Abstract
The conversion of circular genomes to linear chromosomes during molecular evolution required the invention of telomeres. This entailed the acquisition of factors necessary to fulfill two new requirements: the need to fully replicate terminal DNA sequences and the ability to distinguish chromosome ends from damaged DNA. Here we consider the multifaceted functions of factors recruited to perpetuate and stabilize telomeres. We discuss recent theories for how telomere factors evolved from existing cellular machineries and examine their engagement in nontelomeric functions such as DNA repair, replication, and transcriptional regulation. We highlight the remarkable versatility of protection of telomeres 1 (POT1) proteins that was fueled by gene duplication and divergence events that occurred independently across several eukaryotic lineages. Finally, we consider the relationship between oxidative stress and telomeres and the enigmatic role of telomere-associated proteins in mitochondria. These findings point to an evolving and intimate connection between telomeres and cellular physiology and the strong drive to maintain chromosome integrity.
Keywords: telomerase, shelterin, telomerase reverse transcriptase (TERT), DNA replication, molecular evolution, CST, oxidative damage, POT1, protection Of telomeres 1, genome integrity, chromosome ends, telomere repeat
Introduction
Molecular evolution is opportunistic, enabling novel cellular mechanisms to arise in response to biological challenges. One such challenge was conversion of the circular prokaryotic genome into the multiple linear DNA forms that comprise the eukaryotic genome (1). This challenge necessitated the invention of telomeres. Here we discuss the origin and evolution of telomere-related functions. Although the factors associated with chromosome ends were initially thought to be specific for this locale, in-depth analysis has revealed many such factors having noncanonical, so-called “moonlighting” roles in other transactions within the nucleus and the cytoplasm. We now appreciate that some of the moonlighting contributions may reflect ancestral functions preserved from the dawn of genome linearization, whereas others may be newly emergent.
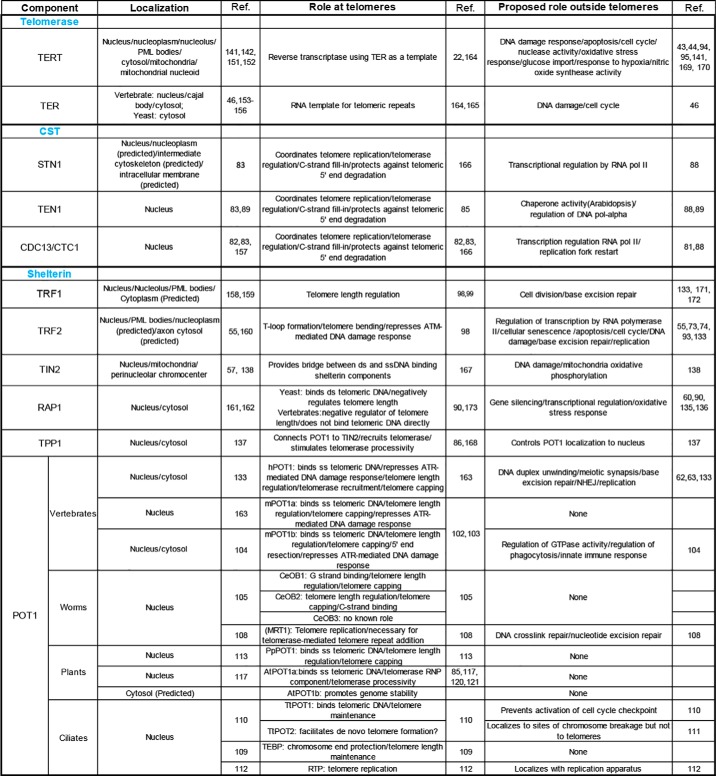
There are several theories for how linear chromosomes evolved from their circular progenitors (2, 3), but one of the more intriguing proposals is that invasion of circular genomes by group II introns (1), via reverse splicing and reverse transcription, led to DNA linearization (4, 5) (Fig. 1). Specifically, it is posited that non-LTR2 retrotransposons targeted to double-strand breaks (DSBs) served as “proto-telomeres” (6). The nascent chromosome ends presented two immediate challenges: the “end replication” problem and need for “end protection” (7, 8). The end replication problem occurs because the DNA replication machinery cannot fully replicate the extreme terminus of the lagging strand, which would lead to the gradual depletion of terminal DNA sequences when the genome is duplicated (9, 10). The chromosome ends may also be perceived as a DSB and must therefore be sequestered to prevent activation of the DNA damage response. Such end protection is also crucial for the avoidance of end-to-end fusions of chromosomes, which would cause improper chromosome segregation during mitosis, cell cycle arrest, genome instability, senescence, and cell death (8, 11). Most eukaryotes cope with these problems by 1) adding long arrays of noncoding DNA repeats to serve as a physical buffer to protect coding regions from attrition and 2) formation of higher-order DNA architecture that helps distinguish chromosome ends from a DSB (i.e. fold-back structures in yeast (12) and t-loops in other species (13, 14)).
Figure 1.
Model for evolution of telomerase and telomere-associated components from group II retrotransposons and DNA repair proteins. After chromosome linearization by the insertion of group II retrotransposons, telomerase and DNA repair proteins evolved roles in telomere maintenance and end protection. Telomere-associated factors also participate genome-wide in transcription, replication, and repair. Other factors function in mitochondria to modulate the response to oxidative stress. Whether the mitochondrial functions of telomere proteins reflect an ancient or newly evolved function is unknown (see text for details).
Emergence of telomerase
To help overcome the telomere end replication problem, a group II intron likely gained the ability to use the 3′ end of linearized chromosomes as a template for reverse transcription (5). There is strong evidence that the telomerase catalytic subunit TERT evolved from a non-LTR class 2 retrotransposon (15–17) (Fig. 1). Fruit flies and silkworms maintain their chromosome ends through a telomerase-independent mechanism that employs a different class of retrotransposons (18, 19), supporting the idea that retrotransposons played an early and critical role in establishing and maintaining telomere architecture (20, 21).
The modern-day enzyme that helps solve the end replication problem is telomerase, a reverse transcriptase that compensates for incomplete replication by continually replenishing terminal DNA using a long noncoding RNA, TER, as template (22). It is possible that TER arose from a transcript derived from the progenitor group II intron (5) (Fig. 1), but TER and TERT are now encoded from separate loci in the genome. TERT can interact with a large array of RNAs in vivo (23). Ultimately, an RNA emerged with a higher affinity for TERT, a short C-rich repeat that could serve as a telomere sequence template, and a stem-loop element abutting the template that could form a functional template boundary element to allow fidelity of telomere repeat addition by TERT (24). Unlike TERT, TER is constrained by structure and not sequence (25, 26). Consequently, TER sequences diverged and expanded to give accessory proteins a foothold in the RNP complex. These new telomerase proteins enabled RNP maturation and both positive and negative regulation of the enzyme (27, 28).
Telomere-associated proteins: Origins and their role in telomere end protection
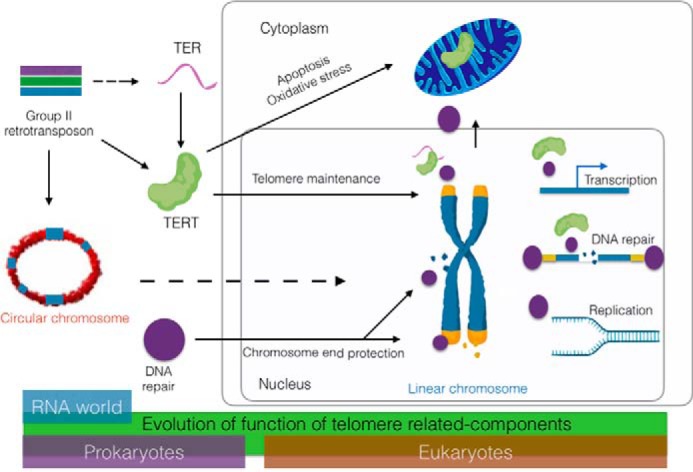
In vertebrates and fission yeast, telomere end protection is mediated by shelterin (29, 30) (Fig. 2 and Table 1). Shelterin physically caps the telomere ends, preventing the termini from being recognized as DNA damage and suffering DNA attrition via nucleolytic processing and DNA damage checkpoint activation. Shelterin is composed of TRF1/TRF2 (SpTAZ1), which binds the duplex DNA, and POT1-TPP1/SpPot1-SpTpz1, which binds the 3′ single-strand extension on the extreme terminus (termed the G-overhang). Additional proteins bridge the two DNA-binding complexes (TIN2(SpPOZ1) and RAP1). In addition to end protection, shelterin controls telomerase access and therefore contributes to telomere length regulation (29).
Figure 2.
Models for chromosome end protection. Human telomeres are protected by the shelterin complex. CST transiently associates with the telomeric G-overhang during S phase to facilitate replication of the C-rich telomeric strand. In budding yeast, CST provides a stable, protective cap on the G-overhang, and RAP1, a shelterin component ortholog, binds the duplex region of telomeric DNA. Arabidopsis telomeres are asymmetrical. Ku maintains a blunt end on one chromosome terminus, whereas the other end harbors a conventional G-overhang that is bound by CST. There are two functional POT1 paralogs in A. thaliana. AtPOT1a is a component of the telomerase RNP, whereas AtPOT1b promotes genome stability and is proposed to reside in the cytoplasm.
Table 1.
Localization and functions of telomere-related components
Tabulated is a summary of published experimental data and in silico predictions for core constituents of the telomerase RNP, the CST complex, and the Shelterin complex. Different functions ascribed to POT1 paralogs from vertebrates, worms, plants, and ciliates are highlighted.
In budding yeast, instead of chromosome end protection by shelterin, the G-overhang is stably bound by the CST complex, comprised of Cdc13(CTC1), STN1, and TEN1 proteins (31) (Fig. 2 and Table 1). Notably, vertebrates also possess CST, but this complex only transiently associates with telomeres during S phase to promote telomeric DNA replication. CST is structurally related to the single-strand DNA-binding complex RPA and had likely evolved from the latter (32, 33). Interestingly, Drosophila lacks canonical telomere repeat arrays at its chromosome termini and yet encodes one or more proteins related to CST subunits (34, 35). Flowering plants, including Arabidopsis, present yet another twist on the telomere protection apparatus wherein one half of the chromosome ends harbor a G-overhang bound by CST, whereas the other half are blunt-ended and bound by Ku, which functions in the nonhomologous DNA end joining (NHEJ) pathway of DSB repair and has high affinity for DNA ends (36) (Fig. 2). The asymmetry of plant telomeres may reflect the absence of a 5′ exonuclease (e.g. Apollo) (37) that normally converts blunt-end telomeres created from leading-strand synthesis into termini with the typical 3′ G-overhang (38).
The shelterin and CST proteins employ one of two DNA binding motifs: the MYB domain for duplex DNA binding and the oligonucleotide/oligosaccharide-binding fold (OB-fold) for interaction with single-strand DNA. The MYB motif is common in transcription factors and may have been predisposed to function at telomeres as it is capable of binding tandemly repeated sequences (39, 40). OB-folds, on the other hand, function in a vast array of nucleic acid transactions and are found in proteins ranging from t-RNA synthetases to nucleases and RPA (41). Thus, the single-strand telomere-binding proteins have likely diverged from a common OB-fold ancestor with a role in DNA repair and/or replication (42).
Telomere protection and DNA repair
DNA damage repair pathways and telomere-associated proteins act collaboratively to promote genome integrity. Both TERT and TER have been linked to the DNA damage response (Table 1). In the presence of a DSB, human TERT relocalizes to the nucleolus (43), an outcome that would decrease the probability of de novo telomere formation at sites of DNA damage. In addition, human cells lacking TERT fail to mount an effective DNA damage response to ionizing radiation (44). Intriguingly, these cells also display altered chromatin structure and fragmented chromosomes, suggesting that TERT plays a role in chromatin reorganization (45). Human TER (hTR) has been proposed to play a TERT-independent role in the response to DNA damage. Inhibition of hTR causes rapid arrest of cell growth, whereas increased hTR, which occurs in response to DNA damage induced by UV light, inhibits the DNA damage checkpoint kinase ATR (46). In contrast, loss of TR in mice does not trigger phenotypes distinct from those of mTERT mutants, suggesting that the core RNA and protein components of telomerase act in the same pathways (47, 48).
Shelterin proteins also modulate the DNA damage response (Table 1). TRF2, for instance, prevents ATM-mediated DNA damage signaling at telomeres (49) and also helps recruit various DNA damage response and repair factors, such as ERCC1, Apollo, the MRE11-RAD50-NBS1 complex, helicases BLM and WRN, Ku, and PARP1/2 (50) (Table 1). The recruitment of these factors facilitates telomeric DNA replication, promotes the formation of a single-strand overhang on the chromosome terminus, and ensures that telomeres are properly sequestered to prevent inappropriate recombination or activation of a DNA damage response (51, 52). TRF2 can also associate with DSBs within the body of the chromosome as part of the early response to DNA damage (53, 54). As such, the ability of TRF2 to engage the machineries concerned with the DNA damage response and DNA repair likely promotes genome stability on a global scale. Interestingly, both TRF1 and TRF2 are modified by MMS21, a SUMO ligase involved in DNA repair and recombination. This modification is associated with alternative lengthening of telomeres (ALT) (55), a mechanism germane for telomere maintenance in cancer cells that lack telomerase (56).
Like TRF2, TIN2 and RAP1 associate with chromosome locales other than the telomeres. TIN2 accumulates at nontelomeric regions (57) associated with HP1 (58), a heterochromatin mark that has been implicated in the DNA damage response (59). Moreover, in human cells, RAP1 interacts with noncoding interstitial TTTAGGG repeats present on some chromosomes, raising the possibility that RAP1 helps prevent fragility and recombination at these sites (60).
POT1 has also been implicated in the DNA damage response (Table 1) (Fig. 3). The association of POT1 with the telomeric G-overhang prevents activation of an ATR-mediated DNA damage response (61), and recent studies indicate that human POT1 increases the fidelity of NHEJ at nontelomeric sites (62). Intriguingly, the C terminus of hPOT1 bears structural similarity to a Holliday junction resolvase domain (63), supporting the notion that POT1 affects other facets of DNA metabolism beyond telomere biology.
Figure 3.
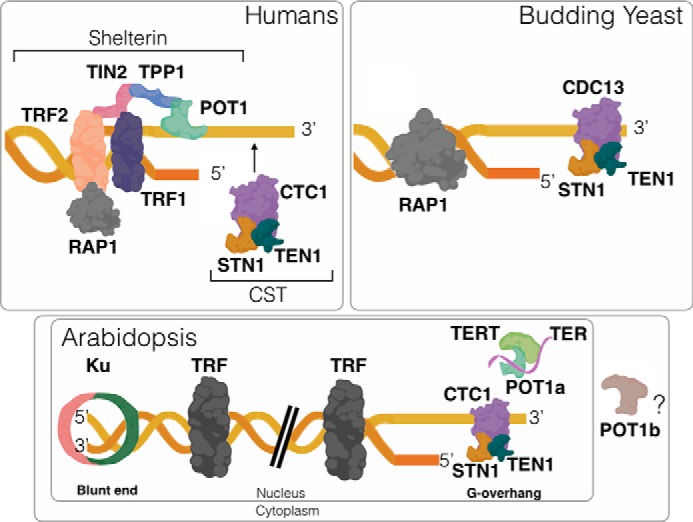
Diverse functions of POT1. Many POT1 orthologs bind single-stranded G-rich telomeric DNA, serving to control telomere length and to protect chromosome ends from eliciting the DNA damage response. Other POT1 proteins are tailored to engage the telomeric C-strand and its replication machinery. There are also examples of POT1 proteins that do not stably engage the chromosome terminus, but rather function to stimulate telomerase activity or to facilitate DNA repair. In addition, several POT1 proteins have been shown to accumulate in the cytoplasm or are predicted to reside here. Cytoplasmic mouse POT1b (mPOT1b) is proposed to promote an innate immunity response. Shown are the A. thaliana POT1a (AtPOT1a) and POT1b (AtPOT1b); C. elegans POT1 proteins CeOB1, CeOB2, and MRT1; human POT1 (hPOT1), mouse POT1a (mPOT1a), and POT1b (mPOT1b); P. patens POT1 (PpPOT1); and T. thermophila POT1 (TtPOT1) and POT2 (TtPOT2).
Ku harbors two subunits (Ku70 and Ku80) and is a core component of the NHEJ pathway (64). Within the context of telomere biology, Ku facilitates telomere protection and telomeric DNA replication (36, 65, 66). Recent studies in budding yeast provide clues for how the DNA repair and telomere protection functions of Ku might be parsed at chromosome termini. Ku harbors two solvent-exposed α-helices on opposite sides of the heterodimer. The surface facing the telomere end is necessary for NHEJ, whereas the inward facing helix is required for telomeric heterochromatin formation (67). In addition to discrete structural boundaries, separation of function can be influenced by cell cycle regulation. For example, the cell cycle regulator CYREN was recently shown to interact with Ku and block NHEJ at telomeres during the S and G2 cell cycle phases (68). Ebrahimi and Cooper (69) have postulated that localization of telomeres within different regions of the nucleus influences a broad range of cellular processes, including meiotic recombination, chromosome segregation, and gene expression. Hence, in a broader sense, both temporal and spatial regulation of telomeres impact cellular physiology.
A role for telomere-associated proteins in DNA replication and transcription
Given that telomere accessory factors have likely evolved from factors that function in DNA repair, DNA replication, and transcription, it is not surprising that some of the telomere-associated factors also function in the aforementioned processes. Because of the highly repetitive nature of G-rich telomeric DNA and its propensity to form higher-order structures, such as the G-quartet, auxiliary factors are needed to ensure timely and proper replication through telomeric tracts. Notably, both POT1 and TRF2 stimulate the helicase activity of WRN (70, 71), and POT1 has been found to promote G-quartet unwinding by the WRN and BLM helicases (72) (Table 1). TRF2 has been proposed to assist in telomeric replication, and it does so by inducing positive supercoiling in DNA that favors enhanced access by DNA topoisomerases and the Apollo nuclease, enzymes critical for replication (73, 74). Furthermore, TRF2 is also hypothesized to assist in the assembly of the prereplication complex during telomere replication (75, 76).
The primary function of the CST heterotrimer appears to be in telomere replication (Table 1). Originally identified as a DNA Pol α accessory factor (77), the vertebrate CST complex was subsequently shown to stimulate synthesis of the telomeric C-strand after telomerase extends the G-strand (78–80). CST plays a crucial role in the restart of stalled replication forks at nontelomeric sites (81), and CST mutations lead to genome-wide instability (82, 83). Vertebrate CST only transiently engages telomeres (84), but in budding yeast and in Arabidopsis thaliana, CST is a constitutive component of telomeres that facilitates both replication of the C-rich telomere strand by Pol α/primase and the G-rich strand by stimulating telomerase activity (82, 84, 85). Hence, some of the POT1-TPP1 functions within the context of shelterin (86) may be fulfilled by CST. Indeed, Lue (87) has provided a compelling argument that POT1-TPP1 evolved from CST. The multifunctional nature of CST is further evidenced by the involvement of components of the yeast complex in transcriptional regulation through interactions with RNA polymerase II and the elongation factor Spt5. The interactions of CST with the transcription machinery are thought to help mitigate the consequences of RNA polymerase II collision with replication forks (88). In addition, studies in Arabidopsis have revealed that the CST component TEN1 possesses protein chaperone activity that is activated in response to heat stress (89) (Table 1).
Besides CST, other telomere-associated proteins also influence transcriptional regulation (Table 1). Yeast RAP1 was originally described as a transcriptional regulator at many promoters (90, 91). Human RAP1 modulates NF-κB expression (92), whereas interaction of TRF2 with the promoter of the cyclin-dependent kinase CDKN1a affects its expression (93). TERT has also been reported to enhance the expression of genes such as cyclin D1 (94) and NF-κB (95).
Gene duplication: Refining the landscape of telomere protein function
Gene duplication has fueled protein evolution, including telomere proteins. The duplication event giving rise to vertebrate TRF1 and TRF2 dates back 540 million years ago (96), at the beginning of the Chordate lineage (97). The conserved C-terminal MYB domain of TRF1/2 facilitates telomeric DNA engagement, whereas divergent N-terminal domains (98) are important for telomeric DNA length regulation (primarily accomplished by TRF1) (99) or chromosome end protection (TRF2) (100) (Table 1). The Candida clade possesses two copies of the gene that encodes the Cdc13 component of CST (101). The two paralogous proteins, Cdc13A and Cdc13B, are significantly smaller than their counterparts in budding yeast and have overlapping but nonredundant functions in telomere length regulation.
One of the most fascinating outcomes of gene duplication is seen with POT1 (Fig. 3) (Table 1). Here, independent gene duplication events occurred repeatedly throughout evolution. Although humans have a single POT1 protein, mice possess two POT1 paralogs, mPOT1a and mPOT1b, that share 72% sequence similarity (102). Recent studies suggest that both mPOT1a and mPOT1b attenuate ATR signaling at chromosome ends (103). However, mPOT1b uniquely contributes to the regulation of 5′ end resection to form the 3′ G-overhang (103) and may also play a cytosolic role in the innate immunity response (104).
The POT1 isoforms in worms and ciliated protozoa exhibit more profound functional divergence. Caenorhabditis elegans encodes four single OB-fold proteins with structural similarity to the OB-folds of mammalian POT1 (105). CeOB1 binds the telomeric G-rich strand, whereas CeOB2 engages the complementary C-rich strand. Mutation in either of these CeOB genes leads to telomere elongation, providing evidence that their encoded proteins serve as a negative regulator of telomerase (106, 107). The function for CeOB3 is unknown; however, CeOB4 (MRT1) was originally identified in a screen for genes required for germ line mortality as a result of telomere shortening (108). CeOB4 is required for telomerase activity in vivo. Intriguingly, CeOB4 also bears a SNM1 family nuclease domain and has been implicated in both DNA cross-link and nucleotide excision repair (108).
In the ciliates Euplotes crassus and Tetrahymena thermophila, there are two POT1 paralogs (109, 110). The Tetrahymena TtPOT1-encoded protein is essential for telomere length maintenance and prevents checkpoint activation much like the vertebrate POT1 proteins (110). However, TtPOT2 protein does not associate with chromosome ends, but instead localizes to internal sites in macronuclear chromosomes that are destined for developmentally programmed cleavage and de novo telomere formation (111). In E. crassus, the telomere end-binding protein caps chromosome ends (109). Replication telomere protein, the other POT1-like protein, is not associated with telomeres, but rather co-localizes with the replication apparatus as it moves through the macronuclear genome (112). This remarkable observation underscores the strong connection between telomere proteins and the DNA replication machinery.
The plant kingdom is replete with large gene families, arising from both localized gene duplication and whole-genome duplication. It is therefore noteworthy that most POT1 genes in plants are not duplicated. The POT1 gene in the early diverging land plant Physcomitrella patens retains the ancestral functions of binding single-stranded G-rich telomeric DNA and protecting chromosome ends from fusion (113). However, at least two independent POT1 duplications occurred in higher plants, one in the grasses and the other in the Brassicaceae family to which A. thaliana belongs (114). There are three POT1 paralogs in A. thaliana, AtPOT1a, AtPOT1b, and AtPOT1c (114, 115). AtPOT1a and AtPOT1b exhibit only 52% sequence similarity. AtPOT1a resembles the mammalian shelterin component TPP1 (86, 116) in that it physically associates with the telomerase RNP and stimulates its repeat addition processivity (84, 117). However, unlike TPP1 (118), AtPOT1a accumulates at telomeres only in S phase (117), indicating that it is not a stable component of the end protection complex. Initially, AtPOT1a was not thought to bind telomeric DNA (119), but a recent study showed that the first OB-fold of AtPOT1a has single-strand telomeric DNA-binding activity (120). Strikingly, the AtPOT1a lineage, but not AtPOT1b, has been subjected to positive selection from an ancestral POT1 protein, leading to enhanced interaction with CST (114). Hence, AtPOT1a appears to have been evolved to be specialized for telomere maintenance through CST interaction. A role for AtPOT1b in telomere biology is not clear. It cannot complement the pot1a mutant (114) and cannot bind telomeric DNA in vitro (120). However, overexpression of the AtPOT1b C-terminal domain leads to massive chromosome fusion (121). Whereas this finding implicates AtPOT1b in chromosome end protection, AtPOT1b probably does so in a manner distinct from the single-copy POT1 proteins from vertebrates and fission yeast. The third POT1 gene in A. thaliana, AtPOT1c, arose only 5 million years ago as a partial duplication of the AtPOT1a locus. The insertion of a transposon into the promoter of AtPOT1c rendered this gene silent almost immediately after its genesis (122). This finding, coupled with the remarkable functional divergence associated with POT1 paralogs across eukarya, argues that POT1 dosage affects the fitness of organisms, and one or more of the duplicated copies must diverge quickly or be silenced.
Telomere proteins and their role in the genome-wide response to oxidative stress
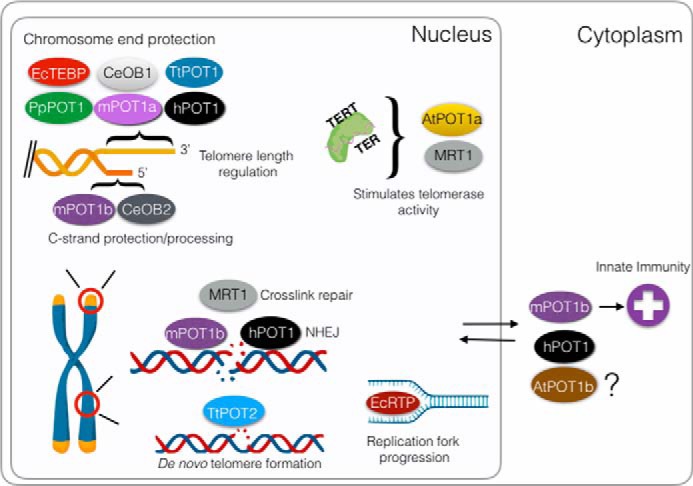
The majority of DNA lesions in mammalian and plant cells can be attributed to oxidative damage (123, 124), and recent data indicate that several shelterin components safeguard the genome against this assault (Fig. 4). Reactive oxygen species (ROS) modifies DNA bases, most commonly resulting in 8-oxo-guanine (8-oxoG) and thymine glycol (Tg) (125). If not repaired, 8-oxoG induces GC-TA transversion mutations as well as single-strand or double-strand breaks, leading to genomic instability (126). Tg is the most prevalent oxidative product of thymine, responsible for 10–20% of ionizing radiation-induced genomic damage (127). Due to their high G-T content, telomeres are a hot spot for oxidative damage (128–130).
Figure 4.
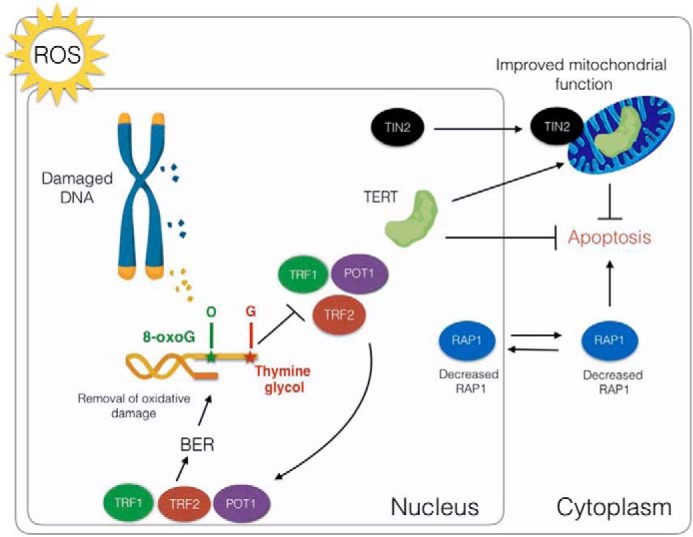
Impact of oxidative stress on telomeres and telomere-associated proteins in mammals. Telomeres are a hot spot for oxidative damage causing base modifications including thymine to thymine glycol and guanine to 8-oxoG. These lesions interfere with DNA binding by TRF1, TRF2, and POT1. These same proteins stimulate BER at telomeres and perhaps elsewhere in the genome, enabling the removal of damaged bases from the DNA. Oxidative DNA damage decreases the abundance of both cytoplasmic and nuclear RAP1, which in turn triggers apoptosis. Conversely, oxidative stress leads to the accumulation in mitochondria of TERT and TIN2, which promote mitochondrial functions that protect against apoptosis.
Base excision repair (BER) is the most important pathway for removing 8-oxoG and Tg lesions (131). Mice lacking the glycosylase NTH1, which removes Tg via BER, exhibit increased telomere fragility (132). Intriguingly, TRF1, TRF2, and POT1 stimulate BER after oxidative damage (133). Interestingly, 8-OxoG and Tg modifications inhibit telomeric DNA binding by TRF1, TRF2, and POT1 in vitro (134). These observations suggest a feedback loop wherein oxidative damage at telomeres leads to the expulsion of the aforementioned telomere proteins, which then become available to assist in the BER-mediated repair of damaged telomeric bases, so as to enable the re-engagement of shelterin at the chromosome terminus (133) (Fig. 4). Because TRF1 and TRF2 can associate with other genomic locales, they may exert a broader impact in the response to oxidative stress.
Recent data reveal an intriguing response of RAP1 to oxidative stress and other types of DNA damage (135). RAP1 levels decrease in the nucleus and the cytoplasm in response to ROS. Diminished levels of cytoplasmic RAP1 appear to promote apoptosis in aging cells (92) (Fig. 4). Notably, in yeast, shortening of telomeres due to senescence releases RAP1, which then becomes associated with extratelomeric sites. Release of RAP1 from telomeres correlates with the down-regulation of genes encoding core histones and the translational apparatus and up-regulation of genes responsive to senescence (136) (Table 1).
Genome protection from a distance: The role of telomere proteins outside the nucleus
The role of telomere proteins in the response to oxidative stress correlates with cytoplasmic activities, but the molecular mechanisms that govern telomere protein function outside the nucleus are largely unexplored. In addition to RAP1, several other telomere-related proteins accumulate in the cytoplasm (Table 1). POT1, TPP1, and trace amounts of TIN2 shuttle in and out of the nucleus and can be detected as subcomplexes in the cytoplasm (137). TTP1 bears a nuclear export signal that is crucial for modulating the levels of the TTP1-POT1 complex within the nucleus. Abrogation of TPP1 nuclear export causes overelongation of telomeres and activates the DNA damage response (137).
TIN2 possesses a mitochondrial targeting sequence (MTS) that enables its transport into the mitochondria, where it is post-translationally modified (138) (Fig. 4). Interestingly, in cells lacking TIN2, glycolysis is inhibited, and ROS production is elevated along with ATP and oxygen consumption. Strikingly, these phenotypes do not correlate with telomeric abnormalities (138), indicating that TIN2's mitochondria-related functions are distinct from its role at telomeres.
TERT proteins from vertebrates and plants also harbor a MTS (Table 1). Extracts prepared from mitochondria are enriched in telomerase activity (139). In addition, TERT is associated with the outer mitochondrial membrane translocators TOM20 and TOM40 (140, 141) as well as tFAM, HSP60, tim23, and a variety of mitochondrial RNAs (23, 142). Notably, oxidative stress triggers hTERT export from the nucleus to mitochondria, and elevated levels of hTERT in this compartment correlate with stabilization of mitochondrial DNA, reduced ROS, increased mitochondrial membrane potential, and enhanced mitochondrial function (143–145) (Fig. 4). There are also reports that mitochondrial TERT not only associates with non-TER RNAs but also possesses noncanonical enzyme activities. These include an RNA-dependent RNA polymerase activity that is implicated in the production of siRNA (23) and reverse transcriptase activity using mitochondrial tRNA as a template (142). The biological relevance of this latter activity is unknown.
TERT in the mitochondria has been proposed to stimulate mitochondrial DNA replication and repair (142). Compared with WT mice, the RNA expression profiles of tert mutants monitored for four consecutive generations (G1–G4) reveal statistically significant changes in the expression of both mitochondrial and nuclear encoded genes required for oxidative phosphorylation, mitochondrial function, and antioxidant defense (146). Similar results were obtained A. thaliana tert mutants of generations G2 and G7 (147). Interestingly, yeast and ciliate TERT proteins lack an MTS (139), raising the possibility that the mitochondrial function of TERT is not conserved in these species or that these TERT proteins are transported into mitochondria via a different mechanism.
Conclusions and future directions
With the advent of linear chromosomes, factors involved in different facets of DNA metabolism were coopted to solve the telomere end protection and end replication problems. Some of these factors retain their functions in DNA replication, DNA repair, and transcriptional regulation. Telomeric DNA is a magnet for oxidative damage, and hence in the drive to maintain genome integrity, telomere proteins may have gained the capacity to protect chromosome ends from this assault by promoting BER proximally, or at a distance by affecting mitochondrial function. Alternatively, some noncanonical functions of telomere proteins may have an older origin. Mitochondria, which possess group II introns (148) and proteins structurally similar to the ancestral OB-folds of RPA (149), emerged 1.45 billion years ago (150). Thus, the building blocks for some of the modern-day telomere proteins and their functions in the oxidative stress response may reflect a mitochondrial ancestry. Finally, the ancient and emerging functions of telomere proteins have been linked to gene duplication events. In particular, POT1 gene duplications that occurred across evolution have given rise to multifaceted roles of telomere proteins in chromosome biology and their integration into the broader context of cellular physiology.
Acknowledgments
We apologize to colleagues whose work we were unable to cite due to space limitations. We thank members of the Shippen laboratory for insightful comments on the manuscript and the American Society for Biochemistry and Molecular Biology for highlighting our research. Components of all of the figures were created using BioRender software.
This work was supported by National Institutes of Health Grants R01 GM065383 and R01 GM127402 and National Science Foundation Grant MCB 1517817 (to D. E. S.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- LTR
- long terminal repeat
- DSB
- double-strand break
- NHEJ
- nonhomologous DNA end joining
- OB-fold
- oligonucleotide/oligosaccharide-binding fold
- ROS
- reactive oxygen species
- 8-oxoG
- 8-oxo-guanine
- Tg
- thymine glycol
- BER
- base excision repair
- MTS
- mitochondrial targeting sequence.
References
- 1. Cavalier-Smith T. (2010) Origin of the cell nucleus, mitosis and sex: roles of intracellular coevolution. Biol. Direct. 5, 7 10.1186/1745-6150-5-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ishikawa F., and Naito T. (1999) Why do we have linear chromosomes? A matter of Adam and Eve. Mutat. Res. 434, 99–107 10.1016/S0921-8777(99)00017-8 [DOI] [PubMed] [Google Scholar]
- 3. Volff J.-N., and Altenbuchner J. (2000) A new beginning with new ends: linearisation of circular chromosomes during bacterial evolution. FEMS Microbiol. Lett. 186, 143–150 10.1111/j.1574-6968.2000.tb09095.x [DOI] [PubMed] [Google Scholar]
- 4. Eickbush T. H. (1997) Molecular biology: telomerase and retrotransposons: which came first? Science 277, 911–912 10.1126/science.277.5322.9k, 10.1126/science.277.5328.911 [DOI] [PubMed] [Google Scholar]
- 5. de Lange T. (2015) A loopy view of telomere evolution. Front. Genet. 6, 321 10.3389/fgene.2015.00321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Garavís M., González C., and Villasante A. (2013) On the origin of the eukaryotic chromosome: The role of noncanonical DNA structures in telomere evolution. Genome Biol. Evol. 5, 1142–1150 10.1093/gbe/evt079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lingner J., Cooper J. P., and Cech T. R. (1995) Telomerase and DNA end replication: no longer a lagging strand problem? Science 269, 1533–1534 10.1126/science.7545310 [DOI] [PubMed] [Google Scholar]
- 8. de Lange T. (2009) How telomeres solve the end-protection problem. Science 326, 948–952 10.1126/science.1170633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Watson J. D. (1972) Origin of concatemeric T7 DNA. Nat. New Biol. 239, 197–201 10.1038/239197a0 [DOI] [PubMed] [Google Scholar]
- 10. Olovnikov A. M. (1973) A theory of marginotomy: the incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. J. Theor. Biol. 41, 181–190 10.1016/0022-5193(73)90198-7 [DOI] [PubMed] [Google Scholar]
- 11. Sfeir A., and de Lange T. (2012) Removal of shelterin reveals the telomere end-protection problem. Science 336, 593–597 10.1126/science.1218498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Bruin D., Kantrow S. M., Liberatore R. A., and Zakian V. A. (2000) Telomere folding is required for the stable maintenance of telomere position effects in yeast. Mol. Cell. Biol. 20, 7991–8000 10.1128/MCB.20.21.7991-8000.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de Lange T. (2004) T-loops and the origin of telomeres. Nat. Rev. Mol. Cell Biol. 5, 323–329 10.1038/nrm1359 [DOI] [PubMed] [Google Scholar]
- 14. Griffith J. D., Comeau L., Rosenfield S., Stansel R. M., Bianchi A., Moss H., and de Lange T. (1999) Mammalian telomeres end in a large duplex loop. Cell 97, 503–514 10.1016/S0092-8674(00)80760-6 [DOI] [PubMed] [Google Scholar]
- 15. Nakamura T. M., and Cech T. R. (1998) Reversing time: origin of telomerase. Cell 92, 587–590 10.1016/S0092-8674(00)81123-X [DOI] [PubMed] [Google Scholar]
- 16. Belfort M., Curcio M. J., and Lue N. F. (2011) Telomerase and retrotransposons: reverse transcriptases that shaped genomes. Proc. Natl. Acad. Sci. U.S.A. 108, 20304–20310 10.1073/pnas.1100269109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pardue M.-L., and DeBaryshe P. G. (2011) Retrotransposons that maintain chromosome ends. Proc. Natl. Acad. Sci. U.S.A. 108, 20317–20324 10.1073/pnas.1100278108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tatsuke T., Sakashita K., Masaki Y., Lee J. M., Kawaguchi Y., and Kusakabe T. (2010) The telomere-specific non-LTR retrotransposons SART1 and TRAS1 are suppressed by Piwi subfamily proteins in the silkworm, Bombyx mori. Cell. Mol. Biol. Lett. 15, 118–133 10.2478/s11658-009-0038-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Silva-Sousa R., López-Panadès E., Casacuberta E. (2012) Drosophila telomeres: an example of co-evolution with transposable elements. Genome Dyn. 7, 46–67 10.1159/000337127 [DOI] [PubMed] [Google Scholar]
- 20. Luan D. D., Korman M. H., Jakubczak J. L., and Eickbush T. H. (1993) Reverse transcription of R2Bm RNA is primed by a nick at the chromosomal target site: a mechanism for non-LTR retrotransposition. Cell 72, 595–605 10.1016/0092-8674(93)90078-5 [DOI] [PubMed] [Google Scholar]
- 21. Han J. S. (2010) Non-long terminal repeat (non-LTR) retrotransposons: mechanisms, recent developments, and unanswered questions. Mob. DNA 1, 15 10.1186/1759-8753-1-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Greider C. W., and Blackburn E. H. (1989) A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature 337, 331–337 10.1038/337331a0 [DOI] [PubMed] [Google Scholar]
- 23. Maida Y., Yasukawa M., Furuuchi M., Lassmann T., Possemato R., Okamoto N., Kasim V., Hayashizaki Y., Hahn W. C., and Masutomi K. (2009) An RNA-dependent RNA polymerase formed by TERT and the RMRP RNA. Nature 461, 230–235 10.1038/nature08283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Podlevsky J. D., and Chen J. J.-L. (2016) Evolutionary perspectives of telomerase RNA structure and function. RNA Biol. 13, 720–732 10.1080/15476286.2016.1205768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bhattacharyya A., and Blackburn E. H. (1994) Architecture of telomerase RNA. EMBO J. 13, 5721–5731 10.1002/j.1460-2075.1994.tb06910.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Webb C. J., and Zakian V. A. (2016) Telomerase RNA is more than a DNA template. RNA Biol. 13, 683–689 10.1080/15476286.2016.1191725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zappulla D. C., and Cech T. R. (2004) Yeast telomerase RNA: a flexible scaffold for protein subunits. Proc. Natl. Acad. Sci. U.S.A. 101, 10024–10029 10.1073/pnas.0403641101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang Q., Kim N.-K., and Feigon J. (2011) Architecture of human telomerase RNA. Proc. Natl. Acad. Sci. U.S.A. 108, 20325–20332 10.1073/pnas.1100279108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. de Lange T. (2005) Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 19, 2100–2110 10.1101/gad.1346005 [DOI] [PubMed] [Google Scholar]
- 30. Moser B. A., and Nakamura T. M. (2009) Protection and replication of telomeres in fission yeast. Biochem. Cell Biol. 87, 747–758 10.1139/O09-037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Price C. M., Boltz K. A., Chaiken M. F., Stewart J. A., Beilstein M. A., and Shippen D. E. (2010) Evolution of CST function in telomere maintenance. Cell Cycle 9, 3157–3165 10.4161/cc.9.15.12521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sun J., Yu E. Y., Yang Y., Confer L. A., Sun S. H., Wan K., Lue N. F., and Lei M. (2009) Stn1-Ten1 is an Rpa2-Rpa3-like complex at telomeres. Genes Dev. 23, 2900–2914 10.1101/gad.1851909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bryan C., Rice C., Harkisheimer M., Schultz D. C., and Skordalakes E. (2013) Structure of the human telomeric Stn1-Ten1 capping complex. PLoS One 8, e66756 10.1371/journal.pone.0066756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Raffa G. D., Raimondo D., Sorino C., Cugusi S., Cenci G., Cacchione S., Gatti M., and Ciapponi L. (2010) Verrocchio, a Drosophila OB fold-containing protein, is a component of the terminin telomere-capping complex. Genes Dev. 24, 1596–1601 10.1101/gad.574810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Biessmann H., and Mason J. M. (1997) Telomere maintenance without telomerase. Chromosoma 106, 63–69 10.1007/s004120050225 [DOI] [PubMed] [Google Scholar]
- 36. Kazda A., Zellinger B., Rössler M., Derboven E., Kusenda B., and Riha K. (2012) Chromosome end protection by blunt-ended telomeres. Genes Dev. 26, 1703–1713 10.1101/gad.194944.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wu P., van Overbeek M., Rooney S., and de Lange T. (2010) Apollo contributes to G-overhang maintenance and protects leading-end telomeres. Mol. Cell. 39, 606–617 10.1016/j.molcel.2010.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nelson A. D. L., and Shippen D. E. (2012) Blunt-ended telomeres: an alternative ending to the replication and end protection stories. Genes Dev. 26, 1648–1652 10.1101/gad.199059.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Du H., Wang Y.-B., Xie Y., Liang Z., Jiang S.-J., Zhang S.-S., Huang Y.-B., and Tang Y.-X. (2013) Genome-wide identification and evolutionary and expression analyses of MYB-related genes in land plants. DNA Res. 20, 437–448 10.1093/dnares/dst021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Horvath M. P. (2013) Evolution of telomere binding proteins. In Madame Curie Bioscience Database [Internet]. Austin, TX: Landes Bioscience; 2000–2013. Available from: http://www.ncbi.nlm.nih.gov/books/NBK5998/ [Google Scholar]
- 41. Theobald D. L., Mitton-Fry R. M., and Wuttke D. S. (2003) Nucleic acid recognition by OB-fold proteins. Annu. Rev. Biophys. Biomol. Struct. 32, 115–133 10.1146/annurev.biophys.32.110601.142506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kerr I. D., Wadsworth R. I. M., Cubeddu L., Blankenfeldt W., Naismith J. H., and White M. F. (2003) Insights into ssDNA recognition by the OB fold from a structural and thermodynamic study of Sulfolobus SSB protein. EMBO J. 22, 2561–2570 10.1093/emboj/cdg272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wong J. M. Y., Kusdra L., and Collins K. (2002) Subnuclear shuttling of human telomerase induced by transformation and DNA damage. Nat. Cell Biol. 4, 731–736 10.1038/ncb846 [DOI] [PubMed] [Google Scholar]
- 44. Masutomi K., Possemato R., Wong J. M. Y., Currier J. L., Tothova Z., Manola J. B., Ganesan S., Lansdorp P. M., Collins K., and Hahn W. C. (2005) The telomerase reverse transcriptase regulates chromatin state and DNA damage responses. Proc. Natl. Acad. Sci. U.S.A. 102, 8222–8227 10.1073/pnas.0503095102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Park J.-I., Venteicher A. S., Hong J. Y., Choi J., Jun S., Shkreli M., Chang W., Meng Z., Cheung P., Ji H., McLaughlin M., Veenstra T. D., Nusse R., McCrea P. D., and Artandi S. E. (2009) Telomerase modulates Wnt signalling by association with target gene chromatin. Nature 460, 66–72 10.1038/nature08137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kedde M., le Sage C., Duursma A., Zlotorynski E., van Leeuwen B., Nijkamp W., Beijersbergen R., and Agami R. (2006) Telomerase-independent regulation of ATR by human telomerase RNA. J. Biol. Chem. 281, 40503–40514 10.1074/jbc.M607676200 [DOI] [PubMed] [Google Scholar]
- 47. Blasco M. A., Lee H. W., Hande M. P., Samper E., Lansdorp P. M., DePinho R. A., and Greider C. W. (1997) Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 91, 25–34 10.1016/S0092-8674(01)80006-4 [DOI] [PubMed] [Google Scholar]
- 48. Strong M. A., Vidal-Cardenas S. L., Karim B., Yu H., Guo N., and Greider C. W. (2011) Phenotypes in mTERT+/− and mTERT−/− mice are due to short telomeres, not telomere-independent functions of telomerase reverse transcriptase. Mol. Cell. Biol. 31, 2369–2379 10.1128/MCB.05312-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Karlseder J., Hoke K., Mirzoeva O. K., Bakkenist C., Kastan M. B., Petrini J. H. J., and de Lange T. (2004) The telomeric protein TRF2 binds the ATM kinase and can inhibit the ATM-dependent DNA damage response. PLoS Biol. 2, E240 10.1371/journal.pbio.0020240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Xin H., Liu D., and Songyang Z. (2008) The telosome/shelterin complex and its functions. Genome Biol. 9, 232 10.1186/gb-2008-9-9-232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Arnoult N., and Karlseder J. (2015) Complex interactions between the DNA-damage response and mammalian telomeres. Nat. Struct. Mol. Biol. 22, 859–866 10.1038/nsmb.3092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Vannier J.-B., Depeiges A., White C., and Gallego M. E. (2009) ERCC1/XPF protects short telomeres from homologous recombination in Arabidopsis thaliana. PLoS Genet. 5, e1000380 10.1371/journal.pgen.1000380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bradshaw P. S., Stavropoulos D. J., and Meyn M. S. (2005) Human telomeric protein TRF2 associates with genomic double-strand breaks as an early response to DNA damage. Nat. Genet. 37, 193–197 10.1038/ng1506 [DOI] [PubMed] [Google Scholar]
- 54. Williams E. S., Stap J., Essers J., Ponnaiya B., Luijsterburg M. S., Krawczyk P. M., Ullrich R. L., Aten J. A., and Bailey S. M. (2007) DNA double-strand breaks are not sufficient to initiate recruitment of TRF2. Nat. Genet. 39, 696–698; author reply 698–699 10.1038/ng0607-696 [DOI] [PubMed] [Google Scholar]
- 55. Potts P. R., and Yu H. (2007) The SMC5/6 complex maintains telomere length in ALT cancer cells through SUMOylation of telomere-binding proteins. Nat. Struct. Mol. Biol. 14, 581–590 10.1038/nsmb1259 [DOI] [PubMed] [Google Scholar]
- 56. Bryan T. M., Englezou A., Gupta J., Bacchetti S., and Reddel R. R. (1995) Telomere elongation in immortal human cells without detectable telomerase activity. EMBO J. 14, 4240–4248 10.1002/j.1460-2075.1995.tb00098.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kaminker P., Plachot C., Kim S.-H., Chung P., Crippen D., Petersen O. W., Bissell M. J., Campisi J., and Lelièvre S. A. (2005) Higher-order nuclear organization in growth arrest of human mammary epithelial cells: a novel role for telomere-associated protein TIN2. J. Cell Sci. 118, 1321–1330 10.1242/jcs.01709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bártová E., Malyšková B., Komůrková D., Legartová S., Suchánková J., Krejčí J., and Kozubek S. (2017) Function of heterochromatin protein 1 during DNA repair. Protoplasma 254, 1233–1240 10.1007/s00709-017-1090-3 [DOI] [PubMed] [Google Scholar]
- 59. Dinant C., and Luijsterburg M. S. (2009) The emerging role of HP1 in the DNA damage response. Mol. Cell. Biol. 29, 6335–6340 10.1128/MCB.01048-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Martinez P., Thanasoula M., Carlos A. R., Gómez-López G., Tejera A. M., Schoeftner S., Dominguez O., Pisano D. G., Tarsounas M., and Blasco M. A. (2010) Mammalian Rap1 controls telomere function and gene expression through binding to telomeric and extratelomeric sites. Nat. Cell Biol. 12, 768–780 10.1038/ncb2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Denchi E. L., and de Lange T. (2007) Protection of telomeres through independent control of ATM and ATR by TRF2 and POT1. Nature 448, 1068–1071 10.1038/nature06065 [DOI] [PubMed] [Google Scholar]
- 62. Yu Y., Tan R., Ren Q., Gao B., Sheng Z., Zhang J., Zheng X., Jiang Y., Lan L., and Mao Z. (2017) POT1 inhibits the efficiency but promotes the fidelity of nonhomologous end joining at non-telomeric DNA regions. Aging 9, 2529–2543 10.18632/aging.101339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rice C., Shastrula P. K., Kossenkov A. V., Hills R., Baird D. M., Showe L. C., Doukov T., Janicki S., and Skordalakes E. (2017) Structural and functional analysis of the human POT1-TPP1 telomeric complex. Nat. Commun. 8, 14928 10.1038/ncomms14928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bertuch A. A., and Lundblad V. (2003) Which end: dissecting Ku's function at telomeres and double-strand breaks. Genes Dev. 17, 2347–2350 10.1101/gad.1146603 [DOI] [PubMed] [Google Scholar]
- 65. Baumann P., and Cech T. R. (2000) Protection of telomeres by the Ku protein in fission yeast. Mol. Biol. Cell. 11, 3265–3275 10.1091/mbc.11.10.3265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gravel S., and Wellinger R. J. (2002) Maintenance of double-stranded telomeric repeats as the critical determinant for cell viability in yeast cells lacking Ku. Mol. Cell. Biol. 22, 2182–2193 10.1128/MCB.22.7.2182-2193.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ribes-Zamora A., Mihalek I., Lichtarge O., and Bertuch A. A. (2007) Distinct faces of the Ku heterodimer mediate DNA repair and telomeric functions. Nat. Struct. Mol. Biol. 14, 301–307 10.1038/nsmb1214 [DOI] [PubMed] [Google Scholar]
- 68. Arnoult N., Correia A., Ma J., Merlo A., Garcia-Gomez S., Maric M., Tognetti M., Benner C. W., Boulton S. J., Saghatelian A., and Karlseder J. (2017) Regulation of DNA repair pathway choice in S and G2 phases by the NHEJ inhibitor CYREN. Nature 549, 548–552 10.1038/nature24023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ebrahimi H., and Cooper J. P. (2016) Finding a place in the SUN: telomere maintenance in a diverse nuclear landscape. Curr. Opin. Cell Biol. 40, 145–152 10.1016/j.ceb.2016.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Opresko P. L., von Kobbe C., Laine J.-P., Harrigan J., Hickson I. D., and Bohr V. A. (2002) Telomere-binding protein TRF2 binds to and stimulates the Werner and Bloom syndrome helicases. J. Biol. Chem. 277, 41110–41119 10.1074/jbc.M205396200 [DOI] [PubMed] [Google Scholar]
- 71. Machwe A., Xiao L., and Orren D. K. (2004) TRF2 recruits the Werner syndrome (WRN) exonuclease for processing of telomeric DNA. Oncogene 23, 149–156 10.1038/sj.onc.1206906 [DOI] [PubMed] [Google Scholar]
- 72. Opresko P. L., Mason P. A., Podell E. R., Lei M., Hickson I. D., Cech T. R., and Bohr V. A. (2005) POT1 stimulates RecQ helicases WRN and BLM to unwind telomeric DNA substrates. J. Biol. Chem. 280, 32069–32080 10.1074/jbc.M505211200 [DOI] [PubMed] [Google Scholar]
- 73. Amiard S., Doudeau M., Pinte S., Poulet A., Lenain C., Faivre-Moskalenko C., Angelov D., Hug N., Vindigni A., Bouvet P., Paoletti J., Gilson E., and Giraud-Panis M.-J. (2007) A topological mechanism for TRF2-enhanced strand invasion. Nat. Struct. Mol. Biol. 14, 147–154 10.1038/nsmb1192 [DOI] [PubMed] [Google Scholar]
- 74. Ye J., Lenain C., Bauwens S., Rizzo A., Saint-Léger A., Poulet A., Benarroch D., Magdinier F., Morere J., Amiard S., Verhoeyen E., Britton S., Calsou P., Salles B., Bizard A., et al. (2010) TRF2 and Apollo cooperate with topoisomerase 2α to protect human telomeres from replicative damage. Cell 142, 230–242 10.1016/j.cell.2010.05.032 [DOI] [PubMed] [Google Scholar]
- 75. Deng Z., Dheekollu J., Broccoli D., Dutta A., and Lieberman P. M. (2007) The origin recognition complex localizes to telomere repeats and prevents telomere-circle formation. Curr. Biol. 17, 1989–1995 10.1016/j.cub.2007.10.054 [DOI] [PubMed] [Google Scholar]
- 76. Tatsumi Y., Ezura K., Yoshida K., Yugawa T., Narisawa-Saito M., Kiyono T., Ohta S., Obuse C., and Fujita M. (2008) Involvement of human ORC and TRF2 in pre-replication complex assembly at telomeres. Genes Cells 13, 1045–1059 10.1111/j.1365-2443.2008.01224.x [DOI] [PubMed] [Google Scholar]
- 77. Casteel D. E., Zhuang S., Zeng Y., Perrino F. W., Boss G. R., Goulian M., and Pilz R. B. (2009) A DNA polymerase α primase cofactor with homology to replication protein A-32 regulates DNA replication in mammalian cells. J. Biol. Chem. 284, 5807–5818 10.1074/jbc.M807593200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Chen L.-Y., Redon S., and Lingner J. (2012) The human CST complex is a terminator of telomerase activity. Nature 488, 540–544 10.1038/nature11269 [DOI] [PubMed] [Google Scholar]
- 79. Nakaoka H., Nishiyama A., Saito M., and Ishikawa F. (2012) Xenopus laevis Ctc1-Stn1-Ten1 (xCST) protein complex is involved in priming DNA synthesis on single-stranded DNA template in Xenopus egg extract. J. Biol. Chem. 287, 619–627 10.1074/jbc.M111.263723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Feng X., Hsu S.-J., Bhattacharjee A., Wang Y., Diao J., and Price C. M. (2018) CTC1-STN1 terminates telomerase while STN1-TEN1 enables C-strand synthesis during telomere replication in colon cancer cells. Nat. Commun. 9, 2827 10.1038/s41467-018-05154-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Stewart J. A., Wang F., Chaiken M. F., Kasbek C., Chastain P. D. 2nd, Wright W. E., and Price C. M. (2012) Human CST promotes telomere duplex replication and general replication restart after fork stalling. EMBO J. 31, 3537–3549 10.1038/emboj.2012.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Surovtseva Y. V., Churikov D., Boltz K. A., Song X., Lamb J. C., Warrington R., Leehy K., Heacock M., Price C. M., and Shippen D. E. (2009) Conserved telomere maintenance component 1 interacts with STN1 and maintains chromosome ends in higher eukaryotes. Mol. Cell. 36, 207–218 10.1016/j.molcel.2009.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Miyake Y., Nakamura M., Nabetani A., Shimamura S., Tamura M., Yonehara S., Saito M., and Ishikawa F. (2009) RPA-like mammalian Ctc1-Stn1-Ten1 complex binds to single-stranded DNA and protects telomeres independently of the Pot1 pathway. Mol. Cell 36, 193–206 10.1016/j.molcel.2009.08.009 [DOI] [PubMed] [Google Scholar]
- 84. Renfrew K. B., Song X., Lee J. R., Arora A., and Shippen D. E. (2014) POT1a and components of CST engage telomerase and regulate its activity in Arabidopsis. PLoS Genet. 10, e1004738 10.1371/journal.pgen.1004738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Grandin N., Damon C., and Charbonneau M. (2001) Ten1 functions in telomere end protection and length regulation in association with Stn1 and Cdc13. EMBO J. 20, 1173–1183 10.1093/emboj/20.5.1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Nandakumar J., Bell C. F., Weidenfeld I., Zaug A. J., Leinwand L. A., and Cech T. R. (2012) The TEL patch of telomere protein TPP1 mediates telomerase recruitment and processivity. Nature 492, 285–289 10.1038/nature11648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Lue N. F. (2018) Evolving linear chromosomes and telomeres: a C-strand-centric view. Trends Biochem. Sci. 43, 314–326 10.1016/j.tibs.2018.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Calvo O., Grandin N., Jordán-Pla A., Miñambres E., González-Polo N., Pérez-Ortín J. E., and Charbonneau M. (2019) The telomeric Cdc13–Stn1–Ten1 complex regulates RNA polymerase II transcription. Nucleic Acids Res. 47, 6250–6268 10.1093/nar/gkz279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lee J. R., Xie X., Yang K., Zhang J., Lee S. Y., and Shippen D. E. (2016) Dynamic interactions of Arabidopsis TEN1: stabilizing telomeres in response to heat stress. Plant Cell 28, 2212–2224 10.1105/tpc.16.00408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Shore D., and Nasmyth K. (1987) Purification and cloning of a DNA binding protein from yeast that binds to both silencer and activator elements. Cell 51, 721–732 10.1016/0092-8674(87)90095-X [DOI] [PubMed] [Google Scholar]
- 91. Shore D. (1994) RAP1: a protean regulator in yeast. Trends Genet. 10, 408–412 10.1016/0168-9525(94)90058-2 [DOI] [PubMed] [Google Scholar]
- 92. Teo H., Ghosh S., Luesch H., Ghosh A., Wong E. T., Malik N., Orth A., de Jesus P., Perry A. S., Oliver J. D., Tran N. L., Speiser L. J., Wong M., Saez E., Schultz P., Chanda S. K., Verma I. M., and Tergaonkar V. (2010) Telomere-independent Rap1 is an IKK adaptor and regulates NF-κB-dependent gene expression. Nat. Cell Biol. 12, 758–767 10.1038/ncb2080 [DOI] [PubMed] [Google Scholar]
- 93. Hussain T., Saha D., Purohit G., Kar A., Kishore Mukherjee A., Sharma S., Sengupta S., Dhapola P., Maji B., Vedagopuram S., Horikoshi N. T., Horikoshi N., Pandita R. K., Bhattacharya S., Bajaj A., et al. (2017) Transcription regulation of CDKN1A (p21/CIP1/WAF1) by TRF2 is epigenetically controlled through the REST repressor complex. Sci. Rep. 7, 11541 10.1038/s41598-017-11177-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Hong J., Lee J. H., and Chung I. K. (2016) Telomerase activates transcription of cyclin D1 gene through an interaction with NOL1. J. Cell Sci. 129, 1566–1579 10.1242/jcs.181040 [DOI] [PubMed] [Google Scholar]
- 95. Ghosh A., Saginc G., Leow S. C., Khattar E., Shin E. M., Yan T. D., Wong M., Zhang Z., Li G., Sung W.-K., Zhou J., Chng W. J., Li S., Liu E., and Tergaonkar V. (2012) Telomerase directly regulates NF-κB-dependent transcription. Nat. Cell Biol. 14, 1270–1281 10.1038/ncb2621 [DOI] [PubMed] [Google Scholar]
- 96. Poulet A., Pisano S., Faivre-Moskalenko C., Pei B., Tauran Y., Haftek-Terreau Z., Brunet F., Le Bihan Y.-V., Ledu M.-H., Montel F., Hugo N., Amiard S., Argoul F., Chaboud A., Gilson E., and Giraud-Panis M.-J. (2012) The N-terminal domains of TRF1 and TRF2 regulate their ability to condense telomeric DNA. Nucleic Acids Res. 40, 2566–2576 10.1093/nar/gkr1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Dehal P., and Boore J. L. (2005) Two rounds of whole genome duplication in the ancestral vertebrate. PLoS Biol. 3, e314 10.1371/journal.pbio.0030314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Broccoli D., Smogorzewska A., Chong L., and de Lange T. (1997) Human telomeres contain two distinct Myb-related proteins, TRF1 and TRF2. Nat. Genet. 17, 231–235 10.1038/ng1097-231 [DOI] [PubMed] [Google Scholar]
- 99. Smogorzewska A., van Steensel B., Bianchi A., Oelmann S., Schaefer M. R., Schnapp G., and de Lange T. (2000) Control of human telomere length by TRF1 and TRF2. Mol. Cell. Biol. 20, 1659–1668 10.1128/MCB.20.5.1659-1668.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. van Steensel B., Smogorzewska A., and de Lange T. (1998) TRF2 protects human telomeres from end-to-end fusions. Cell 92, 401–413 10.1016/S0092-8674(00)80932-0 [DOI] [PubMed] [Google Scholar]
- 101. Lue N. F., and Chan J. (2013) Duplication and functional specialization of the telomere-capping protein Cdc13 in Candida species. J. Biol. Chem. 288, 29115–29123 10.1074/jbc.M113.506519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Hockemeyer D., Daniels J.-P., Takai H., and de Lange T. (2006) Recent expansion of the telomeric complex in rodents: two distinct POT1 proteins protect mouse telomeres. Cell 126, 63–77 10.1016/j.cell.2006.04.044 [DOI] [PubMed] [Google Scholar]
- 103. Kratz K., and de Lange T. (2018) ATR repression by POT1a and POT1b 1 Both protection of telomeres 1 proteins POT1a and POT1b can repress ATR signaling by RPA exclusion but binding to CST limits ATR repression by POT1b. J. Biol. Chem. 293, 14384–14392 10.1074/jbc.RA118.004598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Hagiwara M., Komatsu T., Sugiura S. S., Isoda R., Tada H., Tanigawa N., Kato Y., Ishida N., Kobayashi K., and Matsushita K. (2013) POT1b regulates phagocytosis and NO production by modulating activity of the small GTPase Rab5. Biochem. Biophys. Res. Commun. 439, 413–417 10.1016/j.bbrc.2013.08.018 [DOI] [PubMed] [Google Scholar]
- 105. Raices M., Verdun R. E., Compton S. A., Haggblom C. I., Griffith J. D., Dillin A., and Karlseder J. (2008) C. elegans telomeres contain G-strand and C-strand overhangs that are bound by distinct proteins. Cell 132, 745–757 10.1016/j.cell.2007.12.039 [DOI] [PubMed] [Google Scholar]
- 106. Cheng C., Shtessel L., Brady M. M., and Ahmed S. (2012) Caenorhabditis elegans POT-2 telomere protein represses a mode of alternative lengthening of telomeres with normal telomere lengths. Proc. Natl. Acad. Sci. U.S.A. 109, 7805–7810 10.1073/pnas.1119191109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Shtessel L., Lowden M. R., Cheng C., Simon M., Wang K., and Ahmed S. (2013) Caenorhabditis elegans POT-1 and POT-2 repress telomere maintenance pathways. G3 3, 305–313 10.1534/g3.112.004440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Meier B., Barber L. J., Liu Y., Shtessel L., Boulton S. J., Gartner A., and Ahmed S. (2009) The MRT-1 nuclease is required for DNA crosslink repair and telomerase activity in vivo in Caenorhabditis elegans. EMBO J. 28, 3549–3563 10.1038/emboj.2009.278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Wang W., Skopp R., Scofield M., and Price C. (1992) Euplotes crassus has genes encoding telomere-binding proteins and telomere-binding protein homologs. Nucleic Acids Res. 20, 6621–6629 10.1093/nar/20.24.6621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Jacob N. K., Lescasse R., Linger B. R., and Price C. M. (2007) Tetrahymena POT1a regulates telomere length and prevents activation of a cell cycle checkpoint. Mol. Cell. Biol. 27, 1592–1601 10.1128/MCB.01975-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Cranert S., Heyse S., Linger B. R., Lescasse R., and Price C. (2014) Tetrahymena Pot2 is a developmentally regulated paralog of Pot1 that localizes to chromosome breakage sites but not to telomeres. Eukaryot. Cell 13, 1519–1529 10.1128/EC.00204-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Skopp R., Wang W., and Price C. (1996) rTP: a candidate telomere protein that is associated with DNA replication. Chromosoma 105, 82–91 10.1007/BF02509517 [DOI] [PubMed] [Google Scholar]
- 113. Shakirov E. V., Perroud P.-F., Nelson A. D., Cannell M. E., Quatrano R. S., and Shippen D. E. (2010) Protection of telomeres 1 is required for telomere integrity in the moss Physcomitrella patens. Plant Cell. 22, 1838–1848 10.1105/tpc.110.075846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Beilstein M. A., Renfrew K. B., Song X., Shakirov E. V., Zanis M. J., and Shippen D. E. (2015) Evolution of the telomere-associated protein POT1a in Arabidopsis thaliana is characterized by positive selection to reinforce protein–protein interaction. Mol. Biol. Evol. 32, 1329–1341 10.1093/molbev/msv025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Rossignol P., Collier S., Bush M., Shaw P., and Doonan J. H. (2007) Arabidopsis POT1A interacts with TERT-V(I8), an N-terminal splicing variant of telomerase. J. Cell Sci. 120, 3678–3687 10.1242/jcs.004119 [DOI] [PubMed] [Google Scholar]
- 116. Wang F., Podell E. R., Zaug A. J., Yang Y., Baciu P., Cech T. R., and Lei M. (2007) The POT1–TPP1 telomere complex is a telomerase processivity factor. Nature 445, 506–510 10.1038/nature05454 [DOI] [PubMed] [Google Scholar]
- 117. Surovtseva Y. V., Shakirov E. V., Vespa L., Osbun N., Song X., and Shippen D. E. (2007) Arabidopsis POT1 associates with the telomerase RNP and is required for telomere maintenance. EMBO J. 26, 3653–3661 10.1038/sj.emboj.7601792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Latrick C. M., and Cech T. R. (2010) POT1–TPP1 enhances telomerase processivity by slowing primer dissociation and aiding translocation. EMBO J. 29, 924–933 10.1038/emboj.2009.409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Shakirov E. V., McKnight T. D., and Shippen D. E. (2009) POT1-independent single-strand telomeric DNA binding activities in Brassicaceae. Plant J. 58, 1004–1015 10.1111/j.1365-313X.2009.03837.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Arora A., Beilstein M. A., and Shippen D. E. (2016) Evolution of Arabidopsis protection of telomeres 1 alters nucleic acid recognition and telomerase regulation. Nucleic Acids Res. 44, 9821–9830 10.1093/nar/gkw807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Shakirov E. V., Surovtseva Y. V., Osbun N., and Shippen D. E. (2005) The Arabidopsis Pot1 and Pot2 proteins function in telomere length homeostasis and chromosome end protection. Mol. Cell. Biol. 25, 7725–7733 10.1128/MCB.25.17.7725-7733.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Kobayashi C. R., Castillo-González C., Survotseva Y., Canal E., Nelson A. D. L., and Shippen D. E. (2019) Recent emergence and extinction of the protection of telomeres 1c gene in Arabidopsis thaliana. Plant Cell Rep. 38, 1081–1097 10.1007/s00299-019-02427-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Sharma P., Jha A. B., Dubey R. S., and Pessarakli M. (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 217037 10.1155/2012/217037 [DOI] [Google Scholar]
- 124. Liguori I., Russo G., Curcio F., Bulli G., Aran L., Della-Morte D., Gargiulo G., Testa G., Cacciatore F., Bonaduce D., and Abete P. (2018) Oxidative stress, aging, and diseases. Clin. Interv. Aging 13, 757–772 10.2147/CIA.S158513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Lee H.-T., Bose A., Lee C.-Y., Opresko P. L., and Myong S. (2017) Molecular mechanisms by which oxidative DNA damage promotes telomerase activity. Nucleic Acids Res. 45, 11752–11765 10.1093/nar/gkx789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Fouquerel E., Barnes R. P., Uttam S., Watkins S. C., Bruchez M. P., and Opresko P. L. (2019) Targeted and persistent 8-oxoguanine base damage at telomeres promotes telomere loss and crisis. Mol. Cell. 75, 117–130.e6 10.1016/j.molcel.2019.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Frenkel K., Goldstein M. S., Duker N. J., and Teebor G. W. (1981) Identification of the cis-thymine glycol moiety in oxidized deoxyribonucleic acid. Biochemistry 20, 750–754 10.1021/bi00507a014 [DOI] [PubMed] [Google Scholar]
- 128. Coluzzi E., Leone S., and Sgura A. (2019) Oxidative stress induces telomere dysfunction and senescence by replication fork arrest. Cells 8, E19 10.3390/cells8010019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Suram A., and Herbig U. (2014) The replicometer is broken: telomeres activate cellular senescence in response to genotoxic stresses. Aging Cell 13, 780–786 10.1111/acel.12246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Tian R., Zhang L.-N., Zhang T.-T., Pang H.-Y., Chen L.-F., Shen Z.-J., Liu Z., Fang Q., and Zhang S.-Y. (2017) Association between oxidative stress and peripheral leukocyte telomere length in patients with premature coronary artery disease. Med. Sci. Monit. 23, 4382–4390 10.12659/MSM.902106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Bohr V. A. (2002) Repair of oxidative DNA damage in nuclear and mitochondrial DNA, and some changes with aging in mammalian cells. Free Radic. Biol. Med. 32, 804–812 10.1016/S0891-5849(02)00787-6 [DOI] [PubMed] [Google Scholar]
- 132. Vallabhaneni H., O'Callaghan N., Sidorova J., and Liu Y. (2013) Defective repair of oxidative base lesions by the DNA glycosylase Nth1 associates with multiple telomere defects. PLoS Genet. 9, e1003639 10.1371/journal.pgen.1003639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Miller A. S., Balakrishnan L., Buncher N. A., Opresko P. L., and Bambara R. A. (2012) Telomere proteins POT1, TRF1 and TRF2 augment long-patch base excision repair in vitro. Cell Cycle 11, 998–1007 10.4161/cc.11.5.19483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Opresko P. L., Fan J., Danzy S., Wilson D. M. 3rd, Bohr V. A., and Bohr V. A. (2005) Oxidative damage in telomeric DNA disrupts recognition by TRF1 and TRF2. Nucleic Acids Res. 33, 1230–1239 10.1093/nar/gki273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Swanson M. J., Baribault M. E., Israel J. N., and Bae N. S. (2016) Telomere protein RAP1 levels are affected by cellular aging and oxidative stress. Biomed. Rep. 5, 181–187 10.3892/br.2016.707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Platt J. M., Ryvkin P., Wanat J. J., Donahue G., Ricketts M. D., Barrett S. P., Waters H. J., Song S., Chavez A., Abdallah K. O., Master S. R., Wang L.-S., and Johnson F. B. (2013) Rap1 relocalization contributes to the chromatin-mediated gene expression profile and pace of cell senescence. Genes Dev. 27, 1406–1420 10.1101/gad.218776.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Chen L.-Y., Liu D., and Songyang Z. (2007) Telomere maintenance through spatial control of telomeric proteins. Mol. Cell. Biol. 27, 5898–5909 10.1128/MCB.00603-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Chen L.-Y., Zhang Y., Zhang Q., Li H., Luo Z., Fang H., Kim S. H., Qin L., Yotnda P., Xu J., Tu B. P., Bai Y., and Songyang Z. (2012) Mitochondrial localization of telomeric protein TIN2 links telomere regulation to metabolic control. Mol. Cell 47, 839–850 10.1016/j.molcel.2012.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Santos J. H., Meyer J. N., Skorvaga M., Annab L. A., and Van Houten B. (2004) Mitochondrial hTERT exacerbates free-radical-mediated mtDNA damage. Aging Cell 3, 399–411 10.1111/j.1474-9728.2004.00124.x [DOI] [PubMed] [Google Scholar]
- 140. Gabriel K., Egan B., and Lithgow T. (2003) Tom40, the import channel of the mitochondrial outer membrane, plays an active role in sorting imported proteins. EMBO J. 22, 2380–2386 10.1093/emboj/cdg229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Haendeler J., Dröse S., Büchner N., Jakob S., Altschmied J., Goy C., Spyridopoulos I., Zeiher A. M., Brandt U., and Dimmeler S. (2009) Mitochondrial telomerase reverse transcriptase binds to and protects mitochondrial DNA and function from damage. Arterioscler. Thromb. Vasc. Biol. 29, 929–935 10.1161/ATVBAHA.109.185546 [DOI] [PubMed] [Google Scholar]
- 142. Sharma N. K., Reyes A., Green P., Caron M. J., Bonini M. G., Gordon D. M., Holt I. J., and Santos J. H. (2012) Human telomerase acts as a hTR-independent reverse transcriptase in mitochondria. Nucleic Acids Res. 40, 712–725 10.1093/nar/gkr758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Passos J. F., Saretzki G., and von Zglinicki T. (2007) DNA damage in telomeres and mitochondria during cellular senescence: is there a connection? Nucleic Acids Res. 35, 7505–7513 10.1093/nar/gkm893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Kovalenko O. A., Caron M. J., Ulema P., Medrano C., Thomas A. P., Kimura M., Bonini M. G., Herbig U., and Santos J. H. (2010) A mutant telomerase defective in nuclear-cytoplasmic shuttling fails to immortalize cells and is associated with mitochondrial dysfunction. Aging Cell 9, 203–219 10.1111/j.1474-9726.2010.00551.x [DOI] [PubMed] [Google Scholar]
- 145. Monaghan R. M., and Whitmarsh A. J. (2015) Mitochondrial proteins moonlighting in the nucleus. Trends Biochem. Sci. 40, 728–735 10.1016/j.tibs.2015.10.003 [DOI] [PubMed] [Google Scholar]
- 146. Sahin E., Colla S., Liesa M., Moslehi J., Müller F. L., Guo M., Cooper M., Kotton D., Fabian A. J., Walkey C., Maser R. S., Tonon G., Foerster F., Xiong R., Wang Y. A., et al. (2011) Telomere dysfunction induces metabolic and mitochondrial compromise. Nature 470, 359–365 10.1038/nature09787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Amiard S., Da Ines O., Gallego M. E., and White C. I. (2014) Responses to telomere erosion in plants. PLoS One 9, e86220 10.1371/journal.pone.0086220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Novikova O., and Belfort M. (2017) Mobile group II introns as ancestral eukaryotic elements. Trends Genet. 33, 773–783 10.1016/j.tig.2017.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Webster G., Genschel J., Curth U., Urbanke C., Kang C., and Hilgenfeld R. (1997) A common core for binding single-stranded DNA: structural comparison of the single-stranded DNA-binding proteins (SSB) from E. coli and human mitochondria. FEBS Lett. 411, 313–316 10.1016/S0014-5793(97)00747-3 [DOI] [PubMed] [Google Scholar]
- 150. Martin W. F., Tielens A. G. M., Mentel M., Garg S. G., and Gould S. B. (2017) The physiology of phagocytosis in the context of mitochondrial origin. Microbiol. Mol. Biol. Rev. 81, e00008–17 10.1128/MMBR.00008-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Oh W., Ghim J., Lee E.-W., Yang M.-R., Kim E. T., Ahn J.-H., and Song J. (2009) PML-IV functions as a negative regulator of telomerase by interacting with TERT. J. Cell Sci. 122, 2613–2622 10.1242/jcs.048066 [DOI] [PubMed] [Google Scholar]
- 152. Keo P., Choi J. S., Bae J., Shim Y.-H., and Oh B.-K. (2015) Increased stability of nucleolar PinX1 in the presence of TERT. Mol. Cells 38, 814–820 10.14348/molcells.2015.0144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Freund A., Zhong F. L., Venteicher A. S., Meng Z., Veenstra T. D., Frydman J., and Artandi S. E. (2014) Proteostatic control of telomerase function through TRiC-mediated folding of TCAB1. Cell 159, 1389–1403 10.1016/j.cell.2014.10.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Shukla S., Schmidt J. C., Goldfarb K. C., Cech T. R., and Parker R. (2016) Inhibition of telomerase RNA decay rescues telomerase deficiency caused by dyskerin or PARN defects. Nat. Struct. Mol. Biol. 23, 286–292 10.1038/nsmb.3184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Grozdanov P. N., Roy S., Kittur N., and Meier U. T. (2009) SHQ1 is required prior to NAF1 for assembly of H/ACA small nucleolar and telomerase RNPs. RNA 15, 1188–1197 10.1261/rna.1532109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Teixeira M. T., Forstemann K., Gasser S. M., and Lingner J. (2002) Intracellular trafficking of yeast telomerase components. EMBO Rep. 3, 652–659 10.1093/embo-reports/kvf133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Bourns B. D., Alexander M. K., Smith A. M., and Zakian V. A. (1998) Sir proteins, Rif proteins, and Cdc13p bind Saccharomyces telomeres in vivo. Mol. Cell. Biol. 18, 5600–5608 10.1128/MCB.18.9.5600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Yoo J. E., Oh B.-K., and Park Y. N. (2009) Human PinX1 mediates TRF1 accumulation in nucleolus and enhances TRF1 binding to telomeres. J. Mol. Biol. 388, 928–940 10.1016/j.jmb.2009.02.051 [DOI] [PubMed] [Google Scholar]
- 159. Yu J., Lan J., Wang C., Wu Q., Zhu Y., Lai X., Sun J., Jin C., and Huang H. (2010) PML3 interacts with TRF1 and is essential for ALT-associated PML bodies assembly in U2OS cells. Cancer Lett. 291, 177–186 10.1016/j.canlet.2009.10.009 [DOI] [PubMed] [Google Scholar]
- 160. Zhu X.-D., Küster B., Mann M., Petrini J. H. J., and de Lange T. (2000) Cell-cycle-regulated association of RAD50/MRE11/NBS1 with TRF2 and human telomeres. Nat. Genet. 25, 347–352 10.1038/77139 [DOI] [PubMed] [Google Scholar]
- 161. O'Connor M. S., Safari A., Liu D., Qin J., and Songyang Z. (2004) The human Rap1 protein complex and modulation of telomere length. J. Biol. Chem. 279, 28585–28591 10.1074/jbc.M312913200 [DOI] [PubMed] [Google Scholar]
- 162. Lian S., Meng L., Liu C., Xing X., Song Q., Dong B., Han Y., Yang Y., Peng L., Qu L., and Shou C. (2013) PRL-3 activates NF-κB signaling pathway by interacting with RAP1. Biochem. Biophys. Res. Commun. 430, 196–201 10.1016/j.bbrc.2012.11.036 [DOI] [PubMed] [Google Scholar]
- 163. Baumann P., Podell E., and Cech T. R. (2002) Human Pot1 (protection of telomeres) protein: cytolocalization, gene structure, and alternative splicing. Mol. Cell. Biol. 22, 8079–8087 10.1128/MCB.22.22.8079-8087.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Greider C. W., and Blackburn E. H. (1987) The telomere terminal transferase of tetrahymena is a ribonucleoprotein enzyme with two kinds of primer specificity. Cell 51, 887–898 10.1016/0092-8674(87)90576-9 [DOI] [PubMed] [Google Scholar]
- 165. Singer M. S., and Gottschling D. E. (1994) TLC1: template RNA component of Saccharomyces cerevisiae telomerase. Science 266, 404–409 10.1126/science.7545955 [DOI] [PubMed] [Google Scholar]
- 166. Grandin N., Reed S. I., and Charbonneau M. (1997) Stn1, a new Saccharomyces cerevisiae protein, is implicated in telomere size regulation in association with Cdc13. Genes Dev. 11, 512–527 10.1101/gad.11.4.512 [DOI] [PubMed] [Google Scholar]
- 167. Kim S. H., Kaminker P., and Campisi J. (1999) TIN2, a new regulator of telomere length in human cells. Nat. Genet. 23, 405–412 10.1038/70508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Ye J. Z.-S., Hockemeyer D., Krutchinsky A. N., Loayza D., Hooper S. M., Chait B. T., and de Lange T. (2004) POT1-interacting protein PIP1: a telomere length regulator that recruits POT1 to the TIN2/TRF1 complex. Genes Dev. 18, 1649–1654 10.1101/gad.1215404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Minamino T., Mitsialis S. A., and Kourembanas S. (2001) Hypoxia extends the life span of vascular smooth muscle cells through telomerase activation. Mol. Cell. Biol. 21, 3336–3342 10.1128/MCB.21.10.3336-3342.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Minamino T., Miyauchi H., Yoshida T., Ishida Y., Yoshida H., and Komuro I. (2002) Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation 105, 1541–1544 10.1161/01.CIR.0000013836.85741.17 [DOI] [PubMed] [Google Scholar]
- 171. Pendlebury D. F., Fujiwara Y., Tesmer V. M., Smith E. M., Shibuya H., Watanabe Y., and Nandakumar J. (2017) Dissecting the telomere–inner nuclear membrane interface formed in meiosis. Nat. Struct. Mol. Biol. 24, 1064–1072 10.1038/nsmb.3493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Kishi S., Wulf G., Nakamura M., and Lu K. P. (2001) Telomeric protein Pin2/TRF1 induces mitotic entry and apoptosis in cells with short telomeres and is down-regulated in human breast tumors. Oncogene 20, 1497–1508 10.1038/sj.onc.1204229 [DOI] [PubMed] [Google Scholar]
- 173. Li B., Oestreich S., and de Lange T. (2000) Identification of human Rap1: implications for telomere evolution. Cell 101, 471–483 10.1016/S0092-8674(00)80858-2 [DOI] [PubMed] [Google Scholar]