Abstract
Objective
The aim of this study was to investigate the clinical characteristics of young patients with stage I clear-cell carcinoma (CCC) and evaluate the prognostic factors and effects of fertility-sparing surgery (FSS) using propensity score (PS) adjustment.
Methods
We conducted a regional multi-institutional study between 1986 and 2017. Among 4,277 patients with ovarian tumor, clinical and pathological data of 103 fertile women with stage I unilateral CCC were collected. We evaluated survival and reproductive outcomes in these patients. Additionally, to analyze the effects of FSS, baseline imbalance between patients with and those without FSS was adjusted with an inverse probability of treatment weighting using PSs involving independent clinical variables.
Results
The mean patient age was 39.4 years, and the median follow-up period for surviving patients was 55.6 months. In multivariate analysis, stage IC2/IC3 (vs. IA/IC1) was the only independent prognostic factor for recurrence-free survival (RFS) and overall survival (OS). FSS was not associated with poorer prognosis when compared to the prognosis with non-preserving surgery with regard to both RFS and OS. No statistical difference in survival outcomes between FSS and other approaches was confirmed after PS adjustment. Among patients who underwent FSS, four deliveries with healthy neonates were noted without any gestational complications.
Conclusion
FSS can be considered in stage I CCC, specifically in stage IA and IC1 patients who strongly desire to have children in the future. Further clinical research is needed to clarify the optimal application of FSS for CCC.
Keywords: Ovarian Neoplasms; Adenocarcinoma, Clear Cell; Fertility Preservation; Pregnancy
INTRODUCTION
Clear-cell carcinoma (CCC) of the ovary is the second most frequent subtype, accounting for around 25% of epithelial ovarian cancer (EOC) cases in Japan [1,2]. However, it is relatively rare in the US and in European countries [3,4,5]. It usually arises in premenopausal women around the age of 40–50 years, and it is also noted in women of childbearing age [3,6]. Previous studies have suggested that resistance to anti-neoplastic agents contributed to poorer prognosis in this subtype when compared to the prognosis for other histological subtypes [6,7], despite the fact that most CCC cases are identified in the early stage [5]. In the literature, the recurrence rate of CCC has been reported to range from 18% to 29%, even for stage I disease [6,8]. Therefore, a radical approach for complete tumor resection is required initially owing to its clinical features.
A previous report mentioned that approximately 12% of ovarian cancers arise in patients of reproductive age [9], and therefore, long-term management is a matter of growing concern. Uterine and ipsilateral ovarian preservation surgery, which is referred to as fertility-sparing surgery (FSS), can be considered for patients of reproductive age, especially in the case of germ cell tumors and in early-stage EOC. According to primary practical guidelines, FSS is mainly accepted in patients with stage IA/grade 1–2 tumors, except for CCC [10,11]. FSS for early-stage CCC is controversial because of the lack of clinical evidence and ethical issues with the organization of trials. In this study, we retrospectively identified the clinical and pathological features of International Federation of Gynecology and Obstetrics (FIGO) stage I CCC patients of reproductive age and analyzed various prognostic indicators. Additionally, we focused on FSS and analyzed its effects on the prognosis of patients, including oncologic and reproductive outcomes, with a propensity score (PS)-based method to minimize the effects of possible confounding factors and maximize the therapeutic impact.
MATERIALS AND METHODS
1. Study participants
We conducted a regional multi-institutional retrospective study between January 1986 and March 2017. This study was approved by the Ethics Committee of Nagoya University (No. 357) in accordance with the principles of the Declaration of Helsinki. Data were accumulated from medical records and clinical follow-up visits. Using data of patients with unilateral stage I CCC, those aged 20–45 years were included under the central pathological review system based on the Tokai Ovarian Tumor Study Group, which consisted of Nagoya University Hospital and affiliated institutions. Patients who had insufficient clinical data and those who were lost to follow-up immediately after surgery were excluded. The histological types of EOC were assigned according to the criteria of the World Health Organization. Tumors were classified as CCC when typical clear or hobnail cells existed within papillary, solid, or tubule-cystic structures [12]. All histological slides were reviewed by one of the authors (a pathologist), who was blinded to the clinical data of the included patients.
2. Surgery, chemotherapy, and follow-up
Patients were eligible for FSS, if they 1) had CCC limited to the unilateral ovary (FIGO stage I) [13], 2) were aged ≤45 years at the initial diagnosis, 3) strongly desired to retain fertility, and 4) underwent salpingo-oophorectomy on the side affected by CCC, with at least full peritoneal staging (ascitic cytology, palpation and inspection throughout the peritoneal cavity, and peritoneal biopsy, if necessary). Systematic retroperitoneal lymphadenectomy, wedge resection of the contralateral ovary, and omentectomy could be selected in patients who did not have lymph nodes greater than 1 cm in diameter on computed tomography (CT). All patients eligible for FSS were informed of the possible risks of FSS, and they signed a consent form. We defined radical surgery as total hysterectomy and bilateral salpingo-oophorectomy, involving whole peritoneal staging (peritoneal washing, omentectomy, peritoneal biopsies, and removal of peritoneal implants) with retroperitoneal lymphadenectomy or sampling. Details of the chemotherapy regimen in each period have been described previously [14]. After the initial treatment, all patients were strictly followed-up with pelvic examination, ultrasonography, evaluation of tumor markers such as cancer antigen-125 (CA-125), and periodic CT.
3. Statistical analysis
To assess the effects of FSS in this non-randomized experiment, we introduced a PS method in which the scores were estimated by fitting a multivariate logistic regression model to the original population of patients receiving FSS (FSS group) and those not receiving FSS (non-FSS group) [15]. The independent variables that appeared to be strongly associated with the choice of FSS were considered clinically and statistically relevant. Because of the characteristics of young women with EOC and the small sample size of retrospective cohorts, we included the following independent variables: age, parity, body mass index, presence of endometriosis, tumor size, CA-125 level at the initial diagnosis, FIGO sub-classification (IA/IC1 or IC2/3), and chemotherapy performance. Missing values were substituted with average variable numbers when creating each PS. We adjusted the cohorts for the probability of treatment with the inverse probability weighting of treatment (IPTW) approach. Each individual was weighted by the inverse probability of receiving the treatment, equal to 1/PS for treated individuals and 1/(1−PS) for control individuals. No sample loss occurs with the IPTW method; therefore, the design was considered beneficial for this small observational study [16]. Standardized differences [17] of the independent variables before and after adjustment were calculated to evaluate the balance of the variables and the effectiveness of the PS-based IPTW analysis.
Comparisons between groups were analyzed using Student's t-test for continuous variables and the χ2 or Fisher's exact test for categorical variables as appropriate. The overall survival (OS) duration was considered as the period from the date of initial surgery to the last visit date or death from any cause. The recurrence-free survival (RFS) duration was considered as the period from the date of initial surgery to the last visit date or confirmation of recurrence. Univariate and multivariable Cox regression analyses were performed to identify each predictor of RFS and OS in the original population. Kaplan-Meier curves were calculated to compare RFS and OS between patients who underwent FSS and those who did not undergo FSS with and without IPTW adjustment [18]. The log-rank test was used to assess equality of survival in the two groups. Statistical significance was determined two-sided with a p-value <0.05. All statistical analyses were performed using SPSS 25.0 (IBM Corp., Armonk, NY, USA) and SAS 9.4 software (SAS Institute, Cary, NC, USA).
RESULTS
1. Baseline characteristics of the patients
A total of 4,277 patients with ovarian tumors were identified during the study period. Among 2,703 patients with EOC, 658 (24.3%) were of reproductive age (20–45 years). CCC accounted for 804 cases (28.1%) among all EOC cases. According to the study criteria, we finally identified 103 eligible patients with stage I unilateral CCC (Supplementary Fig. 1). The baseline characteristics of the patients are presented in Table 1. The mean patient age was 39.4 years. FIGO stages IA, IC1, IC2, and IC3 were noted in 27.2%, 47.6%, 15.5%, and 9.7% (IA/IC1, 74.8%; IC2/IC3, 25.2%) of patients, respectively. More than half of the patients underwent radical surgery, and 89 patients (86.4%) received adjuvant chemotherapy.
Table 1. Baseline data and survival outcomes of all patients.
| Characteristic | All (n=103) | RFS | OS | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||||
| HR | p-value | HR | p-value | HR | p-value | HR | p-value | |||
| Age (yr) | 39.4 (3.9) | 1.001 | 0.988 | 0.991 | 0.940 | 1.059 | 0.399 | 1.393 | 0.209 | |
| BMI (kg/m2) | 21.2 (3.5) | 0.901 | 0.393 | 0.862 | 0.380 | 0.896 | 0.503 | 0.953 | 0.845 | |
| Endometriosis | 45 (57.0) | 1.080 | 0.900 | 0.763 | 0.739 | 1.000 | 1.000 | 3.504 | 0.306 | |
| FIGO stage | ||||||||||
| IA/IC1 | 77 (74.8) | Reference | Reference | Reference | Reference | |||||
| IC2/IC3 | 26 (25.2) | 5.509 | <0.001 | 20.840 | 0.006 | 6.392 | <0.001 | 38.258 | 0.021 | |
| Tumor size (cm) | 12.0 (4.6) | 0.972 | 0.692 | 0.946 | 0.577 | 1.066 | 0.451 | 1.189 | 0.351 | |
| CA-125 (IU/mL)* | 127.7 (274.5) | 1.147 | 0.499 | 0.662 | 0.304 | 1.112 | 0.641 | 0.543 | 0.335 | |
| Surgery | - | - | ||||||||
| Standard surgery | 15 (14.6) | Reference | Reference | Reference | ||||||
| FSS | 21 (20.4) | 0.662 | 0.615 | 0.335 | 0.372 | |||||
| Radical surgery | 67 (65.0) | 0.982 | 0.977 | 0.794 | 0.810 | 1.387 | 0.669 | |||
| Adjuvant chemotherapy | - | - | - | - | ||||||
| None | 14 (13.6) | Reference | Reference | |||||||
| Platinum-based regimen | 27 (26.2) | 4.442 | 0.158 | 0.327 | 0.410 | |||||
| Taxane plus platinum | 62 (60.2) | 2.128 | 0.474 | |||||||
Data are presented as mean (standard deviation) or number (percentage).
BMI, body mass index; CA-125, cancer antigen-125; FIGO, International Federation of Gynecology and Obstetrics; FSS, fertility-sparing surgery; HR, hazard ratio; RFS, recurrence-free survival; OS, overall survival.
*Logarithmically transformed.
2. Prognostic factors of stage I CCC
Survival curves were constructed with stratification according to FIGO sub-classification (Supplementary Fig. 2). The median follow-up period of the surviving patients was 55.6 months. The p-values in the log-rank test were <0.001 for both RFS and OS, indicating that these two sub-classes could be prognostic factors in this study cohort. In univariate and multivariate analyses with a Cox regression hazard model, stage IC2/IC3 was the only independent risk factor for both RFS and OS. Surgery and adjuvant chemotherapy were omitted from the target variables when estimating the hazard ratio for OS owing to missing data and its distribution.
3. PS-based approach
The baseline characteristics are re-illustrated in Table 2, with patients divided into those who underwent FSS (n=21) and those who did not undergo FSS (n=82). The PS was estimated for each individual using a multivariate logistic regression model with eight predetermined variables. Using the IPTW approach, pseudo-populations were added for each cohort by weighting cases. Standardized differences and imbalances of independent variables between non-adjusted and adjusted cohorts were small enough and were almost same or reduced after adjustment (Supplementary Table 1).
Table 2. Baseline characteristic of the young patients with stage I CCC.
| Characteristic | FSS (n=21) | Non-FSS (n=82) | p-value* | |
|---|---|---|---|---|
| Age (yr) | 36.2 (3.5) | 40.3 (3.6) | <0.001 | |
| Multipara | 3 (20.0) | 21 (38.2) | 0.233 | |
| BMI (kg/m2) | 21.7 (3.9) | 21.0 (3.4) | 0.498 | |
| Endometriosis | 13 (68.4) | 32 (53.3) | 0.296 | |
| FIGO stage | 0.265 | |||
| IA/IC1 | 18 (85.7) | 59 (72.0) | ||
| IC2/IC3 | 3 (14.3) | 23 (28.0) | ||
| Tumor size (cm) | 11.3 (4.7) | 12.2 (4.5) | 0.477 | |
| CA-125 (IU/mL) | 74.0 (67.8) | 141.9 (305.3) | 0.315 | |
| Surgery | - | |||
| Standard surgery | - | 15 (18.3) | ||
| Radical surgery | - | 67 (81.7) | ||
| Adjuvant chemotherapy | 0.713 | |||
| None | 4 (19.0) | 10 (12.2) | ||
| Platinum-based regimen | 5 (23.8) | 22 (26.8) | ||
| Taxane plus platinum | 12 (57.1) | 50 (61.0) | ||
Data are presented as mean (standard deviation) or number (percentage).
BMI, body mass index; CA-125, cancer antigen-125; CCC, clear-cell carcinoma; FIGO, International Federation of Gynecology and Obstetrics FSS, fertility-sparing surgery.
*Student t-test, χ2 test, or Fisher's exact test was used as appropriate.
4. Effects of FSS
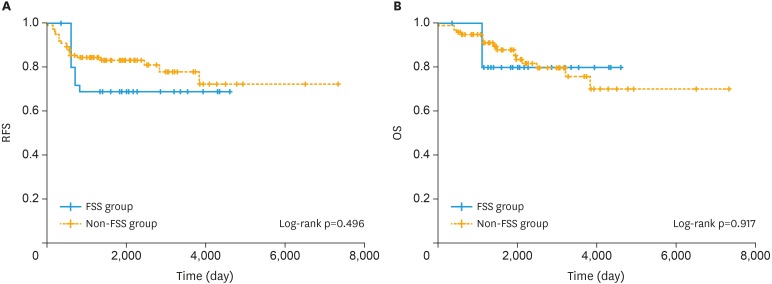
Survival curves estimated by using the Kaplan-Meier method for RFS and OS are presented in Fig. 1. For both RFS and OS, there were no differences in the survival trends between the two groups with PS adjustment. The survival outcomes are presented in Table 3. With IPTW adjustment, the 10-year RFS rates were 68.8% in the FSS group and 72.1% in the non-FSS group, and the 10-year OS rates were 79.8% in the FSS group and 70.1% in the non-FSS group. FSS was not associated with poorer prognosis when compared to the prognosis with non-preserving surgery with regard to both RFS and OS rates.
Fig. 1. Survival curves of IPTW-adjusted Kaplan-Meier analysis for RFS (A) and OS (B) in patients who underwent FSS vs. non-FSS for stage I clear-cell carcinoma of the ovary. The p-values were estimated using the IPTW-adjusted log-rank test.
FSS, fertility-sparing surgery; IPTW, inverse probability of treatment weighting; OS, overall survival; RFS, recurrence-free survival.
Table 3. Survival outcomes of the FSS and non-FSS groups with and without IPTW adjustment.
| Characteristic | Recurrence | Death | IPTW-adjusted cohorts | |
|---|---|---|---|---|
| 10-year RFS rate | 10-year OS rate | |||
| FSS group (n=21) | 3 (14.3) | 1 (4.8) | 68.8 (45.2–92.3) | 79.8 (57.4–100.0) |
| Non-FSS group (n=82) | 16 (19.5) | 14 (17.1) | 72.1 (55.7–88.5) | 70.1 (52.8–87.5) |
Data are expressed as proportion (%) and percentage (95% CI).
CI, confidential interval; FSS, fertility-sparing surgery; IPTW, inverse probability weighting of treatment.
5. Reproductive outcomes
We conducted an additional investigation to clarify the reproductive and obstetric outcomes of patients who underwent FSS. In the additional study, we excluded patients without sufficient reproductive data after surgery and finally identified 11 patients who underwent FSS (Supplementary Fig. 1). Baseline data of these patients are shown in Table 4 and Supplementary Table 2. Of the four normal pregnancies (three patients), 2 concluded with vaginal delivery and two required cesarean section. All the mothers had healthy babies, and no abnormalities were detected. Table 5 presents the three patients who delivered after surgery. All of them received adjuvant chemotherapy, and one of the patients had stage IC1 disease. In patient 1, no issues were noted. In patient 2, preterm delivery was noted, but the baby was healthy, except for a slightly small body. In patient 3, FSS was performed at 17 weeks of gestation without any complications during the perioperative period. The first baby had fetal distress in the second stage of labor at 40 weeks of gestation, and the patient underwent emergency cesarean section. The baby was healthy without complications. The second baby was delivered via repeat cesarean section.
Table 4. Reproductive outcomes of the patients who underwent FSS (n=11).
| Characteristic | Value | |
|---|---|---|
| Age (yr) | 35.1 (31–39) | |
| BMI (kg/m2) | 21.2 (3.6) | |
| Endometriosis | 6 (54.5) | |
| Stage | ||
| IA | 4 (36.4) | |
| IC1 | 6 (54.5) | |
| IC3 | 1 (9.1) | |
| Chemotherapy (taxane plus platinum) | 9 (81.8) | |
| Menstruation | 11 (100.0) | |
| POF | 1 (9.1) | |
| Infertility treatment (IVF) | 3 (27.3) | |
| Pregnancy* | ||
| Total | 7 | |
| Normal | 4 | |
| Spontaneous abortion | 2 | |
| Artificial abortion | 1 | |
| Delivery* | ||
| Vaginal delivery | 2 | |
| Cesarean section | 2 | |
| Neonate* | ||
| Healthy | 4 | |
| Abnormal | 0 | |
Data are presented as mean (standard deviation), mean (range), or number (percentage).
BMI, body mass index; FSS, fertility-sparing surgery; IVF, in vitro fertilization; POF, premature ovarian failure.
*Cumulative total number.
Table 5. Obstetric outcomes of the 3 patients with stage I CCC.
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Age (yr) | 31 | 35 | 37 |
| Gestation before surgery | G0P0 | G2P1 | G1P0 |
| Surgical procedure | Right salpingo-oophorectomy | Left salpingo-oophorectomy | Left salpingo-oophorectomy |
| Pelvic lymph node biopsy | Partial omentectomy (performed 17 weeks of gestation) | ||
| FIGO stage | IC1 | IA | IA |
| Chemotherapy | Taxane plus platinum | Taxane plus platinum | Taxane plus platinum |
| Infertility treatment | None | None | None |
| Delivery | 37 weeks of gestation, normal vaginal delivery | 35 weeks of gestation, normal vaginal delivery | 40 weeks of gestation, C/S* |
| 37 weeks of gestation, C/S | |||
| Neonate | Healthy female, 3,400 g | Healthy female, 2,475 g | Healthy male, 2,980 g* |
| Healthy male, 2,838 g | |||
| Gestational complications | Uneventful | Uneventful | Fetal distress in the second stage of labor |
| Uneventful |
CCC, clear-cell carcinoma; C/S, cesarean section; FIGO, International Federation of Gynecology and Obstetrics.
*The initial operation for CCC was performed during pregnancy.
DISCUSSION
In the present study, FIGO sub-classification of stage IC2/IC3 was the only independent prognostic factor for patients with CCC at reproductive age. In addition, FSS was not a significant prognostic factor for the survival outcomes of fertile patients with stage I CCC. Moreover, we noted some pregnancies and deliveries among patient who underwent FSS, indicating a certain benefit of FSS in young patients. To our knowledge, this is the first retrospective cohort study to use an adjusted population with the IPTW method to determine the effects of FSS in patients with stage I CCC of the ovary.
Stage IC2/IC3, which involves preoperative capsule rupture and/or positive ascitic cytology, was the only independent prognostic factor for patients with stage I CCC in our study cohort. A preoperative break in the tumor capsule is equivalent to persistent leakage of cancer cells into the ascites [19]. Peritoneal dissemination of EOC is consider to arise from the spread of cancer cells via the ascites [20], which contributes to poor prognosis regarding both PFS and OS among patients with stage IC2/3. Accordingly, definite staging in the initial surgery can determine the fate of patients with stage I CCC at reproductive age.
In this study cohort, there were no differences in survival outcomes between the FSS and non-FSS groups, indicating that FSS can be considered for stage I CCC patients with strong hope for future pregnancy. Statistical adjustment using the IPTW method strengthened the relevance of the results, as this approach canceled the effects of confounding factors as much as possible. The guidelines of the American College of Obstetrics and Gynecology and European Society for Medical Oncology illustrate the requirement for FSS regarding unilateral, localized (stage IA or IC1), and favorable histology (grade 1 and 2) [10,11]. On the other hand, in the current guidelines of the National Comprehensive Cancer Network, patients with early-stage EOC, regardless of the histological type, are acceptable candidates for FSS [21]. There is no age restriction as long as the patient strongly desires to become pregnant and accepts the resumption of menstruation. In this study, the age requirement was kept at 20–45 years, as in previous reports [8,22]. Recently, a study reported that some women aged ≥40 years got pregnant and achieved live birth [23]. Because of advancements in artificial reproductive technology, including egg donation, fertility-sparing treatments can be performed for women in their 40s, and the demand will continue to increase. In patients with stage I CCC who underwent FSS, previous analyses showed no significant effects with regard to their prognosis [24]. Some findings have recognized stage I CCC as a candidate for FSS. Satoh et al. [25] reported a multi-institutional retrospective study involving the evaluation of 211 patients with stage I unilateral EOC and mentioned the absence of a difference in survival between stage IA CCC and other histologies. Park et al. [22] reported that among 47 patients with stage I unilateral CCC, no difference in survival outcomes was present between patients who underwent FSS and those who did not undergo FSS. Moreover, the recent study of Nasioudis et al. [26] analyzed 741 premenopausal women with stage IA or IC unilateral CCC and showed that FSS had no negative impact on oncologic outcomes. Based on these findings, Park [27] also described that the indication for FSS for patients with CCC needs not to be different from other histologic types. In this study, we confirmed a similar survival tendency for patients with stage IA and IC1 CCC and found that stage IC2/IC3 is an independent prognostic factor for survival. Consequently, FSS can be optional in patients with stage I CCC, especially stage IA1 and IC1, where careful observation and adequate understanding of the disease and prognosis are ensured.
The limitations of this study include the small cohort population and the existence of possible confounding factors (e.g., chemotherapy regimens and year of treatment) between the groups. As for chemotherapy, we principally treated patients with stage I CCC with adjuvant chemotherapy according to the guideline of Japan Society of Gynecologic Oncology [28]. On the other hand, adjuvant chemotherapy was omitted in some patients with stage IA and IC1 CCC. Based on our previous report, adjuvant chemotherapy does not contribute to the improving prognosis of stage IA and IC1 CCC [29]. Although we canceled the effect of chemotherapy by using PS-based approach, there might be another confounding factor related to chemotherapy regimens. In addition, retroperitoneal lymphadenectomy was abbreviated in several cases in this study; thus, there might have been occult lymph node metastasis. Some studies have supported the non-superiority of lymphadenectomy in stage I CCC [14,30]. However, we could not attribute the results to unnecessity of lymphadenectomy in FSS owing to the retrospective study design. Because of the difficulty in extrapolating the findings of this retrospective analysis directly to therapy recommendations, the results of this study should be used as the basis for additional studies, including prospective trials, to examine the effects of FSS for CCC, especially in stage IA and IC1 disease.
In conclusion, stage IC2/IC3 of the FIGO sub-classification, which indicates preoperative capsule rupture and positive ascitic cytology, was the only independent prognostic factor for stage I CCC in women of childbearing age. Additionally, FSS was not a prognostic factor for patients with stage I CCC. Therefore, FSS can be considered in stage I CCC, specifically in stage IA and IC1 patients who strongly desire to have children in the future. In stage I CCC patients who have undergone FSS, normal pregnancies can be achieved with healthy neonates, and this may provide encouragement to patients with this disease. Further clinical research is needed to clarify the optimal application of FSS for CCC. Prospective trials with sufficient power to confirm the results of this study are required.
ACKNOWLEDGMENTS
We sincerely thank members belonging to Tokai Ovarian Tumor Study Group-affiliated institutions for collaborating in data collection.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: Y.M., K.H.
- Data curation: Y.M., K.H., T.S., S.S.
- Formal analysis: Y.M., K.H., T.K., M.S.
- Investigation: K.H.
- Methodology: Y.M., K.H., T.S., S.S., T.K., M.S.
- Project administration: K.H.
- Supervision: Y.M., K.H., M.S., K.F.
- Validation: Y.M., T.S., S.S.
- Visualization: T.K.
- Writing - original draft: Y.M.
- Writing - review & editing: K.H.
SUPPLEMENTARY MATERIALS
Standardized differences of independent variables
Baseline data of the patients who underwent FSS
Flowchart of the selection of fertile patients with stage I unilateral CCC of the ovary and those who underwent FSS from the database of Tokai Ovarian Tumor Study Group (1986 to 2017).
Survival curves of Kaplan-Meier analysis for OS (A, B) and RFS (C, D) stratified according to FIGO sub-classification. The p-values were estimated using the log-rank test.
References
- 1.Fujiwara K, Shintani D, Nishikawa T. Clear-cell carcinoma of the ovary. Ann Oncol. 2016;27(Suppl 1):i50–i52. doi: 10.1093/annonc/mdw086. [DOI] [PubMed] [Google Scholar]
- 2.Katanuchi H. The patient annual report for 2015. Acta Obstet Gynaecol Jpn. 2017;69:1171–1216. [Google Scholar]
- 3.Banks E. The epidemiology of ovarian cancer. Methods Mol Med. 2001;39:3–11. doi: 10.1385/1-59259-071-3:3. [DOI] [PubMed] [Google Scholar]
- 4.Chan JK, Teoh D, Hu JM, Shin JY, Osann K, Kapp DS. Do clear cell ovarian carcinomas have poorer prognosis compared to other epithelial cell types? A study of 1411 clear cell ovarian cancers. Gynecol Oncol. 2008;109:370–376. doi: 10.1016/j.ygyno.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Köbel M, Kalloger SE, Huntsman DG, Santos JL, Swenerton KD, Seidman JD, et al. Differences in tumor type in low-stage versus high-stage ovarian carcinomas. Int J Gynecol Pathol. 2010;29:203–211. doi: 10.1097/PGP.0b013e3181c042b6. [DOI] [PubMed] [Google Scholar]
- 6.Sugiyama T, Kamura T, Kigawa J, Terakawa N, Kikuchi Y, Kita T, et al. Clinical characteristics of clear cell carcinoma of the ovary: a distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy. Cancer. 2000;88:2584–2589. [PubMed] [Google Scholar]
- 7.Goff BA, Sainz de la Cuesta R, Muntz HG, Fleischhacker D, Ek M, Rice LW, et al. Clear cell carcinoma of the ovary: a distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy in stage III disease. Gynecol Oncol. 1996;60:412–417. doi: 10.1006/gyno.1996.0065. [DOI] [PubMed] [Google Scholar]
- 8.Kajiyama H, Mizuno M, Shibata K, Umezu T, Suzuki S, Yamamoto E, et al. A recurrence-predicting prognostic factor for patients with ovarian clear-cell adenocarcinoma at reproductive age. Int J Clin Oncol. 2014;19:921–927. doi: 10.1007/s10147-013-0645-3. [DOI] [PubMed] [Google Scholar]
- 9.National Cancer Institute. Cancer stat facts: ovarian cancer [Internet] Bethesda, MD: National Cancer Institute; 2016. [cited 2018 Dec 15]. Available from: https://seer.cancer.gov/statfacts/html/ovary.html. [Google Scholar]
- 10.ACOG practice bulletin No. 83: management of adnexal masses. Obstet Gynecol. 2007;110:201–214. doi: 10.1097/01.AOG.0000263913.92942.40. [DOI] [PubMed] [Google Scholar]
- 11.Ledermann JA, Raja FA, Fotopoulou C, Gonzalez-Martin A, Colombo N, Sessa C, et al. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi24–vi32. doi: 10.1093/annonc/mdt333. [DOI] [PubMed] [Google Scholar]
- 12.Takano M, Kikuchi Y, Yaegashi N, Kuzuya K, Ueki M, Tsuda H, et al. Clear cell carcinoma of the ovary: a retrospective multicentre experience of 254 patients with complete surgical staging. Br J Cancer. 2006;94:1369–1374. doi: 10.1038/sj.bjc.6603116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mutch DG, Prat J. 2014 FIGO staging for ovarian, fallopian tube and peritoneal cancer. Gynecol Oncol. 2014;133:401–404. doi: 10.1016/j.ygyno.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki S, Kajiyama H, Shibata K, Ino K, Nawa A, Sakakibara K, et al. Is there any association between retroperitoneal lymphadenectomy and survival benefit in ovarian clear cell carcinoma patients? Ann Oncol. 2008;19:1284–1287. doi: 10.1093/annonc/mdn059. [DOI] [PubMed] [Google Scholar]
- 15.Rosenbaum PR, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score. Am J Stat Assoc. 1984;79:516–524. [Google Scholar]
- 16.Yoshihara M, Uno K, Tano S, Mayama M, Ukai M, Kondo S, et al. The efficacy of recombinant human soluble thrombomodulin for obstetric disseminated intravascular coagulation: a retrospective study. Crit Care. 2015;19:369. doi: 10.1186/s13054-015-1086-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39:33–38. [Google Scholar]
- 18.Xie J, Liu C. Adjusted Kaplan-Meier estimator and log-rank test with inverse probability of treatment weighting for survival data. Stat Med. 2005;24:3089–3110. doi: 10.1002/sim.2174. [DOI] [PubMed] [Google Scholar]
- 19.Kim HS, Ahn JH, Chung HH, Kim JW, Park NH, Song YS, et al. Impact of intraoperative rupture of the ovarian capsule on prognosis in patients with early-stage epithelial ovarian cancer: a meta-analysis. Eur J Surg Oncol. 2013;39:279–289. doi: 10.1016/j.ejso.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Leminen A, Lehtovirta P. Spread of ovarian cancer after laparoscopic surgery: report of eight cases. Gynecol Oncol. 1999;75:387–390. doi: 10.1006/gyno.1999.5596. [DOI] [PubMed] [Google Scholar]
- 21.National Comprehensive Cancer Network (US) NCCN clinical practice guideline in oncology. Ovarian cancer including fallopian tube cancer and primary peritoneal cancer, version 1 [Internet] Plymouth Meeting, PA: National Comprehensive Cancer Network; 2017. [cited 2017 Jun 22]. Available from: https://www.nccn.org/professionals/physician_gls/f_guidelines.asp. [Google Scholar]
- 22.Park JY, Suh DS, Kim JH, Kim YM, Kim YT, Nam JH. Outcomes of fertility-sparing surgery among young women with FIGO stage I clear cell carcinoma of the ovary. Int J Gynaecol Obstet. 2016;134:49–52. doi: 10.1016/j.ijgo.2015.10.022. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention; American Society for Reproductive Medicine; Society for Assisted Reproductive Technology. 2014 assisted reproductive technology national summary report. Atlanta, GA: US Department of Health and Human Services; 2016. [Google Scholar]
- 24.Kajiyama H, Shibata K, Mizuno M, Hosono S, Kawai M, Nagasaka T, et al. Fertility-sparing surgery in patients with clear-cell carcinoma of the ovary: is it possible? Hum Reprod. 2011;26:3297–3302. doi: 10.1093/humrep/der342. [DOI] [PubMed] [Google Scholar]
- 25.Satoh T, Hatae M, Watanabe Y, Yaegashi N, Ishiko O, Kodama S, et al. Outcomes of fertility-sparing surgery for stage I epithelial ovarian cancer: a proposal for patient selection. J Clin Oncol. 2010;28:1727–1732. doi: 10.1200/JCO.2009.24.8617. [DOI] [PubMed] [Google Scholar]
- 26.Nasioudis D, Chapman-Davis E, Frey MK, Witkin SS, Holcomb K. Could fertility-sparing surgery be considered for women with early stage ovarian clear cell carcinoma? J Gynecol Oncol. 2017;28:e71. doi: 10.3802/jgo.2017.28.e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park JY. Safety of fertility-sparing surgery for stage I ovarian clear cell carcinoma. J Gynecol Oncol. 2017;28:e91. doi: 10.3802/jgo.2017.28.e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Komiyama S, Katabuchi H, Mikami M, Nagase S, Okamoto A, Ito K, et al. Japan Society of Gynecologic Oncology guidelines 2015 for the treatment of ovarian cancer including primary peritoneal cancer and fallopian tube cancer. Int J Clin Oncol. 2016;21:435–446. doi: 10.1007/s10147-016-0985-x. [DOI] [PubMed] [Google Scholar]
- 29.Mizuno M, Kajiyama H, Shibata K, Mizuno K, Yamamuro O, Kawai M, et al. Adjuvant chemotherapy for stage I ovarian clear cell carcinoma: is it necessary for stage IA? Int J Gynecol Cancer. 2012;22:1143–1149. doi: 10.1097/IGC.0b013e31825c7cbe. [DOI] [PubMed] [Google Scholar]
- 30.Chan JK, Munro EG, Cheung MK, Husain A, Teng NN, Berek JS, et al. Association of lymphadenectomy and survival in stage I ovarian cancer patients. Obstet Gynecol. 2007;109:12–19. doi: 10.1097/01.AOG.0000249610.95885.ef. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Standardized differences of independent variables
Baseline data of the patients who underwent FSS
Flowchart of the selection of fertile patients with stage I unilateral CCC of the ovary and those who underwent FSS from the database of Tokai Ovarian Tumor Study Group (1986 to 2017).
Survival curves of Kaplan-Meier analysis for OS (A, B) and RFS (C, D) stratified according to FIGO sub-classification. The p-values were estimated using the log-rank test.