Abstract
Amyotrophic lateral sclerosis (ALS) was recently recognized as a neurovascular disease. Accumulating evidence demonstrated blood-spinal-cord barrier (BSCB) impairment mainly via endothelial cell (EC) degeneration in ALS patients and animal models. BSCB repair may be a therapeutic approach for ALS. We showed benefits of human bone marrow endothelial progenitor cell (hBMEPC) transplantation into symptomatic ALS mice on barrier restoration; however, cellular mechanisms remain unclear. The study aimed to characterize hBMEPCs in vitro under normogenic conditions. hBMEPCs were cultured at different time points. Enzyme-linked immunosorbent assay (ELISA) was used to detect concentrations of angiogenic factors (VEGF-A, angiogenin-1, and endoglin) and angiogenic inhibitor endostatin in conditioned media. Double immunocytochemical staining for CD105, ZO-1, and occludin with F-actin was performed. Results showed predominantly gradual significant post-culture increases of VEGF-A and angiogenin-1 levels. Cultured cells displayed distinct rounded or elongated cellular morphologies and positively immunoexpressed for CD105, indicating EC phenotype. Cytoskeletal F-actin filaments were re-arranged according to cell morphologies. Immunopositive expressions for ZO-1 were detected near inner cell membrane and for occludin on cell membrane surface of adjacent hBMEPCs. Together, secretion of angiogenic factors by cultured cells provides evidence for a potential mechanism underlying endogenous EC repair in ALS through hBMEPC transplantation, leading to restored barrier integrity. Also, ZO-1 and occludin immunoexpressions, confirming hBMEPC interactions in vitro, may reflect post-transplant cell actions in vivo.
Keywords: human bone marrow endothelial progenitor cells, in vitro, angiogenic factors, F-actin, tight junction proteins
1. Introduction
Impairment of the 1blood-brain barrier (BBB) and blood-spinal cord barrier (BSCB) has been shown in amyotrophic lateral sclerosis (ALS), a fatal neurodegenerative disease, mainly by capillary endothelial cell (EC) degeneration. Additionally, alteration of astrocyte end-feet processes, downregulation of tight junction protein expressions, and microvascular leakage were found in the brain and spinal cord of ALS patients (Garbuzova-Davis et al., 2012; Henkel et al., 2009; Winkler et al., 2013) and in animal models of disease (Garbuzova-Davis et al., 2007a, 2007b; Meister et al., 2015; Miyazaki et al., 2011; Nicaise et al., 2010, 2009; Zhong et al., 2008). Based on these and other findings on vascular pathology in ALS (reviewed in (Garbuzova-Davis et al., 2011; Rodrigues et al., 2012)), the BBB/BSCB damage may represent an additional pathogenic disease mechanism identifying ALS as a neurovascular disease. Moreover, molecular biomarkers of disease such as aquaporin-4 and potassium channel at barrier level, particularly at an astroglial-vascular complex, were emphasized (Bataveljic et al., 2014; Bataveljić et al., 2012; Wolburg et al., 2009). Thus, a structurally and functionally impaired barrier, allowing entry of harmful factors from the systemic circulation to the CNS, could foster motor neuron degeneration and death in ALS (Garbuzova-Davis et al., 2008). Additionally, motor neuron death is exacerbated by neuroinflammation mainly via non-motor autonomous cell responses (reviewed in (Boillée et al., 2006; Ilieva et al., 2009)). Based on this discovery, comprehensive research by Teng et al. (Teng et al., 2012) provides an important meta-analysis of 11 independent studies from ALS investigators on the effects of neural stem cell (NSC) transplantation into a transgenic mouse model of ALS. The authors showed multimodal cell actions including production of trophic factors and reduction of inflammation and concluded that transplantation of NSCs may be a viable therapeutic approach for treating ALS.
Despite intensive efforts towards an effective therapy for ALS, the altered BBB/BSCB may be a novel therapeutic target (Fang, 2018) and barrier repair via cell administration could be one promising therapeutic strategy for this devastating disease. Recently, we showed that intravenous transplantation of human bone marrow-derived endothelial progenitor cells (hBMEPCs) into symptomatic G93A SOD1 (superoxide dismutase 1) mutant mice significantly enhanced spinal cord motor neuron survival and improved behavioral disease outcomes (Garbuzova-Davis et al., 2019). This beneficial effect likely occurred by replacement of damaged ECs with “healthy” administered cells. Supporting our notion, widespread engraftment of transplanted hBMEPCs into capillary lumen of the gray/white matter spinal cord and brain motor cortex/brainstem was detected. Also, substantial restoration of capillary ultrastructure, significant decrease in capillary permeability, and enhancement of perivascular astrocyte end-feet eminence in the spinal cord were found. These results demonstrated that transplantation of hBMEPCs into symptomatic ALS mice provides superior barrier restoration. However, the mechanisms of transplanted cell actions are still unclear.
Various angiogenic growth factors such as vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), transforming growth factor (TGF)-β, angiogenin, endoglin, platelet-derived growth factor (PDGF) and others play essential roles in EC proliferation, maturation, and integration for promoting angiogenesis, vasculogenesis, and/or revascularization (reviewed in (Crivellato, 2011; Díaz-Flores et al., 1994; Draoui et al., 2017; Rafii and Lyden, 2003; Ribatti and Crivellato, 2012; Risau, 1990; Urbich and Dimmeler, 2004)). Studies demonstrated that several factors (i.e. VEGF (Hoeben et al., 2004; Tammela et al., 2005) and FGF (Esser et al., 2015)), which stimulate the proliferation of ECs in vitro, also induce angiogenesis in vivo. During vasculature development, ECs display heterogeneous morphology and can be categorized as tip cells, stalk cells, or phalanx cells (Adams and Alitalo, 2007; Potente et al., 2011). Cell phenotypes dynamically change during angiogenesis and are tightly regulated by VEGF as well as Dll4 (Delta-like 4) and Notch signaling molecular pathways (Eilken and Adams, 2010; Jakobsson et al., 2010, 2009; Lobov and Mikhailova, 2018; Siekmann et al., 2008; Suchting et al., 2007). Additionally, there is a strong interaction of VEGF-A with reorganization of endothelial cytoskeletal properties for maintenance of vascular morphogenesis (Morales-Ruiz et al., 2000; Nagy and Senger, 2006; Rousseau et al., 2000).
The cytoskeleton is comprised of three major filament types: microtubules (tubulin), intermediate filaments (lamin), and microfilaments (actin), which are essential for cellular homeostasis through proper establishment of cytoplasmic intracellular organization (reviewed in (Fletcher and Mullins, 2010)). Actin, composing roughly 5-10% of all proteins in eukaryotic cells, has a critical role in cell motility and shape (Cooper, 2000; Dominguez and Holmes, 2011). This protein is comprised of monomeric G-actin, which can be polymerized to filamentous F-actin. Cytoskeleton structure is highly dependent on the dynamic nature of F-actin through polymerization and depolymerization of filamentous actin isoforms controlled by a large number of actin-binding proteins (Kozuka et al., 2007; Oda and Maéda, 2010; Waschke et al., 2005). Dynamic actin filaments contribute to cytosolic organelle organization and even new filament formation (Zigmond, 2004) for promoting flexibility of the cellular structure and plasma membrane (Cooper, 2000). The distribution of F-actin is associated with cell morphology during life cycle processes, differentiation, and growth cone development (Stricker et al., 2010), indicating cytoskeletal arrangement. F-actin is also vital for formation and maintenance of intercellular connections between adjacent cells via tight/adherens junctions (Cooper, 2000; Fanning et al., 1998; Itoh et al., 1997; Sakakibara et al., 2018). In vitro and in vivo studies, for instance, have shown that F-actin interacts with the tight junction scaffolding protein zonula occludens-1 (ZO-1) (Fanning et al., 1998; Odenwald et al., 2017), a protein implicated in endothelial cell tight junctions for proper function of the BBB (Huber et al., 2001).
We have previously demonstrated that hBMEPCs enhance the repair of the BSCB in symptomatic ALS mice (Garbuzova-Davis et al., 2019). What needs elucidation are the constituents of these angiocompetent cells that could contribute to repair mechanisms. The aim of the present study was to examine hBMEPCs in vitro at different time points under normogenic conditions as the first step in defining the relevant mechanisms. Levels of angiogenic factors in collected conditioned media were evaluated; tight junction protein expressions were examined; and cytoskeletal F-actin filament arrangements were characterized. These collectively have the potential to restore the integrity of the BSCB.
2. Results
2.1. Angiogenic factors detection in condition media from cultured hBMEPCs
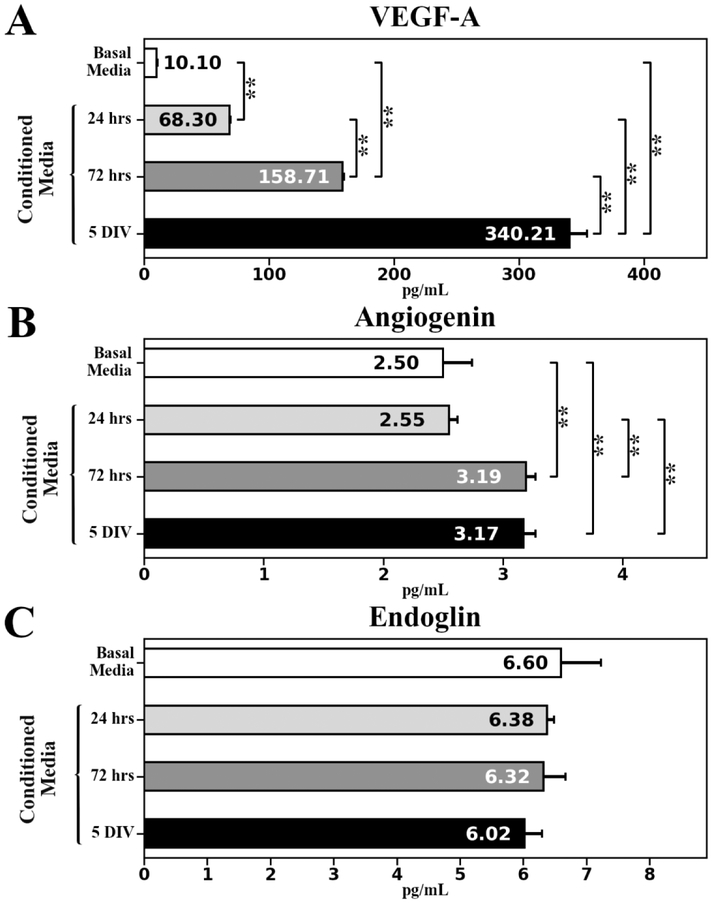
Angiogenic factors (VEGF-A, angiogenin, and endoglin) and angiogenic inhibitor (endostatin) secreted by cultured hBMEPCs at different time points (24 hrs, 72 hrs, and 5 days in vitro (DIV)) were quantified in conditioned media using enzyme-linked immunosorbent assay (ELISA). Results showed a gradually significant (p < 0.01) increase of VEGF-A protein levels from 24 hrs (68.30 ± 0.89 pg/mL) to 5 DIV (340.21 ± 14.16 pg/mL) (Figure 1A). Quantification of angiogenin demonstrated significantly (p < 0.01) elevated protein concentrations at 72 hrs (3.19 ± 0.08 pg/mL) and 5 DIV (3.17 ± 0.10 pg/mL) compared to 24 hrs (2.55 ± 0.07 pg/mL) or basal media (2.50 ± 0.24 pg/mL) (Figure 1B). Of note, endoglin levels in conditioned media at all measured time points (24 hrs: 6.38 ± 0.11 pg/mL; 72 hrs: 6.32 ± 0.35 pg/mL; 5 DIV: 6.02 ± 0.28 pg/mL) did not significantly differ from level in basal media (6.60 ± 0.63 pg/mL) (Figure 1C). Also, endostatin concentration was below the quantification level in conditioned media at each time point in cultured cells and in basal media.
Figure 1. Angiogenic factors ELISA analysis in conditioned media collected at different time points from cultured hBMEPCs.
(A) Significant gradual increase of VEGF-A level was detected in conditioned media from 24 hrs to 5 DIV of cultured hBMEPCs. (B) Significantly elevated angiogenin levels were determined in conditioned media at 72 hrs and 5 DIV of cultured hBMEPCs vs. 24 hrs or basal media. (C) There were no significant differences in endoglin levels between conditioned media at all measured time points and basal media. **p < 0.01.
2.2. Phenotypic and F-actin cytoskeletal characteristics of hBMEPCs in vitro
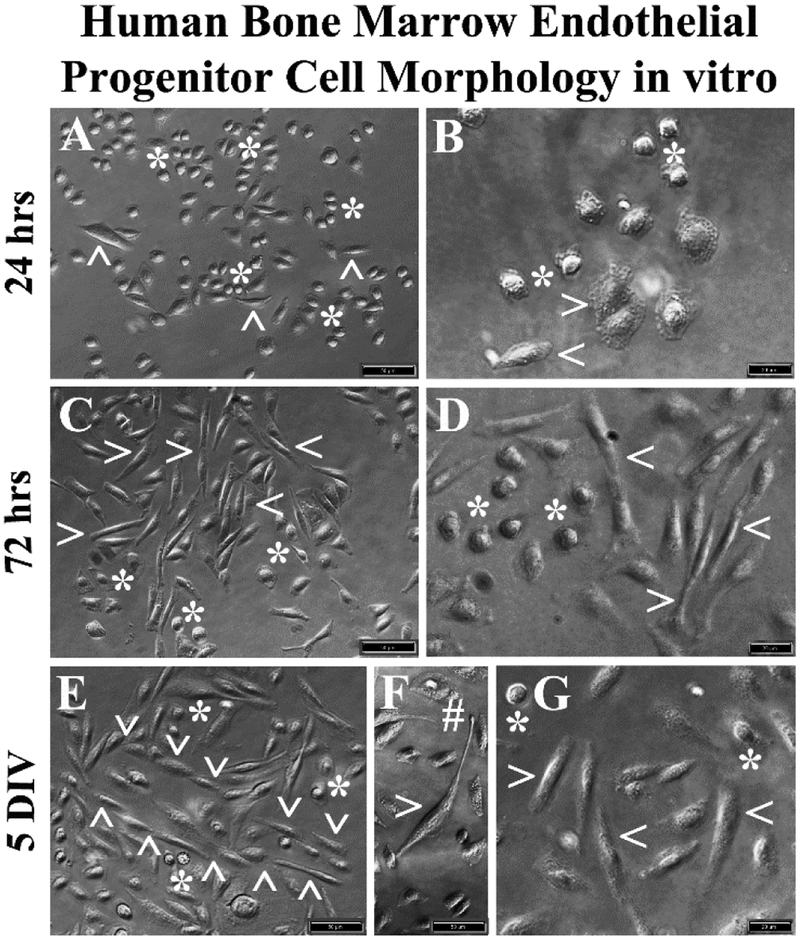
The hBMEPCs cultured in basal media revealed two distinct cell morphologies: rounded and elongated. Rounded cells were predominant at 24 hrs after initial seeding (Figure 2A, 2B). Only a few elongated cells were observed at this time. Of note, numerous vesicles were found within cell cytoplasm (Figure 2B). At 72 hrs, elongated cells were more apparent in the presence of rounded cells (Figure 2C, 2D). In prolonged cultures (5 DIV), oriented elongated cell linings with stalk-like morphology and tubular-like vessel formation were detected (Figure 2E). Some elongated cells demonstrated long processes with notable tip development (Figure 2F). Only a small number of rounded cells were observed at 5 DIV (Figure 2E, 2G).
Figure 2. Phase-contrast images of hBMEPCs in vitro at different time points.
(A, B) The hBMECs showed mainly morphologically rounded cells (asterisks) and a few elongated cells (arrowheads) were observed at 24 hrs of culture in basal media. (C, D) More elongated cells (arrowheads) were detected in cultures at 72 hrs. Numerous rounded cells (asterisks) were noticed. (E) At 5 DIV, a tubular-like vessel formation (arrowheads) composed of elongated cells was determined. (F) Elongated cells also displayed long processes with tip development (hashtag). (G) A few rounded cells (arrowheads) were still observed at 5 DIV. Scale bar in A, C, E, F is 50 μm; in B, D, G is 20 μm.
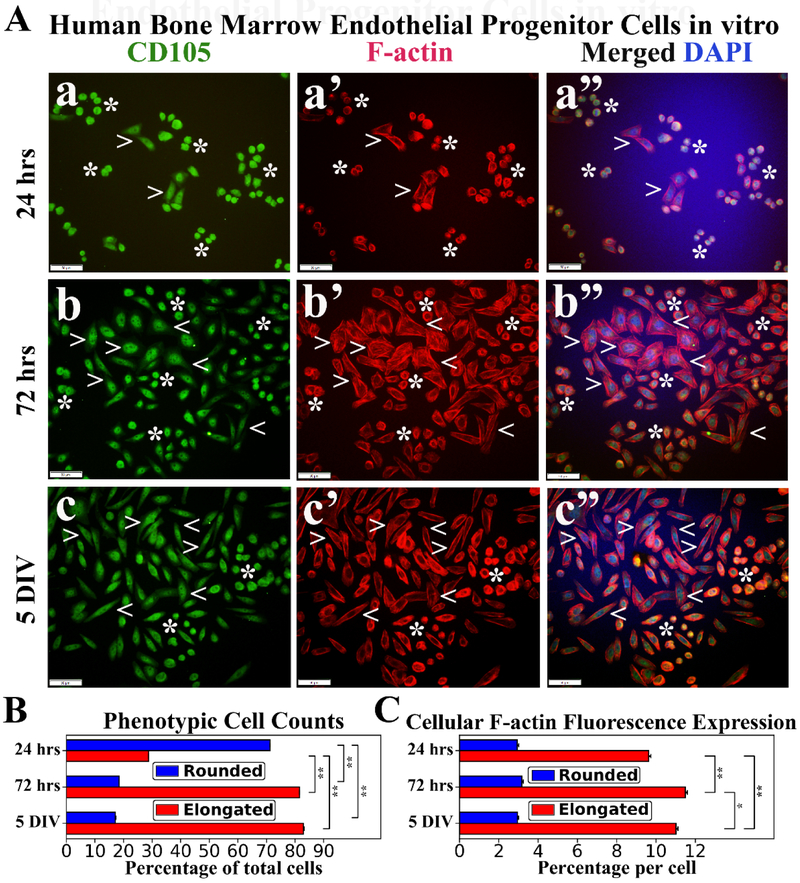
Immunocytochemical staining of hBMEPCs for CD105 and F-actin was performed at different culture time points. CD105 immunoexpression was detected in all rounded and elongated cells at each time point (Figure 3a, 3b, 3c), indicating endothelial cell phenotype. Disperse F-actin immunoexpression was displayed within the cytosol of rounded hBMEPCs and remained stable at different time points of cultured cells (Figure 3a’, 3b’, 3c’). Morphologically elongated cells demonstrated progressively organized filamentous F-actin strips with large distribution in cell cytoplasm during post-culture period (Figure 3a’, 3b’, 3c’). Elongated hBMEPCs were noticeably enlarged compared to rounded cells and some of these elongated cells exhibited probing filopodia-like structures at 72 hrs. At 5 DIV, F-actin filaments were more localized near the inner elongated cell membrane. The prevalence of rounded hBMEPCs significantly (p < 0.01) decreased from 24 hrs (71.22 ± 0.03%) to 72 hrs (18.48 ± 0.02%) and remained low at 5 DIV (17.10 ± 0.03%) (Figure 3B). In contrast, the percentage of elongated cells significantly (p < 0.01) increased from 24 hrs (28.78 ± 0.03%) to 5 DIV (82.90 ± 0.30%) (Figure 3B). Of note, the occurrence of elongated cells at 72 hrs was similar to that at 5 DIV. Cellular F-actin immunoexpression levels in rounded cells held constant across culture time periods: 24 hrs (2.94 ± 0.07%), 72 hrs (3.17 ± 0.08%), and 5 DIV (2.95 ± 0.05%) (Figure 3C). However, significantly (p < 0.01) increased expressions of F-actin filaments were determined in elongated cells from 24 hrs (9.63 ± 0.11%) to 72 hrs (11.49 ± 0.11%) (Figure 3C). Interestingly, F-actin filament expressions were significantly (p < 0.05) decreased at 5 DIV vs. 72 hrs.
Figure 3. Immunocytochemical analyses of hBMEPCs in vitro at different time points for CD105 and F-actin expressions.
(A) CD105 immunoexpression was detected in all cells at each time point (green, a, b, c). During post-culture, F-actin immunoexpression (red) displayed diffusely within cytosol of rounded hBMEPCs (arrowheads) and elongated cells (arrowheads) exhibited organized filamentous strips in cell cytoplasm (a’, b’, c’). Merged images (a”, b”, c”) are shown with DAPI (blue). Scale bar in a-c” is 50 μm. (B) Rounded hBMEPC prevalence significantly decreased in conjunction with significantly increased occurrence of elongated hBMEPC from 24 hrs to 5 DIV. (C) Although F-actin immunoexpression levels remained stable over time, a significant increase of cytoskeletal filaments was determined in elongated cells from 24 hrs to 5 DIV. *p < 0.05, **p < 0.01.
2.3. Immunocytochemical characteristics of hBMEPCs in vitro for tight junction protein expression
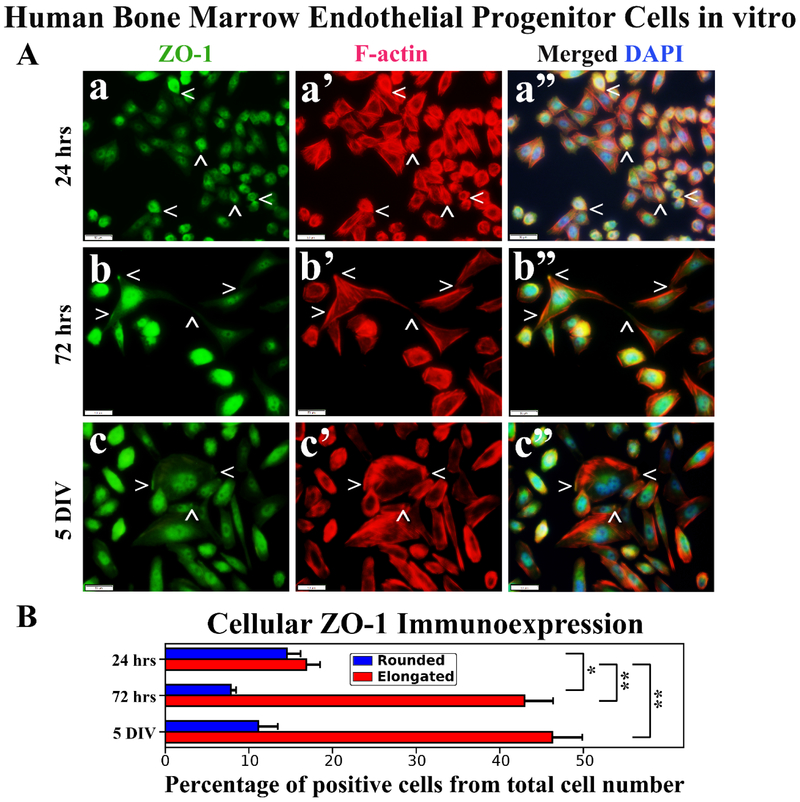
Immunocytochemical staining and analysis of hBMEPCs in vitro for tight junction ZO-1 and occludin proteins in conjunction with F-actin at different culture time points were performed. Results showed diffuse ZO-1 immunoexpressions in rounded hBMEPCs (Figure 4Aa). ZO-1 immunoexpressions were mainly detected within elongated cell filopodia tips (Figure 4Ab) or near inner cell membrane (Figure 4Ac). In numerous elongated hBMEPCs, ZO-1 co-localized with F-actin filaments (Figure 4Ab’, 4Ac’). Percentages of ZO-1 immunopositive hBMEPCs were similar for rounded (14.56 ± 1.63%) and elongated cells (16.88 ± 1.66%) in 24 hrs culture (Figure 4B). Percentage of rounded cells expressing ZO-1 significantly (p < 0.05) decreased to 7.85 ± 0.63% at 72 hrs and no significant differences were detected compared to 5 DIV (11.13 ± 2.34%). In contrast, the numbers of ZO-1 immunopositive elongated hBMEPCs significantly (p < 0.01) increased over time: 24 hrs - 16.88 ± 1.66%, 72 hrs - 42.99 ± 3.40%, 5 DIV - 46.30 ± 3.61% (Figure 4B).
Figure 4. Immunocytochemical analysis of hBMEPCs in vitro at different time points for ZO-1 and F-actin.
(A) Rounded hBMEPCs (a) displayed diffuse presence of ZO-1 (green, arrowheads) in contrast to ZO-1 localization in elongated hBMEPCs (b, c). ZO-1 immunoexpression was mainly detected within elongated cell filopodia tips (b) or near inner cell membrane (c). Merged images (a”, b”, c”) are shown with DAPI (blue). Scale bar in a-c” is 50 μm. (B) Rounded ZO-1 immunopositive hBMEPCs prevalence decreased significantly from 24 hrs to 72 hrs and no significant differences were detected between 72 hrs and 5 DIV cultures. Prevalence of ZO-1 immunopositive elongated hBMEPCs significantly increased across all time points. *p < 0.05, **p < 0.01.
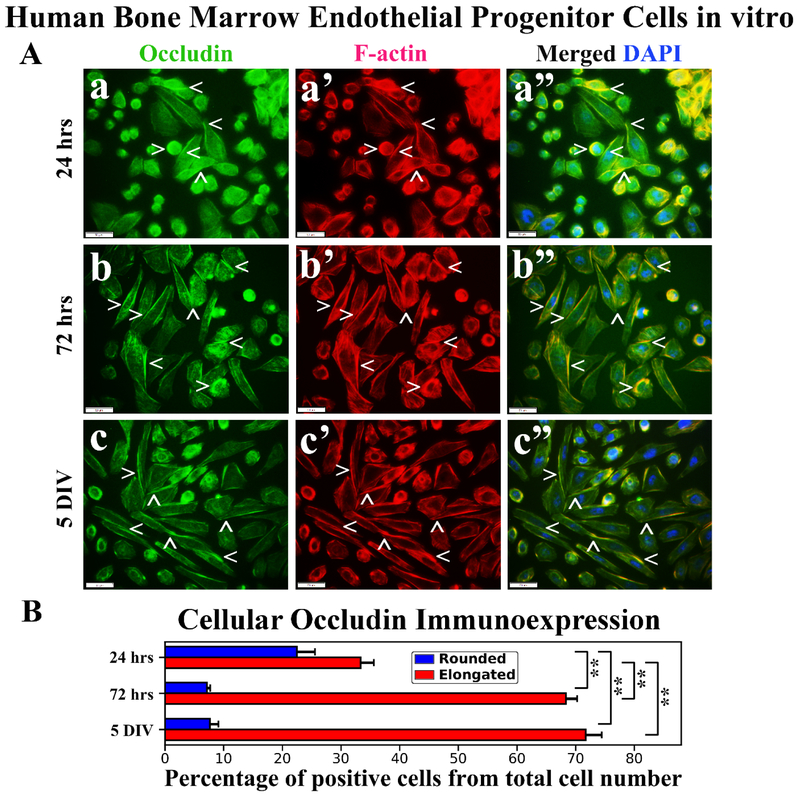
Immunocytochemical analysis of hBMEPCs for occludin immunoexpressions demonstrated protein localization mainly on cell membrane surfaces of rounded hBMEPCs (Figure 5Aa). Elongated cells displayed occludin immunoexpressions as strips between adjacent cells (Figure 5Ab, 5Ac). Occludin immunoexpressions appeared to be in association with F-actin in rounded cells (Figure 5Aa’) and distal from F-actin in numerous elongated cells (Figure 5a’, 5b’, 5c’). A higher percentage of rounded occludin immunopositive hBMEPCs was determined at 24 hrs (22.46 ± 3.10%) and prevalence of these cells was significantly (p < 0.01) decreased at 72 hrs (7.15 ± 0.57%) and 5 DIV (7.65 ± 1.45%) (Figure 5B). The percentage of elongated hBMEPCs immunoexpressing occludin significantly (p < 0.01) increased from 24 hrs (33.35 ± 2.27%) to 5 DIV (71.71 ± 2.75%) (Figure 5B). Of note, there was no significant difference in occludin immunopositive cell percentages between 72 hrs (68.37 ± 1.87%) and 5 DIV cultures.
Figure 5. Immunocytochemical analysis of hBMEPCs in vitro at different time points for occludin and F-actin.
(A) Occludin immunoexpression in rounded hBMEPCs localized on cell membrane surfaces and elongated cells showed this protein immunoexpression as strips between adjacent cells (green, arrowheads, a, b, c). Occludin immunoexpression appeared to be distal from F-actin in numerous cells (red, arrowheads, a’, b’, c’). Merged images (a”, b”, c”) are shown with DAPI (blue). Scale bar in a-c” is 50 μm. (B) Percentage of rounded occludin immunopositive hBMEPCs substantially decreased from 24 hrs to 5 DIV. Significantly increased percentage of elongated hBMEPCs immunoexpressing occludin was determined from 24 hrs to 5 DIV. **p < 0.01.
3. Discussion
In the present study, human bone marrow endothelial progenitor cells (hBMEPCs) as an effective cell type for repair of altered BBB/BSCB in ALS were characterized in vitro at different time points under normogenic conditions. The major study findings were that hBMEPCs: (1) significantly increased VEGF-A and angiogenin secretions; (2) classified phenotypically as rounded or elongated cells; (3) displayed re-arrangement of intracellular F-actin filaments; and (4) immunoexpressed ZO-1 and occludin. These findings provide evidence for potential endogenous endothelium repair in ALS through hBMEPC transplantation by detection of angiogenic factor secretions and tight junction protein immunoexpressions in cultured cells. Furthermore, this in vitro study may be indicative of post-transplant hBMEPC actions in vivo.
Over the last decade, our knowledge about cellular and molecular mechanisms during angiogenesis under physiological and pathological conditions has dramatically increased (reviewed in (Adams and Alitalo, 2007; De Bock et al., 2013; De Smet et al., 2009; Eilken and Adams, 2010; Herbert and Stainier, 2011; Ribatti and Crivellato, 2012; Vandekeere et al., 2015; Yang et al., 2018)). There are specialized endothelial cell phenotypes such as tip cells, stalk cells, and phalanx cells with distinct functionality in vessel morphogenesis. Endothelial tip cells are considered migratory cells, which extend filopodia, spiky plasma membrane protrusions, towards the angiogenic stimuli (Dorrell et al., 2002; Gerhardt et al., 2003; Mattila and Lappalainen, 2008). Stalk cells are located behind the leading tip cell and are characterized as proliferating cells resulting in sprouts elongation and vascular lumen formation (Gerhardt et al., 2003; Jakobsson et al., 2010). Phalanx cells are referred to as quiescent endothelial cells lining vessels after new vessel branches are composed (De Smet et al., 2009). A comprehensive study by Blancas et al., (Blancas et al., 2013), analyzing various markers of these distinct endothelial cell subpopulations derived from embryonic stem cells in vitro, demonstrated high expression levels of CXCR4 in tip-like cells and Notch-1 in proliferative stalk-like cells whereas the phalanx cell type mainly expressed Flt-1, Tie-1, and Tie-2 markers. The authors concluded that functionally different cell subtypes may be separated based on their specific markers for cell-based therapeutic approaches. In our study, hBMEPCs in vitro displayed distinct phenotypic morphologies and were classified as rounded or elongated cells and immunoexpressed CD105 antigen at different culture time points, confirming an endothelial cell phenotype. Over time, prevalence of elongated cells in cultures increased. However, rounded cells were still present at each time point although their relative prevalence decreased through 5 DIV and may have presented a proliferative stage during cell development. Although this suggestion needs confirmation, the detection of numerous vesicles mostly in rounded cells at 24 hrs (Figure 2B) may indicate active cell functionality such as pinocytosis. Importantly, elongated cells upon their growth (5 DIV) showed tips with filopodia and stalk-like morphology forming tubular structures. This observation highlights the potency of hBMEPCs in angiogenesis by promoting the sprout of endothelial cells and their growth as discussed previously (Gerhardt et al., 2003; Jakobsson et al., 2010).
Numerous studies have demonstrated the critical role of angiogenic factors for ECs functional maintenance during angiogenesis (reviewed in (Díaz-Flores et al., 1994; Gianni-Barrera et al., 2011; Hoeben et al., 2004; Ribatti and Crivellato, 2012; Risau, 1990; Tammela et al., 2005)). For instance, pro-angiogenic VEGF stimulates EC proliferation, motility, and filipodia extension promoting angiogenesis (Eilken and Adams, 2010; Hoeben et al., 2004; Tammela et al., 2005). Moreover, changes in EC phenotypes are closely regulated by VEGF and Notch signaling molecular pathways (Eilken and Adams, 2010; Gridley, 2010, 2007; Jakobsson et al., 2009; Siekmann et al., 2008). Our study showed gradual significant increases of VEGF-A concentrations in conditioned media from cultured hBMEPCs at different time points: from 24 hrs to 5 DIV – about 5-fold, from 72 hrs to 5 DIV – more than a 2-fold increase. The elevation of this factor over culture time is potentially associated with the increased percentage of elongated cells from 24 hrs (~29%) to 5 DIV (~83%) and induction of tip-like cell phenotype. Also, angiogenin-1 levels significantly increased from 24 hrs to 5 DIV. Angiogenin is potent stimulator of angiogenesis by inducing EC proliferation and formation of tubular structures as well as mediating interactions between ECs (reviewed in (Lyons et al., 2017; Sheng and Xu, 2016)). Formation of tubular-like structures was detected in cultured hBMEPCs at 5 DIV, possibly linked to the high level of angiogenin present at this time point. Importantly, angiogenin mutations have been identified in ALS patients, which result in reduced angiogenesis by angiogenin deficiency in ribonuclease activity and nuclear translocation (Padhi et al., 2013, 2012; Wu et al., 2007).
Although VEGF-A and angiogenin-1 concentrations were significantly elevated in conditioned media from cultured hBMEPCs during prolonged post-culture time, surprisingly endoglin levels at all measured time points did not differ from levels in basal media. It has been shown that endoglin may be involved in cytoskeletal F-actin organization affecting EC morphology and migration (Conley et al., 2004; Sanz-Rodriguez et al., 2004). Since MMP-14 can cleave membrane bound endoglin to produce a soluble form (ten Dijke et al., 2008), it is possible that endoglin secretion by hBMEPCs was undetected due to the lack of enzymatic protein shedding. However, more studies are needed to confirm or deny this possibility. Of note, a significant reduction of endoglin concentrations in sera from ALS patients was found, potentially leading to motor neuron degeneration in ALS (Iłzecka, 2008). Additionally, angiogenic inhibitor endostatin concentrations in conditioned media were below quantification level at each time point. There is a balance between pro- and anti-angiogenic factors for maintaining proper physiological vascular homeostasis (reviewed in (Rao et al., 2015)). Angiogenic inhibitors are involved in controlling blood vessel establishment by interfering with EC formation, migration, and endothelial tube morphogenesis. Endostatin, as one angiogenic inhibitor, suppresses EC proliferation, migration, and survival (Dixelius et al., 2000; Kim et al., 2002; O’Reilly et al., 1997). Although the molecular mechanisms of this anti-angiogenic protein are still unclear, it has been shown that endostatin directly interacts with tyrosine kinase receptor KDR/Flk-1 and inhibits actions of VEGF, the most important protein in mediation of angiogenesis (Kim et al., 2002). As high VEGF-A levels and substantial phenotypic EC activity were detected, we do not expect endostatin secretion by hBMEPCs.
Furthermore, we demonstrated re-arrangement of cytoskeletal F-actin filaments according to cultured cell morphologies. F-actin immunoexpressions were diffusely detected within rounded cell cytosol, whereas elongated cells showed organized filamentous F-actin strips in cell cytoplasm, predominantly located near the inner cell membrane at 5 DIV. The distinctive F-actin filamentous pattern in elongated hBMEPCs vs. rounded cells supports the importance of cytoskeleton actin for cytosolic organelle distribution during EC differentiation, sprouting, and motility (Kozuka et al., 2007; Stricker et al., 2010; Waschke et al., 2005; Zigmond, 2004). In a comprehensive review by De Smet et al. (De Smet et al., 2009), specific functions, including molecular pathways, of morphologically specialized ECs (tip, stalk, and phalanx cells) in conjunction with cellular cytoskeleton organization were detailed. The authors noted that endothelial tip cells form lamellipodia and filopodia that correct cell movement by probing the microenvironment. These highly dynamic cellular protrusions contain tight filamentous F-actin, which connects to the extracellular matrix (De Smet et al., 2009). The authors also noted that reorganization of endothelial cytoskeletal properties robustly interacts with VEGF-A for proper maintenance of vascular morphogenesis (Nagy and Senger, 2006; Rousseau et al., 2000).
Finally, our results demonstrated positive immunoexpressions of hBMEPCs for tight junction ZO-1 and occludin proteins at different times in culture. However, tight junction expression profiles varied according to cell morphology. Rounded hBMEPCs showed diffuse ZO-1 immunoexpressions and this protein was mainly detected in elongated cells near the inner cell membrane or within cell filopodia tips. Also, in numerous elongated hBMEPCs scaffold protein ZO-1 were co-localized with F-actin filaments. Our findings support previously described important interaction of ZO-1 with F-actin filaments to stabilize cellular integrity and cell-cell connectivity during angiogenesis (Fanning et al., 2002, 1998; Fanning and Anderson, 2009; Odenwald et al., 2017). In contrast, tight junction occludin immunoexpressions were determined mainly on cell membrane surfaces of rounded hBMEPCs whereas elongated cells displayed this protein immunoexpressions as strips between adjacent cells. Similarly to ZO-1, immunoexpressions of occludin appeared to be associated with F-actin in rounded cells and distal from F-actin in numerous elongated cells. However, elevated occludin within the cell membrane may stabilize the tight junction complex in cell-cell connections and likely play a non-initial role in tight junction formation. It has been established that ZO-1 interactions with F-actin and ZO-1 bindings with occludin are required for cell-intrinsic signaling during lumen formation (Fanning et al., 1998; Odenwald et al., 2017). Although our study was restricted to investigating immunoexpressions of individual ZO-1 and occludin proteins in conjunction with F-actin in cultured hBMEPCs, determining concurrence of these two tight junction proteins simultaneously is important and one of our future studies will address this point.
Alternatively to hBMEPCs, umbilical cord blood-derived EPCs could serve as a source of transplant cells, having shown high proliferation in vitro and the ability in vivo to induce neovascularization in a murine model of hind limb ischemia (Vanneaux et al., 2010). Also, EPCs can be derived from pluripotent human embryonic stem cells using small molecule induction, which is a clinically-relevant method (Parsons et al., 2011). Moreover, microvesicles derived from EPCs could substitute for cells in therapeutic transplantation (Cantaluppi et al., 2012). This approach will be examined in our future study. Importantly, impeding neuroinflammation via diminishing production of pro-inflammatory cytokines and other harmful factors should be considered for selection of an appropriate cell type for targeted ALS therapy. In this context, a recent review (Teng, 2019) discussed in detail the multifunctional potency of stem cells, emphasizing their essential role for therapeutic translational research.
Together, the in vitro study results demonstrate that hBMEPCs are angiocompetent cells showing distinct phenotypic morphologies, secretion of angiogenic factors, endothelial cytoskeletal F-actin dynamics, and expression of tight junction proteins. This evidence may suggest post-transplant hBMEPC actions in vivo for potential endogenous maintenance of endothelium in ALS, leading to restoration of barrier integrity. However, one limitation in our study is that the in vitro design corresponded to normogenic culture conditions, which may not fully recapitulate the disease pathology. Adding, for example, sera/plasma from ALS mice and/or patients may better provide translational results. This study is currently underway.
4. Conclusions
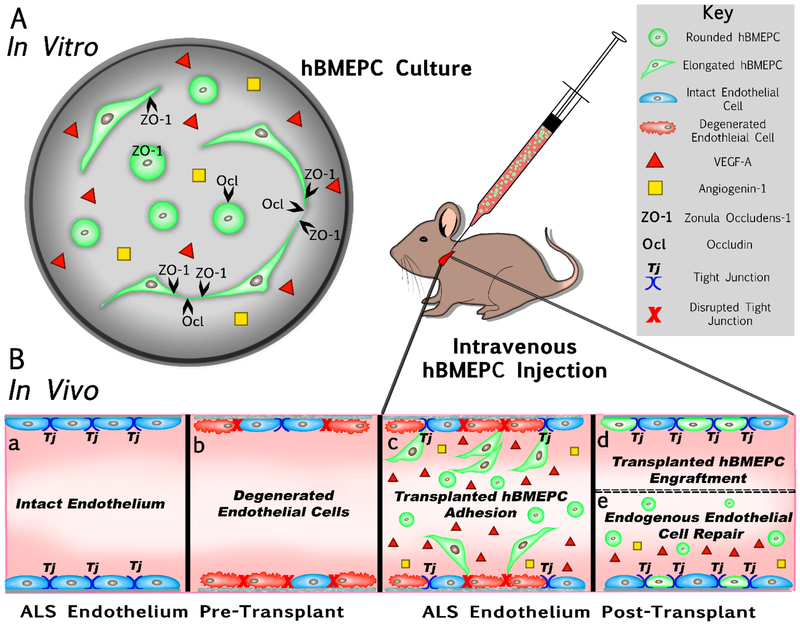
The present study characterized human bone marrow endothelial progenitor cells (hBMEPCs) in vitro at different time points as an effective cell type for repair of damaged CNS endothelium in ALS. Our schematic diagram (Figure 6) briefly summarizes in vitro results and provides potential therapeutic mechanism(s) of transplanted hBMEPCs into a mouse modeling ALS. Mainly, cultured hBMEPCs (Figure 6A) morphologically changed phenotype from rounded to elongated cells, secreted VEGF-A and angiogenin-1, and expressed tight junction ZO-1 and occludin proteins during post-culture. It has been shown that the CNS endothelium in ALS mice may transition from an intact (Figure 6Ba) to a degenerated (Figure 6Bb) status, leading to a dysfunctional BBB/BSCB in ALS (Garbuzova-Davis et al., 2007a, 2007b; Miyazaki et al., 2011; Nicaise et al., 2009; Zhong et al., 2008). We recently demonstrated (Garbuzova-Davis et al., 2019) that intravenous transplantation of hBMEPCs into early symptomatic G93A SOD1 mutant mice substantially repair BSCB, leading to enhancement of motor neuron survival in the spinal cord and improvement in behavioral disease outcomes. Widespread adhesion (Figure 6Bc) followed by engraftment (Figure 6Bd) of transplanted hBMEPCs into capillary lumen of the gray/white matter spinal cord and brain motor cortex/brainstem in ALS mice supports potential replacement of damaged ECs with “healthy” administered cells. Our study also showed the presence of administered cells in blood circulation of mice four weeks after cell treatment, likely evidence of continuing processes in re-establishing BSCB integrity. Based on current in vitro study results, we hypothesize that hBMEPCs, as angiocompetent cells, not only contribute to endogenous restoration of damaged endothelium via secretion of angiogenic factors, but also facilitate proper maintenance of vascular homeostasis by increasing endothelial cell-cell “tightness” (Figure 6Be). We believe that transplantation of hBMEPCs synergistically aids in repair of the altered BSCB in ALS through replacement of damaged ECs and endogenous endothelium restoration.
Figure 6. Schematic diagram summarizing hBMEPCs in vitro results and providing effective therapeutic mechanism(s) of transplanted cells into a mouse modeling ALS.
(A) hBMEPCs in vitro showed changes in morphological phenotype from rounded to elongated cells, secreted VEGF-A and angiogenin-1, and expressed tight junction ZO-1 and occludin proteins during post-culture. (B) Dysfunction of BBB/BSCB in ALS is mainly characterized by endothelial cells transition from an intact (a) to a degenerated (b) status. Intravenous transplantation of hBMEPCs into early symptomatic G93A SOD1 mice significantly repaired BSCB by widespread cell adhesion (c) followed by cell engraftment (d) into capillary lumen of the spinal cords and brains. The presence of administered cells in blood circulation of mice was also noted at four weeks after cell treatment. We hypothesized that hBMEPCs may not only contribute to endogenous restoration of damaged endothelium via secretion of angiogenic factors, but also facilitate proper maintenance of vascular homeostasis by increasing endothelial cell-cell “tightness” (e).
Together, our in vitro results elucidate potential functional mechanism(s) of hBMEPC transplantation for repair of damaged BBB/BSCB in ALS. Also, our results strongly indicate that hBMEPC is an excellent candidate cell type for barrier repair in ALS. However, since hBMEPCs in vitro were characterized under normogenic conditions at baseline, providing pathological ALS systemic content in culture design will better mimic post-transplant cell milieu. Also, detection of angiogenic factors in blood of cell-treated vs. non-treated ALS mice may determine additional transplanted cell actions. Studies addressing these issues are currently under development.
5. Experimental Procedure
5.1. Cell preparation and culture procedure
Cryopreserved human bone marrow-derived endothelial progenitor cells (hBMEPCs, CELPROGEN, Torrance, CA, USA) were thawed rapidly at 37°C and then transferred slowly with a pipette into a centrifuge tube containing 10 mL of phosphate buffered saline 1X (PBS), pH 7.2 (Cat. No. SH30256.01, HyClone Laboratories, Logan, Ut, USA). The cells were centrifuged (100 g/7 min) at room temperature (RT), the supernatant discarded, and the process repeated. After the final wash, cell viability was assessed using the 0.4% trypan blue dye exclusion method before culture. The hBMEPCs were cultured in a 24-well plate (2 X 104 cells/500 μl commercial basal media/well) for 24 hrs, 72 hrs, and 5 days in vitro (DIV). Conditioned media were collected at each time point for detection of angiogenic factors using ELISA. Media was renewed after collection at 24 and 72 hrs. Cells were fixed by 4% paraformaldehyde in PBS solution for immunocytochemistry.
5.2. Enzyme-linked immunosorbent assay (ELISA)
ELISA was used to detect the concentrations of human vascular endothelial growth factor A (VEGF-A, Cat. No. BMS277/2), angiogenin (Cat. No. EHANG), endoglin (Cat. No. EHENG), and endostatin (Cat. No. EHCOL18A1), purchased from Thermo Fisher Scientific (Waltham, MA, USA) in cell conditioned media collected upon incubation of hBMEPCs at each time point vs. basal media. All assay procedures were performed according to the manufacturer’s protocol. Briefly, lyophilized standards were reconstituted and diluted with the supplied assay diluent to generate standard curves specific for each assay. Samples and appropriate standards for each assay were then loaded (100 μL) into pre-coated 96-well ELISA plates in duplicate. Adhesive plate sealers were used to cover the wells and the plates were incubated overnight at 4°C with gentle shaking. On the following day, each plate was washed four times using the supplied wash buffer. After the last wash, an assay specific biotinylated detection antibody was then added to each well (100 μL) and incubated for 1 hour at RT with gentle shaking. The plates were again washed and a streptavidin-HRP solution (100 μL) was added to each well for 45 min at RT. After a final wash, the tetramethylbenzidine substrate (100 μL) was added to each well and incubated for 30 min at RT. A stop solution consisting of 0.2M sulfuric acid (50 μL) was added to each well to inactivate the HRP enzyme and convert any catalyzed substrate from blue to yellow. Absorbance was measured at 450nm using a Perkin Elmer 2300 EnSpire Multimode plate reader (Waltham, MA) within 30 min of adding the stop solution. Data for each angiogenic factor was reported as pg/mL.
5.3. Immunocytochemical staining of hBMEPC in vitro
Double immunocytochemical staining of hBMEPCs for CD105, Zonula Occludens-1 (ZO-1) and occludin, with F-actin, was performed. Briefly, the rabbit polyclonal primary antibodies: CD105 (1:200, Cat. No. ab107595, Abcam, USA), ZO-1 (1:200, Cat. No. 61-7300, Invitrogen, USA) or occludin (1:500, Cat. No. ab168986, Abcam, USA) were applied separately into cell culture wells after pre-incubation in a blocking solution (10% normal goat serum/3% Triton 100X/PBS) for 60 min at RT. After incubation overnight at 4°C, cells were rinsed in PBS and incubated with goat anti-rabbit secondary antibody conjugated to fluorescein isothiocyanate (1:500, Cat. No. A11008, Molecular Probes, USA) for 2 hrs at RT. Cells were then rinsed with PBS and ActinRed 555 ReadyProbes conjugated to fluorescent tetramethylrhodamine (Cat. No. R37112, Thermo Fisher Scientific USA) was applied to culture wells for 30 min at RT. Next, after additional cell washes with PBS, Vectashield containing DAPI (Vector Laboratories, USA) was applied. Cell cultures were examined under an epifluorescent inverted Olympus BX60 microscope and randomly selected images were captured for further analysis of immunoexpression and phenotypic cell characteristics.
5.4. Analyses of hBMEPC immunoexpressions
Phenotypic characterization and analyses of F-actin, ZO-1 and occludin immunoexpressions in hBMEPC cultures were performed using NIH ImageJ software (version 1.46). For Hbmepc morphological characterization and analyses, fluorescence images (n=5 images/time point) were used and cell counts were performed. Cytoskeletal F-actin fluorescence intensity, according to cell morphology, was measured within individual hBMEPC (n=10 images/time point) concurrently with the percentage of fluorescent cell area and presented as %/mm2. Thresholds for detection of F-actin fluorescent expressions were adjusted for each cell image to eliminate background noise. ZO-1 and occludin immunoexpressions in hBMEPCs (n=5 images/time point) were determined by cellular localization of analyzed proteins and subsequently the percentage of immunopositive cells from total cell number was counted in entire image.
5.5. Statistical analysis
Data are presented as means ± S.E.M. One-way ANOVA with post-hoc Tukey HSD (Honesty Significant Difference) multiple comparison test using online statistical software (astatsa.com, 2016 Navendu Vasavada) was performed for statistical analysis. Significance was defined as p < 0.05.
Highlights:
Cultured hBMEPCs gradually changed phenotype from rounded to elongated cells
hBMEPCs significantly increased VEGF-A and angiogenin secretions in vitro
hBMEPCs expressed tight junction ZO-1 and occludin proteins
hBMEPCs showed re-arrangement of intracellular F-actin
hBMEPCs may aid repair of BSCB in ALS by endogenous endothelium restoration
Acknowledgments
This study was supported by the NIH, NINDS (1R01NS090962) grant.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Abbreviations: ALS, amyotrophic lateral sclerosis; BBB, blood-brain barrier; BSCB, blood-spinal cord barrier; DIV, days in vitro; EC, endothelial cell; ELISA, enzyme-linked immunosorbent assay; F-actin, filamentous actin; hBMEPC, human bone marrow-derived endothelial progenitor cell; SOD1, superoxide dismutase 1; VEGF, vascular endothelial growth factor.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams RH, Alitalo K, 2007. Molecular regulation of angiogenesis and lymphangiogenesis. Nat. Rev. Mol. Cell Biol 8, 464–478. 10.1038/nrm2183 [DOI] [PubMed] [Google Scholar]
- Bataveljic D, Milosevic M, Radenovic L, Andjus P, 2014. Novel molecular biomarkers at the blood-brain barrier in ALS. Biomed. Res. Int 2014, 907545 10.1155/2014/907545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bataveljić D, Nikolić L, Milosević M, Todorović N, Andjus PR, 2012. Changes in the astrocytic aquaporin-4 and inwardly rectifying potassium channel expression in the brain of the amyotrophic lateral sclerosis SOD1(G93A) rat model. Glia 60, 1991–2003. 10.1002/glia.22414 [DOI] [PubMed] [Google Scholar]
- Blancas AA, Wong LE, Glaser DE, McCloskey KE, 2013. Specialized tip/stalk-like and phalanx-like endothelial cells from embryonic stem cells. Stem Cells Dev. 22, 1398–1407. 10.1089/scd.2012.0376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boillée S, Vande Velde C, Cleveland DW, 2006. ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron 52, 39–59. 10.1016/j.neuron.2006.09.018 [DOI] [PubMed] [Google Scholar]
- Cantaluppi V, Biancone L, Figliolini F, Beltramo S, Medica D, Deregibus MC, Galimi F, Romagnoli R, Salizzoni M, Tetta C, Segoloni GP, Camussi G, 2012. Microvesicles Derived from Endothelial Progenitor Cells Enhance Neoangiogenesis of Human Pancreatic Islets. Cell Transplant 21, 1305–1320. 10.3727/096368911X627534 [DOI] [PubMed] [Google Scholar]
- Conley BA, Koleva R, Smith JD, Kacer D, Zhang D, Bernabéu C, Vary CPH, 2004. Endoglin controls cell migration and composition of focal adhesions: function of the cytosolic domain. J. Biol. Chem 279, 27440–27449. 10.1074/jbc.M312561200 [DOI] [PubMed] [Google Scholar]
- Cooper GM, 2000. Structure and Organization of Actin Filaments, in: The Cell: A Molecular Approach. 2nd Edition. Sinauer Associates, Inc. [Google Scholar]
- Crivellato E, 2011. The role of angiogenic growth factors in organogenesis. Int. J. Dev. Biol 55, 365–375. 10.1387/ijdb.103214ec [DOI] [PubMed] [Google Scholar]
- De Bock K, Georgiadou M, Carmeliet P, 2013. Role of endothelial cell metabolism in vessel sprouting. Cell Metab. 18, 634–647. 10.1016/j.cmet.2013.08.001 [DOI] [PubMed] [Google Scholar]
- De Smet F, Segura I, De Bock K, Hohensinner PJ, Carmeliet P, 2009. Mechanisms of vessel branching: filopodia on endothelial tip cells lead the way. Arterioscler. Thromb. Vasc. Biol. 29, 639–649. 10.1161/ATVBAHA.109.185165 [DOI] [PubMed] [Google Scholar]
- Díaz-Flores L, Gutiérrez R, Varela H, 1994. Angiogenesis: an update. Histol. Histopathol 9, 807–843. [PubMed] [Google Scholar]
- Dixelius J, Larsson H, Sasaki T, Holmqvist K, Lu L, Engström A, Timpl R, Welsh M, Claesson-Welsh L, 2000. Endostatin-induced tyrosine kinase signaling through the Shb adaptor protein regulates endothelial cell apoptosis. Blood 95, 3403–3411. [PubMed] [Google Scholar]
- Dominguez R, Holmes KC, 2011. Actin structure and function. Annu. Rev. Biophys 40, 169–186. 10.1146/annurev-biophys-042910-155359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrell MI, Aguilar E, Friedlander M, 2002. Retinal vascular development is mediated by endothelial filopodia, a preexisting astrocytic template and specific R-cadherin adhesion. Invest. Ophthalmol. Vis. Sci 43, 3500–3510. [PubMed] [Google Scholar]
- Draoui N, de Zeeuw P, Carmeliet P, 2017. Angiogenesis revisited from a metabolic perspective: role and therapeutic implications of endothelial cell metabolism. Open Biol. 7 10.1098/rsob.170219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilken HM, Adams RH, 2010. Dynamics of endothelial cell behavior in sprouting angiogenesis. Curr. Opin. Cell Biol 22, 617–625. 10.1016/j.ceb.2010.08.010 [DOI] [PubMed] [Google Scholar]
- Esser JS, Rahner S, Deckler M, Bode C, Patterson C, Moser M, 2015. Fibroblast growth factor signaling pathway in endothelial cells is activated by BMPER to promote angiogenesis. Arterioscler. Thromb. Vasc. Biol 35, 358–367. 10.1161/ATVBAHA.114.304345 [DOI] [PubMed] [Google Scholar]
- Fang X, 2018. Impaired tissue barriers as potential therapeutic targets for Parkinson’s disease and amyotrophic lateral sclerosis. Metab. Brain Dis 33, 1031–1043. 10.1007/s11011-018-0239-x [DOI] [PubMed] [Google Scholar]
- Fanning AS, Anderson JM, 2009. Zonula occludens-1 and −2 are cytosolic scaffolds that regulate the assembly of cellular junctions. Ann. N. Y. Acad. Sci 1165, 113–120. 10.1111/j.1749-6632.2009.04440.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM, 1998. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J. Biol. Chem 273, 29745–29753. 10.1074/jbc.273.45.29745 [DOI] [PubMed] [Google Scholar]
- Fanning AS, Ma TY, Anderson JM, 2002. Isolation and functional characterization of the actin binding region in the tight junction protein ZO-1. FASEB J. 16, 1835–1837. 10.1096/fj.02-0121fje [DOI] [PubMed] [Google Scholar]
- Fletcher DA, Mullins RD, 2010. Cell mechanics and the cytoskeleton. Nature 463, 485–492. 10.1038/nature08908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbuzova-Davis S, Haller E, Saporta S, Kolomey I, Nicosia SV, Sanberg PR, 2007a. Ultrastructure of blood-brain barrier and blood-spinal cord barrier in SOD1 mice modeling ALS. Brain Res. 1157, 126–137. 10.1016/j.brainres.2007.04.044 [DOI] [PubMed] [Google Scholar]
- Garbuzova-Davis S, Hernandez-Ontiveros DG, Rodrigues MCO, Haller E, Frisina-Deyo A, Mirtyl S, Sallot S, Saporta S, Borlongan CV, Sanberg PR, 2012. Impaired blood-brain/spinal cord barrier in ALS patients. Brain Res. 1469, 114–128. 10.1016/j.brainres.2012.05.056 [DOI] [PubMed] [Google Scholar]
- Garbuzova-Davis S, Kurien C, Haller E, Eve DJ, Navarro S, Steiner G, Mahendrasah A, Hailu S, Khatib M, Boccio KJ, Borlongan CV, Van Loveren HR, Appel SH, Sanberg PR, 2019. Human Bone Marrow Endothelial Progenitor Cell Transplantation into Symptomatic ALS Mice Delays Disease Progression and Increases Motor Neuron Survival by Repairing Blood-Spinal Cord Barrier. Sci. Rep 9, 5280 10.1038/s41598-019-41747-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbuzova-Davis S, Rodrigues MCO, Hernandez-Ontiveros DG, Louis MK, Willing AE, Borlongan CV, Sanberg PR, 2011. Amyotrophic lateral sclerosis: a neurovascular disease. Brain Res. 1398, 113–125. 10.1016/j.brainres.2011.04.049 [DOI] [PubMed] [Google Scholar]
- Garbuzova-Davis S, Saporta S, Haller E, Kolomey I, Bennett SP, Potter H, Sanberg PR, 2007b. Evidence of compromised blood-spinal cord barrier in early and late symptomatic SOD1 mice modeling ALS. PLoS ONE 2, e1205 10.1371/journal.pone.0001205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbuzova-Davis S, Saporta S, Sanberg PR, 2008. Implications of blood-brain barrier disruption in ALS. Amyotroph. Lateral Scler. 9, 375–376. 10.1080/17482960802160990 [DOI] [PubMed] [Google Scholar]
- Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C, 2003. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol 161, 1163–1177. 10.1083/jcb.200302047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianni-Barrera R, Trani M, Reginato S, Banfi A, 2011. To sprout or to split? VEGF, Notch and vascular morphogenesis. Biochem. Soc. Trans 39, 1644–1648. 10.1042/BST20110650 [DOI] [PubMed] [Google Scholar]
- Gridley T, 2010. Notch signaling in the vasculature. Curr. Top. Dev. Biol 92, 277–309. 10.1016/S0070-2153(10)92009-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gridley T, 2007. Notch signaling in vascular development and physiology. Development 134, 2709–2718. 10.1242/dev.004184 [DOI] [PubMed] [Google Scholar]
- Henkel JS, Beers DR, Wen S, Bowser R, Appel SH, 2009. Decreased mRNA expression of tight junction proteins in lumbar spinal cords of patients with ALS. Neurology 72, 1614–1616. 10.1212/WNL.0b013e3181a41228 [DOI] [PubMed] [Google Scholar]
- Herbert SP, Stainier DYR, 2011. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat. Rev. Mol. Cell Biol 12, 551–564. 10.1038/nrm3176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA, 2004. Vascular endothelial growth factor and angiogenesis. Pharmacol. Rev 56, 549–580. 10.1124/pr.56.4.3 [DOI] [PubMed] [Google Scholar]
- Huber JD, Egleton RD, Davis TP, 2001. Molecular physiology and pathophysiology of tight junctions in the blood-brain barrier. Trends Neurosci. 24, 719–725. [DOI] [PubMed] [Google Scholar]
- Ilieva H, Polymenidou M, Cleveland DW, 2009. Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J Cell Biol 187, 761–772. 10.1083/jcb.200908164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iłzecka J, 2008. Decreased serum endoglin level in patients with amyotrophic lateral sclerosis: a preliminary report. Scand. J. Clin. Lab. Invest 68, 348–351. 10.1080/00365510701604628 [DOI] [PubMed] [Google Scholar]
- Itoh M, Nagafuchi A, Moroi S, Tsukita S, 1997. Involvement of ZO-1 in cadherin-based cell adhesion through its direct binding to alpha catenin and actin filaments. J. Cell Biol. 138, 181–192. 10.1083/jcb.138.1.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson L, Bentley K, Gerhardt H, 2009. VEGFRs and Notch: a dynamic collaboration in vascular patterning. Biochem. Soc. Trans 37, 1233–1236. 10.1042/BST0371233 [DOI] [PubMed] [Google Scholar]
- Jakobsson L, Franco CA, Bentley K, Collins RT, Ponsioen B, Aspalter IM, Rosewell I, Busse M, Thurston G, Medvinsky A, Schulte-Merker S, Gerhardt H, 2010. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat. Cell Biol 12, 943–953. 10.1038/ncb2103 [DOI] [PubMed] [Google Scholar]
- Kim Young-Mi, Hwang S, Kim Young-Myoeng, Pyun B-J, Kim T-Y, Lee S-T, Gho YS, Kwon Y-G, 2002. Endostatin blocks vascular endothelial growth factor-mediated signaling via direct interaction with KDR/Flk-1. J. Biol. Chem 277, 27872–27879. 10.1074/jbc.M202771200 [DOI] [PubMed] [Google Scholar]
- Kozuka J, Yokota H, Arai Y, Ishii Y, Yanagida T, 2007. Dynamic polymorphism of actin as activation mechanism for cell motility. BioSystems 88, 273–282. 10.1016/j.biosystems.2006.07.012 [DOI] [PubMed] [Google Scholar]
- Lobov I, Mikhailova N, 2018. The Role of Dll4/Notch Signaling in Normal and Pathological Ocular Angiogenesis: Dll4 Controls Blood Vessel Sprouting and Vessel Remodeling in Normal and Pathological Conditions. J. Ophthalmol 2018, 3565292 10.1155/2018/3565292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons SM, Fay MM, Akiyama Y, Anderson PJ, Ivanov P, 2017. RNA biology of angiogenin: Current state and perspectives. RNA Biol. 14, 171–178. 10.1080/15476286.2016.1272746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattila PK, Lappalainen P, 2008. Filopodia: molecular architecture and cellular functions. Nat. Rev. Mol. Cell Biol 9, 446–454. 10.1038/nrm2406 [DOI] [PubMed] [Google Scholar]
- Meister S, Storck SE, Hameister E, Behl C, Weggen S, Clement AM, Pietrzik CU, 2015. Expression of the ALS-causing variant hSOD1(G93A) leads to an impaired integrity and altered regulation of claudin-5 expression in an in vitro blood-spinal cord barrier model. J. Cereb. Blood Flow Metab 35, 1112–1121. 10.1038/jcbfm.2015.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki K, Ohta Y, Nagai M, Morimoto N, Kurata T, Takehisa Y, Ikeda Y, Matsuura T, Abe K, 2011. Disruption of neurovascular unit prior to motor neuron degeneration in amyotrophic lateral sclerosis. J. Neurosci. Res 89, 718–728. 10.1002/jnr.22594 [DOI] [PubMed] [Google Scholar]
- Morales-Ruiz M, Fulton D, Sowa G, Languino LR, Fujio Y, Walsh K, Sessa WC, 2000. Vascular endothelial growth factor-stimulated actin reorganization and migration of endothelial cells is regulated via the serine/threonine kinase Akt. Circ. Res 86, 892–896. [DOI] [PubMed] [Google Scholar]
- Nagy JA, Senger DR, 2006. VEGF-A, cytoskeletal dynamics, and the pathological vascular phenotype. Exp. Cell Res 312, 538–548. 10.1016/j.yexcr.2005.10.017 [DOI] [PubMed] [Google Scholar]
- Nicaise C, Mitrecic D, Demetter P, De Decker R, Authelet M, Boom A, Pochet R, 2009. Impaired blood-brain and blood-spinal cord barriers in mutant SOD1-linked ALS rat. Brain Res. 1301, 152–162. 10.1016/j.brainres.2009.09.018 [DOI] [PubMed] [Google Scholar]
- Nicaise C, Soyfoo MS, Delporte C, Pochet R, 2010. Aquaporin-4 as a potential marker of BBB disruption in ALS models. Amyotroph. Lateral Scler 11, 253–254. 10.3109/17482960902803457 [DOI] [PubMed] [Google Scholar]
- Oda T, Maéda Y, 2010. Multiple Conformations of F-actin. Structure 18, 761–767. 10.1016/j.str.2010.05.009 [DOI] [PubMed] [Google Scholar]
- Odenwald MA, Choi W, Buckley A, Shashikanth N, Joseph NE, Wang Y, Warren MH, Buschmann MM, Pavlyuk R, Hildebrand J, Margolis B, Fanning AS, Turner JR, 2017. ZO-1 interactions with F-actin and occludin direct epithelial polarization and single lumen specification in 3D culture. J. Cell. Sci 130, 243–259. 10.1242/jcs.188185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J, 1997. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 88, 277–285. [DOI] [PubMed] [Google Scholar]
- Padhi AK, Jayaram B, Gomes J, 2013. Prediction of functional loss of human angiogenin mutants associated with ALS by molecular dynamics simulations. Sci. Rep 3, 1225 10.1038/srep01225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padhi AK, Kumar H, Vasaikar SV, Jayaram B, Gomes J, 2012. Mechanisms of loss of functions of human angiogenin variants implicated in amyotrophic lateral sclerosis. PLoS ONE 7, e32479 10.1371/journal.pone.0032479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons XH, Teng YD, Parsons JF, Snyder EY, Smotrich DB, Moore DA, 2011. Efficient derivation of human neuronal progenitors and neurons from pluripotent human embryonic stem cells with small molecule induction. J Vis Exp e3273 10.3791/3273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potente M, Gerhardt H, Carmeliet P, 2011. Basic and therapeutic aspects of angiogenesis. Cell 146, 873–887. 10.1016/j.cell.2011.08.039 [DOI] [PubMed] [Google Scholar]
- Rafii S, Lyden D, 2003. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat. Med 9, 702–712. 10.1038/nm0603-702 [DOI] [PubMed] [Google Scholar]
- Rao N, Lee YF, Ge R, 2015. Novel endogenous angiogenesis inhibitors and their therapeutic potential. Acta Pharmacol. Sin 36, 1177–1190. 10.1038/aps.2015.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribatti D, Crivellato E, 2012. “Sprouting angiogenesis”, a reappraisal. Dev. Biol 372, 157–165. 10.1016/j.ydbio.2012.09.018 [DOI] [PubMed] [Google Scholar]
- Risau W, 1990. Angiogenic growth factors. Prog. Growth Factor Res 2, 71–79. [DOI] [PubMed] [Google Scholar]
- Rodrigues MCO, Hernandez-Ontiveros DG, Louis MK, Willing AE, Borlongan CV, Sanberg PR, Voltarelli JC, Garbuzova-Davis S, 2012. Neurovascular aspects of amyotrophic lateral sclerosis. Int. Rev. Neurobiol 102, 91–106. 10.1016/B978-0-12-386986-9.00004-1 [DOI] [PubMed] [Google Scholar]
- Rousseau S, Houle F, Huot J, 2000. Integrating the VEGF signals leading to actin-based motility in vascular endothelial cells. Trends Cardiovasc. Med 10, 321–327. [DOI] [PubMed] [Google Scholar]
- Sakakibara S, Maruo T, Miyata M, Mizutani K, Takai Y, 2018. Requirement of the F-actin-binding activity of l-afadin for enhancing the formation of adherens and tight junctions. Genes Cells 23, 185–199. 10.1111/gtc.12566 [DOI] [PubMed] [Google Scholar]
- Sanz-Rodriguez F, Guerrero-Esteo M, Botella L-M, Banville D, Vary CPH, Bernabéu C, 2004. Endoglin regulates cytoskeletal organization through binding to ZRP-1, a member of the Lim family of proteins. J. Biol. Chem 279, 32858–32868. 10.1074/jbc.M400843200 [DOI] [PubMed] [Google Scholar]
- Sheng J, Xu Z, 2016. Three decades of research on angiogenin: a review and perspective. Acta Biochim. Biophys. Sin. (Shanghai) 48, 399–410. 10.1093/abbs/gmv131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekmann AF, Covassin L, Lawson ND, 2008. Modulation of VEGF signalling output by the Notch pathway. Bioessays 30, 303–313. 10.1002/bies.20736 [DOI] [PubMed] [Google Scholar]
- Stricker J, Falzone T, Gardel ML, 2010. Mechanics of the F-actin cytoskeleton. J. Biomech 43, 9–14. 10.1016/j.jbiomech.2009.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchting S, Freitas C, le Noble F, Benedito R, Bréant C, Duarte A, Eichmann A, 2007. The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc. Natl. Acad. Sci. U.S.A 104, 3225–3230. 10.1073/pnas.0611177104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tammela T, Enholm B, Alitalo K, Paavonen K, 2005. The biology of vascular endothelial growth factors. Cardiovasc. Res 65, 550–563. 10.1016/j.cardiores.2004.12.002 [DOI] [PubMed] [Google Scholar]
- ten Dijke P, Goumans M-J, Pardali E, 2008. Endoglin in angiogenesis and vascular diseases. Angiogenesis 11, 79–89. 10.1007/s10456-008-9101-9 [DOI] [PubMed] [Google Scholar]
- Teng YD, Benn SC, Kalkanis SN, Shefner JM, Onario RC, Cheng B, Lachyankar MB, Marconi M, Li J, Yu D, Han I, Maragakis NJ, Lládo J, Erkmen K, Redmond DE, Sidman RL, Przedborski S, Rothstein JD, Brown RH, Snyder EY, 2012. Multimodal actions of neural stem cells in a mouse model of ALS: a meta-analysis. Sci Transl Med 4, 165ra164 10.1126/scitranslmed.3004579 [DOI] [PubMed] [Google Scholar]
- Urbich C, Dimmeler S, 2004. Endothelial progenitor cells: characterization and role in vascular biology. Circ. Res 95, 343–353. 10.1161/01.RES.0000137877.89448.78 [DOI] [PubMed] [Google Scholar]
- Vandekeere S, Dewerchin M, Carmeliet P, 2015. Angiogenesis Revisited: An Overlooked Role of Endothelial Cell Metabolism in Vessel Sprouting. Microcirculation 22, 509–517. 10.1111/micc.12229 [DOI] [PubMed] [Google Scholar]
- Vanneaux V, El-Ayoubi F, Delmau C, Driancourt C, Lecourt S, Grelier A, Cras A, Cuccuini W, Soulier J, Lataillade J-J, Lebousse-Kerdiles M-C, Oury JF, Sibony O, Marolleau J-P, Benbunan M, Uzan G, Larghero J, 2010. In Vitro and in Vivo Analysis of Endothelial Progenitor Cells from Cryopreserved Umbilical Cord Blood: Are We Ready for Clinical Application? Cell Transplant 19, 1143–1155. 10.3727/096368910X504487 [DOI] [PubMed] [Google Scholar]
- Waschke J, Curry FE, Adamson RH, Drenckhahn D, 2005. Regulation of actin dynamics is critical for endothelial barrier functions. Am. J. Physiol. Heart Circ. Physiol 288, H1296–1305. 10.1152/ajpheart.00687.2004 [DOI] [PubMed] [Google Scholar]
- Winkler EA, Sengillo JD, Sullivan JS, Henkel JS, Appel SH, Zlokovic BV, 2013. Blood-spinal cord barrier breakdown and pericyte reductions in amyotrophic lateral sclerosis. Acta Neuropathol. 125, 111–120. 10.1007/s00401-012-1039-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolburg H, Noell S, Mack A, Wolburg-Buchholz K, Fallier-Becker P, 2009. Brain endothelial cells and the glio-vascular complex. Cell Tissue Res. 335, 75–96. 10.1007/s00441-008-0658-9 [DOI] [PubMed] [Google Scholar]
- Wu D, Yu W, Kishikawa H, Folkerth RD, Iafrate AJ, Shen Y, Xin W, Sims K, Hu G-F, 2007. Angiogenin loss-of-function mutations in amyotrophic lateral sclerosis. Ann. Neurol 62, 609–617. 10.1002/ana.21221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J-X, Pan Y-Y, Wang X-X, Qiu Y-G, Mao W, 2018. Endothelial progenitor cells in age-related vascular remodeling. Cell Transplant 27, 786–795. 10.1177/0963689718779345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z, Deane R, Ali Z, Parisi M, Shapovalov Y, O’Banion MK, Stojanovic K, Sagare A, Boillee S, Cleveland DW, Zlokovic BV, 2008. ALS-causing SOD1 mutants generate vascular changes prior to motor neuron degeneration. Nat. Neurosci 11, 420–422. 10.1038/nn2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond SH, 2004. Beginning and ending an actin filament: control at the barbed end. Curr. Top. Dev. Biol 63, 145–188. 10.1016/S0070-2153(04)63005-5 [DOI] [PubMed] [Google Scholar]