Pseudomonas aeruginosa is an opportunistic pathogen responsible for acute nosocomial infections and chronic pulmonary infections. P. aeruginosa strain PA14 is known to be hypervirulent in different hosts. Despite several studies in the field, the underlining molecular mechanisms sustaining this phenotype remain enigmatic. Here we provide evidence that the PA14 strain has faster quorum sensing (QS) kinetics than the PAO1 strain, due to the lack of QslA expression, an antiactivator of QS. QS is a major regulator of virulence factors in P. aeruginosa; therefore, we propose that the hypervirulent phenotype of the PA14 strain is, at least partially, due to the lack of QslA expression. This mechanism could be of great importance, as it could be conserved among other P. aeruginosa isolates.
KEYWORDS: Pseudomonas aeruginosa, pyocyanin, quorum sensing, T2SS, T6SS
ABSTRACT
Two clinical isolates of the opportunist pathogen Pseudomonas aeruginosa named PAO1 and PA14 are commonly studied in research laboratories. Despite the isolates being closely related, PA14 exhibits increased virulence compared to that of PAO1 in various models. To determine which players are responsible for the hypervirulence phenotype of the PA14 strain, we elected a transcriptomic approach through RNA sequencing. We found 2,029 genes that are differentially expressed between the two strains, including several genes that are involved with or regulated by quorum sensing (QS), known to control most of the virulence factors in P. aeruginosa. Among them, we chose to focus our study on QslA, an antiactivator of QS whose expression was barely detectable in the PA14 strain according our data. We hypothesized that lack of expression of qslA in PA14 could be responsible for higher QS expression in the PA14 strain, possibly explaining its hypervirulence phenotype. After confirming that QslA protein was highly produced in PAO1 but not in the PA14 strain, we obtained evidence showing that a PAO1 deletion strain of qslA has faster QS gene expression kinetics than PA14. Moreover, known virulence factors activated by QS, such as (i) pyocyanin production, (ii) H2-T6SS (type VI secretion system) gene expression, and (iii) Xcp-T2SS (type II secretion system) machinery production and secretion, were all lower in PAO1 than in PA14, due to higher qslA expression. However, biofilm formation and cytotoxicity toward macrophages, although increased in PA14 compared to PAO1, were independent of QslA control. Together, our findings implicated differential qslA expression as a major determinant of virulence factor expression in P. aeruginosa strains PAO1 and PA14.
IMPORTANCE Pseudomonas aeruginosa is an opportunistic pathogen responsible for acute nosocomial infections and chronic pulmonary infections. P. aeruginosa strain PA14 is known to be hypervirulent in different hosts. Despite several studies in the field, the underlining molecular mechanisms sustaining this phenotype remain enigmatic. Here we provide evidence that the PA14 strain has faster quorum sensing (QS) kinetics than the PAO1 strain, due to the lack of QslA expression, an antiactivator of QS. QS is a major regulator of virulence factors in P. aeruginosa; therefore, we propose that the hypervirulent phenotype of the PA14 strain is, at least partially, due to the lack of QslA expression. This mechanism could be of great importance, as it could be conserved among other P. aeruginosa isolates.
INTRODUCTION
Pseudomonas aeruginosa is a ubiquitous Gram-negative opportunistic pathogen responsible for various nosocomial infections in immunocompromised or intubated, ventilated patients, as well as chronic respiratory infections in cystic fibrosis sufferers (1). Its genome encodes a myriad of virulence factors and regulatory mechanisms that allow the pathogen to adapt efficiently to various hostile environments and to cause distinct infections (2). Virulence gene expression in P. aeruginosa is under the control of a sophisticated and dynamic regulation network and responds to largely unidentified environmental signals. This complex regulatory network involves alternative sigma factors, dozens of two-component systems, and quorum sensing (QS) systems (3).
QS is a cell density-based interbacterial communication system that involves the diffusion of small autoinducer molecules that are used to coordinate gene expression (4–6). Once a crucial threshold concentration of signal molecules has been reached, QS target genes can be either activated or repressed. In P. aeruginosa there are two QS systems based on acyl homoserine lactone (HSL) signaling: the LasR/3-oxo-C12-HSL and RhlR/C4-HSL systems. The las system is placed above the rhl system in the HSL-dependent QS hierarchy, since LasR/3-oxo-C12-HSL activates both rhlR and rhlI (7, 8). These two QS systems control the expression of about 6% of genes in the P. aeruginosa genome (9–11). Among them, QS activates the expression of many genes encoding virulence factors, like the elastase LasB secreted by the type II secretion system (T2SS) (12, 13), two type VI secretion systems (T6SS) named H2- and H3-T6SS (14–16), pyocyanin (17), and biofilm maturation (18). In addition to HSL-mediated QS, P. aeruginosa also produces the Pseudomonas quinolone signal (PQS; 2-heptyl-3-hydroxy-4-quinolone) (19). The quinolone signaling system is intertwined in a hierarchical manner to the HSL-based QS systems of P. aeruginosa, as LasR and RhlR, respectively, positively and negatively control the levels of PQS by binding the promoter region of pqsR regulator gene (20, 21). With regard to regulation of the QS systems, there are several QS regulators described in the literature, such as MvaT (22), CdpR (23), the IQS system (24), QscR (25), QteE (26), and QslA (27). QscR, QteE, and QslA are thought to play crucial roles in determining the activation threshold of QS (25–27).
In laboratory settings, two clinically isolated strains of P. aeruginosa, named PAO1 and PA14, are principally studied and are noteworthy for their differences in virulence. While the PAO1 strain displays moderate virulence in most model systems, PA14 is highly virulent in mouse, nematode, and plant models of infection (28); for the historical discovery of PA14 strain, see reference 29. Studies aimed at identifying the determinants mediating PA14 hypervirulence have found that the PA14 genome has two pathogenicity islands (108 kb and 11 kb), called PAPI-1 and PAPI-2 (P. aeruginosa pathogenicity islands 1 and 2, respectively) (30). These pathogenicity islands encode virulence factors such as the type III secretion system (T3SS) effector ExoU, a potent and detrimental cytotoxin producing rapid cell death (31, 32). Nonetheless, enhanced PA14 virulence is not only a consequence of genomic acquired virulence determinants (33). In addition to the pathogenicity islands, the PA14 strain has a mutated version of the ladS gene (34), encoding a sensor of the RetS/LadS/GacS signaling cascade, which is associated with pathogenicity and the switch between acute and chronic infections of P. aeruginosa (35, 36). This mutation leads to derepression of the T3SS regulon and thus higher cytotoxicity toward mammalian cells (34). More recently, differences in the expression of the three T6SS have also been highlighted in PA14 compared to PAO1 and PAK backgrounds, presumably because of the ladS mutation (37). Finally, PA14 has been shown to secrete high levels of pyocyanin compared to those secreted by PAO1; pyocyanin is another important virulence factor of P. aeruginosa (38).
To better understand the divergent virulence profiles between PAO1 and PA14 and determine if there are other unknown players that are involved in this complex mechanism, we chose to perform an unbiased transcriptomic approach using high-throughput RNA sequencing (RNA-seq). From RNA-seq data, we found significant differences in the expression of many QS targets; overall, QS-activated genes were overexpressed in PA14, while QS-repressed genes were overexpressed in PAO1. Interestingly, the expression of qslA, encoding a QS inhibitor (27), is detectable only in PAO1. QslA (QS LasR antiactivator) is known to bind LasR and disrupt LasR dimerization, preventing its binding to target promoters (27, 39). Since many virulence factors are activated by QS in P. aeruginosa, we hypothesized that decreased expression of qslA in PA14 would drive increased QS gene expression and subsequently overexpression of QS target genes, thus eventually leading to the hypervirulent phenotype of PA14. To test this hypothesis, we first demonstrated that the QslA protein is indeed readily produced in PAO1, while it is undetectable in PA14. We further confirmed that genes encoding QS systems are upregulated in PA14 compared to PAO1 and that this is due to a lower level of QslA. Next, we obtained evidence that many QS-activated genes encoding important P. aeruginosa virulence factors are differentially regulated between these two strains, such as pyocyanin production, H2-T6SS gene expression, XcpP production, and Xcp-dependent secretion of the elastase LasB. Finally, we observed that whereas biofilm formation and cytotoxicity toward macrophages were higher in PA14, this was independent of qslA expression. The level of the QslA protein, and presumably the expression level of the qslA gene, is a key player in QS target gene expression, which consequently contributes to higher pathogenic potential of the PA14 strain.
RESULTS
Global comparison of PAO1 and PA14 RNA-seq transcriptomes.
To determine if virulence factor genes are differentially expressed between the PAO1 and PA14 P. aeruginosa strains, we performed RNA-seq on mRNAs extracted from cultures grown in rich medium at the transition between exponential and stationary phases, in which most of the virulence factors are expressed (see Materials and Methods). Based on the transcriptomic data, 2,029 genes were identified as significantly and differentially regulated between the two strains (Fig. 1A and Table 1), which represents about 39% of the P. aeruginosa genome, suggesting a massive gene expression variation between the two isolates. Among the genes differentially expressed, PA3431 and PA3432 expression levels were increased, respectively, 108.7- and 105.3-fold in PAO1 (see Table S1 in the supplemental material). However, we decided not to follow up on these genes encoding hypothetical proteins in this study. Further work will be required to address this interesting observation, more particularly as PA3431 and PA3432 may constitute a holin/antiholin-like system, as PF04172 and PF03788 domains, are, respectively, present in PA3431 and PA3432, suggesting a potential role in bacterial lysis (40). Two other genes whose levels of expression were very different between the two strains drew our attention: PA4685 and PA1244 (qslA). Expression of PA4685 was not detectable in PAO1, while expression of qslA was barely detectable in PA14 (Table 1 and Table S1).
FIG 1.
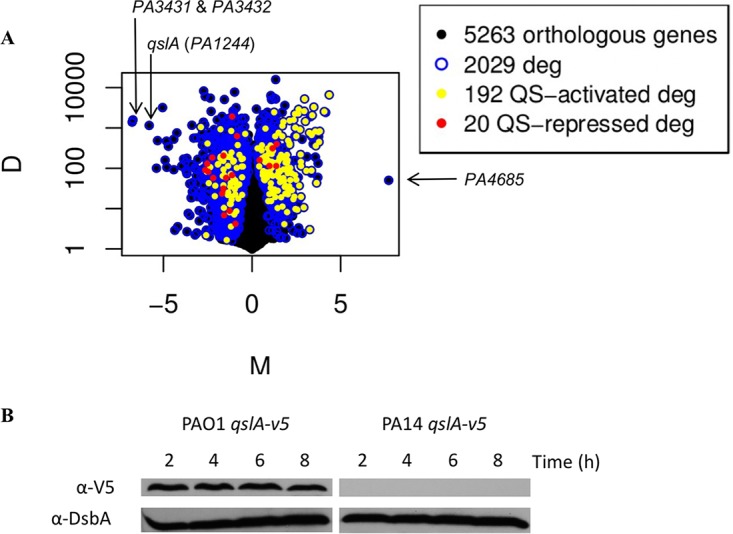
Transcriptomes of PAO1 and PA14 reveal differential QS target genes and qslA expression. (A) M&D plot representing differentially expressed genes (deg) between PA14 and PAO1 strains of P. aeruginosa. QS target genes are more expressed in PA14. The M axis represents the log2 ratio of the two conditions (PAO1/PA14), while the D axis represents the value of the difference between conditions. (B) QslA is produced only in the PAO1 strain. QslAV5 protein was immunodetected using V5 antibody in cellular extracts obtained after 2, 4, 6, and 8 h of growth in TSB at 37°C with aeration. DsbA immunodetection was used as a loading control. In order to standardize the protein samples, the equivalent of 0.1 OD600 unit was loaded.
TABLE 1.
Highlights from Table S1 of genes of interest
| Gene name | Function | Ratio of expressiona |
Activated by QS? | |
|---|---|---|---|---|
| PA14/PAO1 | PAO1/PA14 | |||
| qslA | QS regulator | / | 55.8 | |
| qscR | QS regulator | / | 1.2 | |
| rsaL | QS regulator | / | 2.1 | |
| mvaT | QS regulator | / | 1.6 | |
| cpdR | QS regulator | / | 3.9 | |
| qteE | QS regulator | / | 1.9 | |
| rhlR | Rhl QS system | 2.5 | / | X |
| rhlI | Rhl QS system | 1.8 | / | X |
| lasR | Las QS system | 1.9 | / | X |
| lasI | Las QS system | 1.5 | / | X |
| PA1656 | H2-T6SS machinery | 1.5 | / | X |
| lasB | LasB elastase | 7.7 | / | X |
/, not calculated.
Since we did not detect any PA4685 transcript in our PAO1 transcriptomic data (Table S1), we first decided to test whether the gene was present in the PAO1 genome. PAO1 is one of the most commonly used P. aeruginosa strains in research and is derived from the original Australian PAO isolate, isolated from a wound 50 years ago in Melbourne and distributed worldwide to laboratories. Over decades, discordant phenotypes of PAO1 subcultures emerged (41). Notably, there is a 1-kb deletion within the PA4684 and PA4685 genes present in the Washington Genome Center PAO1 strain compared to the published PAO1 sequence (42) (which served as a reference for our in silico analysis). To test whether this locus deletion is present in the PAO1 strain used in this study (named PAO1 Marseilles), PCR was performed using pairs of oligonucleotides hybridizing to the coding sequence of PA4685 (TSO116-TSO117) or outside thereof (CCO1 and CCO2) (Fig. S1A). Our results revealed that PA4685 is absent in the PAO1 Marseilles strain but present in the PA14 strain used in this study (Fig. S1B). This explains the nonexpression of PA4685 in PAO1 Marseilles observed in our RNA-seq transcriptome.
The expression of qslA gene is increased 55.8-fold in the PAO1 strain (Table 1). Interestingly, this gene encodes an antiactivator of QS, named QslA (27), and led us to hypothesize that the levels of expression of QS-regulated genes may be different between the two isolates. As a result, we decided to look at the expression of all of the QS-regulated genes in our RNA-seq transcriptomic data. Interestingly, among the 353 genes known to be regulated by QS in P. aeruginosa (315 activated and 38 repressed [9]), 212 were significantly and differentially regulated between both strains (Fig. 1A). Overall, QS-activated genes were overexpressed in PA14 (shown as yellow dots in Fig. 1A), whereas QS repressed genes were overexpressed in PAO1 (shown as red dots), suggesting a major differential QS-dependent expression pattern. All together, these observations led us to hypothesize that the difference in QS target genes expression between these two strains could be mediated by the modulation of qslA expression levels. As QS activation is dependent on the growth phase, it is important to note that PAO1 and PA14 strains have the same growth profile under the conditions we used (data not shown).
To first demonstrate a correlation between levels of qslA expression and QslA production, we engineered chromosomally encoded QslAV5 translational fusions in both the PAO1 and PA14 strains. The production of QslAV5 could be monitored and analyzed by Western blotting and immunodetection with an anti-V5 antibody (Fig. 1B). The results for the PAO1 strain reflected a constant level of QslA during growth, whereas QslA was not detected under the same conditions in PA14, which coincides with our transcriptomic data.
qslA expression level drives gene expression of HSL-based QS regulators.
We asked whether this QS-dependent global differential expression could be due to differential expression of qslA. We reasoned that abolishing qslA expression in the PAO1 background may mimic the PA14 profile, whereas QslA overproduction in the PA14 strain may resemble the PAO1 pattern. Our transcriptomic data indicate an increased expression of genes encoding the two HSL-based QS systems in PA14. Indeed, rhlR, rhlI, lasR, and lasI were, respectively, 2.5-, 1.8-, 1.9-, and 1.5-fold more expressed in the PA14 strain (Table 1).
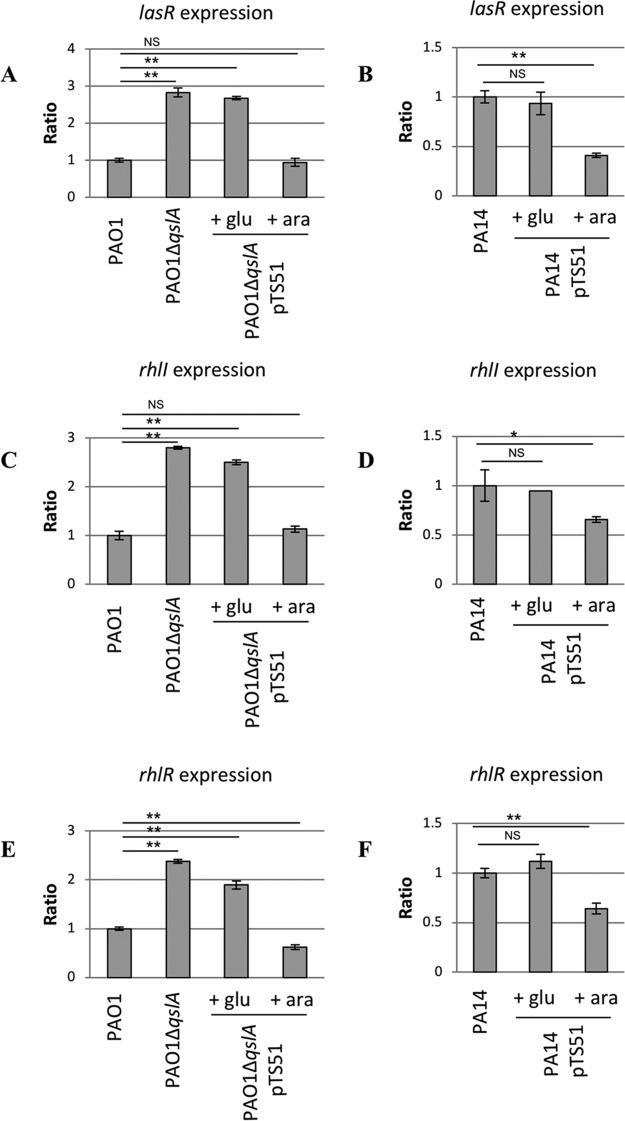
Therefore, we decided to measure the relative levels of lacZ transcriptional fusions to lasR, rhlR, and rhlI promoters (7) in strains PAO1, PA14, PAO1 ΔqslA, PAO1 ΔqslA carrying plasmid pTS51 bearing qslA to complement the qslA deletion, and PA14 overproducing QslA from pTS51 (Table 2). In PAO1, deletion of the qslA gene increased the expression level of QS regulator genes 2- to 3-fold (Fig. 2A, C, and E). The wild-type (WT) level expression was restored by complementation of the mutation in trans (compare columns 3 and 4, repression and activation, respectively, of qslA, in Fig. 2A, C, and E). Overproduction of QslA caused a significant decrease (1.5- to 2.5-fold) in β-galactosidase activities measured for all three of the transcriptional fusions made in the PA14 strain (Fig. 2B, D, and F). Thus, the expression levels of lasR, rhlR, and rhlI were correlated in a dose-dependent manner with QslA production in both P. aeruginosa strains. Interestingly, the increased fold expression in PA14 measured for lasR, rhlR, and rhlI (Fig. 2) recapitulated the increased fold expression of these genes in our transcriptomic data between two strains (Table S1), suggesting that modulation of qslA expression is solely responsible for this differential expression of QS genes.
TABLE 2.
Strains, plasmids, and oligonucleotides used in this study
| Strain, plasmid or oligonucleotide | Genotype, description, or sequence | Source or reference |
|---|---|---|
| E. coli strains | ||
| TG1 | supE hsdΔR thiΔ(lac-proAB) F′ (traD36 proAB+ lacIq lacZΔM15) | Laboratory collection |
| CC118 λpir | (λpir) Δ(ara-leu) araD ΔlacX74 galE galK phoA-20 thi-1 rpsE rpoB Arg(Am) recA1 Rfr (λpir) | Laboratory collection |
| TOP10F′ | F− mcrA Δ(mrr-hsdRMS-mcrBC) ϕ80lacZΔM15 ΔlacX74 nupG recA1 araD139 Δ(ara-leu)7697 galE15 galK16 rpsL (Strr) endA1 λ− | Laboratory collection |
| P. aeruginosa strains | ||
| PAO1 | Wild type, prototroph, chl-2 | B. Holloway collection |
| PAO1 qslA-v5 | Chromosomally encoded QslAV5 translational fusion in PAO1 | This work |
| PAO1 ΔqslA | qslA deletion mutant in PAO1 | This work |
| PAO1TS2 | PH2-T6SS integrated at att site in PAO1 | 15 |
| PAO1TS2 ΔqslA | PH2-T6SS integrated at att site in PAO1ΔqslA | This work |
| PA14 | Clinical isolate | F. Ausubel collection |
| PA14 qslA-v5 | Chromosomally encoded QslAV5 translational fusion in PA14 | This work |
| PA14TS2 | PH2-T6SS integrated at att site in PA14 | This work |
| D40ZQ | xcp locus deletion in PAO1 | 67 |
| PA14 ΔxcpT | xcpT deletion mutant in PA14 | 68 |
| Plasmids | ||
| pCR2.1 | TA cloning; lacZα ColE1 f1 ori Apr Kmr | Invitrogen |
| pMini-CTX::lacZ | Ω-FRT-attP-MCS ori int oriT Tcr | 63 |
| pRK2013 | Tra+ Mob+ ColE1 Kmr | Laboratory collection |
| pKNG101 | oriR6K mobRK2 sacBR+ Smr (suicide vector) | 69 |
| pJN105 | Gmr araC-pBAD | Laboratory collection |
| pTS2 | 722-bp upstream region of H2-T6SS in pMini-CTX::lacZ | 15 |
| pTS48 | 500-bp upstream and 500 bp downstream regions of qslA in pCR2.1 | This work |
| pTS49 | qslA gene in pCR2.1 | This work |
| pTS50 | 500-bp upstream and 500-bp downstream regions of qslA in pKNG101 | This work |
| pTS51 | qslA gene in pJN105 | This work |
| pTS52 | qslAV5 in pCR2.1 | This work |
| pTS53 | qslAV5 in pKNG101 | This work |
| pMAL.R | PlasR-lacZ transcriptional fusion in pMP220 | 7 |
| pMAL.V | PrhlR-lacZ transcriptional fusion in pMP220 | 7 |
| pMAL.I | PrhlI-lacZ transcriptional fusion in pMP220 | 7 |
| Oligonucleotides | ||
| TSO58 | 5′-CCTATCCCTAACCCTCTCCTCGGT-3′ | 45 |
| TSO103 | 5′-CGACCGCAGTTTTCCAACTGCGGGCCTTCATGGCGG-3′ | This work |
| TSO104 | 5’TCACGTAGAATCGAGACCGAGGAGAGGGTTAGGGATAGGCTTACCACCGGAACGTCGAGCGGCTACCAGGCGCTGCTGC-3′ | This work |
| TSO105 | 5’GGTAAGCCTATCCCTAACCCTCTCCTCGGTCTCGATTCTACGTGACCCGGCCATGGCGAATGACGCCGGTGGCGTCG-3′ | This work |
| TSO106 | 5′-ATGCTCGGCGCGTAGGCATCGTTGTACAGGGCGACG-3′ | This work |
| TSO107 | 5′-TGCTTGTCGCCGATGCTCGGC-3′ | This work |
| TSO108 | 5′-CAGCGCCCTCTTCGAAGAAGC-3′ | This work |
| TSO109 | 5′-ATGGCCGGGTCACACATGACCTGCCGCCTTCGC-3′ | This work |
| TSO110 | 5′-GCAGGTCATGTGTGACCCGGCCATGGCGAATGACG-3′ | This work |
| TSO111 | 5′-TGCTTGTCGCCGATGCTCGGCGC-3′ | This work |
| TSO112 | 5′-AGAAAGGGTTATATCCTTATGC-3′ | This work |
| TSO113 | 5′-GGTTCGAGGTCATCCCACAGC-3′ | This work |
| TSO114 | 5′-CTCCATCGATTGACAGCGAAGG-3′ | This work |
| TSO115 | 5′-TCAACCGGAACGTCGAGAGGC-3′ | This work |
| TSO116 | 5′-TCCTCGCACCAGGACCAGTACC-3′ | This work |
| TSO117 | 5′-GCGTCTTCCTCGCTCTCTTCGG-3′ | This work |
FIG 2.
β-Galactosidase activities of transcriptional fusions PlasR-′lacZ (A and B), PrhlI-′lacZ (C and D), and PrhlR-′lacZ (E and F). Shown is the ratio of expression in parental PAO1 (A, C, and E) or PA14 (B, D, and F) versus expression in the reference strain. Results were obtained after 6 h of growth at 37°C with aeration. Glucose (glu) and arabinose (ara) were added in the culture medium after 1 h 30 min of growth at a final concentration of 0.5%, allowing, respectively, repression or induction of the PBAD promoter of pTS51 bearing qslA. **, P < 0.01; *, P < 0.05. NS, not significant.
Biosynthesis of pyocyanin depends on qslA expression levels.
We next hypothesized that differential expression of qslA could explain the differential expression of QS-regulated genes between the PAO1 and PA14 strains. To test this hypothesis, we chose to compare the expression levels or production profiles of several QS-activated virulence factor genes in strains producing and not producing QslA.
Pyocyanin is a blue, redox-active phenazine that contributes to P. aeruginosa virulence by inhibiting the oxidative burst of host phagocytic cells, by inducing apoptosis in host cells, and through antibiotic activities. This respiratory pigment also participates in the reduction of iron and functions as an intracellular redox buffer (43). As the regulation of pyocyanin expression is mediated by both HSL- and PQS-based QS (17, 20), the study of its production is a perfect example of the intrinsic and complex relationship between regulations by HSL- and PQS-based QS. Furthermore, in our transcriptomic data, expression of the operon phz, including the genes involved in the production of pyocyanin, was 10- to 20-fold increased in PA14 (Table S1).
The production of pyocyanin was monitored after 3.5 and 24 h of growth (Fig. 3). Pyocyanin concentrations were higher in PA14 than in PAO1, reflecting the lack of expression of qslA. Moreover, the overproduction of QslA in PA14 led to a major decrease in pyocyanin production (5.2- and 22.4-fold at 3.5 and 24 h), whereas the qslA deletion in PAO1 produced a significant increase (2- and 1.6-fold at 3.5 and 24 h) that could be restored to WT PAO1 levels by complementation. Therefore, the increased pyocyanin production in PA14 (38) is due to low qslA expression in this strain.
FIG 3.
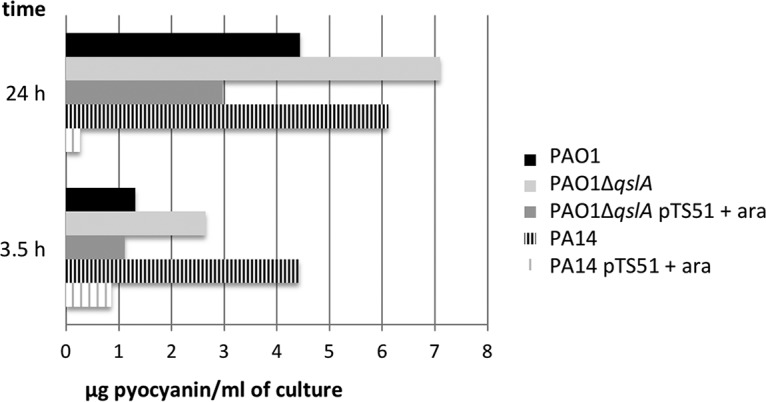
Pyocyanin quantification in the extracellular medium. Pyocyanin was extracted from culture medium as described in Materials and Methods after 3.5 and 24 h of growth at 37°C in LB with aeration. Arabinose was added in the culture medium after 1 h 30 min of growth at a final concentration of 0.5% to induce the PBAD promoter of pTS51 bearing qslA. The data are representative of those from a typical experiment done in triplicate.
H2-T6SS expression is driven by QslA levels.
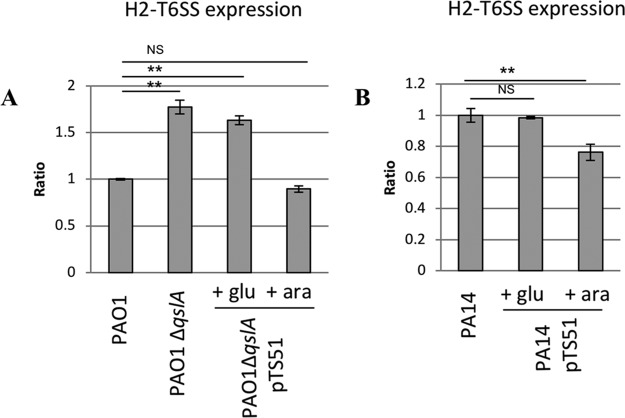
The H2-T6SS machinery is a virulence factor of P. aeruginosa that is known to be activated by QS in both the PA14 and PAO1 strains (14, 15). Our transcriptomic data indicate a 1.5-fold-increased expression of the first gene of the H2-T6SS operon (PA1656) in PA14 (Table 1). P. aeruginosa utilizes H2-T6SS to invade epithelial cells by manipulating the microtubular network and host kinase pathways (44–46) and to promote autophagy (44, 47–49). H2-T6SS also mediates antibacterial activity (44, 47–49), making it a trans-kingdom cell targeting machinery (48, 50). We used a lacZ transcriptional fusion of H2-T6SS promoter region to evaluate its expression in these strains (Fig. 4). Strain PAO1 ΔqslA presented a 1.8-fold increase in the expression of H2-T6SS compared to the PAO1 WT strain. Moreover, production of QslA in the mutant strain was able to decrease H2-T6SS expression to a level comparable to that in the WT strain. Similarly, in PA14, overproduction of QslA significantly reduced the expression of H2-T6SS. The expression of H2-T6SS machinery genes was thus controlled at a transcriptional level by QslA in both strains.
FIG 4.
β-Galactosidase activities of transcriptional fusions PH2-T6SS-′lacZ. Shown is the ratio of expression in parental PAO1 (A) or PA14 (B) versus expression in the reference strain. Results were obtained after 4 h of growth at 37°C with aeration. Glucose and arabinose were added in the culture medium after 1 h 30 min of growth at a final concentration of 0.5%, allowing, respectively, repression or induction of the PBAD promoter of pTS51 bearing qslA. **, P < 0.01.
Production of XcpP depends on qslA expression levels.
XcpP is an inner membrane protein of the P. aeruginosa Xcp T2SS machinery, known to secrete various virulence factors (51). XcpP interacts with the outer membrane secretin XcpQ, which forms the pore through which the secreted protein reaches the extracellular medium (52–54) and is therefore an essential component of the T2SS process. The two operons encoding the Xcp machinery are activated by HSL-based QS (13), and our transcriptomic data indicate that xcp genes are upregulated in PA14 in comparison to PAO1 in transition phase (Table S1). We thus sought to determine whether QslA levels could modulate XcpP production, using immunodetection at different times of growth (Fig. 5). Whereas XcpP could be readily detected after 2 h of growth of the PA14 strain, the same level of proteins was obtained 2 h later in PAO1. Moreover, the deletion of the qslA gene in PAO1 led to an earlier synthesis of XcpP and to higher levels of the protein that could be restored to a PAO1 WT profile by complementation. Accordingly, overproduction of QslA in the PA14 strain showed a PAO1-like pattern of XcpP. These data corroborated our hypothesis that the T2SS machinery is synthesized earlier and presumably assembled when QslA levels are low, as in PA14.
FIG 5.
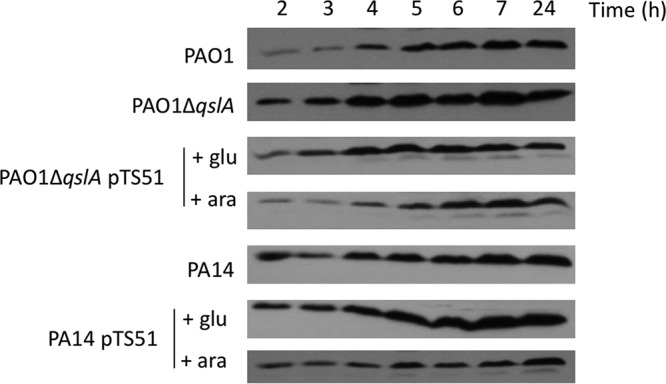
Immunodetection of the T2SS Xcp machinery component XcpP in cellular extracts. Shown is immunodetection of XcpP at 25 kDa after 2, 3, 4, 5, 6, 7, and 24 h of growth of indicated strains at 37°C with aeration. Glucose and arabinose were added in the culture medium after 1 h 30 min of growth at a final concentration of 0.5%, allowing, respectively, repression or induction of the PBAD promoter of pTS51 bearing qslA. In order to standardize the protein samples, the equivalent of 0.1 OD600 unit was loaded.
Elastase secretion is dependent on qslA expression level.
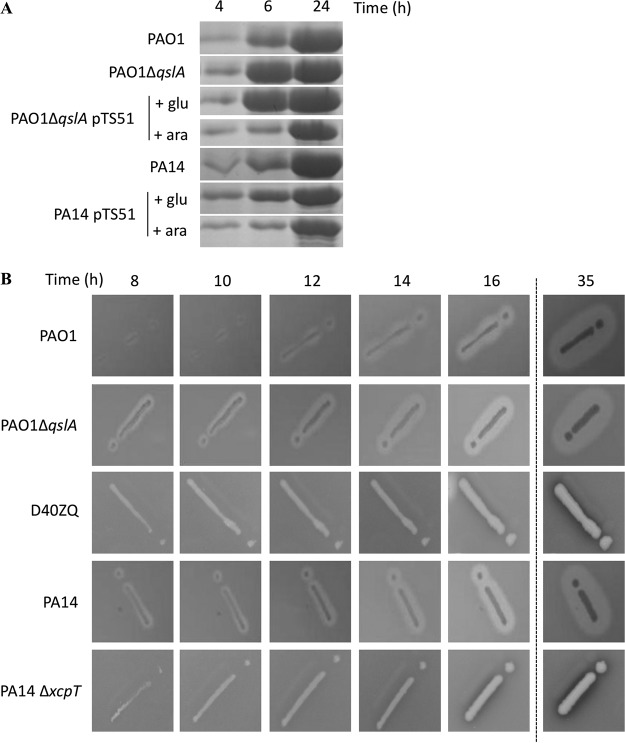
The elastase is an Xcp T2SS-secreted protease, encoded by the lasB gene. It degrades elastin, a major component of lung tissue, and cleaves a surfactant protein (SP-D) involved in several immune functions (55). The expression of elastase coding gene is activated by QS (12), and we measured a 7.7-fold-increased expression of lasB in PA14 in comparison to that in PAO1during early stationary phase in our RNA-seq analysis (Table 1). To measure the impact of QslA on this T2SS substrate, the secretion into the extracellular medium and the extracellular activity of elastase were monitored (Fig. 6). The secretion of LasB was analyzed by Coomassie blue staining of the extracellular fraction at different growth times (Fig. 6A). As expected, the deletion of qslA in PAO1 altered the kinetics, allowing earlier LasB secretion; after 4 and 6 h of growth, LasB secretion was significantly higher in the mutant PAO1 ΔqslA than in the WT PAO1 strain. This phenotype was restored to a WT phenotype by trans-complementation since the protein profile observed for the PAO1 ΔqslA strain producing QslA was identical to that observed for the WT PAO1 strain (Fig. 6A, compare the first and fourth lines). In contrast, in PA14, overproduction of QslA led to a delayed and decreased secretion of LasB compared to that for the PA14 WT strain (Fig. 6A, compare the fifth and sixth lines).
FIG 6.
(A) Detection of elastase in the extracellular medium. Shown is a Coomassie blue-stained gel of extracellular medium of different P. aeruginosa strains. LasB (or elastase) was observed at 33 kDa after 4, 6, and 24 h of growth at 37°C with aeration. Glucose and arabinose were added in the culture medium after 1 h 30 min of growth at a final concentration of 0.5%, allowing, respectively, repression or induction of the PBAD promoter of pTS51 bearing qslA. In order to standardize the protein samples, the equivalent of 1 OD600 unit was loaded. (B) Kinetics of proteolytic activity of elastase secreted by P. aeruginosa. Proteolysis of proteins contained in skim milk allowed formation of a halo all around the colonies of P. aeruginosa, depending on the quantity of elastase secreted. D40ZQ (PAO1 strain lacking all the xcp genes) and PA14 ΔxcpT were used as controls, showing that protease activity observed was massively dependent on this T2SS effector. Elastase activity was observed after 8, 10, 12, 14, 16, and 35 h of growth at 30°C.
These data were also confirmed by monitoring the LasB protease activity by observing the formation of protein degradation halos on skim-milk plates (Fig. 6B). We noticed an earlier halo in PA14 than in PAO1 (Fig. 6B, compare the first and fourth lines). In addition, the delay of halo formation in PAO1 could be visualized in a qslA mutant strain (Fig. 6B, compare the first and second lines). As negative controls, we used mutants in T2SS Xcp machinery for both strains (Fig. 6B, third and fifth lines). As shown, the halo was weak after 35 h.
Together, these data demonstrated that the functionality of the Xcp T2SS machinery measured by the secretion and extracellular activity of the protease LasB was higher in the PA14 strain, due to the decreased expression of qslA in this strain.
QslA does not control biofilm formation by P. aeruginosa.
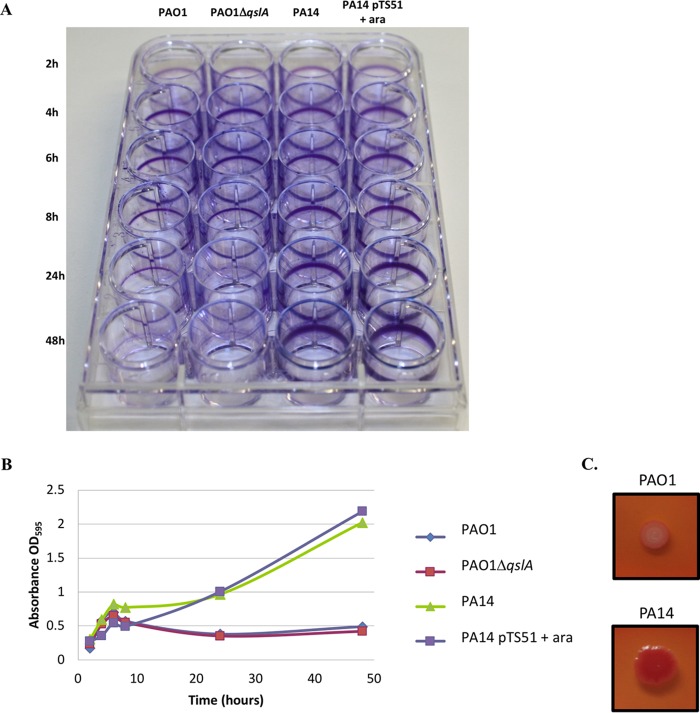
The ability of P. aeruginosa to form biofilm is a crucial virulence determinant, mainly regulated by QS (18). To assess if QslA has a role during biofilm formation, bacterial adherence to abiotic surfaces of various strains was visualized (Fig. 7A) and quantified using crystal violet staining (Fig. 7B). The production of exopolysaccharides, a main biofilm component, was also visualized on Congo red-containing plates (Fig. 7C). As expected from the literature, the ability of the PA14 strain to form biofilm was higher than for PAO1, the exopolysaccharide staining (Fig. 7C; the PA14 strain is red on Congo red plates) being consistent with the adherence assay (Fig. 7A and B). A clear difference in the adherence capacities was observable after 8 h (Fig. 7A, compare line 1 with line 3 at 8 h) and was even greater at 48 h (Fig. 7A, compare line 1 with line 3). However, profiles of the PAO1 strains, WT or ÄqslA mutant, and of the PA14 strains, WT or mutant overproducing QslA, were the same, suggesting that QslA is dispensable for biofilm formation (Fig. 7A and B). Therefore, these results tend to suggest that QslA is not implicated in controlling biofilm formation of P. aeruginosa.
FIG 7.
Bacterial adherence to plastic to infer the ability of strains to form biofilm. (A) Adherence assay with the indicated P. aeruginosa strains grown at 30°C from 2 to 48 h in minimal medium. Arabinose at 0.5% was added in the culture medium to induce the PBAD promoter of pTS51 bearing qslA. (B) Crystal violet quantification of biofilm formation (OD595) over time from two independent experiments. (C) Bacterial colony staining on Congo red-containing agar plates. Red color indicates exopolysaccharide production.
Cytotoxicity toward macrophages is not dependent on QslA.
Finally, we tested the cytotoxicity of the various strains toward J774 macrophages. Even if other virulence factors are linked to P. aeruginosa cytotoxicity, the T3SS, through its effectors, is its major determinant. Indeed, while PA14 is highly cytotoxic due to the T3SS effector ExoU (31), PAO1 is considered an invasive and poorly cytotoxic P. aeruginosa strain. Nevertheless, both strains display T3SS-mediated antiphagocytic functions and the T3SS machinery and effector encoding genes are regulated by HSL-mediated QS (56) and PQS. As QS represses the T3SS regulon (56), we hypothesized that the T3SS-dependent cytotoxicity of the PAO1 should increase in the PAO1 ΔqslA mutant, while it should decrease in the PA14 strain overproducing QslA. Due to the hypercytotoxic phenotype of the PA14 strain, we used two infection conditions according to the two backgrounds (PAO1 and PA14) (Fig. 8). As expected even with longer infection times, the PAO1 strain is poorly cytotoxic compared to PA14 (Fig. 8, compare lines 1 and 3). However, the cytotoxicity levels of the PAO1 strains, the WT or ÄqslA mutant, and of the PA14 strains, the WT or mutant overproducing QslA, were the same, suggesting that the cytotoxicity of P. aeruginosa is not dependent on QslA.
FIG 8.
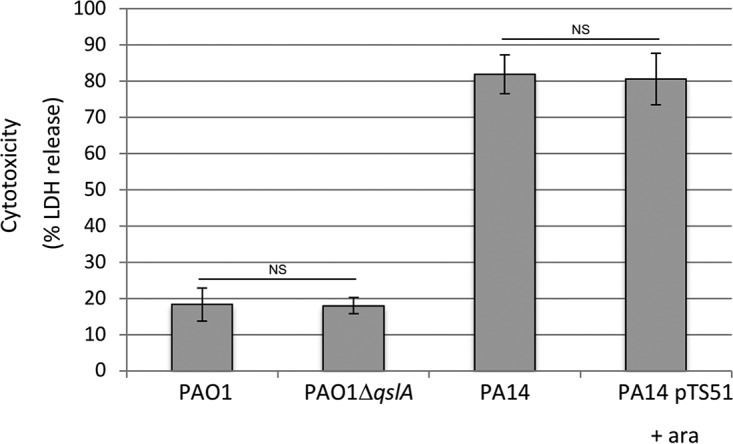
Cytotoxicity toward J774 macrophages evaluated using an LDH assay after 2 h of infection with PAO1 (WT and qslA mutant) and after 45 min of infection with PA14 (WT and QslA overproduction). Arabinose at 0.5% was added in the culture medium after 1 h 30 min of growth to induce the PBAD promoter of pTS51 bearing qslA. Error bars correspond to SDs from three independent experiments done in triplicate. No statistical differences were observed (P values calculated with Student’s t test).
DISCUSSION
PAO1 and PA14 are two stains of P. aeruginosa that are broadly studied throughout the Pseudomonas research community. Interestingly, the PA14 strain is more virulent than the PAO1 strain in different model infection systems (28, 33, 57, 58). Several studies have been conducted to try to decipher why PA14 strain exhibits higher virulence than PAO1. While previous groups have found that pathogenicity islands 1 and 2 are present only in the PA14 genome and encode virulence factors (33), and the ladS gene is mutated in PA14, making T3SS more effective (34, 37), we speculated that other determinants might be involved in the differential virulence between these two strains.
As a result, we decided to use a global transcriptome RNA sequencing approach to highlight new factors that might explain the virulence divergence between these two strains. The transcriptomic data revealed lower expression of the QS regulator qslA in PA14 strain than in PAO1, which we further confirmed at a protein level. Given the fact that QslA is highly produced in PAO1 (Fig. 1B) and plays a crucial role in determining the activation threshold of QS (27), we hypothesized that QS regulon expression occurs earlier and is higher in PA14 than in PAO1. To test this hypothesis, we constructed a deletion mutant of qslA in PAO1 as well as a plasmid allowing overexpression of qslA from a PBAD promoter in PA14 strain (Table 2). This allowed us to investigate whether deletion of qslA in PAO1 can mimic the higher QS activation found in PA14 and, conversely, if overproduction of QslA in PA14 leads to the lower QS activation observed in PAO1. We then focused our study on well-known QS-regulated virulence factors. We were able to confirm that the expression levels of genes encoding the QS system as well as QS gene targets are differentially regulated depending on the expression level of qslA in both strains, except for biofilm and cytotoxicity phenotypes. Considering that a large set of genes encoding virulence factors is regulated by QS in P. aeruginosa, and that their expression levels are increased in PA14 compared to those in PAO1, this could partially explain the hypervirulent phenotype of PA14. Moreover, a qslA mutant in PAO1 strain is more virulent than the wild-type PAO1 strain in a Caenorhabditis elegans model (27). These results combined with our findings suggest that the high expression of qslA in the PAO1 strain is one of the major factors of its decreased virulence compared to PA14 and vice versa for the PA14 strain.
From our results, we questioned whether such mechanisms could be conserved among other isolates of P. aeruginosa. A previous study showed significant differences in QS regulon expression between seven clinical and environmental strains of P. aeruginosa, supporting the notion that different isolates of P. aeruginosa have defined regulation networks (59). These findings indicate a role for QS in the extension of the range of habitats in which a species can thrive, including the host. Based on this, one can ask whether the differential regulation of QS between the different strains of P. aeruginosa causes different levels of virulence that can be observed between different isolates (33). If so, it would be interesting to investigate qslA expression levels in different clinical isolates of P. aeruginosa. However, our data concerning biofilm formation and cytotoxicity toward macrophages suggest that the QslA regulon does not overlap completely the QS regulon. One explanation could be that another QS regulator is important for controlling biofilm and cytotoxicity.
Interestingly, the expression of other known QS regulator genes such as qscR, rsaL, mvaT, cdpR, and qteE was, respectively, 1.2-, 2.1-, 1.6-, 3.9-, and 1.9-fold increased in PA14 (Table 1), which is not comparable to the 55.8-fold-increased expression of qslA in PAO1. This, along with our data concerning the differential modulation of QS, H2-T6SS, pyocyanin, Xcp-T2SS, and LasB, suggests that the modulation of QS between two strains is mostly due to the differential expression of qslA. One could ask, what is the molecular mechanism underlining this differential expression of qslA? We performed a simple BLASTn comparing the upstream 500-bp sequences of qslA in PAO1 and PA14, but there was only one nucleotide difference, hardly explaining the differential expression of qslA in these strains. Likewise, the two proteins are identical (see Fig. S2 in the supplemental material). Further studies will be needed to elucidate the exact mechanism of qslA expression in these two strains. We propose that differential expression of qslA in PAO1 and PA14 leads to modulation of QS signaling. Since QS is a major regulator of virulence factors in P. aeruginosa, it is tempting to speculate that QslA could be a key player in the hypervirulence phenotype of PA14, along with LadS (34) and PAPI-1 and PAPI-2 (30).
MATERIALS AND METHODS
Bacterial strains, culture conditions, plasmids, and oligonucleotides.
The bacterial strains, plasmids, and oligonucleotides used in this study are described in Table 2. LB (lysogeny broth), Pseudomonas isolation agar (PIA), and tryptic soy broth (TSB) were used for the growth of P. aeruginosa and Escherichia coli strains at 37°C. Cultures were inoculated at an optical density at 600 nm (OD600) of 0.1 with overnight cultures, and strains were grown at 37°C with aeration in TSB. Recombinant plasmids were introduced into P. aeruginosa strains PAO1 and PA14 by conjugation using pRK2013 mobilization properties, as described previously (60). The following antibiotics were used: for E. coli, ampicillin (50 μg ml−1), kanamycin (25 μg ml−1), tetracycline (15 μg ml−1), gentamicin (10 μg ml−1), and streptomycin (100 μg ml−1); for P. aeruginosa, tetracycline (200 μg ml−1 for plates or 50 mg ml−1 for liquid growth), gentamicin (50 μg ml−1), and streptomycin (1000 μg ml−1). Expression of the qslA gene under the control of a PBAD promoter in pTS51 was induced or repressed by addition of arabinose or glucose, respectively, at a final concentration of 0.5% after 1 h 30 min of growth.
Transcriptional analysis of Pseudomonas aeruginosa PA14 and PAO1.
To determine if virulence factor genes were differentially expressed between the PAO1 and PA14 P. aeruginosa strains, we performed RNA-seq on mRNA extracted from cultures of both strains grown in rich media (LB) to early stationary phase. Total RNA was isolated from cell pellets equivalent to 2 OD600 units of bacterial culture using the SV total RNA isolation system (Promega) according to the manufacturer’s instructions. Once isolated and prior to the library preparation, the integrity of RNA samples was assessed with a Bioanalyzer system. Barcoded, strand-specific cDNA libraries were constructed, pooled, and sequenced in an Illumina HiSeq 2000, single-end 50-bp reads by BGI-Hong Kong. Illumina reads were mapped to the P. aeruginosa genome PAO1 (GenBank accession number AE004091.2 [61]) and PA14 (GenBank accession number NC_008463.1 [33]) by Bowtie (version Bowtie1 v0.12.9 [62]), indicating strand-specific sequencing. Quantification of gene expression was determined by the HTSeq package (63) using the GenBank P. aeruginosa PA14 and PAO1 annotation files and discarding multimapped reads. Data were normalized by reads per kilobase per million (RPKM) and filtered to the 5,263 orthologous genes conserved between P. aeruginosa strains PA14 and PAO1. Two biological replicates were performed per condition. Differential expression analysis was analyzed using the Bioconductor package NOISeq version 2.22.1 (64), a nonparametric approach suitable for lowly replicated data, and using a q value of 0.99 for strong control of false positives.
cDNA library preparation.
The RNA was fragmented with RNase III. Then the 5′ PPP structures were removed from the RNA samples using RNA 5′ polyphosphatase (Epicentre) and the RNA was poly(A) tailed using poly(A) polymerase. Then an RNA adapter was ligated to the 5′ phosphate of the RNA fragments. First-strand cDNA synthesis was performed using an oligo(dT)-adapter primer and Moloney murine leukemia virus (M-MLV) reverse transcriptase. The resulting cDNA was PCR amplified to about 30 ng/μl using a high-fidelity DNA polymerase. The cDNA was purified using the Agencourt AMPure XP kit (Beckman Coulter Genomics).
Construction of the ΔqslA mutant.
To generate the qslA deletion strain, 500-bp upstream and 500-bp downstream sequences of the qslA gene were amplified by overlapping PCR with high-fidelity DNA polymerase (Roche Applied Science) using primers TSO108, TSO109, TSO110, and TSO111 (Table 2). The PCR product was cloned in pCR2.1 (TA cloning kit; Invitrogen), giving pTS48, which was then sequenced (GATC) and subcloned into pKNG101 suicide vector, giving the mutator pTS50. pTS50, maintained in E. coli CC118 λpir, was further conjugated in P. aeruginosa strain PAO1 using a protocol previously described (60). The mutant, in which the double-recombination events occurred and resulted in the nonpolar deletion of qslA gene, was verified by PCR using external primers TSO112 and TSO113.
Construction of the chromosomic qslAV5 recombinant gene.
PAO1 strains chromosomally encoding QslAV5 translational fusion were engineered by exchanging the qslA stop codon with the sequence encoding the V5 tag followed by a stop codon. This was achieved by overlapping PCR of the 500-bp upstream and downstream regions of the native stop codon of qslA with high-fidelity DNA polymerase (Roche) using primers TSO103, TSO104, TSO105, and TSO106 (Table 2). The PCR product was cloned in pCR2.1 (TA cloning kit; Invitrogen), which was then sequenced (GATC) and subcloned into pKNG101 suicide vector, giving pTS52. pTS52, maintained in the E. coli CC118 λpir strain, was mobilized in wild-type P. aeruginosa strain PAO1 or PA14. The resulting strains, in which the double-recombination events occurred and resulted in the chromosomic tagging of qslA with V5, were verified by PCR using primers TSO58 and TSO107.
lacZ reporter fusion and β-galactosidase assay.
For PH2-T6SS-′lacZ, the promoter fragment was integrated at the CTX phage attachment site in PAO1 or PA14 and isogenic mutants using established protocols (65). For PrhlR-′lacZ, PrhlI-′lacZ, and PlasR-′lacZ, plasmids carrying promoter fragment were conjugated in PAO1 or PA14 and isogenic mutants using established protocols (60). An overnight culture, grown in TSB supplemented with tetracycline, was diluted in TSB to an OD600 of 0.1. Growth and β-galactosidase activity were monitored by harvesting samples at different time intervals. β-Galactosidase activity was measured according to the method of Miller, as described previously (16), and based on o-nitrophenyl-β-d-galactopyranoside hydrolysis. β-Galactosidase activities were expressed in Miller units.
Pyocyanin production.
Pyocyanin was extracted from the extracellular medium by adding an equal volume of chloroform (CHCl3) and vigorous vortexing as previously described (43). The lower pyocyanin-containing organic layer was then taken and vortexed with an equal volume of 0.2 M HCl. The pink pyocyanin-containing aqueous layer resulting from the previous step was then taken, and its absorbance at 520 nm (OD520) was read. Concentrations, expressed as micrograms of pyocyanin produced per milliliter of culture supernatant, were determined by multiplying the OD520 by 17.072.
SDS-PAGE and immunoblotting.
Proteins from the extracellular medium were loaded at an equivalent of 1.0 OD600 unit, while proteins from cellular extracts were loaded at an equivalent of 0.1 OD600 unit. Proteins were then separated on SDS gels containing 11 or 15% acrylamide depending on the size of the proteins being further detected.
For Western blotting, proteins were transferred from gels onto nitrocellulose membranes. After 30-min to overnight saturation in Tris-buffered saline (TBS) (0.1 M Tris, 0.1 M NaCl [pH 7.5]), 0.05% (vol/vol) Tween 20, and 5% (wt/vol) skim milk, the membrane was incubated for 1 h with anti-V5 (diluted 1:2,500), anti-XcpP (1/2,000), or anti-DsbA (1:25,000), washed three times with TBS–0.05% Tween 20, incubated for 45 min with goat anti-rabbit immunoglobulin G (IgG) antibodies (Sigma) diluted 1:5,000, washed three times with TBS–0.05% Tween 20, and then revealed with a Super Signal chemiluminescence system (Pierce).
Adherence assay on inert surfaces and exopolysaccharide production.
The adherence assay was performed as previously described previously (66), with some modifications. Cultures inoculated at an OD600 of 0.05 with overnight cultures were grown at 37°C with aeration in LB with appropriate antibiotics. Induction of the promoter arabinose (PBAD) was done at an OD600 of 0.6 with 0.2% arabinose for 1.5 h. Twenty-four-well polystyrene microtiter plates were then inoculated at an OD600 of 0.2 in 1 ml of MM63 medium supplemented with Casamino Acids (0.5%), 1 mM MgSO4, and glucose or arabinose (0.2%) for 2 to 48 h at 30°C. Bacteria were stained with 0.1% crystal violet for a period of 10 min and washed twice with water. The stain was then dissolved in ethanol, and absorbance was measured at 595 nm.
The Congo red assay was performed as previously described to measure the production of exopolysaccharides (67).
Cytotoxicity toward macrophages.
The cytotoxicity of P. aeruginosa strains grown to mid-log phase in LB broth was assayed using J774 macrophages as described previously (68), except that macrophages were infected for 45 min or 2.5 h at a multiplicity of infection (MOI) of 20 for the PAO1 or PA14 strains, respectively. The percentage of lactate dehydrogenase (LDH) release was calculated relative to that of the uninfected control, which was set at 0% LDH release, and that of uninfected cells lysed with Triton X-100, which was set at 100% LDH release.
Statistical analysis.
For multigroup comparisons, a main P value was calculated by analysis of variance (ANOVA) (Stat Plus software), and unpaired Student’s t tests were performed using Excel software (Microsoft) for two-group comparisons.
Supplementary Material
ACKNOWLEDGMENTS
We are very grateful to Kyler A. Lugo and Lilian H. Lam for careful reading of the manuscript. We also thank Steve Garvis for advice on RNA preparation and Vertis Biotechnologie AG (Freising-Weihenstephan, Germany) for RNA sequencing. We are grateful to I. Bringer, A. Brun, and O. Uderso for technical assistance.
T.G.S. and M.R.G. were supported by a Ph.D. fellowship from the French Research Ministry and with a teaching and research fellowship from Aix-Marseille University. A.L. was financed by a Ph.D. fellowship from Vaincre La Mucoviscidose (RF20120600685 and RF20130500911). This work was supported by recurrent funding from the CNRS and Aix-Marseille University and by Pathomics ERA-net PATHO grant ANR-08-PATH-004-01.
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
T.G.S. and S.B. designed and conceived the experiments. T.G.S., R.L., M.R.G., A.L., C.S., C.C., and B.I. performed the experiments. T.G.S. and S.B. supervised the execution of the experiments. T.G.S., R.L., A.C., B.I., and S.B. analyzed the data and discussed them with M.R.G., A.L., and R.V. T.G.S. and S.B. wrote the paper with contribution from B.I. and reading from R.L., A.C., M.R.G., and R.V.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JB.00362-19.
REFERENCES
- 1.Govan JRW, Deretic V. 1996. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol Rev 60:539–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stover CK, Pham XQ, Erwin AL, Mizoguchi SD, Warrener P, Hickey MJ, Brinkman FSL, Hufnagle WO, Kowalik DJ, Lagrou M, Garber RL, Goltry L, Tolentino E, Westbrock-Wadman S, Yuan Y, Brody LL, Coulter SN, Folger KR, Kas A, Larbig K, Lim R, Smith K, Spencer D, Wong GK-S, Wu Z, Paulsen IT, Reizer J, Saier MH, Hancock REW, Lory S, Olson MV. 2000. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406:959–964. doi: 10.1038/35023079. [DOI] [PubMed] [Google Scholar]
- 3.Balasubramanian D, Schneper L, Kumari H, Mathee K. 2013. A dynamic and intricate regulatory network determines Pseudomonas aeruginosa virulence. Nucleic Acids Res 41:1–20. doi: 10.1093/nar/gks1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuqua WC, Winans SC. 1994. A LuxR-LuxI type regulatory system activates Agrobacterium Ti plasmid conjugal transfer in the presence of a plant tumor metabolite. J Bacteriol 176:2796–2806. doi: 10.1128/jb.176.10.2796-2806.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams P, Cámara M. 2009. Quorum sensing and environmental adaptation in Pseudomonas aeruginosa: a tale of regulatory networks and multifunctional signal molecules. Curr Opin Microbiol 12:182–191. doi: 10.1016/j.mib.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Schuster M, Joseph Sexton D, Diggle SP, Peter Greenberg E. 2013. Acyl-homoserine lactone quorum sensing: from evolution to application. Annu Rev Microbiol 67:43–63. doi: 10.1146/annurev-micro-092412-155635. [DOI] [PubMed] [Google Scholar]
- 7.Latifi A, Foglino M, Tanaka K, Williams P, Lazdunski A. 1996. A hierarchical quorum-sensing cascade in Pseudomonas aeruginosa links the transcriptional activators LasR and RhlR (VsmR) to expression of the stationary-phase sigma factor RpoS. Mol Microbiol 21:1137–1146. doi: 10.1046/j.1365-2958.1996.00063.x. [DOI] [PubMed] [Google Scholar]
- 8.Pesci EC, Pearson JP, Seed PC, Iglewski BH. 1997. Regulation of las and rhl quorum sensing in Pseudomonas aeruginosa. J Bacteriol 179:3127–3132. doi: 10.1128/jb.179.10.3127-3132.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schuster M, Lostroh CP, Ogi T, Greenberg EP. 2003. Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: a transcriptome analysis. J Bacteriol 185:2066–2079. doi: 10.1128/jb.185.7.2066-2079.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagner VE, Bushnell D, Passador L, Brooks AI, Iglewski BH. 2003. Microarray analysis of Pseudomonas aeruginosa quorum-sensing regulons: effects of growth phase and environment. J Bacteriol 185:2080–2095. doi: 10.1128/jb.185.7.2080-2095.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vasil ML. 2003. DNA microarrays in analysis of quorum sensing: strengths and limitations. J Bacteriol 185:2061–2065. doi: 10.1128/jb.185.7.2061-2065.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gambello MJ, Iglewski BH. 1991. Cloning and characterization of the Pseudomonas aeruginosa lasR gene, a transcriptional activator of elastase expression. J Bacteriol 173:3000–3009. doi: 10.1128/jb.173.9.3000-3009.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chapon-Herve V, Akrim M, Latifi A, Williams P, Lazdunski A, Bally M. 1997. Regulation of the xcp secretion pathway by multiple quorum-sensing modulons in Pseudomonas aeruginosa. Mol Microbiol 24:1169–1178. doi: 10.1046/j.1365-2958.1997.4271794.x. [DOI] [PubMed] [Google Scholar]
- 14.Lesic B, Starkey M, He J, Hazan R, Rahme LG. 2009. Quorum sensing differentially regulates Pseudomonas aeruginosa type VI secretion locus I and homologous loci II and III, which are required for pathogenesis. Microbiology 155:2845–2855. doi: 10.1099/mic.0.029082-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sana TG, Hachani A, Bucior I, Soscia C, Garvis S, Termine E, Engel J, Filloux A, Bleves S. 2012. The second type VI secretion system of Pseudomonas aeruginosa strain PAO1 is regulated by quorum sensing and fur and modulates internalization in epithelial cells. J Biol Chem 287:27095–27105. doi: 10.1074/jbc.M112.376368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sana TG, Soscia C, Tonglet CM, Garvis S, Bleves S. 2013. Divergent control of two type VI secretion systems by RpoN in Pseudomonas aeruginosa. PLoS One 8:e76030. doi: 10.1371/journal.pone.0076030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brint JM, Ohman DE. 1995. Synthesis of multiple exoproducts in Pseudomonas aeruginosa is under the control of RhlR-RhlI, another set of regulators in strain PAO1 with homology to the autoinducer-responsive LuxR-LuxI family. J Bacteriol 177:7155–7163. doi: 10.1128/jb.177.24.7155-7163.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fazli M, Almblad H, Rybtke ML, Givskov M, Eberl L, Tolker-Nielsen T. 2014. Regulation of biofilm formation in Pseudomonas and Burkholderia species: regulation of biofilm formation. Environ Microbiol 16:1961–1981. doi: 10.1111/1462-2920.12448. [DOI] [PubMed] [Google Scholar]
- 19.Pesci EC, Milbank JBJ, Pearson JP, McKnight S, Kende AS, Greenberg EP, Iglewski BH. 1999. Quinolone signaling in the cell-to-cell communication system of Pseudomonas aeruginosa. Proc Natl Acad Sci 96:11229–11234. doi: 10.1073/pnas.96.20.11229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diggle SP, Winzer K, Chhabra SR, Worrall KE, Cámara M, Williams P. 2003. The Pseudomonas aeruginosa quinolone signal molecule overcomes the cell density-dependency of the quorum sensing hierarchy, regulates rhl-dependent genes at the onset of stationary phase and can be produced in the absence of LasR. Mol Microbiol 50:29–43. doi: 10.1046/j.1365-2958.2003.03672.x. [DOI] [PubMed] [Google Scholar]
- 21.McGrath S, Wade DS, Pesci EC. 2004. Dueling quorum sensing systems in Pseudomonas aeruginosa control the production of the Pseudomonas quinolone signal (PQS). FEMS Microbiol Lett 230:27–34. doi: 10.1016/S0378-1097(03)00849-8. [DOI] [PubMed] [Google Scholar]
- 22.Diggle SP, Winzer K, Lazdunski A, Williams P, Camara M. 2002. Advancing the quorum in Pseudomonas aeruginosa: MvaT and the regulation of N-acylhomoserine lactone production and virulence gene expression. J Bacteriol 184:2576–2586. doi: 10.1128/jb.184.10.2576-2586.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao J, Yu X, Zhu M, Kang H, Ma J, Wu M, Gan J, Deng X, Liang H. 2016. Structural and molecular mechanism of CdpR involved in quorum-sensing and bacterial virulence in Pseudomonas aeruginosa. PLoS Biol 14:e1002449. doi: 10.1371/journal.pbio.1002449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee J, Wu J, Deng Y, Wang J, Wang C, Wang J, Chang C, Dong Y, Williams P, Zhang L-H. 2013. A cell-cell communication signal integrates quorum sensing and stress response. Nat Chem Biol 9:339–343. doi: 10.1038/nchembio.1225. [DOI] [PubMed] [Google Scholar]
- 25.Ledgham F, Ventre I, Soscia C, Foglino M, Sturgis JN, Lazdunski A. 2003. Interactions of the quorum sensing regulator QscR: interaction with itself and the other regulators of Pseudomonas aeruginosa LasR and RhlR: interactions of QscR. Mol Microbiol 48:199–210. doi: 10.1046/j.1365-2958.2003.03423.x. [DOI] [PubMed] [Google Scholar]
- 26.Siehnel R, Traxler B, An DD, Parsek MR, Schaefer AL, Singh PK. 2010. A unique regulator controls the activation threshold of quorum-regulated genes in Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 107:7916–7921. doi: 10.1073/pnas.0908511107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seet Q, Zhang L-H. 2011. Anti-activator QslA defines the quorum sensing threshold and response in Pseudomonas aeruginosa: quorum sensing anti-activator QslA. Mol Microbiol 80:951–965. doi: 10.1111/j.1365-2958.2011.07622.x. [DOI] [PubMed] [Google Scholar]
- 28.Rahme L, Stevens E, Wolfort S, Shao J, Tompkins R, Ausubel F. 1995. Common virulence factors for bacterial pathogenicity in plants and animals. Science 268:1899–1902. doi: 10.1126/science.7604262. [DOI] [PubMed] [Google Scholar]
- 29.Mathee K. 2018. Forensic investigation into the origin of Pseudomonas aeruginosa PA14—old but not lost. J Med Microbiol 67:1019–1021. doi: 10.1099/jmm.0.000778. [DOI] [PubMed] [Google Scholar]
- 30.He J, Baldini RL, Deziel E, Saucier M, Zhang Q, Liberati NT, Lee D, Urbach J, Goodman HM, Rahme LG. 2004. The broad host range pathogen Pseudomonas aeruginosa strain PA14 carries two pathogenicity islands harboring plant and animal virulence genes. Proc Natl Acad Sci U S A 101:2530–2535. doi: 10.1073/pnas.0304622101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Finck-Barbançon V, Goranson J, Zhu L, Sawa T, Wiener-Kronish JP, Fleiszig SMJ, Wu C, Mende-Mueller L, Frank DW. 1997. ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial injury. Mol Microbiol 25:547–557. doi: 10.1046/j.1365-2958.1997.4891851.x. [DOI] [PubMed] [Google Scholar]
- 32.Hauser AR, Kang PJ, Engel JN. 1998. PepA, a secreted protein of Pseudomonas aeruginosa, is necessary for cytotoxicity and virulence. Mol Microbiol 27:807–818. doi: 10.1046/j.1365-2958.1998.00727.x. [DOI] [PubMed] [Google Scholar]
- 33.Lee DG, Urbach JM, Wu G, Liberati NT, Feinbaum RL, Miyata S, Diggins LT, He J, Saucier M, Déziel E, Friedman L, Li L, Grills G, Montgomery K, Kucherlapati R, Rahme LG, Ausubel FM. 2006. Genomic analysis reveals that Pseudomonas aeruginosa virulence is combinatorial. Genome Biol 7:R90. doi: 10.1186/gb-2006-7-10-r90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mikkelsen H, McMullan R, Filloux A. 2011. The Pseudomonas aeruginosa reference strain PA14 displays increased virulence due to a mutation in ladS. PLoS One 6:e29113. doi: 10.1371/journal.pone.0029113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ventre I, Goodman AL, Vallet-Gely I, Vasseur P, Soscia C, Molin S, Bleves S, Lazdunski A, Lory S, Filloux A. 2006. Multiple sensors control reciprocal expression of Pseudomonas aeruginosa regulatory RNA and virulence genes. Proc Natl Acad Sci U S A 103:171–176. doi: 10.1073/pnas.0507407103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chambonnier G, Roux L, Redelberger D, Fadel F, Filloux A, Sivaneson M, de Bentzmann S, Bordi C. 2016. The hybrid histidine kinase LadS forms a multicomponent signal transduction system with the GacS/GacA two-component system in Pseudomonas aeruginosa. PLoS Genet 12:e1006032. doi: 10.1371/journal.pgen.1006032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allsopp LP, Wood TE, Howard SA, Maggiorelli F, Nolan LM, Wettstadt S, Filloux A. 2017. RsmA and AmrZ orchestrate the assembly of all three type VI secretion systems in Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 114:7707–7712. doi: 10.1073/pnas.1700286114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dietrich LEP, Price-Whelan A, Petersen A, Whiteley M, Newman DK. 2006. The phenazine pyocyanin is a terminal signalling factor in the quorum sensing network of Pseudomonas aeruginosa. Mol Microbiol 61:1308–1321. doi: 10.1111/j.1365-2958.2006.05306.x. [DOI] [PubMed] [Google Scholar]
- 39.Fan H, Dong Y, Wu D, Bowler MW, Zhang L, Song H. 2013. QsIA disrupts LasR dimerization in antiactivation of bacterial quorum sensing. Proc Natl Acad Sci U S A 110:20765–20770. doi: 10.1073/pnas.1314415110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saier MH, Reddy BL. 2015. Holins in bacteria, eukaryotes, and archaea: multifunctional xenologues with potential biotechnological and biomedical applications. J Bacteriol 197:7–17. doi: 10.1128/JB.02046-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klockgether J, Munder A, Neugebauer J, Davenport CF, Stanke F, Larbig KD, Heeb S, Schock U, Pohl TM, Wiehlmann L, Tummler B. 2010. Genome diversity of Pseudomonas aeruginosa PAO1 laboratory strains. J Bacteriol 192:1113–1121. doi: 10.1128/JB.01515-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dötsch A, Pommerenke C, Bredenbruch F, Geffers R, Häussler S. 2009. Evaluation of a microarray-hybridization based method applicable for discovery of single nucleotide polymorphisms (SNPs) in the Pseudomonas aeruginosa genome. BMC Genomics 10:29. doi: 10.1186/1471-2164-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Essar DW, Eberly L, Hadero A, Crawford IP. 1990. Identification and characterization of genes for a second anthranilate synthase in Pseudomonas aeruginosa: interchangeability of the two anthranilate synthases and evolutionary implications. J Bacteriol 172:884–900. doi: 10.1128/jb.172.2.884-900.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang F, Waterfield NR, Yang J, Yang G, Jin Q. 2014. A Pseudomonas aeruginosa type VI secretion phospholipase D effector targets both prokaryotic and eukaryotic cells. Cell Host Microbe 15:600–610. doi: 10.1016/j.chom.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 45.Sana TG, Baumann C, Merdes A, Soscia C, Rattei T, Hachani A, Jones C, Bennett KL, Filloux A, Superti-Furga G, Voulhoux R, Bleves S. 2015. Internalization of Pseudomonas aeruginosa strain PAO1 into epithelial cells is promoted by interaction of a T6SS effector with the microtubule network. mBio 6:e00712-15. doi: 10.1128/mBio.00712-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sana TG, Berni B, Bleves S. 2016. The T6SSs of Pseudomonas aeruginosa strain PAO1 and their effectors: beyond bacterial-cell targeting. Front Cell Infect Microbiol 6:61. doi: 10.3389/fcimb.2016.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang F, Wang X, Wang B, Chen L, Zhao Z, Waterfield NR, Yang G, Jin Q. 2016. The Pseudomonas aeruginosa type VI secretion PGAP1-like effector induces host autophagy by activating endoplasmic reticulum stress. Cell Rep 16:1502–1509. doi: 10.1016/j.celrep.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 48.Russell AB, LeRoux M, Hathazi K, Agnello DM, Ishikawa T, Wiggins PA, Wai SN, Mougous JD. 2013. Diverse type VI secretion phospholipases are functionally plastic antibacterial effectors. Nature 496:508–512. doi: 10.1038/nature12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burkinshaw BJ, Liang X, Wong M, Le ANH, Lam L, Dong TG. 2018. A type VI secretion system effector delivery mechanism dependent on PAAR and a chaperone–co-chaperone complex. Nat Microbiol 3:632–640. doi: 10.1038/s41564-018-0144-4. [DOI] [PubMed] [Google Scholar]
- 50.Bleves S. 2016. Game of trans-kingdom effectors. Trends Microbiol 24:773–774. doi: 10.1016/j.tim.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 51.Bleves S, Viarre V, Salacha R, Michel GPF, Filloux A, Voulhoux R. 2010. Protein secretion systems in Pseudomonas aeruginosa: a wealth of pathogenic weapons. Int J Med Microbiol 300:534–543. doi: 10.1016/j.ijmm.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 52.Bleves S, Lazdunski A, Filloux A. 1996. Membrane topology of three Xcp proteins involved in exoprotein transport by Pseudomonas aeruginosa. J Bacteriol 178:4297–4300. doi: 10.1128/jb.178.14.4297-4300.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Douzi B, Ball G, Cambillau C, Tegoni M, Voulhoux R. 2011. Deciphering the Xcp Pseudomonas aeruginosa type II secretion machinery through multiple interactions with substrates. J Biol Chem 286:40792–40801. doi: 10.1074/jbc.M111.294843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Douzi B, Trinh NTT, Michel-Souzy S, Desmyter A, Ball G, Barbier P, Kosta A, Durand E, Forest KT, Cambillau C, Roussel A, Voulhoux R. 2017. Unraveling the self-assembly of the Pseudomonas aeruginosa XcpQ secretin periplasmic domain provides new molecular insights into type II secretion system secreton architecture and dynamics. mBio 8:e01185-17. doi: 10.1128/mBio.01185-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kuang Z, Hao Y, Walling BE, Jeffries JL, Ohman DE, Lau GW. 2011. Pseudomonas aeruginosa elastase provides an escape from phagocytosis by degrading the pulmonary surfactant protein-A. PLoS One 6:e27091. doi: 10.1371/journal.pone.0027091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bleves S, Soscia C, Nogueira-Orlandi P, Lazdunski A, Filloux A. 2005. Quorum sensing negatively controls type III secretion regulon expression in Pseudomonas aeruginosa PAO1. J Bacteriol 187:3898–3902. doi: 10.1128/JB.187.11.3898-3902.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Choi JY, Sifri CD, Goumnerov BC, Rahme LG, Ausubel FM, Calderwood SB. 2002. Identification of virulence genes in a pathogenic strain of Pseudomonas aeruginosa by representational difference analysis. J Bacteriol 184:952–961. doi: 10.1128/jb.184.4.952-961.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tan M-W, Mahajan-Miklos S, Ausubel FM. 1999. Killing of Caenorhabditis elegans by Pseudomonas aeruginosa used to model mammalian bacterial pathogenesis. Proc Natl Acad Sci U S A 96:715–720. doi: 10.1073/pnas.96.2.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chugani S, Kim BS, Phattarasukol S, Brittnacher MJ, Choi SH, Harwood CS, Greenberg EP. 2012. Strain-dependent diversity in the Pseudomonas aeruginosa quorum-sensing regulon. Proc Natl Acad Sci U S A 109:E2823–E2831. doi: 10.1073/pnas.1214128109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sana TG, Laubier A, Bleves S. 2014. Gene transfer: conjugation, p 17–22. In Filloux A, Ramos J-L (ed), Pseudomonas methods and protocols. Springer New York, New York, NY. [DOI] [PubMed] [Google Scholar]
- 61.Benson DA, Clark K, Karsch-Mizrachi I, Lipman DJ, Ostell J, Sayers EW. 2014. GenBank. Nucleic Acids Res 42:D32–D37. doi: 10.1093/nar/gkt1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Langmead B, Trapnell C, Pop M, Salzberg SL. 2009. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Anders S, Pyl PT, Huber W. 2015. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 31:166–169. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tarazona S, Furió-Tarí P, Turrà D, Pietro AD, Nueda MJ, Ferrer A, Conesa A. 2015. Data quality aware analysis of differential expression in RNA-seq with NOISeq R/Bioc package. Nucleic Acids Res 43:e140. doi: 10.1093/nar/gkv711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hoang TT, Kutchma AJ, Becher A, Schweizer HP. 2000. Integration-proficient plasmids for Pseudomonas aeruginosa: site-specific integration and use for engineering of reporter and expression strains. Plasmid 43:59–72. doi: 10.1006/plas.1999.1441. [DOI] [PubMed] [Google Scholar]
- 66.Bordi C, Lamy M-C, Ventre I, Termine E, Hachani A, Fillet S, Roche B, Bleves S, Méjean V, Lazdunski A, Filloux A. 2010. Regulatory RNAs and the HptB/RetS signalling pathways fine-tune Pseudomonas aeruginosa pathogenesis: Pseudomonas aeruginosa signalling via HptB, RetS and sRNA. Mol Microbiol 76:1427–1443. doi: 10.1111/j.1365-2958.2010.07146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Soscia C, Hachani A, Bernadac A, Filloux A, Bleves S. 2007. Cross talk between type III secretion and flagellar assembly systems in Pseudomonas aeruginosa. J Bacteriol 189:3124–3132. doi: 10.1128/JB.01677-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Figurski DH, Helinski DR. 1979. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A 76:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaniga K, Delor I, Cornelis GR. 1991. A wide-host-range suicide vector for improving reverse genetics in Gram-negative bacteria: inactivation of the BlaA gene of Yersinia enterocolitica. Gene 109:137–141. doi: 10.1016/0378-1119(91)90599-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.