Abstract
The global prevalence of obesity continues to increase, suggesting a need for alternative treatment approaches. Targeting brown fat function to promote energy expenditure represents one such approach. Brown adipocytes and the related beige adipocytes oxidize fatty acids and glucose to generate heat and are activated by cold exposure or consumption of high calorie diets. Alternative, more practical, means to activate thermogenic fat are needed. Here, we review emerging data suggesting new roles for lipids in activating thermogenesis that extend beyond their serving as a fuel source for heat generation. Lipids have also been implicated in mediating inter-organ communication, crosstalk between organelles, and cellular signaling regulating thermogenesis. Understanding how lipids regulate thermogenesis could identify innovative therapeutic interventions for obesity.
Keywords: Obesity, mitochondria, brown adipose tissue, beige fat, plasmalogen, thermogenesis
Thermogenic fat as a target for obesity therapy
The prevalence of obesity continues to rise worldwide. Body weight has increased consistently since 1975, resulting in an estimated 1.9 billion overweight people globally, a third with obesity [1]. Obesity leads to diabetes, which is associated with premature death from many causes including vascular disease, cancer, and infections [2]. Currently approved medications to treat obesity primarily target energy intake by blocking appetite or intestinal absorption of fat and are not ideal, suggesting a need for alternative treatment approaches. Increasing energy expenditure by activating brown adipose tissue (BAT) (see Glossary) function represents one such approach. White adipose tissue (WAT) stores excess energy, but BAT and the related beige adipocytes, which appear within WAT under certain conditions, oxidize fatty acids and glucose to generate heat through non-shivering thermogenesis (see Box 1), a form of adaptive thermogenesis that is distinct from shivering thermogenesis mediated by skeletal muscle. Brown fat cells and beige fat cells are activated by prolonged cold exposure or consumption of high calorie diets rich in fat and carbohydrates [3]. Considerable effort has been devoted to identifying more practical means to activate thermogenic fat. Toward this end, recent studies have revealed an array of lipids that regulate thermogenesis.
Lipids are the preferred fuel source for thermogenesis, but growing evidence suggests that their function in brown and beige adipocytes extends well beyond this role. Here, we review the molecular mechanism through which various lipids mediate inter-organ communication, organelle crosstalk, and intercellular or intracellular signaling to influence adipose tissue thermogenesis. The article will conclude with a discussion of pertinent directions for future research and the translational potential of lipid regulators of brown fat activation. A better understanding of how lipids control these processes could lead to a novel strategy for treating metabolic disorders.
Lipid regulators of inter-organ communication involved in thermogenesis
Brown fat-mediated thermogenesis contributes to systemic metabolism by promoting resting energy expenditure, whole-body glucose disposal and insulin sensitivity [4]. Conversely, the thermogenic function of BAT is influenced by its crosstalk with other organs (Figure 1). Lipids have emerged as key mediators of this inter-organ communication. In this section, we discuss the mechanisms through which lipids metabolized by other organs promote BAT-mediated thermogenesis and energy homeostasis. Structures and functions of various lipids discussed in this review are depicted in Table 1.
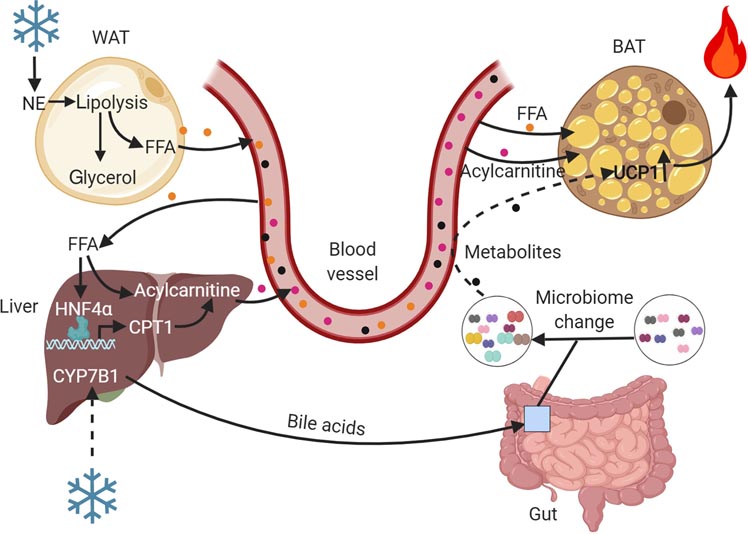
Figure 1. Inter-organ communication regulating brown fat-mediated thermogenesis.
Cold promotes lipolysis in WAT resulting in the release of free fatty acids (FFA) that directly serve as substrates for BAT-mediated thermogenesis or are delivered to the liver, where they undergo conversion to acylcarnitines due to an increased CPT1 gene expression mediated by HNF4α activation. Liver-derived acylcarnitines are an alternative fuel source for BAT-mediated thermogenesis. Cold exposure also activates the alternative pathway of bile acid synthesis in the liver. Bile acids induce changes in the gut microbiome, resulting in production of microbial metabolites with thermogenic activity. This figure was created using BioRender (https://biorender.com/).
Table 1.
Structures and functions of lipid regulators of thermogenic fat.
Role of lipolysis in WAT and heart in BAT-mediated thermogenesis
Lipolysis of intracellular lipid stores in thermogenic fat is activated by cold exposure or β3-adrenergic receptor (β3-AR) agonism, which stimulates the sympathetic nerves innervating adipose tissue to release norepinephrine (NE), initiating hydrolysis of endogenous triglycerides through a cAMP- and protein kinase A (PKA)-dependent pathway [5, 6]. Lipolysis requires adipose triglyceride lipase (ATGL), an NE-responsive enzyme that catalyzes the first and rate-limiting step in the process, hydrolyzing triglycerides to diglycerides [6]. Conventional wisdom holds that lipolysis in brown adipocyte lipid droplet is a prerequisite for thermogenesis through release of free fatty acids (FFA) that activate uncoupling protein 1 (UCP1) and serve as a fuel for heat generation [5]. This assumption was challenged by two recent studies designed to assess the role of lipolysis specifically in BAT [7,8]. BAT-specific knockout of ATGL or the ATGL-activating protein comparative gene identification-58 (CGI-58) in mice resulted in accumulation of lipid droplets in brown adipocytes, reflecting impaired lipolysis, as would be expected. Surprisingly, however, neither of these interventions affected energy expenditure or cold tolerance, suggesting that intracellular lipolysis in BAT is dispensable to elicit non-shivering thermogenesis [7, 8]. Nevertheless, knockout of ATGL or CGI-58 in both BAT and WAT severely impaired cold tolerance in the fasted state, suggesting that lipolysis in WAT fuels BAT-mediated thermogenesis in the absence of dietary lipids [7–9]. Beyond serving as a fuel for BAT-mediated thermogenesis, FFA released by WAT lipolysis following acute cold exposure or stimulation of β3-AR are thought to also promote insulin secretion from beta cells, permitting uptake of lipids into BAT for thermogenesis [10].
Apart from WAT, lipolysis in the heart may also be involved in inter-organ communication regulating thermogenesis [7]. Mice with global knockout of ATGL manifest severe triglyceride accumulation in the heart, progressive dilated cardiomyopathy, and impaired thermogenesis. Unlike adipose-specific ATGL knockout mice, the global knockout animals exhibit cold intolerance even in the fed state [11], suggesting that lipolysis in tissues besides adipose tissue contributes to thermogenesis. To explore the role of cardiac lipolysis in thermogenesis, Schreiber et al. [7] generated mice with tamoxifen-inducible heart-specific knockout of ATGL. The authors discovered that, like the global knockout animals, the cardiac-specific knockout mice developed impaired heart function and cold intolerance, despite having elevated circulating FFA levels. Although it is possible that the hypothermia in these mice is due to reduced availability of cardiac-metabolized substrates for thermogenesis, it more likely reflects the fact that normal cardiac function is required for delivery of circulating substrates for combustion in BAT. Indeed, progressive dilated cardiomyopathy due to knockout of very long-chain acyl-CoA dehydrogenase (VLCAD) also results in cold intolerance [12].
Hepatic acylcarnitines and adipose tissue thermogenesis
Besides directly serving as a fuel source for BAT-mediated heat generation, FFA derived from WAT lipolysis have also been shown to take a detour through the liver for conversion to acylcarnitines, which are then taken up by BAT for thermogenesis [13]. This circuitous route was uncovered when untargeted mass spectrometry-based lipidomic analysis indicated that plasma acylcarnitines are increased in response to cold exposure in mice. Acylcarnitine is a fatty acid oxidation intermediate derived from fatty acyl-CoA, the activated form of FFA. Production of acylcarnitines requires Cpt1, a mitochondrial enzyme that exchanges the CoA group for carnitine [14]. Simcox et al.[13] found that the gene expression of Cpt1, as well as its upstream transcriptional regulator HNF4α was significantly elevated in the liver of mice subjected to cold exposure. Inactivation of Cpt1 or Hnf4α in liver reduced circulating levels of acylcarnitines and impaired cold tolerance. The authors also discovered that adipose-specific knockout of ATGL inhibited β3-adrenergic receptor activation-mediated increase in hepatic Cpt1 expression and acylcarnitine secretion, suggesting that HNF4α activation requires release of FFA from adipose tissue lipolysis. Consistent with this possibility, time course studies suggested that lipolysis of WAT precedes hepatic acylcarnitine release. Finally, the authors demonstrated that cold-induced uptake of circulating acylcarnitines mainly occurs in BAT, where they are metabolized through the TCA cycle [13].
An important finding of the study by Simcox et al. is the identification of a novel role for the liver in BAT-mediated thermogenesis. However, additional work is needed to understand the mechanism through which liver-derived acylcarnitines are taken up by BAT and the relative contribution of acylcarnitines versus other fuel sources in brown adipocyte thermogenesis.
Gut microbiota-metabolized lipids and adipose thermogenesis
Emerging evidence also implicates gut microbiota, the trillions of microorganisms living in the gastrointestinal tract, in mediating inter-organ communication involved in thermogenic fat activation. The gut microbiota evolves with the host and can modulate the host energy metabolism. Given this symbiotic relationship, there is an intense interest in understanding how the gut microbiota can be reshaped to benefit the host [15]. With regard to thermogenesis, recent studies suggest cold temperature can alter the gut microbiota in mice in a manner that promotes adipose tissue thermogenesis and increases energy expenditure. Fecal transplant from cold-treated mice to germ-free recipient mice housed at room temperature resulted in increased thermogenic gene expression, improved cold tolerance and decreased adiposity [16, 17]. These results were linked to changes in the host bile acid metabolism [17].
Bile acids are important for postprandial emulsification and absorption of dietary lipids. They are produced exclusively by the liver from cholesterol by either the classic pathway, which starts with the ER-enzyme 7α-hydroxylase (CYP7a1)-mediated hydroxylation of the steroid nucleus of cholesterol at the C-7 position, or by the alternative pathway, in which the synthesis is initiated by the mitochondrial enzyme sterol 27-hydroxylase (CYP27A1)-mediated hydroxylation at the 27 position, followed by hydroxylation at the 7 position by oxysterol 7α-hydroxylase [18]. Recent work revealed that cold promotes selective activation of the alternative pathway, resulting in a marked increase in fecal bile acid secretion that was accompanied by distinctly altered gut microbiome [19]. Global inactivation of Cyp7B1 in mice decreased cold-induced fecal bile acid secretion in the setting of impaired cold tolerance and reduced energy expenditure. Conversely, Cyp7b1 overexpression had the opposite effects, suggesting that bile acid synthesis through the alternative pathway is involved in thermogenesis [19]. However, it is unclear whether the bile acid-mediated regulation of thermogenesis occurs through modulation of gut microbiota or via direct effects on adipose tissue. In support of the former possibility, bile acid-induced changes in gut microbiome could result in production of microbial metabolites with thermogenic activity, such as 10-oxo-12(Z)-octadecenoic acid [20]. Alternatively, bile acids have been shown to directly promote energy expenditure by increasing BAT activity through activation of type 2 iodothyronine deiodinase, which converts the inactive thyroid hormone thyroxine to active 3-5-3’-triiodothyronine [21]. Future research will be necessary to better understand how lipids metabolized by gut microbes influence thermogenesis.
Lipid mediators of organelle crosstalk in the regulation of thermogenesis
Owing to their fundamental role in energy metabolism, mitochondria are critical for thermogenesis. Recent studies suggest that mitochondria, however, do not carry out this function in isolation and instead require cooperation with other organelles. In this regard, mitochondrial membrane lipids have emerged as key mediators of crosstalk between mitochondria and other organelles, including the nucleus and peroxisomes, in the regulation of adipose tissue thermogenesis.
Plasmalogens in peroxisome-mitochondria crosstalk regulating thermogenesis
Peroxisomes are versatile organelles that perform a variety of metabolic functions, including catabolic and anabolic roles related to lipid metabolism [22]. Catabolic functions of peroxisomes include β-oxidation of very long chain fatty acids (VLCFA) and α-oxidation of branched chain fatty acids, both of which cannot be oxidized in mitochondria. Peroxisomal fatty acid oxidation generates reactive oxygen species, which are rapidly catabolized due to the abundance of catalase in the organelle. Anabolic functions of peroxisomes include synthesis of bile acids and ether lipids, a special class of phospholipids in which the hydrocarbon chain at the sn-1 position of the glycerol backbone is linked by ether bond, in contrast to ester bond in the conventional phospholipids (Box 2).
Peroxisomes, like mitochondria, have long been known to be abundant in BAT [23, 24]. Both are highly dynamic organelles that can modify their morphology, abundance and function in response to physiological signals. For example, mitochondria undergo cycles of fission and fusion and the morphology is dependent on the metabolic context [25]. In brown adipocytes, activation of mitochondrial fission in response to thermogenic stimuli is thought to potentiate FFA-induced uncoupling and energy expenditure [26]. Our recent work identified a role for peroxisomal lipid metabolism in thermogenesis through regulation of mitochondrial dynamics in BAT [27]. Cold exposure promoted peroxisomal biogenesis in brown and beige adipocytes through activation of the thermogenic transcription factor PRDM16 (Figure 2). Inhibition of peroxisomal biogenesis through adipose-specific knockout of the critical peroxisomal biogenesis factor Pex16 impaired cold-induced mitochondrial fission, decreased mitochondrial DNA content and caused mitochondrial dysfunction, resulting in severe cold intolerance and increased diet-induced obesity. Mechanistically, these effects were attributed to impaired peroxisomal synthesis of ethanolamine plasmalogens, the ether lipid equivalent of phosphatidylethanolamine, which we demonstrated are present in mitochondrial membrane. Of note, dietary supplementation of plasmalogens rescued cold-induced mitochondrial division and thermogenesis in the Pex16 deficient mice, suggesting that peroxisomes channel lipids to mitochondria in brown and beige adipocytes to regulate mitochondrial dynamics and thermogenesis [27].
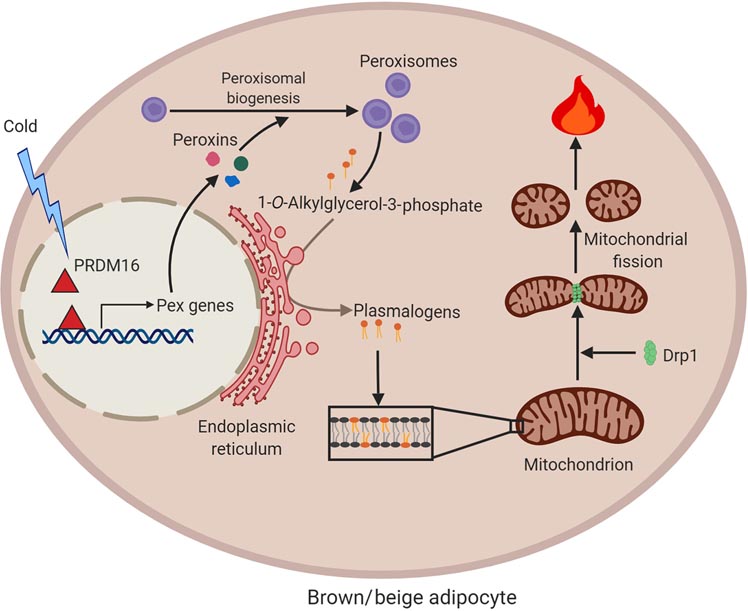
Figure 2. Crosstalk between peroxisomes and mitochondria in the regulation of mitochondrial dynamics and thermogenesis.
Cold promotes peroxisomal biogenesis in brown and beige fat through activation of the thermogenic co-regulatory protein PRDM16. Peroxisomes are required for synthesis of plasmalogens, ether-linked phosphophlipids, which are present in mitochondria. Plasmalogens are necessary for mitochondria to undergo cold-induced fission, presumably due to their effects on recruitment and/or activity of fission factors, such as Drp1. Mitochondrial fission is linked to increased thermogenic activity. This figure was created using BioRender (https://biorender.com/).
Plasmalogens have been hypothesized to regulate membrane dynamics, owing to the presence of a vinyl ether bond at the sn-1 position of their glycerol backbone, which promotes the formation of non-lamellar lipid structures [28–30]. However, the molecular mechanism through which plasmalogens might regulate mitochondrial fission remains to be defined and could potentially involve effects on mitochondrial localization and/or activity of fission factors, such as dynamin-related protein 1 (Drp1), a cytosolic GTPase that is recruited to the mitochondrial outer membrane to mediate fission [31].
Cardiolipin-mediated mitochondria-to-nucleus signaling in brown fat
Cardiolipin, another mitochondrial membrane lipid implicated in thermogenesis [32–34], is a unique dimeric phospholipid synthesized from phosphatidylglycerol and CDP-DAG using the enzyme cardiolipin synthase 1 (CRLS1). Originally discovered in bovine heart, which is the basis of their name, cardiolipins are a key component of the inner mitochondrial membrane, comprising up to 20% of the total mitochondrial membrane phospholipid content [35]. Recently, two separate groups demonstrated that cold exposure promotes a marked increase in the levels of cardiolipins in brown and beige adipocytes, suggesting that this unique phospholipid may be involved in thermogenesis [36, 37]. In support of this possibility, Sustarsic et al. [37] demonstrated that mice with adipose-specific knockout of CRLS1 have reduced NE-induced uncoupled respiration and impaired cold tolerance. Surprisingly, the CRLS1 inactivation also strikingly decreased the gene expression of UCP1. Conversely, ectopic expression of CRLS1 in adipocytes increased UCP1 gene expression and promoted NE-stimulated oxygen consumption, suggesting the cardiolipins might be involved in retrograde communication from the mitochondria to the nucleus. To understand the potential mechanism underlying cardiolipin-mediated regulation of thermogenic gene expression, the authors performed global gene expression analysis. This indicated that several downstream targets of C/EBPα homologous protein 10 (CHOP-10), a transcription factor involved in ER stress [38] were markedly elevated in the BAT from CLRS1 mutant mice. Interestingly, knockdown of CHOP-10 prevented the CRLS1 inactivation-mediated decrease in UCP1 and other nuclear-encoded mitochondrial genes in brown adipocytes, suggesting that cardiolipins might mediate mitochondria-to-nucleus signaling to regulate thermogenesis.
The precise mechanism through which cardiolipins might regulate mitonuclear signaling and thermogenesis remains unclear. As noted above, cardiolipins are an important component of the mitochondrial membrane. They have been shown to interact with various mitochondrial membrane proteins, including components of the oxidative phosphorylation-associated electron transport chain complex [39, 40]. Importantly, cardiolipins have been shown to directly interact with UCP1, promoting its proper folding, stabilizing it in the mitochondrial membrane and preventing its interaction with inhibitory purine nucleotides [33, 34]. It is conceivable that cardiolipin deficiency disrupts mitochondrial membranes, resulting in the mitochondrial unfolded protein response (UPRmt). In addition to its role in ER stress, CHOP-10 has been implicated in UPRmt [41] and might mediate mitochondrial retrograde signaling to restore mitochondria proteostasis in the face of cardiolipin deficiency. It is possible that other mechanisms might also be involved in cardiolipin-mediated regulation of thermogenesis. For instance, cardiolipins have been shown to regulate mitochondrial dynamics through their ability to recruit Drp1 [42, 43].
In addition to regulating thermogenesis, cardiolipin synthesis in adipose tissue is important for maintaining insulin sensitivity. Adipose-specific knockout of CRLS1 in mice was associated with glucose intolerance and insulin resistance in the context of lipodystrophy characterized by markedly decreased adipose tissue weight and increased liver weight [37]. It remains to be determined whether this reflects a potential role for cardiolipins in adipose tissue development and/or lipolysis. It is also unknown whether these effects are related to or independent of the role of cardiolipins in mitochondria. Future research will be required to explore these possibilities.
Cell signaling roles of lipids related to thermogenesis
Increasing evidence suggests that lipids impact cell signaling through a variety of mechanisms [44, 45]. These include interacting with cell surface receptors, such as G-protein coupled receptors (GPCR) to initiate downstream signaling, serving as endogenous ligands of nuclear receptor to regulate gene expression, and modulating localization and/or function of proteins through post-translational modifications. Recent studies have also identified roles for lipids in intercellular and intracellular signaling related to brown and beige fat activation. In this section, we discuss mechanisms through which lipid signals activate thermogenesis to impact adiposity and metabolism.
Regulation of fatty acid uptake in BAT by 12,13-diHOME
WAT is recognized as an important endocrine organ that secretes a variety of adipokines that influence systemic metabolism. Growing evidence suggests that BAT also has a prominent secretory role [46]. Recently, a mass spectrometry-based lipidomics analysis led to the identification of 12,13-dihydroxy-9Z-octadecenoic acid (12,13-diHOME) as a BAT-enriched secreted lipid involved in thermogenesis [47]. Biosynthesis of 12,13-diHOME involves the enzymes epoxide hydrolase 1 and epoxide hydrolase 2 (Ephx1 and Ephx2), which use epoxide derivatives of the essential fatty acid linoleic acid as substrate. Lynes et al. [47] demonstrated that the gene expression of Ephx1 and Ephx2 in BAT increases with cold exposure in mice. The authors also showed that the plasma levels of 12,13-diHOME increase in humans and mice after acute cold exposure and correlate positively with BAT activity according to positron emission tomography (PET)/computed tomography (CT) analysis of [18F]fluorodeoxyglucose (FDG) uptake and negatively with obesity and insulin resistance in humans. Administration of 12,13-diHOME enhanced the ability of mice to tolerate a cold challenge, suggesting that this lipokine promotes thermogenesis. With regard to the mechanism, it appears that 12,13-diHOME acts in an autocrine or paracrine manner to promote uptake of FFA into brown adipocytes by increasing plasma membrane localization of the fatty acid transporters FATP1 and CD36 (Figure 3) [47]. Interestingly, BAT-derived circulating 12,13-diHOME also increases in response to exercise and promotes fatty acid uptake and oxidation in skeletal muscle [48], suggesting that the lipokine has other roles besides regulation of heat generation.
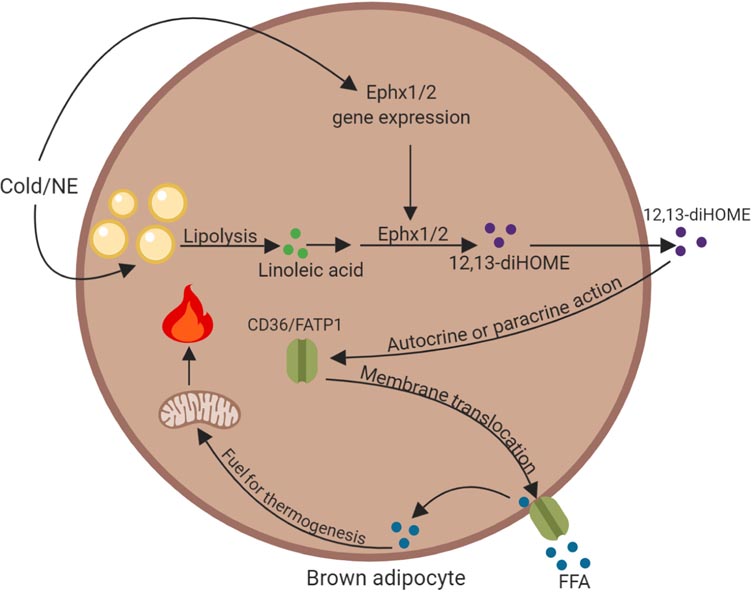
Figure 3. Role of the cold-induced lipokine 12,13-diHOME in fatty acid uptake in brown adipocytes.
Cold exposure or norepinephrine (NE) stimulation activates the production of 12,13-diHOME by increasing the gene expression of epoxide hydrolase 1 and epoxide hydrolase 2 (Ephx1/2) and promoting lipolysis to release linoleic acid. The epoxide derivative of this essential fatty acid is used by Ephx1/2 as a substrate to synthesize 12,13-diHOME. It is possible that linoleic acid might also be derived directly from diet. Through autocrine or paracrine action, 12,13-diHOME promotes plasma membrane translocation of fatty acid transporters CD36 and FATP1, allowing uptake of free fatty acids (FFA), which are a critical fuel source for thermogenesis. This figure was created using BioRender (https://biorender.com/).
Additional work is required to determine precisely how 12,13-diHOME regulates FFA transport and to understand other aspects of its biology. Of note, the circulating levels of 12,13-diHOME are not decreased following genetic ablation of BAT [47], suggesting that beige fat or other tissues might also contribute to the circulating levels of the lipokine. It is also noteworthy that 12,13-diHOME is also synthesized by the peripheral nervous tissue and may be involved in mediating thermal hyperalgesia during inflammatory pain by promoting calcium transients via the transient receptor potential vanilloid 1 (TRPV1) ion channel in sensory neurons [49]. TRPV1 has also been implicated in regulating energy expenditure [50]. Whether the effects of 12,13-diHOME on BAT-mediated thermogenesis involve signaling through TRPV1 remains to be determined. Moreover, it will be of great interest to determine the physiological relevance of the endogenous 12,13-diHOME production in systemic energy metabolism using mice with knockout of Ephx1 or Ephx2.
Mevalonate pathway in the regulation of adipocyte browning through protein prenylation
Endogenous lipid synthesis has also been linked to adipose tissue browning [51, 52]. In this line of investigation, a recent translational study identified a signaling role for the mevalonate pathway of cholesterol synthesis in adipocyte browning [53]. The mevalonate pathway is the target of statin family of cholesterol-lowering drugs, which specifically inhibit HMG-CoA reductase, the enzyme that catalyzes rate-limiting step in cholesterol synthesis. To understand mechanisms regulating themogenic fat function in humans, Balaz et al. [53] performed transcriptome analysis on deep neck BAT and subcutaneous WAT biopsies and observed that HMG-CoA synthase (HMGCS2), the enzyme just upstream of HMG-CoA reductase in the cholesterol synthetic pathway, was highly enriched in BAT and its expression correlated strongly with UCP1 gene expression. The authors found that inhibition of the mevalonate pathway through knockdown of HMGCS2 or treatment with statins significantly reduced UCP1 gene expression in cultured human or mouse adipocytes. Treatment of mice with statins also blocked cold-induced browning of iWAT. The authors translated these results to humans by conducting a retrospective study of patients undergoing FDG-PET/CT analysis of BAT glucose uptake, which showed that statin use inversely correlates with BAT activity. They also performed a small prospective trial and observed that statin use decreases thermogenic gene expression in human BAT. Mechanistically, the authors discovered that the inhibitory effects of statins on UCP1 gene expression could be rescued by the mevalonate pathway intermediate geranylgeranyl pyrophosphate (GGPP), a substrate of geranylgeranyltransferase I (GGTase I). This enzyme mediates protein prenylation, a post-translational modification that affects subcellular localization of proteins [54]. Balaz et al. showed that pharmacological or genetic inactivation of GGTase I blocks adipocyte browning, mimicking the effects of statins. Finally, the authors presented data suggesting that GGTase I mediates geranylgeranylation of small GTP-binding proteins to promote F-actin formation, resulting in the stability of mechanosensitive transcriptional coactivators YAP/TAZ. How YAP/TAZ activation leads to increased browning remains to be determined. Due to the pleiotropic effects of small G-proteins, it is possible that other mechanisms might also be at play.
Omega-3 fatty acid-mediated regulation of thermogenesis
Lipid signaling involved in thermogenic fat activation is not exclusively mediated by endogenously synthesized lipids. Polyunsaturated fatty acids (PUFA), primarily obtained through diet, may also have a signaling role related to thermogenesis. In mice, a diet rich in PUFA has also been shown to promote the thermogenic capacity of brown fat by increasing the UCP1 content and amplifying NE-stimulated oxygen consumption [55]. These beneficial effects are linked to omega-3 fatty acids, such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) rather than other forms of PUFA, such as omega-6 fatty acids, such as arachidonic acid (ARA), which has reported to inhibit adipocyte browning [56]. EPA has been shown to reverse the inhibitory effect of ARA, promote brown adipocyte differentiation, increase UCP1 gene expression, improve the thermogenic response of BAT and inguinal WAT to β3-AR stimulation, and decrease adiposity in mice [57, 58].
Omega-3 fatty acids preferentially interact with GRP120, a GPCR that is also called free fatty acid receptor 4 (FFAR4) [59, 60]. Loss-of-function mutations in GRP120 are associated with an increased risk for obesity in humans [61]. The receptor is thought to mediate obesity-protective, anti-inflammatory, and insulin-sensitizing effects of dietary lipid signals, including omega-3 fatty acids [59, 61]. Recently, a study by Quaesada-Lopez et al. [62] demonstrated that GPR120 is enriched in BAT, where its expression further increases with cold exposure or β3-AR stimulation. The authors observed that pharmacological activation of GPR120 induces brown adipocyte differentiation, increases UCP1 gene expression and promotes energy expenditure through induction of FGF21 release, while knockout of the GPCR impairs cold-induced browning of white fat in mice. Notably, the GPR120 knockout abolishes thermogenic effects of EPA, including increased brown adipogenesis and elevated UCP1 gene expression [62]. It is unclear whether EPA directly regulates thermogenic gene expression or if this is related to the increased brown adipogenesis. Moreover, additional work will be required to determine if the effects of EPA on brown adipocytes are mediated by FGF21.
Breast milk alkylglycerols and control of beige fat development
In addition to the increasing prevalence of obesity in adults, childhood obesity is currently a serious public health problem [63]. Emerging studies suggest a link between breastfeeding and a decreased risk of obesity in children [64, 65]. However, the underlying mechanisms have remained unclear. A recent study by Yu et al. [66] provides some important insight. The authors studied the lipid contents of human and mouse breast milk and discovered that several species of alkylglycerol were abundantly present. As discussed in Box 2, these peroxisome-derived lipids are precursors of ether-linked phospholipids. The authors observed that treatment of neonatal mice with alkylglycerols resulted in decreased weight of inguinal WAT with increased appearance of multilocular beige adipocytes exhibiting elevated UCP1 gene expression and mitochondrial content. Using biopsy samples from human infants, the authors showed that breast-fed infants had a higher beige fat amount as compared to formula-fed children. Interestingly, the authors observed that the increased mitochondrial content, UCP1 expression, and energy expenditure in adipocytes were dependent on adipose tissue macrophages, as treatment of cultured adipocytes directly with alkylglycerols did not elicit these effects, while coculture of adipocytes with macrophages promoted adipocyte browning by alkyglycerols. Mechanistically, the authors discovered that macrophages convert alkylglycerols to a bioactive lipid called platelet activating factor (PAF), which acts in a paracrine manner to induce IL-6 secretion from macrophages, leading to JAK/STAT3 pathway activation in adipocytes and promotion of beige fat development (Figure 4) [66].
Figure 4. Breast milk alkylglycerols regulate beige fat development.
Alkylglycerols derived from breast milk are converted to platelet activating factors (PAF) in macrophages. PAF act in an autocrine manner to induce IL-6 secretion from macrophages, leading to JAK/STAT3 pathway activation in adipocyte precursors and promotion of beige fat development. This figure was created using BioRender (https://biorender.com/).
Additional work is needed to understand how alkylglycerol-mediated activation of the JAK/STAT3 pathway leads to the appearance of beige adipocytes. Moreover, it is unclear whether these lipids promote browning of existing white fat or development of beige fat through differentiation of precursors. Given that alkylglycerols are transferred via breast milk during early postnatal life when adipose tissue is rapidly developing and that treatment of adult mice with these lipids promotes the appearance of beige adipocytes only after prolonged high fat feeding [66], at which point the adipose tissue is thought to further expand by adipocyte hyperplasia [67], it is likely that alkylglycerols regulate de novo beige fat development. Nevertheless, this work has uncovered an important role for these naturally occurring lipids with therapeutic potential to treat obesity in children and adults.
Concluding remarks and future perspectives
It is now apparent that under certain conditions, physiologically relevant amount of brown-like fat exists in adult humans [68–70]. With the increasing evidence positively linking thermogenic adipose tissue to metabolic health [4, 71], the possibility of targeting brown fat function in prevention or treatment of obesity and the associated type-2 diabetes becomes appealing. Remarkably, lipids have emerged as key regulators of brown fat function. Recent work underscores the notion that lipids not only serve as a substrate for heat generation in brown fat, but also play regulatory roles in mitochondrial dynamics and bioenergetics, brown adipocyte gene expression, adipocyte differentiation, and cellular signaling involved in thermogenesis. While the importance of lipids as a fuel source for thermogenesis is well appreciated, emerging studies suggest that these other roles of lipids are also critical in the biology of brown and beige adipocytes. Understanding the diverse roles of lipids in thermogenic fat represents a fertile area for future research and has the potential for rapid translation to human obesity. Significant progress has been made in identifying lipid signals controlling brown fat activation, but additional work is required to elucidate the underlying mechanisms and to determine whether the thermogenesis regulatory activity of lipids could be leveraged for obesity prevention or treatment (see Outstanding Questions Box). In this regard, ether lipids, such as alkylglycerols and plasmalogens, appear to be promising candidates for future diet interventions studies. A better understanding of how these and other lipids control brown fat function could lead to a novel strategy for treating metabolic disorders.
Outstanding Questions Box.
Since lipolysis in BAT is dispensable for thermogenesis, what is its physiological function? How exactly is UCP1 activated in brown adipocytes?
How do cardiolipins mediate mitonuclear signaling involved in thermogenic gene expression?
What is the physiological relevance of the endogenous 12,13-diHOME production in systemic energy metabolism? How does this lipokine regulate fatty acid transport?
How do changes in mitochondrial morphology affect the thermogenic function of brown adipocytes? What role do plasmalogens play in regulating mitochondrial dynamics?
Could dietary interventions incorporating ether lipids, such as alkylglycerols and plasmalogens, be used to effectively treat obesity?
Box 1. Adipose tissue thermogenesis
Brown adipose tissue (BAT) is specialized to conduct non-shivering thermogenesis (NST) due to its ability to burn lipids and glucose to generate heat via uncoupled respiration [3]. The thermogenic activity of BAT is primarily induced by cold exposure, but can also be activated by high-calorie diet enriched in fat and carbohydrates [72]. These stimuli promote the sympathetic nerves innervating the adipose tissue to release norepinephrine (NE), which bind to the β3-adrenergic receptors (β3-AR) present on the plasma membrane of adipocytes, initiating a signaling cascade that causes release of free fatty acids (FFA) from stored triglycerides. NE also promotes uptake of glucose and lipids into brown adipocytes and increases the gene expression of proteins involved in thermogenesis, including uncoupling protein 1 (UCP1), the hallmark of BAT [73]. UCP1 is a transmembrane protein residing in the inner mitochondrial membrane of brown adipocyte and is the primary mediator of NST. It dissipates chemical energy as heat by allowing protons to leak across the inner mitochondrial membrane, bypassing ATP synthase. In addition to brown adipocytes, brown-like beige adipocytes are involved in thermogenesis. Beige adipocytes appear within white fat in response to cold exposure or β3-AR activation. Like brown adipocytes, beige adipocytes are enriched in mitochondria and express UCP1. Beige adipocytes express UCP1 at levels comparable to the classical brown adipocytes when fully stimulated, suggesting that these brown-like adipocytes play a physiologically relevant role in thermogenesis [74–76]. Beige adipocytes are also involved in UCP1-independent mechanisms of thermogenesis, including ATP-consuming futile cycles, namely calcium cycle mediated by the sarcoendoplasmic reticulum calcium ATPase (SERCA) family of calcium pumps [77, 78] or a futile cycle of creatine metabolism [79]. Because thermogenesis promotes energy expenditure, enhancing the activity of thermogenic fat represents a potential strategy to treat obesity.
Box 2. Ether lipids
Conventional phospholipids, such as phosphatidylcholine (PC) and phosphatidylethanolamine (PE), have fatty acyl chains attached to the sn-1 and sn-2 position of their glycerol backbone by ester bonds. Ether lipids, such as plasmalogens and alkylether phospholipids, are a unique class of phospholipids in which the sn-1 substituent is attached by an ether bond. The sn-2 position is generally an ester-linked acyl chain as in diacylphospholipids. The head group of ether-linked phospholipids is usually choline or ethanolamine and occasionally serine or inositol. Plasmalogens are the most common form of ether lipids and are a component of biological membranes [80]. They have a cis double bond adjacent to the ether bond. The loss of carbonyl oxygen confers unique structural and functional properties to plasmalogens. Notably, plasmalogens are thought to play a role in membrane dynamics due to their tendency to form non-lamellar structures [28]. Platelet activating factor (PAF), a bioactive ether lipid, is an analog of PC with an acetyl group at the sn-2 position. PAF can be synthesized de novo or through remodeling of alkylether analog of PC [81]. The initial steps of ether lipid synthesis take place in peroxisomes, generating a precursor called 1-O-alkylglycerol-3-phosphate (AGP), the phosphorylated form of alkylglycerol. AGP is the ether lipid equivalent of lysophosphatidic acid (i.e., 1-acyl-glycerol-3-phosphate) [82, 83]. Although the detailed mechanism remains unclear, subsequent steps of the synthesis, including addition of the sn-2 substituent and completion of the phospholipid synthesis, occurs in the ER, presumably through the Kennedy pathway of PC and PE synthesis [84].
Highlights.
Targeting brown and beige fat activity to stimulate energy expenditure and reduce adiposity could be therapeutically attractive to treat obesity.
Lipids mediate inter-organ communication, organelle crosstalk, and cellular signaling to impact adipose tissue thermogenesis.
Free fatty acids derived from WAT lipolysis, acylcarnitines produced by liver and lipids metabolized by gut microbiota impinge on BAT to regulate thermogenesis.
Cardiolipins and plasmalogens, lipids present in mitochondrial membranes and required for thermogenesis, are at the center of crosstalk between mitochondria and other organelles, including the nucleus and peroxisomes.
Signaling lipids affect thermogenesis through a variety of mechanisms, including regulation of fatty acid transport into BAT, post-translational modification of factors involved in thermogenic gene expression, and control of beige fat development.
Acknowledgements
This work was supported by NIH grants DK115867, DK118333, DK020579, and DK007120 and by funds from the Washington University-Centene Corporation Personalized Medicine Initiative.
Glossary
- Adipose tissue browning
a process that represents the conversion of white adipocytes into brown-like beige adipocytes
- Alkylglycerol
an ether lipid with a long chain alkyl moiety attached at the first carbon position of a glycerol molecule. It is a precursor of ether-linked phospholipids, such as plasmalogens and PAF
- Beige adipocytes
also called brite adipocytes, an inducible form of UCP-1 positive adipocytes that appear in WAT depots under specific conditions, such as cold exposure or β3-adrenergic receptor activation
- Bile acids
steroid carboxylic acids derived from cholesterol that assist in digestion and absorption of dietary lipids and vitamins in gut
- Brown adipose tissue
an adipose tissue in mammals that is enriched in mitochondria and is involved in adaptive non-shivering thermogenesis. It is activated by cold and produces heat using carbohydrates and lipids as substrates
- Docosahexaenoic acid (DHA)
an omega-3 polyunsaturated fatty acid composed of 22 carbons with 6 double bonds (C22:6); enriched in krill and fish oils
- Eicosapentaenoic acid (EPA)
an omega-3 polyunsaturated fatty acid composed of 20 carbons with 5 double bonds (C20:5); enriched in krill and fish oils
- Ether lipid
a phospholipid characterized by an ether linkage of a long-chain alkyl moiety at one or more carbons of the glycerol backbone
- Lipokine
a lipid hormone secreted from a tissue that affects metabolic function locally or systemically
- Lipolysis
a process through which triglycerides stored in lipid droplets are hydrolyzed, generating free fatty acids and glycerol
- Omega-3 fatty acid
an essential polyunsaturated fatty acid, characterized by the presence of a double bond three atoms away from the end farthest from the carboxyl group (i.e., the omega end)
- Omega-6 fatty acid
an essential polyunsaturated fatty acid, characterized by the presence of a double bond six atoms away from the omega end
- Plasmalogen
A type of ether lipid characterized by a vinyl ether linkage at the sn-1 position of the glycerol backbone
- Platelet activating factor (PAF)
an ether lipid composed of an alkyl chain at the first position, an acetyl group at the second position and a phosphocholine at the third position of the glycerol backbone. It is involved in inflammation and derives its name from its role in platelet aggregation
- Polyunsaturated fatty acids
fatty acids that have more than one double bond in their backbone
- Protein prenylation
a post-translational modification (also known as lipidation) of proteins that involves addition a farnesyl or a geranyl-geranyl moiety to a C-terminal cysteine residue of the target protein
- Uncoupling protein 1 (UCP1)
mitochondrial membrane protein that allows protons to leak across the inner mitochondrial membrane, bypassing ATP synthase, and is the primary mediator of non-shivering thermogenesis
- White adipose tissue
a fat tissue specialized to store excess energy as triglycerides and release them as free fatty acids during times of need, such as during fasting
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bluher M (2019) Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol 15 (5), 288–298. [DOI] [PubMed] [Google Scholar]
- 2.Rao Kondapally Seshasai S et al. (2011) Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 364 (9), 829–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chouchani ET et al. (2019) New Advances in Adaptive Thermogenesis: UCP1 and Beyond. Cell Metab 29 (1), 27–37. [DOI] [PubMed] [Google Scholar]
- 4.Chondronikola M et al. (2014) Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes 63 (12), 4089–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cannon B and Nedergaard J (2004) Brown adipose tissue: function and physiological significance. Physiol Rev 84 (1), 277–359. [DOI] [PubMed] [Google Scholar]
- 6.Zechner R (2015) FAT FLUX: enzymes, regulators, and pathophysiology of intracellular lipolysis. EMBO Mol Med 7 (4), 359–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schreiber R et al. (2017) Cold-Induced Thermogenesis Depends on ATGL-Mediated Lipolysis in Cardiac Muscle, but Not Brown Adipose Tissue. Cell Metab 26 (5), 753–763 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shin H et al. (2017) Lipolysis in Brown Adipocytes Is Not Essential for Cold-Induced Thermogenesis in Mice. Cell Metab 26 (5), 764–777 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmadian M et al. (2011) Desnutrin/ATGL is regulated by AMPK and is required for a brown adipose phenotype. Cell Metab 13 (6), 739–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heine M et al. (2018) Lipolysis Triggers a Systemic Insulin Response Essential for Efficient Energy Replenishment of Activated Brown Adipose Tissue in Mice. Cell Metab 28 (4), 644–655 e4. [DOI] [PubMed] [Google Scholar]
- 11.Haemmerle G et al. (2006) Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science 312 (5774), 734–7. [DOI] [PubMed] [Google Scholar]
- 12.Xiong D et al. (2014) Cardiac-specific VLCAD deficiency induces dilated cardiomyopathy and cold intolerance. Am J Physiol Heart Circ Physiol 306 (3), H326–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simcox J et al. (2017) Global Analysis of Plasma Lipids Identifies Liver-Derived Acylcarnitines as a Fuel Source for Brown Fat Thermogenesis. Cell Metab 26 (3), 509–522 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lundsgaard AM et al. (2018) Molecular Regulation of Fatty Acid Oxidation in Skeletal Muscle during Aerobic Exercise. Trends Endocrinol Metab 29 (1), 18–30. [DOI] [PubMed] [Google Scholar]
- 15.Barratt MJ et al. (2017) The Gut Microbiota, Food Science, and Human Nutrition: A Timely Marriage. Cell Host Microbe 22 (2), 134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chevalier C et al. (2015) Gut Microbiota Orchestrates Energy Homeostasis during Cold. Cell 163 (6), 1360–74. [DOI] [PubMed] [Google Scholar]
- 17.Zietak M et al. (2016) Altered Microbiota Contributes to Reduced Diet-Induced Obesity upon Cold Exposure. Cell Metab 23 (6), 1216–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuipers F et al. (2014) Beyond intestinal soap--bile acids in metabolic control. Nat Rev Endocrinol 10 (8), 488–98. [DOI] [PubMed] [Google Scholar]
- 19.Worthmann A et al. (2017) Cold-induced conversion of cholesterol to bile acids in mice shapes the gut microbiome and promotes adaptive thermogenesis. Nat Med 23 (7), 839–849. [DOI] [PubMed] [Google Scholar]
- 20.Kim M et al. (2017) 10-oxo-12(Z)-octadecenoic acid, a linoleic acid metabolite produced by gut lactic acid bacteria, enhances energy metabolism by activation of TRPV1. FASEB J 31 (11), 5036–5048. [DOI] [PubMed] [Google Scholar]
- 21.Broeders EP et al. (2015) The Bile Acid Chenodeoxycholic Acid Increases Human Brown Adipose Tissue Activity. Cell Metab 22 (3), 418–26. [DOI] [PubMed] [Google Scholar]
- 22.Lodhi IJ and Semenkovich CF (2014) Peroxisomes: a nexus for lipid metabolism and cellular signaling. Cell Metab 19 (3), 380–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahlabo I and Barnard T (1971) Observations on peroxisomes in brown adipose tissue of the rat. J Histochem Cytochem 19 (11), 670–5. [DOI] [PubMed] [Google Scholar]
- 24.Bagattin A et al. (2010) Transcriptional coactivator PGC-1alpha promotes peroxisomal remodeling and biogenesis. Proc Natl Acad Sci U S A 107 (47), 20376–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liesa M and Shirihai OS (2013) Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab 17 (4), 491–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wikstrom JD et al. (2014) Hormone-induced mitochondrial fission is utilized by brown adipocytes as an amplification pathway for energy expenditure. EMBO J 33 (5), 418–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park H et al. (2019) Peroxisome-derived lipids regulate adipose thermogenesis by mediating cold-induced mitochondrial fission. J Clin Invest 129 (2), 694–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glaser PE and Gross RW (1994) Plasmenylethanolamine facilitates rapid membrane fusion: a stopped-flow kinetic investigation correlating the propensity of a major plasma membrane constituent to adopt an HII phase with its ability to promote membrane fusion. Biochemistry 33 (19), 5805–12. [DOI] [PubMed] [Google Scholar]
- 29.Koivuniemi A (2017) The biophysical properties of plasmalogens originating from their unique molecular architecture. FEBS Lett 591 (18), 2700–2713. [DOI] [PubMed] [Google Scholar]
- 30.Lohner K (1996) Is the high propensity of ethanolamine plasmalogens to form non-lamellar lipid structures manifested in the properties of biomembranes? Chem Phys Lipids 81 (2), 167–84. [DOI] [PubMed] [Google Scholar]
- 31.Chen H and Chan DC (2017) Mitochondrial Dynamics in Regulating the Unique Phenotypes of Cancer and Stem Cells. Cell Metab 26 (1), 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chouchani ET et al. (2016) Mitochondrial ROS regulate thermogenic energy expenditure and sulfenylation of UCP1. Nature 532 (7597), 112–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoang T et al. (2013) Expression, folding, and proton transport activity of human uncoupling protein-1 (UCP1) in lipid membranes: evidence for associated functional forms. J Biol Chem 288 (51), 36244–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee Y et al. (2015) Uncoupling protein 1 binds one nucleotide per monomer and is stabilized by tightly bound cardiolipin. Proc Natl Acad Sci U S A 112 (22), 6973–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schlame M and Greenberg ML (2017) Biosynthesis, remodeling and turnover of mitochondrial cardiolipin. Biochim Biophys Acta Mol Cell Biol Lipids 1862 (1), 3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lynes MD et al. (2018) Cold-Activated Lipid Dynamics in Adipose Tissue Highlights a Role for Cardiolipin in Thermogenic Metabolism. Cell Rep 24 (3), 781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sustarsic EG et al. (2018) Cardiolipin Synthesis in Brown and Beige Fat Mitochondria Is Essential for Systemic Energy Homeostasis. Cell Metab 28 (1), 159–174 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marciniak SJ et al. (2004) CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev 18 (24), 3066–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fry M and Green DE (1981) Cardiolipin requirement for electron transfer in complex I and III of the mitochondrial respiratory chain. J Biol Chem 256 (4), 1874–80. [PubMed] [Google Scholar]
- 40.Paradies G et al. (2002) Reactive oxygen species affect mitochondrial electron transport complex I activity through oxidative cardiolipin damage. Gene 286 (1), 135–41. [DOI] [PubMed] [Google Scholar]
- 41.Arnould T et al. (2015) Mitochondria Retrograde Signaling and the UPR mt: Where Are We in Mammals? Int J Mol Sci 16 (8), 18224–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stepanyants N et al. (2015) Cardiolipin’s propensity for phase transition and its reorganization by dynamin-related protein 1 form a basis for mitochondrial membrane fission. Mol Biol Cell 26 (17), 3104–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ugarte-Uribe B et al. (2014) Dynamin-related protein 1 (Drp1) promotes structural intermediates of membrane division. J Biol Chem 289 (44), 30645–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lodhi IJ et al. (2011) Lipoexpediency: de novo lipogenesis as a metabolic signal transmitter. Trends Endocrinol Metab 22 (1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wymann MP and Schneiter R (2008) Lipid signalling in disease. Nat Rev Mol Cell Biol 9 (2), 162–76. [DOI] [PubMed] [Google Scholar]
- 46.Villarroya F et al. (2017) The Lives and Times of Brown Adipokines. Trends Endocrinol Metab 28 (12), 855–867. [DOI] [PubMed] [Google Scholar]
- 47.Lynes MD et al. (2017) The cold-induced lipokine 12,13-diHOME promotes fatty acid transport into brown adipose tissue. Nat Med 23 (5), 631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stanford KI et al. (2018) 12,13-diHOME: An Exercise-Induced Lipokine that Increases Skeletal Muscle Fatty Acid Uptake. Cell Metab 27 (5), 1111–1120 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zimmer B et al. (2018) The oxidized linoleic acid metabolite 12,13-DiHOME mediates thermal hyperalgesia during inflammatory pain. Biochim Biophys Acta Mol Cell Biol Lipids 1863 (7), 669–678. [DOI] [PubMed] [Google Scholar]
- 50.Christie S et al. (2018) Involvement of TRPV1 Channels in Energy Homeostasis. Front Endocrinol (Lausanne) 9, 420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guilherme A et al. (2017) Adipocyte lipid synthesis coupled to neuronal control of thermogenic programming. Mol Metab 6 (8), 781–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lodhi IJ et al. (2012) Inhibiting adipose tissue lipogenesis reprograms thermogenesis and PPARgamma activation to decrease diet-induced obesity. Cell Metab 16 (2), 189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Balaz M et al. (2019) Inhibition of Mevalonate Pathway Prevents Adipocyte Browning in Mice and Men by Affecting Protein Prenylation. Cell Metab 29 (4), 901–916 e8. [DOI] [PubMed] [Google Scholar]
- 54.Wang M and Casey PJ (2016) Protein prenylation: unique fats make their mark on biology. Nat Rev Mol Cell Biol 17 (2), 110–22. [DOI] [PubMed] [Google Scholar]
- 55.Sadurskis A et al. (1995) Polyunsaturated fatty acids recruit brown adipose tissue: increased UCP content and NST capacity. Am J Physiol 269 (2 Pt 1), E351–60. [DOI] [PubMed] [Google Scholar]
- 56.Pisani DF et al. (2014) The omega6-fatty acid, arachidonic acid, regulates the conversion of white to brite adipocyte through a prostaglandin/calcium mediated pathway. Mol Metab 3 (9), 834–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ghandour RA et al. (2018) Impact of dietary omega3 polyunsaturated fatty acid supplementation on brown and brite adipocyte function. J Lipid Res 59 (3), 452–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim M et al. (2015) Fish oil intake induces UCP1 upregulation in brown and white adipose tissue via the sympathetic nervous system. Sci Rep 5, 18013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oh DY et al. (2014) A Gpr120-selective agonist improves insulin resistance and chronic inflammation in obese mice. Nat Med 20 (8), 942–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ulven T and Christiansen E (2015) Dietary Fatty Acids and Their Potential for Controlling Metabolic Diseases Through Activation of FFA4/GPR120. Annu Rev Nutr 35, 239–63. [DOI] [PubMed] [Google Scholar]
- 61.Ichimura A et al. (2012) Dysfunction of lipid sensor GPR120 leads to obesity in both mouse and human. Nature 483 (7389), 350–4. [DOI] [PubMed] [Google Scholar]
- 62.Quesada-Lopez T et al. (2016) The lipid sensor GPR120 promotes brown fat activation and FGF21 release from adipocytes. Nat Commun 7, 13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Woo Baidal JA et al. (2016) Risk Factors for Childhood Obesity in the First 1,000 Days: A Systematic Review. Am J Prev Med 50 (6), 761–779. [DOI] [PubMed] [Google Scholar]
- 64.Uwaezuoke SN et al. (2017) Relationship Between Exclusive Breastfeeding and Lower Risk of Childhood Obesity: A Narrative Review of Published Evidence. Clin Med Insights Pediatr 11, 1179556517690196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yan J et al. (2014) The association between breastfeeding and childhood obesity: a meta-analysis. BMC Public Health 14, 1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yu H et al. (2019) Breast milk alkylglycerols sustain beige adipocytes through adipose tissue macrophages. J Clin Invest 130, 2485–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jo J et al. (2009) Hypertrophy and/or Hyperplasia: Dynamics of Adipose Tissue Growth. PLoS Comput Biol 5 (3), e1000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cypess AM et al. (2009) Identification and importance of brown adipose tissue in adult humans. N Engl J Med 360 (15), 1509–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nedergaard J et al. (2007) Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 293 (2), E444–52. [DOI] [PubMed] [Google Scholar]
- 70.van Marken Lichtenbelt WD et al. (2009) Cold-activated brown adipose tissue in healthy men. N Engl J Med 360 (15), 1500–8. [DOI] [PubMed] [Google Scholar]
- 71.Cypess AM et al. (2015) Activation of human brown adipose tissue by a beta3-adrenergic receptor agonist. Cell Metab 21 (1), 33–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rothwell NJ and Stock MJ (1979) A role for brown adipose tissue in diet-induced thermogenesis. Nature 281 (5726), 31–5. [DOI] [PubMed] [Google Scholar]
- 73.Harms M and Seale P (2013) Brown and beige fat: development, function and therapeutic potential. Nat Med 19 (10), 1252–63. [DOI] [PubMed] [Google Scholar]
- 74.Altshuler-Keylin S et al. (2016) Beige Adipocyte Maintenance Is Regulated by Autophagy-Induced Mitochondrial Clearance. Cell Metab 24 (3), 402–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shabalina IG et al. (2013) UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell Rep 5 (5), 1196–203. [DOI] [PubMed] [Google Scholar]
- 76.Wu J et al. (2012) Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150 (2), 366–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ikeda K et al. (2017) UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nat Med 23 (12), 1454–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ukropec J et al. (2006) UCP1-independent thermogenesis in white adipose tissue of cold-acclimated Ucp1−/−mice. J Biol Chem 281 (42), 31894–908. [DOI] [PubMed] [Google Scholar]
- 79.Kazak L et al. (2015) A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell 163 (3), 643–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Braverman NE and Moser AB (2012) Functions of plasmalogen lipids in health and disease. Biochim Biophys Acta 1822 (9), 1442–52. [DOI] [PubMed] [Google Scholar]
- 81.Snyder F (1995) Platelet-activating factor: the biosynthetic and catabolic enzymes. Biochem J 305 (Pt 3), 689–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dean JM and Lodhi IJ (2018) Structural and functional roles of ether lipids. Protein Cell 9 (2), 196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hajra AK and Das AK (1996) Lipid biosynthesis in peroxisomes. Ann N Y Acad Sci 804, 129–41. [DOI] [PubMed] [Google Scholar]
- 84.Gibellini F and Smith TK (2010) The Kennedy pathway--De novo synthesis of phosphatidylethanolamine and phosphatidylcholine. IUBMB Life 62 (6), 414–28. [DOI] [PubMed] [Google Scholar]