Abstract
目的
探讨凋亡抑制蛋白Survivin和PI3K、AKT在寻常型银屑病(PV)进行期斑块状皮损角质形成细胞(KCs)中的表达水平及作用。
方法
临床上收集22例PV患者进行期斑块状皮损以及18例正常皮肤组织。采用免疫组织化学、Western blot和real-time quantitative PCR等方法检测22例PV患者进行期斑块状皮损和18例正常皮肤组织中KCs的Survivin、PI3K和AKT表达,并分析它们在PV皮损KCs中的相关性。使用小干扰RNA(siRNA)在HaCaT细胞中沉默AKT,并通过Western blot实验检测Survivin的表达水平。
结果
PV皮损KCs中Survivin、PI3K和AKT的蛋白表达水平均高于正常皮肤组织。PV皮损KCs中Survivin和PI3K mRNA表达成正相关(r=0.4510;P=0.0351);PV皮损KCs中Survivin和AKT mRNA表达成正相关(r=0.4423;P=0.0393);在HaCaT细胞中,沉默AKT后可引起Survivin的表达下调。
结论
凋亡抑制蛋白Survivin与PI3K/AKT信号通路可能参与了PV的发生与发展。
Keywords: Survivin, PI3K, AKT, 寻常型银屑病
Abstract
Objective
To explore the role of survivin and PI3K/AKT pathway in the pathogenesis of psoriasis vulgaris (PV).
Methods
Plaque-like lesions collected from 22 patients with PV in progressive stage and 18 normal control skin specimens were examined using immunohistochemical staining, Western blotting and real-time quantitative PCR for expressions of survivin, PI3K and AKT in the keratinocytes, and their correlation was analyzed. A small interfering RNA (siRNA) was used to knock down AKT in cultured HaCaT cells, and Western blotting was used to detect the changes in the expression of survivin.
Results
Compared with normal skin, PV lesions showed obviously up-regulated expressions of survivin, PI3K and AKT in the keratinocytes. Survivin expression was positively correlated with PI3K (r=0.4510, P=0.0351) and AKT (r=0.4423, P=0.0393) in the keratinocytes in PV lesions. In cultured HaCaT cells, siRNA-mediated knockdown of AKT caused down-regulation of survivin expression.
Conclusion
Survivin and PI3K/AKT signaling pathway may participate in the occurrence and progression of PV.
Keywords: Survivin, PI3K, AKT, psoriasis vulgaris
银屑病是临床常见的一种慢性复发性的炎症性皮肤病,超过90%的银屑病患者为寻常型银屑病(PV),其组织病理表现主要为表皮角质增厚、真皮乳头层血管增生和炎症细胞浸润[1-3]。然而,银屑病的病因及发病机制尚不完全清楚。存活素(Survivin)作为一种凋亡蛋白抑制因子(IAP),广泛表达于各种肿瘤组织中,如乳腺癌、口腔癌、肺癌和肝癌等[4-7]。然而,目前Survivin在银屑病中的表达水平仍存在争议。磷脂酰肌醇3-激酶(PI3K)/蛋白激酶B(AKT)信号通路是肿瘤研究的热门信号通路,它参与了肿瘤的增殖、转移、耐药以及凋亡抑制等过程[8]。目前研究发现其与银屑病的发生发展可能相关[9-10]。为进一步探索Survivin、PI3K、AKT在银屑病发病机制中的作用,本研究在基因、蛋白、细胞水平并应用小干扰RNA(siRNA)策略,检测人银屑病皮损角质形成细胞(KCs)中Survivin、PI3K和AKT蛋白和mRNA的表达水平并探讨其相关性和意义。
1. 材料和方法
1.1. 临床资料
寻常型银屑病(PV)患者22例,均来自西安交通大学第二附属医院皮肤科门诊患者,患者均具有典型的临床症状及病理特征,符合PV进行期的诊断标准,其中男性12例,女性10例,年龄24~60岁,平均41.68岁;病程3月~22年,平均7.63年。以同期我院皮肤外科18例美容整形患者切除的正常皮肤组织作为对照组,其中男性10例,女性8例,年龄15~51岁,平均39.81岁。所有银屑病患者取材前3月未服用或外用糖皮质激素、免疫抑制剂及维A酸类药物,取材前2周内未使用任何药物治疗,且不伴有其他皮肤及系统疾病。所有标本取材均按常规皮肤活检手术进行。银屑病患者组与健康对照组在年龄、性别等方面比较差异无统计学意义(P>0.05)。
1.2. 免疫组织化学
采用EnVision二步法:65 ℃烤片,二甲苯脱蜡,酒精梯度水化,高压锅抗原修复5 min,阻断内源性过氧化物酶10 min,一抗(Survivin、PI3K、AKT均购买于北京博奥森生物公司)(浓度参考抗体说明书)孵育4 ℃过夜,二抗常温孵育30 min,DAB显色,苏木素复染,盐酸分化和氨水反蓝,酒精梯度脱水,二甲苯透明和中性树胶封片,最后再显微镜下观察和拍片。免疫组织化学染色评分:根据着色强度(无、弱、中等、强)分别评0、1、2、3分;根据阳性细胞百分比[(0~25)%、(25~50)%、(50~ 75)%、(75~100)%]分别评1、2、3、4分,总分为着色强度评分和阳性细胞百分比评分的乘积。
1.3. 细胞培养
将HaCaT细胞株培养于含10%胎牛血清的1640培养基(Gibco)中,并将细胞置于37 ℃、5% CO2的培养箱中进行培养。
1.4. siRNA转染
AKT siRNA(吉玛生物),siAKT序列为:正义链5'-UUGUAGCCAAUGAAGGUGCCA-3',反义链5'-GC ACCUUCAUUGGCUACAATT-3',转染试剂X-tremeGENEsiRNA transfection reagents购自Roche公司。将1.5×105 HaCaT细胞种植于6孔细胞培养板。24 h后采用X-tremeGENE siRNA transfection reagents将2 µg/孔siRNA转染到细胞内,继续培养48 h后提取细胞总蛋白。
1.5. Western blot
常规提取组织和细胞总蛋白,使用10%的SDSPAGE凝胶进行电泳分离蛋白,电泳后采用湿转的方法将蛋白转移至PVDF膜上,5%脱脂牛奶常温封闭1 h,一抗(Survivin、PI3K、AKT和GAPDH均购买于北京博奥森生物公司)孵育4 ℃过夜,TBST洗涤3次,5 min/次,HRP标记的二抗(CST)常温孵育1 h,TBST洗涤3次,5 min/次,在暗室中将PVDF膜蛋白面浸入HRP-ECL发光液中,并使用ChemiDoc XRS成像系统显影。使用GAPDH作为内参。
1.6. Real-time PCR
使用Trizol试剂提取组织和细胞中的总RNA。使用PrimeScriptTM RT Master Mix(Perfect Real-time)反转录试剂盒对所提取的RNA进行反转录。将反应物混匀后,放入MyCyclerTM普通PCR仪中进行反转录。当成功反转录为cDNA后,使用SYBR® Premix Ex TaqTM Ⅱ(TliRNaseH Plus)试剂盒进行Real-time quantitative PCR实验。其中引物序列为:Survivin,上游引物5'-CACCCCGGAGCGGATGG-3',下游引物5'-GTCATC TGGCTCCCAGCCTTC-3';AKT,上游引物5'-AGCG ACGTGGCTATTGTGAAG-3',下游引物5'-GCCATC ATTCTTGAGGAGGAAGT-3';PI3K,上游引物5'-TAT TTGGACTTTGCGACAAGACT-3',下游引物5'-TCG AACGTACTGGTCTGGATAG-3';GAPDH,上游引物5'-GGAGCGAGATCCCTCCAAAAT-3',下游引物5'-GAACCTGGAAGAGTCCGAAGTA-3'。其中GAPDH作为内参。
1.7. 统计学方法
使用Graphpad Prism 5.0版本软件分析两组之间的差异(two-tailed student's t检验),采用Pearson correlation分析Survivin与PI3K、AKT表达的相关性。当P<0.05认为差异有统计学意义。
2. 结果
2.1. 免疫组织化学和Western blot检测
与正常皮肤相比,Survivin、PI3K和AKT蛋白在PV皮损组织KCs中表达上调(图 1)。
1.
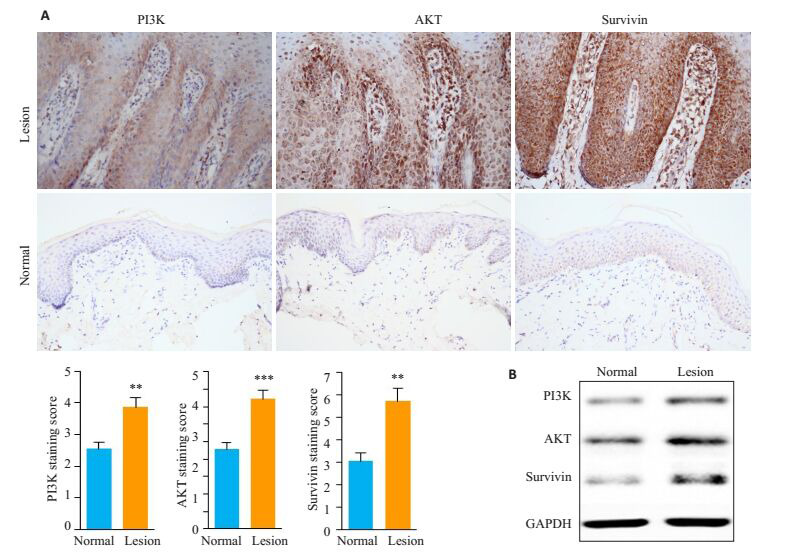
Survivin、PI3K和AKT在PV皮损KCs中的表达
Expression of survivin, PI3K and AKT in the keratinocytes inPV lesions. A: Immunohistochemical staining of survivin, PI3K and AKT in the keratinocytes in normal skin and PV lesions (Original magnification: × 400); B: Western blot analysis of survivin, PI3K and AKT in the keratinocytes in normal skin and PV lesions. **P < 0.01, ***P < 0.001 vs normal skin.
2.2. Real-time quantitative PCR检测及相关性分析
Survivin和PI3K mRNA的表达呈正相关(r=0.4510;P=0.0351);Survivin和AKT mRNA表达呈正相关(r= 0.4423;P=0.0393,图 2)。
2.
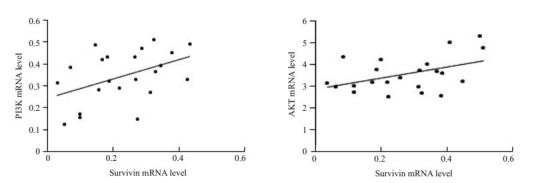
PV皮损KCs中Survivin、PI3K和AKT的相关性分析
Correlation analysis of survivin, PI3K and AKT in the keratinocytes in PV lesions. Left: Correlation analysis between survivin and PI3K mRNA; Right: Correlation analysis between survivin and AKT mRNA.
2.3. siRNA干扰技术
在HaCaT细胞中,沉默AKT可以下调Survivin的表达(图 3)。
3.
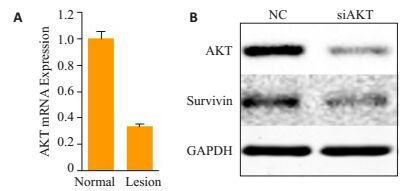
PV皮损KCs中PI3K/AKT信号通路和Survivin的相关性
Correlation between PI3K/AKT signaling pathway and survivin in the keratinocytes in PV lesions. A: Real-time quantitative PCR of AKT in HaCaT NC and AKT-deficient sublines; B: Western blot analysis of AKT and survivin in HaCaT NC and AKT deficient sublines.
3. 讨论
Survivin作为凋亡蛋白抑制因子(IAP)家族成员中抗凋亡作用最强的蛋白之一,目前广泛的在各种肿瘤组织中表达,如乳腺癌、口腔癌、肺癌和肝癌等[4-7]。大量研究发现Survivin在肿瘤中高表达且可以抑制肿瘤细胞的凋亡,从而促进肿瘤的发生、增殖和对放射治疗以及化学治疗的抵抗[6, 11-13]。PV是一种慢性炎症性、增殖性皮肤病,角质形成细胞增殖过度和凋亡的缺失不足是PV发生发展的机制之一[1-2]。目前有关Survivin在PV皮损中的表达水平尚有争议。Bowen等[14]发现最先报道Survivin在皮肤肿瘤和角质细胞增生皮损中表达增高,而在正常皮肤中表达较低。Simonetti等[15]报道Survivin和血管内皮生成因子(VEGF)在PV皮损中均表达上调。同样,Wang等[16]发现Survivin不仅在PV皮损中表达上调,且与抗菌肽β-defensin-3表达呈正相关。然而,Gunduz K等[17]报道PV皮损中survivin蛋白表达水平与正常皮肤中的表达水平没有差异。为了进一步明确survivin在PV皮损中的表达情况,我们在蛋白和mRNA水平上进行探究,发现survivin mRNA和蛋白水平在PV皮损中的表达水平均高于正常皮肤。
PI3K/AKT信号通路在细胞生长、增殖等功能中起重要的作用[18-19]。AKT是PI3K下游的靶蛋白,可以通过磷酸化其下游mTOR、Bad等蛋白,从而调控细胞的增殖和凋亡[20, 23]。大量研究证明,PI3K/AKT信号通路在肿瘤增殖和凋亡异常中起到重要作用[24-25]。在PV发生发展过程中,角质形成细胞的增殖和凋亡失衡起着重要的作用。Chamcheu等[9-10]发现在Imiquimod诱导的鼠PV样皮炎模型中,PI3K/AKT信号活性是增强的,进一步发现抑制PI3K/AKT信号通路可以缓解Imiquimod诱导的鼠PV样皮损。Jeon等[26]报道抑制PI3K/ AKT信号通路可以缓解皮肤的炎症反应。然而,PI3K和AKT在人PV皮损中的表达尚不清楚。在我们的研究中发现,与正常皮肤相比,PV皮损KCs中的PI3K和AKT的表达均上调,提示PI3K/AKT信号通路在PV发生发展中具有重要的作用。
PI3K/AKT信号通路参与了肿瘤细胞的增殖和凋亡异常。然而,在PV中,尚不清楚PI3K/AKT信号通路是否参与了促进角质形成细胞(KCs)增殖、抑制KCs凋亡的过程。本研究检测了PV皮损中Survivin、PI3K和AKTmRNA的表达,并对Survivin和PI3K以及Survivin和AKT进行了相关性分析。我们发现Survivin和PI3K的表达呈正相关;Survivin和AKT的表达也呈正相关,表明PI3K/AKT信号通路可能通过Survivin参与了PV皮损中KCs的凋亡受抑。另外,在体外细胞实验中,我们发现沉默HaCaT细胞AKT可以下调其Survivin蛋白的表达,提示KCs中AKT可以正向调控其Survivin的表达。
目前普遍认为,IL-23/Th17(白介素-23/辅助性T细胞17)轴的失调引起并加剧PV患者皮损中慢性炎症反应[27]。随着对银屑病发病机制研究的深入,人们发现各种细胞因子及炎性细胞、炎性介质在银屑病的发病中都不是单一发挥作用的,而是相互制约、相互影响,构成一个复杂的网络。在银屑病发病过程中,炎症、增殖与凋亡密切相关。
总之,我们的研究初步发现Survivin与PI3K、AKT在PV皮损KCs中蛋白表达均升高,并在mRNA表达水平呈正相关,沉默KCsAKT可以下调其Survivin蛋白的表达,表明PI3K/AKT信号通路可能通过凋亡抑制蛋白Survivin参与了PV的发生与发展,提示Survivin与PI3K/AKT信号通路可为银屑病治疗潜在的新靶点,可能为银屑病的治疗提供新的思路。
Funding Statement
国家自然科学基金(30960349)
Supported by National Natural Science Foundation of China (30960349)
References
- 1.Teraki Y, Shiohara T. Apoptosis and the skin. http://d.wanfangdata.com.cn/OAPaper/oai_doaj-articles_fd5fdd4d7e1c8cc022cc830a94cc4819. Eur J Dermatol. 1999;9(5):413–25. [Teraki Y, Shiohara T. Apoptosis and the skin[J]. Eur J Dermatol, 1999, 9(5): 413-25.] [PubMed] [Google Scholar]
- 2.Hawkes JE, Chan TC, Krueger JG. Psoriasis pathogenesis and the development of novel targeted immune therapies. J Allergy Clin Immunol. 2017;140(3):645–53. doi: 10.1016/j.jaci.2017.07.004. [Hawkes JE, Chan TC, Krueger JG. Psoriasis pathogenesis and the development of novel targeted immune therapies[J]. J Allergy Clin Immunol, 2017, 140(3): 645-53.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boehncke WH, Brembilla NC. Unmet needs in the field of psoriasis: pathogenesis and treatment[J]. Clin Rev Allergy Immunol, 2017, [Epub ahead of print].
- 4.Huang Q, Zeng Y, Lin H, et al. Transfection with Livin and SurvivinshRNA inhibits the growth and proliferation of non-small cell lung cancer cells. https://www.spandidos-publications.com/mmr/16/5/7086. Mol Med Rep. 2017;16(5):7086–91. doi: 10.3892/mmr.2017.7490. [Huang Q, Zeng Y, Lin H, et al. Transfection with Livin and SurvivinshRNA inhibits the growth and proliferation of non-small cell lung cancer cells[J]. Mol Med Rep, 2017, 16(5): 7086-91.] [DOI] [PubMed] [Google Scholar]
- 5.Li SX, Yang YQ, Ding YP, et al. Impacts of survivin and caspase-3 on apoptosis and angiogenesis in oral cancer. https://www.spandidos-publications.com/10.3892/ol.2017.6626/download. Oncol Lett. 2017;14(3, B):3774–9. doi: 10.3892/ol.2017.6626. [Li SX, Yang YQ, Ding YP, et al. Impacts of survivin and caspase-3 on apoptosis and angiogenesis in oral cancer[J]. Oncol Lett, 2017, 14(3, B): 3774-9.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taglieri L, De Iuliis F, Giuffrida AA, et al. Resistance to the mTOR inhibitor everolimus is reversed by the downregulation of survivin in breast cancer cells. https://www.spandidos-publications.com/10.3892/ol.2017.6597. Oncol Lett. 2017;14(3, B):3832–8. doi: 10.3892/ol.2017.6597. [Taglieri L, De Iuliis F, Giuffrida AA, et al. Resistance to the mTOR inhibitor everolimus is reversed by the downregulation of survivin in breast cancer cells[J]. Oncol Lett, 2017, 14(3, B): 3832-8.] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Silke J, Vince J. IAPs and cell death. http://d.wanfangdata.com.cn/Periodical/wjg200714004. Curr Top Microbiol Immunol. 2017;403:95–117. doi: 10.1007/82_2016_507. [Silke J, Vince J. IAPs and cell death[J]. Curr Top Microbiol Immunol, 2017, 403: 95-117.] [DOI] [PubMed] [Google Scholar]
- 8.Guerrero-Zotano A, Mayer IA, Arteaga CL. PI3K/AKT/mTOR: role in breast cancer progression, drug resistance, and treatment. Cancer Metastasis Rev. 2016;35(4):515–24. doi: 10.1007/s10555-016-9637-x. [Guerrero-Zotano A, Mayer IA, Arteaga CL. PI3K/AKT/mTOR: role in breast cancer progression, drug resistance, and treatment[J]. Cancer Metastasis Rev, 2016, 35(4): 515-24.] [DOI] [PubMed] [Google Scholar]
- 9.Chamcheu JC, Chaves-Rodriquez MI, Adhami VM, et al. Upregulation of PI3K/AKT/mTOR, FABP5 and PPAR beta/delta in Human Psoriasis and Imiquimod-induced Murine Psoriasiform Dermatitis Model. https://www.medicaljournals.se/acta/content/html/10.2340/00015555-2359. Acta DermVenereol. 2016;96(6):854–6. doi: 10.2340/00015555-2359. [Chamcheu JC, Chaves-Rodriquez MI, Adhami VM, et al. Upregulation of PI3K/AKT/mTOR, FABP5 and PPAR beta/delta in Human Psoriasis and Imiquimod-induced Murine Psoriasiform Dermatitis Model [J]. Acta DermVenereol, 2016, 96(6): 854-6.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chamcheu JC, Adhami VM, Esnault S, et al. Dual inhibition of PI3K/Akt and mTOR by the dietary antioxidant, delphinidin, ameliorates psoriatic features in vitro and in an Imiquimod-Induced Psoriasis-Like disease in mice. Antioxid Redox Signal. 2017;26(2):49–69. doi: 10.1089/ars.2016.6769. [Chamcheu JC, Adhami VM, Esnault S, et al. Dual inhibition of PI3K/Akt and mTOR by the dietary antioxidant, delphinidin, ameliorates psoriatic features in vitro and in an Imiquimod-Induced Psoriasis-Like disease in mice[J]. Antioxid Redox Signal, 2017, 26 (2): 49-69.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee EY, Gong EY, Shin JS, et al. Human breast cancer cells display different sensitivities to ABT-263 based on the level of survivin. http://www.sciencedirect.com/science/article/pii/S0887233317302758. Toxicol in vitro. 2017;46:229–36. doi: 10.1016/j.tiv.2017.09.023. [Lee EY, Gong EY, Shin JS, et al. Human breast cancer cells display different sensitivities to ABT-263 based on the level of survivin[J]. Toxicol in vitro, 2017, 46: 229-36.] [DOI] [PubMed] [Google Scholar]
- 12.Gu F, Li L, Yuan QF, et al. Down-regulation of survivin enhances paclitaxel-induced Hela cell apoptosis. http://precedings.nature.com/documents/2729/version/1/html. Eur Rev Med Pharmacol Sci. 2017;21(15):3504–9. [Gu F, Li L, Yuan QF, et al. Down-regulation of survivin enhances paclitaxel-induced Hela cell apoptosis[J]. Eur Rev Med Pharmacol Sci, 2017, 21(15): 3504-9.] [PubMed] [Google Scholar]
- 13.Zhang ZX, Wang TY, Liu ZQ, et al. Small interfering RNA target ing of the survivin gene inhibits human tumor cell growth in vitro. ExpTher Med. 2017;14(1, A):35–42. doi: 10.3892/etm.2017.4501. [Zhang ZX, Wang TY, Liu ZQ, et al. Small interfering RNA target ing of the survivin gene inhibits human tumor cell growth in vitro [J]. ExpTher Med, 2017, 14(1, A): 35-42.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bowen AR, Hanks AN, Murphy KJ, et al. Proliferation, apoptosis, and survivin expression in keratinocytic neoplasms and Hyperplasias. Am J Dermatopathol. 2004;26(3):177–81. doi: 10.1097/00000372-200406000-00001. [Bowen AR, Hanks AN, Murphy KJ, et al. Proliferation, apoptosis, and survivin expression in keratinocytic neoplasms and Hyperplasias[J]. Am J Dermatopathol, 2004, 26(3): 177-81.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simonetti O, Lucarini G, Campanati AA, et al. VEGF, survivin and NOS overexpression in psoriatic skin: Critical role of nitric oxide synthases. J Dermatol Sci. 2009;54(3):205–8. doi: 10.1016/j.jdermsci.2008.12.012. [Simonetti O, Lucarini G, Campanati AA, et al. VEGF, survivin and NOS overexpression in psoriatic skin: Critical role of nitric oxide synthases[J]. J Dermatol Sci, 2009, 54(3): 205-8.] [DOI] [PubMed] [Google Scholar]
- 16.Wang F, Zhang X, Xia P, et al. Enhancement of mRNA expression of survivin and human beta-defensin-3 in lesions of psoriasis vulgaris. http://www.ncbi.nlm.nih.gov/pubmed/26771657. Eur J Dermatol. 2016;26(1):28–33. doi: 10.1684/ejd.2015.2698. [Wang F, Zhang X, Xia P, et al. Enhancement of mRNA expression of survivin and human beta-defensin-3 in lesions of psoriasis vulgaris[J]. Eur J Dermatol, 2016, 26(1): 28-33.] [DOI] [PubMed] [Google Scholar]
- 17.Gunduz K, Temiz P, Gencoglan G, et al. Expression of nuclear factor kappa B and survivin in psoriasis. http://d.wanfangdata.com.cn/OAPaper/oai_pubmedcentral.nih.gov_3512308. ISRN Dermatol. 2012;2012:257059. doi: 10.5402/2012/257059. [Gunduz K, Temiz P, Gencoglan G, et al. Expression of nuclear factor kappa B and survivin in psoriasis[J]. ISRN Dermatol, 2012, 2012: 257059.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keppler-Noreuil KM, Parker VE, Darling TN, et al. Somatic overgrowth disorders of the PI3K/AKT/mTOR pathway & therapeutic strategies. Am J Med Genet C Semin Med Genet. 2016;172(4):402–21. doi: 10.1002/ajmg.c.31531. [Keppler-Noreuil KM, Parker VE, Darling TN, et al. Somatic overgrowth disorders of the PI3K/AKT/mTOR pathway & therapeutic strategies[J]. Am J Med Genet C Semin Med Genet, 2016, 172(4): 402-21.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu JS, Cui W. Proliferation, survival and metabolism: the role of PI3K/AKT/mTORsignalling in pluripotency and cell fate determination. Development. 2016;143(17):3050–60. doi: 10.1242/dev.137075. [Yu JS, Cui W. Proliferation, survival and metabolism: the role of PI3K/AKT/mTORsignalling in pluripotency and cell fate determination[J]. Development, 2016, 143(17): 3050-60.] [DOI] [PubMed] [Google Scholar]
- 20.Hu BW, Lv X, Gao F, et al. Downregulation of DEPTOR inhibits the proliferation, migration, and survival of osteosarcoma through PI3K/Akt/mTOR pathway. Onco Targets Ther. 2017;10:4379–91. doi: 10.2147/OTT. [Hu BW, Lv X, Gao F, et al. Downregulation of DEPTOR inhibits the proliferation, migration, and survival of osteosarcoma through PI3K/Akt/mTOR pathway[J]. Onco Targets Ther, 2017, 10: 4379-91.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang ZY, Zhou LQ, Zheng XT, et al. Autophagy protects against PI3K/Akt/mTOR-mediated apoptosis of spinal cord neurons after mechanical injury. Neurosci Lett. 2017;656:158–64. doi: 10.1016/j.neulet.2017.07.036. [Wang ZY, Zhou LQ, Zheng XT, et al. Autophagy protects against PI3K/Akt/mTOR-mediated apoptosis of spinal cord neurons after mechanical injury[J]. Neurosci Lett, 2017, 656: 158-64.] [DOI] [PubMed] [Google Scholar]
- 22.Feng XN, Jiang JJ, Shi SH, et al. Knockdown of miR-25 increases the sensitivity of liver cancer stem cells to TRAIL-induced apoptosis via PTEN/PI3K/Akt/Bad signaling pathway. Int J Oncol. 2016;49(6):2600–10. doi: 10.3892/ijo.2016.3751. [Feng XN, Jiang JJ, Shi SH, et al. Knockdown of miR-25 increases the sensitivity of liver cancer stem cells to TRAIL-induced apoptosis via PTEN/PI3K/Akt/Bad signaling pathway[J]. Int J Oncol, 2016, 49(6): 2600-10.] [DOI] [PubMed] [Google Scholar]
- 23.Ma YF, Qin HD, Cui YF. MiR-34a targets GAS1 to promote cell proliferation and inhibit apoptosis in papillary thyroid carcinoma via PI3K/Akt/Bad pathway. BiochemBiophys Res Commun. 2013;441(4):958–63. doi: 10.1016/j.bbrc.2013.11.010. [Ma YF, Qin HD, Cui YF. MiR-34a targets GAS1 to promote cell proliferation and inhibit apoptosis in papillary thyroid carcinoma via PI3K/Akt/Bad pathway[J]. BiochemBiophys Res Commun, 2013, 441(4): 958-63.] [DOI] [PubMed] [Google Scholar]
- 24.Wang YY, Zhao M, Liu JY, et al. miRNA-125b regulates apoptosis of human non-small cell lung cancer via the PI3K/Akt/GSK3 beta signaling pathway. Oncol Rep. 2017;38(3):1715–23. doi: 10.3892/or.2017.5808. [Wang YY, Zhao M, Liu JY, et al. miRNA-125b regulates apoptosis of human non-small cell lung cancer via the PI3K/Akt/GSK3 beta signaling pathway[J]. Oncol Rep, 2017, 38(3): 1715-23.] [DOI] [PubMed] [Google Scholar]
- 25.Lin YT, Wang HC, Hsu YC, et al. Capsaicin induces autophagy and apoptosis in human nasopharyngeal carcinoma cells by downregulating the PI3K/AKT/mTOR pathway. http://www.mdpi.com/1422-0067/18/7/1343/pdf. Int J MolSci. 2017;18(7):E1343. doi: 10.3390/ijms18071343. [Lin YT, Wang HC, Hsu YC, et al. Capsaicin induces autophagy and apoptosis in human nasopharyngeal carcinoma cells by downregulating the PI3K/AKT/mTOR pathway[J]. Int J MolSci, 2017, 18(7): E1343.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeon YJ, Kim BH, Kim S, et al. Rhododendrin ameliorates skin inflammation through inhibition of NF-kappa B, MAPK, and PI3K/ Akt signaling. https://www.ncbi.nlm.nih.gov/pubmed/23764465. Eur J Pharmacol. 2013;714(1/3):7–14. doi: 10.1016/j.ejphar.2013.05.041. [Jeon YJ, Kim BH, Kim S, et al. Rhododendrin ameliorates skin inflammation through inhibition of NF-kappa B, MAPK, and PI3K/ Akt signaling[J]. Eur J Pharmacol, 2013, 714(1/3): 7-14.] [DOI] [PubMed] [Google Scholar]
- 27.Song HS, Kim SJ, Park TI, et al. Immunohistochemical comparison of IL-36 and the IL-23/Th17 axis of generalized pustular psoriasis and acute generalized exanthematous pustulosis. Ann Dermatol. 2016;28(4):451–6. doi: 10.5021/ad.2016.28.4.451. [Song HS, Kim SJ, Park TI, et al. Immunohistochemical comparison of IL-36 and the IL-23/Th17 axis of generalized pustular psoriasis and acute generalized exanthematous pustulosis[J]. Ann Dermatol, 2016, 28(4): 451-6.] [DOI] [PMC free article] [PubMed] [Google Scholar]


