Abstract
Background:
The period of college represents a particularly risky developmental stage with regards to alcohol use, as college students engage in more risky drinking behaviors than their non-college peers, and such problematic alcohol use is associated with far-reaching negative consequences. Existing findings from genome-wide association studies (GWAS) indicate that alcohol consumption has a complex polygenic etiology. Currently, there is a lack of studies examining genetic risk for alcohol consumption using polygenic risk scores (PRS) in college samples. In this study, we examined whether alcohol-specific and risky-behavior-related PRS were longitudinally associated with alcohol consumption among college students and whether this effect might be partially mediated by impulsivity domains.
Method:
The sample included n = 2,385 European ancestry (EA) and n = 1,153 African ancestry (AA) college students assessed over the course of 4 years. To indicate genetic risk, two PRS were created based on recent large-scale GWAS: alcohol consumption (Liu et al., 2019) – DPW (drinks per week)-PRS, and risky behaviors (Linnér et al., 2019) – RISK-PRS. The main outcome was alcohol consumption, measured across four waves of follow-up data. The UPPS-P impulsivity subscales were examined as mediators of the genetic effect on alcohol consumption.
Results:
The results from structural equation modeling showed that among European ancestry students, both DPW-PRS and RISK-PRS had significant positive effects on alcohol consumption above and beyond UPPS dimensions and control variables. RISK-PRS explained larger portion of variance in alcohol consumption than DPW-PRS. RISK-PRS showed a significant indirect effect on alcohol consumption through sensation seeking; lack of perseverance; no significant indirect effect of DPW-PRS was found. No significant association of either PRS and alcohol consumption was found for African ancestry participants.
Conclusions:
The current results found that PRS related to more broadly-defined risky behaviors predicted alcohol consumption across college years, and that this association was partially mediated via dimensions of impulsivity.
Keywords: polygenic risk scores, alcohol consumption, risky behaviors, impulsivity, college
Introduction
Risky alcohol use is widespread on college campuses and associated with serious consequences among young adults. Risk for alcohol use disorders generally peaks during young adulthood (Grant et al., 2015). College students are more likely to drink heavily and develop alcohol use disorders than their non-college-attending peers (Barnes et al., 2010; Slutske et al., 2004). The latest data from Monitoring the Future showed that the prevalence of alcohol use, binge drinking, and drunkenness is higher among college students as compared to non-college youth (Schulenberg et al., 2018). College students also face a unique set of environmental pressures and social, developmental contexts (Evans et al., 2009b) such as leaving home and forming new peer groups. These changes and transitions usually create increased opportunity for alcohol involvement, leading to elevated risk for alcohol problems among college students. Problematic alcohol use is associated with far-reaching consequences, including decreased academic performance (Meda et al., 2017), unwanted sexual encounters, legal consequences, assault, injury, suicide, and even death (Hingson et al., 2009; Perkins, 2002). In this way, college years represent a particularly critical period for the development and establishment of problematic alcohol use behaviors that persist into adulthood (Dick et al., 2018b). Understanding pathways of risk to alcohol use problems among college students is critically important for developing effective prevention and intervention.
Genetics of Alcohol Use
A large number of studies in the past two decades have shown that alcohol-related phenotypes are strongly affected by genetic factors (Dick and Bierut, 2006). The latest meta-analysis of behavior genetic studies of alcohol use disorder (AUD) estimated that AUD is approximately 50% heritable (Verhulst et al., 2015). Twin studies also robustly demonstrate that the genetic predisposition to alcohol use outcomes is shared with other outcomes that reflect behavioral disinhibition or impulsivity (Hicks et al., 2004; Kendler et al., 2003; Khemiri et al., 2016; Krueger et al., 2002; Slutske et al., 2002, 1998). In fact, as much as 2/3 of the heritability of alcohol dependence is estimated to reflect genetic influences shared across externalizing outcomes (Kendler et al., 2003).
Other alcohol-related phenotypes, such as alcohol consumption, also show a substantial genetic component (Dick et al., 2011; Dick and Bierut, 2006). Advances in gene identification indicate that alcohol-related phenotypes have a complex polygenic etiology affected by a large number of genes with very small effects (Agrawal and Bierut, 2012; Hart and Kranzler, 2015; Liu et al., 2019; Sanchez-Roige et al., 2018; Walters et al., 2018). For this reason, large-scale genome-wide association studies (GWAS) have become the prominent method of estimating genetic risk for behavioral phenotypes. GWAS analyze common genetic variants across the genome for associations with a phenotype of interest. The aggregate measure of these weighted associations is then expressed in the form of a polygenic risk score (PRS), quantifying the relative genetic risk for each individual for the specific phenotype (Bogdan et al., 2018; Dudbridge, 2016). With expanding GWAS sample sizes, the number of genetic variants identified has been increasing and, along with it, the explained variance in the phenotype. A recent study on alcohol consumption (drinks per week) employed a combined sample of approximately 941,000 individuals to identify 111 common loci associated with alcohol consumption. The PRS explained 2.5% of variance in drinks per day in an independent sample (Liu et al., 2019). A GWAS conducted on ~316,000 individuals by Linnér et al. (2019) combined drinks per week with other behaviors characterized by behavioral disinhibition (automobile speeding propensity, whether one has ever been smoker, and number of sexual partners) that all loaded onto a single principal component and likely reflect the general disinhibition toward impulsive behavior identified in twin studies, as described above. Their results showed a substantial genetic overlap for the indicators of risky behaviors and with item reflecting general risk tolerance.
Despite growing sample sizes, GWAS of alcohol phenotypes continue to yield only small effects. This may reflect, in part, the notion that there are no genes specifically “for” alcohol phenotypes but rather, the genetic factors impact these distal outcomes through intermediary traits and pathways (Dick & Hancock, 2015; Dick, 2018).
Externalizing Pathway
One of the most prominent etiological models of problematic alcohol use has been the externalizing pathway to alcohol-related problems (Hussong et al., 2007; Zucker, 2008). The externalizing pathway framework emphasizes that the risk for the development of alcohol-related problems is largely affected by an underlying predisposition toward impulsivity/behavioral disinhibition. This predisposition is first manifested in childhood as a difficult temperament, characterized by conduct problems and aggressive behavior (McGue, Iacono, & Krueger, 2006; Slutske et al., 1998). Developmental research showed that conduct disorders in childhood predicted later development of alcohol problems (Brown et al., 1996; Dick et al., 2006). During adolescence, this propensity might progress into risky behaviors such as delinquency or substance use, including alcohol use. This risk is exacerbated in risk environments, including high parental negativity, low parental warmth and monitoring, affiliation with delinquent peers, and living in poor neighborhood, among others (Dick, 2011; Kendler, Gardner, & Dick, 2011). A large number of previous studies documented the high overlap between externalizing symptoms and alcohol-related problems (King et al., 2004; Kokkevi et al., 2007; Sartor et al., 2007), or, more specifically, between impulsive behaviors and alcohol use (Dick et al., 2010; Lejuez et al., 2010; Stautz and Cooper, 2013).
There have been various approaches to conceptualizing impulsivity. The UPPS scale was created as an instrument that seeks to integrate different approaches to impulsivity or impulsive-like traits into a common system. Through factor analyzing a large number of impulsivity scales, the UPPS is an integration of selected items from multiple instruments (Whiteside and Lynam, 2001). The measure, in its latest version as UPPS-P, comprises five interrelated but distinct factors – negative urgency, which refers to the tendency to act rashly as a result of negative affect, positive urgency or the tendency to act rashly as a result of positive affect, lack of premeditation, reflecting inability to “delay action in favor of careful thinking and planning”, lack of perseverance or the inability to remain with a task and see it finished, and sensation seeking or the tendency to seek out excitement and adventure (Lynam et al., 2007). Previous studies have found that UPPS factors significantly predict different alcohol phenotypes, including drinking quantity and alcohol dependence (Coskunpinar et al., 2013; Stautz and Cooper, 2013), as well as being related to a variety of externalizing behaviors (Berg et al., 2015).
Genetics and Racial/Ethnic Diversity
One caveat to the current genetic findings has been the lack of ethnic/racial diversity as the vast majority of existing studies on genetic risk for alcohol use have been conducted on samples with European ancestry (Hart & Kranzler, 2015). The reason for this has been mostly the lack of non-European samples with adequate sample size. However, European-centered GWAS results might not easily translate to other populations due to different patterns of linkage disequilibrium (Martin et al., 2017). As such, there is a pressing need to expand studies using genome-wide risk scores to non-European populations to provide results that might be more generalizable and inclusive to other populations.
The Current Study
The aim of the current study was to assess whether dimensions of impulsivity, measured by UPPS-P, would serve as mediators between genetic risk and alcohol use. Acknowledging the complex nature of alcohol-related outcomes and grounded in the literature suggesting that the externalizing pathway is related to alcohol consumption, we employed findings from two recent large-scale meta-analytic GWAS to create polygenic risk scores (PRS) to test the idea that genetic factors may influence alcohol use via impulsive personality traits. These GWAS focused on the following phenotypes: drinks per week – DPW-PRS (Liu et al., 2019), and risky behaviors – RISK-PRS (Linnér et al., 2019). We selected these particular GWAS to evaluate and compare the effects of genetic risk for alcohol consumption indexed by a narrowly-defined PRS (drinks per week [DPW] PRS) with PRS reflecting a broader, less-alcohol specific risky propensity (risky behaviors [RISK] PRS). Both DPW-PRS and RISK-PRS stem from the largest available discovery samples to date and as such, reflect the best current genetic approximation of alcohol consumption and behavioral disinhibition, respectively.
Using data from a large, longitudinal study of college students, we examined a primary research question of whether dimensions of impulsivity mediate the effect of genetic risk on alcohol consumption across the college years. Using data from the Spit for Science study, we assessed this research question within two ancestral groups – African and European.
We hypothesized that:
DPW-PRS and RISK-PRS will be associated with more alcohol consumption.
The associations between DPW-PRS and RISK-PRS and alcohol consumption will be mediated through various dimensions of impulsivity as measured by the UPPS subscales.
Materials and Methods
Sample
The Spit for Science (S4S) project is an ongoing, longitudinal, university-wide project at a large, urban university focused on genetic and environmental influences on college students’ substance use and behavioral health outcomes (Dick et al., 2014). S4S invites incoming students aged 18 or older to participate in an online survey at the beginning of the fall semester of their freshman year and provide a saliva sample for genotyping. Participants subsequently complete a follow-up online survey each spring while attending college. Data collection for S4S began in the fall of 2011, and five cohorts of incoming freshman students have been enrolled in the study. Study data were collected and managed using REDCap (Research Electronic Data Capture). REDCap is a secure, web-based application designed to support data capture for research studies, providing 1) an intuitive interface for validated data entry; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for importing data from external sources (Harris et al., 2009). Of the more than 12,000 participants, the sample size was narrowed down for the present study using the following inclusion criteria: First, the genotypic data for analyses were available only for cohorts 1 – 3 (N = 5,943). For this reason, only those cohorts were included in the analytic sample. Furthermore, we focused our analyses on European and African ancestry subsamples, as these were the only ancestral subsamples with adequate sample sizes required for the longitudinal analyses, resulting in 3,010 European ancestry (EA) and 1,339 African ancestry (AA) participants. Lastly, we removed all participants who had no data on alcohol use in any of the spring follow-ups (n = 571 European and n = 162 African participants). After removing outliers (see Plan of Analysis), the final analytic sample size was N = 2,385 European ancestry and N = 1,156 African ancestry participants at the first assessment.
Genotyping.
Participants’ DNA samples were obtained via saliva and genotyped on the Affymetrix BioBank Array that contains 653k both common and rare genetic variants. Genotyping was performed at Rutgers University Cell and DNA Repository, with quality control performed locally following procedures from the Psychiatric Genomics Consortium. Imputation was conducted using the HapMap 1000 genomes Phase 3 reference panel. Single nucleotide polymorphisms (SNPs) with a genotyping rate <0.95 or that violated Hardy–Weinberg equilibrium (p< 10−6) or with minor allele frequency <0.005 were excluded from analysis.
Measures
Polygenic risk scores (PRS).
We used genome-wide association estimates from two of the most recent and largest genome-wide association studies (GWAS) related to alcohol outcomes and risky behavior to calculate PRS in our target sample. These discovery GWAS included a GWAS of alcohol consumption (i.e., drinks per week) conducted by the GWAS & Sequencing Consortium of Alcohol and Nicotine use (GSCAN), with a discovery sample of 941,000 participants (Liu et al., 2019) and a GWAS of risky behaviors with the available discovery sample of n ~ 316,000 from UKBiobank (Linnér et al., 2019). In the discovery GWAS, the GSCAN drinks per week (DPW) was computed for the number of drinks participants had in a week; risky behaviors (RISK) was computed in the original article as the first principal component of four self-reported risky behaviors available in UKBiobank: automobile speeding propensity, drinks per week, ever smoker, and lifetime number of sexual partners.
After removing palindromic SNPs (which can be ambiguous with respect to the reference allele when going across samples), we used the clump and score procedures in PLINK 1.9 to sum each individual’s total number of minor alleles from the score SNPs, with each SNP weighted by the negative log of the GWAS association p value and sign of the association (beta) statistic (Chang et al., 2015; Purcell et al., 2007). Clumping was done with respect to the linkage disequilibrium (LD) pattern in the 1000 Genomes Phase 3 reference panel using a 500kb physical distance and an LD threshold of r2 greater than or equal to 0.25. Thus, the polygenic scores were constructed of SNPs that capture independent genetic association signals from the discovery GWAS. We calculated a series of scores in Spit for Science for each PRS that included SNPs meeting increasingly stringent p-value thresholds from the discovery GWAS sample (p < 0.50, p < 0.40, p < 0.30, p < 0.20, p < 0.10, p < 0.05, p < 0.01, p < 0.001, p < 0.0001). Given there are no set criteria for selecting the PRS threshold with the highest predictive ability, we selected the most predictive one by regressing each one of them on the outcome while controlling for sex, age, their interaction term, and ancestral segregation by using 10 ancestry principal components, which adjusts for genetic differences by superpopulation (Peterson et al., 2017; Su et al., 2018). The PRS with the largest explained variance (indicated by largest ΔR2 explained by the predictor as compared to other thresholds) was selected to be used in subsequent structural models (Evans et al., 2009a).
Given that the above-mentioned PRS were derived from GWAS conducted on primarily European ancestry participants, applying them to participants with African ancestry (AA) might result in biased estimation due to different patterns of linkage disequilibrium (Martin et al., 2017). Thus, for the AA subsample, we employed the multiPRS method described in Márquez-Luna, Loh, and Price (2017) to create PRS; this method has been shown to improve predictive accuracy in samples of non-European ancestry (see Appendix I for the description of multiPRS approach).
Alcohol consumption (Assessed: spring freshman, sophomore, junior, senior year).
Alcohol consumption was operationalized as grams of ethanol consumed per month. We used data on alcohol consumption from participants’ four assessments, from spring freshman year to spring senior year. For the first assessment (spring freshman year) of the first cohort, alcohol consumption was assessed by asking participants about the number of days they had drunk alcohol during the past 30 days, with one-day interval responses ranging from 0 to 30. The participants that answered anything but zero were then asked about the number of drinks they had on a typical day, with a one drink interval responses ranging from 1 through 20 to “more than 20.” For subsequent cohorts and follow-up assessments, alcohol consumption was computed based on items from the Alcohol Use Disorders Identification Test (AUDIT; Bohn, Babor, & Kranzler, 1995). These participants were asked to rate the frequency of their alcohol use with the following categories: “never”, “monthly or less”, “2 to 4 times a month”, “2 to 3 times a week”, “4 or more times a week.” Further, they were asked to specify the number of drinks they had on a typical day when drinking, using the following categories: “1 or 2”, “3 or 4”, “5 or 6”, “7, 8, or 9”, and “10 or more”. The responses on the number of drinking days were converted to the midpoints of ranges for each option, i.e., never = 0, monthly or less = 0.5, 2 to 4 times a month = 3, 2 to 3 times a week = 10.7, four or more times a week = 23.54. Similarly, the responses to the number of drinks per drinking occasion were converted to their midpoints, i.e., 1 or 2 drinks = 1.5, 3 or 4 = 3.5, 5 or 6 = 5.5, 7, 8, or 9 = 8, 10 or more = 15.5 (21 used as an upper bound to match first cohort). Then, the number of days participants were drinking was multiplied by the number of drinks per occasion, and this was multiplied by 14, reflecting 14 grams of pure alcohol, which is roughly the amount of alcohol included in a standard drink. This scale was first used in Salvatore et al. (2016).
Dimensions of impulsivity (Fall freshman year1).
An abbreviated 15-item version of the original UPPS-P scale (Lynam et al., 2007) was used. This version was created and provided to us by the authors of the original UPPS-P scale. This self-reported scale comprises 5 subscales – lack of perseverance (sample item: “I generally like to see things through to the end”[reverse-scored]), lack of premeditation (“My thinking is usually careful and purposeful” [reverse-scored]), negative urgency (“When I am upset I often act without thinking”), positive urgency (“I tend to lose control when I am in a great mood”), and sensation seeking (“I quite enjoy taking risks”), with 3 items per each subscale. The items are Likert-type, rated on a 1–4 scale, ranging from 1 = disagree strongly, to 4 = agree strongly. We tested the psychometric validity of the short UPPS scale in a multigroup CFA model with 5 intercorrelated factors. The model showed a good fit to the data, χ2(160) = 502.639, CFI = .96, RMSEA = .04, 90% CI RMSEA [.03, .04], RMSEA p close = 1.00, SRMR = .04, and has shown metric invariance across EA and AA, Δχ2(10) = 13.09, p = .219, suggesting that the factor structure and item loadings are invariant for EA and AA subsamples.
Covariates (Fall freshman year).
These included sex (coded as 0 = male, 1 = female), age, and ancestral segregation (a total of ten principal components were used in all analyses to control for genetic variation within ancestry).
Plan of Analysis
The hypotheses of the study were analyzed within a structural equation modeling framework employing latent variables. Capitalizing on the longitudinal nature of the design, we used the reported alcohol consumption in each follow-up wave as indicators of levels of alcohol consumption across four years of college in the full structural model, along with the CFA model for UPPS. In this way, latent variables were used to represent the UPPS subscales and alcohol consumption, with indicators represented as items (in the case of UPPS) or sum of grams of ethanol per month across four timepoints.
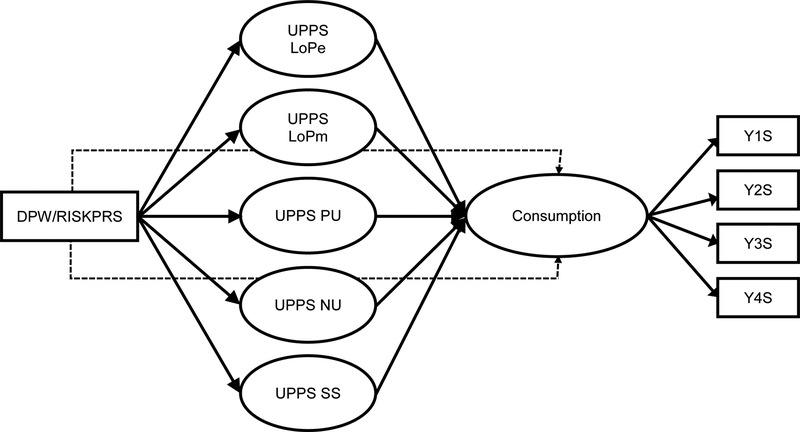
The structural models regressed the alcohol consumption on five UPPS subscales as well as the two PRS of interest (DPW-PRS, RISK-PRS) in separate models for each. The UPPS subscales were regressed on each PRS as well to serve as hypothesized mediators between PRS and alcohol outcomes. The full model is shown in Figure 1. The model was estimated separately for EA and AA subsamples. All models controlled for age, sex, and principal components of subancestral groups. All models were estimated in Mplus 8.0. To handle missing data, maximum likelihood with robust standard errors (MLR) was used.
Figure 1.
The proposed mediation model. DPW-PRS, drinks per week PRS; RISK-PRS, first principal component of risky behavior PRS; SS = sensation seeking; PU = positive urgency; NU = negative urgency; LoPe = lack of perseverance; LoPm = lack of premeditation; Y1S = year 1 spring; Y2S = year 2 spring; Y3S = year 3 spring, Y4S = year 4 spring.
We checked for outliers on our predictors (UPPS) as well as dependent variables. For UPPS, we removed cases based on Mahalanobis distance with p <.001 (21 cases) as well as cases that showed patterned responses (defined as more than 12 identical values across the 15 UPPS items = 52 cases altogether). For dependent variables, we removed participants who indicated that they consumed more than 5,000 grams of alcohol per month at any of the four timepoints (29 cases), as this was a value we considered highly improbable (this would equal 12 drinks each day for 30 days in a row). As an estimator of choice, MLR is robust to non-normality of the data, which is especially pertinent to our alcohol use outcomes, which are expected to be skewed (Muthén & Muthén, 1998-2017). A bootstrapping procedure with 10,000 resamples was used to obtain bias-corrected standard errors of the indirect effects.
Results
First, we computed descriptive statistics and zero-order polychoric correlations for the five UPPS subscales, alcohol consumption, and covariates including sex and age. These are shown in Table 1, with EA estimates below diagonal and AA above diagonal. They showed that UPPS subscales were significantly positively intercorrelated with the exception of sensation seeking, which was not correlated with lack of perseverance in the European subsample (EA) and negatively correlated in the African subsample (AA). Alcohol consumption showed relative stability from one year to another (rs ranging .54 – 62). Higher levels of baseline sensation seeking were significantly associated with more alcohol consumption across all four waves for both groups. positive urgency showed positive associations with alcohol consumption for AA across all four waves, whereas for EA, it was significantly associated for only the first two follow-ups. lack of perseverance showed significant positive associations with consumption for first three waves among EA, but associations for AA were not significant. Conversely, higher levels of negative urgency were associated with more alcohol consumption for three waves among AA, but only for the first follow-up among EA. lack of premeditation showed significant positive associations with alcohol consumption for all four follow-ups among EA and for the first three among AA. Women showed significantly lower levels of positive urgency and sensation seeking, and higher levels of negative urgency in EA sample only.
Table 1.
Means, Standard Deviations, and Polychoric Correlations
| Variable | M | SD | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Negative urgency | 2.19/2.02 | 0.75/0.78 | – | .75*** | .24*** | .44*** | .18*** | .13*** | .15** | .20** | .09 | .02 | .03 |
| 2. Positive urgency | 1.99/1.85 | 0.72/0.74 | .72*** | – | .21*** | .47*** | .35*** | .13*** | .18*** | .30*** | .24*** | −.14*** | .07 |
| 3. Lack of perseverance | 1.71/1.60 | 0.57/0.57 | .18*** | .20*** | – | .60*** | −.18*** | .05 | .02 | −.03 | .02 | −.05 | −.07 |
| 4. Lack of premeditation | 1.83/1.66 | 0.60/0.59 | .43*** | .50*** | .48*** | – | .15*** | .15*** | .15*** | .13* | .07 | −.06 | −.09** |
| 5. Sensation seeking | 2.96/2.82 | 0.69/0.69 | .07* | .32*** | −.03 | .28*** | – | .18*** | .17*** | .20*** | .27*** | −.14** | .06 |
| 6. Alc. consumption Y1S | 280.28/138.23 | 554.37/352.15 | .11*** | .16*** | .09*** | .18*** | .26*** | – | .55*** | .42*** | .33*** | −.13*** | −.02 |
| 7. Alc. consumption Y2S | 304.23/154.69 | 527.72/374.40 | .06 | .11** | .11** | .15*** | .18*** | .54*** | – | .62*** | .48*** | −.20*** | .05 |
| 8. Alc. consumption Y3S | 356.10/183.72 | 593.06/415.91 | .01 | .04 | .09* | .14** | .22*** | .53*** | .56*** | – | .54*** | −.11* | .14 |
| 9. Alc. consumption Y4S | 390.26/205.62 | 563.68/403.45 | .07 | .07 | .06 | .10* | .16*** | .54*** | .53*** | .61*** | – | −.13* | .07 |
| 10. Female | 0.60/0.74 | 0.49/0.45 | .11*** | −.11*** | −.03 | −.01 | −.18*** | −.14*** | −.14*** | −.19*** | −.17*** | – | −.13*** |
| 11. Age | 18.61/18.55 | 0.60/0.58 | −.03 | −.02 | −.01 | −.02 | .02 | .03 | .02 | .14** | .03 | −.10*** | – |
Note. Correlations for European ancestry sample below diagonal, for African ancestry above diagonal. For M and SD, the first number reflects scores for European ancestry, the second for African ancestry. Alc. consumption = alcohol consumption, Y1S = year 1 spring, Y2S = year 2 spring, Y3S = year 3 spring, Y4S = year 4 spring.
indicates p < .05.
indicates p < .01,
indicates p < .001.
Next, we tested nine p-value thresholds for each PRS on alcohol consumption to select the most predictive threshold within each, estimated as increase in R2 when the PRS is added to the baseline model (baseline R2 = .056 in EA and .069 in AA). Table 2 shows the standardized results across all thresholds. This showed that in EA sample, both DPW-PRS and RISK-PRS were positively predictive of alcohol consumption. For DPW-PRS, the PRS with p <.20 (β = .081, p = .001) and for RISK, the PRS with p < .10 (β = .115, p < .001) was the strongest predictor, explaining an additional .6% and 1.3% of variance respectively in alcohol consumption above and beyond baseline model including sex, age, their interaction, and principal components. For AA sample, no significant associations between either DPW-PRS or RISK-PRS and alcohol consumption were found (the only statistically significant association was in negative direction). In this way, the results did not provide support for the expected associations of PRS and our outcome of interest, as observed in the EA sample. For this reason, we decided to carry on with the further analyses employing the full structural model using only the EA sample.
Table 2.
Most Predictive P-value Thresholds for Each PRS
| African ancestry sample | European ancestry sample | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictor | P-value threshold | β | SE | p | R2full | ΔR2full-baseline | β | SE | p | R2full | ΔR2full-baseline |
| DPW-PRS | p <.0001 | 0.049 | 0.044 | .269 | .071 | .002 | 0.019 | 0.026 | .462 | .056 | .000 |
| p <.001 | 0.029 | 0.044 | .517 | .069 | .000 | 0.022 | 0.027 | .424 | .056 | .000 | |
| p <.01 | 0.009 | 0.036 | .800 | .069 | .000 | 0.044 | 0.026 | .091 | .058 | .002 | |
| p <.05 | −0.022 | 0.036 | .536 | .069 | .000 | 0.069 | 0.025 | .006 | .061 | .005 | |
| p <.10 | −0.040 | 0.037 | .276 | .070 | .001 | 0.069 | 0.025 | .005 | .061 | .005 | |
| p <.20 | −0.020 | 0.040 | .612 | .069 | .000 | 0.080 | 0.025 | .001 | .062 | .006 | |
| p <.30 | −0.018 | 0.041 | .664 | .069 | .000 | 0.078 | 0.024 | .001 | .062 | .006 | |
| p <.40 | −0.021 | 0.041 | .601 | .069 | .000 | 0.078 | 0.024 | .001 | .062 | .006 | |
| p <.50 | −0.018 | 0.040 | .662 | .069 | .000 | 0.076 | 0.024 | .002 | .062 | .006 | |
| RISK-PRS | p <.0001 | 0.015 | 0.037 | .685 | .069 | .000 | 0.072 | 0.027 | .009 | .061 | .005 |
| p <.001 | 0.063 | 0.047 | .179 | .071 | .002 | 0.073 | 0.028 | .008 | .061 | .005 | |
| p <.01 | −0.051 | 0.051 | .316 | .070 | .001 | 0.098 | 0.026 | <.001 | .065 | .009 | |
| p <.05 | −0.044 | 0.041 | .283 | .070 | .001 | 0.106 | 0.026 | <.001 | .067 | .011 | |
| p <.10 | −0.067 | 0.036 | .066 | .073 | .004 | 0.114 | 0.026 | <.001 | .069 | .013 | |
| p <.20 | −0.088 | 0.044 | .045 | .075 | .006 | 0.112 | 0.026 | <.001 | .068 | .012 | |
| p <.30 | −0.078 | 0.047 | .094 | .074 | .005 | 0.110 | 0.026 | <.001 | .068 | .012 | |
| p <.40 | −0.071 | 0.047 | .129 | .073 | .004 | 0.112 | 0.026 | <.001 | .068 | .012 | |
| p <.50 | −0.068 | 0.046 | .138 | .073 | .004 | 0.112 | 0.026 | <.001 | .068 | .012 | |
Note. DPW = drinks per week, RISK = first principal component of risky behaviors. Bolded are the most predictive p-value thresholds for each PRS. ΔR2full-baseline refers to a change in R2 when specific PRS score was added to the baseline model, which included sex, age, their interaction term and 10 PCs.
We selected the two most predictive thresholds and expanded the models by adding the five interrelated UPPS factors to each model. The model fit of both models (for DPW-PRS and RISK-PRS) was adequate, χ2(319) = 814.487/808.748, CFI = .95/.95, RMSEA = .026/.025, SRMR = .025/.025. lack of perseverance (β = .09 and .08, p <.040) and sensation seeking (β = .29 and .29, p <.001) significantly and positively predicted alcohol consumption. No significant effects of lack of premeditation, negative or positive urgency were found for alcohol consumption. Further, RISK-PRS significantly predicted all UPPS subscales, except for lack of perseverance; but DPW-PRS did not significantly predict any UPPS subscales. The direct effects of both DPW-PRS (β = .07, p =.007) and RISK-PRS (β = .09, p <.001) remained significant, but decreased in size when the UPPS-P subscales were included in the model. The full models explained 17% of variance in alcohol consumption.
The results from the mediation analyses are presented in Table 3. They show that the longitudinal effect of RISK-PRS on alcohol consumption was partially mediated by sensation seeking, β = .02, 95% BcCI [.002, .032], as well as lack of perseverance, β = .01, 95% BcCI [.001, .015]. On the other hand, no significant mediation effects were found for DPW-PRS and alcohol consumption. This was due to DPW-PRS not being significantly predictive of any of the UPPS-P subscales, while remaining a significant predictor of alcohol consumption above and beyond UPPS subscales and covariates.
Table 3.
Standardized Effects from the Full Structural Models Predicting Alcohol Consumption for European Ancestry Subsample
| PRS | Mediator | Path from PRS to UPPS-P subscale (a) | Path from UPPS-P subscale to Alcohol consumption (b) | Mediated effect (a*b) | |||
|---|---|---|---|---|---|---|---|
| β | p | β | p | β | 95% BcCI | ||
| DPW-PRS | Negative urgency | 0.014 | .585 | 0.132 | .047 | 0.002 | [−.004, .013] |
| Positive urgency | 0.035 | .153 | −0.107 | .135 | −0.004 | [−.017, .001] | |
| Lack of perseverance | 0.028 | .273 | 0.087 | .027 | 0.002 | [−.001, .010] | |
| Lack of premeditation | 0.039 | .111 | 0.063 | .191 | 0.002 | [−.001, .011] | |
| Sensation seeking | 0.032 | .194 | 0.290 | <.001 | 0.009 | [−.005, .025] | |
| RISK-PRS | Negative urgency | 0.048 | .076 | 0.131 | .049 | 0.006 | [.000, .020] |
| Positive urgency | 0.065 | .010 | −0.109 | .131 | −0.007 | [−.023, .001] | |
| Lack of perseverance | 0.064 | .013 | 0.082 | .037 | 0.005 | [.001, .015] | |
| Lack of premeditation | 0.065 | .009 | 0.064 | .185 | 0.004 | [−.001, .014] | |
| Sensation seeking | 0.053 | .031 | 0.288 | <.001 | 0.015 | [.002, .032] | |
Note. The mediated effect refers to the indirect effect of PRS (DPW or RISK) through the five UPPS subscales.
Discussion
The current study investigated the longitudinal effect of alcohol consumption and risky behaviors PRS on alcohol consumption in a sample of European and African ancestry college students. Impulsivity dimensions, assessed through UPPS-P, were proposed as phenotypic mediators of the hypothesized genetic effect on alcohol consumption. The main findings from the European subsample can be summarized in the following way: 1) Both polygenic risk scores reflecting alcohol consumption (DPW-PRS) as well as broader risky behaviors (RISK-PRS) were shown to be positively predictive of alcohol consumption among college students; 2) The RISK-PRS was a stronger predictor of alcohol use than DPW-PRS based on the amount of variance explained; 3) RISK-PRS was significantly associated with UPPS-P dimensions, with sensation seeking, lack of perseverance mediating part of its effect on alcohol consumption; and 4) The effect of DPW-PRS on alcohol consumption was not mediated by dimensions of impulsivity. No significant effects of either PRS were found for the African subsample. These findings are discussed below in more detail.
Consistent with our hypothesis, DPW-PRS and RISK-PRS were associated with higher levels of alcohol consumption. However, both PRS explained only a small portion of variance in alcohol consumption. Although the discovery samples of both PRS were large, PRS for alcohol-related traits continue to account for only small proportions of variance (Evangelou et al., 2018; Liu et al., 2019; Walters et al., 2018). It is likely that moderate heritability and significant heterogeneity in pathways that contribute to alcohol consumption will necessitate extremely large GWAS discovery sample sizes to reliably detect associated SNPs that account for more substantial portions of variance as well as more well-refined phenotypic measurements (such as larger samples of individuals diagnosed with AUD, for example).
Based on the amount of variance explained in the current study, the RISK-PRS proved to be a stronger predictor of alcohol consumption than DPW-PRS, explaining double the portion of variance (1.3% vs .6%). This indicates that among college students, the genotypic risk score based on broader risk-taking behaviors was a stronger predictor of alcohol consumption as compared to a risk score based solely on variants associated with alcohol consumption. This is perhaps unsurprising since alcohol consumption reflects one phenotypic manifestation of a series of externalizing behaviors that have been shown to share underlying genetic variance (Kendler et al., 2011; Slutske et al., 2002). Employing risk scores reflecting broader risky behaviors might be a viable option for capturing more variance in alcohol outcomes than what might be indexed by risk scores based on alcohol phenotypes alone (Agrawal and Bierut, 2012; Hart and Kranzler, 2015).
We also hypothesized that both PRS would be significantly positively associated with dimensions of impulsivity, which would in turn predict alcohol consumption. We found that lack of perseverance, sensation seeking, and negative urgency were predictive of higher amounts of alcohol consumed. sensation seeking or the tendency to seek out thrills has been consistently found to be predictive of higher substance use (Sargent et al., 2010), including alcohol consumption (Hittner and Swickert, 2006; MacPherson et al., 2010). In this study, sensation seeking was comparatively the strongest predictor of alcohol consumption from all UPPS-P subscales, confirming the findings from previous studies (Adams et al., 2012; Cyders et al., 2009; Stautz and Cooper, 2013). lack of perseverance emerged as the second significant predictor of alcohol consumption. lack of perseverance refers to individual’s inability to see through finishing a boring or difficult task and has been closely related to self-discipline (Whiteside and Lynam, 2003). A previous meta-analysis by Coskunpinar et al. (2013) found that lack of perseverance was the strongest predictor of drinking frequency. Furthermore, negative urgency or the tendency to act rashly as a reaction to negative mood was found to be predictive of alcohol consumption. This result was unexpected as previous studies have generally found that both types of Urgency, but especially negative urgency, were more predictive of alcohol problem behaviors, such as alcohol dependency, as opposed to alcohol consumption, as was the focus here (Adams et al., 2012; Coskunpinar et al., 2013; Dick et al., 2010). No significant associations were found between lack of premeditation and positive urgency and alcohol consumption in our study.
Furthermore, the RISK-PRS was significantly positively associated with four out of five UPPS-P subscales, suggesting that the genetic basis for risky behaviors indexed by this PRS might be partially phenotypically manifested as impulsive traits (Sanchez-Roige et al., 2019). Importantly, part of the main effect of RISK-PRS on alcohol consumption was found to be significantly mediated through sensation seeking and lack of perseverance. These results provide evidence for a genetically-influenced externalizing pathway to alcohol use where individuals with elevated levels of impulsivity traits are more likely to consume higher volume of alcohol (Hussong et al., 2007; Zucker, 2008). A previous study also found that sensation seeking mediated the effect of alcohol dependence PRS on alcohol use problems in adolescence (Li et al., 2017).
Contrary to our hypothesis, DPW-PRS did not show any significant association with impulsivity dimensions and mainly for this reason, no significant indirect effects of DPW-PRS on alcohol consumption via impulsivity were found. However, both DPW-PRS and RISK-PRS remained significantly associated with alcohol consumption in the full model where impulsivity dimensions were considered. In this sense, it is possible that the genetic basis of DPW-PRS, as indexed by the GSCAN DPW-PRS is related to more alcohol-specific genetic risk that does not overlap with impulsive behaviors (Kendler et al., 2011).
Strengths and Limitations
The current study has several strengths worth mentioning. First, it employed a large sample of college students with alcohol consumption assessed at multiple timepoints, spanning the college years. College students are a particularly high-risk group for the development of many problem behaviors, including alcohol-related phenotypes (Schulenberg et al., 2018). Exploring the antecedents of alcohol consumption in this age group is critical for informing prevention and intervention efforts at college campuses (Dick and Hancock, 2015).
Second, the study tested genetic effects on alcohol consumption using PRS from GWAS with the largest available discovery samples for alcohol consumption and general risk behavior. The results showed that the genetic etiology of alcohol phenotypes can be reliably detected with more broadly defined PRS reflecting risky behavior, and it might potentially explain more systematic variance than PRS reflecting alcohol phenotypes only. These findings suggest that on-going efforts using multivariate genetic techniques (Grotzinger et al., 2019) to identify genetic variants associated with broad externalizing behaviors (The Externalizing Consortium; Dick et al., 2018a) may further advance our ability to study genetically-influenced pathways of risk in college students.
Finally, the study proposed found partial confirmation for the hypothesis that impulsivity dimensions would mediate the association between genetic risk and alcohol consumption. This suggests that genetic basis for broadly-defined risky behaviors might find phenotypic expression in a variety of behaviors and traits, with elevated levels of impulsivity and alcohol consumption as phenotypic manifestations of this underlying vulnerability. In this way, the current study suggests that personality factors such as impulsivity might serve as an earlier manifestation of genetic risk for the development of alcohol-related problems. As the estimations of polygenic risk score become more precise in the near future, information from such PRS might inform alcohol prevention efforts by identifying at-risk individuals early on in their lifespan, preferably before the onset of alcohol use. This would lead to creating more targeted prevention and intervention efforts characterized by personalized feedback stratified based on individual’s estimated genetic risk for high impulsivity. Such tailored prevention efforts might, for instance, focus on motivational processes underlying the impulsive traits and provide high-impulsivity individuals with coping skills to offer alternatives to using alcohol as a means of coping (Conrod et al., 2011; Newton et al., 2016).
The study also has several limitations worth mentioning. First, given the specificity of the college environment and young adulthood as an age group with regards to alcohol use, the results might not be generalizable to other, non-college samples (Evans et al., 2009b; Schulenberg et al., 2018). Second, for our analyses, we used a subset of the data which had complete genotypic information and included at least one follow-up data point. Although the missing data from subsequent timepoints were estimated using full-information maximum likelihood utilizing all available information from baseline (Enders and Bandalos, 2001), the analytic sample remains a subset of the dataset and might not be fully representative of the college population as a whole.
Finally, our analysis in the African ancestry subsample found no significant associations between the proposed PRS and alcohol consumption. The lack of association might be due to the fact that the discovery sample of our PRS of interest included predominantly individuals with European ancestry, which, given the divergent patterns of linkage disequilibrium across populations, potentially compromises the predictive power of PRS when applied to a non-European ancestry population. Even though we tried to ameliorate this issue by employing a multiPRS approach (Márquez-Luna et al., 2017), the observed effects were lower and much less stable across the p-value thresholds when compared to the European subsample. The lack of large non-European discovery samples in the existing GWAS (Popejoy and Fullerton, 2016) posits a serious issue for studies with diverse populations like our sample. More importantly, research not actively addressing this limitation creates a potential for perpetuating existing health disparities (Dick et al., 2017). In this sense, it is essential for the field as a whole to include large samples of individuals of diverse ancestry to make sure that the genetic findings are generalizable and can be of benefit to everyone (Su et al., 2018).
Conclusions
This study examined the longitudinal effect of genetic risk indicated by two PRS, one related to drinks per week and one to risky behaviors, on alcohol consumption in a college sample followed across four years. Furthermore, it tested whether dimensions of impulsivity would mediate this association. The results showed that among European ancestry participants, both DPW-PRS and RISK-PRS were significantly associated with college alcohol consumption, with RISK-PRS explaining more variance than DPW-PRS. The effects of the RISK-PRS were partially mediated by sensation seeking, lack of perseverance, whereas DPW-PRS showed only a direct longitudinal effect on alcohol consumption. The current results underscore the complex nature by which genetic predispositions impact alcohol consumption through a number of intermediary traits.
Supplementary Material
Acknowledgements:
Spit for Science has been supported by Virginia Commonwealth University, P20 AA017828, R37AA011408, K02AA018755, and P50 AA022537 from the National Institute on Alcohol Abuse and Alcoholism, and UL1RR031990 from the National Center for Research Resources and National Institutes of Health Roadmap for Medical Research. We would like to thank the Spit for Science participants for making this study a success, as well as the many University faculty, students, and staff who contributed to the design and implementation of the project.
Footnotes
Conflict of interest: none
For Cohort 1, the UPPS was not assessed in Fall semester of the freshman year but instead, in the Spring semester. In order to include Cohort 1, we decided to use their spring scores (while using fall scores for cohorts 2 and 3).
References
- Adams ZW, Kaiser AJ, Lynam DR, Charnigo RJ, Milich R (2012) Drinking motives as mediators of the impulsivity-substance use relation: Pathways for negative urgency, lack of premeditation, and sensation seeking. Addict Behav 37:848–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, Bierut LJ (2012) Identifying genetic variation for alcohol dependence. Alcohol Res Curr Rev 34:274–281. [PMC free article] [PubMed] [Google Scholar]
- Barnes GM, Welte JW, Hoffman JH, Tidwell M-CO (2010) Comparisons of gambling and alcohol use among college students and noncollege young people in the United States. J Am Coll Health 58:443–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg JM, Latzman RD, Bliwise NG, Lilienfeld SO (2015) Parsing the heterogeneity of impulsivity: A meta-analytic review of the behavioral implications of the UPPS for psychopathology. Psychol Assess 27:1129–1146. [DOI] [PubMed] [Google Scholar]
- Bogdan R, Baranger DAA, Agrawal A (2018) Polygenic risk scores in clinical psychology: Bridging genomic risk to individual differences. Annu Rev Clin Psychol 14:119–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn MJ, Babor TF, Kranzler HR (1995) The Alcohol Use Disorders Identification Test (AUDIT): Validation of a screening instrument for use in medical settings. J Stud Alcohol 56:423–432. [DOI] [PubMed] [Google Scholar]
- Brown SA, Gleghorn A, Schuckit MA, Myers MG, Mott MA (1996) Conduct disorder among adolescent alcohol and drug abusers. J Stud Alcohol 57:314–324. [DOI] [PubMed] [Google Scholar]
- Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ (2015) Second-generation PLINK: Rising to the challenge of larger and richer datasets. Gigascience 4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrod PJ, Castellanos-Ryan N, Mackie C (2011) Long-term effects of a personality-targeted intervention to reduce alcohol use in adolescents. J Consult Clin Psychol 79:296–306. [DOI] [PubMed] [Google Scholar]
- Coskunpinar A, Dir AL, Cyders MA (2013) Multidimensionality in impulsivity and alcohol use: A meta-analysis using the UPPS model of impulsivity. Alcohol Clin Exp Res 37:1441–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyders MA, Flory K, Rainer S, Smith GT (2009) The role of personality dispositions to risky behavior in predicting first-year college drinking. Addiction 104:193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick D, Koellinger P, Harden KP, Tucker-Drob EM, Waldman I, Linner RK, Mallard T, de Vlaming R, Grotzinger A, Barr P (2018a) Using the genetic architecture of externalizing disorders to aid in gene identification In: BEHAVIOR GENETICS, pp 466–466. SPRINGER; 233 SPRING ST, NEW YORK, NY 10013 USA. [Google Scholar]
- Dick DM (2011) Gene-environment interaction in psychological traits and disorders. Annu Rev Clin Psychol 7:383–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Barr P, Guy M, Nasim A, Scott D (2017) Review: Genetic research on alcohol use outcomes in African American populations: A review of the literature, associated challenges, and implications. Am J Addict 26:486–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Barr PB, Cho SB, Cooke ME, Kuo SI-C, Lewis TJ, Neale Z, Salvatore JE, Savage J, Su J (2018b) Post-GWAS in psychiatric genetics: A developmental perspective on the “other” next steps. Genes Brain Behav 17:e12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Bierut L, Hinrichs A, Fox L, Bucholz KK, Kramer J, Kuperman S, Hesselbrock V, Schuckit M, Almasy L, Tischfield J, Porjesz B, Begleiter H, Nurnberger J, Xuei X, Edenberg HJ, Foroud T (2006) The Role of GABRA2 in risk for conduct disorder and alcohol and drug dependence across developmental stages. Behav Genet 36:577–590. [DOI] [PubMed] [Google Scholar]
- Dick DM, Bierut LJ (2006) The genetics of alcohol dependence. Curr Psychiatry Rep 8:151–157. [DOI] [PubMed] [Google Scholar]
- Dick DM, Hancock LC (2015) Integrating basic research with prevention/intervention to reduce risky substance use among college students. Front Psychol 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Meyers JL, Rose RJ, Kaprio J, Kendler KS (2011) Measures of current alcohol consumption and problems: Two independent twin studies suggest a complex genetic architecture. Alcohol Clin Exp Res 35:2152–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Nasim A, Edwards AC, Salvatore JE, Cho SB, Adkins A, Meyers J, Yan J, Cooke M, Clifford J, Goyal N, Halberstadt L, Ailstock K, Neale Z, Opalesky J, Hancock L, Donovan KK, Sun C, Riley B, Kendler KS (2014) Spit for Science: Launching a longitudinal study of genetic and environmental influences on substance use and emotional health at a large US university. Front Genet 5:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Smith G, Olausson P, Mitchell SH, Leeman RF, O’Malley SS, Sher K (2010) Understanding the construct of impulsivity and its relationship to alcohol use disorders. Addict Biol 15:217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudbridge F (2016) Polygenic epidemiology. Genet Epidemiol 40:268–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders CK, Bandalos DL (2001) The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Struct Equ Model Multidiscip J 8:430–457. [Google Scholar]
- Evangelou E, Gao H, Chu C, Ntritsos G, Blakeley P, Butts AR, Pazoki R, Suzuki H, Koskeridis F, Yiorkas AM, Karaman I, Elliott J, Aeschbacher S, Bartz TM, Baumeister SE, Braund PS, Brown MR, Brody JA, Clarke T-K, Dimou N, Faul JD, Homuth G, Jackson AU, Kentistou KA, Joshi PK, Lemaitre RN, Lind PA, Lyytikainen L-P, Mangino M, Milaneschi Y, Nelson CP, Nolte IM, Perala M-M, Polasek O, Porteous D, Ratliff SM, Smith JA, Stancakova A, Teumer A, Tuominen S, Theriault S, Vangipurapu J, Whitfield JB, Wood A, Yao J, Yu B, Zhao W, Arking DE, Auvinen J, Liu C, Mannikko M, Risch L, Rotter JI, Snieder H, Veijola J, Blakemore AI, Boehnke M, Campbell H, Conen D, Eriksson JG, Grabe HJ, Guo X, van de Harst P, Hartman CA, Hayward C, Heath AC, Jarvelin M-R, Kahonen M, Kardia SL, Kuhne M, Kuusisto J, Laakso M, Lahti J, Lehtimaki T, McIntosh AM, Mohlke KL, Morrison AC, Martin NG, Oldehinkel AJ, Penninx BW, Psaty BM, Raitakari OT, Rudan I, Samani NJ, Scott LJ, Spector TD, Verweij N, Weir DR, Wilson JF, Levy D, Tzoulaki I, Bell JD, Matthews P, Rothenfluh A, Desrivieres S, Schumann G, Elliott P (2018) Genome-wide association and functional studies identify 46 novel loci for alcohol consumption and suggest common genetic mechanisms with neuropsychiatric disorders. bioRxiv 453332. [Google Scholar]
- Evans DM, Visscher PM, Wray NR (2009a) Harnessing the information contained within genome-wide association studies to improve individual prediction of complex disease risk. Hum Mol Genet 18:3525–3531. [DOI] [PubMed] [Google Scholar]
- Evans NJ, Forney DS, Guido FM, Patton LD, Renn KA (2009b) Student development in college: Theory, research, and practice. John Wiley & Sons. [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B, Hasin DS (2015) Epidemiology of DSM-5<alcohol use disorder: results from the national epidemiologic survey on alcohol and related conditions iii. JAMA Psychiatry 72:757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotzinger AD, Rhemtulla M, de Vlaming R, Ritchie SJ, Mallard TT, Hill WD, Ip HF, Marioni RE, McIntosh AM, Deary IJ, Koellinger PD, Harden KP, Nivard MG, Tucker-Drob EM (2019) Genomic structural equation modelling provides insights into the multivariate genetic architecture of complex traits. Nat Hum Behav 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG (2009) Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart AB, Kranzler HR (2015) Alcohol dependence genetics: Lessons learned from genome-wide association studies (GWAS) and post-GWASanalyses. Alcohol Clin Exp Res 39:1312–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks BM, Krueger RF, Iacono WG, McGue M, Patrick CJ (2004) Family transmission and heritability of externalizing disorders: A twin-family study. Arch Gen Psychiatry 61:922–928. [DOI] [PubMed] [Google Scholar]
- Hingson RW, Zha W, Weitzman ER (2009) Magnitude of and trends in alcohol-related mortality and morbidity among U.S. college students ages 18–24, 1998–2005. J Stud Alcohol Drugs Suppl 12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hittner JB, Swickert R (2006) Sensation seeking and alcohol use: A meta-analytic review. Addict Behav 31:1383–1401. [DOI] [PubMed] [Google Scholar]
- Hussong AM, Wirth RJ, Edwards MC, Curran PJ, Chassin LA, Zucker RA (2007) Externalizing symptoms among children of alcoholic parents: Entry points for an antisocial pathway to alcoholism. J Abnorm Psychol 116:529–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Gardner C, Dick DM (2011) Predicting alcohol consumption in adolescence from alcohol-specific and general externalizing genetic risk factors, key environmental exposures and their interaction. Psychol Med 41:1507–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC (2003) The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry 60:929–937. [DOI] [PubMed] [Google Scholar]
- Khemiri L, Kuja-Halkola R, Larsson H, Jayaram-Lindström N (2016) Genetic overlap between impulsivity and alcohol dependence: A large-scale national twin study. Psychol Med 46:1091–1102. [DOI] [PubMed] [Google Scholar]
- King SM, Iacono WG, McGue M (2004) Childhood externalizing and internalizing psychopathology in the prediction of early substance use. Addiction 99:1548–1559. [DOI] [PubMed] [Google Scholar]
- Kokkevi A, Richardson C, Florescu S, Kuzman M, Stergar E (2007) Psychosocial correlates of substance use in adolescence: A cross-national study in six European countries. Drug Alcohol Depend 86:67–74. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M (2002) Etiologic connections among substance dependence, antisocial behavior and personality: Modeling the externalizing spectrum. J Abnorm Psychol 111:411–424. [PubMed] [Google Scholar]
- Lejuez CW, Magidson JF, Mitchell SH, Sinha R, Stevens MC, Wit HD (2010) Behavioral and biological indicators of impulsivity in the development of alcohol use, problems, and disorders. Alcohol Clin Exp Res 34:1334–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JJ, Savage JE, Kendler KS, Hickman M, Mahedy L, Macleod J, Kaprio J, Rose RJ, Dick DM (2017) Polygenic risk, personality dimensions, and adolescent alcohol use problems: A longitudinal study. J Stud Alcohol Drugs 78:442–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnér RK, Biroli P, Kong E, Meddens SFW, Wedow R, Fontana MA, Lebreton M, Abdellaoui A, Hammerschlag AR, Nivard MG, Okbay A, Rietveld CA, Timshel PN, Tino SP, Trzaskowski M, de Vlaming R Zünd CL, Bao Y, Buzdugan L, Caplin AH, Chen C-Y, Eibich P, Fontanillas P, Gonzalez JR, Joshi PK, Karhunen V, Kleinman A, Levin RZ, Lill CM, Meddens GA, Muntané G, Sanchez-Roige S, van Rooij FJ, Taskesen E, Wu Y, Zhang F, Team 23andMe Research, Consortium eQTLgen, Consortium IC, Consortium PG, Consortium SSGA, Auton A, Boardman JD, Clark DW, Conlin A, Dolan CC, Fischbacher U, Groenen PJ, Harris KM, Hasler G, Hofman A, Ikram MA, Jain S, Karlsson R, Kessler RC, Kooyman M, MacKillop J, Männikkö M, Morcillo-Suarez C, McQueen MB, Schmidt KM, Smart MC, Sutter M, Thurik AR, Uitterlinden AG, White J, de Wit H, Yang J, Bertram L, Boomsma D, Esko T, Fehr E, Hinds DA, Johannesson M, Kumari M, Laibson D, Magnusson PK, Meyer MN, Navarro A, Palmer AA, Pers TH, Posthuma D, Schunk D, Stein MB, Svento R, Tiemeier H, Timmers PR, Turley P, Ursano RJ, Wagner GG, Wilson JF, Gratten J, Lee JJ, Cesarini D, Benjamin DJ, Koellinger P, Beauchamp JP (2018) Genome-wide study identifies 611 loci associated with risk tolerance and risky behaviors. bioRxiv 261081. [Google Scholar]
- Linnér RK, Biroli P, Kong E, Meddens SFW, Wedow R, Fontana MA, Lebreton M, Tino SP, Abdellaoui A, Hammerschlag AR, Nivard MG, Okbay A, Rietveld CA, Timshel PN, Trzaskowski M, de Vlaming R. Zünd CL, Bao Y, Buzdugan L, Caplin AH, Chen C-Y, Eibich P, Fontanillas P, Gonzalez JR, Joshi PK, Karhunen V, Kleinman A, Levin RZ, Lill CM, Meddens GA, Muntané G, Sanchez-Roige S, van Rooij FJ, Taskesen E, Wu Y, Zhang F, Auton A, Boardman JD, Clark DW, Conlin A, Dolan CC, Fischbacher U, Groenen PJF, Harris KM, Hasler G, Hofman A, Ikram MA, Jain S, Karlsson R, Kessler RC, Kooyman M, MacKillop J, Männikkö M, Morcillo-Suarez C, McQueen MB, Schmidt KM, Smart MC, Sutter M, Thurik AR, Uitterlinden AG, White J, de Wit H, Yang J, Bertram L, Boomsma DI, Esko T, Fehr E, Hinds DA, Johannesson M, Kumari M, Laibson D, Magnusson PKE, Meyer MN, Navarro A, Palmer AA, Pers TH, Posthuma D, Schunk D, Stein MB, Svento R, Tiemeier H, Timmers PRHJ, Turley P, Ursano RJ, Wagner GG, Wilson JF, Gratten J, Lee JJ, Cesarini D, Benjamin DJ, Koellinger PD, Beauchamp JP (2019) Genome-wide association analyses of risk tolerance and risky behaviors in over 1 million individuals identify hundreds of loci and shared genetic influences. Nat Genet 51:245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Jiang Y, Wedow R, Li Y, Brazel DM, Chen F, Datta G, Davila-Velderrain J, McGuire D, Tian C, Zhan X, Choquet H, Docherty AR, Faul JD, Foerster JR, Fritsche LG, Gabrielsen ME, Gordon SD, Haessler J, Hottenga J-J, Huang H, Jang S-K, Jansen PR, Ling Y, Mägi R, Matoba N, McMahon G, Mulas A, Orrù V, Palviainen T, Pandit A, Reginsson GW, Skogholt AH, Smith JA, Taylor AE, Turman C, Willemsen G, Young H, Young KA, Zajac GJM, Zhao W, Zhou W, Bjornsdottir G, Boardman JD, Boehnke M, Boomsma DI, Chen C, Cucca F, Davies GE, Eaton CB, Ehringer MA, Esko T, Fiorillo E, Gillespie NA, Gudbjartsson DF, Haller T, Harris KM, Heath AC, Hewitt JK, Hickie IB, Hokanson JE, Hopfer CJ, Hunter DJ, Iacono WG, Johnson EO, Kamatani Y, Kardia SLR, Keller MC, Kellis M, Kooperberg C, Kraft P, Krauter KS, Laakso M, Lind PA, Loukola A, Lutz SM, Madden PAF, Martin NG, McGue M, McQueen MB, Medland SE, Metspalu A, Mohlke KL, Nielsen JB, Okada Y, Peters U, Polderman TJC, Posthuma D, Reiner AP, Rice JP, Rimm E, Rose RJ, Runarsdottir V, Stallings MC, Stančáková A, Stefansson H, Thai KK, Tindle HA, Tyrfingsson T, Wall TL, Weir DR, Weisner C, Whitfield JB, Winsvold BS, Yin J, Zuccolo L, Bierut LJ, Hveem K, Lee JJ, Munafò MR, Saccone NL, Willer CJ, Cornelis MC, David SP, Hinds DA, Jorgenson E, Kaprio J, Stitzel JA, Stefansson K, Thorgeirsson TE, Abecasis G, Liu DJ, Vrieze S (2019) Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynam D, Smith GT, Cyders MA, Fischer S, Whiteside SA (2007) The UPPS-P: A multidimensional measure of risk for impulsive behavior. Unpubl Tech Rep. [Google Scholar]
- MacPherson L, Magidson JF, Reynolds EK, Kahler CW, Lejuez CW (2010) Changes in sensation seeking and risk-taking propensity predict increases in alcohol use among early adolescents. Alcohol Clin Exp Res 34:1400–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Márquez-Luna C, Loh P-R, Price AL (2017) Multiethnic polygenic risk scores improve risk prediction in diverse populations. Genet Epidemiol 41:811–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AR, Gignoux CR, Walters RK, Wojcik GL, Neale BM, Gravel S, Daly MJ, Bustamante CD, Kenny EE (2017) Human demographic history impacts genetic risk prediction across diverse populations. Am J Hum Genet 100:635–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGue M, Iacono WG, Krueger R (2006) The association of early adolescent problem behavior and adult psychopathology: A multivariate behavioral genetic perspective. Behav Genet 36:591–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meda SA, Gueorguieva RV, Pittman B, Rosen RR, Aslanzadeh F, Tennen H, Leen S, Hawkins K, Raskin S, Wood RM, Austad CS, Dager A, Fallahi C, Pearlson GD (2017) Longitudinal influence of alcohol and marijuana use on academic performance in college students. PLOS ONE 12:e0172213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO (1998) Mplus User’s Guide. Eighth Edition. Los Angeles, CA, Muthén and Muthén. [Google Scholar]
- Newton NC, Conrod PJ, Slade T, Carragher N, Champion KE, Barrett EL, Kelly EV, Nair NK, Stapinski L, Teesson M (2016) The long-term effectiveness of a selective, personality-targeted prevention program in reducing alcohol use and related harms: A cluster randomized controlled trial. J Child Psychol Psychiatry 57:1056–1065. [DOI] [PubMed] [Google Scholar]
- Perkins HW (2002) Surveying the damage: A review of research on consequences of alcohol misuse in college populations. J Stud Alcohol Suppl 91–100. [DOI] [PubMed] [Google Scholar]
- Peterson RE, Edwards AC, Bacanu S-A, Dick DM, Kendler KS, Webb BT (2017) The utility of empirically assigning ancestry groups in cross-population genetic studies of addiction. Am J Addict 26:494–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popejoy AB, Fullerton SM (2016) Genomics is failing on diversity. Nat News 538:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, De Bakker PI, Daly MJ (2007) PLINK: A tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81:559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore JE, Thomas NS, Cho SB, Adkins A, Kendler KS, Dick DM (2016) The role of romantic relationship status in pathways of risk for emerging adult alcohol use. Psychol Addict Behav 30:335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Roige S, Fontanillas P, Elson SL, Gray JC, de Wit H, MacKillop J, Palmer AA (2019) Genome-wide association studies of impulsive personality traits (BIS-11 and UPPSP) and drug experimentation in up to 22,861 adult research participants identify loci in the CACNA1I and CADM2 genes. J Neurosci 2662–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Roige S, Palmer AA, Fontanillas P, Elson SL, 23andMe Research Team, Substance Use Disorder Working Group of the Psychiatric Genomics Consortium, Adams MJ, Howard DM, Edenberg HJ, Davies G, Crist RC, Deary IJ, McIntosh AM, Clarke T-K (2018) Genome-wide association study meta-analysis of the Alcohol Use Disorders Identification Test (AUDIT) in two population-based cohorts. Am J Psychiatry appiajp201818040369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent JD, Tanski S, Stoolmiller M, Hanewinkel R (2010) Using sensation seeking to target adolescents for substance use interventions. Addiction 105:506–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor CE, Lynskey MT, Heath AC, Jacob T, True W (2007) The role of childhood risk factors in initiation of alcohol use and progression to alcohol dependence. Addiction 102:216–225. [DOI] [PubMed] [Google Scholar]
- Schulenberg JE, Johnston LD, O’Malley PM, Bachman JG, Miech RA, Patrick ME (2018) Monitoring the Future National Survey Results on Drug Use, 1975–2017. Volume II, College Students & Adults Ages 19–55. Inst Soc Res. [Google Scholar]
- Slutske WS, Heath AC, Dinwiddie SH, Madden PA, Bucholz KK, Dunne MP, Statham DJ, Martin NG (1998) Common genetic risk factors for conduct disorder and alcohol dependence. J Abnorm Psychol 107:363–374. [DOI] [PubMed] [Google Scholar]
- Slutske WS, Heath AC, Madden PA, Bucholz KK, Statham DJ, Martin NG (2002) Personality and the genetic risk for alcohol dependence. J Abnorm Psychol 111:124. [PubMed] [Google Scholar]
- Slutske WS, Hunt-Carter EE, Nabors-Oberg RE, Sher KJ, Bucholz KK, Madden PA, Anokhin A, Heath AC (2004) Do college students drink more than their non-college-attending peers? Evidence from a population-based longitudinal female twin study. J Abnorm Psychol 113:530. [DOI] [PubMed] [Google Scholar]
- Stautz K, Cooper A (2013) Impulsivity-related personality traits and adolescent alcohol use: A meta-analytic review. Clin Psychol Rev 33:574–592. [DOI] [PubMed] [Google Scholar]
- Su J, Kuo SI-C, Meyers JL, Guy MC, Dick DM (2018) Examining interactions between genetic risk for alcohol problems, peer deviance, and interpersonal traumatic events on trajectories of alcohol use disorder symptoms among African American college students. Dev Psychopathol 30:1749–1761. [DOI] [PubMed] [Google Scholar]
- Verhulst B, Neale MC, Kendler KS (2015) The heritability of alcohol use disorders: A meta-analysis of twin and adoption studies. Psychol Med 45:1061–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters RK, Polimanti R, Johnson EC, McClintick JN, Adams MJ, Adkins AE, Aliev F, Bacanu S-A, Batzler A, Bertelsen S, Biernacka JM, Bigdeli TB, Chen L-S, Clarke T-K, Chou Y-L, Degenhardt F, Docherty AR, Edwards AC, Fontanillas P, Foo JC, Fox L, Frank J, Giegling I, Gordon S, Hack LM, Hartmann AM, Hartz SM, Heilmann-Heimbach S, Herms S, Hodgkinson C, Hoffmann P, Hottenga JJ, Kennedy MA, Alanne-Kinnunen M, Konte B, Lahti J, Lahti-Pulkkinen M, Lai D, Ligthart L, Loukola A, Maher BS, Mbarek H, McIntosh AM, McQueen MB, Meyers JL, Milaneschi Y, Palviainen T, Pearson JF, Peterson RE, Ripatti S, Ryu E, Saccone NL, Salvatore JE, Sanchez-Roige S, Schwandt M, Sherva R, Streit F, Strohmaier J, Thomas N, Wang J-C, Webb BT, Wedow R, Wetherill L, Wills AG, Boardman JD, Chen D, Choi D-S, Copeland WE, Culverhouse RC, Dahmen N, Degenhardt L, Domingue BW, Elson SL, Frye MA, Gäbel W, Hayward C, Ising M, Keyes M, Kiefer F, Kramer J, Kuperman S, Lucae S, Lynskey MT, Maier W, Mann K, Männistö S, Müller-Myhsok B, Murray AD, Nurnberger JI, Palotie A, Preuss U, Räikkönen K, Reynolds MD, Ridinger M, Scherbaum N, Schuckit MA, Soyka M, Treutlein J, Witt S, Wodarz N, Zill P, Adkins DE, Boden JM, Boomsma DI, Bierut LJ, Brown SA, Bucholz KK, Cichon S, Costello EJ, de Wit H, Diazgranados N, Dick DM, Eriksson JG, Farrer LA, Foroud TM, Gillespie NA, Goate AM, Goldman D, Grucza RA, Hancock DB, Harris KM, Heath AC, Hesselbrock V, Hewitt JK, Hopfer CJ, Horwood J, Iacono W, Johnson EO, Kaprio JA, Karpyak VM, Kendler KS, Kranzler HR, Krauter K, Lichtenstein P, Lind PA, McGue M, MacKillop J, Madden PAF, Maes HH, Magnusson P, Martin NG, Medland SE, Montgomery GW, Nelson EC, Nöthen MM, Palmer AA, Pedersen NL, Penninx BWJH, Porjesz B, Rice JP, Rietschel M, Riley BP, Rose R, Rujescu D, Shen P-H, Silberg J, Stallings MC, Tarter RE, Vanyukov MM, Vrieze S, Wall TL, Whitfield JB, Zhao H, Neale BM, Gelernter J, Edenberg HJ, Agrawal A (2018) Transancestral GWAS of alcohol dependence reveals common genetic underpinnings with psychiatric disorders. Nat Neurosci 21:1656–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside SP, Lynam DR (2003) Understanding the role of impulsivity and externalizing psychopathology in alcohol abuse: Application of the UPPS Impulsive Behavior Scale. Exp Clin Psychopharmacol 11:210–2017. [DOI] [PubMed] [Google Scholar]
- Whiteside SP, Lynam DR (2001) The Five Factor Model and impulsivity: Using a structural model of personality to understand impulsivity. Personal Individ Differ 30:669–689. [Google Scholar]
- Zucker RA (2008) Anticipating problem alcohol use developmentally from childhood into middle adulthood: What have we learned? Addiction 103:100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.