Abstract
Background:
Chronic ethanol exposure induces neurobehavioral maladaptations in the brain though the precise changes have not been fully explored. The central amygdala (CEA) regulates anxiety-like behavior induced by withdrawal from chronic intermittent ethanol (CIE) exposure and the arginine vasopressin (AVP) system within the CEA regulates many anxiety-like behaviors. Thus, adaptations occur in the CEA AVP system due to chronic ethanol exposure which lead to anxiety-like behaviors in rats.
Methods:
Chronic exposure to a low dose ethanol (4.5% wt/vol) induces anxiety-like behavior in rats. Wistar or Sprague-Dawley rats were exposed to a modified CIE or CIE while intra-CEA microinjections of AVP or a V1b receptor antagonist were used to elicit or block withdrawal induced anxiety. Additionally, AVP microinjections into the CEA were given 24 hours following 15 days of continuous high dose ethanol (7% wt/vol), a time period when rats no longer express anxiety. Chemogenetics was also used to activate the basolateral amygdala or deactivate the dorsal periaqueductal grey (dm/dlPAG) to elicit or block withdrawal induced anxiety.
Results:
AVP microinjected into the CEA in lieu of exposure to the first two cycles of CIE was sufficient to induce anxiety-like behavior in these commonly used rat strains. The V1b receptor antagonist, but not an oxytocin receptor agonist, into the CEA during the first two withdrawal cycles suppressed anxiety. However, activation of the basolateral amygdala in lieu of exposure to the first two cycles of CIE was insufficient to induce anxiety-like behavior. AVP microinjection into the CEA 24 hours into withdrawal re-elicited anxiety-like behavior and deactivation of the dm/dlPAG reduced this effect of CEA AVP.
Conclusions:
Taken together, this study demonstrates a role of CEA AVP and a CEA-dm/dlPAG circuit in the development of anxiety induced by CIE. Such information is valuable for identifying novel therapeutic targets for alcohol and anxiety associated disorders.
Keywords: central nucleus of the amygdala, basolateral amygdala, dorsal periaqueductal gray, alcohol, chemogenetics, alcohol withdrawal induced anxiety
1.0. Introduction
Withdrawal from chronic alcohol can lead to detrimental behavioral adaptations including increased anxiety in humans and rodents (Breese et al., 2005, Overstreet et al., 2002, Sinha, 2008). The underpinning neural adaptations which lead to anxiety as a result of alcohol withdrawal are unclear. One possible locus for alcohol withdrawal-induced anxiety is the arginine vasopressin (AVP) receptor system. Through the use of pharmacological techniques it has been shown that AVP regulates anxiety and the AVP system can be a target for anxiolytics (Neumann and Landgraf, 2012, Zelena, 2012, Morales-Medina et al., 2016, Harper et al., 2018a). There are past and ongoing clinical trials to determine the efficacy of vasopressin receptor antagonism on individuals suffering from anxiety (Harper et al., 2018a, Griebel et al., 2012).
The amygdala, and more specifically the central nucleus of the amygdala (CEA), has been a focus of the effects of AVP on anxiety as it contains V1b arginine vasopressin receptors (Young et al., 2006, Hernando et al., 2001, Stemmelin et al., 2005) and one study found blocking V1b receptors in the CEA blocked the anxiogenic effects of AVP in the shock probe burying test (Hernandez-Perez et al., 2018). Interestingly, a V1b receptor antagonist given systemically also blocked the anxiety in the social interaction test produced in rats by protracted withdrawal from adolescent intermittent ethanol (Dannenhoffer et al., 2018). In that study they also found the oxytocin (OT) agonist, like the V1b antagonist, was able to block anxiety due to protracted withdrawal from adolescent intermittent ethanol. OT receptors are commonly found in subregions adjacent to those containing AVP receptors, which is true of the CEA as well (Huber et al., 2005, Song and Albers, 2017, Veinante and Freund-Mercier, 1997). As neurons in one subregion can inhibit the other, this allows OT agonists to commonly have the same effects as AVP antagonists (Huber et al., 2005).
AVP and the V1b receptor have also been implicated in alcohol consumption in rodents. Three separate studies have shown a reduction in alcohol consumption when the V1b antagonist was given (Zhou et al., 2011, Edwards et al., 2012, Zhou et al., 2018). Results such as these have led investigators to speculate about the clinical significance of the AVP system as a possible treatment for alcohol disorders. A recent clinical trial demonstrated a significant increase in the number of days of alcohol abstinence following V1b antagonism for individuals who met the criteria for DSM-IV alcohol dependence (Ryan et al., 2017). Additionally, an instigator for relapse in alcoholic patients is anxiety that often occurs during withdrawal and this emotional experience may escalate alcohol consumption to compensate. Therefore, studies that investigate anxiety following alcohol withdrawal are critical to gain an understanding of the etiology of alcoholism. This premise extends to the idea that a V1b antagonist might be useful to treat multiple symptoms associated with alcohol misuse (alcohol consumption and alcohol withdrawal induced anxiety) as well as useful to treat a common comorbid disorder to alcohol abuse, i.e. anxiety.
The CEA has been implicated in alcohol withdrawal induced anxiety on the social interaction test using the chronic intermittent alcohol paradigm (Huang et al., 2010). This paradigm uses three 5-day bouts of low dose alcohol to cause anxiety-like behavior on the social interaction test that is not produced from withdrawal from one 5-day bout of low dose alcohol (Overstreet et al., 2002). Given that repeated withdrawals are required to achieve anxiety, there are likely adaptations occurring in the brain due to the repeated exposure to withdrawals. The AVP system might be engaged as part of these adaptations in this model as it was suggested to occur in the adolescent intermittent model (Dannenhoffer et al., 2018), but what these adaptations are and whether they occur in the CEA is unknown. Further, if the CEA AVP system is adapting which brain regions upstream and downstream of the CEA are related to this adaptation or affected by this adaptation remains to be examined. The CEA receives afferent input from the basolateral amygdala (BLA) and this circuit has been implicated in a number of anxiety/fear-related behaviors (for review see Calhoon and Tye (2015); Kim et al. (2016)). Furthermore, the BLA to CEA circuit has been implicated in the suppression of negative states induced by withdrawal from chronic alcohol consumption (for review see Janak and Tye (2015); Koob and Volkow (2010); Koob and Volkow (2016);Ron and Barak (2016)). These studies strongly suggest that the BLA to CEA circuit is at least partially responsible for anxiety due to alcohol withdrawal. Additionally, the dorsal periaqueductal grey (dm/dlPAG) receives input from the CEA and numerous studies have shown the neural underpinnings in the dm/dlPAG for the expression of alcohol withdrawal-induced anxiety (Bertotto et al., 2010, Bonassoli et al., 2013, Bonassoli et al., 2011, Cabral et al., 2006).
While AVP regulates anxiety, questions persist as to whether the AVP system in the CEA adapts as a result of chronic alcohol and, if so, whether this adaptation is part of the anxiogenic BLA to CEA to dm/dlPAG pathway. It is hypothesized that the AVP receptor system in the CEA mediates alcohol withdrawal-induced anxiety and that this is predicated upon activation of a circuit involving the BLA, CEA and dm/dlPAG
2.0. Materials and Methods
2.1. Animals
Adult, male Wistar or Sprague-Dawley rats (Charles-River, Raleigh, NC, USA) weighing 180–200 g upon arrival were group housed and fed RMH3000 rat chow (Test Diets, Richmond, IN, USA) (temperatures 70–72 °F; humidity 40%–60%; and light/dark cycle 12 h:12 h with lights from 7:00 a.m. to 7:00 p.m.). All rats were singly-housed after surgery or at the start of the diet procedure and remained singly housed for the duration of experimental procedures. Wistar rats were used to test the role of AVP in alcohol withdrawal induced anxiety (Figure 1A-C), the role of AVP antagonists in alcohol withdrawal induced anxiety (Figure 2), and the role of BLA activation in alcohol withdrawal induced anxiety (Figure 3). Sprague-Dawley rats were used to test the role of AVP in alcohol withdrawal induced anxiety (Figure 1D-F) and the role of AVP and the dm/dlPAG in reinstatement of alcohol withdrawal induced anxiety (Figure 4). Methods used in this study were approved by Institutional Animal Care and Use Committee (IACUC, protocol numbers: 14–125 and 17–017) at the University of North Carolina (Chapel Hill, NC, USA).
2.2. Liquid Diet for Controls and for Chronic Ethanol Exposure
A nutritionally-complete and calorically-balanced liquid diet was used for these studies. The liquid control diet (CD) was calorically balanced to the ethanol diet by adjustments of the amount of dextrose. Rats were fed either CD or the ethanol diet (ED) with a modified pair-feeding strategy (Frye et al., 1983). The chronic intermittent ethanol protocol involved three 5-day bouts of low dose ED (4.5% wt/vol) separated by 2 days of CD and rats were tested for social interaction 5–6 hours after ED was replaced with CD (Overstreet et al., 2002, Knapp et al., 1998). Blood alcohol levels at peak drinking average from 63 to 107 mg% across the drinking paradigm and blood alcohol levels are at zero around 4 hours into withdrawal which precedes time of behavioral testing (Wills et al., 2008, Harper et al., 2018b). Previous research has indicated that though 3 withdrawals from 4.5% wt/vol 5-day bouts of ED causes anxiety-like behavior on the social interaction test, withdrawal from a single 4.5% wt/vol bout of ED does not (Overstreet et al., 2002). Therefore, to test the ability of AVP to mimic the effects of withdrawal from ED, two injections of AVP were given one week apart followed by a single 5-day bout of 4.5% wt/vol ED (Figure 1). To test the role of the CEA AVP system in anxiety-like behavior induced by alcohol withdrawal a V1b receptor antagonist or an OT agonist was given during the first two withdrawals (Figure 2). Additionally, to assess the role of the BLA in this behavior, the BLA was chemogenetically activated twice, one week apart, followed by a single 5-day bout of 4.5% wt/vol ED (Figure 3). The continuous ethanol (CE) protocol involved 15 continuous days of 7% wt/vol ED. Blood alcohol levels at peak drinking the day of withdrawal average 173 mg% (Harper et al., 2018b, Harper et al., 2015). Withdrawal from this higher dose of ethanol induces anxiety-like behavior on the social interaction test when given continuously, but this effect dissipates within 24–48 hours into withdrawal (Overstreet et al., 2002). Previous work has shown that 7% wt/vol is more likely to allow for re-elicitation of anxiety-like behavior when paired with a stressor or certain drug treatments. To test whether the CEA AVP system can reinstate anxiety-like behavior, microinjections of AVP were given into the CEA 24 hours into withdrawal and the dm/dlPAG was chemogenetically deactivated at the same time to test for the role of the dm/dlPAG in this reinstatement (Figure 4). During the CE protocol rats drank on average 5.18 g/kg/day and there was no significant difference in alcohol consumption between groups. The amount of alcohol consumed for all other experiments is listed in tables and discussed in results.
2.3. Social interaction test
The social interaction test is a validated index of anxiety-like states in rats, and this test has been adapted for general use in our laboratory (Breese et al., 2004, Breese et al., 2005, Knapp et al., 2016, Knapp et al., 2005, Overstreet et al., 2002, Overstreet et al., 2004). In the 5-min social interaction test, rats of approximately similar sizes were placed into a 60 × 60 cm square black Plexiglas open field with 15 × 15 cm squares marked on a paper floor under low lighting conditions (30 lux). The amount of aggregate time each rat was engaged in social behavior (conspecific grooming, sniffing, following, crawling over/under its partner) and the number of line crosses by two paws were recorded by a blinded observer. An elevated anxiety-like response was inferred from reduced social interaction behavior.
2.4. Surgeries
Rats were anesthetized with isoflurane, the skulls exposed, and holes drilled for a 26 gauge stainless steel cannula implanted into the brain to a point directly above the bilateral CEA (coordinates: AP −2.3, ML ±4.0, and DV−5.5). Three additional holes for machine screws facilitated securing the cannuli with dental cement. The placements were such that during the subsequent drug injections, the injector needle protruded 2.5 mm deeper (DV) than the cannula tip. 32-gauge stainless steel wire plugs kept the cannuli closed when not in use for injections. Animals were allowed to recover at least 1 week prior to proceeding to other treatments. Rats that received viral DREADD (designer receptors exclusively activated by designer drugs) injections in addition to cannulation received bilateral injection of 0.3 µL pAAV-CaMKIIa-hM3D(Gq)-mCherry (addgene, Cambridge, MA or UNC viral core) into the BLA (coordinates: AP −2.8, ML ±5, and DV−8.4) or 0.3 µL ipAAV-syn-hM4D(Gi)-mCherry (UNC viral core) into the dm/dlPAG (coordinates: AP −7.0, ML ±2.3, and DV−5.0 angled 23.8˚). Rats were allowed approximately 1 month for the virus to extend through the neurons before being started on diet protocol. After administration of the alcohol paradigm and measurement of behavior, rats were sacrificed, and the brains collected to verify placement. Representative dye spots derived from post-mortem histological assessments of CEA injections can be seen in supplemental Figure 1. Representative images of BLA and dm/dlPAG expressing fluorescent proteins from the DREADD virus can be seen in supplemental Figure 2.
2.5. Drugs/chemicals
CEA injections were made over 1 min via a 32-gauge stainless steel injector guided to the site of interest via the indwelling cannula. Injectors were left in place for 1 min to accommodate drug dispersal at the injection site. Vehicles for all control injections were identical to those used to prepare the respective drugs. The V1b receptor antagonist (SSR 149415 Axon Medchem, Groningen, Netherlands) was dissolved in aCSF with 5% dimethyl sulfoxide and injected bilaterally at 5µg in .5µl 2–5 hours into withdrawal if given during the first and second withdrawal or 15 minutes prior to social interaction testing if given during the third withdrawal. The oxytocin receptor agonist (WAY-267464 Tocris, Minneapolis, MN) was dissolved in aCSF with 5% dimethyl sulfoxide and injected bilaterally at 1.5ng in .5µl volume at 2–5 hours into withdrawal during the first and second withdrawal. Vasopressin (Sigma, St. Louis, MO) was dissolved in aCSF and injected bilaterally at 4.34µg or .434µg in .5 µl volume at what would have been 2–5 hours into withdrawal or 15 minutes prior to social interaction testing in reinstatement experiments. All drugs were made and used immediately or stored in ready-to-use aliquots for up to 24 h at 4 °C or up to 3 months at −80 °C. Clozapine-N-oxide (CNO received from the Research Triangle Institute and the National Institute on Drug Abuse Drug Inventory Supply and Control) was dissolved in 0.9% saline and injections were given intraperitoneally 45 minutes before social interaction at a dose of 1mg/kg. This concentration and timing of intraperitoneal CNO injections when used with a Gq DREADD was able to alter consummatory behavior (Jennings et al., 2015).
2.6. Statistical Analysis
Data were analyzed using Student’s t-test, one way, two way (CD vs ED and Veh vs drug), and three way (CD vs ED, Veh vs drug, and Saline vs CNO) ANOVA followed by a Fisher’s least significant difference posthoc on Statview (SAS Institute Inc, Cary, North Carolina). All graphs were made on Graphpad Prism (Graphpad, La Jolla, CA). A repeated measure ANOVA was used for analyzing alcohol consumption over multiple 5 day blocks. P<0.05 was considered significant and data were reported as mean±SEM.
3.0. Results
3.1. Intra-amygdala arginine vasopressin administration can induce alcohol withdrawal-induced anxiety in a subthreshold chronic intermittent ethanol paradigm in two commonly used rat strains
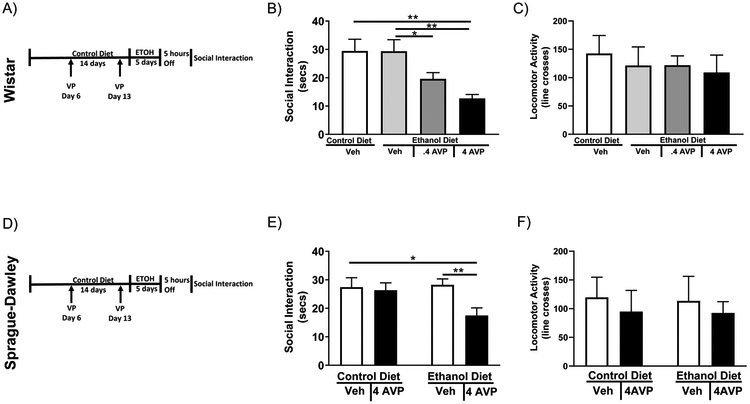
Using a modified chronic intermittent ethanol paradigm, the effects of administration of two doses of AVP (0.4µg and 4µg per side) into the CEA bilaterally were investigated (Figure 1A & D). One-way ANOVA revealed a significant effect of bilateral administration of AVP into the CEA of Wistar rats on anxiety (Figure 1B F3,33=5.909; P<0.01). Fisher’s post-hoc comparison revealed that in vehicle-treated rats, there was no effect of ED-treatment (equivalent of withdrawal from a single-cycle of ED). In ED-treated rats, Fisher’s post-hoc comparison revealed a significant decrease in the amount of social interaction (increase in anxiety-like behavior) in rats which received high dose (4µg per side) intra-CEA AVP compared to vehicle control and vehicle ethanol (CD-Veh vs ED-4AVP P<0.01; ED-Veh vs ED-4AVP P<0.01). On the other hand, Fisher’s post-hoc comparison revealed that low dose AVP (.4 µg per side) did not cause a significant increase in anxiety-like behavior compared to vehicle controls (CD-Veh vs ED-.4AVP, n.s.). One-way ANOVA revealed no significant effect of any treatment on locomotor activity (Figure 1C) or on alcohol consumed (g/kg/day) (Table 1).
Figure 1: CEA AVP can substitute for alcohol withdrawal to cause anxiety-like behavior.
A) Diagram of the AVP injections/alcohol withdrawal paradigm. Wistar rats were given two intra-CEA microinjections of AVP one week apart followed by a 5-day bout of 4.5% w/v ED. Five hours into withdrawal anxiety-like behavior was tested on the social interaction test. B) Wistar rats showed increased anxiety-like behavior when the highest dose of AVP microinjections into the CEA were paired with subthreshold alcohol withdrawal. C) Wistar rats showed no difference in locomotor activity when AVP microinjections into the CEA were paired with subthreshold alcohol withdrawal (CD-Veh N=9, ED-Veh N=11, ED-.4AVP N=8, ED-4AVP N=9). D) Diagram of the AVP injections/alcohol withdrawal paradigm. Sprague-Dawley rats were given two intra-CEA microinjections of AVP one week apart followed by a 5-day bout of 4.5% w/v ED. Five hours into withdrawal anxiety-like behavior was tested on the social interaction test. E) Sprague-Dawley rats show increased anxiety-like behavior when AVP microinjections in to the CEA were paired with subthreshold alcohol withdrawal. F) Sprague-Dawley rats showed no difference in locomotor activity when AVP microinjections into the CEA were paired with subthreshold alcohol withdrawal (CD-Veh N=9, ED-Veh N=8, CD-4AVP N=9, ED-4AVP N=10). Data are presented as mean±SEM. *=p<0.05 post-hoc. **=p<0.01 post-hoc.
Table 1:
AVP microinjections into the CEA did not alter the amount of alcohol consumed.
| Strain | Group | Ave g/kg/day |
|---|---|---|
| Wistar | ED-Veh | 7.68±.34 |
| Wistar | ED-.4 AVP | 7.41±.16 |
| Wistar | ED-4 AVP | 7.71±.27 |
| Sprague-Dawley | ED-Veh | 7.85±.24 |
| Sprague-Dawley | ED-4 AVP | 8.20±.43 |
Data are presented as mean±SEM. No significant difference was found in alcohol consumption between the Wistar experimental groups or between the Sprague-Dawley experimental groups.
To test the generalizability of these results the higher and most effective dose of AVP (4µg per side) was bilaterally injected into the CEA of Sprague-Dawley rats. Two-way ANOVA revealed a significant effect of bilateral administration of AVP into the CEA of Sprague-Dawley rats on anxiety (Figure 1E F1,32=3.14; P<0.05). Once again, Fisher’s post-hoc comparison revealed a significant decrease in the amount of social interaction in Sprague-Dawley rats which received intra-CEA AVP compared to vehicle control and vehicle ethanol (CD-Veh vs ED-AVP P<0.05; ED-Veh vs ED-AVP P<0.01). Two-way ANOVA revealed no significant effect of any treatment on locomotor activity (Figure 1F) and Student’s t-test revealed no significant effect of AVP on alcohol consumed (g/kg/day) (Table 1).
3.2. The arginine vasopressin receptor system in the CEA mediates alcohol withdrawal-induced anxiety
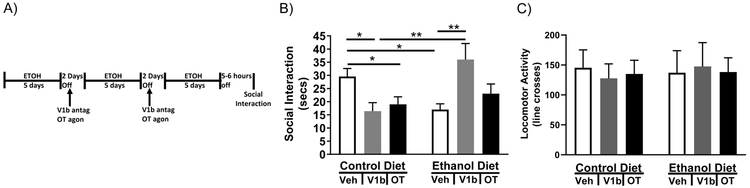
To test the ability of CEA V1b and OT receptors to alter anxiety after repeated withdrawal, a modified chronic intermittent ethanol paradigm was utilized whereby a V1b receptor antagonist or an OT receptor agonist was administered bilaterally to the CEA at the beginning of the first two withdrawal cycles (Figure 2A). Two-way ANOVA (CD/ED x Veh/V1b antagonist/OT agonist) revealed a significant interaction between diet and drug on anxiety (Figure 2B F2,60=9.09; P<0.01). Fisher’s multiple comparisons test revealed that ED-treated rats displayed significantly less social interaction than CD-treated rats (CD-Veh vs ED-Veh P<0.05) and that V1b antagonism, but not OT agonism, during the first two withdrawal cycles attenuated the decrease in social interaction (ED-Veh vs ED-V1b P<0.01; ED-Veh vs ED-OT, n.s.). Surprisingly, V1b antagonism and OT agonism increased social interaction time in CD-treated rats (CD-Veh vs CD-V1b P<0.05; CD-Veh vs CD-OT, P<0.05). Two-way ANOVA revealed no significant effect of any treatment on locomotor activity (Figure 2C). Two-way repeated measure ANOVA revealed a significant effect of cycle on alcohol consumption (F2,60=13.36; P<0.01), but no significant effect of drug or interaction between drug and cycle on alcohol consumption (g/kg/day) (Table 2).
Figure 2: A V1b antagonists, but not an OT agonist, can block alcohol withdrawal induced anxiety-like behavior.
A) Diagram of microinjections/alcohol withdrawal paradigm. Rats were given three 5-day bouts of 4.5%w/v ED separated by 2 days of withdrawal. During the first and second withdrawals rats received intra-CEA injections of either a V1b antagonist, OT agonist, or vehicle. Five to six hours into the third (final) withdrawal rats were tested for anxiety-like behavior on the social interaction test. B) Intra-CEA V1b antagonist microinjections reversed the alcohol withdrawal induced decrease in social interaction, but microinjections of the antagonist causes a decrease in social interaction in rats receiving CD. C) Neither alcohol withdrawal, V1b antagonist microinjections into the CEA, nor OT agonist microinjections into the CEA caused a change in locomotor activity. (CD-Veh N=12, CD-V1b N=10, CD-OT N=11, ED-Veh N=13, ED-V1b N=11, ED-OT N=10). Data are presented as mean±SEM. *=p<0.05 post-hoc. **=p<0.01 post-hoc.
Table 2:
AVP antagonist and OT agonists did not alter the amount of alcohol consumed.
| Group | First block (Ave g/kg/day) |
Second block (Ave g/kg/day) |
Third block (Ave g/kg/day) |
|---|---|---|---|
| ED-Veh | 8.20±.29 | 8.12±.27 | 7.28±.27 |
| ED-V1b | 7.87±.19 | 6.80±.38 | 6.81±.24 |
| ED-OT | 8.06±.43 | 7.76±.30 | 7.07±.20 |
Data are presented as mean±SEM.
3.3. Chemogenetic activation of BLA projection neurons instead of alcohol diet is not sufficient to substitute for alcohol withdrawal to induce alcohol withdrawal-induced anxiety
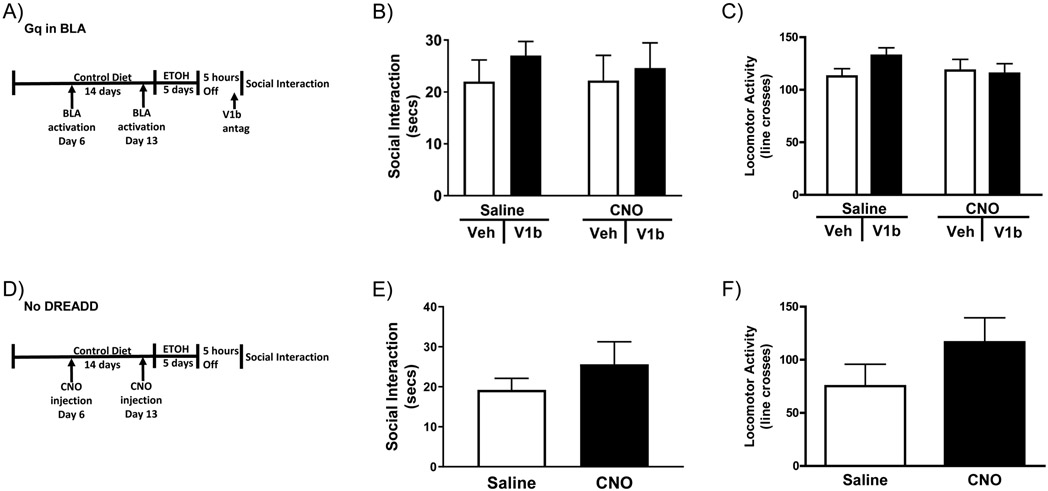
The prior section demonstrated that anxiety-like behavior can be instigated through pharmacological manipulation of the AVP system in the CEA instead of the first two alcohol withdrawals. It has been shown that projections from the BLA are involved in activation of the AVP system in the CEA (Huber et al., 2005). Therefore, chemogenetics was utilized to determine if selective activation of the projection neurons in the BLA (Ca2+/calmodulin-dependent protein kinase II (CAMKII)-positive) could facilitate anxiety-like behavior in rats when used in place of the first two alcohol withdrawals. Additionally, a V1b antagonist was injected into the CEA to see if it could block the effects of activation of the BLA on anxiety-like behavior (Figure 3A). A two-way ANOVA (Sal/CNO x Veh/V1b) revealed no significant effect of BLA activation or V1b antagonism on anxiety-like behavior (Figure 3B). Two-way ANOVA revealed no significant effect of any treatment on locomotor activity (Figure 3C) or on alcohol consumption (g/kg/day) (Table 3).
Figure 3: Activation of the BLA is not sufficient to substitute for alcohol withdrawal to cause alcohol withdrawal induced anxiety-like behavior.
A) Diagram of the injections/alcohol withdrawal paradigm. Rats with Gq DREADDs in their BLA received two CNO or Veh injects one week apart before being exposed to one 5-day bout of 4.5% w/v ED. During withdrawal rats received microinjections of a V1b antagonist into the CEA before testing for anxiety-like behavior 5-6 hours into withdrawal using the social interaction test. B) Neither BLA activation nor V1b antagonists altered behavior in the social interaction test. C) Neither BLA activation nor V1b antagonists altered locomotor activity (Sal-Veh N=11, Sal-V1b N=13, CNO-Veh N=13, CNO-V1b N=11). D) Diagram of the injections/alcohol withdrawal paradigm. Rats with no DREADDs were give two CNO or Veh injections one week apart before being exposed to one 5-day bout of 4.5% w/v ED. Rats were tested for anxiety-like behavior on the social interaction test 5-6 hours into withdrawal. E) CNO history did not alter anxiety-like behavior in rats that did not received the DREADD virus. F) CNO history did not alter locomotor activity in rats that did not receive the DREADD virus. (Sal-Veh N=4, Sal-V1b N=7, CNO-Veh N=6, CNO-V1b N=6). Data are presented as mean±SEM.
Table 3:
Neither BLA activation nor CNO alone altered the amount of alcohol consumed.
| Group | Ave g/kg/day |
|
|---|---|---|
| DREADD | ED-Sal-Veh | 5.35±.31 |
| DREADD | ED-Sal-V1b | 5.88±.38 |
| DREADD | ED-CNO-Veh | 5.73±.29 |
| DREADD | ED-CNO-V1b | 5.17±.36 |
| No DREADD | ED-Sal | 8.12±.22 |
| No DREADD | ED-CNO | 7.54±.29 |
Data are presented as mean±SEM. No significant difference was found in alcohol consumption between the DREADD experimental groups or between the No DEADD experimental groups.
An additional cohort of rats that underwent the same paradigm, but did not receive DREADD viral injections, was used to test for interactions between CNO history and ED that are not related to the effects of CNO on the Gq receptor (Figure 3D). Student’s t-test revealed no effect of CNO history on anxiety-like behavior in ED consuming rats if rats do not have a DREADD virus (Figure 3E). Additionally, there was no effect of CNO history on locomotor activity or alcohol consumed (g/kg/day) in ED consuming rats without DREADDs (Figure 3F & Table 3).
3.4. Arginine vasopressin in the CEA re-elicits anxiety after recovery from withdrawal through dm/dlPAG-dependent mechanisms
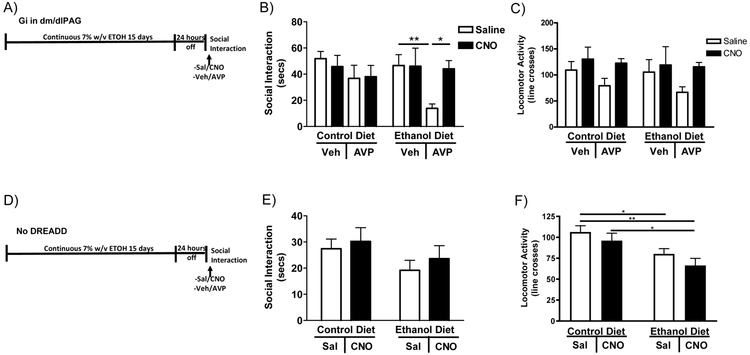
AVP can substitute for alcohol withdrawal to cause anxiety-like behavior, therefore it was predicted that activation of AVP receptors could also re-elicit anxiety. To test this hypothesis AVP was microinjected into the CeA 24 hours after withdrawal, a period of time when adult rats no longer show CE withdrawal induced anxiety. Additionally, as CEA neurons thought to be involved in the negative affect of alcohol withdrawal project to the dm/dlPAG (George et al., 2019), chemogenetics was utilized to determine if selective deactivation of the neurons in the dm/dlPAG could block AVP induced re-elicitation of anxiety-like behavior in rats (Figure 4A). Three-way ANOVA (CD/ED x Sal/CNO x Veh/AVP) revealed only a significant effect of AVP on anxiety-like behavior (Figure 4B F1,32=6.416; P<0.05). Twenty-four hours following withdrawal from continuous ED, ED-consuming rats displayed no significant difference in social interaction compared to CD-treated rats (CD-Sal-Veh vs. ED-Sal-ACSF; n.s.). Fisher’s post-hoc test revealed a highly significant decrease in social interaction time between ED-Sal-Veh vs. ED-Sal-AVP rats (P<0.01) suggesting that AVP reinstated the social interaction deficits. Fisher’s test also revealed a significant increase in social interaction time when dm/dlPAG was inactivated by CNO while AVP was injected into the CEA (ED-CNO-Veh vs ED-CNO-AVP; P<0.05) suggesting that inactivation of the dm/dlPAG blocks the effects of CEA AVP. Three-way ANOVA revealed a significant effect of CNO treatment on locomotor activity most likely caused by slightly elevated levels of activity across all rats receiving CNO injections, but individual comparisons did not reach significance (Figure 4C).
Figure 4: AVP into the CEA can reinstate anxiety-like behavior, but not if the dm/dlPAG is inactivated.
A) Diagram of the injections/alcohol withdrawal paradigm. Twenty-four hours into withdrawal from 15 continuous days of 7% w/v ED, rats with Gi DREADDs in their dm/dlPAG were given injections into the CEA and IP injections of CNO or vehicle before being tested for anxiety on the social interaction test. B) After 24 hours of withdrawal rats no longer demonstrated anxiety-like behavior unless given a microinjection of AVP into the CEA. Deactivation of the dm/dlPAG blocked the effects of CEA AVP microinjections on anxiety-like behavior. C) Rats receiving CNO injections had higher locomotor activity (CD-Sal-Veh N=7, CD-CNO-Veh N=5, CD-Sal-AVP N=4, CD-CNO-AVP N=5, ED-Sal-Veh N=5, ED-CNO-Veh N=4, ED-Sal-AVP N=5, ED-CNO-AVP N=5). D) Diagram of the injections/alcohol withdrawal paradigm. Twenty-four hours into withdrawal from 15 continuous days of 7% w/v ED, rats with no DREADD were given acute IP injections of CNO or vehicle. E) Neither acute CNO injection, alcohol withdrawal nor the combination altered anxiety-like behavior in rats that did not receive the DREADD virus. F) Rats receiving acute CNO injections had the lowest locomotor activity levels. (CD-Sal N=9, CD-CNO N=9, ED-Sal N=9, ED-CNO N=10). Data are presented as mean±SEM. *=p<0.05 post-hoc. **=p<0.01 post-hoc.
To check for the effects of acute CNO not related to its action on the Gi receptor, rats that had no DREADD virus were injected acutely with CNO and exposed to ED in the same paradigm as the dm/dlPAG inactivation study discussed above (Figure 4D). Two-way ANOVA (CD/ED x Sal/CNO) revealed no effect of acute CNO on ED consuming rats if rats do not have a DREADD virus (Figure 4E). Additionally, two-way ANOVA revealed there was a significant effect of acute CNO on locomotor activity in ED consuming rats without DREADDs (Figure 4F F1,32=12.37, P<0.01) most likely caused by a decrease in locomotor activity in all rats that received acute CNO injections.
4.0. Discussion
Previous studies have shown the importance of both AVP and the CEA in the modulation of anxiety-like behaviors. However, the importance of AVP in the CEA and specific input and output pathways surrounding the CEA have not been determined. In this study, multiple modifications of the chronic intermittent ethanol paradigm in rats were utilized to determine the role of the AVP receptor system along a withdrawal-induced anxiety circuit that potentially contains the BLA, the CEA, and the dm/dlPAG. These results confirm that while 5 days of alcohol consumption of a 4.5% alcohol liquid diet is not sufficient to cause anxiety 5–6 hrs into withdrawal, three cycles of 5-days ethanol exposure and withdrawal induce a social interaction deficit in rats following the third withdrawal (Overstreet et al., 2002). AVP microinjection into the CEA instead of the first two cycles of ethanol was also able to induce anxiety-like behavior. On the other hand, AVP microinjections did not cause changes in locomotor activity or alcohol consumption. Stress exposure and corticotropin releasing factor (CRF) microinjected into the CEA have both been shown to subsitute for the first two withdrawals (Breese et al., 2004, Huang et al., 2010). Stress is known to alter AVP and CRF levels (Pinnock and Herbert, 2001, Bartanusz et al., 1993, de Goeij et al., 1992, Ma and Lightman, 1998, Makino et al., 1995, Sawchenko et al., 1993) suggesting the mechanism by which alcohol alters AVP and CRF during withdrawal is most likely similar. Additionally, future work needs to be done to identify the relationship between V1b positive neurons and CRFR positive neurons in the CEA and how activation of both neurons leads to the same behavioral outcome.
V1b antagonism was able to block alcohol withdrawal induced anxiety-like behavior, but caused no significant changes to locomotor activity or alcohol consumption. However, in mice with a V1b receptor knock-out there is still an acute effect of alcohol on anxiety-like behavior in the elevated plus maze (Caldwell et al., 2006). This finding gives weight to the idea that the V1b receptor is related to neuroadaptive changes that occur after repeated exposures to withdrawal rather than the direct effect of ethanol. In agreement with that idea, Dannenhoffer et al. (2018) used a model of adolescent intermittent alcohol exposure which allowed for repeated exposure to withdrawals and long lasting effects into adulthood, and also found that systemic V1b antagonist blocked anxiety caused by exposure to adolescent intermittent alcohol. Therefore, the V1b role likely is related to adaptive changes and our results suggest that at least one of these adaptive changes is in the CEA. The results of Dannenhoffer et al. (2018) might also be mediated by changes in the AVP system in the amygdala, but that idea has yet to be tested. Their report did show, however, that there were adaptive changes in V1b receptors in the hypothalamus, which might be another brain region involved in withdrawal induced anxiety.
A limitation of this study is the use of the social interaction task, as the sole measure of anxiety-like behavior as behavior on this test can be altered by a number of factors in addition to changes in anxiety (File and Seth, 2003). In the case of AVP and OT there is a chance of changes in social motivation which is indistinguishable from changes in anxiety-like behavior on the social interaction task. Therefore, a future direction of this study would be to test other types of anxiety-like behavior effected by alcohol withdrawal. With regard to non-alcohol related anxiety, microinjections of the V1b antagonist into the CEA were not able to alter behavior on the elevated plus maze (Salome et al., 2006), but when the V1b antagonist was adminstered simultaneously with AVP into the CEA it was able to block the effects of AVP on the shock-probe burying test (Hernandez-Perez et al., 2018). Altogether these results suggest that V1b receptors in the CEA cannot account for all the effects of AVP on anxiety, but can account for some effects of AVP on types of anxiety other than social anxiety. Therefore, more work should be done to identify the characteristics of the V1b receptor containing neurons in the CEA. Additionally, these results suggest other brain regions should be explored as well as the CEA in models of AVP related anxiety.
This study found no effect of the V1b antagonist on alcohol consumption. While three other studies did find that the V1b antagonist decreased alcohol drinking in rodents (Edwards et al., 2012, Zhou et al., 2011, Zhou et al., 2018), it is important to keep in mind that this study was not designed to measure changes in alcohol drinking. Accordingly, the three previous studies gave the V1b antagonist shortly before testing for alcohol drinking while this study gave the V1b antagonists during the withdrawal periods. Moreover, the alcohol consumption in this case was not voluntary as the liquid ethanol diet included the animal’s food source.
Unlike Dannenhoffer et al. (2018), the present work did not find a significant effect of the OT agonist on this model of alcohol related anxiety. While the same OT agonist was used in both studies, the dispersion patterns varied between studies with the earlier study using systemic injections. This suggests that it is still worth exploring if OT receptors outside of the CEA play a role in this model of alcohol withdrawal related anxiety.
Surprisingly, input from the BLA was not sufficient to induce adptations associated with alcohol withdrawal induced anxiety in the studies presented here. CRF microinjections into the BLA have previously been shown to be sufficient to subsitute for the first two withdrawals in this paradigm and induce anxiety-like behavior (Huang et al., 2010). It is possible that the Gq DREADDS did not excite the BLA to the same degree as the CRF or perhaps CRF has a different effect in the BLA than the excitatory DREADDs. For example, it is possible that a specific pattern of neural activation in the BLA as produced by CRF microinjections is required for anxiogenesis, whereas the nonspecific activation of excitatory DREADDs was not sufficient. Another potential brain region with AVP neurons that project to the CEA is the hypothalamus, and this circuit is thought to underlie anxiety induced by water deprivation (Hernandez et al., 2016). The levels of AVP mRNA and protein in the hypothalamus change with chronic alcohol (Carmona-Calero et al., 1995, Gulya et al., 1991, Gulya et al., 1993, Hoffman and Dave, 1991, Ishizawa et al., 1990, Madeira et al., 1997, Sanna et al., 1993), which could be related to adaptations in the AVP system in the downstream CEA.
On the other hand, inactivation of the dm/dlPAG was sufficient to block reinstatement of anxiety-like behavior. This effect is unlikely to be related to effects of acute CNO alone or acute CNO interaction with ED as rats without the DREADD virus show no significant changes in social interaction with the same paradigm of acute CNO injection (Figure 4E). Additionally it is unlikely to be related to changes in locomotor activity even though there is a significant effect of CNO (dm/dlPAG deactivation) on locomotor activity. The post-hoc individual comparisons in the locomotor data failed to reach significance suggesting that the differences between groups in locomotor activity is not sufficient enough to cause the differences seen in social interaction between the same groups. AVP in the CEA has been shown to excite inhibitory (GABAergic) neurons (Huber et al., 2005), suggesting a potential multi-synaptic connection. Most likely the V1b receptor containing CEA neurons are inhibiting inhibitory interneurons in the dm/dlPAG (Oka et al., 2008, da Costa Gomez and Behbehani, 1995). Removal of the inhibition of these interneurons allows activity of dm/dlPAG projection neurons, resulting in disinhibition of downstream pathways. Otherwise an excited inhibitory neuron in the CEA would inhibit the dm/dlPAG and inactivating the dm/dlPAG would not block the effects of AVP in the CEA. CEA neuronal inhibition of ventrolateral PAG (vlPAG) interneurons was found to be an important pathway for hyperalgesia in alcohol-dependent rats (Avegno et al., 2018) though it is important to keep in mind this is a different pathway from the one examined in this paper. Additionally, it was shown that a decrease in GABAergic signaling due to altered melanocortin signaling in alcohol dependent rats altered vlPAG activity and caused hyperalgesia (Avegno et al., 2018). A potential alteration in V1b receptors in the CEA due to repeated withdrawals from chronic alcohol exposure might have similar effects on the potential CEA to dm/dlPAG pathway that is related to alcohol withdrawal induced anxiety. As it is currently unknown if changes in the levels of V1b receptors occur in the CEA after the CIE paradigm employed in this paper, future work should focus on identifying adaptative changes in the AVP/OT system in the CEA that could explain changes in the CEA to dm/dlPAG pathway with withdrawal from CIE. Other potential downstream brain regions should also be investigated including the bed nucleus of the stria terminalis which recieves projections from the CEA and has been implicated in alcohol related anxiety (Kash, 2012).
The locomotor data in most of the experiments (see Figure 1 and 2) was not significant in spite of the fact that there was significant differences in social interaction. On the reverse side, the locomotor data in Figure 4F was significant despite a lack of significant change in social interaction. This shows once again that reductions in social interaction and locomotor activity seem to be independently manipulatable (Wills et al., 2010, Breese et al., 2004, Overstreet et al., 2002, Overstreet et al., 2004).
Altogether these data show a role for the CEA system in the neuroadaptive changes that lead to chronic alcohol induced anxiety. This work suggests the AVP system as a potential target for alcohol abuse and its comorbid disorder anxiety.
Supplementary Material
Acknowledgements
The authors would like to acknowledge technical assistance from Natajha Phillips and Edith Rivera.
Funding and Disclosure
Declarations of conflict of interest: none. This work was supported by the National Institutes of Health, National Institute on Alcohol Abuse and Alcoholism (R01-AA022234, R01-AA021275, P60-AA011605), and the Bowles Center for Alcohol Studies.
References
- Avegno EM, Lobell TD, Itoga CA, Baynes BB, Whitaker AM, Weera MM, Edwards S, Middleton JW, Gilpin NW (2018) Central Amygdala Circuits Mediate Hyperalgesia in Alcohol-Dependent Rats. J Neurosci 38:7761–7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartanusz V, Aubry JM, Jezova D, Baffi J, Kiss JZ (1993) Up-regulation of vasopressin mRNA in paraventricular hypophysiotrophic neurons after acute immobilization stress. Neuroendocrinology 58:625–629. [DOI] [PubMed] [Google Scholar]
- Bertotto ME, Bussolino DF, Molina VA, Martijena ID (2010) Increased voluntary ethanol consumption and c-Fos expression in selected brain areas induced by fear memory retrieval in ethanol withdrawn rats. Eur Neuropsychopharmacol 20:568–581. [DOI] [PubMed] [Google Scholar]
- Bonassoli VT, Contardi EB, Milani H, de Oliveira RM (2013) Effects of nitric oxide synthase inhibition in the dorsolateral periaqueductal gray matter on ethanol withdrawal-induced anxiety-like behavior in rats. Psychopharmacology (Berl) 228:487–498. [DOI] [PubMed] [Google Scholar]
- Bonassoli VT, Milani H, de Oliveira RM (2011) Ethanol withdrawal activates nitric oxide-producing neurons in anxiety-related brain areas. Alcohol 45:641–652. [DOI] [PubMed] [Google Scholar]
- Breese GR, Knapp DJ, Overstreet DH (2004) Stress sensitization of ethanol withdrawal-induced reduction in social interaction: inhibition by CRF-1 and benzodiazepine receptor antagonists and a 5-HT1A-receptor agonist. Neuropsychopharmacology 29:470–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Overstreet DH, Knapp DJ, Navarro M (2005) Prior multiple ethanol withdrawals enhance stress-induced anxiety-like behavior: inhibition by CRF1- and benzodiazepine-receptor antagonists and a 5-HT1a-receptor agonist. Neuropsychopharmacology 30:1662–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral A, Isoardi N, Salum C, Macedo CE, Nobre MJ, Molina VA, Brandao ML (2006) Fear state induced by ethanol withdrawal may be due to the sensitization of the neural substrates of aversion in the dPAG. Exp Neurol 200:200–208. [DOI] [PubMed] [Google Scholar]
- Caldwell HK, Stewart J, Wiedholz LM, Millstein RA, Iacangelo A, Holmes A, Young WS 3rd, Wersinger SR (2006) The acute intoxicating effects of ethanol are not dependent on the vasopressin 1a or 1b receptors. Neuropeptides 40:325–337. [DOI] [PubMed] [Google Scholar]
- Calhoon GG, Tye KM (2015) Resolving the neural circuits of anxiety. Nature neuroscience 18:1394–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona-Calero E, del Mar Perez-Delgado M, Banuelos-Pineda J, Marrero-Gordillo N, Ferres-Torres R, Castaneyra-Perdomo A (1995) Effects of chronic alcohol intake on the vasopressin content in the hypothalamic paraventricular and supraoptic nuclei of the mouse. An immunohistochemical and morphometric study. Drug and alcohol dependence 38:19–24. [DOI] [PubMed] [Google Scholar]
- da Costa Gomez TM, Behbehani MM (1995) An electrophysiological characterization of the projection from the central nucleus of the amygdala to the periaqueductal gray of the rat: the role of opioid receptors. Brain Res 689:21–31. [DOI] [PubMed] [Google Scholar]
- Dannenhoffer CA, Kim EU, Saalfield J, Werner DF, Varlinskaya EI, Spear LP (2018) Oxytocin and vasopressin modulation of social anxiety following adolescent intermittent ethanol exposure. Psychopharmacology (Berl). [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Goeij DC, Jezova D, Tilders FJ (1992) Repeated stress enhances vasopressin synthesis in corticotropin releasing factor neurons in the paraventricular nucleus. Brain Res 577:165–168. [DOI] [PubMed] [Google Scholar]
- Edwards S, Guerrero M, Ghoneim OM, Roberts E, Koob GF (2012) Evidence that vasopressin V1b receptors mediate the transition to excessive drinking in ethanol-dependent rats. Addict Biol 17:76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- File SE, Seth P (2003) A review of 25 years of the social interaction test. Eur J Pharmacol 463:35–53. [DOI] [PubMed] [Google Scholar]
- Frye GD, McCown TJ, Breese GR (1983) Characterization of susceptibility to audiogenic seizures in ethanol-dependent rats after microinjection of gamma-aminobutyric acid (GABA) agonists into the inferior colliculus, substantia nigra or medial septum. J Pharmacol Exp Ther 227:663–670. [PMC free article] [PubMed] [Google Scholar]
- George DT, Ameli R, Koob GF (2019) Periaqueductal gray sheds light on dark areas of psychopathology. Trends in neurosciences. [DOI] [PubMed] [Google Scholar]
- Griebel G, Beeske S, Stahl SM (2012) The vasopressin V(1b) receptor antagonist SSR149415 in the treatment of major depressive and generalized anxiety disorders: results from 4 randomized, double-blind, placebo-controlled studies. J Clin Psychiatry 73:1403–1411. [DOI] [PubMed] [Google Scholar]
- Gulya K, Dave JR, Hoffman PL (1991) Chronic ethanol ingestion decreases vasopressin mRNA in hypothalamic and extrahypothalamic nuclei of mouse brain. Brain Res 557:129–135. [PubMed] [Google Scholar]
- Gulya K, Orpana AK, Sikela JM, Hoffman PL (1993) Prodynorphin and vasopressin mRNA levels are differentially affected by chronic ethanol ingestion in the mouse. Brain Res Mol Brain Res 20:1–8. [DOI] [PubMed] [Google Scholar]
- Harper KM, Knapp DJ, Breese GR (2015) Withdrawal from Chronic Alcohol Induces a Unique CCL2 mRNA Increase in Adolescent But Not Adult Brain—Relationship to Blood Alcohol Levels and Seizures. Alcohol Clin Exp Res 39:2375–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper KM, Knapp DJ, Criswell HE, Breese GR (2018a) Vasopressin and alcohol: a multifaceted relationship. Psychopharmacology (Berl) 235:3363–3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper KM, Knapp DJ, Park MA, Breese GR (2018b) Differential effects of single versus repeated minocycline administration-Lack of significant interaction with chronic alcohol history. Pharmacol Biochem Behav 168:33–42. [DOI] [PubMed] [Google Scholar]
- Hernandez-Perez OR, Crespo-Ramirez M, Cuza-Ferrer Y, Anias-Calderon J, Zhang L, Roldan-Roldan G, Aguilar-Roblero R, Borroto-Escuela DO, Fuxe K, Perez de la Mora M (2018) Differential activation of arginine-vasopressin receptor subtypes in the amygdaloid modulation of anxiety in the rat by arginine-vasopressin. Psychopharmacology (Berl) 235:1015–1027. [DOI] [PubMed] [Google Scholar]
- Hernandez VS, Hernandez OR, Perez de la Mora M, Gomora MJ, Fuxe K, Eiden LE, Zhang L (2016) Hypothalamic Vasopressinergic Projections Innervate Central Amygdala GABAergic Neurons: Implications for Anxiety and Stress Coping. Frontiers in neural circuits 10:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernando F, Schoots O, Lolait SJ, Burbach JP (2001) Immunohistochemical localization of the vasopressin V1b receptor in the rat brain and pituitary gland: anatomical support for its involvement in the central effects of vasopressin. Endocrinology 142:1659–1668. [DOI] [PubMed] [Google Scholar]
- Hoffman PL, Dave JR (1991) Chronic ethanol exposure uncouples vasopressin synthesis and secretion in rats. Neuropharmacology 30:1245–1249. [DOI] [PubMed] [Google Scholar]
- Huang MM, Overstreet DH, Knapp DJ, Angel R, Wills TA, Navarro M, Rivier J, Vale W, Breese GR (2010) Corticotropin-releasing factor (CRF) sensitization of ethanol withdrawal-induced anxiety-like behavior is brain site specific and mediated by CRF-1 receptors: relation to stress-induced sensitization. J Pharmacol Exp Ther 332:298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber D, Veinante P, Stoop R (2005) Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science 308:245–248. [DOI] [PubMed] [Google Scholar]
- Ishizawa H, Dave JR, Liu LI, Tabakoff B, Hoffman PL (1990) Hypothalamic vasopressin mRNA levels in mice are decreased after chronic ethanol ingestion. Eur J Pharmacol 189:119–127. [DOI] [PubMed] [Google Scholar]
- Janak PH, Tye KM (2015) From circuits to behaviour in the amygdala. Nature 517:284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JH, Ung RL, Resendez SL, Stamatakis AM, Taylor JG, Huang J, Veleta K, Kantak PA. Aita M, Shilling-Scrivo K, Ramakrishnan C, Deisseroth K, Otte S, Stuber GD (2015) Visualizing hypothalmis network dynamics for appetitive and consummatory behaviors. Cell 160:516–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash TL (2012) The role of biogenic amine signaling in the bed nucleus of the stria terminals in alcohol abuse. Alcohol 46:303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Pignatelli M, Xu S, Itohara S, Tonegawa S (2016) Antagonistic negative and positive neurons of the basolateral amygdala. Nature neuroscience 19:1636–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp DJ, Duncan GE, Crews FT, Breese GR (1998) Induction of Fos-like proteins and ultrasonic vocalizations during ethanol withdrawal: further evidence for withdrawal-induced anxiety. Alcohol Clin Exp Res 22:481–493. [PubMed] [Google Scholar]
- Knapp DJ, Harper KM, Whitman BA, Zimomra Z, Breese GR (2016) Stress and Withdrawal from Chronic Ethanol Induce Selective Changes in Neuroimmune mRNAs in Differing Brain Sites. Brain Sci 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp DJ, Overstreet DH, Breese GR (2005) Modulation of ethanol withdrawal-induced anxiety-like behavior during later withdrawals by treatment of early withdrawals with benzodiazepine/gamma-aminobutyric acid ligands. Alcohol Clin Exp Res 29:553–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND (2010) Neurocircuitry of addiction. Neuropsychopharmacology 35:217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND (2016) Neurobiology of addiction: a neurocircuitry analysis. The lancet. Psychiatry 3:760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XM, Lightman SL (1998) The arginine vasopressin and corticotrophin-releasing hormone gene transcription responses to varied frequencies of repeated stress in rats. The Journal of physiology 510 (Pt 2):605–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeira MD, Andrade JP, Lieberman AR, Sousa N, Almeida OF, Paula-Barbosa MM (1997) Chronic alcohol consumption and withdrawal do not induce cell death in the suprachiasmatic nucleus, but lead to irreversible depression of peptide immunoreactivity and mRNA levels. J Neurosci 17:1302–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino S, Smith MA, Gold PW (1995) Increased expression of corticotropin-releasing hormone and vasopressin messenger ribonucleic acid (mRNA) in the hypothalamic paraventricular nucleus during repeated stress: association with reduction in glucocorticoid receptor mRNA levels. Endocrinology 136:3299–3309. [DOI] [PubMed] [Google Scholar]
- Morales-Medina JC, Witchey SK, Caldwell HK (2016) The Role of Vasopressin in Anxiety and Depression, in Melatonin, Neuroprotective Agents and Antidepressant Therapy, Melatonin, Neuroprotective Agents and Antidepressant Therapy, pp 667–685, Springer. [Google Scholar]
- Neumann ID, Landgraf R (2012) Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends Neurosci 35:649–659. [DOI] [PubMed] [Google Scholar]
- Oka T, Tsumori T, Yokota S, Yasui Y (2008) Neuroanatomical and neurochemical organization of projections from the central amygdaloid nucleus to the nucleus retroambiguus via the periaqueductal gray in the rat. Neuroscience research 62:286–298. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR (2002) Accentuated decrease in social interaction in rats subjected to repeated ethanol withdrawals. Alcohol Clin Exp Res 26:1259–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR (2004) Modulation of multiple ethanol withdrawal-induced anxiety-like behavior by CRF and CRF1 receptors. Pharmacol Biochem Behav 77:405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinnock SB, Herbert J (2001) Corticosterone differentially modulates expression of corticotropin releasing factor and arginine vasopressin mRNA in the hypothalamic paraventricular nucleus following either acute or repeated restraint stress. Eur J Neurosci 13:576–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D, Barak S (2016) Molecular mechanisms underlying alcohol-drinking behaviours. Nature reviews. Neuroscience 17:576–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan ML, Falk DE, Fertig JB, Rendenbach-Mueller B, Katz DA, Tracy KA, Strain EC, Dunn KE, Kampman K, Mahoney E, Ciraulo DA, Sickles-Colaneri L, Ait-Daoud N, Johnson BA, Ransom J, Scott C, Koob GF, Litten RZ (2017) A Phase 2, Double-Blind, Placebo-Controlled Randomized Trial Assessing the Efficacy of ABT-436, a Novel V1b Receptor Antagonist, for Alcohol Dependence. Neuropsychopharmacology 42:1012–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salome N, Stemmelin J, Cohen C, Griebel G (2006) Differential roles of amygdaloid nuclei in the anxiolytic- and antidepressant-like effects of the V1b receptor antagonist, SSR149415, in rats. Psychopharmacology (Berl) 187:237–244. [DOI] [PubMed] [Google Scholar]
- Sanna PP, Folsom DP, Barizo MJ, Hirsch MD, Melia KR, Maciejewski-Lenoir D, Bloom FE (1993) Chronic ethanol intake decreases vasopressin mRNA content in the rat hypothalamus: a PCR study. Brain Res Mol Brain Res 19:241–245. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Arias CA, Mortrud MT (1993) Local tetrodotoxin blocks chronic stress effects on corticotropin-releasing factor and vasopressin messenger ribonucleic acids in hypophysiotropic neurons. Journal of neuroendocrinology 5:341–348. [DOI] [PubMed] [Google Scholar]
- Sinha R (2008) Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci 1141:105–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Albers HE (2017) Cross-talk among oxytocin and arginine-vasopressin receptors: Relevance for basic and clinical studies of the brain and periphery. Frontiers in neuroendocrinology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemmeline J, Lukovic L, Salome N, Griebel G (2005) Evidence that the lateral septum is involved in the antidepressant-like effects of the vasopressin V1b receptor antagonist, SSR149415. Neuropsychopharmacology 30:35–42. [DOI] [PubMed] [Google Scholar]
- Veinante P, Freund-Mercier MJ (1997) Distribution of oxytocin- and vasopressin-binding sites in the rat extended amygdala: a histoautoradiographic study. J Comp Neurol 383:305–325. [PubMed] [Google Scholar]
- Wills TA, Knapp DJ, Overstreet DH, Breese GR (2008) Differential dietary ethanol intake and blood ethanol levels in adolescent and adult rats: effects on anxiety-like beahvior and seizure thresholds. Alcohol Clin Exp Res 32:1350–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills TA, Knapp DJ, Overstreet DH, Breese GR (2010) Interactions of stress and CRF in ethanol-withdrawal induced anxiety in adolescent and adult rats. Alcohol Clin Exp Res 34:1603–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young WS, Li J, Wersinger SR, Palkovits M (2006) The vasopressin 1b receptor is prominent in the hippocampal area CA2 where it is unaffected by restraint stress or adrenalectomy. Neuroscience 143:1031–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelena D (2012) Vasopressin in health and disease with a focus on affective disorders. Central nervous system agents in medicinal chemistry 12:286–303. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Colombo G, Carai MA, Ho A, Gessa GL, Kreek MJ (2011) Involvement of arginine vasopressin and V1b receptor in alcohol drinking in Sardinian alcohol-preferring rats. Alcohol Clin Exp Res 35:1876–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Rubinstein M, Low MJ, Kreek MJ (2018) V1b Receptor Antagonist SSR149415 and Naltrexone Synergistically Decrease Excessive Alcohol Drinking in Male and Female Mice. Alcohol Clin Exp Res 42:195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.