Abstract
Objective
Persistent infection of HPV increases the chance of carcinoma in situ of cervix through stages of cervical intraepithelial neoplasia (CIN) 1, 2, and 3, and finally progresses into cervical cancer. We aimed to explore the safety and efficacy of BLS-M07 which is orally administered agent expressing human papillomavirus (HPV) 16 E7 antigen on the surface of Lactobacillus casei in patients with CIN 3.
Methods
Patients with CIN 3 were recruited in our clinical trial. Reid Colposcopic Index (RCI) grading and serum HPV16 E7 specific antibody production were used to evaluate efficacy of BLS-M07. In phase 1, BLS-M07 was administered orally, 5 times a week, on weeks 1, 2, 4, and 8 with dosages of 500 mg, 1,000 mg, and 1,500 mg. In phase 2a, patients were treated with 1,000 mg. The primary endpoints were the safety and the pathologic regression on colposcopic biopsy.
Results
Nineteen patients were enrolled in the CIN 3 cohort. In phase 1, no patients experienced dose limiting toxicity. No grade 3 or 4 treatment-related adverse events or deaths were observed. At 16 weeks after treatment, RCI grading was improved and serum HPV16 E7 specific antibody production increased (p<0.05). Six of 8 (75%) patients with CIN 3 were cured in phase 2a.
Conclusions
Oral immunization with BLS-M07 increases production of serum HPV16 E7 specific antibody which induces protective humoral immunity. The safety of this oral vaccine was proved and could be a competitive non-surgical therapeutic agent of CIN 3.
Trial Registration
ClinicalTrials.gov Identifier: NCT02195089
Keywords: Papillomavirus Vaccines, Papillomavirus E7 Proteins, Cervical Intraepithelial Neoplasia
INTRODUCTION
Cervical cancer is one of the leading cause of death in female cancer patients, and occurs in approximately 75% of females with persistent infection of high-risk type human papillomavirus (HPV) [1,2]. Persistent infection of HPV increases the chance of developing high-grade cervical intraepithelial neoplasia (CIN). In CIN 3 which represents severe dysplasia, undifferentiated neoplastic cells that span more than 2/3 of the epithelium can be found on the surface of cervix. If left untreated, these cells develop into infiltrating lesions, metastasizing to nearby healthy tissues and ultimately develop into invasive cervical cancer [3,4,5].
Current treatment of CIN 3 is limited to surgical removal, which puts patients into risks of adverse pregnancy related side-effects. Due to this issue, there is an increasing need for non-surgical treatments for CIN 3. Based from the disease's characteristics, immune-related therapeutic medicine has been developed worldwide [6]. To treat various stages of CIN from HPV infection, there is a need for improvement of immune system ranging from cytokine production, cytotoxic T cell response, and inducement of antibody production. For instance, stimulating cell-mediated immune response will lead to increased production of cytokines such as interleukin (IL)-12 p70 and interferon (IFN)-γ, resulting in increased killing of HPV infected target cells by cytotoxic T cells. Furthermore, target-specific cluster of differentiation (CD) 4 T helper cells are activated by dendritic cells to induce humoral immune response which produces HPV specific antibodies. These 2 processes allow our immune system to eliminate HPV infected cells [7,8,9].
HPV E7 protein is a viral oncoprotein that causes carcinogenesis by binding to and inactivating tumor suppressor protein Rb. We developed a HPV E7-targeted antigen that has modifications in the amino acid sequence of the binding site of Rb, to inhibit carcinogenicity while maintaining immunogenicity [10,11].
By using our MucoMax® Platform Technology (BioLeaders, Yongin, Korea), which expresses target antigen on the surface of Lactobacillus casei, combined with gene recombination technology, we are able to transform HPV E7 protein's gene into L. casei. Subsequent expression of HPV E7 protein on the surface of L. casei are recognized by antigen presenting cells (APCs) and presented to cytotoxic T cells, resulting in cellular mediated immune response [12]. This process can explain development of BLS-M07 and its role as a therapeutic agent for cervical intraepithelial neoplasia.
BLS-M07 is orally administered therapeutic agent that induces gut-associated lymphoid tissue immune response by entering through Peyer's patches in the small intestine. This immune response transits into mucous membrane of cervix, thus illustrating BLS-M07 as an effective therapeutic agent for CIN 3. With ease of oral administration and use of safe microorganism L. casei as a host, BLS-M07 (BLS-ILB-E710c) has advantages of easy production and low potential for adverse side effects. BLS-M07 also induces humoral antibody to other antigens that has similar homology to HPV E7 due to mechanism of cross-reactivity and epitope spreading [13].
We designed phase 1/2a clinical trial to examine the safety and efficacy of BLS-M07 in patients with CIN3. In phase 1, dose escalation test was performed with an increasing dosage of 500 mg, 1,000 mg, and 1,500 mg per day. In phase 2a, clinical efficacy of BLS-M07 was assessed and the systemic immunogenicity of BLS-M07 was evaluated by HPV16 E7-specific antibody.
MATERIALS AND METHODS
1. Patients
Our study is a multicenter, phase 1/2a trial investigating the safety and efficacy of BLS-M07 in CIN 3 with HPV16 infection. Informed consent was obtained for all patients. Eligibility for our study were; 1) histologically confirmed CIN 3, 2) infected only with HPV16 assessed by HPV Liquid Bead Microarray or AnyplexTM II HPV 28 (Seegene, Seoul, Korea), 3) Women aged 20–50 years, 4) normal laboratory blood values and electrocardiogram, 5) satisfactory colposcopic findings, and 6) signed informed consent. Satisfactory colposcopic finding was defined visualization of the entire squamo-columnar junction and margins of any visible lesions. Exclusion criteria were; 1) patients with self-immune disease or use of immunosuppressive medications, 2) hyperactive reaction to lactobacillus, 3) acute or chronic pancreatitis, 4) pregnancy or lactation, and 5) symptomatic infection with hepatitis B or C.
2. Study design
This was a phase 1/2a, open label, dose-escalation study in subjects diagnosed with cervical intraepithelial neoplasia stage 3 with HPV16 single infection to test the safety and the efficacy of oral drug BLS-M07. BLS-M07 was administered orally 5 times week, on weeks 1, 2, 4, and 8. During phase 1 of the trial to assess initial safety, subjects were enrolled in 3 cohorts. We designed the starting dose of 500 mg per day. Three patients enrolled at each dose proceeded to the next stage if the dose-limiting toxicity (DLT) did not occur. Subjects in the first cohort received 500 mg of BLS-M07 (2 capsules, 250mg/capsule) and in no adverse events (AEs) were observed in first cohort, sequential cohorts received escalating doses of BLS-M07 (1,000 mg, 4 capsules, in the second cohort, and 1,500 mg, 6 capsules, in the third cohort). The primary endpoints were the safety of BLS-M07 and the pathologic regression on colposcopic biopsy. And the secondary endpoints were Reid Colposcopic Index (RCI) score and systemic inducement of HPV16 E7-specific immunoglobulin G (IgG).
If toxicity does not occur in phase 1 dose of 1,500 mg, proceed to phase 2a with and appropriate dose of 1,000 mg. Based on the safety test of phase 1, dose of 1,000 mg for phase 2a trial was determined. During phase 2a of the trial, treatment efficacy of BLS-M07 (BLS-ILB-E710c) was defined as histological regression to CIN 1 of less by colposcopy with diagnostic biopsies at 16 weeks.
All study procedures were reviewed and approved by Ministry of Food and Drug Safety and Institutional Review Boards (IRBs) of all participating sites. Approval was obtained from the IRB of the Korea University, College of Medicine (approval number, KUGH14014-001).
3. AEs assessment
AEs included events reported by the subjects, as well as clinically significant abnormal findings on clinical examination or laboratory evaluation. A new illness, symptom, sign or clinically significant clinical laboratory abnormality or worsening of a pre-existing condition or abnormality was considered as an AEs. Severity of AE was evaluated as grade 1 to 5 according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Dose Limiting Toxicities to BLS-M07 (BLS-ILB-E710c) were defined as grade ≥3 toxicity as per CTCAE, and grade 4 or 5 was considered as severe adverse event (SAE).
4. Clinical efficacy
Histological assessment was examined by colposcopy-guided biopsy. Colposcopic biopsy were obtained at initial screening and 16 weeks after initial treatment (visit 6). Efficacy was defined as histological regression to CIN 1 or less after treatment by colposcopic biopsies at 16 weeks compared to CIN 3 at baseline. As the secondary endpoints, the visual examination of colposcopy were evaluated using RCI score at 4, 9, 12, 16 weeks after initial treatment.
5. Liquid-based Pap (LBP) test and HPV Test
For LBP test, cytology specimens were collected using liquid-based system with a broom-type sampling tool (SurePath, BD Corp., Franklin Lakes, NJ, USA). Slides were prepared based on the manufacturer's instructions and interpreted by cytopathologists according to the Bethesda 2001 system. For HPV test, cone-shaped cervical brush (Cervical Sampler, Digene Corp., Gaithersburg, MD, USA) was used to obtain samples from the cervix by means of Hybrid Capture 2 (HC2; Digene Diagnostics, Gaithersburg, MD, USA). During the study period, the HPV genotyping techniques were used according to each institution. Two techniques were used, HPV Liquid Bead Microarray or Anyplex™ II HPV 28 (Seegene). Followed by genotyping test, we selected patients who were infected only with HPV16.
6. Titration of HPV16 E7-specific IgG in plasma
Blood samples were collected at week 1, 9, and 16 and plasma were used to detect HPV16 E7-specific IgG. Molecular Devices Spectramax 190 (USA) was used to detect ELISA assay. A 0.5 mg of purified HPV16 E7 protein was coated onto 96-well flat bottom Maxisorb™ ELISA plate (Nunc, Roskilde, Denmark) and incubated at 4°C overnight. Plates were washed 3 times with phosphate-buffered saline (PBS). Plates were blocked with 5% skim milk at 37°C for 1 hour. Plates were washed three times. Two-fold serial dilution of plasma in 1% Bovine serum albumin (BSA) was added to the wells and incubated at 37°C for 2 hours. After wash, horseradish peroxidase-conjugated anti-human IgG antibody was diluted 1:5,000 in 1% BSA/PBS and 1:100 of dilution was added per well. Plates were incubated at 37°C for 1 hour and washed 5 times. Plates were developed with peroxidase substrate tetramethylbenzidine solution and stopped with 0.5 N hydrochloric acid. Plates were read at optical density (OD) 450 nm. Titers were found by determining the OD = 0.2, using a regression analysis of titer data.
7. Statistical analysis
An analysis of the study data was performed on the subjects who had been administered investigational product at least once and whose data is available for analysis for primary endpoint. For the safety evaluations, baseline was defined as the last available assessment before day 1; and any AE with an onset date that was the same as the start of study treatment or later was reported. The safety issues included all patients who received at least 1 dose of BLS-M07. Disease response was based on all patients who had received at least 1 dose of study drug and from whom at least 1 efficacy assessment evaluation was attained. All analysis including demographic, efficacy, and safety assessments were set at a significance level of 0.05. Continuous data were analyzed using either a parametric (paired t-test) or nonparametric (Wilcoxon signed rank test) test. Categorical data were analyzed using McNemar's test as needed.
RESULTS
1. Clinical patient selection and study design
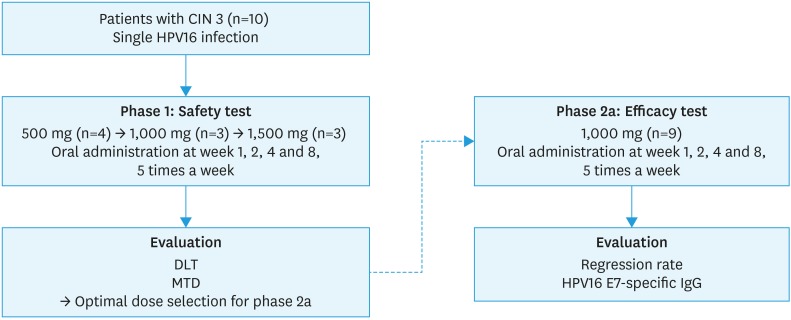
Between April 2014 and March 2016, 19 patients with single infection of HPV16 were enrolled in the study. HPV 16 single infection was confirmed through HPV DNA test (Fig. 1). Baseline characteristics of the patients participating in the study are shown in Table 1. Mean age of patients enrolled in phase 1 study were 28.3, 31.3, and 30.7 per 500 mg, 1,000 mg, 1,500 mg dosage levels respectively, and in phase 2, mean age of patients enrolled was 35.0.
Fig. 1. Scheme of the phase I/IIa clinical trial. In the phase I trial, DLT and MTD were assessed after the first dose of treatment and each subsequent cohort was enrolled once each subject had completed the first dose of treatment. In the phase II trial, efficacy was assessed based on CIN by colposcopic biopsy.
CIN, cervical intraepithelial neoplasia; DLT, dose-limiting toxicity; HPV, human papillomavirus; IgG, immunoglobulin G; MTD, maximum tolerated dose.
Table 1. Baseline characteristics of the study population.
| Patients ID | Age | BMI (kg/m2) | Dose (mg) | HPV16 infection | Cytology | Histology | RCI grading | |
|---|---|---|---|---|---|---|---|---|
| Phase 1 | ||||||||
| 1-1 (A0201) | 26 | 17.31 | 500 | + | HSIL | CIN 3 | 7 | |
| 1-2 (A0401) | 35 | 22.52 | 500 | + | ASC-H | CIN 3 | 5 | |
| 1-3 (A0402) | 24 | 17.58 | 500 | + | LSIL | CIN 3 | 5 | |
| 1-4 (A0403) | 28 | 17.86 | 500 | + | HSIL | CIN 3 | 2 | |
| 1-5 (B0401) | 33 | 33.27 | 1,000 | + | HSIL | CIN 3 | 5 | |
| 1-6 (B0402) | 27 | 22.31 | 1,000 | + | HSIL | CIN 3 | 6 | |
| 1-7 (B0403) | 34 | 25.85 | 1,000 | + | HSIL | CIN 3 | 5 | |
| 1-8 (C0201) | 34 | 32.74 | 1,500 | + | HSIL | CIN 3 | 3 | |
| 1-9 (C0401) | 25 | 21.88 | 1,500 | + | ASC-US | CIN 3 | 5 | |
| 1-10 (C0501) | 33 | 23.71 | 1,500 | + | HSIL | CIN 3 | 3 | |
| Phase 2a | ||||||||
| 2-1 (D0201) | 39 | 26.31 | 1,000 | + | HSIL | CIN 3 | 3 | |
| 2-2 (D0401) | 47 | 18.14 | 1,000 | + | ASC-US | CIN 3 | 2 | |
| 2-3 (D0402) | 27 | 23.46 | 1,000 | + | ASC-US | CIN 3 | 4 | |
| 2-4 (D0501) | 37 | 20.57 | 1,000 | + | Negative | CIN 3 | 7 | |
| 2-5 (D0502) | 47 | 24.91 | 1,000 | + | Negative | CIN 3 | 3 | |
| 2-6 (D0503) | 25 | 16.82 | 1,000 | + | ASC-H | CIN 3 | 6 | |
| 2-7 (D0504) | 32 | 18.82 | 1,000 | + | ASC-US | CIN 3 | 4 | |
| 2-8 (D0505) | 30 | 22.43 | 1,000 | + | ASC-H | CIN 3 | 3 | |
| 2-9 (D0506) | 30 | 20.02 | 1,000 | + | LSIL | CIN 3 | 3 | |
ASC-H, atypical squamous cells, cannot exclude; ASC-US, atypical squamous cells of undetermined significance; CIN, cervical intraepithelial neoplasia; BMI, body mass index; HSIL, high-grade squamous intraepithelial lesion; HPV, human papillomavirus; LSIL, Low-grade squamous intraepithelial lesion; RCI, Reid Colposcopic Index.
To test the safety of BLS-M07 (BLS-ILB-E710c), 4 clinical patients received oral administration of BLS-M07 (BLS-ILB-E710c) in dosage of 500 mg per day. After DLT was not experienced in first 4 patients (Table 2), 3 additional clinical patients received higher dosage of BLS-M07 (BLS-ILB-E710c). This dose escalation cycle was repeated until 1,500 mg concentration was tested. In conclusion, phase 1 study administered BLS-M07 (BLS-ILB-E710c) 5 times a week, on weeks 1, 2, 4, and 8, to 4 patients with 500 mg, 3 patients with 1,000 mg, and 3 patients with 1,500 mg.
Table 2. Summary of BLS-M07 dose escalation.
| BLS-M07 dose (mg) | No. of patients | DLTs* and treatment-related SAEs |
|---|---|---|
| 500 | 4 | None |
| 1,000 | 3 | None |
| 1,500 | 3 | None, grade 1 premenstrual pain (2 patients) |
DLS, dose-limiting toxicity; SAE, severe adverse event.
*DLT: any drug related grade 3 or higher toxicities.
In phase 1, maximum tolerated dose (MTD) was determined as 1,500 mg per day. Phase 2a study was performed with dosage of 1,000 mg per day, one dosage level below MTD in phase 1. Out of 9 patients enrolled in phase 2a, 8 patients completed the study, while 1 patient withdrew prematurely due to progression of the disease. BLS-M07's (BLS-ILB-E710c) clinical evaluation was assessed firstly, through histological analysis before and after treatment to evaluate overall response rate and secondly, through RCI grading and HPV 16 E7-sepcific IgG levels in plasma.
2. Safety
DLT was not experienced in phase 1 due to no observation of drug related grade 3 or higher toxicities and MTD was established as 1,500 mg per day. At dose level of 1,500 mg, 2 of 3 patients experienced a grade 1 premenstrual pain (Table 2). Immunological efficacy was also observed through correlation of E7-specific IFN-γ-producing cells with pathological responses. In non-clinical toxicity test, human equivalent dose of no observed adverse effect level (NOAEL) was 960 mg, therefore administered dosage of phase 2a' was chosen as 1,000 mg.
All 19 patients treated with BLS-M07 (BLS-ILB-E710c) reported a total of 9 AEs during the study. In phase 1, 2 AEs occurred in the 500 mg cohort, and in phase 2a, 7 AEs occurred in the 1,000 mg cohort. None of the AEs were related to the study drug BLS-M07 (BLS-ILB-E710c). To be specific, in phase 1, all of AEs were categorized as reproductive system and breast disorders (100%). In phase 2a, 2 AEs were categorized each as gastrointestinal disorders, nervous system disorders, respiratory, thoracic and mediastinal disorder (28.57%), and one as skin and subcutaneous tissue disorder (14.29%). Most of the AEs were single occurrences, and all of the patients recovered from AEs without any side-effects.
3. Clinical efficacy
The histologic response in patients with CIN 3 was showed in Table 3. Histologic responses were evaluated at 16 weeks after initial medication (7 weeks after final medication with 1,000 mg). Six of 8 (75%) patients experienced a clinical efficacy. In the efficient group of 6 patients, 3 patients experienced complete remission to normal and another 3 had down grading to CIN 1.
Table 3. Summary of clinical efficacy and adverse event in phase 2a trial.
| Patients ID | Dose (mg) | Cytology | Histology | AEs | ||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 16 week | Response | Baseline | 16 week | Response | |||
| 2-1 (D0201) | 1,000 | HSIL | HSIL | No interval change | CIN 3 | CIN 3 | No interval change | Nasopharyngitis |
| 2-2 (D0401) | 1,000 | ASC-US | ASC-H | Progression | CIN 3 | CIN 1 | Regression | None |
| 2-3 (D0402) | 1,000 | ASC-US | ASC-US | No interval change | CIN 3 | CIN 1 | Regression | None |
| 2-5 (D0502) | 1,000 | Negative | Negative | No interval change | CIN 3 | Normal | Remission | Headache |
| 2-6 (D0503) | 1,000 | ASC-H | Negative | Remission | CIN 3 | Normal | Remission | Headache |
| 2-7 (D0504) | 1,000 | ASC-US | Negative | Remission | CIN 3 | Normal | Remission | Abdominal pain lower, Ileus |
| 2-8 (D0505) | 1,000 | ASC-H | Negative | Remission | CIN 3 | CIN 1 | Regression | None |
| 2-9 (D0506) | 1,000 | LSIL | HSIL | Progression | CIN 3 | CIN 3 | - | Psoriasis |
AE, adverse event; ASC-H, atypical squamous cells, cannot exclude; ASC-US, atypical squamous cells of undetermined significance; CIN, cervical intraepithelial neoplasia; HSIL, high-grade squamous intraepithelial lesion; LSIL, Low-grade squamous intraepithelial lesion.
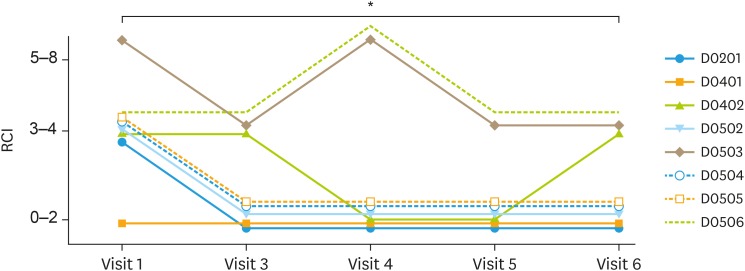
To observe efficacy of BLS-M07 (BLS-ILB-E710c) in phase 2a, RCI was used to compare baseline grade with visit 6 grade (Fig. 2). RCI grade at the time of screening consisted of 1 patient from grade of 0–2, 6 patients from grade 3–4, and 1 patient from grade 5–8. In visit 6 (7 weeks after final medication), there were 5 patients from grade 0–2, 3 patients from grade 3–4, and none from grade 5–8. There were 7 patients with grade of 3–4 or higher (87.50%) at baseline; however, RCI scoring were declining during follow-up and patients with RCI grade of 3–4 or higher were reduced at visit 6. The mean RCI score of 8 patients was significantly lower at visit 6 than initial visit (p<0.05).
Fig. 2. Treatment of CIN 3 with oral vaccination BLS-M07. The effect of BLS-M07 was evaluated by RCI on colposcopic finding. The RCI score at 16 weeks after initial treatment (visit 6) was significantly decreased compared to initial assessment.
CIN, cervical intraepithelial neoplasia; RCI, Reid Colposcopic Index.
*p<0.05.
4. Mechanism of actions (E7 specific immune response)
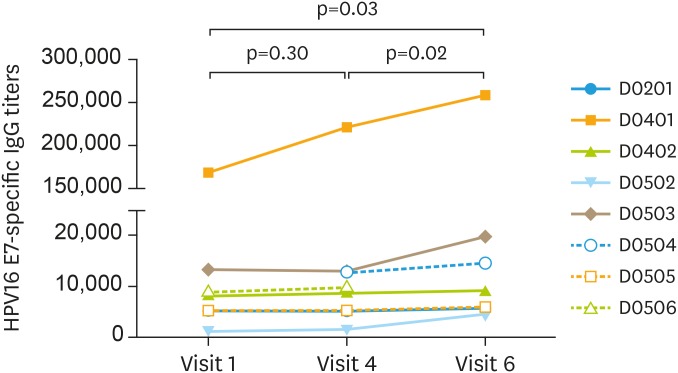
To evaluate the systemic immunologic response to BLS-M07, we examined the HPV16 E7-specific IgG level in plasma. At initial screening, specific IgG level varied in each patient. The level of IgG was higher at visit 6 (16 week) than at screening in all of seven patients, excluding one patient who was dropped out at visit 6. The mean titer of plasma IgG in 7 patients were significantly increased at 7 weeks after completion of treatment (visit 6) compared with initial visit (p<0.05); however, there was no significant difference of IgG level at immediately after completion of treatment (visit 4) and initial visit (p=0.03) (Fig. 3). These results show that oral administration of BLS-M07 induces systemic humoral immune response through increasing HPV16 E7-specific IgG level in plasma, which was steadily than abruptly.
Fig. 3. The effect of oral vaccination of BLS-M07 on HPV16 E7-specific IgG. IgG titers were assessed using a log-based slope equation where the OD = 0.2. The titers of HPV16 E7-specific IgG titers at initial visit (visit 1), at completion of treatment (visit 4 at 9 week) and follow up at 16 week (visit 6). Antibody titers were increased significantly at 16 week (visit 6).
HPV, human papillomavirus; IgG, immunoglobulin G; OD, optical density.
DISCUSSION
Current worldwide design of therapeutic agent for the treatment of CIN 3 is DNA vaccine administered through intramuscular injection. However, BLS-M07 (BLS-ILB-E710c) is a first-in-class orally administered drug that uses protein displaying technology (MucoMax®), which expresses targeted antigen on the surface of L. casei. Oncoprotein E7 of HPV which is responsible for cervical cancer, is expressed on the surface of Lactobacillus casei. Enteric capsule is used to deliver E7 displayed Lactobacillus casei through intestinal mucosa to antigen-presenting-cells, eventually inducing immune response. Lactobacillus casei, the host of BLS-M07, is the most widely used lactic acid bacteria, and its safety has been approved worldwide. 19 female patients aged 20 to 50 with histologically confirmed CIN 3 participated in our study. BLS-M07 was orally administered to confirm the safety and MTD of the drug in phase 1 trial, and to observe the efficacy of the drug through transition of pathological findings, and to validate inducement of systemic humoral immunity in phase 2a trial.
In phase 1, BLS-M07 was evaluated using three different dosages: 500 mg, 1,000 mg, 1,500 mg. Primary purpose of phase 1 was to determine the MTD and confirm that no patients experienced DLT. Throughout the phase 1 trial, none of the patients experienced DLT and grade 3 or higher drug-related toxicities. The MTD was established as 1,500 mg at the maximal administered dosage. Although there were various reported AEs, they were neither serious AEs nor drug-induced AEs, and all of the patients with AEs resolved without chronic toxicity. This revealed the safety of BLS-M07 as an orally-administered therapeutic agent.
Secondary purpose of this study was to validate the efficacy of BLS-M07. Previous study with 1,000 mg dosage level showed pathological efficacy in 2 out of 3 patients [14]. Drug dosing was initiated with 500 mg and when 3 patients were completed at each dose without DLT, proceeded to the next stage. If DLT does not occur at the 1,500 mg dose of phase 1, proceed to phase 2a with a dose of 1,000 mg. Six out of 8 patients experienced down-grade to CIN 1 or normal following the treatment, thus pathological cure rate of BLS-M07 was 75%.
In order to treat patients diagnosed with CIN 3 caused by HPV infection, HPV infected cells in cervical lesions must be eliminated. Eradication of HPV infected cells in cervical lesions requires stimulation of mucosal immunity. Kawana et al. [14] from Tokyo University conducted Phase 1/2a clinical trial with BLS-M07 for the treatment of CIN patients. Each patient's cervical lymphocyte, collected from cervical mucosal membrane, was separated and assayed with enzyme-linked immunospot [14]. After 9 weeks of treatment, the results showed an increase of HPV16 E7 specific IFN-γ producing cells and illustrated positive correlation with clinical treatment response.
The RCI is one of the most verified colposcopic scoring system for evaluating the precancerous cervical lesions. According to the previous studies investigating correlation of RCI and CIN lesions, there was good correlation between RCI and histopathology [15,16]. It consists of 4 colposcopic signs including color, vessels, margin, and iodine staging, and each sign is assigned between 0 and 2 according to colposcopic findings. The 4 scores are summed and total scores with 0 to 2 represent CIN 1 or immature metaplasia, 3 to 5 represent CIN 1 to CIN 2, and 6 to 8 represent CIN 3. In this study, percentage of CIN 3 patients that were diagnosed with equal to or higher than 3–4 RCI grading was 87.5%. However, at visit 6, percentage of CIN 3 patients with equal to or higher than 3–4 RCI grading was 37.5%. This implies that BLS-M07 had a clinical efficacy in cervical lesions scored with RCI.
Our study revealed the oral vaccination induces the improvement through systemic immune response, which was confirmed by HPV16 E7-specific IgG in plasma. The mechanism by which oral immunization induces intestinal mucosa and systemic immunity have not clearly revealed. APCs are considered to deliver E7 protein to lymphoid tissues of mucosal surface followed by inducing specific B cells [17,18]. Immunization with viral antigen induce protective cellular immunities as well as humoral immunities; however, we did not show the cellular immunity. Although our study did not evaluate the local cell-mediated immune response such as cervical lymphocytes, previous non-clinical data reported oral administration of BLS-M07 to mice induced E7-specific serum IgG and mucosal IgA production in systemic and vaginal immune system [12,19,20]. In addition, BLS-M07 induced intestinal mucosal HPV16 E7 specific cell-mediated immune response, and phase 1/2a results from Tokyo University confirmed BLS-M07 treatment increases E7 specific IFN-γ producing cells, thus inducing HPV16 E7 specific cell-mediated immune response [12,14,19]. Especially, cervical lesions of patients treated with oral administration of BLS-M07 showed cytotoxic T lymphocyte (CTL) response. These findings confirmed positive correlation of CTL response and cure rate.
Current treatment for CIN is surgical removal, which includes lesion excision and focal therapy [6]. Therefore, there is an increasing demand for new therapeutic method to treat CIN that avoids side-effects from surgical removal. Most important factor that patients evaluate before deciding drug for treatment is safety and efficacy. BLS-M07 is confirmed as a non-invasive drug without any negative side-effects. Furthermore, with ease of orally administered capsule, patients are able to treat themselves without visiting hospital. This will improve the quality of life in the CIN patients. In conclusion, BLS-M07 proved to be the first-in-class therapeutic agent that uses L. casei, and this technology may become the platform to treat other mucosal diseases.
ACKNOWLEDGMENTS
We thank all members of research team in BioLeaders, Corp., for technical support.
Footnotes
Funding: This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number : HI14C1023).
Conflict of Interest: Young-Chul Park, Moon-Hee Sung, Hong-Gyu Park from BioLeaders, Corp. are employees of and/or shareholders of the company, which is developing BLS-M07 vaccine. The remaining authors declare no competing financial interests.
- Conceptualization: P.Y.C., C.C.H., P.J.S., L.J.K.
- Data curation: P.Y.C., S.M.H., P.H.G., K.T.J.
- Formal analysis: O.Y.T.
- Methodology: P.Y.C., S.M.H., P.H.G.
- Resources: P.Y.C.
- Validation: O.Y.T., S.M.H., P.H.G.
- Writing - original draft: P.Y.C., S.M.H., P.H.G., C.C.H., L.J.K.
- Writing - review & editing: P.Y.C., O.Y.T., S.M.H., C.C.H., P.J.S., L.J.K.
References
- 1.Einstein MH, Schiller JT, Viscidi RP, Strickler HD, Coursaget P, Tan T, et al. Clinician's guide to human papillomavirus immunology: knowns and unknowns. Lancet Infect Dis. 2009;9:347–356. doi: 10.1016/S1473-3099(09)70108-2. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F. Chapter 2: the burden of HPV-related cancers. Vaccine. 2006;24(Suppl 3):S3/11–25. doi: 10.1016/j.vaccine.2006.05.111. [DOI] [PubMed] [Google Scholar]
- 3.Cox JT. The development of cervical cancer and its precursors: what is the role of human papillomavirus infection? Curr Opin Obstet Gynecol. 2006;18(Suppl 1):s5–s13. doi: 10.1097/01.gco.0000216315.72572.fb. [DOI] [PubMed] [Google Scholar]
- 4.Dalstein V, Riethmuller D, Prétet JL, Le Bail Carval K, Sautière JL, Carbillet JP, et al. Persistence and load of high-risk HPV are predictors for development of high-grade cervical lesions: a longitudinal French cohort study. Int J Cancer. 2003;106:396–403. doi: 10.1002/ijc.11222. [DOI] [PubMed] [Google Scholar]
- 5.Snijders PJ, Steenbergen RD, Heideman DA, Meijer CJ. HPV-mediated cervical carcinogenesis: concepts and clinical implications. J Pathol. 2006;208:152–164. doi: 10.1002/path.1866. [DOI] [PubMed] [Google Scholar]
- 6.Kyrgiou M, Koliopoulos G, Martin-Hirsch P, Arbyn M, Prendiville W, Paraskevaidis E. Obstetric outcomes after conservative treatment for intraepithelial or early invasive cervical lesions: systematic review and meta-analysis. Lancet. 2006;367:489–498. doi: 10.1016/S0140-6736(06)68181-6. [DOI] [PubMed] [Google Scholar]
- 7.Deligeoroglou E, Giannouli A, Athanasopoulos N, Karountzos V, Vatopoulou A, Dimopoulos K, et al. HPV infection: immunological aspects and their utility in future therapy. Infect Dis Obstet Gynecol. 2013;2013:540850. doi: 10.1155/2013/540850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu XS, Abdul-Jabbar I, Qi YM, Frazer IH, Zhou J. Mucosal immunisation with papillomavirus virus-like particles elicits systemic and mucosal immunity in mice. Virology. 1998;252:39–45. doi: 10.1006/viro.1998.9442. [DOI] [PubMed] [Google Scholar]
- 9.Woo YL, van den Hende M, Sterling JC, Coleman N, Crawford RA, Kwappenberg KM, et al. A prospective study on the natural course of low-grade squamous intraepithelial lesions and the presence of HPV16 E2-, E6- and E7-specific T-cell responses. Int J Cancer. 2010;126:133–141. doi: 10.1002/ijc.24804. [DOI] [PubMed] [Google Scholar]
- 10.Smahel M, Síma P, Ludvíková V, Vonka V. Modified HPV16 E7 genes as DNA vaccine against E7-containing oncogenic cells. Virology. 2001;281:231–238. doi: 10.1006/viro.2000.0794. [DOI] [PubMed] [Google Scholar]
- 11.Yugawa T, Kiyono T. Molecular mechanisms of cervical carcinogenesis by high-risk human papillomaviruses: novel functions of E6 and E7 oncoproteins. Rev Med Virol. 2009;19:97–113. doi: 10.1002/rmv.605. [DOI] [PubMed] [Google Scholar]
- 12.Poo H, Pyo HM, Lee TY, Yoon SW, Lee JS, Kim CJ, et al. Oral administration of human papillomavirus type 16 E7 displayed on Lactobacillus casei induces E7-specific antitumor effects in C57/BL6 mice. Int J Cancer. 2006;119:1702–1709. doi: 10.1002/ijc.22035. [DOI] [PubMed] [Google Scholar]
- 13.Nakagawa M, Greenfield W, Moerman-Herzog A, Coleman HN. Cross-reactivity, epitope spreading, and de novo immune stimulation are possible mechanisms of cross-protection of nonvaccine human papillomavirus (HPV) types in recipients of HPV therapeutic vaccines. Clin Vaccine Immunol. 2015;22:679–687. doi: 10.1128/CVI.00149-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawana K, Adachi K, Kojima S, Taguchi A, Tomio K, Yamashita A, et al. Oral vaccination against HPV E7 for treatment of cervical intraepithelial neoplasia grade 3 (CIN3) elicits E7-specific mucosal immunity in the cervix of CIN3 patients. Vaccine. 2014;32:6233–6239. doi: 10.1016/j.vaccine.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 15.Durdi GS, Sherigar BY, Dalal AM, Desai BR, Malur PR. Correlation of colposcopy using Reid colposcopic index with histopathology- a prospective study. J Turk Ger Gynecol Assoc. 2009;10:205–207. [PMC free article] [PubMed] [Google Scholar]
- 16.Kaban I, Cengiz H, Kaban A, Yildiz S, Ekin M, Avci E. Agreement between colposcopy results using the Reid Colposcopic Index and histopathology. Ginekol Pol. 2015;86:537–540. [PubMed] [Google Scholar]
- 17.Massa S, Paolini F, Curzio G, Cordeiro MN, Illiano E, Demurtas OC, et al. A plant protein signal sequence improved humoral immune response to HPV prophylactic and therapeutic DNA vaccines. Hum Vaccin Immunother. 2017;13:271–282. doi: 10.1080/21645515.2017.1264766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taguchi A, Kawana K, Yokoyama T, Adachi K, Yamashita A, Tomio K, et al. Adjuvant effect of Japanese herbal medicines on the mucosal type 1 immune responses to human papillomavirus (HPV) E7 in mice immunized orally with Lactobacillus-based therapeutic HPV vaccine in a synergistic manner. Vaccine. 2012;30:5368–5372. doi: 10.1016/j.vaccine.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 19.Adachi K, Kawana K, Yokoyama T, Fujii T, Tomio A, Miura S, et al. Oral immunization with a Lactobacillus casei vaccine expressing human papillomavirus (HPV) type 16 E7 is an effective strategy to induce mucosal cytotoxic lymphocytes against HPV16 E7. Vaccine. 2010;28:2810–2817. doi: 10.1016/j.vaccine.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Nardelli-Haefliger D, Roden RB, Benyacoub J, Sahli R, Kraehenbuhl JP, Schiller JT, et al. Human papillomavirus type 16 virus-like particles expressed in attenuated Salmonella typhimurium elicit mucosal and systemic neutralizing antibodies in mice. Infect Immun. 1997;65:3328–3336. doi: 10.1128/iai.65.8.3328-3336.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]