Abstract
Background
The optimal choice of antibiotics is challenging in culture-negative pyogenic spondylitis (PS). The empiric use of glycopeptides is suggested depending on various risk factors, although clinical data are sparse. This study aimed to analyze the clinical characteristics and outcomes of patients with culture-negative PS and evaluate the effect of empiric glycopeptide use on clinical outcomes in these patients.
Materials and Methods
Data on the characteristics, treatment, and outcomes of 175 patients diagnosed with PS were retrospectively obtained from the electronic database of a tertiary referral hospital from 2009 to 2016. Patients with negative culture results were grouped by the duration of glycopeptide treatment: glycopeptide therapy <28 days (Group A) and glycopeptide therapy ≥28 days (Group B).
Results
Of 89 patients with negative culture results, 78 were included in the analysis (Group A, n = 66; Group B, n = 12). The mean age of patients with negative culture results was 65.5 years, and 52.6% were male. The median follow-up duration was 573 (interquartile range [IQR], 83 – 1,037) days. The duration of intravenous glycopeptide therapy was 0.0 (IQR, 0.0 – 0.0) days and 55.5 (IQR, 37.0 – 75.7) days for Groups A and B, respectively. Patients who used glycopeptide longer empirically (Group B) had more commonly undergone a previous spinal procedure, including surgery (P = 0.024). The length of hospitalization, erythrocyte sedimentation rate, and C-reactive protein level were significantly higher in Group B compared with those in Group A (P <0.001, P <0.001, and P = 0.006, respectively). Regarding treatment modalities, patients in Group B underwent surgery more frequently (P = 0.017). The duration of parenteral antibiotic treatment was longer in Group B (P <0.001). Recurrence was noted in 7 patients (9.0%), and the recurrence rate was not significantly different between the 2 groups (Group A, 5/66 [7.6%]; Group B, 2/12 [16.7%]; P = 0.293).
Conclusion
The recurrence rate among patients with culture-negative PS was not different based on the duration of empiric glycopeptide use. However, considering the small sample size and heterogeneity of our study population, we suggest that it is reasonable to administer glycopeptide antibiotics in these patients depending on clinical risk factors. Further large-scale prospective studies are needed to obtain more evidence for appropriate antibiotic treatment.
Keywords: Antibiotics, Culture, Glycopeptide, Pyogenic spondylodiscitis
Introduction
Infectious spondylitis, also known as vertebral osteomyelitis, is classified as pyogenic, granulomatous (tuberculous, brucellar, or fungal), or parasitic according to its etiology [1]. Pyogenic spondylitis (PS) refers to a bacterial infection of vertebral bodies and their adjacent intervertebral disc spaces [2].
Microbiologic confirmation is key to the treatment of PS. Staphylococci are the most common bacteria that cause PS [3,4]. The prevalence of methicillin-resistant Staphylococcus aureus infection has increased in recent decades [5,6]; hence, glycopeptide (GP) antibiotics are more frequently considered in clinical practice.
Some studies have described the clinical features and outcomes of culture-negative PS [7,8,9]. However, the guidelines for proper antibiotic therapy in patients with culture-negative PS have not been well established. A few recent studies noted an increasing incidence of PS with no identified pathogen [8,9,10].
In the present study, we analyzed the clinical characteristics and treatment regimens of patients with culture-negative PS and determined whether empiric GP antibiotic use could influence clinical outcomes in these patients.
Materials and Methods
1. Study setting and subjects
We retrospectively reviewed the medical records of patients diagnosed with infectious spondylitis who visited Seoul St. Mary's Hospital (a tertiary referral teaching hospital in Seoul, Korea) between January 2009 and December 2016.
The diagnosis of PS was based on clinical features, laboratory and imaging findings, tissue pathological findings, and culture results, which led to the medical decision on antibiotic therapy and the compatible response. Cases in which specimen culture results and/or histological findings were consistent with those of granulomatous inflammation or fungal infection were excluded. PS was classified as culture-positive or culture-negative according to the results of bacterial cultures obtained using blood and/or tissue specimens. Some patients presumed to have PS were excluded from our analyses if they refused treatment, were noncompliant with medical decisions, had incomplete information regarding antibiotic treatment, or did not undergo both blood and tissue cultures.
Patients with negative culture results were grouped according to the duration of GP antibiotic (vancomycin [VAN] or teicoplanin) treatment: GP therapy <28 days (Group A) and GP therapy ≥28 days (Group B). GP antibiotic treatment included both first-line (initial empiric) and second-line therapy containing GP antibiotics.
Empiric antibiotics were selected based on the physician's preference or consultation with infectious disease specialists, which was probably influenced by clinical risk factors such as recent hospitalization, invasive device use, hemodialysis, and surgery. The reasons for changing antibiotics, including GP antibiotics during the treatment period, were largely due to suspected treatment failure; the microorganisms cultured, including colonizers from other sites; and adverse drug effects. The doses of GP antibiotics were adjusted according to estimated glomerular filtration rates and serum GP levels were monitored. Decisions regarding surgical treatment options were based on the surgeon's discretion according to the characteristics of individual patients.
The protocol of the present study was reviewed and approved by the Institutional Review Board of Seoul St. Mary's Hospital, The Catholic University of Korea (approval no. KC19RESI0232). The requirements for informed consent were waived by the board.
2. Data collection
During the study period, we extracted data from the electronic hospital database. We reviewed patient-related data regarding demographics, underlying diseases, admission, presenting symptoms (fever, back pain, and neurologic deficit), radiologic findings (involved vertebras and presence of abscesses), laboratory data (white blood cell count, C-reactive protein [CRP] level, and erythrocyte sedimentation rate [ESR]), blood and tissue culture results, spinal tissue histopathologic findings, medical treatment including GP antibiotic infusion and percutaneous drainage, surgery, recurrence, and death during follow-up.
Previous spinal procedures included acupuncture, spinal injections, nerve block, neuroplasty, vertebroplasty, and surgery. Symptom duration was defined as the time from the onset of symptoms to the day of admission or first outpatient visit. Laboratory data were obtained at the time of admission (or first outpatient visit) or radiologic imaging if patients were already hospitalized due to another disease.
Recurrent infection was defined as one that recurred with clinical features, laboratory values, and/or imaging findings consistent with those of PS after completion of treatment and in which a second course of antibiotics was administered. Time to recurrence denoted the time between the last day of antibiotic administration and the next hospital visit with the same illness or the first day of administration of a second course of antibiotics.
3. Statistical analysis
Variables are expressed as the frequency (%), mean ± standard deviation, or median (interquartile range [IQR]). The Mann-Whitney test was used to compare continuous variables between the 2 groups and Pearson's chi-squared test or Fisher's exact test for categorical variables, as appropriate. All statistical analyses were performed using SPSS version 21.0 (SPSS Korea, Seoul, Korea). A two-tailed P-value <0.05 was considered statistically significant.
Results
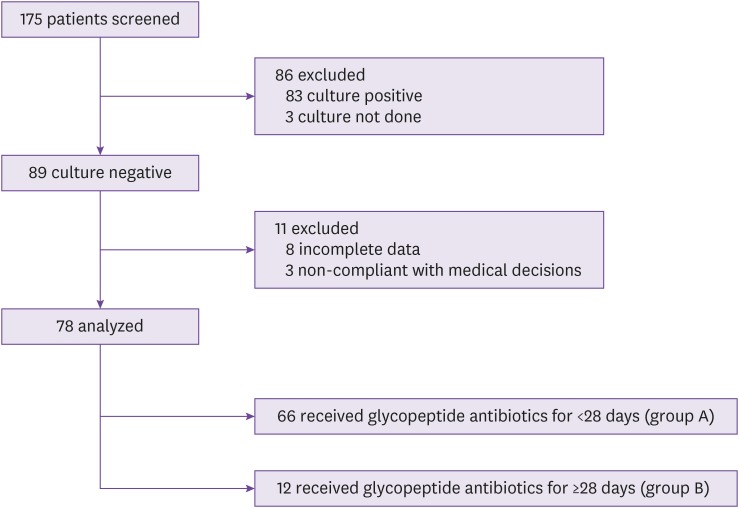
During the study period, a total of 175 patients with PS were screened (Fig. 1). Of 78 eligible patients with negative culture results, 66 patients received GP antibiotic treatment for <28 days (Group A) and 12 patients for ≥28 days (Group B). Fifty-three of the patients in Group A received no GP antibiotics.
Figure 1. Study flow chart.
1. Clinical characteristics
Table 1 shows the demographics and clinical characteristics of patients with culture-negative PS. The mean age of these patients was 65.5 years, and 52.6% were male. Five of 66 patients (7.5%) in Group A were treated with oral antibiotics alone without admission. The length of hospitalization was significantly longer in Group B compared with that in Group A (Group A, 21.5 [IQR, 11.2 – 34.0] days; Group B, 57.0 [IQR, 28.7 – 78.5] days; P <0.001). Based on the underlying diseases, the rates of previous spinal procedures were significantly different between Groups A and B (Group A, 37/66 [56.1%]; Group B, 11/12 [91.7%]; P = 0.024). Back pain was noted in 75/78 (96.2%) patients and fever in only 7/77 (9.1%). There were no differences in the incidences of associated symptoms of fever, back pain, and neurologic deficit between the 2 groups. Regarding laboratory data, ESRs were significantly higher in Group B than in Group A (Group A, 61.1 ± 26.3 mm/h; Group B, 93.8 ± 24.4 mm/h; P <0.001). CRP levels were also significantly higher in Group B (Group A, 3.4 [IQR, 1.2 – 7.5] mg/dL; Group B, 8.8 [IQR, 3.7 – 18.8] mg/dL; P = 0.006).
Table 1. Demographic and clinical characteristics of patients with culture-negative pyogenic spondylitis.
| Characteristics | Total (n = 78) | Group A (n = 66) | Group B (n = 12) | P-value | |
|---|---|---|---|---|---|
| Male | 41 (52.6) | 35 (53.0) | 6 (50) | 0.847 | |
| Age, mean ± SD, years | 65.5 ± 13.3 | 64.5 ± 14.1 | 71.3 ± 5.7 | 0.108 | |
| Duration of admission, median (IQR), days | 23.0 (14.5–36.0) | 21.5 (11.2–34.0) | 57.0 (28.7–78.5) | <0.001 | |
| Transfer from other hospitals | 21 (26.9) | 15 (22.7) | 6 (50) | 0.075 | |
| Underlying disease | |||||
| Diabetes mellitus | 15 (19.2) | 12 (18.2) | 3 (25) | 0.691 | |
| Cardiovascular disease/Congestive heart failure | 9 (11.5) | 7 (10.6) | 2 (16.7) | 0.622 | |
| Malignancy | 7 (9.0) | 4 (6.1) | 3 (25) | 0.069 | |
| Liver cirrhosis | 3 (3.8) | 2 (3.0) | 1 (8.3) | 0.398 | |
| End-stage renal disease | 2 (2.6) | 2 (3.0) | 0 (0) | >0.999 | |
| Symptoms at diagnosis | |||||
| Fever, ≥38°C | 7/77a (9.1) | 5/65a (7.7) | 2 (16.7) | 0.298 | |
| Back pain | 75 (96.2) | 65 (98.5) | 10 (83.3) | 0.060 | |
| Neurologic deficit | 30 (38.5) | 23 (34.8) | 7 (58.3) | 0.196 | |
| Symptom durationb, median (IQR), days | 14.0 (5.0–39.0) | 22.0 (5.0–43.0) | 12.5 (5.0–19.2) | 0.403 | |
| Antibiotic initiation before obtaining cultures | 42/75a (56.0) | 34/64a (53.1) | 8/11a (72.7) | 0.328 | |
| Laboratory data | |||||
| WBC, median (IQR), /mm3 | 8,030 (6,527–9,575) | 8,030 (6,570–9,492) | 8,315 (5,942–10,177) | 0.967 | |
| CRPc, median (IQR), mg/dL | 4.1 (1.3–8.1) | 3.4 (1.2–7.5) | 8.8 (3.7–18.8) | 0.006 | |
| ESRd, mean ± SD, mm/h | 66.3 ± 28.5 | 61.1 ± 26.3 | 93.8 ± 24.4 | <0.001 | |
| Location | >0.999 | ||||
| Cervical | 1 (1.3) | 1 (1.5) | 0 (0) | ||
| Thoracic | 8 (10.3) | 7 (10.6) | 1 (8.3) | ||
| Lumbar | 50 (64.1) | 41 (62.1) | 9 (75) | ||
| Sacral | 1 (1.3) | 1 (1.5) | 0 (0) | ||
| Cervicothoracic | 0 (0) | 0 (0) | 0 (0) | ||
| Thoracolumbar | 4 (5.1) | 4 (6.1) | 0 (0) | ||
| Lumbosacral | 14 (17.9) | 12 (18.2) | 2 (16.7) | ||
| No. of involved vertebrae, mean ± SD | 2.3 ± 0.9 | 2.3 ± 0.9 | 2.3 ± 0.7 | 0.633 | |
| Associated abscess | 23 (29.5) | 18 (27.3) | 5 (41.7) | 0.322 | |
| Paraspinal abscess | 18 (23.1) | 14 (21.2) | 4 (33.3) | 0.457 | |
| Epidural abscess | 2 (2.6) | 2 (3.0) | 0 (0) | >0.999 | |
| Psoas abscess | 6 (7.7) | 5 (7.6) | 1 (8.3) | >0.999 | |
| Previous spinal procedure | 48 (61.5) | 37 (56.1) | 11 (91.7) | 0.024 | |
| Previous spinal surgery | 38 (48.7) | 28 (42.4) | 10 (83.3) | 0.009 | |
| Presence of spinal implant | 22 (28.2) | 16 (24.2) | 6 (50) | 0.087 | |
Data are presented as n (%) unless otherwise specified.
an/total with available information (%).
bMissing data in 31/78 (39.7%) patients: 27/66 (40.9%) in Group A, and 4/12 (33.3%) in Group B.
cMissing data in 1/78 (1.3%) patients: 1/66 (1.5%) in Group A, and 0/12 (0%) in Group B.
dMissing data in 3/78 (3.8%) patients: 3/66 (4.5%) in Group A, and 0/12 (0%) in Group B.
SD, standard deviation; IQR, interquartile range; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate.
Regarding imaging modalities for the diagnosis of PS, magnetic resonance imaging was performed in 74/78 (94.9%) patients, and computed tomography alone without magnetic resonance imaging was used to localize the spinal lesions in 4/78 (5.1%) patients. The mean number of involved vertebrae was 2.3 ± 0.9, and the lumbar vertebra was the most frequently affected region (64.1%), followed by the lumbosacral and thoracic vertebrae (17.9% and 10.3%, respectively). Accompanying abscesses were found in 29.5% (23/78) of patients. At the time of diagnosis, 22/78 (28.2%) patients had spinal implants.
2. Microbiologic tests
Blood cultures were performed in 82.1% (64/78) of patients with negative culture results, tissue cultures in 61.5% (48/78), and both blood and tissue cultures in only 43.6% (34/78). Tissue samples were obtained via percutaneous (18/48, 37.5%) or surgical (30/48, 62.5%) biopsy. No second biopsies were obtained for patients with negative culture results. Prior to culture sampling, 42/75 (56.0%) patients had been exposed to antibiotics.
The pathogens identified in patients with culture-positive PS are shown in Supplementary Table 1. Staphylococci were the most commonly isolated pathogens (50.6%, 42 of 83 culture-positive cases), and 54.7% of these cases were methicillin-resistant. The next most common pathogens were Streptococcus species (14.5%, 12/83) and Enterobacteriaceae (10.8%, 9/83).
3. Treatment and outcomes
Table 2 shows the treatment and clinical outcomes among patients with culture-negative PS. The median follow-up duration was 573 (IQR, 83 – 1,037) days. Of 78 patients with negative culture results, 44 (56.4%) received only medical therapy, and the rest (43.6%) underwent both medical and surgical treatment. Percutaneous abscess drainage was performed in only 1 of 41 medically treated patients in Group A. Surgical treatment included debridement, discectomy, laminectomy, spinal instrumentation, bone graft, and implant replacement. The treatment modalities were significantly different between the groups (P = 0.017); Group B (75%) had a higher proportion of patients with combined treatment of medical therapy and surgery than Group A (37.9%).
Table 2. Treatment and outcomes of culture-negative pyogenic spondylitis.
| Treatment/Outcomes | Total (n = 78) | Group A (n = 66) | Group B (n = 12) | P-value | |
|---|---|---|---|---|---|
| Treatment | 0.017 | ||||
| Medical | 44 (56.4) | 41 (62.1) | 3 (25) | ||
| Medical + Surgical | 34 (43.6) | 25 (37.9) | 9 (75) | ||
| Follow-up duration, median (IQR), days | 573 (83–1,037) | 621 (77–1,083) | 302 (127–872) | 0.879 | |
| Recurrence | 7 (9.0) | 5 (7.6) | 2 (16.7) | 0.293 | |
| In-hospital mortality | 0 (0) | 0 (0) | 0 (0) | ||
Data are presented as n (%) unless otherwise specified.
IQR, interquartile range.
The types of antibiotics used and the duration of antibiotic treatment are shown in Table 3, which includes those from referring hospitals. Antibiotic therapies administered before sampling included GP-containing regimens (47.6%), third-generation cephalosporins (30.9%), first-generation cephalosporins (21.4%), second-generation cephalosporins (14.2%), beta-lactam/beta-lactamase inhibitor (11.9%), quinolone ± beta-lactam (9.5%), and others (21.4%). During the entire treatment period, 8 of 77 (10.4%) patients received only parenteral antibiotics, and 6 of 78 (7.7%) patients received only oral antibiotics. Apart from GP-containing regimens, the intravenous antibiotics administered were cefazolin (42.3%), ampicillin/sulbactam (32.1%), third-generation cephalosporin (19.2%), piperacillin/tazobactam (12.8%), etc., in descending order of frequency. Carbapenem was more commonly used in Group B (P = 0.011). Therapeutic regimens containing GP included combinations of GP antibiotics, broad-spectrum cephalosporin, and others (20.5%); GP antibiotics alone (12.8%); combinations of GP antibiotics and quinolone (7.7%); combinations of GP antibiotics and beta-lactam/beta-lactamase inhibitor (7.7%); and combinations of GP antibiotics and a carbapenem (6.4%). Infused GP antibiotics comprised VAN (63.6%) and teicoplanin (36.4%). Among 12 patients in Group B, 7 patients (58.3%) received GP antibiotics as initial empiric therapy, while 5 patients (41.7%) received them as second-line therapy. Except for oral treatment alone, almost all oral antibiotics were administered as a switch therapy from intravenous antibiotics. The most commonly used oral antibiotic was amoxicillin/clavulanate (23.4%) followed by quinolone (22.1%), second-generation cephalosporin (22.1%), third-generation cephalosporin (20.8%), and first-generation cephalosporin (11.7%).
Table 3. Therapeutic antibiotics in patients with culture-negative pyogenic spondylitis.
| Antibiotic regimens | Total (n = 78) | Group A (n = 66) | Group B (n = 12) | P-value | ||
|---|---|---|---|---|---|---|
| Intravenous antibiotics | ||||||
| Monotherapy | ||||||
| Cefazolin | 33 (42.3) | 29 (43.9) | 4 (33.3) | 0.494 | ||
| Ampicillin/sulbactam | 25 (32.1) | 24 (36.4) | 1 (8.3) | 0.090 | ||
| 3rd-generation cephalosporin | 15 (19.2) | 14 (21.2) | 1 (8.3) | 0.443 | ||
| Piperacillin/tazobactam | 10 (12.8) | 8 (12.1) | 2 (16.7) | 0.647 | ||
| Quinolone | 4 (5.1) | 3 (4.5) | 1 (8.3) | 0.495 | ||
| 2nd-generation cephalosporin | 4 (5.1) | 4 (6.1) | 0 (0) | >0.999 | ||
| Carbapenem | 4 (5.1) | 1 (1.5) | 3 (25) | 0.011 | ||
| Combination therapy | ||||||
| Beta-lactam + quinolone | 5 (6.4) | 4 (6.1) | 1 (8.3) | 0.577 | ||
| Beta-lactam + aminoglycoside | 4 (5.1) | 3 (4.5) | 1 (8.3) | 0.495 | ||
| 3rd-generation cephalosporin + metronidazole | 3 (3.8) | 3 (4.5) | 0 (0) | >0.999 | ||
| Glycopeptide-containing regimens | ||||||
| Glycopeptide + broad-spectrum cephalosporin ± others | 16 (20.5) | 7 (10.6) | 9 (75) | <0.001 | ||
| Glycopeptide alone | 10 (12.8) | 5 (7.6) | 5 (41.7) | 0.006 | ||
| Glycopeptide + quinolone | 6 (7.7) | 2 (3.0) | 4 (33.3) | 0.004 | ||
| Glycopeptide + beta-lactam/beta-lactamase inhibitor | 6 (7.7) | 2 (3.0) | 4 (33.3) | 0.004 | ||
| Glycopeptide + carbapenem | 5 (6.4) | 1 (1.5) | 4 (33.3) | 0.002 | ||
| Glycopeptide + others | 3 (3.8) | 1 (1.5) | 2 (16.7) | 0.060 | ||
| Others | 7 (9.0) | 7 (10.6) | 0 (0) | 0.587 | ||
| No intravenous antibiotic use | 6 (7.7) | 6 (9.1) | 0 (0) | 0.582 | ||
| Oral antibiotics | ||||||
| Amoxicillin/clavulanate | 18/77a (23.4) | 15/65a (23.1) | 3 (25) | >0.999 | ||
| Quinolone | 17/77a (22.1) | 14/65a (21.5) | 3 (25) | 0.721 | ||
| 2nd-generation cephalosporin | 17/77a (22.1) | 16/65a (24.6) | 1 (8.3) | 0.282 | ||
| 3rd-generation cephalosporin | 16/77a (20.8) | 14/65a (21.5) | 2 (16.7) | >0.999 | ||
| 1st-generation cephalosporin | 9/77a (11.7) | 9/65a (13.8) | 0 (0) | 0.340 | ||
| Amoxicillin | 2/77a (2.6) | 2/65a (3.1) | 0 (0) | >0.999 | ||
| Amoxicillin/clavulanate + quinolone | 2/77a (2.6) | 2/65a (3.1) | 0 (0) | >0.999 | ||
| Others | 5/77a (6.5) | 2/65a (3.1) | 3 (25) | 0.025 | ||
| No oral antibiotic use | 8/77a (10.4) | 5/65a (7.7) | 3 (25) | 0.104 | ||
| Antibiotic duration, totalb, median (IQR), days | 73.0 (47.0–104.0) | 67.0 (44.0–94.5) | 113.0 (67.0–172.2) | 0.005 | ||
| Intravenousc | 32.0 (20.0–56.0) | 28.0 (16.5–40.0) | 75.5 (56.0–95.5) | <0.001 | ||
| Orald | 41.0 (19.0–63.5) | 41.0 (19.5–63.5) | 32.5 (0.0–81.2) | 0.757 | ||
Data are presented as n (%) unless otherwise specified.
an/total with available information (%).
b, c, dMissing data in 1/78 (1.2%) patients: 1/66 (1.5%) in Group A, and 0/12 (0%) in Group B.
IQR, interquartile range.
The duration of pre-sampling antibiotic use was 11.0 (IQR, 2.0 – 22.0) days. The total antibiotic treatment duration, including periods of both parenteral and oral administration, was significantly different between the 2 groups (Group A, 67.0 [IQR, 44.0 – 94.5] days; Group B, 113.0 [IQR, 67.0 – 172.2] days; P = 0.005). The duration of intravenous antibiotic therapy was significantly longer in Group B than in Group A (Group A, 28.0 [IQR, 16.5 – 40.0] days; Group B, 75.5 [IQR, 56.0 – 95.5] days; P <0.001), but there was no difference in the duration of oral antibiotic treatment (P = 0.757). The median duration of intravenous GP antibiotic therapy was 0.0 (IQR, 0.0 – 0.0) days and 55.5 (IQR, 37.0 – 75.7) days for Groups A and B, respectively (P <0.001).
Recurrence was noted in 7 (9.0%) of 78 patients with culture-negative PS. The recurrence rate was not significantly different between the 2 groups, but was higher in Group B than in Group A (Group A, 5/66 [7.6%]; Group B, 2/12 [16.7%]; P = 0.293). The median time to recurrence was 68 (range, 9 – 193) days. Spondylitis-related deaths did not occur during hospitalization, but 2 patients with malignancy and 1 with end-stage renal disease died during the follow-up period.
Discussion
This single-center retrospective study described the clinical characteristics and outcomes in patients with culture-negative PS who were divided into 2 groups according to the GP treatment duration: GP antibiotic treatment for <28 days, and GP antibiotic treatment for ≥28 days.
In our study, pathogens were not identified in 51.7% of patients with PS. As many as 56% of patients with culture-negative PS received antibiotics before specimen collection. Although GP antibiotic monotherapy was used in some cases, most of our empiric GP-containing regimens comprised combinations covering Gram-positive organisms including staphylococci and streptococci and Gram-negative bacilli.
The recurrence rate of PS was reported to be 0 – 15% in previous studies [11]. In our study, the recurrence rate of culture-negative PS was 9.0% (7/78), which was not different from that of culture-positive PS (11.0% [8/73], P = 0.684). The mortality rate of vertebral osteomyelitis has been reported to be 0 – 30% with a recent decline [12,13]. In a study comparing culture-negative PS with culture-positive PS, when spinal surgery cases were excluded, a higher CRP level was found to be a risk factor for treatment failure in culture-negative PS [9]. Some authors have reported that culture-negative PS is related to lower inflammation levels compared with culture-positive PS, possibly due to small inocula of pathogens [8,9]. However, studies comparing culture-negative and culture-positive groups showed mixed outcomes [8,9,14,15].
There has been insufficient research about the optimal selection of empirical antibiotics in patients with culture-negative PS. There was a report of a favorable outcome when cefazolin was used in hematogenous PS and when VAN was used in post-procedural PS [15]. Some studies revealed that empiric antibiotic regimens comprising levofloxacin and rifampicin in culture-negative PS were as effective as treatments tailored according to culture results in microbiologically confirmed PS [16]. Moreover, patients with spontaneous PS without microbiological diagnoses who were treated with amoxicillin/clavulanate and ciprofloxacin showed results similar to those of patients who received targeted antimicrobial therapy in a retrospective study [17]. Although the data were not presented, antibiotic regimens containing rifampicin were infrequently used in our study.
A high percentage of patients in the present study previously underwent spinal procedures including surgery, especially in the group with longer GP antibiotic treatment (spinal procedure, 11/12 [91.7%]; spinal surgery, 10/12 [83.3%]). PS that occurs after spinal surgery or procedure is usually associated with more resistant microorganisms and removal of implants may be unavoidable [9]. Coagulase-negative staphylococci and Propionibacterium acnes are found mostly in PS after spinal surgery or instrumentation [18]. In prosthetic device infection, biofilm formation is frequently associated with treatment failure [19]. Treating physicians tend to choose GP antibiotics in patients with prior spinal surgery.
Our study showed that in the group with longer GP antibiotic treatment, the length of hospital stay and the duration of antibiotic treatment were longer; the levels of inflammatory markers such as CRP or ESR were higher; and more surgical procedures were performed. Moreover, this group showed more frequent recurrence, although this trend was not statistically significant. This may be due to the severity of spondylitis and the risk factors of patients. This result implies that the main determinants of clinical outcomes are not only GP antibiotic use and the duration of GP antibiotic treatment, but also the severity and associated risk factors. In addition, the shorter follow-up duration in the group that received GP antibiotics for a longer period, although not significant, may have led to this non-significant difference in recurrence rates.
The present study had several limitations. The relatively small sample size reduced the power of the evidence, and the retrospective nature conferred some inherent selection and misclassification biases. There were no standardized criteria among physicians, surgeons, treating departments, and referring clinics as regards the selection of medical or surgical treatment options in our study population. Antimicrobial regimens were too diverse in antibiotic type and treatment length between groups to compare the exact effects of specific antibiotics among patients with PS. Furthermore, the comparison of outcomes such as recurrence via multivariate analyses was not performed due to low event rates. The enrolled cases covered healthcare associated PS, including post-surgical infection. Thus, our results do not purely and simply reflect the characteristics of community-acquired PS. Due to the heterogeneity in the groups, our results should be interpreted with caution. In addition, data regarding therapeutic monitoring of GP antibiotics and adverse drug events were not evaluated.
Therefore, it is not clear from this study whether the use or duration of empiric GP antibiotic treatment can influence clinical outcomes in patients with PS that has not been confirmed via microbiology. However, it is prudent to consider GP antibiotics in patients at high risk for infection with resistant pathogens. Further large-scale prospective studies are needed to obtain more evidence for appropriate antibiotic treatment.
Footnotes
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest: No conflicts of interest.
- Conceptualization: YJK, YHJ, KYH.
- Data curation: KYH, YHK, KSR, JSK, JWH.
- Formal analysis: YDL, YJK.
- Methodology: YJK, YHJ.
- Resources: KYH, YHK, KSR, JSK, JWH.
- Writing - original draft: YDL.
- Writing - review & editing: YJK, YHJ, YDL.
SUPPLEMENTARY MATERIAL
Microorganisms isolated from the blood and/or tissue of patients with pyogenic spondylitis
References
- 1.Gouliouris T, Aliyu SH, Brown NM. Spondylodiscitis: update on diagnosis and management. J Antimicrob Chemother. 2010;65(Suppl 3):iii11–iii24. doi: 10.1093/jac/dkq303. [DOI] [PubMed] [Google Scholar]
- 2.Boody BS, Jenkins TJ, Maslak J, Hsu WK, Patel AA. Vertebral osteomyelitis and spinal epidural abscess: an evidence-based review. J Spinal Disord Tech. 2015;28:E316–E327. doi: 10.1097/BSD.0000000000000294. [DOI] [PubMed] [Google Scholar]
- 3.Nickerson EK, Sinha R. Vertebral osteomyelitis in adults: an update. Br Med Bull. 2016;117:121–138. doi: 10.1093/bmb/ldw003. [DOI] [PubMed] [Google Scholar]
- 4.Korean Society for Chemotherapy; Korean Society of Infectious Diseases; Korean Orthopaedic Association. Clinical guidelines for the antimicrobial treatment of bone and joint infections in Korea. Infect Chemother. 2014;46:125–138. doi: 10.3947/ic.2014.46.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park KH, Chong YP, Kim SH, Lee SO, Choi SH, Lee MS, Jeong JY, Woo JH, Kim YS. Clinical characteristics and therapeutic outcomes of hematogenous vertebral osteomyelitis caused by methicillin-resistant Staphylococcus aureus . J Infect. 2013;67:556–564. doi: 10.1016/j.jinf.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 6.Agency for Healthcare Research and Quality. Statistical brief #35: Infections with methicillin-resistant Staphylococcus aureus (MRSA) in U.S. hospitals, 1993–2005. [Accessed 4 September 2019]. Available at: http://hcup-us.ahrq.gov/reports/statbriefs/sb35.jsp.
- 7.Bhagat S, Mathieson C, Jandhyala R, Johnston R. Spondylodiscitis (disc space infection) associated with negative microbiological tests: comparison of outcome of suspected disc space infections to documented non-tuberculous pyogenic discitis. Br J Neurosurg. 2007;21:473–477. doi: 10.1080/02688690701546155. [DOI] [PubMed] [Google Scholar]
- 8.Lora-Tamayo J, Euba G, Narváez JA, Murillo O, Verdaguer R, Sobrino B, Narváez J, Nolla JM, Ariza J. Changing trends in the epidemiology of pyogenic vertebral osteomyelitis: the impact of cases with no microbiologic diagnosis. Semin Arthritis Rheum. 2011;41:247–255. doi: 10.1016/j.semarthrit.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Kim J, Kim YS, Peck KR, Kim ES, Cho SY, Ha YE, Kang CI, Chung DR, Song JH. Outcome of culture-negative pyogenic vertebral osteomyelitis: comparison with microbiologically confirmed pyogenic vertebral osteomyelitis. Semin Arthritis Rheum. 2014;44:246–252. doi: 10.1016/j.semarthrit.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Kehrer M, Pedersen C, Jensen TG, Lassen AT. Increasing incidence of pyogenic spondylodiscitis: a 14-year population-based study. J Infect. 2014;68:313–320. doi: 10.1016/j.jinf.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 11.Pigrau C, Almirante B, Flores X, Falco V, Rodriguez D, Gasser I, Villanueva C, Pahissa A. Spontaneous pyogenic vertebral osteomyelitis and endocarditis: incidence, risk factors, and outcome. Am J Med. 2005;118:1287. doi: 10.1016/j.amjmed.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 12.Mustapić M, Višković K, Borić I, Marjan D, Zadravec D, Begovac J. Vertebral osteomyelitis in adult patients-characteristics and outcome. Acta Clin Croat. 2016;55:9–15. doi: 10.20471/acc.2016.55.01.2. [DOI] [PubMed] [Google Scholar]
- 13.Berbari EF, Kanj SS, Kowalski TJ, Darouiche RO, Widmer AF, Schmitt SK, Hendershot EF, Holtom PD, Huddleston PM, III, Petermann GW, Osmon DR Infectious Diseases Society of America. 2015 Infectious Diseases Society of America (IDSA) clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adults. Clin Infect Dis. 2015;61:e26–e46. doi: 10.1093/cid/civ482. [DOI] [PubMed] [Google Scholar]
- 14.Hopkinson N, Stevenson J, Benjamin S. A case ascertainment study of septic discitis: clinical, microbiological and radiological features. QJM. 2001;94:465–470. doi: 10.1093/qjmed/94.9.465. [DOI] [PubMed] [Google Scholar]
- 15.Yoon SH, Chung SK, Kim KJ, Kim HJ, Jin YJ, Kim HB. Pyogenic vertebral osteomyelitis: identification of microorganism and laboratory markers used to predict clinical outcome. Eur Spine J. 2010;19:575–582. doi: 10.1007/s00586-009-1216-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Viale P, Furlanut M, Scudeller L, Pavan F, Negri C, Crapis M, Zamparini E, Zuiani C, Cristini F, Pea F. Treatment of pyogenic (non-tuberculous) spondylodiscitis with tailored high-dose levofloxacin plus rifampicin. Int J Antimicrob Agents. 2009;33:379–382. doi: 10.1016/j.ijantimicag.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 17.Luzzati R, Giacomazzi D, Danzi MC, Tacconi L, Concia E, Vento S. Diagnosis, management and outcome of clinically- suspected spinal infection. J Infect. 2009;58:259–265. doi: 10.1016/j.jinf.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Zimmerli W. Clinical practice. Vertebral osteomyelitis. N Engl J Med. 2010;362:1022–1029. doi: 10.1056/NEJMcp0910753. [DOI] [PubMed] [Google Scholar]
- 19.Parikh MS, Antony S. A comprehensive review of the diagnosis and management of prosthetic joint infections in the absence of positive cultures. J Infect Public Health. 2016;9:545–556. doi: 10.1016/j.jiph.2015.12.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Microorganisms isolated from the blood and/or tissue of patients with pyogenic spondylitis