Abstract
Background
The nociceptin/orphanin FQ opioid peptide (NOP) receptor and its endogenous ligand N/OFQ have been implicated in the regulation of drug and alcohol use disorders. In particular, evidence demonstrated that NOP receptor activation blocks reinforcing and motivating effects of alcohol across a range of behavioral measures, including alcohol intake, conditioned place preference and vulnerability to relapse.
Methods
Here, we show the effects of pharmacological activation and inhibition of NOP receptors on binge-like alcohol consumption, as measured by the “drinking in the dark” (DID) model in C57BL/6J mice.
Results
We found that two potent and selective NOP agonists AT-202 (0, 0.3, 1, 3 mg/kg) and AT-312 (0, 0.3, 1 mg/kg) did not affect binge alcohol drinking at doses that do not affect locomotor activity. AT-202 also failed to alter DID behavior when administered to mice previously exposed to chronic alcohol treatment with an alcohol-containing liquid diet. Conversely, treatment with either the high affinity NOP receptor antagonist SB-612111 (0, 3, 10, 30 mg/kg) or the selective antagonist LY2817412 (0, 3, 10, 30 mg/kg) decreased binge drinking. SB-612111 was effective at all doses examined, LY2817412 was effective at 30 mg/kg. Consistently, NOP receptor knockout mice consumed less alcohol compared to wild type. SB-612111 reduced DID and increased sucrose consumption at doses that do not appear to affect locomotor activity. However, the high dose of SB-612111 (30 mg/kg) reduced alcohol intake but failed to inhibit preference in a two-bottle choice DID model that can assess moderate alcohol intake.
Conclusions
The present results suggest that NOP receptor inhibition rather than activation may represent a valuable approach for treatment of alcohol use disorders characterized by excessive alcohol consumption such as binge drinking.
Keywords: Alcohol, drinking in the dark, SB-612111, NOP receptor, N/OFQ
1. INTRODUCTION
Alcoholism and alcohol use disorders (AUD) represent an enormous public health concern in the United States and worldwide. The prevalence of alcohol abuse and dependence in the United States is about 7% of the adult population (SAMHSA, 2013) with an estimated annual economic burden of 249 billion dollars (Sacks et al., 2015). Due to the universal availability of alcohol, abuse in minors is also significant with 2.8% of adolescents having an alcohol use disorder, 35% of high school students reporting drinking and 21% binge drinking in the last 30 days (Kann et al., 2014). Nearly 88,000 people die from alcohol related causes annually, with roughly 10,000 driving-related fatalities (NHTSA, 2016), making it the third leading preventable cause of death in the United States (Stahre et al., 2014, CDC, 2015). Current FDA-approved pharmacotherapies to reduce alcohol consumption include disulfiram, which does not diminish craving and is known to cause peripheral neuropathy, acamprosate, and the opiate antagonist naltrexone, both of which have shown mixed results in clinical trials (Volkow and Skolnick, 2012, Liang and Olsen, 2014). Although various additional medications for treatment of alcohol dependence are under clinical investigation (Heilig and Egli, 2006, Johnson, 2008, Swift and Aston, 2015), new medications are still a high priority.
One of the target systems that preclinical pharmacology has identified as a candidate for new medications for AUD is the NOP-Nociceptin/Orphanin FQ (NOP-N/OFQ) system. N/OFQ is the endogenous ligand for the nociceptin opioid receptor (NOP), the fourth member of the opioid receptor family (Cox et al., 2015, Toll et al., 2016, Meunier et al., 1995). Consistent with the wide distribution of NOP receptors throughout the brain (Ozawa et al., 2015, Neal et al., 1999, Narendran et al., 2018), the N/OFQ-NOP receptor signal is involved in a multitude of physiological actions including pain, memory, anxiety, depression, obesity and reward associated with opiates, psychostimulants and alcohol (Witkin et al., 2014, Lambert, 2008). In particular, evidence has shown that activation of NOP receptors with N/OFQ and other NOP receptor agonists blocked acquisition, expression and reinstatement of alcohol-induced conditioned place preference (CPP) in mice (Kuzmin et al., 2003) and operant, as well as home cage alcohol self-administration and relapse in rats (Ciccocioppo et al., 2004, Ciccocioppo et al., 1999, Kuzmin et al., 2007, Witkin et al., 2014). However, many of these studies were conducted in an alcohol-preferring rat line characterized by overexpression of the N/OFQ-NOP receptor system in limbic regions (Economidou et al., 2008). Other studies found NOP agonists to be effective in decreasing alcohol drinking and relapse into alcohol seeking in rats with a previous history of alcohol dependence, but not in non-dependent rats (de Guglielmo et al., 2015, Martin-Fardon et al., 2010, Ciccocioppo et al., 2014). This overall evidence, along with findings that NOP receptor antagonism or genetic deletion of NOP receptor increased CPP and drug-induced CPP, suggest that the N/OFQ signal counteracts the basal or the positive hedonic state induced by rewarding drugs including alcohol (Sakoori and Murphy, 2008). In contrast, a lower voluntary alcohol consumption in NOP receptor knockout (KO) mice compared to wild type (WT) was shown by the same authors in a two-bottle choice test (Sakoori and Murphy, 2008). Consistent with this finding, genetically modified rats carrying constitutive deletion of NOP receptor show reduced motivation for alcohol and other drugs of abuse (Kallupi et al., 2017). Moreover, it was recently reported that a selective and orally-bioavailable NOP receptor antagonist from Eli Lilly reduced alcohol intake, motivation to consume alcohol, and relapse to alcohol seeking in alcohol-preferring rats (Rorick-Kehn et al., 2016). Clinically, a proof-of-concept study showed that the NOP antagonist, LY2940094, was effective in reducing the number of heavy drinking days in patients diagnosed with alcohol dependence (Post et al., 2016). Concurrently with this emerging evidence, we also have shown that NOP receptor antagonists have anti-drug abuse properties (Cippitelli et al., 2016). Together, these findings indicate that blockade of endogenous N/OFQ signaling also displays a modulatory function in attenuating alcohol use disorders. The understanding of the role of the N/OFQ-NOP receptor system in alcohol reward and addiction is still inconclusive.
To help clarify the involvement of this system in alcohol related processes, we sought to determine how the pharmacological manipulation of the NOP receptor with agonists and antagonists affects excessive alcohol consumption in a mouse model of binge drinking. Binge drinking refers to having five or more drinks for men and having four or more drinks for women in about 2 hours (Campo and Cameron, 2006). This can be modeled by a straightforward voluntary drinking paradigm whereby singly-housed C57BL/6J mice are exposed to 20% alcohol for 2 or 4 h, beginning 3 hours into the dark cycle (Rhodes et al., 2005, Thiele et al., 2014). Termed “drinking in the dark” (DID), this paradigm reliably produces pharmacologically relevant blood alcohol concentrations, even upon first exposure. Using this model we first tested the effects of two NOP receptor agonists and two antagonists on binge drinking behavior of C57BL/6J mice. Then, to verify whether alcohol-induced neuroadaptations elicited differential sensitivity to the effects of NOP-targeted compounds, we replicated these experiments in mice chronically exposed to an alcohol-containing liquid diet. Finally, we established behavioral specificity to the observed effects that altered binge-like alcohol consumption.
2. MATERIALS AND METHODS
2.1. Animals.
Male C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) used in the experiments were between 6 and 8 weeks of age and were housed three to four animals per cage up until the start of each experiment. During acclimation, all animals were kept on a reverse 12-h light/dark cycle with lights off at 7:00 AM and on at 7:00 PM. The animals were given food and water ad libitum, except where specified. NOP receptor (−/−) mice (Clarke et al., 2001), maintained on C57BL/6J background, were housed and maintained under identical environmental conditions throughout the study. All experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All methods used were pre-approved by the Institutional Animal Care and Use Committee at Florida Atlantic University and Torrey Pines Institute for Molecular Studies.
2.2. Drugs.
20% (v/v) alcohol solution was prepared by diluting 95% alcohol in water. Sucrose was dissolved in water to make a 0.5% (w/v) concentration. SB-612111, (5S,7S)-7-[[4-(2,6-dichlorophenyl)piperidin-1-yl]methyl]-1-methyl-6,7,8,9-tetrahydro-5H-benzo[7]annulen-5-ol hydrochloride, was provided by the National Institute of Drug Abuse. AT-202, previously known as SR16835, 1-(1-(2,3,3a,4,5,6-hexahydro-1H-phenalen-1-yl)piperidinl-4-yl)-indolin-2-one (Toll et al., 2009) and AT-312, (1-(1-((cis)-4-isopropylcyclohexyl)piperidin-4-yl)-1H-indol-2-yl)methanol) (Zaveri et al., 2018) were synthesized and provided by Astraea Therapeutics (Mountain View, CA). LY2817412 [2′-chloro-4′,4′-difluoro-1-{[1-(3-fluoropyridin-2-yl)-3-methyl-1H-pyrazol-4-yl]methyl}−4′,5′-dihydrospiro[piperidine-4,7′-thieno[2,3-C]pyran]2,3-dihydroxybutanedioate] was synthesized and provided by Eli Lilly (Indianapolis, IN). All compounds were suspended in a vehicle containing 2% DMSO and 98% hydroxypropyl cellulose (0.5% in distilled water). All compounds were administered in a 5 ml/kg volume injection and given by intraperitoneal (ip) route of administration.
2.3. DID procedure.
Except where specified, all experiments used a standard 4-day DID procedure that has been previously described (Rhodes et al., 2005, Thiele et al., 2014). Mice were singly housed in experimental chambers for 1 week prior to the beginning of the DID sessions. During this period mice were handled three times, habituated to ip 0.9% saline injection and exposed to 10 ml graduated plastic cylinders (fitted with a stainless-steel drinking spout) containing water for 2 days to acclimate the mice to the different drinking source. On days 1–3, beginning 3 h into the dark cycle, water bottles were removed from all cages and replaced with the graduated cylinders containing a 20% (v/v) alcohol solution. Mice had 2 h access to alcohol, after which alcohol cylinders were removed from cages and replaced with water bottles. The same procedure was followed on day 4 except that alcohol access was extended to 4 h. The effects of the NOP receptor agonists AT-202 (0, 0.3, 1, 3 mg/kg) and AT-312 (0, 0.3, 1 mg/kg), as well as the NOP antagonists LY2817412 (0, 3, 10, 30 mg/kg) and SB-612111 (0, 3, 10, 30 mg/kg) were examined in this assay in four different groups of mice of N=8, N=7, N=8 and N=10, respectively. NOP receptor targeted ligands were administered 30 min prior to the 4-h DID exposure according to a counterbalanced Latin square design. Drug treatment groups were initially equated based on alcohol consumption on days 1–3. Drug testing was performed every other day. Test days included a 4-h access to alcohol, with measurements taken at 2 h and 4 h. Non test days included 2-h access to alcohol. Drug doses were chosen according to previous literature (Zaratin et al., 2004, Toll et al., 2009, Zaveri et al., 2018) and preliminary observations. The amount of alcohol consumed was recorded immediately after each session and converted to grams/kilogram per each animal’s alcohol consumption and body weight. NOP KO (−/−, N=10) and WT (+/+, N=8) mice were subjected to DID procedure using identical methods as described above.
2.4. Blood Alcohol Concentrations (BACs).
To test if SB-612111 treatment affects alcohol metabolism, a 20% (v/v) alcohol dose of 3 g/kg was ip administered 30 min after pre-treatment with either SB-611121 10 mg/kg (N=6) or vehicle (N=6). Blood samples were taken from the mouse tail vein at 1, 2.5 and 4 h following alcohol administration (Livy et al., 2003), using heparinized capillary tubes with plungers (Polymer Technology Systems, Inc., Indianapolis, IN). Additional mice (N=6) were used to take blood samples at the end of the 4-h exposure period. Samples were immediately centrifuged (10 min, 1500g at 5 ºC), plasma was separated and kept at −20 ºC. Alcohol content was then assayed using ab65343 Ethanol Assay Kit (ABCAM, Cambridge, MA).
2.5. Chronic alcohol treatment by liquid diet.
To examine the effect of NOP receptor activation and blockade in binge-like alcohol drinking of mice previously exposed to a chronic alcohol treatment, an alcohol-containing liquid diet procedure was used as previously described (Anji and Kumari, 2008). The mice (N=10/group) were individually housed and lab chow was removed from cages immediately prior to the start of liquid diet exposure. From that point forward, the sole source of nutrition available to mice in the home cage was alcohol or control diet. The liquid diet was prepared fresh daily (9:00 AM) and composed by a high-protein chocolate-flavored nutritional energy drink (Boost®, Nestlé, Florham Park, NJ), alcohol (95% v/v), a vitamin/mineral mixture (3 g/L MP Biomedicals Vitamin Diet fortification mixture and 5 g/L U.S.P. XIV Salt Mixture) and water. Control liquid diet was similar except that it contained sucrose, calorically matching the alcohol liquid diet. Upon a 2-day acclimation with control diet, one group of mice (N=10) was switched to alcohol-containing liquid diet, whereas other mice (N=10) continued with the control diet. Alcohol concentration was progressively increased during the chronic treatment from 2.3% on day 1 and 2, to 4.7% on day 3 and 4 and 7% on days 5–15. To attenuate body weight lost during the alcohol treatment mice received a restricted amount of chow (~1 g/day) beginning day 7 through the end of the procedure. The amount of alcohol and control liquid diet consumed was tracked every day at 9:00 AM. Signs of alcohol withdrawal were assessed by observing handling-induced convulsion (HIC) at various time points into withdrawal (0, 1, 2, 4, 6, 8 and 24 h). HIC scoring was performed as previously described (Crabbe et al., 1991, Anji and Kumari, 2008) by two experimenters blind to the animals’ experimental history. One week following termination of the liquid diet procedure, mice were exposed to the standard DID procedure. The NOP receptor agonist AT-202 (0, 0.3, 1 mg/kg) and the antagonist SB-612111 (0, 3, 10, 30 mg/kg) were tested as described above. The dose of AT-202 (3 mg/kg) elicited observable, clear sedation and was excluded from the experiment. Drug testing with AT-202 and SB-612111 occurred in the same animals with the SB-612111 treatment followed by treatment with AT-202 upon re-establishment of a stable 2-h alcohol drinking pattern.
2.6. Sucrose drinking.
A new cohort of mice received 0.5% (w/v) sucrose instead of 20% (v/v) alcohol according to the DID procedure described above. A low concentration of sucrose (e.g., 0.5% w/v) is thought to provide a good control for the alcohol studies, as 20% (v/v) alcohol solution and 0.5% (w/v) sucrose solutions are consumed by mice at comparable levels (Anderson et al., 2019). Intake of sucrose was calculated as milliliters/kilogram body weight. One group of mice (N=9) was used for AT-202 testing. A second mouse group (N=10) was assigned to SB-612111 testing. AT-202 (0, 0.3, 1, 3 mg/kg) and SB-612111 (0, 3, 10, 30 mg/kg) were administered 30 min prior to the 4-h exposure according to a counterbalanced Latin square design. Drug testing was performed every other day. Test days included a 4-h access to sucrose, with measurements taken at 2 h and 4 h. Non test days included 2-h access to sucrose.
2.7. Open field test.
To determine whether doses of 3 mg/kg AT-202 and 30 mg/kg SB-612111 affected animal locomotion, additional mice (N=8 per group) were tested in the open field. The open field arenas (27.3 × 27.3 × 20.3 cm) were enclosed within sound-attenuating cubicles that were equipped with a fan for ventilation, white noise to mask extraneous sounds and two lights. Locomotor activity was recorded using a photocell-based automated monitoring system (Med Associates Inc., St Albans, Vermont, USA). Horizontal activity was detected by two infrared arrays in the x- and y-axes and measured as total ambulatory counts and ambulatory counts performed in 5-min intervals. Vertical activity was detected by a third array at 6 cm above the arena floor and measured as total number vertical counts and vertical counts performend in 5-min intervals. Med Associates Open Field Activity software was used to track and analyze the mouse movements. Well handled mice received an injection of saline 3 times prior to starting the locomotor activity test that occurred during the light cycle. On the day 1, mice were habituated to the testing room for 1 h, then they were placed in the center of the open field chambers allowed to explore for 30 min. On the day 2, after 30 min habituation to the testing room, mice received an injection of saline and 30 min later they were placed in the open field chambers for 30 min. Drug testing occurred on day 3 and 5 using an identical procedure as day 2 except that the drugs (AT-202 or SB-612111) or veh were administered instead of saline. An additional group of mice (N=8) were used to test locomotor activity 30 min following administration of AT-312 (0, 1, 3 mg/kg, ip) using identical methods. Mice were tested in a counterbalanced order with 1-day washout period between treatments.
2.8. Modification to the DID procedure.
Modification to the standard DID procedure consisted of giving mice (N=8) access to an additional cylinder containing water during the alcohol session, thus allowing the calculation of preference ratios (Giardino and Ryabinin, 2013). In this procedure, mice had 2 h daily access to water and 15% (v/v) alcohol beginning 3 h into the dark cycle for 14 days, in order to ensure stable levels of drinking and high preference (≥ 80% over the last three days) for the 15% alcohol solution. Water intake was calculated as ml/kg body weight. On day 15, mice were administered with the NOP receptor antagonist SB-612111 (0, 3, 10, 30 mg/kg) 30 min prior to exposure and the consumption of water and alcohol solution was monitored for 4 h. Drug testing was performed every other day according to a counterbalanced Latin square design. Test days included a 4-h access to 15% alcohol and water, with measurements taken at 2 h and 4 h. Non test days included 2-h access to 15% alcohol and water.
2.9. Data Analysis.
Statistical analyses were performed using analysis of variance (ANOVA). Effects of NOP receptor agonists and antagonists on DID and open field data were analyzed by means of a two-way ANOVA that used two within-subject factors (e.g., “time interval”, that is 0–2 h vs. 2–4 h epochs or 5-min intervals, and “treatment”). The same approach was used for sucrose drinking and for analysis of alcohol consumption, water consumption and preference in the two-bottle choice paradigm. Cumulative drinking (0–4 h) and total measures for open field parameters were analyzed by repeated measures ANOVA. Two-way ANOVA was also used to analyze HIC score, effects of NOP-directed ligands on DID in mice previously exposed to liquid and control diet, drinking patterns of KO vs. WT mice, and to assess the effects of SB-612111 on alcohol metabolism. The two-bottle free choice training procedure was analyzed by means of repeated measures ANOVA with treatment used as the only within-subject factor. Determination of BACs was carried out by a linear regression analysis. When appropriate, analyses were followed by Fisher’s least significant difference (LSD) post hoc tests.
3. RESULTS
3.1. Effect of NOP receptor agonism and antagonism on binge-like alcohol consumption
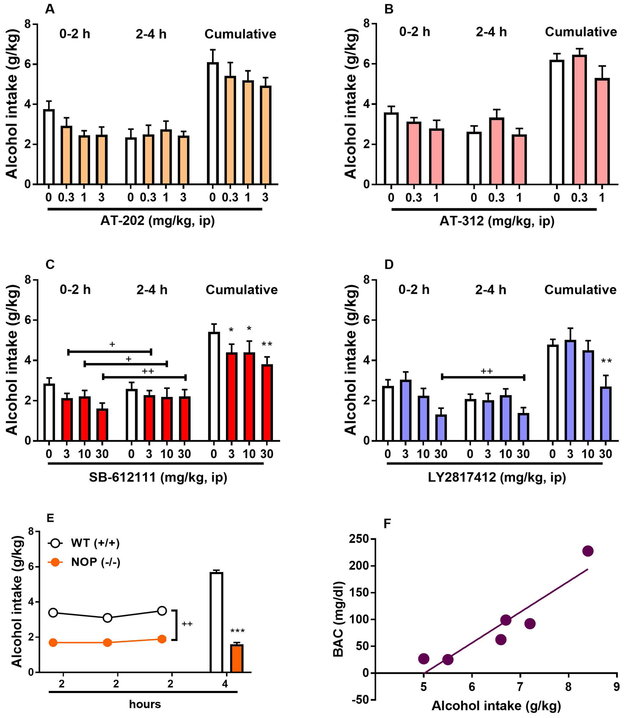
To determine the effects of NOP receptor activation on binge-like alcohol consumption, the potent and selective NOP receptor agonists AT-202 (0, 0.3, 1, 3 mg/kg) and AT-312 (0, 0.3, 1 mg/kg) (Cippitelli et al., 2016, Zaveri et al., 2018) were tested in the DID paradigm. As revealed by ANOVA, average alcohol consumption was not changed following treatment with either AT-202 (main effect of “time” [F(1,7)= 4.1, NS], main effect of “treatment” [F(3,21)=2.0, NS], “time” x “treatment” interaction [F(3,21)=1.9, NS], Figure 1A) or AT-312 (main effect of “time” [F(1,6)= 0.6, NS], main effect of “treatment” [F(2,12)=1.6, NS], “time” x “treatment” interaction [F(2,12)=2.1, NS], Figure 1B). To determine the effects of NOP receptor inhibition on binge-like alcohol consumption, two chemically unrelated selective NOP receptor antagonists, namely LY2817412 (0, 3, 10, 30 mg/kg) and SB-612111 (0, 3, 10, 30 mg/kg) (Kallupi et al., 2017, Witkin et al., 2014, Rizzi et al., 2007, Silva et al., 2019), were tested in the same paradigm. ANOVA revealed a significant main effect of “treatment” with the antagonist SB-612111 [F(3,27)= 3.6, p<0.05] accompanied by lack of “time” x “treatment” interaction [F(3,27)=0.6, NS] with post hoc analysis conducted on the “treatment” effect showing reduced intake at all doses examined (3 & 10 mg/kg, p<0.05; 30 mg/kg, p<0.01), Figure 1C. ANOVA also revealed a significant main effect of LY2817412 on binge-like alcohol consumption [F(3,21)= 6.1, p<0.01] with no “time” x “treatment” interaction [F(3,21)=1.9, NS]. On post hoc analysis the 30 mg/kg dose significantly reduced binge-like alcohol drinking (p<0.01, Figure 1D). One-way repeated measures ANOVA, used to verify the effects of agonists and antagonists on cumulative alcohol drinking, led to the same statistical values detected by two-way ANOVAs for main treatement effects. These results indicate that NOP receptor inhibition rather than activation reduces binge-like alcohol consumption. WT (+/+) and NOP (−/−) mice, both of C57BL/6J background, were exposed to 20% (v/v) alcohol bottles for 2 h/day for three consecutive days. NOP (−/−) mice consumed significantly less alcohol compared with WT (+/+) mice. As shown in Figure 1E, ANOVA showed a significant effect of “genotype” [F(1,16)=15.2; p<0.01] associated with no significant effect of “time” [day, F(2,32)=0.5, NS] and no interaction “genotype” × “time” [F(2,32)=0.2, NS]. After the 3 days of 2-h alcohol availability, alcohol was made available for 4 h to both mouse genotypes. A significant effect of genotype was observed [F(1,16)=32.0; p<0.001]. BACs were assessed in a separate group of mice exposed to 20% alcohol for 4 hours. Mice consumed 6.6 ± 0.5 g/kg with average BAC of 89.0 ± 30.6. The amount of alcohol consumed after 4 hours significantly correlated with the measured BACs (R2=0.86; p=0.0073) Figure 1F).
Figure 1.
Binge-like alcohol consumption in the drinking in the dark (DID) procedure and modulation by NOP receptor agonists and antagonists. Mice were treated acutely with the potent NOP receptor agonists (A) AT-202 (0.0, 0.3, 1.0, 3.0 mg/kg, ip) and (B) AT-312 (0, 0.3, 1 mg/kg, ip) 30 min prior to 20% (v/v) alcohol access. There were no significant effects in either experiment. Mice were treated acutely with the selective NOP receptor antagonists (C) SB-612111 (0, 3, 10, 30 mg/kg, ip) and (D) LY2817412 (0, 3, 10, 30 mg/kg, ip) 30 min prior to 20% (v/v) alcohol access. Both antagonists attenuated alcohol drinking. All data are presented as mean (± SEM) alcohol intake in 2-h fractions (0-2 h & 2-4 h) and as mean (± SEM) cumulative (0-4 h) alcohol intake (g/kg) of N=8-10 mice per group. (E) Drinking patterns of NOP receptor Knockout (NOP −/−) and wild type (WT, +/+) mice in the DID model of binge alcohol drinking. NOP (−/−) mice drink consistently less as compared to WT (+/+) animals. All data are presented as mean (± SEM) alcohol intake (g/kg) in 2 and 4 h of N=8-10 mice per genotype. (F) Blood alcohol concentrations (BACs, mg/dl) were monitored at the end of the 4-h 20% alcohol exposure in (N=6) mice. *p<0.05, **p<0.01, ***p<0.001 difference from control. +p<0.05, ++p<0.01 difference from control. Connecting line with single or double plus (+ or ++) indicates combined post hoc testing. For detailed statistics, see “Results”.
3.2. Effect of NOP receptor activation and inhibition on binge-like alcohol consumption following a history of chronic alcohol exposure
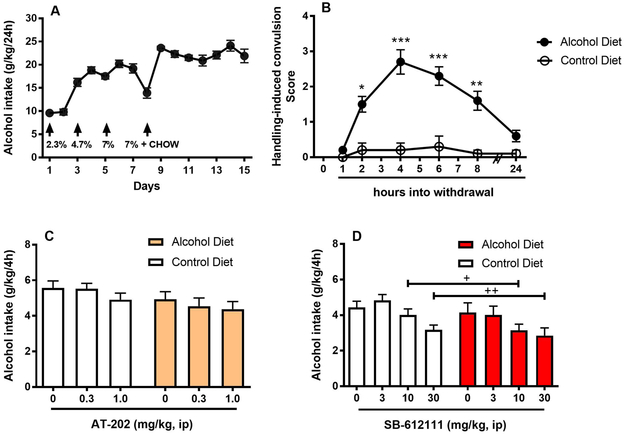
The amount of alcohol-containing liquid diet consumed was measured every day. By gradually increasing alcohol concentration, alcohol intake escalated from 9.6 ± 0.3 g/kg/day to values as high as 24.1 ± 1.2 or 21.9 ± 1.5 g/kg/day observed in the last two days of exposure, respectively (Figure 2A). To verify the successful induction of physical dependence, withdrawal following alcohol discontinuation was examined by determining whether mice showed HICs. ANOVA revealed significant difference in HIC scores between liquid and control diet mice (“group” x “time point” interaction: [F(5,90)=18.2, p<0.001]. As determined by post hoc analysis, the severity of withdrawal increased as a function of time. The peak withdrawal severity score was reached between 4 h and 6h following the onset of the withdrawal (p<0.001 for both time points) and subsided at 24 h (Figure 2B). Because the NOP receptor agonist MT-7716 preferentially inhibits the reinforcing effects of alcohol particularly in subjects with a previous history of alcohol dependence (de Guglielmo et al., 2015), AT-202 was tested on the DID paradigm in mice previously exposed to chronic liquid diet exposure and exposed to control diet to determine whether it was equally effective in blocking binge-like drinking behavior. As shown in Figure 2C, pretreatment with the NOP agonist failed to alter binge-like alcohol drinking in both liquid diet exposed and control diet exposed mice (main effect of “treatment” [F(2,36)=1.6, NS], main effect of “group” [F(1,18)=3.1, NS], interaction between the two factors [F(2,36)=0.2, NS]). When the NOP receptor antagonist SB-612111 was tested, results clearly showed that SB-612111 reduced binge alcohol drinking in both liquid diet-exposed and control diet-exposed mice (main effect of “treatment” [F(3,54)=8.4, p<0.001]; main effect of “group”:[F(1,18)=1.8, NS]). The lack of interaction between factor “treatment” and factor “group” [F(3,54)=0.3, NS] indicated similar efficacy of the antagonist in reducing binge alcohol drinking (Figure 2D). Post hoc analysis of the “treatment” factor displayed efficacy of SB-612111 at 10 mg/kg (p<0.05) and 30 mg/kg dose (p<0.01).
Figure 2.
Effects of NOP receptor directed ligands in mice with a previous history of chronic alcohol by liquid diet. Mice were exposed to an alcohol-containing liquid diet (N=10) or isocaloric control diet (N=10) for 15 days. (A) Upon escalation, alcohol intake stabilized at values over 20 g/kg/day during the last days of treatment. Arrows mark the days when the alcohol concentration of the liquid diet was increased and when mice started receiving a restricted amount of chow (~1 g/day) to attenuate body weight lost during the alcohol treatment. (B) HIC scores were recorded at various time points into withdrawal. The peak withdrawal severity score was reached at 4 h following the onset of the withdrawal and subsided at 24 h. (C) Acute treatment with the NOP receptor agonist AT-202 (0, 0.3, 1 mg/kg, ip) 30 min prior to alcohol exposure failed to alter binge-like alcohol drinking in both liquid diet exposed and control diet exposed mice. (D) Acute treatment with SB-612111 (0, 3, 10, 30 mg/kg, ip) elicited significant reduction of alcohol intake at 10 and 30 mg/kg in both liquid diet-exposed and control diet-exposed mice. All data are presented as mean (± SEM) alcohol intake (g/kg) in 4 h. *p<0.05, **p<0.01, ***p<0.001 difference from control diet group. +p<0.05, ++p<0.01 difference from vehicle groups (0 mg/kg). Connecting line with single or double plus (+ or ++) indicates combined post hoc testing. For detailed statistics, see “Results”.
3.3. Effect of NOP receptor agonism and antagonism on sucrose consumption
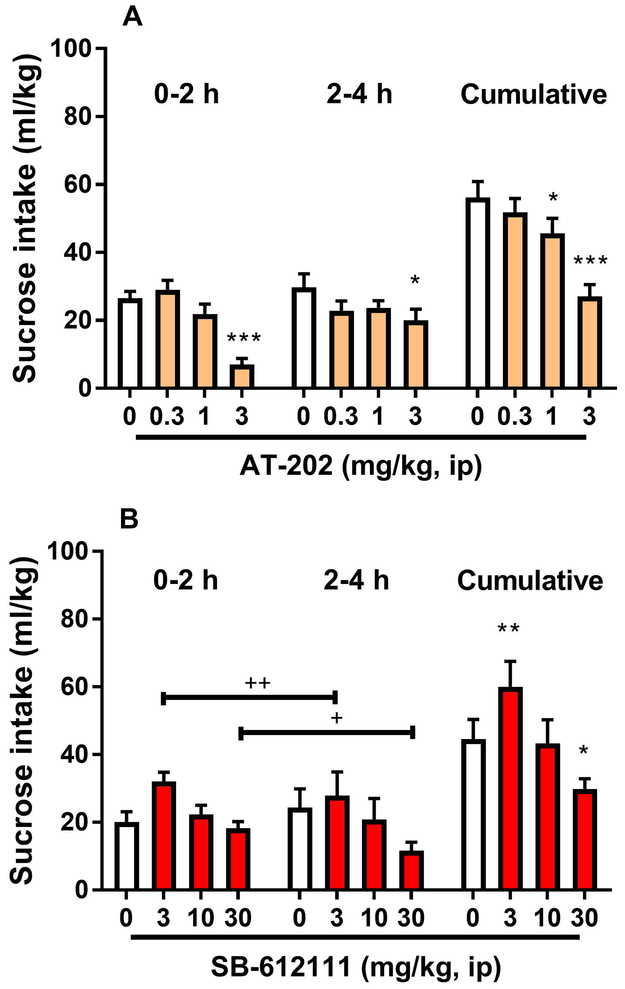
The agonist AT-202 altered sucrose intake (“time” x “treatment” interaction [F(3,24)=4.6, p<0.05]). Post hoc analysis revealed a significant reduction of the intake at 3 mg/kg during the two drinking intervals (0–2 h & 2–4 h, p<0.05). One-way repeated measures ANOVA conducted on the cumulative sucrose intake confirmed this reduction [F(3,24)=16.2, p<0.001]. On post hoc analysis, doses of AT-202 1 and 3 mg/kg were both effective (p<0.05 and p<0.001, respectively; Figure 3A). To control for the observed alcohol suppressive effect of NOP receptor inhibition, SB-612111 (0, 3, 10, 30 mg/kg) was tested on 0.5% (w/v) sucrose intake under the same conditions used in the alcohol DID paradigm. Average sucrose consumption following treatment with SB-612111 was significantly altered as revealed by ANOVA (main effect of “treatment” [F(3,27)=10.5, p<0.001]. No “time” x “treatment” interaction was observed [F(3,27)=1.2, NS]). Post hoc analysis of the “treatment” factor indicated changes at 3 (p<0.01) and 30 mg/kg (p<0.05), with the smallest dose inducing increased sucrose drinking and the highest dose inducing decreased consumption (Figure 3B).
Figure 3.
Effects of administration of the NOP receptor agonist AT-202 and the antagonist SB-612111 on sucrose consumption. Mice received injection (ip) of (A) AT-202 (0, 0.3, 1, 3 mg/kg) or (B) SB-612111 (0, 3, 10, 30 mg/kg) 30 min prior to the start of the 4-h test drinking session of the DID procedure with 0.5% (w/v) sucrose, respectively. AT-202 treatment reduced sucrose consumption. SB-612111 increased sucrose intake at low dose (3 mg/kg) while decreasing consumption at the figh dose examined (30 mg/kg). All data are presented as mean (± SEM) sucrose intake in 2-h fractions (0-2 h & 2-4 h) and as mean (± SEM) cumulative (0-4 h) sucrose intake (ml/kg) of N=9–10 mice per group. * p<0.05, **p<0.01, ***p<0.001 difference from vehicle groups (0 mg/kg). +p<0.05, ++p<0.01 difference from vehicle groups. Connecting line with single or double plus (+ or ++) indicates combined post hoc testing. For detailed statistics, see “Results”.
3.4. Effect of NOP receptor ligands on locomotor activity
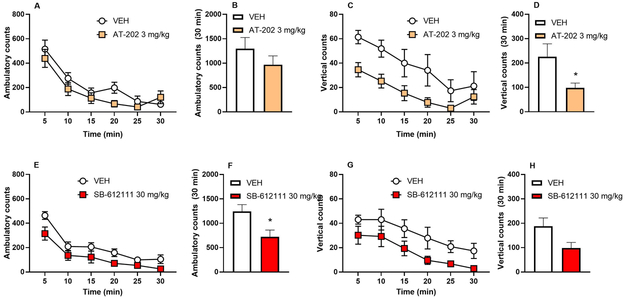
Global (horizontal and verical) locomotor activity was measured in mice treated with AT-202 (0, 3 mg/kg) and SB-612111 (0, 30 mg/kg). AT-202 did not significantly alter the number of horizontal ambulatory counts (main effect of “treatment” [F(1,7)=4.1, NS]; main effect of “time” [F(5,35)=34.1, p<0.001]; interaction [F(5,35)=1.6, NS], Figure 4A & 4B) but did reduce vertical activity (main effect of “treatment” [F(1,7)=8.6, p<0.05]; main effect of “time” [F(5,35)=17.0, p<0.001]; interaction [F(5,35)=1.5, NS], Figure 4C & 4D). In contrast, SB-612111 reduced the number of horizontal ambulatory counts (main effect of “treatment” [F(1,7)=5.6, NS]; main effect of “time” [F(5,35)=30.5, p<0.001]; interaction [F(5,35)=1.0, NS], Figure 4E & 4F) while the number of vertical counts was not significantly reduced (main effect of “treatment” [F(1,7)=4.8, NS]; main effect of “time” [F(5,35)=13.5, p<0.001]; interaction [F(5,35)=0.1, NS], Figure 4G & 4H). Locomotor activity was not tested for SB-612111 at 10 mg/kg, as it is unlikely this dose decreases activity in mice (Rizzi et al., 2007, Silva et al., 2019). Locomotor activity was also assessed in a separate group of mice receiving AT-312 (0, 1, 3 mg/kg). ANOVA revealed potent effects of AT-312 in altering both ambulatory [F(2,14)=6.6, p<0.001] and vertical [F(2,14)=5.8, p<0.05] counts. Post hoc analysis showed that the dose of 3 mg/kg, but not 1 mg/kg, was effective in reducing locomotory parameters (p<0.01 for both parameters).
Figure 4.
Effects of administration of the NOP receptor agonist AT-202 and the antagonist SB-612111 on locomotor activity. Mice received injection (ip) of AT-202 (0, 3 mg/kg) or SB-612111 (0, 30 mg/kg) 30 min prior to 30-min locomotor activity assessment in the open field test conducted under familiarity conditions. (A & B) AT-202 treatment did not affect horizontal activity but (C & D) reduced vertical activity. (E & F) SB-612111 treatment reduced horizontal activity, whereas (G & H) vertical activity was not significantly altered. Data are expressed as mean (± SEM) 5-min fractions and total ambulatory counts (horizontal activity) and as mean (± SEM) 5-min fractions and total vertical counts (vertical activity) of N=8 mice per group. * p<0.05 difference from vehicle groups (0 mg/kg). +p<0.05, ++p<0.01 difference from vehicle groups (0 mg/kg). For detailed statistics, see “Results”.
3.5. Effect of NOP receptor antagonism in a two-bottle choice DID paradigm
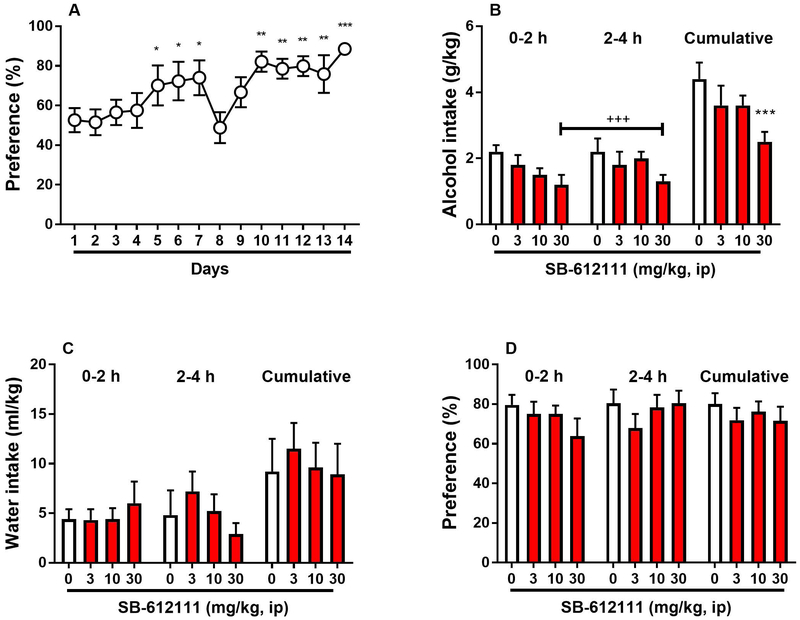
To investigate the behavioral specificity of the anti-alcohol response elicited by SB-612111, the NOP antagonist was tested in a slightly modified DID paradigm in which mice had access to two-bottle free choice between 15% alcohol solution and water rather than access to one 20% alcohol bottle only. The two-bottle free choice procedure produced robust escalation of alcohol preference that switched from 52.6 ± 6.1 % of preference on day 1 to 88.5 ± 2.5 % of preference on day 14 [F(13,91)=4.2, p<0.001]. Post hoc analysis indicated that significantly different intake was found on day 5, 6 and 7 (p<0.05) and from days 10 to 14 (p<0.01 and p<0.001) as compared with the intake of the first exposure (Figure 5A). On day 8 and 9 intake was low because habituation to ip 0.9% saline injection 30 min prior to bottle exposure was started. SB-612111 reduced alcohol intake (main effect of “treatment” [F(3,21)=5.7, p<0.01]; main effect of “time” [F(1,7)=0.8, NS]; interaction [F(3,21)=0.4, NS]) only at the highest dose examined (p<0.001, Figure 5B). Water intake (main effect of “treatment” [F(3,21)=0.3, NS]; main effect of “time” [F(1,7)=0.2, NS]; interaction [F(3,21)=1.8, NS], Figure 5C) and alcohol preference (main effect of “treatment” [F(3,21)=1.1, NS]; main effect of “time” [F(1,7)=4.0, NS]; interaction [F(3,21)=2.0, NS], Figure 5D) were not affected by the antagonist.
Figure 5.
Effect of the NOP receptor antagonist SB-612111 on a two-bottle free choice drinking paradigm that can assess moderate alcohol drinking. (A) 14-day exposure to two bottles containing 15 % (v/v) alcohol and water, respectively, induced escalation of preference for the alcohol bottle. *p<0.05, **p<0.01, ***p<0.001 difference from day 1. On day 15 mice were treated acutely with SB-612111 (0, 3, 10, 30 mg/kg, ip) 30 min prior to 4-h exposure to concomitant 15% (v/v) alcohol solution and water. (B) Administration of 30 mg/kg SB-612111 decreased alcohol consumption. (C) Water intake was not significantly affected by the treatment. (D) Preference ratio during the 4-h two-bottle choice session was not altered. All data are presented as mean (± SEM) alcohol intake (g/kg), water intake (ml/kg) and preference (%) in 2-h fractions (0-2 h & 2-4 h) and as mean (± SEM) cumulative (0-4 h) alcohol intake (g/kg), water intake (ml/kg) and preference (%) of N=8 mice. +++p<0.001, ***p<0.001 difference from vehicle (0 mg/kg). Connecting line with triple plus sign (+++) indicates combined post hoc testing. For detailed statistics, see “Results”.
3.6. Effect of NOP receptor antagonism in alcohol elimination
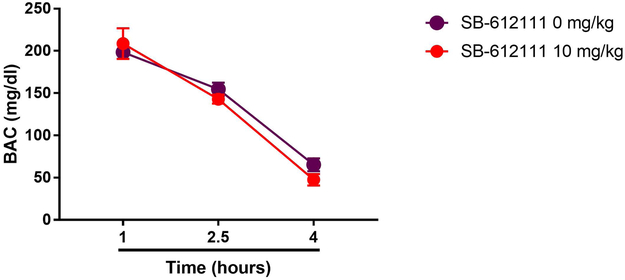
Blood alcohol concentrations decreased over time (Figure 6; main “time” effect: F(2,20)=156.7, p<0.001), but elimination rates were not affected by treatment with SB-612111 (10 mg/kg; main “treatment” effect: F(1,10)=0.4, NS; “time” × “treatment” interaction: F(2,20)=1.5, NS).
Figure 6.
Unaltered kinetics of alcohol elimination after ip treatment with SB-612111 (0 and 10 mg/kg) followed by ip injection of 3.0 g/kg of 20% (v/v) alcohol. Values are reported as mean (±SEM) blood alcohol concentrations (BACs mg/dl). For detailed statistics, see “Results”.
4. DISCUSSION
Here we demonstrate that treatment with the potent and selective NOP receptor agonists AT-202 and AT-312 did not reduce binge-like alcohol consumption in C57BL/6J mice exposed to the DID procedure at doses that did not affect locomotion. AT-202 also failed to affect DID behavior when administered in mice with a previous history of alcohol intoxication. Conversely, treatment with two NOP receptor antagonists, SB-612111 and LY2817412, decreased binge-like alcohol drinking. The effect of SB-612111 in reducing alcohol consumption in the DID model was independent of a previous history of alcohol intoxication. Consistent with our antagonist data, NOP receptor (−/−) mice showed attenuated alcohol drinking as compared to WT (+/+) when subjected to the DID paradigm. SB-612111 reduced DID and increased sucrose consumption at doses that do not appear to affect locomotor activity, suggesting a specific behavioral response. Additionally, alcohol elimination was unaffected by the drug. Finally, only the high dose of SB-612111 (30 mg/kg) reduced alcohol intake and failed to inhibit alcohol preference in a two-bottle choice DID model.
The ability of N/OFQ and small molecule NOP agonists to attenuate alcohol reward and alcohol taking behavior has been repeatedly demonstrated using CPP and self-administration paradigms in rats and mice (Ciccocioppo et al., 1999, Kuzmin et al., 2003, Zaveri et al., 2018, Aziz et al., 2016, Ciccocioppo et al., 2014). Consistently, NOP receptor agonists decrease drug-induced dopamine release in the nucleus accumbens (Murphy and Maidment, 1999, Vazquez-DeRose et al., 2013, Di Giannuario et al., 1999). As shown in previous studies, suppression of alcohol drinking by NOP receptor agonism is particularly pronounced in genetically selected alcohol-preferring msP rats or in unselected rat lines with a previous history of chronic alcohol intoxication (Ciccocioppo et al., 2014, de Guglielmo et al., 2015, Economidou et al., 2008). Noteworthy, both msP rats and post-dependent Wistar rats show upregulation of NOP receptor and N/OFQ transcripts in brain areas that contribute to mediate high anxiety states and increased stress-sensitivity, such as the central nucleus of the amygdala and the bed nucleus of the stria terminalis. In agreement, anxiolytic activity of N/OFQ was more potent in post-dependent rats compared to non-dependent controls (Aujla et al., 2013, Economidou et al., 2011, Economidou et al., 2008). Given this premise, we were surprised to find that two potent NOP receptor agonists, AT-202 and AT-312, failed to decrease drinking at doses that were not sedative, in the DID model that maximizes alcohol drinking largely by recruiting the brain stress systems. Indeed, pharmacological strategies aimed at lowering anxiety states by reducing the brain corticotropin-releasing factor (CRF) acting on CRF receptor 1 signaling (Sparta et al., 2008, Lowery et al., 2010, Kaur et al., 2012) or enhancing neuropeptide Y (Sparrow et al., 2012) signaling have been successful in altering this pattern of alcohol consumption. However, another NOP agonist SR-8993 was shown to decrease alcohol consumption in several paradigms in rats, as demonstrated by Aziz et al (Aziz et al., 2016). It is conceivable that structurally different NOP agonists may show a separation between doses that decrease activity and doses that decrease alcohol consumption.
Here we show that NOP receptor antagonists are able to reduce excessive alcohol consumption in the DID paradigm and mice lacking NOP receptors show reduced alcohol DID relative to WT mice. In support of the present results, previous evidence showed that mice and rats with genetic deletion of NOP receptors consumed less alcohol compared to their WT counterparts (Sakoori and Murphy, 2008, Kallupi et al., 2017). These findings are in contrast with the observation that NOP (−/−) mice showed significant alcohol-induced CPP (Sakoori and Murphy, 2008), suggesting that reduced alcohol consumption does not necessarily correlate with reduced reward. More recently, three studies have reported the efficacy of selective NOP receptor antagonists in reducing alcohol taking and seeking in rats (Rorick-Kehn et al., 2016, Cippitelli et al., 2016, Kallupi et al., 2017). Altogether, these data point to the possibility that NOP receptor inhibition rather than activation is efficacious in treating excessive, binge-like alcohol consumption. However, as discussed above, it is equally true that NOP receptor activation, as well as inhibition, can lead to decreased alcohol consumption depending upon strain and the particular procedure being conducted. The comparable alcohol-attenuating effects of NOP receptor agonists as well as antagonists have been attributed to the ability of NOP agonists to produce desensitization and internalization of NOP receptors in vivo, thus reducing receptor availability and resulting in a “functional” receptor blockade (Rorick-Kehn et al., 2016).
We further examined the effects of the NOP agonist AT-202 and the NOP antagonist SB-612111 using the DID procedure in mice with previous chronic alcohol exposure using an alcohol-containing liquid diet (Anji and Kumari, 2008). Upon alcohol discontinuation, mice exhibited signs of withdrawal, as measured by HIC, with a peak severity score at 4h following the onset of the withdrawal, which confirms that the liquid diet procedure successfully induced a dependence-like state in C57BL/6J mice. Once again, pretreatment with the NOP agonist AT-202 failed to alter binge-like alcohol drinking in alcohol liquid diet- and control diet-exposed mice, whereas SB-612111 reduced drinking in alcohol liquid diet- as well as control diet-exposed mice. These results provide reliability and increase the validity of our findings, as the NOP antagonism approach confirmed efficacy in reducing high alcohol consumption in a post-dependent state. However, unexpectedly, alcohol intake following liquid diet exposure was not increased, as compared to mice exposed to control diet. One possible explanation is that the alcohol intoxication procedure used here was insufficient to induce persistent neuroadaptations that cause a negative affective state that in turn motivates subsequent excessive drinking, which may also account for the lack of effect of AT-202. It has been shown that repeated cycling of alcohol exposure and withdrawal is more effective than continuous alcohol in enhancing anxiety and negative mood, as well as excessive alcohol consumption (Becker and Lopez, 2004, Ghozland et al., 2005, Overstreet et al., 2004). However, the alcohol liquid diet procedure was sufficient to induce withdrawal, which is a negative affective state associated with the development of alcohol dependence. Therefore, similar levels of alcohol intake observed following liquid and control diets are more likely due to a ceiling effect in the 4h DID protocol.
Binge alcohol drinking is lower in mouse strains that exhibit reduced feeding behavior and is attenuated by drugs that reduce food consumption. For example, antagonism of the receptor of the orexigenic peptide ghrelin induced a 20% decrease in alcohol consumption in mice (Kaur and Ryabinin, 2010) and ghrelin receptor deletion reduced binge-like alcohol drinking in rats (Zallar et al., 2018). Similarly, mutant mice lacking normal production of agouti-related protein, an endogenous antagonist of melanocortin function, showed blunted binge-like alcohol consumption (Navarro et al., 2009). Data described here is in apparent agreement, since the endogenous agonist for NOP receptors, N/OFQ, induces hyperphagia, whereas blockade of N/OFQ function reduces excessive alcohol consumption. Furthermore, the recent evidence that SB-612111 and LY2940094 potently decrease high fat diet binge eating and other eating-related disorders may support this view (Hardaway et al., 2016, Statnick et al., 2016). Therefore, the reduction of alcohol drinking observed following SB-612111 treatment may not be related to a modulatory effect of the rewarding properties of alcohol, but may result from decreased need for calories, as it pertains to alcohol’s caloric value. However, SB-612111, at doses lower than those affecting locomotor activity increased intake of a low sucrose concentration in an experiment conducted under identical conditions as those used for the alcohol DID paradigm. More experiments are required to fully understand this observation that may result from other properties of NOP receptor inhibition such as the ability to improve mood-related behaviors (Rizzi et al., 2011, Rizzi et al., 2007, Holanda et al., 2016).
In a two-bottle variant of the DID model (Giardino and Ryabinin, 2013), in which mice had a choice of water or 15% alcohol solution during the 4-h access period, and typically drank less alcohol as compared to a one bottle exposure, treatment with SB-612111 reduced alcohol only at a dose that also decreased locomotor activity and sucrose intake (30 mg/kg). This suggests that blockade of endogenous N/OFQ function can more effectively blunt binge-like ethanol consumption rather than intake of moderate amounts. Alternatively, it is possible that NOP receptor antagonism more effectively reduces voluntary consumption of high alcohol concentrations rather than consumption of lower concentrations, as previously shown for CRF receptor 1 antagonists (Cippitelli et al., 2012).
Further investigation is required to determine the mechanisms through which NOP antagonism blocks binge alcohol consumption. Due to the widespread distribution of NOP receptor throughout the brain it is hard to determine which areas or circuitries of the brain are involved in the observed anti-alcohol effects of NOP antagonists. The ventral tegmental area and the amygdala have been involved in binge-like alcohol consumption associated with DID procedures (Lowery-Gionta et al., 2012, Rinker et al., 2016). Additionally, a population of prepronociceptin-expressing neurons in the central amygdala has been shown to mediate palatable food consumption that contributes to binge eating (Hardaway et al., 2019). Thus, in future studies we will verify if these brain regions may represent neurobiological substrates for the behavioral effects produced by NOP receptor antagonism.
In conclusion, consistent with previous research, C57BL/6J mice exposed to the classic DID procedure show elevated alcohol drinking and achieve BACs that approached 100 mg % on day 4 of DID experiments. Pharmacological blockade of NOP receptors by selective antagonists, as well as genetic depletion of the NOP receptor, attenuated binge drinking, an effect that was behaviorally specific. These experiments provide novel evidence for a role of the N/OFQ-NOP receptor system in mediating binge-like alcohol drinking behavior in mice and suggest that NOP receptor antagonists may represent a viable approach for treatment of alcohol abuse with clinical potential in humans. Furthermore, given the critical role for the endogenous N/OFQ in the modulation of nicotine reinforcement (Cippitelli et al., 2016), NOP receptor antagonism may also represent an attractive strategy for alcohol and nicotine co-addiction pharmacotherapy.
Acknowledgments
This work was supported by NIH grants R01DA023281 and R01AA014351 and by a mobility grant from the Polish Ministry of Science and Higher Education within the programme “Mobilność Plus V” (decision nr 1662/1/MOB/V/17/2018/0) to KT-D.
Footnotes
Conflict of Interest Statement
The authors declare no conflict of interest.
6. References
- ANDERSON RI, LOPEZ MF, GRIFFIN WC, HAUN HL, BLOODGOOD DW, PATI D, BOYT KM, KASH TL & BECKER HC 2019. Dynorphin-kappa opioid receptor activity in the central amygdala modulates binge-like alcohol drinking in mice. Neuropsychopharmacology, 44, 1084–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANJI A & KUMARI M 2008. Supplementing the liquid alcohol diet with chow enhances alcohol intake in C57 BL/6 mice. Drug Alcohol Depend, 97, 86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AUJLA H, CANNARSA R, ROMUALDI P, CICCOCIOPPO R, MARTIN-FARDON R & WEISS F 2013. Modification of anxiety-like behaviors by nociceptin/orphanin FQ (N/OFQ) and time-dependent changes in N/OFQ-NOP gene expression following ethanol withdrawal. Addict Biol, 18, 467–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AZIZ AM, BROTHERS S, SARTOR G, HOLM L, HEILIG M, WAHLESTEDT C & THORSELL A 2016. The nociceptin/orphanin FQ receptor agonist SR-8993 as a candidate therapeutic for alcohol use disorders: validation in rat models. Psychopharmacology (Berl), 233, 3553–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BECKER HC & LOPEZ MF 2004. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res, 28, 1829–38. [DOI] [PubMed] [Google Scholar]
- CAMPO S & CAMERON KA 2006. Differential effects of exposure to social norms campaigns: a cause for concern. Health Commun, 19, 209–19. [DOI] [PubMed] [Google Scholar]
- CDC 2015. Centers for Disease Control and Preventions Alcohol-Related Disease Impact (ARDI), in Series Alcohol-Related Disease Impact (ARDI) (SERVICES. USDOHAH; ed, Atlanta, GA: ). [Google Scholar]
- CICCOCIOPPO R, ECONOMIDOU D, FEDELI A, ANGELETTI S, WEISS F, HEILIG M & MASSI M 2004. Attenuation of ethanol self-administration and of conditioned reinstatement of alcohol-seeking behaviour by the antiopioid peptide nociceptin/orphanin FQ in alcohol-preferring rats. Psychopharmacology (Berl), 172, 170–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CICCOCIOPPO R, PANOCKA I, POLIDORI C, REGOLI D & MASSI M 1999. Effect of nociceptin on alcohol intake in alcohol-preferring rats. Psychopharmacology (Berl), 141, 220–4. [DOI] [PubMed] [Google Scholar]
- CICCOCIOPPO R, STOPPONI S, ECONOMIDOU D, KURIYAMA M, KINOSHITA H, HEILIG M, ROBERTO M, WEISS F & TESHIMA K 2014. Chronic treatment with novel brain-penetrating selective NOP receptor agonist MT-7716 reduces alcohol drinking and seeking in the rat. Neuropsychopharmacology, 39, 2601–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CIPPITELLI A, DAMADZIC R, SINGLEY E, THORSELL A, CICCOCIOPPO R, ESKAY RL & HEILIG M 2012. Pharmacological blockade of corticotropin-releasing hormone receptor 1 (CRH1R) reduces voluntary consumption of high alcohol concentrations in non-dependent Wistar rats. Pharmacology Biochemistry and Behavior, 100, 522–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CIPPITELLI A, SCHOCH J, DEBEVEC G, BRUNORI G, ZAVERI NT & TOLL L 2016. A key role for the N/OFQ-NOP receptor system in modulating nicotine taking in a model of nicotine and alcohol co-administration. Sci Rep, 6, 26594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARKE S, CHEN Z, HSU MS, PINTAR J, HILL R & KITCHEN I 2001. Quantitative autoradiographic mapping of the ORL1, mu-, delta- and kappa-receptors in the brains of knockout mice lacking the ORL1 receptor gene. Brain Res, 906, 13–24. [DOI] [PubMed] [Google Scholar]
- COX BM, CHRISTIE MJ, DEVI L, TOLL L & TRAYNOR JR 2015. Challenges for opioid receptor nomenclature: IUPHAR Review 9. Br J Pharmacol, 172, 317–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRABBE JC, MERRILL C & BELKNAP JK 1991. Acute dependence on depressant drugs is determined by common genes in mice. J Pharmacol Exp Ther, 257, 663–7. [PubMed] [Google Scholar]
- DE GUGLIELMO G, MARTIN-FARDON R, TESHIMA K, CICCOCIOPPO R & WEISS F 2015. MT-7716, a potent NOP receptor agonist, preferentially reduces ethanol seeking and reinforcement in post-dependent rats. Addict Biol, 20, 643–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DI GIANNUARIO A, PIERETTI S, CATALANI A & LOIZZO A 1999. Orphanin FQ reduces morphine-induced dopamine release in the nucleus accumbens: a microdialysis study in rats. Neurosci Lett, 272, 183–6. [DOI] [PubMed] [Google Scholar]
- ECONOMIDOU D, CIPPITELLI A, STOPPONI S, BRACONI S, CLEMENTI S, UBALDI M, MARTIN-FARDON R, WEISS F, MASSI M & CICCOCIOPPO R 2011. Activation of brain NOP receptors attenuates acute and protracted alcohol withdrawal symptoms in the rat. Alcohol Clin Exp Res, 35, 747–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECONOMIDOU D, HANSSON AC, WEISS F, TERASMAA A, SOMMER WH, CIPPITELLI A, FEDELI A, MARTIN-FARDON R, MASSI M, CICCOCIOPPO R & HEILIG M 2008. Dysregulation of nociceptin/orphanin FQ activity in the amygdala is linked to excessive alcohol drinking in the rat. Biol Psychiatry, 64, 211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GHOZLAND S, CHU K, KIEFFER BL & ROBERTS AJ 2005. Lack of stimulant and anxiolytic-like effects of ethanol and accelerated development of ethanol dependence in mu-opioid receptor knockout mice. Neuropharmacology, 49, 493–501. [DOI] [PubMed] [Google Scholar]
- GIARDINO WJ & RYABININ AE 2013. CRF1 Receptor Signaling Regulates Food and Fluid Intake in the Drinking-in-the-Dark Model of Binge Alcohol Consumption. Alcoholism-Clinical and Experimental Research, 37, 1161–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARDAWAY JA, HALLADAY LR, MAZZONE CM, PATI D, BLOODGOOD DW, KIM M, JENSEN J, DIBERTO JF, BOYT KM, SHIDDAPUR A, ERFANI A, HON OJ, NEIRA S, STANHOPE CM, SUGAM JA, SADDORIS MP, TIPTON G, MCELLIGOTT Z, JHOU TC, STUBER GD, BRUCHAS MR, BULIK CM, HOLMES A & KASH TL 2019. Central Amygdala Prepronociceptin-Expressing Neurons Mediate Palatable Food Consumption and Reward. Neuron, 102, 1037–1052 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARDAWAY JA, JENSEN J, KIM M, MAZZONE CM, SUGAM JA, DIBERTO JF, LOWERY-GIONTA EG, HWA LS, PLEIL KE, BULIK CM & KASH TL 2016. Nociceptin receptor antagonist SB 612111 decreases high fat diet binge eating. Behav Brain Res, 307, 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEILIG M & EGLI M 2006. Pharmacological treatment of alcohol dependence: target symptoms and target mechanisms. Pharmacol Ther, 111, 855–76. [DOI] [PubMed] [Google Scholar]
- HOLANDA VAD, MEDEIROS IU, ASTH L, GUERRINI R, CALO G & GAVIOLI EC 2016. Antidepressant activity of nociceptin/orphanin FQ receptor antagonists in the mouse learned helplessness. Psychopharmacology, 233, 2525–2532. [DOI] [PubMed] [Google Scholar]
- JOHNSON BA 2008. Update on neuropharmacological treatments for alcoholism: scientific basis and clinical findings. Biochem Pharmacol, 75, 34–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KALLUPI M, SCUPPA G, DE GUGLIELMO G, CALO G, WEISS F, STATNICK MA, RORICK-KEHN LM & CICCOCIOPPO R 2017. Genetic Deletion of the Nociceptin/Orphanin FQ Receptor in the Rat Confers Resilience to the Development of Drug Addiction. Neuropsychopharmacology, 42, 695–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANN L, KINCHEN S, SHANKLIN SL, FLINT KH, KAWKINS J, HARRIS WA, LOWRY R, OLSEN EO, MCMANUS T, CHYEN D, WHITTLE L, TAYLOR E, DEMISSIE Z, BRENER N, THORNTON J, MOORE J, ZAZA S, CENTERS FOR DISEASE, C. & PREVENTION 2014. Youth risk behavior surveillance--United States, 2013. MMWR Surveill Summ, 63 Suppl 4, 1–168. [PubMed] [Google Scholar]
- KAUR S, LI J, STENZEL-POORE MP & RYABININ AE 2012. Corticotropin-releasing factor acting on corticotropin-releasing factor receptor type 1 is critical for binge alcohol drinking in mice. Alcohol Clin Exp Res, 36, 369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUR S & RYABININ AE 2010. Ghrelin receptor antagonism decreases alcohol consumption and activation of perioculomotor urocortin-containing neurons. Alcohol Clin Exp Res, 34, 1525–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUZMIN A, KREEK MJ, BAKALKIN G & LILJEQUIST S 2007. The nociceptin/orphanin FQ receptor agonist Ro 64–6198 reduces alcohol self-administration and prevents relapse-like alcohol drinking. Neuropsychopharmacology, 32, 902–10. [DOI] [PubMed] [Google Scholar]
- KUZMIN A, SANDIN J, TERENIUS L & OGREN SO 2003. Acquisition, expression, and reinstatement of ethanol-induced conditioned place preference in mice: effects of opioid receptor-like 1 receptor agonists and naloxone. J Pharmacol Exp Ther, 304, 310–8. [DOI] [PubMed] [Google Scholar]
- LAMBERT DG 2008. The nociceptin/orphanin FQ receptor: a target with broad therapeutic potential. Nat Rev Drug Discov, 7, 694–710. [DOI] [PubMed] [Google Scholar]
- LIANG J & OLSEN RW 2014. Alcohol use disorders and current pharmacological therapies: the role of GABA(A) receptors. Acta Pharmacol Sin, 35, 981–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIVY DJ, PARNELL SE & WEST JR 2003. Blood ethanol concentration profiles: a comparison between rats and mice. Alcohol, 29, 165–71. [DOI] [PubMed] [Google Scholar]
- LOWERY-GIONTA EG, NAVARRO M, LI C, PLEIL KE, RINKER JA, COX BR, SPROW GM, KASH TL & THIELE TE 2012. Corticotropin releasing factor signaling in the central amygdala is recruited during binge-like ethanol consumption in C57BL/6J mice. J Neurosci, 32, 3405–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWERY EG, SPANOS M, NAVARRO M, LYONS AM, HODGE CW & THIELE TE 2010. CRF-1 antagonist and CRF-2 agonist decrease binge-like ethanol drinking in C57BL/6J mice independent of the HPA axis. Neuropsychopharmacology, 35, 1241–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN-FARDON R, ZORRILLA EP, CICCOCIOPPO R & WEISS F 2010. Role of innate and drug-induced dysregulation of brain stress and arousal systems in addiction: Focus on corticotropin-releasing factor, nociceptin/orphanin FQ, and orexin/hypocretin. Brain Res, 1314, 145–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEUNIER JC, MOLLEREAU C, TOLL L, SUAUDEAU C, MOISAND C, ALVINERIE P, BUTOUR JL, GUILLEMOT JC, FERRARA P, MONSARRAT B & et al. 1995. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature, 377, 532–5. [DOI] [PubMed] [Google Scholar]
- MURPHY NP & MAIDMENT NT 1999. Orphanin FQ/nociceptin modulation of mesolimbic dopamine transmission determined by microdialysis. J Neurochem, 73, 179–86. [DOI] [PubMed] [Google Scholar]
- NARENDRAN R, CICCOCIOPPO R, LOPRESTI B, PARIS J, HIMES ML & MASON NS 2018. Nociceptin Receptors in Alcohol Use Disorders: A Positron Emission Tomography Study Using [(11)C]NOP-1A. Biol Psychiatry, 84, 708–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAVARRO M, CUBERO I, KO L & THIELE TE 2009. Deletion of agouti-related protein blunts ethanol self-administration and binge-like drinking in mice. Genes Brain Behav, 8, 450–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEAL CR JR., MANSOUR A, REINSCHEID R, NOTHACKER HP, CIVELLI O, AKIL H & WATSON SJ JR. 1999. Opioid receptor-like (ORL1) receptor distribution in the rat central nervous system: comparison of ORL1 receptor mRNA expression with (125)I-[(14)Tyr]-orphanin FQ binding. J Comp Neurol, 412, 563–605. [PubMed] [Google Scholar]
- NHTSA 2016. National Highway Traffic Safety Administration (US Department of Transportation). 2015 Motor Vehicle Crashes: Overview. [Google Scholar]
- OVERSTREET DH, KNAPP DJ & BREESE GR 2004. Modulation of multiple ethanol withdrawal-induced anxiety-like behavior by CRF and CRF1 receptors. Pharmacol Biochem Behav, 77, 405–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OZAWA A, BRUNORI G, MERCATELLI D, WU J, CIPPITELLI A, ZOU B, XIE XS, WILLIAMS M, ZAVERI NT, LOW S, SCHERRER G, KIEFFER BL & TOLL L 2015. Knock-In Mice with NOP-eGFP Receptors Identify Receptor Cellular and Regional Localization. J Neurosci, 35, 11682–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POST A, SMART TS, JACKSON K, MANN J, MOHS R, RORICK-KEHN L, STATNICK M, ANTON R, O’MALLEY SS & WONG CJ 2016. Proof-of-Concept Study to Assess the Nociceptin Receptor Antagonist LY2940094 as a New Treatment for Alcohol Dependence. Alcohol Clin Exp Res. [DOI] [PubMed] [Google Scholar]
- RHODES JS, BEST K, BELKNAP JK, FINN DA & CRABBE JC 2005. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav, 84, 53–63. [DOI] [PubMed] [Google Scholar]
- RINKER JA, MARSHALL SA, MAZZONE CM, LOWERY-GIONTA EG, GULATI V, PLEIL KE, KASH TL, NAVARRO M & THIELE TE 2016. Extended Amygdala to Ventral Tegmental Area Corticotropin-Releasing Factor Circuit Controls Binge Ethanol Intake. Biol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIZZI A, GAVIOLI EC, MARZOLA G, SPAGNOLO B, ZUCCHINI S, CICCOCIOPPO R, TRAPELLA C, REGOLI D & CALO G 2007. Pharmacological characterization of the nociceptin/orphanin FQ receptor antagonist SB-612111 [(−)-cis-1-methyl-7-[[4-(2,6-dichlorophenyl)piperidin-1-yl]methyl]-6,7,8,9-tetrahydro-5H-benzocyclohepten-5-ol]: In vivo studies. Journal of Pharmacology and Experimental Therapeutics, 321, 968–974. [DOI] [PubMed] [Google Scholar]
- RIZZI A, MOLINARI S, MARTI M, MARZOLA G & CALO G 2011. Nociceptin/orphanin FQ receptor knockout rats: In vitro and in vivo studies. Neuropharmacology, 60, 572–579. [DOI] [PubMed] [Google Scholar]
- RORICK-KEHN LM, CICCOCIOPPO R, WONG CJ, WITKIN JM, MARTINEZ-GRAU MA, STOPPONI S, ADAMS BL, KATNER JS, PERRY KW, TOLEDO MA, DIAZ N, LAFUENTE C, JIMENEZ A, BENITO A, PEDREGAL C, WEISS F & STATNICK MA 2016. A Novel, Orally Bioavailable Nociceptin Receptor Antagonist, LY2940094, Reduces Ethanol Self-Administration and Ethanol Seeking in Animal Models. Alcohol Clin Exp Res, 40, 945–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SACKS JJ, GONZALES KR, BOUCHERY EE, TOMEDI LE & BREWER RD 2015. 2010 National and State Costs of Excessive Alcohol Consumption. Am J Prev Med, 49, e73–9. [DOI] [PubMed] [Google Scholar]
- SAKOORI K & MURPHY NP 2008. Endogenous nociceptin (orphanin FQ) suppresses basal hedonic state and acute reward responses to methamphetamine and ethanol, but facilitates chronic responses. Neuropsychopharmacology, 33, 877–91. [DOI] [PubMed] [Google Scholar]
- SAMHSA 2013. Substance Dependence or Abuse in the Past Year among Persons Aged 18 or Older, by Demographic Characteristics: Percentages, 2012 and 2013. . National Survey on Drug Use and Health Table 5.8B. [Google Scholar]
- SILVA EF, SILVA AI, ASTH L, SOUZA LS, ZAVERI NT, GUERRINI R, CALO G, RUZZA C & GAVIOLI EC 2019. Nociceptin/orphanin FQ receptor agonists increase aggressiveness in the mouse resident-intruder test. Behav Brain Res, 356, 120–126. [DOI] [PubMed] [Google Scholar]
- SPARROW AM, LOWERY-GIONTA EG, PLEIL KE, LI C, SPROW GM, COX BR, RINKER JA, JIJON AM, PENA J, NAVARRO M, KASH TL & THIELE TE 2012. Central neuropeptide Y modulates binge-like ethanol drinking in C57BL/6J mice via Y1 and Y2 receptors. Neuropsychopharmacology, 37, 1409–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPARTA DR, SPARROW AM, LOWERY EG, FEE JR, KNAPP DJ & THIELE TE 2008. Blockade of the corticotropin releasing factor type 1 receptor attenuates elevated ethanol drinking associated with drinking in the dark procedures. Alcohol Clin Exp Res, 32, 259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STAHRE M, ROEBER J, KANNY D, BREWER RD & ZHANG X 2014. Contribution of excessive alcohol consumption to deaths and years of potential life lost in the United States. Prev Chronic Dis, 11, E109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STATNICK MA, CHEN Y, ANSONOFF M, WITKIN JM, RORICK-KEHN L, SUTER TM, SONG M, HU C, LAFUENTE C, JIMENEZ A, BENITO A, DIAZ N, MARTINEZ-GRAU MA, TOLEDO MA & PINTAR JE 2016. A Novel Nociceptin Receptor Antagonist LY2940094 Inhibits Excessive Feeding Behavior in Rodents: A Possible Mechanism for the Treatment of Binge Eating Disorder. J Pharmacol Exp Ther, 356, 493–502. [DOI] [PubMed] [Google Scholar]
- SWIFT RM & ASTON ER 2015. Pharmacotherapy for alcohol use disorder: current and emerging therapies. Harv Rev Psychiatry, 23, 122–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THIELE TE, CRABBE JC & BOEHM SL 2ND, 2014. “Drinking in the Dark” (DID): a simple mouse model of binge-like alcohol intake. Curr Protoc Neurosci, 68, 9 49 1–9 49 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOLL L, BRUCHAS MR, CALO G, COX BM & ZAVERI NT 2016. Nociceptin/Orphanin FQ Receptor Structure, Signaling, Ligands, Functions, and Interactions with Opioid Systems. Pharmacol Rev, 68, 419–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOLL L, KHROYAN TV, POLGAR WE, JIANG F, OLSEN C & ZAVERI NT 2009. Comparison of the antinociceptive and antirewarding profiles of novel bifunctional nociceptin receptor/mu-opioid receptor ligands: implications for therapeutic applications. J Pharmacol Exp Ther, 331, 954–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAZQUEZ-DEROSE J, STAUBER G, KHROYAN TV, XIE XS, ZAVERI NT & TOLL L 2013. Retrodialysis of N/OFQ into the nucleus accumbens shell blocks cocaine-induced increases in extracellular dopamine and locomotor activity. Eur J Pharmacol, 699, 200–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOLKOW ND & SKOLNICK P 2012. New medications for substance use disorders: challenges and opportunities. Neuropsychopharmacology, 37, 290–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WITKIN JM, STATNICK MA, RORICK-KEHN LM, PINTAR JE, ANSONOFF M, CHEN Y, TUCKER RC & CICCOCIOPPO R 2014. The biology of Nociceptin/Orphanin FQ (N/OFQ) related to obesity, stress, anxiety, mood, and drug dependence. Pharmacol Ther, 141, 283–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZALLAR LJ, BEURMANN S, TUNSTALL BJ, FRASER CM, KOOB GF, VENDRUSCOLO LF & LEGGIO L 2018. Ghrelin receptor deletion reduces binge-like alcohol drinking in rats. J Neuroendocrinol, e12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZARATIN PF, PETRONE G, SBACCHI M, GARNIER M, FOSSATI C, PETRILLO P, RONZONI S, GIARDINA GA & SCHEIDELER MA 2004. Modification of nociception and morphine tolerance by the selective opiate receptor-like orphan receptor antagonist (−)-cis-1-methyl-7-[[4-(2,6-dichlorophenyl)piperidin-1-yl]methyl]-6,7,8,9-tetrahy dro-5H-benzocyclohepten-5-ol (SB-612111). J Pharmacol Exp Ther, 308, 454–61. [DOI] [PubMed] [Google Scholar]
- ZAVERI NT, MARQUEZ PV, MEYER ME, POLGAR WE, HAMID A & LUTFY K 2018. A Novel and Selective Nociceptin Receptor (NOP) Agonist (1-(1-((cis)-4-isopropylcyclohexyl)piperidin-4-yl)-1H-indol-2-yl)methanol (AT-312) Decreases Acquisition of Ethanol-Induced Conditioned Place Preference in Mice. Alcohol Clin Exp Res, 42, 461–471. [DOI] [PMC free article] [PubMed] [Google Scholar]