Abstract
Alcohol use disorder (AUD) is a chronic relapsing condition that represents a significant public health concern. Pharmacological treatment development for AUD is a top research priority and many studies are being conducted to evaluate potential AUD treatments. Understanding the brain circuitry impacted by addiction is crucial for the development of efficacious pharmacological interventions. These neuroadaptations can be probed non-invasively using functional magnetic resonance neuroimaging (fMRI). fMRI may be an effective tool to identify biomarkers for AUD pharmacotherapies, evaluating changes associated with pharmacological treatment. Thus, the present qualitative review of the literature focuses on the role of fMRI as a tool for medications development for AUD. The aim of this review was to assemble research across a range of fMRI paradigms to study the effectiveness of pharmacological treatments of adult AUD. First, we present a qualitative review of fMRI AUD pharmacotherapy studies, differentiating studies based on their dosing regimen. Second, we provide recommendations for the field to improve the use of fMRI as a biomarker for AUD pharmacotherapy.
Introduction
Alcohol use disorder (AUD) is a chronic relapsing condition, with consumption continuing despite significant health, social, physical, economic, and legal consequences. AUD represents a significant public health concern; worldwide over 3 million deaths are attributable to alcohol, and the harmful use of alcohol is associated with over 5% of the global disease burden (Organization, 2018). AUD is highly prevalent in the US, with 13.9% of the general population of adults meeting criteria for a current (12-month) AUD and 29.1% of the general population of adults meeting criteria for lifetime AUD (Grant et al., 2015). Despite the prevalence of AUD and the significant public health concerns associated with high-risk drinking, treatment rates for AUD are low. Among those diagnosed with current AUD, less than 10% sought treatment (Grant et al., 2015). Furthermore, pharmacological treatments for AUD are used less often than psychosocial interventions (Ray et al., 2018). The limited use of pharmacotherapies as AUD treatments is due, in part, to the relative lack of available pharmacological treatments for AUD. Currently, there are only four Food and Drug Administration (FDA) approved medications for the treatment of AUD: disulfiram, acamprosate, oral naltrexone, and extended-release injectable naltrexone, and these medications are only modestly effective at treating AUD (Ray et al., 2018). Therefore, treatment development for AUD is a top research priority and many studies are being conducted to evaluate potential AUD pharmacotherapies.
Chronic exposure to alcohol results in pathological neuroadaptations to brain systems involved in reward and motivation, as well as neural circuits involved in executive function and inhibitory control (Koob and Volkow, 2016). Traditionally, these maladaptive changes have been studied using animal models, which provide experimental control that is difficult to attain in studies with human participants. Translational studies investigating AUD-associated neuroadaptations are crucial to provide a full understanding of the pathology of the disorder. Functional magnetic resonance imaging (fMRI) represents an important, non-invasive tool to conduct these translational studies, where preclinical findings can be corroborated in individuals with AUD. Understanding the brain circuitry impacted by addiction is crucial for the development of efficacious pharmacological interventions. Further, fMRI may be an effective tool to identify biomarkers for AUD pharmacotherapies, evaluating changes associated with pharmacological treatment beyond what can be obtained from self-report or clinical outcome measures. To that end, the present qualitative review of the literature focuses on the role of fMRI as a tool for medications development for AUD.
The most common image contrast used in fMRI is the blood-oxygen-level-dependent (BOLD) contrast. fMRI exploits coupling in the brain between neuronal activity and hemodynamics to non-invasively localize and measure brain activity (Heeger and Ress, 2002). Specifically, increases in neuronal activity are associated with increases in regional cerebral blood flow, which are accompanied by small changes in oxygen consumption (Fox and Raichle, 1986, Hoge et al., 1999). Alterations in vascular occupancy, oxygen supply, and oxygen consumption result in an increased concentration of diamagnetic oxyhemoglobin and a decreased quantity of paramagnetic deoxyhemoglobin in the blood (Buxton et al., 2004, Iannetti and Wise, 2007). This produces a net decrease in the magnetic field around the blood vessels which can be detected through the BOLD contrast. While BOLD signal highly correlated with neural activity (Logothetis, 2003, Mukamel et al., 2005), changes in BOLD signal may be influenced by the pharmacological treatment which is being studied (Iannetti and Wise, 2007). For example, a pharmacotherapy may alter the efficiency of the signaling between neurons and blood vessels, which would reduce the BOLD signal response to a stimulus. If the pharmacotherapy’s effect on the hemodynamic response is not taken into account, the results of the study may be incorrectly interrupted as an effect of the medication on neural activity (Iannetti and Wise, 2007). This confound can be evaluated and corrected through the collection of an arterial spin labeling (ASL) scan to evaluate the overall effect of a medication on cerebral blood flow. However, the majority of pharmacotherapy neuroimaging studies do not currently employee this correction method.
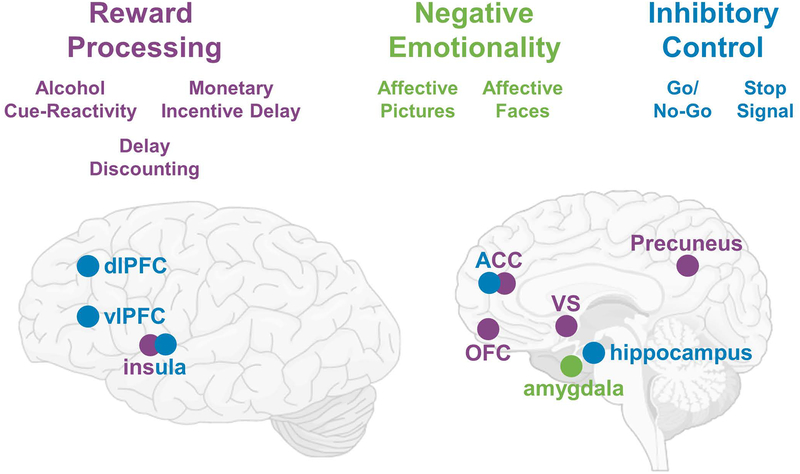
Several fMRI paradigms have been developed to investigate brain circuits putatively involved in AUD. The most commonly used paradigm is alcohol cue-reactivity, where alcohol associated stimuli are presented to induce an alcohol craving response (Monti et al., 1987). Cue-reactivity paradigms have been widely adapted for neuroimaging protocols and studies suggest that cue-reactivity engages learning and memory circuits as well as reward circuitry, and fMRI studies have reliably shown activation in regions including the ventral striatum (VS), prefrontal cortex (PFC), cingulate, insula, and precuneus (Courtney et al., 2016, Schacht et al., 2013a). The neural processing of non-drug reward, which is thought to be maladaptively decreased in individuals with AUD, has been commonly measured using the monetary incentive delay (MID) task (Knutson et al., 2001). In the MID task, participants are presented with abstract stimuli which indicate the reward trial type; participants then view a target, which is presented for a short period of time. During the presentation of the target they must press a button in order to receive the reward. Finally, they view feedback on the success of the trial (Knutson et al., 2001). There have been divergent findings using the MID task in AUD populations; however, several studies have reported a decrease in VS activation in response to monetary reward anticipation in individuals with AUD compared to controls (Balodis and Potenza, 2015). Inhibitory control, which has also been suggested to be pathologically low in individuals with AUD, has been commonly evaluated with the Go/No-Go (GNG) and stop signal tasks (SST). Both the GNG and SS tasks create conflict conditions in which an individual must inhibit a prepotent motor response. Task difficulty is modulated such that withholding the motor response is challenging and thus requires inhibitory control processing. Individuals with addictive disorders consistently show hypoactivation of brain circuits involved in executive control and memory, as well as decreased recruitment of the salience network during conflict processing, when inhibitory control is required (Zilverstand et al., 2018). Yet another dimension that has been implicated in AUD is negative emotionality, where negative affective states such as dysphoria and anhedonia are commonly reported, particularly during alcohol withdrawal and withdrawal-induced alcohol craving. Emotional processing has been evaluated using two fMRI visual stimulus sets: one using positive, negative and neutral stimuli from the International Affective Picture System (IAPS), and the other presenting emotional face stimuli. Typically, participants are asked to passively view images from each group of stimuli; in some studies, participants are asked to press a button during specific times during the scan to ensure attention. Finally, delay discounting tasks measure intertemporal choice behavior, where individuals must choose between smaller immediate rewards and larger delayed rewards, and are considered a behavioral index of impulsivity (MacKillop, 2016). Together, this array of neuroimaging tasks seeks to capture AUD-relevant pathophysiology and associated neural activation, in turn providing an opportunity to test the effects of AUD pharmacotherapies on these processes (see Figure 1).
Figure 1: Brain Circuits Implicated in AUD.
Three domains implicated in AUD are displayed (purple = reward processing; green = negative emotionality; blue = inhibitory control). fMRI tasks used to probe these domains are listed below the domain and major brain regions targeted within these tasks are highlighted. Brain regions that are involved in multiple domains are listed in two-colors.
Abbreviations: dlPFC = dorsolateral prefrontal cortex; vlPFC = ventrolateral prefrontal cortex; ACC = anterior cingulate cortex; OFC = orbitofrontal cortex; VS = ventral striatum
While several reviews and meta-analyses have examined alcohol cue-elicited neural processes (Schacht et al., 2013a, Buhler and Mann, 2011, Yalachkov et al., 2012, Courtney et al., 2016), only two reviews have examined the use of neuroimaging methods to evaluate substance use disorder treatments (Courtney et al., 2016, Cabrera et al., 2016). Both previous reviews of substance disorder treatments focused broadly on all substances of abuse and are not specific to AUD. Further, Courtney and colleagues only included studies which employed a cue-reactivity paradigm, which limited their study selection (Courtney et al., 2016). To our knowledge, there have been no reviews that focus solely on the role of fMRI as a tool to investigate pharmacotherapies in AUD. Therefore, the aim of this review was to assemble research across a range of fMRI paradigms to study the effectiveness of pharmacological treatments of adult AUD. The goal of this review is two-fold. First, we present a qualitative review of fMRI AUD pharmacotherapy studies, describing the main findings from studies that administered pharmacotherapies chronically, i.e. for 6 or more days prior to the fMRI scan, and studies that administered pharmacotherapies in a single dose prior to the fMRI scan. Second, we provide recommendations for the field to improve the use of fMRI as a biomarker for AUD pharmacotherapy.
Methods
Literature Search and Selection
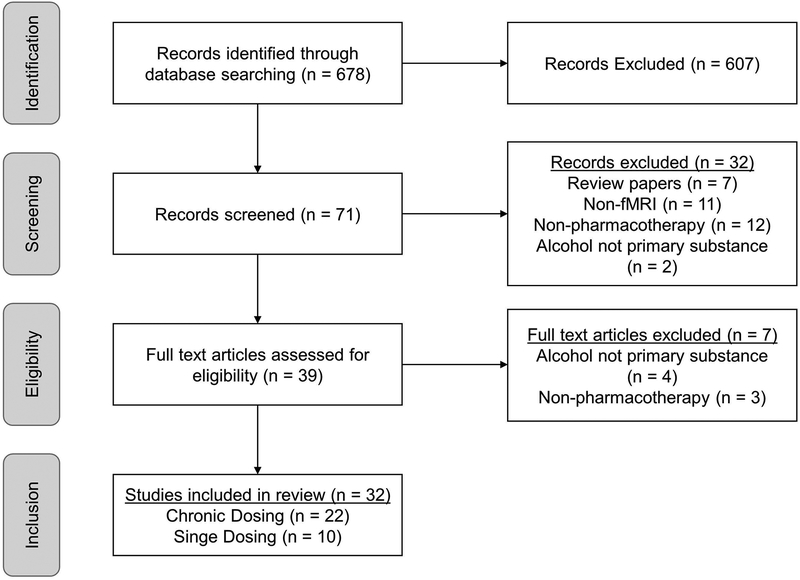
Published papers were identified using the PubMed database (https://www.ncbi.nlm.nih.gov/pubmed). The keywords used in the search were: ‘fMRI’, ‘functional magnetic resonance imaging’ and ‘alcohol’. The reference sections of identified papers were also consulted to identify additional relevant papers. Studies were included in this review if they were original investigations of individuals with AUD, treatment seeking or non-treatment-seeking, or heavy drinkers, with or without an AUD, published in the English language, which used fMRI, and included a pharmacological treatment for AUD. Studies which were excluded from this review include: review papers, studies in languages other than English, studies using animals, studies using other imaging modalities (structural MRI, positron emission tomography, magnetic resonance spectroscopy), studies that did not use an fMRI task (e.g. resting state fMRI), studies that examined the effect of alcohol infusion on brain function without the inclusion of a pharmacological treatment, studies of AUD pharmacotherapies in light drinkers or non-drinkers, and studies that examined psychosocial treatments without the combination of a pharmacotherapy. This search yielded 32 total studies, with 22 studies examining the effect of chronic dosing of AUD pharmacotherapies, with studies evaluating the effect of naltrexone (n=6), varenicline (n=3), baclofen (n=4), aripiprazole (n=3), CRF1 receptor antagonists (n=2), NK1 antagonists (n=2), acamprosate (n=1), and an NMDA agonist (n=1) on neural activation. Ten studies examined the effect of a single dose of AUD pharmacotherapies, with studies evaluating the effect of naltrexone (n=4), nalmefene (n=1), dopamine antagonists (n=2), modafinil (n=2), and oxytocin (n=1) on brain activation (see Figure 2 for PRISMA diagram).
Figure 2: PRISMA Flow Diagram.
The Pubmed search identified 678 studies. Of these studies, 71 were initially screening, and 39 articles were assessed for eligibility. Thirty-two studies combining fMRI and AUD pharmacotherapy were included in this review, which were then further divided based on their pharmacological dosing regimen into 22 studies which employed a chronic dosing approach and 10 studies which employed an acute, single dosing approach
Results
In reviewing the literature, it became clear that from a pharmacological view point, studies differed markedly with regard to the medication dosing regimen prior to the fMRI assessment. Therefore, in this review we differentiate between studies in which pharmacotherapies were administered chronically (defined as medication administration for 6 or more days prior to the fMRI scan), and studies that administered pharmacotherapies in a single dose prior to the fMRI scan. On average, participants in the chronic dosing AUD pharmacotherapy studies were 36.50 ± 8.11 years old. Five studies enrolled non-treatment-seeking individuals with alcohol dependence (AD) or AUD, two studies enrolled non-treatment seeking heavy drinkers, and the remaining fifteen studies enrolled treatment-seeking individuals with AD or AUD. Of note, given that the nomenclature for alcohol use disorder has shifted from DSM-IV-TR to DSM-5, this review uses the diagnostic nomenclature provided by the primary source article. Participants were on active study medication for 16.23± 7.30 days prior to the fMRI scan (range = 6–42 days).
Regarding single dosing studies, on average, participants were 41.59 ± 8.97 years old. The majority of studies (8) enrolled abstinent individuals with AD or AUD, while one study involved alcohol infusion and enrolled non-treatment-seeking individuals with AD and one study enrolled non-treatment seeking heavy drinkers. Participants were administered the study drug 2.08 ± 0.74 hours prior to the MRI scan (range = 45 minutes-4 hours).
Studies of AUD Pharmacotherapies Administered Chronically
We begin with a review of the studies in which pharmacotherapies were administered chronically. In doing so, it is important to recognize that the definition of chronic administration is arbitrary in that from a pharmacological view point, a 6-day administration is fairly short. However, the assumption underlying these studies is that a steady-state on the given medication has been reached, allowing for meaningful testing of neuroimaging parameters. Furthermore, different medications have different titration (i.e., dosing up) protocols which in turn would impact the feasibility of single-dose testing and by definition, would require a lengthier dose escalation procedure. All of the chronic dosing studies employed a between-subjects approach, where participants were randomized to receive the active medication or a matched placebo control. With those considerations in mind, the following is a summary the key findings by each medication administered chronically, across a range of fMRI tasks (see Table 1)
Table 1.
AUD Pharmacotherapy studies with Chronic Dosing
| First author, year | Medication, dose, duration | fMRI task | Active (Drug) N | Control (Placebo) N | Population Type | Scan Timing | Analysis Approach | Results | Clinical Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Naltrexone (NTX) | |||||||||
| Myrick et al., 2008 | 50mg NTX × 7 days 0.5mg OND × 7 days 50mg NTX + 0.5mg OND × 7 days |
Visual Alcohol Cues | 23 23 20 |
24 | NTS AD | Post | Whole Brain and ROI |
|
NS (craving) NS (craving) NTX+OND reduced in-scanner craving ratings. |
| Schacht et al., 2013b | 50mg NTX × 6 days | Visual Alcohol Cues | 35 | 39 | NTS AD | Post | ROI |
|
NP |
| Lukas et al., 2013 | 380mg extended-release NTX (single dose delivered 14 days prior to scanning) | Visual and Olfactory Alcohol Cues | 15 | 13 | TS Detoxed AD | Pre, Post | Whole Brain |
|
XR-NTX reduced in-scanner craving ratings. |
| Schacht et al., 2017 | 50mg NTX × 14 days | Visual Alcohol Cues | 59 | 57 | TS AD | Pre, Post | ROI |
|
NTX treated individuals with large reductions in pre-post VS activation had less heavy drinking compared to placebo. |
| Bach et al., 2019 | Open-label NTX × 14 days | Visual Alcohol Cues | 22 | 13 | TS Detoxed AD | Pre, Post | Whole Brain and ROI |
|
NTX treated individuals with positive cue reactivity in the putamen had a longer time to relapse compared to placebo. |
| Spagnolo et al., 2014 | 50mg NTX × 9 days | Affective Faces + Alcohol Infusion | 31 | 32 | TS AD | Post | ROI |
|
NTX + alcohol infusion increased ratings of intoxicated and high compared to placebo. |
| Varenicline (VAR) | |||||||||
| Schacht et al., 2014 | 2mg varenicline × 14 days | Visual Alcohol Cues | 18 | 17 | NTS AD | Post | ROI |
|
NS (heavy drinking and craving) |
| Vatsalya et al., 2015* | 2mg varenicline × 14 days | Alcohol Food Incentive Delay Task | 17 | 12 | NTS HD | Post | Whole Brain and ROI |
|
NP |
| Gowin et al., 2016* | 2mg varenicline × 14 days | Affective Faces + Alcohol Infusion | 17 | 15 | NTS HD | Post | Whole Brain and ROI |
|
NP |
| Anticonvulsants (Gabapentin and Baclofen) | |||||||||
| Schacht et al., 2013a | 1200mg Gabapentin (maximum dose) × 21 days + 2mg infusions of flumazenil × 2 days |
Visual Alcohol Cues | 28 | 20 | TS AD | Post | Whole Brain |
|
NS (heavy drinking) |
| Beck et al., 2018 | 30–270mg baclofen × 14 days (individually titrated, mean dose = 138mg) | Visual Alcohol Cues | 10 | 13 | TS Detoxed AD | Pre, Post | ROI |
|
BAC reduced relapse rates. fMRI + BAC clinical outcomes NP |
| Holla et al., 2018 | 60mg baclofen × 17 days | Visual Alcohol Cues | 23 | n/a | TS AUD | Pre, Post | Whole Brain and ROI |
|
In BAC-treated individuals, increased ACC activation and decreased insula activation had longer time to alcohol relapse compared to placebo. |
| Logge et al., 2019 | 30mg baclofen (low dose) or 74mg baclofen (high dose) × 17 days | Visual Alcohol Cues | 11 (low dose) 8 (high dose) |
11 | TS AD | Post | Whole Brain |
|
BAC treatment abolished association between activation in caudate and heavy drinking. |
| Aripiprazole (APZ) | |||||||||
| Myrick et al., 2010 | 15mg aripiprazole × 14 days | Visual Alcohol Cues | 14 | 16 | NTS AD | Post | ROI |
|
APZ decreased heavy drinking compared to placebo. fMRI + AP clinical outcomes NP. |
| Han et al., 2013 | 15mg aripiprazole + 20mg Escitalopram × 42 days | Video Alcohol Cues | 14 | 17 | TS Detoxed AD with comorbid MDD | Pre, Post | Whole brain |
|
APZ + ESC reduced ratings of alcohol craving. fMRI + AP clinical outcomes NP. |
| Schacht et al., 2018 | 15mg aripiprazole × 7 days | Visual Alcohol Cues | 38 | 43 | NTS AUD | Post | ROI |
|
APZ interacted with DAT1 genotype, such that it reduced bar-lab drinking in 9R carriers compared to placebo, but not in 10R homozygotes. fMRI + AP clinical outcomes NP. |
| CRF1 Antagonists (Pexacerfont and Verucerfont) | |||||||||
| Kwako et al., 2015b | 1000mg pexacerfont × 21 days | Visual Alcohol Cues IAPS Affective Faces |
29 | 26 | TS Detoxed AD | Post | Whole Brain |
|
NP |
| Schwandt et al., 2016 | 350mg verucerfont × 21 days | Visual Alcohol Cues IAPS Affective Faces |
18 | 21 | TS Detoxed AD | Post | Whole Brain |
|
NP |
| NK1 Antagonists (LY686017 and Aprepitant) | |||||||||
| George et al., 2008 | 50mg LY686017 × 21 days | Visual Alcohol Cues IAPS |
25 | 25 | TS Detoxed AD | Post | Whole Brain |
|
LY686017 decreased ratings of craving compared to placebo. fMRI + LY686017 clinical outcomes NP. |
| Kwako et al., 2015a | 125mg aprepitant × 21 days | Visual Alcohol Cues IAPS Affective Faces |
26 | 27 | TS Detoxed AD with PTSD | Post | Whole Brain |
|
NP |
| NMDA Modulators (Acamprosate and D-Cycloserine) | |||||||||
| Langosch et al., 2012 | 1998mg acamprosate × 14 days | Visual Alcohol Cues | 12 | 10 | TS AD | Pre, Post | Whole Brain and ROI |
|
NP |
| Kiefer et al., 2015 | 50mg D-cycloserine × 21 days | Visual Alcohol Cues | 16 | 16 | TS Detoxed AD | Pre, Post | Whole brain |
|
DCS + CET was more efficacious in individuals with high pre-treatment VS activation and high craving, compared to placebo. |
Abbreviations: NTX – naltrexone; OND – ondansetron; VAR – varenicline; GBP – gabapentin; BAC – baclofen; APZ - aripiprazole; PEX – pexacerfont; VER – verucerfont; APREP – aprepitant; DCS – D-cycloserine; TS – treatment seeking; NTS – non-treatment seeking; AD – alcohol dependence (DSM-IV TR diagnosis); AUD – alcohol use disorder (DSM-5 diagnosis); HD – heavy drinker; MDD – major depressive disorder; PTSD – Posttraumatic Stress Disorder; ROI – region of interest; VS – ventral striatum; mPFC – medial prefrontal cortex; OFC – orbitofrontal cortex; ACC – anterior cingulate cortex; VTA – ventral tegmental area; DLPFC – dorsolateral prefrontal cortex; vmPFC – ventromedial prefrontal cortex; NS = non-significant effect of drug on clinical outcomes; NP = clinical outcomes not present.
studies using the same study population
Naltrexone, an opioid antagonist with the greatest selectivity for μ- and κ-opioid receptors and FDA-approved to treat AUD (Niciu and Arias, 2013), has been the most widely studied pharmacotherapy for AUD in the context of neuroimaging. Six studies examined the ability of naltrexone to modulate reward and affective neural processes in individuals with AUD (Myrick et al., 2008, Schacht et al., 2013c, Schacht et al., 2017, Lukas et al., 2013, Spagnolo et al., 2014, Bach et al., 2019). The first neuroimaging study of naltrexone was conducted by Myrick and colleagues, who investigated the effect of naltrexone (NTX), ondansetron (OND), which is a selective 5HT-3 antagonist (Akbar et al., 2018), and the combination of naltrexone and ondansetron (NTX+OND) on neural alcohol cue reactivity (Myrick et al., 2008). They found that while individuals on the placebo control reliably demonstrated ventral striatal activation in response to alcohol cues, NTX, OND, and NTX+OND abolished this reward activation response. Further, the combination of NTX+OND resulted in a reduction in alcohol cue-induced craving compared to placebo. Schacht and colleagues also found that two-weeks of NTX reduced right VS activation in response to alcohol cues compared to placebo (Schacht et al., 2017). Additionally, they reported an interaction between VS activation and medication in predicting heavy drinking, such that NTX-treated individuals who had the greatest reductions in VS activation experienced the fewest heavy drinking in the 14 weeks following the scan. However, these findings are in contrast with an earlier study conducted by the same group, where they did not find a main effect of NTX on alcohol cue-elicited activation in the VS, or in two other region-of-interests (ROIs): the medial prefrontal cortex (mPFC) and orbitofrontal cortex (OFC) (Schacht et al., 2013c). This study did find a moderating effect of genetic polymorphisms in the μ-opioid receptor gene (OPRM1) and in the dopamine transporter gene (DAT1/SLC6A3) on response to NTX in the VS (Schacht et al., 2013c). Bach and colleagues evaluated the ability of NTX to block the incubation of alcohol-elicited cue reactivity (Bach et al., 2019). Open-label NTX significantly attenuated alcohol-cue elicited activation in the left putamen compared to a standard treatment group. Moreover, NTX-treated patients with positive cue reactivity at baseline, i.e. individuals who had increased activation to alcohol compared to neutral cues, had a longer time to severe relapse than NTX-treated patients with negative cue reactivity at baseline or patients in the standard treatment group (Bach et al., 2019). Lukas and colleagues evaluated the effect of extended-release NTX (XR-NTX), a formulation of naltrexone administered through intramuscular injection once monthly, on neural response to visual and olfactory alcohol cues (Lukas et al., 2013). XR-NTX significantly attenuated activation in brain regions implicated in processing salience (cingulate, inferior frontal gyrus, orbital gyri) to visual and olfactory alcohol cues. However, these regions do not exhibit overlap with other studies of neuroimaging studies of NTX and alcohol cue reactivity. Similar to Myrick and colleagues (Myrick et al., 2008), treatment with XR-NTX reduced subjective reports of wanting alcohol during the fMRI scan (Lukas et al., 2013). One study employed a different methodology, using an intravenous alcohol infusion to investigate the effects of NTX on alcohol-induced activation in response to affective stimuli (Spagnolo et al., 2014). Contrary to earlier studies, Spagnolo and colleagues found that irrespective of alcohol infusion condition, NTX increased activation in the VS compared to placebo across affective stimuli. Also in contrast to other studies, Spagnolo and colleagues found that treatment with NTX increased ratings of alcohol craving after the presentation of alcohol cues compared to placebo. Overall, NTX appears to modulate reward circuitry, with the majority of studies reporting decreased activation in regions responsible for reward and salience during alcohol cue-reactivity.
Varenicline, a full α7 nicotinic acetylcholine receptor agonist and a partial agonist to the α4β2, α3β4, and α6β2 subtypes (Akbar et al., 2018, Mihalak et al., 2006), is FDA-approved for smoking cessation and has shown promise as an AUD pharmacotherapy. Three studies have investigated the effect of varenicline on neural activation to alcohol cues and affective stimuli (Schacht et al., 2014, Vatsalya et al., 2015, Gowin et al., 2016). Schacht and colleagues investigated the effect of varenicline on alcohol-cue elicited activation in regions implicated in reward processing (Schacht et al., 2014). Utilizing an ROI-approach, they found that varenicline significantly reduced alcohol-cue elicited activation in the OFC, but did not modulate activity in the VS or mPFC. Treatment with varenicline did not reduce drinking or smoking during the two-week trial, but did decrease alcohol craving compared to placebo (Schacht et al., 2014). Vatsalya and colleagues also examined the effect of varenicline on reward processing, using a modification of the monetary incentive delay (MID) task, in which alcohol and food rewards were substituted for the traditional monetary rewards (Vatsalya et al., 2015). They found that varenicline significantly reduced activation in the striatum, amygdala, and posterior insula in response to the anticipation of alcohol reward. Finally, Gowin and colleagues investigated the effect of varenicline on affective processing using fearful face stimuli (Gowin et al., 2016). They found that varenicline significantly attenuated amygdala activation to fearful faces compared to placebo. It should be noted that the Vatsalya and Gowin studies report sub-components from the same larger study, i.e. they used the same participants within their analyses. Together these studies indicate that varenicline modulates reward circuitry in response to alcohol cues and may also attenuate negative emotional responses to fearful faces.
Several studies have investigated the effect of anticonvulsant medications on neural alcohol cue-reactivity (Schacht et al., 2013b, Beck et al., 2018, Holla et al., 2018, Logge et al., 2019). Schacht and colleagues investigated the effect of a combination of flumazenil (FMZ), a γ- aminobutyric acid (GABA)A receptor antagonist, and gabapentin (GBP), a GABA analogue which acts on voltage-gated calcium ion channels (Mason et al., 2018), on brain activation to visual alcohol cues (Schacht et al., 2013b). This study did not find a significant main effect of the combination of medications on neural alcohol cue reactivity. There was a moderating effect of alcohol withdrawal on neural alcohol cue-reactivity, such that individuals with high levels of alcohol withdrawal treated with the combination of medications displayed increased activation in the dorsal anterior cingulate cortex (dACC) in response to alcohol cues. Beck and colleagues investigated the effect of high-dose baclofen, a selective GABAB receptor agonist (Agabio et al., 2018), on neural activation to alcohol cues (Beck et al., 2018). Baclofen decreased activation in the OFC, amygdala, and ventral tegmental area (VTA) in response to alcohol cues, compared to placebo. Clinically, treatment with baclofen reduced relapse rates compared to placebo (Beck et al., 2018). Logge and colleagues examined the effect of low (30mg) and high (75mg) dose baclofen on neural response to alcohol cues (Logge et al., 2019). Similarly to Beck and colleagues, they found that high dose baclofen decreased alcohol cue-elicited activation in frontal (mPFC, ACC, and dorsolateral prefrontal cortex (DLPFC)) regions compared to placebo. Moreover, in the placebo group, there was a positive correlation between percent heavy drinking days prior to the fMRI scan and alcohol cue-elicited activation in the caudate and ACC, which was not present in the high or low dose baclofen groups. Holla and colleagues also examined the effect of baclofen on neural response to alcohol cues (Holla et al., 2018). In contrast with the other baclofen studies, they found that baclofen increased activation in the DLPFC and ACC and decreased activation in the insula compared to a non-medication AUD control group. Further, increases and decreases in brain activation associated with baclofen treatment were predictive of time to relapse, such that when treated with baclofen, greater activation of the ACC when viewing alcohol cues reduced the likelihood of early lapse; whereas continued activation of the insula under baclofen increased the likelihood of early lapse (Holla et al., 2018).
The effect of dopamine stabilization through the partial dopamine agonist aripiprazole (Akbar et al., 2018) on alcohol cue-elicited brain response has also been evaluated (Myrick et al., 2010, Han et al., 2013, Schacht et al., 2018). Myrick and colleagues reported that aripiprazole blunted alcohol-elicited reward responses in the left VTA and right VS and reduced heavy drinking days compared to placebo (Myrick et al., 2010). Han and colleagues investigated the effect of the combination of aripiprazole and escitalopram, a selective serotonin reuptake inhibitor (Owens et al., 2001), on neural response to alcohol cues in individuals with co-morbid major depressive disorder and AUD. They found that the combination of aripiprazole and escitalopram resulted in increased activation in the ACC when viewing drinking scenes compared to escitalopram alone. Treatment with the combination of medications also resulted in a reduction in alcohol craving (Han et al., 2013). Finally, Schacht and colleagues investigated the moderating role of variation in dopamine-related genes on aripiprazole’s effect on alcohol cue-elicited brain response (Schacht et al., 2018). They found a significant interaction between medication and DAT1 genotype, such that aripiprazole reduced alcohol cue-elicited activation in the VS among 9R carriers, but increased VS activation in 10R homozygotes, compared to placebo. Furthermore, they found that in a laboratory setting, aripiprazole reduced the number of drinks consumed only in 9R carriers (Schacht et al., 2018).
Preclinical studies have indicated that receptors for corticotrophin-releasing factor 1 (CRF1, also referred to as corticotrophin-releasing hormone (CRH)) and neurokinin-1 receptor (NK1R) are critically involved in AUD and stress (Spierling and Zorrilla, 2017, Petrakis and Simpson, 2017). Two studies evaluated the effect of CRF1 receptor antagonists on modulating neural response to alcohol cues and affective stimuli (Kwako et al., 2015b, Schwandt et al., 2016). Kwako and colleagues evaluated the effect of pexacerfont, a selective CRF1 antagonist, on modulating brain response to alcohol-related and affective stimuli in individuals with AUD and high levels of trait anxiety (Kwako et al., 2015b). There were no significant effects of pexacerfont on neural responses to alcohol cues, negative images, or fearful faces. Furthermore, pexacerfont did not impact stress-induced alcohol craving (Kwako et al., 2015b). Similarly, Schwandt and colleagues evaluated the effect of verucerfont, a selective CRF1 receptor antagonist, on modulating brain response to alcohol-related and affective stimuli in women with AUD and high levels of trait anxiety (Schwandt et al., 2016). In contrast to Kwako et al., this study reported that verucerfont significantly attenuated activation in the right amygdala in response to fearful faces compared to placebo, but did not impact neural responses to negative pictures. However, verucerfont also did not suppress stress-induced alcohol craving responses (Schwandt et al., 2016). Two studies investigated if NK1R antagonism modulates brain responses to alcohol cues and affective stimuli (George et al., 2008, Kwako et al., 2015a). George and colleagues reported that LY686017 (now Tradipitant), a NK1R antagonist, reduced activation to negative images in the insula and increased VS activation to positive images compared to placebo. Moreover, treatment with LY686017 reduced overall alcohol craving as well as reduced stress-induced alcohol craving. NK1R antagonism did not modulate neural responses to alcohol cues (George et al., 2008). In contrast, Kwako and colleagues found that aprepitant, a NKR1 antagonist, potentiated ventromedial prefrontal cortex responses to negative stimuli compared to placebo in individuals with co-morbid AUD and Post-traumatic stress disorder (PSTD). Aprepitant did not affect stress- or alcohol-induced craving (Kwako et al., 2015a). Overall these studies indicate that the preclinical promise of CRF1 and NK1 receptor antagonists have not translated well into the clinical population of treatment-seeking individuals with AUD.
Finally, the effect of N-methyl-D-aspartate (NMDA) modulators on neural alcohol cue reactivity have been evaluated (Langosch et al., 2012, Kiefer et al., 2015). Langosch and colleagues investigated the effect of acamprosate, an FDA-approved pharmacotherapy for AUD thought to be a glutamate modulator (Plosker, 2015), on brain responses to alcohol cues (Langosch et al., 2012). They found no significant effect of acamprosate on modulating neural alcohol cue reactivity compared to placebo. Kiefer and colleagues investigated the effect of the combination of cue-exposure-based extinction training (CET), which is a psychosocial treatment for AUD, and D-cycloserine (DCS), a partial NMDA receptor agonist thought to facilitate memory consolidation (Norberg et al., 2008), on alcohol cue-elicited brain activation (Kiefer et al., 2015). The authors report a reduction in alcohol cue-induced brain activation in the ventral and dorsal striatum in individuals treated with the combination of CET and DCS compared to those treated with CET and placebo. Furthermore, there was an interaction between pre-treatment VS activation, medication, and alcohol craving, such that in individuals with high levels of alcohol craving and high pre-treatment VS activation, treatment with DCS and CET was more efficacious compared to placebo (Kiefer et al., 2015).
Summary
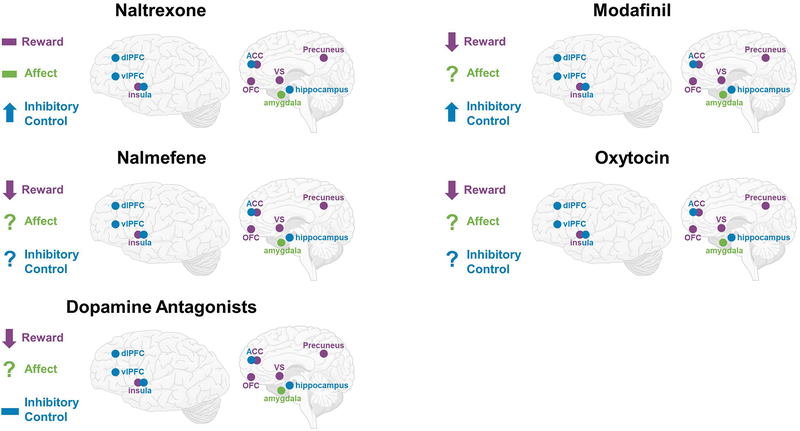
Overall there is considerable variability in sample selection (treatment seeking vs. non-treatment seeking, individuals diagnosed with AUD vs. heavy drinkers), task selection, and analytical methodology (ROI vs. whole brain approaches) in studies investigating the neural effects of AUD pharmacotherapies administered chronically. Despite these extensive differences, a few patterns do emerge, which are summarized in Figure 3. Naltrexone does seem to have an effect on alcohol reward processing, with several studies reporting reductions in activation in regions associated with reward, including the ventral striatum (Myrick et al., 2010, Schacht et al., 2017, Lukas et al., 2013, Bach et al., 2019). Furthermore, two studies demonstrated the predictive utility of fMRI combined with pharmacotherapy for predicting clinical outcomes (Schacht et al., 2017, Bach et al., 2019). Schacht and colleagues found that individuals treated with NTX who had large reductions in VS activation to alcohol cues had the lowest amount of heavy drinking in the weeks following the fMRI scan (Schacht et al., 2017), and Bach and colleagues found that NTX-treated individuals who demonstrated high alcohol cue-reactivity at baseline had a longer time to severe relapse than NTX-treated patients with low alcohol cue-reactivity at baseline (Bach et al., 2019). Varenicline also shows promise as an AUD treatment and appears to impact reward and affective processing; however, there is little overlap in task selection in the reported studies which limits generalizability (Schacht et al., 2014, Vatsalya et al., 2015, Gowin et al., 2016). Two studies reported an increase in ACC activation following treatment with a GABA antagonist (Schacht et al., 2013b, Holla et al., 2018), and this increase in ACC activation after treatment has been associated with lower relapse rates (Holla et al., 2018). Aripiprazole may modulate alcohol cue-elicited brain response in the VS (Myrick et al., 2010), with some evidence indicating a pharmacogenetic interaction between dopamine-related genetic variation and aripiprazole response (Schacht et al., 2018). Medications targeting stress-circuitry (CRF1 and NKR1) do not show consistent effects on brain response to alcohol cues or affective stimuli, and have largely shown null effects clinically (Kwako et al., 2015b, Kwako et al., 2015a, George et al., 2008, Schwandt et al., 2016). Surprisingly, only one study has evaluated the effect of acamprosate on alcohol cue-elicited neural response (Langosch et al., 2012), which reported null effects of the medication. The combination of the pharmacotherapy DCS with CET was effective at reducing alcohol-elicited activation in the VS and dorsal striatum compared to CET alone (Kiefer et al., 2015). Moreover, DCS may work best in individuals with high craving for alcohol who demonstrate high levels of VS-activation before the start of treatment. Together, these studies suggest that fMRI can be a useful tool to identify biomarkers for AUD pharmacotherapy. It is encouraging that for medications that have been studied most often, such as naltrexone and varenicline, a consistent pattern of results emerges. Perhaps most importantly, the literature is beginning to implicate patterns of brain response to pharmacotherapy with clinical outcomes, the gold-standard of clinical care.
Figure 3: Brain Circuits Modulated by AUD Pharmacotherapies Administered Chronically.
The summarized findings of the review are presented for each pharmacotherapy that was investigated using a chronic dosing approach, ↓ = attenuated activation in targeted brain circuitry; ↑ = potentiated activation in targeted brain circuity; - = mixed findings in targeted brain circuitry (attenuation, potentiation, and/or null); ? = has not yet been investigated.
Single Dosing Studies of AUD Pharmacotherapies
Table 2 presents a comprehensive list of single dosing neuroimaging pharmacotherapy studies. Naltrexone is the also the most commonly studied AUD pharmacotherapy using single dosing procedures and neuroimaging (Boettiger et al., 2009, Savulich et al., 2017, Nestor et al., 2017, Nestor et al., 2018). The majority of these studies employed a double-blind, placebo-controlled, crossover design enrolling participants with AUD and a healthy control comparison group. Boettiger and colleagues investigated the effect of acute naltrexone on impulsive decision making in abstinent individuals with AD using a delay discounting paradigm (Boettiger et al., 2009). There was no significant interaction between group and medication conditions; however, NTX administration did increase OFC activation during decision-making for “later” choices in both abstinent individuals with AD and healthy controls.
Table 2.
AUD Pharmacotherapy studies with Acute Dosing
| First author, year | Medication, dose, duration | fMRI task | AUD N | Comparison Group N | Analysis Approach | Results |
|---|---|---|---|---|---|---|
| Naltrexone (NTX) | ||||||
| Boettiger et al., 2009 | 50mg NTX × 2 hours | Delay Discounting | 9 Abstinent AD | 10 Healthy controls | Whole Brain and ROI |
|
| Savulich et al., 2017 | 50mg NTX × 2 hours | IAPS | 18 abstinent AD only 21 abstinent Polysubstance (AD + Cocaine or Opioid Dependence) |
21 Heathy Controls | ROI |
|
| Nestor et al., 2017* | 50mg NTX × 2 hours | Monetary Incentive Delay | 21 abstinent ADD only 25 abstinent Polysubstance (AD + Other Drug Dependence) |
35 Healthy Controls | Whole Brain |
|
| Nestor et al., 2018* | 50mg NTX × 2 hours | Go/No-Go | 21 abstinent AD only 25 abstinent Polysubstance (AD + Other Drug Dependence) |
35 Healthy Controls | Whole Brain |
|
| Nalmefene | ||||||
| Quelch et al., 2017 | 18mg nalmefene × 4 hours | Monetary Incentive Delay + Alcohol Infusion | 18 AUD | N/A | Whole Brain and ROI |
|
| Dopamine Antagonists | ||||||
| Hermann et al., 2006 | 400mg amisulpride × 2 hours | Visual Alcohol Cues | 10 abstinent AD | 10 Healthy Controls | Whole Brain |
|
| Murphy et al., 2017 | 60mg GSK598809 × 2 hours | Monetary Incentive Delay Go/No-Go |
18 abstinent AD Only 32 abstinent Polysubstance (AD + other dependence) |
33 Healthy Controls | Whole brain and ROI |
|
| Modafinil | ||||||
| Schmaal et al., 2013b* | 200mg modafinil × 2 hours | Stop Signal | 16 AD | 16 Healthy Controls | Whole brain |
|
| Schmaal et al., 2014* | 200mg modafinil × 2 hours | Delay Discounting | 14 abstinent AD | 18 Healthy Controls | Whole Brain |
|
| Oxytocin | ||||||
| Hansson et al., 2018 | 24 IU Oxytocin (intranasal) × 45 minutes | Visual Alcohol Cues | 12 NTS-HD | N/A | Whole Brain and ROI |
|
Abbreviations: NTX – naltrexone; ROI – region of interest; OFC – orbitofrontal cortex; DLPFC – dorsolateral prefrontal cortex; vmPFC – ventromedial prefrontal cortex; NTS – non-treatment-seeking; AD – alcohol dependence (DSM-IV TR diagnosis); AUD – alcohol use disorder (DSM-5 diagnosis); HD – heavy drinker
studies using the same study population
The following set of studies investigated NTX through the Imperial College Cambridge Manchester (ICCAM) platform, which is an experimental medicine approach to explore the neuropharmacology of relapse using fMRI techniques (Paterson et al., 2015). The ICCAM studies enrolled abstinent individuals with AD alone, abstinent individuals with AD and other substance use disorders (polysubstance dependent), and a comparison group of healthy controls. Using this platform, Savulich and colleagues investigated the effect of acute naltrexone on negative emotion processing (Savulich et al., 2017). NTX did not significantly modulate activation in the AD only group; however, it did reduce amygdala activation in the polysubstance dependent group compared to the AD only and healthy control groups. Nestor and colleagues investigated effect of acute naltrexone administration on non-drug reward processing using the MID task (Nestor et al., 2017). There was no significant interaction between group and medication on non-drug reward processing. Nestor and colleagues also investigated the effect of acute naltrexone on the neural correlates of motor impulse control using the GNG task (Nestor et al., 2018). There was a significant interaction between group and medication condition, such that in individuals with AD alone, NTX increased activation in the OFC compared to the polysubstance dependent group; in the polysubstance dependent group, NTX increased activation in the anterior insula compared to the AD only group.
Another opioid receptor antagonist nalmefene, which is a μ and δ opioid receptor antagonist and κ opioid receptor partial agonist (Soyka, 2016), has been also investigated using the single dosing approach (Quelch et al., 2017). Quelch and colleagues investigated the effect of nalmefene on the neural correlates of reward processing using the MID task paired with an intravenous alcohol infusion (Quelch et al., 2017). In individuals with AUD, nalmefene reduced activation in the striatum when anticipating rewards during an alcohol infusion compared to placebo.
Two studies have investigated the effect of acute dopamine antagonism on neural responses to alcohol and non-alcohol reward processing (Hermann et al., 2006, Murphy et al., 2017). Hermann and colleagues investigated the effect of amisulpride, a D2/3 dopamine receptor antagonist (Grunder et al., 2003), on neural alcohol cue reactivity (Hermann et al., 2006). Acute treatment with amisulpride reduced alcohol cue-elicited activation in the right thalamus in individuals with AD. Using the ICCAM platform, Murphy and colleagues evaluated the effect of GSK598809, an acute D3 dopamine receptor antagonist (Heidbreder and Newman, 2010), on non-drug reward processing using the MID task, and motor impulse control, using the GNG task (Murphy et al., 2017). Regarding reward processing, there was a significant group by medication interaction during reward anticipation, such that in individuals with AD only GSK598809 increased activation in the dorsolateral PFC compared to the polysubstance and healthy controls groups. There was no significant effect of the drug on the neural correlates of inhibitory control.
Modafinil, a cognitive enhancer used to treat narcolepsy (Leeman et al., 2014), has been investigated as a potential pharmacotherapy to improve impulse control in individuals with AD (Schmaal et al., 2014, Schmaal et al., 2013). Schmaal and colleagues investigated the effect of acute modafinil on response inhibition, evaluated through the SST and impulsive decision-making, evaluated through a delay discounting task, using a double-blind, placebo-controlled, crossover approach. There was a significant group by medication interaction on response inhibition activation in the putamen, such that treatment with modafinil increased activation in the putamen in individuals with AD compared to placebo (Schmaal et al., 2013). Modafinil also modulated the neural correlates of impulsive decision-making; in individuals with AD, acute treatment with modafinil improved impulsive decision making and increased activation in the superior frontal gyrus and decreased activation in the ventromedial PFC (Schmaal et al., 2014). It should be noted that these studies were conducted on the same participants during the same study visits; the differing number of subjects included in the sub-studies reflect differences in motion and task engagement for the individual tasks.
Finally, oxytocin, a neuropeptide that is implicated in social behavior (Lee and Weerts, 2016), has been investigated as a novel pharmacotherapy for AUD. Hansson and colleagues investigated the effect of acute oxytocin, administered intranasally, on neural alcohol cue-reactivity in male heavy drinkers (Hansson et al., 2018). Oxytocin administration resulted in significant reductions in neural alcohol cue-reactivity in the insula, cingulate, and the medial frontal gyrus, compared to placebo.
Summary
Together, single-administration NTX fMRI studies have largely not been effective at identifying NTX-induced modulations in neural circuitry in AUD populations. One study did report an effect of naltrexone on modulating neural activation during inhibitory control processing in AD individuals, with NTX increasing OFC activation in abstinent AD participants (Nestor et al., 2018) One study reported an overall effect of NTX on neural activation during a delay discounting task (i.e. brain activation changes were present in both healthy and AD groups) (Boettiger et al., 2009), while another study have found an effect of NTX on brain activation during negative emotional processing in a polysubstance using AD group (Savulich et al., 2017). Intriguingly, nalmefene, a medication whose putative mechanism is similar to NTX, was effective at reducing reward activation during non-drug reward processing combined with an alcohol infusion in individuals with AUD (Quelch et al., 2017). Modafinil appears to be effective at modulating brain response in individuals with AD during decision-making and impulse control tasks (Schmaal et al., 2014, Schmaal et al., 2013). However, these findings come from one larger study; additional work is needed to replicate and extend upon these findings. Oxytocin also showed initial efficacy at reducing neural responses to alcohol cues in heavy social drinkers (Hansson et al., 2018). However, this pilot study enrolled a small sample of only male participants, which indicates that additional replication and more representative samples will be required. Overall, these studies call into question the future use of single-administration AUD pharmacotherapies, due to the mixed findings and wide variety of fMRI tasks employed, summarized in Figure 4. An important conclusion emerging from the literature reviewed herein, and divided into acute and chronic administering, is that acute (or single dose) drug administration is much less reliable than chronic dosing from the viewpoint of pharmacological effects detected through functional neuroimaging. This is perhaps not surprising, given that organisms adapt to pharmacological agents and chronic dosing is most representative of clinical care models. Nonetheless, this review cautions against the use of acute administration fMRI models on the basis of these mixed results. The exception to this recommendation may be pharmacotherapies used on an as needed (i.e., PRN) basis, such as nalmefeme and oxytocin, for example.
Figure 4: Brain Circuits Modulated by AUD Pharmacotherapies Administered Acutely.
The summarized findings of the review are presented for each pharmacotherapy that was investigated using a single dosing approach. ↓ = attenuated activation in targeted brain circuitry; ↑ = potentiated activation in targeted brain circuity; - = mixed findings in targeted brain circuitry (attenuation, potentiation, and/or null); ? = has not yet been investigated.
While the preponderance of the qualitative findings are displayed in Tables 1 and 2, organized by dosing and pharmacotherapy, readers interested in findings organized by task and targeted brain circuitry (separated by dosing) are directed to Supplementary Tables 1–5. Figures 3 and 4 also provide an integration of task-specific findings grouped by pharmacotherapy and separated by dosing.
Recommendations for Future Research
Overall, while the studies included in this review indicate that fMRI is a promising tool to narrow the pathway of pharmacological treatments for AUD, there are several key recommendations for improvements upon the existing method. These recommendations are meant to maximize the potential for success when conducting neuroimaging pharmacotherapy studies for AUD and are based on commons issues identified in this review.
First, to adequately compare and contrast fMRI pharmacology studies we recommend a standardization of neuroimaging parameters and methods. In regards to neuroimaging methodology, two main approaches are currently used: a data-driven whole-brain method and an a-priori region of interest (ROI) approach. Both approaches have strengths and weaknesses; the whole-brain method is useful for identifying neural circuitry modulated by a pharmacotherapy, especially in cases where the pharmacology is poorly understood; however, this approach comes at the cost of statistical power. The a-priori ROI approach increases statistical power and represents a theory-driven model; this method has been successful in the case of NTX where the medication targets are known. Additionally, the ROI approach allows for a-priori power calculation, whereas whole-brain studies do not. Both the ROI and the whole-brain approach can be applied to examine task-based connectivity, where the relationship between brain activity across time in specific seed-regions (ROI-based approach) or across the brain (whole-brain approach) during specific task-contrasts and under different medication conditions can be explored. Few published pharmacotherapy fMRI studies have employed this approach; however, as the field grows in its understanding of the complex interplay between brain circuits involved in addiction and exposure to alcohol it is likely that functional connectivity approaches will be required to better understand the up- and down-regulation of addiction-related neural circuits. Another area which requires standardization is the timing of the collection of fMRI scans. In order to determine the causal role of a treatment on brain function, neuroimaging should be conducted both pre- and post-treatment. This approaches will enable researches to draw conclusions about neural circuitry changes directly attributable to the pharmacotherapy, and will also provide opportunities for precision medicine approaches, discussed in detail below.
Neuroimaging pharmacology studies should also take into account sample selection and sample size. Several of the studies included in this review were underpowered, potentially due to ethical considerations in early stages of treatment development. In the chronic dosing studies, where results were arguably more consistent, sample size ranged from 10 to 59 individuals per treatment group. In the single dosing studies, sample size ranged from 9 to 32 individuals per treatment group, with the majority of studies being underpowered to find whole-brain treatment effects. Regarding sample selection, studies included in this review enrolled participants who were non-treatment-seeking heavy drinkers, non-treatment-seeking individuals with an AUD, treatment-seeking individuals with an AUD, and abstinent individuals with an AUD. Furthermore, studies included in this review also enrolled participants with co-morbid psychiatric diagnoses (MDD and PTSD) and with high levels of anxiety. Given this heterogeneity, it is not surprising that results do not routinely converge, with the exception of naltrexone where similar results are seen in non-treatment-seeking and treatment-seeking samples (Myrick et al., 2008, Lukas et al., 2013, Schacht et al., 2017, Bach et al., 2019). Recent findings indicate that non-treatment-seekers and treatment-seekers differ on many clinical characteristics, including age, dependence severity, drinking consequences, craving, and alcohol drinking measures (Ray et al., 2017, Rohn et al., 2017). Moreover, the differences between these populations was shown to be predictive of clinical outcomes in a large behavioral pharmacological study (COMBINE study) (Ray et al., 2017), and therefore these differences may also influence neural responses to pharmacotherapy. Given these important differences, we recommend that the field should standardize sample selection for fMRI pharmacotherapy studies. Ideally this standardization should address treatment-seeking vs. non-treatment seeking, AUD diagnosis requirements, alcohol consumption measures, and the date of last drinking prior to study enrollment.
Second, we recommend the standardization of fMRI task selection for AUD pharmacotherapy studies. There was significant heterogeneity in the task selection used for the studies included in this review. The most commonly used task was the alcohol cue-reactivity paradigm, which has strong face validity as a measure for AUD pharmacotherapy. However, even within this paradigm different approaches are employed which may contribute to inconsistent findings. For example, alcohol cue-reactivity studies use different modalities of alcohol cues, including pictures of alcoholic beverages, videos of alcohol, gustatory stimuli, and olfactory stimuli. Other common fMRI paradigms included the MID task, which targets non-drug reward processes, and affective processing visual stimuli sets, including affective faces and negative images. All of these paradigms/visual stimuli sets can be standardized with regard to trial duration and task contrasts analyzed. Moreover, establishing criterion validity with regard to task selection will be key for future studies. fMRI offers the ability to provide an objective measure that can predict treatment response. For example, Mann and colleagues have shown that pre-treatment brain activation during an alcohol-cue reactivity task can predict treatment efficacy of naltrexone on relapse behavior (Mann et al., 2014). Several recent studies included in this review highlight the benefits of these treatment-prediction analyses (Schacht et al., 2017, Bach et al., 2019, Holla et al., 2018). These studies indicate that neural response to alcohol cues has criterion validity for a clinical outcome and indicates that this paradigm should be used in future studies of AUD pharmacotherapy, particularly for medications targeting reward circuitry. Moreover, additional brain-behavior relationships can and should be investigated in future studies. Measurements of alcohol craving, alcohol use, and mood are commonly collected in pharmacotherapy fMRI studies. We recommend that brain-behavior associations should be investigated to better triangulate brain imaging findings onto behavior, particularly in the context of pharmacotherapy.
Third, we recommend studying AUD pharmacotherapies using a chronic dosing regimen. By parsing the literature into chronic and acute dosing, there was mixed support for pharmacotherapy effects in acute dosing (i.e., single dose) studies. Therefore, unless the drug is a PRN or there is another compelling reason to examine single dose effects, it appears as though chronic dosing should be the preferred approach to combining pharmacotherapy and fMRI. Although exactly how long the dosing regimen should last prior to imaging remains an open question, and may in fact vary by medication. For example, naltrexone blocks mu and kappa receptors at high rates and rather quickly (Weerts et al., 2008), such that acute dosing may be useful in this context. Conversely, targeting delta opioid receptors would like necessitate a different dosing and titration schedule. An important first step may be to ensure that the medication under study has reached steady state and is at the target clinical dosing. Lastly, it should be noted that while samples in the chronic and acute dosing studies were of similar age on average, they were quite different in their clinical characteristics; namely, acute dosing studies recruited longer-term abstinent individuals while chronic dosing studies recruited current users or individuals with short-term abstinence.
Additionally, we recommend including neuroimaging in the context of a clinical pharmacotherapy trial. While incorporating neuroimaging into a trial will increase upfront costs, it offers substantial benefits, as it can provide both mechanistic insights into the method of action of a medication, as well as providing biomarkers for prediction of clinical outcomes. Additionally, as clinical trials are generally better-powered than small-scale fMRI trials, the inclusion of neuroimaging in such trials will provide better sample sizes and increased statistical power. In the context of clinical applications, understanding the putative mechanism of action of a given pharmacotherapy may suggest different experimental designs. For example, naltrexone is known to alter the subjective experience of alcohol (Ray et al., 2010), thus an experimental design that includes alcohol administration may be ideally suited to capture these alcohol-dependent effects. Furthermore, we recommend that combined neuroimaging and pharmacogenetic evaluation be conducted whenever possible, particularly in the context of pharmacogenetic clinical trials. Several studies reviewed herein demonstrate the utility of this approach, revealing complex interactions between neural brain activation, genetic phenotype(s), and medication response (Schacht et al., 2013c, Schacht et al., 2018, Schacht et al., 2017). For example, Schacht and colleagues evaluated if variation in dopaminergic genes (DAT1I variable number tandem repeat, and polymorphisms in COMT, DRD2, and DRD4) moderated the effect of aripiprazole on neural alcohol cue reactivity (Schacht et al., 2018). They found that the DAT1 genotype moderated medication effects, such that there were opposing effects of the medication on VS activation to alcohol cues. Due to the opposing direction of these effects, had this sample had been combined and no pharmacogenetic effect been evaluated, the authors may have concluded that the medication produces an overall null effect on brain response.
In conclusion, this qualitative review of the literature examined studies combining AUD pharmacotherapies and functional neuroimaging methods. This review documented imaging tasks, imaging methods, sample characteristics, sample sizes, and pharmacotherapy dosing. Together, findings from this review underscore the utility of fMRI for elucidating mechanisms of action of AUD pharmacotherapies and for predicting clinical response, albeit the later goal has only been pursued in a few recent studies. It appears as though the promise of functional neuroimaging applied to AUD medications development is best served in the context of clinically informative samples, dosing regimens, tasks, and analytic approaches. To that end, this review provides a series of recommendations to advance pharmacotherapy development for AUD by leveraging neuroimaging tools. Lastly, a host of novel methods are constantly being developed for neuroimaging, and for data analysis broadly. Thus it is plausible that new analytic methods, not yet represented in the current literature, may have a major impact in how fMRI is used. Examples include functional connectivity analyses (both task-based and resting-state) and computational psychiatry methods leveraging fMRI data. Moreover, the data and conclusions presented in the current review are drawn from group fMRI studies. As scanners with higher signal to noise ratio, i.e. 7T scanners, become more widely used, the field will need to move to individual level analyses. Analytic methods notwithstanding, strong experimental rigor cannot be replaced by data analytic tools and as such, the recommendations for further standardization of fMRI studies of AUD pharmacotherapies stands as a critical next step for the field.
Supplementary Material
Acknowledgements
We greatly appreciate the assistance of Catherine Trinh in the collection of publications for this review.
This research was supported by a grant from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) to LAR (R01AA021744). LAR has received study medication from Pfizer Medicinova and consulted for GSK and Mitsubishi Tanabe. None of the authors have conflicts of interest to disclose.
References
- Agabio R, Sinclair JM, Addolorato G, Aubin H-J, Beraha EM, Caputo F, Chick JD, de La Selle P, Franchitto N, Garbutt JC (2018) Baclofen for the treatment of alcohol use disorder: the Cagliari Statement. The Lancet Psychiatry 5:957–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbar M, Egli M, Cho YE, Song BJ, Noronha A (2018) Medications for alcohol use disorders: An overview. Pharmacol Ther 185:64–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach P, Weil G, Pompili E, Hoffmann S, Hermann D, Vollstadt-Klein S, Mann K, Perez-Ramirez U, Moratal D, Canals S, Dursun SM, Greenshaw AJ, Kirsch P, Kiefer F, Sommer WH (2019) Incubation of neural alcohol cue reactivity after withdrawal and its blockade by naltrexone. Addict Biol. [DOI] [PubMed] [Google Scholar]
- Balodis IM, Potenza MN (2015) Anticipatory reward processing in addicted populations: a focus on the monetary incentive delay task. Biological psychiatry 77:434–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A, Pelz P, Lorenz RC, Charlet K, Geisel O, Heinz A, Wustenberg T, Muller CA (2018) Effects of high-dose baclofen on cue reactivity in alcohol dependence: A randomized, placebo-controlled pharmaco-fMRI study. Eur Neuropsychopharmacol 28:1206–1216. [DOI] [PubMed] [Google Scholar]
- Boettiger CA, Kelley EA, Mitchell JM, D’Esposito M, Fields HL (2009) Now or Later? An fMRI study of the effects of endogenous opioid blockade on a decision-making network. Pharmacol Biochem Behav 93:291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhler M, Mann K (2011) Alcohol and the human brain: a systematic review of different neuroimaging methods. Alcohol Clin Exp Res 35:1771–1793. [DOI] [PubMed] [Google Scholar]
- Buxton RB, Uludag K, Dubowitz DJ, Liu TT (2004) Modeling the hemodynamic response to brain activation. Neuroimage 23:S220–S233. [DOI] [PubMed] [Google Scholar]
- Cabrera EA, Wiers CE, Lindgren E, Miller G, Volkow ND, Wang GJ (2016) Neuroimaging the Effectiveness of Substance Use Disorder Treatments. J Neuroimmune Pharmacol 11:408–433. [DOI] [PubMed] [Google Scholar]
- Courtney KE, Schacht JP, Hutchison K, Roche DJ, Ray LA (2016) Neural substrates of cue reactivity: association with treatment outcomes and relapse. Addict Biol 21:3–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PT, Raichle ME (1986) Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc Natl Acad Sci U S A 83:1140–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George DT, Gilman J, Hersh J, Thorsell A, Herion D, Geyer C, Peng X, Kielbasa W, Rawlings R, Brandt JE, Gehlert DR, Tauscher JT, Hunt SP, Hommer D, Heilig M (2008) Neurokinin 1 receptor antagonism as a possible therapy for alcoholism. Science 319:1536–1539. [DOI] [PubMed] [Google Scholar]
- Gowin JL, Vatsalya V, Westman JG, Schwandt ML, Bartlett S, Heilig M, Momenan R, Ramchandani VA (2016) The Effect of Varenicline on the Neural Processing of Fearful Faces and the Subjective Effects of Alcohol in Heavy Drinkers. Alcohol Clin Exp Res 40:979–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B (2015) Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA psychiatry 72:757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunder G, Siessmeier T, Piel M, Vernaleken I, Buchholz HG, Zhou Y, Hiemke C, Wong DF, Rosch F, Bartenstein P (2003) Quantification of D2-like dopamine receptors in the human brain with 18F-desmethoxyfallypride. J Nucl Med 44:109–116. [PubMed] [Google Scholar]
- Han DH, Kim SM, Choi JE, Min KJ, Renshaw PF (2013) Adjunctive aripiprazole therapy with escitalopram in patients with co-morbid major depressive disorder and alcohol dependence: clinical and neuroimaging evidence. J Psychopharmacol 27:282–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson AC, Koopmann A, Uhrig S, Buhler S, Domi E, Kiessling E, Ciccocioppo R, Froemke RC, Grinevich V, Kiefer F, Sommer WH, Vollstadt-Klein S, Spanagel R (2018) Oxytocin Reduces Alcohol Cue-Reactivity in Alcohol-Dependent Rats and Humans. Neuropsychopharmacology 43:1235–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeger DJ, Ress D (2002) What does fMRI tell us about neuronal activity? Nat Rev Neurosci 3:142–151. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Newman AH (2010) Current perspectives on selective dopamine D3 receptor antagonists as pharmacotherapeutics for addictions and related disorders. Annals of the New York Academy of Sciences 1187:4–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann D, Smolka MN, Wrase J, Klein S, Nikitopoulos J, Georgi A, Braus DF, Flor H, Mann K, Heinz A (2006) Blockade of cue-induced brain activation of abstinent alcoholics by a single administration of amisulpride as measured with fMRI. Alcohol Clin Exp Res 30:1349–1354. [DOI] [PubMed] [Google Scholar]
- Hoge RD, Atkinson J, Gill B, Crelier GR, Marrett S, Pike GB (1999) Linear coupling between cerebral blood flow and oxygen consumption in activated human cortex. P Natl Acad Sci USA 96:9403–9408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holla B, Karthik S, Biswal J, Viswanath B, Jayarajan D, Bharath RD, Venkatasubramanian G, Benegal V (2018) Brain Functional Magnetic Resonance Imaging Cue-reactivity Can Predict Baclofen Response in Alcohol Use Disorders. Clin Psychopharmacol Neurosci 16:290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannetti GD, Wise RG (2007) BOLD functional MRI in disease and pharmacological studies: room for improvement? Magn Reson Imaging 25:978–988. [DOI] [PubMed] [Google Scholar]
- Kiefer F, Kirsch M, Bach P, Hoffmann S, Reinhard I, Jorde A, von der Goltz C, Spanagel R, Mann K, Loeber S, Vollstadt-Klein S (2015) Effects of D-cycloserine on extinction of mesolimbic cue reactivity in alcoholism: a randomized placebo-controlled trial. Psychopharmacology (Berl) 232:2353–2362. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D (2001) Anticipation of increasing monetary reward selectively recruits nucleus accumbens. Journal of Neuroscience 21:RC159–RC159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND (2016) Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry 3:760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwako LE, George DT, Schwandt ML, Spagnolo PA, Momenan R, Hommer DW, Diamond CA, Sinha R, Shaham Y, Heilig M (2015a) The neurokinin-1 receptor antagonist aprepitant in co-morbid alcohol dependence and posttraumatic stress disorder: a human experimental study. Psychopharmacology (Berl) 232:295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwako LE, Spagnolo PA, Schwandt ML, Thorsell A, George DT, Momenan R, Rio DE, Huestis M, Anizan S, Concheiro M, Sinha R, Heilig M (2015b) The corticotropin releasing hormone-1 (CRH1) receptor antagonist pexacerfont in alcohol dependence: a randomized controlled experimental medicine study. Neuropsychopharmacology 40:1053–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langosch JM, Spiegelhalder K, Jahnke K, Feige B, Regen W, Kiemen A, Hennig J, Olbrich HM (2012) The impact of acamprosate on cue reactivity in alcohol dependent individuals: a functional magnetic resonance imaging study. J Clin Psychopharmacol 32:661–665. [DOI] [PubMed] [Google Scholar]
- Lee MR, Weerts EM (2016) Oxytocin for the treatment of drug and alcohol use disorders. Behavioural pharmacology 27:640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeman RF, Bogart D, Fucito LM, Boettiger CA (2014) “Killing Two Birds with One Stone”: Alcohol Use Reduction Interventions with Potential Efficacy at Enhancing Self-control. Current addiction reports 1:41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logge WB, Morris RW, Baillie AJ, Haber PS, Morley KC (2019) Baclofen attenuates fMRI alcohol cue reactivity in treatment-seeking alcohol dependent individuals. Psychopharmacology (Berl). [DOI] [PubMed] [Google Scholar]
- Logothetis NK (2003) The underpinnings of the BOLD functional magnetic resonance imaging signal. J Neurosci 23:3963–3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas SE, Lowen SB, Lindsey KP, Conn N, Tartarini W, Rodolico J, Mallya G, Palmer C, Penetar DM (2013) Extended-release naltrexone (XR-NTX) attenuates brain responses to alcohol cues in alcohol-dependent volunteers: a bold FMRI study. Neuroimage 78:176–185. [DOI] [PubMed] [Google Scholar]
- MacKillop J (2016) The behavioral economics and neuroeconomics of alcohol use disorders. Alcoholism: Clinical and Experimental Research 40:672–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K, Vollstadt-Klein S, Reinhard I, Lemenager T, Fauth-Buhler M, Hermann D, Hoffmann S, Zimmermann US, Kiefer F, Heinz A, Smolka MN (2014) Predicting naltrexone response in alcohol-dependent patients: the contribution of functional magnetic resonance imaging. Alcohol Clin Exp Res 38:2754–2762. [DOI] [PubMed] [Google Scholar]
- Mason BJ, Quello S, Shadan F (2018) Gabapentin for the treatment of alcohol use disorder. Expert opinion on investigational drugs 27:113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalak KB, Carroll FI, Luetje CW (2006) Varenicline is a partial agonist at alpha 4 beta 2 and a full agonist at alpha 7 neuronal nicotinic receptors. Mol Pharmacol 70:801–805. [DOI] [PubMed] [Google Scholar]
- Monti PM, Binkoff JA, Abrams DB, Zwick WR, Nirenberg TD, Liepman MR (1987) Reactivity of alcoholics and nonalcoholics to drinking cues. J Abnorm Psychol 96:122–126. [DOI] [PubMed] [Google Scholar]
- Mukamel R, Gelbard H, Arieli A, Hasson U, Fried I, Malach R (2005) Coupling between neuronal firing, field potentials, and fMR1 in human auditory cortex. Science 309:951–954. [DOI] [PubMed] [Google Scholar]
- Murphy A, Nestor LJ, McGonigle J, Paterson L, Boyapati V, Ersche KD, Flechais R, Kuchibatla S, Metastasio A, Orban C, Passetti F, Reed L, Smith D, Suckling J, Taylor E, Robbins TW, Lingford-Hughes A, Nutt DJ, Deakin JFW, Elliott R, Platform I (2017) Acute D3 Antagonist GSK598809 Selectively Enhances Neural Response During Monetary Reward Anticipation in Drug and Alcohol Dependence. Neuropsychopharmacology 42:1925–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Randall PK, Voronin K (2008) Effect of naltrexone and ondansetron on alcohol cue-induced activation of the ventral striatum in alcohol-dependent people. Arch Gen Psychiatry 65:466–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrick H, Li X, Randall PK, Henderson S, Voronin K, Anton RF (2010) The effect of aripiprazole on cue-induced brain activation and drinking parameters in alcoholics. J Clin Psychopharmacol 30:365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor LJ, Murphy A, McGonigle J, Orban C, Reed L, Taylor E, Flechais R, Paterson LM, Smith D, Bullmore ET, Ersche KD, Suckling J, Tait R, Elliott R, Deakin B, Rabiner I, Lingford-Hughes A, Nutt DJ, Sahakian B, Robbins TW, Consortium I (2017) Acute naltrexone does not remediate fronto-striatal disturbances in alcoholic and alcoholic polysubstance-dependent populations during a monetary incentive delay task. Addict Biol 22:1576–1589. [DOI] [PubMed] [Google Scholar]
- Nestor LJ, Paterson LM, Murphy A, McGonigle J, Orban C, Reed L, Taylor E, Flechais R, Smith D, Bullmore ET, Ersche KD, Suckling J, Elliott R, Deakin B, Rabiner I, Lingford Hughes A, Sahakian BJ, Robbins TW, Nutt DJ, Consortium I (2018) Naltrexone differentially modulates the neural correlates of motor impulse control in abstinent alcohol-dependent and polysubstance-dependent individuals. Eur J Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niciu MJ, Arias AJ (2013) Targeted opioid receptor antagonists in the treatment of alcohol use disorders. CNS Drugs 27:777–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norberg MM, Krystal JH, Tolin DF (2008) A meta-analysis of D-cycloserine and the facilitation of fear extinction and exposure therapy. Biol Psychiatry 63:1118–1126. [DOI] [PubMed] [Google Scholar]
- Organization WH (2018) Global status report on alcohol and health 2018, World Health Organization. [Google Scholar]
- Owens MJ, Knight DL, Nemeroff CB (2001) Second-generation SSRIs: human monoamine transporter binding profile of escitalopram and R-fluoxetine. Biological psychiatry 50:345–350. [DOI] [PubMed] [Google Scholar]
- Paterson LM, Flechais RS, Murphy A, Reed LJ, Abbott S, Boyapati V, Elliott R, Erritzoe D, Ersche KD, Faluyi Y, Faravelli L, Fernandez-Egea E, Kalk NJ, Kuchibatla SS, McGonigle J, Metastasio A, Mick I, Nestor L, Orban C, Passetti F, Rabiner EA, Smith DG, Suckling J, Tait R, Taylor EM, Waldman AD, Robbins TW, Deakin JF, Nutt DJ, Lingford-Hughes AR, Platform I (2015) The Imperial College Cambridge Manchester (ICCAM) platform study: An experimental medicine platform for evaluating new drugs for relapse prevention in addiction. Part A: Study description. J Psychopharmacol 29:943–960. [DOI] [PubMed] [Google Scholar]
- Petrakis IL, Simpson TL (2017) Posttraumatic Stress Disorder and Alcohol Use Disorder: A Critical Review of Pharmacologic Treatments. Alcohol Clin Exp Res 41:226–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plosker GL (2015) Acamprosate: A Review of Its Use in Alcohol Dependence. Drugs 75:1255–1268. [DOI] [PubMed] [Google Scholar]
- Quelch DR, Mick I, McGonigle J, Ramos AC, Flechais RSA, Bolstridge M, Rabiner E, Wall MB, Newbould RD, Steiniger-Brach B, van den Berg F, Boyce M, Ostergaard Nilausen D, Breuning Sluth L, Meulien D, von der Goltz C, Nutt D, Lingford-Hughes A (2017) Nalmefene Reduces Reward Anticipation in Alcohol Dependence: An Experimental Functional Magnetic Resonance Imaging Study. Biol Psychiatry 81:941–948. [DOI] [PubMed] [Google Scholar]
- Ray LA, Bujarski S, Grodin E, Hartwell E, Green R, Venegas A, Lim AC, Gillis A, Miotto K (2018) State-of-the-art behavioral and pharmacological treatments for alcohol use disorder. The American journal of drug and alcohol abuse:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Bujarski S, Yardley MM, Roche DJO, Hartwell EE (2017) Differences between treatment-seeking and non-treatment-seeking participants in medication studies for alcoholism: do they matter? Am J Drug Alcohol Ab 43:703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Chin PF, Miotto K (2010) Naltrexone for the treatment of alcoholism: clinical findings, mechanisms of action, and pharmacogenetics. CNS Neurol Disord Drug Targets 9:13–22. [DOI] [PubMed] [Google Scholar]
- Rohn MCH, Lee MR, Kleuter SB, Schwandt ML, Falk DE, Leggio L (2017) Differences Between Treatment-Seeking and Nontreatment-Seeking Alcohol-Dependent Research Participants: An Exploratory Analysis. Alcoholism-Clinical and Experimental Research 41:414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savulich G, Riccelli R, Passamonti L, Correia M, Deakin JF, Elliott R, Flechais RS, Lingford-Hughes AR, McGonigle J, Murphy A, Nutt DJ, Orban C, Paterson LM, Reed LJ, Smith DG, Suckling J, Tait R, Taylor EM, Sahakian BJ, Robbins TW, Ersche KD (2017) Effects of naltrexone are influenced by childhood adversity during negative emotional processing in addiction recovery. Transl Psychiatry 7:e1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Myrick H (2013a) Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addict Biol 18:121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Randall PK, Li X, Henderson S, Myrick H (2013b) Effects of a GABA-ergic medication combination and initial alcohol withdrawal severity on cue-elicited brain activation among treatment-seeking alcoholics. Psychopharmacology (Berl) 227:627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Randall PK, Li X, Henderson S, Myrick H (2014) Varenicline effects on drinking, craving and neural reward processing among non-treatment-seeking alcohol-dependent individuals. Psychopharmacology (Berl) 231:3799–3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Voronin KE, Randall PK, Li X, Henderson S, Myrick H (2013c) Interacting effects of naltrexone and OPRM1 and DAT1 variation on the neural response to alcohol cues. Neuropsychopharmacology 38:414–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht JP, Randall PK, Latham PK, Voronin KE, Book SW, Myrick H, Anton RF (2017) Predictors of Naltrexone Response in a Randomized Trial: Reward-Related Brain Activation, OPRM1 Genotype, and Smoking Status. Neuropsychopharmacology 42:2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht JP, Voronin KE, Randall PK, Anton RF (2018) Dopaminergic Genetic Variation Influences Aripiprazole Effects on Alcohol Self-Administration and the Neural Response to Alcohol Cues in a Randomized Trial. Neuropsychopharmacology 43:1247–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaal L, Goudriaan AE, Joos L, Dom G, Pattij T, van den Brink W, Veltman DJ (2014) Neural substrates of impulsive decision making modulated by modafinil in alcohol-dependent patients. Psychol Med 44:2787–2798. [DOI] [PubMed] [Google Scholar]
- Schmaal L, Joos L, Koeleman M, Veltman DJ, van den Brink W, Goudriaan AE (2013) Effects of modafinil on neural correlates of response inhibition in alcohol-dependent patients. Biol Psychiatry 73:211–218. [DOI] [PubMed] [Google Scholar]
- Schwandt ML, Cortes CR, Kwako LE, George DT, Momenan R, Sinha R, Grigoriadis DE, Pich EM, Leggio L, Heilig M (2016) The CRF1 Antagonist Verucerfont in Anxious Alcohol-Dependent Women: Translation of Neuroendocrine, But not of Anti-Craving Effects. Neuropsychopharmacology 41:2818–2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyka M (2016) Nalmefene for the treatment of alcohol use disorders: recent data and clinical potential. Expert opinion on pharmacotherapy 17:619–626. [DOI] [PubMed] [Google Scholar]
- Spagnolo PA, Ramchandani VA, Schwandt ML, Zhang L, Blaine SK, Usala JM, Diamond KA, Phillips MJ, George DT, Momenan R, Heilig M (2014) Effects of naltrexone on neural and subjective response to alcohol in treatment-seeking alcohol-dependent patients. Alcohol Clin Exp Res 38:3024–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spierling SR, Zorrilla EP (2017) Don’t stress about CRF: assessing the translational failures of CRF1antagonists. Psychopharmacology (Berl) 234:1467–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatsalya V, Gowin JL, Schwandt ML, Momenan R, Coe MA, Cooke ME, Hommer DW, Bartlett S, Heilig M, Ramchandani VA (2015) Effects of Varenicline on Neural Correlates of Alcohol Salience in Heavy Drinkers. Int J Neuropsychopharmacol 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerts EM, Kim YK, Wand GS, Dannals RF, Lee JS, Frost JJ, McCaul ME (2008) Differences in delta- and mu-opioid receptor blockade measured by positron emission tomography in naltrexone-treated recently abstinent alcohol-dependent subjects. Neuropsychopharmacology 33:653–665. [DOI] [PubMed] [Google Scholar]
- Yalachkov Y, Kaiser J, Naumer MJ (2012) Functional neuroimaging studies in addiction: multisensory drug stimuli and neural cue reactivity. Neurosci Biobehav Rev 36:825–835. [DOI] [PubMed] [Google Scholar]
- Zilverstand A, Huang AS, Alia-Klein N, Goldstein RZ (2018) Neuroimaging Impaired Response Inhibition and Salience Attribution in Human Drug Addiction: A Systematic Review. Neuron 98:886–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.