Abstract
Background.
Among the neurological consequences of alcoholism is peripheral neuropathy. Relative to HIV or diabetes-related neuropathies, neuropathy associated with Alcohol Use Disorders (AUD) is understudied. In both the diabetes and HIV literature, emerging evidence supports a CNS component to peripheral neuropathy.
Methods.
In seeking a central substrate for AUD-related neuropathy, the current study was conducted in 154 individuals with AUD (43 women, ages 21–74) and 99 healthy controls (41 women, ages 21–77) and explored subjective symptoms (self-report) and objective signs (perception of vibration, deep tendon ankle reflex, position sense, 2-point discrimination) of neuropathy separately. In addition to regional brain volumes, risk factors for AUD-related neuropathy, including age, sex, total lifetime ethanol consumed, nutritional indices (i.e., thiamine, folate), and measures of liver integrity (i.e., γ-glutamyl-transferase) were evaluated.
Results.
The AUD group described more subjective symptoms of neuropathy and were more frequently impaired on bilateral perception of vibration.
From 5 correlates, the number of AUD-related seizures was most significantly associated with subjective symptoms of neuropathy. There were 15 correlates of impaired perception of vibration among the AUD participants: of these, age and volume of frontal precentral cortex were the most robust predictors.
Conclusions.
This study supports CNS involvement in objective signs of neuropathy in AUD.
Keywords: magnetic resonance imaging (MRI), vibration, peripheral neuropathy, volume, gray matter
INTRODUCTION
Estimates of the prevalence of peripheral neuropathy associated with chronic alcohol consumption vary from 9 to 76% (Maiya and Messing, 2014, Julian et al., 2018). Symptoms, typically beginning in the feet, are described as numbness, aching, or burning (Zambelis et al., 2005, Sadowski and Houck, 2019, Koike et al., 2003). Signs of neuropathy in Alcohol Use Disorders (AUD) include bilateral impairment of sensation (heat, pain, vibration), proprioception, and deep tendon ankle reflexes (Koike et al., 2001, Sadowski and Houck, 2019, Ammendola et al., 2001). Both nerve conduction (Ammendola et al., 2000, Koike et al., 2001, D’Amour et al., 2000) and electromyographic (Behse and Buchthal, 1977, Scholz et al., 1986) studies support the diagnoses of axonal neuropathy in AUD. Factors reported to contribute to peripheral neuropathy among individuals with AUD include total lifetime dose and duration of alcohol intake (Vittadini et al., 2001, Monforte et al., 1995, Julian et al., 2018), nutritional deficiencies for thiamine or folate (Chopra and Tiwari, 2012, Mellion et al., 2011, Sanvisens et al., 2017, Hamel and Logigian, 2018), macrocytosis (Zambelis et al., 2005), or liver disease (Agelink et al., 1998, Milovanovic et al., 2009, Gane et al., 2004).
Recent evidence supports Central Nervous System (CNS) involvement in peripheral neuropathy (e.g., Tesfaye et al., 2016, Zahr et al., 2019, Keltner et al., 2017, Pfefferbaum et al., 2009). Diabetes with neuropathy relative to diabetes without neuropathy is associated with compromise in volume of cingulate cortex (Boland et al., 2014), implicated in the affective processing of pain (Coppieters et al., 2017, Buckalew et al., 2008, Absinta et al., 2012). Similarly, distal neuropathic pain symptoms in HIV are associated with deficits in posterior cingulate volume (Keltner et al., 2017).
The current study aimed to determine correlates of subjective symptoms or “objective” signs [perception of vibration (great toes), deep tendon ankle reflexes, position sense (great toes), and 2-point discrimination (feet)] of AUD-related peripheral neuropathy. Relations between measures of neuropathy and demographic (e.g., age, sex), alcohol-related (e.g., total lifetime ethanol consumed, duration of AUD diagnosis), laboratory (e.g., thiamine, folate, γ-glutamyl-transferase, mean corpuscular volume), balance (i.e., ataxia), and regional brain volume variables were evaluated. We hypothesized that subjective symptoms of AUD-neuropathy would be associated with smaller cingulate volume, while “objective” signs would be associated with smaller somatosensory cortex volume (Kim and Choi-Kwon, 1996, Apkarian et al., 1999, Gelnar et al., 1999, Soros et al., 2007, Scholvinck et al., 2008).
MATERIALS AND METHODS
Participants
The current study sample included 99 controls (41 women, 58 men, 50.9±13.3 years) and 154 participants with AUD (43 women, 111 men, 49.8±10.5 years) with signed consent provided in the presence of a staff member. This study, approved by the Institutional Review Boards of SRI International and Stanford University, included data that were collected between January 2007 and March 2018.
Individuals with AUD were recruited from local in- and out- patient treatment centers. Healthy, control participants were recruited from the local community via flyers and online ads (e.g., Nextdoor). The Structured Clinical Interview for DSM-IV (SCID) was used to screen study participants (First et al., 1998). Also collected were structured health questionnaires including Activities of Daily Living questionnaires (Pfeffer et al., 1982), and a semi-structured timeline follow-back to quantify lifetime alcohol consumption (Skinner and Sheu, 1982). Of note, although subjects were screened with DSM-IV, which differentiates alcohol abuse and alcohol dependence, the DSM-V term “AUD” is used herein inclusive of both diagnoses.
Subjects were excluded if they had a significant history of medical (e.g., uncontrolled diabetes), psychiatric (i.e., schizophrenia), or neurological (e.g., stroke) disorders, or recent (i.e., within 3 months) substance dependence.
Evaluation of Peripheral Neuropathy
Laboratory personnel, trained and calibrated to assess neuropathy, included 4 individuals (among them author NMZ). Subjective neuropathy (i.e., self-report) was documented after participants were questioned regarding the bilateral severity – in feet or legs – of 1) pain, aching, or burning; 2) pins and needles; or 3) numbness. Subjective neuropathy scores were 0 for absence of symptoms and 1 for symptoms ranging from mild to severe.
While we acknowledge that the gold standard for objective measurement of neuropathy requires electromyography or nerve conduction testing, the term “objective” is used herein to differentiate from self-report of subjective symptoms of neuropathy. The four “objective” signs of lower-limb neuropathy evaluated included perception of vibration (right or left great toe assessed separately) where 0 = normal, 1 = bilateral impairment (perception of vibration <10 seconds); deep tendon ankle reflexes (right or left ankle assessed separately) where 0 = normal, 1 = bilateral impairment (hypoactive ankle reflex); position sense (right or left great toe assessed separately) where 0 = normal, 1 = bilateral impairment (≥1 errors during evaluation); and 2-point discrimination (right or left soles of feet assessed separately): a 3-point aesthesiometer was used to determine minimal distance detected between 2 points. Raw scores were transformed to age-corrected Z-scores where 0 = normal, 1 = bilateral impairment (≥2 standard deviations from Z-scored mean of controls).
Blood Measures
Samples (~40cc) were extracted and evaluated for complete blood count with differential (whole blood), a comprehensive metabolic panel including liver enzymes (serum), and hepatitis C virus (HCV) screening (serum) by Quest Diagnostics. In addition to standard laboratory testing at Quest Diagnostics, we include a whole blood measure of vitamin B1 (thiamine) conducted by ARUP Laboratories.
Quantitative Ataxia Testing
An ataxia battery assessed balance with eyes open and eyes closed. Age-corrected Z scores of each subtest were calculated separately for eyes open and eyes closed conditions (Sullivan et al., 2000).
MRI Acquisition and Analysis
Image Acquisition
MRI data of the brain were acquired on a 3 Tesla whole body MR system (GE Healthcare, Waukesha, WI) using an 8-channel array head coil. T1-SPoiled Gradient Recall (SPGR) images were used for regional brain volume analyses. Axial SPGR acquisitions used the following parameters: repetition time (TR)=6.55/5.92ms, echo time (TE)=1.56/1.93ms, inversion time (TI)=300ms, 256×256 matrix, field of view (FOV)=24cm, 24 slices at a thickness of 1.25 mm. Imaging data were available for 74/99 of the control and 125/154 AUD participants.
Image Processing
MRI data were processed using a pipeline developed in our laboratory (Pfefferbaum et al., 2018). Processing began with noise removal (Coupe et al., 2008). Gray matter, white matter, and cerebrospinal fluid tissue was segmented using average intensity maps that were rigidly aligned via ANTS (Avants et al., 2008) then segmented via Atropos (Avants et al., 2011). Regions of interest were parcellated based on the SRI24 atlas (Rohlfing et al., 2010). Volume was quantified for 23 bilateral supratentorial, 5 bilateral infratentorial (superior and inferior cerebellum, cerebellar white matter, vermis, pons), and 7 bilateral subcortical (amygdala, caudate, putamen, pallidum, thalamus, parahippocampus, hippocampus) regions, corpus callosum, third and lateral ventricles. Regional brain volumes were adjusted for age and supratentorial brain volume (Pfefferbaum et al., 2018).
Statistical Analysis
Statistics were performed using JMP Pro 14.1.0 (SAS Institute Inc. 2016). Group differences in demographic measures were evaluated by t-test or Pearson’s Chi-square tests. Two-group differences in subjective and “objective” measures of neuropathy were assessed using Pearson’s Chi-square tests. In the AUD group only, relationships between neuropathy measures and demographic, alcohol-related, laboratory, balance, or brain volume variables were assessed using non-parametric Wilcoxon rank sums tests. A nominal logistic regression was conducted to determine the percent of neuropathy (subjective or “objective”) explained by significantly associated variables. Finally, a data-driven approach entered scores on neuropathy (subjective or “objective”) and variables associated with these measures into a JMP-based cluster analysis.
RESULTS
Study Participants
Demographic data, presented in Table 1, demonstrates that AUD and healthy control participants were matched with respect to age, handedness, and body mass index (BMI). The AUD relative to the control group had proportionally more men and African-Americans, lower education and socioeconomic status (SES) (Hollingshead, 1975), more smokers, more depressive symptoms [as assessed with the Beck Depression Inventory-II (BDI-II) (Beck et al., (1996) )], and lower scores on the National Adult Reading Test (NART) (Nelson, 1982) and the Dementia Rating Scale (DRS) (Mattis, 1998).
Table 1.
Characteristics of the study groups: mean±SD / frequency count
| Control (n=99) | AUD (n=154) | p-value* | |
|---|---|---|---|
| N (men / women) | 58 / 41 | 111 / 43 | 0.03 |
| Age (years) | 50.9 ± 13.3 | 49.8 ± 10.5 | 0.50 |
| Handedness (Right / Center) | 87 / 12 | 133 / 21 | 0.73 |
| Ethnicity♩ (Caucasian / African American / Other) | 50 / 24 / 25 | 88 / 60 / 5 | < 0.0001 |
| Body Mass Index | 26.4 ± 4.7 | 27.2 ± 4.6 | 0.18 |
| Education (years) | 15.8 ± 2.5 | 13.1 ± 2.2 | < 0.0001 |
| Socioeconomic Status# | 28.0 ± 13.3 | 43.4 ± 14.8 | < 0.0001 |
| Beck Depression Index (BDI) | 3.1 ± 3.8 | 10.6 ± 8.3 | < 0.0001 |
| National Adult Reading Test (NART) | 112.0 ± 8.4 | 106.3 ± 9.1 | 0.0005 |
| Dementia Rating Scale (DRS) | 139.8 ± 2.6 | 136.2 ± 5.0 | < 0.0001 |
| AUD onset age | - | 24.5 ± 9.2 | - |
| Months since last drink | - | 8.1 ± 21.2 | - |
| Lifetime alcohol consumption (kg) | 35.8 ± 58.5 | 1301.6 ± 1090.8 | < 0.0001 |
| Smoker (never / past / current) | 89 / 2 / 5 | 45 / 25 / 83 | < 0.0001 |
| Nicotine (daily) | 0.9 ± 2.7 | 6.3 ± 7.1 | < 0.0001 |
t-tests used on continuous variables (e.g., age); χ2 used on nominal variables (e.g., handedness)
self-defined, where Other = Native American, Asian, or Islander
lower score = higher status
A subset of the results from standard laboratory testing is presented in Supplementary Table 1. General markers of nutrition - blood urea nitrogen (BUN), creatinine, albumin, folate, and vitamin B12 - were not different between the 2 groups. Prealbumin levels were moderately lower in the AUD relative to the control group (p=.04). Whole blood thiamine levels were slightly higher in the AUD than control group (p=.05).
Neuropathy Measures
The AUD relative to the control group reported a greater incidence of symptoms of neuropathy (χ2=7.2 p=.007) [AUD group: normal n=134, impaired n=20 (mild n=8, moderate n=7, severe n=5); control group: normal n=96, impaired n=3 (mild n=2, moderate n=1)] (Figure 1a). Neuropathy was described by 10 AUD participants as pain, aching, or burning; by 4 as numbness; by 3 as pain, aching, and burning + numbness; by 2 as numbness + pins and needles; and 1 AUD participant reported all 3 symptoms (i.e., pain, aching, and burning; pins and needles; numbness).
Figure 1.
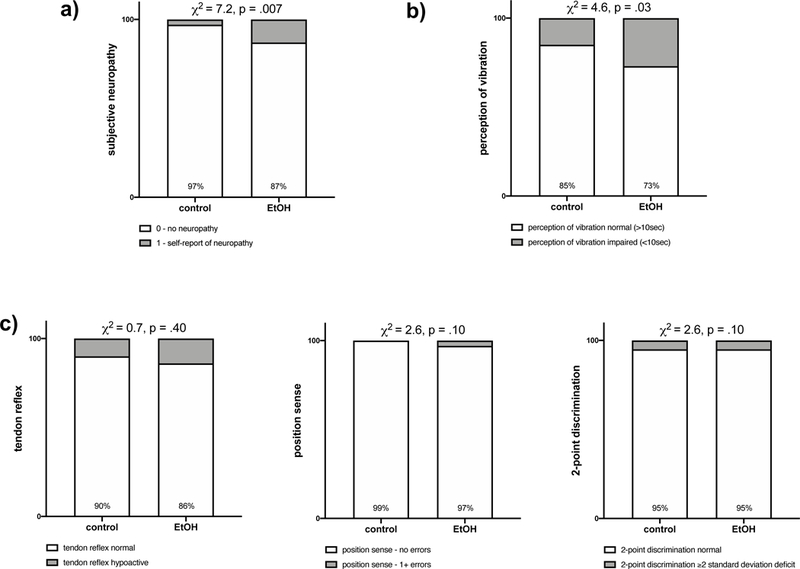
Percent of total healthy control (n=99) or AUD (n=154) group with a) no subjective symptoms (white) or bilateral subjective symptoms (gray) of neuropathy and no “objective” signs (white) or bilateral “objective” signs (gray) of impairment in b) perception of vibration, c) tendon reflex, position sense, and 2-point discrimination.
Among the “objective” neuropathy measures, only perception of vibration distinguished the two groups (χ2=4.6, p=.03) [AUD group: normal (>10 sec) n=113; impaired (<10 sec) n=41; control group: normal (>10 sec) n=84; impaired (<10 sec) n=15] (Figure 1b). Deep tendon ankle reflex (χ2=0.7, p=.40), position sense (χ2=2.6, p=.11), and 2-point discrimination (χ2=0.03, p=.85) scores were not different between the 2 groups (Figure 1c).
To ensure that neuropathy was not related to recency of drinking, AUD participants were triaged into groups based on recency of abstinence: current drinkers were defined as those with under 1 month of abstinence; recent drinkers were defined as those with between 2 and 12 months of recovery; past drinkers were defined as those with over 1 year of sobriety. Of 154 alcoholics, 58 were current drinkers, 81 were recent drinkers, and 14 were past drinkers (with an average of 61.1 ± 3.4 months of sobriety ranging from 13 to 154 months). Chi-squared analyses for abstinence category by subjective neuropathy (χ2=3.2, p=.20: current drinkers, neuropathy absent n=48, neuropathy present n=10; recent drinkers, neuropathy absent n=71, neuropathy present n=10; past drinkers, neuropathy absent n=15, neuropathy present n=0) and “objective” neuropathy (i.e., perception of vibration) (χ2=.02, p=.98: current drinkers, neuropathy absent n=43, neuropathy present n=15; recent drinkers, neuropathy absent n=59, neuropathy present n=22; past drinkers, neuropathy absent n=11, neuropathy present n=4) were not significantly different between groups.
Relationships
Subjective Neuropathy
Among the AUD group, more frequent self-report of symptoms of neuropathy was associated with more frequent impairment in bilateral perception of vibration (χ2=6.4, p=.01, Figure 2a). Self-report of symptoms of neuropathy were not associated with any blood markers. Also contrary to our hypothesis, subjective symptoms of neuropathy in the AUD group did not correlate with cingulate volume: anterior (χ2=0.8, p=.37) or mid-posterior (χ2=1.5, p=.22). Instead, symptoms of neuropathy were associated with a greater frequency of AUD-related seizure history (χ2=15.6, p<.0001) (Figure 2b); impairment in activities of daily living: physical (χ2=4.7 p=.03) & instrumental (χ2=6.2 p=.01); worse performance on ataxia with eyes open (χ2=3.9, p=.05), and smaller frontal inferior volume (χ2=6.6, p=.01) (Supplementary Figure 1). A nominal logistic regression including all 5 correlates was significant (χ2=25.6, p<.0001), explained 29.4% of the variance in neuropathy symptoms, and was driven by a history of AUD-related seizures (χ2=8.2, p=.004).
Figure 2.
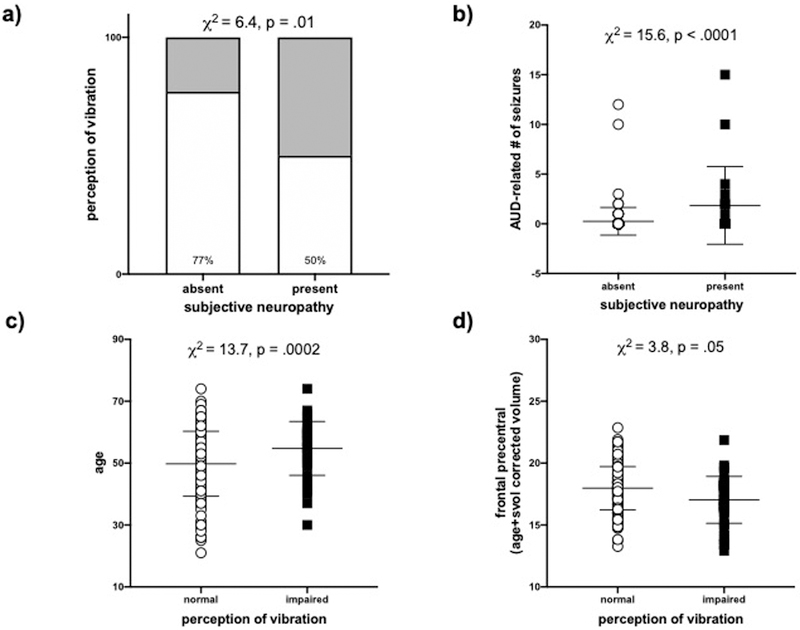
a) Association between subjective symptoms and the “objective” sign (i.e., perception of vibration) of neuropathy. b) Association between subjective symptoms of neuropathy and AUD-related seizures. Associations between the “objective” sign of neuropathy (perception of vibration) and c) age or d) frontal precentral volume (age and supratentorial volume (svol) corrected).
“Objective” Neuropathy
In the AUD group, impaired perception of vibration was associated with older age (χ2=13.7, p=.0002) (Figure 2c); psychosocial stressors (χ2=12.2, p=.0005); depressive symptoms (χ2=7.3 p=.007); greater BMI (χ2=7.1, p=.008); impairments in activities of daily living: physical (χ2=6.4, p=.01) & instrumental (χ2=9.2, p=.002); worse performance on ataxia with eyes open (χ2=5.9, p=.02); nicotine dependence (χ2=3.8, p=.05); and self-report of high blood pressure in the past year (χ2=12.1, p=.0005) (Supplementary Figure 2). There were no relationships between perception of vibration and any blood marker. With respect to regional brain volumes, poorer vibration perception was not related to parietal post-central (i.e., somatosensory cortex) volume (χ2=0.4, p=.53), but did correlate with smaller frontal precentral (χ2=3.8, p=.05) (Figure 2d), frontal medial (χ2=7.3, p=.007), temporal superior (χ2=4.3, p=.04), cingulate mid-posterior (χ2=6.6, p=.01), thalamus (χ2=5.7, p=.02), and pons (χ2=7.2, p=.008) volumes (Supplementary Figure 3). A nominal logistic regression including all 15 correlates was significant (χ2=40.8, p=.0003), explained 50.7% of the variance in perception of vibration, and was driven by age (χ2=5.0, p=.02), psychosocial stressors (χ2=8.4, p=.004), nicotine dependence (χ2=4.8, p=.03), and frontal precentral volume (χ2=4.6, p=.03). These 4 correlates explained 21.6% of the variance in perception of vibration (χ2=30.0, p<.0001).
Cluster Analysis
Subjective neuropathy, perception of vibration, and their correlates [including age, BMI, BDI-II scores, smoking status, psychosocial stressors, self-report of high blood pressure in the past year, AUD-related seizure history, activities of daily living (physical & instrumental), ataxia, and volumes of frontal inferior, frontal precentral, frontal medial, temporal superior, cingulate mid-posterior, thalamus, and pons] were entered into a JMP-based cluster analysis. This data-driven approach assigned the 19 variables into 6 clusters (Table 2): Cluster 1 explained 9.8% of the overall variance and included perception of vibration, age, self-report of high blood pressure in the past year, and volumes of the frontal precentral and cingulate mid-posterior cortices. Cluster 3 explained 8.6% of the overall variance and included subjective neuropathy, number of AUD-related seizures, ataxia with eyes open, and BMI.
Table 2.
Cluster Summary
| Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | Cluster 5 | Cluster 6 | |
|---|---|---|---|---|---|---|
| total proportion of variation explained: | 9.8 | 9.2 | 8.9 | 8.6 | 8.0 | 6.6 |
| cluster members*: | age | BDI-II total | subjective neuropathy | temporal superior volume | pons | smoking status |
| perception of vibration | ADL instrumental | AUD-related seizures | frontal medial volume | thalamus | ADL physical | |
| frontal precentral volume | psychosocial stressors | ataxia (eyes open) | frontal inferior volume | |||
| high blood pressure | BMI | |||||
| cingulate mid-posterior volume | ||||||
listed in order of significance per cluster
DISCUSSION
In the current study, individuals with AUD were more likely than controls to report symptoms of neuropathy; these correlated with a greater frequency of impairment in an “objective” measure of neuropathy, perception of vibration. This relationship in AUD was not identified in HIV, where subjective symptoms and “objective” signs of neuropathy were dissociable (Zahr et al., 2019). This finding, along with impairment in only perception of vibration in AUD, whereas individuals infected with HIV showed deficits in deep tendon ankle reflexes, position sense, and 2-point discrimination, suggests differing pathogenic mechanisms for neuropathy in the 2 disorders.
This study did not identify relationships between general markers of nutrition, non-fasted glucose levels, or any other blood marker evaluated and either subjective or “objective” neuropathy. While there is evidence for peripheral neuropathy specifically associated with thiamine deficiency, most studies in alcoholic neuropathy do not report relationships with markers of nutritional status (e.g., Ammendola et al., 2000, Koike et al., 2001); available data suggest that the pathophysiology of thiamine-deficiency-related and alcoholism-related neuropathy is different (e.g., Chopra and Tiwari, 2012, Koike et al., 2003).
Symptoms of neuropathy in the AUD group correlated with 5 measures: an AUD-related seizure history, activities of daily living (physical & instrumental), ataxia with eyes open, and frontal inferior volume. Compromise in activities of daily living has previously been noted in patients living with diabetic (Le Floch et al., 2014) and chemotherapy-induced (Kanda et al., 2017) neuropathies. Our study of peripheral neuropathy in HIV found a correlation between symptoms of neuropathy and performance on ataxia with eyes closed (Zahr et al., 2019), (but see, Berner et al., 2017). These findings suggest that subjective symptoms of neuropathy in AUD contribute to impaired physical functioning, including deficits in balance and postural stability, as observed in diabetic neuropathy (cf., Riandini et al., 2018). A single study of diabetic retinopathy showed reduced gray matter density in frontal inferior gyrus (Wessels et al., 2006). The frontal inferior gyrus, an area that may be especially sensitive to reduced blood flow (Snowdon et al., 1997), shows reduced perfusion in AUD (Gansler et al., 2000).
AUD-related seizure history was highlighted by both the nominal logistic regression and the cluster-based analysis as relevant to symptoms of AUD-related neuropathy. In a previous study of 46 patients with alcoholic neuropathy, 11 (24%) had experienced seizures (Kemppainen et al., 1982). Beyond this report, there is little in the alcoholism-related literature associating seizures and peripheral neuropathy. By contrast, several reports on the neurological consequences of kidney disease report concomitant seizures and neuropathy (Jabbari and Vaziri, 2018, Baluarte, 2017, Mahoney and Arieff, 1982). Further analysis of the present dataset, however, did not provide evidence of kidney compromise (e.g., elevated creatinine or blood urea, or compromised estimated glomerular filtration rate) in the AUD group.
Previous studies suggest the presence of bilateral impairment of proprioception and deep tendon ankle reflexes in AUD (e.g., Koike et al., 2001, Sadowski and Houck, 2019, Ammendola et al., 2001). Findings in the current group of AUD subjects, however, show only impaired vibration sense. This comports with a recent literature review that reports individuals with AUD are more likely to show impaired vibration sense than altered proprioception (cf., Julian et al., 2018). Fifteen correlates of vibration sense were identified in the AUD group – including high blood pressure, smoking, and BMI, which have high odds ratio as risk factors for diabetic neuropathy (Tesfaye et al., 2016) – but not total lifetime ethanol dose – which a recent meta-analysis indicated is the most valid risk factor for the development of AUD-related peripheral neuropathy (Julian et al., 2018). Of the 15 variables, age and frontal precentral volume were underscored by both the nominal logistic regression and the cluster analysis as significant to vibration sense. Older age has often been reported as a risk factor for neuropathy, regardless of the etiology (Barrell and Smith, 2019, Liu et al., 2019, Brouwer et al., 2015, Agelink et al., 1998).
Brain regions that correlated with perception of vibration included frontal precentral, frontal medial, and temporal superior cortices; the cingulate mid-posterior cortex, implicated in pain perception, and previously reported as relevant to both diabetic and HIV-related neuropathies (Sugimine et al., 2016, Hsieh et al., 2015, Keltner et al., 2017); the thalamus, relevant to perception of pain (Geha and Apkarian, 2005, Peyron et al., 2000), and sometimes directly implicated in neuropathy (Selvarajah et al., 2011); and the pons, which has been reported in case studies of rare neuropathies (cf., Weinmann, 1967, Rossi et al., 1986). Consistent with posterior cingulate involvement, our recent study in HIV found relations between subjective symptoms of neuropathy and smaller parietal precuneus volume (Zahr et al., 2019); both regions support introspection and self-awareness (Kjaer et al., 2002, Jacob and Kostev, 2016).
With respect to frontal regions, hereditary neuropathy has been associated with white matter disturbances in frontal regions (Wang et al., 2015); both chemotherapy-induced (Boland et al., 2014, Nudelman et al., 2016) and diabetic (Li et al., 2018, Manor et al., 2012) neuropathies show altered frontal activity. Furthermore, frontoparietal regions are relevant to encoding vibrotactile stimuli (Woolgar and Zopf, 2017) (see also, Godde et al., 2010, Wu et al., 2018, Fassihi et al., 2017, Ku et al., 2007, Soros et al., 2007). Circuits including frontal and cingulate cortices, thalamus and pons are relevant for distinguishing the position and intensity of painful stimuli (Apkarian et al., 2005) and are commonly affected in alcoholism (Sullivan et al., 2018). Indeed, it is likely that perceptual awareness is the result of interactions between a distributed network of brain regions including primary sensory and higher-order cortical regions (Boly et al., 2007). In AUD, vibratory sense may have an altered network substrate, as brains of individuals with AUD show profound changes in functional connectivity (cf., Chanraud et al., 2013, Chanraud et al., 2011).
Although this study is adequately-powered, limitations include reliance on accurate self-reporting from individuals with AUD with respect to several of the relevant variables, including symptoms of neuropathy, AUD-related seizure history, and high blood pressure (cf., McKay, 1999, Heffernan, 2008). Furthermore, gold standards for the measurement of objective neuropathy such as nerve or muscle conduction studies, computerized quantitative sensory testing, or quantification of nerve fiber densities were not collected. Clinical assessment, as evaluated herein, is nevertheless often used in neuropathy studies (e.g., McArthur et al., 1998, Cherry et al., 2005). The lack of gold standard measures of neuropathy precludes ascribing the neuropathy observed in the current study to fiber gauge. In a study conducted in 98 alcohol-dependent subjects, 58% had neuropathy and of those, 25.5% had both large and small fiber neuropathy; 12.2% had exclusively small fiber and 20.4% exclusively large fiber neuropathy (Zambelis et al., 2005). Indeed, a study published in 1977 suggested that “at least 4 different types of fiber loss could be distinguished” in 27 patients with alcoholic neuropathy (Tackmann et al., 1977). A more recent study reported only small fiber involvement in alcohol-related peripheral neuropathy (Mellion et al., 2014). These discrepancies suggest that alcoholic neuropathy is not clearly understood and requires more attention as a debilitating consequence, potentially contributing to motor impairment, in alcoholism. The current results are endorsed, however, because the correlates identified herein have previously been identified in the diabetes and HIV neuropathy literature.
In conclusion, the present study supports the presence of both subjective and “objective” neuropathy in AUD, recapitulates a number of the risk factors identified in the diabetic literature as relevant to AUD-related neuropathy, and provides further evidence supporting CNS involvement in peripheral neuropathy.
Supplementary Material
Acknowledgments
Funding
This study was supported with grant funding from the National Institute of Alcohol Abuse and Alcoholism (NIAAA): AA010723, AA013521.
REFERENCES
- Absinta M, Rocca MA, Colombo B, Falini A, Comi G, Filippi M (2012) Selective decreased grey matter volume of the pain-matrix network in cluster headache. Cephalalgia : an international journal of headache 32:109–115. [DOI] [PubMed] [Google Scholar]
- Agelink MW, Malessa R, Weisser U, Lemmer W, Zeit T, Majewski T, Klieser E (1998) Alcoholism, peripheral neuropathy (PNP) and cardiovascular autonomic neuropathy (CAN). Journal of the neurological sciences 161:135–142. [DOI] [PubMed] [Google Scholar]
- Ammendola A, Gemini D, Iannaccone S, Argenzio F, Ciccone G, Ammendola E, Serio L, Ugolini G, Bravaccio F (2000) Gender and peripheral neuropathy in chronic alcoholism: a clinical-electroneurographic study. Alcohol Alcohol 35:368–371. [DOI] [PubMed] [Google Scholar]
- Ammendola A, Tata MR, Aurilio C, Ciccone G, Gemini D, Ammendola E, Ugolini G, Argenzio F (2001) Peripheral neuropathy in chronic alcoholism: a retrospective cross-sectional study in 76 subjects. Alcohol Alcohol 36:271–275. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK (2005) Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 9:463–484. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Darbar A, Krauss BR, Gelnar PA, Szeverenyi NM (1999) Differentiating cortical areas related to pain perception from stimulus identification: temporal analysis of fMRI activity. J Neurophysiol 81:2956–2963. [DOI] [PubMed] [Google Scholar]
- Avants BB, Epstein CL, Grossman M, Gee JC (2008) Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Medical image analysis 12:26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC (2011) A reproducible evaluation of ANTs similarity metric performance in brain image registration. NeuroImage 54:2033–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluarte JH (2017) Neurological Complications of Renal Disease. Semin Pediatr Neurol 24:25–32. [DOI] [PubMed] [Google Scholar]
- Barrell K, Smith AG (2019) Peripheral Neuropathy. Med Clin North Am 103:383–397. [DOI] [PubMed] [Google Scholar]
- Beck AT, R.A. S, G.K. B ((1996) ) Manual for the Beck Depression Inventory-II., in Series Manual for the Beck Depression Inventory-II, Psychological Corporation, San Antonio, TX. [Google Scholar]
- Behse F, Buchthal F (1977) Alcoholic neuropathy: Clinical, electrophysiological, and biopsy findings. Annals of Neurology 2:95–110. [Google Scholar]
- Berner K, Morris L, Baumeister J, Louw Q (2017) Objective impairments of gait and balance in adults living with HIV-1 infection: a systematic review and meta-analysis of observational studies. BMC musculoskeletal disorders 18:325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland EG, Selvarajah D, Hunter M, Ezaydi Y, Tesfaye S, Ahmedzai SH, Snowden JA, Wilkinson ID (2014) Central pain processing in chronic chemotherapy-induced peripheral neuropathy: a functional magnetic resonance imaging study. PloS one 9:e96474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boly M, Balteau E, Schnakers C, Degueldre C, Moonen G, Luxen A, Phillips C, Peigneux P, Maquet P, Laureys S (2007) Baseline brain activity fluctuations predict somatosensory perception in humans. Proceedings of the National Academy of Sciences of the United States of America 104:12187–12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer BA, de Greef BT, Hoeijmakers JG, Geerts M, van Kleef M, Merkies IS, Faber CG (2015) Neuropathic Pain due to Small Fiber Neuropathy in Aging: Current Management and Future Prospects. Drugs & aging 32:611–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckalew N, Haut MW, Morrow L, Weiner D (2008) Chronic pain is associated with brain volume loss in older adults: preliminary evidence. Pain medicine 9:240–248. [DOI] [PubMed] [Google Scholar]
- Chanraud S, Pitel AL, Muller-Oehring EM, Pfefferbaum A, Sullivan EV (2013) Remapping the brain to compensate for impairment in recovering alcoholics. Cereb Cortex 23:97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanraud S, Pitel AL, Pfefferbaum A, Sullivan EV (2011) Disruption of functional connectivity of the default-mode network in alcoholism. Cereb Cortex 21:2272–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry CL, Wesselingh SL, Lal L, McArthur JC (2005) Evaluation of a clinical screening tool for HIV-associated sensory neuropathies. Neurology 65:1778–1781. [DOI] [PubMed] [Google Scholar]
- Chopra K, Tiwari V (2012) Alcoholic neuropathy: possible mechanisms and future treatment possibilities. British journal of clinical pharmacology 73:348–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppieters I, De Pauw R, Caeyenberghs K, Danneels L, Kregel J, Pattyn A, Meeus M, Cagnie B (2017) Decreased Regional Grey Matter Volume in Women with Chronic Whiplash-Associated Disorders: Relationships with Cognitive Deficits and Disturbed Pain Processing. Pain physician 20:E1025–E1051. [PubMed] [Google Scholar]
- Coupe P, Yger P, Prima S, Hellier P, Kervrann C, Barillot C (2008) An optimized blockwise nonlocal means denoising filter for 3-D magnetic resonance images. IEEE transactions on medical imaging 27:425–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amour ML, Brissette S, Lavoie J, Butterworth RF (2000) Reduced sensory and motor nerve conduction velocities in moderate drinkers. Addict Biol 5:71–75. [DOI] [PubMed] [Google Scholar]
- Fassihi A, Akrami A, Pulecchi F, Schonfelder V, Diamond ME (2017) Transformation of Perception from Sensory to Motor Cortex. Current biology : CB 27:1585–1596.e1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW (1998) Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) Version 2.0, in Series Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) Version 2.0, Biometrics Research Department, New York State Psychiatric Institute, New York, NY. [Google Scholar]
- Gane E, Bergman R, Hutchinson D (2004) Resolution of alcoholic neuropathy following liver transplantation. Liver Transpl 10:1545–1548. [DOI] [PubMed] [Google Scholar]
- Gansler DA, Harris GJ, Oscar-Berman M, Streeter C, Lewis RF, Ahmed I, Achong D (2000) Hypoperfusion of inferior frontal brain regions in abstinent alcoholics: a pilot SPECT study. Journal of studies on alcohol 61:32–37. [DOI] [PubMed] [Google Scholar]
- Geha PY, Apkarian AV (2005) Brain imaging findings in neuropathic pain. Curr Pain Headache Rep 9:184–188. [DOI] [PubMed] [Google Scholar]
- Gelnar PA, Krauss BR, Sheehe PR, Szeverenyi NM, Apkarian AV (1999) A comparative fMRI study of cortical representations for thermal painful, vibrotactile, and motor performance tasks. NeuroImage 10:460–482. [DOI] [PubMed] [Google Scholar]
- Godde B, Diamond ME, Braun C (2010) Feeling for space or for time: task-dependent modulation of the cortical representation of identical vibrotactile stimuli. Neuroscience letters 480:143–147. [DOI] [PubMed] [Google Scholar]
- Hamel J, Logigian EL (2018) Acute nutritional axonal neuropathy. Muscle Nerve 57:33–39. [DOI] [PubMed] [Google Scholar]
- Heffernan TM (2008) The impact of excessive alcohol use on prospective memory: a brief review. Current drug abuse reviews 1:36–41. [DOI] [PubMed] [Google Scholar]
- Hollingshead A (1975) Four-factor index of social status., in Series Four-factor index of social status., Department of Sociology, Yale University, New Haven, CT. [Google Scholar]
- Hsieh PC, Tseng MT, Chao CC, Lin YH, Tseng WY, Liu KH, Chiang MC, Hsieh ST (2015) Imaging signatures of altered brain responses in small-fiber neuropathy: reduced functional connectivity of the limbic system after peripheral nerve degeneration. Pain 156:904–916. [DOI] [PubMed] [Google Scholar]
- Jabbari B, Vaziri ND (2018) The nature, consequences, and management of neurological disorders in chronic kidney disease. Hemodial Int 22:150–160. [DOI] [PubMed] [Google Scholar]
- Jacob L, Kostev K (2016) Prevalence of depression in type 2 diabetes patients in German primary care practices. J Diabetes Complications 30:432–437. [DOI] [PubMed] [Google Scholar]
- Julian T, Glascow N, Syeed R, Zis P (2018) Alcohol-related peripheral neuropathy: a systematic review and meta-analysis. J Neurol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda K, Fujimoto K, Kyota A (2017) Emotional Responses to Persistent Chemotherapy-induced Peripheral Neuropathy Experienced by Patients with Colorectal Cancer in Japan. Asia-Pacific journal of oncology nursing 4:233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keltner JR, Connolly CG, Vaida F, Jenkinson M, Fennema-Notestine C, Archibald S, Akkari C, Schlein A, Lee J, Wang D, Kim S, Li H, Rennels A, Miller DJ, Kesidis G, Franklin DR, Sanders C, Corkran S, Grant I, Brown GG, Atkinson JH, Ellis RJ, Group C (2017) HIV Distal Neuropathic Pain Is Associated with Smaller Ventral Posterior Cingulate Cortex. Pain medicine 18:428–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemppainen R, Juntunen J, Hillbom M (1982) Drinking habits and peripheral alcoholic neuropathy. Acta neurologica Scandinavica 65:11–18. [DOI] [PubMed] [Google Scholar]
- Kim JS, Choi-Kwon S (1996) Discriminative sensory dysfunction after unilateral stroke. Stroke 27:677–682. [DOI] [PubMed] [Google Scholar]
- Kjaer TW, Nowak M, Lou HC (2002) Reflective self-awareness and conscious states: PET evidence for a common midline parietofrontal core. NeuroImage 17:1080–1086. [PubMed] [Google Scholar]
- Koike H, Iijima M, Sugiura M, Mori K, Hattori N, Ito H, Hirayama M, Sobue G (2003) Alcoholic neuropathy is clinicopathologically distinct from thiamine-deficiency neuropathy. Ann Neurol 54:19–29. [DOI] [PubMed] [Google Scholar]
- Koike H, Mori K, Misu K, Hattori N, Ito H, Hirayama M, Sobue G (2001) Painful alcoholic polyneuropathy with predominant small-fiber loss and normal thiamine status. Neurology 56:1727–1732. [DOI] [PubMed] [Google Scholar]
- Ku Y, Ohara S, Wang L, Lenz FA, Hsiao SS, Bodner M, Hong B, Zhou YD (2007) Prefrontal cortex and somatosensory cortex in tactile crossmodal association: an independent component analysis of ERP recordings. PloS one 2:e771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Floch JP, Doucet J, Bauduceau B, Verny C (2014) Retinopathy, nephropathy, peripheral neuropathy and geriatric scale scores in elderly people with Type 2 diabetes. Diabet Med 31:107–111. [DOI] [PubMed] [Google Scholar]
- Li J, Zhang W, Wang X, Yuan T, Liu P, Wang T, Shen L, Huang Y, Li N, You H, Xiao T, Feng F, Ma C (2018) Functional magnetic resonance imaging reveals differences in brain activation in response to thermal stimuli in diabetic patients with and without diabetic peripheral neuropathy. PloS one 13:e0190699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Xu Y, An M, Zeng Q (2019) The risk factors for diabetic peripheral neuropathy: A meta-analysis. PloS one 14:e0212574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney CA, Arieff AI (1982) Uremic encephalopathies: clinical, biochemical, and experimental features. Am J Kidney Dis 2:324–336. [DOI] [PubMed] [Google Scholar]
- Maiya RP, Messing RO (2014) Peripheral systems: neuropathy. Handb Clin Neurol 125:513–525. [DOI] [PubMed] [Google Scholar]
- Manor B, Newton E, Abduljalil A, Novak V (2012) The relationship between brain volume and walking outcomes in older adults with and without diabetic peripheral neuropathy. Diabetes care 35:1907–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattis S (1998) Dementia rating scale (DRS) professional manual., in Series Dementia rating scale (DRS) professional manual., Psychological Assessment Resources, Inc, Odessa, FL. [Google Scholar]
- McArthur JC, Stocks EA, Hauer P, Cornblath DR, Griffin JW (1998) Epidermal nerve fiber density: normative reference range and diagnostic efficiency. Archives of neurology 55:1513–1520. [DOI] [PubMed] [Google Scholar]
- McKay JR (1999) Studies of factors in relapse to alcohol, drug and nicotine use: a critical review of methodologies and findings. Journal of studies on alcohol 60:566–576. [DOI] [PubMed] [Google Scholar]
- Mellion M, Gilchrist JM, de la Monte S (2011) Alcohol-related peripheral neuropathy: nutritional, toxic, or both? Muscle Nerve 43:309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellion ML, Silbermann E, Gilchrist JM, Machan JT, Leggio L, de la Monte S (2014) Small-fiber degeneration in alcohol-related peripheral neuropathy. Alcohol Clin Exp Res 38:1965–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milovanovic B, Milinic N, Trifunovic D, Krotin M, Filipovic B, Bisenic V, Djuric D (2009) Autonomic dysfunction in alcoholic cirrhosis and its relation to sudden cardiac death risk predictors. Gen Physiol Biophys 28 Spec No:251–261. [PubMed] [Google Scholar]
- Monforte R, Estruch R, Valls-Sole J, Nicolas J, Villalta J, Urbano-Marquez A (1995) Autonomic and peripheral neuropathies in patients with chronic alcoholism. A dose-related toxic effect of alcohol. Archives of neurology 52:45–51. [DOI] [PubMed] [Google Scholar]
- Nelson HE (1982) National Adult Reading Test (NART): Test Manual., in Series National Adult Reading Test (NART): Test Manual., Windsor: NFER-NELSON. [Google Scholar]
- Nudelman KN, McDonald BC, Wang Y, Smith DJ, West JD, O’Neill DP, Zanville NR, Champion VL, Schneider BP, Saykin AJ (2016) Cerebral Perfusion and Gray Matter Changes Associated With Chemotherapy-Induced Peripheral Neuropathy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 34:677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron R, Garcia-Larrea L, Gregoire MC, Convers P, Richard A, Lavenne F, Barral FG, Mauguiere F, Michel D, Laurent B (2000) Parietal and cingulate processes in central pain. A combined positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) study of an unusual case. Pain 84:77–87. [DOI] [PubMed] [Google Scholar]
- Pfeffer RI, Kurosaki TT, Harrah CH Jr., Chance JM, Filos S (1982) Measurement of functional activities in older adults in the community. Journal of gerontology 37:323–329. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Rohlfing T, Sullivan EV (2009) Degradation of association and projection white matter systems in alcoholism detected with quantitative fiber tracking. Biol Psychiatry 65:680–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Zahr NM, Sassoon SA, Kwon D, Pohl KM, Sullivan EV (2018) Accelerated and Premature Aging Characterizing Regional Cortical Volume Loss in Human Immunodeficiency Virus Infection: Contributions From Alcohol, Substance Use, and Hepatitis C Coinfection. Biological psychiatry. Cognitive neuroscience and neuroimaging 3:844–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riandini T, Wee HL, Khoo EYH, Tai BC, Wang W, Koh GCH, Tai ES, Tavintharan S, Chandran K, Hwang SW, Venkataraman K (2018) Functional status mediates the association between peripheral neuropathy and health-related quality of life in individuals with diabetes. Acta diabetologica 55:155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlfing T, Zahr NM, Sullivan EV, Pfefferbaum A (2010) The SRI24 multichannel atlas of normal adult human brain structure. Human brain mapping 31:798–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi A, Ciacci G, Federico A, Mondelli M, Rizzuto N (1986) Sensory and motor peripheral neuropathy in olivopontocerebellar atrophy. Acta neurologica Scandinavica 73:363–371. [DOI] [PubMed] [Google Scholar]
- Sadowski A, Houck RC (2019) Alcoholic Neuropathy, in StatPearls, StatPearls, Treasure Island (FL). [PubMed] [Google Scholar]
- Sanvisens A, Zuluaga P, Pineda M, Fuster D, Bolao F, Junca J, Tor J, Muga R (2017) Folate deficiency in patients seeking treatment of alcohol use disorder. Drug Alcohol Depend 180:417–422. [DOI] [PubMed] [Google Scholar]
- Scholvinck ML, Howarth C, Attwell D (2008) The cortical energy needed for conscious perception. NeuroImage 40:1460–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz E, Diener HC, Dichgans J, Langohr HD, Schied W, Schupmann A (1986) Incidence of peripheral neuropathy and cerebellar ataxia in chronic alcoholics. J Neurol 233:212–217. [DOI] [PubMed] [Google Scholar]
- Selvarajah D, Wilkinson ID, Gandhi R, Griffiths PD, Tesfaye S (2011) Microvascular perfusion abnormalities of the Thalamus in painful but not painless diabetic polyneuropathy: a clue to the pathogenesis of pain in type 1 diabetes. Diabetes care 34:718–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HA, Sheu WJ (1982) Reliability of alcohol use indices. The Lifetime Drinking History and the MAST. Journal of studies on alcohol 43:1157–1170. [DOI] [PubMed] [Google Scholar]
- Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR (1997) Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. Jama 277:813–817. [PubMed] [Google Scholar]
- Soros P, Marmurek J, Tam F, Baker N, Staines WR, Graham SJ (2007) Functional MRI of working memory and selective attention in vibrotactile frequency discrimination. BMC Neurosci 8:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimine S, Ogino Y, Kawamichi H, Obata H, Saito S (2016) Brain morphological alternation in chronic pain patients with neuropathic characteristics. Molecular pain 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Pfefferbaum A (2000) Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcohol Clin Exp Res 24:611–621. [PubMed] [Google Scholar]
- Sullivan EV, Zahr NM, Sassoon SA, Thompson WK, Kwon D, Pohl KM, Pfefferbaum A (2018) The Role of Aging, Drug Dependence, and Hepatitis C Comorbidity in Alcoholism Cortical Compromise. JAMA Psychiatry 75:474–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tackmann W, Minkenberg R, Strenge H (1977) Correlation of electrophysiological and quantitative histological findings in the sural nerve of man. Studies on alcoholic neuropathy. J Neurol 216:289–299. [DOI] [PubMed] [Google Scholar]
- Tesfaye S, Selvarajah D, Gandhi R, Greig M, Shillo P, Fang F, Wilkinson ID (2016) Diabetic peripheral neuropathy may not be as its name suggests: evidence from magnetic resonance imaging. Pain 157 Suppl 1:S72–80. [DOI] [PubMed] [Google Scholar]
- Vittadini G, Buonocore M, Colli G, Terzi M, Fonte R, Biscaldi G (2001) Alcoholic polyneuropathy: a clinical and epidemiological study. Alcohol Alcohol 36:393–400. [DOI] [PubMed] [Google Scholar]
- Wang WW, Song CL, Huang L, Song QW, Liang ZH, Wei Q, Hu JN, Miao YW, Wu B, Xie L (2015) DTI Study of Cerebral Normal-Appearing White Matter in Hereditary Neuropathy With Liability to Pressure Palsies (HNPP). Medicine (Baltimore) 94:e1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinmann RL (1967) Heterogeneous system degeneration of the central nervous system associated with peripheral neuropathy. Neurology 17:597–603. [DOI] [PubMed] [Google Scholar]
- Wessels AM, Simsek S, Remijnse PL, Veltman DJ, Biessels GJ, Barkhof F, Scheltens P, Snoek FJ, Heine RJ, Rombouts SA (2006) Voxel-based morphometry demonstrates reduced grey matter density on brain MRI in patients with diabetic retinopathy. Diabetologia 49:2474–2480. [DOI] [PubMed] [Google Scholar]
- Woolgar A, Zopf R (2017) Multisensory coding in the multiple-demand regions: vibrotactile task information is coded in frontoparietal cortex. J Neurophysiol 118:703–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YH, Uluc I, Schmidt TT, Tertel K, Kirilina E, Blankenburg F (2018) Overlapping frontoparietal networks for tactile and visual parametric working memory representations. NeuroImage 166:325–334. [DOI] [PubMed] [Google Scholar]
- Zahr NM, Pohl KM, Pfefferbaum A, Sullivan EV (2019) Dissociable Contributions of Precuneus and Cerebellum to Subjective and Objective Neuropathy in HIV. J Neuroimmune Pharmacol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambelis T, Karandreas N, Tzavellas E, Kokotis P, Liappas J (2005) Large and small fiber neuropathy in chronic alcohol-dependent subjects. J Peripher Nerv Syst 10:375–381. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


