Abstract
The circadian system, ubiquitous across species, generates ~24-h rhythms in virtually all biological processes and allows them to anticipate and adapt to the 24-h day/night cycle, thus ensuring optimal physiological function. Epidemiological studies show time-of-day variations in adverse cardiovascular events, while controlled laboratory studies demonstrate a circadian influence on key markers of cardiovascular function and risk. Furthermore, circadian misalignment, typically experienced by shift workers, and by individuals who experience late eating, jet lag, or circadian rhythm sleep-wake disturbances, increases cardiovascular risk factors. Therefore, understanding the mechanisms by which the circadian system regulates cardiovascular function, and which of those are affected by circadian disruption, may help to develop intervention strategies to mitigate cardiovascular risk.
Keywords: circadian rhythms, circadian misalignment, shift work, cardiovascular risk
Why time-of-day is important for cardiovascular risk?
Adverse cardiovascular events, including myocardial infarction, stroke, and ventricular arrhythmias, are worldwide leading causes of death [1]. Epidemiological evidence supports heightened risk for these adverse cardiovascular events during the “vulnerable morning hours” (6:00AM-12:00PM) [2, 3]. Importantly, these time-of-day variations are not fully explained by daily rhythms in behavioral triggers, such as physical activity [4], fasting/feeding and sleep schedules. They may instead be due to the influence of the circadian system, and its interaction with behavioral triggers, on specific cardiovascular risk factors. These daily rhythms in acute events are superimposed on the slower rise in overall cardiovascular risk during the development and progression of cardiovascular disease. Such longer-term cardiovascular risk is associated with modifiable risk factors, such as smoking, unhealthy diet, sedentarism and excessive alcohol consumption [5]. Beyond these established risk factors, growing epidemiological evidence indicates shift work as an important risk factor for cardiovascular diseases, including hypertension, ischemic stroke, coronary heart disease and sudden cardiac death [6-11]. Similarly, social jetlag (“jetlag” between week-days and weekends [12]) may be associated with an increased cardiovascular risk, as indicated by lower high-density lipoprotein-cholesterol levels, higher triglyceride levels, and decreased insulin sensitivity [13]. Chronotype (a measure of preferred timing of sleep and activity) may be linked to cardiovascular disease mortality, with evening types at higher risk [14]. Even on a shorter time-scale, the transition to daylight saving time is associated with incidence of myocardial infarction and ischemic stroke hospitalizations [15]. Common to these conditions is the disruption of circadian alignment with behavioral/environmental rhythms (Fig.1), which means that the endogenous circadian timing system loses its optimal alignment with behavioral/environmental cycles (i.e. sleep/wake, light/dark, fasting/feeding). Together, these findings raised the hypothesis that this circadian misalignment may increase cardiovascular risk. Indeed, growing evidence from animal models [16-19] and experimental human studies [20-24] now provide physiological mechanistic insights into the circadian disruption/misalignment effects on cardiovascular risk, and suggest that mitigating circadian disruption may benefit vulnerable populations, such as shift workers. In this review, we present the current state of knowledge regarding the role of the circadian system in cardiovascular function, potential physiological mechanisms underlying the association between shift work and cardiovascular risk factors, implications for cardiovascular disease, and novel targeted cardioprotective treatments. This review will focus primarily on human studies and will be supplemented by data from animal models.
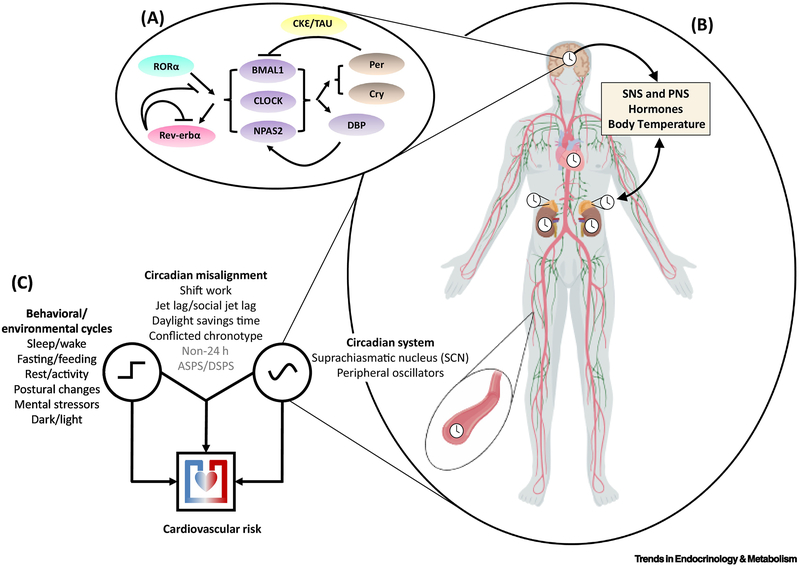
Figure 1: Conceptual framework of circadian misalignment effects on cardiovascular risk.
A: Circadian rhythms are driven by molecular clocks centered around core clock genes such as BMAL1, CLOCK and NPAS2, which regulate rhythms of their own transcription/expression via negative (and positive) feedback loops (see top left inset for schematic, see ref# [71] for review).
B: These molecular clocks are present in almost every cell in the body, including cardiac/vascular tissues, adrenal glands, kidneys, the immune system, and importantly, the “master clock” in the SCN. The SCN clock can affect cardiovascular function independently of peripheral clocks (e.g. via autonomic nervous system outflow/endocrine outflow) and by acting to synchronize peripheral clocks (e.g. via circadian rhythms in body temperature/endocrine outflow). Peripheral circadian clocks provide feedback to each other and the SCN clock via their modulatory effects on cardiovascular and endocrine physiology. Endogenous circadian rhythms also influence daily patterns in behavior/exposure to environmental factors, which in turn also impact cardiovascular physiology.
C: Disruptions to the circadian system can impact cardiovascular risk without necessarily disrupting behavioral/environmental cycles (e.g. via tissue-specific circadian clock-gene mutations). Behavioral/environmental cycles can impact cardiovascular risk even when said rhythms are entrained/aligned to the endogenous circadian cycle (e.g. via disrupted sleep in individuals who maintain regular schedules). However, in the most common/epidemiologically relevant scenarios, behavioral/environmental cycles become misaligned relative to the endogenous circadian cycle and it is this circadian misalignment that impacts cardiovascular risk. Known risk contributors are shown in black, putative ones in gray. “Non 24h” – any scenario with individuals living on non-24 schedules; “ASPS/DSPS” – advanced sleep phase syndrome/delayed sleep phase syndrome.
Circadian rhythms in cardiovascular risk
In humans, there are day/night rhythms in key aspects of cardiovascular function, including blood pressure (higher levels during the day and lower levels at night [25]), and platelet aggregability (higher levels in the morning [26, 27]). Epidemiologic studies report a morning peak in adverse cardiovascular events [2, 28]. However, such data collected during regular sleep/wake cycles cannot determine whether this temporal pattern is due to the circadian system or due to behavioral and environmental factors. Circadian rhythm corresponds to any biological process that displays an endogenous, entrainable oscillation of ~24 hours that continues even in the absence of external time cues. [29]. The distinction between day/night rhythms and circadian rhythms is of critical mechanistic and clinical relevance for a number of reasons. One is the benefit of understanding mechanisms needed to develop targeted preventative and therapeutic strategies to adequately address time-of-day effects in cardiovascular disease. Furthermore, distinguishing the limits of ‘clock time’ vs. circadian time is important because of the large variability in sleep/wake timing [30] and phase angle of entrainment. When considering individuals with comorbidities, medication use, circadian sleep wake disturbances, extreme chronotypes, and/or shift work exposure, these differences are likely to be much larger. Collectively, these findings suggest that differences between clock time and circadian time differ greatly across individuals, and thus day/night rhythms based on clock time cannot be generalized across the population. Therefore, there is a substantial benefit to determining endogenous circadian phase for disease processes and risk factors that are under circadian influence. With the existence of well-validated assessments of the phase of the central circadian pacemaker by dim light melatonin onset [31], as well as the advent of biomarkers for the assessment of circadian phase from one or two blood samples [32], these assessments are expected to be within the reach of clinical implementation in the future.
To adequately assess the role of the circadian system in cardiovascular function, stringently controlled circadian protocols, such as Constant Routine (CR) or Forced Desynchrony (FD) protocols, are needed to determine the effect of the circadian system, both independent from and interacting with the influence of behaviors/environment. The CR protocol is a study paradigm whereby participants remain awake under dim light (typically < 5lux to minimize light effects on the circadian system), and under semi-recumbent posture with isocaloric, evenly-spaced meals for more than 24h [31]. The FD protocol is designed to uncouple circadian cycles from daily behavioral/environmental rhythms, which enables the assessment of the circadian effects, just as for the CR, but it also allows assessment of their interaction with behaviors. This uncoupling is achieved by maintaining participants on a non-24-h behavioral sleep/wake cycle (often 20-h or 28-h cycles) in dim light, thus out of the range of entrainment of the human circadian system. Therefore, the circadian system ‘runs free’ at its inherent rate of ~24h, which allows scheduled behaviors to be uniformly distributed across the circadian cycle. Using these carefully controlled paradigms, circadian rhythms have been observed in blood pressure [21, 33], heart rate [34], heartbeat dynamics [35], circulating epinephrine and norepinephrine [21, 36], vagal modulation [37], platelet aggregability [38], plasminogen activator inhibitor-1 (PAI-1) [39], and immune responses [40]. Collectively, the circadian system influences cardiovascular risk factors, with possible contributions to morning peak in cardiovascular events (Fig.2) (see Text box 1 for the molecular mechanisms of the central circadian clock and peripheral clocks on cardiovascular function).
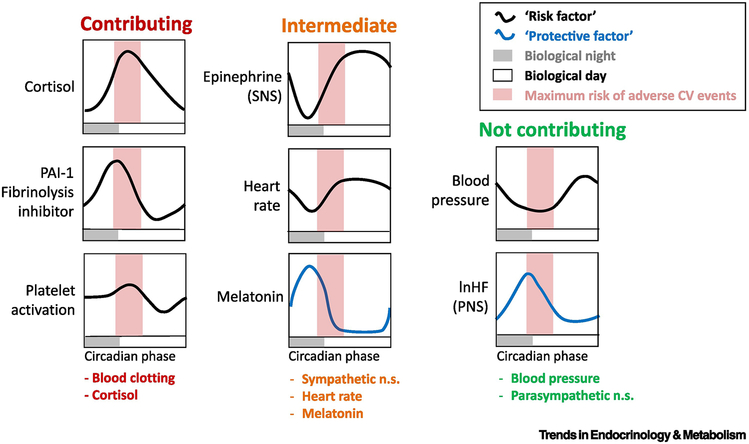
Figure 2: Circadian system influences cardiovascular risk factors: Possible contribution to morning peak in CV events.
Displayed here are cardiovascular risk factors shown to be under endogenous circadian control. They are grouped according to temporal alignment with the window of increased cardiovascular vulnerability in the morning hours. “Risk factors” (red lines) are those whose elevation may be correlated with increased acute risk for adverse cardiovascular events, while elevation of “protective factors” (green lines) are considered to decrease acute risk for adverse cardiovascular events. “Risk factors” are considered contributing if the peak in their circadian rhythm occurs during the vulnerable window (biological morning), intermediate if they rise during the vulnerable window, and not contributing if they are at their minimum during the vulnerable window. “Protective factors” are considered not contributing if the peak in their circadian rhythm occurs during the vulnerable window (biological morning), intermediate if they fall during the vulnerable window during the vulnerable window.
PAI-1 – plasminogen activating factor 1; Epinephrine (SNS) – epinephrine used here as a marker of sympathetic nervous system activation/sympathetic cardiac modulation; lnHF (PNS) – the natural log of high frequency power in the electrocardiogram, used here as a marker of parasympathetic nervous system activation/vagal cardiac modulation.
This a qualitative summary of quantitative data collected during circadian unmasking (forced desynchrony) experiments in human participants [21, 33, 37-39]. These studies suggest rhythms of the hemostatic (blood clotting) system and of cortisol contribute to the morning peak in adverse cardiovascular events, that rhythms in blood pressure and lnHF (PNS) do not contribute and that circadian rhythms in melatonin, heart rate and epinephrine (SNS) may contribute to an intermediate extent.
Text Box 1: Effect of Molecular Clocks and Their Mutations on Cardiovascular Physiology and Risk Factors.
The SCN clock modulates cardiovascular function through two mechanisms: (1) directly via autonomic nervous system [79, 80] and endocrine outflow (e.g. glucocorticoid rhythms) that act primarily on the cardiac muscle/blood vessels via receptors on the myocardium/vascular endothelium [81] and (2) indirectly via synchronization of relevant peripheral clocks, including robust local clocks in cardiac tissues [82]. In rats, cardiomyocytes in culture show rhythmic clock gene expression, including Bmal1, Rev-erb alpha, Per2 and Dbp [75]. In vivo studies of the cardiac transcriptome indicate ~10% of transcribed genes are regulated by the cardiomyocyte circadian clock [58]. Furthermore, in vivo and in vitro studies show rhythmic oscillations in circadian clock gene transcripts (i.e. clock gene mRNA transcripts of Per2, Bmal1, and Dbp) in the mouse aorta/vascular tissue [51, 83, 84]. Importantly, cardiomyocyte-specific Bmal1 knockout mice exhibit disrupted systolic function (fractional shortening and ejection fraction) with increased age, and ultimately decreased lifespan [58].
In mice, cardiac ion-channel expression and QT-interval duration (an index of myocardial repolarization) show circadian rhythms, and both are under the control of a clock-dependent oscillator, responsible for rhythmic expression of the transient outward potassium current [72]. Circadian molecular machinery present in the vascular system, including endothelial cells, plays a central role in vascular function [85], and potentially in vascular dysfunction, including blood clotting. Since the kidney is central to maintaining fluid and ion homeostasis, circadian rhythms in kidney tissue may help explain circadian rhythms in blood pressure [86]. Mice lacking functional Per1 and Per2 genes show dramatic alteration to normal rhythms in mRNA of the renal alphaENaC (alpha subunit of the epithelial sodium channel, linked to renal absorption of sodium). Moreover, mice lacking the circadian Cry1 and Cry2 genes show salt-sensitive hypertension potentially driven by high aldosterone synthesis [77]. Aldosterone (and corticosterone) also modulate levels of Cry1, Per1, Per2 and ReverbA gene expression in the heart over the 24-h day [87].
The circadian timing system may also impact cardiovascular risk through modulation of immune function [88, 89] or potentially via ReverbA-sensitive cholesterol pathways and bile acid regulation [90]. Virtually all immune system measures show rhythmic oscillations, and the circadian system may act on inflammation responses by restraining the expression of inflammatory genes to specific times within the circadian cycle [89].
Behaviors (i.e. physical activity, posture, mental stress) impact cardiovascular function both directly and in interactions with the circadian system. One FD protocol where participants underwent standardized tilt table tests (passive head-up tilt) to assess cardiovascular responses to an orthostatic challenge across all circadian phases [37] revealed a circadian rhythm in the risk for presyncopal events, with a robust peak during the biological night. Furthermore, the circadian system modulates reactivity of numerous cardiovascular risk markers to standardized exercise as tested across all circadian phases during a FD protocol [21], with peaks in catecholamine reactivity at endogenous circadian phase typically equivalent to ~9:00AM, and maximum decrease in cardiac vagal modulation at ~9:00AM. The combination of the maximum increase in catecholamine reactivity and decrease in cardiac vagal modulation simultaneously at a circadian phase equivalent to 9:00AM may contribute to the increased risk for serious cardiovascular events, as observed in epidemiological studies. Recently, using a FD protocol, we showed additive effects of mental stress and the circadian system on hemodynamic function, sympathovagal balance, epinephrine, and cardiac vagal modulation [36]. A key environmental factor that impacts cardiovascular function is light exposure. Light is the most important input to the suprachiasmatic nucleus (SCN) and has robust effects on the circadian system. Moreover, the acute effects of light on cardiovascular function potentially occur through mechanisms independent from phase shifting the circadian system (for a review see [41]). Light exposure shows maximal effects on heart rate, preejection period, and relative sympathetic tone during the night and morning hours [41]. Collectively, the translational value of studies assessing the interaction of circadian and behavioral/environmental effects on cardiovascular function is to identify the time-of-day when increased cardiovascular risk is associated to common behaviors.
Impact of circadian misalignment on cardiovascular risk
If circadian rhythms contribute to cardiovascular health [42] then circadian disruption/misalignment may influence cardiovascular risk. Circadian misalignment effects on circadian factors and cardiovascular risk factors in humans (Fig.3) can be determined using controlled in-laboratory studies, including FD and simulated shift work protocols [43]. In a FD protocol, where participants lived in seven 28-h “days” under dim light [20], waketime blood pressure was higher during circadian misalignment. These misalignment-induced effects on blood pressure did not seem to be explained by changes in waketime HR (unchanged), cardiac vagal modulation (unchanged) nor sympathoadrenal measures (urinary epinephrine decreased and norepinephrine unchanged). The effects on epinephrine were likely due to the inhibitory role of the circadian system on epinephrine during the biological night (when participants were awake as they were misaligned by 12h) [21]. Moreover, circadian misalignment did not impact the 24-h average in plasma cortisol. While FD protocols provide experimental evidence for the adverse circadian misalignment effects on cardiovascular function, its translational value may be limited because shift workers do not live on 28-h days in dim light. Therefore, carefully-controlled laboratory shift work simulation protocols provide a closer approximation to what shift workers typically experience, while still controlling for behavioral/environmental factors. Using a simulated shift work protocol, healthy non-shift workers underwent 3 days under circadian alignment (“day shift work”) or circadian misalignment (“night shift work”) [22]. Consistent with the FD results, circadian misalignment increased waketime blood pressure, as well as 24-h average systolic and diastolic blood pressure and dampened the healthy sleep-associated systolic blood pressure dipping effect linked to increased cardiovascular risk and mortality [44]. Moreover, circadian misalignment decreased waketime cardiac vagal modulation [26], with vagal (parasympathetic) activity typically considered cardioprotective [45]. In contrast, 24-hour average urinary epinephrine excretion rate decreased, while no effects were observed for norepinephrine, suggesting that parasympathetic changes more than sympathetic changes may contribute to increase cardiovascular risk. However, the specific role of the sympathetic and parasympathetic nervous system contributions to cardiovascular risk under circadian misalignment cannot be fully teased apart, as the techniques used under circadian misalignment conditions are typically derived from non-invasive measures (i.e. EKG-based heart rate and heart rate variability; urinary catecholamine levels). The techniques that best mirror the sympathetic and parasympathetic nervous system activity are invasive and not often utilized in clinical research (microneurography, norepinephrine spillover). Therefore, the specificity and reproducibility in capturing the heterogeneous and often regionalized responses of the sympathetic and parasympathetic nervous system activity (in physiological and pathological conditions) under circadian misalignment conditions should be viewed with caution. Furthermore, circadian misalignment increased levels of inflammatory markers [including serum interleukin-6 (IL-6), high-sensitivity C-reactive protein (hsCRP), resistin, and tumor necrosis factor-α (TNF- α)]. A limitation of this study is that non-shift workers were studied, raising the question whether these effects of circadian misalignment translate to shift workers. When the latter were exposed to two 3-day laboratory protocols under circadian alignment or misalignment, profound misalignment effects were observed for markers of cardiovascular risk [23]. Circadian misalignment increased hsCRP, a marker of systemic inflammation. Waketime systolic blood pressure increased, and diastolic blood pressure increased, predominantly during sleep. These findings show that shift workers are not resilient against the adverse cardiovascular effects of circadian misalignment.
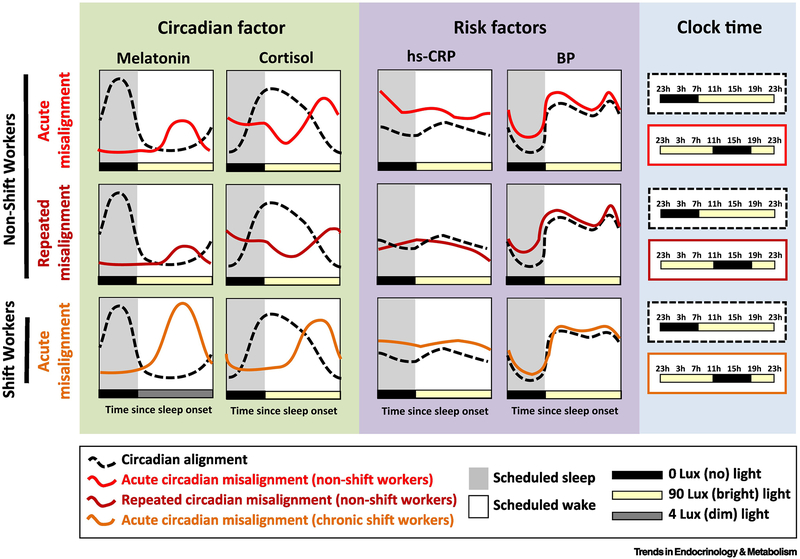
Figure 3: Effects of circadian misalignment on circadian and cardiovascular risk markers.
Shown is a visual, qualitative summary of quantitative data collected during in-lab circadian misalignment (night-shift work simulation) experiments in human participants [22, 23] (see blue panel on the right for clarification of the inverted light-dark schedule, and note that all other panels are plotted by time since scheduled lights-off).
In the green box (left) we show the effect of acute/recurrent circadian misalignment on two classic phase markers of the central circadian pacemaker: melatonin and cortisol. Along with suppression of the amplitude of cortisol/melatonin (in part due to the ~90lux light exposure), acute and even short-term repeated misalignment leads to these two phase markers being ~12hours temporally offset relative to the sleep/wake cycle due to the inertia of the circadian system.
In the purple box (middle) we show the effect of acute/recurrent circadian misalignment on two markers of cardiovascular risk: high sensitivity C-reactive protein (hs-CRP) and blood pressure (BP). Daily rhythms in these two profiles are primarily driven by the behavioral (sleep/wake); thus, unlike melatonin and cortisol, their rhythms are not 12-hours offset during misalignment. However, during misalignment hs-CRP and BP are elevated (in both non-shift workers and chronic shift workers). These observations may help to elucidate the underlying mechanisms by which shiftwork (or other forms of recurrent circadian misalignment) increases cardiovascular risk.
Circadian misalignment causes disrupted sleep, since sleeping during the biological day while being awake at night is at odds with the endogenous circadian system [46]. Disrupted sleep is associated with increased risk of cardiovascular disease, including atherosclerosis [47], and sleep disruption may change heart rate and blood pressure, autonomic nervous system function and fluid homeostasis (for a review see [48]). Therefore, night shift workers, who typically experience the effects of circadian misalignment, may also be exposed to the effects of sleep disturbance. Laboratory studies that induced circadian misalignment and sleep disruption [24, 49] indicate that their combination increased 24-h urinary norepinephrine. During scheduled wake, sleep restriction increased HR in both circadian alignment and misalignment conditions. Similarly, during scheduled sleep, circadian misalignment increased heart rate and LF/HF ratio (a measure of sympathovagal balance), and reduced cardiac vagal modulation, although no changes were observed for blood pressure [24]. Furthermore, circadian misalignment combined with sleep restriction [49] or total sleep deprivation [50] increased the levels of inflammatory markers as compared to circadian alignment and sleep restriction. Collectively, these findings demonstrate changes in sleep cannot fully explain the changes observed with circadian misalignment, and that circadian misalignment has effects above and beyond those of sleep disruption.
Molecular mechanisms of circadian misalignment effects on cardiovascular risk
Animal experimental paradigms to test how circadian disruption adversely impacts cardiovascular function include models of light/dark (LD) cycle manipulation, meal timing models, genetic mutations with a circadian period phenotype (changing circadian cycle length of whole animal or specific tissue), circadian rhythm ablation mutations (complete molecular knock-out of the clock in whole animal or tissue), and SCN-lesion studies [51]. In a LD cycle manipulation model, mice were randomized to a condition with either a 20-hour cycle (LD cycle of 10:10h) or a typical 24-hour cycle (LD cycle of 12:12h), to induce circadian disruption [52]. Accordingly, only mice under a 20-h cycle (circadian disruption) had decreased levels of cardiomyocytes and vascular smooth muscle cells hypertrophy, as well as a compensatory reduced expression of key genes in cardiac hypertrophic pathways (ANF, BNP, ACE, and collagen). When the mice were subsequently exposed to a typical 24-h rhythm, there was a rescue (reversal/attenuation) of abnormal cardiac pathophysiology [52]. Furthermore, circadian disruption induced by a genetic mutation causing a shortening of the circadian period (22h) in hamsters kept on a typical 24-h LD cycle resulted in extensive cardiac fibrosis, impaired contractility, renal disease, and early death due to cardiomyopathy [16]. Intriguingly, when placed on an LD cycle matching their genotype (i.e., 22h), their circadian rhythm patterns, cardiac and renal structure and function were normalized [4]. These observations [16, 52] support the notion that circadian synchrony (“resonance” of internal and external cycles) is integral to compensatory hypertrophy and normal remodeling. Moreover, an experimental ‘shift work’ paradigm in mice (under 22.5-h, 24-h and 27-h LD cycles for 17 weeks) showed that mice synchronized at an adverse circadian phase angle (i.e., misaligned) under 22.5h and 27h LD cycles had decreased metabolic efficiency and disrupted cardiac function, as compared to mice under 24 LD cycle [53]. This suggests that “environmental misalignment” [43] may have adverse cardiovascular health consequences even if the animals remain synchronized, but at an abnormal phase angle. As mentioned previously, clear links between low-grade inflammation and cardiovascular risk have been shown [54], which raises the question whether immune function changes may serve as causal mechanisms contributing to cardiovascular risk during circadian disruption. In a circadian disruption paradigm with mice exposed to once-a-week 6-h phase advance of the light/dark cycle for 4 weeks, immune responses dramatically changed [55]. Endotoxemic shock induced by an immune change (lipopolysaccharide [LPS] from Escherichia coli) was magnified, leading to a ~90% mortality rate as compared to ~20% mortality in unshifted control mice. These immune responses were likely driven by an increased release of proinflammatory cytokines (i.e. IL-1β, IL-12, IL-13, IL-6) in response to LPS challenge in shifted mice. Proinflammatory cytokines mediate immune responses, and increased levels are associated with endothelial dysfunction in patients with coronary artery disease and/or heart failure [56]. Clock-genetic mutations in mice models also impact cardiovascular function. Whole-animal deletion or mutation of core clock genes (i.e., Bmal1, Clock and Npas2) lowers mean arterial pressure and disrupts sympathoadrenal responses (norepinephrine and epinephrine) to stress [57]. Furthermore, cardiomyocyte-specific Bmal1 knockout attenuates glucose utilization, accelerates dilated cardiomyopathy and reduces longevity [58]. Cardiomyocyte-specific Bmal1 knockout or Clock mutation also disrupts gene and protein expression of insulin signaling components in cardiac tissues [18]. Collectively, these mechanistic insights into circadian disruption from animal experimental work - LD cycle manipulations [52, 53, 55], circadian period mutations (by causing period dissonance) [4],[16], SCN lesion as a circadian disruption model ([59]) or whole-animal [57] or cardiomyocyte-specific clock gene disruption [18, 58, 60] - show profound effects of circadian disruption on cardiovascular and immune function, consistent with the human misalignment data..
Clinical implications of circadian misalignment for cardiovascular disease
Human physiological mechanistic work provides translational evidence for the involvement of the circadian system in daily rhythms in adverse cardiovascular events [21, 33-39], as well as the contribution of circadian disruption (e.g. misalignment) to increase cardiovascular risk factors [20, 22-24]. Mechanistic insights from preclinical experimental work have shed light on the existence of circadian clocks throughout the cardiovascular system, and that circadian clocks in myocardial tissue become altered in cardiovascular disease (see Text Box 1). Based on these insights, the effects of the circadian system and of its disruption on cardiovascular risk may hold direct clinical implications. In humans, a randomized trial showed that the timing of aortic valve surgery was associated with adverse cardiac events up to 500 days following surgery, with less events and lower perioperative cardiac troponin T release with afternoon surgery as compared to morning surgery [61]. Ex-vivo analyses of the myocardium indicated alterations in circadian gene expression (Rev-Erbα gene deletion and antagonism), such that nuclear receptor Rev-Erbα was higher in the morning surgery group, which may be connected to increased cardiovascular risk. Importantly, night shift workers are repeatedly exposed to the effects of circadian misalignment, and may be at higher cardiovascular risk due to the progressive increase in blood pressure over time [23]. Together with epidemiological data that show time-of-day variations in adverse cardiovascular events [2, 3], the abovementioned studies provide mounting evidence in favor of a role of circadian disruption/misalignment in cardiovascular risk (Fig.4).
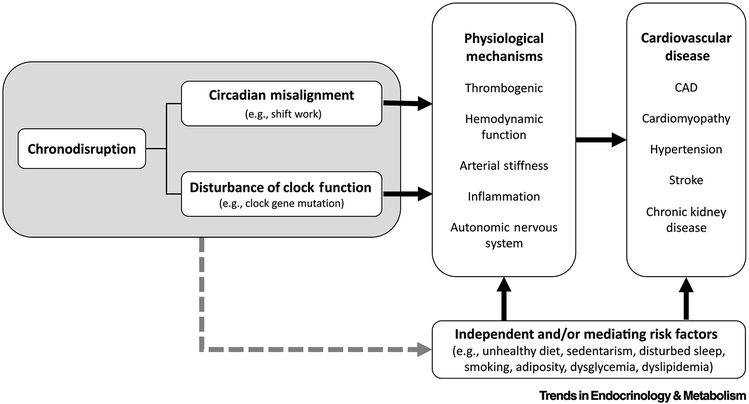
Figure 4: Linking chronodisription to cardiovascular disease.
Chronodisruption (see left figure panel) can take the form of circadian misalignment or disturbance of circadian clock function per se, either of which can lead to increased cardiovascular risk. Chronodisruption leads to physiological changes which are associated with and often precede cardiovascular disease (see middle figure panel), including adverse effects on thrombogenic pathways [22, 23], hemodynamic function [22, 53, 58, 72, 73], arterial stiffness [74], inflammation[22, 23, 49, 55, 75], and autonomic nervous system function [24, 37, 57]. Furthermore, chronodisruption has also been directly linked to many forms of cardiovascular disease (see right figure panel), including coronary artery disease (CAD) [76], cardiomyopathy [16, 18, 52, 58], hypertension [77], stroke [15, 60], and chronic kidney disease [16, 78]. Chronodisruption may also contribute to such physiological changes and/or cardiovascular disease via other classes of risk factors (e.g. alterations to behavioral patterns; see bottom figure panel).
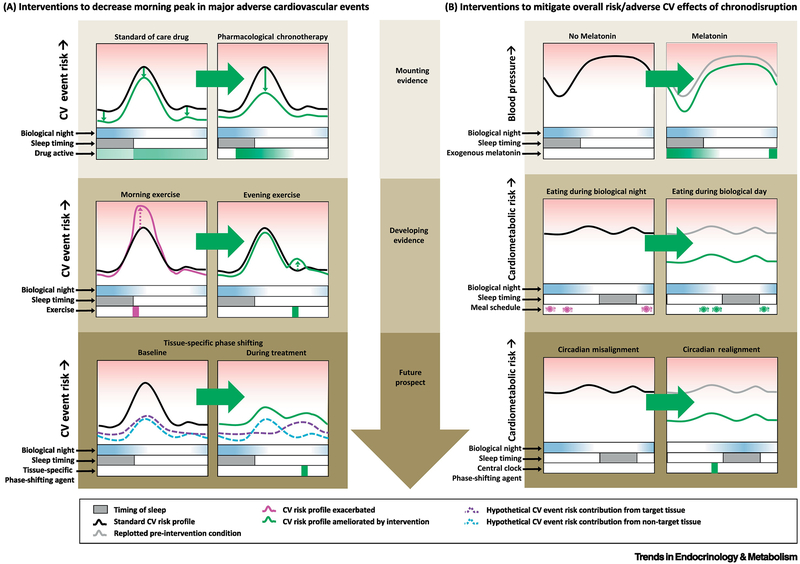
While it does not address the question of cause and effect, there is evidence for decreased neurotransmitter content (both mRNA and protein levels) in the SCN of hypertensive patients [62] that relate to changes in corticotropin releasing hormone-expression neurons in the paraventricular nucleus of the hypothalamus [63]. Initial suggestion for a potential beneficial effect of interventions that can influence circadian function comes from studies using melatonin in clinical populations with increased cardiovascular risk [64, 65], as melatonin affects the circadian system, although additional or alternative mechanisms such as anti-oxidant and/or immunomodulatory effects, may be involved [66]. Repeated use of 2.5mg of melatonin for 3-weeks in a randomized clinical trial reduced blood pressure during sleep, with an increase in the day-night amplitudes of blood pressure rhythms in hypertensive patients [64]. More recently, data on patients with type 2 diabetes (T2D) and essential hypertension who took melatonin (3mg or 5mg) in the evening for 4 weeks indicate that ~30% of non-dippers had an improvement in night blood pressure and night average blood pressure dipping, while no effects were observed in normal dippers with melatonin or in the control groups without melatonin [65]. While the data are limited with respect to other cardiovascular diseases, there is some evidence that melatonin supplementation (10mg nightly) for 12 weeks reduced blood pressure and serum hsCRP in patients with T2D and coronary heart disease [67]. Taken together, these studies raise the question whether normalizing circadian rhythms in patients with cardiovascular disease may offer a new approach in risk reduction. Treatment interventions to the morning peak in adverse cardiovascular events (Fig. 5) could include: i. Pharmacological Chronotherapy, whereby medication would be targeted before the time of highest CV risk (morning), as in the case of β-blockers and aspirin ([68]); ii. modifying timing of behavioral triggers to minimize the impact of behaviors on cardiovascular risk, e.g., when to exercise [21]; and iii. use of small molecules to impact clocks to accelerate entrainment in shift work, jet lag, circadian rhythm sleep disturbances, including extreme chronotypes [69].
Figure 5: Prospects for circadian interventions to mitigate cardiovascular risk.
A: Interventions to decrease morning peak in major adverse cardiovascular events (CV events). Top panel: Pharmacological chronotherapy here refers to timing of cardiovascular medication (via timing administration and/or delayed/slow-release formulations) aimed at maximizing the dose during the vulnerable time while minimizing the dose at other times to decrease adverse side effects. Middle Panel: Although exercise lowers overall/long-term cardiovascular risk, it may carry increased acute risk of an adverse event for patients with cardiovascular disease, which could be mitigated by optimal timing of exercise within the biological day. Bottom panel: There may be a multiplicative effect of CV event risk factors produced by certain tissues in the body at a given circadian phase (e.g., circadian morning peak in PAI-1 and coinciding peak in platelet activation). If so, tissue-specific phase shifting of peripheral clocks in one (but not the other) tissue could serve to blunt the overall CV event risk during the vulnerable time window.
B: Interventions to mitigate overall risk/adverse CV effects of chronodisruption. Top panel: Mounting evidence suggests that optimally timed exogenous melatonin may decrease blood pressure. Middle panel: During circadian misalignment, evidence is developing that confining meals to the biological day may decrease metabolic/glucoregulatory sequelae of circadian misalignment. Bottom panel: A long-term goal of treating circadian misalignment is the development of therapeutics, e.g., small molecules, accelerating re-entrainment of the central and/or peripheral clocks to shifted behavioral and environmental cycles, e.g., for shift workers, jet lag, and circadian rhythm sleep/wake disorders.
Concluding Remarks and Future Perspectives
Time-of-day patterns in the risk of acute myocardial infarction were first described in 1963 [70]. Since then, numerous studies have reported time-of-day variations of system-level markers of cardiovascular function, and molecular and gene control in the heart, vasculature and kidneys, which may play a role in the onset and development of cardiovascular diseases. Central to these time-of-day fluctuations is the modulatory role of the circadian timing system as well as its interaction with behavioral/environmental triggers. Epidemiological data show an association between shift work and cardiovascular disease [6], and controlled experimental human studies indicate that circadian misalignment (typical in shift workers) adversely affects cardiovascular risk factors. These studies strengthen the argument that the circadian system and the deleterious effects of circadian disruption/misalignment need to be considered in the assessment of cardiovascular risk. Despite these advances, there are important gaps of knowledge (see Outstanding Questions). Human studies have focused on measures of system-level cardiovascular function, including HR, BP, ANS, cortisol, and measures of fibrinolysis, platelet activation, cytokines and chemokines, many of which are the typical systemic measures assessed under cardiovascular challenges (e.g. exercise tolerance testing). Additional measures to be considered include, e.g., ion channel involvement, and cardiac electrical conduction and repolarization, which focus on cardiac electrophysiology and are particularly suited for assessing mechanisms underlying ventricular arrhythmia. Furthermore, omics approaches (genomic, transcriptomic, proteomic, metabolomic) may help identify biomarkers of circadian disruption or sub-set susceptibility associated with cardiovascular risk [68], while tissue-specific omics (heart, endothelial function, vasculature smooth muscles, kidneys) may offer in-depth mechanistic insights. While animal models of circadian disruption provide important insights in clock gene expression in the heart and vasculature, these effects remain to be fully established across cardiometabolic tissues, and their relevance needs to be established in humans. Furthermore, in humans it is still unknown which specific exposures underlie the adverse effects of circadian misalignment. In other words, is it the sleep disturbance, and/or the mistiming of food intake, physical activity, posture, and/or the light/dark cycle? Addressing this question will aid in the identification of which facets of misalignment impact cardiovascular risk and help guide preventative and therapeutic interventions. Furthermore, most studies to date have assessed fundamental basic physiological processes in humans, with few (if any) preventative studies on circadian disruption in vulnerable populations. Future studies in humans are needed to establish how potential circadian therapeutic interventions (Fig. 5), such as appropriately timed light exposure, meal timing, exercise, sleep strategies and/or timing of medications, can provide cardioprotective strategies to minimize cardiovascular risk in shift workers and populations with cardiovascular disease.
Outstanding Questions.
What are the metabolic, gene and protein pathways disrupted by circadian misalignment that contribute to increased cardiovascular risk in humans? System biology approaches integrating “omics” techniques, such as transcriptomics, proteomics, metabolomics and genomics, are currently being applied to sleep loss and more recently to circadian misalignment paradigms, yet mechanistic insight into increased cardiovascular risk is limited.
How does circadian disruption affect different organ systems associated with cardiometabolic control, and what is their relative contribution to the increased cardiovascular risk? Animal models using circadian disruption paradigms have provided key insights on the molecular and clock gene expression in heart tissues. However, these effects still remain to be fully established across cardiometabolic tissues.
Which behavioral/environmental exposures underly the adverse consequences of circadian misalignment: is it the sleep disturbance, the mistiming of food intake, of physical activity, of posture, or LD cycle? Addressing this question will help to develop targeted behavioral, environmental and pharmacological approaches to mitigate cardiovascular risk associated with circadian misalignment.
How can we mitigate or prevent the adverse consequences of circadian disruption/misalignment in individuals susceptible to cardiovascular risk? Can we use individualized recommendations using chronotherapeutic approaches, such as light therapy, time of food intake, sleep strategies (e.g. naps), physical activity and/or timing of medication intake (i.e. melatonin supplementation, modafinil, caffeine, sleep aids)?
Trends.
Adverse cardiovascular events, including myocardial infarction, arrythmias and stroke, show time-of-day variations. Underlying factors may include the circadian system control over a plethora of markers associated with cardiovascular function.
Our modern lifestyle, which includes shift work, jet lag and disturbed sleep, has been associated with increased cardiovascular risk.
Misalignment of the endogenous circadian timing system and behavioral/environmental cycles can adversely impact cardiovascular function both in animal models and in human studies. These mechanistic insights may help explain why some aspects of our modern lifestyle increase cardiovascular risk.
Circadian disruption may play a role in the onset and development of cardiovascular disease, and treatments aimed at mitigating circadian disruption may benefit cardiovascular risk.
Acknowledgements
S.L.C was supported by NIH grant R01HL118601. N.V was supported by NIH grant R01 DK099512. J. S. W. was supported by NIH grants R01HL127146, R01HL136567, R01HL141406, P01AG009975, R56HL114765, R01HL144779. F.A.J.L.S. was supported by NIH grants R01HL094806 R01HL118601, R01DK099512, R01DK102696, and R01DK105072 and R01HL140574.
Glossary
- Circadian disruption
Disruption of endogenous circadian rhythms. This can occur from the level of the molecular clock (that temporally regulates cellular activities) to misalignment between behavioral and environmental cycles.
- Circadian misalignment
Misalignment between the endogenous circadian timing system and behavioral/environmental cycles (i.e. sleep/wake, light/dark, fasting/feeding).
- Circadian rhythm
Any biological process with an endogenous, entrainable oscillation of ~24 hours that is persistent under constant environmental conditions. Circadian rhythms can be synchronized to the environmental cycle by the light/dark cycle.
- Day/night rhythms
A rhythm in physiology or behavior over the 24-h light/dark cycle. When environmental and behavioral changes are present (e.g., light/dark cycle), it is virtually impossible to tease apart whether day/night rhythms are endogenously generated or whether they are a consequence of behavioral/environmental changes.
- Dim light melatonin onset
The onset of melatonin secretion when individuals are under dim light (typically < 5lux) exposure. DLMO is useful for determining whether an individual is entrained (synchronized) to a 24-h light/dark cycle, and for assessing phase delays or advances of rhythms in entrained individuals.
- Epinephrine
A hormone, also known as adrenaline, secreted by the adrenal medulla upon stimulation by the central nervous system in response to stress, as anger or fear, and under strong circadian control, and acting to increase heart rate, blood pressure, cardiac output, and carbohydrate metabolism.
- Heart rate variability
The physiological phenomenon of variation in the time interval between heartbeats. It is measured by the variation in the beat-to-beat interval and is of important cardiovascular research value.
- LF/HF power ratio
The ratio of low frequency (LF) to high frequency (HF) power, which allows for an approximation of the ratio between sympathetic nervous system (SNS) and parasympathetic nervous system (PNS) activity, although the validity of the measure depends on the conditions.
- Norepinephrine
A hormone, also known as noradrenaline, secreted by the adrenal medulla. Norepinephrine release is lowest during sleep, rises during wakefulness, with higher levels during situations of stress or danger.
- Presyncopal events
A symptom or prodrome to the sudden and transient loss of consciousness due to transient global cerebral hypoperfusion, which is associated with hypotension and/or bradycardia.
- Phase angle of entrainment
The relationship between the timing of two entrained oscillations. In chronobiology, this is often used to describe the relative time difference between the central circadian clock (often estimated in humans by the dim light melatonin onset) and the timing of an external time cue (i.e., Zeitgeber), or behavior (e.g., bedtime).
- Plasminogen activator inhibitor-1 (PAI-1)
Primary inhibitor of tissue plasminogen activator, and thereby an inhibitor of fibrinolysis (breakdown of blood clots). PAI-1 is thereby associated with increased risk for development of occlusive thrombi (blood clots in the vascular system).
- Platelet aggregability
The likelihood or ability of blood platelets to clump together as part of the sequence of events leading to the formation of a thrombus.
- Shift work
Work schedule outside the traditional 9:00AM – 5:00PM day. It can involve evening or night shifts, early morning shifts, and rotating shifts.
- Sympathovagal balance
Reflects the autonomic state resulting from the sympathetic and parasympathetic influences.
Footnotes
Conflicts of interest
F.A.J.L.S. has received lecture fees from Bayer HealthCare, Sentara HealthCare, Philips, Vanda Pharmaceuticals, and Pfizer Pharmaceuticals. S.L.C, N.V and J.S.W report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roth GA et al. (2017) Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. Journal of the American College of Cardiology 70 (1), 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muller JE et al. (1985) Circadian variation in the frequency of onset of acute myocardial infarction. The New England journal of medicine 313 (21), 1315–22. [DOI] [PubMed] [Google Scholar]
- 3.Suarez-Barrientos A et al. (2011) Circadian variations of infarct size in acute myocardial infarction. Heart 97 (12), 970–6. [DOI] [PubMed] [Google Scholar]
- 4.Krantz DS et al. (1996) Circadian variation of ambulatory myocardial ischemia. Triggering by daily activities and evidence for an endogenous circadian component. Circulation 93 (7), 1364–71. [DOI] [PubMed] [Google Scholar]
- 5.Organization WH (2011) Global status report on noncommunicable diseases 2010. Geneva: World Health Organization. [Google Scholar]
- 6.Vetter C et al. (2016) Association Between Rotating Night Shift Work and Risk of Coronary Heart Disease Among Women. JAMA 315 (16), 1726–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown DL et al. (2009) Rotating night shift work and the risk of ischemic stroke. American journal of epidemiology 169 (11), 1370–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawachi I et al. (1995) Prospective study of shift work and risk of coronary heart disease in women. Circulation 92 (11), 3178–82. [DOI] [PubMed] [Google Scholar]
- 9.Fujino Y et al. (2006) A prospective cohort study of shift work and risk of ischemic heart disease in Japanese male workers. American journal of epidemiology 164 (2), 128–35. [DOI] [PubMed] [Google Scholar]
- 10.Ha M and Park J (2005) Shiftwork and metabolic risk factors of cardiovascular disease. Journal of occupational health 47 (2), 89–95. [DOI] [PubMed] [Google Scholar]
- 11.Murata K et al. (2005) Effects of shift work on QTc interval and blood pressure in relation to heart rate variability. International archives of occupational and environmental health 78 (4), 287–92. [DOI] [PubMed] [Google Scholar]
- 12.Wittmann M et al. (2006) Social jetlag: misalignment of biological and social time. Chronobiology international 23 (1-2), 497–509. [DOI] [PubMed] [Google Scholar]
- 13.Wong PM et al. (2015) Social Jetlag, Chronotype, and Cardiometabolic Risk. The Journal of clinical endocrinology and metabolism 100 (12), 4612–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knutson KL and von Schantz M (2018) Associations between chronotype, morbidity and mortality in the UK Biobank cohort. Chronobiology international 35 (8), 1045–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manfredini R et al. (2018) Daylight saving time, circadian rhythms, and cardiovascular health. Internal and emergency medicine 13 (5), 641–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martino TA et al. (2008) Circadian rhythm disorganization produces profound cardiovascular and renal disease in hamsters. American journal of physiology. Regulatory, integrative and comparative physiology 294 (5), R1675–83. [DOI] [PubMed] [Google Scholar]
- 17.Alibhai FJ et al. (2017) Disrupting the key circadian regulator CLOCK leads to age-dependent cardiovascular disease. Journal of molecular and cellular cardiology 105, 24–37. [DOI] [PubMed] [Google Scholar]
- 18.McGinnis GR et al. (2017) Genetic disruption of the cardiomyocyte circadian clock differentially influences insulin-mediated processes in the heart. Journal of molecular and cellular cardiology 110, 80–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ingle KA et al. (2015) Cardiomyocyte-specific Bmal1 deletion in mice triggers diastolic dysfunction, extracellular matrix response, and impaired resolution of inflammation. American journal of physiology. Heart and circulatory physiology 309 (11), H1827–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scheer FA et al. (2009) Adverse metabolic and cardiovascular consequences of circadian misalignment. Proceedings of the National Academy of Sciences of the United States of America 106 (11), 4453–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scheer FA et al. (2010) Impact of the human circadian system, exercise, and their interaction on cardiovascular function. Proceedings of the National Academy of Sciences of the United States of America 107 (47), 20541–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morris CJ et al. (2016) Circadian misalignment increases cardiovascular disease risk factors in humans. Proceedings of the National Academy of Sciences of the United States of America 113 (10), E1402–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris CJ et al. (2017) Circadian Misalignment Increases C-Reactive Protein and Blood Pressure in Chronic Shift Workers. Journal of biological rhythms 32 (2), 154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grimaldi D et al. (2016) Adverse Impact of Sleep Restriction and Circadian Misalignment on Autonomic Function in Healthy Young Adults. Hypertension 68 (1), 243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morris CJ et al. (2013) Day/night variability in blood pressure: influence of posture and physical activity. American journal of hypertension 26 (6), 822–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tofler GH et al. (1987) Concurrent morning increase in platelet aggregability and the risk of myocardial infarction and sudden cardiac death. The New England journal of medicine 316 (24), 1514–8. [DOI] [PubMed] [Google Scholar]
- 27.Mores N et al. (1994) Platelet alpha 2-adrenoceptors and diurnal changes of platelet aggregability in hypertensive patients. Journal of hypertension 12 (8), 939–45. [PubMed] [Google Scholar]
- 28.Bromfield SG et al. (2016) Cardiovascular Risk Factors and Masked Hypertension: The Jackson Heart Study. Hypertension 68 (6), 1475–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore RY (1997) Circadian rhythms: basic neurobiology and clinical applications. Annual review of medicine 48, 253–66. [DOI] [PubMed] [Google Scholar]
- 30.Roenneberg T et al. (2007) Epidemiology of the human circadian clock. Sleep medicine reviews 11 (6), 429–38. [DOI] [PubMed] [Google Scholar]
- 31.Duffy JF and Dijk DJ (2002) Getting through to circadian oscillators: why use constant routines? Journal of biological rhythms 17 (1), 4–13. [DOI] [PubMed] [Google Scholar]
- 32.Wittenbrink N et al. (2018) High-accuracy determination of internal circadian time from a single blood sample. The Journal of clinical investigation 128 (9), 3826–3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shea SA et al. (2011) Existence of an endogenous circadian blood pressure rhythm in humans that peaks in the evening. Circulation research 108 (8), 980–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krauchi K and Wirz-Justice A (1994) Circadian rhythm of heat production, heart rate, and skin and core temperature under unmasking conditions in men. The American journal of physiology 267 (3 Pt 2), R819–29. [DOI] [PubMed] [Google Scholar]
- 35.Hu K et al. (2004) Endogenous circadian rhythm in an index of cardiac vulnerability independent of changes in behavior. Proceedings of the National Academy of Sciences of the United States of America 101 (52), 18223–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scheer F et al. (2019) Impact of mental stress, the circadian system and their interaction on human cardiovascular function. Psychoneuroendocrinology 103, 125–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu K et al. (2011) Endogenous circadian rhythm in vasovagal response to head-up tilt. Circulation 123 (9), 961–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scheer FA et al. (2011) The human endogenous circadian system causes greatest platelet activation during the biological morning independent of behaviors. PloS one 6 (9), e24549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scheer FA and Shea SA (2014) Human circadian system causes a morning peak in prothrombotic plasminogen activator inhibitor-1 (PAI-1) independent of the sleep/wake cycle. Blood 123 (4), 590–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boivin DB et al. (2003) Circadian clock genes oscillate in human peripheral blood mononuclear cells. Blood 102 (12), 4143–5. [DOI] [PubMed] [Google Scholar]
- 41.Chellappa SL et al. (2017) In a Heartbeat: Light and Cardiovascular Physiology. Frontiers in neurology 8, 541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thosar SS et al. (2018) Role of the circadian system in cardiovascular disease. The Journal of clinical investigation 128 (6), 2157–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qian J and Scheer F (2016) Circadian System and Glucose Metabolism: Implications for Physiology and Disease. Trends in endocrinology and metabolism: TEM 27 (5), 282–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ingelsson E et al. (2006) Diurnal blood pressure pattern and risk of congestive heart failure. JAMA 295 (24), 2859–66. [DOI] [PubMed] [Google Scholar]
- 45.Gourine A and Gourine AV (2014) Neural mechanisms of cardioprotection. Physiology 29 (2), 133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dijk DJ and von Schantz M (2005) Timing and consolidation of human sleep, wakefulness, and performance by a symphony of oscillators. Journal of biological rhythms 20 (4), 279–90. [DOI] [PubMed] [Google Scholar]
- 47.McAlpine CS et al. (2019) Sleep modulates haematopoiesis and protects against atherosclerosis. Nature 566 (7744), 383–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tobaldini E et al. (2018) Short sleep duration and cardiometabolic risk: from pathophysiology to clinical evidence. Nature reviews. Cardiology. [DOI] [PubMed] [Google Scholar]
- 49.Leproult R et al. (2014) Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes 63 (6), 1860–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wright KP Jr. et al. (2015) Influence of sleep deprivation and circadian misalignment on cortisol, inflammatory markers, and cytokine balance. Brain, behavior, and immunity 47, 24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rudic RD et al. (2005) Bioinformatic analysis of circadian gene oscillation in mouse aorta. Circulation 112 (17), 2716–24. [DOI] [PubMed] [Google Scholar]
- 52.Martino TA et al. (2007) Disturbed diurnal rhythm alters gene expression and exacerbates cardiovascular disease with rescue by resynchronization. Hypertension 49 (5), 1104–13. [DOI] [PubMed] [Google Scholar]
- 53.West AC et al. (2017) Misalignment with the external light environment drives metabolic and cardiac dysfunction. Nature communications 8 (1), 417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Danesh J et al. (2000) Low grade inflammation and coronary heart disease: prospective study and updated meta-analyses. BMJ 321 (7255), 199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Castanon-Cervantes O et al. (2010) Dysregulation of inflammatory responses by chronic circadian disruption. Journal of immunology 185 (10), 5796–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaptoge S et al. (2014) Inflammatory cytokines and risk of coronary heart disease: new prospective study and updated meta-analysis. European heart journal 35 (9), 578–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Curtis AM et al. (2007) Circadian variation of blood pressure and the vascular response to asynchronous stress. Proceedings of the National Academy of Sciences of the United States of America 104 (9), 3450–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Young ME et al. (2014) Cardiomyocyte-specific BMAL1 plays critical roles in metabolism, signaling, and maintenance of contractile function of the heart. Journal of biological rhythms 29 (4), 257–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Buijs FN et al. (2014) The suprachiasmatic nucleus is part of a neural feedback circuit adapting blood pressure response. Neuroscience 266, 197–207. [DOI] [PubMed] [Google Scholar]
- 60.Durgan DJ et al. (2010) Short communication: ischemia/reperfusion tolerance is time-of-day-dependent: mediation by the cardiomyocyte circadian clock. Circulation research 106 (3), 546–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Montaigne D et al. (2018) Daytime variation of perioperative myocardial injury in cardiac surgery and its prevention by Rev-Erbalpha antagonism: a single-centre propensity-matched cohort study and a randomised study. Lancet 391 (10115), 59–69. [DOI] [PubMed] [Google Scholar]
- 62.Goncharuk VD et al. (2001) Neuropeptide changes in the suprachiasmatic nucleus in primary hypertension indicate functional impairment of the biological clock. The Journal of comparative neurology 431 (3), 320–30. [DOI] [PubMed] [Google Scholar]
- 63.Goncharuk VD et al. (2007) Corticotropin-releasing hormone neurons in hypertensive patients are activated in the hypothalamus but not in the brainstem. The Journal of comparative neurology 503 (1), 148–68. [DOI] [PubMed] [Google Scholar]
- 64.Scheer FA et al. (2004) Daily nighttime melatonin reduces blood pressure in male patients with essential hypertension. Hypertension 43 (2), 192–7. [DOI] [PubMed] [Google Scholar]
- 65.Mozdzan M et al. (2014) The effect of melatonin on circadian blood pressure in patients with type 2 diabetes and essential hypertension. Archives of medical science : AMS 10 (4), 669–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Favero G et al. (2017) Melatonin as an Anti-Inflammatory Agent Modulating Inflammasome Activation. International journal of endocrinology 2017, 1835195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Raygan F et al. (2017) Melatonin administration lowers biomarkers of oxidative stress and cardio-metabolic risk in type 2 diabetic patients with coronary heart disease: A randomized, double-blind, placebo-controlled trial. Clinical nutrition. [DOI] [PubMed] [Google Scholar]
- 68.Tsimakouridze EV et al. (2015) Therapeutic applications of circadian rhythms for the cardiovascular system. Frontiers in pharmacology 6, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen Z et al. (2018) Development and Therapeutic Potential of Small-Molecule Modulators of Circadian Systems. Annual review of pharmacology and toxicology 58, 231–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pell S and D'Alonzo CA (1963) Acute Myocardial Infarction in a Large Industrial Population: Report of a 6-Year Study of 1,356 Cases. JAMA 185, 831–8. [DOI] [PubMed] [Google Scholar]
- 71.Takahashi JS (2015) Molecular components of the circadian clock in mammals. Diabetes, obesity & metabolism 17 Suppl 1, 6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jeyaraj D et al. (2012) Circadian rhythms govern cardiac repolarization and arrhythmogenesis. Nature 483 (7387), 96–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rutters F et al. (2014) Is social jetlag associated with an adverse endocrine, behavioral, and cardiovascular risk profile? Journal of biological rhythms 29 (5), 377–83. [DOI] [PubMed] [Google Scholar]
- 74.Jankowiak S et al. (2016) Current and cumulative night shift work and subclinical atherosclerosis: results of the Gutenberg Health Study. International archives of occupational and environmental health 89 (8), 1169–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Durgan DJ et al. (2005) The intrinsic circadian clock within the cardiomyocyte. American journal of physiology. Heart and circulatory physiology 289 (4), H1530–41. [DOI] [PubMed] [Google Scholar]
- 76.Havakuk O et al. (2018) Shift Work and the Risk of Coronary Artery Disease: A Cardiac Computed Tomography Angiography Study. Cardiology 139 (1), 11–16. [DOI] [PubMed] [Google Scholar]
- 77.Doi M et al. (2010) Salt-sensitive hypertension in circadian clock-deficient Cry-null mice involves dysregulated adrenal Hsd3b6. Nature medicine 16 (1), 67–74. [DOI] [PubMed] [Google Scholar]
- 78.Uhm JY et al. (2018) The association between shift work and chronic kidney disease in manual labor workers using data from the Korea National Health and Nutrition Examination Survey (KNHANES 2011-2014). Annals of occupational and environmental medicine 30, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Buijs RM et al. (2003) The suprachiasmatic nucleus balances sympathetic and parasympathetic output to peripheral organs through separate preautonomic neurons. The Journal of comparative neurology 464 (1), 36–48. [DOI] [PubMed] [Google Scholar]
- 80.Black N et al. (2019) Circadian rhythm of cardiac electrophysiology, arrhythmogenesis, and the underlying mechanisms. Heart rhythm 16 (2), 298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Burford NG et al. (2017) Hypothalamic-Pituitary-Adrenal Axis Modulation of Glucocorticoids in the Cardiovascular System. International journal of molecular sciences 18 (10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Storch KF et al. (2002) Extensive and divergent circadian gene expression in liver and heart. Nature 417 (6884), 78–83. [DOI] [PubMed] [Google Scholar]
- 83.McNamara P et al. (2001) Regulation of CLOCK and MOP4 by nuclear hormone receptors in the vasculature: a humoral mechanism to reset a peripheral clock. Cell 105 (7), 877–89. [DOI] [PubMed] [Google Scholar]
- 84.Nonaka H et al. (2001) Angiotensin II induces circadian gene expression of clock genes in cultured vascular smooth muscle cells. Circulation 104 (15), 1746–8. [DOI] [PubMed] [Google Scholar]
- 85.Walters JF et al. (2006) Circadian variation in endothelial function is attenuated in postmenopausal women. Maturitas 54 (3), 294–303. [DOI] [PubMed] [Google Scholar]
- 86.Gumz ML et al. (2009) The circadian clock protein Period 1 regulates expression of the renal epithelial sodium channel in mice. The Journal of clinical investigation 119 (8), 2423–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fletcher EK et al. (2019) Cardiomyocyte transcription is controlled by combined MR and circadian clock signalling. The Journal of endocrinology. [DOI] [PubMed] [Google Scholar]
- 88.Rahman SA et al. (2015) Endogenous circadian regulation of pro-inflammatory cytokines and chemokines in the presence of bacterial lipopolysaccharide in humans. Brain, behavior, and immunity 47, 4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Man K et al. (2016) Immunity around the clock. Science 354 (6315), 999–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Duez H et al. (2008) Regulation of bile acid synthesis by the nuclear receptor Rev-erbalpha. Gastroenterology 135 (2), 689–98. [DOI] [PubMed] [Google Scholar]