Abstract
Adolescents are more susceptible to dysregulation in positive social contexts, compared to children, and as such, we investigated whether maternal presence would buffer these effects in adolescence. Fifty-four adolescents and children (age range = 8-17 years, Mage = 13.38 years) completed a social go-nogo task during an fMRI scan alone and in the presence of their mother. We found age related patterns, such that older relative to younger youth displayed more disinhibition toward socially appetitive than socially aversive stimuli, which was buffered by maternal presence. Furthermore, with age, maternal buffering in socially appetitive contexts elicited heightened activation in the ventromedial PFC, and amygdala-prefrontal connectivity. Findings underscore the importance of caregivers in promoting the neural regulation of their offspring during adolescence.
Keywords: fMRI, maternal buffering, regulation, adolescence
Adolescence is characterized by a series of psychobiological changes that coincide with a re-orientation towards social cues as individuals navigate new developmental challenges and goals (Blakemore & Mills, 2014; Crone & Dahl, 2012; Nelson, Jarcho, & Guyer, 2016). Socially appetitive contexts, defined as environments with positive social cues, elicit approach behavior in adolescents given their sensitivity toward rewarding cues (Doremus-Fitzwater, Varlinskaya, & Spear, 2010; Spear, 2012), which can promote developmental goals and prevents negative social interactions. Although approach behaviors can be beneficial during adolescence (e.g., gaining social status, creating new friendships), they can also come at a cost, by orientating youth toward risky contexts, distracting them from educational and occupational goals, and increasing susceptibility to peer pressure to engage in substance use and risky sexual behaviors (Steinberg, 2008). Indeed, adolescents show compromised inhibition in the presence of socially appetitive cues relative to socially aversive cues (Somerville, Jones, & Casey, 2010), such as happy facial expressions (Hare et al., 2008; Somerville, Hare, & Casey, 2011) and positive youth interactions (Perino, Miernicki, & Telzer, 2016), and engage in greater risk taking in the presence of peers (King, McLaughlin, Silk, & Monahan, 2017). Given that adolescents exhibit poorer inhibition in the presence of appetitive stimuli compared to aversive stimuli (Breiner et al., 2018; Perino et al., 2016; Somerville et al., 2011), adolescents may require greater regulation to successfully inhibit actions in appetitive social contexts. This developmental shift in emotion regulation is thought to arise due to altered activation in regulatory (e.g., medial prefrontal cortex (mPFC), and affective (e.g., amygdala, ventral striatum) neural regions (Lee et al., 2018; Somerville et al., 2010). Thus, in the current study, we sought to investigate the neurobiological and social factors that buffer adolescents from dysregulation within social contexts.
Social buffering is the process by which the presence of sensitive parental figures buffer youth from emotional dysregulation (Gee et al., 2013). Effective social buffering occurs via modulated neurobiological processes (Gunnar, Hostinar, Sanchez, Tottenham, & Sullivan, 2015; Hostinar & Gunnar, 2015; Schriber & Guyer, 2016), such that parental presence buffers youth from behavioral disinhibition by promoting neural regulation via the mPFC (e.g., Gee et al., 2014; Schriber et al., 2018). For example, the physical presence of mothers redirects adolescent risk-taking behavior toward safer decision-making (Telzer, Ichien, & Qu, 2015), which occurs via striatum-mPFC coupling (Guassi Moreira & Telzer, 2018). Similarly, the presence of maternal stimuli supports more effective regulation in children via amygdala-mPFC coupling (Gee et al., 2014; Tottenham, 2015). The mPFC is a brain region implicated in detecting social context cues (Dumontheil, Hillebrandt, Apperly, & Blakemore, 2012), social motivation (Somerville, Jones, Ruberry, & Dyke, 2014), and emotion regulation (Etkin, Egner, & Kalisch, 2011). Together, this research underscores the mPFC as a key brain region for effective social buffering via down regulating activation in affective brain regions (e.g. the amygdala and ventral striatum).
Despite the purported decline in the need for maternal buffering in adolescence (Gee et al., 2013; Gunnar et al., 2015), parents remain a highly important source of support for adolescents. Specifically, supportive parent-child relationships and parental involvement are pivotal processes in promoting self-regulation across childhood and adolescence (Grolnick & Kurowski, 1999), with empirical work corroborating the link between these family processes and academic engagement (Grolnick & Slowiaczek, 1994; Melby, Conger, Fang, Wickrama, & Conger, 2008), and overall physical health (Farrell, Simpson, Carlson, Englund, & Sung, 2016) across adolescence. Furthermore, parents buffer adolescents from associating with deviant peers and engaging in substance use (Van Ryzin, Fosco, & Dishion, 2012), and buffer against negative changes in adolescent depression and risk-taking behavior (Qu, Fuligni, Galvan, & Telzer, 2015; Wang, Hill, & Hofkens, 2014). Although adolescents navigate different social challenges as compared to children, parents continue to provide an important influence on their children as they develop across adolescence (for a review, Telzer, van Hoorn, Rogers, & Do, 2018).
In the current study, we investigated age differences across children and adolescents in their inhibition in appetitive and aversive social contexts, and whether maternal presence served as an effective social buffer for adolescents’ dysregulation. Similar to previous findings examining adolescent emotional dysregulation (e.g., Perino et al., 2016), we expected that adolescents would exhibit more disinhibition toward socially appetitive compared to socially aversive stimuli, and that this effect that would not be present in younger participants. We also hypothesized that adolescents would benefit from the presence of their mother, replicating prior effects on maternal buffering in the risk taking literature (e.g., Telzer, Ichien, & Qu, 2015). We examined whether maternal buffering of adolescents’ disinhibition in socially appetitive contexts would occur via heightened activation in brain regions associated with social motivation and regulation (i.e., mPFC; Guassi Moreira & Telzer, 2018; Telzer et al., 2015), as well connectivity between the mPFC and affective regions (i.e., amygdala, ventral striatum; Gee et al., 2014).
Methods
Participants
Participants included 54 youth (Mage = 13.38 years, range = 8.10–16.54; 29 females) who were recruited from a small midwestern community via flyers, word of mouth, and a pool of participants from prior research studies. An additional male participated but was excluded due to excessive head movement (> 2.0 mm interslice movement on ≥10% of slices). Inclusion criteria required that participants were free from MRI contraindications, learning disabilities, clinical diagnoses, and the use of neurological-altering medications. The majority of the sample self-identified as European-American (75.9%), with 13% multiethnic, 3.7% Latin-American, 3.7% African-American, and 3.7% Asian-American participants. Annual family incomes were distributed such that 9.2% earned <$29,000, 24.1% $30,000-$59,000, 11.1% $60,000-$89,000, 29.6% $90,000-$119,000, and 18.6% >$120,000, with 7.4% of parents providing no response. The highest reported maternal and paternal education was: 3.7 % < high school diploma, 5.6% high school diploma, 5.6% some college, 11.1% associate’s degree, 24% bachelor’s degree, 37% master’s degree, and 9.3% professional degree (e.g., M.D., Ph.D.), with 3.7% of parents opting to not report. Informed consent/assent was obtained for all participants in accordance with the University’s Institutional Review Board.
Social Go-NoGo Task
During an fMRI scan, participants completed a social go-nogo task, which couples salient socioemotional stimuli with a cognitive go-nogo task (Perino et al., 2016). We chose a go-nogo task because it allowed us to reliably measure successful task performance (i.e., inhibition), and thus, assess emotion regulation as participants performed the go-nogo task in both socially appetitive and socially aversive contexts. Participants were presented with an image for 300 ms, which included either socially appetitive (e.g., social acceptance and celebration) or socially aversive (e.g., bullying and victimization) scenes. Next, a letter was overlaid on the scene for 500 ms. Participants were instructed to press a button as quickly as possible when the letter appeared (go trial), but to withhold the button press if there was an X (nogo trial). 72% of trials were ‘go’ and 28% were ‘nogo’, thereby producing a prepotent response to press on go trials. Jitters between trials averaged 1200 ms. Across two runs, participants completed eight blocks, including four socially appetitive blocks (Figure 1a) and four socially aversive blocks (Figure 1b). Each block included 25 trials, and block order was randomized for each participant, totaling 100 trials each for the socially appetitive and socially aversive blocks. The social stimuli were balanced in the number of photos of children and adolescents to avoid effects specific to participant inclusion of one of these age groups. Equivalence between appetitive and aversive blocks were validated on valence, physiological impact, and social impact (see Perino et al., 2016).
Figure 1.
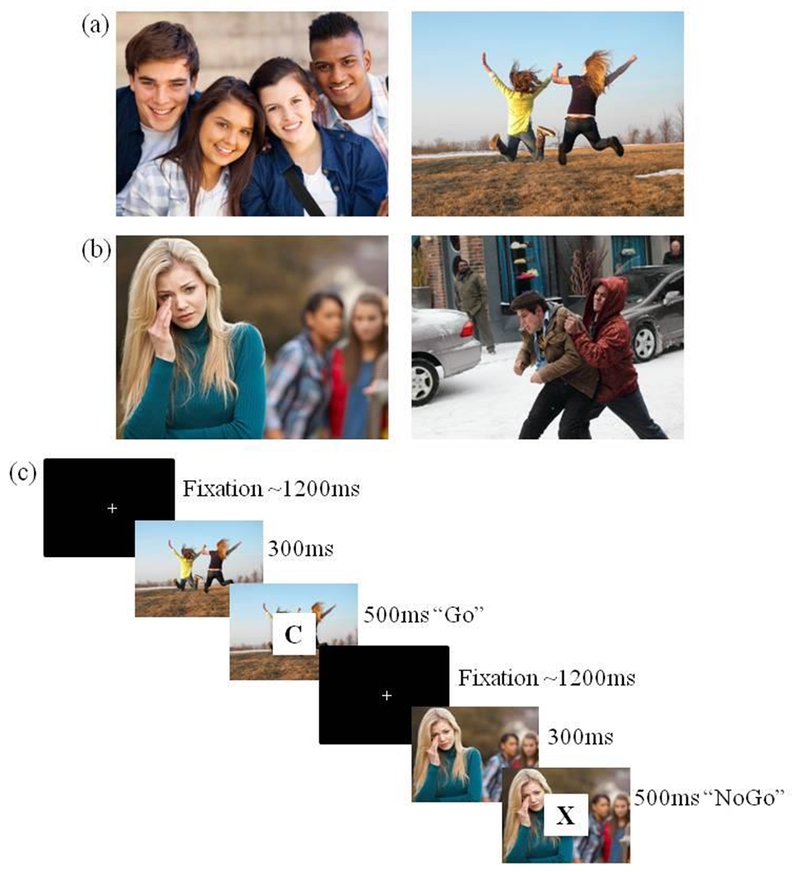
The Social Go-NoGo Task. Examples of the socially appetitive and socially aversive stimuli are shown in panels (a) and (b), respectively. Panel (c) displays the sequence and timing of the social go-nogo task.
Participants completed two runs of the task. In the alone condition, participants were told that “nobody will be watching you”. Consistent with other studies examining maternal buffering (e.g., Telzer, Ichien, & Qu, 2015), in the maternal present condition, participants were told that their mother was coming into the scan room and would be watching them play. Their mother then spoke into the microphone and read the following script verbatim, “Hi [child’s name], I’m here and I just wanted to let you know that I’m looking at these pictures with you!” These two conditions were counterbalanced across participants. We measured behavioral performance across the affective (appetitive and aversive) and maternal presence (alone and mother present) conditions. At both the behavioral and neural level, our variable of interest was successful task performance at the trial-level, which was defined as correctly pressing (on go trials) or correctly inhibiting (on no-go trials) button presses versus incorrect responses on each trial. Hereafter, successful task performance will be referred to as inhibition with the acknowledgement that this construct also embodies cognition beyond inhibition.
Behavioral Analysis
We conducted a multilevel model to test for age differences in the effect of affect and maternal presence on correct responses during the task using HLM 7 (Raudenbush, Bryk, & Congdon, 2010). Trials (Level 1) were nested within participants (Level 2), with the outcome variable defined as correct versus incorrect response on each trial. Level 1 covariates included trial type (go versus nogo trial) to control for whether each trial required a go or nogo response. The model included age as a level 2 factor and two level 1 factors of interest, including the affective and maternal presence conditions. The multilevel model was estimated using the following equations:
| Level-1 Model |
| Level-2 Model |
Successful task performance (i.e., CorrectResponse; 1=correct response, 0=incorrect response) on a particular trial (i) for a particular participant (j) was modeled as a function of the average number of correct responses across the task (β0j) and the control variable, which was whether the current trial was a go versus a nogo trial (β1j; 1=go trial, 0=nogo trial). The variables of interest included the maternal presence condition (β2j, 1=maternal presence, 0=alone), the affective condition (β3j, 1=appetitive, 0=aversive), and the interaction between maternal presence and affective conditions (β4j). All of these variables were modeled as random effects, with continuous age entered as a cross-level interaction term, except the go trial control variable, which was modeled as a fixed effect.
fMRI Acquisition
Brain images were collected using a research dedicated 3T Siemens Trio MRI scanner. The social go⎼nogo task was presented on a computer screen and projected through a mirror. A high⎼resolution T2*-weighted structural matched⎼bandwidth, anatomical scan (TR = 4000ms; TE = 64ms; matrix = 192 × 192; FOV = 230mm; slice thickness = 3mm; 38 slices) and a T1* magnetization prepared rapid acquisition gradient echo (MPRAGE; TR = 1900ms; TE = 2.3ms; matrix = 256 × 256; FOV = 230mm; sagittal plane; slice thickness = 1mm; 192 slices) were acquired. Both affective conditions of the social go-nogo task included 120 high resolution T2* weighted echo-planar images (EPIs; TR = 2000ms; TE = 25ms; matrix = 92 × 92; FOV = 230mm; slice thickness = 3mm; 38 slices; voxel size = 3 × 3 × 3 mm3). The orientation for the EPI and T2 anatomical scans were oblique axial to optimize brain coverage and to reduce noise.
fMRI Data Preprocessing and Analysis
fMRI data was analyzed using Statistical Parametric Mapping software (SPM8; Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK). Functional images were spatially realigned to correct for head motion, and only participants whose absolute slice-to-slice motion was less than 2mm in all directions on 90% or more slices were included. These images were coregistered with the MRPGAGE, segmented into cerebrospinal fluid, gray matter, and white matter, and then normalized into standard sterotactic space as defined by the Montreal Neurological Institute. Smoothing was performed with a FWHM 8mm Gaussian kernel to maximize signal-to-noise ratio, and a high-pass temporal filter of 128s was applied to eliminate low-frequency drift across the time series. Finally, we estimated autocorrelations with a REML algorithm with an autoregressive model order of 1.
At the individual level, a fixed-effects analysis was modeled for each of the 4 conditions of interest, including the 2 affective conditions (appetitive and aversive) and 2 maternal presence conditions (alone and mother present). The task was modeled as an event-related design, such that each trial was modeled individually with a duration of 800ms, and the inter-trial jitter null events were not explicitly modeled. We created a parametric modulator (PM) at the trial-level to assess neural regions that were recruited that differentiated successful task completion from behavioral disruption across the conditions. The PM was modeled such that 1 = correct response (i.e., correct hit, correct inhibition), and 0 = incorrect response (i.e., false alarm, failed hit). We used this PM in our analysis to identify neural regions which tracked successful task performance across the conditions (appetitive, aversive, mother present, alone) of the task, and therefore, captured the neural correlates of successful inhibition relative to failed inhibition. We conducted general linear modeling to obtain parameter estimates, which were used to create linear contrasts at the group level.
Random effects, whole brain analyses were modeled at the group level to examine age differences in maternal buffering (mother present versus alone) on neural activation and neural connectivity. For the connectivity analyses, we conducted psychophysiological interaction (PPI) analyses to examine functional connectivity using a generalized form of the context-dependent PPI (gPPI; McLaren, Ries, Xu, & Johnson, 2012). Our seed regions included affective brain regions (i.e., amygdala, ventral striatum) based on previous studies which identified connectivity within these regions and the mPFC during maternal buffering (Gee et al., 2014; Guassi Moreira & Telzer, 2018). The gPPI toolbox involves a 3-step process: (1) the deconvolved times series was extracted from the seed regions for each participant to create the physiological variable, (2) the psychological regressor was created by convolving each trial type with the canonical HRF, and (3) the time series from the physiological variable and psychological regressor were multiplied to create the PPI interaction terms, which identified regions that covaried with the ventral striatum and amygdala in a task-dependent manner.
We conducted a small volume correction to examine activity within the mPFC to improve specificity and reduce the Type 1 error rate, compared to a whole-brain analysis (e.g., Perino et al., 2016; Telzer, Ichien, & Qu, 2015). We conducted Monte Carlo simulations to correct for multiple comparisons using 3dClustSim in the AFNI software package (Ward, 2000; updated April 2016), and used an anatomical mask of the mPFC for small volume correction. The mPFC mask was defined using an automated meta-analysis mask of the mPFC via Neurosynth (http://neurosynth.org/analyses/terms/mpfc/), which included 1,647 voxels. The mask was broad to encompass both ventral and dorsal portions of the mPFC. The Monte Carlo simulation yielded a voxel-wise threshold of p < .005 and a minimum cluster size of 24 voxels for neural activation and 25 voxels for connectivity analyses within the mPFC, corresponding to p < .05, False Wise Error (FWE) corrected. Correction for whole-brain was also applied, and these results are presented in the tables. All reported results and mPFC mask are available on NeuroVault (Gorgolewski et al., 2015; see /collections/ANPRKZMZ/).
Results
Behavioral Results
We first tested for age differences in the effect of affect (socially appetitive versus socially aversive) and maternal presence (mother present versus alone) on correct responses during the task. As shown in Table 1, there was a significant age x affect interaction, an age x maternal presence interaction, and an age x affect x maternal presence interaction. For exploratory purposes to unpack this 3-way interaction, we divided the sample into pre-teens (8-12 years old) and teens (13-16 years old), and ran two separate models with the same level 1 equations as those described in the methods but excluding age from level 2. The coefficients from these models were used for descriptive purposes to illustrate the rate of correct inhibitory responses by age, in the affective and maternal presence conditions. As shown in Figure 2, teens exhibited significantly more errors in the socially appetitive condition compared to the socially aversive condition (b = −.254, SE = .085, p = .006) whereas pre-teens did not show a difference in performance in response to affective conditions (b =−.007, SE = .074, p = .924). In addition, teens’ disinhibition during the appetitive condition was buffered in the presence of mothers compared to alone (b = .295, SE = .095, p = .004), an effect pre-teens did not display (b = .083, SE = .127, p = .518). These results suggest that adolescents process social stimuli differentially depending on the affective valence (i.e., appetitive vs. aversive) and context (i.e., alone vs. mother present), and that maternal presence buffers adolescence from emotion regulation difficulties, whereas pre-teens did not significantly differ in inhibition across the affective conditions and did not display a social buffering effect.
Table 1.
Within and between person associations between correct responses and variables of interest
| Fixed Effect | b (SE) |
|---|---|
| Intercept | .825 (.094)*** |
| Age | .118 (.029)*** |
| Go Trials | 1.780 (.111)*** |
| Mom Condition | .065 (.069)ns |
| Mom X Age | −.080 (.032)* |
| Appetitive Condition | −.151 (.056)** |
| Appetitive X Age | −.056 (.019)** |
| Mom X Appetitive | .142 (.074)ns |
| Mom X Appetitive X Age | .105 (.027)*** |
Note: Age was a continuous between-individual variable centered around the grand mean. All other variables were within-individual. In addition, the within-individual variables were dummy coded such that the presence of the variable = 1, whereas the absence of the variable = 0.
p < .05.
p < .01.
= nonsignificant value.
Figure 2.
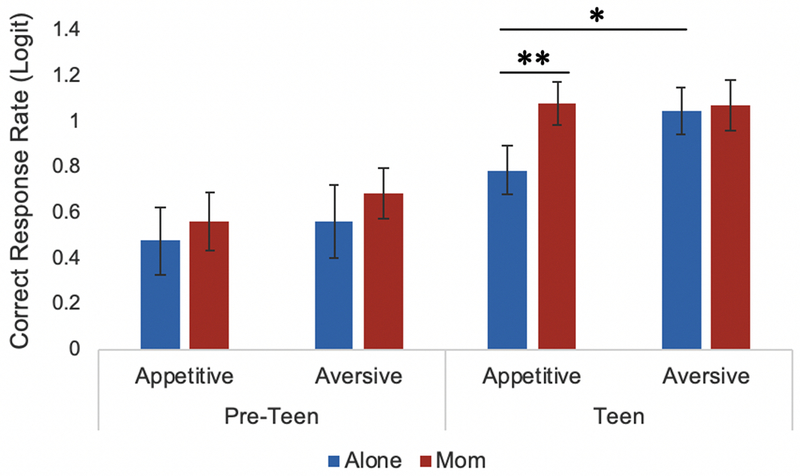
Older participants exhibited significantly more errors toward socially appetitive stimuli than socially aversive stimuli when completing the social go-nogo task alone. Older participants also displayed significantly more errors toward socially appetitive stimuli when completing the task alone, compared to completing the task during maternal presence. ** p < .05, * p < .01.
Neuroimaging Results
At the neural level, we conducted whole brain regression analyses with continuous age as the regressor on the contrast maternal presence > alone in the appetitive and aversive conditions, separately. Paralleling the behavioral model, we included the PM, which identified neural regions that differentiated successful task completion from disinhibition. We found a significant correlation with age in the vmPFC during the socially appetitive condition. Given that the PM identified neural regions based on successful task performance, this analysis suggests that older relative to younger participants exhibited greater vmPFC activation when they responded correctly in the appetitive social context in the presence of their mothers compared to alone (Figure 3a). For descriptive purposes, we extracted parameter estimates of signal intensity from the vmPFC cluster and plotted the estimates by age groups (pre-teens: 8-12 years; teens: 13-16 years; Figure 3b). In the maternal presence relative to alone condition, greater vmPFC activation was recruited to successfully perform the task in the appetitive condition for older participants (for whom maternal buffering also occurred behaviorally), suggesting social buffering occurs via modulation of the vmPFC. In addition, the dACC, fusiform, and temporal pole were each associated with age when individuals responded correctly in the appetitive social context in the maternal presence condition, compared to alone. In the aversive condition, age was not correlated with any regions of interest (see Table 2 for a list of all regions identified in the whole-brain analyses).
Figure 3.
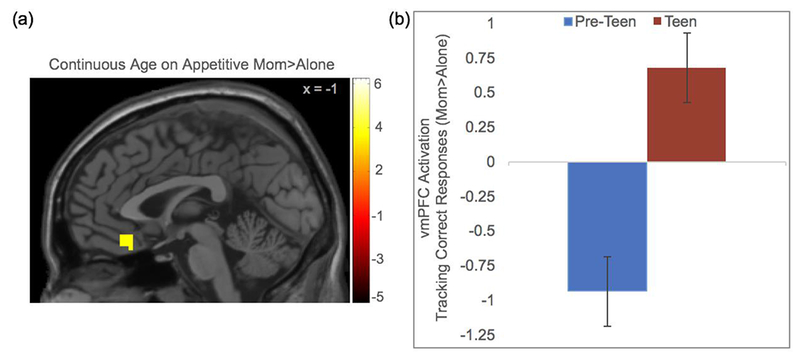
(a) Continuous age was associated with more activation in the vmPFC that tracked correct task performance toward socially appetitive stimuli during maternal presence compared to alone. (b) For descriptive purposes only, parameter estimates of signal intensity were extracted from the vmPFC and plotted separately for younger and older participants.
Table 2.
Neural regions that differed across age during correct responses in the alone compared to the maternal presence condition, separately for appetitive and aversive social stimuli
| Anatomical Region | x | y | z | t | k |
|---|---|---|---|---|---|
| Appetitive mom > alone | |||||
| vmPFC | 3 | 29 | ⎼23 | 3.65 | 81 |
| dACC | ⎼15 | 11 | 40 | 5.20 | 2992a |
| R Middle Frontal Gyrus | 30 | ⎼4 | 61 | 6.02 | a |
| L Precentral Gyrus | ⎼33 | ⎼7 | 58 | 5.29 | a |
| R Fusiform Gyrus | 24 | ⎼7 | ⎼38 | 4.09 | 119b |
| R Temporal Pole | 39 | 14 | ⎼32 | 3.22 | b |
| R Cerebellum (VIII) | 3 | ⎼76 | ⎼41 | 3.94 | 325c |
| L Cerebellum (IX) | ⎼12 | ⎼61 | ⎼41 | 3.88 | c |
| Aversive mom > alone | |||||
| R Calcarine Gyrus | 18 | ⎼73 | 22 | 3.75 | 329d |
| R Cuneus | ⎼12 | ⎼70 | 34 | 3.48 | d |
| PPI (amygdala seed): Appetitive mom > alone | |||||
| mPFC | ⎼12 | 41 | 7 | 3.65 | 22 |
| R Inferior Parietal Lobule | 42 | ⎼58 | 49 | 3.77 | 75 |
Note: L and R refer to left and right hemispheres; k refers to the number of voxels in each significant cluster; t refers to peak activation level in each cluster; and x, y, and z refer to MNI coordinates. Regions that share the same superscript are part of the same cluster. The mPFC was small volume corrected, which yielded a minimum cluster size of 20 for neural activation and 18 for coactivation (PPI). All other regions were based on a whole-brain mask, which yielded a minimum cluster size of 116 voxels for neural activation and 74 voxels for coactivation (PPI). All regions are significant at p < .005.
Next, we ran whole brain regression analyses with continuous age on functional connectivity with our a priori seed regions (amygdala, ventral striatum) on the contrast maternal presence > alone in the appetitive and aversive conditions separately. We found a significant correlation with age and mPFC-amygdala coupling, such that older adolescents showed greater mPFC-amygdala coupling during correct responses in the presence of their mother compared to alone (Figure 4). Although the parameter estimates are not visually displayed given the complex interplay between age, maternal presence condition, affective condition, the PM, and coactivation, the findings suggest that with age, individuals show more connectivity between the mPFC and amygdala in the presence of their mom, compared to alone, as they correctly respond toward appetitive cues. Age was not correlated with mPFC connectivity with the amygdala in the aversive condition, nor with the ventral striatum in the appetitive or aversive conditions. See Table 2 for a list of all regions identified in the PPI whole-brain analyses.
Figure 4.
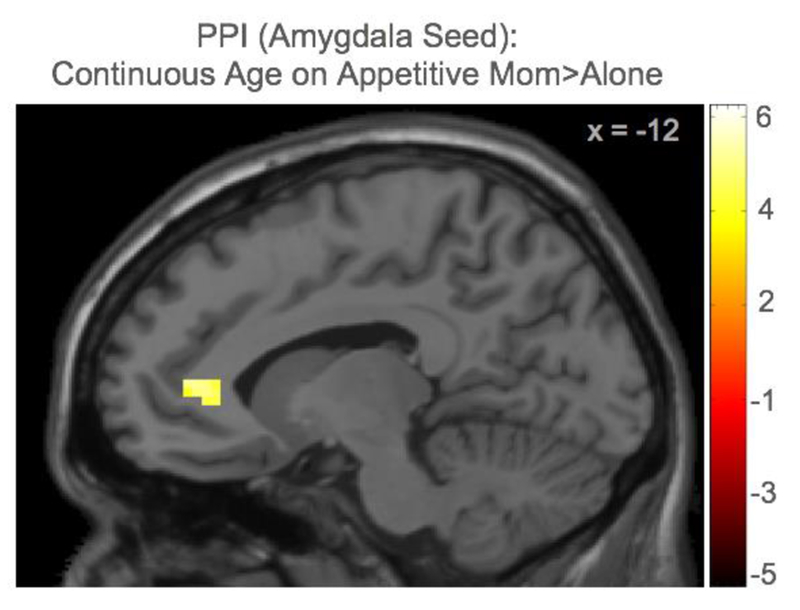
The PPI analyses showed that continuous age was associated with coactivation between the mPFC and amygdala toward socially appetitive stimuli during maternal presence compared to alone.
Discussion
The majority of social buffering research has focused on reward sensitivity and risk taking during adolescence (Guassi Moreira & Telzer, 2018; Telzer et al., 2015), or on early life stress (e.g., Gee et al., 2014), leaving maternal buffering on the neural correlates of emotion regulation across development a relatively unstudied topic. Adolescents’ inhibition is dependent on situational factors, as evidenced by decrements in socially appetitive contexts (Perino et al., 2016; Somerville et al., 2011). Given the protective effect caregivers have on youths’ psychosocial adjustment across adolescence (e.g., Bowes, Maughan, Caspi, Moffitt, & Arseneault, 2010; Gutman & Eccles, 2007; Sapouna & Wolke, 2013), we examined the role of maternal buffering on adolescents’ emotion regulation in appetitive and aversive contexts. Our findings highlight adolescence as a sensitive period for emotion regulation difficulties in positive social contexts, which is amenable to social buffering effects via modulation of the mPFC.
Our findings indicate that socially appetitive contexts disrupt adolescents’ inhibitory performance, which was operationalized based on successful task performance, despite its inclusion of other cognitive processes. Older adolescents performed significantly worse in response to socially appetitive stimuli compared to socially aversive stimuli, whereas younger participants did not demonstrate this disparity. These findings are consistent with developmental theory suggesting that adolescents are neurobiologically sensitive to positive social cues to help them obtain social status and navigate novel social environments (Kuhn, 2006; Telzer, van Hoorn, Rogers, & Do, 2018), as well as empirical work indicating that adolescents experience disinhibition in socially appetitive contexts (Hare et al., 2008; Somerville et al., 2011). While adolescents were more susceptible to disinhibition to appetitive stimuli, we found that this emotional dysregulation was completely buffered in the presence of their mothers. This finding suggests that maternal presence can boost adolescents’ inhibition in socially appetitive contexts where they may be particularly susceptible to emotional dysregulation. This is consistent with prior work showing that maternal presence buffers adolescents’ risk-taking behaviors (Guassi Moreira & Telzer, 2018), but differs from previous work showing effective maternal buffering is specific to children, but not adolescents, in emotional contexts (Gee et al., 2014). One possibility for the discrepancy between studies includes the nature of the go-nogo tasks, such that our task utilized social cues that are particularly salient, such as appetitive and aversive social scenes of same-aged peers, whereas prior work used images of adult woman faces (e.g., happy and sad expressions, Gee et al., 2014). Although maternal buffering during childhood has been shown to promote regulation for basic emotions (i.e., sadness, anger, fear; Gee et al., 2014), adolescents have developed competence discriminating these emotions (Tottenham et al., 2011) and making decisions in more simple contexts (e.g., unknown adult expressing a high valence facial expression; Steinberg, 2005). However, given that adolescents are sensitive to dynamic social contexts, such as interacting with high status peers and risk taking alongside friends (Albert, Chein, & Steinberg, 2013; Telzer et al., 2017), maternal buffering during adolescence may be specific to promoting adolescent regulation in these more complex and socially salient contexts, underscoring adolescence as a time of opportunity for receiving maternal support.
At the neural level, we found that maternal presence boosted activation in the vmPFC when older adolescents made correct relative to incorrect responses in the appetitive social context. Given that the vmPFC is associated with social influence (Welborn et al., 2015) valuation (Etkin et al., 2011; Hare, Camerer, & Rangel, 2009), and reward processing (for a review, Sescousse, Caldú, Segura, & Dreher, 2013), this finding suggests that maternal presence may increase the value of performance goals, and in kind the rewarding nature of performing well, in appetitive contexts for older youth relative to younger youth. Furthermore, the orbitofrontal cortex, a region proximal to the vmPFC, is associated with valuation and reward processing (Van Leijenhorst et al., 2010) and has been implicated in adolescent relational reasoning during peer observation, compared to adults (Dumontheil, Wolf, & Blakemore, 2016). The vmPFC may play a similar role as the orbitofrontal cortex on adolescent emotion regulation, such that these neural regions promote better adolescent inhibition during social monitoring.
Although not primary regions of interest, we also found that older participants recruited several other regions during successful inhibition toward appetitive contexts in the presence of their mother, including the dACC, fusiform, and temporal pole. The dACC is associated with social stress (Schriber et al., 2018), suggesting that the monitoring of a caregiver may induce social motivation to improve performance during adolescence. The fusiform gyrus has been proposed to detect salient social information (Nelson, Leibenluft, McClure, & Pine, 2005), and is implicated during adolescent decision-making in the presence of mothers and peers (Guassi Moreira & Telzer, 2018; van Hoorn, McCormick, Rogers, Ivory, & Telzer, 2018). The fusiform gyrus may be integral for adolescent decision-making in the context of salient social others, particularly for aligning performance toward the observing entity’s desires (i.e., accuracy for mothers, risk taking for risky peers). Older participants also showed increased activation in the temporal pole, which has been associated with social processing, mentalizing, and emotional competence (Lieberman et al., 2007; Telzer et al., 2014). Together, these findings indicate that caregiver monitoring elicits recruitment of brain regions involved in valuation, detection, and social processing during successful inhibitory performance, which is consistent with adolescence as a developmental period of neurobiological sensitivity to social context (Schriber & Guyer, 2016).
We also found that heightened mPFC-amygdala connectivity was related to older adolescents’ emotion regulation toward socially appetitive contexts in the presence of their mother. The connectivity results revealed a more dorsal subregion of the mPFC, which has been implicated in self-referencing within social contexts (Yamawaki et al., 2017) and being observed (Somerville et al., 2014). In addition, the dorsal subregion of the mPFC has been associated with successful emotional regulation (Etkin et al., 2011), particularly as it coactivates with the amygdala (Banks, Eddy, Angstadt, Nathan, & Luan Phan, 2007; Morawetz, Bode, Baudewig, & Heekeren, 2017), and is the identical subregion of the mPFC identified in prior work on amygdala-mPFC connectivity during maternal buffering (Gee et al., 2014). Thus, caregivers may promote recruitment of mPFC to down-regulate affective processing of the amygdala in order to regulate their inhibition within socially appetitive contexts. Our findings are consistent with theories suggesting that adolescence is a period of neurobiological flexibility and potential (Crone & Dahl, 2012; Telzer, van Hoorn, Rogers, & Do, 2018), such that neural plasticity in the adolescent brain is amenable to caregivers’ influence for modulating neural activation and regulation. Of note, the neuroimaging analyses provide novel, but low powered, findings, which should be interpreted with caution and utilized to propel future work investigating the neurobiological underpinnings of maternal buffering during adolescence.
The limitations of this study are noteworthy for future investigations on maternal influences on the neurobiology of adolescent inhibition in affectively salient social contexts. First, the social images used in the go-nogo task may have affected participant behavioral responses differentially given that appetitive stimuli can activate an approach mindset (Keefer & Petrovich, 2017) whereas aversive stimuli can trigger a withdrawal response (Boeke, Moscarello, LeDoux, Phelps, & Hartley, 2017). Prior research examining behavioral responses to positive and negative valence social stimuli have been shown to be different (e.g., Tottenham, Hare, & Casey, 2011), so future examinations should utilize paradigms that can better account for the disruptive effects of social cues that are not confounded by task design. Furthermore, cognitive performance was always measured in an affective context, and thus, future work would benefit from utilizing a non-social context to better distinguish the processes of emotion regulation, attention, and cognitive control. Second, although the pattern of results shown in this study suggest that maternal presence predicts differences in the neural correlates of emotion regulation across age, future research in this area should conduct longitudinal analyses such as latent growth curve modeling (Grimm & Ram, 2012) and cross lagged panel analyses (Selig & Little, 2012) that can finely detect developmental shifts in neural processes across childhood and adolescence. In addition, future research should investigate whether these findings are specific to maternal buffering, or whether these findings are universal across social actors, such as the presence of another adult (e.g., Guassi Moreira & Telzer, 2018). Third, the sample size constrained the power of the analyses. Although we used multi-level analyses at the behavioral level, and our neural analyses examined trial-level parametric differences in brain activation, both of which increase power due to taking advantage of all trials in the task, these findings should be interpreted with caution and replicated for reliability. In addition, the sample size limited the ability to test sex differences in brain and behavior, which provides an opportunity for future approaches to examine how individual differences may explain how adolescents differentially respond to affective stimuli (Blakemore, 2018). Normatively developing adolescents may respond to affective stimuli differently than other populations, such as those experiencing high stress (Gee et al., 2014), highlighting the need for more thorough investigations on the variation in responses to affective stimuli, and the mechanisms that account for such variations.
Together, our findings highlight adolescence as a period of sensitivity toward appetitive social contexts, but also as a time of opportunity for maternal buffering in supporting emotion regulation. Furthermore, our findings provide support for maternal presence as a protective factor on the neurobiology of adolescent emotion regulation in socially appetitive contexts. These findings inform our understanding of adolescence as both a developmental period of susceptibility in appetitive contexts, which might place youth at a greater risk for developing internalizing and externalizing symptoms, but also as a developmental period of social opportunity, as caregiver influence still matters and can boost adolescent emotion regulation in dynamic social contexts.
Acknowledgments
This research was supported by a grant to E.H.T. (R01DA039923) and generous funds from the Department of Psychology at the University of Illinois. M.T.P. was funded in part by the R01 and T32 grant (NIMH 2T32MH100019-06). We greatly appreciate the assistance of the Developmental Social Neuroscience Lab and the Biomedical Imaging Center at the University of Illinois. We also thank Nicholas Ichien and Inge Karosevica for collecting the data.
Footnotes
There are not any conflicts of interest to report.
References
- Albert D, Chein J, & Steinberg L (2013). Peer Influences on Adolescent Decision Making. Current Directions in Psychological Science, 22, 114–120. 10.1177/0963721412471347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, & Luan Phan K (2007). Amygdala-frontal connectivity during emotion regulation. Social Cognitive and Affective Neuroscience, 2, 303–312. 10.1093/scan/nsm029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S-J, & Mills KL (2014). Is adolescence a sensitive period for sociocultural processing? Annual Review of Psychology, 65, 187–207. 10.1146/annurev-psych-010213-115202 [DOI] [PubMed] [Google Scholar]
- Boeke EA, Moscarello JM, LeDoux JE, Phelps EA, & Hartley CA (2017). Active Avoidance: Neural Mechanisms and Attenuation of Pavlovian Conditioned Responding. The Journal of Neuroscience, 37, 4808–4818. 10.1523/JNEUROSCI.3261-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowes L, Maughan B, Caspi A, Moffitt TE, & Arseneault L (2010). Families promote emotional and behavioural resilience to bullying: Evidence of an environmental effect. Journal of Child Psychology and Psychiatry and Allied Disciplines, 51, 809–817. 10.1111/j.1469-7610.2010.02216.x [DOI] [PubMed] [Google Scholar]
- Breiner K, Li A, Cohen AO, Steinberg L, Bonnie RJ, Scott ES, … Adriana Galván C. (2018). Combined effects of peer presence, social cues, and rewards on cognitive control in adolescents. Developmental Psychobiology, 1–11. 10.1002/dev.21599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone EA, & Dahl RE (2012). Understanding adolescence as a period of social-affective engagement and goal flexibility. Nat Rev Neurosci, 13, 636–650. 10.1038/nrn3313 [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, & Spear LP (2010). Motivational systems in adolescence: possible implications for age differences in substance abuse and other risk-taking behaviors. Brain and Cognition, 72(1), 114–123. 10.1016/j.bandc.2009.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumontheil I, Hillebrandt H, Apperly I. a., & Blakemore S-J (2012). Developmental differences in the control of action selection by social information. Journal of Cognitive Neuroscience, 24, 2080–2095. 10.1162/jocn_a_00268 [DOI] [PubMed] [Google Scholar]
- Dumontheil I, Wolf LK, & Blakemore SJ (2016). Audience effects on the neural correlates of relational reasoning in adolescence. Neuropsychologia, 87, 85–95. 10.1016/j.neuropsychologia.2016.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, & Kalisch R (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences, 15, 85–93. 10.1016/j.tics.2010.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell AK, Simpson JA, Carlson EA, Englund MM, & Sung S (2016). The impact of stress at different life stages on physical health and the buffering effects of maternal sensitivity. Health Psychology, 36, 35–44. 10.1037/hea0000424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, … Tottenham N. (2013). Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc Natl Acad Sci U S A, 110(39), 15638–15643. 10.1073/pnas.1307893110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam L, Telzer EH, Humphreys KL, Goff B, Shapiro M, … Tottenham N. (2014). Maternal buffering of human amygdala-prefrontal circuitry during childhood but not during adolescence. Psychological Science, 25, 2067–2078. 10.1177/0956797614550878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm KJ, & Ram N (2012). Modeling change over time In Tennenbaum, Eklund R & Kamata A (Eds.), Measurement in sport and exercise psychology (pp. 65–74). Champaign, IL.: Human Kinetics. [Google Scholar]
- Grolnick W, & Kurowski C (1999). Family processes and the development of children’s self-regulation. Educational Psychologist, 34(1), 3–14. 10.1207/s15326985ep3401_1 [DOI] [Google Scholar]
- Grolnick WS, & Slowiaczek ML (1994). Parents’ involvement in children’s schooling: A multidimensional conceptualization and motivational model. Child Development, 65(1), 237–252. [DOI] [PubMed] [Google Scholar]
- Guassi Moreira JF, & Telzer EH (2018). Mother still knows best: Maternal influence uniquely modulates adolescent reward sensitivity during risk taking. Developmental Science, 21, 1–11. 10.1111/desc.12484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Hostinar CE, Sanchez MM, Tottenham N, & Sullivan RM (2015). Parental buffering of fear and stress neurobiology: Reviewing parallels across rodent, monkey, and human models. Social Neuroscience, 10, 474–478. 10.1126/science.1249098.Sleep [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman LM, & Eccles JS (2007). Stage-environment fit during adolescence: trajectories of family relations and adolescent outcomes. Developmental Psychology, 43, 522–537. 10.1037/0012-1649.43.2.522 [DOI] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, & Rangel A (2009). Self-control in decision-making involves modulation of the vmPFC valuation system. Science, 324, 646–648. [DOI] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, & Casey BJ (2008). Biological Substrates of Emotional Reactivity and Regulation in Adolescence During an Emotional Go-Nogo Task. Biological Psychiatry, 63, 927–934. 10.1016/j.biopsych.2008.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, & Gunnar MR (2015). Social support can buffer against stress and shape brain activity. AJOB Neuroscience, 6, 34–42. 10.1080/21507740.2015.1047054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefer SE, & Petrovich GD (2017). Distinct recruitment of basolateral amygdala-medial prefrontal cortex pathways across Pavlovian appetitive conditioning. Neurobiology of Learning and Memory, 141, 27–32. 10.1016/j.nlm.2017.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King KM, McLaughlin KA, Silk J, & Monahan KC (2017). Peer effects on self-regulation in adolescence depend on the nature and quality of the peer interaction. Development and Psychopathology, 1–13. 10.1017/S0954579417001560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn D (2006). do cognitive changes accompany developments in the adolescent brain? Perspectives in Psychological Science, 1, 59–67. [DOI] [PubMed] [Google Scholar]
- Lee NC, Weeda WD, Insel C, Somerville LH, Krabbendam L, & Huizinga M (2018). Neural substrates of the influence of emotional cues on cognitive control in risk-taking adolescents. Developmental Cognitive Neuroscience, 31, 20–34. 10.1016/j.dcn.2018.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, & Way BM (2007). Putting Feelings Into Words. Psychological Science, 18(5), 421–428. 10.1111/j.1467-9280.2007.01916.x [DOI] [PubMed] [Google Scholar]
- McLaren DG, Ries ML, Xu G, & Johnson SC (2012). A generalized form of context-dependent psychophysiological interactions (gPPI): A comparison to standard approaches. NeuroImage, 61, 1277–1286. 10.1016/j.neuroimage.2012.03.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melby JN, Conger RD, Fang S, Wickrama K a S., & Conger KJ. (2008). Adolescent family experiences and educational attainment during early adulthood. Developmental Psychology, 44, 1519–36. 10.1037/a0013352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawetz C, Bode S, Baudewig J, & Heekeren HR (2017). Effective amygdala-prefrontal connectivity predicts individual differences in successful emotion regulation. Social Cognitive and Affective Neuroscience, 12, 569–585. 10.1093/scan/nsw169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EE, Jarcho JM, & Guyer AE (2016). Social re-orientation and brain development: An expanded and updated view. Developmental Cognitive Neuroscience, 17, 118–127. 10.1016/j.dcn.2015.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure EB, & Pine DS (2005). The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine, 35, 163–174. 10.1017/s0033291704003915 [DOI] [PubMed] [Google Scholar]
- Perino MT, Miernicki ME, & Telzer EH (2016). Letting the good times roll: Adolescence as a period of reduced inhibition to appetitive social cues. Social Cognitive and Affective Neuroscience, 11, 1762–1771. 10.1093/scan/nsw096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y, Fuligni AJ, Galvan A, & Telzer EH (2015). Buffering effect of positive parent–child relationships on adolescent risk taking: A longitudinal neuroimaging investigation. Developmental Cognitive Neuroscience, 15, 26–34. 10.1016/j.dcn.2015.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapouna M, & Wolke D (2013). Resilience to bullying victimization: The role of individual, family and peer characteristics. Child Abuse and Neglect, 37, 997–1006. 10.1016/j.chiabu.2013.05.009 [DOI] [PubMed] [Google Scholar]
- Schriber RA, & Guyer AE (2016). Adolescent neurobiological susceptibility to social context. Developmental Cognitive Neuroscience, 19, 1–18. 10.1016/j.dcn.2015.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schriber RA, Rogers CR, Ferrer E, Conger RD, Robins RW, Hastings PD, & Guyer AE (2018). Do Hostile School Environments Promote Social Deviance by Shaping Neural Responses to Social Exclusion? Journal of Research on Adolescence, 28, 103–120. 10.1111/jora.12340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selig JP, & Little TD (2012). Chapter 16: Autoregressive and Cross-Lagged Panel Analysis for Longitudinal Data In Laursen B, Little TD, & Card NA (Eds.), Handbook of Developmental Research Methods. New York: Guilford Press. [Google Scholar]
- Sescousse G, Caldú X, Segura B, & Dreher JC (2013). Processing of primary and secondary rewards: A quantitative meta-analysis and review of human functional neuroimaging studies. Neuroscience and Biobehavioral Reviews, 37, 681–696. 10.1016/j.neubiorev.2013.02.002 [DOI] [PubMed] [Google Scholar]
- Somerville LH, Hare T, & Casey BJ (2011). Frontostriatal maturation predicts cognitive control failure of appetitive cues in adolescence. Journal of Cognitive Neuroscience, 23, 2123–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Jones RM, & Casey BJ (2010). A time of change: Behavioral and neural correlates of appetitive and aversive environmental cues. Brain and Cognition, 72, 124–133. 10.1016/j.bandc.2009.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Jones RM, Ruberry EJ, & Dyke JP (2014). Medial prefrontal cortex and the emergence of self-conscious emotion in adolescence. Psychological Science, 24, 1554–1562. 10.1177/0956797613475633.Medial [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP (2012). Rewards, aversions, and affect in adolescence: Emerging convergences across laboratory animal and human data. Developmental Cognitive Neuroscience, 1(4), 390–403. 10.1016/j.dcn.2011.08.001.Rewards [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L (2005). Cognitive and affective development in adolescence. Trends Cogn Sci, 9, 69–74. 10.1016/j.tics.2004.12.005 [DOI] [PubMed] [Google Scholar]
- Steinberg L (2008). A social neuroscience perspective on adolescent risk-taking. Developmental Review, 28, 78–106. 10.1016/j.dr.2007.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Ichien N, & Qu Y (2015). The ties that bind: Group membership shapes the neural correlates of in-group favoritism. NeuroImage, 115, 42–51. 10.1016/j.neuroimage.2015.04.035 [DOI] [PubMed] [Google Scholar]
- Telzer EH, Qu Y, Goldenberg D, Fuligni AJ, Galván A, & Lieberman MD (2014). Adolescents’ emotional competence is associated with parents’ neural sensitivity to emotions. Frontiers in Human Neuroscience, 8(July), 558 10.3389/fnhum.2014.00558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Rogers CR, & van Hoorn J (2017). Neural correlates of social influence on risk taking and substance use in adolescents. Addictions Report, 4, 333–341. Retrieved from http://dsnlab.web.unc.edu/files/2017/08/Telzer-E.H.-Rogers-C.R.-Van-Hoorn-J.−2017-Addition-Reports.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, van Hoorn J, Rogers CR, & Do KT (2018). Social Influence on Positive Youth Development: A Developmental Neuroscience Perspective. In Advances in Child Development and Behavior (Vol. 54, pp. 215–258). 10.1016/bs.acdb.2017.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer E, Ichien NT, & Qu Y (2015). Mothers know best: Redirecting adolescent reward sensitivity toward safe behavior during risk taking. Social Cognitive and Affective Neuroscience, 10, 1383–1391. 10.1093/scan/nsv026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N (2015). Social scaffolding of human amygdala-mPFC circuit development. Social Neuroscience, 10, 489–499. 10.1016/j.pediatrneurol.2015.01.016.Pathophysiology [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, & Casey BJ (2011). Behavioral assessment of emotion discrimination, emotion regulation, and cognitive control in childhood, adolescence, and adulthood. Frontiers in Psychology, 2, 1–9. 10.3389/fpsyg.2011.00039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hoorn J, McCormick E, Rogers C, Ivory S, & Telzer E (2018). Differential effects of parent and peer presence on neural correlates of risk taking in adolescence. Social Cognitive and Affective Neuroscience, 1–7. 10.1093/scan/nsy024/4965846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leijenhorst L, Zanolie K, Van Meel CS, Westenberg PM, Rombouts SA, & Crone EA (2010). What motivates the adolescent? Brain regions mediating reward sensitivity across adolescence. Cerebral Cortex, 20, 61–69. 10.1093/cercor/bhp078 [DOI] [PubMed] [Google Scholar]
- Van Ryzin MJ, Fosco GM, & Dishion TJ (2012). Family and peer predictors of substance use from early adolescence to early adulthood: An 11-year prospective analysis. Addiction Behavior, 37, 1314–1324. 10.1038/jid.2014.371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M-T, Hill NE, & Hofkens T (2014). Parental involvement and African American and European American adolescents’ academic, behavioral, and emotional development in secondary school. Child Development, 85(6), 2151–2168. 10.1111/cdev.12284 [DOI] [PubMed] [Google Scholar]
- Welborn BL, Lieberman MD, Goldenberg D, Fuligni AJ, Galván A, & Telzer EH (2015). Neural mechanisms of social influence in adolescence. Social Cognitive and Affective Neuroscience, 11, 100–109. 10.1093/scan/nsv095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamawaki R, Nakamura K, Aso T, Shigemune Y, Fukuyama H, & Tsukiura T (2017). Remembering my friends: Medial prefrontal and hippocampal contributions to the self-reference effect on face memories in a social context. Human Brain Mapping, 38, 4256–4269. 10.1002/hbm.23662 [DOI] [PMC free article] [PubMed] [Google Scholar]


