Abstract
Introduction
Wilms tumour (WT) is the most common childhood abdominal malignancy, with an average annual incidence of 1 in 10,000 children. The study published in 2002 reported lower survival rates of WT in Lithuania in comparison to the data of SIOP-9 study and the European Organization for Research and Treatment of Cancer (EORTC). We aimed to assess current diagnostic approach and treatment results of patients with WT treated at our institution and to compare the results with the previously published study.
Materials and methods
A retrospective single-centre study was performed. 48 patients with WT registered at the institutional data-base from 2000 to 2018 were enrolled. An estimated 5-year overall survival (OS5y) and 2-year event-free survival (EFS2y) by stage and risk groups was calculated using IBM SPSS. A comparative analysis of two time periods – 2000–2008 and 2009–2018 – was carried out.
Results
Forty-two (87.5%) patients presented with localised disease and 6 (12.5%) with primary metastatic disease. The majority of cases were of the intermediate-risk group (77%). The OS5yof all analysed children was 86.4%. The EFS2y was 88.9% in stage I, 91.7% in stage II, 83.3% in stage III, and 50% in stage IV. The EFS2y was 100% in the low-risk group, 86.5% in the intermediate-risk group, and 25% in the high-risk group. Improvement of outcomes was observed over the analysed period: OS5y changed from 81.0% in 2000–2008 to 92.6% in 2009–2018. Among 48 cases, ten patients showed recurrence: eight – early relapse and two – late relapse. Six patients died.
Conclusions
WT was diagnosed at early stages in most cases. The survival was better among the patients diagnosed in earlier stages and with favourable risk group. Better survival rates were observed in patients treated in 2009–2018 compared to the 2000–2008 period.
Keywords: Wilms tumour, children, survival, SIOP
Abstract
VAIKŲ VILMSO NAVIKAS: 18 METŲ PATIRTIS VILNIAUS UNIVERSITETO LIGONINĖS SANTAROS KLINIKOSE
Santrauka
Įvadas. Vilmso navikas (VN) yra labiausiai paplitęs vaikų piktybinis abdominalinis navikas, vidutiniškai nustatomas 1 iš 10 000 vaikų per metus. 2002 m. paskelbto tyrimo duomenimis, VN išgyvenamumo rodikliai Lietuvoje buvo mažesni, palyginti su SIOP-9 tyrimo ir EORTC duomenimis. Mūsų tyrimo tikslas buvo įvertinti šiuo metu taikomus diagnostikos metodus ir pacientų, kuriems diagnozuotas VN, išgyvenamumą ir palyginti gydymo rezultatus su ankstesnio tyrimo duomenimis.
Tikslai ir metodai. Atlikta retrospektyvinė vieno centro duomenų analizė. Analizuoti 2000–2018 m. diagnozuotų ir gydytų pacientų duomenys. IBM SPSS programa apskaičiuotas penkerių metų bendras išgyvenamumas (BI5m) ir dvejų metų išgyvenamumas be komplikacijų (IBNĮ2m), priklausomai nuo ligos stadijos ir rizikos grupės. Palyginti 2000–2008 ir 2009–2018 m. gydymo rezultatai.
Rezultatai. Keturiasdešimt dviem (87,5 %) pacientams diagnozuota lokali ligos forma, o šešiems (12,5 %) – išplitusi liga su atokiomis metastazėmis. Dauguma atvejų buvo priskirti vidutinės rizikos grupei (77 %). Visų tiriamųjų BI5m siekė 86,4 %. Pacientų, kuriems nustatyta I ligos stadija, IBNĮ2m buvo 88,9 %, II – 91,7 %, III – 83,3 %, IV – 50 %. Žemos rizikos grupėje IBNĮ2m buvo 100 %, vidutinės – 86,5 %, aukštos – 25 %. Stebėtas gydymo rezultatų pagerėjimas: BI5m padidėjo nuo 81,0 % 2000–2008 m. iki 92,6 % 2009–2018 metais. Iš 48 pacientų 10-čiai navikas recidyvavo: aštuoniems pacientams nustatytas ankstyvas recidyvas, dviems – vėlyvas recidyvas, šeši pacientai mirė.
Išvados. VN dauguma atvejų buvo nustatytas ankstyvose ligos stadijose. Geresni išgyvenamumo rodikliai buvo tų pacientų, kuriems liga diagnozuota ankstyvose stadijose ir priskirtiems žemesnės rizikos grupėms. Stebėtas gydymo rezultatų pagerėjimas: 2009–2018 m. gydytų pacientų išgyvenamumo rodikliai viršijo pacientų, gydytų 2000–2008 m., rodiklius.
Raktažodžiai: Vilmso navikas, vaikai, išgyvenamumas, SIOP
INTRODUCTION
Wilms tumour (WT) or nephroblastoma is an embryonal kidney tumour, the most common childhood renal tumour, representing around 6–7% of childhood cancer cases (1), affecting approximately one child per 10,000 worldwide (2, 3). Predominantly seen in children under five years of age, the median age at diagnosis is 2–3 years (4, 5). The most common reported symptoms are an abdominal mass or swelling in otherwise clinically healthy children (6). Clinical presentation also includes abdominal pain, fever, haematuria, nausea, loss of appetite, constipation, hypertension, or other symptoms. About 10% of WT cases have haematogenous spread, most commonly to the lungs (85%), liver (10%), and very rarely to the bones and brain (2). Like in many other childhood tumours, genetics is important in the development and outcomes of WT. Loss of heterozygosity at 16q and 1p loci is shown to be related to poorer survival (7). Furthermore, the TP53 gene has been associated with anaplastic histology (8). Reduced expression of WT1 gene is associated with stromal predominant type of WT (9). Wilms tumour associated with syndromes accounts for approximately 10% of all cases (10), including WAGR (WT, aniridia, genitourinary anomalies, and mental retardation), Denys-Drash syndrome, Beckwith-Wiedemann syndrome, asymmetric overgrowth, or a family history of WT (11). Cases associated with congenital syndromes tend to occur earlier.
Worldwide there are two different approaches to treatment. Most children in European countries, including Lithuania, are treated with pre-operative chemotherapy, according to the International Society of Paediatric Oncology Renal Tumour Study Group (SIOP-RTSG) protocols. In North America, patients are treated with upfront nephrectomy prior to administration of chemotherapy, according to the National Wilms’ Tumour Study/Children’s Oncology Group (COG) protocols. Despite different initial treatment strategies, both cooperative groups have reached overall survival of nearly 90% (12, 13). According to the SIOP treatment approach, histopathological features of the tumour stratify patients into three prognostic groups: low risk (completely necrotic, cystic partially differentiated), intermediate risk (regressive, stromal, epithelial, mixed type and focal anaplasia) and high risk (diffuse anaplasia, blastemal type). Although in developed countries, approximately 90% of patients with WT are cured, 5-year overall survival (OS5y) varies between European countries (3). In addition, approximately 10% of patients in the intermediate risk group and up to 25% of patients with high risk tumours experience a relapse (12, 14).
The previous study that analysed epidemiology, diagnostic approach and treatment results of WT in Lithuania was published in 2002. It revealed that in most cases WT was diagnosed at advanced stages (postsurgical stages were distributed as follows: stage I was observed in 28%, stage II in 30%, stage III in 27%, and stage IV in 15% of patients) (15) and OS5y of children treated for WT at our institution from 1991 to 2000 was significantly lower in comparison with data of Northern European countries (4) (69% and 91%, respectively).
In the present study, we report on the institutional data and outcomes of the children with WT treated at the Centre for Paediatric Oncology and Haematology (CPOH) of the Children’s Hospital, Affiliate of Vilnius University Hospital Santaros Klinikos, from 2000 to 2018. Survival rates were compared between subgroups of patients treated from 2000 to 2008 according to the International Society of Paediatric Oncology (SIOP) 93–01 protocol and patients treated from 2009 to 2018 according to the SIOP 2001 protocol. The results were compared with the outcomes observed during the 1991–2000 period in Lithuania.
MATERIALS AND METHODS
A retrospective single-centre medical record review of patients diagnosed with WT at the CPOH was performed. The data were retrieved from patients’ records (when available) and reviewed in April 2019. Totally we identified 57 children diagnosed and treated for WT from 2000 to 2018 at our institution. Nine patients were excluded due to the lack of information needed for the study. The study was approved by the institutional ethics board.
All the patients underwent multimodal treatment according to SIOP protocols: SIOP 93-01 protocol in 2000–2008, and SIOP 2001 protocol in 2009–2018, which investigated the safety of omitting doxorubicin in treating stage II–III intermediate-risk Wilms tumours, thereby avoiding acute and long-term toxicities (16). Treatment included neoadjuvant chemotherapy (a combination of dactinomycin (ACTD) and vincristine (VCR) versus ACTD, VCR and doxorubicin (DOX)), surgical removal of the tumour with involved kidney, restaging and adjuvant chemotherapy after histological diagnosis of the tumour was established (ACTD, VCR, ifosfamide, etoposide (VP-16), carboplatin and (DOX) in patients with metastatic disease). Preoperative fine-needle biopsy was performed in nine patients; otherwise, histological diagnosis was confirmed after surgery.
We analysed the prevalence of patients by age, sex, and clinical presentation at the diagnosis. At the time of diagnosis, the patients were stratified into the low, intermediate or high risk groups based on stage and histological type. The revised SIOP working classification of renal tumours of childhood (2001) was used for stratification. To assess the hypothesized improvement of treatment, the entire cohort was split into two subgroups: patients treated in 2000–2008 according to International Society of Paediatric Oncology (SIOP) 93–01 protocol and patients treated in 2009–2018 according to SIOP 2001 protocol.
An estimated OS5y and 2-year event-free survival (EFS2y) by stage and risk groups were calculated with IBM SPSS Statistics 25.0 work package using the Kaplan-Meier method. An event was defined as relapse, disease progression or toxicity-related death. There was no case of secondary malignancy. The significance of differences in the distribution of survival data was assessed using a log-rank test. The selected level of statistical significance was 0.05.
RESULTS
From 2000 to 2018, 57 patients with the diagnosis of WT were identified in the institutional database. Nine patients (15%) were excluded due incomplete data. Twenty-one out of 48 (43.7%) children were treated during the first study period (2000–2008) and 27 (56.3%) during the second period (2009–2018).
The analysed characteristics of patients are presented in Table 1. Among the 48 cases, girls were more frequently affected than boys: 29 (60.4%) and 19 (39.6%), respectively. Most patients at the time of diagnosis were 2–4 years (52%), median age was 36 months (ranging from minimum of 14 days to maximum of 15 years, interquartile range (IQR) from 19.5 months to 57 months). The median follow-up from diagnosis was 104 months (ranging from 10 months to 218 months, IQR: 69.25–173.75 months).
Table 1.
Characteristics of the patients included in the study
| Characteristics of patients | N (%) | |
|---|---|---|
| Sex | Girls | 29 (60.4) |
| Boys | 19 (39.6) | |
| Stage at the time of diagnosis | I | 18 (37.5) |
| II | 12 (25) | |
| III | 12 (25) | |
| IV | 6 (12.5) | |
| Risk group | Low risk | 7 (15) |
| Intermediate risk | 37 (77) | |
| High risk | 4 (8) | |
The stage distribution was as follows: stage I in 18 (37.5%), stage II in 12 (25%), stage III in 12 (25%) and stage IV in 6 (12.5%) patients.
The risk group distribution according to the histological type revealed the following pattern: low risk group comprised 7 cases (15%), intermediate risk group – 37 (77%) and high risk group – 4 (8%).
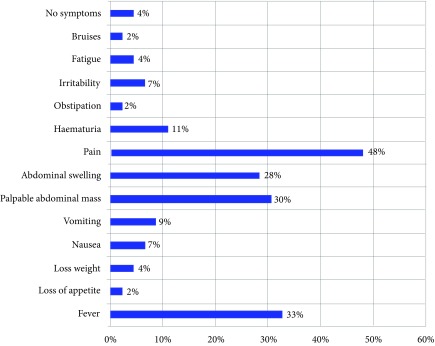
The most frequent tumour manifestation symptoms were pain (n = 23, 47.9%), fever (n = 16, 33%), palpable abdominal mass (n = 14, 30%), abdominal swelling (n = 13, 28%). The distribution of symptoms is shown in Fig. 1.
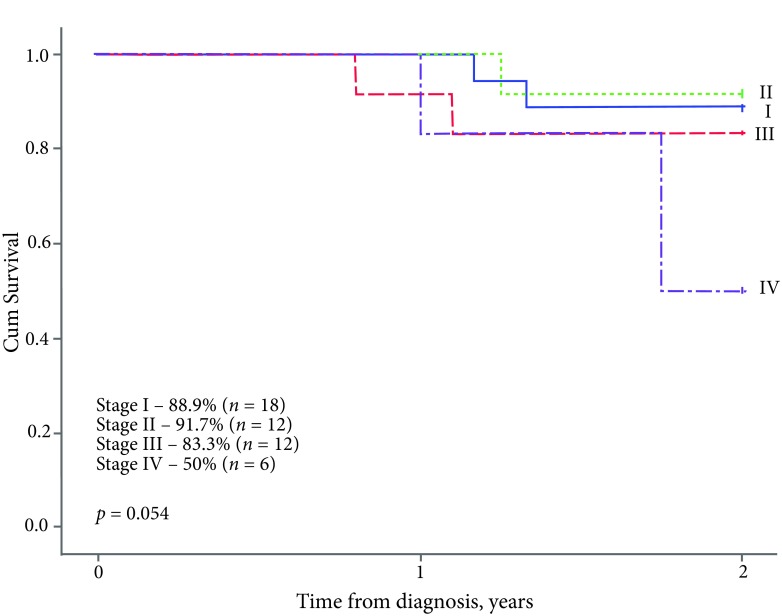
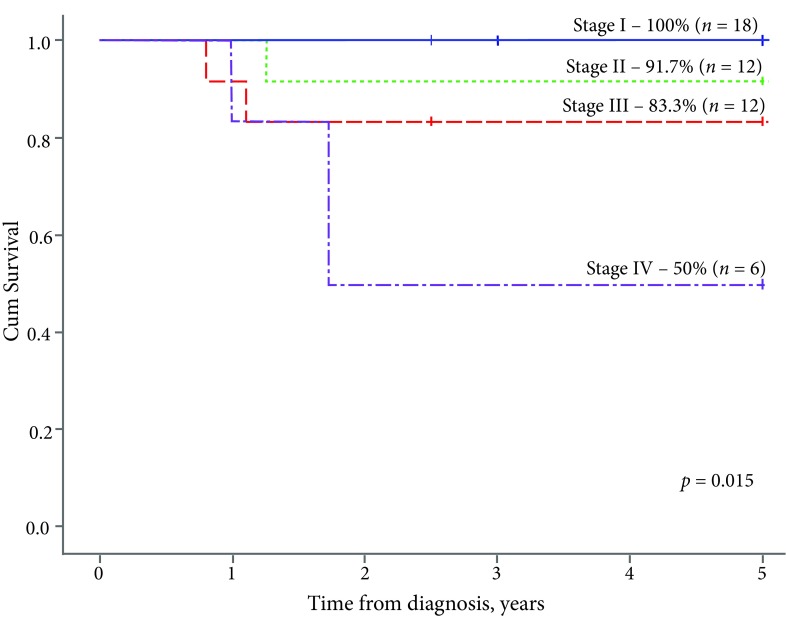
The EFS2y of the entire group was 83.3%, the OS5y – 86.4%. In terms of the disease stage, the EFS2y was significantly lower in patients with metastatic disease (stage IV – 50%) compared to the localised form of WT: stage I – 88.9%, stage II – 91.7%, stage III – 83.3% (p = 0.054, Fig. 2). All the patients in stage I survived: the OS5y rate was 100% in stage I, 91.7% in stage II, 83.3% in stage III, and 50% in stage IV (p = 0.015, Fig. 3).
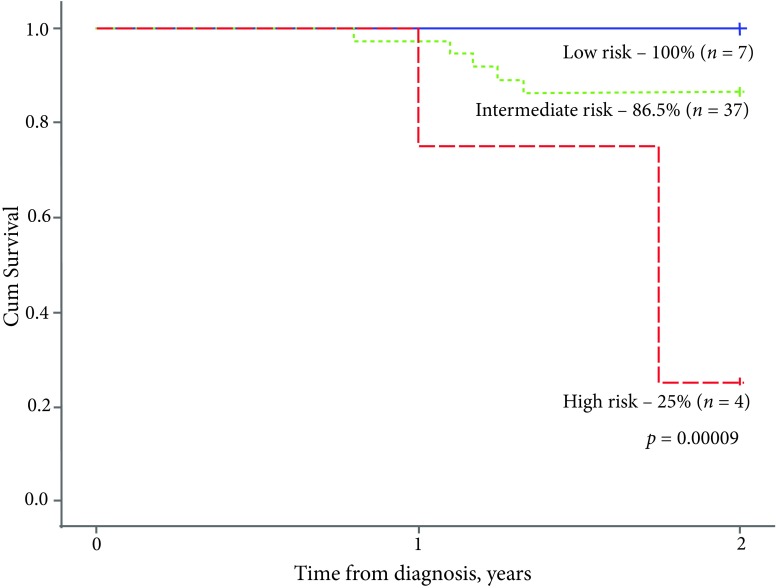
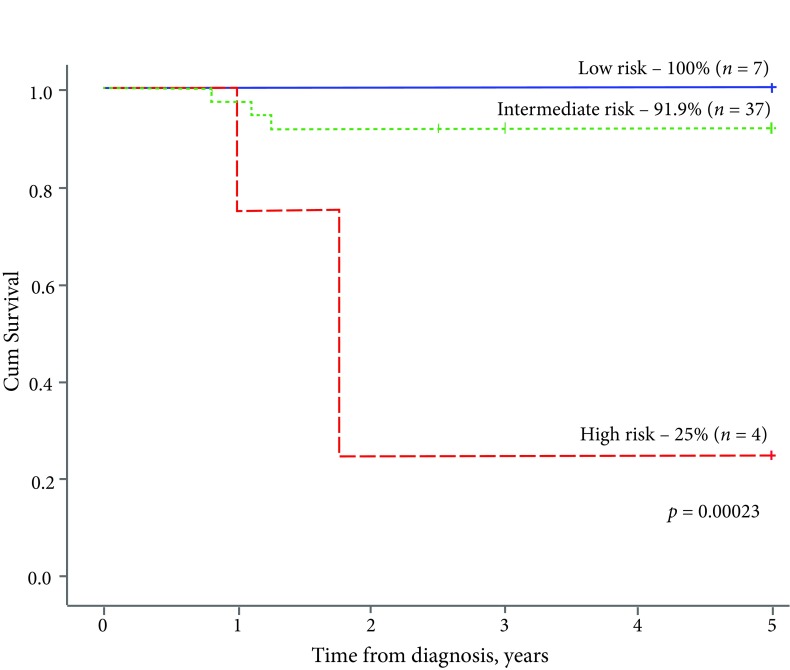
A significant difference in survival rates became evident when analysing the outcome of treatment across different risk groups: both EFS2y and OS5y were inferior for the high risk group as compared to intermediate and low-risk groups (25% vs 86.5% vs 100% (p = 0.00009, Fig. 4) and 25% vs 91.9% vs 100% (p = 0.00023, Fig. 5), respectively).
Fig. 1.
Clinical symptoms at the time of diagnosis
Fig. 2.
The 2-year event-free survival, by stage
During the first period of our study (2000–2008), 21 patients with WT were treated at our institution. The majority of the patients were diagnosed with stage II (n = 7, 33.3%) and stage III (n = 9, 42.9%) at the time of diagnosis. An estimated OS5y was 100% in stage I (n = 2), 85.7% in stage II (n = 7), 77.8% in stage III (n = 9), and 66.7% in stage IV (n = 3). Within the low risk group (n = 1, 4.8%) 5-year overall survival was 100%, in the intermediate risk group (n = 18, 85.7%) OS5y was 83.3%, and in the high risk group (n = 2, 9.5%) OS5y was 50%.
Fig. 3.
The 5-year overall survival, to stage
During the second period of the study (2009–2018), 27 children were identified. Most of the patients were diagnosed with stage I disease (n = 16, 59.3%) at the time of diagnosis. Survival rates were markedly superior in local disease: OS5y in stage I (n = 16), stage II (n = 5), and stage III (n = 3) was 100%, while only 33.3% of children diagnosed with stage IV (n = 3) survived. All the patients in low (n = 6, 22.2%) and intermediate (n = 19, 70.4%) risk groups survived, while lethal outcome occurred among the patients in the high risk group (n = 2, 7.4%).
Fig. 4.
The 2-year event-free survival, by risk group
Fig. 5.
The 5-year overall survival, by risk group
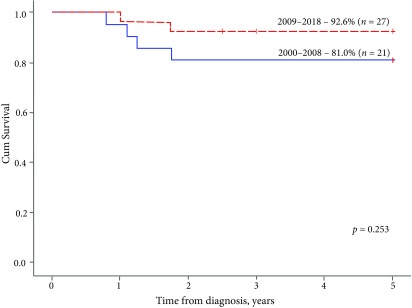
The analysis of survival rates based on treatment periods (2000–2008 and 2009–2018, treated according to SIOP 2001 and SIOP 93–01 protocols, respectively) showed a trend for improvement in terms of event-free and overall survival: EFS2y increased from 76.2% to 88.9% (p = 0.231, data not shown), OS5y – from 81.0% to 92.6% (p = 0.253, Fig. 6). The OS5y of the entire analysed cohort (n = 48) treated from 2000 to 2018 was 87.5%.
Fig. 6.
The 5-year overall survival, by period of time
The main reason of treatment failure was resistant malignancy. Among 48 cases, ten (20.8%) patients experienced disease recurrence: eight early relapses developed within 15 months after nephrectomy and two patients had late relapses at 54 and 96 months after surgery. A median time to the diagnosis of a relapse was 12.5 months (interquartile range – 7.8–25.5 months, range from minimum to maximum – 6–96 months). Two cases experienced a local recurrence; eight children developed a metastatic relapse (three patients in the lungs only, two patients in the liver only, one patient in the lungs, mediastinal lymph nodes, and abdominal lymph nodes, one patient in the retroperitoneal space, and one patient in both the liver and the retroperitoneal space). Two patients had a second relapse: one case in the abdominal wall, peritoneum, and omentum, and one case in the central nervous system. Six (12.5%) patients with systemic relapse died: five of them due to resistant malignancy, and one child was lost because of treatment-related toxicity.
DISCUSSION
A retrospective analysis was performed with the aim to assess survival of WT in one of the two paediatric oncology centres in Lithuania. The purpose of the study was to evaluate the treatment outcome in patients treated from 2000 to 2018 and to compare survival rates between two periods, 2000–2008 and 2009–2018. Our 18-year single centre study demonstrated an improved OS5y from 81.0% in the 2000–2008 period to 92.6% in the 2009–2018 period; also, EFS2y from 76.2% to 88.9%. Furthermore, WT was diagnosed in earlier stages in 2009 to 2018 as compared to the patients treated in 2000 to 2008.
One important issue that could have had a beneficial impact on the treatment outcomes was early detection of tumour. In contrast to the previous study of WT in Lithuania carried out from 1991 to 2000 (15), in 2000–2018 the disease was diagnosed at the earlier stages: stage I was found in majority of patients (37.5%), while in the previous research there were no patients with stage I. There has been a significant improvement in survival rates: the OS5y increased from 69% as previously reported (15) to 87.5% in the current study. Moreover, the OS5y of the children treated in the 2009–2018 period reached 92.6% that was comparable to the currently reported survival rates (18). Notwithstanding, from 2000 to 2018, the recurrence of the disease was detected earlier: the median time to the diagnosis of relapse was 12 months compared to 3.5 years in 1991 to 1997 (15). Several important issues contributed to the positive trend. A cornerstone change in care of solid paediatric tumours in Lithuania was the transition from adult services (where children with WT were treated up to 1998) to specialized paediatric oncology centres. Centralisation of patient care and accumulation of specific knowledge and expertise was crucial for the improvement of cure rates as demonstrated in our previous study in which we analysed the outcome of treatment of paediatric acute myeloid leukaemia (19).
Despite remarkable achievements, prognosis for patients diagnosed with stage IV remained dismal: EFS2y and OS5y did not exceed 50%. In contrast, in the 25-year single-centre UK experience, Fawkner-Corbett et al. reported 76% survival of children presented with metastatic disease at the time of diagnosis (5). Indeed, survival of children with distant metastases at presentation is markedly affected by tumour histology. According to a study of the National Cancer Institute, stage IV tumours with a favourable histology have 4-year overall survival rate that ranges from 86% to 96%, while tumours with diffuse anaplasia confer poor prognosis and 4-year OS of 33–44% (20). A small number of patients with initially advanced disease (only six children were enrolled to the study) did not allow us to make an analysis of histologic subgroups. The limitation of this study, however, was partially reflected in treatment outcome by risk groups – the high risk group had a significantly poorer prognosis with both EFS2y and OS5y of 25%.
According to EUROCARE-5 study, OS5y of children affected by WT in all European countries from 2000 to 2007 was 89.4% (17). EUROCARE-5 study demonstrated considerable difference in childhood cancer survival across European countries. Doganis et al. confirmed specific differences in WT cure rates between Eastern European (OS5y – 84% in the 2000–2007 period) and Central European countries (OS5y – 94% in the 2000–2007 period) (21). Although the survival indicators of Lithuania during the same time period were lower with OS5y only 81%, it has markedly improved in the 2009–2018 period, when OS5y reached 92.6%. The same increasing cure rate in Lithuanian children treated at our institution was demonstrated in other solid tumours such as neuroblastoma (22) and Ewing sarcoma (23). They reflect positive changes in childhood cancer care in the country, gain of knowledge and expertise, and importance of international collaboration.
In summary, our study demonstrated that survival of patients with WT treated at our institution has been improved and comparable to the currently reported survival rates in other developed European countries.
CONCLUSIONS
According to the data retrieved from the CPOH database, from 2000 to 2018 patients were diagnosed with WT at early stages in most cases, with less aggressive histological types of WT prevailing as compared to the previous study that involved patients treated from 1991 to 2000. Survival depended on the stage at the time of diagnosis and on the risk group: OS5y and EFS2y were better among the patients diagnosed in earlier stages and with a favourable risk group. Better survival rates were observed in patients treated in the 2009-2018 period than in the 2000–2008 period. Compared to the previous retrospective study of WT conducted in Lithuania, in the period of 2000–2018 WT was diagnosed at earlier stages and better treatment results were observed, with a significant increase in overall survival. The first two years after diagnosis require thorough follow upsince a relapse was more likely to occur within the first two years after diagnosis. Children under 4 years complaining for abdominal pain or swelling, a palpable mass or unexplained fever should be checked for WT.
Milda Rančelytė, Rolanda Nemanienė, Lina Ragelienė, Jelena Rascon
References
- Erginel B. Wilms tumor and its management in a surgical aspect. In: van den Heuvel-Eibrink MM. editor. Wilms Tumor. Brisbane (AU): Codon Publications; 2016. March Chapter 4. [PubMed] [Google Scholar]
- Breslow N, Olshan A, Beckwith JB, Green DM. Epidemiology of Wilms tumor. Med Pediatr Oncol. 1993; 21(3): 172–81. [DOI] [PubMed] [Google Scholar]
- Brok J, Treger TD, Gooskens SL, van den Heuvel-Eibrink MM, Pritchard-Jones K. Biology and treatment of renal tumours in childhood. European Journal of Cancer. 2016. November 1; 68: 179–95. [DOI] [PubMed] [Google Scholar]
- Plesko I, Kramárová E, Stiller CA, Coebergh JW, Santaquilani M. EUROCARE Working Group. Survival of children with Wilms’ tumour in Europe. Eur J Cancer. 2001. April; 37(6): 736–43. [DOI] [PubMed] [Google Scholar]
- Fawkner-Corbett DW, Howell L, Pizer BL, Dominici C, McDowell HP, Losty PD. Wilms’ tumor – lessons and outcomes – a 25-year single center UK experience. Pediatr Hematol Oncol. 2014. August; 31(5): 400–8. [DOI] [PubMed] [Google Scholar]
- Mullen E, Graf N. Clinical Presentation In: Pritchard-Jones K, Dome J (Hrsg) Renal tumors of childhood: biology and therapy. Pediatric Oncology. Berlin: Springer, 2015, p. 40. [Google Scholar]
- Ahmed HU Arya M Tsiouris A Sellaturay SV Shergill IS Duffy PG et al. . An update on the management of Wilms’ tumour. Eur J Surg Oncol. 2007. September; 33(7): 824–31. [DOI] [PubMed] [Google Scholar]
- Varan A. Wilms’ tumor in children: an overview. NEC. 2008; 108(2): c83–90. [DOI] [PubMed] [Google Scholar]
- Lee SB, Harber DA. Wilms’ tumour and the WT1 gene. Exp Cell Res 2001; 264(1): 74–99. [DOI] [PubMed] [Google Scholar]
- Kumar V, Abbas AK, Aster JC. Robbins and Cotran pathologic basis of disease. Philadelphia, PA: Elsevier-Saunders; 2015. [Google Scholar]
- Dumoucel S Gauthier-Villars M Stoppa-Lyonnet D Parisot P Brisse H Philippe-Chomette P et al. . Malformations, genetic abnormalities, and Wilms tumor. Pediatr Blood Cancer. 2014. January; 61(1): 140–4. [DOI] [PubMed] [Google Scholar]
- Pritchard-Jones K Bergeron C de Camargo B van den Heuvel-Eibrink MM Acha T Godzinski J et al. . Omission of doxorubicin from the treatment of stage II–III, intermediate-risk Wilms’ tumour (SIOP WT 2001): an open-label, non-inferiority, randomised controlled trial. Lancet. 2015. September 19; 386(9999): 1156–64. [DOI] [PubMed] [Google Scholar]
- Grundy PE Breslow NE Li S Perlman E Beckwith JB Ritchey ML et al. . Loss of heterozygosity for chromosomes 1p and 16q is an adverse prognostic factor in favorable-histology Wilms tumor: a report from the National Wilms Tumor Study Group. J Clin Oncol. 2005; 23: 7312–21. [DOI] [PubMed] [Google Scholar]
- Van Tinteren H. Statistical report. RTSG–SIOP annual meeting; Stockholm; June 24–26, 2015. [Google Scholar]
- Pečiulytė V., Ragelienė L., Gricius K. Clinical features and survival of children with Wilms’ tumor in Lithuania. Acta Med Litu. 2002; 9(3): 166–9. [Google Scholar]
- Van den Heuvel-Eibrink MM Hol JA Pritchard-Jones K van Tinteren H Furtwängler R Verschuur AC et al. . Rationale for the treatment of Wilms tumour in the UMBRELLA SIOP–RTSG 2016 protocol. Nature Reviews Urology. 2017. October 31; 14: 743. [DOI] [PubMed] [Google Scholar]
- Gatta G Botta L Rossi S Aareleid T Bielska-Lasota M Clavel J et al. . Childhood cancer survival in Europe 1999–2007: results of EUROCARE-5 – a population-based study. Lancet Oncol. 2014. January; 15(1): 35–47. [DOI] [PubMed] [Google Scholar]
- Murali Chintagumpala, MD, Jodi A Muscal., MD Treatment and prognosis of Wilms tumor. Post TW, ed. UpToDate. Waltham, MA: UpToDate Inc; https://www.uptodate.com [cited 2019. May 4]. Available from: https://www.uptodate.com/contents/treatment-and-prognosis-of-wilms-tumor-#H15 [Google Scholar]
- Kairiene I, Pasauliene R, Lipunova N, Vaitkeviciene G, Rageliene L, Rascon J. Improved outcome of childhood acute myeloid leukemia in an Eastern European country: Lithuanian experience. Eur J Ped. 2017. October; 176(10): 1329–37. [DOI] [PubMed] [Google Scholar]
- Bethesda MD. National Cancer Institute. PDQ® Pediatric Treatment Editorial Board. PDQ Wilms tumor and other childhood kidney tumors treatment [cited 2019. May 4]. Available from: https://www.cancer.gov/types/kidney/hp/wilms-treatment-pdq [Google Scholar]
- Doganis D Panagopoulou P Tragiannidis A Vichos T Moschovi M Polychronopoulou S et al. . Survival and mortality rates of Wilms tumour in Southern and Eastern European countries: Socioeconomic differentials compared with the United States of America. Eur J Cancer. 2018; 101: 38–46. [DOI] [PubMed] [Google Scholar]
- Juškaitė A, Tamulienė I, Rascon J. Results of neuroblastoma treatment in Lithuania: a single centre experience. Acta Med Litu. 2017; 24(2): 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakutis G, Ragelienė L, Rascon J. Survival of children treated for Ewing sarcoma in Lithuania: a single centre experience. Acta Med Litu. 2017; 24(4): 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]