Abstract
Rotavirus is a non-enveloped double-stranded RNA virus that causes severe gastroenteritis in children, but complications are rarely reported. Some reports have shown that rotavirus can induce diverse complications of the central nervous system, such as seizures, encephalopathy with a reversible splenial lesion, encephalitis, cerebral white matter abnormalities, and cerebellitis. Here, we present a 2-year-old patient with seizures, who had an isolated splenial lesion in the corpus callosum on neuroimaging, and the rotavirus antigen detected in faeces.
Keywords: rotavirus, paediatric, seizures, rotavirus, complications
Abstract
ROTAVIRUSO SUKELTI TRAUKULIAI IR LAIKINAS DIDŽIOSIOS SMEGENŲ JUNGTIES PAŽEIDIMAS
Santrauka
Rotavirusas yra dvigrandės RNR virusas be apvalkalo, sukeliantis sunkų vaikų gastroenteritą, tačiau apie komplikacijas pranešama retai. Kai kurie atvejų aprašymai rodo, kad rotavirusas gali sukelti įvairias centrinės nervų sistemos komplikacijas, tokias kaip traukuliai, encefalopatija su trumpalaikiu didžiosios smegenų jungties pažeidimu, encefalitas, smegenų baltosios medžiagos anomalijos ir cerebelitas. Čia pristatome dvejų metų pacientą su traukuliais, kuriam laikinas didžiosios smegenų jungties pažeidimas buvo nustatytas neurovaizdinimo metu, o rotaviruso antigenas aptiktas išmatose.
Raktažodžiai: rotavirusas, vaikų traukuliai, rotaviruso komplikacijos
CASE PRESENTATION
A previously healthy 2-year-old boy was hospitalized in the Emergency Department (ED) of the Children’s Clinical University Hospital in Riga, after suffering several consecutive generalized tonic seizures that lasted approximately three minutes each and were followed by disturbance of consciousness in which the patient initially did not recognize his parents and had difficulties speaking. The seizures were preceded by a 24-hour history of mild diarrhoea, vomiting, and a fever. The patient was afebrile during seizure episode. The patient’s younger sister also had diarrhoea, vomiting, and a fever. He was completely immunized according to the recommendations of the National Immunization Programme (with the exception of the Rotavirus vaccine). On admission, body temperature was 37.4°C, the heart rate 150 beats/min, the respiratory rate 26–30 breaths/min, and SpO2 was 99%. Signs of mild dehydration were present, the abdomen was soft by palpation and meteorized, active bowel sounds were auscultated. There were no significant changes in patient’s full blood count and formula, cerebrospinal fluid and levels of electrolytes that were performed on admission. Neurological examination did not reveal any focal abnormality, and the boy was transferred to the ward of infectious diseases where supportive therapy was initiated. While the boy was in the hospital, he had another afebrile generalized tonic seizure episode followed by disturbance of consciousness, and after the second episode he was transferred to the ICU. After 24 hours of observation in the ICU, the boy was transferred back to the ward. At recommendations of a neurologist, treatment with furosemide and phenobarbital was initiated, and MRI and EEG were prescribed. After the therapy was initiated, the patient had a short episode of facial muscle spasms but no further neurological symptoms developed. The rotavirus antigen was detected in the stool sample at the time of presentation to the ED. Electroencephalography was without pathology. MRI showed a transient intensified signal on the splenium of corpus callosum (Fig. 1). The patient was evaluated by an ophthalmologist and a neurologist and neither examination revealed any abnormalities. The patient was discharged five days later in stable overall condition, with recommendations to stay under the supervision of a general practitioner and to continue therapy with phenobarbital for four days. Repeat MRI was recommended after two months. Follow-up MRI revealed no pathology, and no signs of neurologic complications were evident upon discharge or after two months (Fig. 2).
Fig. 1.
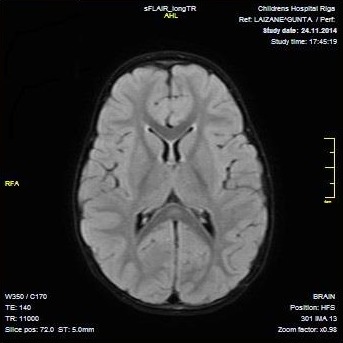
Axial diffusion-weighted magnetic resonance images (DWI) demonstrate high signal intensity on the splenium of the corpus callosum on day 2 of hospitalization
Fig. 2.
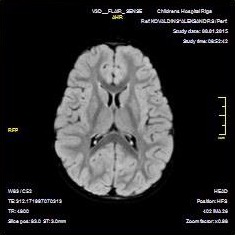
Normal axial diffusion-weighted magnetic resonance image (DWI) two months after discharge
DISCUSSION
Group A rotavirus infection is the leading cause of severe acute gastroenteritis in children (3). While infection is generally not associated with extragastrointestinal complications, rotavirus gastroenteritis has been reported to be accompanied by neurological manifestations, such as febrile seizures, afebrile seizures, encephalopathy, meningoencephalitis, or cerebellitis (1). Transient corpus callosum lesions, similar to the lesion found in our patient, have been reported in the cases of neurological manifestations associated with influenza A virus, parainfluenza virus, mumps, Epstein-Barr virus, rotavirus, and adenovirus infections (4–9). These lesions are described in association with many diverse clinical conditions including the use of antiepileptic drugs, hypoglycaemia, and are related to migraine with aura in children (10–12). Recent case reports illustrate that rotavirus RNA may be found in the cerebrospinal fluid (CSF) of patients with gastroenteritis and neurological symptoms (13, 14). However, the pathophysiology of the involvement of the central nervous system has not been explained yet. There are several hypotheses. Detection of rotavirus RNA or the antigen by PCR from CSF of rotavirus-positive patients with neurological manifestations supports the hypothesis of direct viral invasion (13, 14). Another explanation focuses on possible contamination at the time of lumbar puncture or in the testing laboratory, or carriage of rotavirus RNA in CSF lymphocytes (15), although the case reported by Lynch et al. (15) did not support this hypothesis. In their case report, the detection of rotavirus RNA in two CSF samples obtained three weeks apart makes faecal contamination unlikely. Rotavirus infection of neurons has been demonstrated in vitro and in vivo (16–18). However, the lack of evidence of rotavirus in the CSF in many cases of rotavirus-associated neurological manifestations suggests an alternative mechanism. Another possible explanation of neurological manifestations could be associated with secondary involvement of the CNS. During infection, nonstructural protein-4 (NSP4) destroys enterocytes and toxins entering the bloodstream via damaged intestinal epithelium. In addition, rotavirus infection destroys healthy intestinal brush border enzymes. NSP4 has been shown to have direct toxic effects on the gastrointestinal epithelium and lamina propria (19).
In this case report, the examination of the rotavirus antigen was not performed in CSF. However, imaging results support the association of rotavirus with corpus callosum lesions in children with severe acute gastroenteritis.
CONCLUSIONS
Our case report demonstrates encephalopathy associated with rotavirus infection with transient corpus callosum lesion. The patient’s neurological symptoms resolved after therapy without any complications. This and other reported cases suggest that once the intensified signal of the splenium is detected, a mild course can be expected without major complications and a good prognosis.
Gunta Laizane, Liene Smane, Ieva Nokalna, Dace Gardovska, Kristen A. Feemster
References
- Dennehy PH. Rotavirus infection: an update on management and prevention. Adv Pediatr. 2012; 59(1): 47–74. [DOI] [PubMed] [Google Scholar]
- Takanashi J. Wide range of CNS manifestations of rotavirus infection. Brain Dev. 2011. January; 33(1): 9. [DOI] [PubMed] [Google Scholar]
- Dennehy PH. Rotavirus infection: a disease of the past? Infect Dis Clin North Am. 2015. December; 29(4): 617–35. [DOI] [PubMed] [Google Scholar]
- Iwata A, Matsubara K, Nigami H, Kamimura K, Fukaya T. Reversible splenial lesion associated with novel influenza A (H1N1) viral infection. Pediatr Neurol. 2010. June; 42(6): 447–50. [DOI] [PubMed] [Google Scholar]
- Abenhaim Halpern L, Agyeman P, Steinlin M, El-Koussy M, Grunt S. Mild encephalopathy with splenial lesion and parainfluenza virus infection. Pediatr Neurol. 2013. March; 48(3): 252–4. [DOI] [PubMed] [Google Scholar]
- Hara M Mizuochi T Kawano G Koike T Shibuya I Ohya T Ohbu K Nagai K Nagamitsu S Yamashita Y, Nakayama T Matsuishi T.. A case of clinically mild encephalitis with a reversible splenial lesion (MERS) after mumps vaccination. Brain Dev. 2011. November; 33(10): 842–4. [DOI] [PubMed] [Google Scholar]
- Zhang S, Feng J, Shi Y. Transient widespread cortical and splenial lesions in acute encephalitis/encephalopathy associated with primary Epstein-Barr virus infection. Int J Infect Dis. 2016. January; 42: 7–10. [DOI] [PubMed] [Google Scholar]
- Chen WX, Liu HS, Yang SD, Zeng SH, Gao YY, Du ZH, Li XJ, Lin HS, Liang HC, Mai JN. Reversible splenial lesion syndrome in children: Retrospective study and summary of case series. Brain Dev. 2016. November; 38(10): 915–27. [DOI] [PubMed] [Google Scholar]
- Hibino M, Horiuchi S, Okubo Y, Kakutani T, Ohe M, Kondo T. Transient hemiparesis and hemianesthesia in an atypical case of adult-onset clinically mild encephalitis/encephalopathy with a reversible splenial lesion associated with adenovirus infection. Intern Med. 2014; 53(11): 1183–5. [DOI] [PubMed] [Google Scholar]
- Sawagashira R, Narita H, Hashimoto N, Kurita T, Nakagawa S, Saitoh T, Kusumi I. Transient lesions of the splenium of the corpus callosum following rapid withdrawal of levetiracetam. Epileptic Disord. 2017. September 1; 19(3): 379–82. [DOI] [PubMed] [Google Scholar]
- Lin YJ Ho CS Chiu NC Tseng HS Hsu CH Huang JK. . The reversible corpus callosum splenium lesion in a neonate with hypoglycemia and seizure. Acta Neurol Taiwan. 2015. March; 24(1): 15–8. [PubMed] [Google Scholar]
- Ünver O Kutlubay B Besci T Ekinci G Baltacıoğlu F, Türkdoğan D.. Transient splenial lesion of the corpus callosum related to migraine with aura in a pediatric patient. Acta Medica (Hradec Kralove). 2016; 59(2): 64–6. [DOI] [PubMed] [Google Scholar]
- Iturriza-Gómara M, Auchterlonie IA, Zaw W, Molyneaux P, Desselberger U, Gray J. Rotavirus gastroenteritis and central nervous system (CNS) infection: characterization of the VP7 and VP4 genes of rotavirus strains isolated from paired fecal and cerebrospinal fluid samples from a child with CNS disease. J Clin Microbiol. 2002. December; 40(12): 4797–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalilvand S, Marashi SM, Tafakhori A, Shoja Z. Extraintestinal involvement of rotavirus infection in children. Arch Iran Med. 2015. September; 18(9): 604–5. [PubMed] [Google Scholar]
- Lynch M, Lee B, Azimi P, Gentsch J, Glaser C, Gilliam S, Chang HG, Ward R, Glass RI. Rotavirus and central nervous system symptoms: cause or contaminant? Case reports and review. Clin Infect Dis. 2001. October 1; 33(7): 932–8. [DOI] [PubMed] [Google Scholar]
- Weclewicz K, Svensson L, Kristensson K. Targeting of endoplasmic reticulum-associated proteins to axons and dendrites in rotavirus-infected neurons. Brain Res Bull. 1998. July 1; 46(4): 353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weclewicz K, Svensson L, Billger M, Holmberg K, Wallin M, Kristensson K. Microtubule-associated protein 2 appears in axons of cultured dorsal root ganglia and spinal cord neurons after rotavirus infection. J Neurosci Res. 1993. October 1; 36(2): 173–82. [DOI] [PubMed] [Google Scholar]
- Gregorio L, Sutton CL, Lee DA. Central pontine myelinolysis in a previously healthy 4-year-old child with acute rotavirus gastroenteritis. Pediatrics. 1997. May; 99(5): 738–43. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Díaz J, Banasaz M, Istrate C, Buesa J, Lundgren O, Espinoza F, Sundqvist T, Rottenberg M, Svensson L. Role of nitric oxide during rotavirus infection. J Med Virol. 2006. July; 78(7): 979–85. [DOI] [PubMed] [Google Scholar]


