Abstract
The medial prefrontal cortex (mPFC) coordinates goal-directed behaviors, which may be mediated through mPFC regulation of dopamine release in the nucleus accumbens (NAc). Furthermore, frequency-specific oscillatory activity between the frontal cortex and downstream structures may facilitate inter-region communication. Although high-frequency (e.g., 60 Hz) mPFC stimulation is known to increase basal dopamine levels in the NAc, little is known about how phasic dopamine release is affected by mPFC stimulation. Understanding the frequency-specific control of phasic dopamine release by mPFC stimulation could elucidate mechanisms by which the mPFC modulates other regions. It could also inform optimization of deep brain stimulation for treatment of neurological disorders.
Objective:
The goal of this work was to characterize the frequency response of NAc dopamine release resultant from mPFC stimulation. We hypothesized that the magnitude of dopamine release in the NAc would increase with increasing stimulation frequency.
Methods:
Electrical stimulation of the mPFC of anesthetized rats was delivered at 4 – 60 Hz and at varying durations while measuring NAc dopamine release with fast-scan cyclic voltammetry.
Results:
mPFC stimulation resulted in phasic dopamine release in the NAc. Furthermore, 20 Hz stimulation evoked the largest peak response for stimulation intervals > 5 seconds when compared to higher or lower frequencies.
Conclusions:
Activation of the mPFC drives dopamine release in the NAc in a complex frequency- and duration-dependent manner. This has implications for the use of deep brain stimulation treatment of disorders marked by dopaminergic dysregulation, and suggest that mPFC may exert more specialized control over neuromodulator release than previously understood.
Keywords: medial prefrontal cortex, nucleus accumbens, electrical stimulation, beta, fast-scan cyclic voltammetry, dopamine, deep brain stimulation
Introduction
Dopamine release in the nucleus accumbens (NAc) facilitates critical components of learning and decision making such as motivation, reward valuation, and action selection (for review see [1,2]). Still, little is known about top-down modulatory (cortical) control of dopamine release in the NAc. Dysfunctional cortical control of dopamine release is implicated in many disorders such as depression [3,4], chronic pain [5], and addiction [6,7]. Specifically, the medial prefrontal cortex (mPFC) is often implicated in regulation of mesolimbic activity. The mPFC sends direct glutamatergic projections to the NAc and to the ventral tegmental area (VTA) [8,9]. These projections are involved in goal-directed behaviors and learning [10–13]. While early tracing studies in rats suggested that mPFC efferents to the VTA do not synapse onto cells that then project to the NAc [9], recent evidence indicates that these projections do indeed synapse onto NAc-projecting dopaminergic cells [10]. Further, inactivation of mPFC neurons with tetrodotoxin decreases tonic dopamine levels in the NAc [14]. Electrophysiological evidence also suggests a role of the mPFC in the activation of meso-accumbens-projecting VTA neurons [15] and functional connections have been illustrated through electrical [16–18] and pharmacological [14,19] stimulation of the mPFC.
The potential of the mPFC to regulate dopamine release is also supported by the successful application of deep brain stimulation (DBS) in various prefrontal sub-regions for the treatment of disorders associated with dysregulation of the dopaminergic system. For example, DBS of the mPFC is used clinically for treatment-resistant depression and intractable chronic pain [20,21]. Despite its success, the mechanisms by which DBS in the mPFC exerts its clinical benefit remain poorly understood. While Rea et al. suggest that the mesolimbic dopamine pathway is not engaged during DBS [22], Bruchim-Samuel et al. have shown improvement in depressive behaviors following electrical stimulation of ventral mPFC that may be due to VTA activation [23]. Similar techniques have shown a clear connection between the activation of dopamine neurons and anti-depressive effects. For example, optogenetic stimulation of tyrosine hydroxylase-expressing VTA neurons results in both a behavioral anti-depressive effect and an alteration in NAc single-unit activity (i.e., cell firing) [24]. Additionally, optogenetic stimulation of VTA-projecting glutamatergic mPFC terminals has been shown to affect behavior mediated by NAc dopaminergic activity [10]. Repeated transcranial magnetic stimulation of the prefrontal cortex at 10 Hz and 20 Hz has also been reported to increase extracellular dopamine concertation in the NAc, dorsal striatum, and hippocampus of rats [25] and in the caudate of humans [26]. Together, the findings above suggest that dopamine release is an active component of DBS-mediated relief of depression and/or chronic pain.
Previous studies have illustrated the importance of stimulation frequency by demonstrating that extended (> 30 min) high-frequency (60 Hz) electrical stimulation of the mPFC can increase extracellular concentrations of dopamine in the NAc of rats as measured by microdialysis [16,17] Contrarily, low-frequency (10 Hz) stimulation reduced measured dopamine levels [17]. Although microdialysis has excellent chemical specificity, it has the disadvantage of poor temporal resolution (minutes to hours). Consequently, the effects of mPFC stimulation on second-to-minute changes in dopamine concentration were not resolved. While slow (minutes) “tonic” changes in dopamine concentration have been linked to motivation and value estimation [27], understanding dopamine release with second-to-minute resolution is of equal interest as dopaminergic fluctuations on this time scale are critical for decision making and learning. For example, rapid (sub-second) “phasic” changes in dopamine concentration in the NAc regulate error-driven learning [28] and respond to the proximity of an animal to a reward [29].
Observations of some physiological characteristics of the mPFC (such as cell firing rate and local-field activity) suggest that stimulation frequencies below those typically used for DBS (< 100 Hz) may be optimal for triggering dopamine release. For example, principal neurons in the prefrontal cortex fire at frequencies that are far lower than frequencies used for deep brain stimulation, with peak rates rarely exceeding 30 Hz when animals perform behaviors involving decision making, working memory, and attention [30–35]. Similarly, local-field oscillatory activity in the mPFC in the theta- (5 – 10 Hz) and beta- (15 – 30 Hz) frequency bands is associated with inter-region communication, attention, and decision making [36]. Stimulation frequencies that more closely mirror prefrontal firing patterns and local-field activity may enhance the effectiveness of DBS treatments to normalize or regulate dysfunction.
This study was designed to systematically examine the effects of mPFC stimulation frequency (4 – 60 Hz) and stimulation duration (1 – 30 s) on phasic dopamine release in the NAc using a real-time electrochemical detection method—FSCV. Although we originally hypothesized that NAc dopamine concentration would increase as mPFC stimulation frequency increased, we found instead that stimulation at 10 – 20 Hz for 10 to 20 s triggered the largest response.
Materials and Methods
Electrode fabrication and calibration.
Carbon-fiber microelectrodes were fabricated as previously described [37]. T-40 carbon fibers (Cytec Thornel, Woodland Park, NJ) were first aspirated into 0.68 mm I.D. glass capillary (A-M Systems, Inc., Sequim, WA). Capillaries were then heated and pulled to form a seal around the fiber using a type PE-2 pipette puller (Narishige, Tokyo, Japan). Fibers were cut 70 – 80 μm in length from the glass seal. These electrodes were coated with PEDOT:Nafion following procedures described by Vreeland et al. [38] to minimize bio-fouling and increase electrochemical selectivity and sensitivity. Reference electrodes (Ag/AgCl) were prepared by soaking a silver wire (0.5 mm diameter, Sigma-Aldrich, St. Louis, MO) in chlorine bleach for 24 hours.
Electrodes were calibrated using a flow cell and known concentrations of dopamine. A calibration factor of 22 nA/μM, was used to convert current measurements to dopamine concentration.
Animals and Surgery.
Male Sprague Dawley rats (350 – 500 g) were anesthetized using 3.5 % Isoflurane gas which was subsequently lowered to 0.75 – 1.5% for the duration of the procedure. Oxygen flow rate was kept constant at 1.5 L/min. Holes were drilled and a single carbon-fiber microelectrode was lowered into the NAc (AP: 1.5 mm, ML: 1.4 mm, DV: 6.0 – 7.4 mm for pharmacology experiment and AP: 1.5 mm, ML: 1.4 mm, DV: 6.2 – 7.2 mm for frequency and duration experiments [39]). Bipolar stimulating electrodes (Plastics One) were lowered into the mPFC (AP: 3.2 mm, ML: 0.8 mm, DV: - 2.5.0 to - 4.4 mm for pharmacology experiment and AP: 3.2 mm, ML: 0.8 mm, DV: - 2.5.0 to – 3.5 mm for frequency and duration experiments [39]) and the medial forebrain bundle (AP: −2.5 mm, ML: 1.7 mm, DV: - 7.8 to - 8.9 mm [39]). An Ag/AgCl reference electrode was lowered 5 – 7 mm into cerebral cortex contralateral to the carbon fiber electrode roughly 3 mm lateral to the midline. Upon completion of the experiment, electrolytic lesions were made at both stimulating electrode sites and at the site of the carbon probe. Lesions at the stimulating electrodes were made by passing a constant 200 μA anodic current for up to 20 seconds at each pole of the bipolar stimulating electrode. A lesion at the carbon-fiber electrode was made by passing a constant 1 mA anodic current for up to 20 seconds. Isoflurane was increased to 3.5% prior to lesioning to mitigate any discomfort to the animal. Animals were sacrificed using Fatal-Plus® (350 mg/kg, Vortech Pharmaceutical Ltd., Dearborn, MI) or Euthasol® (350 mg/kg, Virbac AH, Inc., Fort Worth, TX) and perfused using 4 % paraformaldehyde in phosphate-buffered saline. Brains were extracted, sectioned, and a Nissl stain was used to confirm electrode placement (Figure S1). All experiments were approved by the University of Arizona Institutional Animal Care and Use Committee.
Fast-Scan Cyclic Voltammetry.
Voltammetric recordings were made using custom hardware and WCCV 3.0 software (Knowmad Technologies, LLC. Tucson, AZ). Recording was performed by applying a triangular voltage waveform (- 0.4 to 1.3 V) to the carbon-fiber microelectrode 400 V/s (waveform duration = 8.5 ms) once every 100 ms (10 Hz) [37].
Stimulation Procedures.
The position of the carbon-fiber microelectrode was optimized by electrically stimulating the medial forebrain bundle (MFB) and lowering the carbon-fiber microelectrode and stimulation electrode in 200 μm increments until evoked dopamine release in the NAc exceeded 300 nM. The stimulating electrode in the prefrontal cortex was then lowered in 200 μm increments (stimulating with 1 ms/phase, 60 pulses, 60 Hz, ± 300 – 600 μA) until dopamine release in the NAc was detected. In trials that explored stimulation-parameter space, the parameters were varied randomly to control for any possible order effects. Amplitude was varied from ± 200 μA to ± 800 μA in 200 μA increments while pulse width, frequency, and duration were held constant (1 ms/phase, 60 Hz, 60 pulses). Frequency was then varied (10 Hz, 20 Hz, 30 Hz, 40 Hz, and 60 Hz) while pulse width, duration, and amplitude were kept constant (1 ms/phase, 60 pulses, 600 μA). On separate trials, duration was varied across several frequencies (4 Hz, 10 Hz, 20 Hz, and 60 Hz; for 1 s, 2 s, 5 s, 10 s, 20 s, and 30 s) to assess the interaction between frequency and duration. Each stimulation train was separated by –several minutes (5 minutes for the frequency experiments and 7 minutes for the pharmacological validation experiment using GBR-12909) to allow for recovery of releasable dopamine and all stimulations were biphasic with charge balanced pulses to minimize tissue damage. No pharmacological agents were administered when frequency parameters were varied. Pharmacology was only used as described in the following section to characterize the observed signal.
Pharmacology.
To ensure accurate chemical identification, rats were injected with the highly selective, high-affinity dopamine reuptake inhibitor GBR-12909. GBR-12909 was dissolved in 1 – 3 mL saline to yield a dose of 10 mg/kg. Rats were then injected (i.p.) with either GBR-12909 (n = 6 rats) or saline (n = 7 rats) after a 28-minute baseline recording. Measurements were taken up to 70 minutes post-injection using fixed stimulation parameters (60 Hz, 60 pulses, 2 ms/phase pulse width, 600 μA).
Data Analysis.
Analyses were carried out in Graphpad (Prism; La Jolla, CA), Matlab (Matlab R2016, Mathworks Inc., Natick, MA, 2016), and WCCV 3.0 (Knowmad Technologies, LLC.) Principal component regression was used to isolate dopamine measurements from other local pH fluctuations as previously described [37]. Statistical tests used include Grubb’s test, Student’s t-test, and one- and two-way ANOVA.
Results
Evidence that mPFC stimulation evokes dopamine release in the NAc
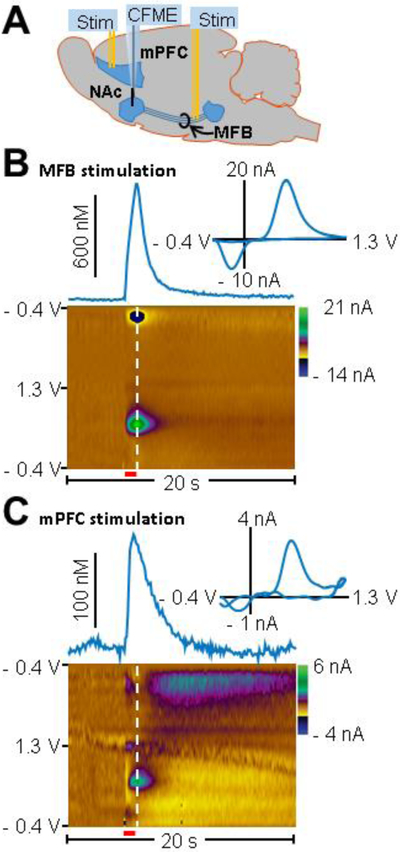
To determine whether electrical stimulation of the mPFC evokes phasic dopamine release in the NAc, voltammetric responses produced by mPFC stimulation were compared to responses produced by direct MFB axonal stimulation (Figure 1). First, a carbon-fiber microelectrode was lowered into the NAc and stimulating electrodes were placed in the MFB and mPFC to evoke dopamine release in the NAc (Figure 1A). Electrode placement was verified histologically. Figure S1 A - C show examples of electrode tracts seen coronal tissue slices from the mPFC, NAc, and MFB. Figure S1 D – E show the electrode placements from other animals not shown in A – C. A brief (8.5 ms) triangular waveform (- 0.4 to 1.3 V) was applied to the working electrode every 100 ms resulting in the oxidation and reduction of dopamine at the electrode surface. The resultant current was background subtracted using pre-stimulus voltammograms and plotted versus the applied potential generating cyclic voltammograms (CVs) that can be used to distinguish dopamine from other analytes (Figure 1B and 1C, insets). The CV shown from mPFC stimulation was highly correlated with that from MFB stimulation (r2 = 0.87) suggesting that dopamine is the analyte released in the NAc upon mPFC stimulation. These data are presented in color plots where time is shown on the x-axis, applied potential on the y-axis, and current in false color (Figure 1B and 1C). Currents due to the oxidation of dopamine were isolated from interfering current changes due to fluctuations in local pH using principal component regression as previously described [37]. Concentration versus time traces are shown above the color plots and the insets show cyclic voltammograms corresponding to the white dashed lines on the color plots. Figure 1B shows representative dopamine release evoked by MFB stimulation (± 300 μA, 60 Hz, 60 pulses, 1 ms/phase pulse width). Figure 1C shows representative dopamine release evoked by mPFC stimulation (± 600 μA, 60 Hz, 60 pulses, 1 ms/phase pulse width).
Figure 1. Representative dopamine release recorded from the NAc using fast-scan cyclic voltammetry during stimulation of the mPFC or MFB.
(A) Schematic of the experimental setup. Bipolar stimulating electrodes (Stim) were placed in both the mPFC and the MFB to electrically evoke dopamine release in the NAc. A carbon-fiber microelectrode (CFME) was placed in the NAc to measure evoked dopamine release. (B) Measured change in extracellular dopamine concentration in the NAc resultant from direct axonal MFB stimulation (± 300 μA, 60 Hz, 60 pulses, 1ms/phase pulse width, stimulation train indicated by red bar below color plot). The inset contains a voltammogram taken from the time point corresponding to the white dashed line on the color plot. Time is shown on the x-axis, applied potential on the y-axis, and current in false color. (C) Measured change in dopamine concentration in the NAc evoked by mPFC stimulation (± 600 μA, 60 Hz, 60 pulses, 1 ms/phase pulse width, stimulation train indicated by red bar below color plot). Inset contains a voltammogram taken from the time point corresponding to the white line on color plot. Color plot indicates dopamine release evoked by mPFC stimulation.
Characterization of dopamine release in the NAc evoked by different amplitudes of mPFC stimulation
To determine the relationship between stimulation intensity and NAc dopamine release, the stimulation current applied to the mPFC electrode was varied from ± 200 to ± 800 μA while holding all other parameters constant (60 Hz, 60 pulses, 1 ms/phase pulse width, Figure S2). It was observed that dopamine release increased with increasing stimulation current up to ± 600 μA (one-way ANOVA; F 4,12 = 28.68, p < 0.003, n = 5 rats). Dopamine release evoked by either ± 600 μA or ± 800 μA was significantly greater than that evoked by ± 200 μA (p < 0.04 and p < 0.02, respectively, Tukey multiple-comparisons test) and ± 400 μA (p < 0.02 and p < 0.01, respectively). No significant difference was observed between ± 600 and ± 800 μA (p > 0.22). Given these data, ± 600 μA stimulation was used for all subsequent experiments.
Pharmacological validation of measured signal
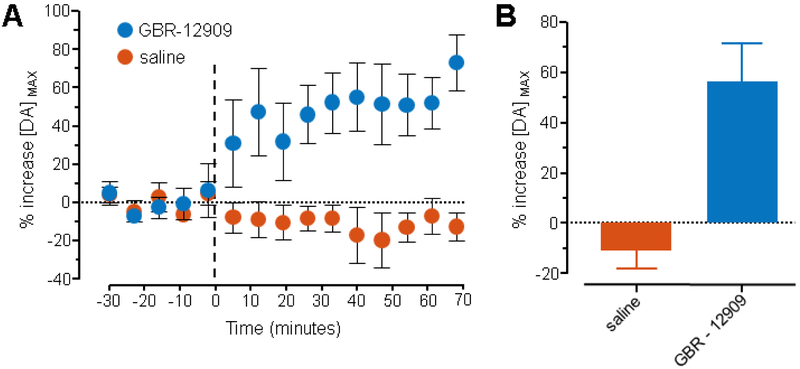
Pharmacological manipulations were performed to validate whether the signal resultant from mPFC stimulation was due to dopamine release. Specifically, a selective dopamine reuptake inhibitor was injected (GBR-12909, 10 mg/kg, i.p., Figure 2) which has been shown to increase the magnitude of stimulated dopamine release [40]. The mPFC was stimulated every 7 minutes (± 600 μA, 60 Hz, 60 pulses, 1 ms/phase pulse width), dopamine release was measured in the NAc, and peak dopamine release ([DA] MAX) was quantified. After recording 28 minutes of baseline data, GBR-12909 or saline was injected i.p. Average data from each measurement are shown (Figure 2A, error bars indicate SEM). A marked increase in the magnitude of stimulated release is observed following drug injection. Grubb’s test for outliers was performed and a single outlier that showed an increase following GBR-12909 injection was removed (alpha = 0.05). Peak stimulated dopamine release increased significantly following injection of GBR-12909 when compared to saline (Figure 2B, Student’s t-test, p < 0.002, average of measurements 6 – 10 post injection were compared, saline n = 7 rats, GBR-12909 n = 5 rats). Because the baseline and saline data were stable throughout the experiment, it can be inferred that changes in dopamine release due to the injection of the drug were not affected by repetitive stimulation.
Figure 2. Pharmacological characterization of measured signal.
Chemical identity of the measured signal as dopamine was confirmed through injection of a dopamine reuptake inhibitor (GBR-12909, 10 mg/kg i.p.). Stimulations were administered to the mPFC once every 7 minutes (± 600 μA, 60 Hz, 60 pulses, 1 ms/phase pulse width) and peak dopamine release, [DA] MAX, was measured in the NAc. (A) After recording a 28-minute baseline (5 measurements), rats were injected with either saline (n = 7 rats) or GBR-12909 (n = 5 rats) at time = 0 minutes (black dashed line). Data were normalized to the average baseline signal for each animal (- 30 to - 2 minutes). Error bars indicate SEM. (B) Summary of the effect of GBR-12909 or saline on measured signal. The average of five measurements (post-injection measurements 6 – 10 which is the 40 – 70 minute post-injection interval) following the injection of GBR-12909 was compared to the average of the same five measurements following the injection of saline. GBR-12909 significantly increased the measured signal when compared to saline (Student’s t-test, p < 0.002).
Effect of mPFC stimulation frequency on NAc dopamine release
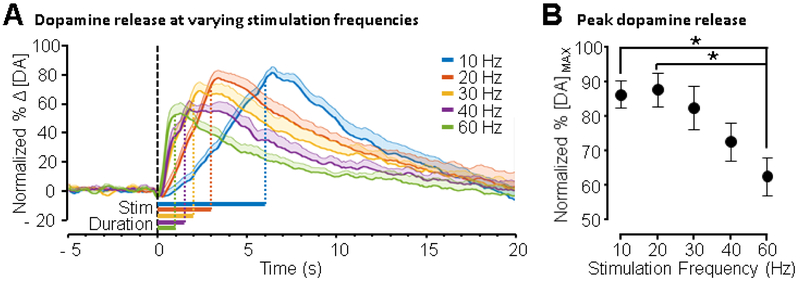
The effect of mPFC stimulation frequency on evoked NAc dopamine release was investigated by varying stimulation frequency while holding total charge passed constant (± 600 μA, 60 pulses, 1 ms/phase pulse width). Interestingly, varying mPFC stimulation frequency (10, 20, 30, 40, and 60 Hz) resulted in greater evoked release for lower (e.g., 10 – 20 Hz) versus higher (e.g., 60 Hz) frequencies (Figure 3). Figure 3A shows average change in dopamine versus time. Data were normalized as percent of maximal release of all mPFC stimulations within animal and smoothed using a moving average with a 500 ms window. Figure 3B shows average, normalized peak release for each frequency tested (± SEM, n = 10 rats). A main effect of frequency was observed (F 4,45 = 4.247, p < 0.006, one-way ANOVA) with post-hoc tests indicating that 10 and 20 Hz stimulation evoked greater release than 60 Hz stimulation (10 versus 60 Hz: p < 0.03, 20 versus 60 Hz: p < 0.02, Tukey multiple-comparisons test).
Figure 3. Effect of mPFC stimulation frequency on NAc dopamine release.
Frequency of mPFC stimulation was varied and total charge passed was held constant (± 600 μA, 60 pulses, 1 ms/phase pulse width, n = 10 rats). (A) Dopamine release was measured while administering 10, 20, 30, 40, and 60 Hz stimulation (data were smoothed using a 500 ms moving average). Data were normalized to maximum peak release recorded for each animal across all parameters. Normalized change in dopamine (Δ [DA]) versus time is shown and shaded regions indicate + SEM. The black dashed line indicates the start of stimulation and the horizontal colored bars under the traces show the stimulation duration. (B) Average normalized peak release is shown as a function of frequency. Dopamine release evoked by 20 Hz and 10 Hz was greater than dopamine release evoked by 60 Hz (p < 0.02 and p < 0.03 respectively, one-way ANOVA, Tukey multiple-comparisons test, error bars represent SEM).
Dopamine release is dependent on both frequency and duration of stimulation
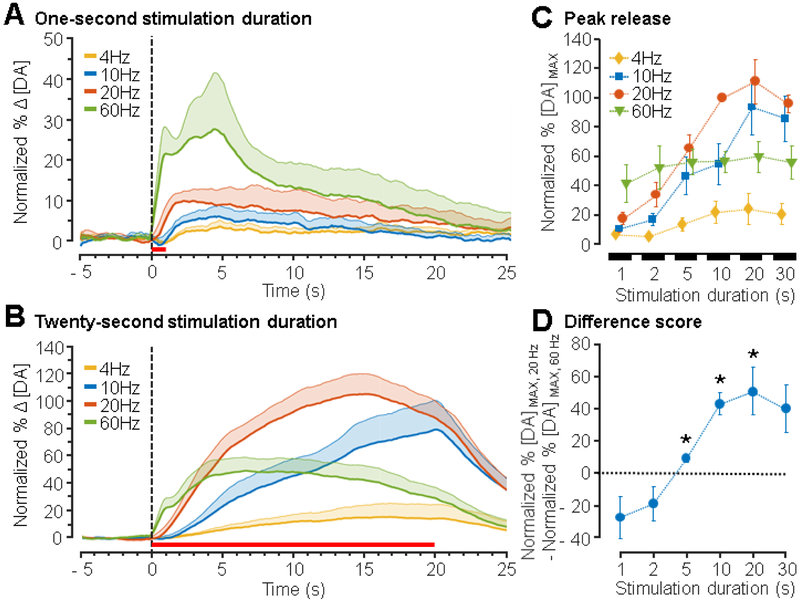
As a consequence of controlling the number of pulses in the stimulation train (total charge entering tissue), the duration of the stimulation is shortened with increasing frequency (e.g., 60 pulses x 20 Hz = 3 s versus 60 pulses x 60 Hz = 1 s). To investigate the effect of stimulation duration, dopamine release was measured in response to different frequencies of mPFC stimulation (4, 10, 20, and 60 Hz), while holding the stimulation duration constant (1, 2, 5, 10, 20, or 30 s; Figure 4). To determine the optimal parameter to use for normalizing the data, the standard deviation of the peak dopamine release for each stimulation parameter across all animals was calculated. The parameter that resulted in the largest standard deviation between animals (20 Hz 10 s, data not shown) was chosen as the normalization factor to account for inter-animal variability. The effects of different frequencies of stimulation when duration was held at 1 s (Figure 4A) and 20 s (Figure 4B) are shown. At a stimulation duration of 1 s, 60 Hz evoked greater dopamine release than other frequencies. In contrast, a 20 s, 20 Hz stimulation evoked greater dopamine release when compared to other frequencies tested. Peak dopamine release depended on both the frequency and duration of stimulation as shown in the responses presented in Figure 4C. A significant interaction between duration and frequency was observed (F15,144 = 3.07, p < 0.0003), along with main effects of frequency (F3,144 = 28.05, p < 0.0001) and duration (F5,144 = 16.69, p < 0.0001, two-way ANOVA).
Figure 4. Dependence of peak dopamine release on the frequency of stimulation.
The mPFC was stimulated at 4, 10, 20, and 60 Hz for 1, 2, 5, 10, 20, and 30 s and dopamine release was recorded from the NAc (± 600 μA, 1 ms/phase pulse width, n = 6 – 8 rats). Peak dopamine release for each animal and for each parameter tested was normalized to peak dopamine release from a 20 Hz, 10 s stimulation within animal. (A) Mean normalized traces of dopamine release from 1 s stimulations. The stimulation period is indicated by the horizontal red line and shaded regions represent + SEM. (B) Mean trace of dopamine release from 20 s stimulations. The stimulation period is indicated by the horizontal red line below the traces. (C) Average normalized peak release as a function of duration for each stimulation frequency. A two-way ANOVA revealed effects of frequency (F 3,144 = 28.05, p < 0.0001), duration (F 5,144 = 16.69, p < 0.0001), and their interaction (F 15,144 = 3.07, p < 0.0003). All post-hoc comparisons are presented in Table S1. (D) Difference scores were then calculated by subtracting normalized dopamine release at 60 Hz stimulation from normalized dopamine release at 20 Hz stimulation for each duration. Stimulation at 20 Hz results in greater dopamine release than 60 Hz stimulation for durations from 5 to 20 s. Values significantly different from 0 are denoted with an asterisk wherein 0 indicates no difference between dopamine release from 20 Hz versus 60 Hz stimulation (multiple Student’s t-tests with Bonferroni-Holm correction, p < 0.05).
When comparing each stimulation frequency within each stimulation duration, post-hoc analysis further indicated that high stimulation frequencies produced greater dopamine release at short durations (1 s), while lower stimulation frequencies (10 – 20 Hz) produced greater dopamine release at durations > 5 s (Figure 4C). For example, at a 2 s stimulation duration, dopamine release from 60 Hz stimulation was larger than that from 4 Hz (p < 0.02, Tukey multiple-comparisons test), but for 20 s, dopamine release from 20 Hz stimulation exceeded 4 Hz (20 Hz p < 0.0001) and 60 Hz (p < 0.002). All post-hoc comparisons are presented in Table S1.
The interaction between frequency and duration was further explored by computing the difference between peak dopamine release at 20 and 60 Hz for each stimulation duration (Figure 4D). Peak dopamine release from 60 Hz stimulation was subtracted from that of 20 Hz stimulation for each duration (1, 2, 5, 10, 20, and 30 s) such that 0 indicates no difference between the two. Values significantly less than 0 indicate that 60 Hz stimulation resulted in greater dopamine release whereas values significantly greater than 0 indicate that 20 Hz stimulation resulted in greater dopamine release. This analysis revealed that dopamine release in response to 20 Hz stimulation exceeded dopamine release in response to 60 Hz stimulation for durations of 5, 10, and 20 s (Multiple Student’s t-tests with Bonferroni-Holm correction for multiple-comparisons, p < 0.05).
Discussion
In the present work, we show that phasic dopamine release can be evoked by mPFC stimulation and validate the observed response as an increase in dopamine using the selective DAT inhibitor, GBR-12909 as other stimulations have been validated as previously reported [41–43]. We also show effects of frequency and duration of mPFC stimulation on NAc dopamine release. Wightman et al. found that increasing the frequency of direct stimulation of the MFB from 10 – 60 Hz results in an increase in dopamine release [44]. In contrast, this work identified a more complex relationship between the frequency of mPFC stimulation and dopamine release in the NAc. Increasing the frequency of MFB stimulation increases measured extracellular dopamine as the rate of stimulated release overtakes uptake kinetics [44,45]. As such, we predicted that increasing stimulation frequencies in the mPFC would result in increased measured dopamine release. Instead, it was observed that NAc dopamine release peaked at 10 – 20 Hz when stimulation durations were ≥ 5 s. In contrast, at short stimulation durations (< 5 s), high frequencies (e.g., 60 Hz) triggered peak dopamine release.
Contrast to microdialysis studies
Tonic (slow) changes in dopamine concentration, typically measured over several seconds to minutes, are implicated in motivation and value estimation [27,46] while phasic (rapid) dopamine release is implicated in error-driven learning and decision making [28,29,47]. Previously, Jackson et al. used microdialysis to measure tonic changes in dopamine concentration at 5-minute intervals in response to repeated mPFC stimulation [17]. They reported that low-frequency stimulation (10 Hz, 100 μA, 5 pulses repeated every 5 s for 30 minutes, total pulses = 1800) resulted in reduced tonic dopamine concentration in the NAc. In contrast, we measured rapid dopamine release in response to individual stimulation trains. We observed that evoked dopamine release in the NAc is affected by both stimulation duration and frequency whereby low-frequency, 10 – 20 Hz, mPFC stimulation resulted in greater NAc dopamine release than high-frequency, 60 Hz, stimulation. Our results suggest a frequency-dependent mechanism by which the mPFC regulates phasic NAc dopamine release. A closer study of the mechanisms by which the mPFC induces changes in phasic release would bolster understanding mPFC-mesolimbic interactions involved in learning and decision making. Taken together our data and the Jackson et al. study suggest that phasic and tonic dopamine signaling are differentially affected by stimulation duration and frequency.
Possible mechanisms underlying peak phasic dopamine release at 10 – 20 Hz stimulation
Because the FSCV signal is background subtracted, it is typically used to measure phasic dopamine release. The timescale typically used to describe phasic dopamine release is sub-seconds to seconds and the timescale for tonic measurement is typically minutes to hours [42,48]. Here we observe an increase in evoked dopamine release that lasted in some cases for several seconds and thus may suggest that tonic and phasic dopamine signaling were altered. Because we cannot measure absolute (tonic) concentrations of dopamine, we will refer to this release pattern as prolonged-phasic dopamine release. We observed that 10 – 20 Hz stimulation is more effective than high-frequency stimulation to drive prolonged-phasic dopamine release in the NAc. Several features of the mPFC and the dopaminergic system may contribute to this phenomenon such as electrophysiological characteristics of neurons in the mPFC and VTA and connectivity between neurons in the mPFC, VTA, and NAc.
VTA cell activity
VTA neurons can fire in burst or tonic modes. Burst firing is associated with phasic dopamine release in the striatum with burst onset defined as at least two action potentials occurring within an 80 ms interval [49] and is thought to be involved in encoding salient events [50]. Furthermore, Hyland et al. reported the intra-burst frequency of VTA neurons to be ~ 20 Hz [51]. This is similar to intervals used here to evoke peak prolonged-phasic dopamine release (10 to 20 Hz ≈ 50 to 100 ms inter-pulse interval). These data suggest that stimulation frequencies near 20 Hz are physiologically optimal for maximizing prolonged-phasic dopamine release driven by the mPFC.
Electrophysiological characteristics of the mPFC
The mPFC is implicated in goal-directed behaviors which may be facilitated by its projections to the NAc and VTA. mPFC neurons fire at frequencies near 20 Hz when animals perform behaviors that require modulation of dopamine release [30,34]. For example, single-neuron and local-field activity within the prefrontal cortex at frequencies between 13 – 30 Hz (beta frequency) have been observed in animals performing working memory, attention, and decision making tasks [30–36,52].
Circuit-level organization/interconnectivity
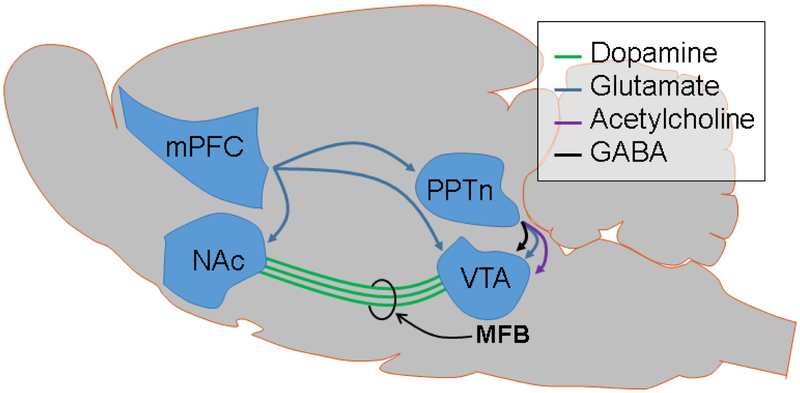
mPFC projections to other regions may also play a role in the observed relationship between mPFC stimulation frequency and NAc dopamine. The mPFC projects to the VTA [10], the NAc [53], and intermediate structures such as the pedunculopontine nucleus [53]. Anatomical studies by Carr and Sesack were unable to find evidence for projections from the mPFC to NAc-projecting dopamine neurons in the VTA [9]. Nevertheless, Beier et al. have shown that direct projections from the mPFC to the VTA are capable of changing NAc-dopamine-mediated behaviors [10]. Specifically, the authors observed that animals preferred nose-poke holes associated with 20 Hz optogenetic stimulation of mPFC terminals in the VTA over lower stimulation frequencies. This effect was abolished by dopamine-receptor blockade in the NAc. Additionally, there are direct mPFC projections to the NAc capable of modulating dopamine release [54]. In a neurochemical investigation in brain slices, Kosillo et al., found that cortical projections to the striatum increase dopamine release via cholinergic interneurons [54]. These studies support both direct and indirect routes by which the mPFC controls NAc dopamine release. Figure 5 offers several established neural circuits by which the mPFC could activate dopamine release in the NAc through direct activation of the VTA [10], through intermediate structures that synapse onto VTA [9], through direct/indirect activation of dopamine terminals in the NAc [55], or through a combination of these (e.g., perhaps direct NAc terminal activation and indirect VTA activation to mediate different patterns of release). Given that VTA burst firing occurs in the range of 20 Hz [51] and that others have shown that activation of mPFC afferents in the VTA are sufficient to induce behavioral changes [10], we propose that direct mPFC activation of the VTA is the most likely connection mediating the findings presented here. Future experiments that explore this circuit through activation or inactivation of its components could distinguish between these different pathways towards mPFC-mediated activation of NAc.
Figure 5. Diagram of established connections that act as potential mediators of the observed evoked dopamine release from mPFC stimulation.
The blue lines represent glutamatergic connections, the green lines represent dopaminergic fibers from the VTA, the black lines represent gamma-aminobutyric acid-ergic (GABA) connections, and the purple lines represent cholinergic connections. VTA: ventral tegmental area, NAc: nucleus accumbens, mPFC: medial prefrontal cortex, and PPTn: pedunculopontine tegmental nucleus.
Cellular mechanisms may also account for the prolonged-phasic dopamine release seen in 20 Hz but not 60 Hz stimulation. Montague et al. offer a model that describes dynamic changes in dopamine release in response to different patterns of stimulation [56]. They found that their model consistently captured short-term facilitation, short-term depression, and long-term depression of dopamine release as governed by both the duration of stimulation and the interstimulus interval. They suggest that the long-lasting depression in dopamine release is due to dopamine biosynthesis and vesicle packaging as the rate-limiting factor. Similarly, short-term depression could be mediated by the inhibition of dopamine release by D2 autoreceptors found in terminals. While evoking dopamine release through mPFC activation could involve a single synapse or multiple synapses through both VTA and/or NAc terminal activation, the phenomenon of depression modeled by Montague et al. may help to explain why 60 Hz stimulation is less optimal than 20 Hz stimulation for long stimulus durations. If 60 Hz stimulation of the VTA results in depression over long durations as suggested by Montague et al., perhaps 60 Hz stimulation of the mPFC afferents drives VTA neurons in a similar manner that curtails dopamine release through autoreceptor activation or by exceeding the rate of vesicle packaging. In contrast, 20 Hz stimulation may result in less frequent autoreceptor stimulation or may allow for vesicle repackaging kinetics to effectively catch up with the rate of release. Experiments observing the effects of stimulation frequency while manipulating autoreceptor activation and vesicular packaging could help to better understand this phenomenon.
The observed data also appears to have a pattern of release that is not typically seen when stimulating dopaminergic cells or axons directly. As can be seen in Figure 4A, there appears to be an immediate rise in release (as would be expected with direct stimulation) and a second late rise after stimulation offset with 60-Hz stimulation. Figure 4B shows a similar pattern of rise-decrease-rise. Individual traces from each animal (Figure S3) show that this unique pattern of release was not observed in all cases. These differences may be due to individual differences among animals or slight perturbations (due to changes in depth—see Methods) in the groups of cells being stimulated. If different groups of cells were stimulated in some animals, perhaps the direct projections from the mPFC to the NAc played a more active role in evoked release while in others, projections to the VTA mediated a majority of the release observed.
Implications for deep brain stimulation
Deep brain stimulation is used to treat neurological disorders associated with dopaminergic dysregulation and high stimulation frequencies are common (> 100 Hz) [57,58]. Our data and results from studies described above indicate that ~ 20 Hz activity has important physiological and behavioral functions, yet few studies have explored the effects of low-frequency stimulation on dopamine release. Nevertheless, there is evidence that low-frequency stimulation can have therapeutic benefits. For example, one study involving stimulation of the sub-thalamic nucleus in Parkinson’s patients found that 10 Hz stimulation improved verbal fluency but reduced motor function, while 130 Hz stimulation reduced verbal fluency but improved motor function [59]. In another study, both low- (20 Hz) and high- (130 Hz) frequency stimulation of the ventral mPFC in rats was shown to increase swimming behavior in a forced swim test which may indicate an anti-depressant effect [60]. Results obtained from these studies suggest that the choice of DBS frequency is particularly important for regulating dopaminergic systems and for maximizing therapeutic benefit. It should be noted, however, that the stimulation amplitude and method used here vary from those used in many DBS protocols. For example, DBS typically varies voltage instead of current to stimulation tissue, it is typically higher frequency than those tested here (> 100 Hz), and it is typically monopolar with a single focal electrode in the brain and a sync some distance away [58,61]. For this reason, further research addressing the effect of DBS frequency on dopamine signaling is needed.
Conclusions
This work investigated the effects of a range of frequencies (4 to 60 Hz) of mPFC stimulation on dopamine release in the NAc. We show that mPFC stimulation evokes phasic and prolonged-phasic dopamine release. We also found that 20 Hz stimulation triggered maximal dopamine release in the NAc for stimulation durations > 5 s. Additionally, we report an interaction between the frequency and the duration of mPFC stimulation. These results are consistent with the observation that endogenous 20 Hz activity in the mPFC facilitates goal-directed behaviors, which may be a consequence of increased dopamine release in the NAc. Importantly, for clinical applications, these data suggest that the therapeutic effect of deep brain stimulation for diseases involving dysregulation of dopaminergic systems may depend on the specific choice of frequency. The potential role of mPFC regulation of mesolimbic dopamine in ameliorating neurological symptoms compels further investigation of low-frequency stimulation.
Supplementary Material
Highlights.
mPFC stimulation evokes phasic dopamine release in the NAc
Dopamine release is maximal at 10 – 20 Hz for stimulation durations > 5 s
At short durations (≤ 5 s), 60 Hz stimulation resulted in maximal dopamine release
A significant interaction between frequency and duration of stimulation was shown
Acknowledgements
Mitchell Bartlett for advising on pharmacology and injection procedures. Hannah Mullins and Matthew Vance for manufacturing carbon-fiber microelectrodes and performing histology. We thank University of Arizona and National Science Foundation Grant 1450767 (S.L.C. and M.L.H.) for funding this work. Kate L. Parent was funded through National Institutes of Health Grant T32-GM00804.
Abbreviations
- NAc
nucleus accumbens
- mPFC
medial prefrontal cortex
- DBS
deep brain stimulation
- LFP
local-field potential
- VTA
ventral tegmental area
- FSCV
fast-scan cyclic voltammetry
- DA
dopamine
- CV
cyclic voltammogram
- CFME
carbon-fiber microelectrode
Footnotes
The authors declare the following competing financial interest(s): Dr. Michael Heien declares an actual or potential financial conflict of interest and is co-founder/equity holder in Knowmad Technologies, a licensee of University of Arizona (UA) intellectual property used in this research. This relationship has been disclosed to the UA Institutional Review Committee and is managed by a Financial Conflict of Interest Management Plan.
References
- [1].Floresco SB. The Nucleus Accumbens: An Interface Between Cognition, Emotion, and Action. Annu Rev Psychol 2015;66:25–52. doi: 10.1146/annurev-psych-010213-115159. [DOI] [PubMed] [Google Scholar]
- [2].Schultz W, Carelli RM, Wightman RM. Phasic dopamine signals: from subjective reward value to formal economic utility. Curr Opin Behav Sci 2015;5:147–54. doi: 10.1016/j.cobeha.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lozano AM, Giacobbe P, Hamani C, Rizvi SJ, Kennedy SH, Kolivakis TT, et al. A multicenter pilot study of subcallosal cingulate area deep brain stimulation for treatment-resistant depression. J Neurosurg 2012;116:315–22. doi: 10.3171/2011.10.JNS102122. [DOI] [PubMed] [Google Scholar]
- [4].Yadid G, Friedman A. Dynamics of the dopaminergic system as a key component to the understanding of depression. Prog Brain Res 2008;172:265–86. doi: 10.1016/S0079-6123(08)00913-8. [DOI] [PubMed] [Google Scholar]
- [5].Russo JF, Sheth S a. Deep brain stimulation of the dorsal anterior cingulate cortex for the treatment of chronic neuropathic pain. Neurosurg Focus 2015;38:1–11. doi: 10.3171/2015.3.FOCUS1543.Disclosure. [DOI] [PubMed] [Google Scholar]
- [6].Pierce RC, Vassoler FM. Deep brain stimulation for the treatment of addiction: Basic and clinical studies and potential mechanisms of action. Psychopharmacology (Berl) 2013;229:487–91. doi: 10.1007/s00213-013-3214-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Levy D, Shabat-Simon M, Shalev U, Barnea-Ygael N, Cooper A, Zangen A. Repeated Electrical Stimulation of Reward-Related Brain Regions Affects Cocaine But Not “Natural” Reinforcement. J Neurosci 2007;27:14179–89. doi: 10.1523/JNEUROSCI.4477-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Francois J, Huxter J, Conway MW, Lowry JP, Tricklebank MD, Gilmour G. Differential contributions of infralimbic prefrontal cortex and nucleus accumbens during reward-based learning and extinction. J Neurosci 2014;34:596–607. doi: 10.1523/JNEUROSCI.2346-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Carr DB, Sesack SR. Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J Neurosci 2000;20:3864–73. doi:http://www.jneurosci.org/content/20/10/3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Beier KT, Steinberg EE, Deloach KE, Xie S, Miyamichi K, Schwarz L, et al. Circuit Architecture of Midbrain Dopamine Neurons Revealed by Systematic Input – Output Mapping. Submitted 2015:622–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Murty VP, Ballard IC, Adcock RA. Hippocampus and Prefrontal Cortex Predict Distinct Timescales of Activation in the Human Ventral Tegmental Area. Cereb Cortex 2016:1–10. doi: 10.1093/cercor/bhw005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Goto Y, Grace A a. Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nat Neurosci 2005;8:805–12. doi: 10.1038/nn1471. [DOI] [PubMed] [Google Scholar]
- [13].Vitay J, Hamker FH. Timing and expectation of reward: A neuro-computational model of the afferents to the ventral tegmental area. Front Neurorobot 2014;8:1–25. doi: 10.3389/fnbot.2014.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Karreman M, Moghaddam B. The prefrontal cortex regulates the basal release of dopamine in the limbic striatum: an effect mediated by ventral tegmental area. J Neurochem 1996;66:589–98. [DOI] [PubMed] [Google Scholar]
- [15].Fujisawa S, Buzsáki G. A 4 Hz Oscillation Adaptively Synchronizes Prefrontal, VTA, and Hippocampal Activities. Neuron 2011;72:153–65. doi: 10.1016/j.neuron.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Taber MT, Fibiger HC. Electrical stimulation of the prefrontal cortex increases dopamine release in the nucleus accumbens of the rat: modulation by metabotropic glutamate receptors. J Neurosci 1995;15:3896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jackson ME, Frost AS, Moghaddam B. Stimulation of prefrontal cortex at physiologically relevant frequencies inhibits dopamine release in the nucleus accumbens. J Neurochem 2001;78:920–3. doi: 10.1046/j.1471-4159.2001.00499.x. [DOI] [PubMed] [Google Scholar]
- [18].Mora F, Cobo M. The neurobiological basis of prefrontal cortex self-stimulation: a review and an integrative hypothesis. Prog Brain Res 1990;85:419–31. [DOI] [PubMed] [Google Scholar]
- [19].Murase S, Grenhoff J, Chouvet G, Gonon FG, Svensson TH. Prefrontal cortex regulates burst firing and transmitter release in rat mesolimbic dopamine neurons studied in vivo. Neurosci Lett 1993;157:53–6. doi: 10.1016/0304-3940(93)90641-W. [DOI] [PubMed] [Google Scholar]
- [20].Boccard SGJ, Pereira E a C, Moir L, Van Hartevelt TJ, Kringelbach ML, FitzGerald JJ, et al. Deep brain stimulation of the anterior cingulate cortex: targeting the affective component of chronic pain. Neuroreport 2014;25:83–8. doi: 10.1097/WNR.0000000000000039. [DOI] [PubMed] [Google Scholar]
- [21].Hamani C, Diwan M, Macedo CE, Brandão ML, Shumake J, Gonzalez-Lima F, et al. Antidepressant-Like Effects of Medial Prefrontal Cortex Deep Brain Stimulation in Rats. Biol Psychiatry 2010;67:117–24. doi: 10.1016/j.biopsych.2009.08.025. [DOI] [PubMed] [Google Scholar]
- [22].Rea E, Rummel J, Schmidt TT, Hadar R, Heinz A, Mathé AA, et al. Anti-anhedonic effect of deep brain stimulation of the prefrontal cortex and the dopaminergic reward system in a genetic rat model of depression: An intracranial self-stimulation paradigm study. Brain Stimul 2014;7:21–8. doi: 10.1016/j.brs.2013.09.002. [DOI] [PubMed] [Google Scholar]
- [23].Bruchim-Samuel M, Lax E, Gazit T, Friedman A, Ahdoot H, Bairachnaya M, et al. Electrical stimulation of the vmPFC serves as a remote control to affect VTA activity and improve depressive-like behavior. Exp Neurol 2016;283:255–63. doi: 10.1016/j.expneurol.2016.05.016. [DOI] [PubMed] [Google Scholar]
- [24].Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai H-C, Finkelstein J, et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature 2012;493:537–41. doi: 10.1038/nature11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Keck M, Welt T, Müller M, Erhardt A, Ohl F, Toschi N, et al. Repetitive transcranial magnetic stimulation increases the release of dopamine in the mesolimbic and mesostriatal system. Neuropharmacology 2002;43:101–9. doi: 10.1016/S0028-3908(02)00069-2. [DOI] [PubMed] [Google Scholar]
- [26].Strafella AP, Paus T, Barrett J, Dagher A. Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J Neurosci 2001;21:1–4. doi:20015457 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Niv Y Cost, benefit, tonic, phasic: What do response rates tell us about dopamine and motivation? Ann. N. Y. Acad. Sci, vol. 1104, 2007, p. 357–76. doi: 10.1196/annals.1390.018. [DOI] [PubMed] [Google Scholar]
- [28].Schultz W Dopamine reward prediction error coding. Dialogues Clin Neurosci 2016;18:23–32. doi: 10.1038/nrn.2015.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Howe MW, Tierney PL, Sandberg SG, Phillips PEM, Graybiel AM. Prolonged dopamine signalling in striatum signals proximity and value of distant rewards. Nature 2013;500:575–9. doi: 10.1038/nature12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Insel N, Barnes CA. Differential activation of fast-spiking and regular-firing neuron populations during movement and reward in the dorsal medial frontal cortex. Cereb Cortex 2015;25:2631–47. doi: 10.1093/cercor/bhu062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Deco G, Rolls ET. Attention and working memory: A dynamical model of neuronal activity in the prefrontal cortex. Eur J Neurosci 2003;18:2374–90. doi: 10.1046/j.1460-9568.2003.02956.x. [DOI] [PubMed] [Google Scholar]
- [32].Kepecs A, Uchida N,Zariwala H a, Mainen ZF Neural correlates, computation and behavioural impact of decision confidence. Nature 2008;455:227–31. doi: 10.1038/nature07200. [DOI] [PubMed] [Google Scholar]
- [33].Baeg EH, Kim YB, Huh K, Mook-Jung I, Kim HT, Jung MW. Dynamics of population code for working memory in the prefrontal cortex. Neuron 2003;40:177–88. doi: 10.1016/S0896-6273(03)00597-X. [DOI] [PubMed] [Google Scholar]
- [34].Euston DR, Gruber AJ, McNaughton BL. The Role of Medial Prefrontal Cortex in Memory and Decision Making. Neuron 2012;76:1057–70. doi: 10.1016/j.neuron.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Cowen SL, McNaughton BL. Selective delay activity in the medial prefrontal cortex of the rat: contribution of sensorimotor information and contingency. J Neurophysiol 2007;98:303–16. doi: 10.1152/jn.00150.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Benchenane K, Tiesinga PH, Battaglia FP. Oscillations in the prefrontal cortex: A gateway to memory and attention. Curr Opin Neurobiol 2011;21:475–85. doi: 10.1016/j.conb.2011.01.004. [DOI] [PubMed] [Google Scholar]
- [37].Heien MLAV, Khan AS, Ariansen JL, Cheer JF, Phillips PEM, Wassum KM, et al. Real-time measurement of dopamine fluctuations after cocaine in the brain of behaving rats. Proc Natl Acad Sci U S A 2005;102:10023–8. doi: 10.1073/pnas.0504657102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Vreeland RF, Atcherley CW, Russell WS, Xie JY, Lu D, Laude ND, et al. Biocompatible PEDOT:Nafion composite electrode coatings for selective detection of neurotransmitters in vivo. Anal Chem 2015;87:2600–7. doi: 10.1021/ac502165f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates; Compact, Sixth Edition. Elsevier; 2009. [Google Scholar]
- [40].Budygin E a., Kilpatrick MR, Gainetdinov RR, Wightman RM. Correlation between behavior and extracellular dopamine levels in rat striatum: Comparison of microdialysis and fast-scan cyclic voltammetry. Neurosci Lett 2000;281:9–12. doi: 10.1016/S0304-3940(00)00813-2. [DOI] [PubMed] [Google Scholar]
- [41].Phillips PEM, Robinson DL, Stuber GD, Carelli RM, Wightman RM. Real-Time Measurements of Phasic Changes in Extracellular Dopamine Concentration in Freely Moving Rats by Fast-Scan Cyclic Voltammetry. Drugs Abus 2003;79:443–64. doi: 10.1385/1-59259-358-5:443. [DOI] [PubMed] [Google Scholar]
- [42].Owesson-White C a., Roitman MF, Sombers L a., Belle AM, Keithley RB, Peele JL, et al. Sources contributing to the average extracellular concentration of dopamine in the nucleus accumbens. J Neurochem 2012;121:252–62. doi: 10.1111/j.1471-4159.2012.07677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].John CE, Jones SR. Voltammetric characterization of the effect of monoamine uptake inhibitors and releasers on dopamine and serotonin uptake in mouse caudate-putamen and substantia nigra slices. Neuropharmacology 2007;52:1596–605. doi: 10.1016/j.neuropharm.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wightman RM, Zimmerman JB. Control of dopamine extracellular concentration in rat striatum by impulse flow and uptake. Brain Res Rev 1990;15:135–44. doi: 10.1016/0165-0173(90)90015-G. [DOI] [PubMed] [Google Scholar]
- [45].Wightman RM, Amatore C, Engstrom RC, Hale PD, Kristensen EW, Kuhr WG, et al. Real-time characterization of dopamine overflow and uptake in the rat striatum. Neuroscience 1988;25:513–23. doi: 10.1016/0306-4522(88)90255-2. [DOI] [PubMed] [Google Scholar]
- [46].Atcherley CW, Laude ND, Parent KL, Heien ML. Fast-scan controlled-adsorption voltammetry for the quantification of absolute concentrations and adsorption dynamics. Langmuir 2013;29:14885–92. doi: 10.1021/la402686s. [DOI] [PubMed] [Google Scholar]
- [47].Sugam J a., Day JJ, Wightman RM, Carelli RM Phasic nucleus accumbens dopamine encodes risk-based decision-making behavior. Biol Psychiatry 2012;71:199–205. doi: 10.1016/j.biopsych.2011.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Robinson DL, Venton BJ, Heien MLAV, Wightman RM. Detecting subsecond dopamine release with fast-scan cyclic voltammetry in vivo. Clin Chem 2003;49:1763–73. doi: 10.1373/49.10.1763. [DOI] [PubMed] [Google Scholar]
- [49].Grace A, Grace A. Grace AA, Bunney BS . The control of firing pattern in nigral dopamine nurons : burst firing. J Neurosci 4 : 2877–2890 1984:2877–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Cooper DC. The significance of action potential bursting in the brain reward circuit. Neurochem Int 2002;41:333–40. doi: 10.1016/S0197-0186(02)00068-2. [DOI] [PubMed] [Google Scholar]
- [51].Hyland BI, Reynolds JNJ, Hay J, Perk CG, Miller R. Firing modes of midbrain dopamine cells in the freely moving rat. Neuroscience 2002;114:475–92. doi: 10.1016/S0306-4522(02)00267-1. [DOI] [PubMed] [Google Scholar]
- [52].Ferenczi EA, Zalocusky KA, Liston C, Grosenick L, Warden MR, Amatya D, et al. Prefrontal cortical regulation of brainwide circuit dynamics and reward-related behavior. Science (80- ) 2016;351:aac9698-aac9698. doi: 10.1126/science.aac9698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Vertes RP. Differential Projections of the Infralimbic and Prelimbic Cortex in the Rat. Synapse 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- [54].Kosillo P, Zhang Y-F, Threlfell S, Cragg SJ. Cortical Control of Striatal Dopamine Transmission via Striatal Cholinergic Interneurons. Cereb Cortex 2016:1–10. doi: 10.1093/cercor/bhw252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Del Arco A, Mora F. Prefrontal cortex-nucleus accumbens interaction: in vivo modulation by dopamine and glutamate in the prefrontal cortex. Pharmacol Biochem Behav 2008;90:226–35. doi: 10.1016/j.pbb.2008.04.011. [DOI] [PubMed] [Google Scholar]
- [56].Montague PR. Dynamic Gain Control of Dopamine Delivery in Freely Moving Animals. J Neurosci 2004;24:1754–9. doi: 10.1523/JNEUROSCI.4279-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kringelbach ML, Jenkinson N, Owen SLF, Aziz TZ. Translational principles of deep brain stimulation. Nat Rev Neurosci 2007;8:623–35. doi: 10.1038/nrn2196. [DOI] [PubMed] [Google Scholar]
- [58].Dostrovsky JO, Lozano AM. Mechanisms of deep brain stimulation. Mov Disord 2002;17:S63–8. doi: 10.1002/mds.10145. [DOI] [PubMed] [Google Scholar]
- [59].Wojtecki L, Timmermann L, Jörgens S, Südmeyer M, Maarouf M, Treuer H, et al. Frequency-dependent reciprocal modulation of verbal fluency and motor functions in subthalamic deep brain stimulation. Arch Neurol 2006;63:1273–6. doi: 10.1001/archneur.63.9.1273. [DOI] [PubMed] [Google Scholar]
- [60].Hamani C, Diwan M, Isabella S, Lozano AM, Nobrega JN. Effects of different stimulation parameters on the antidepressant-like response of medial prefrontal cortex deep brain stimulation in rats. J Psychiatr Res 2010;44:683–7. doi: 10.1016/j.jpsychires.2009.12.010. [DOI] [PubMed] [Google Scholar]
- [61].Brocker DT, Grill WM. Principles of electrical stimulation of neural tissue. vol. 116 1st ed. Elsevier B.V; 2013. doi: 10.1016/B978-0-444-53497-2.00001-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.