Abstract
Background.
Chronic exposure to ethanol and other drugs of abuse can alter the expression and activity of cyclin-dependent kinase 5 (CDK5) and its cofactor p35, but the functional implication of CDK5 signaling in the regulation of ethanol-related behaviors remains unknown. In the present study, we sought to determine whether CDK5 activity plays a role in the escalation of ethanol self-administration triggered by dependence.
Methods.
We tested the effect of systemically administered (S)-CR8, a non-selective CDK inhibitor, on operant responding for ethanol or saccharin, a highly palatable reinforcer, in adult male Wistar rats. Half of the rats were made ethanol-dependent via chronic intermittent ethanol inhalation (CIE). We then sought to identify a possible neuroanatomical locus for the behavioral effect of (S)-CR8 by quantifying protein levels of CDK5 and p35 in subregions of the extended amygdala and prefrontal cortex from ethanol-naïve, non-dependent, and dependent rats at the expected time of ethanol self-administration. We also analyzed the phosphorylation of four CDK5 substrates and of the CDK substrate consensus motif.
Results.
(S)-CR8 dose-dependently reduced ethanol self-administration in dependent rats. It had no effect on water or saccharin self-administration, nor in non-dependent rats. The abundance of CDK5 or p35 was not altered in any of the brain regions analyzed. In the bed nucleus of the stria terminalis, CDK5 abundance was negatively correlated with intoxication levels during ethanol vapor exposure but there was no effect of dependence on the phosphorylation ratio of CDK5 substrates. In contrast, ethanol dependence increased the phosphorylation of low-molecular weight CDK substrates in the basolateral amygdala.
Conclusions.
The selective effect of (S)-CR8 on excessive ethanol intake has potential therapeutic value for the treatment of alcohol use disorders. Our data do not support the hypothesis that this effect would be mediated by the inhibition of upregulated CDK5 activity in the extended amygdala nor prefrontal cortex. However, increased activity of CDKs other than CDK5 in the basolateral amygdala may contribute to excessive ethanol consumption in alcohol dependence. Other (S)-CR8 targets may also be implicated.
Keywords: Alcohol, alcoholism, vapor, roscovitine, immunoblotting
Introduction
Improving our understanding of the neuroadaptations associated with chronic exposure to alcohol and excessive alcohol drinking is a critical step to identify novel targets for the treatment of alcohol use disorders. We previously identified cyclin-dependent kinase 5 (CDK5) signaling as the canonical pathway most significantly associated with genes differentially expressed in the extended amygdala of ethanol-dependent mice (Contet et al., 2011) but the relevance of CDK5 signaling in the regulation of ethanol-related behaviors is currently unknown.
Cyclin-dependent kinases (CDKs) are small serine/threonine kinases that orchestrate the progression through the cell cycle (Morgan, 1997). CDK5 is a unique member of this family, as it is not involved in mitosis but instead plays a critical role in neuronal maturation and migration, synaptic activity and plasticity, and neurodegeneration (Dhavan and Tsai, 2001). CDK5 requires the association of a cofactor, such as p35 or p39, to become activated (Lew et al., 1994; Tsai et al., 1994; Humbert et al., 2000). Calpain-mediated truncation of p35 to p25 leads to hyperactivation of CDK5, which can promote synaptic plasticity if transient, but becomes neurotoxic if prolonged (Patrick et al., 1999; Cruz et al., 2003; Fischer et al., 2005).
Chronic exposure to psychostimulants, opioids, cannabinoids, and ethanol can alter the expression of CDK5, p35, and p25 in local brain regions (Bibb et al., 2001; Rajgopal and Vemuri, 2001; Ferrer-Alcon et al., 2003; Chen and Chen, 2005; Camp et al., 2006; Mlewski et al., 2008; Pascual et al., 2012; Goggin et al., 2014; Zhang et al., 2015; Chen et al., 2017; Lazenka et al., 2017).
Studies that have investigated the functional role of CDK5 in response to drugs of abuse have yielded conflicting results. Some indicated that CDK5 activity opposes cocaine-induced dopamine release and postsynaptic dopaminergic signaling, as well as the locomotor and motivational effects of cocaine and heroin, while others indicated that CDK5 facilitates the effects of psychostimulants on dendritic spine density and locomotor activity (Bibb et al., 2001; Norrholm et al., 2003; Chergui et al., 2004; Chen and Chen, 2005; Takahashi et al., 2005; Taylor et al., 2007; Meyer et al., 2008; Heller et al., 2016; Chen et al., 2017; Ferreras et al., 2017). Moreover, a number of studies have challenged the behavioral relevance of changes in CDK5/p35 abundance for long-lasting adaptations to chronic cocaine and morphine exposure (Lu et al., 2003; Hope et al., 2005; Seiwell et al., 2007; Contet et al., 2008).
In the present study, we sought to determine whether CDK5 activity plays a role in the escalation of ethanol self-administration triggered by dependence. To this end, we tested the effect of systemically administered (S)-CR8 on operant responding for ethanol and water in dependent and non-dependent rats. CR8 is an analog of roscovitine with similar target profile (both molecules inhibit multiple CDKs, including CDK5, as well as a small set of other kinases) and brain penetrance, but CR8 is more potent and has a longer half-life than roscovitine (Bettayeb et al., 2008; McClue and Stuart, 2008; Sallam et al., 2013; Delehouze et al., 2014). The (S) enantiomer of CR8 has the added advantage of not inhibiting glycogen synthase kinase-3β, an enzyme known to regulate ethanol drinking (Bettayeb et al., 2008; Neasta et al., 2011; Cheng et al., 2017; Liu et al., 2017; van der Vaart et al., 2018). (S)-CR8 dose-dependently reduced ethanol self-administration in dependent rats without affecting non-dependent rats or saccharin self-administration.
We then sought to identify a possible neuroanatomical locus for the behavioral effect of (S)-CR8 by quantifying protein levels of CDK5 and p35, as well as the phosphorylation of CDK substrates, in several brain regions of ethanol-naïve, non-dependent, and dependent rats at the expected time of ethanol self-administration (6–8 h into withdrawal for dependent rats). This time point was selected to capture molecular changes associated with differential motivation to drink ethanol and to match the time point at which (S)-CR8 was effective in dependent rats. Based on our previous study, we first analyzed the bed nucleus of the stria terminalis (BNST), central nucleus of the amygdala (CeA), and basolateral amygdala (BLA) (Contet et al., 2011). We then expanded our analysis to other brain regions involved in addiction - nucleus accumbens (Acb), cingulate cortex (Cg), medial prefrontal cortex (mPFC), and orbitofrontal cortex (OFC) (Koob and Volkow, 2016; Um et al., 2019). Chronic ethanol self-administration, with or without CIE, did not alter the abundance of CDK5 nor p35 in any of the brain regions analyzed. The abundance of CDK5 in the BNST negatively correlated with the level of intoxication during ethanol vapor exposure but there was no effect of dependence on the phosphorylation ratio of four CDK5 substrates (DARPP32, PSD95, PAK1, p27Kip1). Ethanol dependence increased the phosphorylation state of low-, but not high-, molecular weight CDK substrates in the basolateral amygdala, indicating that the behavioral effect of (S)-CR8 may result from the inhibition of another CDK than CDK5.
Materials and Methods
Animals
Adult male Wistar rats (Charles River; Raleigh, NC), weighing 225–275 g at the beginning of the experiments, were housed in groups of two per cage in a temperature-controlled vivarium (22 °C) on a 12 h/12 h light/dark cycle (lights on at 8:00 PM) with ad libitum access to food and water. All behavioral tests were conducted during the dark phase of the light/dark cycle. All procedures adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute.
Cohorts
A first cohort of rats was used to test the effect of (S)-CR8 on ethanol self-administration in non-dependent (n=10) and dependent (n=10) rats. A second cohort of rats was used to test the effect of (S)-CR8 on saccharin self-administration in dependent rats (n=12). A third cohort of rats was used to measure the effects of chronic ethanol self-administration in non-dependent (n=12) and dependent (n=11) rats on the abundance of CDK5 and p35, as well as CDK substrate phosphorylation, in discrete brain regions. This cohort also included a subgroup of age-matched, ethanol-naïve rats (n=10).
Ethanol self-administration
Self-administration sessions were conducted in standard operant conditioning chambers (Med Associates; St. Albans, VT). Animals were first trained to self-administer ethanol [10% (v/v)] and water orally, until a stable response was maintained. First, to facilitate the acquisition of self-administration, rats were initially provided free-choice access to ethanol and water for one day in their home cages to habituate them to the taste of ethanol. Second, the rats were subjected to an overnight session in the operant chambers with access to one lever (right lever) that delivered water (0.1 mL) under a fixed ratio 1 (FR1) schedule of reinforcement. Food was available ad libitum during this training. Third, after one day off, the rats were subjected to a 2 h session (FR1) for one day and a 1 h session (FR1) the next day, with one lever delivering ethanol (right lever, 0.1 mL). All of the subsequent sessions lasted 30 min, and two levers were available (left lever: water; right lever: ethanol) and each operant response was reinforced with 0.1 mL of liquid (FR1). Self-administration on an FR1 schedule requires minimal effort by the rat to obtain the reinforcement and herein was considered a measure of intake. Sessions were conducted until stable levels of ethanol intake were reached (±10% over the last three sessions).
Dependence induction and intake escalation
Once stable ethanol self-administration was established, rats were split into two subgroups of equivalent baseline intake (Fig. 1A). Half of the rats were made dependent to ethanol via chronic intermittent inhalation of ethanol vapor (CIE), as previously described (O’Dell et al., 2004; Gilpin et al., 2008). CIE consisted in cycles of 14 h on (i.e., ethanol vapor inhalation) and 10 h off (i.e., air inhalation). Tail blood samples (0.1 mL) were collected once a week to determine blood ethanol concentrations (BECs) using an oxygen-rate ethanol analyzer (Analox Instruments; Stourbridge, England). Overall average BECs were 141.5 ± 9.6 mg/dL in the first cohort and 161.3 ± 4.9 mg/dL in the third cohort, with BECs gradually increasing over the first 3–4 weeks and reaching an average of 206.4 ± 13.0 mg/dL and 213.6 ± 8.0 mg/dL, respectively, during the last week of vapor exposure. The other half of the rats were not exposed to ethanol vapor (air only, non-dependent rats).
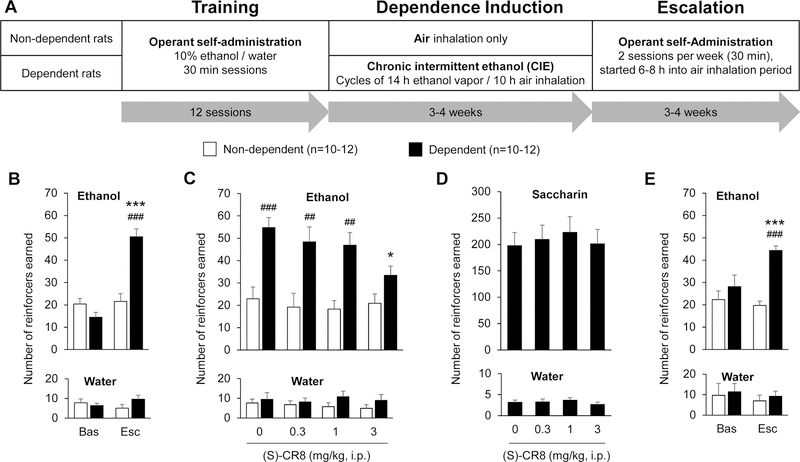
Figure 1.
Systemic administration of (S)-CR8 dose-dependently reduces ethanol self-administration selectively in dependent rats. A. Model of ethanol dependence. Rats were trained to lever press for 10% ethanol or water then divided into two groups: one group was exposed to air while the other group was made dependent to ethanol by chronic intermittent exposure to ethanol (CIE) via vapor inhalation. Self-administration sessions were resumed 3–4 weeks later, every-other-day, until escalation of ethanol intake was achieved by the dependent rats, for a total of 6–8 weeks of CIE. For 0.04% saccharin self-administration, training was initiated after 4 weeks of vapor exposure. B. Ethanol intake escalation. The number of ethanol rewards increased in CIE-exposed rats, both compared to their own baseline (***, p<0.001) and compared to air-exposed counterparts (###, p<0.001). The number of water rewards remained stable over time and between groups. C. Effect of (S)-CR8 on ethanol self-administration. Four doses of (S)-CR8 were administered intraperitoneally (i.p.) 30 min prior to the self-administration session in a counterbalanced order. There was a dose-dependent reduction of ethanol intake in dependent rats. *, p<0.05 versus vehicle; ##, p<0.01; ###, p<0.001 versus non-dependent rats. D. Effect of (S)-CR8 on saccharin self-administration in dependent rats. Four doses of (S)-CR8 were administered intraperitoneally (i.p.) 30 min prior to the self-administration session in a counterbalanced order. There was no effect of (S)-CR8 on saccharin intake. E. Ethanol intake escalation in rats used for molecular analyses. The number of ethanol rewards increased in CIE-exposed rats, both compared to their own baseline (***, p<0.001) and compared to air-exposed counterparts (###, p<0.001). The number of water rewards remained stable over time and between groups. Data are shown as mean ± standard error of the mean.
Dependent rats were exposed to a total of 6–8 weeks of CIE. During the first 3–4 weeks, rats were exposed to CIE (or air only) without concomitant self-administration, while during the following weeks rats were exposed to CIE (or air only) and ethanol self-administration sessions (30 min, FR1, ethanol versus water) were conducted three times per week, 6–8 h after ethanol vapor was turned off (when brain and blood ethanol levels are negligible, Gilpin et al., 2009). Sessions were conducted until ethanol intake had significantly escalated above baseline.
Saccharin self-administration
Rats were exposed to CIE as described above. In this cohort BECs were measured using a gas chromatography system (7820A, Agilent Technologies, Santa Clara, CA) with headspace sampler (7697A, Agilent Technologies), and averaged 175.8 ± 8.5 mg/dL. After 4 weeks of CIE exposure, rats were trained to self-administer 0.04% saccharin under FR1 using the same conditions as for ethanol self-administration (30-min sessions).
Pharmacological experiments
(S)-CR8 (AdipoGen Life Sciences; San Diego, CA) was dissolved in a vehicle composed of DMSO [5% (v/v); Sigma-Aldrich, St. Louis, MO] and Alkamuls EL620 [5% (v/v); Solvay, Brussels, Belgium]. Dependent rats were exposed to at least 6 weeks of CIE by the time (S)-CR8 testing was initiated. The rats were injected with (S)-CR8 intraperitoneally (i.p., 1 mL/kg) 30 min before each test session. Doses 0, 0.3, 1.0, and 3.0 mg/kg were administered according to a Latin square design with a baseline (no injection) self-administration session between tests.
Tissue collection
Brains were collected at the time of expected self-administration (i.e., 6–8 h after last vapor exposure) in an independent cohort of rats. Rats were anesthetized using isoflurane then sacrificed by decapitation. Brains were removed, flash frozen in isopentane (Sigma-Aldrich), then stored at −80 °C. Brain regions of interest were isolated from frozen sections using a CM 1800 cryostat (Leica Biosystems; Nussloch, Germany). Prior to sectioning, brains were equilibrated to −20 °C. Each brain region was punched from 500 μm thick sections using 1 or 2 mm ID sample corers (Fine Science Tools; Foster City, CA) as follows [AP coordinates (mm) relative to bregma]: OFC (four bilateral 2-mm punches; 4.68 to 2.68 mm), mPFC (four 2-mm punches centered on midline; 4.18 to 2.18 mm), Cg (four 2-mm punches centered on midline; 2.18 to 0.18 mm), Acb (two bilateral 2-mm punches; 1.68 to 0.68 mm), BNST (three bilateral 1-mm punches; 0.18 to −1.32 mm), CeA (two bilateral 1-mm punches; −1.82 to −2.82 mm), and BLA (two bilateral 1-mm punches; −2.82 to −3.82 mm). The punches were stored at −80 °C until homogenization.
Tissue homogenization
The homogenization procedure that was used for BNST, CeA, and BLA samples removed the nuclei, while the procedure used for Acb, OFC, mPFC, and Cg samples preserved nuclei. Punches from the BNST, CeA, and BLA were thawed on ice for 10 min then homogenized in an ice-cold homogenization buffer [2% (v/v) Triton X-100, 1% (v/v) SDS, 10 mM Tris-Cl pH 8, 100 mM NaCl, 1 mM EDTA pH 8, and the following inhibitors at 1X: Protease Inhibitor Cocktail, Phosphatase Inhibitor Cocktail 2, and Phosphatase Inhibitor Cocktail 3 (respective catalog numbers: P8340, P5726, and P0044; Sigma-Aldrich). The punches were homogenized by hand over ice using 10 twists of a blue polypropylene pellet pestle (Sigma-Aldrich) then by 10 strokes (up/down = 1 stroke) of an insulin syringe. The homogenates were incubated end-over-end for 15 min at 4 °C using a tube revolver/rotator (ThermoFisher Scientific; Waltham, MA) then centrifuged at 13,600 x g for 10 min at 4 °C. The supernatants were collected, and protein concentrations were determined using the Pierce BCA Protein Assay Kit (ThermoFisher Scientific) before the samples were diluted according to manufacturer’s specifications in 4x Laemmli Sample Buffer with 2-mercaptoethanol (Bio-Rad Laboratories; Hercules, CA).
Punches from the OFC, mPFC, Cg, and Acb were thawed on wet ice for 10 min, then ice-cold buffer (10 mM Tris-Cl pH 8, 100 mM NaCl, 1 mM EDTA pH 8, and 1X inhibitors) was added to the tubes containing the punches. Next, approximately 200 mg of 0.5 mm zirconium oxide beads (Next Advance; Troy, NY) were added and the punches were homogenized using a Bullet Blender Storm 24 tissue homogenizer (Next Advance) at speed 12 for 2 min at 4 °C. An equal volume of another ice-cold buffer [10 mM Tris-Cl pH 8, 100 mM NaCl, 1 mM EDTA pH 8, 4% (v/v) Triton X-100, 2% (v/v) SDS, and 1X inhibitors] was added and the homogenates were incubated end over end for 15 min at 4 °C using a tube revolver/rotator (ThermoFisher Scientific). The homogenates were transferred to new tubes, then protein concentrations were determined using the Pierce BCA Protein Assay Kit (ThermoFisher Scientific) before the samples were diluted in 4x Laemmli Sample Buffer with 2-mercaptoethanol (Bio-Rad Laboratories). Samples were stored at −80 °C until immunoblotting.
Immunoblotting
The diluted samples were thawed to room temperature, heated at 95 °C for 5 min, then cooled back to room temperature. Next, a volume corresponding to 16 μg of protein was loaded into hand-poured 8% or 10% Tris-glycine SDS-polyacrylamide gels topped with a 5% stacking gel and electrophoresed at 75 V and 150 V through the stacking and resolving gels, respectively, using a Mini-PROTEAN Tetra Cell electrophoresis apparatus (Bio-Rad Laboratories). The proteins were transferred to a PVDF Immobilon-P membrane (Merck Millipore; Billerica, MA) using a Mini Trans-Blot cell (Bio-Rad Laboratories) running at 30 V for 18 h in a cold room. After the transfer, the membranes were rinsed 2 × 5 min in TBS (50 mM Tris-Cl pH 8 and 50 mM NaCl), dried for 1 h at room temperature, then stored at 4 °C.
Membranes were reactivated in 100% methanol, rinsed 3 × 5 min in TBST (50 mM Tris-Cl pH 8, 50 mM NaCl, and 0.1% Tween 20), then blocked for 1 h at room temperature in blocking buffer [TBST with 5% bovine serum albumin fraction V (ThermoFisher Scientific) for phospho-specific antibodies, or TBST with 5% (w/v) blotting grade nonfat dry milk (Bio-Rad Laboratories) for non-phospho-specific antibodies]. Next, the membranes were incubated overnight at 4 °C in blocking buffer containing the primary antibody [CDK5, Cell Signaling Technologies (CST) #14145, 1:1,000; p35/p25, CST #2680, 1:1,000; DARPP-32 (phospho-T75), CST #2301, 1:500; PSD95 (phospho-T19), Abcam ab16496, 1:2,000; PAK1 (phospho-T212), Abcam ab75599, 1:500; p27Kip1 (phospho-S10), Abcam ab62364, 1:5,000; phospho-CDK substrate motif [(K/H)pSP] MultiMab mix, CST #9477, 1:1,000]. Next, the membranes were washed at room temperature 3 × 5 min with blocking buffer, incubated for 1 h at room temperature in blocking buffer containing a 1:4,000 (CDK5) or 1:2,000 (all others) dilution of anti-rabbit IgG (H+L) horseradish peroxidase-conjugated antibody (Bio-Rad Laboratories #1706515), then washed at room temperature 3 × 5 min with TBST. Finally, membranes were bathed with 1 mL of SuperSignal West Pico Chemiluminescent Substrate (ThermoFisher Scientific) and imaged using a ChemiDoc MP System (Bio-Rad Laboratories). For phosphorylated CDK5 substrates, the membrane was then stripped by incubating with 5 mL of Restore PLUS Western Blot Stripping Buffer (ThermoFisher Scientific) for 15 min at 37°C with 100 rpm shaking, washed 3 × 5 min in TBST, and the immunoblotting procedure was repeated using the antibody against the total protein (DARPP-32, CST #2306, 1:2,000; PSD95, Abcam ab76115, 1:2,000; PAK1, Abcam ab40852, 1:2,000; p27Kip1, CST #3686, 1:1,000).
To quantify the amount of protein loaded in each lane, the blots were stained with a Coomassie solution [0.5% (w/v) Coomassie Brilliant Blue R-250 Dye, 50% (v/v) methanol, and 10% (v/v) acetic acid], destained with destaining solution [50% (v/v) methanol and 7% (v/v) acetic acid], then imaged using a ChemiDoc MP System (Bio-Rad Laboratories), as described in (Welinder and Ekblad, 2011).
Immunoblot data analysis
The density of immunolabeled bands, as well as Coomassie staining density in corresponding lanes, were acquired using Image Lab Software (version 5.2.1; Bio-Rad Laboratories) with background subtraction enabled, using a disc size of 10 mm for immunolabeled bands and 70 mm for Coomassie staining (Taylor et al., 2013; Ghosh et al., 2014). The immunoblotting signal in each lane was normalized to Coomassie staining to adjust for variability in protein loading. Total protein staining is a superior loading control normalizer compared to a single housekeeping protein whose abundance may be affected by chronic ethanol exposure (Gilda and Gomes, 2013; Ghosh et al., 2014; Moritz, 2017). For CDK5 substrates, the normalized signal of the phosphorylated protein was divided by the normalized signal of the total protein (p/t ratio) for each sample. Since the samples were spread across multiple blots (6–12 samples per blot), the values from non-dependent and dependent rat samples were further normalized to the average signal from naïve rat samples loaded on the same blot to account for differences in transfer efficiency between blots. Finally, normalized values for each treatment condition were averaged across blots and expressed as a percentage of the naïve rat samples. Lanes in which signal was compromised by protein transfer issues were excluded from the analysis.
Statistical analyses
Statistical analysis was conducted in Statistica 13.3 (TIBCO Software Inc.; Palo Alto, CA). Significant escalation of ethanol intake in CIE-exposed rats was verified using a two-way repeated-measures analysis of variance (ANOVA) with time (baseline versus post-vapor) as within-subject variable and treatment (air versus CIE) as between-subject variable. The effect of (S)-CR8 on ethanol (and water) intake was analyzed using a two-way repeated-measures ANOVA with (S)-CR8 dose as the within-subject variable and vapor exposure as the between-subject variable. The effect of (S)-CR8 on saccharin (and water) intake was analyzed using a one-way repeated-measures ANOVA with (S)-CR8 dose as the within-subject variable. When relevant, post hoc analysis was conducted using the Newman-Keuls test. The distribution normality of immunoblotting data was evaluated using a Shapiro-Wilk test and parametric/non-parametric tests were selected accordingly. To analyze the effect of treatment (ethanol-naïve, non-dependent, and dependent) on protein abundance, data were subjected to parametric ANOVA or Kruskal-Wallis ANOVA. The correlation between protein abundance (or phosphorylation) and ethanol intake (non-dependent and dependent rats only) or BECs (dependent rats only) was analyzed using a Pearson product-moment correlation test or Spearman’s rank correlation test.
Results
(S)-CR8 reduces ethanol self-administration selectively in dependent rats
As expected, rats exposed to CIE selectively increased their ethanol intake, as reflected by a significant interaction between time and treatment for ethanol reinforcers (Fig. 1B, top panel; F1,18=58.7, p<0.001), but not for water reinforcers (Fig. 1B, bottom panel; F1,18=3.8, n.s.). Post hoc analysis confirmed that ethanol rewards were higher in dependent rats, both compared to their own baseline (p<0.001) and compared to their non-dependent counterparts (p<0.001). Systemic administration of (S)-CR8 dose-dependently reduced ethanol self-administration in dependent rats without altering ethanol intake in non-dependent rats (Fig. 1C). Two-way repeated-measures ANOVA revealed a significant main effect of (S)-CR8 (F3,18=3.0, p<0.05) and vapor exposure (F1,18=22.5, p<0.001) on ethanol rewards, as well as a trend for an interaction between the two variables (F3,18=2.5, p=0.07). Post hoc analysis indicated that the effect of (S)-CR8 was driven by the highest dose tested, 3 mg/kg, which reduced ethanol intake in dependent rats compared to vehicle (p<0.05) and ablated the effect of CIE such that the intake of non-dependent and dependent rats injected with 3 mg/kg (S)-CR8 was not significantly different. In contrast, there was no effect of (S)-CR8 on water intake (Fig. 1C; F3,18=0.3, n.s.). There was also no effect of (S)-CR8 on saccharin intake measured in an independent cohort of dependent rats (Fig. 1D; F3,33=0.7, n.s.), and again no effect on water intake (Fig. 1D; F3,33=0.6, n.s.).
Chronic ethanol self-administration does not alter CDK5 and p35 abundance in extended amygdala and prefrontal cortex subregions of non-dependent or dependent rats
A third cohort of rats was subjected to ethanol self-administration combined with CIE for molecular analysis, in an attempt to identify the brain locus mediating the inhibitory effect of (S)-CR8 on ethanol intake in dependent rats. In this cohort, there was also a significant interaction between time and treatment for the number of reinforced responses on the ethanol lever (Fig. 1E; F1,21=11.9, p<0.01). Post hoc analysis confirmed that dependent rats escalated their ethanol intake, both compared to their own baseline (p<0.001) and compared to their non-dependent counterparts (p<0.001). As expected, no significant effects of time nor treatment nor an interaction were detected for water intake (F1,21<1.1, n.s.; Fig. 2A). This cohort also included a subgroup of ethanol-naïve rats to evaluate the effect of chronic ethanol self-administration, independent of vapor exposure. Brains were collected at the expected time of ethanol self-administration and the abundance of CDK5 and p35 was quantified in the BNST, CeA, BLA, Acb, Cg, mPFC, and OFC using immunoblotting.
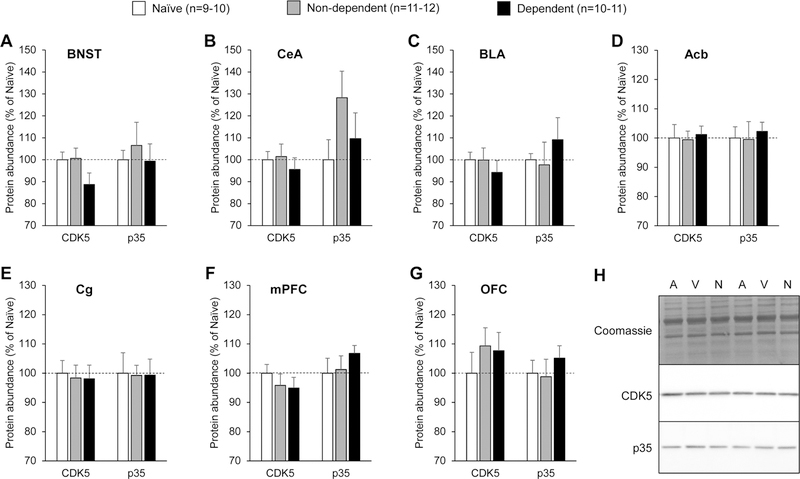
Figure 2.
Abundance of CDK5 and p35 in extended amygdala and prefrontal cortex subregions of ethanol-naïve, non-dependent, and dependent rats. A. Bed nucleus of the stria terminalis (BNST). B. Central nucleus of the amygdala (CeA). C. Basolateral amygdala (BLA). D. Nucleus accumbens (Acb). E. Cingulate cortex (Cg). F. Medial prefrontal cortex (mPFC). G. Orbitofrontal cortex (OFC). Rats were sacrificed 6–8 h into withdrawal from ethanol vapor, at the expected time of self-administration. Naïve rats were age-matched and handled, but never exposed to ethanol. The immunoblotting signal was normalized to the quantity of proteins loaded in the lane, as determined by Coomassie staining. Values obtained in non-dependent and dependent rats were normalized to the values obtained in ethanol-naïve rats. There was no main effect of treatment on the abundance of either CDK5 or p35 in any of the brain regions analyzed. Data are shown as mean ± standard error of the mean. H. Representative images of Coomassie staining and immunoblotting signal (A, Air, non-dependent; V, Vapor, dependent; N, Naïve).
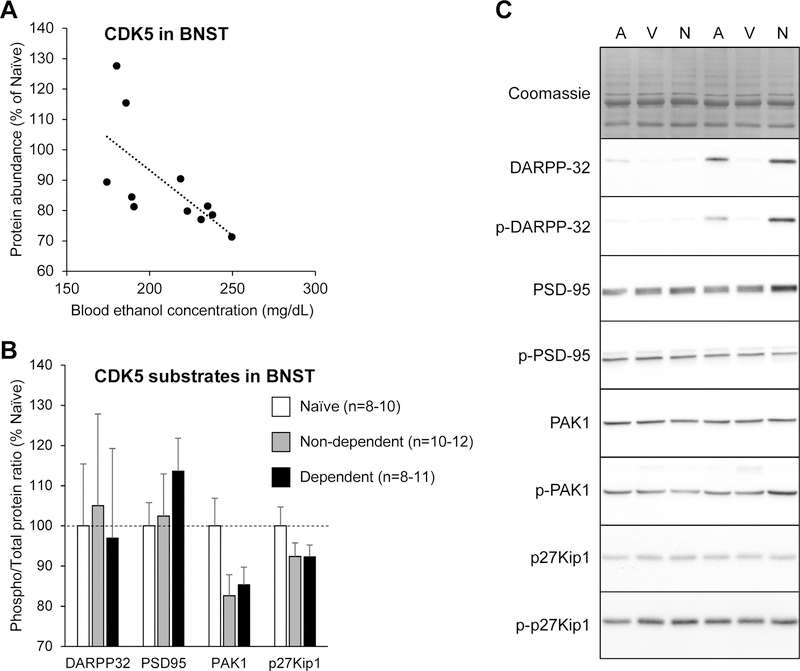
There was no effect of treatment (ethanol self-administration alone or combined with CIE) on the abundance of either protein in any of the brain regions analyzed (Fig. 2A–G; see Table 1 for statistical analyses and Fig. 2H for representative images). Furthermore, there was no significant correlation between the number of ethanol reinforcers earned during the last self-administration session and the abundance of either protein in any of the brain regions analyzed (Table 1). There was also no significant correlation between the BECs measured in CIE-exposed rats during the last period of ethanol vapor inhalation prior to brain collection and the abundance of either protein in any of the brain regions analyzed, except for CDK5 in the BNST, whose abundance was negatively correlated with final BECs (Table 1, Fig. 3A). To examine the potential significance of this correlation, we determined the phosphorylation state of four CDK5 substrates in the BNST: DARPP32, PSD95, PAK1, and p27Kip1 (Nikolic et al., 1998; Bibb et al., 1999; Rashid et al., 2001; Morabito et al., 2004; Kawauchi et al., 2006). There was no effect of treatment (ethanol self-administration alone or combined with CIE) on the phosphorylated over total ratio for any of these proteins (Fig. 3B; see Table 1 for statistical analyses and Fig. 3C for representative images).
Table 1.
Statistical analyses of the main effect of treatment on protein abundance or phosphorylation (parametric ANOVA or Kruskal-Wallis ANOVA in ethanol-naïve, non-dependent, and dependent rats), correlation of protein abundance or phosphorylation with the number of ethanol reinforcers earned during the last self-administration session (Pearson or Spearman test in non-dependent and dependent rats only), and correlation of protein abundance or phosphorylation with the blood ethanol concentrations (BECs) measured during the last period of ethanol vapor inhalation prior to brain collection (Pearson or Spearman test in dependent rats only). Pearson and Spearman correlation coefficients (RP and RS, respectively) are shown as absolute value. p/t, phosphorylated protein over total protein ratio; p-CSM, phosphorylated CDK substrate motif.
| Brain region | Protein | Main effect of treatment on protein abundance or phosphorylation | Correlation with ethanol intake | Correlation with final BECs |
|---|---|---|---|---|
| BNST | CDK5 | F2,30=2.1, n.s. | RP =0.3, n.s. | RP=0.7, p<0.05 |
| p35 | H2=0.3, n.s. | RS=0.2, n.s. | RS=0.01, n.s. | |
| DARPP32 (p/t) | H2=0.4, n.s. | RS=0.06, n.s. | RS=0.2, n.s. | |
| PSD95 (p/t) | H2=2.3, n.s. | RS=0.4, n.s. | RS=0.3, n.s. | |
| PAK1 (p/t) | F2,30=2.8, n.s. | RP =0.2, n.s. | RP=0.8, p<0.01 | |
| P27Kip1 (p/t) | F2,30=1.4, n.s. | RP =0.02, n.s. | RP=0.4, n.s. | |
| p-CSM (150 kDa) | F2,28=0.1, n.s. | RP =0.01, n.s. | RP=0.6, n.s. | |
| p-CSM (37–75 kDa) | F2,29=0.3, n.s. | RP =0.06, n.s. | RP=0.5, n.s. | |
| CeA | CDK5 | H2=1.7, n.s. | RS=0.2, n.s. | RS=0.3, n.s. |
| p35 | F2,29=1.6, n.s. | RP=0.2, n.s. | RP=0.05, n.s. | |
| p-CSM (150 kDa) | F2,29=0.2, n.s. | RP =0.2, n.s. | RP=0.3, n.s. | |
| p-CSM (37–75 kDa) | H2=1.4, n.s. | RS=0.2, n.s. | RS=0.4, n.s. | |
| BLA | CDK5 | H2=1.6, n.s. | RS=0.006, n.s. | RS=0.06, n.s. |
| p35 | H2=2.1, n.s. | RS=0.2, n.s. | RS=0.1, n.s. | |
| p-CSM (150 kDa) | H2=6.6, p<0.05 | RS=0.5, n.s. | RS=0.04, n.s. | |
| p-CSM (37–75 kDa) | F2,18=3.9, p<0.05 | RP =0.3, n.s. | RP=0.5, n.s. | |
| Acb | CDK5 | F2,30=0.1, n.s. | RP=0.1, n.s. | RP=0.08, n.s. |
| p35 | F2,30=0.1, n.s. | RP=0.08, n.s. | RP=0.1, n.s. | |
| p-CSM (150 kDa) | F2,29=1.4, n.s. | RP =0.02, n.s. | RP=0.4, n.s. | |
| Cg | CDK5 | F2,29=1.0, n.s. | RP=0.05, n.s. | RP=0.5, n.s. |
| p35 | F2,27=0.01, n.s. | RP=0.002, n.s. | RP=0.1, n.s. | |
| p-CSM (150 kDa) | F2,17=0.6, n.s. | RP =0.1, n.s. | RP=0.8, n.s. | |
| mPFC | CDK5 | F2,29=0.6, n.s. | RP=0.05, n.s. | RP=0.3, n.s. |
| p35 | F2,29=0.7, n.s. | RP=0.3, n.s. | RP=0.2, n.s. | |
| p-CSM (150 kDa) | F2,19=0.4, n.s. | RP =0.2, n.s. | RP=0.5, n.s. | |
| OFC | CDK5 | F2,30=0.6, n.s. | RP=0.08, n.s. | RP=0.2, n.s. |
| p35 | F2,30=0.5, n.s. | RP=0.2, n.s. | RP=0.3, n.s. | |
| p-CSM (150 kDa) | F2,28=0.6, n.s. | RP =0.1, n.s. | RP=0.3, n.s. |
Figure 3.
Measure of CDK5 abundance and activity in the bed nucleus of the stria terminalis (BSNT). A. Correlation between CDK5 abundance in the BNST and blood ethanol concentrations measured during the last period of ethanol vapor exposure prior to brain collection in dependent rats (R2=0.45, p<0.05). B. Phosphorylation levels of four CDK5 substrates in the BNST. Rats were sacrificed 6–8 h into withdrawal from ethanol vapor, at the expected time of self-administration. Naïve rats were age-matched and handled, but never exposed to ethanol. The immunoblotting signal was normalized to the quantity of proteins loaded in the lane, as determined by Coomassie staining. For each sample, the immunoblotting signal of the phosphorylated protein was divided by the immunoblotting signal of the total protein. Values obtained in non-dependent and dependent rats were normalized to the values obtained in ethanol-naïve rats. There was no main effect of treatment on the phosphorylation of any of the CDK5 substrates analyzed. Data are shown as mean ± standard error of the mean. C. Representative images of Coomassie staining and immunoblotting signal (A, Air, non-dependent; V, Vapor, dependent; N, Naïve).
The phosphorylation of some CDK substrates is increased in the basolateral amygdala of dependent rats
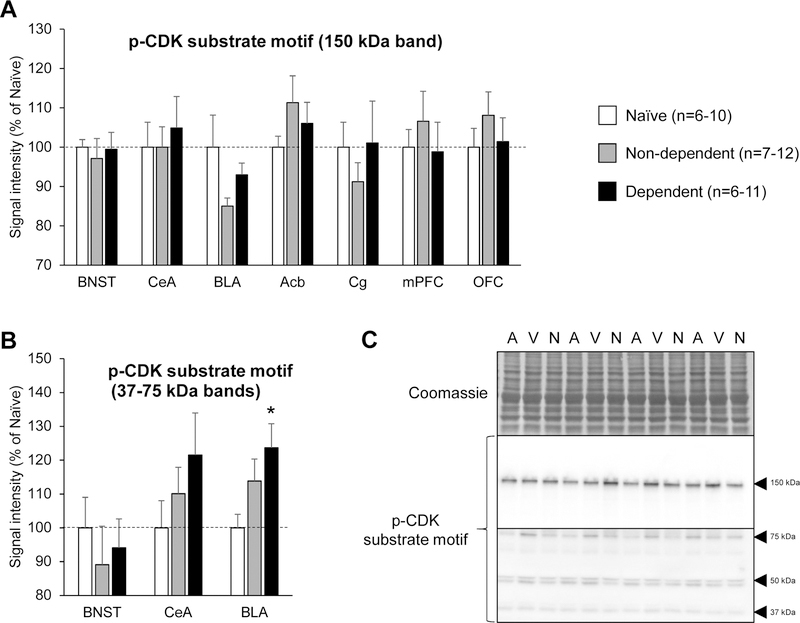
We then examined the possibility that the effect of (S)-CR8 on ethanol intake in dependent rats could result from the inhibition of upregulated CDKs other than CDK5. We used a monoclonal antibody mix recognizing the phosphorylated consensus motif of CDK substrates to get a general measure of CDK activity. This antibody mix labeled a band at ≈150 kDa, which we could quantify in BNST, CeA, BLA, Acb, Cg, mPFC and OFC samples. There was a significant effect of treatment on the intensity of this band in the BLA but post-hoc tests did not detect differences between groups (Fig. 4A; see Table 1 for statistical analyses and Fig. 4C for representative images). The antibody mix also labeled fainter bands of lower molecular weight (≈37–75 kDa), which we could only quantify in the BNST, CeA, and BLA. There was again a significant effect of treatment in the BLA, where the cumulated density of these bands was higher in the dependent rats compared to ethanol-naïve rats (p<0.05; Fig. 4B; see Table 1 for statistical analyses and Fig. 4C for representative images). A similar trend was seen in the CeA but did not reach significance. No effect of treatment was detected in the BLA or CeA when these low-molecular weight bands were analyzed individually (Supplementary Figure S1).
Figure 4.
CDK substrate motif phosphorylation in extended amygdala and prefrontal cortex subregions of ethanol-naïve, non-dependent, and dependent rats. A. Quantification of high-molecular weight band (150 kDa). B. Quantification of low-molecular weight bands (37–75 kDa). Rats were sacrificed 6–8 h into withdrawal from ethanol vapor, at the expected time of self-administration. Naïve rats were age-matched and handled, but never exposed to ethanol. The immunoblotting signal was normalized to the quantity of proteins loaded in the lane, as determined by Coomassie staining. Values obtained in non-dependent and dependent rats were normalized to the values obtained in ethanol-naïve rats. There was a main effect of treatment on the phosphorylation of high- and low-molecular weight CDK substrates in the basolateral amygdala. *, p<0.05, versus ethanol-naïve rats. Data are shown as mean ± standard error of the mean. C. Representative images of Coomassie staining and immunoblotting signal (A, Air, non-dependent; V, Vapor, dependent; N, Naïve).
Discussion
Our study shows that systemic administration of (S)-CR8, a potent, brain-penetrant, non-selective CDK inhibitor, dose-dependently reduces ethanol intake in dependent rats. Importantly, (S)-CR8 did not impact ethanol intake in non-dependent rats, nor saccharin intake in dependent rats, nor water intake in either group of rats, which indicates that, even at the highest dose tested (3 mg/kg), (S)-CR8 does not disrupt operant performance or sweet taste perception. The selective effect of (S)-CR8 on excessive, but not moderate, ethanol intake has potential therapeutic value for the treatment of alcohol use disorders. This compound also showed neuroprotective properties in a mouse model of traumatic brain injury (Kabadi et al., 2012) in addition to its potent antitumoral activity (Bettayeb et al., 2008; Bettayeb et al., 2010; Delehouze et al., 2014). The behavioral effect of (S)-CR8 may result from its ability to reduce microglial activation induced by CIE (Kabadi et al., 2012; Wu et al., 2014; Bajo et al., 2016; Sanchez-Alavez et al., 2019). The present study was conducted in male rats and future studies will be needed to determine whether (S)-CR8 can also reduce excessive alcohol drinking in female subjects and in different species.
Based on CDK5 being one of the kinases inhibited by (S)-CR8 and published evidence that CDK5 modulates addiction-related behaviors, we hypothesized that the inhibitory effect of (S)-CR8 on ethanol self-administration in dependent rats may result from CDK5 inhibition. We therefore sought to identify a brain region in which CDK5 and/or p35 would be upregulated in dependent rats compared to ethanol-naïve or non-dependent rats. We analyzed subregions of the extended amygdala (BNST, CeA, BLA, and Acb) and prefrontal cortex (Cg, mPFC, and OFC) based on previous evidence of CDK5 alterations in these areas and their involvement in positive and negative reinforcement mechanisms (Koob and Volkow, 2016; Um et al., 2019). However, the abundance of CDK5 and p35 was not altered by chronic ethanol self-administration, alone or in combination with CIE, at the time of expected access to ethanol in any of the brain regions analyzed. Although studies that have demonstrated a functional implication of CDK5 in the acute or chronic effects of drugs of abuse have generally observed concomitant changes in the abundance of CDK5 or its activators, we cannot rule out that a functional assay would have detected an effect of chronic ethanol exposure on CDK5 activity (see for instance Rajgopal and Vemuri, 2001). Alternatively, it is possible that substantial changes in CDK5 or p35 abundance would have been detected in brain regions that we did not collect (e.g., hippocampus, see Zhang et al., 2015). Based on the inhibitory effect of (S)-CR8 on ethanol intake in dependent rats, the negative correlation of CDK5 abundance in the BNST with BECs during vapor exposure suggests that CDK5 downregulation in that brain region may represent a compensatory adaptation opposing intake escalation in CIE-exposed rats. However, this negative correlation was not accompanied by a significant reduction in the phosphorylation ratio of four CDK5 substrates in dependent rats. Anesthetics are known to alter protein phosphorylation and we cannot rule out that phosphorylation changes consistent with reduced CDK5 activity would have been detected if the brains had been collected without isoflurane anesthesia (Kohtala et al., 2016; Ko et al., 2019).
Importantly, CDK5 is not the only target of (S)-CR8. Both roscovitine and CR8 enantiomers inhibit several CDKs (CDK1, CDK2, CDK5, CDK7, and CDK9) with similar potencies, as well as enzymes from the casein kinase 1 (CK1α1, CK1γ2, CK1γ3, CK1ε, and CK1δ), cdc2 like kinase (CLK1, CLK2, and CLK4) and dual specificity, tyrosine phosphorylation-regulated kinase (DYRK1A and DYRK1B) families (Bettayeb et al., 2008; Delehouze et al., 2014). It is therefore possible that the effect of (S)-CR8 on ethanol self-administration in dependent rats was mediated by the inhibition of a non-CDK5 kinase, which would be consistent with the lack of changes in CDK5 and p35 abundance.
We explored the possibility that this kinase would be a CDK other than CDK5 (e.g., CDK1, CDK2, CDK7, or CDK9). We detected an increase in the phosphorylation of some, but not all, CDK substrates in the BLA of dependent rats compared to ethanol-naïve rats, with intermediate levels in non-dependent rats. A similar trend was observed in the CeA but did not reach significance. The identity of the different proteins labeled by the antibody mix is unknown and future studies will be needed to determine which CDK(s) may be upregulated in the amygdala of dependent rats. We did not find evidence in the literature for an effect of chronic ethanol exposure on the expression or activity of CDK1, CDK2, CDK7, or CDK9 in the brain.
(S)-CR8 targets other than CDKs may also be implicated. Systemic administration of a CK1ε/δ inhibitor was previously shown to reduce ethanol consumption in long-term ethanol-drinking rats upon re-exposure to ethanol after deprivation (alcohol deprivation effect, Perreau-Lenz et al., 2012). Furthermore, CK1γ3, CK1ε, CLK 1, CLK4, and DYRK1A were downregulated, while DYRK1B was upregulated, in the amygdala and frontal cortex of alcoholic subjects (Ponomarev et al., 2012). CK1δ was also upregulated in the frontal cortex of mice subjected to chronic ethanol drinking (Osterndorff-Kahanek et al., 2013). Based on the existing literature, we can therefore speculate that increased activity of CK1δ or DYRK1B may contribute to the escalation of ethanol self-administration in CIE-exposed rats, such that the effect of (S)-CR8 on ethanol intake may have resulted from the inhibition of one (or both) of these enzymes.
Altogether, our study shows that systemic administration of (S)-CR8 selectively reduces excessive ethanol intake in a rat model of ethanol dependence, without affecting the intake of their non-dependent counterparts. Our molecular analysis does not support the hypothesis that this effect would be mediated by the inhibition of upregulated CDK5 activity in the extended amygdala or prefrontal cortex but suggests that increased activity of other CDKs in the BLA may contribute to intake escalation in dependent rats. Furthermore, the target profile of (S)-CR8 along with previous pharmacological and gene expression studies suggest that the behavioral effect of (S)-CR8 could be mediated by CK1δ or DYRK1B inhibition. Future studies using selective inhibitors of these enzymes will be needed to test this hypothesis.
Supplementary Material
Acknowledgements
This work was supported by the following grants from the National Institute on Alcohol Abuse and Alcoholism: AA006420 (CC and OG), AA007456 (SPG), AA024198 (CC), AA020608 (OG), and AA026685 (CC). LLGC is supported by a postdoctoral fellowship from the Research Foundation – Flanders.
Footnotes
Conflict of interest
None
References
- Bajo M, Montgomery SE, Cates LN, Nadav T, Delucchi AM, Cheng K, Yin H, Crawford EF, Roberts AJ, Roberto M (2016) Evaluation of TLR4 Inhibitor, T5342126, in Modulation of Ethanol-Drinking Behavior in Alcohol-Dependent Mice. Alcohol Alcohol 51(5): 541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettayeb K, Baunbaek D, Delehouze C, Loaec N, Hole AJ, Baumli S, Endicott JA, Douc-Rasy S, Benard J, Oumata N, Galons H, Meijer L (2010) CDK Inhibitors Roscovitine and CR8 Trigger Mcl-1 Down-Regulation and Apoptotic Cell Death in Neuroblastoma Cells. Genes Cancer 1(4): 369–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettayeb K, Oumata N, Echalier A, Ferandin Y, Endicott JA, Galons H, Meijer L (2008) CR8, a potent and selective, roscovitine-derived inhibitor of cyclin-dependent kinases. Oncogene 27(44): 5797–5807. [DOI] [PubMed] [Google Scholar]
- Bibb JA, Chen J, Taylor JR, Svenningsson P, Nishi A, Snyder GL, Yan Z, Sagawa ZK, Ouimet CC, Nairn AC, Nestler EJ, Greengard P (2001) Effects of chronic exposure to cocaine are regulated by the neuronal protein Cdk5. Nature 410(6826): 376–380. [DOI] [PubMed] [Google Scholar]
- Bibb JA, Snyder GL, Nishi A, Yan Z, Meijer L, Fienberg AA, Tsai LH, Kwon YT, Girault JA, Czernik AJ, Huganir RL, Hemmings HC Jr., Nairn AC, Greengard P (1999) Phosphorylation of DARPP-32 by Cdk5 modulates dopamine signalling in neurons. Nature 402(6762): 669–671. [DOI] [PubMed] [Google Scholar]
- Camp MC, Mayfield RD, McCracken M, McCracken L, Alcantara AA (2006) Neuroadaptations of Cdk5 in cholinergic interneurons of the nucleus accumbens and prefrontal cortex of inbred alcohol-preferring rats following voluntary alcohol drinking. Alcohol Clin Exp Res 30(8): 1322–1335. [DOI] [PubMed] [Google Scholar]
- Chen PC, Chen JC (2005) Enhanced Cdk5 activity and p35 translocation in the ventral striatum of acute and chronic methamphetamine-treated rats. Neuropsychopharmacology 30(3): 538–549. [DOI] [PubMed] [Google Scholar]
- Chen ZG, Liu X, Wang W, Geng F, Gao J, Gan CL, Chai JR, He L, Hu G, Zhou H, Liu JG (2017) Dissociative role for dorsal hippocampus in mediating heroin self-administration and relapse through CDK5 and RhoB signaling revealed by proteomic analysis. Addict Biol 22(6): 1731–1742. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Huang CCY, Ma T, Wei X, Wang X, Lu J, Wang J (2017) Distinct Synaptic Strengthening of the Striatal Direct and Indirect Pathways Drives Alcohol Consumption. Biol Psychiatry 81(11): 918–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chergui K, Svenningsson P, Greengard P (2004) Cyclin-dependent kinase 5 regulates dopaminergic and glutamatergic transmission in the striatum. Proc Natl Acad Sci U S A 101(7): 2191–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contet C, Filliol D, Matifas A, Kieffer BL (2008) Morphine-induced analgesic tolerance, locomotor sensitization and physical dependence do not require modification of mu opioid receptor, cdk5 and adenylate cyclase activity. Neuropharmacology 54(3): 475–486. [DOI] [PubMed] [Google Scholar]
- Contet C, Gardon O, Filliol D, Becker JA, Koob GF, Kieffer BL (2011) Identification of genes regulated in the mouse extended amygdala by excessive ethanol drinking associated with dependence. Addict Biol 16(4): 615–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz JC, Tseng HC, Goldman JA, Shih H, Tsai LH (2003) Aberrant Cdk5 activation by p25 triggers pathological events leading to neurodegeneration and neurofibrillary tangles. Neuron 40(3): 471–483. [DOI] [PubMed] [Google Scholar]
- Delehouze C, Godl K, Loaec N, Bruyere C, Desban N, Oumata N, Galons H, Roumeliotis TI, Giannopoulou EG, Grenet J, Twitchell D, Lahti J, Mouchet N, Galibert MD, Garbis SD, Meijer L (2014) CDK/CK1 inhibitors roscovitine and CR8 downregulate amplified MYCN in neuroblastoma cells. Oncogene 33(50): 5675–5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhavan R, Tsai LH (2001) A decade of CDK5. Nat Rev Mol Cell Biol 2(10): 749–759. [DOI] [PubMed] [Google Scholar]
- Ferrer-Alcon M, La Harpe R, Guimon J, Garcia-Sevilla JA (2003) Downregulation of neuronal cdk5/p35 in opioid addicts and opiate-treated rats: relation to neurofilament phosphorylation. Neuropsychopharmacology 28(5): 947–955. [DOI] [PubMed] [Google Scholar]
- Ferreras S, Fernandez G, Danelon V, Pisano MV, Masseroni L, Chapleau CA, Krapacher FA, Mlewski EC, Masco DH, Arias C, Pozzo-Miller L, Paglini MG (2017) Cdk5 Is Essential for Amphetamine to Increase Dendritic Spine Density in Hippocampal Pyramidal Neurons. Front Cell Neurosci 11: 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Pang PT, Lu B, Tsai LH (2005) Opposing roles of transient and prolonged expression of p25 in synaptic plasticity and hippocampus-dependent memory. Neuron 48(5): 825–838. [DOI] [PubMed] [Google Scholar]
- Ghosh R, Gilda JE, Gomes AV (2014) The necessity of and strategies for improving confidence in the accuracy of western blots. Expert Rev Proteomics 11(5): 549–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilda JE, Gomes AV (2013) Stain-Free total protein staining is a superior loading control to beta-actin for Western blots. Anal Biochem 440(2): 186–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Cole M, Koob GF (2008) Vapor inhalation of alcohol in rats. Curr Protoc Neurosci Chapter 9: Unit 9 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Smith AD, Cole M, Weiss F, Koob GF, Richardson HN (2009) Operant behavior and alcohol levels in blood and brain of alcohol-dependent rats. Alcohol Clin Exp Res 33(12): 2113–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goggin SL, Caldwell KK, Cunningham LA, Allan AM (2014) Prenatal alcohol exposure alters p35, CDK5 and GSK3beta in the medial frontal cortex and hippocampus of adolescent mice. Toxicol Rep 1: 544–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller EA, Hamilton PJ, Burek DD, Lombroso SI, Pena CJ, Neve RL, Nestler EJ (2016) Targeted Epigenetic Remodeling of the Cdk5 Gene in Nucleus Accumbens Regulates Cocaine- and Stress-Evoked Behavior. J Neurosci 36(17): 4690–4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope BT, Crombag HS, Jedynak JP, Wise RA (2005) Neuroadaptations of total levels of adenylate cyclase, protein kinase A, tyrosine hydroxylase, cdk5 and neurofilaments in the nucleus accumbens and ventral tegmental area do not correlate with expression of sensitized or tolerant locomotor responses to cocaine. J Neurochem 92(3): 536–545. [DOI] [PubMed] [Google Scholar]
- Humbert S, Dhavan R, Tsai L (2000) p39 activates cdk5 in neurons, and is associated with the actin cytoskeleton. J Cell Sci 113 ( Pt 6): 975–983. [DOI] [PubMed] [Google Scholar]
- Kabadi SV, Stoica BA, Hanscom M, Loane DJ, Kharebava G, Ii MG Murray, Cabatbat RM, Faden AI (2012) CR8, a selective and potent CDK inhibitor, provides neuroprotection in experimental traumatic brain injury. Neurotherapeutics 9(2): 405–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawauchi T, Chihama K, Nabeshima Y, Hoshino M (2006) Cdk5 phosphorylates and stabilizes p27kip1 contributing to actin organization and cortical neuronal migration. Nat Cell Biol 8(1): 17–26. [DOI] [PubMed] [Google Scholar]
- Ko MJ, Mulia GE, van Rijn RM (2019) Commonly Used Anesthesia/Euthanasia Methods for Brain Collection Differentially Impact MAPK Activity in Male and Female C57BL/6 Mice. Front Cell Neurosci 13: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohtala S, Theilmann W, Suomi T, Wigren HK, Porkka-Heiskanen T, Elo LL, Rokka A, Rantamaki T (2016) Brief Isoflurane Anesthesia Produces Prominent Phosphoproteomic Changes in the Adult Mouse Hippocampus. ACS Chem Neurosci 7(6): 749–756. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND (2016) Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry 3(8): 760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazenka MF, Kang M, De DD, Selley DE, Sim-Selley LJ (2017) Delta(9)-Tetrahydrocannabinol Experience Influences DeltaFosB and Downstream Gene Expression in Prefrontal Cortex. Cannabis Cannabinoid Res 2(1): 224–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew J, Huang QQ, Qi Z, Winkfein RJ, Aebersold R, Hunt T, Wang JH (1994) A brain-specific activator of cyclin-dependent kinase 5. Nature 371(6496): 423–426. [DOI] [PubMed] [Google Scholar]
- Liu F, Laguesse S, Legastelois R, Morisot N, Hamida S Ben, Ron D (2017) mTORC1-dependent translation of collapsin response mediator protein-2 drives neuroadaptations underlying excessive alcohol-drinking behaviors. Mol Psychiatry 22(1): 89–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Shaham Y, Hope BT (2003) Molecular neuroadaptations in the accumbens and ventral tegmental area during the first 90 days of forced abstinence from cocaine self-administration in rats. J Neurochem 85(6): 1604–1613. [DOI] [PubMed] [Google Scholar]
- McClue SJ, Stuart I (2008) Metabolism of the trisubstituted purine cyclin-dependent kinase inhibitor seliciclib (R-roscovitine) in vitro and in vivo. Drug Metab Dispos 36(3): 561–570. [DOI] [PubMed] [Google Scholar]
- Meyer DA, Richer E, Benkovic SA, Hayashi K, Kansy JW, Hale CF, Moy LY, Kim Y, O’Callaghan JP, Tsai LH, Greengard P, Nairn AC, Cowan CW, Miller DB, Antich P, Bibb JA (2008) Striatal dysregulation of Cdk5 alters locomotor responses to cocaine, motor learning, and dendritic morphology. Proc Natl Acad Sci U S A 105(47): 18561–18566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlewski EC, Krapacher FA, Ferreras S, Paglini G (2008) Transient enhanced expression of Cdk5 activator p25 after acute and chronic d-amphetamine administration. Ann N Y Acad Sci 1139: 89–102. [DOI] [PubMed] [Google Scholar]
- Morabito MA, Sheng M, Tsai LH (2004) Cyclin-dependent kinase 5 phosphorylates the N-terminal domain of the postsynaptic density protein PSD-95 in neurons. J Neurosci 24(4): 865–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan DO (1997) Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu Rev Cell Dev Biol 13: 261–291. [DOI] [PubMed] [Google Scholar]
- Moritz CP (2017) Tubulin or Not Tubulin: Heading Toward Total Protein Staining as Loading Control in Western Blots. Proteomics 17(20). [DOI] [PubMed] [Google Scholar]
- Neasta J, Hamida S Ben, Yowell QV, Carnicella S, Ron D (2011) AKT signaling pathway in the nucleus accumbens mediates excessive alcohol drinking behaviors. Biol Psychiatry 70(6): 575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolic M, Chou MM, Lu W, Mayer BJ, Tsai LH (1998) The p35/Cdk5 kinase is a neuron-specific Rac effector that inhibits Pak1 activity. Nature 395(6698): 194–198. [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Bibb JA, Nestler EJ, Ouimet CC, Taylor JR, Greengard P (2003) Cocaine-induced proliferation of dendritic spines in nucleus accumbens is dependent on the activity of cyclin-dependent kinase-5. Neuroscience 116(1): 19–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell LE, Roberts AJ, Smith RT, Koob GF (2004) Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res 28(11): 1676–1682. [DOI] [PubMed] [Google Scholar]
- Osterndorff-Kahanek E, Ponomarev I, Blednov YA, Harris RA (2013) Gene expression in brain and liver produced by three different regimens of alcohol consumption in mice: comparison with immune activation. PLoS One 8(3): e59870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Do Couto BR, Alfonso-Loeches S, Aguilar MA, Rodriguez-Arias M, Guerri C (2012) Changes in histone acetylation in the prefrontal cortex of ethanol-exposed adolescent rats are associated with ethanol-induced place conditioning. Neuropharmacology 62(7): 2309–2319. [DOI] [PubMed] [Google Scholar]
- Patrick GN, Zukerberg L, Nikolic M, de la Monte S, Dikkes P, Tsai LH (1999) Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature 402(6762): 615–622. [DOI] [PubMed] [Google Scholar]
- Perreau-Lenz S, Vengeliene V, Noori HR, Merlo-Pich EV, Corsi MA, Corti C, Spanagel R (2012) Inhibition of the casein-kinase-1-epsilon/delta/ prevents relapse-like alcohol drinking. Neuropsychopharmacology 37(9): 2121–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponomarev I, Wang S, Zhang L, Harris RA, Mayfield RD (2012) Gene coexpression networks in human brain identify epigenetic modifications in alcohol dependence. J Neurosci 32(5): 1884–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajgopal Y, Vemuri MC (2001) Ethanol induced changes in cyclin-dependent kinase-5 activity and its activators, P35, P67 (Munc-18) in rat brain. Neurosci Lett 308(3): 173–176. [DOI] [PubMed] [Google Scholar]
- Rashid T, Banerjee M, Nikolic M (2001) Phosphorylation of Pak1 by the p35/Cdk5 kinase affects neuronal morphology. J Biol Chem 276(52): 49043–49052. [DOI] [PubMed] [Google Scholar]
- Sallam H, El-Serafi I, Meijer L, Hassan M (2013) Pharmacokinetics and biodistribution of the cyclin-dependent kinase inhibitor -CR8- in mice. BMC Pharmacol Toxicol 14: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Alavez M, Nguyen W, Mori S, Wills DN, Otero D, Ehlers CL, Conti B (2019) Time course of microglia activation and brain and blood cytokine/chemokine levels following chronic ethanol exposure and protracted withdrawal in rats. Alcohol 76: 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiwell AP, Reveron ME, Duvauchelle CL (2007) Increased accumbens Cdk5 expression in rats after short-access to self-administered cocaine, but not after long-access sessions. Neurosci Lett 417(1): 100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Ohshima T, Cho A, Sreenath T, Iadarola MJ, Pant HC, Kim Y, Nairn AC, Brady RO, Greengard P, Kulkarni AB (2005) Increased activity of cyclin-dependent kinase 5 leads to attenuation of cocaine-mediated dopamine signaling. Proc Natl Acad Sci U S A 102(5): 1737–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JR, Lynch WJ, Sanchez H, Olausson P, Nestler EJ, Bibb JA (2007) Inhibition of Cdk5 in the nucleus accumbens enhances the locomotor-activating and incentive-motivational effects of cocaine. Proc Natl Acad Sci U S A 104(10): 4147–4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SC, Berkelman T, Yadav G, Hammond M (2013) A defined methodology for reliable quantification of Western blot data. Mol Biotechnol 55(3): 217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai LH, Delalle I, Caviness VS Jr., Chae T, Harlow E (1994) p35 is a neural-specific regulatory subunit of cyclin-dependent kinase 5. Nature 371(6496): 419–423. [DOI] [PubMed] [Google Scholar]
- Um M, Whitt ZT, Revilla R, Hunton T, Cyders MA (2019) Shared Neural Correlates Underlying Addictive Disorders and Negative Urgency. Brain Sci 9(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vaart A, Meng X, Bowers MS, Batman AM, Aliev F, Farris SP, Hill JS, Green TA, Dick D, Wolstenholme JT, Miles MF, Consortium C (2018) Glycogen synthase kinase 3 beta regulates ethanol consumption and is a risk factor for alcohol dependence. Neuropsychopharmacology [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welinder C, Ekblad L (2011) Coomassie staining as loading control in Western blot analysis. J Proteome Res 10(3): 1416–1419. [DOI] [PubMed] [Google Scholar]
- Wu J, Zhao Z, Sabirzhanov B, Stoica BA, Kumar A, Luo T, Skovira J, Faden AI (2014) Spinal cord injury causes brain inflammation associated with cognitive and affective changes: role of cell cycle pathways. J Neurosci 34(33): 10989–11006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Li S, Wang W, Xu C, Liang S, Liu M, Hao W, Zhang R (2015) Beneficial effects of polydatin on learning and memory in rats with chronic ethanol exposure. Int J Clin Exp Pathol 8(9): 11116–11123. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.