Abstract
Background
Cerebral palsy (CP) is the most common cause of physical disabilities in children in high‐income countries. Spasticity is the most common motor disturbance in CP. Botulinum toxin type A (BoNT‐A) is considered the first‐line treatment for focal spasticity in people with CP.
Objectives
To evaluate the effectiveness and safety of BoNT‐A compared to other treatments used in the management of lower limb spasticity in children with CP.
Search methods
We searched CENTRAL, PubMed, four other databases, and two trial registers in October 2018. We also searched the reference lists of relevant studies and reviews and contacted experts in the field. We did not apply any date or language restrictions.
Selection criteria
Randomised controlled trials of children with CP, aged between birth and 19 years, treated with BoNT‐A injections in the lower limb muscles compared to other interventions. The primary outcomes were gait analysis and function. The secondary outcomes were joint range of motion, quality of life, satisfaction, spasticity, and adverse events.
Data collection and analysis
Two review authors independently selected studies, extracted data, assessed risk of bias, and rated the quality of the evidence using GRADE. A third review author arbitrated in case of disagreements. We conducted meta‐analyses of available data whenever possible, analysing dichotomous data with risk ratios (RR), and continuous data with mean differences (MD) or standardised mean differences (SMD), with 95% confidence intervals (CI). We considered a 5% significance level for all analyses.
Whenever possible, we analysed outcomes at the time points at which they were assessed: short term (2 to 8 weeks); medium term (12 to 16 weeks); and long term (> 24 weeks).
Main results
We included 31 randomised controlled trials assessing 1508 participants. Most studies included ambulatory patients with more than one motor type of CP, and with a mean age of between three and seven years. There was a slight predominance of males.
Studies compared BoNT‐A in the lower limb muscles to usual care or physiotherapy (14 studies), placebo or sham (12 studies), serial casting (4 studies), or orthoses (1 study).
We rated studies as at high or unclear risk of bias mainly due to random sequence generation, allocation concealment, blinding of participants and personnel, and blinding of outcome assessment.
BoNT‐A versus usual care or physiotherapy
BoNT‐A might improve overall gait scores at medium‐term follow‐up (MD 2.80, 95% CI 1.55 to 4.05; 1 study, 40 children; very low‐quality evidence) and is moderately effective at improving function at short‐term (SMD 0.59, 95% CI 0.23 to 0.95; 2 studies, 123 children) and medium‐term (SMD 1.04, 95% CI 0.16 to 1.91; 4 studies, 191 children) follow‐up (all very low‐quality evidence).
BoNT‐A improves ankle range of motion, satisfaction, and ankle plantarflexors spasticity at one or more time points (very low‐quality evidence).
The proportion of adverse events in the BoNT‐A group was 0.37 (95% CI 0.08 to 0.66; I2 = 95%; very low‐quality evidence). No adverse events were reported in the control group.
BoNT‐A versus placebo or sham
BoNT‐A improves overall gait scores at short‐term (RR 1.66, 95% CI 1.16 to 2.37, P = 0.006; 4 studies, 261 assessments) and medium‐term (RR 1.90, 95% CI 1.32 to 2.74, P < 0.001; 3 studies, 248 assessments) follow‐up, and may improve peak ankle dorsiflexion in stance (MD 15.90 degrees, 95% CI 4.87 to 26.93, P = 0.005; 1 study, 19 children) and in swing (MD 10.20 degrees, 95% CI 4.01 to 16.39, P = 0.001; 1 study, 19 children) at short‐term follow‐up (all moderate‐quality evidence).
BoNT‐A is not more effective than placebo or sham at improving function at short‐term (SMD 0.24, 95% CI −0.35 to 0.83, P = 0.42; 4 studies, 305 children) or long‐term (SMD −0.07, 95% CI −0.48 to 0.35, P = 0.76; 2 studies, 91 children) follow‐up, but has a small positive effect at medium‐term follow‐up (SMD 0.28, 95% CI 0.06 to 0.49, P = 0.01; 5 studies, 327 children) (all moderate‐quality evidence).
BoNT‐A improves passive ankle range of motion, satisfaction, and ankle plantarflexors spasticity at one or more time points (moderate‐quality evidence).
There was no difference between groups in the rate of adverse events at short‐term follow‐up (RR 1.29, 95% CI 0.87 to 1.93, P = 0.21; 12 studies, 918 children; moderate‐quality evidence).
BoNT‐A versus serial casting
There was no difference between groups for overall gait scores at short‐term (MD 0.00, 95% CI −1.66 to 1.66); medium‐term (MD 0.65, 95% CI −1.21 to 2.51); or long‐term (MD 0.46, 95% CI −1.33 to 2.25) follow‐up in one study with 18 children (moderate‐quality evidence).
BoNT‐A improved instrumented gait analysis only in terms of ankle dorsiflexion at initial contact (MD 6.59 degrees, 95% CI 1.39 to 11.78, P = 0.01; 2 studies, 47 children). There was no difference between groups for peak ankle dorsiflexion in stance and swing, and gait speed at any time point (moderate‐ and low‐quality evidence).
BoNT‐A is not more effective than serial casting at improving function, ankle range of motion, and spasticity at any time point (moderate‐ and low‐quality evidence).
BoNT‐A is not associated with a higher risk of adverse events than serial casting (RR 0.59, 95% CI 0.03 to 11.03; 3 studies, 64 children; low‐quality evidence).
BoNT‐A versus orthoses
There was no difference between groups for function at medium‐term follow‐up (MD 11.14, 95% CI −0.05 to 22.33; 1 study, 43 children), but BoNT‐A is more effective than orthoses at improving hip range of motion and hip adductors spasticity (all very low‐quality evidence).
Authors' conclusions
The quality of the evidence was low or very low for most of the outcomes analysed. We found limited evidence that BoNT‐A is more effective than placebo or a non‐placebo control at improving gait, joint range of motion, satisfaction, and lower limb spasticity in children with CP, whereas the results for function were contradictory. The rate of adverse events with BoNT‐A is similar to placebo. BoNT‐A is not more effective than ankle serial casting to treat ankle contractures for any of the assessed outcomes, but is more effective than orthotics at improving range of motion and spasticity.
Plain language summary
Botulinum toxin type A injections for the treatment of lower limb spasticity in cerebral palsy
Background
Cerebral palsy (CP) is a non‐progressive, lifelong condition resulting from damage to the developing brain. Over time, most children with CP will develop abnormal muscle activity and stiffness/overactivity (spasticity) that affects at least one limb and interferes with their normal movement. Treatments for spasticity include physiotherapy, oral antispasticity drugs (a type of medication that works to relax the muscles and relieve spasticity), casts, splints, orthopaedic surgery, and botulinum toxin A (BoNT‐A; a poisonous biological substance that is thought to relieve spasticity by reducing muscle overactivity when injected into the muscle). This review looked at the effects of BoNT‐A.
Review question
The aim of this review was to assess and summarise scientific studies comparing BoNT‐A injections to other treatments for lower limb spasticity in children with CP.
Study characteristics
We found 31 studies assessing 1508 participants. The use of BoNT‐A in the lower limb muscles was compared to: (1) children's regular care or physiotherapy, (2) placebo (fake injections), (3) a series of below‐knee plaster casts, and (4) leg splints.
Key results
Children receiving BoNT‐A injections tended to have improved walking pattern (gait), joint range of motion, satisfaction with outcome of treatment, and muscle spasms compared with their usual programme of care or physiotherapy, or placebo. Measures of function tended to show only modest improvements in children receiving BoNT‐A injections. The rate of side effects was similar when comparing BoNT‐A injections to placebo. BoNT‐A injections and plaster casts below the knee produced similar benefits in walking and joint motion and relieving spasms. In addition, BoNT‐A provided better results in terms of joint range of motion compared to a specific type of splinting (Johnstone pressure splints).
Quality of the evidence
We considered the quality of the evidence as very low for the comparison BoNT‐A versus usual care or physiotherapy; moderate for the comparison BoNT‐A versus placebo; moderate and low for the comparison BoNT‐A versus plaster casts; and very low for the comparison BoNT‐A versus splints.
Conclusion
There is limited evidence that, compared to placebo or regular care, BoNT‐A improves walking, joint motion, satisfaction with the outcome of treatment, and muscle spasticity in children with CP. The rate of side effects with BoNT‐A was similar to placebo. BoNT‐A was no better than plaster casts in any of our analyses, but was better than splints at improving range of motion and spasticity.
Summary of findings
Summary of findings for the main comparison. BoNT‐A compared to usual care or physiotherapy in the treatment of lower limb spasticity in children with cerebral palsy: short‐term results.
| BoNT‐A compared to usual care or physiotherapy in the treatment of lower limb spasticity in children with cerebral palsy: short‐term results | ||||||
| Patient or population: children with CP Setting: short‐term follow‐up (2 to 8 weeks) Intervention: BoNT‐A injections into the lower limb muscles Comparison: usual care or physiotherapy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with usual care or physiotherapy | Risk with BoNT‐A | |||||
| Instrumented gait analysis (kinematics) (not measured) | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured in any trial |
| Observational gait analysis (not measured) | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured in any trial |
| Function Assessed with: various scales (GMFM total scores and GMFM goal scores) Follow‐up: range 2 to 8 weeks | ‐ | The SMD in the intervention group was 0.59 SD higher (0.23 higher to 0.95 higher). | ‐ | 123 (2 RCTs) | ⊕⊝⊝⊝ Very lowa | Favours BoNT‐A group (higher functional scores in BoNT‐A group). Rule of thumb to interpret the magnitude of effect for the SMD: 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect (Cohen 1988). |
| Range of motion (passive ankle dorsiflexion) Assessed with: goniometry (degrees) Follow‐up: range 2 to 8 weeks | The mean passive ankle dorsiflexion in the control group ranged from −0.30 to 13.40 degrees. | The mean passive ankle dorsiflexion in the intervention group was 8.34 degrees higher (1.19 higher to 15.50 higher). | ‐ | 186 (2 RCTs**) | ⊕⊝⊝⊝ Very lowb | **1 study reported on this outcome per lower limb. Favours BoNT‐A group (higher passive ankle dorsiflexion in BoNT‐A group). High statistical heterogeneity |
| Satisfaction (not measured) | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured in any trial |
| Spasticity (ankle plantarflexors) Assessed with: various scales Follow‐up: range 2 to 8 weeks | ‐ | The SMD in the intervention group was 1.19 SD lower (2.62 lower to 0.24 higher). | ‐ | 186 (2 RCTs***) | ⊕⊝⊝⊝ Very lowb | ***1 study reported on this outcome per lower limb. No difference between groups. High statistical heterogeneity. Rule of thumb to interpret the magnitude of effect for the SMD: 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect (Cohen 1988). |
| Adverse events | Study population | 0.37 proportion of AE in the BoNT‐A group (0.08 to 0.66) | 206 (5 RCTs) | ⊕⊝⊝⊝ Very lowc | AE for all studies (multiple follow‐up times). Favours control group (higher number of AE in BoNT‐A group) | |
| 0 per 1000 | Not estimable due to the lack of events in the control group | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AE: adverse events; BoNT‐A: botulinum toxin type A; CP: cerebral palsy; CI: confidence interval; GMFM: Gross Motor Function Measure; RCT: randomised controlled trial; SD: standard deviation; SMD: standardised mean difference. | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded two levels for several potential sources of bias and one level for imprecision due to the small sample size. bDowngraded two levels for several potential sources of bias and one level due to high statistical heterogeneity and imprecision due to the small sample size. cDowngraded two levels for several potential sources of bias and high statistical heterogeneity and one level for imprecision, since most studies in this comparison did not report on adverse events.
Summary of findings 2. BoNT‐A compared to usual care or physiotherapy in the treatment of lower limb spasticity in children with cerebral palsy: medium‐term results.
| BoNT‐A compared to usual care or physiotherapy in the treatment of lower limb spasticity in children with cerebral palsy: medium‐term results | ||||||
|
Patient or population: children with CP Setting: medium‐term follow‐up (12 to 16 weeks) Intervention: BoNT‐A injections into the lower limb muscles Comparison: usual care or physiotherapy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with usual care or physiotherapy | Risk with BoNT‐A | |||||
| Instrumented gait analysis (kinematics) (not measured) | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured in any trial |
| Observational gait analysis Assessed with: PRS Follow‐up: range 12 to 16 weeks | The mean gait score in the control group was 8.93. | The mean gait score in the intervention group was 2.80 higher (1.55 higher to 4.05 higher). | ‐ | 40 (1 RCT) | ⊕⊝⊝⊝ Very lowa | Favours BoNT‐A group (higher gait scores in BoNT‐A group) |
| Function Assessed with: various scales (GMFM total scores,GMFM goal scores, and GAS) Follow‐up: range 12 to 16 weeks | ‐ | The SMD in the intervention group was 1.04SD higher (0.16 higher to 1.91 higher). | ‐ | 191 (4 RCTs) | ⊕⊝⊝⊝ Very lowb | Favours BoNT‐A group (higher function scores in BoNT‐A group). High statistical heterogeneity. Rule of thumb to interpret the magnitude of effect for the SMD: 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect (Cohen 1988). |
| Range of motion (passive ankle dorsiflexion) Assessed with: goniometry (degrees) Follow‐up: range 12 to 16 weeks | The mean passive ankle dorsiflexion in the control groups ranged from 3.10 to 26.1 degrees. | The mean passive ankle dorsiflexion in the intervention groups was 6.36 degrees higher (4.03 higher to 8.69 higher). | ‐ | 272 (5 RCTs**) | ⊕⊝⊝⊝ Very lowc | **1 study reported on this outcome per lower limb. Favours BoNT‐A group (higher passive ankle dorsiflexion in BoNT‐A group). Note: 2 studies reported this outcome as changes from baseline. |
| Satisfaction (not measured) | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured in any trial |
| Spasticity (ankle plantarflexors) Assessed with: various scales Follow‐up: range 12 to 16 weeks | ‐ | The SMD in the intervention group was 1.66 SD lower (2.88 lower to 0.43 lower). | ‐ | 226 (3 RCTs***) | ⊕⊝⊝⊝ Very lowc | ***1 study reported on this outcome per lower limb. Favours BoNT‐A group (lower ankle plantarflexors spasticity in BoNT‐A group). High statistical heterogeneity. Rule of thumb to interpret the magnitude of effect for the SMD: 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect (Cohen 1988). |
| Adverse events | ‐ | ‐ | ‐ | ‐ | ‐ | See Table 1 |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BoNT‐A: botulinum toxin type A; CI: confidence interval; CP: cerebral palsy; GAS: Goal Attainement Scale;GMFM: Gross Motor Function Measure; PRS: Physician Rating Scale; RCT: randomised controlled trial; SD: standard deviation; SMD: standardised mean difference. | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded two levels for several potential sources of bias and one level for imprecision, since this outcome was reported by a single study with a relatively small sample size. bDowngraded two levels for several potential sources of bias and one level due to high statistical heterogeneity. cDowngraded two levels for several potential sources of bias and one level for imprecision, as the unit of analysis in one study was each lower limb.
Summary of findings 3. BoNT‐A compared to usual care or physiotherapy in the treatment of lower limb spasticity in children with cerebral palsy: long‐term results.
| BoNT‐A compared to usual care or physiotherapy in the treatment of lower limb spasticity in children with cerebral palsy: long‐term results | ||||||
|
Patient or population: children with CP Setting: long‐term follow‐up (more than 24 weeks) Intervention: BoNT‐A injections into the lower limb muscles Comparison: usual care or physiotherapy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with usual care or physiotherapy | Risk with BoNT‐A | |||||
| Instrumented gait analysis (kinematics) | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured in any trial |
| Observational gait analysis (not measured) | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured in any trial |
| Function Assessed with: various scales (GMFM total scores and GMFM goal scores) Follow‐up: range 6 to 24 months | ‐ | The SMD in the intervention group was 0.34 SD higher (0.33 lower to 1.01 higher). | ‐ | 199 (4 RCTs) | ⊕⊝⊝⊝ Very lowa | No difference between groups. High statistical heterogeneity. Rule of thumb to interpret the magnitude of effect for the SMD: 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect (Cohen 1988). |
| Range of motion (passive ankle dorsiflexion) Assessed with: goniometry (degrees) Follow‐up: range 6 to 24 months | The mean change in passive ankle dorsiflexion in the control groups ranged from −5.20 to −2.02 degrees (changes from baseline). | The mean passive ankle dorsiflexion in the intervention groups was 6.48 degrees higher (4.42 higher to 8.53 higher). | ‐ | 250 (4 RCTs**) | ⊕⊝⊝⊝ Very lowb | **1 study reported on this outcome per lower limb. Favours BoNT‐A group (higher passive ankle dorsiflexion in BoNT‐A group). Note: 3 studies reported this outcome as changes from baseline. |
| Satisfaction Assessed with: visual analogue scale (scale from 0 to 10) Follow‐up: range 6 to 24 months | The mean satisfaction score in the control group was 6.31. | The mean satisfaction score in the intervention group was 1.57 higher (0.76 higher to 2.38 higher). | ‐ | 24 (1 RCT) | ⊕⊝⊝⊝ Very lowc | Favours BoNT‐A group (higher satisfaction scores in BoNT‐A group) |
| Spasticity (ankle plantarflexors) Assessed with: various scales Follow‐up: range 6 to 24 months | ‐ | The SMD in the intervention group was 0.77 SD lower (1.13 lower to 0.40 lower). | ‐ | 258 (4 RCTs***) | ⊕⊝⊝⊝ Very lowb | ***1 study reported on this outcome per lower limb. Favours BoNT‐A group (lower spasticity in BoNT‐A group). Note: 2 studies reported this outcome as changes from baseline. Rule of thumb to interpret the magnitude of effect for the SMD: 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect (Cohen 1988). |
| Adverse events | ‐ | ‐ | ‐ | ‐ | ‐ | See Table 1 |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BoNT‐A: botulinum toxin type A; CI: confidence interval; CP: cerebral palsy; GMFM: Gross Motor Function Measure; RCT: randomised controlled trial; SD: standard deviation; SMD: standardised mean difference. | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded two levels for several potential sources of bias and imprecision and one level due to high statistical heterogeneity. bDowngraded two levels for several potential sources of bias and one level for imprecision, as the unit of analysis in one study was each lower limb. cDowngraded two levels for several potential sources of bias and one level for imprecision, as this outcome was reported by a single study with a small sample size.
Summary of findings 4. BoNT‐A compared to placebo or sham in the treatment of lower limb spasticity in children with cerebral palsy: short‐term results.
| BoNT‐A compared to placebo or sham in the treatment of lower limb spasticity in children with cerebral palsy: short‐term results | ||||||
| Patient or population: children with CP Setting: short‐term follow‐up (2 to 8 weeks) Intervention: BoNT‐A injections into the lower limb muscles Comparison: placebo or sham injections | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo or sham | Risk with BoNT‐A | |||||
|
Instrumented gait analysis (peak ankle dorsiflexion in stance) Assessed with: (degrees) Follow‐up: range 2 to 8 weeks |
The mean change in peak ankle dorsiflexion in stance in the control group was −3.40 (changes from baseline). | The mean change in peak ankle dorsiflexion in stance in the intervention group was 15.90 higher (4.87 higher to 26.93 higher). | ‐ | 19 (1 RCT) | ⊕⊕⊕⊝ Moderatea | Favours BoNT‐A group (higher peak ankle dorsiflexion in stance in the BoNT‐A group) |
| Observational gait analysis (improvement) Assessed with: various scales (PRS, VGA) Follow‐up: range 2 to 8 weeks | Study population | RR 1.66 (1.16 to 2.37) | 261 (4 RCTs**) | ⊕⊕⊕⊝ Moderateb | **1 study reported on this outcome per lower limb. Favours BoNT‐A group (higher improvement on observational gait analysis in BoNT‐A group) | |
| 26 per 100 | 42 per 100 (30 to 61) | |||||
| Function Assessed with: various scales (COPM performance, GMFM goal scores, PGA) Follow‐up: range 2 to 8 weeks | ‐ | The SMD in the intervention group was 0.24 SD higher (0.35 lower to 0.83 higher). | ‐ | 305 (4 RCTs) | ⊕⊕⊕⊝ Moderatec | No difference between groups. High statistical heterogeneity. Rule of thumb to interpret the magnitude of effect for the SMD: 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect (Cohen 1988). |
| Range of motion (passive ankle dorsiflexion) Assessed with: goniometry (degrees) Follow‐up: range 2 to 8 weeks | The mean change in passive ankle dorsiflexion in the control groups ranged from −0.60 to 1.70 (changes from baseline). | The mean change in passive ankle dorsiflexion in the intervention groups was 2.68 degrees higher (0.12 higher to 5.23 higher). | ‐ | 291 (3 RCTs***) | ⊕⊕⊕⊝ Moderateb | ***1 study reported on this outcome per lower limb. Favours BoNT‐A group (higher passive ankle dorsiflexion in BoNT‐A group). Data were reported as changes from baseline. |
| Satisfaction Assessed with: COPM satisfaction Follow‐up: range 2 to 8 weeks | The mean satisfaction score in the control group was 4.40 points. | The mean satisfaction score in the intervention group was 1.81 higher (0.25 higher to 3.37 higher). | ‐ | 41 (1 RCT) | ⊕⊕⊕⊝ Moderatea | Favours BoNT‐A group (higher satisfaction in BoNT‐A group) |
| Spasticity (ankle plantarflexors) Assessed with: MAS Follow‐up: range 2 to 8 weeks | The mean change in the ankle plantarflexors score in the control group was −0.48 (changes from baseline). | The mean change in the ankle plantarflexors score in the intervention group was 0.49 lower (0.78 lower to 0.2 lower). | ‐ | 156 (1 RCT) | ⊕⊕⊕⊝ Moderated | Favours BoNT‐A group (lower spasticity in BoNT‐A group). This outcome was reported as changes from baseline. |
| Adverse events | Study population | RR 1.29 (0.87 to 1.93) | 918 (12 RCTs) | ⊕⊕⊕⊝ Moderatec | No difference between groups. Adverse events for all studies (multiple follow‐up times). High statistical heterogeneity | |
| 269 per 1000 | 347 per 1000 (234 to 519) | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BoNT‐A: botulinum toxin type A; CI: confidence interval; COPM: Canadian Occupational Performance Measure; CP: cerebral palsy; GMFM: Gross Motor Function Measure; MAS: Modified Ashworth Scale; PGA: Physician's Global Assessment; PRS: Physician Rating Scale; RCT: randomised controlled trial; RR: risk ratio; SD: standard deviation; SMD: standardised mean difference; VGA: video gait analysis. | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for imprecision, as the outcome was reported by a single study with a small sample size. bDowngraded one level for imprecision, as the results of one study considered each lower limb as the unit of analysis. cDowngraded one level due to high statistical heterogeneity amongst studies. dDowngraded one level for imprecision, as the outcome was reported by a single study.
Summary of findings 5. BoNT‐A compared to placebo or sham in the treatment of lower limb spasticity in children with cerebral palsy: medium‐term results.
| BoNT‐A compared to placebo or sham in the treatment of lower limb spasticity in children with cerebral palsy: medium‐term results | ||||||
| Patient or population: children with CP Setting: medium‐term follow‐up (12 to 16 weeks) Intervention: BoNT‐A injections into the lower limb muscles Comparison: placebo or sham injections | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo or sham | Risk with BoNT‐A | |||||
| Instrumented gait analysis (kinematics) (not measured) | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured in any trial |
| Observational gait analysis (improvement) Assessed with: various scales (PRS, VGA) Follow‐up: range 12 to 16 weeks | Study population | RR 1.90 (1.32 to 2.74) | 248 (3 RCTs**) | ⊕⊕⊕⊝ Moderatea | **1 study reported on this outcome per lower limb. Favours BoNT‐A group (higher improvement on observational gait analysis in BoNT‐A group) | |
| 24 per 100 | 45 per 100 (31 to 65) | |||||
| Function Assessed with: various scales (COPM performance, GMFM goal scores, PGA) Follow‐up: range 12 to 16 weeks | ‐ | The SMD in the intervention groups was 0.28 SD higher (0.06 higher to 0.49 higher). | ‐ | 327 (5 RCTs) | ⊕⊕⊕⊝ Moderateb | Favours BoNT‐A group (higher motor function in BoNT‐A group). Rule of thumb to interpret the magnitude of effect for the SMD: 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect (Cohen 1988). |
| Range of motion (passive ankle dorsiflexion) Assessed with: goniometry (degrees) Follow‐up: range 12 to 16 weeks | The mean change in passive ankle dorsiflexion in the control groups ranged from −2.80 to −0.30 degrees (changes from baseline). | The mean change in passive ankle dorsiflexion in the intervention groups was 1.57 degrees higher (2.12 lower to 5.25 higher). | ‐ | 150 (2 RCTs***) | ⊕⊕⊕⊝ Moderatea | ***1 study reported on this outcome per lower limb. No difference between groups. Data were reported as changes from baseline. |
| Satisfaction Assessed with: COPM satisfaction Follow‐up: range 12 to 16 weeks | The mean satisfaction score in the control group was 3.89 in 1 study. | The mean satisfaction score in the intervention groups was 0.96 higher (0.04 higher to 1.88 higher). | ‐ | 74 (2 RCTs) | ⊕⊕⊕⊝ Moderateb | Favours BoNT‐A group (higher satisfaction in BoNT‐A group). 1 of the studies reported this outcome as changes from baseline. |
| Spasticity (ankle plantarflexors) Assessed with: MAS Follow‐up: range 12 to 16 weeks | The mean change in the ankle plantarflexors score in the control group was −0.50 (changes from baseline). | The mean change in the ankle plantarflexors score in the intervention group was 0.50 lower (0.78 lower to 0.22 lower). | ‐ | 144 (1 RCT) | ⊕⊕⊕⊝ Moderatec | Favours BoNT‐A group (lower spasticity in BoNT‐A group). This outcome was reported as changes from baseline. |
| Adverse events | ‐ | ‐ | ‐ | ‐ | ‐ | See Table 4 |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BoNT‐A: botulinum toxin type A; CI: confidence interval; COPM: Canadian Occupational Performance Measure; CP: cerebral palsy; GMFM: Gross Motor Function Measure; MAS: Modified Ashworth Scale;PGA: Physician's Global Assessment; PRS: Physician Rating Scale; RCT: randomised controlled trial; RR: risk ratio; SD: standard deviation; SMD: standardised mean difference; VGA: video gait analysis. | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for imprecision, as the results of one study considered each lower limb as the unit of analysis. bDowngraded one level for imprecision, as different types of scales were used (individualised function measures and standard motor scales). cDowngraded one level for imprecision, as this outcome was reported by a single study.
Summary of findings 6. BoNT‐A compared to placebo or sham in the treatment of lower limb spasticity in children with cerebral palsy: long‐term results.
| BoNT‐A compared to placebo or sham in the treatment of lower limb spasticity in children with cerebral palsy: long‐term results | ||||||
| Patient or population: children with CP Setting: long‐term follow‐up (> 24 weeks) Intervention: BoNT‐A injections into the lower limb muscles Comparison: placebo or sham injections | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo or sham | Risk with BoNT‐A | |||||
| Instrumented gait analysis (kinematics) (not measured) | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured in any trial |
| Observational gait analysis (not measured) | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured in any trial |
| Function Assessed with: various scales (COPM performance, GMFM total scores) Follow‐up: range 6 to 24 months | ‐ | The SMD in the intervention groups was 0.07 SD lower (0.48 lower to 0.35 higher). | ‐ | 91 (2 RCTs) | ⊕⊕⊕⊝ Moderatea | No difference between groups. Rule of thumb to interpret the magnitude of effect for the SMD: 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect (Cohen 1988). |
| Range of motion (passive ankle dorsiflexion) Assessed with: goniometry (degrees) Follow‐up: range 6 to 24 months | The mean change in passive ankle dorsiflexion in the control group was −0.30 degrees (changes from baseline). | The mean change in passive ankle dorsiflexion in the intervention group was0.20 degrees higher (4.88 lower to 5.28 higher). | ‐ | 40 (1 RCT) | ⊕⊕⊕⊝ Moderateb | No difference between groups. Data were reported as changes from baseline. |
| Satisfaction Assessed with: COPM satisfaction Follow‐up: range 6 to 24 months | The mean change in the satisfaction score in the control group was 1.70 (changes from baseline). | The mean change in the satisfaction score in the intervention group was 0.10 higher (1.27 lower to 1.47 higher). | ‐ | 33 (1 RCT) | ⊕⊕⊕⊝ Moderateb | No difference between groups. Data were reported as changes from baseline. |
| Spasticity (ankle plantarflexors) Assessed with: MAS Follow‐up: range 6 to 24 months | The mean change in the ankle plantarflexors score in the control group was 0.40 (changes from baseline). | The mean change in the ankle plantarflexors score in the intervention group was 0.10 higher (0.58 lower to 0.78 higher). | ‐ | 42 (1 RCT) | ⊕⊕⊕⊝ Moderateb | No difference between groups. Data were reported as changes from baseline. |
| Adverse events | ‐ | ‐ | ‐ | ‐ | ‐ | See Table 4 |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BoNT‐A: botulinum toxin type A; CI: confidence interval; COPM: Canadian Occupational Performance Measure; CP: cerebral palsy; GMFM: Gross Motor Function Measure; MAS: Modified Ashworth Scale; RCT: randomised controlled trial; SD: standard deviation; SMD: standardised mean difference. | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for imprecision due to the small sample size. bDowngraded one level for imprecision, as this outcome was reported by a single study with a relatively small sample size.
Summary of findings 7. BoNT‐A compared to serial casting in the treatment of lower limb spasticity in children with cerebral palsy: short‐term results.
| BoNT‐A compared to serial casting in the treatment of lower limb spasticity in children with cerebral palsy: short‐term results | ||||||
| Patient or population: children with CP Setting: short‐term follow‐up (2 to 8 weeks) Intervention: BoNT‐A injections into the ankle plantarflexors Comparison: short‐leg serial casting for ankle equinus deformity | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with casts | Risk with BoNT‐A | |||||
| Instrumented gait analysis (peak ankle dorsiflexion in stance) (degrees) Follow‐up: range 2 to 8 weeks | The mean peak ankle dorsiflexion in stance in the control group was 8.70. | The mean peak ankle dorsiflexion in stance in the intervention group was 0.60 lower (5.78 lower to 4.58 higher). | ‐ | 21 (1 RCT) | ⊕⊕⊝⊝ Lowa | No difference between groups |
| Observational gait analysis Assessed with: PRS Follow‐up: range 2 to 8 weeks | The mean gait score in the control group was 2.87. | The mean gait score in the intervention group was 0 higher (1.66 lower to 1.66 higher). | ‐ | 18 (1 RCT) | ⊕⊕⊕⊝ Moderateb | No difference between groups |
| Function Assessed with: GMFM (goal scores) Follow‐up: range 2 to 8 weeks | The mean function score in the control group was 60.15. | The mean function score in the intervention group was 2.01 higher (23.31 lower to 27.33 higher). | ‐ | 18 (1 RCT) | ⊕⊕⊕⊝ Moderateb | No difference between groups |
| Range of motion (ankle dorsiflexion) Assessed with: goniometry (degrees) Follow‐up: range 2 to 8 weeks | The mean passive ankle dorsiflexion in the control group was 13.70. | The mean passive ankle dorsiflexion in the intervention group was 1.77 higher (4.25 lower to 7.79 higher). | ‐ | 18 (1 RCT**) | ⊕⊕⊝⊝ Lowc | No difference between groups. **1 study reported on this outcome per lower limb. |
| Satisfaction (not measured) | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured in any trial |
| Spasticity (ankle plantarflexors) Assessed with: MAS Follow‐up: range 2 to 8 weeks | The mean ankle plantarflexors score in the control group was 1.60. | The mean ankle plantarflexors score in the intervention group was 0.20 lower (0.81 lower to 0.41 higher). | ‐ | 18 (1 RCT***) | ⊕⊕⊝⊝ Lowc | No difference between groups. ***1 study reported on this outcome per lower limb. |
| Adverse events | Study population | RR 0.59 (0.03 to 11.03) | 64 (3 RCTs) | ⊕⊕⊝⊝ Lowd | No difference between groups. Adverse events for all studies (multiple follow‐up times). High statistical heterogeneity | |
| 667 per 1000 | 393 per 1000 (20 to 1000) | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BoNT‐A: botulinum toxin type A; CI: confidence interval; CP: cerebral palsy; GMFM: Gross Motor Function Measure; MAS: Modified Ashworth Scale; PRS: Physician Rating Scale; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded two levels for imprecision, as this outcome was reported by a single study with a small sample size and was available only for some of the participants. bDowngraded one level for imprecision, as this outcome was reported by a single study with a small sample size. cDowngraded two levels for imprecision, as this outcome was reported by a single study with a small sample size, and the study reported each lower limb as the unit of analysis for this outcome. dDowngraded one level for high statistical heterogeneity amongst studies and one level for imprecision due to a relatively small sample size and small number of events.
Summary of findings 8. BoNT‐A compared to serial casting in the treatment of lower limb spasticity in children with cerebral palsy: medium‐term results.
| BoNT‐A compared to serial casting in the treatment of lower limb spasticity in children with cerebral palsy: medium‐term results | ||||||
| Patient or population: children with CP Setting: medium‐term follow‐up (12 to 16 weeks) Intervention: BoNT‐A injections into the ankle plantarflexors Comparison: short‐leg serial casting for ankle equinus deformity | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with casts | Risk with BoNT‐A | |||||
|
Instrumented gait analysis (peak ankle dorsiflexion in stance) Assessed with: (degrees) Follow‐up: range 12 to 16 weeks |
The mean peak ankle dorsiflexion in stance in the control groups ranged from 6.30 to 6.90 in 2 studies. | The mean peak ankle dorsiflexion in stance in the intervention groups was 3.03 higher (3.56 lower to 9.62 higher). | ‐ | 61 (3 RCTs**) | ⊕⊕⊝⊝ Lowa | **2 studies reported on this outcome per lower limb. No difference between groups. Note: 1 study reported on this outcome as changes from baseline. High statistical heterogeneity |
| Observational gait analysis Assessed with: PRS Follow‐up: range 12 to 16 weeks | The mean gait score in the control group was 2.73. | The mean gait score in the intervention group was 0.65 higher (1.21 lower to 2.51 higher). | ‐ | 18 (1 RCT) | ⊕⊕⊕⊝ Moderateb | No difference between groups |
| Function Assessed with: GMFM (goal scores) Follow‐up: range 12 to 16 weeks | The mean function score in the control group was 61.41 in 1 study. | The mean function score in the intervention group was 3.64 higher (1.55 lower to 8.82 higher). | ‐ | 41 (2 RCTs) | ⊕⊕⊕⊝ Moderatec | No difference between groups. Note: 1 study reported on this outcome as changes from baseline. |
| Range of motion (passive ankle dorsiflexion) Assessed with: goniometry (degrees) Follow‐up: range 12 to 16 weeks | The mean passive ankle dorsiflexion in the control groups ranged from 14.62 to 18.00 in 2 studies. | The mean passive ankle dorsiflexion in the intervention groups was 1.82 higher (2.26 lower to 5.91 higher). | ‐ | 67 (3 RCTs***) | ⊕⊕⊝⊝ Lowd | ***2 studies reported on this outcome per lower limb. No difference between groups. Note: 1 study reported on this outcome as changes from baseline. |
| Satisfaction (not measured) | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured in any trial |
| Spasticity (ankle plantarflexors) Assessed with: MAS Follow‐up: range 12 to 16 weeks | The mean ankle plantarflexors score in the control groups ranged from 1.78 to 2.1. | The mean ankle plantarflexors score in the intervention groups was 0.13 higher (0.25 lower to 0.52 higher). | ‐ | 67 (3 RCTs****) | ⊕⊕⊝⊝ Lowd | ****2 studies reported on this outcome per lower limb. No difference between groups |
| Adverse events | ‐ | ‐ | ‐ | ‐ | ‐ | See Table 7 |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BoNT‐A: botulinum toxin type A; CI: confidence interval; CP: cerebral palsy; GMFM: Gross Motor Function Measure; MAS: Modified Ashworth Scale; PRS: Physician Rating Scale; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for imprecision and one level due to statistical heterogeneity amongst studies. bDowngraded one level for imprecision, as this outcome was reported by a single study with a small sample size. cDowngraded one level for imprecision due to the small sample size. dDowngraded two levels for imprecision due to the small sample size and because two studies evaluated each limb as an independent unit of analysis.
Summary of findings 9. BoNT‐A compared to serial casting in the treatment of lower limb spasticity in children with cerebral palsy: long‐term results.
| BoNT‐A compared to serial casting in the treatment of lower limb spasticity in children with cerebral palsy: long‐term results | ||||||
| Patient or population: children with CP Setting: long‐term follow‐up (> 24 weeks) Intervention: BoNT‐A injections into the ankle plantarflexors Comparison: short‐leg serial casting for ankle equinus deformity | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with casts | Risk with BoNT‐A | |||||
|
Instrumented gait analysis (peak ankle dorsiflexion in stance) Assessed with: degrees Follow‐up: range 6 to 24 months |
The mean peak ankle dorsiflexion in stance in the control group was 5.20. | The mean peak ankle dorsiflexion in stance in the intervention group was 2.00 lower (8.50 lower to 4.50 higher). | ‐ | 26 (1 RCT) | ⊕⊕⊕⊝ Moderatea | No difference between groups |
| Observational gait analysis Assessed with: PRS Follow‐up: range 6 to 24 months | The mean gait score in the control group was 2.67. | The mean gait score in the intervention group was 0.46 higher (1.33 lower to 2.25 higher). | ‐ | 18 (1 RCT) | ⊕⊕⊕⊝ Moderatea | No difference between groups |
| Function Assessed with: GMFM (goal scores) Follow‐up: range 6 to 24 months | The mean function score in the control group was 64.57. | The mean function score in the intervention group was 2.02 lower (26.85 lower to 22.81 higher). | ‐ | 18 (1 RCT) | ⊕⊕⊕⊝ Moderatea | No difference between groups |
| Range of motion (passive ankle dorsiflexion) Assessed with: goniometry (degrees) Follow‐up: range 6 to 24 months | The mean passive ankle dorsiflexion in the control groups ranged from 14.00 to 14.87. | The mean passive ankle dorsiflexion in the intervention groups was 1.02 lower (5.63 lower to 3.58 higher). | ‐ | 44 (2 RCTs**) | ⊕⊕⊝⊝ Lowb | **2 studies reported on this outcome per lower limb. No difference between groups |
| Satisfaction (not measured) | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured in any trial |
| Spasticity (ankle plantarflexors) Assessed with: MAS Follow‐up: range 6 to 24 months | The mean ankle plantarflexors score in the control groups ranged from 1.87 to 2.30. | The mean ankle plantarflexors score in the intervention groups was 0.17 higher (0.34 lower to 0.67 higher). | ‐ | 44 (2 RCTs***) | ⊕⊕⊝⊝ Lowb | ***2 studies reported on this outcome per lower limb. No difference between groups |
| Adverse events | ‐ | ‐ | ‐ | ‐ | ‐ | See Table 7 |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BoNT‐A: botulinum toxin type A; CI: confidence interval; CP: cerebral palsy; GMFM: Gross Motor Function Measure; MAS: Modified Ashworth Scale; PRS: Physician Rating Scale; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for imprecision, as this outcome was reported by a single study with a small sample size. bDowngraded two levels for imprecision due to the small sample size and because two studies evaluated each limb as an independent unit of analysis.
Summary of findings 10. BoNT‐A compared to orthoses in the treatment of lower limb spasticity in children with cerebral palsy: medium‐term results.
| BoNT‐A compared to orthoses in the treatment of lower limb spasticity in children with cerebral palsy: medium‐term results | ||||||
| Patient or population: children with CP Setting: medium‐term follow‐up (12 to 16 weeks) Intervention: BoNT‐A injections into the hip adductors and hamstrings Comparison: Johnstone pressure splints | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with orthoses | Risk with BoNT‐A | |||||
| Instrumented gait analysis (not reported) | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Observational gait analysis (not reported) | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
|
Function Assessed with: GMFM (total scores) Follow‐up: range 12 to 16 weeks |
The mean function score in the control group was 36.81. | The mean motor function score in the intervention group was 11.14 higher (0.05 lower to 22.33 higher). | ‐ | 43 (1 RCT) | ⊕⊝⊝⊝ Very lowa | No difference between groups |
| Range of motion (passive hip abduction) Assessed with: goniometry (degrees) Follow‐up: range 12 to 16 weeks | The mean passive hip abduction in the control group was 53.17. | The mean passive hip abduction in the intervention group was 10.61 higher (2.53 higher to 18.69 higher). | ‐ | 43 (1 RCT) | ⊕⊝⊝⊝ Very lowa | Favours BoNT‐A group (higher hip abduction in BoNT‐A group) |
| Satisfaction (not reported) | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
|
Spasticity (hip adductors)
Assessed with: MAS Follow‐up: range 12 to 16 weeks |
The mean hip adductors score in the control group was 3.52. | The mean hip adductors score in the intervention group was 0.70 lower (1.10 lower to 0.3 lower). | ‐ | 43 (1 RCT) | ⊕⊝⊝⊝ Very lowa | Favours BoNT‐A (lower hip adductors spasticity in BoNT‐A group) |
| Adverse events (not reported) | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BoNT‐A: botulinum toxin type A; CI: confidence interval; CP: cerebral palsy; GMFM: Gross Motor Function Measure; MAS: Modified Ashworth Scale; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded two levels for several potential sources of bias and one level for imprecision, as this comparison included only one study with a relatively small sample size.
Background
Description of the condition
Cerebral palsy (CP) is considered the most common cause of physical disabilities in children in high‐income countries, affecting around 2:1000 live births (Blair 2010). The term is actually used to describe a number of motor impairment disorders with a wide range of aetiologies and clinical presentations. One of the most comprehensive definitions of CP states that: “Cerebral palsy (CP) describes a group of permanent disorders of the development of movement and posture, causing activity limitation, that are attributed to non‐progressive disturbances that occurred in the developing fetal or infant brain. The motor disorders of CP are often accompanied by disturbances of sensation, perception, cognition, communication, and behaviour, by epilepsy, and by secondary musculoskeletal problems” (Rosenbaum 2007).
Spasticity is the most common tone abnormality in CP, affecting 80% to 90% of individuals with CP. People with spasticity experience increased tone with rapid stretch of the muscle fibers (Sanger 2003). This prevents normal movement, often hampering locomotion. It can also cause contractures and deformities as the child grows, due to the failure of the spastic muscles to grow as rapidly as neighbouring bone and soft tissue (Graham 2013).
Description of the intervention
The treatment of spastic CP usually involves a multimodal approach, using a combination of oral medications, neuromuscular blocks, physical therapy, orthotics, casting, neurosurgery, and orthopaedic surgery (Graham 2013).
Botulinum toxin type A (BoNT‐A) is produced by the bacterium Clostridium botulinum. It is one of seven distinct serological types of the toxin known to be produced by this bacterium. Human botulism usually occurs following ingestion of contaminated food or after wound infection, mainly by BoNT types A, B, and E (Brin 1997; Kostrzewa 2007). The symptoms of botulism include nausea and vomiting, blurred vision, diplopia, dysphagia, dysarthria, respiratory insufficiency, and limb weakness (Brin 1997).
The toxin consists of a heavy chain and a light chain connected by disulphide bridges. The heavy chain promotes binding of the BoNT‐A molecule to high‐affinity recognition sites on the cholinergic nerve terminals at the neuromuscular junction. The light chain acts in the cytosol of nerve endings and inhibits the release of acetylcholine by cleaving the synaptosomal‐associated protein 25 (SNAP‐25), which is required for vesicle docking and, consequently, neurotransmitter release (Brin 1997; Kostrzewa 2007; Simpson 2008).
Brooks suggested in the 1950s that BoNT‐A might be used to reduce activity in hyperactive muscles (Brooks 1954). Scott published the results of the first clinical trial of BoNT‐A for strabismus in 1980 (Scott 1980), and in the years that followed, treated patients for several other disorders of muscle function, including spasticity of the legs (Scott 1994).
How the intervention might work
Since the initial reports by Koman 1993, there is a growing body of evidence showing the effectiveness of BoNT‐A to reduce focal spasticity in individuals with CP, with little or negligible generalised effect (Simpson 2008).
Most non‐randomised studies support the concept that by controlling spasticity, BoNT‐A improves joint range of motion, function, and gait pattern in children with CP (Desloovere 2007; Pascual‐Pascual 1997; Sánchez‐Carpintero 1997). Furthermore, some authors suggest that BoNT‐A may actually reduce or delay the need for orthopaedic surgery (Desloovere 2007; Ruiz 2004; Zmanovskaia 2014).
Over the past two decades, several randomised studies have been conducted to evaluate the effectiveness and safety of BoNT‐A injections to treat lower limb spasticity in children with CP. However, even though most studies showed some benefit after the neuromuscular block, a few studies failed to show more favourable outcomes in the BoNT‐A group when compared with control (Kay 2004; Mall 2006; Moore 2008). Factors related to better outcomes have yet to be identified.
Why it is important to do this review
The substantial heterogeneity of clinical presentations in children with CP poses a challenge for the clinician trying to devise a treatment plan for these patients. Focal lower limb spasticity management with BoNT‐A injections is one of the available treatment methods. However, the wide range of injected muscles, doses, and location methods used in the published studies makes it difficult to summarise the best evidence on this subject. For this reason, there was a need for a systematic review to identify whether the control of lower limb spasticity provided by BoNT‐A promotes significant benefits for children with CP. This is an update of the original review on this subject published by Ade‐Hall 2000.
Objectives
To evaluate the effectiveness and safety of BoNT‐A compared to other treatments used in the management of lower limb spasticity in children with CP.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) only.
We considered a trial eligible for inclusion if it:
evaluated the effectiveness of BoNT‐A;
included concurrent control groups (e.g. receiving placebo or sham injections, surgery, casts, physiotherapy, etc.); and
allocated children through a randomisation procedure.
Types of participants
Eligible trials must have involved children (defined for the purposes of this review as individuals aged between birth and 19 years old) with CP who had been treated for lower limb spasticity. We included studies involving mixed diagnoses including CP only if data from the group with CP could be extracted separately. We made no restrictions regarding functional level or topographic distribution.
Types of interventions
Trials involving BoNT‐A injections into the lower limb muscles (treatment group) compared to a different intervention (control group). We included trials with different doses and different muscle injections. Other interventions were permitted (e.g. a study was considered eligible even if it did not exclude other interventions, such as physiotherapy, casts, etc.) provided that they were not systematically applied differently to the two groups. Finally, the treatment group must have received BoNT‐A, and the control group must not have received BoNT‐A.
Types of outcome measures
Based on the International Classification of Functioning, Disability and Health (WHO 2001), significant outcomes to assess the effectiveness of interventions for people with CP should involve both measures of body function and structure (e.g. range of motion, spasticity) as well as activity and participation (e.g. gait analysis, function). Other measures, such as quality of life and satisfaction, are also considered relevant in this group of patients, and were included in the original review (Ade‐Hall 2000).
As such, we identified the primary and secondary outcomes described below as relevant to measure the effectiveness of BoNT‐A to treat lower limb spasticity in CP.
Primary outcomes
Gait analysis, including observational gait analysis, assessed by subjective assessments and standardised scales such as the Physician Rating Scale (PRS) and the Edinburgh Visual Gait Score (Koman 1994; Read 2003), as well as instrumented gait analysis, including spatio‐temporal parameters, kinematic and kinetic data.
Function, measured (preferably) by validated scales, including the Gross Motor Function Measure (GMFM; Russell 1989), the mobility domain of the Pediatric Evaluation of Disability Inventory (PEDI; Feldman 1990), and the performance domain of the Canadian Occupational Performance Measure (COPM; Law 1990).
Secondary outcomes
Range of motion, notably passive range of motion of the joints measured in degrees.
Quality of life, measured (preferably) by scores on tests developed specifically for children with CP, such as the Cerebral Palsy Quality of Life Questionnaire (CPQOL; Waters 2007).
Satisfaction with the outcome of treatment, measured by scales such as the satisfaction domain of the COPM (Law 1990). We also considered caregivers' perceptions of the effectiveness of interventions as a measure of satisfaction with the outcome of treatment, either by subjective questionnaires or by validated scales such as the Caregiver Priorities and Child Health Index of Life with Disabilities (CPCHILD; Narayanan 2006).
Spasticity, measured by validated scales such as the Modified Ashworth Scale (MAS) and the Modified Tardieu Scale (MTS) (Bohannon 1987; Boyd 1999).
Adverse events, such as muscle weakness, upper respiratory tract symptoms, and pain in the injection site, in order to evaluate the safety of BoNT‐A.
Search methods for identification of studies
Electronic searches
We searched the following databases and trial registers in August 2014, June 2017, and October 2018. We did not limit our search by language or publication year.
Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 10) in the Cochrane Library, which includes the Cochrane Developmental, Psychosocial and Learning Problems Specialised Register (searched 15 October 2018).
MEDLINE PubMed (www.ncbi.nlm.nih.gov/pubmed; searched 15 October 2018).
Embase Ovid (1980 to 2018 Week 41).
Cumulative Index to Nursing and Allied Health Literature EBSCOhost (CINAHL; 1980 to 15 October 2018).
Science Citation Index Web of Science (SCI; 1970 to 8 October 2018).
Conference Proceedings Citation Index‐Science Web of Science (CPCI‐S; 1990 to 8 October 2018).
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov; searched 15 October 2018).
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; www.who.int/ictrp/en; searched 15 October 2018).
We searched MEDLINE using a sensitive search strategy developed in consultation with the Cochrane Information Specialist for Developmental, Psychosocial and Learning Problems, which was then adapted for each of the other databases using appropriate indexing terms and syntax (Appendix 1).
Searching other resources
We contacted authors and experts in the field in order to identify any ongoing or unpublished studies. In addition, we searched for additional studies in the the reference lists of the included studies and any systematic and non‐systematic reviews identified through our Electronic searches,
Data collection and analysis
Selection of studies
Two review authors (FB, MT) independently screened the titles and abstracts of all records retrieved by the Electronic searches to determine if they met the inclusion criteria (Criteria for considering studies for this review). We then obtained and assessed the full‐text reports of those studies that appeared to meet the inclusion criteria or for which additional information was needed to assess eligibility. Where required, disagreements were resolved through negotiation with a third review author (JB), who served as an arbitrator. We depicted the study inclusion process in a PRISMA diagram (Moher 2009).
Data extraction and management
Two review authors (FB, MT) independently extracted the following data from each included study, using a standardised form specifically developed for this review and tested prior to review commencement.
Methods: randomisation; allocation; blinding; incomplete data.
Participants: country of origin; sample size; age; gender; inclusion and exclusion criteria; motor distribution; Gross Motor Function Classification System (GMFCS) level; follow‐up time; loss to follow‐up.
Intervention: dose; location method; muscle groups injected; number of injection cycles; duration; adjuvant treatments.
Control group: placebo; usual care; physiotherapy; orthoses; surgery; others.
Outcomes: primary and secondary (see Types of outcome measures).
Where data were not available in the published trial reports, we attempted to contact the study investigators to obtain the missing information (see Dealing with missing data). We entered all available data into Review Manager 5 (RevMan 5) (Review Manager 2014).
Any disagreements were resolved by consensus and by consulting a third review author (JB) for further discussion when necessary.
Assessment of risk of bias in included studies
Two review authors (FB, MT) independently assessed the risk of bias in each included study using Cochrane's 'Risk of bias' tool (Higgins 2011). We evaluated each trial as being at low, high, or unclear (uncertain) risk of bias in the following domains: sequence generation; allocation concealment; blinding of participants and personnel; blinding of outcome assessors; incomplete outcome data; selective reporting; and other sources of bias (Appendix 2). In cases where the information in the trial report was insufficient to permit a judgement, we attempted to contact the trial investigators for further information (see Dealing with missing data) before assigning a judgement of unclear risk of bias. A third review author (JB) was consulted in the case of disagreements that could not be resolved by consensus.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented the results for each study as a risk ratio (RR) with 95% confidence intervals (95% CIs), and whenever possible pooled the data in a meta‐analysis using a random‐effects model.
Continuous data
For continuous data, we reported the mean post‐treatment/intervention values and standard deviation for each group, and calculated the mean difference (MD) with 95% CI in the meta‐analysis. Where different scales measured the same variable, we calculated the standardised mean difference (SMD) with 95% CI. We used a rule of thumb to interpret the magnitude of effect for the SMD, whereby 0.20 represents a small effect, 0.50 a moderate effect, and 0.80 a large effect (Cohen 1988).
Minimal clinically important differences
Minimal clinically important differences (MCID) have been established for a small number of outcome measures used for children with CP. These include the Edinburgh Visual Gait Score (Gupta 2012), GMFM and Wee Functional Independence Measure (WeeFIM) (Oeffinger 2008), and PEDI (Iyer 2003). However, these apply mostly to ambulatory patients. Whenever possible, we planned to analyse our results taking the MCID into account, which amongst the outcomes measures found and reported in this review would be available only for the GMFM. However, all analyses involving the GMFM either found no differences between groups, or involved pooling data from different scales and reporting the SMD, which made interpreting the MCID difficult.
Unit of analysis issues
Cluster‐randomised trials
We used the child as the unit of analysis in most cases (each randomised child contributed to a single measurement for each outcome assessed at a specific time point). A few studies reported each lower limb as the unit of analysis, mainly for local outcome measures in bilateral interventions. In such situations, whenever we were unable to obtain the original raw data, we used the available data as reported by the studies.
Multiple intervention trials
For studies with more than one type of intervention and only one control group, we selected the intervention group that was closest to our inclusion criteria (Criteria for considering studies for this review), as recommended in Section 16.5.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
Dealing with missing data
We contacted study authors with a request to supply any data missing from the included studies. We performed an intention‐to‐treat analysis whenever possible. We did not impute missing continuous data, as the strategies for imputation are subjective (Higgins 2011a). When results were presented only in graphs and the study authors did not provide the original raw data after personal contact, we attempted to manually extract graphic data using the online software WebPlotDigitizer version 3.10 (Rohatgi 2016).
Assessment of heterogeneity
We assessed heterogeneity by examining study characteristics, including differences in methods, participants, and interventions, as well as the resulting forest plots. We used the I2 statistic to assess the impact of statistical heterogeneity, interpreting an I2 of 50% or more as significant. Whenever we found substantial statistical heterogeneity, we conducted sensitivity analyses and reported Tau2, an estimate of the between‐study variance.
Assessment of reporting biases
We had insufficient studies to assess reporting bias using a funnel plot. See Table 11 (Unused review methods).
1. Unused review methods.
| Methods | Description |
| Assessment of reporting biases | We planned to assess reporting bias using a funnel plot whenever possible. However, due to the small number of studies assessing each specific outcome measure in each comparison, we were unable to perform this type of analysis. |
| Subgroup analyses | We planned to conduct subgroup analyses according to cerebral palsy severity level and age group. However, due to the small number of studies in each meta‐analysis, this was not possible. |
Data synthesis
Qualitative data
We summarised qualitative data pertaining to the methods, risk of bias, and the description of participants and interventions in Characteristics of included studies tables.
Quantitative data
For each of the main comparisons, we combined study data in a meta‐analysis, provided there was sufficient clinical homogeneity in the intervention delivered and the characteristics of the study participants and outcome measures. Irrespective of the nature of data, we used the random‐effects model to pool the data, since we expected substantial clinical and methodological heterogeneity.
For comparisons involving dichotomous data where the rate of events in one group was zero, we performed a meta‐analysis of proportions using the Metaprop function in the RStudio software package (RStudio 2018). We performed the analysis using a random‐effects model, considering the raw proportions and a restricted maximum likelihood model. We used the RevMan 5 layout preset as a basis.
Whenever the studies did not present the required data for the forest plots (e.g. means and standard deviations for continuous data), we attempted to contact the authors directly (see Dealing with missing data). When we were unable to obtain the required data, we provided a narrative description of the reported results under each specific comparison.
Whenever possible, we analysed the outcomes according to the time points at which they were assessed, as follows:
short term: outcomes assessed at two to eight weeks postintervention, when the peak effect of botulinum toxin is evaluated;
medium term: outcomes assessed at 12 to 16 weeks postintervention, when the effect of botulinum toxin is wearing off; and
long term: outcomes assessed at 24 weeks or more postintervention, when the residual benefit of previous botulinum toxin use may still be perceived.
Subgroup analysis and investigation of heterogeneity
Whenever we observed substantial heterogeneity, we conducted an additional, detailed analysis to explore possible causes (see Sensitivity analysis).
We were unable to conduct our preplanned subgroup analyses according to CP severity level and age group due to the small number of studies in each analysis. See Table 11.
Sensitivity analysis
We conducted sensitivity analyses to investigate the impact of including studies at high risk of bias or with missing data, or both, on the effect estimates. Whenever we observed substantial statistical heterogeneity, we conducted a sensitivity analysis to investigate the impact of including studies of lower methodological quality.
'Summary of findings’ tables
We summarised our main findings in 'Summary of findings’ tables, which provide a simple, tabular format to present the results for the most relevant outcomes for the review. We used GRADEpro GDT software to construct the tables (GRADEpro GDT). The tables present:
information about the number of studies and participants;
judgements about the underlying quality of the evidence;
the main statistical results; and
a final grade (rating) for the quality of evidence for each outcome.
Whenever continuous outcome measures were reported both as absolute means and changes from baseline values in a given analysis, we presented data in the 'Risk with placebo' column considering only studies reporting the absolute means.
We used the GRADE approach to assess the quality of the evidence and the strength of recommendations on a four‐point nominal scale ranging from 'high’ to 'very low’ (Schünemann 2013). All assessments were conducted by two review authors (FB, MT) working independently, with a third review author (JB) consulted in case of disagreement. Since all data presented in this review were extracted from RCTs, we initially rated the quality of the evidence as 'high', downgrading it by one level for every GRADE domain considered inadequate, up to a maximum of three levels. The points considered for this assessment were: clinically important heterogeneity, risk of bias, indirectness, imprecision, and publication bias.
According to Cochrane methodology (Schünemann 2017), up to seven outcome measures may be included in each table, ranked in order of importance, as defined by the review authors. We considered the most relevant primary and secondary outcomes that were available from most studies. We created three tables for each comparison in order to reflect the short‐, medium‐, and long‐term time points defined previously (see Data synthesis). We considered the ankle joint for all local outcome measures for comparisons one to three, since BoNT‐A was used to treat ankle flexors spasticity in most studies. The exception was comparison four, which evaluated spasticity of the muscles around the hip exclusively. For the purposes of this review, we reported the following seven outcome measures in the 'Summary of findings' tables:
instrumented gait analysis;
observational gait analysis;
function;
passive range of motion;
spasticity;
satisfaction; and
adverse events. Since we were unable to determine the exact time points at which the adverse events had occurred, we described these under the short‐term tables for all comparisons, with the exception of comparison four, which only included a medium‐term table.
Results
Description of studies
Results of the search
We conducted the initial search for this review in August 2014, and updated it again in June 2017 and October 2018. Our search strategy retrieved 1715 records in August 2014, and an additional 469 records in June 2017 and 230 records in October 2018. After excluding 1017 duplicates, we screened a total of 1397 records, of which 101 were deemed potentially relevant. We obtained the full‐text reports of these records and excluded a further 59 reports that did not meet the inclusion criteria (see Excluded studies; Characteristics of excluded studies tables; Criteria for considering studies for this review). We included 31 studies from 36 reports (see Included studies; Characteristics of included studies tables) and identified five ongoing trials (Characteristics of ongoing studies tables). One potentially relevant study was published in abstract form only and was assessed as awaiting classification (Characteristics of studies awaiting classification tables). The manual search identified no additional studies (see Figure 1).
1.
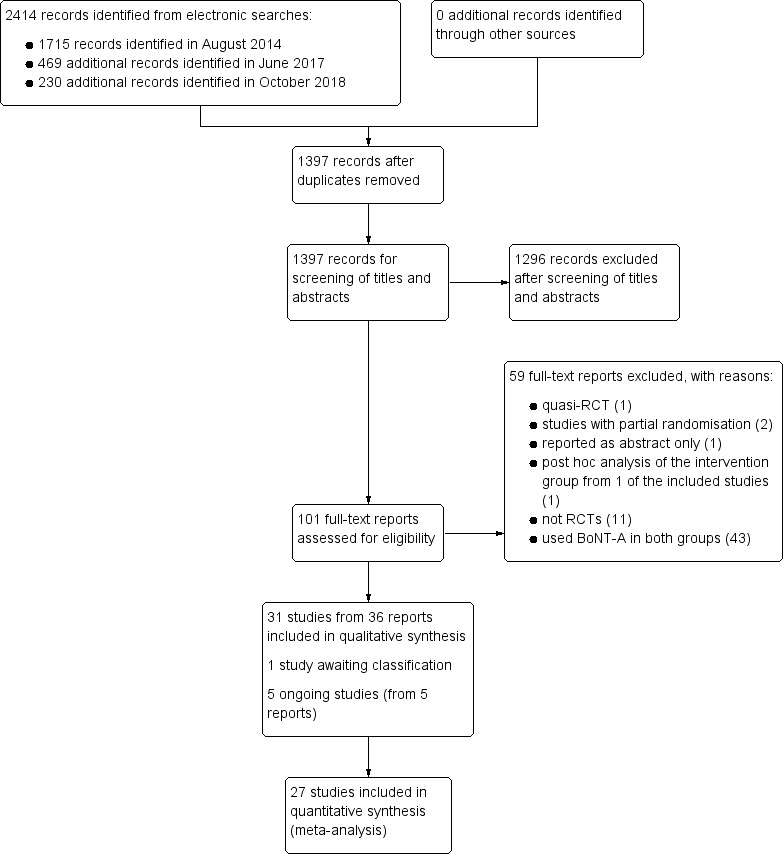
Study flow diagram.
Included studies
We included 31 studies (from 36 reports) in the review (Ackman 2005; Baker 2002; Barwood 2000; Bjornson 2007; Boyd 2001; Çağlar 2019; Chaturvedi 2013; Copeland 2014; Corry 1998; Delgado 2016; El‐Etribi 2004; Flett 1999; Hazneci 2006; Ibrahim 2007; Jozwiak 2007; Kanovsky 2004; Kay 2004; Koman 1994; Koman 2000; Love 2001; Mall 2006; Moore 2008; Navarrete 2010; Reddihough 2002; Scholtes 2006; Steenbeek 2005; Sutherland 1999; Tedroff 2010; Ubhi 2000; Xu 2006; Zhu 2016). Only three of these studies, Corry 1998, Flett 1999, and Koman 1994, were included in the original review (Ade‐Hall 2000).
We grouped studies with more than one report as follows:
Boyd 2001, comprising two reports (Boyd 2001a; Graham 2008);
Copeland 2014, comprising two reports (Copeland 2014a; Edwards 2015);
Delgado 2016, comprising two reports (Delgado 2016a; Tilton 2017); and
Scholtes 2006, comprising three reports (Scholtes 2006a; Scholtes 2007; Van der Houwen 2011).
Most studies were published in English, except for four studies reported in the following languages: Spanish (Navarrete 2010), Chinese (Xu 2006; Zhu 2016), and Polish (Jozwiak 2007).
See Characteristics of included studies tables.
Study design
We considered all included studies to be RCTs. The Reddihough 2002 study had a cross‐over design. The Steenbeek 2005 study had a multiple baseline/treatment phase design. One of the included studies had a previously published protocol (Copeland 2014). The protocols for two other studies were available from international clinical trial registries (Delgado 2016; Zhu 2016).
Location/setting
Twenty‐three studies were single‐centre trials: five from Australia (Barwood 2000; Copeland 2014; Flett 1999; Love 2001; Reddihough 2002); four from the USA (Bjornson 2007; Kay 2004; Koman 1994; Sutherland 1999); three from the UK (Corry 1998; Moore 2008; Ubhi 2000); two from China (Xu 2006; Zhu 2016); two from Turkey (Çağlar 2019; Hazneci 2006); and one apiece from Chile (Navarrete 2010), Egypt (El‐Etribi 2004), India (Chaturvedi 2013), Jordan (Ibrahim 2007), Poland (Jozwiak 2007), Sweden (Tedroff 2010), and the Netherlands (Steenbeek 2005).
The remaining eight studies were multicentre trials, involving one or more countries. The Ackman 2005 study was conducted in five centres in the USA. The Baker 2002 study was conducted in six centres in the UK, five in Poland, and one in Ireland. The Boyd 2001 study was conducted in two centres in Australia. The Delgado 2016 study was conducted in 23 centres in six countries (Chile, France, Mexico, Poland, Turkey, and the USA). The Kanovsky 2004 study was conducted in three centres in the Czech Republic and two centres in Slovakia. The Koman 2000 study was conducted in multiple centres in the USA, Canada, Italy, and Spain. The Mall 2006 study was conducted in nine centres in Germany and one in Austria. The Scholtes 2006 study was conducted in four centres in the Netherlands.
Sample size
The included studies randomised a total of 1567 children and assessed 1508 children.
Some studies included multiple intervention groups. For the purposes of this review, we selected only one intervention group and one control group for most of the main outcomes (see Unit of analysis issues). For the Ackman 2005 study, we included the BoNT‐A group and the placebo + serial casts group. For the Baker 2002 study, we included the intermediate‐dose BoNT‐A group (20 international units (IU)/body weight) and the placebo group for most analyses, except for adverse events, for which we analysed the entire sample. For the Delgado 2016 study, we included the higher‐dose group (15 units/kg) versus placebo, except for adverse events, which we also analysed for all groups.
Participants
Age
Most studies included participants with a mean age at study entry of between three and seven years old. The study by Tedroff 2010 included participants with the lowest mean age (BoNT‐A group: mean = 16.7 months (standard deviation (SD) = 5.1); control group: mean = 15.6 months (SD = 3.0)). The study by Çağlar 2019 included participants with the highest mean age (BoNT‐A group: mean = 9.01 years (SD = 2.47); control group: mean = 9.46 (SD = 2.89)).
Gender
We found a slight predominance of males in most studies. One study, Bjornson 2007, described a disproportionate gender distribution between the BoNT‐A group (70% males) and the placebo group (37% males). Seven studies did not report on the participants' gender (El‐Etribi 2004; Hazneci 2006; Ibrahim 2007; Koman 1994; Love 2001; Navarrete 2010; Steenbeek 2005).
Motor distribution
Five studies included only children with spastic diplegia (Baker 2002; Bjornson 2007; El‐Etribi 2004; Hazneci 2006; Kanovsky 2004); two studies included only children with hemiplegia (Ibrahim 2007; Love 2001); and one study included only children with quadriplegia (Copeland 2014). Three studies did not report on motor distribution (Çağlar 2019; Chaturvedi 2013; Xu 2006), and the remaining studies included children with more than one type of motor dysfunction.
Gross Motor Function Classification System (GMFCS) level
Sixteen studies included only ambulatory patients with GMFCS levels I, II, and III (Ackman 2005; Baker 2002; Bjornson 2007; Delgado 2016; Flett 1999; Hazneci 2006; Ibrahim 2007; Kanovsky 2004; Kay 2004; Koman 1994; Koman 2000; Love 2001; Sutherland 1999; Tedroff 2010; Ubhi 2000; Xu 2006). Three studies included only GMFCS level IV and V patients (Barwood 2000; Copeland 2014; Jozwiak 2007). Seven studies included children with both GMFCS levels I to III and levels IV and V (Boyd 2001; Mall 2006; Navarrete 2010; Reddihough 2002; Scholtes 2006; Steenbeek 2005; Zhu 2016). In four studies, the GMFCS level was not reported and could not be clearly determined (Chaturvedi 2013; Corry 1998; El‐Etribi 2004; Moore 2008). In one study, the GMFCS was inadequately used as an outcome measure, rather than being used to describe the baseline characteristics of the participants (Çağlar 2019).
Interventions
Target muscles
All included studies used BoNT‐A injections, which we considered as the main intervention for the purposes of this review. Most studies (n = 16) involved isolated BoNT‐A injections into the ankle plantar flexors (gastrocnemius or soleus, or both) (Ackman 2005; Baker 2002; Bjornson 2007; Corry 1998; Delgado 2016; Flett 1999; Kanovsky 2004; Kay 2004; Koman 1994; Koman 2000; Love 2001; Sutherland 1999; Tedroff 2010; Ubhi 2000; Xu 2006; Zhu 2016). Six studies specifically described BoNT‐A injections into the hip adductors, in addition to the gastrocnemius muscle, in Ibrahim 2007, the hamstrings muscles, in Boyd 2001; Hazneci 2006; Jozwiak 2007; Mall 2006, or both the gastrocnemius and hamstrings muscles, in El‐Etribi 2004. Eight studies involved multilevel BoNT‐A injections with muscle groups individually determined for each child according to the study protocol (Çağlar 2019; Chaturvedi 2013; Copeland 2014; Moore 2008; Navarrete 2010; Reddihough 2002; Scholtes 2006; Steenbeek 2005).
Toxin type and dose
Nineteen studies used onabotulinumtoxinA (Barwood 2000; Bjornson 2007; Boyd 2001; Çağlar 2019; Copeland 2014; Corry 1998; El‐Etribi 2004; Flett 1999; Ibrahim 2007; Jozwiak 2007; Kay 2004; Koman 1994; Koman 2000; Love 2001; Navarrete 2010; Scholtes 2006; Sutherland 1999; Tedroff 2010; Zhu 2016). Seven studies used abobotulinumtoxinA (Baker 2002; Chaturvedi 2013; Delgado 2016; Kanovsky 2004; Mall 2006; Moore 2008; Ubhi 2000). In addition, in one study (Corry 1998), two children also received abobotulinumtoxinA. Only one study used the HengLi002‐type toxin (Xu 2006). Three studies did not describe the toxin type, but onabotulinumtoxinA was most likely used based on the publication year and dose (Ackman 2005; Hazneci 2006; Reddihough 2002).
For studies involving onabotulinumtoxinA, the maximum reported dose ranged from 4 units/kg body weight, Flett 1999; Sutherland 1999, to 30 units/kg body weight (Scholtes 2006), with the average dose per muscle varying from 2 to 8 units/kg. For studies involving abobotulinumtoxinA, the maximum reported dose ranged from 10 IU/kg body weight (one group in the Baker 2002 study and in the Delgado 2016 study) to 30 IU/kg body weight (another group in the Baker 2002 study and in Chaturvedi 2013; Kanovsky 2004; Mall 2006; Moore 2008). Two studies compared different BoNT‐A doses (Baker 2002; Delgado 2016).
Injection cycles
Six studies used multiple cycles of BoNT‐A injections (see Table 12) (Ackman 2005; Boyd 2001; Copeland 2014; Jozwiak 2007; Moore 2008; Tedroff 2010). The interval between serial injections ranged from three to six months, which represents the usual time for neuromuscular blockade reversal. Three initial studies, Koman 1994, Koman 2000, and Sutherland 1999, described two low‐dose BoNT‐A injections given two to four weeks apart. Since the interval was too short, we considered this as a single cycle of BoNT‐A. The remaining studies assessed the effectiveness of BoNT‐A after a single treatment session.
2. Study grouping: single versus multiple cycles.
BoNT‐A: botulinum toxin type A
Location method
In 15 studies, the location method for the injection sites was manual palpation (see Table 13) (Baker 2002; Barwood 2000; Boyd 2001; Corry 1998; El‐Etribi 2004; Flett 1999; Ibrahim 2007; Kanovsky 2004; Koman 1994; Koman 2000; Love 2001; Moore 2008; Reddihough 2002; Tedroff 2010; Ubhi 2000). Five studies used electrical stimulation to identify the desired motor points (Bjornson 2007; Copeland 2014; Navarrete 2010; Scholtes 2006; Xu 2006). Four studies described the use of ultrasound as the location method (Çağlar 2019; Copeland 2014; Delgado 2016; Zhu 2016). Seven studies did not describe the location method (Ackman 2005; Chaturvedi 2013; Hazneci 2006; Kay 2004; Mall 2006; Steenbeek 2005; Sutherland 1999).
3. Study grouping: location method.
Adjunctive treatment
Most studies described adjunctive treatment methods both for the intervention and control groups. One study, Boyd 2001, used a specific type of orthosis (SWASH ‐ Sitting Walking And Standing Hip orthosis) in the intervention group only in addition to the BoNT‐A injections. We decided to include this study in the review as we assumed that the potential impact of the orthoses on our primary outcome measures would be minimal. The Barwood 2000 study compared children who underwent hip‐adductor release surgery and had prior BoNT‐A or placebo injections to control pain. The remaining studies described similar adjunctive methods, both for the intervention and control groups, as clinically indicated (e.g. physiotherapy, orthoses, serial casting, and walking aids).
Comparisons
All of the included studies compared BoNT‐A injections into the lower limb muscles to other treatment methods. We grouped the studies into four main comparisons based on the control interventions, as described below.
Usual care, physiotherapy, or both
In 14 included studies the comparison group did not involve a placebo or sham intervention (Boyd 2001; Çağlar 2019; Chaturvedi 2013; El‐Etribi 2004; Ibrahim 2007; Jozwiak 2007; Love 2001; Navarrete 2010; Reddihough 2002; Scholtes 2006; Steenbeek 2005; Tedroff 2010; Xu 2006; Zhu 2016). Five studies compared BoNT‐A injections to a non‐standardised rehabilitation programme considered as usual care for patients with CP (e.g. use of orthoses, walking aids, and physiotherapy) (Boyd 2001; Love 2001; Reddihough 2002; Scholtes 2006; Steenbeek 2005). The remaining nine studies described a specific rehabilitation programme (Çağlar 2019; Chaturvedi 2013; El‐Etribi 2004; Ibrahim 2007; Jozwiak 2007; Navarrete 2010; Tedroff 2010; Xu 2006; Zhu 2016). For the purposes of this review, we grouped these studies in 'Comparison 1: BoNT‐A versus usual care or physiotherapy'.
Placebo and sham
Eleven studies described the use of placebo injections in the control group (Baker 2002; Barwood 2000; Bjornson 2007; Delgado 2016; Kanovsky 2004; Koman 1994; Koman 2000; Mall 2006; Moore 2008; Sutherland 1999; Ubhi 2000). One study, Copeland 2014, described a sham procedure performed with a blunt needle (not penetrating the skin). The injection sites were covered with iodine and dressings to ensure blinding. In the Barwood 2000 study, BoNT‐A was compared to placebo to control pain after hip‐adductor release surgery. For the purposes of this review, we grouped these studies in 'Comparison 2: BoNT‐A versus placebo'.
Serial casting
One study, Ackman 2005, compared isolated BoNT‐A injections to two control groups: (1) placebo + serial casting and (2) BoNT‐A + serial casting, to treat ankle equinus contracture. We decided to include only the isolated BoNT‐A and placebo + serial casting groups. We did this in order to comply with the methods in the original review (Ade‐Hall 2000), in which it was established that we would exclude studies where both intervention groups received BoNT‐A injections. Three studies used a serial casting protocol to treat ankle equinus only for the control group (Corry 1998; Flett 1999; Kay 2004). For the purposes of this review, we grouped these studies in 'Comparison 3: BoNT‐A versus serial casting'.
Orthoses
Only one study, Hazneci 2006, described the use of orthoses, specifically in the control group. Hazneci 2006 compared Johnstone pressure splints to BoNT‐A injections. We included this study in 'Comparison 4: BoNT‐A versus orthoses'. Several other studies described the use of orthoses both in the intervention and control groups, but only as an adjunctive method.
Primary outcomes
Gait analysis
Fourteen studies used gait parameters as outcome measures (Ackman 2005; Corry 1998; El‐Etribi 2004; Flett 1999; Ibrahim 2007; Kanovsky 2004; Kay 2004; Koman 1994; Koman 2000; Scholtes 2006; Sutherland 1999; Tedroff 2010; Ubhi 2000; Xu 2006).
Seven studies analysed objective gait data, including linear parameters (e.g. speed, step length) and kinematic data derived from instrumented gait analysis (Ackman 2005; Corry 1998; Ibrahim 2007; Kay 2004; Sutherland 1999; Tedroff 2010; Xu 2006). The Tedroff 2010 study used the Gillette Gait Index to quantify deviations from normal gait based on kinematic parameters (Schutte 2000).
Five studies performed observational gait analysis using a version of the Physician Rating Scale (PRS), originally described in the Koman 1994 study (Corry 1998; El‐Etribi 2004; Flett 1999; Koman 1994; Koman 2000).
The Scholtes 2006 study assessed gait using the Edinburgh Visual Gait Analysis Interval Testing (GAIT; Read 2003).
The Flett 1999 study assessed change in gait pattern after the interventions using a Global Scoring Scale for Gait (GSS). Children could present with a normal gait pattern post‐treatment (score = 0), significant or partial improvement (scores = 1 or 2), no change (score = 3), or worsening of the gait pattern (score = 4).
Both the Kanovsky 2004 and Ubhi 2000 studies used video gait analysis (VGA) to classify the child's gait pattern into five categories, according to foot initial contact.
Function
Most studies included a direct measure of function. Eighteen studies used the Gross Motor Function Measure (GMFM; Russell 1989): Baker 2002; Bjornson 2007; Boyd 2001; Chaturvedi 2013; Flett 1999; Hazneci 2006; Kanovsky 2004; Kay 2004; Love 2001; Mall 2006; Moore 2008; Navarrete 2010; Reddihough 2002; Scholtes 2006; Tedroff 2010; Ubhi 2000; Xu 2006; Zhu 2016.
Other outcome measures of function were: the Pediatric Evaluation of Disability Inventory (PEDI; Feldman 1990), reported by two studies (Moore 2008; Tedroff 2010); the Wee Functional Independence Measure (WeeFIM; Msall 1994), reported by one study (Navarrete 2010); the Physician's Global Assessment of the treatment response (PGA), reported by one study (Delgado 2016); and the Goal Attainment Scaling (GAS; Kiresuk 1968), reported by four studies (Bjornson 2007; Çağlar 2019; Delgado 2016; Steenbeek 2005).
In addition, two studies, Bjornson 2007 and Copeland 2014, described the performance domain of the Canadian Occupational Performance Measure (COPM; Law 1990), which we also considered as a measure of function for the purposes of this review.
Secondary outcomes
Range of motion
Nineteen studies described the joint range of motion around the target muscles as an outcome measure (Ackman 2005; Baker 2002; Bjornson 2007; Boyd 2001; El‐Etribi 2004; Flett 1999; Hazneci 2006; Kay 2004; Koman 2000; Love 2001; Mall 2006; Moore 2008; Reddihough 2002; Scholtes 2006; Sutherland 1999; Tedroff 2010; Ubhi 2000; Xu 2006; Zhu 2016). We considered the passive range of motion for all analyses in this review. For the ankle joint, we preferably considered passive dorsiflexion with the knee extended, which tightens the gastrocnemius muscle.
Quality of life
Only one study, Copeland 2014, assessed quality of life, using the Cerebral Palsy Quality of Life Questionnaire for Children (CPQOL‐Child; Waters 2007).
Satisfaction
Two studies used the satisfaction domain of the COPM, Law 1990, as a measure of satisfaction (Bjornson 2007; Copeland 2014).
Four studies reported a subjective assessment of caregiver perception on treatment benefit (Baker 2002; Kanovsky 2004; Koman 1994; Reddihough 2002).
One study, Copeland 2014, also used the Caregiver Priorities and Child Health Index of Life with Disabilities (CPCHILD), which evaluates the caregiver's perception of their child's health status (Narayanan 2006). We determined that this could be used as a measure of satisfaction postintervention.
One study, Love 2001, used a visual analogue scale to assess satisfaction.
Spasticity
Sixteen studies measured spasticity using the Modified Ashworth Scale (MAS; Bohannon 1987): Ackman 2005; Bjornson 2007; Boyd 2001; Çağlar 2019; Corry 1998; Delgado 2016; El‐Etribi 2004; Flett 1999; Hazneci 2006; Ibrahim 2007; Kay 2004; Love 2001; Mall 2006; Reddihough 2002; Tedroff 2010; Xu 2006). Six studies used the Modified Tardieu Scale (MTS; Boyd 1999): Ackman 2005; Çağlar 2019; Delgado 2016; Love 2001; Scholtes 2006; Zhu 2016.
One study, Baker 2002, used a computerised system to measure the dynamic component of gastrocnemius muscle length, which reflects spasticity.
One study, Bjornson 2007, used an electromechanical system to measure spasticity (spasticity measurement system).
Adverse events and complications
Only 20 studies evaluated adverse events (Ackman 2005; Baker 2002; Barwood 2000; Bjornson 2007; Boyd 2001; Çağlar 2019; Copeland 2014; Corry 1998; Delgado 2016; Flett 1999; Kanovsky 2004; Koman 1994; Koman 2000; Mall 2006; Moore 2008; Reddihough 2002; Sutherland 1999; Tedroff 2010; Ubhi 2000; Zhu 2016). We accounted for all adverse events, irrespective of severity, that were reported by the authors.
Other outcomes
Additional outcomes not contemplated in the methods of the original review were addressed in the included studies (Ade‐Hall 2000), such as: pain (Barwood 2000; Çağlar 2019; Copeland 2014); hip migration percentage (Boyd 2001; Jozwiak 2007); electromyography (Bjornson 2007; El‐Etribi 2004; Koman 2000; Scholtes 2006; Sutherland 1999; Zhu 2016); diffusion tensor imaging (Chaturvedi 2013); dynamometry (Koman 1994); selective motor control (Çağlar 2019); and energy cost (Bjornson 2007; Ubhi 2000). We did not consider these as outcomes of interest in this review and did not include them in our analyses.
Funding sources
A few trials received funding from the drug company, either in the form of direct funding (Baker 2002; Delgado 2016; Kanovsky 2004); unrestricted educational grants (Ackman 2005; Copeland 2014; Kay 2004; Koman 2000; Sutherland 1999; Tedroff 2010); or medication supply at no charge (Bjornson 2007; Tedroff 2010; Ubhi 2000). See Characteristics of included studies tables for further details.
Excluded studies
We excluded 59 full‐text reports; for details see Characteristics of excluded studies tables.
Most of the excluded studies (43 in total) used BoNT‐A injections for all participants (e.g. comparing different doses or injection methods) (Amirsalari 2011; Barber 2013; Bostock 2009; Bottos 2003; Carraro 2016; Chang 2017; Dai 2017; Dalvand 2012; Desloovere 2001; Detrembleur 2002; Dincer 2008; Flemban 2018; Hansen 2011; Hastings‐Ison 2016; Hong 2017; Hu 2009; Javadzadeh 2006; Jianjun 2013; Kang 2007; Kanovsky 2009; Kelly 2019; Kim 2011; Kurenkov 2017; Kwon 2010; Lee 2009; Lee 2011; Love 2009; Newman 2007; Niu 2014; Park 2010; Picelli 2017; Polak 2002; Sätilä 2005; Sätilä 2008; Thomas 2012; Van Campenhout 2013; Van Campenhout 2015; Wang 2007; Wang 2008; Williams 2013; Wissel 1999; Xu 2009; Zier 2008). None of these studies included a control group with an intervention that differed from BoNT‐A injections, which was an inclusion criterion for this review (Criteria for considering studies for this review).
Eleven excluded studies were not RCTs (Ackman 1998; Ayllón 2003; Hawamdeh 2007; Mohamed 2001; Richman 1996; Robertshaw 2005; Sätilä 2006; Thorley 2012; Wong 2005; YaJie 2008; Zonta 2013). Two studies described randomisation for only a subset of participants (Schasfoort 2017; Schasfoort 2018), and one study was considered to be a quasi‐RCT (Dimitrijević 2007). One study presented a subgroup analysis for a subset of participants from the Delgado 2016 study, including only those participants who had previous treatment with BoNT‐A (Dabrowski 2018). One study was published only in abstract form; it remains unclear whether it was an RCT, and it appears that no outcomes of interest for this review were assessed (Peeters 2018).
Studies awaiting classification
One study, Kim 2018, is awaiting classification. This study is an RCT that compared two different doses of BoNT‐A to placebo. It was published as an abstract, and the final publication was not available; however, we extracted preliminary data from the abstract and from ClinicalTrials.gov (NCT01603628). For details see Characteristics of studies awaiting classification table.
Ongoing studies
We identified five relevant ongoing studies. Two studies are comparing BoNT‐A to a control group receiving usual care (ACTRN12615001162505; TCTR20150803003); one is comparing BoNT‐A to orthopaedic surgery (CTRI/2015/03/005642); one is comparing BoNT‐A to shockwave therapy (NCT02400619); and one is comparing BoNT‐A to placebo (NCT02546999). For details see Characteristics of ongoing studies tables.
Risk of bias in included studies
The risk of bias of the included studies is discussed below. For further information see the 'Risk of bias’ tables in the Characteristics of included studies tables for each study. A graphical representation of the review authors' judgements about each risk of bias item as percentages and a summary of risk of bias detailing each item across all studies are shown in Figure 2 and Figure 3, respectively.
2.
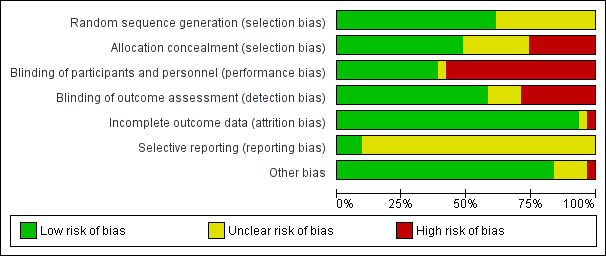
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
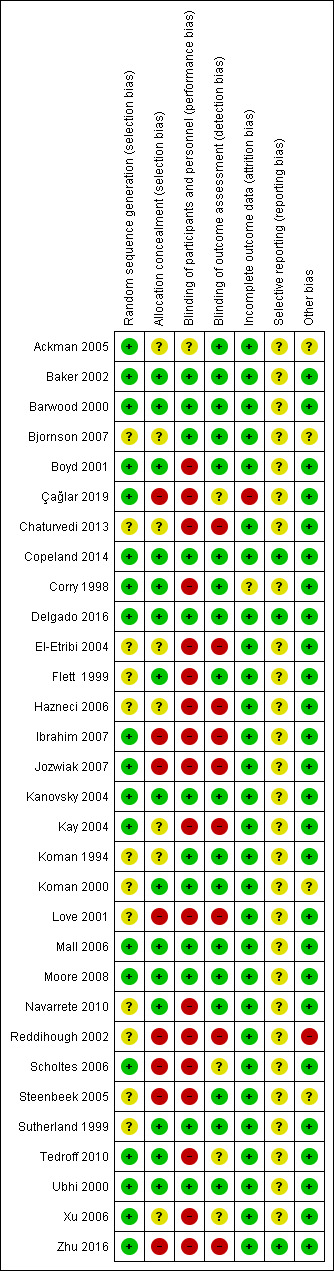
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
We assessed 19 studies as at low risk of bias as the randomisation methods were clearly described (Ackman 2005; Baker 2002; Barwood 2000; Boyd 2001; Çağlar 2019; Copeland 2014; Corry 1998; Delgado 2016; Ibrahim 2007; Jozwiak 2007; Kanovsky 2004; Kay 2004; Mall 2006; Moore 2008; Scholtes 2006; Tedroff 2010; Ubhi 2000; Xu 2006; Zhu 2016). A subset of these studies (n = 14) stratified randomisation by specific criteria, including motor distribution of CP, spasticity severity, age, GMFM score, and GMFCS level (Ackman 2005; Baker 2002; Bjornson 2007; Boyd 2001; Chaturvedi 2013; Copeland 2014; Delgado 2016; Love 2001; Moore 2008; Navarrete 2010; Reddihough 2002; Scholtes 2006; Tedroff 2010; Xu 2006).
The remaining 12 studies provided no detail on the randomisation method and were therefore rated as at unclear risk of bias (Bjornson 2007; Chaturvedi 2013; El‐Etribi 2004; Flett 1999; Hazneci 2006; Koman 1994; Koman 2000; Love 2001; Navarrete 2010; Reddihough 2002; Steenbeek 2005; Sutherland 1999).
Allocation concealment
We deemed the method of allocation as adequate in 15 studies (Baker 2002; Barwood 2000; Boyd 2001; Chaturvedi 2013; Copeland 2014; Corry 1998; Delgado 2016; Flett 1999; Kanovsky 2004; Koman 2000; Moore 2008; Navarrete 2010; Sutherland 1999; Tedroff 2010; Ubhi 2000). Eight studies were rated as at unclear risk of bias as allocation methods were not clearly described (Ackman 2005; Bjornson 2007; Chaturvedi 2013; El‐Etribi 2004; Hazneci 2006; Kay 2004; Koman 1994; Xu 2006). We judged the remaining eight studies to be at high risk of selection bias for this domain, as either there was no central allocation or no allocation concealment, or neither was present (Çağlar 2019; Ibrahim 2007; Jozwiak 2007; Love 2001; Reddihough 2002; Scholtes 2006; Steenbeek 2005; Zhu 2016).
Blinding
Most studies either described no blinding or partial blinding, due in many cases to the nature of the interventions (e.g. comparison between BoNT‐A injections and serial casting). Only 12 studies adequately described blinding of participants and personnel and blinding of outcome assessors (Baker 2002; Barwood 2000; Bjornson 2007; Copeland 2014; Delgado 2016; Kanovsky 2004; Koman 1994; Koman 2000; Mall 2006; Moore 2008; Sutherland 1999; Ubhi 2000).
Performance bias
Due to the nature of the interventions, only studies involving a placebo or a sham group described adequate blinding of participants and research personnel. We judged 12 studies that described blinding for all included participants to be at low risk of performance bias (Baker 2002; Barwood 2000; Bjornson 2007; Copeland 2014; Delgado 2016; Kanovsky 2004; Koman 1994; Koman 2000; Mall 2006; Moore 2008; Sutherland 1999; Ubhi 2000). We judged one study to be at unclear risk of bias (Ackman 2005). The authors of this study describe blinding of participants and personnel as if adequate, however the treatment protocol involved serial casting for two of the experimental groups, which makes full blinding less likely. Nevertheless, the study authors reported that participants in the isolated BoNT‐A group were instructed not to discuss their treatment with the research personnel. We rated the remaining 18 included studies as at high risk of performance bias (Boyd 2001; Çağlar 2019; Chaturvedi 2013; Corry 1998; El‐Etribi 2004; Flett 1999; Hazneci 2006; Ibrahim 2007; Jozwiak 2007; Kay 2004; Love 2001; Navarrete 2010; Reddihough 2002; Scholtes 2006; Steenbeek 2005; Tedroff 2010; Xu 2006; Zhu 2016).
Detection bias
Most of the included studies (n = 18) reported adequate blinding of outcome assessors and were judged to be at low risk of detection bias. Nine studies did not describe adequate blinding and were rated as at high risk of bias (Chaturvedi 2013; El‐Etribi 2004; Hazneci 2006; Ibrahim 2007; Jozwiak 2007; Kay 2004; Love 2001; Reddihough 2002; Zhu 2016). We judged four studies to be at unclear risk of bias (Çağlar 2019; Scholtes 2006; Tedroff 2010; Xu 2009). In two of these studies, only part of the outcome assessments (gait analysis) had adequate blinding (Scholtes 2006; Tedroff 2010), and although Xu 2009 reported blinding of outcome assessors, the means by which they ensured this was achieved was not described. Çağlar 2019 reported that the physical therapists involved in the rehabilitation protocol were blinded to the treatment, but provided no clear description of the blinding of outcome assessments.
Incomplete outcome data
Most studies did not present significant problems regarding loss to follow‐up: either it was non‐existent, minimal and/or balanced amongst the experimental groups. In addition, studies adequately assessed the primary and secondary outcomes at the prespecified time points (see Data synthesis) for most of the randomised participants.
We considered one study to be at unclear risk of attrition bias (Corry 1998), since one of the primary outcomes (gait analysis) was only available for eight of the 20 included participants due to age, co‐operation restraints, or both. We rated one study that did not present spasticity data for all muscle groups that were presumably treated as at high risk of bias (Çağlar 2019). The remaining outcome measures were adequately reported for all children, thus we considered all other studies (n = 29) to be at low risk of attrition bias.
Selective reporting
We judged one study that had a previously published protocol to be at low risk of reporting bias (Copeland 2014). Two other studies for which a protocol was available from international clinical trial registries were also considered to be at low risk of bias for this domain (Delgado 2016; Zhu 2016). The remaining studies (n = 28) apparently reported on relevant outcome measures that were consistent with their purpose; however, since we did not have access to their full protocol, we assessed these studies as at unclear risk of reporting bias.
Other potential sources of bias
The Ackman 2005 study reported that the initial power calculations determined the need for 25 children in each group. However, even though 90 children met the inclusion criteria, the study authors reported a refusal rate higher than 50%, and only 39 participants were included. A similar problem was reported by Bjornson 2007, which described an inclusion rate of 55% of all eligible patients. We considered both studies to be at unclear risk of bias, since we could not determine whether the low enrolment rate could have influenced the outcomes.
The Koman 2000 study reported that a total of 145 children were enrolled initially and included in the safety evaluation. The study authors excluded data from one centre (15 children) from the efficacy analysis because regulations in that country prohibited the use of placebo in children; consequently, data from this centre were not blinded. We rated this study as at unclear risk of bias for this domain.
The Reddihough 2002 had a cross‐over design. In one group, children were assigned to six months of BoNT‐A injections, followed by physical therapy. In the other group, children had six months of physical therapy, followed by BoNT‐A injections. It is possible that the order of the interventions may have played a role in the results, therefore we rated this study as at high risk of bias for this domain.
The Steenbeek 2005 study had a multiple baseline/treatment phase design. All children received the intervention with two different control and treatment phases. However, we could not extract data to perform direct comparisons between the control and treatment phases of the study. We considered this study to be at unclear risk of bias for this domain.
We identified no other sources of bias, thus all remaining studies (n = 26) were judged to be at low risk of other bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9; Table 10
Our findings below are divided into four main comparisons according to the control intervention, as described in the Included studies section.
We have described the number of assessments when study authors reported each lower limb as the unit of analysis. In all other cases we have reported the number of children.
Where possible, we have presented exact P values as calculated by us or as reported by study authors. Where the P value is less than 0.001, we have reported P < 0.001.
Comparison 1: BoNT‐A versus usual care or physiotherapy
We included 14 studies in this comparison, one of which had a cross‐over design (Reddihough 2002), and another that had a multiple baseline/treatment phase design (Steenbeek 2005).
Ten (out of 14) studies provided adequate numerical data for pooling (Boyd 2001; Çağlar 2019; Chaturvedi 2013; El‐Etribi 2004; Ibrahim 2007; Love 2001; Reddihough 2002; Tedroff 2010; Xu 2006; Zhu 2016). Of these 10 studies, one presented a portion of the data in graphs, however digital extraction was possible and we were able to include these data in the analyses (Tedroff 2010), and in one study it was only possible to extract numerical data for the GAS (Çağlar 2019). Regarding the studies where data could not be extracted, one presented their data as medians and interquartile intervals (Navarrete 2010); one presented the comparisons between groups, reporting only the significance level for function scores (GAS) and medians/interquartile intervals for the spasticity scale (Steenbeek 2005); and one presented their data as the mean difference (MD) between groups, considering the changes from baseline (Scholtes 2006). Note that one included study did not address any of the primary or secondary outcomes of interest for this review and is thus not presented in our results below (Jozwiak 2007).
1.1 Primary outcomes
1.1.1 Gait analysis
Five studies assessed gait parameters as an outcome measure (El‐Etribi 2004; Ibrahim 2007; Scholtes 2006; Tedroff 2010; Xu 2006). One study, El‐Etribi 2004, performed observational gait analysis using a modified version of the Physician Rating Scale (PRS), and another study, Scholtes 2006, using the Edinburgh Visual Gait Analysis Interval Testing (GAIT). Two studies reported on the linear parameters of gait, including speed, in Ibrahim 2007; Xu 2006, and step length, in Ibrahim 2007. In both studies, data were obtained by observational gait analysis. We elected to only use data from the 'BoNT‐A gastrocnemius group' in the Ibrahim 2007 study, to match the interventions performed in Xu 2006. Ibrahim 2007 reported gait speed as metres/minute, which we converted to the more common metres/second. One study, Tedroff 2010, performed instrumented gait analysis and reported the results of the Gillette Gait Index (GGI).
One study, Scholtes 2006, presented data only as MD between groups, considering changes from baseline values.
1.1.1.1 Pooled results
We were unable to combine studies in a meta‐analysis due to the different time points and outcome measures used.
1.1.1.2 Single‐study results
El‐Etribi 2004 (40 children) reported higher PRS scores in the BoNT‐A group at medium‐term follow‐up (MD 2.80, 95% confidence interval (CI) 1.55 to 4.05; see the illustrative forest plot in Analysis 1.1).
1.1. Analysis.
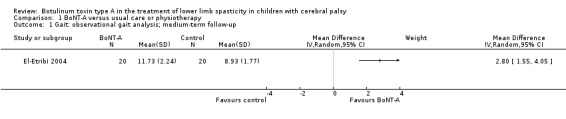
Comparison 1 BoNT‐A versus usual care or physiotherapy, Outcome 1 Gait: observational gait analysis; medium‐term follow‐up.
Scholtes 2006 (47 children) reported lower mean total gait scores in the BoNT‐A group at short‐term follow‐up (reported MD −1.74, 95% CI −2.76 to −0.72, P < 0.01; analysis not shown), which represents a better overall gait pattern in the intervention group. This difference was not sustained at long‐term follow‐up (reported MD −1.02, 95% CI −2.47 to 0.43, P = 0.17; analysis not shown).
Xu 2006 (43 children) reported no difference between the groups in gait speed (m/s) at short‐term follow‐up (MD 0.07 m/s, 95% CI −0.02 to 0.16; see the illustrative forest plot in Analysis 1.2), but found a higher mean gait speed in the BoNT‐A group at medium‐term follow‐up (MD 0.11 m/s, 95% CI 0.03 to 0.19; see the illustrative forest plot in Analysis 1.3). Ibrahim 2007 (30 children) reported no difference between groups in gait speed (MD 0.01 m/s, 95% CI −0.06 to 0.08; see the illustrative forest plot in Analysis 1.4) and step length (cm) (MD 1.00 cm, 95% CI −3.67 to 5.67; see the illustrative forest plot in Analysis 1.5) at long‐term follow‐up.
1.2. Analysis.
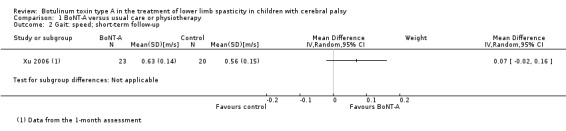
Comparison 1 BoNT‐A versus usual care or physiotherapy, Outcome 2 Gait: speed; short‐term follow‐up.
1.3. Analysis.
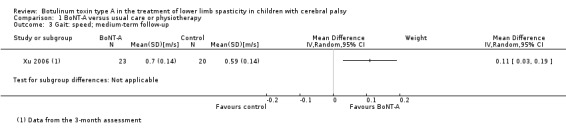
Comparison 1 BoNT‐A versus usual care or physiotherapy, Outcome 3 Gait: speed; medium‐term follow‐up.
1.4. Analysis.
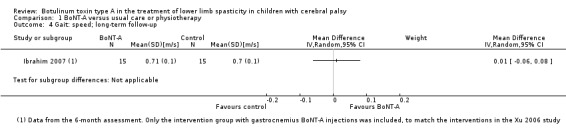
Comparison 1 BoNT‐A versus usual care or physiotherapy, Outcome 4 Gait: speed; long‐term follow‐up.
1.5. Analysis.
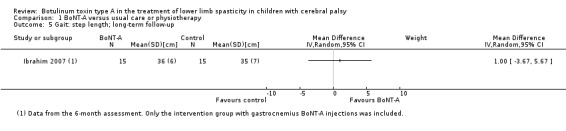
Comparison 1 BoNT‐A versus usual care or physiotherapy, Outcome 5 Gait: step length; long‐term follow‐up.
Tedroff 2010 (13 children) reported no difference between the groups on the GGI at long‐term follow‐up (MD −315.00, 95% CI −808.32 to 178.32; see the illustrative forest plot in Analysis 1.6).
1.6. Analysis.
Comparison 1 BoNT‐A versus usual care or physiotherapy, Outcome 6 Gait (GGI); long‐term follow‐up.
1.1.2 Function
Nine studies measured function (Boyd 2001; Chaturvedi 2013; Love 2001; Navarrete 2010; Reddihough 2002; Scholtes 2006; Steenbeek 2005; Xu 2006; Zhu 2016). Four studies assessed function using GMFM total scores (Chaturvedi 2013; Reddihough 2002; Scholtes 2006, Zhu 2016); two studies using GMFM goal scores (Love 2001; Xu 2006); one study using both GMFM goal and total scores (Boyd 2001); and two studies using GAS (Çağlar 2019; Steenbeek 2005). One study assessed function using both GMFM total scores and WeeFIM (Navarrete 2010).
Two studies presented data as MDs between groups for the changes from baseline values (Love 2001; Scholtes 2006). One study presented data as medians and interquartile intervals (Navarrete 2010), and one study did not present data for the comparisons between groups (Steenbeek 2005).
1.1.2.1 Pooled results
We were able to combine data from five studies in meta‐analyses (Çağlar 2019; Chaturvedi 2013; Reddihough 2002; Xu 2006; Zhu 2016). When both GMFM total scores and goal scores were available, we elected to use the GMFM goal scores for data pooling. We conducted the analyses using the standardised mean difference (SMD), since different scales were used.
The mean function scores were 0.59 SD higher in the BoNT‐A group at short‐term follow‐up (95% CI 0.23 to 0.95, P = 0.001; 2 studies, 123 children; I2 = 0%; Analysis 1.7). The mean function scores were 1.04 SD higher in the BoNT‐A group at medium‐term follow‐up (95% CI 0.16 to 1.91, P = 0.02; 4 studies, 191 children; I2 = 86%; Analysis 1.8). There was no difference in function scores between the groups at long‐term follow‐up (SMD 0.34, 95% CI −0.33 to 1.01, P = 0.32; 4 studies, 199 children; I2 = 81%; Analysis 1.9). We further explored the high heterogeneity found in the analyses at medium‐ and long‐term follow‐up below, under 'Sensitivity analysis'.
1.7. Analysis.
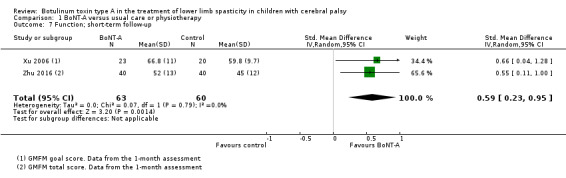
Comparison 1 BoNT‐A versus usual care or physiotherapy, Outcome 7 Function; short‐term follow‐up.
1.8. Analysis.
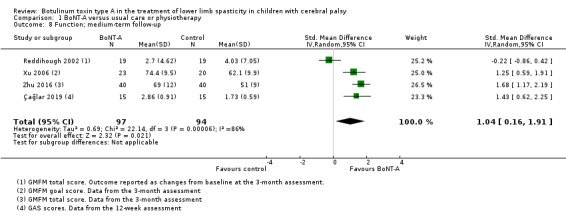
Comparison 1 BoNT‐A versus usual care or physiotherapy, Outcome 8 Function; medium‐term follow‐up.
1.9. Analysis.
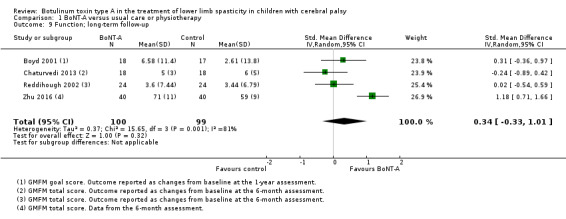
Comparison 1 BoNT‐A versus usual care or physiotherapy, Outcome 9 Function; long‐term follow‐up.
1.1.2.2 Single‐study results
Navarrete 2010 (24 children) reported no differences between groups in terms of function at short‐, medium‐ and long‐term follow‐up when assessed with the GMFM (reported P = 0.14, P = 0.12, P = 0.01, respectively; analyses not shown), and the WeeFIM (reported P = 0.32, P = 0.56, P = 0.44, respectively; analyses not shown).
Scholtes 2006 (47 children) reported no difference in GMFM scores at short‐term follow‐up (reported MD −0.77, 95% CI −3.07 to 1.52, P = 0.50; analysis not shown). However, their study found greater changes in GMFM scores in the BoNT‐A group at medium‐term follow‐up (reported MD 2.07, 95% CI 0.31 to 3.83, P = 0.02) and long‐term follow‐up (reported MD 3.48, 95% CI 1.34 to 5.61, P < 0.01); analyses not shown.
Love 2001 (24 children) reported greater changes in GMFM scores in the BoNT‐A group at medium‐term (reported MD 4.79, 95% CI 1.3 to 8.3, P < 0.05) and long‐term follow‐up (reported MD 4.96, 95% CI 2.3 to 7.6, P < 0.05); analyses not shown.
Steenbeek 2005 (11 children) reported significant improvements on the GAS for children in the BoNT‐A phase of the study (reported P = 0.01; analysis not shown).
1.2 Secondary outcomes
1.2.1 Range of motion
Range of motion of the ankle joint was evaluated in seven studies (El‐Etribi 2004; Love 2001; Reddihough 2002; Scholtes 2006; Tedroff 2010; Xu 2006; Zhu 2016), of the knee in three studies (Reddihough 2002; Scholtes 2006; Tedroff 2010), and of the hip in two studies (Reddihough 2002; Scholtes 2006). For the ankle joint, we considered the passive dorsiflexion with the knee extended. For the knee joint, we considered the popliteal angle.
Data from Tedroff 2010 were only available in graphs, however digital data extraction was possible. Love 2001, Reddihough 2002, and Tedroff 2010 presented this outcome as changes from baseline values. Scholtes 2006 reported only the MDs between the groups with their respective CI and significance levels. Reddihough 2002 presented data pertaining to the ankle range of motion, but not the hip and knee, which were also assessed in the study.
1.2.1.1 Pooled results
We were able to combine data from six studies in meta‐analyses (El‐Etribi 2004; Love 2001; Reddihough 2002; Tedroff 2010; Xu 2006; Zhu 2016).
The mean passive ankle dorsiflexion was 8.34 degrees higher in the BoNT‐A group at short‐term follow‐up (95% CI 1.19 to 15.50, P = 0.02; 2 studies, 186 assessments; I2 = 93%; Analysis 1.10); 6.36 degrees higher in the BoNT‐A group at medium‐term follow‐up (95% CI 4.03 to 8.69, P < 0.001; 5 studies, 272 assessments; I2 = 50%; Analysis 1.11); and 6.48 degrees higher in the BoNT‐A group at long‐term follow‐up (95% CI 4.42 to 8.53, P < 0.001; 4 studies, 250 assessments; I2 = 5%; Analysis 1.12). We further explored the high heterogeneity found in the analysis at short‐term follow‐up below, under 'Sensitivity analysis'.
1.10. Analysis.
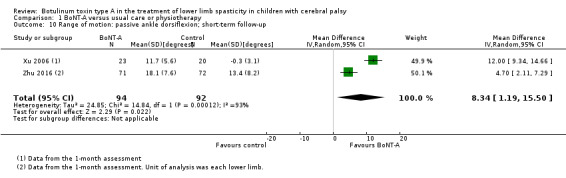
Comparison 1 BoNT‐A versus usual care or physiotherapy, Outcome 10 Range of motion: passive ankle dorsiflexion; short‐term follow‐up.
1.11. Analysis.
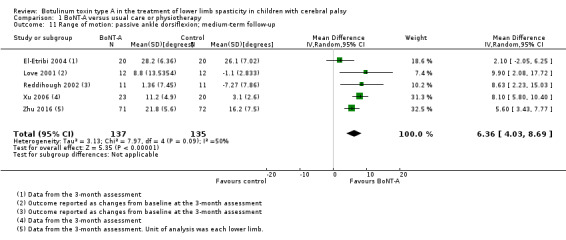
Comparison 1 BoNT‐A versus usual care or physiotherapy, Outcome 11 Range of motion: passive ankle dorsiflexion; medium‐term follow‐up.
1.12. Analysis.
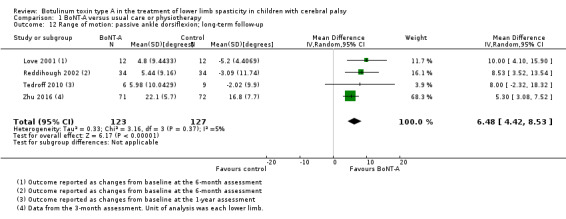
Comparison 1 BoNT‐A versus usual care or physiotherapy, Outcome 12 Range of motion: passive ankle dorsiflexion; long‐term follow‐up.
1.2.1.2 Single‐study results
Tedroff 2010 (15 children) reported that the mean decrease (improvement) in popliteal angle was higher in the BoNT‐A group at long‐term follow‐up (MD −18.54, 95% CI −30.31 to −6.77; see the illustrative forest plot in Analysis 1.13).
1.13. Analysis.
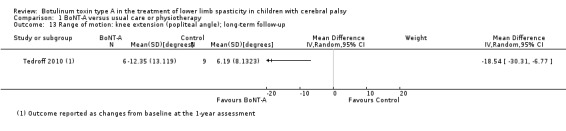
Comparison 1 BoNT‐A versus usual care or physiotherapy, Outcome 13 Range of motion: knee extension (popliteal angle); long‐term follow‐up.
Scholtes 2006 (47 children) reported a higher increase in ankle dorsiflexion in the BoNT‐A group at short‐term (reported MD 4.76, 95% CI 2.04 to 7.47, P < 0.01), medium‐term (reported MD 3.57, 95% CI 0.47 to 6.67, P = 0.02), and long‐term follow‐up (reported MD 4.66, 95% CI 0.57 to 8.75, P = 0.03); analyses not shown. In addition, the study authors reported a greater improvement in knee extension in the BoNT‐A group at short‐term (reported MD −8.87, 95% CI −12.87 to −4.88, P < 0.01), medium‐term (reported MD −9.68, 95% CI −14.24 to −5.12, P < 0.01), and long‐term follow‐up (reported MD −10.10, 95% CI 16.12 to 4.08, P < 0.01); analyses not shown. However, the study authors reported no differences in hip abduction between groups at short‐term (reported MD 3.10, 95% CI −0.05 to 6.25, P = 0.05), medium‐term (reported MD 1.34, 95% CI −2.25 to 4.94, P = 0.46), and long‐term follow‐up (reported MD 2.94, 95% CI −1.80 to 7.69, P = 0.22); analyses not shown.
Reddihough 2002 reported no differences in hip and knee range of motion between groups (significance level not available; analyses not shown).
1.2.2 Quality of life
No study in this comparison assessed quality of life.
1.2.3 Satisfaction with the outcome of treatment: single‐study results
Only one study (24 children) measured satisfaction with the outcome of treatment (Love 2001). The authors used a visual analogue scale (VAS) on which parents and caregivers rated their opinion regarding the intervention. The study provided raw data for each participant, which allowed us to calculate the mean scores and SD for each group. The mean satisfaction score was higher in the BoNT‐A group (MD 1.57, 95% CI 0.76 to 2.38; see the illustrative forest plot in Analysis 1.14).
1.14. Analysis.
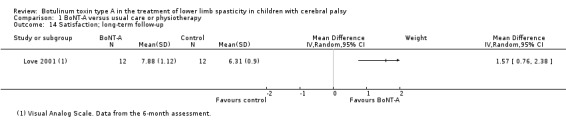
Comparison 1 BoNT‐A versus usual care or physiotherapy, Outcome 14 Satisfaction; long‐term follow‐up.
1.2.4 Spasticity
Nine studies measured spasticity (Çağlar 2019; El‐Etribi 2004; Ibrahim 2007; Love 2001; Reddihough 2002; Scholtes 2006; Tedroff 2010; Xu 2006; Zhu 2016). Six studies assessed spasticity using the Modified Ashworth Scale (MAS) in different muscle groups, including the ankle plantarflexors (El‐Etribi 2004; Ibrahim 2007; Love 2001; Reddihough 2002; Tedroff 2010; Xu 2006), hamstrings (Tedroff 2010), and hip adductors (Ibrahim 2007; Reddihough 2002). One study measured spasticity in the ankle plantarflexors using the Modified Tardieu Scale (MTS) (Zhu 2016). One study reported both MAS and MTS scores but did not describe the muscle groups to which these measures pertained (Çağlar 2019). One study measured the joint angle (ankle dorsiflexion, knee extension, and hip abduction) in which a catch occurred in response to a single fast passive stretch (Scholtes 2006), which is comparable to part of the MTS and was defined as such for the purposes of this review.
Two studies presented data as changes from baseline (Reddihough 2002; Tedroff 2010). One study, Reddihough 2002, provided only numerical data for hip adductors and ankle plantarflexors spasticity at long‐term follow‐up. Two studies reported only the MD between groups (Love 2001; Scholtes 2006). One study, Ibrahim 2007, combined the MAS scores of the ankle plantarflexors with the hip adductors, yielding a composite spasticity index score. To isolate the effect of BoNT‐A in each muscle group, we performed the analyses as follows: for the ankle plantarflexors spasticity outcome, we included the isolated gastrocnemius and control groups, and for the hip adductors spasticity outcome, we included the isolated hip adductors and control groups.
1.2.4.1 Pooled results
We were able to combine six studies in meta‐analyses (El‐Etribi 2004; Ibrahim 2007; Reddihough 2002; Tedroff 2010; Xu 2006; Zhu 2016).
There was no difference between groups in mean ankle plantarflexors spasticity scores at short‐term follow‐up (SMD −1.19, 95% CI −2.62 to 0.24, P = 0.10; 2 studies, 186 assessments; I2 = 92%; Analysis 1.15). The mean ankle plantarflexors spasticity scores were 1.66 SD lower in the BoNT‐A group at medium‐term follow‐up (95% CI −2.88 to −0.43, P = 0.008; 3 studies, 226 assessments; I2 = 91%; Analysis 1.16) and 0.79 SD lower in the BoNT‐A group at long‐term follow‐up (95% CI −1.04 to −0.53, P < 0.001; 4 studies, 258 assessments; I2 = 0%; Analysis 1.17). We further explored the high heterogeneity found in the analyses at short‐ and medium‐term follow‐up below, under 'Sensitivity analysis'.
1.15. Analysis.
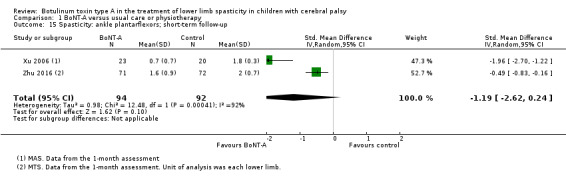
Comparison 1 BoNT‐A versus usual care or physiotherapy, Outcome 15 Spasticity: ankle plantarflexors; short‐term follow‐up.
1.16. Analysis.
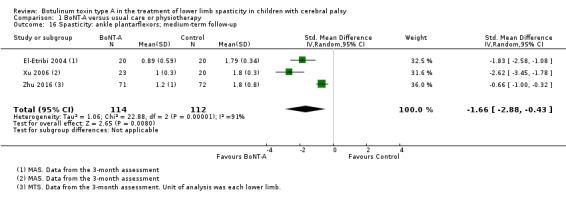
Comparison 1 BoNT‐A versus usual care or physiotherapy, Outcome 16 Spasticity: ankle plantarflexors; medium‐term follow‐up.
1.17. Analysis.
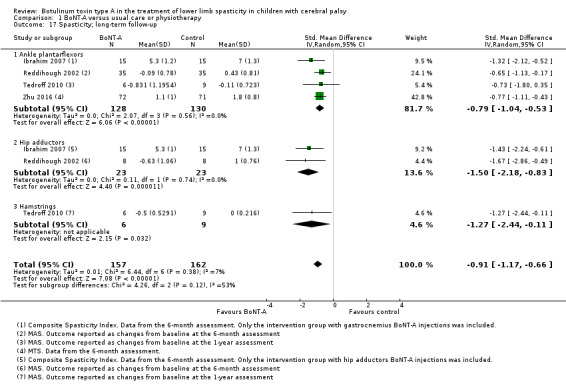
Comparison 1 BoNT‐A versus usual care or physiotherapy, Outcome 17 Spasticity; long‐term follow‐up.
The mean hip adductors spasticity score was 1.50 SD lower in the BoNT‐A group at long‐term follow‐up (95% CI −2.18 to −0.83, P < 0.001; 2 studies, 46 children; I2 = 0%; Analysis 1.17).
1.2.4.2 Single‐study results
Tedroff 2010 (15 children) reported lower hamstrings spasticity in the BoNT‐A group at long‐term follow‐up (SMD −1.27, 95% CI −2.44 to −0.11; see the illustrative forest plot in Analysis 1.17). However, when we calculated the MD, the mean hamstrings spasticity score was only −0.50 lower in the BoNT‐A group (MD −0.50, 95% CI −0.95 to −0.05; analysis not shown).
Love 2001 (24 children) reported lower ankle plantarflexors spasticity at medium‐term (reported MD −1.17, 95% CI −0.76 to −1.58, P < 0.05) and long‐term follow‐up (reported MD −0.54, 95% CI −0.26 to −0.82, P < 0.05); analyses not shown.
Scholtes 2006 (47 children) reported lower ankle plantarflexors spasticity in the BoNT‐A group (higher 'catch' joint angle) at short‐term (reported MD 5.69 degrees, 95% CI 1.40 to 9.98, P = 0.01) and medium‐term follow‐up (reported MD 10.03 degrees, 95% CI 5.12 to 14.93, P < 0.01), but not at long‐term follow‐up (reported MD 6.31 degrees, 95% CI −0.18 to 12.80, P = 0.06); analyses not shown. In addition, the study authors reported lower hamstrings spasticity in the BoNT‐A group at short‐term (reported MD −11.40 degrees, 95% CI −17.37 to −5.43, P < 0.01) and medium‐term follow‐up (reported MD −11.68 degrees, 95% CI 18.50 to 4.87, P < 0.01), but not at long‐term follow‐up (reported MD −5.70 degrees, 95% CI −14.70 to 3.30, P = 0.21); analyses not shown. There were no differences between groups in terms of hip adductor spasticity at short‐term (reported MD 2.62 degrees, 95% CI 1.37 to 6.62, P = 0.20), medium‐term (reported MD 2.92 degrees, 95% CI 1.63 to 7.48, P = 0.21), or long‐term follow‐up (reported MD 3.41, 95% CI 2.61 to 9.42, P = 0.27); analyses not shown.
Çağlar 2019 (30 children) reported lower MAS and MTS scores in the BoNT‐A group at 4 and 12 weeks follow‐up (reported P < 0.01). However, the muscle groups from which the measures were obtained were not described.
1.2.5 Adverse events
Only six studies in this comparison objectively described the occurrence or absence of adverse events (Boyd 2001; Çağlar 2019; Reddihough 2002; Tedroff 2010; Xu 2006; Zhu 2016). In these studies, the control group underwent a rehabilitation programme only, which included physical therapy and the use of orthotics. For this reason, the number of adverse events in the control group was null.
1.2.5.1 Pooled results
Since no events were reported in the control group, we performed a meta‐analysis of proportions of the rate of adverse events in the BoNT‐A group from the six studies (Boyd 2001; Çağlar 2019; Reddihough 2002; Tedroff 2010; Xu 2006; Zhu 2016).
The proportion of adverse events in the BoNT‐A group was a 0.37 (95% CI 0.08 to 0.66; I2 = 95%; Figure 4).
4.
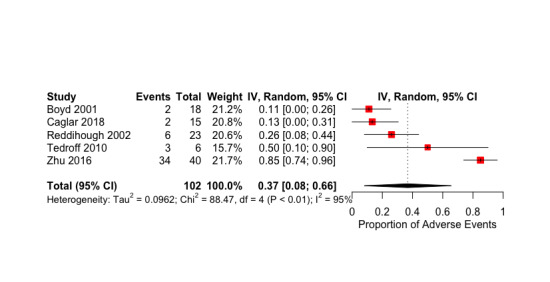
Meta‐analysis of proportions for the rate of adverse events in the BoNT‐A group from Comparison 1 (BoNT‐A versus usual care or physiotherapy).
1.2.5.2 Single‐study results
Çağlar 2019 reported skin rashes on the injection site in two children in the BoNT‐A group. The study authors did not detect any other adverse events.
The Boyd 2001a report from the Boyd 2001 study described two children in the BoNT‐A group with respiratory tract infection two weeks after treatment. One of these children also presented with pseudobulbar palsy and was withdrawn from the injections protocol. The Graham 2008 report from the Boyd 2001 study presented the total number of adverse events in all 204 BoNT‐A injections. Since the study authors did not provide the actual number of children experiencing at least one adverse event, and considering that all children received multiple injection cycles, we could not use their data in our meta‐analysis. The study authors reported 12 major adverse events that required additional treatment, consultations, or hospital admission: two deaths (considered to be unrelated to the injections); four children with respiratory tract infection; two with bronchospasm when recovering from general anaesthesia; three with transient urinary incontinence; and one with flu‐like illness. The study authors also reported 33 minor adverse events not requiring additional treatment or consultations: six children with mild fever; four with urticarial rash; 10 with upper respiratory tract symptoms; five with muscle weakness; three with pain and bruising; two with vomiting; two with diarrhoea; and one with both vomiting and diarrhoea.
In Reddihough 2002, the parents of six children reported adverse events in a questionnaire, given six months after the intervention. The most common adverse events were related to urinary loss, muscle weakness, and non‐specific complaints.
Tedroff 2010 reported adverse events in three children in the BoNT‐A group, which included muscle weakness, decreased sensation, and pain at the injection site.
Xu 2006 reported a few children with mild muscle fatigue, but the the exact number of adverse events in each group was not provided.
Zhu 2016 reported 25 cases of children crying after the injection (likely due to pain according to the study authors), 33 children with decreased muscle strength, and one child with a rash. As the total number of children experiencing at least one adverse event was not provided, we included only muscle weakness and rash in the meta‐analysis.
1.3 Sensitivity analyses
For all meta‐analyses, we verified the effect of excluding studies with several sources of bias, as well as the Reddihough 2002 study, which used a cross‐over design. We also explored the reasons for statistical heterogeneity when this was present, and the impact of using the SMD in some of the analyses. Only the three analyses considered relevant are detailed below.
1.3.1 Primary outcome: function
We found high statistical heterogeneity for this outcome at medium‐term (Tau2 = 0.69; Chi2 = 22.14, df = 3, P < 0.001; I2 = 86%) and long‐term follow‐up (Tau2 = 0.37; Chi2 = 15.65, df = 3, P = 0.001; I2 = 81%).
The exclusion of the Reddihough 2002 study (cross‐over design) from the medium‐term follow‐up analysis reduced statistical heterogeneity, and the mean function scores increased in the BoNT‐A group (SMD 1.50, 95% CI 1.14 to 1.86, P < 0.001; 3 studies, 153 children; I2 = 0%; Analysis 1.18).
1.18. Analysis.

Comparison 1 BoNT‐A versus usual care or physiotherapy, Outcome 18 Sensitivity analysis: function; medium‐term follow‐up.
In the long‐term follow‐up analysis, only the exclusion of the Zhu 2016 study reduced statistical heterogeneity, whilst maintaining no difference between groups in function scores (SMD 0.03, 95% CI −0.33 to 0.39, P = 0.88; 3 studies, 119 children; I2 = 0%; Analysis 1.19). This was the only study in this analysis in which function scores were higher in the BoNT‐A group. We evaluated the methodological aspects and specific details of the children and interventions in each study, and noted that the Zhu 2016 study used BoNT‐A exclusively for the ankle plantarflexors, whereas the other studies involved multilevel injections. We could not determine a clear reason for this difference.
1.19. Analysis.
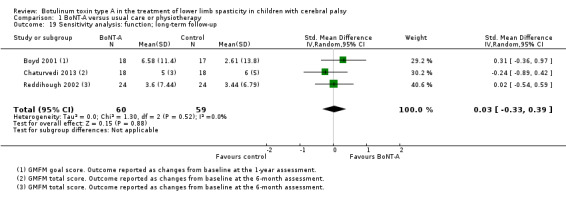
Comparison 1 BoNT‐A versus usual care or physiotherapy, Outcome 19 Sensitivity analysis: function; long‐term follow‐up.
1.3.2 Secondary outcomes
1.3.2.1 Range of motion
We found high statistical heterogeneity in a meta‐analysis of ankle dorsiflexion range of motion at short‐term follow‐up (Tau2 = 24.85; Chi2 = 14.84, df = 1, P < 0.001; I2 = 93%).
Both studies included in this meta‐analysis, Xu 2006 and Zhu 2016, individually reported higher ankle range of motion in the BoNT‐A group, although the effect magnitude was higher in the Xu 2006 study. Using a fixed‐effect model did not decrease statistical heterogeneity (analysis not shown). The Xu 2006 study included older children, who are more likely to present with decreased range of motion pre‐treatment. Furthermore, the brand of toxin differed between studies.
1.3.2.2 Spasticity
We found high statistical heterogeneity for the meta‐analysis of ankle plantarflexors spasticity at short‐term (Tau2 = 0.98; Chi2 = 12.48, df = 1, P < 0.001; I2 = 92%) and medium‐term follow‐up (Tau2 = 1.06; Chi2 = 22.88, df = 2, P < 0.001; I2 = 91%).
Both studies in the meta‐analysis at short‐term follow‐up individually reported lower spasticity scores in the BoNT‐A group (Xu 2006; Zhu 2016). When using a fixed‐effect model instead of a random‐effects model, the difference between groups became significant (SMD −0.74, 95% CI −1.04 to −0.44, P < 0.001; 2 studies, 186 children; I2 = 92%; Analysis 1.20); however, statistical heterogeneity remained high. This could be related to the different spasticity scales used in each study (MAS in Xu 2006 and MTS in Zhu 2016).
1.20. Analysis.
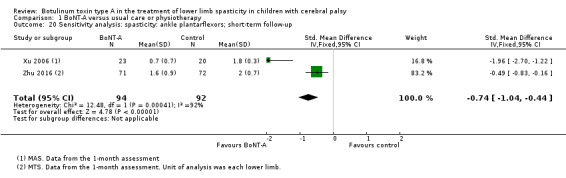
Comparison 1 BoNT‐A versus usual care or physiotherapy, Outcome 20 Sensitivity analysis: spasticity: ankle plantarflexors; short‐term follow‐up.
All three studies in the meta‐analysis at medium‐term follow‐up individually reported lower spasticity scores in the BoNT‐A group (El‐Etribi 2004; Xu 2006; Zhu 2016). By removing the Zhu 2016 study, which used a different spasticity scale (MTS), statistical heterogeneity decreased, and the effect estimate remained in favour of the BoNT‐A group (MD −0.83, 95% CI −0.98 to −0.67, P < 0.001; 2 studies, 83 children; I2 = 0%; Analysis 1.21).
1.21. Analysis.
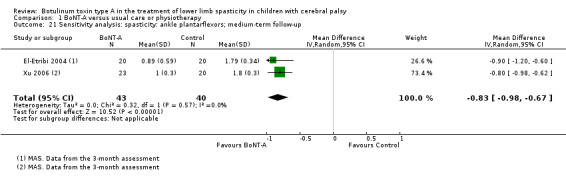
Comparison 1 BoNT‐A versus usual care or physiotherapy, Outcome 21 Sensitivity analysis: spasticity: ankle plantarflexors; medium‐term follow‐up.
1.3.2.3 Adverse events
We found high statistical heterogeneity for the meta‐analysis of proportions of the rate of adverse events in the BoNT‐A group, notably because the Zhu 2016 study reported a higher rate of adverse events than the other studies included in this comparison. The authors of this study reported on 33 children presenting with transient muscle weakness, considered by the study authors to be a mild adverse event. Having analysed the specific details of this study, including the type of BoNT‐A used as well as dose and muscles injected, we could not find a clear reason for this rate of adverse event. By removing the Zhu 2016 study, heterogeneity decreased to 8.01%, and the rate of adverse events in the BoNT‐A group was 0.18 (95% CI 0.08 to 0.28; analysis not shown).
1.4 Quality of the evidence
See Table 1; Table 2; Table 3.
1.4.1 Primary outcomes
1.4.1.1 Gait analysis
There is very low‐quality evidence that BoNT‐A improves overall gait pattern (observational gait analysis) at medium‐term follow‐up when compared to usual care or physical therapy.
1.4.1.2 Function
There is very low‐quality evidence that BoNT‐A improves function at short‐ and medium‐term follow‐up, but not at long‐term follow‐up, when compared to usual care or physical therapy.
1.4.2 Secondary outcomes
1.4.2.1 Range of motion
There is very low‐quality evidence that BoNT‐A improves ankle range of motion at short‐, medium‐, and long‐term follow‐up, when compared to usual care or physical therapy.
1.4.2.2 Satisfaction with the outcome of treatment
There is very low‐quality evidence that BoNT‐A is associated with higher satisfaction with the outcome of treatment at long‐term follow‐up, when compared to usual care or physical therapy.
1.4.2.3 Spasticity
There is very low‐quality evidence that BoNT‐A improves ankle plantarflexors spasticity at medium‐ and long‐term follow‐up, but not at short‐term follow‐up, when compared to usual care or physical therapy.
1.4.2.4 Adverse events
There is very low‐quality evidence that there is a higher risk of adverse events with BoNT‐A than with usual care or physical therapy.
Comparison 2: BoNT‐A versus placebo or sham
We included 12 studies in this comparison (Baker 2002; Barwood 2000; Bjornson 2007; Copeland 2014; Delgado 2016; Kanovsky 2004; Koman 1994; Koman 2000; Mall 2006; Moore 2008; Sutherland 1999; Ubhi 2000).
Two studies presented some of their results in graphs (Koman 2000; Sutherland 1999), however we were able to digitally extract their data. One study, Bjornson 2007, presented most of their data in graphs, but we were unable to extract means and SD for the relevant outcome measures. In that study, numerical data were mostly presented as medians with no measure of dispersion and therefore the data could not be pooled; only the COPM scores were reported as means and SD, and the number of adverse events could be extracted and included in the meta‐analyses. One study, Mall 2006, presented some of their data in graphs, but digital data extraction was not possible. The non‐significant findings were only mentioned in the text, with no P value or CI reported, and only the number of adverse events could be extracted from this study. The Barwood 2000 study did not address any of the outcomes of interest for this review except for adverse events.
2.1 Primary outcomes
2.1.1 Gait analysis
Five studies assessed gait parameters as an outcome measure (Kanovsky 2004; Koman 1994; Koman 2000; Sutherland 1999; Ubhi 2000). Two studies performed observational gait analysis using the PRS (Koman 1994; Koman 2000), and two studies used video gait analysis (VGA) (Kanovsky 2004; Ubhi 2000). One study, Sutherland 1999, reported on the linear parameters of gait and also presented ankle kinematics data derived from instrumented gait analysis.
Four studies presented this outcome as dichotomous data, with positive events representing the number of children who presented with an improvement greater than two points on the PRS in two studies (Koman 1994; Koman 2000), and positive events representing the number of children who presented improved initial foot contact after the intervention in two studies (Kanovsky 2004; Ubhi 2000). Of note, Kanovsky 2004 presented the rate of improvement by lower limb (n = total number of lower limbs treated). One study, Sutherland 1999, presented ankle kinematics data in graphs as changes from baseline values. We performed digital data extraction and calculated the means and SDs for both groups. Regarding the linear parameters of gait, the study authors only presented the significance level for their analyses.
2.1.1.1 Pooled results
We were able to combine data from four studies in meta‐analyses (Kanovsky 2004; Koman 1994; Koman 2000; Ubhi 2000).
The estimated risk ratio (RR) for gait improvement in the BoNT‐A group was 1.66 (95% CI 1.16 to 2.37, P = 0.006; 4 studies, 261 assessments; I2 = 0%; Analysis 2.1) at short‐term follow‐up and 1.90 (95% CI 1.32 to 2.74, P < 0.001; 3 studies, 248 assessments; I2 = 0%; Analysis 2.2) at medium‐term follow‐up. In both cases, the results represented a greater rate of improvement in the BoNT‐A group. No study reported data on this outcome at long‐term follow‐up.
2.1. Analysis.
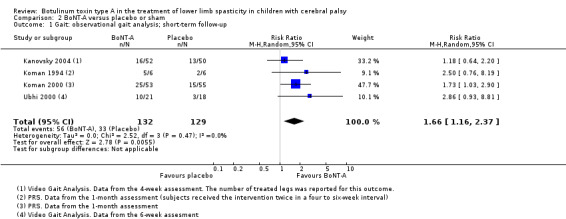
Comparison 2 BoNT‐A versus placebo or sham, Outcome 1 Gait: observational gait analysis; short‐term follow‐up.
2.2. Analysis.
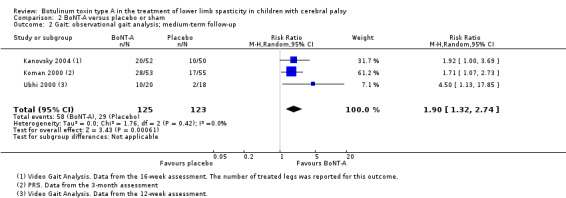
Comparison 2 BoNT‐A versus placebo or sham, Outcome 2 Gait: observational gait analysis; medium‐term follow‐up.
2.1.1.2 Single‐study results
Sutherland 1999 (19 children) reported a higher mean peak ankle dorsiflexion in stance (MD 15.90 degrees, 95% CI 4.87 to 26.93, P = 0.005) and a higher mean peak ankle dorsiflexion in swing (MD 10.20 degrees, 95% CI 4.01 to 16.39, P = 0.001) in the BoNT‐A group at short‐term follow‐up. See the illustrative forest plot in Analysis 2.3.
2.3. Analysis.
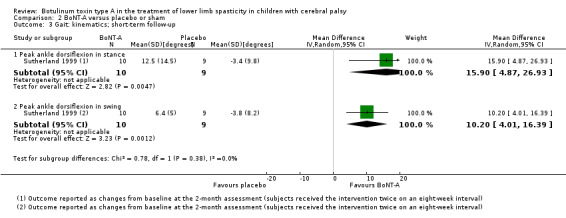
Comparison 2 BoNT‐A versus placebo or sham, Outcome 3 Gait: kinematics; short‐term follow‐up.
Sutherland 1999 (19 children) reported no differences between groups in step length (reported P = 0.33), stride length (reported P = 0.37), cadence (reported P = 0.61), and speed (reported P = 0.40); analyses not shown.
2.1.2 Function
Eight studies measured function (Baker 2002; Bjornson 2007; Copeland 2014; Delgado 2016; Kanovsky 2004; Mall 2006; Moore 2008; Ubhi 2000). Four studies assessed function with GMFM total scores (Baker 2002; Bjornson 2007; Kanovsky 2004; Moore 2008), three studies with GAS (Bjornson 2007; Delgado 2016; Mall 2006), two studies with COPM performance scores (Bjornson 2007; Copeland 2014), two studies with GMFM goal scores (Kanovsky 2004; Ubhi 2000), one study with the PEDI mobility subscale (Moore 2008), and one study with the PGA (Delgado 2016).
One study presented the GMFM walking dimension as a dichotomous outcome measure (Ubhi 2000), where positive events were represented by the number of children with an improvement higher than 6%. One study presented both final GMFM scores and changes from baseline values (Kanovsky 2004). For the purposes of this review, we used changes from baseline values for two reasons: (1) the study authors performed all of their analyses in the same way; and (2) the baseline GMFM values differed between groups, with higher scores in the BoNT‐A group. Four other studies also presented data as changes from baseline values (Baker 2002; Bjornson 2007; Delgado 2016; Moore 2008). One study did not present GMFM scores in adequate numerical form for data pooling (Bjornson 2007), and one study presented their data only in graphs, and digital extraction was not possible (Mall 2006).
2.1.2.1 Pooled results
We were able to combine data from six studies in meta‐analyses (Baker 2002; Bjornson 2007; Copeland 2014; Delgado 2016; Kanovsky 2004; Moore 2008). When the same study reported on two different functional outcome measures, we elected only one for inclusion in our meta‐analyses. When both GMFM total and goal scores were reported, we elected to use GMFM goal scores only.
There were no differences in overall mean function scores at short‐term (SMD 0.24, 95% CI −0.35 to 0.83, P = 0.42; 4 studies, 305 children; I2 = 82%; Analysis 2.4) or long‐term follow‐up (SMD −0.07, 95% CI −0.48 to 0.35, P = 0.76; 2 studies, 91 children; I2 = 0%; Analysis 2.6), but the mean function scores were higher in the BoNT‐A group at medium‐term follow‐up (SMD 0.28, 95% CI 0.06 to 0.49, P = 0.01; 5 studies, 327 children; I2 = 0%; Analysis 2.5).
2.4. Analysis.
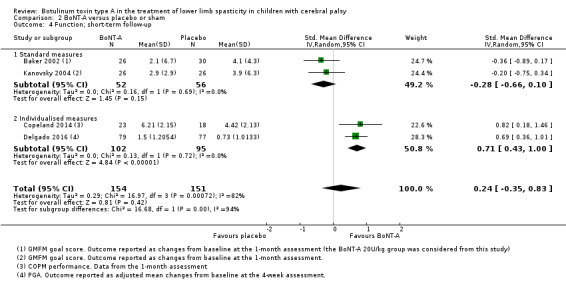
Comparison 2 BoNT‐A versus placebo or sham, Outcome 4 Function; short‐term follow‐up.
2.6. Analysis.
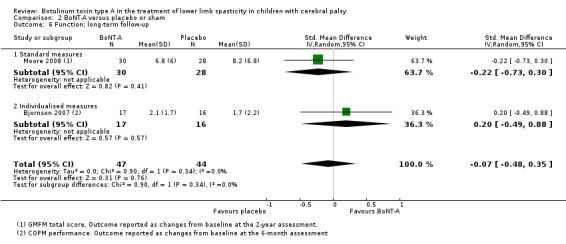
Comparison 2 BoNT‐A versus placebo or sham, Outcome 6 Function; long‐term follow‐up.
2.5. Analysis.
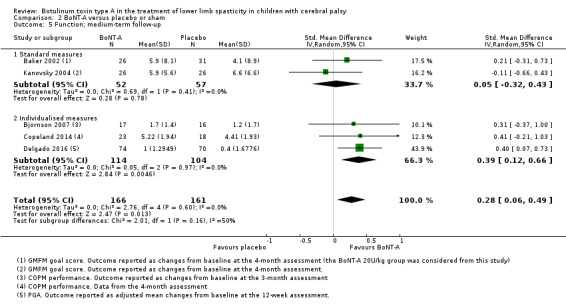
Comparison 2 BoNT‐A versus placebo or sham, Outcome 5 Function; medium‐term follow‐up.
The high heterogeneity identified for the analysis at short‐term follow‐up appeared to be related to the different types of outcome measures used (e.g. standard motor scales and individualised measures). We divided our analyses into subgroups according to the type of outcome measure used: standard measures (e.g. GMFM; PEDI) and individualised measures (e.g. COPM; GAS; PGA). Considering standard measures, there were no differences in function scores at short‐term (SMD −0.28, 95% CI −0.66 to 0.10, P = 0.15; 2 studies, 108 children; I2 = 0%; Analysis 2.4), medium‐term (SMD 0.05, 95% CI −0.32 to 0.43, P = 0.78; 2 studies, 109 children; I2 = 0%; Analysis 2.5), or long‐term follow‐up (SMD −0.22, 95% CI −0.73 to 0.30, P = 0.41; 1 study, 58 children; Analysis 2.6). Considering individualised measures, the mean function scores were higher in the BoNT‐A group at short‐term (SMD 0.71, 95% CI 0.43 to 1.00, P < 0.001; 2 studies, 197 children; I2 = 0%;Analysis 2.4) and medium‐term follow‐up (SMD 0.39, 95% CI 0.12 to 0.66, P = 0.005; 3 studies, 218 children; I2 = 0%; Analysis 2.5). There were no differences between groups at long‐term follow‐up (SMD 0.20, 95% CI −0.49 to 0.88, P = 0.57; 1 study, 33 children; Analysis 2.6).
2.1.2.2 Single‐study results
Ubhi 2000 (34 children) reported that the estimated RR for GMFM improvement after BoNT‐A injections at medium‐term follow‐up was 5.53 (95% CI 0.76 to 40.14; see the illustrative forest plot in Analysis 2.7). The number of children with a significant improvement in GMFM was higher in the BoNT‐A group, but this was not considered statistically significant since the CI crossed the no‐effect line.
2.7. Analysis.
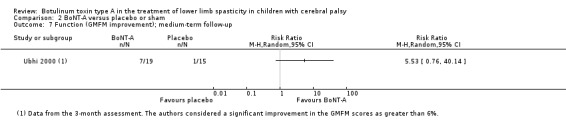
Comparison 2 BoNT‐A versus placebo or sham, Outcome 7 Function (GMFM improvement); medium‐term follow‐up.
Bjornson 2007 (33 children) reported no differences in GMFM scores between groups at short‐term (reported P = 0.07) and medium‐term follow‐up (reported P = 0.70); analyses not shown. The GMFM scores were higher in the BoNT‐A group at long‐term follow‐up (reported P < 0.01; analysis not shown). The same study reported no difference in GAS scores between groups at medium‐term (reported P = 0.92) and long‐term follow‐up (reported P = 0.80); analyses not shown.
Delgado 2016 (156 children) reported higher GAS scores in the BoNT‐A group at medium‐term follow‐up (MD 4.65, 95% CI 1.59 to 7.71, reported as P < 0.01 in the report; analysis not shown).
Mall 2006 (61 children) reported no difference between groups in GMFM scores at short‐term follow‐up, but the level of statistical significance was not provided. The same study reported higher GAS scores in the BoNT‐A group at short‐term follow‐up (reported P = 0.04; analysis not shown).
Moore 2008 (58 children) reported no difference between groups in scores on the PEDI mobility subscale at long‐term follow‐up (MD −0.70, 95% CI −3.45 to 2.05, P = 0.62; analysis not shown).
2.2 Secondary outcomes
2.2.1 Range of motion
Range of motion was measured of the ankle joint in six studies (Baker 2002; Bjornson 2007; Delgado 2016; Moore 2008; Sutherland 1999; Ubhi 2000), of the knee joint in one study (Moore 2008), and of the hip joint in one study (Moore 2008). For ankle range of motion, we considered passive dorsiflexion with the knee extended, when described. In one study, Delgado 2016, we extracted ankle range of motion data from the angle of arrest measure (V1) of the Tardieu Scale.
Five studies reported on this outcome using changes from baseline values (Baker 2002; Bjornson 2007; Delgado 2016; Moore 2008; Ubhi 2000). One of these studies, Baker 2002, also provided the absolute range of motion measures at different follow‐up times, however we decided to use the reported changes from baseline values in order to maintain consistency with the other studies in this meta‐analysis. One study, Baker 2002, reported their results considering each side as the unit of analysis (n = total number of assessed lower limbs); one study, Bjornson 2007, presented range of motion data as medians with no measures of dispersion; and one study, Sutherland 1999, presented only the changes from baseline values and used the range as the measure of dispersion.
2.2.1.1 Pooled results
We were able to combine data from four studies in meta‐analyses (Baker 2002; Delgado 2016; Moore 2008; Ubhi 2000).
The mean passive ankle dorsiflexion was 2.68 degrees higher in the BoNT‐A group at short‐term follow‐up (95% CI 0.12 to 5.23, P = 0.04; 3 studies, 291 children; I2 = 0%; Analysis 2.8). There was no difference in passive ankle dorsiflexion between groups at medium‐term follow‐up (MD 1.57 degrees, 95% CI −2.12 to 5.25, P = 0.40; 2 studies, 150 children; I2 = 0%; Analysis 2.9).
2.8. Analysis.
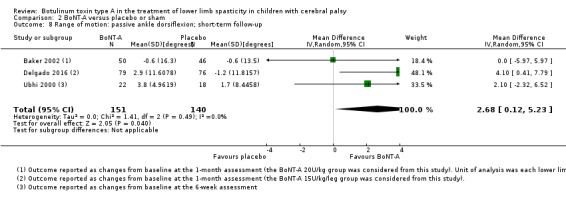
Comparison 2 BoNT‐A versus placebo or sham, Outcome 8 Range of motion: passive ankle dorsiflexion; short‐term follow‐up.
2.9. Analysis.
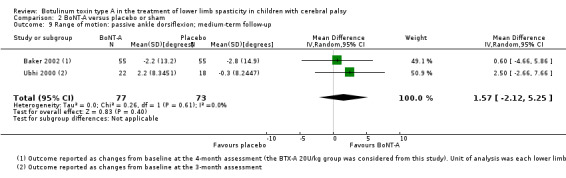
Comparison 2 BoNT‐A versus placebo or sham, Outcome 9 Range of motion: passive ankle dorsiflexion; medium‐term follow‐up.
2.2.1.2 Single‐study results
Moore 2008 reported no differences between groups at long‐term follow‐up with regard to ankle dorsiflexion (MD 0.20, 95% CI −4.88 to 5.28, P = 0.94; 40 children); knee extension (MD −5.60, 95% CI −13.49 to 2.29, P = 0.16; 19 children); or hip abduction (MD 9.60, 95% CI −9.97 to 29.17, P = 0.34; 11 children). See the illustrative forest plot in Analysis 2.10.
2.10. Analysis.
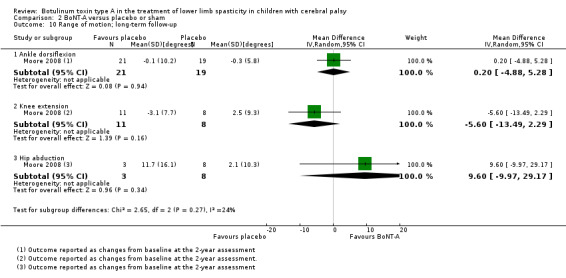
Comparison 2 BoNT‐A versus placebo or sham, Outcome 10 Range of motion; long‐term follow‐up.
Bjornson 2007 (33 children) reported a higher improvement in ankle range of motion in the BoNT‐A group at medium‐term follow‐up (reported MD 4.2 degrees, P < 0.01; analysis not shown). The authors found no differences between groups in ankle range of motion at short‐term (reported P = 0.76) or long‐term follow‐up (reported P = 0.71); analyses not shown.
Sutherland 1999 (20 children) reported that the BoNT‐A group had an average decrease of 0.56 degrees in ankle dorsiflexion at short‐term follow‐up (range: −10 to 8), whilst the placebo group had an average decrease of 6.3 degrees (range: −20 to 6.3).
2.2.2 Quality of life: single‐study results
Only one study with 41 children measured quality of life, using the CPQOL (Copeland 2014); consequently no meta‐analysis was possible. Ths study authors reported no difference between groups in CPQOL scores at short‐term (MD −1.33, 95% CI −6.77 to 4.11; Analysis 2.11) or medium‐term follow‐up (MD −3.00, 95% CI −8.04 to 2.04; Analysis 2.12).
2.11. Analysis.
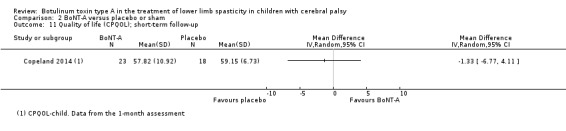
Comparison 2 BoNT‐A versus placebo or sham, Outcome 11 Quality of life (CPQOL); short‐term follow‐up.
2.12. Analysis.
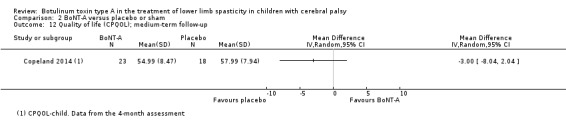
Comparison 2 BoNT‐A versus placebo or sham, Outcome 12 Quality of life (CPQOL); medium‐term follow‐up.
2.2.3 Satisfaction with the outcome of treatment
Two studies measured satisfaction with the outcome of treatment with COPM satisfaction scores (Bjornson 2007; Copeland 2014), three studies with a subjective questionnaire (Baker 2002; Kanovsky 2004; Koman 1994), and one study with the CPCHILD score (Copeland 2014).
We analysed data from the studies using subjective questionnaires dichotomously, with positive events represented by the number of children for whom caregivers reported significant improvement postintervention. In Baker 2002, caregivers completed a subjective assessment 16 weeks after treatment. In Kanovsky 2004, caregivers evaluated treatment outcomes after four, eight, and 16 weeks, and rated the response as good, minimal, or absent; we considered good responses as a positive event. The Koman 1994 study performed a subjective assessment after four to six weeks regarding improvement of children's gait pattern.
2.2.3.1 Pooled results
We were able to combine data from five studies in meta‐analyses (Baker 2002; Bjornson 2007; Copeland 2014; Kanovsky 2004; Koman 1994). When a study reported on two or more measures of satisfaction with the outcome of treatment, we elected to use only one in our pooled analyses.
There were no differences between groups in the subjective satisfaction scores at short‐term (RR 1.20, 95% CI 0.72 to 2.01, P = 0.49; 2 studies, 64 children; I2 = 0%; Analysis 2.13) or medium‐term follow‐up (RR 1.32, 95% CI 0.94 to 1.83, P = 0.11; 2 studies, 111 children; I2 = 0%; Analysis 2.14).
2.13. Analysis.
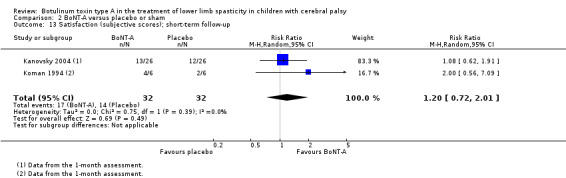
Comparison 2 BoNT‐A versus placebo or sham, Outcome 13 Satisfaction (subjective scores); short‐term follow‐up.
2.14. Analysis.
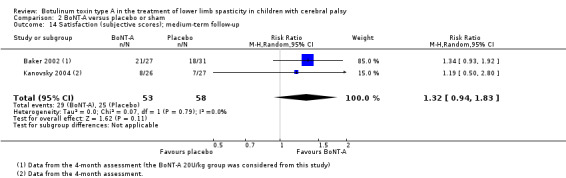
Comparison 2 BoNT‐A versus placebo or sham, Outcome 14 Satisfaction (subjective scores); medium‐term follow‐up.
The mean COPM satisfaction scores were 0.96 points higher in the BoNT‐A group at medium‐term follow‐up (95% CI 0.04 to 1.88, P = 0.04; 2 studies, 74 children; I2 = 0%; Analysis 2.16).
2.16. Analysis.
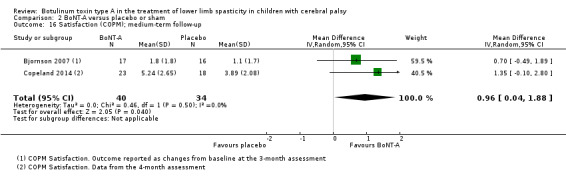
Comparison 2 BoNT‐A versus placebo or sham, Outcome 16 Satisfaction (COPM); medium‐term follow‐up.
2.2.3.2 Single‐study results
Copeland 2014 (41 children) reported that the mean COPM satisfaction scores were 1.81 points higher in the BoNT‐A group at short‐term follow‐up (95% CI 0.25 to 3.37; see the illustrative forest plot in Analysis 2.15).
2.15. Analysis.
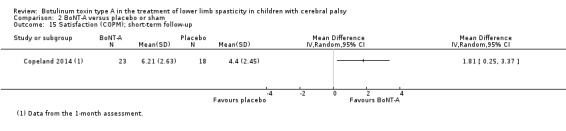
Comparison 2 BoNT‐A versus placebo or sham, Outcome 15 Satisfaction (COPM); short‐term follow‐up.
Bjornson 2007 (33 children) reported no difference between groups at long‐term follow‐up (MD 0.10, 95% CI −1.27 to 1.47; see the illustrative forest plot in Analysis 2.17).
2.17. Analysis.
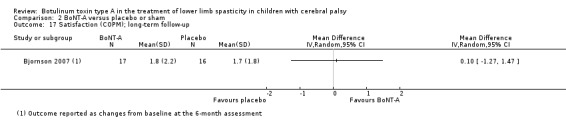
Comparison 2 BoNT‐A versus placebo or sham, Outcome 17 Satisfaction (COPM); long‐term follow‐up.
Copeland 2014 (41 children) reported no difference between groups in CPCHILD scores at short‐term (MD 1.10, 95% CI −5.67 to 7.87) or medium‐term follow‐up (MD 4.22, 95% CI −2.15 to 10.59, P = 0.19); analyses not shown.
2.2.4 Spasticity
Four studies measured spasticity using the MAS (Bjornson 2007; Delgado 2016; Mall 2006; Moore 2008), one study using the MTS (Delgado 2016), and one study using an electromechanical method (spasticity measurement system (SMS)) (Bjornson 2007). Three studies evaluated the ankle plantarflexors (Bjornson 2007; Delgado 2016; Moore 2008); one study the hamstrings (Moore 2008); and two studies the hip adductors (Mall 2006; Moore 2008).
One study only presented data as medians (Bjornson 2007), and one study presented data in graphs only as medians and interquartile intervals (Mall 2006).
2.2.4.1 Pooled results
No meta‐analyses were possible.
2.2.4.2 Single‐study results
Bjornson 2007 (33 children) reported no difference between groups in ankle plantarflexors assessed by the MAS at short‐term (reported P = 0.38), medium‐term (reported P = 0.71), or long‐term follow‐up (reported P = 0.66); analyses not shown. However, the study authors reported lower spasticity in the BoNT‐A group at short‐term follow‐up for total SMS (reported P < 0.04) and SMS elastic length (reported P < 0.05); analyses not shown. There were no differences between groups in total SMS and elastic length at medium‐term (reported P = 0.10 and P = 0.06, respectively) and long‐term follow‐up (reported P = 0.82 and P = 0.55, respectively); analyses not shown.
Delgado 2016 (156 children) reported lower ankle plantarflexors scores in the BoNT‐A group at short‐term follow‐up when assessed with the MAS (MD 0.49, 95% CI −0.78 to −0.20; see the illustrative forest plot in Analysis 2.18) and the MTS (reported MD −0.40, 95% CI −0.60 to −0.30, P < 0.01; analysis not shown), and at medium‐term follow‐up when assessed with the MAS (MD −0.50, 95% CI −0.78 to −0.22; see the illustrative forest plot in Analysis 2.19).
2.18. Analysis.
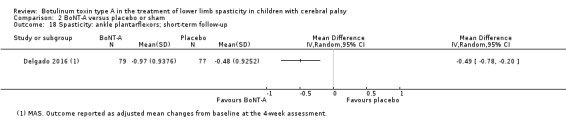
Comparison 2 BoNT‐A versus placebo or sham, Outcome 18 Spasticity: ankle plantarflexors; short‐term follow‐up.
2.19. Analysis.
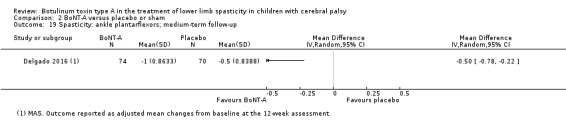
Comparison 2 BoNT‐A versus placebo or sham, Outcome 19 Spasticity: ankle plantarflexors; medium‐term follow‐up.
Mall 2006 (61 children) reported that the mean hip adductors score, assessed with the MAS, was lower in the BoNT‐A group at short‐term follow‐up (reported P < 0.01), but not at medium‐term follow‐up (reported P = 0.08); analyses not shown.
Moore 2008 reported no differences in MAS scores at long‐term follow‐up for ankle plantarflexors (MD 0.10, 95% CI −0.58 to 0.78, P = 0.77; 42 children); hamstrings (MD −1.00, 95% CI −2.06 to 0.06, P = 0.06; 10 children); and hip adductors (MD 0.10, 95% CI −0.79 to 0.99, P = 0.83; 13 children). See the illustrative forest plot in Analysis 2.20.
2.20. Analysis.
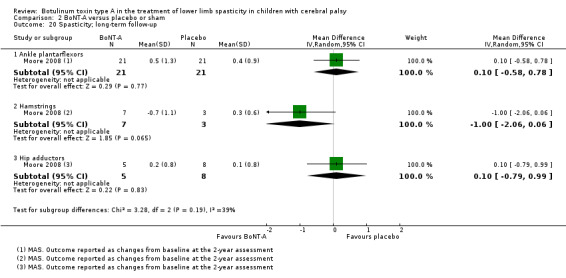
Comparison 2 BoNT‐A versus placebo or sham, Outcome 20 Spasticity; long‐term follow‐up.
2.2.5 Adverse events
All studies included in this comparison described the occurrence or absence of adverse events. We included all reported adverse events, with no distinction regarding severity. A detailed description of each study is provided below under 'Single‐study results'.
For the meta‐analyses, we considered the number of children who had at least one adverse event as positive events, rather than the total number of adverse events reported in the studies.
2.2.5.1 Pooled analysis
There was no difference between groups in the number of patients with adverse events (RR 1.29, 95% CI 0.87 to 1.93, P = 0.21; 12 studies, 918 children; I2 = 80%; Analysis 2.21). We further explored the high heterogeneity found in this analysis below, under 'Sensitivity analysis'.
2.21. Analysis.
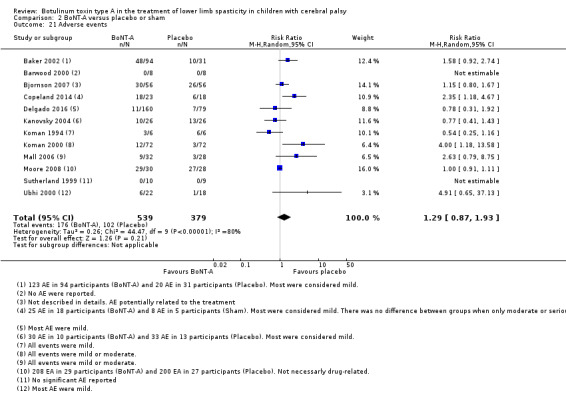
Comparison 2 BoNT‐A versus placebo or sham, Outcome 21 Adverse events.
2.2.5.2 Single‐study results
Barwood 2000 reported no adverse events following BoNT‐A or placebo injections. The children subsequently underwent surgical treatment (hip adductors release), but no further adverse events related to the initial treatment were reported.
From Baker 2002, we considered all children undergoing BoNT‐A treatment (10 IU/kg, 20 IU/kg, 30 IU/kg groups), and not only the intermediate‐dose group as in the other analyses. The study authors reported 123 adverse events in 94 children in the BoNT‐A groups and 20 adverse events in 31 children in the placebo group. The authors considered most adverse events to be mild (60% in the placebo group and 69% in the BoNT‐A group). In a detailed analysis, the study authors concluded that 24% of adverse events in the BoNT‐A group were related to the study medication, whereas no adverse events in the placebo group appeared to be related to the study. The most frequent adverse events in the BoNT‐A group were pain, falls, and asthenia.
Bjornson 2007 did not detail the severity of the adverse events. The study authors only reported that six children had pain at the injection site, and three reduced their activity level for 24 hours after treatment.
Copeland 2014 reported 25 adverse events in 18 children in the BoNT‐A group and eight adverse events in five children in the sham group. Most adverse events were considered mild (60%) in the BoNT‐A group and moderate (50%) in the sham group. The number of events considered as severe, including drooling, temporary vocal loss, prolonged seizures, pneumonia, diarrhoea and vomiting, was similar between the BoNT‐A (three adverse events in three children) and sham (two adverse events in one child) groups.
Delgado 2016 reported a total of 144 children with at least one adverse event, most of mild intensity. For this outcome, we considered all children undergoing BoNT‐A treatment (10 units/kg and 15 units/kg groups), and not only the high‐dose group as in other analyses. The most frequent adverse events were weakness, pain, and fever.
Kanovsky 2004 reported 30 adverse events in 10 children in the BoNT‐A group and 33 adverse events in 13 children in the placebo group. Most adverse events were considered mild in the BoNT‐A group (80%) and in the placebo group (79%). The most frequent adverse events were rhinitis, bronchitis, pharyngitis, and viral infection in both groups.
Koman 1994 reported adverse events in three children in the BoNT‐A group and six children in the placebo group, which included pain, loss of balance, fatigue, and headache.
Koman 2000 reported adverse events in 12 children in the BoNT‐A group and three children in the placebo group. The study authors considered all adverse events to be mild or moderate. The most common adverse events were muscle weakness, pain, and falls.
Mall 2006 reported adverse events in nine children in the BoNT‐A group and three children in the placebo group. The study authors considered all adverse events to be mild or moderate. The most frequent adverse events were muscle weakness, dysphagia, and increased urinary frequency.
Moore 2008 reported all adverse events (not necessarily related to treatment) over the two‐year study duration. They observed a total of 208 adverse events in 29 children in the BoNT‐A group and 200 adverse events in 27 children in the placebo group. The most frequent adverse events were related to airway disorders (cough, infection), gastrointestinal disorders, and changes in mobility.
Sutherland 1999 only reported in the study summary that no adverse events were observed.
Ubhi 2000 reported adverse events in six children in the BoNT‐A group (pain, falls, rhinitis, and convulsion) and one child in the placebo group (vomiting).
2.3 Sensitivity analyses
For all meta‐analyses, we verified the effect of excluding studies with potential sources of bias, although no study in this comparison presented with a high risk of bias in any domain. We also explored the reasons for statistical heterogeneity when this was present, and the impact of using the SMD in some analyses. The results of the only two analyses we considered to be relevant are described below.
2.3.1 Secondary outcomes: adverse events
For this outcome, we found high statistical heterogeneity in our meta‐analysis (Tau2 = 0.26; Chi2 = 44.47, df = 9, P < 0.001; I2 = 80%). Statistical heterogeneity remained high after the exclusion of studies with potential sources of bias (i.e. those at unclear risk of bias) (Tau2 = 0.19; Chi2 = 19.70, df = 8, P = 0.01; I2 = 59%; Analysis 2.21).
We noted that one study with 58 children that analysed several cycles of BoNT‐A injections reported that 56 children experienced adverse events in both groups (Moore 2008). This led to a disproportionately large weight for this study in the analysis. By excluding Moore 2008, statistical heterogeneity decreased (Tau2 = 0.19; Chi2 = 19.70, df = 8, P = 0.01; I2 = 59%), but was still considered high (Analysis 2.22).
2.22. Analysis.

Comparison 2 BoNT‐A versus placebo or sham, Outcome 22 Sensitivity analysis: adverse events.
Two studies in this analysis reported a significantly higher rate of adverse events in the BoNT‐A group (Copeland 2014; Koman 2000). We explored the individual characteristics of all studies, including BoNT‐A dosage, treated muscle groups, and children's clinical details. The Copeland 2014 study involved only non‐ambulatory children with CP, who are potentially at a higher risk of adverse events with BoNT‐A treatment. We were not able to identify the reasons for a higher number of adverse events in the Koman 2000 study.
2.4 Quality of the evidence
See Table 4; Table 5; Table 6.
2.4.1 Primary outcomes
2.4.1.1 Gait analysis
There is moderate‐quality evidence that BoNT‐A improves ankle kinematics (instrumented gait analysis) at short‐term follow‐up, and overall gait pattern (observational gait analysis) at short‐ and medium‐term follow‐up, when compared to placebo or sham.
2.4.1.2 Function
There is moderate‐quality evidence that BoNT‐A improves function at medium‐term follow‐up, but not at short‐ or long‐term follow‐up, when compared to placebo or sham.
2.4.2 Secondary outcomes
2.4.2.1 Range of motion
There is moderate‐quality evidence that BoNT‐A improves passive ankle range of motion at short‐term follow‐up, but not at medium‐ or long‐term follow‐up, when compared to placebo or sham.
2.4.2.2 Satisfaction with the outcome of treatment
There is moderate‐quality evidence that BoNT‐A is associated with higher satisfaction with the outcome of treatment at short‐ and medium‐term follow‐up, but not at long‐term follow‐up, when compared to placebo or sham.
2.4.2.3 Spasticity
There is moderate‐quality evidence that BoNT‐A reduces ankle plantarflexors spasticity at short‐ and medium‐term follow‐up, but not at long‐term follow‐up, when compared to placebo or sham.
2.4.2.4 Adverse events
There is moderate‐quality evidence that BoNT‐A is not associated with a higher rate of adverse events than placebo or sham.
Comparison 3: BoNT‐A versus serial casting
We included four studies in this comparison (Ackman 2005; Corry 1998; Flett 1999; Kay 2004).
One study, Ackman 2005, presented their results in graphs only. However, the authors provided a table with their numerical data through personal contact by email.
Ackman 2005 compared isolated BoNT‐A in the treatment of fixed ankle equinus to two different control groups: one with placebo injections followed by serial casting, and one with BoNT‐A injections also followed by serial casting. To comply with the methods in the original review (Ade‐Hall 2000), which stated that we would not include studies if BoNT‐A was used in the control group, we only considered the placebo + serial casting group as the comparator for our analyses. Corry 1998 and Flett 1999 compared the application of BoNT‐A into the ankle plantarflexors to a serial casting protocol, whilst Kay 2004 compared the application of combined BoNT‐A + serial casting to a control group with serial casting only.
3.1 Primary outcomes
3.1.1 Gait analysis
All four studies assessed gait parameters as an outcome measure (Ackman 2005; Corry 1998; Flett 1999; Kay 2004). Two studies performed observational gait analysis using a version of the PRS (Corry 1998; Flett 1999); two studies assessed gait speed with instrumented gait analysis (Ackman 2005; Corry 1998); and three studies reported on ankle kinematics (Ackman 2005; Corry 1998; Kay 2004). For the purposes of this review, we analysed the following ankle kinematic data: peak ankle dorsiflexion in stance and in swing, and ankle dorsiflexion at initial contact.
One study reported only the medians and range for each group in the PRS scores, and the mean change in gait speed, but did not present the significance level for their findings (Corry 1998). Two studies, Corry 1998 and Kay 2004, considered each lower limb as the unit of analysis for ankle kinematics (n = total number of assessed lower limbs).
3.1.1.1 Pooled results
We were able to combine data from three studies in meta‐analyses (Ackman 2005; Corry 1998; Kay 2004), but only for ankle kinematics. See Analysis 3.5.
3.5. Analysis.
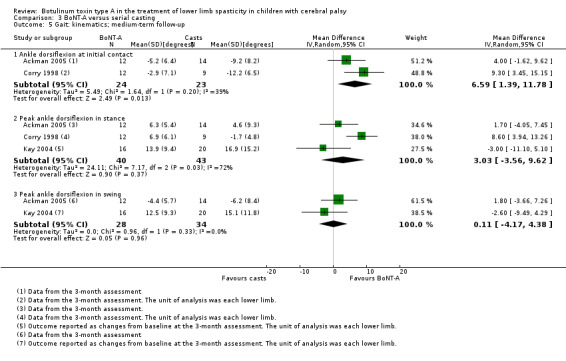
Comparison 3 BoNT‐A versus serial casting, Outcome 5 Gait: kinematics; medium‐term follow‐up.
The mean ankle dorsiflexion at initial contact was 6.59 degrees higher in the BoNT‐A group at medium‐term follow‐up (95% CI 1.39 to 11.78, P = 0.01; 2 studies, 47 assessments; I2 = 39%).
There was no difference between groups in peak ankle dorsiflexion in stance at medium‐term follow‐up (MD 3.03 degrees, 95% CI −3.56 to 9.62, P = 0.37; 3 studies, 83 assessments; I2 = 72%). We further explored the high heterogeneity found in this analysis below, under 'Sensitivity analysis'.
There was no difference between groups in peak ankle dorsiflexion in swing at medium‐term follow‐up (MD 0.11 degrees, 95% CI −4.17 to 4.38, P = 0.96; 2 studies, 62 assessments; I2 = 0%).
3.1.1.2 Single‐study results
Ackman 2005 (26 children) reported no differences between groups in terms of gait speed at medium‐term (MD 0.13, 95% CI −0.06 to 0.32; Analysis 3.7) or long‐term follow‐up (MD 0.02, 95% CI −0.11 to 0.15; Analysis 3.8). Ackman 2005 also reported no differences in ankle dorsiflexion at initial contact at long‐term follow‐up (MD −0.60 degrees, 95% CI −6.37 to 5.17, P = 0.84); peak ankle dorsiflexion in stance (MD −2.00 degrees, 95% CI −8.50 to 4.50, P = 0.55); and peak ankle dorsiflexion in swing (MD 3.70 degrees, 95% CI −0.80 to 8.20, P = 0.55); see Analysis 3.6.
3.7. Analysis.
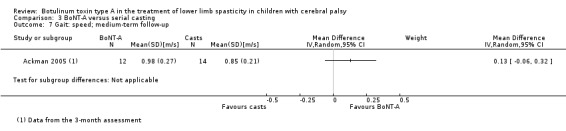
Comparison 3 BoNT‐A versus serial casting, Outcome 7 Gait: speed; medium‐term follow‐up.
3.8. Analysis.
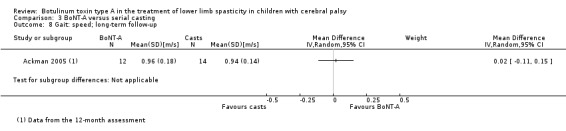
Comparison 3 BoNT‐A versus serial casting, Outcome 8 Gait: speed; long‐term follow‐up.
3.6. Analysis.
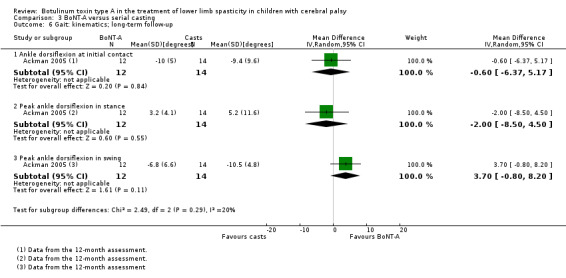
Comparison 3 BoNT‐A versus serial casting, Outcome 6 Gait: kinematics; long‐term follow‐up.
Corry 1998 (21 children) reported no differences between groups in terms of PRS scores at short‐ and medium‐term follow‐up (level of significance not reported; analyses not shown), and no differences between groups at short‐term follow‐up regarding ankle dorsiflexion at initial contact (MD 2.90 degrees, 95% CI −2.90 to 8.70, P = 0.33) and peak ankle dorsiflexion in stance (MD −0.60 degrees, 95% CI −5.78 to 4.58, P = 0.82); see Analysis 3.4. However, Corry 1998 reported a greater increase in gait speed in the BoNT‐A group (mean decrease of 0.14 m/s in the serial casting group and mean increase of 0.05 m/s in the BoNT‐A group) (level of significance not reported; analysis not shown).
3.4. Analysis.
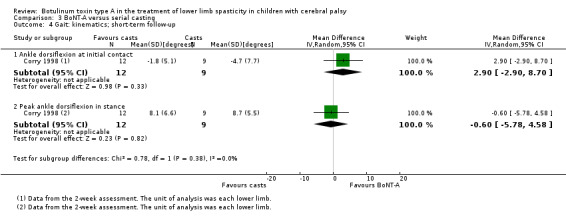
Comparison 3 BoNT‐A versus serial casting, Outcome 4 Gait: kinematics; short‐term follow‐up.
Flett 1999 (18 children) reported no differences between groups in terms of PRS scores at short‐term (MD 0.00, 95% CI −1.66 to 1.66; Analysis 3.1); medium‐term (MD 0.65, 95% CI −1.21 to 2.51; Analysis 3.2); and long‐term follow‐up (MD 0.46, 95% CI −1.33 to 2.25; Analysis 3.3).
3.1. Analysis.
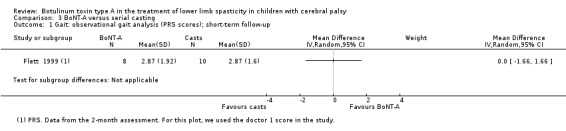
Comparison 3 BoNT‐A versus serial casting, Outcome 1 Gait: observational gait analysis (PRS scores); short‐term follow‐up.
3.2. Analysis.
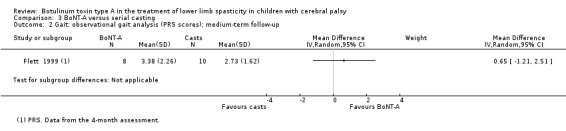
Comparison 3 BoNT‐A versus serial casting, Outcome 2 Gait: observational gait analysis (PRS scores); medium‐term follow‐up.
3.3. Analysis.
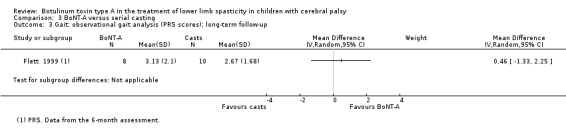
Comparison 3 BoNT‐A versus serial casting, Outcome 3 Gait: observational gait analysis (PRS scores); long‐term follow‐up.
3.1.2 Function
Two studies measured function using the GMFM goal scores (Flett 1999; Kay 2004).
One study reported this outcome as changes from baseline values (Kay 2004).
3.1.2.1 Pooled results
We were able to combine the data from both studies in a meta‐analysis of the medium‐term assessments (Flett 1999; Kay 2004).
There was no difference between groups in function scores at medium‐term follow‐up (MD 3.64, 95% CI −1.55 to 8.82, P = 0.17; 41 children; I2 = 0%; Analysis 3.9).
3.9. Analysis.
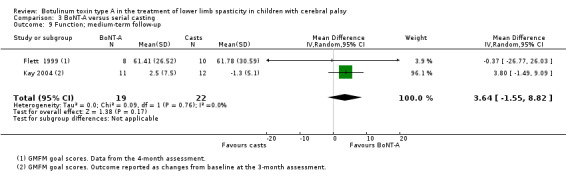
Comparison 3 BoNT‐A versus serial casting, Outcome 9 Function; medium‐term follow‐up.
3.1.2.2 Single‐study results
Flett 1999 (18 children) reported no differences between groups in GMFM goal scores at short‐term (MD 2.01, 95% CI −23.31 to 27.33; Analysis 3.10) or long‐term follow‐up (MD −2.02, 95% CI −26.85 to 22.81; Analysis 3.11).
3.10. Analysis.
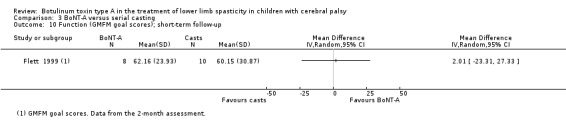
Comparison 3 BoNT‐A versus serial casting, Outcome 10 Function (GMFM goal scores); short‐term follow‐up.
3.11. Analysis.
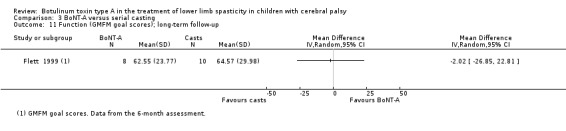
Comparison 3 BoNT‐A versus serial casting, Outcome 11 Function (GMFM goal scores); long‐term follow‐up.
3.2 Secondary outcomes
3.2.1 Range of motion
All four studies in this comparison measured ankle range of motion (Ackman 2005; Corry 1998; Flett 1999; Kay 2004).
One study reported only the medians and range for each group, therefore we could not pool their data in a meta‐analysis (Corry 1998). One study presented data as changes from baseline values (Kay 2004). Two studies, Flett 1999 and Kay 2004, considered each side as the unit of analysis (n = total number of assessed lower limbs).
3.2.1.1 Pooled results
We were able to combine data from three studies in meta‐analyses (Ackman 2005; Flett 1999; Kay 2004).
There were no differences between groups in ankle dorsiflexion at medium‐term (MD 1.82 degrees, 95% CI −2.26 to 5.91, P = 0.38; 3 studies, 93 assessments; I2 = 0%; Analysis 3.13) or long‐term follow‐up (MD −1.02 degrees, 95% CI −5.63 to 3.58, P = 0.66; 2 studies, 57 assessments; I2 = 0%; Analysis 3.14).
3.13. Analysis.
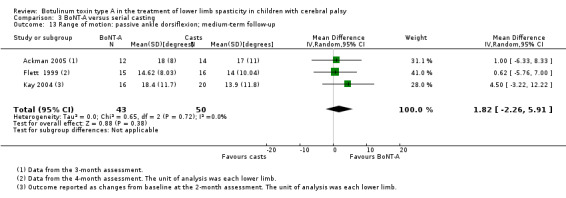
Comparison 3 BoNT‐A versus serial casting, Outcome 13 Range of motion: passive ankle dorsiflexion; medium‐term follow‐up.
3.14. Analysis.
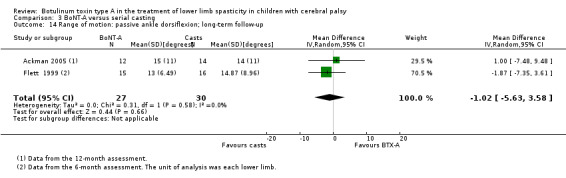
Comparison 3 BoNT‐A versus serial casting, Outcome 14 Range of motion: passive ankle dorsiflexion; long‐term follow‐up.
3.2.1.2 Single‐study results
Flett 1999 (18 children; 31 assessments) reported no difference between groups in passive ankle dorsiflexion at short‐term follow‐up (MD 1.77 degrees, 95% CI −4.25 to 7.79; Analysis 3.12).
3.12. Analysis.
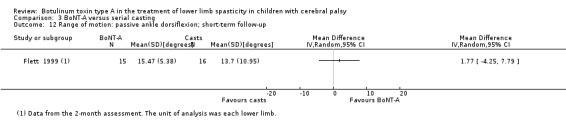
Comparison 3 BoNT‐A versus serial casting, Outcome 12 Range of motion: passive ankle dorsiflexion; short‐term follow‐up.
Corry 1998 (21 children) reported no differences between groups in passive ankle dorsiflexion at short‐ or medium‐term follow‐up (level of significance not reported; analyses not shown).
3.2.2 Quality of life
No study in this comparison measured quality of life.
3.2.3 Satisfaction with the outcome of treatment
No study in this comparison measured satisfaction with the outcome of treatment.
3.2.4 Spasticity
All four studies in this comparison measured ankle plantarflexors spasticity using the MAS (Ackman 2005; Corry 1998; Flett 1999; Kay 2004).
One study reported only the medians and range for each group, therefore we could not pool their data in a meta‐analysis (Corry 1998). One study, Kay 2004, presented data as changes from baseline values. Two studies, Flett 1999 and Kay 2004, considered each side as the unit of analysis (n = total number of assessed lower limbs).
3.2.4.1 Pooled results
We were able to combine data from three studies in meta‐analyses (Ackman 2005; Flett 1999; Kay 2004).
There were no differences between groups in ankle plantarflexors spasticity at medium‐term (MD 0.13, 95% CI −0.25 to 0.52, P = 0.49; 3 studies, 93 assessments; I2 = 0%; Analysis 3.16) or long‐term follow‐up (MD 0.17, 95% CI −0.34 to 0.67, P = 0.52; 2 studies, 57 assessments; I2 = 0%; Analysis 3.17).
3.16. Analysis.
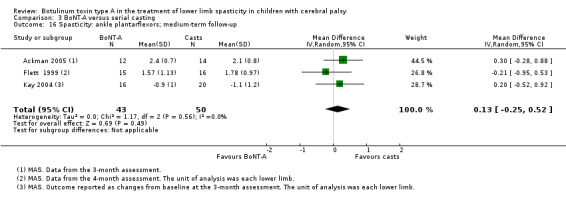
Comparison 3 BoNT‐A versus serial casting, Outcome 16 Spasticity: ankle plantarflexors; medium‐term follow‐up.
3.17. Analysis.
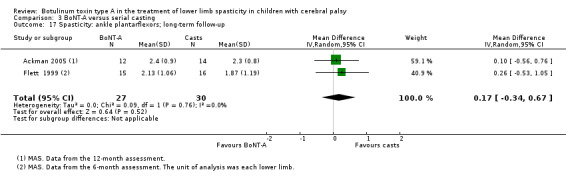
Comparison 3 BoNT‐A versus serial casting, Outcome 17 Spasticity: ankle plantarflexors; long‐term follow‐up.
3.2.4.2 Single‐study results
Flett 1999 (18 children; 31 assessments) reported no difference between groups in ankle plantarflexors spasticity at short‐term follow‐up (MD −0.20, 95% CI −0.81 to 0.41; see the illustrative forest plot in Analysis 3.15).
3.15. Analysis.
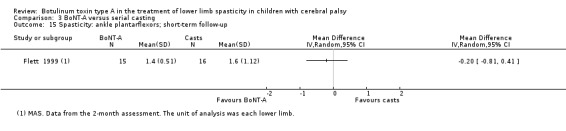
Comparison 3 BoNT‐A versus serial casting, Outcome 15 Spasticity: ankle plantarflexors; short‐term follow‐up.
Corry 1998 (21 children) reported a higher recurrence of spasticity in the serial casting group at medium‐term follow‐up compared to the BoNT‐A group (reported P = 0.03; analysis not shown).
3.2.5 Adverse events
Three studies in this comparison reported the occurrence or absence of adverse events (Ackman 2005; Corry 1998; Flett 1999).
3.2.5.1 Pooled results
We were able to combine data from all three studies in a meta‐analysis (Ackman 2005; Corry 1998; Flett 1999).
There was no difference between groups with respect to the number of adverse events (RR 0.59, 95% CI 0.03 to 11.03, P = 0.72; 64 children; I2 = 62%; Analysis 3.18). The high heterogeneity found in this analysis seemed to be related to the discrepancy of findings between the two studies reporting positive events (Ackman 2005; Corry 1998). We could not evaluate the impact of excluding each study individually because Flett 1999 had no positive events in either group. Consequently, statistical heterogeneity could not be calculated for any combination of two studies in this analysis.
3.18. Analysis.
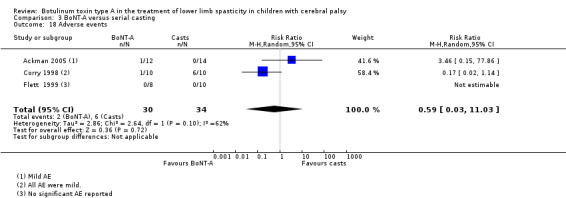
Comparison 3 BoNT‐A versus serial casting, Outcome 18 Adverse events.
3.2.5.2 Single‐study results
Kay 2004 (23 children) did not state whether or not any adverse events occurred.
From Ackman 2005 (26 children), we only considered the isolated BoNT‐A group compared to the placebo + serial casting group for this outcome. The study authors reported an increase in the number of falls for one child in the BoNT‐A group.
Corry 1998 (20 children) reported only one adverse event in the BoNT‐A group (pain in the injection site) and six in the serial casting group (pain and skin lesions). All adverse events were considered to be mild.
Flett 1999 (18 children) only mentioned that there were no systemic or treatment‐related adverse events.
3.3 Sensitivity analysis
For all meta‐analyses, we verified the effect of excluding studies with potential sources of bias. We also explored the reasons for statistical heterogeneity when this was present. Only the analysis considered to be relevant is described below.
3.3.1 Primary outcome: gait analysis
For the outcome peak ankle dorsiflexion in stance, we found high statistical heterogeneity in the meta‐analysis at medium‐term follow‐up (Tau2 = 24.11; Chi2 = 7.17, df = 2, P = 0.03; I2 = 72%). We initially attempted to exclude Kay 2004, for the following reasons: (1) it was the only study reporting changes from baseline; (2) the study authors did not describe how the outcome assessment was blinded; (3) the effect estimate was in the opposite direction of the other studies; and (4) it was the only study to include the use of serial casting in both groups. However, statistical heterogeneity remained high (Tau2 = 16.67; Chi2 = 3.34, df = 1, P = 0.07; I2 = 70%; results not shown), and the forest plot diamond still crossed the no‐effect line. But by excluding Corry 1998, which had a small sample but still reported a difference in favour of the BoNT‐A group, statistical heterogeneity became null (Tau2 = 0.00; Chi2 = 0.86, df = 1, P = 0.35; I2 = 0%), whilst keeping the estimate close to the no‐effect line (Analysis 3.19).
3.19. Analysis.
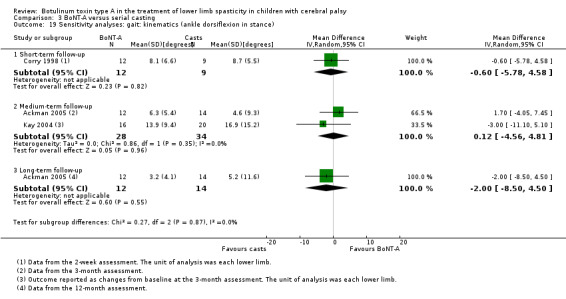
Comparison 3 BoNT‐A versus serial casting, Outcome 19 Sensitivity analyses: gait: kinematics (ankle dorsiflexion in stance).
3.4 Quality of the evidence
See Table 7; Table 8; Table 9.
3.4.1 Primary outcomes
3.4.1.1 Gait analysis
There is low‐quality evidence that there is no difference between BoNT‐A and serial casting for ankle kinematics (instrumented gait analysis) at short‐ and medium‐term follow‐up, and moderate‐quality evidence that there is no difference between treatments at long‐term follow‐up.
There is moderate‐quality evidence that there is no difference between BoNT‐A and serial casting for overall gait pattern (observational gait analysis) at short‐, medium‐, and long‐term follow‐up.
3.4.1.2 Function
There is moderate‐quality evidence that there is no difference between BoNT‐A and serial casting for function at short‐, medium‐, and long‐term follow‐up.
3.4.2 Secondary outcomes
3.4.2.1 Range of motion
There is low‐quality evidence that there is no difference between BoNT‐A and serial casting for passive ankle range of motion at short‐, medium‐, and long‐term follow‐up.
3.4.2.2 Spasticity
There is low‐quality evidence that there is no difference between BoNT‐A and serial casting for ankle plantarflexors spasticity at short‐, medium‐, and long‐term follow‐up.
3.4.2.3 Adverse events
There is low‐quality evidence that BoNT‐A is not associated with a higher risk of adverse events than serial casting.
Comparison 4: BoNT‐A versus orthoses
We included only one study (43 children) in this comparison (Hazneci 2006), which compared BoNT‐A injections to Johnstone pressure splints in the treatment of spasticity in children with CP. The study only reported data on the primary outcome of function and the secondary outcomes of range of motion and spasticity of the hip at medium‐term follow‐up. Given that there was only one study, no meta‐analysis was possible.
4.1 Primary outcomes: function
There was no difference between groups in terms of function (MD 11.14, 95% CI −0.05 to 22.33), as measured by GMFM total scores at medium‐term follow‐up (see the illustrative forest plot in Analysis 4.1).
4.1. Analysis.
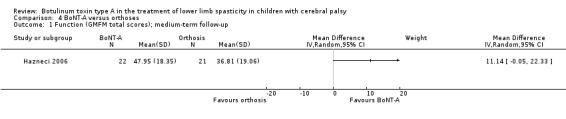
Comparison 4 BoNT‐A versus orthoses, Outcome 1 Function (GMFM total scores); medium‐term follow‐up.
4.2 Secondary outcomes
4.2.1 Range of motion
The mean hip abduction, as measured by the hip range of motion (passive hip abduction), was higher in the BoNT‐A group at medium‐term follow‐up: MD 10.61 degrees, 95% CI 2.53 to 18.69; see the illustrative forest plot in Analysis 4.2.
4.2. Analysis.
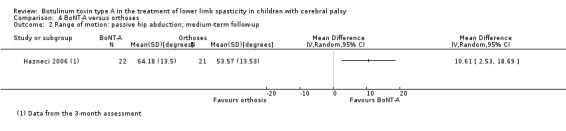
Comparison 4 BoNT‐A versus orthoses, Outcome 2 Range of motion: passive hip abduction; medium‐term follow‐up.
4.2.2 Spasticity
Hip adductor spasticity, as measured by the MAS, was lower in the BoNT‐A group at medium‐term follow‐up: MD −0.70, 95% CI −1.10 to −0.30; see the illustrative forest plot in Analysis 4.3.
4.3. Analysis.
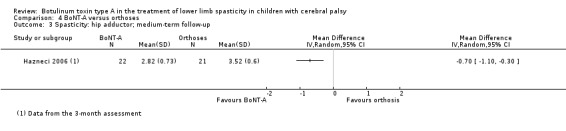
Comparison 4 BoNT‐A versus orthoses, Outcome 3 Spasticity: hip adductor; medium‐term follow‐up.
4.2.3 Adverse events
The study did not report on the occurrence or absence of adverse events.
4.3 Quality of the evidence
There is very low‐quality evidence that there is no difference between BoNT‐A and Johnstone pressure splints for function, but there is a difference in favour of BoNT‐A for hip range of motion (passive abduction) and hip adductors spasticity at medium‐term follow‐up. See Table 10.
Discussion
Summary of main results
Overall
We included 31 studies, from 36 reports, assessing a total of 1508 children with CP who underwent lower limb spasticity treatment with BoNT‐A injections. Most studies involved the use of BoNT‐A into the ankle plantarflexors, either isolated or associated with other muscle groups. The number of injection cycles, location method, follow‐up time, and adjunctive therapies varied considerably amongst the included studies. Individually, most studies involved a small number of participants who presented with high variability in their clinical presentations, which reflects the diverse nature of CP. For this reason, and to make it easier to analyse the results, we established four comparison groups according to the control intervention. Within each comparison, we analysed the outcomes at short‐, medium‐, and long‐term follow‐up.
Comparison 1: BoNT‐A versus usual care or physiotherapy
We included 14 studies (582 children assessed) in this comparison, but only 10 studies provided adequate numerical data for pooling. We included studies comparing lower‐limb BoNT‐A injections to usual rehabilitation care or a specific physical therapy protocol. Due to the nature of the interventions, adequate blinding of participants and personnel was usually not performed, but adequate allocation concealment and blinding of outcome assessors was still possible. One study in this comparison did not address any of the outcomes of interest for this review (Jozwiak 2007). We found evidence of the following.
Primary outcomes
BoNT‐A improved overall gait scores at medium‐term follow‐up (very low‐quality evidence). Single‐study results suggested higher gait speed in the BoNT‐A group at medium‐term follow‐up (Xu 2006), but no difference in step length, Ibrahim 2007, and GGI, Tedroff 2010, at long‐term follow‐up. BoNT‐A was moderately effective at improving function at short‐ and medium‐term follow‐up (very low‐quality evidence), but not at long‐term follow‐up. Other single‐study results were conflicting. One study found no difference in function scores at any time point (Navarrete 2010), whilst three other studies reported higher scores in the BoNT‐A group (Love 2001; Scholtes 2006; Steenbeek 2005).
Secondary outcomes
There was very low‐quality evidence that BoNT‐A improved ankle range of motion, satisfaction with the outcome of treatment, and ankle plantarflexors spasticity at one or more time points. The rate of adverse events was higher in the BoNT‐A group (very low‐quality evidence). However, as all studies reporting this outcome had no specific intervention in the control group other than physical therapy, it is unlikely that adverse events would have been monitored.
Comparison 2: BoNT‐A versus placebo or sham
We included 12 studies (788 children assessed) comparing BoNT‐A injections into the lower limb to placebo or a sham procedure. We rated all studies included in this comparison as at low risk of bias for most domains. One study in this comparison did not address any of the outcomes of interest for this review, except for adverse events (Barwood 2000). We found evidence of the following.
Primary outcomes
BoNT‐A improved overall gait scores at short‐ and medium‐term follow‐up (moderate‐quality evidence) and improved ankle kinematics at short‐term follow‐up compared to placebo (moderate‐quality evidence). Additionally, single‐study results suggested no difference in step length between groups (Sutherland 1999). There was moderate‐quality evidence that BoNT‐A was not more effective than placebo or sham at improving overall function at short‐ and long‐term follow‐up, but it did improve function in the medium term. Considering standard scales (e.g. GMFM, PEDI), there were no differences between groups at any time point. However, considering individualised measures (e.g. COPM, GAS, PGA), the function scores were higher in the BoNT‐A group at short‐ and medium‐term follow‐up, but not at long‐term follow‐up.
Secondary outcomes
There was moderate‐quality evidence that BoNT‐A improved passive ankle range of motion, satisfaction with the outcome of treatment, and ankle plantarflexors spasticity at one or more time points. One study, Copeland 2014, reported no differences in quality of life between groups at short‐term follow‐up. There was no difference between groups in the rate of adverse events (moderate‐quality evidence).
Comparison 3: BoNT‐A versus serial casting
We included four studies (95 children assessed) in this comparison. We included studies comparing BoNT‐A injections into the ankle plantarflexors to the use of serial casting on the treatment of a fixed ankle equinus deformity. One of the studies also used serial casting in the intervention group. We included this study in this comparison for simplicity, since it ultimately evaluates the effect of adding BoNT‐A to a serial casting protocol in comparison to serial casting alone. One study only provided adequate numerical data for pooling for some of the reported outcomes (Corry 1998). We found evidence of the following.
Primary outcomes
BoNT‐A was not more effective than serial casting at improving overall gait pattern at short‐, medium‐, and long‐term follow‐up (moderate‐quality evidence) or most of the ankle kinematics parameters at short‐, medium‐, and long‐term follow‐up (low‐ and moderate‐quality evidence). BoNT‐A was not more effective than serial casting at improving function at short‐, medium‐, and long‐term follow‐up (moderate‐quality evidence).
Secondary outcomes
There was low‐quality evidence that BoNT‐A was not more effective than serial casting at improving ankle range of motion and spasticity at any time point. In fact, one study reported higher spasticity recurrence in the serial casting group at medium‐term follow‐up (Corry 1998). There was low‐quality evidence that BoNT‐A was not associated with a higher risk of adverse events than serial casting. One of the studies reported a higher number of adverse events in the BoNT‐A group (Ackman 2005), and one study reported more adverse events in the serial casting group (Corry 1998).
Comparison 4: BoNT‐A versus orthoses
Only one study (43 children assessed) performed a direct comparison of BoNT‐A injections versus orthosis, in this case the Johnstone pressure splint. This type of orthosis is rarely used in current clinical practice, which makes this comparison less relevant. Only measures of function, hip range of motion, and hip adductors spasticity were available for this comparison, assessed at medium‐term follow‐up. There was no evidence of a difference between Johnstone pressure splints and BoNT‐A for function. However, compared to Johstone pressure splints, BoNT‐A improved hip range of motion (passive abduction) and hip adductor spasticity at medium‐term follow‐up. We rated this evidence as very low quality due to very serious risk of bias and imprecision. This study did not report on adverse events.
Overall completeness and applicability of evidence
We only included RCTs in the review, which represent the highest level of evidence among primary intervention studies. We developed our search strategy with the aim of high sensitivity to maximise the number of results obtained in order to avoid missing relevant studies. We searched several electronic databases and clinical trial registries. We actively sought information on unpublished studies and studies published in the grey literature through internet searches, the bibliographies of the included studies, and contact with experts in the field.
Current standards in the treatment of spasticity in children with CP usually apply a multimodal approach, involving a combination of oral medications, neuromuscular blocks, physical therapy, orthotics, casting, and neurosurgery (intrathecal baclofen pump and selective dorsal rhyzotomy) (Simpson 2008). However, the selection criteria described in the previous version of this review, Ade‐Hall 2000, established that only studies comparing BoNT‐A injections to other interventions or to no intervention should be included. We excluded studies in which BoNT‐A was used in all groups, which could potentially include studies evaluating different treatment combinations (e.g. BoNT‐A with physical therapy versus BoNT‐A without physical therapy). These comparisons were not within the scope of this review.
All of the included studies were published within the past 20 years, which encompasses the entire period since the introduction of BoNT‐A as a treatment method for people with CP. However, it is important to note that there has been a significant change in clinical practice in the past two decades, since BoNT‐A was initially used predominantly in the lower limbs and for ambulant children. After 2000, its use was expanded to more severely affected children and to the upper limbs. Most of the studies involving a placebo comparison group were published in the first few years after BoNT‐A introduction into clinical practice. Some of the included studies compared BoNT‐A injections to a group with no specific treatment (e.g. undergoing their usual rehabilitation care) or with a given physical therapy protocol. We analysed these studies separately from those involving a placebo or sham procedure, which tended to have better overall methodological quality. Studies involving other interventions mainly focused on the application of serial casting for the treatment of ankle plantarflexors spasticity with some degree of fixed equinus deformity.
In our literature search, we noted that most of the recent RCTs involving a control group with no BoNT‐A injections were focused on rather specific issues. For instance, Copeland 2014 included only individuals who were severely affected by CP, classified as GMFCS levels IV and V. Other recent studies that did not meet the inclusion criteria for this review analysed specific aspects of BoNT‐A application, such as different location methods (Kwon 2010), injection techniques (Van Campenhout 2013), interval between applications (Hastings‐Ison 2016), dose ranging (Niu 2014), and even alternative commercial brands (Carraro 2016). These issues were not the object of, and hence not answered by, this review.
Several of our meta‐analyses allowed us to draw conclusions with a moderate degree of confidence in the effect estimate. This was particularly true for outcomes related to the ankle joint, since the treatment of ankle plantarflexors spasticity was addressed by most authors. However, due to relatively small sample sizes in all our analyses, we downgraded the quality of the evidence due to imprecision by at least one level for all of the outcome measures considered in this review.
Given the number of included studies, we were able to perform a quantitative analysis for most of the primary and secondary outcomes, especially in 'Comparison 1: BoNT‐A versus usual care or physiotherapy' and 'Comparison 2: BoNT‐A versus placebo or sham'. However, some of our attempts to pool data were accompanied by significant statistical heterogeneity. In general, we were able to perform sensitivity analyses to identify potential causes for these findings, with no significant changes in the direction or magnitude of the effect estimate. We believe that the variability in presentation of people with CP, different BoNT‐A application techniques, and associated treatment methods can account for some degree of heterogeneity. During the review process, we attempted to investigate the impact of several factors on our findings, including those related to participants (age, functional level, and motor distribution) and to the intervention (dose, brand, location method, application regimen, and muscle groups treated). However, due to the small number of studies in each analysis, no relevant conclusions could be drawn.
One key issue is the apparent disparity of results between some studies in Comparisons 1 and 2. Studies using a non‐placebo control tended to present better outcomes after BoNT‐A treatment. Most of these studies had high or unclear risk of bias for several domains, including lack of blinding of outcome assessment. It is important to note that some of the assessments performed, such as observational gait analysis, manual spasticity measurement, and satisfaction scales, are relatively subjective and depend on the examiner's experience and on patient collaboration. On the one hand, the absence of blinding could imply a more condescending judgement by the examiner. Likewise, it is also possible that in knowing the treatment received, a participant could be induced to provide, for example, more positive responses on a questionnaire. On the other hand, it is the case that many of the above‐mentioned outcome measures and scales are validated, have good reliability, and are widely used in the literature.
Associated interventions may also play a role in the final outcome and may have influenced some of the favourable results observed in Comparison 1. As an example, a study comparing BoNT‐A injections associated with an intensive physiotherapy protocol versus isolated intensive physiotherapy failed to show differences in GMFM scores between groups (Navarrete 2010). Other studies involving the same rehabilitation protocol in the intervention and control groups failed to show differences between groups for the same outcome measure (Boyd 2001; Chaturvedi 2013; Reddihough 2002). However, a study comparing the multilevel application of BoNT‐A associated with intensive physical therapy versus usual care observed higher functional scores in the intervention group (Scholtes 2006). The current standards for spasticity treatment in children with CP focus on a combined approach, whereby different methods are used simultaneously to improve treatment outcomes. We believe that BoNT‐A may potentiate the effect of a comprehensive physical therapy protocol, for example by helping the child to achieve their preset rehabilitation goals. However, focal BoNT‐A injections alone may not be enough to change global motor function scores significantly.
Another issue relates to our choice of primary and secondary outcomes. This review is an update of a previously published review, Ade‐Hall 2000, in which these outcomes had already been established but were retained as still being current and relevant. Our assessments focused on gait pattern, function, joint range of motion, satisfaction, quality of life, and spasticity. These outcomes encompass most domains of the International Classification of Functioning, Disability and Health (WHO 2001), although the actual method used to measure these outcomes may be questionable, since the sensitivity of some of the tools employed may not have been adequate to detect changes postintervention. For example, in Comparison 2, standard motor scales such as the GMFM were not able to detect differences between groups, whilst studies using individualised measures, such as COPM, reported a short‐term improvement in performance in the intervention group.
Since treatment goals are so distinct between individuals at GMFCS levels I to III and those at GMFCS levels IV to V, different primary outcomes might have been more appropriate for each group. For example, gait analysis is only an adequate primary outcome for individuals at GMFCS levels I to III. In addition, since non‐ambulatory patients tend to present with other medical comorbidities, one could assume that the rate of adverse events would be higher in this group. However, a separate analysis of adverse events between GMFCS levels I to III and GMFCS levels IV or V was not possible, since data could not be extracted separately from studies involving individuals from both groups. Only one study, Copeland 2014, analysed the rate of adverse events after BoNT‐A injections compared to placebo solely in children at GMFCS levels IV and V. The study authors found a higher rate of adverse events in the BoNT‐A group when evaluating all reported events (most were considered mild); however, when only moderate and serious adverse events were considered, there was no difference in the rate of adverse events. A detailed analysis of the safety profile of BoNT‐A in their trial was published as a separate report (Edwards 2015).
Finally, we found some studies in our electronic search that addressed other outcomes that are not of specific interest to this review, such as the evaluation of pain (Barwood 2000), prevention of hip dislocation (Graham 2008; Jozwiak 2007), and surface electromyography patterns (Van der Houwen 2011). These issues were not addressed in our review.
Quality of the evidence
In general, there were a few, small studies per comparison and outcome, which directly impacts our confidence on the effect estimates. Furthermore, a large number of studies had high risk of bias for some of the domains assessed. The main methodological problems amongst the 31 studies included in this review related to randomisation sequence generation, allocation concealment, blinding of participants and personnel, and blinding of outcome assessment; see Figure 2. These criteria were particularly flawed in the studies included in Comparison 1. To a certain extent, the nature of the interventions in this group made it impossible to implement blinding of participants. However, measures to ensure the impartiality of the outcome assessors could have been taken, as was done in most studies included in Comparison 3. The studies included in Comparison 2 were for the most part those with better methodological quality and at low risk of bias in most domains.
These observations are reflected in our evaluations of the quality of the evidence, which we performed using the GRADE approach (Schünemann 2013). The main reasons for downgrading levels of quality were: (1) imprecision due to relatively small sample size; (2) potential sources of bias; and (3) high degree of statistical heterogeneity. In certain situations, where blinding of participants was not possible due to the nature of the interventions, we did not downgrade the quality of the evidence.
Overall, we rated the quality of the evidence for the outcomes assessed in Comparison 1 as very low. We graded the quality of the evidence for the outcomes in Comparison 2 as moderate. In Comparison 3, moderate‐ and low‐quality ratings predominated. We considered the evidence from Comparison 4 to be of very low quality. Our confidence in the effect estimate for the analysed outcomes is thus greater for the studies included in Comparisons 2 and 3.
Potential biases in the review process
We conducted this systematic review in accordance with the criteria and methods prespecified in the Methods section of the previous version of this review (Ade‐Hall 2000). We discussed with the editorial team a few required changes to the original review, to ensure the methods adhered to current Cochrane standards. These differences are described in the Differences between protocol and review section. Amongst them was the decision to subdivide all analyses by time points whenever possible (i.e. short‐, medium‐, and long‐term follow‐up).
In some of our analyses, each lower limb was assessed independently, as reported by the original studies. In such cases we evaluated the potential impact of excluding these studies from the analyses; however, there was no change in the direction of the effect estimate. We downgraded the quality of the evidence by one level in these instances. Another issue relates to the statistical heterogeneity found in some of our forest plots, which may be inadequately estimated and may be related to the small number of studies and participants included in each analysis.
Our search strategy was very comprehensive and initially resulted in the identification of a very large number of studies. Two review authors independently screened titles and abstracts, thus reducing the risk of missing a relevant study. Nevertheless, the possibility remains, particularly for unpublished reports, or those published in the grey literature or presented at conferences.
Contact with the study authors provided additional information for the Ackman 2005 study only. Specifically, the study authors provided a table with their detailed results, which had only been available in graphs in the published report.
Agreements and disagreements with other studies or reviews
Since the initial studies on the use of BoNT‐A in CP by Koman and colleagues (Koman 1993; Koman 1994), a significant body of evidence has been published on this subject.
Studies with lower levels of evidence presented mixed results, but tended to demonstrate the effectiveness of BoNT‐A in the treatment of lower limb spasticity in CP, especially in younger patients (Gormley 2001; Lee 2014; Molenaers 2013; Wissel 1999; Wong 1998; Yang 1999). In one of the largest retrospective series analysing the safety profile of BoNT‐A, Naidu 2010 evaluated a total of 1980 applications in 1147 children, and reported a low rate of severe adverse events; however, their incidence was associated with GMFCS levels and BoNT‐A dose.
In a broad review of the literature, we found five other relevant, but less comprehensive, systematic reviews (Cardoso 2006; Druschel 2013; García Salazar 2015; Koog 2010; Ryll 2011).
Cardoso 2006 conducted a systematic review to assess the effectiveness of BoNT‐A in the treatment of spastic equinus in CP. They included six double‐blinded clinical trials, all comparing BoNT‐A injections into the ankle plantarflexors to a placebo group. The review authors found that BoNT‐A was more effective than placebo at improving observational gait analysis and caregivers' perception. Adverse events were observed more frequently in the BoNT‐A group, but were considered mild and self‐limited. The results of this study were similar to those of Comparison 2 in our review, except for the incidence of adverse events, which we found to be similar across groups. During our quantitative data synthesis, we noted that some of the studies included in the Cardoso 2006 review were those reporting a significantly higher number of adverse events in the BoNT‐A group, which, in turn, contributed to high levels of statistical heterogeneity in our analysis.
Druschel 2013 conducted a systematic review to assess the safety and effectiveness of BoNT‐A in children with CP younger than two years of age. They included three RCTs of average methodological quality, only one of which involved children exclusively from this age group at study onset. The review authors stated that it was not possible to conclude whether BoNT‐A promotes the achievement of developmental milestones in this age group. Nevertheless, they found positive evidence regarding the reduction of spasticity, a lower incidence of contractures, and a slower rate of progression to surgical treatment. We found a very small number of studies involving this age group in our review, thus preventing us from carrying out specific analyses of effectiveness and safety for younger patients.
García Salazar 2015 conducted a systematic review to assess the intrinsic muscle properties and functional changes seen after BoNT‐A injections in children with CP. They included 17 studies, both RCTs and prospective non‐randomised studies. The review authors concluded that BoNT‐A does not appear to alter the intrinsic properties of spastic muscles in CP. In addition, they found contradictory results regarding functional improvement, which could be related to the difficulty in performing high‐quality RCTs. We agree with their opinion that studies with a higher risk of bias seem to report more favourable results after BoNT‐A treatment, but we also believe that standardised motor scales may not be sensitive enough to detect changes in these patients. In our review, studies involving individualised function measures had a tendency to report more favourable results for children receiving BoNT‐A treatment.
Koog 2010 conducted a systematic review to evaluate the effectiveness of BoNT‐A in the treatment of ankle plantarflexors spasticity in CP. The review authors included 15 RCTs comparing BoNT‐A injections to a sham or non‐sham control. When analysing the studies comparing BoNT‐A with a non‐sham control, the review authors found differences in favour of the intervention group for the following outcomes: spasticity, range of motion, gait speed, and GMFM. However, in their analysis of studies comparing BoNT‐A with a sham control, only the GMFM scores presented more favourable results in the intervention group. These findings are, in part, similar to our review, which found BoNT‐A to be more effective than usual care or physiotherapy (or both) for most of the outcomes in Comparison 1. However, we also found BoNT‐A to be more effective than placebo or sham (Comparison 2) for a few additional outcome measures not reported by Koog 2010, such as visual gait assessment and satisfaction. On the other hand, we found no difference between groups for standard motor scales (e.g. GMFM) for Comparison 2, which differs from the Koog 2010 findings mentioned above. This difference may be related to the fact that Koog 2010 did not include studies involving treatment of other lower limb muscle groups.
Ryll 2011 conducted a systematic review to assess the effectiveness of BoNT‐A at improving gait in people with CP. They included four RCTs comparing the use of BoNT‐A to usual care, and four others comparing the use of BoNT‐A to ankle serial casting. Regarding the studies that compared BoNT‐A to usual care or physical therapy (or both), the review authors found moderate‐quality evidence in favour of the BoNT‐A group with respect to functional outcomes (visual gait analysis and GMFM according to the study). However, that review was methodologically flawed, as it only considered data reported by the authors of the primary studies; the review authors did not perform a quantitative synthesis by means of a meta‐analysis. Regarding the studies that compared BoNT‐A to the use of serial casting, the review authors found strong evidence suggesting no difference between groups, which is similar to our findings.
Authors' conclusions
Implications for practice.
There is very low‐quality evidence that botulinum toxin type A (BoNT‐A) is more effective than a non‐placebo control at improving gait, function, joint range of motion, spasticity, and caregiver satisfaction in the treatment of lower limb spasticity in children with cerebral palsy (CP), mostly when assessed in the short or medium term.
There is moderate‐quality evidence that BoNT‐A is more effective than placebo or sham at improving gait, range of motion, spasticity, and caregiver satisfaction in this group, mostly when assessed in the short or medium term. There is also moderate‐quality evidence that BoNT‐A is more effective than placebo or sham at improving individualised function measures, but not standard motor function scales.
There is moderate‐ and low‐quality evidence that BoNT‐A is not more effective than serial casting in treating fixed ankle equinus contractures (with regard to gait, function, joint range of motion, spasticity, and caregiver satisfaction) in children with CP.
There is very low‐quality evidence that BoNT‐A is not more effective than orthotics at improving function at medium‐term follow‐up, but is more effective at improving hip joint range of motion and hip adductors spasticity.
Regarding safety, there is moderate‐ and low‐ quality evidence that the overall rate of adverse events with BoNT‐A is similar to placebo and ankle serial casting, but higher than a non‐placebo control group. No separate comparison regarding the rate of adverse events in different Gross Motor Function Classification System (GMFCS) levels was possible.
Implications for research.
Considering a multimodal approach to the management of spasticity in children with CP, we believe that future studies should aim to determine the most effective treatment combinations, rather than performing individual comparisons between BoNT‐A and other methods. The GMFCS level of the participant should be taken into account in order to set goals and to define the most relevant outcomes for each group, perhaps including individualised functional measures and quality of life assessments. Future versions of this review might eventually be split into two different reviews, involving either ambulatory (GMFCS I‐III) or non‐ambulatory (GMFCS IV‐V) children with CP. In addition, a number of specific questions regarding BoNT‐A treatment remain unanswered, including the following.
What age range and functional level benefit most?
What is the best muscle location technique?
What is the best application regimen?
What is the difference in effectiveness between different commercial brands?
What are the potential consequences for muscle structure (muscle atrophy, change in the number and length of muscle fibers, change in the elastic properties, change in strength)?
What are the long‐term effects of these interventions, including in the prevention of muscle contractures?
What's new
| Date | Event | Description |
|---|---|---|
| 7 October 2019 | New search has been performed | Review updated following another top‐up search in October 2018. |
| 29 November 2017 | New citation required and conclusions have changed | Review updated following a top‐up search in June 2017. |
| 3 March 2017 | New search has been performed | Review updated following a new search in August 2014. |
History
Review first published: Issue 1, 2000
| Date | Event | Description |
|---|---|---|
| 12 November 2008 | Amended | Converted to new review format. |
| 18 October 1999 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We acknowledge the Cochrane Developmental, Psychosocial and Learning Problems Review Group, particularly Joanne Duffield, for all the editorial support and invaluable comments, and Margaret Anderson, for her help with our initial search and subsequent update.
We acknowledge Peter Moore and Ruth Ade‐Hall for their previous contributions in the development of the original review and for accepting the change in lead authorship, allowing us to update this review.
We would like to thank Dr Adam Scheinberg, The Royal Children's Hospital, Australia; Dr Dimitris Mavridis, University of Ioannina, Greece; and two other, anonymous reviewers for their helpful comments on an earlier version of this review.
Appendices
Appendix 1. Search strategies
Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library
#1 MeSH descriptor: [Cerebral Palsy] explode all trees #2 cerebral pals* #3 (#1 or #2) #4 MeSH descriptor: [Botulinum Toxins] explode all trees #5 (botulinum or botulinium) and (toxin or toxins) #6 botulin* #7 Botox #8 Dysport #9 Prosigne #10 Xeomin #11 OnabotulinumtoxinA #12 AbobotulinumtoxinA #13 IncobotulinumtoxinA #14 (#4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13) #15 (#3 and #14) in Trials
MEDLINE PubMed
#1 Cerebral palsy[MeSH Terms] #2 cerebral pals* #3 (#1 OR #2) #4 Botulinum toxins[MeSH Terms] #5 (botulinum OR botulinium) AND (toxin OR toxins) #6 botulin* #7 Botox #8 Dysport #9 Prosigne #10 Xeomin #11 OnabotulinumtoxinA #12 AbobotulinumtoxinA #13 IncobotulinumtoxinA #14 (#4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13) #15 (#3 AND #14) #16 randomized controlled trial[Publication Type] #17 controlled clinical trial[Publication Type] #18 (randomized[tiab] or randomised[tiab]) #19 placebo[tiab] #20 drug therapy[sh] #21 randomly[tiab] #22 trial[tiab] #23 groups[tiab] #24 (#16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23) #25 (animals[mh] NOT humans[mh]) #26 (#24 NOT #25) #27 (#15 AND #26)
Embase Ovid
1 cerebral palsy/ 2 cerebral pals$.tw. 3 or/1‐2 4 botulinum toxin/ 5 botulinum toxin A/ 6 ((botulinum or botulinium) adj3 toxin$).tw. 7 botulin$.tw. 8 Botox.tw. 9 Dysport.tw. 10 Prosigne.tw. 11 Xeomin.tw. 12 OnabotulinumtoxinA.tw. 13 AbobotulinumtoxinA.tw. 14 IncobotulinumtoxinA.tw. 15 or/4‐14 16 3 and 15 17 Randomized controlled trial/ 18 controlled clinical trial/ 19 Single blind procedure/ 20 Double blind procedure/ 21 triple blind procedure/ 22 Crossover procedure/ 23 (crossover or cross‐over).tw. 24 ((singl$ or doubl$ or tripl$ or trebl$) adj1 (blind$ or mask$)).tw. 25 Placebo/ 26 placebo.tw. 27 prospective.tw. 28 factorial$.tw. 29 random$.tw. 30 assign$.ab. 31 allocat$.tw. 32 volunteer$.ab. 33 or/17‐32 34 16 and 33
Cumulative Index to Nursing and Allied Health Literature EBSCOhost (CINAHL)
S1 (MH "Cerebral palsy") S2 Cerebral pals* S3 S1 OR S2 S4 (MH "Botulinum toxins") S5 (botulinum OR botulinium) AND (toxin OR toxins) S6 botulin* S7 botox S8 dysport S9 prosigne S10 xeomin S11 OnabotulinumtoxinA S12 AbobotulinumtoxinA S13 IncobotulinumtoxinA S14 S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 S15 S3 AND S14 S16 (MH "Clinical Trials+") S17 PT Clinical trial S18 TX clinic* n1 trial* S19 TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) S20 TX randomi* control* trial* S21 (MH "Random Assignment") S22 TX random* allocat* S23 TX placebo* S24 (MH "Placebos") S25 (MH "Quantitative Studies") S26 TX allocat* random* S27 S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 S28 S15 AND S27
Science Citation Index (SCI); Conference Proceedings Citation Index ‐ Science (CPCI‐S); both Web of Science
# 7 #6 AND #5 # 6 TS=(random* or placebo* or assign* or allocat* or "cross‐ove"r or crossover or control or controls or ((singl* or doubl* or tripl* or trebl*) near/1 (blind* or mask*)) or prospective or factorial*) # 5 #4 AND #1 # 4 #3 OR #2 # 3 TS=(botulin or botox or Dysport or Prosigne or Xeomin or OnabotulinumtoxinA or AbobotulinumtoxinA or IncobotulinumtoxinA) # 2 TS= ((botulinum OR botulinium) NEAR/3 (toxin OR toxins)) # 1 TS=("cerebral pals*")
ClinicalTrials.gov
(clinicaltrials.gov)
"botulinum toxin" AND "cerebral palsy"
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP)
"botulinum toxin" AND "cerebral palsy"
Appendix 2. Criteria for judging risk of bias
| Selection bias | ||
| Random sequence generation | Low risk of bias | The investigators describe a random component in the sequence generation process, such as:
|
| High risk of bias | The investigators describe a non‐random component in the sequence generation process, such as:
The investigators describe other non‐random approaches, such as:
|
|
| Unclear risk of bias | There is insufficient information about the sequence generation process to permit a judgement of low or high risk of bias. | |
| Allocation concealment | Low risk of bias | Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation:
|
| High risk of bias | Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on:
|
|
| Unclear risk of bias | There is insufficient information to permit a judgement of low or high risk of bias. | |
| Performance bias | ||
| Blinding of participants and personnel | Low risk of bias | Any one of the following:
|
| High risk of bias | Any one of the following:
|
|
| Unclear risk of bias | Any one of the following:
|
|
| Detection bias | ||
| Blinding of outcome assessment | Low risk of bias | Any one of the following:
|
| High risk of bias | Any one of the following:
|
|
| Unclear risk of bias | Any one of the following:
|
|
| Reporting bias | ||
| Selective outcome reporting | Low risk of bias | The study protocol is available, and all of the study’s prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way. |
| High risk of bias | Any one of the following:
|
|
| Unclear risk of bias | The study protocol is not available, and it is unclear whether the published reports include all expected outcomes. | |
| Other bias | ||
| Bias due to problems not covered elsewhere in the table | Low risk of bias | The study appears to be free of other sources of bias. |
| High risk of bias | There is at least one important risk of bias. | |
| Unclear risk of bias | There may be a risk of bias, but there is either:
|
|
Data and analyses
Comparison 1. BoNT‐A versus usual care or physiotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Gait: observational gait analysis; medium‐term follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2 Gait: speed; short‐term follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3 Gait: speed; medium‐term follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4 Gait: speed; long‐term follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5 Gait: step length; long‐term follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6 Gait (GGI); long‐term follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7 Function; short‐term follow‐up | 2 | 123 | Std. Mean Difference (IV, Random, 95% CI) | 0.59 [0.23, 0.95] |
| 8 Function; medium‐term follow‐up | 4 | 191 | Std. Mean Difference (IV, Random, 95% CI) | 1.04 [0.16, 1.91] |
| 9 Function; long‐term follow‐up | 4 | 199 | Std. Mean Difference (IV, Random, 95% CI) | 0.34 [‐0.33, 1.01] |
| 10 Range of motion: passive ankle dorsiflexion; short‐term follow‐up | 2 | 186 | Mean Difference (IV, Random, 95% CI) | 8.34 [1.19, 15.50] |
| 11 Range of motion: passive ankle dorsiflexion; medium‐term follow‐up | 5 | 272 | Mean Difference (IV, Random, 95% CI) | 6.36 [4.03, 8.69] |
| 12 Range of motion: passive ankle dorsiflexion; long‐term follow‐up | 4 | 250 | Mean Difference (IV, Random, 95% CI) | 6.48 [4.42, 8.53] |
| 13 Range of motion: knee extension (popliteal angle); long‐term follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 14 Satisfaction; long‐term follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 15 Spasticity: ankle plantarflexors; short‐term follow‐up | 2 | 186 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.19 [‐2.62, 0.24] |
| 16 Spasticity: ankle plantarflexors; medium‐term follow‐up | 3 | 226 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.66 [‐2.88, ‐0.43] |
| 17 Spasticity; long‐term follow‐up | 4 | 319 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.91 [‐1.17, ‐0.66] |
| 17.1 Ankle plantarflexors | 4 | 258 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.79 [‐1.04, ‐0.53] |
| 17.2 Hip adductors | 2 | 46 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.50 [‐2.18, ‐0.83] |
| 17.3 Hamstrings | 1 | 15 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.27 [‐2.44, ‐0.11] |
| 18 Sensitivity analysis: function; medium‐term follow‐up | 3 | 153 | Std. Mean Difference (IV, Random, 95% CI) | 1.50 [1.14, 1.86] |
| 19 Sensitivity analysis: function; long‐term follow‐up | 3 | 119 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.33, 0.39] |
| 20 Sensitivity analysis: spasticity: ankle plantarflexors; short‐term follow‐up | 2 | 186 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.74 [‐1.04, ‐0.44] |
| 21 Sensitivity analysis: spasticity: ankle plantarflexors; medium‐term follow‐up | 2 | 83 | Mean Difference (IV, Random, 95% CI) | ‐0.83 [‐0.98, ‐0.67] |
Comparison 2. BoNT‐A versus placebo or sham.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Gait: observational gait analysis; short‐term follow‐up | 4 | 261 | Risk Ratio (M‐H, Random, 95% CI) | 1.66 [1.16, 2.37] |
| 2 Gait: observational gait analysis; medium‐term follow‐up | 3 | 248 | Risk Ratio (M‐H, Random, 95% CI) | 1.90 [1.32, 2.74] |
| 3 Gait: kinematics; short‐term follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Peak ankle dorsiflexion in stance | 1 | 19 | Mean Difference (IV, Random, 95% CI) | 15.9 [4.87, 26.93] |
| 3.2 Peak ankle dorsiflexion in swing | 1 | 19 | Mean Difference (IV, Random, 95% CI) | 10.2 [4.01, 16.39] |
| 4 Function; short‐term follow‐up | 4 | 305 | Std. Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.35, 0.83] |
| 4.1 Standard measures | 2 | 108 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.66, 0.10] |
| 4.2 Individualised measures | 2 | 197 | Std. Mean Difference (IV, Random, 95% CI) | 0.71 [0.43, 1.00] |
| 5 Function; medium‐term follow‐up | 5 | 327 | Std. Mean Difference (IV, Random, 95% CI) | 0.28 [0.06, 0.49] |
| 5.1 Standard measures | 2 | 109 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.32, 0.43] |
| 5.2 Individualised measures | 3 | 218 | Std. Mean Difference (IV, Random, 95% CI) | 0.39 [0.12, 0.66] |
| 6 Function; long‐term follow‐up | 2 | 91 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.48, 0.35] |
| 6.1 Standard measures | 1 | 58 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.73, 0.30] |
| 6.2 Individualised measures | 1 | 33 | Std. Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.49, 0.88] |
| 7 Function (GMFM improvement); medium‐term follow‐up | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8 Range of motion: passive ankle dorsiflexion; short‐term follow‐up | 3 | 291 | Mean Difference (IV, Random, 95% CI) | 2.68 [0.12, 5.23] |
| 9 Range of motion: passive ankle dorsiflexion; medium‐term follow‐up | 2 | 150 | Mean Difference (IV, Random, 95% CI) | 1.57 [‐2.12, 5.25] |
| 10 Range of motion; long‐term follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.1 Ankle dorsiflexion | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐4.88, 5.28] |
| 10.2 Knee extension | 1 | 19 | Mean Difference (IV, Random, 95% CI) | ‐5.6 [‐13.49, 2.29] |
| 10.3 Hip abduction | 1 | 11 | Mean Difference (IV, Random, 95% CI) | 9.6 [‐9.97, 29.17] |
| 11 Quality of life (CPQOL); short‐term follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 12 Quality of life (CPQOL); medium‐term follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 13 Satisfaction (subjective scores); short‐term follow‐up | 2 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.72, 2.01] |
| 14 Satisfaction (subjective scores); medium‐term follow‐up | 2 | 111 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.94, 1.83] |
| 15 Satisfaction (COPM); short‐term follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 16 Satisfaction (COPM); medium‐term follow‐up | 2 | 74 | Mean Difference (IV, Random, 95% CI) | 0.96 [0.04, 1.88] |
| 17 Satisfaction (COPM); long‐term follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 18 Spasticity: ankle plantarflexors; short‐term follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 19 Spasticity: ankle plantarflexors; medium‐term follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 20 Spasticity; long‐term follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 20.1 Ankle plantarflexors | 1 | 42 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.58, 0.78] |
| 20.2 Hamstrings | 1 | 10 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐2.06, 0.06] |
| 20.3 Hip adductors | 1 | 13 | Mean Difference (IV, Random, 95% CI) | 0.1 [‐0.79, 0.99] |
| 21 Adverse events | 12 | 918 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.87, 1.93] |
| 22 Sensitivity analysis: adverse events | 11 | 860 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.90, 1.99] |
Comparison 3. BoNT‐A versus serial casting.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Gait: observational gait analysis (PRS scores); short‐term follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2 Gait: observational gait analysis (PRS scores); medium‐term follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3 Gait: observational gait analysis (PRS scores); long‐term follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4 Gait: kinematics; short‐term follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Ankle dorsiflexion at initial contact | 1 | 21 | Mean Difference (IV, Random, 95% CI) | 2.90 [‐2.90, 8.70] |
| 4.2 Peak ankle dorsiflexion in stance | 1 | 21 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐5.78, 4.58] |
| 5 Gait: kinematics; medium‐term follow‐up | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Ankle dorsiflexion at initial contact | 2 | 47 | Mean Difference (IV, Random, 95% CI) | 6.59 [1.39, 11.78] |
| 5.2 Peak ankle dorsiflexion in stance | 3 | 83 | Mean Difference (IV, Random, 95% CI) | 3.03 [‐3.56, 9.62] |
| 5.3 Peak ankle dorsiflexion in swing | 2 | 62 | Mean Difference (IV, Random, 95% CI) | 0.11 [‐4.17, 4.38] |
| 6 Gait: kinematics; long‐term follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Ankle dorsiflexion at initial contact | 1 | 26 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐6.37, 5.17] |
| 6.2 Peak ankle dorsiflexion in stance | 1 | 26 | Mean Difference (IV, Random, 95% CI) | ‐2.0 [‐8.50, 4.50] |
| 6.3 Peak ankle dorsiflexion in swing | 1 | 26 | Mean Difference (IV, Random, 95% CI) | 3.70 [‐0.80, 8.20] |
| 7 Gait: speed; medium‐term follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8 Gait: speed; long‐term follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9 Function; medium‐term follow‐up | 2 | 41 | Mean Difference (IV, Random, 95% CI) | 3.64 [‐1.55, 8.82] |
| 10 Function (GMFM goal scores); short‐term follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 11 Function (GMFM goal scores); long‐term follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 12 Range of motion: passive ankle dorsiflexion; short‐term follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 13 Range of motion: passive ankle dorsiflexion; medium‐term follow‐up | 3 | 93 | Mean Difference (IV, Random, 95% CI) | 1.82 [‐2.26, 5.91] |
| 14 Range of motion: passive ankle dorsiflexion; long‐term follow‐up | 2 | 57 | Mean Difference (IV, Random, 95% CI) | ‐1.02 [‐5.63, 3.58] |
| 15 Spasticity: ankle plantarflexors; short‐term follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 16 Spasticity: ankle plantarflexors; medium‐term follow‐up | 3 | 93 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.25, 0.52] |
| 17 Spasticity: ankle plantarflexors; long‐term follow‐up | 2 | 57 | Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.34, 0.67] |
| 18 Adverse events | 3 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.03, 11.03] |
| 19 Sensitivity analyses: gait: kinematics (ankle dorsiflexion in stance) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 19.1 Short‐term follow‐up | 1 | 21 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐5.78, 4.58] |
| 19.2 Medium‐term follow‐up | 2 | 62 | Mean Difference (IV, Random, 95% CI) | 0.12 [‐4.56, 4.81] |
| 19.3 Long‐term follow‐up | 1 | 26 | Mean Difference (IV, Random, 95% CI) | ‐2.0 [‐8.50, 4.50] |
Comparison 4. BoNT‐A versus orthoses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Function (GMFM total scores); medium‐term follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2 Range of motion: passive hip abduction; medium‐term follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Spasticity: hip adductor; medium‐term follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ackman 2005.
| Methods |
Method of randomisation: a block design randomisation sequence was used, in a way that each child enrolled in any centre was randomly assigned to 1 of the 3 groups. Randomisation stratified by topographic pattern of CP. Blinding: the evaluating clinician, parents, and children were blinded. The physician or site co‐ordinator was aware of the group to which the child had been randomised. Intention‐to‐treat analysis: yes Loss of follow‐up: 5 children
Unit of analysis: child |
|
| Participants |
Place: 5 centres in the USA Period of study: not described Number assigned: 39 Number assessed: 34
Inclusion criteria:
Exclusion criteria:
Age:
Gender:
Motor distribution:
GMFCS:
|
|
| Interventions | All children received a total of 3 treatments, at baseline and 3 and 6 months. BoNT‐A‐only group:
Placebo/cast group:
BoNT‐A/cast group:
|
|
| Outcomes |
Length of follow‐up:
Primary outcomes:
Secondary outcomes:
|
|
| Notes |
Comments:
Source of funding: funded by an unrestricted educational grant by Allergan Inc. Conflicts of interest: not reported, but the grant mentioned above could be considered as a possible conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: not described clearly, but the block sequence is likely to have been computer generated |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described clearly, but the study authors state that the randomisation list was provided only to the individual responsible for co‐ordinating the injection procedure |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: evaluating clinicians and parents were not aware whether children received BoNT‐A or placebo injections. The study authors state that parents of children in the BoNT‐A‐only group were instructed not to discuss the treatment with the assessing clinician. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: individuals responsible for data analysis were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study protocol was not available. The relevant outcomes seem to have been reported. |
| Other bias | Unclear risk | Comment: the initial power calculations determined the need for 25 children in each group. However, even though 90 children met the inclusion criteria, the authors reported a refusal rate higher than 50%. |
Baker 2002.
| Methods |
Method of randomisation: computer‐generated sequence with block randomisation, stratified by centre and baseline spasticity component Blinding: all participants and personnel blinded to the intervention Intention‐to‐treat analysis: yes Loss of follow‐up: 2
Unit of analysis: lower limb for local measures and child for global measures |
|
| Participants |
Place: 6 centres in the UK, 1 in Ireland, and 5 in Poland Period of study: not described Number assigned: 126 Number assessed: 124
Inclusion criteria:
Exclusion criteria:
Age:
Gender:
Motor distribution:
GMFCS:
|
|
| Interventions |
BoNT‐A groups:
Placebo group:
|
|
| Outcomes |
Length of follow‐up:
Primary outcomes:
Secondary outcomes:
|
|
| Notes |
Comments:
Source of funding: Ipsen Limited Conflicts of interest: not reported, but the grant mentioned above could be considered as a possible conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Comment: allocation concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: group assignments were not released until the final assessment and completion of data analysis |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: study protocol not available. The relevant outcomes seem to have been reported. |
| Other bias | Low risk | Comment: no other sources of bias identified |
Barwood 2000.
| Methods |
Method of randomisation: randomisation performed using envelopes prepared in blocks of 8, with equal numbers of each instruction in each group (restricted randomisation) Blinding: the clinician who injected BoNT‐A was not blinded. There was blinding of children, parents, carers, and other investigators. Intention‐to‐treat analysis: no Loss of follow‐up: no losses to follow‐up Unit of analysis: child |
|
| Participants |
Place: 1 centre in Australia Period of study: not described Number assigned: 16 Number assessed: 16
Inclusion criteria:
Exclusion criteria:
Age:
Gender:
Motor distribution:
GMFCS:
|
|
| Interventions |
BoNT‐A group:
Placebo group:
|
|
| Outcomes |
Length of follow‐up:
Primary outcomes:
Secondary outcomes:
|
|
| Notes |
Comments: none Source of funding: 1 of the study authors, S Barwood, is funded by the Brockhoff Foundation, and another, R Boyd, is funded in part by a National Health and Medical Research Council Grant (no. 980753) Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: predetermined list (block randomisation) |
| Allocation concealment (selection bias) | Low risk | Comment: allocation done using opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: participants blinded to intervention. The clinician who injected the BoNT‐A was not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: other investigators were unaware of group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no incomplete outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: study protocol not available. Most of the relevant outcomes were reported. |
| Other bias | Low risk | Comment: no other sources of bias |
Bjornson 2007.
| Methods |
Method of randomisation: block randomisation stratified by age and the use of oral baclofen. Sequence generation method not described Blinding: the investigators, study co‐ordinators, physical therapists, and participants were blinded to the intervention Intention‐to‐treat analysis: no Loss of follow‐up: no Unit of analysis: child |
|
| Participants |
Place: 1 centre in the USA Period of study: October 1997 to September 2001 Number assigned: 33 Number assessed: 33
Inclusion criteria:
Exclusion criteria:
Age:
Gender:
Motor distribution:
GMFCS:
|
|
| Interventions |
BoNT‐A group:
Placebo group:
|
|
| Outcomes |
Length of follow‐up:
Primary outcomes:
Secondary outcomes:
|
|
| Notes |
Comments:
Source of funding: National Center for Medical Rehabilitation Research and National Institutes of Health (RO1 HD35750). BoNT‐A was provided without charge by Allergan. Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation method not clearly described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: the investigators, study co‐ordinators, physical therapists, and participants were blinded to the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: the outcome assessors were blinded to the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no incomplete outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: study protocol not available. Most of the relevant outcomes were reported. |
| Other bias | Unclear risk | Comment: the study authors describe an inclusion rate of 55% of all eligible patients |
Boyd 2001.
| Methods |
Method of randomisation: computer‐generated predetermined list. Randomisation stratified by motor distribution, age at the time of entry, and migration percentage. Blinding: no blinding of participants and personnel. GMFM assessment was videotaped and analysed by 2 observers blinded to treatment. Decision to exit study to surgery done by a blinded observer. Intention‐to‐treat analysis: yes Loss of follow‐up:
Unit of analysis: child for global measures. Hip migration percentage analysed per limb. |
|
| Participants |
Place: 2 centres in Australia, Boyd 2001a, and 4 centres in Australia, Graham 2008 Period of study: patients recruited between 1997 and 2001 Number assigned: 39 Boyd 2001a; 91 Graham 2008 Number assessed: 35 Boyd 2001a; 85 Graham 2008
Inclusion criteria:
Exclusion criteria:
Age:
Gender:
Motor distribution:
GMFCS:
|
|
| Interventions |
BoNT‐A group:
Control group:
|
|
| Outcomes |
Length of follow‐up:
Primary outcomes:
Secondary outcomes:
|
|
| Notes |
Comments:
Source of funding: supported by the National Health and Medical Research Council of Australia (NHMRC; grant no. 980753) and the Murdoch Children's Research Institute, The Royal Hobart Hospital Research Foundation, Tascare Society for Children, and the St John's Rehabilitation service. SWASH orthoses for the study were provided by Camp Ltd, Sweden. Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: computer‐generated, predetermined list |
| Allocation concealment (selection bias) | Low risk | Comment: central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding of participants and personnel involved in treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: GMFM assessment was videotaped and analysed by 2 observers blinded to treatment. Hip migration percentage assessment was not blinded, but these data were not used for the purposes of this review. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no incomplete outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: study protocol not available. It appears that the relevant outcomes were addressed. |
| Other bias | Low risk | Comment: no other sources of bias identified |
Chaturvedi 2013.
| Methods |
Method of randomisation: randomisation stratified by GMFCS and age. Sequence generation method unclear Blinding: no blinding described Intention‐to‐treat analysis: no Loss of follow‐up: no Unit of analysis: child |
|
| Participants |
Place: 1 centre in India Period of study: not described Number assigned: 36 Number assessed: 36
Inclusion criteria:
Exclusion criteria:
Age:
Gender:
Motor distribution:
GMFCS:
|
|
| Interventions |
BoNT‐A group:
Control group:
|
|
| Outcomes |
Length of follow‐up:
Primary outcomes:
Secondary outcomes:
|
|
| Notes |
Comments:
Source of funding: supported by a grant from the Department of Science and Technology (DST No. SR/HO/HS‐125/2007) Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding described |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no blinding described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no incomplete outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: study protocol not available. It appears that the relevant outcomes were addressed. |
| Other bias | Low risk | Comment: no other sources of bias identified |
Copeland 2014.
| Methods |
Method of randomisation: randomisation stratified by the primary goal area (upper limb or lower limb), as described in the previously published study protocol. Sequence generation method not described, but concealed allocation was reported. Blinding: participants, parents, and assessors masked to group allocation Intention‐to‐treat analysis: yes Loss of follow‐up: no Unit of analysis: child |
|
| Participants |
Place: 1 centre in Australia Period of study: March 2009 to August 2011 Number assigned: 41 Number assessed: 41
Inclusion criteria:
Exclusion criteria:
Age:
Gender:
Motor distribution:
GMFCS:
|
|
| Interventions |
BoNT‐A group:
Placebo group:
|
|
| Outcomes |
Length of follow‐up:
Primary outcomes:
Secondary outcomes:
|
|
| Notes |
Comments:
Source of funding: supported by Queensland CP Health Service, which received an unrestricted educational grant (10268 to SD and LGP) through the Royal Children’s Hospital Foundation from Allergan Australia. Conflicts of interest: the study authors declare no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: sequence generation not clearly described, but the study authors described in their previously published protocol that "randomization would be performed using concealed allocation" (quote), and block randomisation would be done. Likely computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Comment: concealed allocation is described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: participants and parents were masked to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: assessors were masked to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | Comment: no selective reporting. The study protocol was previously published and available. |
| Other bias | Low risk | Comment: no other sources of bias identified |
Corry 1998.
| Methods |
Method of randomisation: 1 of 20 cards containing the instruction BoNT‐A or cast was drawn for each participant. Randomisation was restricted to ensure 10 participants in each group. Blinding: no blinding of participants and personnel providing treatment. The surgeon assessing the postintervention gait videotapes was blinded to the treatment received. Intention‐to‐treat analysis: no Loss of follow‐up: no Unit of analysis: lower limb |
|
| Participants |
Place: 1 centre in Ireland Period of study: not described Number assigned: 20 Number assessed: 20
Inclusion criteria:
Exclusion criteria:
Age:
Gender:
Motor distribution:
GMFCS:
|
|
| Interventions |
BoNT‐A group:
Control group:
|
|
| Outcomes |
Length of follow‐up:
Primary outcomes:
|
|
| Notes |
Comments:
Source of funding: supported by The Royal Belfast Hospital for Sick Children, the Medical Research Council, the MITRE trust, the Canon Gribben Legacy, and the Helen C Young Trust Fund Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: 1 of 20 cards containing instructions for BoNT‐A or cast was drawn for each child (10 cards for each group) |
| Allocation concealment (selection bias) | Low risk | Comment: adequate allocation concealment. Instructions written in identical, sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding of participants and personnel providing treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: the surgeon assessing the postintervention gait videotapes was blinded to the treatment received |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: gait analysis data were not available for 3 children in the BoNT‐A group and 5 children in the cast group, as described in the 'Notes' section above. |
| Selective reporting (reporting bias) | Unclear risk | Comment: study protocol not available. It appears that the relevant outcomes were addressed. |
| Other bias | Low risk | Comment: no other sources of bias identified |
Delgado 2016.
| Methods |
Method of randomisation: randomisation done using the sponsor's computer‐generated scheme, and stratified according to age and to BoNT‐A naive/non‐naive status Blinding: participants, parents, and assessors blinded to group allocation Intention‐to‐treat analysis: yes Loss of follow‐up: 13 children discontinued treatment
Unit of analysis: child |
|
| Participants |
Place: 23 centres from 6 countries (USA, Chile, France, Mexico, Poland, and Turkey) Period of study: July 2011 to February 2014 Number assigned: 241 Number assessed: 228
Inclusion criteria:
Exclusion criteria:
Age:
Gender:
Motor distribution:
GMFCS:
|
|
| Interventions |
BoNT‐A groups:
Placebo group:
|
|
| Outcomes |
Length of follow‐up:
Primary outcomes:
Secondary outcomes:
|
|
| Notes |
Comments:
Source of funding: Ipsen Conflicts of interest: all study authors (external from Ipsen) have been investigators in Ipsen‐sponsored clinical trials (including the current study), and they or their institutions have received payment for participation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: computer‐generated list |
| Allocation concealment (selection bias) | Low risk | Comment: not clearly described, but randomisation was made by the sponsor. Presumably central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: participants and personnel were blinded to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: outcome assessors were blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing outcome data |
| Selective reporting (reporting bias) | Low risk | Comment: no selective reporting. Study protocol previously registered and available at ClinicalTrials.gov (NCT01249417). |
| Other bias | Low risk | Comment: no other sources of bias |
El‐Etribi 2004.
| Methods |
Method of randomisation: not described Blinding: not described. Participants and personnel were likely not blinded due to the nature of the interventions. Intention‐to‐treat analysis: no Loss of follow‐up: no Unit of analysis: not described clearly, but likely lower limb for local measures and child for global measures |
|
| Participants |
Place: 1 centre in Egypt Period of study: March 2001 to March 2003 Number assigned: 40 Number assessed: 40
Inclusion criteria:
Exclusion criteria:
Age:
Gender:
Motor distribution:
GMFCS:
|
|
| Interventions |
BoNT‐A group:
Control group:
|
|
| Outcomes |
Length of follow‐up:
Primary outcomes:
|
|
| Notes |
Comments:
Source of funding: not reported Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and personnel likely not blinded due to the nature of the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: not described, but likely not given the nature of the interventions and assessments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no incomplete outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: study protocol not available. It appears that the relevant outcomes were addressed. |
| Other bias | Low risk | Comment: no other sources of bias identified |
Flett 1999.
| Methods |
Method of randomisation: sequence generation not clearly described. Allocation carried out by the hospital pharmacy. Blinding: participants and person administering treatment were aware of treatment allocation. Research clinicians were blinded to the intervention arms. Intention‐to‐treat analysis: no Loss of follow‐up: 2 dropouts from the BoNT‐A group ("one for social reasons, and the other at parental request because of the perceived need to treat differently by a combination of botulinum toxin A and fixed plaster treatment") Unit of analysis: lower limb for local measures and likely child for global measures |
|
| Participants |
Place: 1 centre in Australia Period of study: not described Number assigned: 20 Number assessed: 18
Inclusion criteria:
Exclusion criteria:
Age:
Gender:
Motor distribution:
GMFCS:
|
|
| Interventions |
BoNT‐A group:
Casts group:
|
|
| Outcomes |
Length of follow‐up:
Primary outcomes:
|
|
| Notes |
Comments: none Source of funding: Crippled Children’s Association Research and Development Fund Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not clearly described |
| Allocation concealment (selection bias) | Low risk | Comment: central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: study protocol not available. It appears that the relevant outcomes were addressed. |
| Other bias | Low risk | Comment: no other sources of bias identified |
Hazneci 2006.
| Methods |
Method of randomisation: randomisation procedure not described Blinding: not described, but presumably not Intention‐to‐treat analysis: not described Loss of follow‐up: not described Unit of analysis: not clearly described, but likely lower limb for local measures and child for global measures |
|
| Participants |
Place: 1 centre in Turkey Period of study: not described Number assigned: 43 Number assessed: 43
Inclusion criteria:
Exclusion criteria:
Age:
Gender:
Motor distribution:
GMFCS:
|
|
| Interventions |
BoNT‐A group:
Johnstone pressure splint group:
|
|
| Outcomes |
Length of follow‐up:
Primary outcomes:
|
|
| Notes |
Comments: none Source of funding: not reported Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not described, but likely not given the nature of the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: not described, but likely not given the nature of interventions and assessments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: study protocol not available. It appears that the relevant outcomes were addressed. |
| Other bias | Low risk | Comment: no other sources of bias identified |
Ibrahim 2007.
| Methods |
Method of randomisation: randomisation done by selecting a group from a hat Blinding: presumably there was no blinding Intention‐to‐treat analysis: no Loss of follow‐up: no losses to follow‐up Unit of analysis: child |
|
| Participants |
Place: 1 centre in Jordan Period of study: patients recruited from March 2002 to June 2004 Number assigned: 60 Number assessed: 60
Inclusion criteria:
Exclusion criteria:
Age:
Gender:
Motor distribution:
GMFCS:
|
|
| Interventions |
BoNT‐A groups:
Control group:
|
|
| Outcomes |
Length of follow‐up:
Primary outcomes:
|
|
| Notes |
Comments:
Source of funding: not reported Conflict of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: the groups were assigned by drawing the group number from a hat |
| Allocation concealment (selection bias) | High risk | Comment: no allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not described, but likely not given the nature of the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: not described, but likely not given the nature of the interventions and assessments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: study protocol not available. It appears that the relevant outcomes were addressed. |
| Other bias | Low risk | Comment: no other sources of bias identified |
Jozwiak 2007.
| Methods |
Method of randomization: randomisation done by retrieving white or black balls drawn from lots when patients were enrolled into the study Blinding: no blinding Intention‐to‐treat analysis: no, as there were no losses Loss of follow‐up: no losses to follow‐up Unit of analysis: lower limb |
|
| Participants |
Place: 1 centre in Poland Period of study: between 1998 and 2002 Number assigned: 67 Number assessed: 67
Inclusion criteria:
Exclusion criteria:
Age:
Gender:
Motor distribution:
GMFCS:
|
|
| Interventions |
BoNT‐A group:
Control group:
|
|
| Outcomes |
Length of follow‐up: variable
Primary outcomes:
|
|
| Notes |
Comments:
Source of funding: partially supported by grant KBN no. 3PO5C 038 24 Conflicts of interest: the study authors declared no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: randomisation was done by drawing white or black balls from lots |
| Allocation concealment (selection bias) | High risk | Comment: no allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: study protocol not available. It appears that the relevant outcomes were addressed. |
| Other bias | Low risk | Comment: no other sources of bias identified |
Kanovsky 2004.
| Methods |
Method of randomisation: children were randomly allocated "using a computer system" Blinding: participants and study personnel blinded to the intervention Intention‐to‐treat analysis: no Loss of follow‐up: no losses to follow‐up Unit of analysis: lower limb for local measures and child for global measures |
|
| Participants |
Place: 3 centres in the Czech Republic and 2 in Slovakia Period of study: not described Number assigned: 52 Number assessed: 52
Inclusion criteria:
Exclusion criteria:
Age:
Gender:
Motor distribution:
GMFCS:
|
|
| Interventions |
BoNT‐A group:
Placebo group:
|
|
| Outcomes |
Length of follow‐up:
Primary outcomes:
|
|
| Notes |
Comment: none Source of funding: sponsored by Ipsen Limited Conflicts of interest: not reported, but the funding mentioned above could be considered as a possible conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: randomisation was done "using a computer system" |
| Allocation concealment (selection bias) | Low risk | Comment: central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: both participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: study protocol not available. It appears that the relevant outcomes were addressed. |
| Other bias | Low risk | Comment: no other sources of bias identified |
Kay 2004.
| Methods |
Method of randomisation: children randomly assigned with use of a random number generator Blinding: all investigators except the study co‐ordinator and the physician performing the BoNT‐A injections were blinded to children's group assignments Intention‐to‐treat analysis: no Loss of follow‐up: no losses to follow‐up Unit of analysis: lower limb |
|
| Participants |
Place: 1 centre in the USA Period of study: not described Number assigned: 23 Number assessed: 23
Inclusion criteria:
Exclusion criteria:
Age:
Gender:
Motor distribution:
GMFCS:
|
|
| Interventions |
BoNT‐A + casts group:
Casts group:
|
|
| Outcomes |
Length of follow‐up:
Primary outcomes:
|
|
| Notes |
Comments:
Source of funding: in support of their research or preparation of this manuscript, 1 or more of the study authors received grants or outside funding from Allergan Conflicts of interest: despite the funding mentioned above, the study authors declared no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: children were randomly assigned with the use of a random number generator |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation was not clearly described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding of participants |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: the study authors state that all investigators except the study co‐ordinator and the physician performing the BoNT‐A injections were blinded to children's group assignments. However, we noticed that the study co‐ordinator was responsible for many of the assessments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: study protocol not available. It appears that the relevant outcomes were addressed. |
| Other bias | Low risk | Comment: no other sources of bias identified |
Koman 1994.
| Methods |
Method of randomisation: not described Blinding: double‐blind study Intention‐to‐treat analysis: no Loss of follow‐up: no losses to follow‐up Unit of analysis: lower limb for local measures and child for global measures |
|
| Participants |
Place: 1 centre in the USA Period of study: not described Number assigned: 12 Number assessed: 12
Inclusion criteria:
Exclusion criteria:
Age:
Gender:
Motor distribution:
GMFCS:
|
|
| Interventions |
BoNT‐A group:
Placebo group:
|
|
| Outcomes |
Length of follow‐up:
Primary outcomes:
|
|
| Notes |
Comments:
Source of funding: not reported Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: outcome assessors were blind to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: study protocol not available. It appears that the relevant outcomes were addressed. |
| Other bias | Low risk | Comment: no other sources of bias identified |
Koman 2000.
| Methods |
Method of randomisation: not described Blinding: double‐blind study Intention‐to‐treat analysis: not described, but apparently yes Loss of follow‐up: BoNT‐A group (3 discontinued) and placebo group (3 discontinued). Reasons not stated Unit of analysis: child |
|
| Participants |
Place: multiple centres in the USA, Canada, Italy, and Spain Period of study: not described Number assigned: 114 Number assessed: 108
Inclusion criteria:
Exclusion criteria:
Age:
Gender:
Motor distribution:
GMFCS:
|
|
| Interventions |
BoNT‐A group:
Placebo group:
|
|
| Outcomes |
Length of follow‐up:
Primary outcomes:
|
|
| Notes |
Comments:
Source of funding: this study was supported by a grant from Allergan Inc. The research at Bowman Gray School of Medicine of Wake Forest University was supported by the General Clinical Research Center of the Bowman Gray School of Medicine, grant number M01RR07122. Conflict of interest: not reported, but the grant mentioned above could be considered as a possible conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Low risk | Comment: multicentre study, likely central allocation by the description |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: double‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: the outcome assessors were blind to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing data |
| Selective reporting (reporting bias) | Unclear risk | Comment: study protocol not available. It appears that the relevant outcomes were addressed. |
| Other bias | Unclear risk | Comment: Data from 1 centre (n = 15) were excluded from the efficacy analysis because regulations in that country prohibited the use of placebo in children. |
Love 2001.
| Methods |
Method of randomisation: sequence generation method not described. Pair randomised controlled trial matched by age, GMFM, and MAS Blinding: no blinding Intention‐to‐treat analysis: not described, but apparently yes Loss of follow‐up: BoNT‐A group (3 discontinued) and placebo group (3 discontinued). Reasons not stated Unit of analysis: child |
|
| Participants |
Place: 1 centre in Australia Period of study: not clearly described. Enrolment for 25 months Number assigned: 24 Number assessed: 24
Inclusion criteria:
Exclusion criteria:
Age:
Gender:
Motor distribution:
GMFCS:
|
|
| Interventions |
BoNT‐A group:
Control group:
|
|
| Outcomes |
Length of follow‐up:
Primary outcomes:
|
|
| Notes |
Comments: none Source of funding: supported by a PMH Clinical Research Committee Seeding Grant and the EV Clarke Scholarship Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | High risk | Comment: no allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding due to the nature of the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: not described, but presumably not |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: study protocol not available. It appears that the relevant outcomes were addressed. |
| Other bias | Low risk | Comment: no other sources of bias identified |
Mall 2006.
| Methods |
Method of randomisation: randomisation done using a predetermined list Blinding: both participants and assessors blinded to the intervention Intention‐to‐treat analysis: yes Loss of follow‐up: 4 children lost to follow‐up BoNT‐A group:
Placebo group:
Unit of analysis: not described |
|
| Participants |
Place: 9 centres in Germany and 1 centre in Austria Period of study: not described Number assigned: 61 Number assessed: 57
Inclusion criteria:
Exclusion criteria:
Age:
Gender:
Motor distribution:
GMFCS:
|
|
| Interventions |
BoNT‐A group:
Placebo group:
|
|
| Outcomes |
Length of follow‐up:
Primary outcome:
Secondary outcomes:
|
|
| Notes |
Comments: none Source of funding: not reported Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: randomisation was done using a predetermined list |
| Allocation concealment (selection bias) | Low risk | Quote: "The master list was kept confidential by the drug supplier" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: both participants and personnel were blinded to the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all predetermined outcomes were described in the results, but no means, SD, or P values were given for most of the non‐significant results. This was likely due to space restrictions in the journal. |
| Selective reporting (reporting bias) | Unclear risk | Comment: study protocol not available. It appears that the relevant outcomes were addressed. |
| Other bias | Low risk | Comment: no other sources of bias identified |
Moore 2008.
| Methods |
Method of randomisation: randomisation done using a computer‐generated sequence. Randomisation stratified in blocks of 4 by unilateral or bilateral spasticity. Blinding: both participants and assessors blinded to the intervention Intention‐to‐treat analysis: yes Loss of follow‐up: 6 children lost to follow‐up and 19 children "withdrew from treatment during the study because they discerned no benefits from the injections" BoNT‐A group:
Placebo group:
Unit of analysis: child |
|
| Participants |
Place: 1 centre in the UK Period of study: patients recruited from October 1997 to July 1999 Number assigned: 64 Number assessed: 58
Inclusion criteria:
Exclusion criteria:
Age:
Gender:
Motor distribution:
GMFCS:
|
|
| Interventions |
BoNT‐A group:
Placebo group:
|
|
| Outcomes |
Length of follow‐up:
Primary outcomes:
Secondary outcomes:
Note: range of motion was not considered an outcome by the study author, although it was described and was used for the purposes of this review. |
|
| Notes |
Comments: none Source of funding: the charity Action Medical Research funded this study (grant AP0622) Conflicts of interest: APM has received payment from Ipsen, UK, and other companies promoting botulinum toxins for advice and for educational help. His unit has received funds from them to support other research with botulinum toxins. The remaining study authors report no conflicts. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: randomisation was done using a computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Comment: central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: participants and personnel were blinded to the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: outcome assessors were blinded to the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: study protocol not available. It appears that the relevant outcomes were addressed. |
| Other bias | Low risk | Comment: no other sources of bias identified |
Navarrete 2010.
| Methods |
Method of randomisation: randomisation stratified by GMFCS, age, and gender. Sequence generation not described Blinding: only the outcome assessors were blinded to the intervention Intention‐to‐treat analysis: no Loss of follow‐up: no Unit of analysis: child |
|
| Participants |
Place: 1 centre in Chile Period of study: March to November 2007 Number assigned: 36 Number assessed: 36
Inclusion criteria:
Exclusion criteria:
Age:
Gender:
Motor distribution:
GMFCS:
|
|
| Interventions |
BoNT‐A group:
Control group:
|
|
| Outcomes |
Length of follow‐up:
Primary outcomes:
|
|
| Notes |
Comments: none Source of funding: Rehabilitacion Infantil Teleton, Chile Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomisation was stratified by GMFCS, age, and gender, but sequence generation method not described |
| Allocation concealment (selection bias) | Low risk | Comment: allocation was done by an external evaluator using opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding due to the nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: study protocol not available. It appears that the relevant outcomes were addressed. |
| Other bias | Low risk | Comment: no other sources of bias identified |
Reddihough 2002.
| Methods |
Method of randomisation: sequence generation not described. Children were matched by age and GMFCS level. "Children were randomly assigned to a study group, or allocated to the corresponding group if they could be matched on age and severity to an existing study participant" Blinding: no blinding Intention‐to‐treat analysis: no Loss of follow‐up: 12 children lost to follow‐up ("7 required surgery during the study period and were withdrawn, and 5 left the study due to an inability to continue with the assessment protocol") Unit of analysis: child |
|
| Participants |
Place: 1 centre in Australia Period of study: not described Number assigned: 61 Number assessed: 49
Inclusion criteria:
Exclusion criteria:
Age:
Gender:
Motor distribution:
GMFCS:
|
|
| Interventions | Cross‐over trial Group 1:
Group 2:
|
|
| Outcomes |
Length of follow‐up:
Primary outcomes:
|
|
| Notes |
Comment: none Source of funding: the study authors acknowledge the support of the Royal Children’s Hospital Research Institute, the Murdoch Children's Research Institute (Theme Grant), the Financial Markets Foundation for Children, and the Hugh DT Williamson Foundation, who provided funds for the study Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: sequence generation not described |
| Allocation concealment (selection bias) | High risk | Comment: not described. Likely not, due to the study design and nature of intervention |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding due to the study design (cross‐over) and nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: study protocol not available. It appears that the relevant outcomes were addressed. |
| Other bias | High risk | Comment: cross‐over study design may represent a source of bias |
Scholtes 2006.
| Methods |
Method of randomisation: computer‐generated random blocks of 4 stratified per centre Blinding: no blinding Intention‐to‐treat analysis: no Loss of follow‐up: 1 child from the control group dropped out at the parent's request Unit of analysis: child |
|
| Participants |
Place: 4 centres in the Netherlands Period of study: patients recruited from October 2001 and March 2003 Number assigned:
Number assessed:
Inclusion criteria:
Exclusion criteria:
Age:
Gender:
Motor distribution:
GMFCS
|
|
| Interventions |
BoNT‐A group:
Control group:
|
|
| Outcomes |
Length of follow‐up:
Primary outcomes:
Secondary outcomes:
|
|
| Notes |
Comments:
Source of funding: supported by the Johanna Kinderfonds (grant no. 2000/0145); Prinses Beatrix Fonds (grant no. PGO01‐134), and Stichting Bio‐Kinderrevalidatie, the Netherlands. Study medication was self‐supported by the Department of Rehabilitation Medicine, VU University Medical Center. Conflicts of interest: the study authors declare no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: computer‐generated sequence |
| Allocation concealment (selection bias) | High risk | Comment: not described. Likely no concealed allocation due to the nature of the interventions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: the gait assessment was done by an independent researcher who had no knowledge of the group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: energy cost measures were available only for a subset of children. However, this outcome was not addressed in our review. |
| Selective reporting (reporting bias) | Unclear risk | Comment: study protocol not available. It appears that the relevant outcomes were addressed. |
| Other bias | Low risk | Comment: no other sources of bias identified |
Steenbeek 2005.
| Methods |
Method of randomisation: not described Blinding: no blinding Intention‐to‐treat analysis: no Loss of follow‐up: no Unit of analysis: child |
|
| Participants |
Place: 1 centre in the Netherlands Period of study: not described Number assigned: 11 Number assessed: 11
Inclusion criteria:
Exclusion criteria:
Age:
Gender:
Motor distribution:
GMFCS:
|
|
| Interventions |
Intervention phase:
Baseline phase:
|
|
| Outcomes |
Length of follow‐up:
Primary outcome:
Secondary outcomes:
|
|
| Notes |
Comments:
Source of funding: not reported Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | High risk | Comment: no allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: outcome assessors blinded for the primary outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: study protocol not available. It appears that the relevant outcomes were addressed. |
| Other bias | Unclear risk | Comment: randomised, multiple baseline/treatment phase trial design may be a source of bias. All children received the intervention with 2 different control and treatment phases. |
Sutherland 1999.
| Methods |
Method of randomisation: not described, but central allocation was performed Blinding: double‐blind study Intention‐to‐treat analysis: no Loss of follow‐up: 1 child from the placebo group was unable to complete the second gait study in the time frame allotted due to an unrelated illness Unit of analysis: child |
|
| Participants |
Place: 1 centre in the USA. This study was part of a multicentre trial that evaluated different outcome measures. Period of study: not described Number assigned: 20 Number assessed: 19
Inclusion criteria:
Exclusion criteria:
Age:
Gender:
Motor distribution:
GMFCS:
|
|
| Interventions |
BoNT‐A group:
Placebo group:
|
|
| Outcomes |
Length of follow‐up:
Primary outcomes:
|
|
| Notes |
Comments:
Source of funding: funds were received to support this research from Allergan Inc, Irvine, CA, USA Conflicts of interest: not reported, but the grant mentioned above could be considered as a possible conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Low risk | Comment: central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: both participants and research personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: plantar flexor strength not available for all children, however we considered this study at low risk of bias for this domain since this was not an outcome of interest for this review |
| Selective reporting (reporting bias) | Unclear risk | Comment: study protocol not available. It appears that the relevant outcomes were addressed. |
| Other bias | Low risk | Comment: no other sources of bias identified |
Tedroff 2010.
| Methods |
Method of randomisation: sequence generated by an external statistician. 20 sealed envelopes were produced in blocks of 4. Blinding: no blinding Intention‐to‐treat analysis: no Loss of follow‐up: 1 child in the BoNT‐A group moved abroad prior to the first injection Unit of analysis: child |
|
| Participants |
Place: 1 centre in Sweden Period of study: not described Number assigned: 16 Number assessed: 15
Inclusion criteria:
Exclusion criteria:
Age:
Gender:
Motor distribution:
GMFCS:
|
|
| Interventions |
BoNT‐A group:
Control group:
|
|
| Outcomes |
Length of follow‐up:
Primary outcome:
Secondary outcomes:
|
|
| Notes |
Comments:
Source of funding: supported through the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet, and educational grants from Stiftelsen Frimurare barnhuset i Stockholm, Norrbacka‐Eugeniastiftelsen, Stiftelsen Kempe‐Carlgrenska Fonden, Stiftelsen Sunnerdahls Handikappfond and RBU:s forskningsstiftelse. Allergan Inc sponsored the study with onabotulinumtoxinA and an unrestricted grant. Conflicts of interest: not reported, but the grant mentioned above could be considered as a possible conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: likely adequate sequence generation. Described as a randomisation process that included 20 sealed envelopes, in blocks of 4, produced by an external statistician |
| Allocation concealment (selection bias) | Low risk | Comment: concealed allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding due to the nature of the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no blinding of most of the outcome measures. The outcome assessors of the final 3‐dimensional gait analysis were blinded to the interventions. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing outcome data for the "treatment‐phase" of the study |
| Selective reporting (reporting bias) | Unclear risk | Comment: study protocol not available. It appears that the relevant outcomes were addressed. |
| Other bias | Low risk | Comment: no other sources of bias identified |
Ubhi 2000.
| Methods |
Method of randomisation: individual randomisation code produced by the statistics department. Central allocation Blinding: blinding of participants and personnel Intention‐to‐treat analysis: no Loss of follow‐up: no Unit of analysis: child |
|
| Participants |
Place: 1 centre in the UK Period of study: patients recruited from September 1996 to March 1998 Number assigned: 40 Number assessed: 40
Inclusion criteria:
Exclusion criteria:
Age:
Gender:
Motor distribution:
GMFCS:
|
|
| Interventions |
BoNT‐A group:
Placebo group:
|
|
| Outcomes |
Length of follow‐up:
Primary outcome:
Secondary outcomes:
|
|
| Notes |
Comments: none Sources of funding: Northern & Yorkshire Health Authority and the Special Trustees at St James’s University Hospital. Ipsen supplied abobotulinumtoxinA and placebo.Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: individual randomisation code produced by the statistics department |
| Allocation concealment (selection bias) | Low risk | Comment: central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: both participants and research personnel were blinded to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: outcome assessors were blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: similar missing video gait analysis scores amongst groups |
| Selective reporting (reporting bias) | Unclear risk | Comment: study protocol not available. It appears that the relevant outcomes were addressed. |
| Other bias | Low risk | Comment: no other sources of bias identified |
Xu 2006.
| Methods |
Method of randomisation: computer‐generated sequence stratified by Modified Ashworth Scale, Gross Motor Function Measure, and walking speed Blinding: no blinding Intention‐to‐treat analysis: no Loss of follow‐up: no Unit of analysis: lower limb |
|
| Participants |
Place: 1 centre in China Period of study: June 2004 to August 2005 Number assigned: 43 Number assessed: 43
Inclusion criteria:
Exclusion criteria:
Age:
Gender:
Motor distribution:
GMFCS:
|
|
| Interventions |
BoNT‐A group:
Control group:
|
|
| Outcomes |
Length of follow‐up:
Primary outcomes:
|
|
| Notes |
Comments: none Source of funding: not reported Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: computer‐generated sequence |
| Allocation concealment (selection bias) | Unclear risk | Comment: not clearly described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: the study authors describe blinded assessment, but provide no detailed description as to the methods used to mask the assessors. The direct contact with the child makes it difficult to achieve this goal with the specific outcomes used in this study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing data |
| Selective reporting (reporting bias) | Unclear risk | Comment: study protocol not available. It appears that the relevant outcomes were addressed. |
| Other bias | Low risk | Comment: no other sources of bias identified |
Zhu 2016.
| Methods |
Method of randomisation: random number table was used for randomisation Blinding: no blinding Intention‐to‐treat analysis: no Loss of follow‐up: no Unit of analysis: lower limb for local measures and child for global measures |
|
| Participants |
Place: 1 centre in China Period of study: not described Number assigned: 40 Number assessed: 40
Inclusion criteria:
Exclusion criteria:
Age:
Gender:
Motor distribution:
GMFCS
|
|
| Interventions |
BoNT‐A group:
Control group:
|
|
| Outcomes |
Length of follow‐up:
Primary outcomes:
Secondary outcomes:
|
|
| Notes |
Comments: none Source of funding: funded by general science and technology project of Zhengzhou Science and Technology Bureau (153PKJGG164) Conflict of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: random number table |
| Allocation concealment (selection bias) | High risk | Comment: no allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing outcome data |
| Selective reporting (reporting bias) | Low risk | Comment: no selective reporting. Study protocol previously registered and available at www.chictr.org.cn (ChiCTR‐IPR‐15006318) |
| Other bias | Low risk | Comment: no other sources of bias |
Çağlar 2019.
| Methods |
Method of randomisation: block randomisation, no further details given Blinding: all participants and personnel blinded to the intervention Intention‐to‐treat analysis: not described Loss of follow‐up: 0 Unit of analysis: not described clearly, but likely each child was the unit of analysis |
|
| Participants |
Place: 1 centre in Turkey Period of study: not described Number assigned: 30 Number assessed: 30
Inclusion criteria:
Exclusion criteria:
Age:
Gender:
Motor distribution:
GMFCS:
|
|
| Interventions |
BoNT‐A group:
Control group:
|
|
| Outcomes |
Length of follow‐up:
Primary outcomes:
Secondary outcomes:
|
|
| Notes |
Comments:
Source of funding: the study authors report that this study received no outside funding Conflict of interest: the study authors report no conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: the children were divided into the BoNT‐A and control groups by block randomisation (sequences of AB and BA) |
| Allocation concealment (selection bias) | High risk | Comment: no allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants were not blinded due to the nature of the interventions. All rehabilitation protocols were performed by blinded physiotherapists. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: blinding of outcome assessment was not clearly described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: data were not presented for all muscle groups. It is unclear to which muscles the provided MAS and Tardieu Scale data refer. |
| Selective reporting (reporting bias) | Unclear risk | Comment: study protocol not available. Most of the relevant outcomes were reported. |
| Other bias | Low risk | Comment: no other sources of bias identified |
AFO: ankle foot orthosis; BoNT‐A: botulinum toxin type A; CP: cerebral palsy; DQ/IQ: Development Quotient/Intelligence Quotient; GMFCS: Gross Motor Function Classification System; GMFM: Gross Motor Function Measure; IU: international units; MAS: Modified Ashworth Scale; MP: Migration percentage; PEDI: Pediatric Evaluation of Disability Inventory; PT: physical therapy; SD: standard deviation;SE: standard error; SWASH: standing, walking and sitting hip orthosis; U: units.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ackman 1998 | Published as abstract; non‐randomised prospective trial |
| Amirsalari 2011 | BoNT‐A injected in both groups. There was no control group receiving a different intervention. The study compared BoNT‐A injections to BoNT‐A injections + serial casting. |
| Ayllón 2003 | Published as abstract; non‐randomised prospective trial |
| Barber 2013 | BoNT‐A was injected in both groups. There was no control group receiving a different intervention. The study compared single injection with multiple‐injection techniques for children with CP, and a control group of typically developing children. |
| Bostock 2009 | Abstract from conference proceedings. BoNT‐A was injected in both groups. There was no control group receiving a different intervention. |
| Bottos 2003 | BoNT‐A was injected in both groups. There was no control group receiving a different intervention. The study compared BoNT‐A injections to BoNT‐A injections + serial casting. |
| Carraro 2016 | BoNT‐A was injected in both groups. There was no control group receiving a different intervention. The study compared 2 commercial brands of BoNT‐A. |
| Chang 2017 | BoNT‐A was injected in both groups. There was no control group receiving a different intervention. The study compared 2 commercial brands of BoNT‐A. |
| Dabrowski 2018 | Subgroup analysis for a subset of children from the Delgado 2016 study, including only the children who had been previously treated with BoNT‐A |
| Dai 2017 | BoNT‐A was injected in both groups. There was no control group receiving a different intervention. The study compared BoNT‐A + serial casting to BoNT‐A alone. |
| Dalvand 2012 | BoNT‐A was injected in both groups. There was no control group receiving a different intervention. The study compared BoNT‐A injections to BoNT‐A injections + serial casting. |
| Desloovere 2001 | BoNT‐A was injected in both groups. There was no control group receiving a different intervention. The study compared casting before and after BoNT‐A injections. |
| Detrembleur 2002 | BoNT‐A was injected in both groups. There was no control group receiving a different intervention. The study evaluated BoNT‐A injections with and without electrical stimulation. |
| Dimitrijević 2007 | Quasi‐randomised controlled trial comparing BoNT‐A injections to placebo. The randomisation was done as described in personal communication by the author: "The parents selected numbers; children with odd numbers were included in the experimental group and children with even numbers were included in control group". |
| Dincer 2008 | BoNT‐A was injected in both groups. There was no control group receiving a different intervention. The study compared BoNT‐A injections + physiotherapy to BoNT‐A injections + orthosis. |
| Flemban 2018 | BoNT‐A was injected in both groups. There was no control group receiving a different intervention. The study compared BoNT‐A + physiotherapy to BoNT‐A alone. |
| Hansen 2011 | BoNT‐A was injected in both groups. There was no control group receiving a different intervention. The study evaluated BoNT‐A injections with or without the presence of a clown. |
| Hastings‐Ison 2016 | BoNT‐A was injected in both groups. There was no control group receiving a different intervention. The study compared 4‐monthly to 12‐monthly BoNT‐A injections. |
| Hawamdeh 2007 | Non‐randomised study comparing BoNT‐A injections to a control group |
| Hong 2017 | BoNT‐A was injected in both groups. There was no control group receiving a different intervention. The study compared 2 commercial brands of BoNT‐A. |
| Hu 2009 | BoNT‐A was injected in both groups. There was no control group receiving a different intervention. The study compared low‐dose and high‐dose BoNT‐A injections. |
| Javadzadeh 2006 | Abstract from conference proceedings. BoNT‐A was injected in both groups. There was no control group receiving a different intervention. The study compared 3 different doses of BoNT‐A. |
| Jianjun 2013 | BoNT‐A was injected in both groups. There was no control group receiving a different intervention. The study evaluated BoNT‐A injections with and without rehabilitation. |
| Kang 2007 | BoNT‐A was injected in both groups. There was no control group receiving a different intervention. The study evaluated BoNT‐A with and without electrical stimulation. |
| Kanovsky 2009 | BoNT‐A was injected in both groups. There was no control group receiving a different intervention. The study compared yearly to 4‐monthly BoNT‐A injections. |
| Kelly 2019 | BoNT‐A was injected in both groups. There was no control group receiving a different intervention. The study compared a single below‐knee cast to serial casting in children who had received BoNT‐A. |
| Kim 2011 | BoNT‐A was injected in both groups. There was no control group receiving a different intervention. The study compared 2 commercial brands of BoNT‐A. |
| Kurenkov 2017 | BoNT‐A was injected in both groups. There was no control group receiving a different intervention. The study compared 2 commercial brands of BoNT‐A. |
| Kwon 2010 | BoNT‐A was injected in both groups. There was no control group receiving a different intervention. The study compared BoNT‐A injections to BoNT‐A injections + serial casting. |
| Lee 2009 | BoNT‐A was injected in both groups. There was no control group receiving a different intervention. The study compared 2 BoNT‐A dilution techniques. |
| Lee 2011 | BoNT‐A was injected in both groups. There was no control group receiving a different intervention. The study compared BoNT‐A injections to BoNT‐A injections + serial casting. |
| Love 2009 | Abstract from conference proceedings. BoNT‐A was injected in both groups. There was no control group receiving a different intervention. The study compared 2 starting ages for BoNT‐A injections. |
| Mohamed 2001 | Retrospective study |
| Newman 2007 | BoNT‐A was injected in both groups. There was no control group receiving a different intervention. The study compared BoNT‐A injections with immediate or delayed casting. |
| Niu 2014 | BoNT‐A was injected in both groups. There was no control group receiving a different intervention. The study compared low‐dose and high‐dose BoNT‐A injections. |
| Park 2010 | BoNT‐A was injected in both groups. There was no control group receiving a different intervention. The study compared BoNT‐A injections to BoNT‐A injections + serial casting. |
| Peeters 2018 | Abstract from conference proceedings. It is unclear whether the study is a randomised controlled trial. The study assessed muscle length after BoNT‐A injections using ultrasound. No outcomes of interest for this review were analysed. |
| Picelli 2017 | BoNT‐A was injected in both groups. There was no control group receiving a different intervention. The study compared BoNT‐A injections to BoNT‐A + shockwave therapy. |
| Polak 2002 | BoNT‐A was injected in both groups. There was no control group receiving a different intervention. The study compared low‐dose and high‐dose BoNT‐A injections. |
| Richman 1996 | Published in abstract form only; not a randomised controlled trial |
| Robertshaw 2005 | Article is a letter to the editor reporting the results of a non‐randomised trial. |
| Schasfoort 2017 | Only some of the participants were randomised. |
| Schasfoort 2018 | Only some of the participants were randomised. |
| Sätilä 2005 | BoNT‐A was injected in both groups. There was no control group receiving a different intervention. The study compared 2 BoNT‐A injection sites. |
| Sätilä 2006 | Non‐randomised study comparing 2 doses of BoNT‐A |
| Sätilä 2008 | BoNT‐A was injected in both groups. There was no control group receiving a different intervention. The study compared a single injection with multiple‐injection techniques. |
| Thomas 2012 | Abstract from conference proceedings. BoNT‐A was injected in both groups. There was no control group receiving a different intervention. |
| Thorley 2012 | Published protocol for a randomised controlled trial |
| Van Campenhout 2013 | BoNT‐A was injected in all groups. There was no control group receiving a different intervention. The study compared 2 different BoNT‐A injection techniques. |
| Van Campenhout 2015 | BoNT‐A was injected in all groups. There was no control group receiving a different intervention. The study compared 2 different BoNT‐A injection techniques. |
| Wang 2007 | BoNT‐A was injected in all groups. There was no control group receiving a different intervention. The study compared low‐, medium‐, and high‐dose BoNT‐A injections. |
| Wang 2008 | BoNT‐A was injected in all groups. There was no control group receiving a different intervention. The study compared low‐, medium‐, and high‐dose BoNT‐A injections. |
| Williams 2013 | BoNT‐A was injected in all groups. There was no control group receiving a different intervention. The study compared strength training either before or after BoNT‐A injections. |
| Wissel 1999 | BoNT‐A was injected in all groups. There was no control group receiving a different intervention. The study compared low‐dose and high‐dose BoNT‐A injections. |
| Wong 2005 | Non‐randomised study comparing BoNT‐A to selective dorsal rhizotomy |
| Xu 2009 | BoNT‐A was injected in both groups. The study compared 2 BoNT‐A injection techniques. |
| YaJie 2008 | Article is a letter to the editor reporting the results of a non‐randomised prospective study. |
| Zier 2008 | BoNT‐A was injected in all groups. There was no control group receiving a different intervention. The study compared 2 sedation techniques for the injection of BoNT‐A. |
| Zonta 2013 | Non‐randomised prospective study |
BoNT‐A: botulinum toxin type A; CP: cerebral palsy.
Characteristics of studies awaiting assessment [ordered by study ID]
Kim 2018.
| Methods |
Method of randomisation: automated randomisation stratified by baseline Modified Ashworth Scale scores and age Blinding: all participants and personnel were blinded to the intervention Intention‐to‐treat analysis: yes, according to the initial protocol Loss to follow‐up: 8 (2.1%)
Unit of analysis: not described |
| Participants |
Place: multiple centres in Hungary, Italy, Korea, the Philippines, Poland, Russian, Thailand, Turkey, and the USA Period of study: 2012 to 2017 Number assigned: 384 Number assessed: 376
Inclusion criteria:
Exclusion criteria:
Age:
Gender:
Motor distribution:
GMFCS:
|
| Interventions |
BoNT‐A groups:
Placebo group:
|
| Outcomes |
Length of follow‐up:
Primary outcomes:
Secondary outcomes:
|
| Notes |
Comment:
|
BoNT‐A: botulinum toxin type A; CP: cerebral palsy; SD: standard deviation.
Characteristics of ongoing studies [ordered by study ID]
ACTRN12615001162505.
| Trial name or title |
Public title: The impact of Botox on muscle structure and function in children with CP Scientific title: A RCT of the efficacy of first intramuscular botulinum toxin type‐A treatment on muscle structure and function in children with CP: a multicenter trial |
| Methods | Multicentre RCT with 1 group receiving treatment at baseline and the other after a 6‐month waiting period (control) |
| Participants | Ambulatory children with CP and lower limb spasticity Target sample size: 50 Inclusion criteria:
Exclusion criteria:
|
| Interventions | BoNT‐A injections into gastrocnemius, soleus, and tibialis posterior muscles versus control group where no BoNT‐A was offered for a waiting period of 6 months |
| Outcomes | Muscle growth, gait quality, walking speed/step length, lower limb anaerobic power: ramp test, habitual physical activity |
| Starting date | 9 November 2015 |
| Contact information | Lee Barber (email: l.barber@uq.edu.au) |
| Notes |
Comment: none Status: recruiting Date completed: not reported Last updated: 2 June 2017 Sponsor: CP Alliance Research Foundation |
CTRI/2015/03/005642.
| Trial name or title |
Public title: Clinical trial to compare the effect of botulinum toxin versus surgical management of lower limb deformities in children with spastic CP Scientific title: Botulinum toxin versus single event multilevel surgery (SEMLS) in children with lower limb deformities in spastic CP ‐ a randomized comparative trial |
| Methods | RCT |
| Participants |
Sample: children with CP and dynamic deformities of the lower limbs Target sample size: 50 Inclusion criteria:
Exclusion criteria:
|
| Interventions | BoNT‐A injections versus multilevel orthopaedic surgery |
| Outcomes | Improvement in gait pattern, correction of deformity |
| Starting date | 10 August 2012 (recruitment completed, study not published) |
| Contact information | Shah Alam Khan (email: shahalamkhan70@gmail.com) Mohammed Sadiq (email: sadiq22288@yahoo.com) |
| Notes |
Comment: none Status: completed Date completed: not reported. Estimated duration of trial was 2 years. Last updated: 17 March 2017 Sponsor: other (participants would not incur any expense other than the treatment costs) |
NCT02400619.
| Trial name or title |
Public title: Shockwaves therapy and botulinum toxin for the treatment of spasticity in patients with CP: a cross‐over RCT Scientific title: Efficacy of radial extracorporeal shock waves compared to botulinum toxin type A in the treatment of spasticity of the lower extremities in patients with CP: a crossover randomized clinical trial |
| Methods | Cross‐over RCT |
| Participants |
Sample: individuals with CP and lower limb spasticity Target sample size: 70 Inclusion criteria:
Exclusion criteria:
|
| Interventions | BoNT‐A injections versus shockwave therapy |
| Outcomes | Muscular elongation of spastic muscle, participant's perceived pain, if type of GMFCS influences either treatment, and participant and family perception and experience |
| Starting date | September 2014 |
| Contact information | Xavi Vidal Novellas (email not provided) |
| Notes |
Comment:
Status: completed (June 2015) Date completed: December 2016, not published at the time of last electronic search Last updated: 13 March 2017 Sponsor: Xavi Vidal Novellas (email not provided) |
NCT02546999.
| Trial name or title |
Public title: Does botulinum toxin A make walking easier in children with CP? (WE) Scientific title: Does botulinum toxin A make walking easier in children with CP? |
| Methods | RCT |
| Participants |
Sample: ambulatory children with CP and lower limb spasticity Target sample size: 96 Inclusion criteria:
Exclusion criteria:
|
| Interventions | BoNT‐A injections versus placebo |
| Outcomes | Energy cost during walking, activity, perceived improved performance and satisfaction, recurrent musculoskeletal pain, walking capacity, gait pattern, ankle strength, spasticity, self‐perceived effect on walking |
| Starting date | September 2015 |
| Contact information | Siri M Braendvik (email: siri.brendvik@stolav.no) Torstein Vik (email: torstein.vik@ntnu.no) |
| Notes |
Comment: none Status: recruiting Date completed: estimated 31 December 2020 Last updated: 21 August 2019 Sponsor: St Olavs Hospital |
TCTR20150803003.
| Trial name or title |
Public title: Efficacy of botulinum toxin in pediatric spasticity: a randomized trial comparing 2‐ to 4‐sites hamstring injection Scientific title: Efficacy of botulinum toxin in pediatric spasticity: a randomized trial comparing 2‐ to 4‐sites hamstring injection |
| Methods | RCT |
| Participants |
Sample: children with CP and lower limb spasticity Target sample size: 80 Inclusion criteria:
Exclusion criteria:
|
| Interventions | BoNT‐A injections into the hamstrings (2 groups with different injection methods, using either 2 or 4 points) versus control with standard treatment |
| Outcomes | Range of motion, spasticity, gait, motor function, quality of life, satisfaction |
| Starting date | 7 August 2015 |
| Contact information | Chuenchom Chueluecha (email: puaichuenchom@hotmail.com) |
| Notes |
Comment: none Status: recruiting Date completed: not reported Last updated: 16 November 2015 Sponsor: Thammasat University |
BoNT‐A: botulinum toxin type A; CP: cerebral palsy; GMFCS: Gross Motor Function Classification System; RCT: randomised controlled trial.
Differences between protocol and review
Differences between protocol and this review
We did not have access to the original review protocol. All methods used in the current version of this review were based on the methods of the original review (Ade‐Hall 2000).
Differences between original review and this update
Authorship. The leading author of this current review version changed to FB, three other authors were added (JB, MT, JP).
We divided the Background section into subheadings, according to RevMan 5 format (Review Manager 2014), and expanded upon it.
We divided the Types of outcome measures section into subheadings (Primary outcomes and Secondary outcomes), and expanded on it, providing further detail on the outcome measures considered for this review.
We updated our search strategies and databases searched in accordance with current standards (Appendix 1).
We assessed the risk of bias in the included studies using Cochrane's 'Risk of bias' tool (Higgins 2011).
We reported our analyses according to the time points at which outcomes were assessed (Measures of treatment effect).
In accordance with current guidance, we included a 'Summary of findings' table for each comparison (Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9; Table 10). In addition, we used the GRADE approach to rate the quality of the evidence for the outcomes included in these tables (Schünemann 2013).
Contributions of authors
Francesco C Blumetti conceived the necessary changes to the methods in the original review (Ade‐Hall 2000), implemented the search strategy, applied eligibility criteria, assessed studies, extracted and analysed data, analysed risk of bias, assessed the quality of the evidence using the GRADE approach, led the review writing, and has overall responsibility for the review (guarantor).
João Carlos Belloti helped to conceive the necessary changes to the original protocol, acted as an arbitrator when required, and assisted with review writing.
Marcel Jun Tamaoki applied eligibility criteria, assessed studies, extracted data, analysed risk of bias, and assessed the quality of the evidence using the GRADE approach.
José Antonio Pinto oversaw the data analyses and helped to interpret the results, and assisted with review writing (particularly with the introduction, discussion and conclusion).
Sources of support
Internal sources
None, Other.
External sources
None, Other.
Declarations of interest
Francesco C Blumetti ‐ none known. João Carlos Belloti ‐ none known. Marcel Jun Tamaoki ‐ none known. José A Pinto ‐ none known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Ackman 2005 {published and unpublished data}
- Ackman JD, Russman BS, Thomas SS, Buckon CE, Sussman MD, Masso P, et al. Comparing botulinum toxin A with casting for treatment of dynamic equinus in children with cerebral palsy. Developmental Medicine & Child Neurology 2005;47(9):620‐7. [PUBMED: 16138670] [PubMed] [Google Scholar]
- Thomas SS. Systematic review [personal communication]. Email to: FC Blumetti 2 February 2016.
Baker 2002 {published data only}
- Baker R, Jasinski M, Maciag‐Tymecka I, Michalowska‐Mrozek J, Bonikowski M, Carr L, et al. Botulinum toxin treatment of spasticity in diplegic cerebral palsy: a randomized, double‐blind, placebo‐controlled, dose‐ranging study. Developmental Medicine & Child Neurology 2002;44(10):666‐75. [DOI: 10.1017/s0012162201002730; PUBMED: 12418791] [DOI] [PubMed] [Google Scholar]
Barwood 2000 {published data only}
- Barwood S, Baillieu C, Boyd R, Brereton K, Low J, Nattrass G, et al. Analgesic effects of botulinum toxin A: a randomized, placebo‐controlled clinical trial. Developmental Medicine & Child Neurology 2000;42(2):116‐21. [DOI: 10.1017/s0012162200000220; PUBMED: 10698329] [DOI] [PubMed] [Google Scholar]
Bjornson 2007 {published data only}
- Bjornson K, Hays R, Graubert C, Price R, Won F, McLaughlin JF, et al. Botulinum toxin for spasticity in children with cerebral palsy: a comprehensive evaluation. Pediatrics 2007;120(1):49‐58. [DOI: 10.1542/peds.2007-0016; PMC1920182; PUBMED: 17606561] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boyd 2001 {published data only}
- Boyd RN, Dobson F, Parrott J, Love S, Oates J, Larson A, et al. The effect of botulinum toxin type A and a variable hip abduction orthosis on gross motor function: a randomized controlled trial. European Journal of Neurology 2001;8 Suppl 5:109‐19. [PUBMED: 11851739] [DOI] [PubMed] [Google Scholar]
- Graham HK, Boyd R, Carlin JB, Dobson F, Lowe K, Nattrass G, et al. Does botulinum toxin A combined with bracing prevent hip displacement in children with cerebral palsy and "hips at risk"? A randomized, controlled trial. Journal of Bone & Joint Surgery 2008;90(1):23‐33. [DOI: 10.2106/JBJS.F.01416; PUBMED: 18171954] [DOI] [PubMed] [Google Scholar]
Çağlar 2019 {published data only}
Chaturvedi 2013 {published data only}
- Chaturvedi SK, Rai Y, Chourasia A, Goel P, Paliwal VK, Garg RK, et al. Comparative assessment of therapeutic response to physiotherapy with or without botulinum toxin injection using diffusion tensor tractography and clinical scores in term diplegic cerebral palsy children. Brain & Development 2013;35(7):647‐53. [DOI: 10.1016/j.braindev.2012.10.012; PUBMED: 23165172] [DOI] [PubMed] [Google Scholar]
Copeland 2014 {published data only}
- Copeland L, Edwards P, Thorley M, Donaghey S, Gascoigne‐Pees L, Kentish M, et al. Botulinum toxin A for nonambulatory children with cerebral palsy: a double blind randomized controlled trial. Journal of Pediatrics 2014;165(1):140‐6.e4. [DOI: 10.1016/j.jpeds.2014.01.050; PUBMED: 24630348] [DOI] [PubMed] [Google Scholar]
- Edwards P, Sakzewski L, Copeland L, Gascoigne‐Pees L, McLennan K, Thorley M, et al. Safety of botulinum toxin type A for children with nonambulatory cerebral palsy. Pediatrics 2015; Vol. 136, issue 5:895‐904. [DOI: 10.1542/peds.2015-0749; PUBMED: 26482662] [DOI] [PubMed]
Corry 1998 {published and unpublished data}
- Corry IS, Cosgrove AP, Duffy CM, McNeill S, Taylor TC, Graham HK. Botulinum toxin A compared with stretching casts in the treatment of spastic equinus: a randomised prospective trial. Journal of Pediatric Orthopaedics 1998;18(3):304‐11. [PUBMED: 9600553] [PubMed] [Google Scholar]
Delgado 2016 {published data only}
- Delgado MR, Tilton A, Russman B, Benavides O, Bonikowski M, Carranza J, et al. AbobotulinumtoxinA for equinus foot deformity in cerebral palsy: a randomized controlled trial. Pediatrics 2016;137(2):e20152830. [DOI: 10.1542/peds.2015-2830; PUBMED: 26812925] [DOI] [PubMed] [Google Scholar]
- Tilton A, Russman B, Aydin R, Dincer U, Escobar RG, Kutlay S, et al. AbobotulinumtoxinA (Dysport®) improves function according to goal attainment in children with dynamic equinus due to cerebral palsy. Journal of Child Neurology 2017;32(5):482‐7. [DOI: 10.1177/0883073816686910; PMC5405835; PUBMED: 28068857] [DOI] [PMC free article] [PubMed] [Google Scholar]
El‐Etribi 2004 {published data only}
- El‐Etribi MA, Salem ME, El‐Shakankiry HM, El‐Kahky AM, El‐Mahboub SM. The effect of botulinum toxin type‐A injection on spasticity, range of motion and gait patterns in children with spastic diplegic cerebral palsy: an Egyptian study. International Journal of Rehabilitation Research 2004;27(4):275‐81. [PUBMED: 15572990] [DOI] [PubMed] [Google Scholar]
Flett 1999 {published data only}
- Flett PJ, Stern LM, Waddy H, Connell TM, Seeger JD, Gibson SK. Botulinum toxin A versus fixed cast stretching for dynamic calf tightness in cerebral palsy. Journal of Paediatrics and Child Health 1999;35(1):71‐7. [DOI: 10.1046/j.1440-1754.1999.00330.x; PUBMED: 10234640] [DOI] [PubMed] [Google Scholar]
Hazneci 2006 {published data only}
- Hazneci B, Tan AK, Guncikan MN, Dincer K, Kalyon TA. Comparison of the efficacies of botulinum toxin A and Johnstone pressure splints against hip adductor spasticity among patients with cerebral palsy: a randomized trial. Military Medicine 2006;171(7):653‐6. [DOI: 10.7205/milmed.171.7.653; PUBMED: 16895135] [DOI] [PubMed] [Google Scholar]
Ibrahim 2007 {published data only}
- Ibrahim AI, Hawamdeh ZM, Al‐Qudah AA. Functional outcome of botulinum toxin injection of gastrocnemius and adductors in spastic hemiplegic cerebral palsied children. Europa Medicophysica 2007;43(1):13‐20. [PUBMED: 17021584] [PubMed] [Google Scholar]
Jozwiak 2007 {published data only}
- Jozwiak M, Harasymczuk P, Ciemniewska‐Gorzela K. The use of botulinum toxin in the treatment of spastic hip joint instability in children with cerebral palsy. Chirurgia Narzadow Ruchu i Ortopedia Polska 2007;72(3):205‐9. [PUBMED: 17941584] [PubMed] [Google Scholar]
Kanovsky 2004 {published data only}
- Kanovsky P, Bares M, Severa S, Benetin J, Kraus J, Richardson A, et al. Functional benefit of botulinum toxin (Dysport®) in the treatment of dynamic equinus cerebral palsy spasticity: a prospective, multicentre, double‐blind, placebo‐controlled study. Ceska a Slovenska Neurologie a Neurochirurgie 2004;67:16‐23. [Google Scholar]
Kay 2004 {published data only}
- Kay RM, Rethlefsen SA, Fern‐Buneo A, Wren TA, Skaggs DL. Botulinum toxin as an adjunct to serial casting treatment in children with cerebral palsy. Journal of Bone and Joint Surgery 2004;86‐a:2377‐84. [DOI] [PubMed] [Google Scholar]
Koman 1994 {published data only}
- Koman LA, Mooney JF, Smith BP, Goodman A, Mulvaney T. Management of spasticity in cerebral palsy with botulinum‐A toxin: report of a preliminary, randomized, double‐blind trial. Journal of Pediatric Orthopaedics 1994;14(3):299‐303. [DOI] [PubMed] [Google Scholar]
Koman 2000 {published data only}
- Koman LA, Mooney JF 3rd, Smith BP, Walker F, Leon JM. Botulinum toxin type A neuromuscular blockade in the treatment of lower extremity spasticity in cerebral palsy: a randomized, double‐blind, placebo‐controlled trial. BOTOX Study Group. Journal of Pediatric Orthopaedics 2000;20:108‐15. [PubMed] [Google Scholar]
Love 2001 {published data only}
- Love SC, Valentine JP, Blair EM, Price CJ, Cole JH, Chauvel PJ. The effect of botulinum toxin type A on the functional ability of the child with spastic hemiplegia a randomized controlled trial. European Journal of Neurology 2001;8 Suppl 5:50‐8. [PUBMED: 11851734] [DOI] [PubMed] [Google Scholar]
Mall 2006 {published data only}
- Mall V, Heinen F, Siebel A, Bertram C, Hafkemeyer U, Wissel J, et al. Treatment of adductor spasticity with BTX‐A in children with CP: a randomized, double‐blind, placebo‐controlled study. Developmental Medicine & Child Neurology 2006;48(1):10‐3. [DOI: 10.1017/S0012162206000041; PUBMED: 16359588] [DOI] [PubMed] [Google Scholar]
Moore 2008 {published data only}
- Moore AP, Ade‐Hall RA, Smith CT, Rosenbloom L, Walsh HP, Mohamed K, et al. Two‐year placebo‐controlled trial of botulinum toxin A for leg spasticity in cerebral palsy. Neurology 2008;71(2):122‐8. [DOI: 10.1212/01.wnl.0000316801.74683.c0; PUBMED: 18606966] [DOI] [PubMed] [Google Scholar]
Navarrete 2010 {published data only}
- Navarrete ÁA, Peters D, Ruz S. Functional outcome of multilevel botulinum toxin infiltration of the lower limbs and comprehensive therapy in children with spastic cerebral palsy [Resultado funcional de infiltración de toxina botulínica multinivel de las extremidades inferiores y terapia integral en niños con parálisis cerebral espástica]. Rehabilitación 2010;44(3):236‐43. [DOI: 10.1016/j.rh.2009.11.010] [DOI] [Google Scholar]
Reddihough 2002 {published data only}
- Reddihough DS, King JA, Coleman GJ, Fosang A, McCoy AT, Thomason P, et al. Functional outcome of botulinum toxin A injections to the lower limbs in cerebral palsy. Developmental Medicine & Child Neurology 2002;44(12):820‐7. [DOI: 10.1017/s0012162201002997; PUBMED: 12455858] [DOI] [PubMed] [Google Scholar]
Scholtes 2006 {published data only}
- Scholtes VA, Dallmeijer AJ, Knol DL, Speth LA, Maathuis CG, Jongerius PH, et al. Effect of multilevel botulinum toxin A and comprehensive rehabilitation on gait in cerebral palsy. Pediatric Neurology 2007;36(1):30‐9. [DOI: 10.1016/j.pediatrneurol.2006.09.010; PUBMED: 17162194] [DOI] [PubMed] [Google Scholar]
- Scholtes VA, Dallmeijer AJ, Knol DL, Speth LA, Maathuis CG, Jongerius PH, et al. The combined effect of lower‐limb multilevel botulinum toxin type A and comprehensive rehabilitation on mobility in children with cerebral palsy: a randomized clinical trial. Archives of Physical Medicine and Rehabilitation 2006;87(12):1551‐8. [DOI: 10.1016/j.apmr.2006.08.342; PUBMED: 17141633] [DOI] [PubMed] [Google Scholar]
- Houwen LE, Scholtes VA, Becher JG, Harlaar J. Botulinum toxin A injections do not improve surface EMG patterns during gait in children with cerebral palsy ‐ a randomized controlled study. Gait Posture 2011;33(2):147‐51. [DOI: 10.1016/j.gaitpost.2010.11.001; PUBMED: 21190858] [DOI] [PubMed] [Google Scholar]
Steenbeek 2005 {published data only}
- Steenbeek D, Meester‐Delver A, Becher JG, Lankhorst GJ. The effect of botulinum toxin type A treatment of the lower extremity on the level of functional abilities in children with cerebral palsy: evaluation with goal attainment scaling. Clinical Rehabilitation 2005;19(3):274‐82. [DOI: 10.1191/0269215505cr859oa; PUBMED: 15859528] [DOI] [PubMed] [Google Scholar]
Sutherland 1999 {published data only}
- Sutherland DH, Kaufman KR, Wyatt MP, Chambers HG, Mubarak SJ. Double‐blind study of botulinum A toxin injections into the gastrocnemius muscle in patients with cerebral palsy. Gait Posture 1999;10(1):1‐9. [DOI: 10.1016/S0966-6362(99)00012-0; PUBMED: 10469936] [DOI] [PubMed] [Google Scholar]
Tedroff 2010 {published data only}
- Tedroff K, Löwing K, Haglund‐Akerlind Y, Gutierrez‐Farewik E, Forssberg H. Botulinum toxin A treatment in toddlers with cerebral palsy. Acta Paediatrica 2010;99(8):1156‐62. [DOI: 10.1111/j.1651-2227.2010.01767.x; PUBMED: 20222884] [DOI] [PubMed] [Google Scholar]
Ubhi 2000 {published data only}
- Ubhi T, Bhakta BB, Ives HL, Allgar V, Roussounis SH. Randomised double blind placebo controlled trial of the effect of botulinum toxin on walking in cerebral palsy. Archives of Disease in Childhood 2000;83(6):481‐7. [DOI: 10.1136/adc.83.6.481; PMC1718586; PUBMED: 11087280] [DOI] [PMC free article] [PubMed] [Google Scholar]
Xu 2006 {published data only}
- Xu KS, Yan TB, Mai JN. Effects of botulinum toxin guided by electric stimulation on spasticity in ankle plantar flexor of children with cerebral palsy: a randomized trial [Diàn cìjī dìngwèi yǐndǎo ròu dú dúsù zhìliáo nǎoxìngtānhuàn huànér huái zhí qū jī qún jìngluán de duìzhào yánjiū]. Zhonghua Er Ke Za Zhi [Chinese Journal of Pediatrics] 2006;44(12):913‐7. [PUBMED: 17254459] [PubMed] [Google Scholar]
Zhu 2016 {published data only}
- Zhu DN, Wang MM, Wang J, Zhang W, Li HZ, Yang P, et al. Effect of botulinum toxin A injection in the treatment of gastrocnemius spasticity in children aged 9‐36 months with cerebral palsy: a prospective study [Ròu dú gǎnjùn dúsù A zhùshè yè zhìliáo 9‐36 gè yuè nǎotān huàn ér féichángjī jìngluán de qiánzhān xìng yánjiū]. Zhongguo Dang Dai Er Ke Za Zhi [Chinese Journal of Contemporary Pediatrics] 2016;18(2):123‐9. [DOI: 10.7499/j.issn.1008-8830.2016.02.006; PUBMED: 26903058] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Ackman 1998 {published data only}
- Ackman J, Abu‐Faraj Z, Chambers C, Philips B, Davids J. Botulinum toxin treatment of dynamic deformities in an ambulatory spastic cerebral palsy population: a multi‐center study. Gait & Posture 1998;7(2):167. [DOI: 10.1016/S0966-6362(98)90245-4] [DOI] [Google Scholar]
Amirsalari 2011 {published data only}
- Amirsalari S, Dalvand H, Dehghan L, Feizy A, Hosseini SAA, Shamsodini A. The efficacy of botulinum toxin type A injection in the hamstring and calf muscles with and without serial foot casting in gait improvement in children with cerebral palsy. Tehran University Medical Journal 2011; Vol. 69, issue 8:509‐17. [tumj.tums.ac.ir/article‐1‐208‐en.html]
Ayllón 2003 {published data only}
- Ayllón C, Prieto F, Tsuruoka M, Cermignani E, Guerrini de Larrañaga N. Botulin toxin A ‐ treatment of spastic cerebral palsy. Pediatric Research 2003;53:873. [DOI: 10.1203/00006450-200305000-00056] [DOI] [Google Scholar]
Barber 2013 {published data only}
- Barber L, Hastings‐Ison T, Baker R, Kerr Graham H, Barrett R, Lichtwark G. The effects of botulinum toxin injection frequency on calf muscle growth in young children with spastic cerebral palsy: a 12‐month prospective study. Journal of Children's Orthopaedics 2013;7(5):425‐33. [DOI: 10.1007/s11832-013-0503-x; PMC3838523; PUBMED: 24432106] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bostock 2009 {published data only}
- Bostock S, Gibson S, Russo R, Flett P. Who needs serial casting? The profile of children with cerebral palsy who did not require serial casting following botulinum toxin (A) treatment for dynamic equinus. Developmental Medicine & Child Neurology 2009;51(Suppl 2):9. [DOI: 10.1111/j.1469-8749.2008.03237.x] [DOI] [Google Scholar]
Bottos 2003 {published data only}
- Bottos M, Benedetti MG, Salucci P, Gasparroni V, Giannini S. Botulinum toxin with and without casting in ambulant children with spastic diplegia: a clinical and functional assessment. Developmental Medicine & Child Neurology 2003;45(11):758‐62. [DOI: 10.1017/s0012162203001403; PUBMED: 14580131] [DOI] [PubMed] [Google Scholar]
Carraro 2016 {published data only}
- Carraro E, Trevisi E, Martinuzzi A. Safety profile of incobotulinum toxin A (Xeomin®) in gastrocnemious muscles injections in children with cerebral palsy: randomized double‐blind clinical trial. European Journal of Paediatric Neurology 2016;20(4):532‐7. [DOI: 10.1016/j.ejpn.2016.04.008] [DOI] [PubMed] [Google Scholar]
Chang 2017 {published data only}
- Chang HJ, Hong BY, Lee SJ, Lee S, Park JH, Kwon JY. Efficacy and safety of letibotulinum toxin A for the treatment of dynamic equinus foot deformity in children with cerebral palsy: a randomized controlled trial. Toxins 2017;9(8):250. [DOI: 10.3390/toxins9080252; PMC5577586; PUBMED: 28820439] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dabrowski 2018 {published data only}
Dai 2017 {published data only}
- Dai AI, Demiryürek AT. Serial casting as an adjunct to botulinum toxin type A treatment in children with cerebral palsy and spastic paraparesis with scissoring of the lower extremities. Journal of Child Neurology 2017; Vol. 32, issue 7:671‐5. [DOI: 10.1177/0883073817701526; PUBMED: 28393669] [DOI] [PubMed]
Dalvand 2012 {published data only}
- Dalvand H, Dehghan L, Feizi A, Amir Salari S, Hosseini SA, Shamsoddini A. The effect of foot serial casting along with botulinum toxin type‐A injection on spasticity in children with cerebral palsy. Journal of Kerman University of Medical Sciences 2012;19(6):562‐73. [www.sid.ir/en/journal/ViewPaper.aspx?id=288007] [Google Scholar]
Desloovere 2001 {published data only}
- Desloovere K, Molenaers G, Jonkers I, Cat J, Borre L, Nijs J, et al. A randomized study of combined botulinum toxin type A and casting in the ambulant child with cerebral palsy using objective outcome measures. European Journal of Neurology 2001;8 Suppl 5:75‐87. [PUBMED: 11851736] [DOI] [PubMed] [Google Scholar]
Detrembleur 2002 {published data only}
- Detrembleur C, Lejeune TM, Renders A, Bergh PY. Botulinum toxin and short‐term electrical stimulation in the treatment of equinus in cerebral palsy. Movement Disorders 2002;17(1):162‐9. [DOI: 10.1002/mds.1282; PUBMED: 11835456] [DOI] [PubMed] [Google Scholar]
Dimitrijević 2007 {published data only}
- Dimitrijević L, Stanković I, Živković V, Mikov A, Čolović H, Janković I. Botulinum toxin type A for the treatment of spasticity in children with cerebral palsy [Primena botulinskog toksina tip A u lečenju spasticiteta kod dece sa cerebralnom paralizom]. Vojnosanitetski Pregled 2007;64(8):513‐8. [DOI: 10.2298/VSP0708513D; PUBMED: 17874717] [DOI] [PubMed] [Google Scholar]
Dincer 2008 {published data only}
- Dincer Ü, Cakar E, Kiralp MZ, Dursun H. Comparison of the effectiveness of physiotherapy and ankle foot orthosis after botulinum toxin injection in diplegic cerebral palsy patients [Diplejik serebral palsili hastalarda botulinum toksin uygulamasi sonrasinda fizyoterapi ve alt ekstremite ortezinin etkinliginin karsilastirilmasi]. Turkiye Fiziksel Tip ve Rehabilitasyon Dergisi 2008;54(2):41‐5. [www.ftrdergisi.com/uploads/sayilar/235/buyuk/41‐451.pdf] [Google Scholar]
Flemban 2018 {published data only}
- Flemban A, Elsayed W. Effect of combined rehabilitation program with botulinum toxin type A injections on gross motor function scores in children with spastic cerebral palsy. Journal of Physical Therapy Science 2018; Vol. 30, issue 7:902‐5. [DOI: 10.1589/jpts.30.902; PMC6047965; PUBMED: 30034093] [DOI] [PMC free article] [PubMed]
Hansen 2011 {published data only}
- Hansen LK, Kibaek M, Martinussen T, Kragh L, Hejl M. Effect of a clown's presence at botulinum toxin injections in children: a randomized, prospective study. Journal of Pain Research 2011;4:297‐300. [DOI: 10.2147/JPR.S23199; PMC3191928; PUBMED: 22003302 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hastings‐Ison 2016 {published data only}
Hawamdeh 2007 {published data only}
- Hawamdeh ZM, Ibrahim AI, Al‐Qudah AA. Long‐term effect of botulinum toxin (A) in the management of calf spasticity in children with diplegic cerebral palsy. Europa Medicophysica 2007;43(3):311‐8. [PUBMED: 17268388] [PubMed] [Google Scholar]
Hong 2017 {published data only}
- Hong BY, Chang HJ, Lee SJ, Lee S, Park JH, Kwon JY. Efficacy of repeated botulinum toxin type A injections for spastic equinus in children with cerebral palsy ‐ a secondary analysis of the randomized clinical trial. Toxins 2017; Vol. 9, issue 8:253. [DOI: 10.3390/toxins9080253; PMC5577587; PUBMED: 28825663] [DOI] [PMC free article] [PubMed]
Hu 2009 {published data only}
- Hu GC, Chuang YC, Liu JP, Chien KL, Chen YM, Chen YF. Botulinum toxin (Dysport) treatment of the spastic gastrocnemius muscle in children with cerebral palsy: a randomized trial comparing two injection volumes. Clinical Rehabilitation 2009;23(1):64‐71. [DOI: 10.1177/0269215508097861; PUBMED: 19114438] [DOI] [PubMed] [Google Scholar]
Javadzadeh 2006 {published data only}
- Javadzadeh M, Akbari A, Zadeh‐Vakili A, Moghtaderi A, Mahvelati F. Comparing the effectiveness of different doses of botulinum toxin on improvement of walking and reducing the spasticity in spastic diplegic cerebral palsy. European Journal of Pediatrics 2006; Vol. 165, issue Suppl 1:216. [DOI: 10.1007/s00431-006-0349-z] [DOI]
Jianjun 2013 {published data only}
- Jianjun L, Shurong J, Weihong W, Yan Z, Fanyong Z, Nanling L. Botulinum toxin‐A with and without rehabilitation for the treatment of spastic cerebral palsy. Journal of International Medical Research 2013;41(3):636‐41. [DOI: 10.1177/0300060513488515; PUBMED: 23696596] [DOI] [PubMed] [Google Scholar]
Kang 2007 {published data only}
- Kang BS, Bang MS, Jung SH. Effects of botulinum toxin A therapy with electrical stimulation on spastic calf muscles in children with cerebral palsy. American Journal of Physical Medicine & Rehabilitation 2007;86(11):901‐6. [DOI: 10.1097/PHM.0b013e3181520449; PUBMED: 17873826] [DOI] [PubMed] [Google Scholar]
Kanovsky 2009 {published data only}
- Kanovský P, Bares M, Severa S, Richardson A, Dysport Paediatric Limb Spasticity Study Group. Long‐term efficacy and tolerability of 4‐monthly versus yearly botulinum toxin type A treatment for lower‐limb spasticity in children with cerebral palsy. Developmental Medicine & Child Neurology 2009;51(6):436‐45. [DOI: 10.1111/j.1469-8749.2008.03264.x; PUBMED: 19563586] [DOI] [PubMed] [Google Scholar]
Kelly 2019 {published data only}
- Kelly B, MacKay‐Lyons M, Berryman S, Hyndman J, Wood E. Casting protocols following BoNT‐A injections to treat spastic hypertonia of the triceps surae in children with cerebral palsy and equinus gait: a randomized controlled trial. Physical & Occupational Therapy Pediatrics 2019; Vol. 39, issue 1:77‐93. [DOI: 10.1080/01942638.2018.1471015; PUBMED: 29771161] [DOI] [PubMed]
Kim 2011 {published data only}
- Kim K, Shin HI, Kwon BS, Kim SJ, Jung IY, Bang MS. Neuronox versus BOTOX for spastic equinus gait in children with cerebral palsy: a randomized, double‐blinded, controlled multicentre clinical trial. Developmental Medicine & Child Neurology 2011;53(3):239‐44. [DOI: 10.1111/j.1469-8749.2010.03830.x; PUBMED: 21087238] [DOI] [PubMed] [Google Scholar]
Kurenkov 2017 {published data only}
- Kurenkov AL, Klochkova OA, Bursagova BI, Karimova HM, Kuzenkova LM, Mamedyarov AM, et al. Efficacy and safety of botulinum toxin type A (IncobotulinumtoxinA) in the treatment of patients with cerebral palsy [Èffektivnostʹ i bezopasnostʹ primenenija botuliničeskogo toksina tipa A (IncobotulinumtoxinA) pri lečenii pacientov s detskim cerebralʹnym paraličom]. Zhurnal Nevrologii i Psikhiatrii imeni SS Korsakova 2017; Vol. 117, issue 11:37‐44. [DOI: 10.17116/jnevro201711711137-44; PUBMED: 29265085] [DOI] [PubMed]
Kwon 2010 {published data only}
- Kwon JY, Hwang JH, Kim JS. Botulinum toxin A injection into calf muscles for treatment of spastic equinus in cerebral palsy: a controlled trial comparing sonography and electric stimulation‐guided injection techniques: a preliminary report. American Journal of Physical Medicine & Rehabilitation 2010;89(4):279‐86. [DOI: 10.1097/PHM.0b013e3181ca24ac; PUBMED: 20068435] [DOI] [PubMed] [Google Scholar]
Lee 2009 {published data only}
- Lee JH, Sung IY, Yoo JY, Park EH, Park SR. Effects of different dilutions of botulinum toxin type A treatment for children with cerebral palsy with spastic ankle plantarflexor: a randomized controlled trial. Journal of Rehabilitation Medicine 2009;41(9):740‐5. [DOI: 10.2340/16501977-0418; PUBMED: 19774308] [DOI] [PubMed] [Google Scholar]
Lee 2011 {published data only}
- Lee SJ, Sung IY, Jang DH, Yi JH, Lee JH, Ryu JS. The effect and complication of botulinum toxin type A injection with serial casting for the treatment of spastic equinus foot. Annals of Rehabilitation Medicine 2011;35(3):344‐53. [DOI: 10.5535/arm.2011.35.3.344; PMC3309222; PUBMED: 22506143] [DOI] [PMC free article] [PubMed] [Google Scholar]
Love 2009 {published data only}
- Love S, Blair E, Valentine J, Cole J. Should we be using BoNT‐A earlier?. Developmental Medicine & Child Neurology 2009;51(Suppl 2):8. [DOI: 10.1111/j.1469-8749.2008.03237.x] [DOI] [Google Scholar]
Mohamed 2001 {published data only}
- Mohamed KA, Moore AP, Rosenbloom L. Adverse events following repeated injections with botulinum toxin A in children with spasticity. Developmental Medicine & Child Neurology 2001;43(11):791. [DOI: 10.1017/s0012162201211426; PUBMED: 11730154] [DOI] [PubMed] [Google Scholar]
Newman 2007 {published data only}
- Newman CJ, Kennedy A, Walsh M, O'Brien T, Lynch B, Hensey O. A pilot study of delayed versus immediate serial casting after botulinum toxin injection for partially reducible spastic equinus. Journal of Pediatric Orthopaedics 2007;27(8):882‐5. [DOI: 10.1097/BPO.0b013e31815b4d7d; PUBMED: 18209608] [DOI] [PubMed] [Google Scholar]
Niu 2014 {published data only}
- Niu GH, Zhang XL, Zhu DN, Cai ZJ, Li SS, Zhang W. Therapeutic effects of different doses of botulinum toxin A injection on tiptoe deformation in children with cerebral palsy [Bùtóng jìliàng A xíng ròu dú dúsù zhùshè zhìliáo nǎo xìng tānhuàn jiān zú de liáoxiào duìbǐ yánjiū]. Zhongguo Dang Dai Er Ke Za Zhi [Chinese Journal of Contemporary Pediatrics] 2014;16(7):720‐4. [DOI: 10.7499/j.issn.1008-8830.2014.07.013; PUBMED: 25008880] [DOI] [PubMed] [Google Scholar]
Park 2010 {published data only}
- Park ES, Rha DW, Yoo JK, Kim SM, Chang WH, Song SH. Short‐term effects of combined serial casting and botulinum toxin injection for spastic equinus in ambulatory children with cerebral palsy. Yonsei Medical Journal 2010;51(4):579‐84. [DOI: 10.3349/ymj.2010.51.4.579; PMC2880273; PUBMED: 20499426] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peeters 2018 {published data only}
- Peeters N, Hanssen B, Cenni F, Schless SH, Beukelaer N, Degelaen M, et al. O 019 ‐ do botulinum toxin‐A and lower leg casting alter calf muscle and tendon lengths in children with spastic cerebral palsy?. Gait & Posture 2018; Vol. 65 Suppl 1:36‐8. [DOI: 10.1016/j.gaitpost.2018.06.037] [DOI]
Picelli 2017 {published data only}
- Picelli A, Marchina E, Gajofatto F, Pontillo A, Vangelista A, Filippini R, et al. Sonographic and clinical effects of botulinum toxin type A combined with extracorporeal shock wave therapy on spastic muscles of children with cerebral palsy. Developmental Neurorehabilitation 2017; Vol. 20, issue 3:160‐4. [DOI: 10.3109/17518423.2015.1105320; PUBMED: 26890193] [DOI] [PubMed]
Polak 2002 {published data only}
- Polak F, Morton R, Ward C, Wallace WA, Doderlein L, Siebel A. Double‐blind comparison study of two doses of botulinum toxin A injected into calf muscles in children with hemiplegic cerebral palsy. Developmental Medicine & Child Neurology 2002;44(8):551‐5. [DOI: 10.1017/s0012162201002547; PUBMED: 12206622] [DOI] [PubMed] [Google Scholar]
Richman 1996 {published data only}
- Richman DA, Gaebler‐Spira DJ. The use of botulinum A toxin to improve quality of life of children with cerebral palsy in a long term health care facility. Archives of Physical Medicine and Rehabilitation 1996;77(9):923. [DOI: 10.1016/S0003-9993(96)90288-9] [DOI] [Google Scholar]
Robertshaw 2005 {published data only}
- Robertshaw K, Watson L, Parkin T, Morton RE. Botulinum toxin A dosage: autonomic function as a measure of side effects. Developmental Medicine & Child Neurology 2005;47(11):792. [DOI: 10.1017/S0012162205001659; PUBMED: 16225746] [DOI] [PubMed] [Google Scholar]
Sätilä 2005 {published data only}
- Sätilä H, Iisalo T, Pietikäinen T, Seppänen RL, Salo M, Koivikko M, et al. Botulinum toxin treatment of spastic equinus in cerebral palsy: a randomized trial comparing two injection sites. American Journal of Physical Medicine & Rehabilitation 2005;84(5):355‐65; quiz 366‐7, 392. [PUBMED: 15829782] [DOI] [PubMed] [Google Scholar]
Sätilä 2006 {published data only}
- Sätilä HK, Pietikäinen T, Lehtonen‐Räty P, Koivikko M, Autti‐Rämö I. Treatment of spastic equinus gait with botulinum toxin A: does dose matter? Analysis of a clinical cohort. Neuropediatrics 2006;37(6):344‐9. [DOI: 10.1055/s-2007-964998; PUBMED: 17357036] [DOI] [PubMed] [Google Scholar]
Sätilä 2008 {published data only}
- Sätilä H, Pietikäinen T, Iisalo T, Lehtonen‐Räty P, Salo M, Haataja R, et al. Botulinum toxin type A injections into the calf muscles for treatment of spastic equinus in cerebral palsy: a randomized trial comparing single and multiple injection sites. American Journal of Physical Medicine & Rehabilitation 2008;87(5):386‐94. [DOI: 10.1097/PHM.0b013e31816ddaa9; PUBMED: 18427220] [DOI] [PubMed] [Google Scholar]
Schasfoort 2017 {published data only}
- Schasfoort FC, Pangalila RF, Dallmeijer AJ, Catsman CE, Sneekes EM, Eline E, et al. Evaluation of the added value of botulinum toxin treatment and intensive physiotherapy to improve body function and structure in children with spastic cerebral palsy. Developmental Medicine & Child Neurology 2017; Vol. 59, issue Suppl 2:21. [DOI: 10.1111/dmcn.13455; oral presentation 48] [DOI]
Schasfoort 2018 {published data only}
- Schasfoort F, Pangalila R, Sneekes EM, Catsman C, Becher J, Horemans H, et al. Intramuscular botulinum toxin prior to comprehensive rehabilitation has no added value for improving motor impairments, gait kinematics and goal attainment in walking children with spastic cerebral palsy. Journal of Rehabilitation Medicine 2018; Vol. 50, issue 8:732‐42. [DOI: 10.2340/16501977-2369; PUBMED: 30080235] [DOI] [PubMed]
Thomas 2012 {published data only}
- Thomas R, Johnston LM, Boyd R, Sakzewski L, Case V, Kentish M. GR IN: randomised trial of group versus individual models of physiotherapy for ambulant children with cerebral palsy following lower limb botulinum toxin‐A. Developmental Medicine & Child Neurology 2012;54(Suppl 5):46‐7. [DOI: 10.1111/j.1469-8749.2012.04289.x; F3.F2] [DOI] [Google Scholar]
Thorley 2012 {published data only}
- Thorley M, Donaghey S, Edwards P, Copeland L, Kentish M, McLennan K, et al. Evaluation of the effects of botulinum toxin A injections when used to improve ease of care and comfort in children with cerebral palsy whom are non‐ambulant: a double blind randomized controlled trial. BMC Pediatrics 2012;12:120. [DOI: 10.1186/1471-2431-12-120; PMC3472230; PUBMED: 22873758] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Campenhout 2013 {published data only}
- Campenhout A, Verhaegen A, Pans S, Molenaers G. Botulinum toxin type A injections in the psoas muscle of children with cerebral palsy: muscle atrophy after motor end plate‐targeted injections. Research in Developmental Disabilities 2013;34(3):1052‐8. [DOI: 10.1016/j.ridd.2012.11.016; PUBMED: 23295965] [DOI] [PubMed] [Google Scholar]
Van Campenhout 2015 {published data only}
- Campenhout A, Bar‐On L, Desloovere K, Huenaerts C, Molenaers G. Motor endplate‐targeted botulinum toxin injections of the gracilis muscle in children with cerebral palsy. Developmental Medicine & Child Neurology 2015; Vol. 57, issue 5:476‐83. [DOI: 10.1111/dmcn.12667; PUBMED: 25557985] [DOI] [PubMed]
Wang 2007 {published data only}
- Wang YJ, Yan GY, Gao BQ. Management of spastic cerebral palsy with botulinum‐A toxin: relationship between dosage and efficacy. Zhongguo Dang Dai Er Ke Za Zhi [Chinese journal of contemporary pediatrics] 2007;9(3):247‐8. [PUBMED: 17582266] [PubMed] [Google Scholar]
Wang 2008 {published data only}
- Wang Y, Gao B. A dose‐response relationship research on botulinum toxin type A local intramuscular injections of lower extremity spasticity in children with cerebral palsy. Child's Nervous System 2008;24(5):545‐7. [DOI: 10.1007/s00381-007-0571-7; PUBMED: 18297290] [DOI] [PubMed] [Google Scholar]
Williams 2013 {published data only}
- Williams SA, Elliott C, Valentine J, Gubbay A, Shipman P, Reid S. Combining strength training and botulinum neurotoxin intervention in children with cerebral palsy: the impact on muscle morphology and strength. Disability and Rehabilitation 2013;35(7):596‐605. [DOI: 10.3109/09638288.2012.711898; PUBMED: 22928803] [DOI] [PubMed] [Google Scholar]
Wissel 1999 {published data only}
- Wissel J, Heinen F, Schenkel A, Doll B, Ebersbach G, Müller J, et al. Botulinum toxin A in the management of spastic gait disorders in children and young adults with cerebral palsy: a randomized, double‐blind study of "high‐dose" versus "low‐dose" treatment. Neuropediatrics 1999;30(3):120‐4. [DOI: 10.1055/s-2007-973475; PUBMED: 10480205] [DOI] [PubMed] [Google Scholar]
Wong 2005 {published data only}
- Wong AM, Pei YC, Lui TN, Chen CL, Wang CM, Chung CY. Comparison between botulinum toxin type A injection and selective posterior rhizotomy in improving gait performance in children with cerebral palsy. Journal of Neurosurgery 2005;102(4 Suppl):385‐9. [DOI: 10.3171/ped.2005.102.4.0385; PUBMED: 15926389] [DOI] [PubMed] [Google Scholar]
Xu 2009 {published data only}
- Xu K, Yan T, Mai J. A randomized controlled trial to compare two botulinum toxin injection techniques on the functional improvement of the leg of children with cerebral palsy. Clinical Rehabilitation 2009;23(9):800‐11. [DOI: 10.1177/0269215509335295; PUBMED: 19482892] [DOI] [PubMed] [Google Scholar]
YaJie 2008 {published data only}
- YaJie W, BaoQin G. Botulinum toxin A injection for children with spastic cerebral palsy. Developmental Medicine & Child Neurology 2008;50(8):640. [DOI: 10.1111/j.1469-8749.2008.03023.x] [DOI] [PubMed] [Google Scholar]
Zier 2008 {published data only}
- Zier JL, Rivard PF, Krach LE, Wendorf HR. Effectiveness of sedation using nitrous oxide compared with enteral midazolam for botulinum toxin A injections in children. Developmental Medicine & Child Neurology 2008;50(11):854‐8. [DOI: 10.1111/j.1469-8749.2008.03069.x; PUBMED: 19046178] [DOI] [PubMed] [Google Scholar]
Zonta 2013 {published data only}
- Zonta MB, Bruck I, Puppi M, Muzzolon S, Neto Ade C, Coutinho dos Santos LH. Effects of early spasticity treatment on children with hemiplegic cerebral palsy: a preliminary study. Arquivos de Neuro‐Psiquiatria 2013;71(7):453‐61. [DOI: 10.1590/0004-282X20130061; PUBMED: 23857615] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Kim 2018 {published data only}
- Kim H, Meilahn J, Liu C, Chambers HG, Dimitrova R. Efficacy and safety of onabotulinumtoxinA for the treatment of pediatric lower limb spasticity: primary results (S29.007). Neurology 2018;90(15 Suppl). [ISSN: 1526‐632X; n.neurology.org/content/90/15_Supplement/S29.007] [Google Scholar]
References to ongoing studies
ACTRN12615001162505 {unpublished data only}
- ACTRN12615001162505. The impact of Botox on muscle structure and function in children with cerebral palsy [A RCT of the efficacy of the first intramuscular botulinum toxin type‐A treatment on muscle structure and function in children with cerebral palsy: a multi‐centre trial]. www.anzctr.org.au/ACTRN12615001162505.aspx (registered 30 october 2015).
CTRI/2015/03/005642 {unpublished data only}
- CTRI/2015/03/005642. Clinical trial to compare the effect of botulinum toxin vs surgical management of lower limb deformities in children with spastic cerebral palsy [Botulinum toxin versus singe event multi‐level surgery (SEMLS) in children with lower limb deformities in spastic cerebral palsy ‐ a randomised comparative trial]. www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=8038 (registered 19 March 2015).
NCT02400619 {unpublished data only}
- NCT02400619. Shockwaves therapy and botulinum toxin for the treatment of spasticity in patients with cerebral palsy: a cross over RCT [Efficacy of radial extracorporeal shock waves compared to botulinum toxin type A in the treatment of spasticity of the lower extremities in patients with cerebral palsy: a crossover randomized clinical trial]. clinicaltrials.gov/show/NCT02400619 (registered 27 March 2015).
NCT02546999 {unpublished data only}
- NCT02546999. Does botulinum toxin A make walking easier in children with cerebral palsy?. clinicalTrials.gov/show/NCT02546999 (registered 11 September 2015).
TCTR20150803003 {unpublished data only}
- TCTR20150803003. Efficacy of botulinum toxin in pediatric spasticity: a randomized trial comparing 2‐ to 4‐ sites hamstring injection. www.clinicaltrials.in.th/index.php?tp=regtrials&menu=trialsearch&smenu=fulltext&task=search&task2=view1&id=1496 (registered 3 August 2015).
Additional references
Blair 2010
- Blair E. Epidemiology of the cerebral palsies. Orthopedic Clinics of North America 2010;41(4):441–55. [DOI: 10.1016/j.ocl.2010.06.004; PUBMED: 20868877] [DOI] [PubMed] [Google Scholar]
Bohannon 1987
- Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Physical Therapy 1987;67(2):206‐7. [DOI: 10.1093/ptj/67.2.206; PUBMED: 3809245] [DOI] [PubMed] [Google Scholar]
Boyd 1999
- Boyd RN, Graham HK. Objective measurement of clinical findings in the use of botulinum toxin type A for the management of children with cerebral palsy. European Journal of Neurology 1999;6(Suppl 4):s23–35. [DOI: 10.1111/j.1468-1331.1999.tb00031.x] [DOI] [Google Scholar]
Boyd 2001a
- Boyd RN, Dobson F, Parrott J, Love S, Oates J, Larson A, et al. The effect of botulinum toxin type A and a variable hip abduction orthosis on gross motor function: a randomized controlled trial. European Journal of Neurology 2001;8 Suppl 5:109‐19. [PUBMED: 11851739] [DOI] [PubMed] [Google Scholar]
Brin 1997
- Brin MF. Botulinum toxin: chemistry, pharmacology, toxicity, and immunology. Muscle & Nerve. Supplement 1997;6:S146‐68. [PUBMED: 9826987] [PubMed] [Google Scholar]
Brooks 1954
- Brooks VB. The action of botulinum toxin on motor‐nerve filaments. Journal of Physiology 1954;123(3):501‐15. [DOI: 10.1113/jphysiol.1954.sp005067; PMC1366222; PUBMED: 13152695] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cardoso 2006
- Cardoso ES, Rodrigues BM, Barroso M, Menezes CJ, Lucena RS, Nora DB, et al. Botulinum toxin type A for the treatment of the spastic equinus foot in cerebral palsy. Pediatric Neurology 2006;34(2):106‐9. [DOI: 10.1016/j.pediatrneurol.2005.08.010; PUBMED: 16458821] [DOI] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis in the Behavioural Sciences. 2nd Edition. Hillsdale (NJ): Lawrence Erlbaum Associates Inc, 1988. [Google Scholar]
Copeland 2014a
- Copeland L, Edwards P, Thorley M, Donaghey S, Gascoigne‐Pees L, Kentish M, et al. Botulinum toxin A for nonambulatory children with cerebral palsy: a double blind randomized controlled trial. Journal of Pediatrics 2014;165(1):140‐6.e4. [DOI: 10.1016/j.jpeds.2014.01.050; PUBMED: 24630348] [DOI] [PubMed] [Google Scholar]
Delgado 2016a
- Delgado MR, Tilton A, Russman B, Benavides O, Bonikowski M, Carranza J, et al. AbobotulinumtoxinA for equinus foot deformity in cerebral palsy: a randomized controlled trial. Pediatrics 2016;137(2):e20152830. [DOI: 10.1542/peds.2015-2830; PUBMED: 26812925] [DOI] [PubMed] [Google Scholar]
Desloovere 2007
- Desloovere K, Molenaers G, Cat J, Pauwels P, Campenhout A, Ortibus E, et al. Motor function following multilevel botulinum toxin type A treatment in children with cerebral palsy. Developmental Medicine & Child Neurology 2007;49(1):56‐61. [DOI: 10.1017/s001216220700014x.x; PUBMED: 17209978] [DOI] [PubMed] [Google Scholar]
Druschel 2013
- Druschel C, Althuizes HC, Funk JF, Placzek R. Off label use of botulinum toxin in children under two years of age: a systematic review. Toxins 2013;5(1):60‐72. [DOI: 10.3390/toxins5010060; PMC3564068; PUBMED: 23296386] [DOI] [PMC free article] [PubMed] [Google Scholar]
Edwards 2015
- Edwards P, Sakzewski L, Copeland L, Gascoigne‐Pees L, McLennan K, Thorley M, et al. Safety of botulinum toxin type A for children with nonambulatory cerebral palsy. Pediatrics 2015;136(5):895‐904. [DOI: 10.1542/peds.2015-0749; PUBMED: 26482662] [DOI] [PubMed] [Google Scholar]
Feldman 1990
- Feldman AB, Haley SM, Coryell J. Concurrent and construct validity of the Pediatric Evaluation of Disability Inventory. Physical Therapy 1990;70(10):602–10. [DOI: 10.1093/ptj/70.10.602; PUBMED: 2217539] [DOI] [PubMed] [Google Scholar]
García Salazar 2015
- García Salazar LF, Dos Santos GL, Pavão SL, Rocha NA, Russo TL. Intrinsic properties and functional changes in spastic muscle after application of BTX‐A in children with cerebral palsy: systematic review. Developmental Neurorehabilitation 2015;18(1):1‐14. [DOI: 10.3109/17518423.2014.948640; PUBMED: 25180438] [DOI] [PubMed] [Google Scholar]
Gormley 2001
- Gormley ME, Gaebler‐Spira D, Delgado MR. Use of botulinum toxin type A in pediatric patients with cerebral palsy: a three‐center retrospective chart review. Journal of Child Neurology 2001;16(2):113‐8. [DOI: 10.1177/088307380101600209; PUBMED: 11292216] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime, Inc.). GRADEpro GDT: GRADEpro Guideline Development Tool. Version accessed 16 August 2019. Hamilton (ON): McMaster University (developed by Evidence Prime, Inc.), 2015.
Graham 2008
- Graham HK, Boyd R, Carlin JB, Dobson F, Lowe K, Nattrass G, et al. Does botulinum toxin A combined with bracing prevent hip displacement in children with cerebral palsy and "hips at risk"? A randomized, controlled trial. Journal of Bone & Joint Surgery 2008;90(1):23‐33. [DOI: 10.2106/JBJS.F.01416; PUBMED: 18171954] [DOI] [PubMed] [Google Scholar]
Graham 2013
- Graham HK, Thomason P, Novacheck TF. Cerebral palsy. In: Weinstein SL, Flynn JM editor(s). Lovell & Winter's Pediatric Orthopaedics. 7th Edition. Philadelphia: Lippincott Williams & Wilkins, Wolters Kluwer Health, 2013:484‐554. [Google Scholar]
Gupta 2012
- Gupta S, Raja K. Responsiveness of Edinburgh Visual Gait Score to orthopedic surgical intervention of the lower limbs in children with cerebral palsy. American Journal of Physical Medicine & Rehabilitation 2012;91(9):761‐7. [DOI: 10.1097/PHM.0b013e31825f1c4d; PUBMED: 22790796] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC, editor(s), on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011a
- Higgins JPT, Deeks JJ, Altman DG, editor(s), on behalf of the Cochrane Statistical Methods Group. Chapter 16: Special topics in statistics. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Iyer 2003
- Iyer LV, Haley SM, Watkins MP, Dumas HM. Establishing minimal clinically important differences for scores on the Pediatric Evaluation of Disability Inventory for inpatient rehabilitation. Physical Therapy 2003;83(10):888‐98. [PUBMED: 14519060] [PubMed] [Google Scholar]
Kiresuk 1968
- Kiresuk TJ, Sherman RE. Goal attainment scaling: a general method of evaluating comprehensive community mental health programs. Community Mental Health Journal 1968;4(6):443‐53. [DOI: 10.1007/BF01530764; PUBMED: 24185570] [DOI] [PubMed] [Google Scholar]
Koman 1993
- Koman LA, Mooney JF 3rd, Smith B, Goodman A, Mulvaney T. Management of cerebral palsy with botulinum‐A toxin: preliminary investigation. Journal of Pediatric Orthopaedics 1993;13(4):489‐95. [DOI: 10.1097/01241398-199307000-00013; PUBMED: 8370782] [DOI] [PubMed] [Google Scholar]
Koog 2010
- Koog YH, Min BI. Effects of botulinum toxin A on calf muscles in children with cerebral palsy: a systematic review. Clinical Rehabilitation 2010;24(8):685‐700. [DOI: 10.1177/0269215510367557; PUBMED: 20554641] [DOI] [PubMed] [Google Scholar]
Kostrzewa 2007
- Kostrzewa RM, Segura‐Aguilar J. Botulinum neurotoxin: evolution from poison, to research tool ‐ onto medicinal therapeutic and future pharmaceutical panacea. Neurotoxicity Research 2007;12(4):275‐90. [PUBMED: 18201955] [DOI] [PubMed] [Google Scholar]
Law 1990
- Law M, Baptiste S, McColl M, Opzoomer A, Polatajko H, Pollock N. The Canadian Occupational Performance Measure: an outcome measure for occupational therapy. Canadian Journal of Occupational Therapy 1990;57(2):82–7. [DOI: 10.1177/000841749005700207; PUBMED: 10104738] [DOI] [PubMed] [Google Scholar]
Lee 2014
- Lee WY, Park GY, Kwon DR. Comparison of treatment effects between children with spastic cerebral palsy under and over five years after botulinum toxin type a injection. Annals of Rehabilitation Medicine 2014;38(2):200‐8. [DOI: 10.5535/arm.2014.38.2.200; PMC4026606; PUBMED: 24855614] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097; PMC2707599; PUBMED: 19621072] [DOI] [PMC free article] [PubMed] [Google Scholar]
Molenaers 2013
- Molenaers G, Fagard K, Campenhout A, Desloovere K. Botulinum toxin A treatment of the lower extremities in children with cerebral palsy. Journal of Children's Orthopedics 2013;7(5):383‐7. [DOI: 10.1007/s11832-013-0511-x; PMC3838518; PUBMED: 24432099] [DOI] [PMC free article] [PubMed] [Google Scholar]
Msall 1994
- Msall ME, DiGaudio K, Rogers BT, LaForest S, Catanzaro NL, Campbell J, et al. The Functional Independence Measure for Children (WeeFIM). Conceptual basis and pilot use in children with developmental disabilities. Clinical Pediatrics 1994;33(7):421‐30. [DOI: 10.1177/000992289403300708; PUBMED: 7525140] [DOI] [PubMed] [Google Scholar]
Naidu 2010
- Naidu K, Smith K, Sheedy M, Adair B, Yu X, Graham HK. Systemic adverse events following botulinum toxin A therapy in children with cerebral palsy. Developmental Medicine & Child Neurology 2010;52(2):139‐44. [DOI: 10.1111/j.1469-8749.2009.03583.x; PUBMED: 20412252] [DOI] [PubMed] [Google Scholar]
Narayanan 2006
- Narayanan UG, Fehlings D, Weir S, Knights S, Kiran S, Campbell K. Initial development and validation of the Caregiver Priorities and Child Health Index of Life with Disabilities (CPCHILD). Developmental Medicine & Child Neurology 2006;48(10):804‐12. [DOI] [PubMed] [Google Scholar]
Oeffinger 2008
- Oeffinger D, Bagley A, Rogers S, Gorton G, Kryscio R, Abel M, et al. Outcome tools used for ambulatory children with cerebral palsy: responsiveness and minimum clinically important differences. Developmental Medicine & Child Neurology 2008;50(12):918‐25. [DOI: 10.1111/j.1469-8749.2008.03150.x; PMC2990955; PUBMED: 19046185] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pascual‐Pascual 1997
- Pascual‐Pascual SI, Sánchez de Muniain P, Roche MC, Pascual‐Castroviejo I. Botulinum toxin as a treatment for infantile cerebral palsy. Revista de Neurologica 1997;25(145):1369‐75. [PUBMED: 9377292] [PubMed] [Google Scholar]
Read 2003
- Read HS, Hazlewood ME, Hillman SJ, Prescott RJ, Robb JE. Edinburgh visual gait score for use in cerebral palsy. Journal of Pediatric Orthopaedics 2003;23(3):296‐301. [PUBMED: 12724590] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rohatgi 2016 [Computer program]
- Rohatgi A. WebPlotDigitizer. Version 3.10. Austin (TX): Rohatgi A, 2016.
Rosenbaum 2007
- Rosenbaum P, Paneth N, Leviton A, Goldstein M, Bax M, Damiano D, et al. A report: the definition and classification of cerebral palsy April 2006. Developmental Medicine & Child Neurology. Supplement 2007;109:8‐14. [PUBMED: 17370477] [PubMed] [Google Scholar]
RStudio 2018 [Computer program]
- RStudio, Inc. RStudio: Integrated Development for R.. Version 1.2.1335. Boston, MA: RStudio, Inc, 2018.
Ruiz 2004
- Ruiz FJ, Guest JF, Lehmann A, Davie AM, Güttler K, Schlüter O, et al. Costs and consequences of botulinum toxin type A use. Management of children with cerebral palsy in Germany. European Journal of Health Economics 2004;5(3):227‐35. [DOI: 10.1007/s10198-004-0224-7; PUBMED: 15714343] [DOI] [PubMed] [Google Scholar]
Russell 1989
- Russell DJ, Rosenbaum PL, Cadman DT, Gowland C, Hardy S, Jarvis S. The Gross Motor Function Measure: a means to evaluate the effects of physical therapy. Developmental Medicine & Child Neurology 1989;31(3):341‐52. [DOI: 10.1111/j.1469-8749.1989.tb04003.x; PUBMED: 2753238] [DOI] [PubMed] [Google Scholar]
Ryll 2011
- Ryll U, Bastiaenen C, Bie R, Staal B. Effects of leg muscle botulinum toxin A injections on walking in children with spasticity‐related cerebral palsy: a systematic review. Developmental Medicine & Child Neurology 2011;53(3):210‐6. [DOI: 10.1111/j.1469-8749.2010.03890.x; PUBMED: 21291464] [DOI] [PubMed] [Google Scholar]
Sanger 2003
- Sanger TD. Pathophysiology of pediatric movement disorders. Journal of Child Neurology 2003;18 Suppl 1:S9‐24. [DOI: 10.1177/0883073803018001S0401; PUBMED: 13677568] [DOI] [PubMed] [Google Scholar]
Scholtes 2006a
- Scholtes VA, Dallmeijer AJ, Knol DL, Speth LA, Maathuis CG, Jongerius PH, et al. The combined effect of lower‐limb multilevel botulinum toxin type A and comprehensive rehabilitation on mobility in children with cerebral palsy: a randomized clinical trial. Archives of Physical Medicine & Rehabilitation 2006;87(12):1551‐8. [DOI: 10.1016/j.apmr.2006.08.342; PUBMED: 17141633] [DOI] [PubMed] [Google Scholar]
Scholtes 2007
- Scholtes VA, Dallmeijer AJ, Knol DL, Speth LA, Maathuis CG, Jongerius PH, et al. Effect of multilevel botulinum toxin A and comprehensive rehabilitation on gait in cerebral palsy. Pediatric Neurology 2007;36(1):30‐9. [DOI: 10.1016/j.pediatrneurol.2006.09.010; PUBMED: 17162194] [DOI] [PubMed] [Google Scholar]
Schutte 2000
- Schutte LM, Narayanan U, Stout JL, Selber P, Gage JR, Schwartz MH. An index for quantifying deviations from normal gait. Gait & Posture 2000;11(1):25‐31. [PUBMED: 10664482] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Schünemann 2017
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Akl E, et al. on behalf of the Cochrane GRADEing Methods Group and the Cochrane Statistical Methods Group. Chapter 11: Completing ‘Summary of findings’ tables and grading the confidence in or quality of the evidence. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Scott 1980
- Scott AB. Botulinum toxin injection into extraocular muscles as an alternative to strabismus surgery. Ophthalmology 1980;87(10):1044‐9. [DOI: 10.1016/s0161-6420(80)35127-0; PUBMED: 7243198] [DOI] [PubMed] [Google Scholar]
Scott 1994
- Scott AB. Forward. In: Jankovic J, Hallet M editor(s). Therapy with Botulinum Toxin. New York (NY): Marcel Dekker Inc, 1994:vii‐ix. [Google Scholar]
Simpson 2008
- Simpson DM, Gracies JM, Graham HK, Miyasaki JM, Naumann M, Russman B, et al. Assessment: botulinum neurotoxin for the treatment of spasticity (an evidence‐based review): report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2008;70(19):1691‐8. [PUBMED: 18458229] [DOI] [PubMed] [Google Scholar]
Sánchez‐Carpintero 1997
- Sánchez‐Carpintero R, Narbona J. Botulinum toxin in spastic infantile cerebral palsy: results in 27 cases during one year. Revista de Neurología 1997;25(140):531‐5. [PubMed] [Google Scholar]
Tilton 2017
- Tilton A, Russman B, Aydin R, Dincer U, Escobar RG, Kutlay S, et al. AbobotulinumtoxinA (Dysport®) improves function according to goal attainment in children with dynamic equinus due to cerebral palsy. Journal of Child Neurology 2017;32(5):482‐7. [DOI: 10.1177/0883073816686910; PMC5405835; PUBMED: 28068857] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van der Houwen 2011
- Houwen LE, Scholtes VA, Becher JG, Harlaar J. Botulinum toxin A injections do not improve surface EMG patterns during gait in children with cerebral palsy ‐ a randomized controlled study. Gait & Posture 2011;33(2):147‐51. [DOI: 10.1016/j.gaitpost.2010.11.001; PUBMED: 21190858] [DOI] [PubMed] [Google Scholar]
Waters 2007
- Waters E, Davis E, Mackinnon A, Boyd R, Graham HK, Kai Lo S, et al. Psychometric properties of the Quality of Life Questionnaire for Children with CP. Developmental Medicine & Child Neurology 2007;49(1):49‐55. [DOI: 10.1017/s0012162207000126.x; PUBMED: 17209977] [DOI] [PubMed] [Google Scholar]
WHO 2001
- World Health Organization. International Classification of Functioning, Disability and Health (ICF). Geneva: World Health Organization, 2001. [Google Scholar]
Wong 1998
- Wong V. Use of botulinum toxin injection in 17 children with spastic cerebral palsy. Pediatric Neurology 1998;18(2):124‐31. [DOI: 10.1016/s0887-8994(97)00164-1; PUBMED: 9535297] [DOI] [PubMed] [Google Scholar]
Yang 1999
- Yang TF, Chan RC, Chuang TY, Liu TJ, Chiu JW. Treatment of cerebral palsy with botulinum toxin: evaluation with Gross Motor Function Measure. Journal of the Formosan Medical Association 1999;98(12):832‐6. [PUBMED: 10634023] [PubMed] [Google Scholar]
Zmanovskaia 2014
- Zmanovskaia VA, Levitina EV, Popkov DA, Butorina MN, Pavlova OL. Botulinum toxin type A (Dysport) in the complex rehabilitation of children with spastic forms of cerebral palsy [Botulinicheskij toksin tipa A (disport) v kompleksnoj reabilitacii detej so spasticheskimi formami cerebral'nogo paralicha]. Zhurnal Nevrologii i Psikhiatrii Imeni SS Korsakova 2014;114(7):33‐6. [www.mediasphera.ru/issues/zhurnal‐nevrologii‐i‐psikhiatrii‐im‐s‐s‐korsakova/2014/7/031997‐7298201476/annotation] [PubMed] [Google Scholar]
References to other published versions of this review
Ade‐Hall 2000
- Ade‐Hall RA, Moore AP. Botulinum toxin type A in the treatment of lower limb spasticity in cerebral palsy. Cochrane Database of Systematic Reviews 2000, Issue 1. [DOI: 10.1002/14651858.CD001408] [DOI] [PubMed] [Google Scholar]


