Abstract
Background
Vertebral osteomyelitis (VO) is a rare but serious condition, and a potentially significant cause of morbidity. Methicillin-susceptible Staphylococcus aureus (MSSA) is the most common microorganism in native VO. Long-term administration of parenteral and oral antibiotics with good bioavailability and bone penetration is required for therapy. Use of oral β-lactams against staphylococcal bone and joint infections in adults is not generally recommended, but some experts recommend oral switching with β-lactams. This study aimed to describe the current status of antibiotic therapy and treatment outcomes of oral switching with β-lactams in patients with MSSA VO, and to assess risk factors for treatment failure.
Materials and Methods
This retrospective study included adult patients with MSSA VO treated at nine university hospitals in Korea between 2005 and 2014. Treatment failure was defined as infection-related death, microbiological relapse, neurologic deficits, or unplanned surgical procedures. Clinical characteristics and antibiotic therapy in the treatment success and treatment failure groups were compared. Risk factors for treatment failure were identified using the Cox proportional hazards model.
Results
A total of 100 patients with MSSA VO were included. All patients were treated, initially or during antibiotic therapy, with one or more parenteral antibiotics. Sixty-nine patients received one or more oral antibiotics. Antibiotic regimens were diverse and durations of parenteral and oral therapy differed, depending on the patient and the hospital. Forty-two patients were treated with parenteral and/or oral β-lactams for a total duration of more than 2 weeks. Compared with patients receiving parenteral β-lactams only, no significant difference in success rates was observed in patients who received oral β-lactams for a relatively long period. Sixteen patients had treatment failure. Old age (adjusted hazard ratio [HR] 5.600, 95% confidence interval [CI] 1.402 – 22.372, P = 0.015) and failure to improve C-reactive protein levels at follow-up (adjusted HR 3.388, 95% CI 1.168 – 9.829, P = 0.025) were independent risk factors for treatment failure.
Conclusion
In the study hospitals, diverse combinations of antibiotics and differing durations of parenteral and oral therapy were used. Based on the findings of this study, we think that switching to oral β-lactams may be safe in certain adult patients with MSSA VO. Since limited data are available on the efficacy of oral antibiotics for treatment of staphylococcal VO in adults, further evaluation of the role of oral switch therapy with β-lactams is needed.
Keywords: Staphylococcus aureus, Vertebral osteomyelitis, Treatment outcome, Beta-Lactams, Oral administration
Introduction
Vertebral osteomyelitis (VO) is a relatively rare infection [1], but has been reported to have increased in recent years [2]. Without timely and careful management, this disease can have devastating consequences. Staphylococcus aureus is the most common causative microorganism in VO [3,4,5,6,7]. Methicillin-susceptible strains of S. aureus (MSSA) are still the leading cause of VO, especially in the community setting [3,8,9], although the frequency of methicillin-resistant S. aureus (MRSA) has been increasing. VO requires prolonged antibiotic therapy and potential surgical intervention for cure [1]. Surgical intervention should be considered if progressive neurologic deficits, large epidural abscess, significant spinal instability or deformity, intractable back pain, or failure of medical treatment occurs [1]. If uncomplicated, VO can be cured with antibiotic therapy alone [1].
When treating VO with antibiotics, a total duration of treatment of 4–6 weeks up to 3 months is generally recommended [1]. Prolonged antibiotic therapy is recommended in patients with undrained abscesses or spinal implants [1]. Parenteral administration remains the mainstay of antibiotic therapy, but an early switch to oral administration is appropriate for antibiotics with good bioavailability and bone penetration [1]. Recent practice guidelines recommend a total duration of 6 weeks of parenteral or highly bioavailable oral antibiotic therapy for most adult patients with bacterial native VO [10].
Choice of oral antibiotics after the initial parenteral therapy for staphylococcal VO is a clinical challenge. Oral β-lactams are not generally recommended for use against staphylococcal bone and joint infections in adults, particularly those with implant retention [11]. However, some experts recommend oral switching with β-lactams in adults with acute osteomyelitis [5,12,13,14]. There is a paucity of evidence regarding oral antibiotic therapy to guide choice, and data on treatment outcomes of oral β-lactams for staphylococcal VO in patients without implant retention are scarce.
The aim of this study was to describe the current status of antibiotic therapy in adult patients with MSSA VO, to characterize treatment outcomes of oral switching with β-lactams, and to assess risk factors for treatment failure.
Materials and Methods
1. Study setting
This retrospective study was conducted at nine university hospitals in Korea, between January 2005 and December 2014. All patients with VO treated during study period were detected according to use of the relevant codes (M46.0 ‒ M46.9) in the ninth or tenth revision of the International Classification of Diseases (ICD 9/10). After reviewing the medical records, adult patients (≥18 years old) with MSSA VO were enrolled in the study. Patients with postoperative wound infections, prior surgery or penetrating trauma, infective endocarditis, infections caused by other microorganisms or unknown etiology, polymicrobial infections, or unknown clinical outcome due to inadequate medical records were excluded. This study protocol (SGPAIK 2016-08-026) was approved by the Institutional Review Board of the hospital, and was exempted from the requirement for informed consent.
2. Data collection and definition
We collected data on demographics, comorbid conditions, clinical, laboratory, or radiographic findings, culture specimens, biopsy or surgery procedures, antibiotic treatment, and clinical course or outcome. Comorbid conditions at the time of diagnosis included solid tumor, diabetes mellitus, liver cirrhosis, alcoholism, rheumatoid arthritis, end-stage renal disease on renal replacement therapy, immunosuppressive therapy within 30 days, spinal trauma or fracture within 6 months, or prior VO at the same site. Radiographic findings at the time of diagnosis included which part of the spine was involved, presence of involvement of ≥3 vertebral segments, or presence of infectious complications around the spinal column (such as paravertebral extension, psoas abscess, or epidural or meningeal involvements). Paravertebral extension, psoas abscess, epidural or meningeal involvements were defined as the presence of any of the enhancement, phlegmon, or abscess in pararvertebral area, psoas abscess, epidural space, or meninges, respectively, as determined by magnetic resonance imaging, computed tomography, or intraoperative findings [4].
VO was defined as having symptoms or signs consistent with spinal column infection and one or more positive culture results from samples obtained from the corresponding sites, including pus, surgical or other tissue specimens, or blood. The use of antibiotics to which MSSA isolates were susceptible was considered to be appropriate, whereas the use of antibiotics to which MSSA isolates were not susceptible or use of antibiotics with unknown susceptibility were considered to be inappropriate.
The clinical course included improvement of inflammatory markers, which were defined as 25% or more reduction in C-reactive protein (CRP) or erythrocyte sedimentation rate (ESR) compared with the baseline value, during the period 4 – 8 weeks after diagnosis. If no baseline values were available, but follow-up values were, a CRP of <1 mg/dL or ESR of <40 mm/h were considered improved [4]. Clinical outcomes included length of hospital stay, treatment failure, and death. Microbiological failure included infection-related death, microbiologically confirmed relapse with the same or a different organism, development of acute neurological deficits, or having an unplanned surgical procedure with positive intraoperative microbiology [4]. Mechanical failure included undergoing unplanned surgical procedures for purely mechanical reasons (severe pain, spinal instability, or deformity), with negative intraoperative microbiology. If the patient did not have treatment failure within one year after the end of antibiotic therapy, treatment was considered successful.
3. Microbiological testing
Identification of and antimicrobial susceptibility testing for isolates from the urine and blood were performed with standardized methods at each hospital.
4. Statistical analysis
We compared the characteristics in the treatment success and treatment failure groups. Dichotomous variables were compared using Pearson's χ2 or Fisher's exact tests, and their results were presented as the number and proportion. Continuous variables were compared using the Mann–Whitney U or Kruskal-Wallis test, and their results were presented as the median and interquartile range (IQR). To identify risk factors for treatment failure in patients with MSSA VO, all candidate variables that were significant at P-value <0.2 on univariate analysis were analyzed using the Cox proportional hazards model, and results were presented as hazard ratios (HRs) and 95% confidence intervals (CIs). Missing values were imputed using multiple imputation techniques assuming missing at random. The IBM SPSS Statistics 22.0 program (IBM Corp., Armonk, NY, USA) was used for all statistical analyses. A two-tailed P-value of <0.05 was considered as indicating statistical significance.
Results
1. Clinical characteristics of patients with MSSA VO
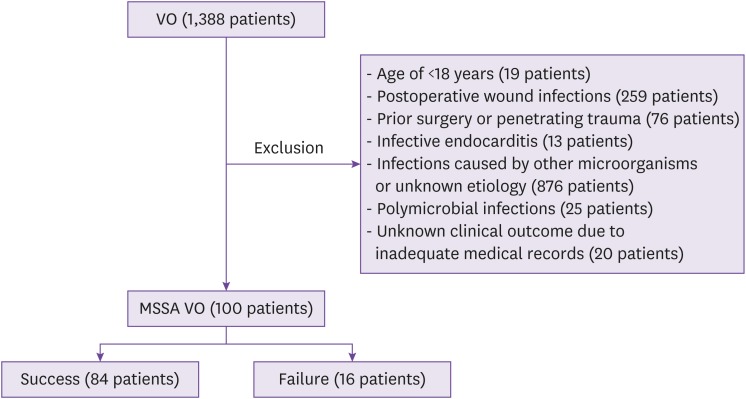
Of a total of 1,388 patients whose diagnosis corresponded to the codes for VO (M46.0 ‒ M46.9) in the ICD 9/10, 1,288 were excluded; finally, 100 adult patients with MSSA VO were included in the study (Fig. 1). The clinical characteristics of the patients in the study are shown in Table 1. Of the 100 patients, 58 were male, and the median age was 67 years (IQR 56 – 73). Fifty-three patients had one or more comorbid conditions. Of note was that 32 patients had diabetes mellitus, and 14 had a history of spinal trauma or fracture within the previous 6 months.
Figure 1. Flow diagram for eligibility of cases with vertebral osteomyelitis caused by methicillin-susceptible Staphylococcus aureus.
VO, vertebral osteomyelitis; MSSA, methicillin-susceptible Staphylococcus aureus.
Table 1. Clinical characteristics and treatment outcomes of patients with vertebral osteomyelitis caused by methicillin-susceptible Staphylococcus aureus.
| Characteristics | No. (%) | P-value | ||||
|---|---|---|---|---|---|---|
| Total (N = 100) | Treatment success (N = 84) | Treatment failure (N = 16) | ||||
| Demography | ||||||
| Age >65 years | 56 (56.0) | 43 (51.2) | 13 (81.3) | 0.026 | ||
| Male gender | 58 (58.0) | 48 (57.1) | 10 (62.5) | 0.691 | ||
| Obesity (BMI >25 kg/m2) | 26 (26.0) | 22 (26.2) | 4 (25.0) | 1.000 | ||
| Comorbid conditions at the time of diagnosis | ||||||
| Presence of one or more comorbid conditions | 53 (53.0) | 45 (53.6) | 8 (53.0) | 0.793 | ||
| Solid tumor | 4 (4.0) | 4 (4.8) | 0 | 1.000 | ||
| Diabetes mellitus | 32 (32.0) | 26 (32.0) | 6 (37.5) | 0.607 | ||
| Liver cirrhosis | 5 (5.0) | 4 (4.8) | 1 (6.3) | 1.000 | ||
| Alcoholism | 6 (6.0) | 6 (7.1) | 0 | 0.586 | ||
| Rheumatoid arthritis | 3 (3.0) | 2 (2.4) | 1 (6.3) | 0.411 | ||
| ESRD on renal replacement therapy | 3 (3.0) | 3 (3.6) | 0 | 1.000 | ||
| Immunosuppressive therapy within 30 days | 2 (2.0) | 2 (2.4) | 0 | 1.000 | ||
| History of spinal trauma or fracture within 6 months | 14 (14.0) | 13 (15.5) | 1 (6.3) | 0.458 | ||
| Prior VO at the same site | 2 (2.0) | 2 (2.4) | 0 | 1.000 | ||
| Clinical or laboratory findings at the time of diagnosis | ||||||
| Duration of symptoms before presentation (median [IQR], days) | 7 (3–19) | 7 (3–18) | 7 (2–21) | 0.819 | ||
| Back pain | 93 (93.0) | 78 (92.9) | 15 (93.8) | 1.000 | ||
| Fever or a history of fever | 53 (53.0) | 44 (52.4) | 9 (56.3) | 0.776 | ||
| Neurologic symptoms | 41 (41.0) | 36 (42.9) | 5 (31.3) | 0.387 | ||
| Leukocytosis (WBC >10,000 cells/mm3) | 66 (66.0) | 54 (64.3) | 12 (66.0) | 0.407 | ||
| Elevated CRP (>1 mg/dL) | 95 (95.0) | 80 (95.2) | 15 (93.8) | 1.000 | ||
| Elevated ESR (>20 mm/h) | 79 (79.0) | 66 (78.6) | 13 (81.3) | 1.000 | ||
| Azotemia (BUN >20 mg/dL or serum creatinine >1.5 mg/dL) | 40 (40.0) | 32 (38.1) | 8 (50.0) | 0.373 | ||
| Radiographic findings at the time of diagnosis | ||||||
| Involved spines | ||||||
| Cervical spine | 10 (10.0) | 10 (11.9) | 0 | 0.358 | ||
| Thoracic spine | 27 (27.0) | 21 (25.0) | 6 (37.5) | 0.359 | ||
| Lumbosacral spine | 76 (76.0) | 65 (77.4) | 11 (68.8) | 0.525 | ||
| Multifocal involvement | 13 (13.0) | 12 (14.3) | 1 (6.3) | 0.687 | ||
| Involvement of ≥3 vertebral segments | 34 (34.0) | 28 (33.3) | 6 (37.5) | 0.747 | ||
| Infectious complications around the spinal column | 93 (93.0) | 77 (91.7) | 16 (100.0) | 0.594 | ||
| Paravertebral extension | 52 (52.0) | 39 (46.4) | 13 (81.3) | 0.011 | ||
| Psoas abscess | 44 (44.0) | 36 (42.9) | 8 (50.0) | 0.598 | ||
| Epidural extension | 63 (63.0) | 55 (65.5) | 8 (50.0) | 0.240 | ||
| Meningeal involvement | 1 (1.0) | 1 (1.2) | 0 | 1.000 | ||
| Positive culture specimens | ||||||
| Blood | 71 (71.0) | 60 (71.4) | 11 (68.8) | 1.000 | ||
| Purulent discharge | 14 (14.0) | 13 (15.5) | 1 (6.3) | 0.458 | ||
| Aspiration or biopsy specimen | 11 (11.0) | 11 (13.1) | 0 | 0.204 | ||
| Surgical specimen | 46 (46.0) | 38 (45.2) | 8 (50.0) | 0.726 | ||
| Biopsy or surgical procedure after initial presentation | ||||||
| Biopsy | 33 (33.0) | 26 (31.0) | 7 (43.8) | 0.318 | ||
| Surgical procedure | 63 (63.0) | 54 (64.3) | 9 (56.3) | 0.542 | ||
| Multiple surgical procedures | 15 (15.0) | 12 (14.3) | 3 (18.8) | 0.704 | ||
| Implant insertion during surgical procedures | 24 (24.0) | 20 (23.8) | 4 (25.0) | 1.000 | ||
| Antibiotic therapy | ||||||
| Interval from presentation to appropriate antibiotic therapy (median [IQR], days) | 2 (0–6) | 3 (0–6) | 1 (0–6) | 0.499 | ||
| Duration of appropriate parenteral antibiotic therapy (median [IQR], days) | 36 (21–51) | 36 (21–52) | 32 (7–48) | 0.486 | ||
| Duration of appropriate oral antibiotic therapy (median [IQR], days) | 20 (0–45) | 28 (0–46) | 0 (0–32) | 0.074 | ||
| Duration of appropriate total antibiotic therapy (median [IQR], days) | 61 (33–97) | 61 (35–102) | 48 (7–91) | 0.165 | ||
| Clinical courses | ||||||
| Improvement of the follow-up CRP | 66 (66.0) | 60 (71.4) | 6 (37.5) | 0.009 | ||
| Improvement of the follow-up ESR | 45 (45.0) | 40 (47.6) | 5 (31.3) | 0.228 | ||
| Clinical outcomes | ||||||
| Length of hospital stay (median [IQR], days) | 45 (30–66) | 46 (34–66) | 37 (13–86) | 0.275 | ||
| Interval from discontinuation of antibiotic therapy to end of follow-up (median [IQR], days) | 152 (41–694) | 159 (46–693) | 65 (0–929) | 0.268 | ||
BMI, body mass index; ESRD, end-stage renal disease; VO, vertebral osteomyelitis; IQR, interquartile range; WBC, white blood cells; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; BUN, blood urea nitrogen.
The median duration of symptoms before presentation was 7 days (IQR 3 – 19), and it was less than 2 weeks in 66% of the patients. Back pain (93%) was the most common symptom at the time of presentation, followed by fever (53%) and neurologic symptoms (41%). At the time of diagnosis, 95 patients showed elevated CRP, 79 elevated ESR, and 66 leukocytosis.
The lumbar spine (76 patients) was the most common site of involvement, followed by the thoracic (27) and cervical spine (10). At the time of diagnosis, 93% of the patients had one or more infectious complications around the spinal column: paravertebral extension in 52 patients, psoas abscess in 44, epidural involvement in 63, and meningeal involvement in 1.
2. Antibiotic susceptibility of MSSA isolates
The causative microorganism was isolated from the blood (71 patients) or surgical specimens (46). All 100 MSSA isolates were susceptible to trimethoprim/sulfamethoxazole (100%), followed, in descending order, by rifampicin (99%), clindamycin (97%), ciprofloxacin (96%), erythromycin (93%), tetracycline (93%), and fusidic acid (36%).
3. Antibiotic therapy for MSSA VO
The median interval from presentation to appropriate antibiotic therapy was 2 days (IQR 0 –6), as shown in Table 1. The median duration of total appropriate antibiotic therapy was 61 days (IQR 33 ‒ 97), with median durations of 36 days (IQR 21 ‒ 51) and 20 days (IQR 0 ‒ 45), respectively, for parenteral and oral antibiotic therapy. During antibiotic therapy, the median number of antibiotics given to each patient was 3.5 (IQR 2‒5). All 100 patients were treated, initially or during antibiotic treatment, with one or more parenteral antibiotics (Table 2). Sixty-nine patients received one or more oral antibiotics; in these 69 patients, the median durations of parenteral and oral antibiotic therapy were 38 days (IQR 28 ‒ 50) and 40 days (IQR 28 ‒ 66), respectively. The most commonly used antibiotics for oral switch therapy were first-generation cephalosporins (30 patients); in these patients, the median durations of parenteral and oral antibiotic therapy were 37 days (IQR 28 ‒ 52) and 43 days (IQR 29 ‒ 62), respectively. A wide range of combinations of parenteral and oral antibiotics was given to the study patients; antibiotic regimens were diverse, and durations of parenteral and oral therapy were different, depending on the patient and the hospital.
Table 2. Antibiotic therapy in patients with vertebral osteomyelitis caused by methicillin-susceptible Staphylococcus aureus.
| Route | Appropriate antibiotics used in each patienta | |
|---|---|---|
| Parenteral (N = 100) | Oral (N = 69) | |
| Antibiotic | First-generation cephalosporins (76), penicillinase-resistant penicillins (43), glycopeptides (38), penicillins/β-lactamase inhibitors (23), carbapenems (9), clindamycin (7), first-generation cephalosporins + fluoroquinolones (6), fourth-generation cephalosporins (5), penicillinase-resistant penicillins + fluoroquinolones (3), fluoroquinolones (3), second-generation cephalosporins (3), glycopeptides + carbapenems (2), first-generation cephalosporins + aminoglycosides (1), first-generation cephalosporins + carbapenems (1), glycopeptides + penicillins/β-lactamase inhibitors (1), glycopeptides + fluoroquinolones (1), macrolides (1), penicillins/β-lactamase inhibitors + rifampicin (1), fluoroquinolones + aminoglycosides (1) | First-generation cephalosporins (31), penicillins/β-lactamase inhibitors (15), fluoroquinolones + rifampicin (6), fluoroquinolones (5), second-generation cephalosporins (4), clindamycin (4), first-generation cephalosporins + fluoroquinolones (3), first-generation cephalosporins + rifampicin (2), trimethoprim-sulfamethoxazole (2), fusidic acid (1), fluoroquinolones + clindamycin (1) |
aTotal number of antibiotics used might be over 100 because one or more antibiotics were used in each patient.
4. Treatment outcomes in patients with MSSA VO
The median interval from discontinuation of antibiotic therapy to the end of follow-up was 152 days (IQR 41 ‒ 694). The median length of hospital stay was 45 days (IQR 30 ‒ 66). The follow-up CRP was improved in 66 patients, while the follow-up ESR was improved in only 45 patients. Surgery, in combination with antibiotic therapy, was performed in 63 patients, of whom 15 (23.8%) underwent two or more surgical procedures and 24 (38.1%) had spinal implants inserted. Of the 100 patients with MSSA VO, 16 had treatment failures – 13 microbiological failure and 3 mechanical failure. Eleven patients died, and 8 (72.7%) of these deaths were related to MSSA VO.
5. Risk factors for treatment failure in patients with MSSA VO
Using variables that were significant at P-value <0.2, the Cox proportional hazards model demonstrated that old age (adjusted HR 5.600, 95% CI 1.402 – 22.372, P = 0.015) and failure of improvement in CRP at follow-up (adjusted HR 3.388, 95% CI 1.168 – 9.829, P = 0.025) were independent risk factors for treatment failure in patients with MSSA VO (Table 3).
Table 3. Cox proportional hazards regression of risk factors for treatment failure in patients with vertebral osteomyelitis caused by methicillin-susceptible Staphylococcus aureus .
| Variables | Unadjusted HR (95% CI) | P-value | Adjusted HR (95% CI) | P-value |
|---|---|---|---|---|
| Old age (>65 years) | 3.956 (1.114–14.053) | 0.033 | 5.600 (1.402–22.372) | 0.015 |
| Paravertebral extension | 2.433 (0.666–8.891) | 0.179 | - | - |
| Duration of appropriate oral antibiotic therapy (days) | 0.985 (0.967–1.004) | 0.132 | - | - |
| Duration of appropriate total antibiotic therapy (days) | 0.991 (0.979–1.003) | 0.135 | - | - |
| Failure of improvement of CRP at follow-up | 4.185 (1.505–11.637) | 0.006 | 3.388 (1.168–9.829) | 0.025 |
HR, hazard ratio; CI, confidence interval; CRP, C-reactive protein.
6. Antibiotic therapy with parenteral and/or oral β-lactams
Of the study patients with MSSA VO, 42 were treated with parenteral and/or oral β-lactams, including first-generation cephalosporins, penicillinase-resistant penicillins, and penicillins/β-lactamase inhibitors, for a total duration of more than 2 weeks (Table 4). Only 13 patients were treated with parenteral antibiotics, including β-lactams, for the whole period. In contrast, only 5 patients were treated with parenteral β-lactams for less than 2 weeks and oral β-lactams for the remaining total period of antibiotic therapy (group I in Table 4).
Table 4. Comparison of parenteral vs. parenteral to oral switch therapy with β-lactams in patients with vertebral osteomyelitis caused by methicillin-susceptible Staphylococcus aureus .
| Treatment group | Parenteral therapy with β-lactam aloneb (N = 13) | Parenteral to oral switch therapy with β-lactamsa | |||||
|---|---|---|---|---|---|---|---|
| Group Ic (N = 5) | P-value | Group IId (N = 13) | P-value | Group IIIe (N = 11) | P-valuef | ||
| Median duration of oral β-lactam therapy, days (IQR) | - | Oral 44 (30–53) | - | Oral 37 (29–66) | - | Oral 40 (24–67) | - |
| Median duration of total β-lactam therapy, days (IQR) | Total 47 (27–55) | Total 55 (39–57) | 0.503 | Total 61 (47–92) | 0.029 | Total 80 (62–102) | 0.005 |
| Median length of hospital stay, days (IQR) | 60 (45–80) | 34 (24–53) | 0.046 | 34 (28–41) | 0.009 | 44 (37–52) | 0.207 |
| Treatment success rate | 100% (13/13) | 100% (5/5) | 1.000 | 92.3% (12/13) | 1.000 | 72.7% (8/11) | 0.082 |
aOral β-lactams included first-generation cephalosporins (cefadroxil, cefatrizine, cefroxadine, cephalexin) and penicillins/β-lactamase inhibitors (amoxicillin-clavulanate).
bParenteral β-lactam used for ≥2 weeks and more than half of the period of total parenteral therapy without switch to oral antibiotics.
cParenteral β-lactam used for <2 weeks and oral β-lactam used for the remaining period of total antibiotic therapy.
dParenteral β-lactam used for 2–4 weeks and oral β-lactam used for the remaining period of total antibiotic therapy.
eParenteral β-lactam used for 4–6 weeks and oral β-lactam used for the remaining period of total antibiotic therapy.
fAll P-values compared with patients who received parenteral therapy alone.
IQR, interquartile range.
Compared with patients who received only parenteral β-lactams, no significant difference in success rate was observed in patients who received oral β-lactams for a relatively long period (groups I and II). The longer the duration of total antibiotic therapy with β-lactams, the lower the treatment success rates; this difference was not statistically significant. Of the 11 patients who received longer parenteral and oral β-lactam therapy (group III), 3 showed treatment failure. These 3 cases were complicated: they were elderly, had a long duration of symptoms before admission, neurologic symptoms, paravertebral or epidural abscess at the time of presentation, or a short duration of antibiotic therapy.
Discussion
A wide range of combinations of parenteral and oral antibiotics were given to the study patients. Among 42 patients treated with parenteral and/or oral β-lactams for a total duration of more than 2 weeks, no significant difference in success rate was observed in those receiving oral β-lactams compared with patients receiving parenteral β-lactams only. Treatment failure was observed in 16% of the study patients, and was significantly associated with old age and no improvement in CRP at follow-up.
A high prevalence of infectious complications at the time of diagnosis was found: 93% of patients with MSSA VO showed one or more infectious complications, including paravertebral involvement in 52%, psoas abscess in 44%, and epidural involvement in 63%. Of note is that paravertebral involvement was more common in the treatment failure group than in the treatment success group. The rate of infectious complications was comparable to that found in other studies of bacterial VO [4,15]. Consistent with the findings of a previous study of VO [15], our study showed that VO caused by S. aureus is associated with a higher rate of complications. The reason for the high prevalence of infectious complications at the time of diagnosis in this study seems to be the high virulence of S. aureus.
In this study, 63% of patients with MSSA VO underwent surgery. The high rate of surgical procedures in this study seems to be due to the fact that many patients had neurologic symptoms and infectious complications around the spinal column at the time of diagnosis. These factors might also affect length of hospital stay and duration of antibiotic therapy; however, no statistically significant difference in these was found between the treatment success and treatment failure groups.
It has been found that S. aureus might be associated with a higher rate of treatment failure in patients with bacterial VO [4]. In a cohort study including 260 patients with bacterial VO, in 71 (27.3%) of whom treatment failed, S. aureus and duration of symptoms before diagnosis were identified as risk factors for treatment failure [4]. In that study, MRSA infection did not have a worse outcome compared with MSSA infection. Other studies showed that adverse outcomes in patients with VO are associated with persistent or progressive pain, neurologic deficits, systemic symptoms of infection, delayed diagnosis, undrained or partially drained large epidural abscess, end-stage renal disease, MRSA infections, persistently elevated inflammatory markers, or recurrent bacteremia [9,10,16,17,18].
Antibiotic therapy of long duration is generally recommended for the treatment of bacterial VO. In our patients with MSSA VO, the median duration of total antibiotic therapy was 61 days (IQR 33 ‒ 97). Sixty-nine per cent of our patients received oral antibiotic therapy after parenteral antibiotic therapy. The duration of total antibiotic therapy in the treatment success and failure groups showed no statistically significant difference. In a cohort of 255 patients with bacterial VO who received parenteral antibiotic therapy for a median duration of 42 days (IQR 38 – 53), plus adjunctive oral antibiotic therapy for a median duration of 41 days (IQR 26 – 60) in 31% [4], there was no significant difference in treatment failure rates between those receiving oral antibiotic therapy and those who did not (20% vs. 20%; P = 0.87). There are no data from randomized, controlled trials to guide decisions about specific antibiotic regimens or the duration of therapy for VO [10]. According to the recent practice guidelines, a total duration of 6 weeks of antibiotic therapy is recommended for adult patients with bacterial VO when not associated with implants [10]. A recent prospective randomized trial revealed that 6-week therapy is not inferior to 12-week therapy for bacterial VO [8].
Parenteral antibiotic therapy is still the standard treatment for VO, but can be switched to oral antibiotics with high bioavailability. However, data on oral antibiotic therapy for bone and joint infections in adults are lacking. The recent practice guidelines recommend that oral β-lactams should not be prescribed for the “initial” treatment of native VO, given their low bioavailability [10]. Non-β-lactams such as linezolid, levofloxacin plus rifampin, or clindamycin can be used for oral switch therapy for native VO in adults [10]. Oral β-lactams have been widely used in children with acute hematogenous osteomyelitis [19,20,21]. In contrast, oral therapy with β-lactams is generally not recommended in adults with staphylococcal osteoarticular infections; mostly parenteral administration of β-lactams, if needed, is used.
According to the national guidelines in Korea, parenteral β-lactams including nafcillin or cefazolin should be administrated to treat osteomyelitis caused by MSSA, but oral first-generation cephalosporins such as cefadroxil, cephalexin, and cephradine are generally not recommended [22]. However, some experts recommend oral switching with β-lactams in adults with acute osteomyelitis [5,12,13,14]. In this study, we found that of 69 patients with a switch from parenteral to oral antibiotics, 52 (75.4%) received oral β-lactam monotherapy for treatment of MSSA VO. Compared with patients receiving parenteral β-lactams only, no significant difference in success rate was observed in those who received oral β-lactams for a relatively long period, as shown in Table 4. Based on the findings of this study, and in contrast to previous thoughts on this matter, we can say that switching to oral β-lactams may be safe in certain adult patients with MSSA VO. Since limited oral agents are available for the treatment of staphylococcal osteomyelitis, including native VO, whether oral β-lactams can be used for the treatment of VO in adults remains to be elucidated.
The present study has some limitations. First of all, antibiotic therapy at each study hospital was not controlled, although only acute VO cases were included. Because the number of patients included in the study was relatively small, whereas a large number of antibiotics was used and duration of antibiotic therapy varied, the clinical outcome of MSSA VO could not be evaluated according to the antibiotic classes or regimens. This kind of difficulty in classifying antibiotic therapy, especially with oral antibiotics, has often been found in retrospective studies of VO [3,4,5,6,23]. Mostly, efficacy based on the type of oral antibiotic regimens was not described at all or was poorly described [3,4,5,6,23]. To overcome these problems, a standardized protocol for treatment of VO should be used at each hospital, and large-scale prospective studies need to be carried out. Second, this study was retrospective in nature, so we could not evaluate details of the clinical course of MSSA VO. Third, since we screened the electronic databases of hospital information systems and searched for MSSA VO cases at each hospital, a certain number of culture-negative cases were excluded. If patients were referred from primary care clinics or other hospitals where antibiotics had already been prescribed, the results of bacterial cultures performed at the study hospitals were likely to be negative. Finally, follow-up periods after discontinuation of antibiotic therapy varied, depending on the patient. Therefore, rates of treatment success might not be sufficiently valid.
In summary, we revealed the current status of antibiotic therapy for MSSA VO in Korea. No significant difference in treatment success rate was observed in patients who received oral β-lactams for a relatively long period, compared with patients who received parenteral β-lactams only. Based on the findings of this study, we think that switching to oral β-lactams may be safe in certain adult patients with MSSA VO. Since limited data are available on the efficacy of oral antibiotics for the treatment of staphylococcal VO in adults, we think that further evaluation of the role of oral switch therapy with β-lactams in adults with osteomyelitis is needed, so that more evidence-based conclusions may be developed in the future.
Footnotes
Funding: None.
Conflict of Interest: BN Kim is an associate editor of the journal Infect Chemother. However, he did not involve in the reviewer selection, evaluation, and decision making of this article. Otherwise, no conflicts of interest were reported.
- Conceptualization: WSO, BNK.
- Data curation: WSO, CM, JWC, EJC, YGK, SHK, SYR, SYP, BNK.
- Formal analysis: WSO.
- Investigation: BNK.
- Supervision: BNK.
- Writing - original draft: WSO, BNK.
- Writing - review & editing: WSO, CM, JWC, EJC, YGK, SHK, SYR, SYP, BNK.
References
- 1.Zimmerli W. Clinical practice. Vertebral osteomyelitis. N Engl J Med. 2010;362:1022–1029. doi: 10.1056/NEJMcp0910753. [DOI] [PubMed] [Google Scholar]
- 2.Issa K, Diebo BG, Faloon M, Naziri Q, Pourtaheri S, Paulino CB, Emami A. The epidemiology of vertebral osteomyelitis in the United States from 1998 to 2013. Clin Spine Surg. 2018;31:E102–E108. doi: 10.1097/BSD.0000000000000597. [DOI] [PubMed] [Google Scholar]
- 3.Mylona E, Samarkos M, Kakalou E, Fanourgiakis P, Skoutelis A. Pyogenic vertebral osteomyelitis: a systematic review of clinical characteristics. Semin Arthritis Rheum. 2009;39:10–17. doi: 10.1016/j.semarthrit.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Gupta A, Kowalski TJ, Osmon DR, Enzler M, Steckelberg JM, Huddleston PM, Nassr A, Mandrekar JM, Berbari EF. Long-term outcome of pyogenic vertebral osteomyelitis: a cohort study of 260 patients. Open Forum Infect Dis. 2014;1:ofu107. doi: 10.1093/ofid/ofu107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McHenry MC, Easley KA, Locker GA. Vertebral osteomyelitis: long-term outcome for 253 patients from 7 Cleveland-area hospitals. Clin Infect Dis. 2002;34:1342–1350. doi: 10.1086/340102. [DOI] [PubMed] [Google Scholar]
- 6.Chang WS, Ho MW, Lin PC, Ho CM, Chou CH, Lu MC, Chen YJ, Chen HT, Wang JH, Chi CY. Clinical characteristics, treatments, and outcomes of hematogenous pyogenic vertebral osteomyelitis, 12-year experience from a tertiary hospital in central Taiwan. J Microbiol Immunol Infect. 2018;51:235–242. doi: 10.1016/j.jmii.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Park KH, Kim DY, Lee YM, Lee MS, Kang KC, Lee JH, Park SY, Moon C, Chong YP, Kim SH, Lee SO, Choi SH, Kim YS, Woo JH, Ryu BH, Bae IG, Cho OH. Selection of an appropriate empiric antibiotic regimen in hematogenous vertebral osteomyelitis. PLoS One. 2019;14:e0211888. doi: 10.1371/journal.pone.0211888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernard L, Dinh A, Ghout I, Simo D, Zeller V, Issartel B, Le Moing V, Belmatoug N, Lesprit P, Bru JP, Therby A, Bouhour D, Dénes E, Debard A, Chirouze C, Fèvre K, Dupon M, Aegerter P, Mulleman D Duration of Treatment for Spondylodiscitis (DTS) study group. Antibiotic treatment for 6 weeks versus 12 weeks in patients with pyogenic vertebral osteomyelitis: an open-label, non-inferiority, randomised, controlled trial. Lancet. 2015;385:875–882. doi: 10.1016/S0140-6736(14)61233-2. [DOI] [PubMed] [Google Scholar]
- 9.Park KH, Cho OH, Lee JH, Park JS, Ryu KN, Park SY, Lee YM, Chong YP, Kim SH, Lee SO, Choi SH, Bae IG, Kim YS, Woo JH, Lee MS. Optimal duration of antibiotic therapy in patients with hematogenous vertebral osteomyelitis at low risk and high risk of recurrence. Clin Infect Dis. 2016;62:1262–1269. doi: 10.1093/cid/ciw098. [DOI] [PubMed] [Google Scholar]
- 10.Berbari EF, Kanj SS, Kowalski TJ, Darouiche RO, Widmer AF, Schmitt SK, Hendershot EF, Holtom PD, Huddleston PM, 3rd, Petermann GW, Osmon DR Infectious Diseases Society of America. 2015 Infectious Diseases Society of America (IDSA) clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adults. Clin Infect Dis. 2015;61:e26–46. doi: 10.1093/cid/civ482. [DOI] [PubMed] [Google Scholar]
- 11.Lew DP, Waldvogel FA. Osteomyelitis. Lancet. 2004;364:369–379. doi: 10.1016/S0140-6736(04)16727-5. [DOI] [PubMed] [Google Scholar]
- 12.Rao N, Ziran BH, Lipsky BA. Treating osteomyelitis: antibiotics and surgery. Plast Reconstr Surg. 2011;127(Suppl 1):177S–187S. doi: 10.1097/PRS.0b013e3182001f0f. [DOI] [PubMed] [Google Scholar]
- 13.Davis JS. Management of bone and joint infections due to Staphylococcus aureus . Intern Med J. 2005;35(Suppl 2):S79–S96. doi: 10.1111/j.1444-0903.2005.00982.x. [DOI] [PubMed] [Google Scholar]
- 14.Haas DW, McAndrew MP. Bacterial osteomyelitis in adults: evolving considerations in diagnosis and treatment. Am J Med. 1996;101:550–561. doi: 10.1016/s0002-9343(96)00260-4. [DOI] [PubMed] [Google Scholar]
- 15.Loibl M, Stoyanov L, Doenitz C, Brawanski A, Wiggermann P, Krutsch W, Nerlich M, Oszwald M, Neumann C, Salzberger B, Hanses F. Outcome-related co-factors in 105 cases of vertebral osteomyelitis in a tertiary care hospital. Infection. 2014;42:503–510. doi: 10.1007/s15010-013-0582-0. [DOI] [PubMed] [Google Scholar]
- 16.Yoon SH, Chung SK, Kim KJ, Kim HJ, Jin YJ, Kim HB. Pyogenic vertebral osteomyelitis: identification of microorganism and laboratory markers used to predict clinical outcome. Eur Spine J. 2010;19:575–582. doi: 10.1007/s00586-009-1216-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kowalski TJ, Berbari EF, Huddleston PM, Steckelberg JM, Osmon DR. Do follow-up imaging examinations provide useful prognostic information in patients with spine infection? Clin Infect Dis. 2006;43:172–179. doi: 10.1086/505118. [DOI] [PubMed] [Google Scholar]
- 18.Solis Garcia del Pozo J, Vives Soto M, Solera J. Vertebral osteomyelitis: long-term disability assessment and prognostic factors. J Infect. 2007;54:129–134. doi: 10.1016/j.jinf.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 19.Peltola H, Pääkkönen M. Acute osteomyelitis in children. N Engl J Med. 2014;370:352–360. doi: 10.1056/NEJMra1213956. [DOI] [PubMed] [Google Scholar]
- 20.DeRonde KJ, Girotto JE, Nicolau DP. Management of pediatric acute hematogenous osteomyelitis, Part I: Antimicrobial stewardship approach and review of therapies for Methicillin-Susceptible Staphylococcus aureus, Streptococcus pyogenes, and Kingella kingae. Pharmacotherapy. 2018;38:947–966. doi: 10.1002/phar.2160. [DOI] [PubMed] [Google Scholar]
- 21.Le Saux N. Diagnosis and management of acute osteoarticular infections in children. Paediatr Child Health. 2018;23:336–343. doi: 10.1093/pch/pxy049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korean Society for Chemotherapy; Korean Society of Infectious Diseases; Korean Orthopaedic Association. Clinical guidelines for the antimicrobial treatment of bone and joint infections in Korea. Infect Chemother. 2014;46:125–138. doi: 10.3947/ic.2014.46.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daver NG, Shelburne SA, Atmar RL, Giordano TP, Stager CE, Reitman CA, White AC., Jr Oral step-down therapy is comparable to intravenous therapy for Staphylococcus aureus osteomyelitis. J Infect. 2007;54:539–544. doi: 10.1016/j.jinf.2006.11.011. [DOI] [PubMed] [Google Scholar]