Abstract
Pseudomonas aeruginosa is a pathogenic bacterium known to cause serious human infections, especially in immune-compromised patients. This is due to its unique ability to transform from a drug-tolerant planktonic to a more dangerous and treatment-resistant sessile life form, called biofilm. Recently, two derivatives of the frog skin antimicrobial peptide esculentin-1a, i.e. Esc(1-21) and its D-amino acids containing diastereomer Esc(1-21)-1c, were characterized for their powerful anti-Pseudomonal activity against both forms. Prevention of biofilm formation already in its early stages could be even more advantageous for counteracting infections induced by this bacterium. In this work, we studied how the diastereomer Esc(1-21)-1c can inhibit Pseudomonas biofilm formation in comparison to the parent peptide and two clinically-used conventional antibiotics, i.e. colistin and aztreonam, when applied at dosages below the minimal growth inhibitory concentration. Biofilm prevention was correlated to the peptides’ ability to inhibit Pseudomonas motility and to reduce the production of virulent metabolites e.g. pyoverdine and rhamnolipids. Furthermore, the molecular mechanism underlying these activities was evaluated by studying the peptides’ effect on the expression of key genes involved in the virulence and motility of bacteria, as well as by monitoring the peptides’ binding to the bacterial signaling nucleotide ppGpp. Our results demonstrate that the presence of only two D-amino acids in Esc(1-21)-1c is sufficient to downregulate ppGpp-mediated expression of biofilm-associated genes, presumably as a result of higher peptide stability and therefore prolonged interaction with the nucleotide. Overall, these studies should assist efficient design and optimization of new anti-infective agents with multiple pharmacologically beneficial properties.
Keywords: antimicrobial peptides, biofilm inhibition, Pseudomonas aeruginosa, virulence genes, amino acids epimerization
Graphical Abstract
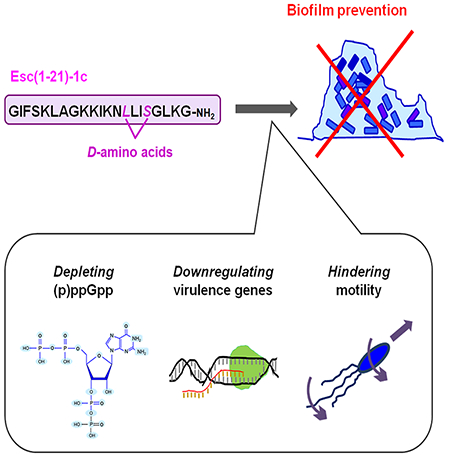
In aqueous environment, the AMP Esc(1-21)-1c prevents bacterial biofilm formation, by reducing the availability of the free nucleotide ppGpp. This leads to downregulation of the expression of virulence genes, thus hindering bacterial motility.
Introduction
A large variety of microorganisms, including the opportunistic Gram-negative bacterium Pseudomonas aeruginosa, have the ability to grow on various types of inert materials or biological tissues, forming multicellular sessile communities known as biofilms [1–3]. It is proposed that biofilm development in vitro proceeds through a cycle of distinct stages including a triggering attachment of bacteria to surfaces; an immobilization and formation of micro-colonies accompanied by production of the extracellular matrix [4,5]; a maturation complemented by activation of quorum sensing (QS) intercellular communication. In P. aeruginosa, the principal QS circuits are the las, rhl and pqs. They coordinate the response of bacterial cells to the surrounding environment via the production of signaling molecules. Once production of these molecules has reached a significant level, they activate their regulatory genes to enhance the expression of several virulence factors (e.g. toxins, proteases) [6,7]. Maturation of biofilm is then followed by selected cell death and a matrix degradation process which allows viable cells to dissociate, revert to the planktonic form and proceed with infectious dissemination [8–11].
According to recent studies, P. aeruginosa biofilms developed in vivo during chronic soft-tissue infections have generally a smaller size than those formed in vitro. Here, bacterial cell agglomerates become embedded into the host material and are then continuously exposed to the host’s own nutrients flows, as well as an entire range of the circulating communication cues, and to various host defense mechanisms [12,13].
Once established, biofilm communities are very difficult to be eradicated due to their high resistance to the available drugs and their ability to evade the host immune clearance [14–16]. These features explain why biofilm formation is associated with more than 80% of human clinical infections [17–19] including chronic infections of the lung, especially in cystic fibrosis (CF) [20–22], burn wounds, and otitis [17,18,23].
Importantly, even though biofilms represent a serious challenge to the medical field [18,24,25], there are currently no effective treatments for biofilm-related infections, and only limited studies have been conducted to date with the aim to identify novel compounds and therapies capable of selectively eradicating, preventing or inhibiting biofilm growth [26–35].
Naturally occurring cationic antimicrobial peptides (AMPs) hold promise for the development of a new class of antibiotics, due to their biocompatibility, wide spectrum of antimicrobial activity and immunomodulatory properties [24,36–41]. Their main mechanism of action generally involves destabilization of the target microbial membrane, and can also include action on multiple non-membranous targets (e.g. intracellular enzymes, proteins), altogether making AMPs less prone to induce resistance relative to conventional, single target-directed antibiotics [42,43]. During the past years, we found that a derivative of the frog skin AMP esculentin-1a, named Esc(1-21) (GIFSKLAGKKIKNLLISGLKG-CONH2 [44]), exhibits strong bactericidal activity. Indeed, it is active against both planktonic bacteria and existing biofilms of P. aeruginosa, with a membrane-perturbing activity as its major killing mechanism [35,45–47]. However, little is known about its potential to inhibit the formation of P. aeruginosa biofilm.
Remarkably, a common concern around the use of AMPs as new therapeutic agents is their high susceptibility to enzymatic degradation by ubiquitous host proteases [38]. One of the strategies pursued to increase AMPs biostability is the selective incorporation of non-natural (e.g. D-epimers) amino acids within the peptide sequence. This is because D-amino acids are not recognized by proteases, thus protecting the peptide from enzymatic cleavage [48,49]. Hence, we recently designed and synthesized a diastereomer of Esc(1-21), namely Esc(1-21)-1c, by replacing two L-amino acids, i.e. Leu14 and Ser17, with the corresponding D-enantiomers, and found out that it exhibited better activity than the all-L isomer in eradicating preformed Pseudomonas biofilms [50] and also in reducing lung bacterial burden in mouse models of acute P. aeruginosa lung infection [41]. Furthermore, Esc(1-21)-1c was shown to be more efficient in promoting migration of airway epithelial cells and preserving antipseudomonal activity in artificial sputum medium without inducing resistance [43]. Here, we have studied the ability of this peptide to prevent Pseudomonas biofilm formation and explored its underlying molecular mechanisms. These results were compared to those of the all-L parent peptide and demonstrated that the presence of two D-amino acids in Esc(1-21)-1c is sufficient to make this peptide not only more effective in downregulating the expression of virulence factors of P. aeruginosa, but also to hinder bacterial motility. This would hamper the formation of a sessile bacterial community. In addition, we also confirmed that the diastereomer exhibits reduced in vivo toxicity in a zebrafish (Danio rerio) embryo model, demonstrating Esc(1-21)-1c to be a more environmentally friendly and pharmacologically attractive candidate compared to the parent peptide.
Results
Inhibition of biofilm formation
To examine the ability of the two Esc peptides in inhibiting biofilm formation when used at dosages below the minimum inhibitory concentration (MIC), we first determined their MICs against the planktonic form of this pathogen (Table 1). The standard strain P. aeruginosa ATCC 27853 and clinical isolates (either from early or late stages of chronic lung infection in CF patients) were used for MIC and biofilm formation assays. Furthermore, the minimum peptide concentration causing 95% eradication of biofilm (BEC) formed by the four P. aeruginosa strains [50] was included in Table 1. The D-amino acid containing isomer was able to prevent biofilm formation of the selected strains at concentrations ranging from ½ to 1/16 MIC (Fig. 1). In contrast, the all-L peptide appeared to have no effect or it slightly stimulated biofilm formation. The only exception was observed at the highest concentration of Esc(1-21), i.e. ½ and ¼ MIC, against AA43 and the reference strain ATCC 27853, where the peptide was able to cause ~ 50% biofilm inhibition. It is notable that the biofilm inhibitory effect by Esc(1-21) and Esc(1-21)-1c was not due to inhibition of planktonic growth, as indicated by the absorbance values from bacterial cultures of the four P. aeruginosa strains being challenged with Esc peptides at sub-MICs, compared to untreated control cells (Fig. 2). Next, we analyzed the effect of two clinically-used antibiotics, aztreonam and colistin [51] at their sub-MIC levels (Table 2) on the CF clinical isolates. As indicated in Fig. 3, no inhibition, but rather stimulation of the biofilm formation, primarily for the KK1 strain, was observed for both antibiotics at all concentrations examined. These results are in line with what has been shown for different classes of commonly-used antibiotics [52–54] and even other AMPs [55]. As reported in Fig. 1, higher biofilm formation of P. aeruginosa AA43 and AA11 was shown at higher concentrations of Esc(1-21)-1c. Additionally, higher biofilm formation of KK1 and AA11 strains was observed upon treatment with ½ MIC of colistin and aztreonam, respectively, compared to lower antibiotics concentration (Fig. 3). As notes by Kaplan et al., some conventional antibiotics can act as inhibitors of biofilm formation at low concentration levels, promoters at higher levels, and once again as inhibitors at still higher concentration [54]. The precise biofilm-modulating action at sub-MIC depends on many factors including the type of antimicrobial agent used, its mode of action, level of persistency and age of the bacterial culture, the bacterial strain and its susceptibility to various classes of antibiotics, etc. Therefore, it does not seem unusual that AMPs can also have inhibitory activity on different bacterial strains which are not linearly dose-dependent (Fig. 1).
Table 1.
Antibacterial activity of the Esc peptides against various P. aeruginosa strains in the planktonic and biofilm forms.
| Antibacterial concentrations (μM) | ||||
|---|---|---|---|---|
| Esc(1-21) | Esc(1-21)-1c | |||
| Bacterial strains | MIC | BEC | MIC | BEC |
| P. aeruginosa ATCC 27853 | 4 | 12.5* | 8 | 12.5* |
| P. aeruginosa AA11 | 2 | 25* | 8 | 12.5* |
| P. aeruginosa AA43 | 2 | 12.5* | 4 | 12.5 |
| P. aeruginosa KK1 | 4 | 12.5* | 8 | 12.5* |
The reported MICs are the values obtained from three identical readings out of four independent experiments, and differences by a single dilution step are therefore significant.
Data taken from [50]
Fig. 1.
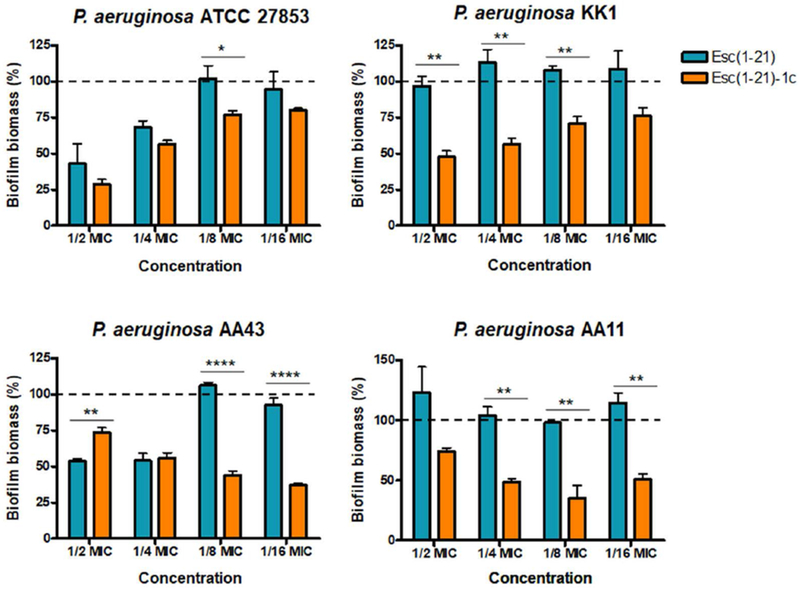
P. aeruginosa bacterial strains were incubated for 20 h in the presence of Esc peptides at concentrations below the MIC (from ½ to 1/16 MIC). Surface-associated biofilm after peptide treatment was evaluated by 0.05% CV staining followed by absorbance measurements at 590 nm. Results are reported as percentage of biofilm biomass with respect to untreated samples. The experiments were performed three times with 3 technical repeats and the data are reported as mean ± SEM. Dotted line indicates biofilm biomass of untreated bacteria. Statistical significance between the two peptides was determined by the Student’s t-test. *, p<0.05; **, p<0.01; ****, p<0.0001.
Fig. 2.
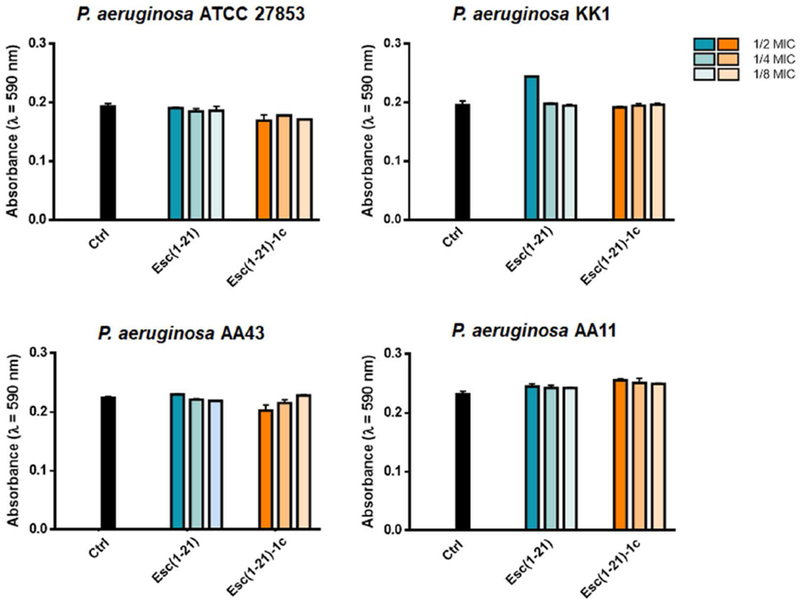
Bacterial growth of different P. aeruginosa strains in the presence of sub-MIC levels of Esc peptides with respect to the untreated control samples (Ctrl). Absorbance of the bacterial culture was measured at 590 nm after 20 h incubation at 37 °C. Data points are the mean ± SD of three different experiments.
Table 2.
Antibacterial activity of aztreonam and colistin against various P. aeruginosa strains in the planktonic and biofilm forms.
| Antibacterial concentrations (μM) | ||||
|---|---|---|---|---|
| Aztreonam | Colistin | |||
| Bacterial strains | MIC | BEC | MIC | BEC |
| P. aeruginosa ATCC 27853 | 16 | ND | 1 | 6.25 |
| P. aeruginosa AA11 | 4 | ND | 2 | 12.5 |
| P. aeruginosa AA43 | 32 | ND | 1 | 12.5 |
| P. aeruginosa KK1 | 16 | ND | 2 | 12.5 |
The reported values were obtained from three identical readings out of four independent experiments, and differences by a single dilution step are therefore significant. ND, not determined
Fig. 3.
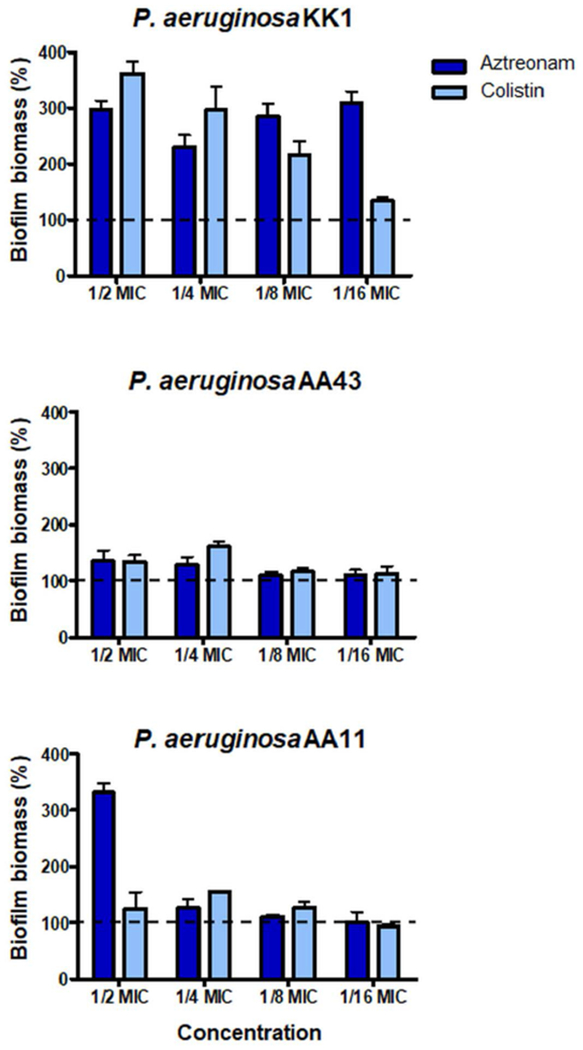
P. aeruginosa bacterial strains were incubated for 20 h in the presence of aztreonam and colistin at concentrations ranging from ½ to 1/16 MIC. Surface-associated biofilm after antibiotic treatment was evaluated by 0.05% CV staining followed by absorbance measurements at 590 nm. Results are reported as percentage of biofilm biomass with respect to untreated samples. The experiments were performed three times with 3 technical repeats and the data are reported as mean ± SEM. Dotted line indicates biofilm biomass of untreated bacteria.
Peptides’ effect on bacterial swimming, swarming and twitching motility
Since a more marked difference in inhibiting biofilm formation between the two isomers was observed against P. aeruginosa AA11, especially at 1/8 MIC, we selected this strain for subsequent experiments aimed at understanding the mechanism(s) underlying the inhibition of biofilm formation by Esc(1-21)-1c in comparison to the all-L parent peptide.
It is known that the initial step in the formation of a biofilm community in vitro is the adherence of bacterial cells to a surface. This is favored by bacterial movement induced by polar flagella in aqueous environments (swimming motility) [33]. Afterward, bacteria start to proliferate and to produce extracellular polymeric substances in which they remain entrapped. Type IV pili are involved in twitching motility, which is the bacterial movement inside the biofilm community resulting from extension and retraction of pili [56]. It is notable that twitching activity plays a direct role in the cell-to-cell interactions necessary for micro-colony formation and for the establishment of cell agglomerates characteristic of mature biofilms [57]. In addition to swimming and twitching motility, P. aeruginosa is able to swarm across viscous environments. It is a complex type of motility characterized by a coordinated progression of cells across a semi-solid surface and is dependent on both flagella and type IV pili. Swarming contributes to colonization of surfaces and plays a crucial role in the early biofilm development [58].
To study whether the inhibition of biofilm formation by Esc peptides was associated to their ability to impede bacterial swimming/swarming and/or twitching motility, AA11 growth was evaluated in 0.3%, 0.5% and 1% agarose plates, respectively, supplemented with the peptide at 1/8 MIC, according to a previously published procedure [33]. The D-amino acid containing peptide markedly decreased swimming, swarming as well as twitching motility, as evident from the significant reduction of the readouts (Fig. 4 A). In contrast, the all-L peptide did not influence the three kinds of cell motion until 48 h of co-incubation as shown by representative images of the bacterial growth zones (Fig. 4 B). To exclude the possibility that the negligible effect of Esc(1-21) on AA11 motility was due to a lower absolute concentration (i.e. 0.25 μM) compared to that of Esc(1-21)-1c (1 μM), motility assays were also performed at 1 μM for both Esc peptides. However, similar data were obtained (data not shown), confirming that the different behavior between the two peptides cannot be attributed to their different concentrations.
Fig. 4.
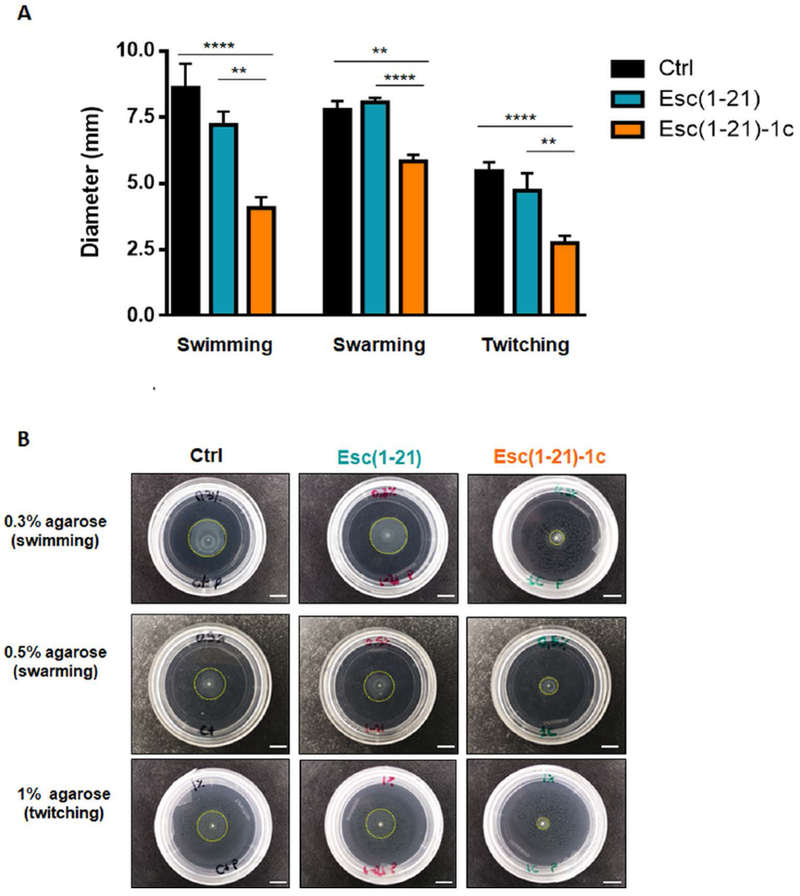
(A) Effect of Esc(1-21) and Esc(1-21)-1c at 1/8 MIC on the swimming, swarming or twitching motility of P. aeruginosa AA11. Bacterial cells were inoculated in 0.3 %, 0.5% or 1% agarose plates (for swimming, swarming or twitching motility assays, respectively). Swimming, swarming and twitching zones were determined after 24 h of incubation at 30 °C. Control samples (Ctrl) were plates inoculated with bacteria in the absence of peptide. All experiments were performed in triplicates with 3 technical repeats and statistical significance was determined using the Student’s t test. Data are shown as mean ± SEM. **, p<0.01; ****, p<0.0001. (B): Representative images of swimming, swarming and twitching activity of P. aeruginosa in the presence of each peptide compared to Ctrl, 48 h after incubation at 30 °C. Dotted circle line indicates the zonal extent of bacterial growth. Images are at 1x magnification. All scale bars represent 0.5 cm.
Gene-expression analysis
To specifically address the difference in inhibition of bacterial swimming, swarming and twitching motility by the Esc peptides, we studied the effect of the two peptides on the expression of selected genes known to be involved in the control of bacterial motility and in the biofilm formation [59]. As shown in Fig. 5, when applied at a sub-MIC level (i.e. 1 μM), Esc(1-21)-1c was found to significantly down-regulate the expression of fleN, fimT and fimX genes, encoding for the flagellar synthesis regulator FleN, the type IV fimbrial biogenesis protein FimT, and the twitching motility regulating phosphodiesterase FimX, respectively. Also lasI gene encoding for the quorum sensing molecule acyl-homoserine-lactone synthase as well as lasB gene encoding the elastase LasB (pseudolysin precursor involved in the pathogenicity of P. aeruginosa) were down-regulated (~95% reduction of mRNA levels). In addition, treatment with the non-natural diastereomer led to ~98% lowering in the level of mRNA encoding for rhamnosyltransferase subunits, i.e. RhlA and RhlB, both being key enzymes in the biosynthesis of rhamnolipids – bacterial surfactants which modulate P. aeruginosa swarming motility and biofilm formation/maintenance [60]. Moreover, upon treatment with the non-natural diastereomer, the expression level of rpoS gene was reduced by half compared to the level in bacteria exposed to Esc(1-21). This gene is reported to affect the production of extracellular alginate, exotoxin A and biofilm formation [61]. In contrast, both peptides did not significantly alter the expression of fliC gene (encoding the structural subunit of flagella, B-type flagellin).
Fig. 5.
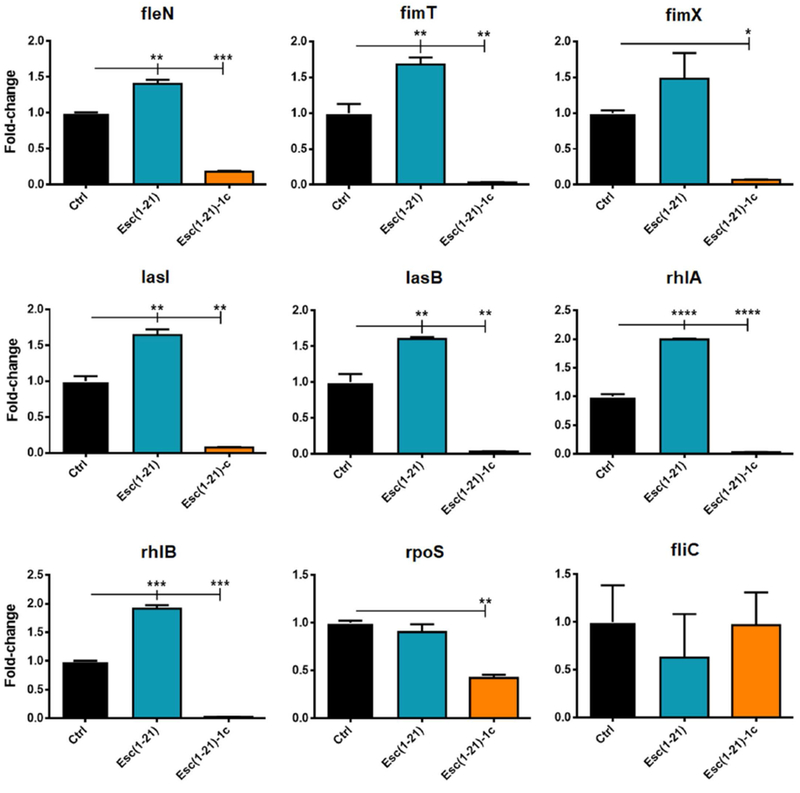
Effect of Esc peptides on the expression of the following genes in P. aeruginosa AA11: fleN, flagellar synthesis regulator fleN; fimT, type IV fimbrial biogenesis protein FimT; fimX, twitching motility regulating phosphodiesterase; lasI, autoinducer synthesis protein LasI; lasB, elastase LasB; rhlA, rhamnosyltransferase chain A; rhlB, rhamnosyltransferase chain B; rpoS sigma factor RpoS; fliC, flagellin type B. Ten mL of bacterial planktonic cells culture at a density of 108 CFU/mL was incubated with 1 μM peptide for 20 h in an orbital shaker at 37 °C. Total RNA was extracted immediately after the incubation. Results are representative of three independent experiments. Data are shown as mean ± SEM. Statistical analyses were performed by one-way ANOVA and Dunnett’s multiple comparisons test. *, p<0.05; **, p<0.01; ***, p<0.001; ****, p<0.0001 compared to untreated control cells, Ctrl (otherwise non-significant).
Production of pyoverdine and rhamnolipids
We also explored whether the two peptides differentially affected the production of virulence factors i.e, pyoverdine, the primary siderophore of P. aeruginosa which facilitates pathogenic growth at the infection site. As reported in Fig. 6 A, only Esc(1-21)-1c led to a significant reduction in the amount of siderophore (~3-fold lower than that of control samples), corroborating its significant antivirulent activity compared to Esc(1-21). In parallel, the production of rhamnolipids was investigated and found to be significantly reduced after the exposure to Esc(1-21)-1c (Fig. 6 B). This is in line with the results of rhlA and rhlB genes expression profile and with the reduced swarming activity, which is known to be dependent upon the self-produced biosurfactant rhamnolipid [62].
Fig. 6.
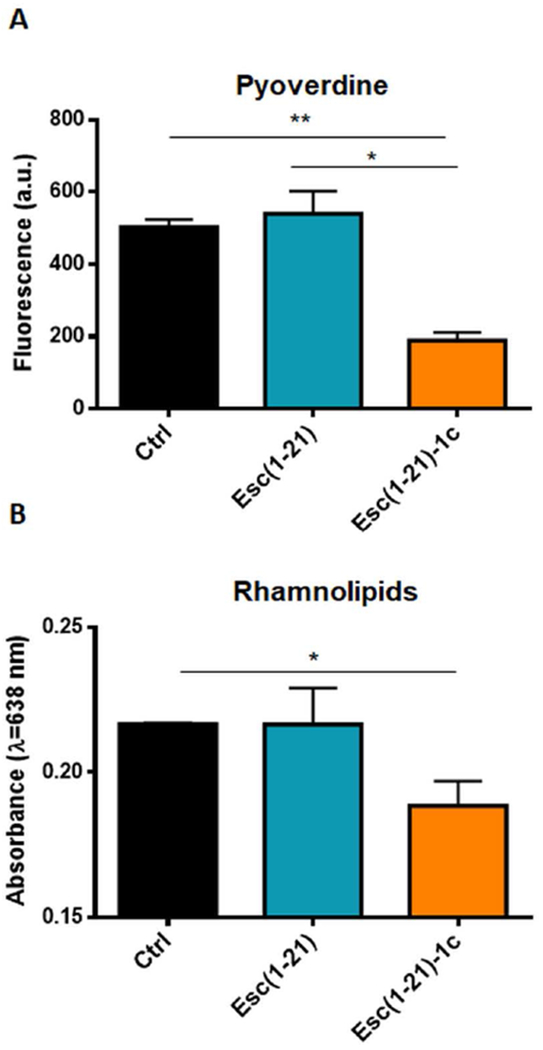
(A): Effect of Esc peptides on the production of pyoverdine in P. aeruginosa AA11 after 20 h incubation, compared to the cells not treated with the peptide (Ctrl). Bacteria were grown in 10% LB in water and pyoverdine was measured in the supernatant of the bacterial culture after 20 h treatment with the peptides at 37 °C. Samples were excited at 400 nm and fluorescence emission was measured at 460 nm and reported as arbitrary units. All values are the mean of three independent experiments ± SEM. Statistical significance was determined using the Student’s t test. *, p<0.05; **, p<0.01. (B): Effect of Esc peptides on the production of rhamnolipids by P. aeruginosa AA11 after 20 h incubation, compared to the bacterial cells not treated with the peptide (Ctrl). Bacteria were grown in 10% LB in water, and rhamnolipids were quantified by colorimetric assay as described in Materials and Methods. Absorbance was measured at 638 nm. The values are the mean ± SD of three independent experiments. Statistical significance was determined using the Student’s t test. *, p<0.05.
Confocal microscopy
For direct visualization of the peptides’ distribution within bacterial cells when used at sub-MIC, we incubated P. aeruginosa AA11 with the rhodamine-labeled peptides. Note that conjugation of rhodamine to these peptides did not affect their antimicrobial activity. Indeed, the MICs of Esc(1-21) and Esc(1-21)-1c against P. aeruginosa AA11 were equal to 2 μM and 8 μM, respectively. Examination of the samples revealed that both peptides accumulated at the membrane level mainly at the cell poles and division septa and that they diffused into the cytosol already within the first 5 min (Fig. 7). No pronounced variation was noted between the two isomers, despite a more homogeneous intracellular distribution of the non-natural diastereomer compared to Esc(1-21). The latter appeared to be located mostly on and beneath the plasma membrane. Furthermore, to exclude that rhodamine itself could drive the peptide distribution, bacterial cells were treated with the free dye at the same concentration as labeled peptides. No fluorescence was detected after washes (Fig. 7, control), confirming that rhodamine alone would not enter bacterial cells.
Fig. 7.
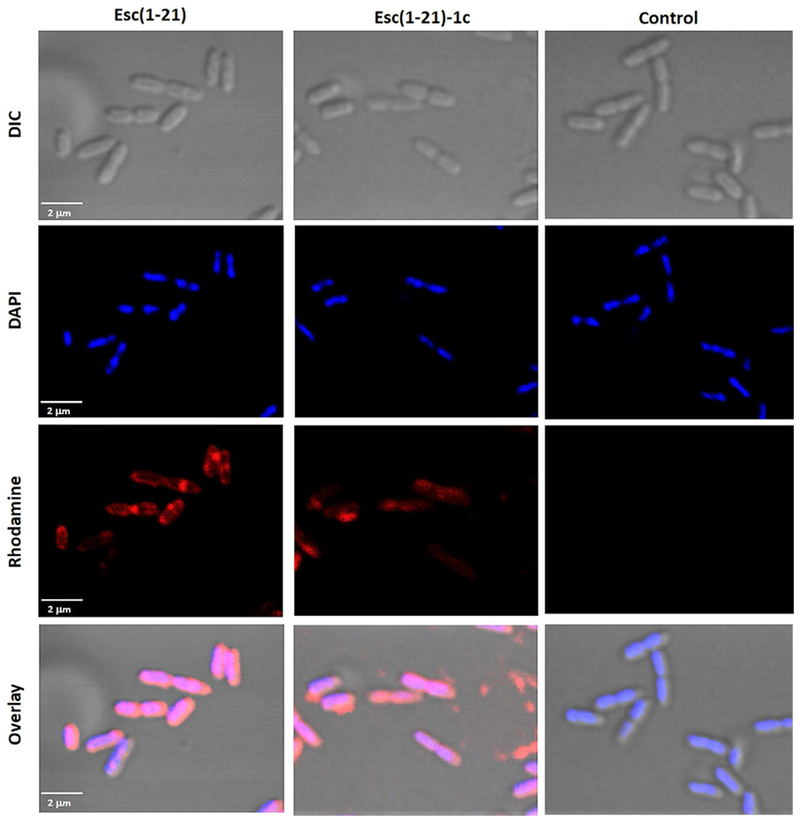
Confocal images of P. aeruginosa cells treated with rhodamine-labeled Esc peptides for 5 min. After peptide treatment, cells were stained with DAPI for DNA detection. DIC shows differential interference contrast images; DAPI shows DNA staining; rhodamine shows signal from labeled peptide and the free dye; overlay shows the merged channels. All images represent confocal single planes taken at the mid-cell height. Control samples are bacterial cells treated with the peptide-free rhodamine.
Involvement of ppGpp in peptides’ activity
Since an invariant mRNA transcription level was detected for the fliC gene, 20 h upon addition of Esc(1-21)-1c, we ruled out a general inhibition of RNA transcription by this peptide. In line with the literature, the bacterial alarmone (p)ppGpp is a nucleotide with a distinctive role in the stress-induced biofilm development and maintenance [63]. We therefore examined the ability of Esc peptides to interact with ppGpp by 31P-NMR, as has been previously described for other AMPs [64]. The 31P-NMR spectra in Fig. 8 shows that the resonances of the four phosphate groups in ppGpp were shifted by up to 3 ppm upfield upon adding increasing amounts of peptide. Interestingly, the α-phosphate resonances experienced the largest shifts, suggesting that the nucleoside part of the molecule is directly involved in binding. At the tested concentrations of up to a 2-fold excess of peptide over nucleotide, we did not observe any co-precipitation and/or concomitant disappearance of the ppGpp NMR signals. Furthermore, in the presence of sub-stoichiometric amounts of peptide, an 8-fold excess of ppGpp showed only a single set of signals, indicating fast exchange between the free and bound forms of ppGpp on the NMR timescale. Therefore, we can rule out an irreversible sequestration of the nucleotide by the peptide as a mechanism of Esc peptides action, which would have led to a broadening and depletion of the 31P-NMR signals, as recently reported for the antibiofilm-active peptide gramicidin S [64]. In the case of Esc peptides, we note that the wild type peptide and its non-natural diastereomer bound ppGpp in virtually the same way, hence the observed difference in their interference with biofilm signaling is not related to any differential affinity towards ppGpp.
Fig. 8.
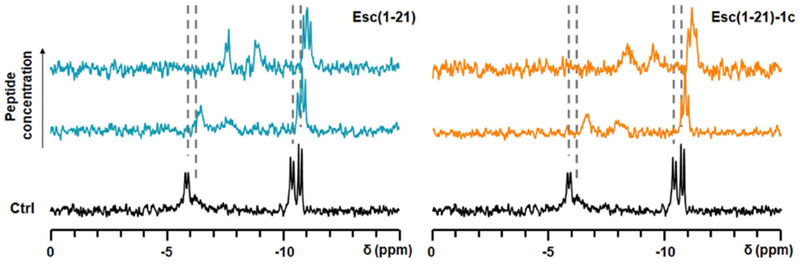
Interaction of Esc peptides with ppGpp, observed by 31P-NMR spectroscopy. Resonances from the four phosphate groups of ppGpp after 2 h incubation with the peptide in aqueous environment (10% D2O) are shifted compared to the Ctrl (without peptide). Dashed lines indicate the underlying chemical shifts of the nucleotide (without splittings).
In vivo acute toxicity on a zebrafish embryo model
For consideration of pharmacological use of AMPs it is important to demonstrate a high degree of selectivity towards bacteria versus animal cells, both in vitro and in vivo, to ensure safety. It was previously shown that the diastereomer Esc(1-21)-1c has a lower in vitro cytotoxic effect than the all-L peptide on a range of mammalian cell cultures, and that it is harmless to mice, when instilled intra-tracheally [41,50,65]. However, no information on its potential toxicity on other vertebrate organisms is known to date. Zebrafish embryotoxicity tests (FET) generally provide an economic and ethical way of expanding the range of vertebrate in vivo safety endpoint evaluations [66]. Here, they were used as a straightforward model to address the safety of Esc peptides in the treatment of P. aeruginosa caused airway-associated infections, because the FET LC50 values of environmental toxicants are known to correlate well with the inhalation LC50 values in rodents [67]. Both peptides were tested in a 2-fold dilution series for the whole-organism survival of wild type zebrafish embryos at the 72 hpf stage (Fig. 9). The D-amino acid containing isomer showed an approximately 5-times lower toxicity than the parent peptide (LC50 of 123.7 μM and 18.2 μM for Esc(1-21)-1c and Esc(1-21), respectively). Interestingly, the LC50 values remained unchanged between 1 h and 24 h exposure, suggesting that lethality is caused by a rapid mechanism common to both peptide isomers.
Fig. 9.
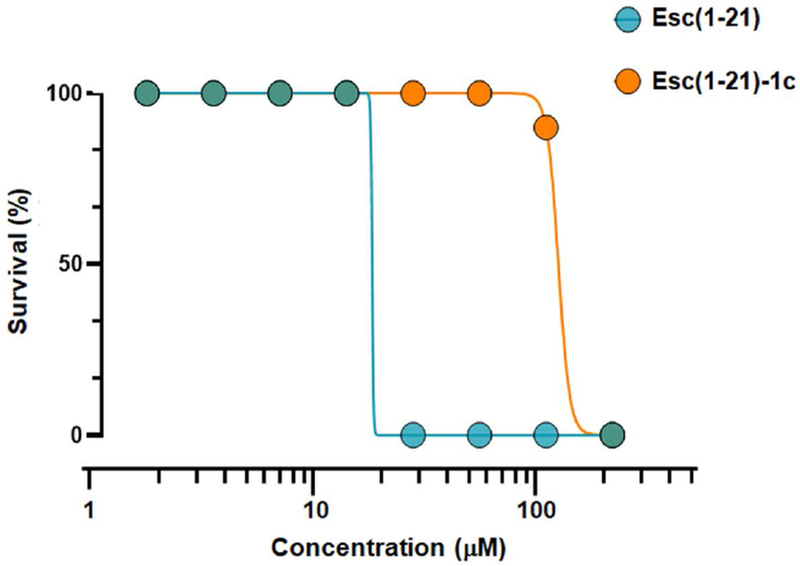
Survival of zebrafish embryos after peptide treatment at different concentrations. DMSO (2%) was used as vehicle and did not cause any toxicity. Data are the average of three independent experiments.
Discussion
P. aeruginosa is among the most clinically relevant pathogens leading to chronic biofilm infections that are highly resilient to physical disruption by external stresses and to immune clearance mechanisms [68]. Accordingly, it is challenging to gain insight into the ability of new antimicrobial agents to inhibit the development of sessile communities which protect bacterial cells during infection.
In this study, we first show that the presence of two D-amino acids in the AMP Esc(1-21)-1c is sufficient to significantly enhance its ability to prevent biofilm formation of Pseudomonas already within 1 day of peptide treatment at concentrations below those that hamper bacterial growth. The inhibited biofilm formation by Esc(1-21)-1c likely occurs through three different mechanisms. First, by inhibiting flagella-mediated swimming motility of P. aeruginosa cells as suggested by either motility assays (showing a reduction in the swimming diameter) or gene expression analysis (showing down-expression of fleN gene which controls the number of flagella). This would lead to a smaller number of bacteria to reach the surface for the initial attachment or to diffuse deep into the host tissues where in vivo biofilm formation starts [13]. Second, we observed that Esc(1-21)-1c decreased the mRNA level of genes responsible for type IV pili biosynthesis and function. As a result, this would hinder pili-mediated twitching motility (Fig. 4), and therefore also micro-colony formation and colonization of surfaces [69]. Third, we were able to demonstrate that Esc(1-21)-1c affects the two major quorum sensing systems, namely the lasI and rhl systems, by down-regulating genes encoding for virulence factors that are essential for biofilm development and host tissues damage e.g. RhlA, RhlB and LasB. In line with these findings is the observed reduced production of important molecules involved in the pathogenicity and in the biofilm architecture of P. aeruginosa, such as pyoverdine and rhamnolipids [70].
It is worthwhile emphasizing that both Esc(1-21) and its diastereomer are membrane-active peptides [17,71]. Hence, it is conceivable that they could perturb or destabilize the bacterial membrane at sub-MIC levels without compromising the bacterial viability [72]. The peptide would partition to the inner leaflet of the membrane phospholipid bilayer and diffuse into the cytosol. This is supported by confocal microscopy studies confirming a subcellular localization of the peptides underneath the membrane already after 5 min from their addition to the cells. However, both molecules appeared to be more concentrated at the level of apical ends and cell septa, where the negatively-charged cardiolipin (to which cationic peptides would have a high binding affinity) resides [73,74]. Most likely, cell poles are initially targeted by Esc peptides which would then translocate across the plasma membrane and distribute into the cytosol.
Previously report indicated that bacterial cells are known to respond to stressful environmental conditions by activating the stringent response [75]. Activation involves synthesis of small signaling nucleotides, guanosine 5′-diphosphate 3′-diphosphate (ppGpp) and guanosine 5′-triphosphate 3′-diphosphate (pppGpp), collectively denoted (p)ppGpp which regulate the expression of a plethora of genes promoting biofilm formation [63,76–79]. Targeting of (p)ppGpp had been described as a major mechanism of biofilm inhibition by the AMP IDR-1018 [80]. In our case, 31P-NMR studies indicated that both Esc peptides interact similarly though weakly with the nucleotide. Based on these data, we hypothesize that the different phenotypic outcome of the treatment with the two Esc isomers, within one day of bacterial exposure, is the consequence of a higher biostability of the D-amino acids containing diastereomer than the all-L peptide. This would lead to an extended antimicrobial efficacy of Esc(1-21)-1c compared to Esc(1-21) which could be more rapidly degraded by numerous bacterial proteases e.g. elastase, according to earlier studies [50].
As reported by Schafhauser and colleagues, the absence of ppGpp is linked to a reduced expression of lasI and rhl quorum-sensing associated genes and to a de-repressed expression of pqsA [81]. In our case, a prolonged interaction of Esc(1-21)-1c with ppGpp would not just lead to a transient depletion of the alarmone, but rather a constantly reduced availability of the free nucleotides. Hence, (p)ppGpp-mediated signaling pathways, which collectively regulate bacterial virulence (e.g. quorum sensing lasI and rhl systems), would be steadily slowed down. This is consistent with the reduced expression of lasI and rhl genes in bacterial cells upon exposure to this peptide (Fig. 5), along with the invariant expression levels of pqsA and pqsR (Fig. 10). A schematic representation of a possible mechanism of bacterial biofilm inhibition by Esc(1-21)-1c is reported in Fig. 11.
Fig. 10.
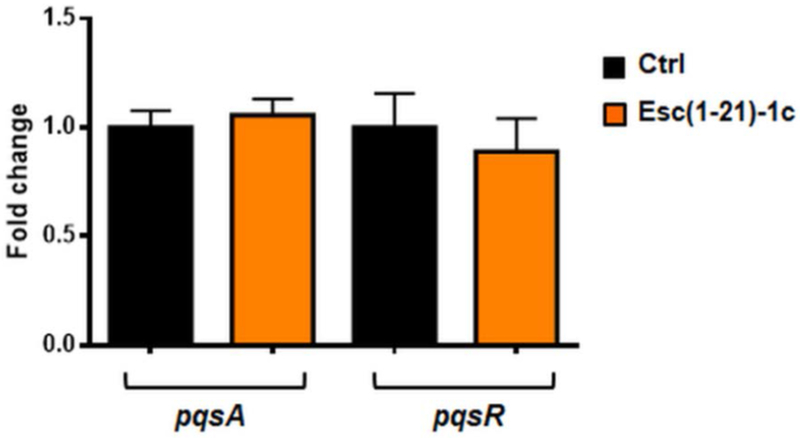
Effect of Esc(1-21)-1c on the expression of pqsA and pqsR genes in P. aeruginosa AA11. Ten mL of bacterial planktonic cells culture at a density of 108 CFU/mL was incubated with 1 μM peptide for 20 h in an orbital shaker at 37 °C. Total RNA was extracted immediately after the incubation. Results are representative of three independent experiments. Data are shown as mean ± SEM.
Fig. 11.
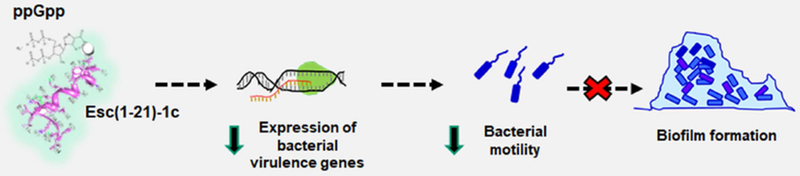
Cartoon representing one possible mechanism of bacterial biofilm inhibition activity by Esc(1-21)-1c. Prolonged peptide interaction with ppGpp reduces the availability of free nucleotides, lowering expression of virulence genes (including those controlling bacterial motility) which leads to preventative inhibition of biofilm formation.
Although the exact molecular mechanism is not yet known and would need to be further explored, a stereospecific mechanism mediated by selective inhibition or activation of other intracellular targets by the two peptides may be involved.
Notably, besides a direct reduction of the biofilm biomass or limiting its formation, an additional strategy would be to impede microbial growth in a human host and to delay the emergence of chronic infections. This strategy stands in contrast with the properties of currently used antibiotics, e.g. colistin and aztreonam, among others, which do not prevent but rather stimulate biofilm formation of the selected strains at sub-MIC levels (Fig. 3). As described in the literature, only few AMPs have shown the ability either to display a bactericidal activity or to arrest biofilm formation of P. aeruginosa [27,33,60]. Among them, LL-37 was reported to inhibit Pseudomonas biofilm formation, regardless of its chirality, but no data on the mechanism of action were provided [82].
We have demonstrated here for the first time that the presence of only two D-amino acids in Esc(1-21)-1c is sufficient to confer an ability to prevent biofilm formation of P. aeruginosa and to modulate expression of its virulence genes, presumably via an interaction of the peptide with the bacterial alarmone ppGpp. This activity becomes highly pronounced likely due to the enhanced resistance of the D-amino acid containing peptide to bacterial proteases e.g. elastase [65] and thereby altered pharmacokinetics compared to the all-L parent peptide. Altogether, the enhanced antibiofilm properties of Esc(1-21)-1c combined with its wound healing activity, higher biostability [65] and lower in vivo toxicity, when compared to the all-L counterpart, render this peptide a more attractive candidate for the development of novel antibiotics. In addition to other potential indications we envision that these agents will help fight Pseudomonas infections, especially in CF subjects where the compromised ability to clear bacteria by mucociliary action favors the establishment of chronic lung bacterial biofilm [83]. In addition to broadening our knowledge on the impact of D-amino acids on the ability of peptide antibiotics to control biofilm formation by interfering with the signal pathways of human pathogens, these studies should assist the design and optimization of new anti-infective agents with multiple therapeutically valuable properties.
Materials and Methods
Reagents
Crystal violet (CV), 4',6-diamidino-2-phenylindole (DAPI), colistin, aztreonam, rhodamine, Mowiol 4-88 and molecular biology grade dimethyl sulfoxide (DMSO), methylene blue solution 1.4% (w:v) in 95% ethanol were purchased from Sigma-Aldrich (St.Luis, MO). Guanosine-3',5'-bisdiphosphate (ppGpp) was from TriLink BioTechnologies (San Diego, CA). All the other chemicals were reagent grade.
Peptides
The synthetic Esc(1-21) and Esc(1-21)-1c (Esc peptides) as well as the rhodamine-labeled Esc peptides were purchased from Chematek Spa (Milan, Italy) and synthesized by solid-phase synthesis using standard Fmoc strategy. A purity of 98% was achieved via reverse-phase high-performance liquid chromatography; the molecular mass was verified by mass spectrometry.
Microorganisms
P. aeruginosa strains used in the assays were the reference ATCC 27853 and the CF clinical isolates KK1, AA43 and AA11, from the collection of the Cystic Fibrosis Outpatients Clinic at Hannover Medical School, Germany [84,85].
Determination of antimicrobial activities
Bacteria were grown in Luria-Bertani (LB) medium at 37 °C to a mid-log phase. Susceptibility testing was performed by adapting the microbroth dilution method outlined by the Clinical and Laboratory Standards Institute, using sterile Falcon® 96-well plates. Aliquots (50 μL) of bacteria at a concentration of 1 × 106 colony-forming units (CFU) in LB broth diluted 1:10 in water (10% LB) were added to 50 μL of diluted LB containing the peptide/antibiotic in serial 2-fold dilutions. Antimicrobial activities were expressed as the minimum inhibitory concentration (MIC), the concentration of peptide at which 100% inhibition of microbial growth is visually observed after 20 h of incubation at 37 °C. In parallel, the minimum concentration of peptides/antibiotics causing 95% eradication of Pseudomonas biofilm (BEC) was taken from [50] or determined according to the procedure described in [50].
Biofilm quantification
Pseudomonas strains were allowed to grow in LB medium at 37 °C until reaching an optical density of 0.8 at λ = 590 nm. They were then diluted to 10% LB in water and 50 μL were added into the wells of a 96-well plate containing 50 μL of 2-fold serial dilutions of peptides/antibiotics in 10% LB, to reach a final cell density of 5 × 105 CFU/mL. The plate was then incubated for 20 h at 37 °C in a humidified incubator. After 20 h, the bacterial biomass was evaluated by CV staining, as already reported [45].
Motility assays
P. aeruginosa AA11 was allowed to grow as described above. For swimming, swarming and twitching assays, 5 μL aliquots of the bacterial culture grown for 20 h in the presence of each peptide at a sub-MIC level (1/8 of MIC) were inoculated onto 3.5 cm dish plates containing 10% LB medium supplemented with 0.3%, 0.5% and 1% agarose plus the peptide at 1/8 MIC. Plates with 0.3%, 0.5% and 1% agarose were used to, respectively, simulate bacterial movement across an aqueous environment (i.e. swimming motility) or across a moist surface of low and moderate viscosity (i.e. swarming and twitching motility, respectively) [86]. The diameters of the swimming, swarming and twitching zones were determined after an incubation at 30 °C for 24 h.
Gene expression analysis
P. aeruginosa AA11 (1 × 108 cells/mL) was incubated in 10% LB supplemented with each peptide at a concentration of 1 μM for 20 h at 37 °C. Total bacterial RNA was extracted using TRI Reagent® (Sigma-Aldrich). The cDNA was synthesized by High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Fast SYBR Green Master Mix (Applied Biosystems) and 7900HT Fast Real-Time PCR System (Applied Biosystems) were used for quantification. The constitutively expressed rplU gene encoding 50S ribosomal protein was used as housekeeping gene to normalize qPCR data. Note that the expression of rplU gene did not vary under the described experimental conditions. Fold change was calculated by ΔΔCT values. Primer sequences are listed in Table 3.
Table 3.
Sequence of primers
| Gene | Primer sequence |
|---|---|
| fleN | Forward 5’-TGTTCGCCAAACTGACCA-3’ Reverse 5’-GTATACGGCTCGCTGTTTCT-3’ |
| fimT | Forward 5’-CGCTTGCAAAGAAGGAAAGG-3’ Reverse 5’-CCTTCCGCAGAGCAGAAA-3’ |
| fimX | Forward 5’-CCTGGCCTATATCCATCTCAAC-3’ Reverse 5’-ACTGTTCACGCATCAGTCC-3’ |
| lasI | Forward 5’-GGCGCGAAGAGTTCGATAAA-3’ Reverse 5’-CCATCTCGTCGATGACACTAAC-3’ |
| lasB | Forward 5’-CAACCAGAAGATCGGCAAGTA-3’ Reverse 5’-GTTCATGTCGACGGTGATGA-3’ |
| rhlA | Forward 5’-CGAGACCGTCGGCAAATAC-3’ Reverse 5’-GCACCTGGTCGATGTGAAA-3’ |
| rhlB | Forward 5’-CTCACGAGAAGTACGGGATTC-3’ Reverse 5’-CTCGGGCACGTTGAACT-3’ |
| rpoS | Forward 5’-CGGCGAGTTGGTCATCATCAAACA-3’ Reverse 5’-ATCGATTGCCCTACCTTGACCTGTC-3’ |
| fliC | Forward 5’-GTCAACACGAACATTGCTTCCC-3’ Reverse 5’-TTGCTGCCGACCTGGTAAGAAC-3’ |
| pqsA | Forward 5’-GACCGGCTGTATTCGATTC-3’ Reverse 5’-GCTGAACCAGGGAAAGAAC-3’ |
| pqsR | Forward 5’-CTGATCTGCCGGTAATTGG-3’ Reverse 5’-ATCGACGAGGAACTGAAGA-3’ |
| rplU | Forward 5’-CGCAGTGATTGTTACCGGTG-3’ Reverse 5’-AGGCCTGAATGCCGGTGATC-3’ |
Production of pyoverdine
P. aeruginosa AA11 cells (5 × 105 CFU/mL) were grown with mild agitation in 10% LB supplemented with each peptide at a concentration of 1 μM, for 20 h at 37 °C. The relative concentration of pyoverdine was quantified in the supernatants by measurement of the fluorescence intensity at 460 nm after excitation at 400 nm with a Tecan Infinite M200 microplate reader [87].
Production of rhamnolipids
The production of rhamnolipids was evaluated according to [88]. Briefly, P. aeruginosa AA11 (1 × 108 CFU/mL) in 10% LB (5 mL) was incubated for 20 h at 37 °C with 1 µM of Esc(1-21) and Esc(1-21)-1c and then centrifuged at 3,000 x g for 5 min. The supernatant was filtered (with 0.1 µm filter) to remove suspended cells and the pH was adjusted to 2.3 ± 0.2 using 1 N HCl. The supernatant was mixed with 4 mL of ethyl acetate for rhamnolipids extraction. The non-miscible mixture was vortexed and centrifuged (1 min at 100 x g) to allow phase separation. The upper organic phase was collected in a glass reaction tube, and the extraction procedure was repeated two more times. The collected organic phases were dried with a SpeedVac concentrator for the lipid quantification. The dry extract was dissolved in 4 mL chloroform and mixed with 400 µL methylene blue solution. The tubes were vortexed for 5 min and subsequently incubated at room temperature to stain the lipids coloration and allow phase separation. Then, 1 mL of the lower phase was mixed with 500 µL of 0.2 N HCl and phase separation was achieved by centrifugation (1 min at 100 x g). The mixture was left 10 min at room temperature; then 200 µl of the upper acidic phase was transferred in a 96-well microplate and the absorbance was measured at 638 nm with a Tecan Infinite M200 microplate reader.
Confocal fluorescence microscopy
Bacterial cells (1 × 108 CFU/mL) in 10% LB were incubated for 5 min at 37 °C with 5 µM of rhodamine-labeled Esc peptides. Afterwards, cells were washed with phosphate buffered saline (PBS) and seeded on round glass coverslips for about 30 minutes at 37 °C. Samples were then washed with PBS and fixed with 4% formaldehyde for 10 min at room temperature. Subsequently, they were washed with PBS, stained with 10 μg/mL of DAPI for 30 min at room temperature, for DNA detection. The coverslips were placed on a glass slide with a drop of mounting medium Mowiol 4-88 and visualized under a fluorescence confocal microscope (Olympus iX83-FV1200). DAPI and rhodamine-labeled peptides were visualized using laser excitation at 405 and 559 nm, respectively. All images were taken using a 60x NA 1.35 objective with zoom 3x.
Phosphorus-31 nuclear magnetic resonance (31P-NMR) spectroscopy
Freshly prepared stock solutions of Esc peptides and ppGpp in 10% D2O (pH 7.2) were combined in NMR tubes and adjusted to a final volume of 600 μL. A constant concentration of 0.5 mM ppGpp was maintained in all samples, whereas the amount of peptides was varied over a range of concentrations, from an excess of peptide (2:1 mol/mol) to an excess of nucleotide (1:8 mol/mol). To probe for binding, aggregation, and potentially even precipitation, each NMR sample was allowed to remain without perturbation at ambient temperature for at least 2 h. Proton-decoupled 31P-NMR spectra were acquired on a Bruker AVANCE 400 MHz spectrometer operating at a 31P frequency of 161.974 MHz. The experiments were performed without temperature control (room temperature) using a standard Bruker 5 mm BB-PABBO probe. For 1H decoupling, a WALTZ16 pulse sequence was used. A total of 3500 scans were accumulated for each spectrum, with an interpulse delay of 2 s. Spectra were processed with TopSpin 3.5 (Bruker), using line broadening of 5 Hz.
Acute toxicity in zebrafish embryos
All experimental procedures were performed in accordance with the Organization for Economic Cooperation and Development Test Guideline [89] and German animal protection standards. Freshly hatched wild type D. rerio embryos were obtained from the European Zebrafish Resource Center at Karlsruhe Institute of Technology. Fertilized eggs (6 hpf, hours post fertilization) were washed once with E3 medium to avoid any microorganism contamination. Then 20 eggs per well (6-well plate) were raised in 4 mL E3 medium at 28 °C until 72 hpf. For toxicity evaluation, the embryos were transferred into flat-bottom glass vials, containing embryonic medium E3 (15-20 embryos/vial). The volume in each bottle was adjusted to 980 µL, and peptides were added as a single 20 µL aliquot in 10% DMSO in serial 2-fold dilutions. Toxicity was expressed as the LC50 - the calculated concentration of peptide at which 50% of the embryos die - after visual viability readout at 1, 3 and 24 h (the values did not change) of incubation at room temperature. Each LC50 value is the average of three independent experiments.
Statistical analysis
Statistical analyses were performed by one-way analysis of variance and Dunnett’s multiple comparisons test or Student’s t test. Data are expressed as mean ± standard errors of the mean (SEM). Statistical significance is achieved at p<0.05.
Acknowledgments
This work was supported by grants from Sapienza University of Rome (projects RM116154C8434109 and RM11816436113D8A) and the Italian Cystic Fibrosis Foundation (project FFC#11/2014 adopted by FFC Delegations from Siena, Sondrio Valchiavenna, Cerea Il Sorriso di Jenny, and Pavia and project FFC 15/2017 Adopted by Delegations of Palermo, Vittoria, Ragusa, Siracusa, Catania Mascalucia, Messina, Gruppo di Sostegno di Tremestieri). M.L.M thanks Alessandra Bragonzi (San Raffaele Institute, Milan, Italy) and Burkhard Tummler (Klinische Forschergruppe, OE 6710, Medizinische Hochschule Hannover, Germany) for the P. aeruginosa clinical isolates. S.A. and A.S.U. thank the German Research Society for grant INST 121384/58-1 FUGG. This research was also partly supported by National Institutes of Health awards R01 HL-125128 and AI-133351.
Abbreviations:
- AMP
antimicrobial peptide
- BEC
biofilm eradication concentration
- CF
cystic fibrosis
- CFU
colony-forming units
- Ctrl
control
- CV
crystal violet
- DAPI
4',6-diamidino-2-phenylindole
- DIC
differential interference contrast (microscopy)
- DMSO
dimethyl sulfoxide
- Esc peptides
collectively Esc(1-21) and Esc(1-21)-1c
- LB
Luria-Bertani (medium)
- FET
fish embryotoxicity test
- hpf
hours post fertilization
- LC50
50% lethal concentration
- MIC
minimal inhibitory concentration
- NMR
nuclear magnetic resonance (spectroscopy)
- PBS
phosphate buffered saline
- ppGpp
guanosine-3',5'-bisdiphosphate
- QS
quorum sensing
Footnotes
Conflict of interest. The authors declare that they have no conflicts of interest with the contents of this article
References
- 1.Stoodley P, Sauer K, Davies DG, Costerton JW (2002) Biofilms as complex differentiated communities. Annu Rev Microbiol 56, 187–209. [DOI] [PubMed] [Google Scholar]
- 2.Rasamiravaka T, Labtani Q, Duez P, El Jaziri M (2015) The formation of biofilms by Pseudomonas aeruginosa: a review of the natural and synthetic compounds interfering with control mechanisms. Biomed Res Int 2015, 759348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moradali MF, Ghods S, Rehm BH (2017) Pseudomonas aeruginosa lifestyle: A paradigm for adaptation, survival, and persistence. Front Cell Infect Microbiol 7, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopez D, Vlamakis H, Kolter R (2010) Biofilms. Cold Spring Harb Perspect Biol 2, a000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romero D, Aguilar C, Losick R, Kolter R (2010) Amyloid fibers provide structural integrity to Bacillus subtilis biofilms. Proc Natl Acad Sci U S A 107, 2230–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pearson JP, Gray KM, Passador L, Tucker KD, Eberhard A, Iglewski BH, Greenberg EP (1994) Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc Natl Acad Sci U S A 91, 197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Shaer S, Shaaban M, Barwa R, Hassan R (2016) Control of quorum sensing and virulence factors of Pseudomonas aeruginosa using phenylalanine arginyl beta-naphthylamide. J Med Microbiol 65, 1194–1204. [DOI] [PubMed] [Google Scholar]
- 8.Hall-Stoodley L, Stoodley P (2002) Developmental regulation of microbial biofilms. Curr Opin Biotechnol 13, 228–233. [DOI] [PubMed] [Google Scholar]
- 9.Webb JS, Thompson LS, James S, Charlton T, Tolker-Nielsen T, Koch B, Givskov M, Kjelleberg S (2003) Cell death in Pseudomonas aeruginosa biofilm development. J Bacteriol 185, 4585–4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Webb JS, Givskov M, Kjelleberg S (2003) Bacterial biofilms: prokaryotic adventures in multicellularity. Curr Opin Microbiol 6, 578–585. [DOI] [PubMed] [Google Scholar]
- 11.Karatan E, Watnick P (2009) Signals, regulatory networks, and materials that build and break bacterial biofilms. Microbiol Mol Biol Rev 73, 310–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sonderholm M, Kragh KN, Koren K, Jakobsen TH, Darch SE, Alhede M, Jensen PO, Whiteley M, Kuhl M, Bjarnsholt T (2017) Pseudomonas aeruginosa aggregate formation in an alginate bead model system exhibits in vivo-like characteristics. Appl Environ Microbiol 83, e00113–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bjarnsholt T, Alhede M, Alhede M, Eickhardt-Sorensen SR, Moser C, Kuhl M, Jensen PO, Hoiby N (2013) The in vivo biofilm. Trends Microbiol 21, 466–474. [DOI] [PubMed] [Google Scholar]
- 14.Pestrak MJ, Chaney SB, Eggleston HC, Dellos-Nolan S, Dixit S, Mathew-Steiner SS, Roy S, Parsek MR, Sen CK, Wozniak DJ (2018) Pseudomonas aeruginosa rugose small-colony variants evade host clearance, are hyper-inflammatory, and persist in multiple host environments. PLoS Pathog 14, e1006842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romling U, Balsalobre C (2012) Biofilm infections, their resilience to therapy and innovative treatment strategies. J Intern Med 272, 541–561. [DOI] [PubMed] [Google Scholar]
- 16.Pang Z, Raudonis R, Glick BR, Lin TJ, Cheng Z (2019) Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and alternative therapeutic strategies. Biotechnol Adv 37, 177–192. [DOI] [PubMed] [Google Scholar]
- 17.Davies D (2003) Understanding biofilm resistance to antibacterial agents. Nat Rev Drug Discov 2, 114–122. [DOI] [PubMed] [Google Scholar]
- 18.Fux CA, Costerton JW, Stewart PS, Stoodley P (2005) Survival strategies of infectious biofilms. Trends Microbiol 13, 34–40. [DOI] [PubMed] [Google Scholar]
- 19.Akers KS, Cardile AP, Wenke JC, Murray CK (2015) Biofilm formation by clinical isolates and its relevance to clinical infections. Adv Exp Med Biol 830, 1–28. [DOI] [PubMed] [Google Scholar]
- 20.Govan JR, Deretic V (1996) Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol Rev 60, 539–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Filkins LM, O’Toole GA (2015) Cystic fibrosis lung infections: Polymicrobial, complex, and hard to Treat. PLoS Pathog 11, e1005258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silva Filho LV, Ferreira Fde A, Reis FJ, Britto MC, Levy CE, Clark O, Ribeiro JD (2013) Pseudomonas aeruginosa infection in patients with cystic fibrosis: scientific evidence regarding clinical impact, diagnosis, and treatment. J Bras Pneumol 39, 495–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kostakioti M, Hadjifrangiskou M, Hultgren SJ (2013) Bacterial biofilms: development, dispersal, and therapeutic strategies in the dawn of the postantibiotic era. Cold Spring Harb Perspect Med 3, a010306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Casciaro B, Cappiello F, Cacciafesta M, Mangoni ML (2017) Promising approaches to optimize the biological properties of the antimicrobial peptide esculentin-1a(1–21)NH2: Amino acids substitution and conjugation to nanoparticles. Front Chem 5, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arciola CR, Campoccia D, Montanaro L (2018) Implant infections: adhesion, biofilm formation and immune evasion. Nat Rev Microbiol 16, 397–409. [DOI] [PubMed] [Google Scholar]
- 26.Johansson EM, Crusz SA, Kolomiets E, Buts L, Kadam RU, Cacciarini M, Bartels KM, Diggle SP, Camara M, Williams P, Loris R, Nativi C, Rosenau F, Jaeger KE, Darbre T, Reymond JL (2008) Inhibition and dispersion of Pseudomonas aeruginosa biofilms by glycopeptide dendrimers targeting the fucose-specific lectin LecB. Chem Biol 15, 1249–1257. [DOI] [PubMed] [Google Scholar]
- 27.Junker LM, Clardy J (2007) High-throughput screens for small-molecule inhibitors of Pseudomonas aeruginosa biofilm development. Antimicrob Agents Chemother 51, 3582–3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kolodkin-Gal I, Romero D, Cao S, Clardy J, Kolter R, Losick R (2010) D-amino acids trigger biofilm disassembly. Science 328, 627–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu TK, Collins JJ (2007) Dispersing biofilms with engineered enzymatic bacteriophage. Proc Natl Acad Sci U S A 104, 11197–11202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muh U, Schuster M, Heim R, Singh A, Olson ER, Greenberg EP (2006) Novel Pseudomonas aeruginosa quorum-sensing inhibitors identified in an ultra-high-throughput screen. Antimicrob Agents Chemother 50, 3674–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riera E, Macia MD, Mena A, Mulet X, Perez JL, Ge Y, Oliver A (2010) Anti-biofilm and resistance suppression activities of CXA-101 against chronic respiratory infection phenotypes of Pseudomonas aeruginosa strain PAO1. J Antimicrob Chemother 65, 1399–1404. [DOI] [PubMed] [Google Scholar]
- 32.Valle J, Da Re S, Henry N, Fontaine T, Balestrino D, Latour-Lambert P, Ghigo JM (2006) Broad-spectrum biofilm inhibition by a secreted bacterial polysaccharide. Proc Natl Acad Sci U S A 103, 12558–12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de la Fuente-Nunez C, Korolik V, Bains M, Nguyen U, Breidenstein EB, Horsman S, Lewenza S, Burrows L, Hancock RE (2012) Inhibition of bacterial biofilm formation and swarming motility by a small synthetic cationic peptide. Antimicrob Agents Chemother 56, 2696–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Segev-Zarko L, Saar-Dover R, Brumfeld V, Mangoni ML, Shai Y (2015) Mechanisms of biofilm inhibition and degradation by antimicrobial peptides. Biochem J 468, 259–270. [DOI] [PubMed] [Google Scholar]
- 35.Casciaro B, Dutta D, Loffredo MR, Marcheggiani S, McDermott AM, Willcox MD, Mangoni ML (2017) Esculentin-1a derived peptides kill Pseudomonas aeruginosa biofilm on soft contact lenses and retain antibacterial activity upon immobilization to the lens surface. Biopolymers, e23074. [DOI] [PubMed] [Google Scholar]
- 36.Hilpert K, Volkmer-Engert R, Walter T, Hancock RE (2005) High-throughput generation of small antibacterial peptides with improved activity. Nat Biotechnol 23, 1008–1012. [DOI] [PubMed] [Google Scholar]
- 37.Hancock RE, Sahl HG (2006) Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol 24, 1551–1557. [DOI] [PubMed] [Google Scholar]
- 38.Fjell CD, Hiss JA, Hancock RE, Schneider G (2012) Designing antimicrobial peptides: form follows function. Nat Rev Drug Discov 11, 37–51. [DOI] [PubMed] [Google Scholar]
- 39.Easton DM, Nijnik A, Mayer ML, Hancock RE (2009) Potential of immunomodulatory host defense peptides as novel anti-infectives. Trends Biotechnol 27, 582–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cherkasov A, Hilpert K, Jenssen H, Fjell CD, Waldbrook M, Mullaly SC, Volkmer R, Hancock RE (2009) Use of artificial intelligence in the design of small peptide antibiotics effective against a broad spectrum of highly antibiotic-resistant superbugs. ACS Chem Biol 4, 65–74. [DOI] [PubMed] [Google Scholar]
- 41.Chen C, Mangoni ML, Di YP (2017) In vivo therapeutic efficacy of frog skin-derived peptides against Pseudomonas aeruginosa-induced pulmonary infection. Sci Rep 7, 8548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peschel A, Sahl HG (2006) The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat Rev Microbiol 4, 529–536. [DOI] [PubMed] [Google Scholar]
- 43.Casciaro B, Loffredo MR, Luca V, Verrusio W, Cacciafesta M, Mangoni ML (2018) Esculentin-1a derived antipseudomonal peptides: Limited induction of resistance and synergy with aztreonam. Protein Pept Lett 25, 1155–1162 [DOI] [PubMed] [Google Scholar]
- 44.Islas-Rodriguez AE, Marcellini L, Orioni B, Barra D, Stella L, Mangoni ML (2009) Esculentin 1–21: a linear antimicrobial peptide from frog skin with inhibitory effect on bovine mastitis-causing bacteria. J Pept Sci 15, 607–614. [DOI] [PubMed] [Google Scholar]
- 45.Luca V, Stringaro A, Colone M, Pini A, Mangoni ML (2013) Esculentin(1–21), an amphibian skin membrane-active peptide with potent activity on both planktonic and biofilm cells of the bacterial pathogen Pseudomonas aeruginosa. Cell Mol Life Sci 70, 2773–2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kolar SS, Luca V, Baidouri H, Mannino G, McDermott AM, Mangoni ML (2015) Esculentin-1a(1–21)NH2: a frog skin-derived peptide for microbial keratitis. Cell Mol Life Sci 72, 617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mangoni ML, Luca V, McDermott AM (2015) Fighting microbial infections: A lesson from amphibian skin-derived esculentin-1 peptides. Peptides 71, 286–295 [DOI] [PubMed] [Google Scholar]
- 48.Li H, Anuwongcharoen N, Malik AA, Prachayasittikul V, Wikberg JE, Nantasenamat C (2016) Roles of d-amino acids on the bioactivity of host defense peptides. Int J Mol Sci 17, e1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grieco P, Carotenuto A, Auriemma L, Saviello MR, Campiglia P, Gomez-Monterrey IM, Marcellini L, Luca V, Barra D, Novellino E, Mangoni ML (2013) The effect of d-amino acid substitution on the selectivity of temporin L towards target cells: identification of a potent anti-Candida peptide. Biochim Biophys Acta 1828, 652–660. [DOI] [PubMed] [Google Scholar]
- 50.Di Grazia A, Cappiello F, Cohen H, Casciaro B, Luca V, Pini A, Di YP, Shai Y, Mangoni ML (2015) D-Amino acids incorporation in the frog skin-derived peptide esculentin-1a(1–21)NH2 is beneficial for its multiple functions. Amino Acids 47, 2505–2519. [DOI] [PubMed] [Google Scholar]
- 51.Izadpanah M, Khalili H (2015) Antibiotic regimens for treatment of infections due to multidrug-resistant Gram-negative pathogens: An evidence-based literature review. J Res Pharm Pract 4, 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davies J, Spiegelman GB, Yim G (2006) The world of subinhibitory antibiotic concentrations. Curr Opin Microbiol 9, 445–453. [DOI] [PubMed] [Google Scholar]
- 53.Andersson DI, Hughes D (2014) Microbiological effects of sublethal levels of antibiotics. Nat Rev Microbiol 12, 465–478. [DOI] [PubMed] [Google Scholar]
- 54.Kaplan JB (2011) Antibiotic-induced biofilm formation. Int J Artif Organs 34, 737–751. [DOI] [PubMed] [Google Scholar]
- 55.Marina Berditsch SA, Tatiana Vladimirova, Parvesh Wadhwani and Anne S Ulrich (2012) Antimicrobial peptides can enhance the risk of persistent infections. Front. Immunol 3, 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Skerker JM, Berg HC (2001) Direct observation of extension and retraction of type IV pili. Proc Natl Acad Sci U S A 98, 6901–6904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Giraud C, Bernard CS, Calderon V, Yang L, Filloux A, Molin S, Fichant G, Bordi C, de Bentzmann S (2011) The PprA-PprB two-component system activates CupE, the first non-archetypal Pseudomonas aeruginosa chaperone-usher pathway system assembling fimbriae. Environ Microbiol 13, 666–683. [DOI] [PubMed] [Google Scholar]
- 58.Overhage J, Lewenza S, Marr AK, Hancock RE (2007) Identification of genes involved in swarming motility using a Pseudomonas aeruginosa PAO1 mini-Tn5-lux mutant library. J Bacteriol 189, 2164–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Winsor GL, Griffiths EJ, Lo R, Dhillon BK, Shay JA, Brinkman FS (2016) Enhanced annotations and features for comparing thousands of Pseudomonas genomes in the Pseudomonas genome database. Nucleic Acids Res 44, D646–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin Q, Deslouches B, Montelaro RC, Di YP (2018) Prevention of ESKAPE pathogen biofilm formation by antimicrobial peptides WLBU2 and LL37. Int J Antimicrob Agents 52, 667–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suh SJ, Silo-Suh L, Woods DE, Hassett DJ, West SE, Ohman DE (1999) Effect of rpoS mutation on the stress response and expression of virulence factors in Pseudomonas aeruginosa. J Bacteriol 181, 3890–3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Caiazza NC, Shanks RM, O’Toole GA (2005) Rhamnolipids modulate swarming motility patterns of Pseudomonas aeruginosa. J Bacteriol 187, 7351–7361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu X, Yu H, Zhang D, Xiong J, Qiu J, Xin R, He X, Sheng H, Cai W, Jiang L, Zhang K, Hu X (2016) Role of ppGpp in Pseudomonas aeruginosa acute pulmonary infection and virulence regulation. Microbiol Res 192, 84–95. [DOI] [PubMed] [Google Scholar]
- 64.Berditsch M, Trapp M, Afonin S, Weber C, Misiewicz J, Turkson J, Ulrich AS (2017) Antimicrobial peptide gramicidin S is accumulated in granules of producer cells for storage of bacterial phosphagens. Sci Rep 7, 44324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cappiello F, Di Grazia A, Segev-Zarko LA, Scali S, Ferrera L, Galietta L, Pini A, Shai Y, Di YP, Mangoni ML (2016) Esculentin-1a-derived peptides promote clearance of Pseudomonas aeruginosa internalized in bronchial cells of cystic fibrosis patients and lung cell migration: Biochemical properties and a plausible mode of action. Antimicrob Agents Chemother 60, 7252–7262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee KY, Jang GH, Byun CH, Jeun M, Searson PC, Lee KH (2017) Zebrafish models for functional and toxicological screening of nanoscale drug delivery systems: promoting preclinical applications. Biosci Rep 37, BSR20170199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ducharme NA, Reif DM, Gustafsson JA, Bondesson M (2015) Comparison of toxicity values across zebrafish early life stages and mammalian studies: Implications for chemical testing. Reprod Toxicol 55, 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Singh S, Singh SK, Chowdhury I, Singh R (2017) Understanding the mechanism of bacterial biofilms resistance to antimicrobial agents. Open Microbiol J 11, 53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chow L, Lin PC, Chang JS, Chu PY, Lee PK, Chen SN, Cheng YM, Lee JC, Chang JY, Liu TW (2012) Differences in the frequencies of K-ras c12–13 genotypes by gender and pathologic phenotypes in colorectal tumors measured using the allele discrimination method. Environ Mol Mutagen 53, 22–31. [DOI] [PubMed] [Google Scholar]
- 70.Skariyachan S, Sridhar VS, Packirisamy S, Kumargowda ST, Challapilli SB (2018) Recent perspectives on the molecular basis of biofilm formation by Pseudomonas aeruginosa and approaches for treatment and biofilm dispersal. Folia Microbiol (Praha) 63, 413–432. [DOI] [PubMed] [Google Scholar]
- 71.Loffredo MR, Ghosh A, Harmouche N, Casciaro B, Luca V, Bortolotti A, Cappiello F, Stella L, Bhunia A, Bechinger B, Mangoni ML (2017) Membrane perturbing activities and structural properties of the frog-skin derived peptide Esculentin-1a(1–21)NH2 and its diastereomer Esc(1–21)-1c: Correlation with their antipseudomonal and cytotoxic activity. Biochim Biophys Acta 1859, 2327–2339. [DOI] [PubMed] [Google Scholar]
- 72.Powers JP, Martin MM, Goosney DL, Hancock RE (2006) The antimicrobial peptide polyphemusin localizes to the cytoplasm of Escherichia coli following treatment. Antimicrob Agents Chemother 50, 1522–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Renner LD, Weibel DB (2011) Cardiolipin microdomains localize to negatively curved regions of Escherichia coli membranes. Proc Natl Acad Sci U S A 108, 6264–6269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Swain J, El Khoury M, Kempf J, Briee F, Van Der Smissen P, Decout JL, Mingeot-Leclercq MP (2018) Effect of cardiolipin on the antimicrobial activity of a new amphiphilic aminoglycoside derivative on Pseudomonas aeruginosa. PLoS One 13, e0201752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Potrykus K, Cashel M (2008) (p)ppGpp: still magical? Annu Rev Microbiol 62, 35–51. [DOI] [PubMed] [Google Scholar]
- 76.Srivatsan A, Wang JD (2008) Control of bacterial transcription, translation and replication by (p)ppGpp. Curr Opin Microbiol 11, 100–105. [DOI] [PubMed] [Google Scholar]
- 77.Costerton JW, Stewart PS, Greenberg EP (1999) Bacterial biofilms: a common cause of persistent infections. Science 284, 1318–1322. [DOI] [PubMed] [Google Scholar]
- 78.Hauryliuk V, Atkinson GC, Murakami KS, Tenson T, Gerdes K (2015) Recent functional insights into the role of (p)ppGpp in bacterial physiology. Nat Rev Microbiol 13, 298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Petchiappan A, Chatterji D (2017) Antibiotic resistance: Current perspectives. ACS Omega 2, 7400–7409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.de la Fuente-Nunez C, Reffuveille F, Haney EF, Straus SK, Hancock RE (2014) Broad-spectrum anti-biofilm peptide that targets a cellular stress response. PLoS Pathog 10, e1004152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schafhauser J, Lepine F, McKay G, Ahlgren HG, Khakimova M, Nguyen D (2014) The stringent response modulates 4-hydroxy-2-alkylquinoline biosynthesis and quorum-sensing hierarchy in Pseudomonas aeruginosa. J Bacteriol 196, 1641–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dean SN, Bishop BM, van Hoek ML (2011) Susceptibility of Pseudomonas aeruginosa biofilm to alpha-helical peptides: D-enantiomer of LL-37. Front Microbiol 2, 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Drenkard E, Ausubel FM (2002) Pseudomonas biofilm formation and antibiotic resistance are linked to phenotypic variation. Nature 416, 740–743. [DOI] [PubMed] [Google Scholar]
- 84.Bragonzi A, Paroni M, Nonis A, Cramer N, Montanari S, Rejman J, Di Serio C, Doring G, Tummler B (2009) Pseudomonas aeruginosa microevolution during cystic fibrosis lung infection establishes clones with adapted virulence. Am J Respir Crit Care Med 180, 138–145. [DOI] [PubMed] [Google Scholar]
- 85.Lore NI, Cigana C, De Fino I, Riva C, Juhas M, Schwager S, Eberl L, Bragonzi A (2012) Cystic fibrosis-niche adaptation of Pseudomonas aeruginosa reduces virulence in multiple infection hosts. PLoS One 7, e35648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mattick JS (2002) Type IV pili and twitching motility. Annu Rev Microbiol 56, 289–314. [DOI] [PubMed] [Google Scholar]
- 87.Deziel E, Comeau Y, Villemur R (2001) Initiation of biofilm formation by Pseudomonas aeruginosa 57RP correlates with emergence of hyperpiliated and highly adherent phenotypic variants deficient in swimming, swarming, and twitching motilities. J Bacteriol 183, 1195–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tsiry Rasamiravaka OMV, Mondher El Jaziri (2016) Procedure for rhamnolipids quantification using methylene-blue. Bio Protocol 6, DOI: 10.21769/BioProtoc.21783. [DOI] [Google Scholar]
- 89.Busquet FH, Braunbeck T, et al. (2013) 236 - Fish Embryo Acute Toxicity (FET) Test. . OECD Guidelines for the Testing of Chemicals. Organization for Economic Cooperation and Development. [Google Scholar]


