for the larva-to-adult metamorphosis. Rescue analysis showed that RNase catalytic activity, but not zinc-finger motif, of Regnase-1 is required for the larva-to-adult metamorphosis. Thus, this study defined the biological role of Regnase-1 RNase in the larva-to-adult metamorphosis.
Small RNA-seq and mRNA-seq analyses revealed that miRNA and mRNA profiles in regnase-1 mutant early pupae differ from those in control early pupae while those in regnase-1 mutant third instar larvae are similar to those in control third instar larvae (Figure 2–6). Thus, although regnase-1 mutant early pupae did not exhibit apparent phenotypic defects (Figure 1(e)), they exhibited significant molecular dysregulation. The dysregulation of miRNA and mRNA profiles at the early pupal stage in regnase-1 mutant likely underlies the failure in the larva-to-adult metamorphosis, and more specifically, in the pupa-to-adult transition.
Furthermore, we identified large-scale remodelling of miRNA and mRNA profiles upon the larva-to-pupa transition in the wild-type flies. miRNA and mRNA profiles in regnase-1 mutant early pupae exhibited a greater similarity to those in third instar larvae than control early pupae did. These results indicate that Regnase-1 is required for the large-scale remodelling of miRNA and mRNA profiles during the larva-to-pupa transition.
We identified 1,146 genes upregulated and 1,679 genes downregulated in regnase-1 mutant early pupae compared with two control early pupae. Since Regnase-1 is thought to cleave and degrade target mRNAs, loss of Regnase-1 function is expected to cause accumulation (upregulation) of the target mRNAs. Therefore, some of the upregulated genes may be direct targets of the Regnase-1 RNase activity, while dysregulation of other genes including downregulated ones may be an indirect consequence of loss of Regnase-1 function. Defining direct mRNA binding targets of Regnase-1 is required to fully understand the roles of Regnase-1 in miRNA and mRNA profile remodelling. For this purpose, our RNase catalytic-dead Regnase-1 transgenic strains, especially Regnase-1D138N, might be useful to capture bound mRNAs. The corresponding human Regnase-1 D141N mutant retains the ability to recognise and bind RNA while it cannot cleave the RNA [8,19].
No single miRNA or endo-siRNA showed a significant, more than two-fold abundance change in regnase-1-/- third instar larvae compared with control third instar larvae (Figure 2(a)). mRNA profile in regnase-1-/- third instar larvae was overall very similar to those in control third instar larvae (Figure 3–6). However, among ~17,000 genes examined, there were some genes that exhibited significant differential expression in regnase-1-/- third instar larvae, while they may well be false positives (Figure 5(a)). 62 genes were upregulated and 32 genes were downregulated in regnase-1-/- third instar larvae compared with the two control third instar larvae (Supplementary Tables S3 and S4). Expression levels of top 5 each of upregulated (CG11843, CG15570, CG5070, CG13065, Ccp84Ab) and downregulated (CG43315, CG8087, CG14852, CG137134, Iris) genes are shown in Supplementary Figure S6. When the 62 upregulated genes were used for the GO term enrichment analysis, GO term biological processes of cuticle development, GO term cellular components of extracellular matrix, and GO term molecular functions of structural molecule activity were enriched (Supplementary Tables S5). Cuticle and extracellular matrix are important for pupation process. No GO term was significantly enriched when the 32 downregulated genes were used for analysis.
Twenty-one out of the 62 genes upregulated in regnase-1-/- third instar larvae compared with the two control third instar larvae were upregulated also in regnase-1-/- early pupae compared with the two control pupae (Supplementary Table S3). Twenty out of the 32 genes downregulated in regnase-1-/- third instar larvae compared with the two control third instar larvae were downregulated also in regnase-1-/- early pupae compared with the two control early pupae (Supplementary Tables S4). The 4 out of the top 5 genes upregulated in regnase-1-/- third instar larvae also exhibited higher expression in regnase-1-/- early pupae compared with control early pupae (Supplementary Figure S6A). All of the top 5 genes downregulated in regnase-1-/- third instar larvae that were expressed in control third instar larvae but at a much lower level in regnase-1-/- third instar larvae, exhibited similarly low expression levels in control and regnase-1-/- early pupae (Supplementary Figure S6C). These genes exhibiting differential expression in regnase-1-/- third instar larvae might be ‘early responders’ to loss of Regnase-1 function. If so, some of these genes, especially upregulated genes, may be direct Regnase-1 RNase targets in third instar larvae. Although regnase-1-/- third instar larvae and early pupae did not exhibit apparent phenotypic defects, the dysregulation of the small number of genes identified in regnase-1-/- third instar larvae might cause the observed downstream failure in the larger scale mRNA profile remodelling during the larva-to-pupa transition resulting in death in puparium case.
This study uncovers the biological roles of Drosophila Regnase-1 RNase in the larva-to-adult metamorphosis. We determined that Drosophila Regnase-1 remodels miRNA and mRNA profiles during the larva-to-pupa transition. This study supports a general theme that Regnase-1 orthologues play crucial roles in RNA profile remodelling during important biological processes, while which biological processes Regnase-1 orthologues are involved differ among different species. Our RNA-seq analysis did not reveal dysregulation of innate immune and cellular defence systems in regnase-1 mutant larvae and early pupae. Future study is warranted to examine if Regnase-1 is important to fight against external immune stimuli at various stages of development.
Further studies should define direct target miRNAs and mRNAs of Drosophila Regnase-1 and uncover its substrate specificity mechanism. Human Regnase-1 cooperates with another CCCH-type zinc finger domain protein Roquin in T cells to target specific mRNAs for degradation [30]. Since Drosophila has a Roquin ortholog (Drosophila Roquin), it will be interesting to examine if Drosophila Regnase-1 and Roquin cooperate to achieve specific RNA degradation. The Regnase-1 zinc-motif was not required for larva-to-adult metamorphosis (Figure 1). The zinc finger motif in Roquin may have compensated the loss of Regnase-1 zinc-motif during larva-to-adult metamorphosis.
Materials and methods
Fly strains
We generated the regnase-1KO allele by introducing a deletion within the Regnase-1 coding region by genome editing using a CRISPR/Cas9 system [31,32]. The deletion removed the five nt residues at the 246-250th nt positions of the 1620 nt-long coding sequence of Regnase-1, creating an in-frame, premature stop codon, resulting in a production of only N-terminal 83 amino acid residue fragment of the total 540 aa residue Regnase-1 protein followed by additional frameshifted 42 amino acid residues. This short fragment lacks the Zc3h12a-like Ribonuclease NYN domain and CCCH-type zinc finger domain and thus is considered to be nonfunctional. The control fly strains used were not isogenic.
The transgenic Regnase-1 fly strains were produced by inserting the coding sequence of wild-type and point mutants of Regnase-1 with a 3xHA-HRV3Csite-3xFLAG epitope tag in a pUASPattB plasmid vector and integrating the transgenes site-specifically within fly genome using the PhiC31 system [33–35].
Western blot
The third instar larvae were homogenised in RIPA buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1% [v/v] IGEPAL CA-630, 0.1% [w/v] sodium dodecyl sulfate (SDS), 0.5% [w/v] sodium deoxycholate, 1 mM ethylenediaminetetraacetic acid (EDTA), 5 mM dithiothreitol, and 0.5 mM phenylmethylsulfonyl fluoride (PMSF). The homogenates were clarified by centrifugation at 21,000 × g at 4°C for 10 min, and the protein concentration was determined using the BCA protein assay kit (Pierce). Fifteen micrograms of total protein was loaded per lane for Western blot. Mouse anti-Flag (1/3000, Sigma, F1804) and Mouse anti-alpha-Tubulin (1/1500, Sigma, T9026) were used as primary antibodies. IRDye 800CW goat anti-Mouse Igg (1/10,000, Li-cor, 827–08364) was used as the secondary antibody. The membranes were scanned on an Odyssey imaging system (Licor).
Microscope imaging
Stereomicroscope images of larvae, pupae, and adult were taken using Leica M125 stereomicroscope and IC80 HD Camera.
RNA preparation
Three biological replicates each of total RNA from third instar larvae and early pupae were purified using miRVana kit (ThermoFisher). Larvae and pupae of a mix of both sexes were directly homogenised in the lysis buffer in miRVana kit. For each biological replicate, tens of larvae or pupae were used. The genotypes used were (1) w1118; +; +, (2) w1118; +; regnase-1KO/+, and (3) w1118; +; regnase-1KO/Df(3R)ED6027.
qRT-PCR
RNA was treated with Turbo DNase (Thermo Fisher Scientific), and then was reverse-transcribed into cDNA using an oligo-dT primer and AMV Reverse Transcriptase (NEB). qPCR was performed using SsoAdvanced Universal SYBR Green Supermix on CFX96 (Biorad) and the primers listed in Table S6.
Northern blot
Northern blot was performed as described previously [36,37]: 7 μg total RNA was denatured in formamide loading buffer (98% v/v formamide, 0.1% w/v bromophenol blue and xylene cyanol, 10 mM EDTA) at 95 C for 2 min and was resolved on a 0.4 mm thick, 12% denaturing polyacrylamide 7 M urea sequencing gel in 0.5× TBE (Tris-Borate-EDTA) buffer. After electrophoresis, RNA was transferred at 20 V for 1 hr to a Hybond-N+ membrane (GE healthcare) in 0.5× TBE buffer using a semi-dry transfer system (Transblot SD, Bio-Rad). The RNA was UV cross-linked (HL2000, UVP) to the membrane and pre-hybridised in Church buffer for at least 60 min at 37 C. DNA oligonucleotide probes (Supplementary Table 6) were 5′ 32P-radiolabeled with T4 polynucleotide kinase (NEB) and γ-32P-ATP. After labelling, non-incorporated nucleotides were removed using a Sephadex G-25 spin column (GE healthcare) and the probes were added to the Church buffer and hybridised for at least 3 hrs at 37 C. Membranes were washed three times for 10 min in 2× SSC containing 0.05% (w/v) SDS at 37 C, subsequently exposed to Storage Phosphor Screens (GE healthcare) and analysed using FLA-9500 (GE healthcare). Probes were stripped from the membranes in boiling 0.1% SDS solution. The membranes were re-probed with the next probe.
High-throughput sequencing of small RNAs (small RNA-seq)
Small RNA libraries were prepared using size-selected 18–30 nt long RNAs by gel purification, sequenced using HiSeq2500 platform (Illumina), and analysed as previously described [32,36,38–44]. 2S RNA was depleted at the size selection step and the 5ʹ adapter ligation step. After adapter sequence was removed using cutadapt 1.18, piPipes was used for analysis.
Approximately 8–12 million reads were obtained for each library. Approximately 54–88% of the reads were mapped to the dm6 Drosophila genome. Approximately 67–90% of the non-rRNA-mapping reads were mapped to miRNA hairpins. The abundance of miRNAs and siRNAs normalised by the sequencing depth (non-rRNA-mapping reads) in each library was calculated. Then, their mean abundance among the three biological replicates was calculated. To eliminate miRNAs with very low expression levels, which are unlikely to have a physiological role, only miRNAs whose mean abundance was more than 100 reads per million total reads in either +/+, -/+ or -/- samples and three endo-siRNAs (esi-1.1, esi-1.2, and esi-2.1) were analysed.
High-throughput sequencing of mRNAs (mRNA-seq)
Poly-A+ mRNA-seq libraries were constructed as previously described [36,44,45,46,47]. Paired-end 100 nt sequencing (2x 100 bp) was performed using HiSeq2500 platform (Illumina). Approximately 9–15 million reads were obtained for each library. After quality and adapter trimming using Trim Galore! and quality check using FastQC, reads were mapped to the dm3 Drosophila genome using HISAT2 [48] on the Galaxy platform [49]. Approximately 93–95% of the paired reads were mapped to the dm3 Drosophila genome. The differential expression was analysed using featureCounts [50] and DESeq2 [51] on the Galaxy platform [52,53]. GO term and KEGG pathway enrichment analyses were performed with WebGestalt (http://www.webgestalt.org) using significantly differentially expressed genes.
Deposited sequenced libraries
The SRA accession number for the small RNA-seq and mRNA-seq libraries reported in this manuscript is PRJNA510420.
Statistical test
Unpaired two-tailed Student’s t-test was performed to determine the statistical significance of changes in small RNA and mRNA abundance. The Benjamini–Hochberg method was used to adjust p-values for multiple comparisons in mRNA-seq analysis.
Supplementary Material
Funding Statement
This work was supported by the National Institute of General Medical Sciences (US) [R01GM116841].
Acknowledgments
This work was supported by the grant from the National Institutes of Health [R01GM116841] to RF.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Thummel CS. Molecular mechanisms of developmental timing in C. elegans and Drosophila. Dev Cell. 2001;1(4): 453–465. PubMed PMID: 11703937. [DOI] [PubMed] [Google Scholar]
- [2].White KP, Rifkin SA, Hurban P, et al. Microarray analysis of Drosophila development during metamorphosis. Science. 1999;286(5447): 2179–2184. PubMed PMID: 10591654. [DOI] [PubMed] [Google Scholar]
- [3].Arbeitman MN, Furlong EE, Imam F, et al. Gene expression during the life cycle of Drosophila melanogaster. Science. 2002;297(5590):2270–2275. PubMed PMID: 12351791. [DOI] [PubMed] [Google Scholar]
- [4].Cheadle C, Fan J, Cho-Chung YS, et al. Stability regulation of mRNA and the control of gene expression. Ann N Y Acad Sci. 2005;1058:196–204. PubMed PMID: 16394137. [DOI] [PubMed] [Google Scholar]
- [5].Ling L, Ge X, Li Z, et al. MicroRNA let-7 regulates molting and metamorphosis in the silkworm, bombyx mori. Insect Biochem Mol Biol. 2014;53:13–21. PubMed PMID: 25016132. [DOI] [PubMed] [Google Scholar]
- [6].Wu W, Xiong W, Li C, et al. MicroRNA-dependent regulation of metamorphosis and identification of microRNAs in the red flour beetle, Tribolium castaneum. Genomics. 2017;109(5–6):362–373. PubMed PMID: 28624536. [DOI] [PubMed] [Google Scholar]
- [7].Matsushita K, Takeuchi O, Standley DM, et al. Zc3h12a is an RNase essential for controlling immune responses by regulating mRNA decay. Nature. 2009;458(7242):1185–1190. PubMed PMID: 19322177. [DOI] [PubMed] [Google Scholar]
- [8].Yokogawa M, Tsushima T, Noda NN, et al. Structural basis for the regulation of enzymatic activity of Regnase-1 by domain-domain interactions. Sci Rep. 2016;6:22324 PubMed PMID: 26927947; PubMed Central PMCID: PMC4772114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Uehata T, Akira S. mRNA degradation by the endoribonuclease Regnase-1/ZC3H12a/MCPIP-1. Biochim Biophys Acta. 2013;1829(6–7):708–713. PubMed PMID: 23500036. [DOI] [PubMed] [Google Scholar]
- [10].Habacher C, Guo Y, Venz R, et al. Ribonuclease-mediated control of body fat. Dev Cell. 2016;39(3):359–369. PubMed PMID: 27746047. [DOI] [PubMed] [Google Scholar]
- [11].Mizgalska D, Wegrzyn P, Murzyn K, et al. Interleukin-1-inducible MCPIP protein has structural and functional properties of RNase and participates in degradation of IL-1beta mRNA. Febs J. 2009;276(24):7386–7399. PubMed PMID: 19909337. [DOI] [PubMed] [Google Scholar]
- [12].Iwasaki H, Takeuchi O, Teraguchi S, et al. The IkappaB kinase complex regulates the stability of cytokine-encoding mRNA induced by TLR-IL-1R by controlling degradation of regnase-1. Nat Immunol. 2011;12(12):1167–1175. PubMed PMID: 22037600. [DOI] [PubMed] [Google Scholar]
- [13].Li M, Cao W, Liu H, et al. MCPIP1 down-regulates IL-2 expression through an ARE-independent pathway. PloS One. 2012;7(11):e49841 PubMed PMID: 23185455; PubMed Central PMCID: PMC3504106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mino T, Murakawa Y, Fukao A, et al. Regnase-1 and roquin regulate a common element in inflammatory mRNAs by spatiotemporally distinct mechanisms. Cell. 2015;161(5):1058–1073. PubMed PMID: 26000482. [DOI] [PubMed] [Google Scholar]
- [15].Dobosz E, Wilamowski M, Lech M, et al. MCPIP-1, alias Regnase-1, controls epithelial inflammation by posttranscriptional regulation of IL-8 production. J Innate Immun. 2016;8(6):564–578. PubMed PMID: 27513529; PubMed Central PMCID: PMC5089914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Monin L, Gudjonsson JE, Childs EE, et al. MCPIP1/Regnase-1 restricts IL-17A- and IL-17C-dependent skin inflammation. J Iimmunol. 2017;198(2):767–775. PubMed PMID: 27920272; PubMed Central PMCID: PMC5225040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Roy A, Zhang M, Saad Y, et al. Antidicer RNAse activity of monocyte chemotactic protein-induced protein-1 is critical for inducing angiogenesis. Am J Physiol Cell Physiol. 2013;305(10):C1021–32. PubMed PMID: 24048733; PubMed Central PMCID: PMC3840202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Suzuki HI, Arase M, Matsuyama H, et al. MCPIP1 ribonuclease antagonizes dicer and terminates microRNA biogenesis through precursor microRNA degradation. Mol Cell. 2011;44(3):424–436. PubMed PMID: 22055188. [DOI] [PubMed] [Google Scholar]
- [19].Lipert B, Wegrzyn P, Sell H, et al. Monocyte chemoattractant protein-induced protein 1 impairs adipogenesis in 3T3-L1 cells. Biochim Biophys Acta. 2014;1843(4):780–788. PubMed PMID: 24418043. [DOI] [PubMed] [Google Scholar]
- [20].Lu W, Ning H, Gu L, et al. MCPIP1 selectively destabilizes transcripts associated with an antiapoptotic gene expression program in breast cancer cells that can elicit complete tumor regression. Cancer Res. 2016;76(6):1429–1440. PubMed PMID: 26833120; PubMed Central PMCID: PMC4794406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Marona P, Gorka J, Mazurek Z, et al. MCPIP1 downregulation in clear cell renal cell carcinoma promotes vascularization and metastatic progression. Cancer Res. 2017;77(18):4905–4920. PubMed PMID: 28716897. [DOI] [PubMed] [Google Scholar]
- [22].Yoshinaga M, Nakatsuka Y, Vandenbon A, et al. Regnase-1 maintains iron homeostasis via the degradation of transferrin receptor 1 and prolyl-hydroxylase-domain-containing protein 3 mRNAs. Cell Rep. 2017;19(8):1614–1630. PubMed PMID: 28538180. [DOI] [PubMed] [Google Scholar]
- [23].Kidoya H, Muramatsu F, Shimamura T, et al. Regnase-1-mediated post-transcriptional regulation is essential for hematopoietic stem and progenitor cell homeostasis. Nat Commun. 2019;10(1):1072 10.1038/s41467-019-09028-w. PubMed PMID: 30842549; PubMed Central PMCID: PMC6403248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Nagahama Y, Shimoda M, Mao G, et al. Regnase-1 controls colon epithelial regeneration via regulation of mTOR and purine metabolism. Proc Natl Acad Sci U S A. 2018;115(43):11036–11041. PubMed PMID: 30297433; PubMed Central PMCID: PMC6205455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lin RJ, Chien HL, Lin SY, et al. MCPIP1 ribonuclease exhibits broad-spectrum antiviral effects through viral RNA binding and degradation. Nucleic Acids Res. 2013;41(5):3314–3326. PubMed PMID: 23355615; PubMed Central PMCID: PMC3597685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].An P, Yamaguchi M, Fukusaki E. Metabolic profiling of Drosophila melanogaster metamorphosis: a new insight into the central metabolic pathways. Metabolomics. 2017;13:29. [Google Scholar]
- [27].Ohhara Y, Kobayashi S, Yamanaka N. Nutrient-dependent endocycling in steroidogenic tissue dictates timing of metamorphosis in Drosophila melanogaster. PLoS Genet. 2017;13(1):e1006583 PubMed PMID: 28121986; PubMed Central PMCID: PMC5298324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Aly H, Akagi K, Ueda H. Proteasome activity determines pupation timing through the degradation speed of timer molecule blimp-1. Dev Growth Differ. 2018;60(8):502–508. PubMed PMID: 30368781. [DOI] [PubMed] [Google Scholar]
- [29].Baehrecke EH. Ecdysone signaling cascade and regulation of Drosophila metamorphosis. Arch Insect Biochem Physiol. 1996;33(3–4):231–244. PubMed PMID: 8913033 DOI: [DOI] [PubMed] [Google Scholar]
- [30].Jeltsch KM, Hu D, Brenner S, et al. Cleavage of roquin and regnase-1 by the paracaspase MALT1 releases their cooperatively repressed targets to promote T(H)17 differentiation. Nat Immunol. 2014;15(11):1079–1089. PubMed PMID: 25282160. [DOI] [PubMed] [Google Scholar]
- [31].Kondo S, Ueda R. Highly improved gene targeting by germline-specific Cas9 expression in Drosophila. Genetics. 2013;195(3):715–721. PubMed PMID: 24002648; PubMed Central PMCID: PMC3813859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhu L, Kandasamy SK, Fukunaga R. Dicer partner protein tunes the length of miRNAs using base-mismatch in the pre-miRNA stem. Nucleic Acids Res. 2018. PubMed PMID: 29373753 DOI: 10.1093/nar/gky043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bischof J, Maeda RK, Hediger M, et al. An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc Natl Acad Sci U S A. 2007;104(9):3312–3317. PubMed PMID: 17360644; PubMed Central PMCID: PMC1805588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Takeo S, SK S, Nandanan K, et al. Shaggy/glycogen synthase kinase 3beta and phosphorylation of sarah/regulator of calcineurin are essential for completion of Drosophila female meiosis. Proc Natl Acad Sci U S A. 2012;109(17):6382–6389. PubMed PMID: 22421435; PubMed Central PMCID: PMC3340032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Zhu L, Kandasamy SK, Liao SE, et al. LOTUS domain protein MARF1 binds CCR4-NOT deadenylase complex to post-transcriptionally regulate gene expression in oocytes. Nat Commun. 2018;9(1): 4031 10.1038/s41467-018-06404-w. PubMed PMID: 30279526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Fukunaga R, Han BW, Hung JH, et al. Dicer partner proteins tune the length of mature miRNAs in flies and mammals. Cell. 2012;151(3):533–546. PubMed PMID: 23063653; PubMed Central PMCID: PMC3609031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lin X, Steinberg S, Kandasamy SK, et al. Common miR-590 variant rs6971711 present only in African Americans reduces miR-590 biogenesis. PloS One. 2016;11(5):e0156065 PubMed PMID: 27196440; PubMed Central PMCID: PMC4873136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Fukunaga R, Colpan C, Han BW, et al. Inorganic phosphate blocks binding of pre-miRNA to dicer-2 via its PAZ domain. Embo J. 2014;33(4):371–384. PubMed PMID: 24488111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Han BW, Wang W, Li C, et al. Noncoding RNA. piRNA-guided transposon cleavage initiates Zucchini-dependent, phased piRNA production. Science. 2015;348(6236):817–821. PubMed PMID: 25977554; PubMed Central PMCID: PMC4545291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Han BW, Wang W, Zamore PD, et al. piPipes: a set of pipelines for piRNA and transposon analysis via small RNA-seq, RNA-seq, degradome- and CAGE-seq, ChIP-seq and genomic DNA sequencing. Bioinformatics. 2015;31(4):593–595. PubMed PMID: 25342065; PubMed Central PMCID: PMC4325541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kandasamy SK, Fukunaga R. Phosphate-binding pocket in dicer-2 PAZ domain for high-fidelity siRNA production. Proc Natl Acad Sci U S A. 2016;113(49):14031–14036. PubMed PMID: 27872309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kandasamy SK, Zhu L, Fukunaga R. The C-terminal dsRNA-binding domain of Drosophila dicer-2 is crucial for efficient and high-fidelity production of siRNA and loading of siRNA to argonaute2. Rna. 2017;23(7):1139–1153. PubMed PMID: 28416567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Fukunaga R. Loquacious-PD removes phosphate inhibition of dicer-2 processing of hairpin RNAs into siRNAs. Biochem Biophys Res Commun. 2018;498(4):1022–1027. PubMed PMID: 29550490; PubMed Central PMCID: PMC5881606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Vakrou S, Fukunaga R, Foster DB, et al. Allele-specific differences in transcriptome, miRNome, and mitochondrial function in two hypertrophic cardiomyopathy mouse models. JCI Insight. 2018;3(6). PubMed PMID: 29563334 DOI: 10.1172/jci.insight.94493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zhang Z, Theurkauf WE, Weng Z, et al. Strand-specific libraries for high throughput RNA sequencing (RNA-Seq) prepared without poly(A) selection. Silence. 2012;3(1):9 PubMed PMID: 23273270; PubMed Central PMCID: PMC3552703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zhu L, Liao SE, Ai Y, Fukunaga R. RNA methyltransferase BCDIN3D is crucial for female fertility and miRNA and mRNA profiles in Drosophila ovaries. PLoS One. 2019. May 30;14(5):e0217603. doi: 10.1371/journal.pone.0217603 eCollection 2019. PMID: 31145769 [DOI] [PMC free article] [PubMed]
- [47].Liao SE, Kandasamy SK, Zhu L, Fukunaga R. DEAD-box RNA helicase Belle post-transcriptionally promotes gene expression in an ATPase activity-dependent manner. RNA. 2019;25:825-839. PMID: 30979781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 2015;12(4):357–360. PubMed PMID: 25751142; PubMed Central PMCID: PMC4655817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Afgan E, Baker D, Batut B, et al. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Res. 2018;46(W1):W537–W44. PubMed PMID: 29790989; PubMed Central PMCID: PMC6030816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30(7):923–930. PubMed PMID: 242276770. [DOI] [PubMed] [Google Scholar]
- [51].Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550 PubMed PMID: 25516281; PubMed Central PMCID: PMC4302049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Trapnell C, Roberts A, Goff L, et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc. 2012;7(3):562–578. PubMed PMID: 22383036; PubMed Central PMCID: PMC3334321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Afgan E, Baker D, van Den Beek M, et al. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2016 update. Nucleic Acids Res. 2016;44(W1):W3–w10. Epub 2016/05/04 PubMed PMID: 27137889; PubMed Central PMCID: PMCPMC4987906. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


