Abstract
Metformin is the most widely-prescribed oral hypoglycemic medication for type 2 diabetes worldwide. Metformin also retards aging in model organisms and reduces the incidence of aging-related diseases such as neurodegenerative disease and cancer in humans. In spite of its widespread use, the mechanisms by which metformin exerts favorable effects on aging remain largely unknown. Further, not all individuals prescribed metformin derive the same benefit, and some develop side effects. Before metformin finds its way to mainstay therapy for anti-aging, a more granular understanding of the effects of the drug in humans is needed. This review provides an overview of recent findings from metformin studies in aging and longevity and discusses the use of metformin to combat aging and aging-related diseases.
Keywords: metformin, aging, type 2 diabetes, mitochondria, lysosome, personalized medicine
Metformin Usage Beyond Type 2 Diabetes: Aging and Aging-Related Disease
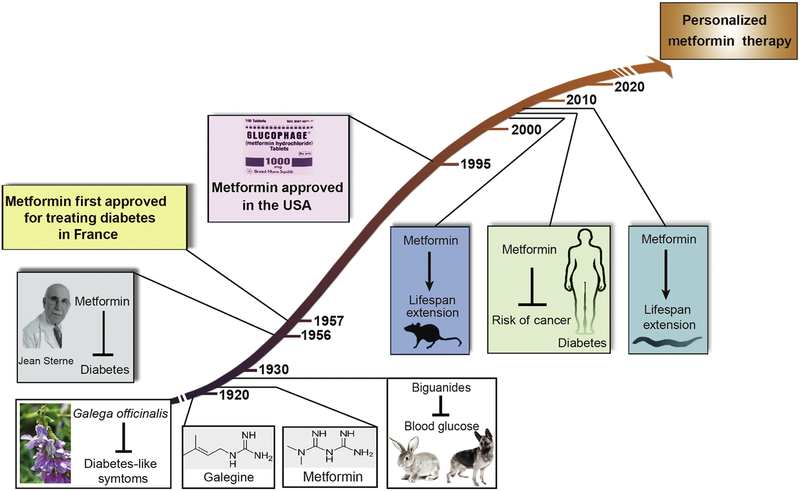
The history of the antidiabetic drug metformin dates to the 17th century, where extracts of the leaves of the French lilac Galega officinalis, which contain metformin-like guanidine compounds, were used to treat plague, fever, snake bites, and other ailments. The anti-glycemic property of G. officinalis was first described in Culpeper’s Complete Herbal in 1653 [1]. Although guanidine-containing compounds are responsible for the plant’s anti-glycemic effect in animals, these agents proved too toxic for use in humans. In 1922, synthesis of metformin and related biguanide compounds phenformin and buformin was achieved by Werner and Bell [2], paving the way for metformin to attain widespread use in humans as first-line therapy for type 2 diabetes (T2D) worldwide [3] (Figure 1, Key Figure). Metformin also has proven roles in prevention of diabetes [4], in treatment of the polycystic ovary syndrome (PCOS) [5], and in helping individuals with diabetes prevent weight gain or even lose weight [6].
Figure 1. Significant events in metformin use in diabetes and aging-related diseases.
Metformin-like compounds such as galegine are the active compounds in the French lilac Galega officinalis that has been used since medieval times to treat diabetes-like symptoms. Metformin, phenformin and buformin were synthesized by Werner and Bell in 1922, and studies determined that biguanides lowered blood glucose in laboratory animals in the mid 1920s. Owing to studies in humans by the French physician-scientist Dr. Jean Sterne, metformin went into use in Europe in the 1950s and was later approved in the US in 1995. Its US approval was delayed due to concerns over lactic acidosis, far more likely with its sister drugs phenformin and buformin. In the early 2000s, studies at the National Institutes of Health determined that metformin extends lifespan and healthspan in laboratory mice, and shortly thereafter metformin was found in observational studies to reduce morbidity and mortality from aging-associated diseases such as cancer in humans. Metformin extends the lifespan of the roundworm Caenorhabditis elegans by up to 50%, a discovery that has enabled genetic dissection of the pathways necessary for metformin longevity effects. We predict that the future of metformin use to combat aging in humans will involve the use of personalized medicine approaches.
The first milestone step for the use of metformin to treat diabetes was taken by the French physician Jean Sterne in 1957, who achieved approval for metformin use under the brand name Glucophage [7]. Metformin was slow to gain approval in the USA due to concerns over lactic acidosis that were far greater with sister-compounds buformin and phenformin (the latter two are no longer in clinical use). Metformin went into use in the USA in 1995, boosting its use and stimulating research targeted at elucidating its mechanism of action.
Emerging evidence indicates that metformin has favorable effects on health beyond those associated with improvement in glycemia. Observational studies suggest that diabetic individuals treated with metformin manifest a survival benefit even when compared to non-diabetic controls [8, 9]. Metformin not only reduces cardiovascular disease incidence in patients with type 2 diabetes [10], it similarly reduces atherosclerotic burden in non-diabetic individuals at risk for the disease [11]. Observational data in humans further support a role for metformin in prevention of aging related decline and cancer [9, 12], an area of immense clinical interest. Molecular analyses of septagenarians treated with metformin indicate that the drug elicits metabolic and non-metabolic effects consistent with multiple effects on aging [13]. In this article, recent progress on our understanding of metformin actions in aging are reviewed and explored with a concluding proposal that precision medicine approaches may be needed to apply metformin broadly as an anti-aging therapy in humans.
Recognition of Aging as a Disease
Aging is often referred to as a risk factor for age-related diseases and is sometimes described as the “sum of age-related diseases” [14]. Although it has been a long time coming, the World Health Organization (WHO) now formally recognizes aging as a disease in the latest version of the International Classification of Diseases (ICD-11, code ‘Ageing-related’ XT9T). The formal recognition of aging as a disease is meaningful for the development of future therapeutic interventions or strategies targeting aging and aging-related diseases [15]. It is also likely to raise interest in repurposing drugs to treat aging, such as metformin. Metformin has been explored as an anti-aging agent in model organisms and humans [16, 17], given its excellent safety record for over six decades in the clinic, well-documented beneficial properties in cardioprotection and potential value in cancer prevention and treatment [18, 19].
Metformin Prolongs Lifespan and Healthspan in the Invertebrate Caenorhabditis elegans
C. elegans is a powerful model organism for mechanistic study of longevity, having aided in identification of more than 200 longevity-affecting genes and regimens [20]. The lifespan prolonging effects of metformin in C. elegans were first reported in 2010 [21]. This study demonstrated that metformin also prolonged healthspan, the portion of the lifespan where animals are active, suggesting that metformin promotes both lifespan and healthy aging (Figure 1).
Metformin-mediated lifespan extension in C. elegans is genetically dependent upon the cellular energy sensor adenosine monophosphate-activated protein kinase (AMPK) and its upstream activating kinase liver kinase B1 (Lkb1, par-4 in the worm), as well as the stress-induced transcription factor skn-1/nuclear factor erythroid 2-related factor 2 (Nrf2). This is in contrast to effects on glycemia and cell growth, suggesting that the glycemic and anti-aging effects of the drug have distinct mechanisms of action. Subsequent work confirmed these observations but indicated that the effects of metformin on lifespan are far from straightforward [22–25]. Our studies indicate a requirement for the nuclear pore complex (NPC) and acyl-CoA dehydrogenase family member 10 (ACAD10) in lifespan extension, a pathway that is activated by direct action of metformin on C. elegans [24]. Other work suggests that metformin prolongs lifespan in C. elegans through direct action on lysosomes [25]. And yet another study suggests that metformin slows aging of C. elegans through metabolic modulation of the E. coli food source [22]. The potential mechanisms by which metformin exerts its anti-aging effects are discussed in detail below.
Metformin Extends Lifespan and Healthspan in Mice
In the early 2000s, studies at the NIH and elsewhere determined that metformin extends the lifespan and healthspan of genetically outbred and inbred laboratory mice [26–30] (Figure 1). Some, but not all these studies indicate a sexual dimorphism suggestive of a greater benefit for female mice.
In contrast to observations in C. elegans, mice, and observational studies in humans, lifespan extension is not evident with metformin treatment in the fruit fly Drosophila [31] or rats [32], although AMPK activation in flies and body weight loss in rats was detected. The exact explanation for these disparate effects of metformin in different organisms remains elusive. Numerous individual factors affect aging, such as nutrient availability and the intensity of exercise [33]. Thus, factors such as activity, may explain why metformin promotes lifespan of certain organisms (worm, caged mice) but not others (fruit fly). Further, metformin may not further extend longevity of already long-lived species such as the F344 rat strain [32]. Finally, dosing regimens that are not optimized for each organism may also explain failure to achieve lifespan extension in some cases.
Current Knowledge of Metformin Targets and Its Mode of Action
Metformin has been used to treat T2D for more than 60 years, and yet even its antihyperglycemic mode of action remains incompletely characterized. Recent advances have revealed multiple cellular effects of metformin that may be relevant for its effects both on metabolism and aging. Under different circumstances, effects of metformin may be mediated by molecular targets as disparate as mitochondrial complex I [34], mitochondrial glycerol-3-phosphate dehydrogenase [35], and the H3K27me3 demethylase KDM6A/UTX [36]. It should be noted that very few studies attempt to discriminate between direct and indirect metformin response pathways. Here we will make an effort to illuminate direct versus indirect metformin targets with available evidence.
Metformin Targets the Mitochondrial Respiratory Chain
It is widely accepted that the mitochondrion is a primary target of metformin responsible for its anti-glycemic effect [34, 35, 37, 38]. In line with early studies indicating a primary effect of metformin on complex I of the mitochondrial electron transport chain, recent work also provides strong genetic evidence that metformin inhibits cancer cell growth through its actions on complex I [34, 37, 39–41]. Our own work also shows that rotenone (a complex I inhibitor) and metformin both activate the same signaling cascade in the same manner in C. elegans and mammals [24]. Ectopic expression of the metformin-resistant S. cerevisiae NADH dehydrogenase NDI1 in place of complex I renders HCT 116 p53−/− colon cancer cells resistant to killing by metformin in vitro and in tumor allografts in vivo [34]. However, mitochondria continue to be challenged as a primary target of metformin, mainly because experimentally discernible inhibition of mitochondrial function by metformin can require millimolar levels of drug. It remains an unanswered question as to whether levels of drug that are achievable in humans also mediate metformin’s benefit on aging and prevention of aging-related diseases through modest effects on mitochondria. It is little appreciated that metformin inhibits production of reactive oxygen at far lower doses than those required to affect respiratory capacity [42]. Thus, it remains extremely plausible that mitochondrial effects of metformin dominate even at the micromolar levels obtainable in humans in vivo [43].
By targeting complex I, metformin lowers the relative energy charge of the cell, raising adenosine monophosphate (AMP) levels relative to adenosine triphosphate (ATP) [41]. Among other effects, the rise in AMP allosterically primes activation of the energy sensor AMPK [44], the significance of which in metformin’s antihyperglycemic, prolongevity, and anti-cancer effects remains unclear (discussed below). An additional immediate consequence of the rise in AMP levels is inhibition of the gluconeogenic enzyme fructose-1–6-bisphosphatase (FBP1) [45]. Recent elegant genetic work in mice demonstrates that metformin lowers glucose levels through allosteric inhibition of FBP1 [45]. Thus, while there is some debate on the relevance of the action of metformin on mitochondrial energetics, the data on FBP1 provide serious credence to the idea that metformin at attainable levels in vivo manifests important effects via modulation of cellular energy charge.
Metformin and Mechanistic Target of Rapamycin Complex 1 (mTORC1)
The heteromultimeric protein kinase mTORC1 plays a central role in regulating cell growth, proliferation and survival in response to nutrient and energy availability [46–48]. Metformin inhibits mTORC1 activity in cells in culture independently of AMPK [49, 50]. From a longevity standpoint, metformin effects on mTORC1 and longevity are in line with the well-known ability of genetic and pharmacologic inhibition of mTORC1 to extend lifespan across multiple model systems [51–57]. In support of the idea that metformin treatment modulates certain downstream cellular effects by blocking mTORC1, both metformin and canonical mTOR inhibitors have similar molecular effects, decreasing translation of mRNAs encoding cell-cycle and growth regulators [58].
Cellular mTORC1 signaling is regulated by several, distinct pathways, including the TSC-Rheb pathway [47, 59] and Ras-related GTP-binding protein (Rag) GTPase-mediated amino acid signaling [60, 61]. Our work builds upon the observation that metformin inhibits mTORC1 via the Rag GTPases [50], as we have identified the molecular mechanism by which this occurs: 1) metformin action at mitochondria leads to restricted transport through the nuclear pore complex (NPC); 2) reduced NPC transport restricts the entrance of small GTPase RagC into the nucleus, thus preventing its full activation; 3) as RagC activation is critical for normal mTORC1 activity, inactivation of RagC leads to inhibition of mTORC1 [24].
Metformin and Mitochondrial Glycerol-3-Phosphate Dehydrogenase (mGPDH)
mGPDH is localized on the outer layer of the inner mitochondrial membrane, where metformin has been found to bind directly to the enzyme and inhibit its function, converting glycerol-3-phosphate to dihydroxyacetone phosphate [35]. mGPDH plays a role in the glycerol-phosphate shuttle, which is responsible in part for shuttling NADH reducing equivalents into the mitochondrial matrix, in the process regenerating cytosolic NAD+ [62]. The inhibition of mGPDH by metformin decreases the cytoplasmic NAD/NADH ratio and reduces hepatic glucose production in mice. Downregulation of GPD2 (the gene encoding mGPDH) mimics the antihyperglycemic effects of metformin, while metformin does not lower glucose in mice genetically lacking GPD2. More recent work demonstrates that metformin at therapeutic concentrations impedes gluconeogenesis by inhibiting mGPDH activity in a redox-dependent manner [63, 64]. Metformin’s ability to inhibit mGPDH may also contribute to the drug’s anti-cancer effects by altering cellular redox potential [65]. Whether mGPDH mediates other anti-aging properties of metformin requires further exploration.
Metformin-Mediated Activation of AMPK
Mitochondrial inhibition by metformin results in depletion of ATP and elevation of cellular AMP [34, 39, 40], activating the master cellular energy sensor, AMPK [44]. However, multiple studies demonstrate that AMPK is dispensable for the beneficial effects of metformin, particularly in lowering of blood glucose [63, 66, 67]. Although mice genetically deficient in AMPK in liver respond normally to the antihyperglycemic effects of metformin, some work suggests that AMPK activation that occurs at therapeutically attainable metformin levels may be important for certain effects of biguanides [68, 69].
An overwhelming amount of data support the conclusion that metformin blocks cancer growth in a manner dependent upon inhibition of mitochondrial complex I, but independently of AMPK [34]. Metformin inhibits mTORC1 in a manner dependent upon the Rag GTPases, independently of AMPK [50]. Further, AMPK and its upstream activating kinase LKB1 are dispensable for metformin to inhibit cancer growth, albeit in the limited numbers of cancer cell types investigated [67, 70]. Our work shows that AMPK is completely dispensable for metformin effects on C. elegans growth, and that the pathway defined by metformin-NPC-RagC-mTORC1-ACAD10 is not affected by AMPK in C. elegans or in human cancer cell lines [24]. Thus, AMPK is unlikely to be a major effector of metformin action in cancer.
These observations of metformin action in cancer contrast sharply with metformin action in aging. Seemingly paradoxically, at least in C. elegans, AMPK is genetically required for the prolongevity effects of metformin, an effect that has been reproduced by multiple research groups [21, 22, 25]. Thus, metformin response pathways, while they may begin at the mitochondrion, are complex and branching. More work is needed to determine how the disparate effects of metformin are mediated by common versus distinct effector mechanisms.
Metformin and Lysosomes
The lysosome, an acidified, membrane-bound cellular organelle that participates in nutrient sensing and recycling, is a central hub in control of cell signaling and metabolism. Alterations in AMPK and mTORC1 signaling in response to metformin both require biochemical events that converge on the lysosome [25, 71]. AMPK phosphorylation and activation by LKB1 occurs on the surface of lysosomes in response to starvation [35]. Metformin activates AMPK via a similar mechanism on lysosomes, possibly through the lysosomal V-ATPase [71, 72]. Early work in isolated lysosomes also suggests that metformin may act to coordinate AMPK activation and mTORC1 inhibition via direct effects [25]. However, the exact link between metformin action and lysosomes remains elusive.
Lysosomes and lysosome-related organelles, which play important roles in modulation of aging and longevity [73], also house cellular stores of metal ions such as copper, zinc and iron [74]. Curiously, metformin has metal binding properties [75, 76]. Accordingly, metformin can affect cellular copper homeostasis, particularly in mitochondria [77, 78]. These findings suggest that metformin, by virtue of its concentration in mitochondria, could set up a copper competition between mitochondria and lysosomes, which could connect metformin action at mitochondria to lysosomal regulation on AMPK and mTORC1. Earlier work also suggests that metformin’s zinc-binding activity might target lysosomal zinc stores, thereby promoting the drug’s known anti-inflammatory activity [79]. Most recently, relying on an assay called hdPCA, Stynen et al. uncovered that metformin induces an iron deficiency-like state in cells [80]. Together, it appears that metformin has the potential to modulate effects on mitochondrial function, lysosomal function, cellular signaling, and inflammation on the basis of alterations in lysosomal metal homeostasis. Further investigation is also needed to fully reveal whether metformin action on lysosomal ions contributes to the prolongevity and health-promoting effects of the drug.
Epigenetic Modulation by Metformin
Advances in machine learning have enabled next-generation studies of drug-target interactions [36, 81]. A recent study examined 300,000 chemical compounds and more than 9,000 protein binding cavities, yielding up to 41 putative metformin-binding targets. Among these potential metformin targets, the H3K27me3 demethylase KDM6A/UTX contains an experimentally validated unique metformin direct-binding motif.
A second computational modeling study provided evidence that SIRT1, a NAD+-dependent histone deacetylase, may also be a direct target of metformin [82]. SIRT1 plays a vital role in growth regulation, stress response and aging modulation. It is plausible that metformin extends lifespan by activating SIRT1. Metformin may also impact the epigenome indirectly by modulation of metabolite levels known to alter the activity of histone and DNA modulating enzymes. Metformin is known to affect cellular NAD+, ATP, and tricarboxylic acid intermediate levels as well as AMPK, all of which impact the activity of epigenome-modifying enzymes [83]. Thus, it remains a compelling, but largely untested possibility that metformin may exert some of its health-promoting effects through epigenomic alterations.
Metformin and the Microbiome
An increasing amount of evidence suggests that epigenetics and environmental factors (including diet and the gut microbiota) may trump genetics as the major determinant of longevity (Figure 2). The microbiota has been reported to have strong associations with many age-related disorders, such as T2D, obesity, and cancer [84, 85]. In certain organisms, metformin may impede aging and age-related disorders by modulating the microbiome [22, 86, 87]. We and others propose microbiome-independent mechanisms for the anti-aging effect of metformin in C. elegans [24], but microbiome-dependent effects remain a possibility. Cabreiro et al. first reported that metformin promotes C. elegans lifespan by changing microbial folate and methionine metabolism [22]. Studies in rodents have focused on whether metformin modulation of the microbiota affects metabolism rather than aging per se [86, 88]. Studies in humans have yielded important findings on metformin’s microbiotal effects: 1) metformin increases the population of bacteria good at producing short-chain fatty acids that contribute to weight loss and inflammation suppression in T2D individuals [87], and 2) microbial shifts following metformin exposure may account for the anti-glycemic effect of metformin and its accompanied side effects in people with T2D [89]. However, the precise mechanisms by which metformin modulates the microbiome is still largely unknown.
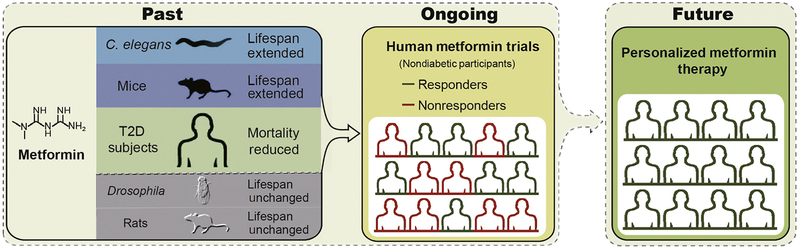
Figure 2. Metformin effects on longevity in model organisms and in humans.
Metformin has been shown to have pro-longevity and healthspan extending properties in the roundworm Caenorhabditis elegans, mice, and humans. In other model organisms such as Drosophila (fruit fly) and rats, similar benefit has not been identified. Although data from prospective clinical trials in humans on metformin in aging are only just planned or beginning to emerge, widespread use of the drug in aging in otherwise healthy individuals requires far more granular understanding of its effects, and the genetic and environmental determinants of its success in promoting aging versus potential detrimental effects. T2D, type 2 diabetes.
Metformin and Extension of Human Longevity
In addition to the lifespan-promoting activity of metformin in various model organisms, metformin has the capability to reduce the mortality rate of diabetic patients from all causes independent of its effect on diabetes control [9] (Figures 1 and 2). These findings have prompted great interest among researchers and physicians in setting up human trials with non-diabetic participants to evaluate metformin as an agent to extend human longevity.
The Metformin in Longevity Study (MILES) is a double-blind, placebo-controlled crossover clinical trial with 14 human participants launched in 2014 to determine whether taking metformin 1700 mg/day can restore more youthful gene expression in elderly people with impaired glucose tolerance (https://clinicaltrials.gov/ct2/show/NCT02432287). Recent publication of gene expression profiling of skeletal muscle and adipose tissue from the MILES study provided the first direct evidence that metformin modulates metabolic and non-metabolic gene expression linked to aging [13]. The far larger double-blind, placebo-controlled multicenter trial Targeting Aging with Metformin (TAME), plans to enroll 3,000 individuals aged 65–79 with a primary endpoint of the time until presence of any aging-related morbidity (including coronary heart disease, stroke, congestive heart failure, peripheral arterial disease, cancer, T2D, cognitive impairment, and mortality, Figure 2). Subjects will take 1500 mg of metformin daily for 6 years, with a mean follow-up time of more than 3.5 years [16]. Results from the TAME will provide a widely expected answer to the question whether metformin reduces aging-associated disease and disability in non-diabetic individuals. Further, the trial will set the stage as a paradigm of investigating anti-aging therapies, using disease and biomarkers as surrogates of the aging process [90].
An extremely important consideration is that the doses of metformin used in preclinical studies of aging in vitro and in vivo, are, in most cases, not comparable to doses achievable in humans. Levels of the drug used in vitro to elicit molecular effects discussed below are 10 – 100 fold higher than maximal serum levels of metformin achieved in clinical studies in humans (reviewed in [43]). It remains a distinct possibility that the aging-related benefits of metformin in humans are manifest at lower doses via chronic, low-level effects on pathways affected at higher concentrations in cells in culture. Early evidence in support of this possibility is suggested by gene expression changes manifest in human muscle and adipose tissue from metformin-treated individuals [13]. None the less, further testing is required to determine what the optimal levels of metformin required to maximize benefits in aging, and at that dose, which molecular effects predominate.
In spite of the lack of randomized prospective trial data on aging in humans, many in Silicon Valley have embraced metformin in attempts to live longer and healthier (https://www.cnbc.com/2019/03/23/metformin-for-cancer-prevention-longevity-popular-in-silicon-valley.html). While it is likely based upon data in diabetics that the drug is safe and generally well tolerated, it is unclear whether healthy individuals will manifest a net benefit on aging in the same way that diabetic subjects do. Below we highlight the possible issues with widespread metformin use to promote healthy aging in humans.
Uncertainty in the Widespread Use of Metformin
Metformin and Vitamin B12 Deficiency
Evidence indicates that long-term use of metformin can cause vitamin B12 deficiency in T2D patients [11, 91, 92]. The mechanism underlying this vitamin deficiency and its clinical consequences are still unclear. Unlike other, more severe forms of vitamin B12 deficiency [93], the vitamin B12 deficiency associated with metformin use is typically less severe and generally not accompanied by neuropathy or anemia [11]. However, whether metformin-induced B12 deficiency could be more clinically significant if the drug is taken by a larger group of people for a more substantial period of the lifespan remains unknown.
Metformin and Increased Risk of Lactic Acidosis
Metformin increases levels of lactate in mice [35] and humans [94]. While this is generally not clinically significant, in the setting of abnormal kidney function, the body is not able to eliminate metformin, leading to accumulation of the drug and risk for lactic acidosis. Generally metformin-associated lactic acidosis is extremely rare even in individuals with substantial renal dysfunction [95], but, when evident, carries a 50% mortality rate [96]. Phenformin, a member from the same biguanide family as metformin, was withdrawn from clinical use in the 1970s due to a 10-fold higher rate of severe lactic acidosis versus metformin [97, 98]. It is not known whether the incidence of clinically important lactic acidosis will increase if the drug is taken on a more widespread basis.
Uncertainty Surrounding Metformin and Its Cellular Targets
In addition to multiple targets discussed above for metformin action, Stynen et al. also identified 745 proteins that are altered by metformin treatment [80]. There is still uncertainty on whether those proteins represent beneficial or potentially detrimental off-target effects when metformin is taken across the lifespan. Strong consideration should be given to additional possible targets of the drug before it attains widespread use for anti-aging in humans.
Viability of Metformin as an Anti-Aging Therapy in Humans
Beyond the uncertainty surrounding metformin mechanisms of action in aging, additional uncertainty exists in potential side effects of metformin. Typically, gastrointestinal (GI) side effects, including diarrhea, nausea, flatulence, indigestion, vomiting and abdominal discomfort, dominate in individuals taking metformin. In most patients these effects are not evident or disappear over time, and only a minority have to reduce the dose or stop the drug altogether (<5% of people) [99]. Generally, the effects are minimized by starting metformin with food at a low dose and increasing gradually.
A second major area of concern is whether metformin will have efficacy across the population for aging. Even though metformin is first-line therapy for T2D treatment, with regard to glycemic effects of the drug, there are responders and non-responders [100]. Metformin is effective in restoring ovulation to a much greater extent in PCOS patients with overweight and impaired glucose tolerance versus patients with a lean body habitus [101]. The mechanisms underlying differential responses to metformin in humans remain largely unknown, although some variability may be explained by genetic variations in the metformin transporter organic cation transporter (OCT1) [102]. A recently published clinical trial suggests that metformin may negate some of the benefits of exercise on muscle, though this study was small in size and results were highly variable between individuals [103]. Further, we do not know, at the present time, whether individuals without diabetes will manifest longevity and reduction in aging-associated disease in response to metformin as individuals with or at risk for T2D. Given the uncertainty surrounding the full spectrum of metformin effects, whether the drug will benefit all who take it for aging, and the possibility of negative pleiotropies, we suggest that precision metformin therapy may be needed to apply metformin as an anti-aging drug in humans (Figure 2).
Concluding Remarks and Future Perspectives
Metformin has over 6 decades of use in diabetes with an outstanding safety record in treating human T2D. Mounting evidence in preclinical models and in humans suggests beneficial effects in reducing the risk of aging-related diseases, such as neurodegeneration and cancer. These properties of metformin have attracted an enormous amount of attention from research and industry to develop indications for metformin as an anti-aging therapeutics in humans. Although on the surface this would appear to be justified based upon the safety and tolerability of the drug, there is still much we don’t know on the mode of metformin action, especially in aging. Aging is a heterogeneous phenomenon, and different individuals in the same population also respond metformin differently. Therefore, large scale, multicenter, randomized, placebo-controlled trials are necessary to further elucidate the anti-aging effects of metformin. We also suggest that individualized, precision approaches may be needed to implement metformin in aging. These could be developed once we have better biomarkers of metformin effects in humans that correlate with favorable effects on healthspan and lifespan. Last but not least, if hundreds of millions of humans take metformin, we will need to understand the consequences of unmanaged discharge into ecosystem, as metformin is eliminated from the human body unchanged, and has already been detected in the environment and surface water.
Outstanding Questions.
Does metformin have a sole and direct target for all effects on aging in model organisms and human? Alternatively, does it manifest favorable effects on metabolism and aging through distinct mechanistic targets? What targets or pathways would support its use in human to combat aging?
How exactly does metformin work at the organellar level, such as at the mitochondrion and lysosome?
How does metformin change the epigenetic landscape, and are these changes responsible for the effects of metformin on aging? Are the epigenetic effects heritable?
Why does metformin promote the lifespan of certain but not all organisms?
What are the human determinants of metformin action in aging and metabolism? Are the major determinants genetic or environmental (microbiota, diet)? What biomarkers can be leveraged to achieve precision metformin therapy in aging in humans?
What is the fate of metformin in the ecosystem? Is metformin or its derivatives harmful to the future of the planet?
Highlights.
With continuous improvement in living conditions, interest and investment in antiaging therapies are vastly growing. The antidiabetic drug metformin has garnered tremendous interest owing to its position as first-line therapy for type 2 diabetes treatment and exhibition of anti-aging properties in model organisms.
In spite of its widespread use, the mode of metformin action is not fully understood. Multiple targets and distinct mechanisms have been proposed by which its anti-aging effects are mediated.
Many uncertainties exist in metformin mechanisms and side effects that may prevent its widespread use in aging in otherwise healthy individuals.
Studies on metformin’s antidiabetic effects demonstrate that metformin does not affect all users in the same fashion. Thus, precision metformin therapy may be needed to fully realize the benefit of metformin to combat aging-related diseases in humans.
Acknowledgements
This work was supported by NIH grants R01AG058256 and R01DK101522 (to A.A.S.), awards from the Glenn Foundation for Medical Research and the American Federation for Aging Research (to A.A.S.), by the Weissman Family MGH Research Scholar Award (to A.A.S.), and by institutional funds from the Westlake Institute for Advanced Study / Westlake University (to L.W).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bailey CJ (2017) Metformin: historical overview. Diabetologia 60 (9), 1566–1576. [DOI] [PubMed] [Google Scholar]
- 2.Werner A and Bell J (1922) The preparation of methylguanidine, and of ββ-dimethylguanidine by the interaction of dicyandiamide, and methylammonium and dimethylammonium chlorides respectively. Journal of the Chemical Society, Transactions 121, 1790–1794. [Google Scholar]
- 3.Inzucchi SE et al. (2015) Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 38 (1), 140–9. [DOI] [PubMed] [Google Scholar]
- 4.Knowler WC et al. (2002) Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346 (6), 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abu Hashim H (2016) Twenty years of ovulation induction with metformin for PCOS; what is the best available evidence? Reprod Biomed Online 32 (1), 44–53. [DOI] [PubMed] [Google Scholar]
- 6.Yerevanian A and Soukas AA (2019) Metformin: Mechanisms in Human Obesity and Weight Loss. Curr Obes Rep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jean S (1957) Du nouveau dans les antidiabetiques. La NN dimethylamine guanyl guanide (NNDG). Maroc Medical 36, 1295–1296. [Google Scholar]
- 8.Bannister CA et al. (2014) Can people with type 2 diabetes live longer than those without? A comparison of mortality in people initiated with metformin or sulphonylurea monotherapy and matched, non-diabetic controls. Diabetes Obes Metab 16 (11), 1165–73. [DOI] [PubMed] [Google Scholar]
- 9.Campbell JM et al. (2017) Metformin reduces all-cause mortality and diseases of ageing independent of its effect on diabetes control: A systematic review and meta-analysis. Ageing Res Rev 40, 31–44. [DOI] [PubMed] [Google Scholar]
- 10.Johnson JA et al. (2005) Reduced cardiovascular morbidity and mortality associated with metformin use in subjects with Type 2 diabetes. Diabet Med 22 (4), 497–502. [DOI] [PubMed] [Google Scholar]
- 11.Aroda VR et al. (2016) Long-term Metformin Use and Vitamin B12 Deficiency in the Diabetes Prevention Program Outcomes Study. J Clin Endocrinol Metab 101 (4), 1754–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castillo-Quan JI and Blackwell TK (2016) Metformin: Restraining Nucleocytoplasmic Shuttling to Fight Cancer and Aging. Cell 167 (7), 1670–1671. [DOI] [PubMed] [Google Scholar]
- 13.Kulkarni AS et al. (2018) Metformin regulates metabolic and nonmetabolic pathways in skeletal muscle and subcutaneous adipose tissues of older adults. Aging Cell 17 (2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gavrilov LA and Gavrilova NS (2017) [Is aging a disease? Biodemographers’ point of view.]. Adv Gerontol 30 (6), 841–842. [PMC free article] [PubMed] [Google Scholar]
- 15.The Lancet Diabetes E (2018) Opening the door to treating ageing as a disease. Lancet Diabetes Endocrinol 6 (8), 587. [DOI] [PubMed] [Google Scholar]
- 16.Barzilai N et al. (2016) Metformin as a Tool to Target Aging. Cell Metab 23 (6), 1060–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Novelle MG et al. (2016) Metformin: A Hopeful Promise in Aging Research. Cold Spring Harb Perspect Med 6 (3), a025932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Argun M et al. (2016) Cardioprotective effect of metformin against doxorubicin cardiotoxicity in rats. Anatol J Cardiol 16 (4), 234–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vancura A et al. (2018) Metformin as an Anticancer Agent. Trends Pharmacol Sci 39 (10), 867–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tissenbaum HA (2015) Using C. elegans for aging research. Invertebr Reprod Dev 59 (sup1), 59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Onken B and Driscoll M (2010) Metformin induces a dietary restriction-like state and the oxidative stress response to extend C. elegans Healthspan via AMPK, LKB1, and SKN-1. PLoS One 5 (1), e8758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cabreiro F et al. (2013) Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell 153 (1), 228–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Haes W et al. (2014) Metformin promotes lifespan through mitohormesis via the peroxiredoxin PRDX-2. Proc Natl Acad Sci U S A 111 (24), E2501–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu L et al. (2016) An Ancient, Unified Mechanism for Metformin Growth Inhibition in C. elegans and Cancer. Cell 167 (7), 1705–1718 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen J et al. (2017) Metformin extends C. elegans lifespan through lysosomal pathway. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anisimov VN et al. (2008) Metformin slows down aging and extends life span of female SHR mice. Cell Cycle 7 (17), 2769–73. [DOI] [PubMed] [Google Scholar]
- 27.Anisimov VN et al. (2011) If started early in life, metformin treatment increases life span and postpones tumors in female SHR mice. Aging (Albany NY) 3 (2), 148–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anisimov VN et al. (2005) Metformin decelerates aging and development of mammary tumors in HER-2/neu transgenic mice. Bull Exp Biol Med 139 (6), 721–3. [DOI] [PubMed] [Google Scholar]
- 29.Anisimov VN et al. (2010) Gender differences in metformin effect on aging, life span and spontaneous tumorigenesis in 129/Sv mice. Aging (Albany NY) 2 (12), 945–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin-Montalvo A et al. (2013) Metformin improves healthspan and lifespan in mice. Nat Commun 4, 2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slack C et al. (2012) Activation of AMPK by the putative dietary restriction mimetic metformin is insufficient to extend lifespan in Drosophila. PLoS One 7 (10), e47699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith DL Jr. et al. (2010) Metformin supplementation and life span in Fischer-344 rats. J Gerontol A Biol Sci Med Sci 65 (5), 468–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liochev SI (2015) Which Is the Most Significant Cause of Aging? Antioxidants (Basel) 4 (4), 793–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wheaton WW et al. (2014) Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. Elife 3, e02242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Madiraju AK et al. (2014) Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature 510 (7506), 542–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cuyas E et al. (2018) Metformin directly targets the H3K27me3 demethylase KDM6A/UTX. Aging Cell, e12772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Owen MR et al. (2000) Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J 348 Pt 3, 607–14. [PMC free article] [PubMed] [Google Scholar]
- 38.Miller RA et al. (2013) Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature 494 (7436), 256–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andrzejewski S et al. (2014) Metformin directly acts on mitochondria to alter cellular bioenergetics. Cancer Metab 2, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Birsoy K et al. (2014) Metabolic determinants of cancer cell sensitivity to glucose limitation and biguanides. Nature 508 (7494), 108–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El-Mir MY et al. (2000) Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem 275 (1), 223–8. [DOI] [PubMed] [Google Scholar]
- 42.Kane DA et al. (2010) Metformin selectively attenuates mitochondrial H2O2 emission without affecting respiratory capacity in skeletal muscle of obese rats. Free Radic Biol Med 49 (6), 1082–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He L and Wondisford FE (2015) Metformin action: concentrations matter. Cell Metab 21 (2), 159–162. [DOI] [PubMed] [Google Scholar]
- 44.Zhou G et al. (2001) Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108 (8), 1167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hunter RW et al. (2018) Metformin reduces liver glucose production by inhibition of fructose-1–6-bisphosphatase. Nat Med 24 (9), 1395–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim DH et al. (2002) mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110 (2), 163–75. [DOI] [PubMed] [Google Scholar]
- 47.Jewell JL et al. (2013) Amino acid signalling upstream of mTOR. Nat Rev Mol Cell Biol 14 (3), 133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmelzle T and Hall MN (2000) TOR, a central controller of cell growth. Cell 103 (2), 253–62. [DOI] [PubMed] [Google Scholar]
- 49.Kickstein E et al. (2010) Biguanide metformin acts on tau phosphorylation via mTOR/protein phosphatase 2A (PP2A) signaling. Proc Natl Acad Sci U S A 107 (50), 21830–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kalender A et al. (2010) Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab 11 (5), 390–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robida-Stubbs S et al. (2012) TOR signaling and rapamycin influence longevity by regulating SKN-1/Nrf and DAF-16/FoxO. Cell Metab 15 (5), 713–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vellai T et al. (2003) Genetics: influence of TOR kinase on lifespan in C. elegans. Nature 426 (6967), 620. [DOI] [PubMed] [Google Scholar]
- 53.Harrison DE et al. (2009) Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460 (7253), 392–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bjedov I et al. (2010) Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metab 11 (1), 35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lamming DW et al. (2012) Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science 335 (6076), 1638–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Filer D et al. (2017) RNA polymerase III limits longevity downstream of TORC1. Nature 552 (7684), 263–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Swindell WR (2017) Meta-Analysis of 29 Experiments Evaluating the Effects of Rapamycin on Life Span in the Laboratory Mouse. J Gerontol A Biol Sci Med Sci 72 (8), 1024–1032. [DOI] [PubMed] [Google Scholar]
- 58.Larsson O et al. (2012) Distinct perturbation of the translatome by the antidiabetic drug metformin. Proc Natl Acad Sci U S A 109 (23), 8977–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saucedo LJ et al. (2003) Rheb promotes cell growth as a component of the insulin/TOR signalling network. Nat Cell Biol 5 (6), 566–71. [DOI] [PubMed] [Google Scholar]
- 60.Kim E et al. (2008) Regulation of TORC1 by Rag GTPases in nutrient response. Nat Cell Biol 10 (8), 935–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sancak Y et al. (2008) The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 320 (5882), 1496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baur JA and Birnbaum MJ (2014) Control of gluconeogenesis by metformin: does redox trump energy charge? Cell Metab 20 (2), 197–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Madiraju AK et al. (2018) Metformin inhibits gluconeogenesis via a redox-dependent mechanism in vivo. Nat Med 24 (9), 1384–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alshawi A and Agius L (2019) Low metformin causes a more oxidized mitochondrial NADH/NAD redox state in hepatocytes and inhibits gluconeogenesis by a redox-independent mechanism. J Biol Chem 294 (8), 2839–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thakur S et al. (2018) Metformin Targets Mitochondrial Glycerophosphate Dehydrogenase to Control Rate of Oxidative Phosphorylation and Growth of Thyroid Cancer In Vitro and In Vivo. Clin Cancer Res 24 (16), 4030–4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Foretz M et al. (2010) Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest 120 (7), 2355–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Griss T et al. (2015) Metformin Antagonizes Cancer Cell Proliferation by Suppressing Mitochondrial-Dependent Biosynthesis. PLoS Biol 13 (12), e1002309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Howell JJ et al. (2016) Metformin Inhibits Hepatic mTORC1 Signaling via Dose-Dependent Mechanisms Involving AMPK and the TSC Complex. Cell Metab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cao J et al. (2014) Low concentrations of metformin suppress glucose production in hepatocytes through AMP-activated protein kinase (AMPK). J Biol Chem 289 (30), 20435–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu X et al. (2014) Discrete mechanisms of mTOR and cell cycle regulation by AMPK agonists independent of AMPK. Proc Natl Acad Sci U S A 111 (4), E435–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang CS et al. (2016) Metformin Activates AMPK through the Lysosomal Pathway. Cell Metab 24 (4), 521–522. [DOI] [PubMed] [Google Scholar]
- 72.Zhang CS et al. (2017) Fructose-1,6-bisphosphate and aldolase mediate glucose sensing by AMPK. Nature 548 (7665), 112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carmona-Gutierrez D et al. (2016) The crucial impact of lysosomes in aging and longevity. Ageing Res Rev 32, 2–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Blaby-Haas CE and Merchant SS (2014) Lysosome-related organelles as mediators of metal homeostasis. J Biol Chem 289 (41), 28129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Quan X et al. (2015) The copper binding properties of metformin--QCM-D, XPS and nanobead agglomeration. Chem Commun (Camb) 51 (97), 17313–6. [DOI] [PubMed] [Google Scholar]
- 76.Logie L et al. (2012) Cellular responses to the metal-binding properties of metformin. Diabetes 61 (6), 1423–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Muller S et al. (2018) Metformin reveals a mitochondrial copper addiction of mesenchymal cancer cells. PLoS One 13 (11), e0206764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lanza V et al. (2018) Repurposing of Copper(II)-chelating Drugs for the Treatment of Neurodegenerative Diseases. Curr Med Chem 25 (4), 525–539. [DOI] [PubMed] [Google Scholar]
- 79.Lockwood TD (2010) The lysosome among targets of metformin: new anti-inflammatory uses for an old drug? Expert Opin Ther Targets 14 (5), 467–78. [DOI] [PubMed] [Google Scholar]
- 80.Stynen B et al. (2018) Changes of Cell Biochemical States Are Revealed in Protein Homomeric Complex Dynamics. Cell 175 (5), 1418–1429 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nascimento AC et al. (2016) A multiple kernel learning algorithm for drug-target interaction prediction. BMC Bioinformatics 17, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cuyas E et al. (2018) Metformin Is a Direct SIRT1-Activating Compound: Computational Modeling and Experimental Validation. Front Endocrinol (Lausanne) 9, 657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bridgeman SC et al. (2018) Epigenetic effects of metformin: From molecular mechanisms to clinical implications. Diabetes Obes Metab 20 (7), 1553–1562. [DOI] [PubMed] [Google Scholar]
- 84.Hand TW et al. (2016) Linking the Microbiota, Chronic Disease, and the Immune System. Trends Endocrinol Metab 27 (12), 831–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Caesar R (2019) Pharmacologic and Nonpharmacologic Therapies for the Gut Microbiota in Type 2 Diabetes. Can J Diabetes 43 (3), 224–231. [DOI] [PubMed] [Google Scholar]
- 86.Lee H et al. (2018) Modulation of the gut microbiota by metformin improves metabolic profiles in aged obese mice. Gut Microbes 9 (2), 155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu H et al. (2017) Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med 23 (7), 850–858. [DOI] [PubMed] [Google Scholar]
- 88.Pyra KA et al. (2012) Prebiotic fiber increases hepatic acetyl CoA carboxylase phosphorylation and suppresses glucose-dependent insulinotropic polypeptide secretion more effectively when used with metformin in obese rats. J Nutr 142 (2), 213–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Forslund K et al. (2015) Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature 528 (7581), 262–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Justice JN et al. (2018) A framework for selection of blood-based biomarkers for geroscience-guided clinical trials: report from the TAME Biomarkers Workgroup. Geroscience 40 (5–6), 419–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.de Jager J et al. (2010) Long term treatment with metformin in patients with type 2 diabetes and risk of vitamin B-12 deficiency: randomised placebo controlled trial. BMJ 340, c2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Reinstatler L et al. (2012) Association of biochemical B(1)(2) deficiency with metformin therapy and vitamin B(1)(2) supplements: the National Health and Nutrition Examination Survey, 1999–2006. Diabetes Care 35 (2), 327–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Langan RC and Goodbred AJ (2017) Vitamin B12 Deficiency: Recognition and Management. Am Fam Physician 96 (6), 384–389. [PubMed] [Google Scholar]
- 94.Huang W et al. (2017) Lactate Levels with Chronic Metformin Use: A Narrative Review. Clin Drug Investig 37 (11), 991–1007. [DOI] [PubMed] [Google Scholar]
- 95.Bakris GL and Molitch ME (2016) Should Restrictions Be Relaxed for Metformin Use in Chronic Kidney Disease? Yes, They Should Be Relaxed! What’s the Fuss? Diabetes Care 39 (7), 1287–91. [DOI] [PubMed] [Google Scholar]
- 96.Kalantar-Zadeh K and Kovesdy CP (2016) Should Restrictions Be Relaxed for Metformin Use in Chronic Kidney Disease? No, We Should Never Again Compromise Safety! Diabetes Care 39 (7), 1281–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Silvestre J et al. (2007) Metformin-induced lactic acidosis: a case series. J Med Case Rep 1, 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gershkovich B et al. (2018) A Unique Case of Metformin-Associated Lactic Acidosis. Case Rep Nephrol 2018, 4696182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McCreight LJ et al. (2018) Pharmacokinetics of metformin in patients with gastrointestinal intolerance. Diabetes Obes Metab 20 (7), 1593–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Florez JC (2017) The pharmacogenetics of metformin. Diabetologia 60 (9), 1648–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.McCartney CR and Marshall JC (2016) CLINICAL PRACTICE. Polycystic Ovary Syndrome. N Engl J Med 375 (1), 54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shu Y et al. (2007) Effect of genetic variation in the organic cation transporter 1 (OCT1) on metformin action. J Clin Invest 117 (5), 1422–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Konopka AR et al. (2019) Metformin inhibits mitochondrial adaptations to aerobic exercise training in older adults. Aging Cell 18 (1), e12880. [DOI] [PMC free article] [PubMed] [Google Scholar]