Abstract
Background
High-flow oxygen therapy via nasal cannula (HFOTNASAL) increases airway pressure, ameliorates oxygenation and reduces work of breathing. High-flow oxygen can be delivered through tracheostomy (HFOTTRACHEAL), but its physiological effects have not been systematically described. We conducted a cross-over study to elucidate the effects of increasing flow rates of HFOTTRACHEAL on gas exchange, respiratory rate and endotracheal pressure and to compare lower airway pressure produced by HFOTNASAL and HFOTTRACHEAL.
Methods
Twenty-six tracheostomized patients underwent standard oxygen therapy through a conventional heat and moisture exchanger, and then HFOTTRACHEAL through a heated humidifier, with gas flow set at 10, 30 and 50 L/min. Each step lasted 30 min; gas flow sequence during HFOTTRACHEAL was randomized. In five patients, measurements were repeated during HFOTTRACHEAL before tracheostomy decannulation and immediately after during HFOTNASAL. In each step, arterial blood gases, respiratory rate, and tracheal pressure were measured.
Results
During HFOTTRACHEAL, PaO2/FiO2 ratio and tracheal expiratory pressure slightly increased proportionally to gas flow. The mean [95% confidence interval] expiratory pressure raise induced by 10-L/min increase in flow was 0.2 [0.1–0.2] cmH2O (ρ = 0.77, p < 0.001). Compared to standard oxygen, HFOTTRACHEAL limited the negative inspiratory swing in tracheal pressure; at 50 L/min, but not with other settings, HFOTTRACHEAL increased mean tracheal expiratory pressure by (mean difference [95% CI]) 0.4 [0.3–0.6] cmH2O, peak tracheal expiratory pressure by 0.4 [0.2–0.6] cmH2O, improved PaO2/FiO2 ratio by 40 [8–71] mmHg, and reduced respiratory rate by 1.9 [0.3–3.6] breaths/min without PaCO2 changes. As compared to HFOTTRACHEAL, HFOTNASAL produced higher tracheal mean and peak expiratory pressure (at 50 L/min, mean difference [95% CI]: 3 [1–5] cmH2O and 4 [1–7] cmH2O, respectively).
Conclusions
As compared to standard oxygen, 50 L/min of HFOTTRACHEAL are needed to improve oxygenation, reduce respiratory rate and provide small degree of positive airway expiratory pressure, which, however, is significantly lower than the one produced by HFOTNASAL.
Keywords: Oxygen inhalation therapy, Tracheostomy, Respiratory insufficiency, Mechanical ventilator weaning, Positive end-expiratory pressure
Background
Nasal high-flow oxygen therapy (HFOTNASAL) has been proposed to treat acute hypoxemic respiratory failure [1–4], to facilitate weaning from mechanical ventilation [5–8] and to prevent hypoxemia during endotracheal intubation [9, 10].
With HFOTNASAL, up to 60 L/min of heated and humidified air/oxygen mixture are continuously delivered to the patient through specifically designed nasal prongs [11]. Unlike standard oxygen, high flows limit dilution of inhaled gas mixture, thus enabling more accurate delivery of the set fraction of inspired oxygen (FiO2) [12]. HFOTNASAL increases end-expiratory lung volume due to the generation of flow-dependent airway positive pressure, with highest values reached at end-expiration with closed mouth [13–15]. The continuous high flow washes CO2 out from upper airways, reducing anatomical dead space and work of breathing [16]. Active heating/humidification and the comfortable interface improve comfort related to airway dryness and optimize device tolerability [16–18].
High-flow oxygen can be delivered also through tracheostomy (HFOTTRACHEAL), but its mechanism of action and physiological effects appear different and have not been thoroughly elucidated [19, 20]. We conducted a randomized cross-over study to assess the effects of HFOTTRACHEAL administered at different gas flow rates on gas exchange, tracheal pressure, and respiratory rate, and to establish whether the increase in airway pressure generated by high-flow oxygen is different when administered by nasal cannula or tracheostomy.
Methods
The present study was carried out in the general intensive care unit (ICU) of a tertiary-care university hospital in Rome between September 2016 and September 2017, after a preliminary study conducted on a previous cohort of patients to assess the feasibility of tracheal pressure measurement in critically ill patients [21]. The study protocol was approved by the local institutional review board; written informed consent was obtained by all patients or next of kin, according to the ethics committee recommendations.
Patients
We studied critically ill tracheostomized patients with no hemodynamic instability who had been weaned from mechanical ventilation, had been spontaneously breathing with no ventilatory support for at least 24 h and were receiving tracheal oxygen according to the prescription of the attending physician. All enrolled patients had received single-dilator percutaneous tracheostomy with PercuTwist® technique (Rüsch, Kernen, Germany): the procedure was performed by an intensivist under bronchoscopy, which confirmed that the puncture was taking place between the first and second, or second and third, tracheal rings [22, 23]. Non-inclusion criteria were age < 18 years, pregnancy, recent tracheal, esophageal, neck or thoracic surgery, presence of pneumothorax/chest drainage. For safety reasons, patients with partial pressure of arterial oxygen to nominal FiO2 ratio (PaO2/FiO2) below 100 mmHg and/or respiratory rate > 45 breaths per minute during standard oxygen were not enrolled.
Procedures
After study inclusion, each patient received for 30 min standard oxygen through tracheostomy with a heat and moisture exchanger (Tracheolife II HME, Mallinckrodt, United Kingdom), with oxygen flow set by the attending physician (standard oxygen step, maximal O2 flow 8 L/min).
Patients subsequently underwent high-flow oxygen: gas flow was provided by the dedicated module of an ICU ventilator (EvitaXL or EvitaInfinity, Drager, Lubeck, Germany), inspired gas was actively conditioned by heated humidifier set at 37 °C (HH MR850, Fisher & Paykel Healthcare, New-Zealand, absolute humidity provided 44 mgH2O/L) and delivered through the specifically designed interface (Optiflow™ Tracheostomy interface OPT870, Fisher & Paykel Healthcare, New-Zealand). Three oxygen flow rates with the HFOTTRACHEAL device were tested in random order, for 30 min each: 10 L/min, 30 L/min, and 50 L/min. No wash-out period was applied between these interventions. Although 10 L/min cannot be considered as ‘high-flow therapy’, this step allowed (A) to better characterize the effects of increasing flow rate with the same device on analyzed endpoints, and (B) to compare standard oxygenation device (closed system through a heat a moisture exchanger) and HFOTTRACHEAL (open system) at similar gas flow rate, highlighting the difference between these techniques. The randomization sequence was provided by S.A.S. random allocation software. FiO2 was set to obtain a SpO2 between 92 and 98% (88–92% in patients with PaCO2 ≥ 45 mmHg during standard oxygen). Changes in the FiO2 over the course of the study were discouraged and allowed only whether clinically unavoidable.
Measurements
At the end of each step, hemodynamic parameters, arterial blood gases and SpO2 were recorded. To estimate PaO2/FiO2 during standard oxygen, delivered FiO2 was calculated using a previously described formula [24]:
At study entry, a sterile, disposable 18-gauge catheter (15/25-cm length according to patient’s height; 1-mm diameter; BD, CareFusion corporation, San Diego, CA, USA) connected to a differential pressure transducer was inserted in the trachea (2 cm away from carina, with the distance between tracheal stoma and carina measured on the chest X-ray) and secured to the skin with an adhesive tape. At the end of each study step, endotracheal pressure was recorded continuously for 3 min by a dedicated software at a sample rate of 200 Hz (Kleis-Tek, ICU lab, Bari, Italy). Pressure signals were offline-reviewed to assess respiratory rate and compute mean expiratory pressure (between the end of inspiration and the beginning of the following inspiration), peak expiratory and inspiratory pressure (maximal and minimal pressure achieved over the whole respiratory cycle, respectively). All these parameters were measured for all breaths in the 3-min recording and values were averaged for each study step.
In a subgroup of five patients who underwent tracheostomy decannulation after study inclusion and during the ICU stay, the experimental protocol was repeated on the day of decannulation, both during HFOTTRACHEAL and during HFOTNASAL after decannulation. Briefly, when the tracheal cannula was removed, the catheter for tracheal pressure measurement was hold in situ and the stoma was covered with gauze and adherent sealing tape (percutaneous tracheostomy maintains subcutaneous tissue integrity and elasticity) [25]. After medication, absence of leaks through the stoma was assessed by hand while the patient spontaneously vocalized and coughed. This approach was clinically useful for assessing patient’s tolerance to mouth/nose breathing and represented a unique opportunity to evaluate lower airway pressure during HFOTNASAL. In these 5 patients, HFOTTRACHEAL and HFOTNASAL with three flow settings (10, 30 and 50 L/min) were applied for 20-min periods in sequential order, just before and immediately after tracheostomy decannulation. No wash-out period was applied between the interventions. Heated humidifier settings were kept unchanged. Towards the end of each period, tracheal pressure tracings were recorded and were offline-analyzed to compute mean and peak expiratory pressure, as previously described.
End-points
Primary endpoint was to compare ratio of arterial oxygen partial pressure to nominal FiO2 (PaO2/FiO2) in the different study steps. Main secondary endpoints were to analyze the effects of the tested settings on respiratory rate, endotracheal pressure and PaCO2. Furthermore, we aimed at establishing whether tracheal pressure is different when high-flow oxygen is delivered through tracheostomy or nasal cannula, at similar flow rates.
Statistical analysis
Descriptive data are expressed as number and percentage and continuous data as median [interquartile range]. Because of the limited sample, adopting a conservative approach, all data were analyzed with non-parametric tests. Paired comparisons between the study steps were performed with the Wilcoxon sum of ranks test and mean differences [95% confidence interval] are displayed for most significant results. Correlation was assessed with Spearman’s rank-order correlation: ρ and the p value are reported. Analysis on the mean expiratory pressure rise induced by increasing gas flow was performed with linear regression: the slope and the p value of the relationship are reported. Inter-individual variability was rated with the coefficient of variation, computed as the ratio of standard deviation to mean of the measurements [26]. Results with two-tail p ≤ 0.05 were considered significant. Statistical analysis was performed with SPSS 20.0 (IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY, USA).
Sample size
Clinical data on the effects of HFOTTRACHEAL are limited to a single exploratory study [20]: this hampered any estimation of the adequate sample needed to provide sufficient statistical power to the study. Because previous investigations with similar design demonstrate that 15–20 patients studied in a cross-over fashion represent an adequate sample to draw conclusions on similar physiological endpoints [13, 15, 18, 20, 27], adopting a conservative approach, we planned to enroll 25 patients.
Results
Twenty-six patients were enrolled and analyzed. Demographics and most relevant clinical characteristics are reported in Table 1. In the standard oxygen step, median oxygen flow was 4 [3, 4] L/min and median estimated FiO2 was 0.33 [0.33–0.37]. No patient experienced changes in heart rate or arterial blood pressure over the course of the study. The sequence of HFOTTRACHEAL interventions did not affect PaO2/FiO2 (ρ = 0.05, p = 0.69) nor respiratory rate (ρ = 0.002, p = 0.99).
Table 1.
Baseline characteristics of enrolled patients
| No. of patients | 26 |
| Age, years | 57 [48–71] |
| Female sex, no. (%) | 4 (15) |
| Height, cm | 175 [168–180] |
| Body weight, kg | 75 [70–85] |
| Body mass index, kg/m2 | 25 [24–28] |
| SAPS II | 46 [41–60] |
| Patients with history of COPD, no. (%) | 5 (19) |
| ICU admission, no. (%) | |
| Medical | 12 (46) |
| Surgical | 7 (27) |
| Trauma | 7 (27) |
| Cause of prolonged need for mechanical ventilation, no (%) | |
| Respiratory failure | 8 (31) |
| Traumatic brain injury | 7 (27) |
| Non-traumatic brain injury | 11 (42) |
| Length of mechanical ventilation before enrollment, days | 11 [8–13] |
| Glasgow coma scale at enrollment | 10 [6–15] |
| PaO2/FiO2 during standard oxygen, mmHga | 238 [197–311] |
| Tracheal cannula inner diameter, mm | 9 [8.5–10] |
| Tracheal cannula external diameter, mm | 12.3 [12.3–12.3] |
| Length of ICU stay, days | 20 [14–26] |
| In-ICU mortality, no. (%) | 3 (12) |
Results are displayed as medians [interquartile range], if not otherwise specified
SAPSII simplified acute physiology score 2 at ICU admission, COPD chronic obstructive pulmonary disease, ICU intensive care unit
aMeasured during the standard oxygen step of the experiment
Gas exchange and respiratory rate
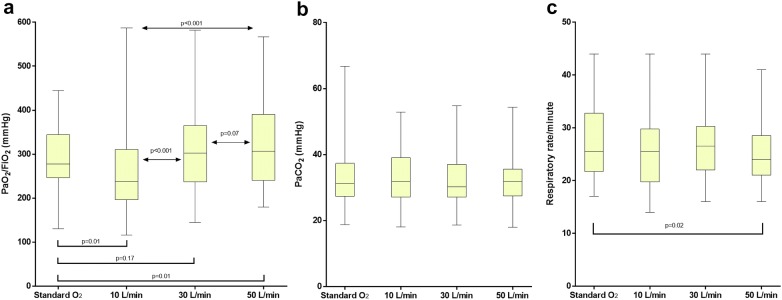
These results are displayed in Fig. 1.
Fig. 1.
PaO2/FiO2 (a), PaCO2 (b) and respiratory rate (c) in the four study steps. Results are displayed as median, interquartile range, maximum and minimum. With HFOTTRACHEAL device, PaO2/FiO2 increases proportionally to gas flow, especially between 10 and 30 L/min. As compared to standard oxygen, 50 L/min, but not 30 L/min nor 10 L/min, ameliorate oxygenation and reduce respiratory rate in isocapnic conditions
During HFOTTRACHEAL, increasing flow rates yielded improvement in oxygenation, markedly between 10 and 30 L/min (p < 0.001) and mildly between 30 and 50 L/min (p = 0.07).
As compared to standard oxygen, HFOTTRACHEAL 50 L/min, but not 30 nor 10 L/min, increased PaO2/FiO2 ratio: median [Interquartile range] 307 [241–390] mmHg vs. 277 [247–344] mmHg, p = 0.01; mean difference [95% CI] 40 [8–71] mmHg) (Fig. 1a).
When compared to standard oxygen, HFOTTRACHEAL 50 L/min led to a slight reduction in respiratory rate (24 [21–29] breaths/min vs. 26 [22–33] breaths/min, p = 0.02), without changes in PaCO2 (32 [26–36] mmHg vs. 31 [27–37] mmHg, p = 0.43) (Fig. 1b, c). The mean reduction [95% CI] in respiratory rate yielded by HFOTTRACHEAL 50 L/min was 1.9 [0.3–3.6] breaths/min and was proportional to respiratory rate during standard oxygen (i.e., greater in patients with higher respiratory rate, ρ = 0.43 p = 0.03). No differences in PaCO2 were detected between the studied conditions (Fig. 1b, c).
Tracheal pressure
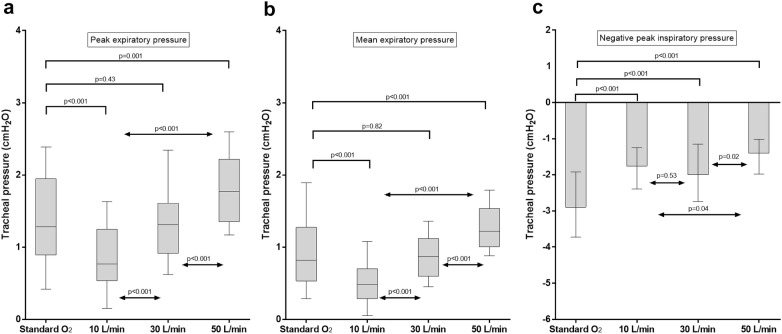
These results are displayed in Fig. 2.
Fig. 2.
Peak (a), mean expiratory pressure (b) and negative peak of inspiratory pressure. Results are displayed as median, interquartile range, maximum and minimum. During HFOTTRACHEAL, tracheal expiratory pressure increases proportionally to the gas flow. All HFOTTRACHEAL settings limit the negative inspiratory pressure, especially as flow is set at 50 L/min, likely due to the capability of the high gas flow in an open system to match patient’s peak inspiratory flow. As compared to standard oxygen, 50 L/min, but not 30 L/min nor 10 L/min, increase tracheal peak and mean tracheal expiratory pressure
In the three HFOTTRACHEAL steps, mean and peak expiratory pressures were proportional to the delivered gas flow (p < 0.001 for all comparisons). The mean [95% CI] expiratory pressure rise induced by 10-L/min increase in flow was 0.2 [0.1–0.2] cmH2O (ρ = 0.77, p < 0.001). As compared to standard oxygen, 50 L/min, but not other HFOTTRACHEAL settings, led to an increase in peak and mean expiratory pressures: peak pressure 1.8 [1.4–2.2] cmH2O vs. 1.3 [0.9–2] cmH2O, p = 0.001; mean pressure 1.2 [1–1.5] cmH2O vs. 0.8 [0.5–1.3] cmH2O, p < 0.001 (Fig. 2a, b). Mean differences [95% CI] in peak and mean expiratory pressure between HFOTTRACHEAL 50 L/min and standard oxygen were 0.4 [0.2–0.6] cmH2O and 0.4 [0.3–0.6] cmH2O, respectively. Both peak and mean expiratory pressures were lower at HFOTTRACHEAL 10 L/min than during standard oxygen (both p < 0.001).
All HFOTTRACHEAL settings yielded less negative tracheal peak inspiratory pressure, as compared to standard oxygen (p < 0.001 for all the comparisons): this effect was magnified at 50 L/min (Fig. 2c).
Comparison with HFOTNASAL
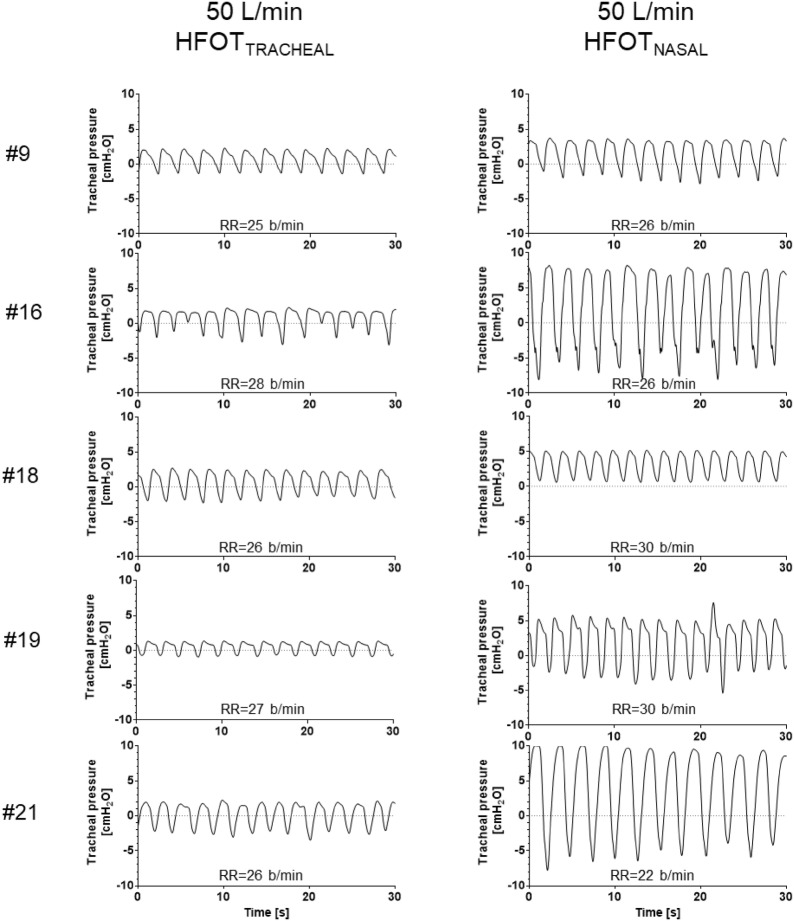
Five patients underwent tracheostomy decannulation within their stay in ICU, and received HFOTTRACHEAL and HFOTNASAL before and after the procedure. Samples of tracheal pressure tracings are displayed in Fig. 3. Inter-individual variability in peak and mean expiratory pressure at 50 L/min was greater during HFOTNASAL (both 35%) than during HFOTTRACHEAL (21 and 20%, respectively). Inspiratory pressure during HFOTNASAL 50 L/min fell below 0 during inspiration in 4/5 patients. With all the tested flow settings, peak and mean expiratory tracheal pressures during HFOTNASAL were significantly higher than during HFOTTRACHEAL (Fig. 4; p = 0.05 for all comparisons). In particular, with flow set at 50 L/min: median peak expiratory pressure was 5.1 [4.2–7.7] cmH2O during HFOTNASAL vs. 1.8 [1.6–2.3] cmH2O during HFOTTRACHEAL (p = 0.05); mean expiratory pressure was 3.9 [3.1–6] cmH2O during HFOTNASAL vs. 1.5 [1.2–1.7] cmH2O during HFOTTRACHEAL (p = 0.05). The mean difference [95% CI] in tracheal peak and mean expiratory pressure between HFOTNASAL and HFOTTRACHEAL was 4 [1–7] cmH2O and 3 [1–5] cmH2O, respectively.
Fig. 3.
Thirty-second recordings of tracheal pressure tracings during HFOTTRACHEAL and HFOTNASAL in 5 patients who underwent tracheostomy decannulation over the course of ICU stay. In both conditions gas flow was set at 50 L/min. Average respiratory rate for the 30-s recording is reported for all conditions. During HFOTNASAL lower airway pressure during expiration is higher and more inter-individually variable than HFOTTRACHEAL, despite a non-dissimilar respiratory rate, which was calculated on the same 30-s recording. This suggests that the HFOTNASAL-induced increase in expiratory pressure depends not only on gas flow, but also on patient’s expiratory pattern and, likely, on individual respiratory system mechanical properties. Please note that, under this condition, tracheal pressure was not constant over the course of the respiratory cycle and became negative during inspiration in 4 patients, which is different from what previously reported for pharyngeal pressure [14]
Fig. 4.
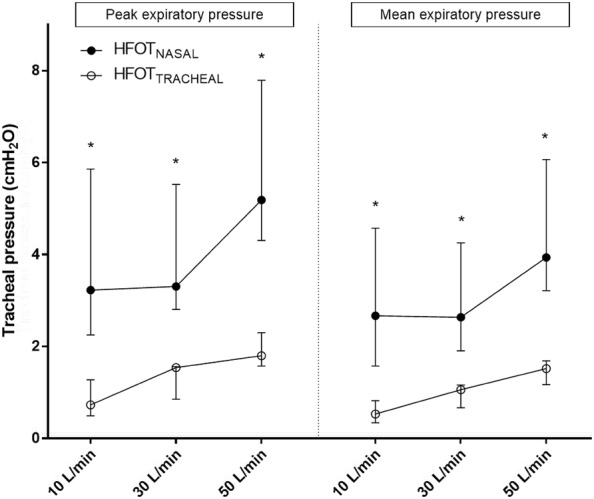
Peak and mean expiratory pressure during HFOTTRACHEAL and HFOTNASAL and different gas flows delivered. Results are displayed as median and interquartile range; *indicates p ≤ 0.05 for HFOTTRACHEAL vs. HFOTNASAL comparisons
Discussion
In the present cross-over study, we show that, as compared to standard oxygen, HFOTTRACHEAL mitigates the negative swing in airway pressure during inspiration, and, when flow is set at 50 L/min, ameliorates oxygenation and slightly reduces respiratory rate. With similar flow rates, tracheal expiratory pressure is significantly lower with HFOTTRACHEAL than with HFOTNASAL, suggesting that the physiologic effects of HFOTTRACHEAL are milder than HFOTNASAL. A gas flow of 50 L/min should be set with the tracheal interface to slightly improve oxygenation and reduce respiratory rate.
Several studies addressed the effects of HFOTNASAL in a variety of clinical scenarii [1]. Although high-flow oxygen can be delivered through tracheostomy, few data elucidate its mechanisms of action, which can be different from HFOTNASAL [20].
Oxygenation
During HFOTTRACHEAL, PaO2/FiO2 ratio increases proportionally to gas flow. However, when compared to standard oxygen via heat and moisture exchangers, only 50 L/min generate improvement in PaO2/FiO2 ratio. These data are partially consistent with what has been reported for HFOTNASAL [18] and may be explained by the following mechanisms:
Increasing flow rate up to 50 L/min can limit air dilution of inhaled gas mixture, enabling more accurate delivery of set FiO2. This can be demonstrated by the reduction of the inspiratory airway pressure swing during HFOTTRACHEAL.
Increasing flow rate yields a concomitant increase in peak and mean expiratory pressure. Although the increase in tracheal pressure generated by HFOTTRACHEAL is lower than the one reported during HFOTNASAL [11, 14, 15, 28], this rise in expiratory pressure may still contribute to increase end-expiratory lung volume, reduce shunt fraction, optimize lung mechanics and improve oxygenation [11, 13, 18, 29].
One previous report showed that, when compared to T-Piece with a Venturi generator in tracheostomized patients, airway pressure and SpO2/FiO2 slightly increase during 50 L/min HFOTTRACHEAL [20]. However, because of the entrainment effect, Venturi systems can provide flows up to 30–50 L/min and cannot be considered standard oxygen devices [30]. Standard oxygen through heat and moisture exchangers represents a widely used alternative for oxygen therapy in tracheostomized patients.
We have shown that standard oxygen through heat and moisture exchangers produces positive expiratory pressure, which is comparable to the one obtained with 30 L/min of high-flow oxygen through an open system. In fact, oxygenation between these two settings was similar. For the same gas flow (≈ 10 L/min), oxygenation and tracheal expiratory pressure were higher with the standard oxygenation (closed system) than with the HFOTTRACHEAL device (open system). This suggests that the oxygenation changes are dependent on the amount of tracheal expiratory pressure. However, mechanisms of airway pressure generation may be different between the two devices: with standard oxygen, the increase in pressure depends on the expiratory resistance produced by the heat and moisture exchanger; while, during HFOTTRACHEAL, positive expiratory pressure is produced by patient’s expiration against the delivered gas flow in an open system and airway pressure is more stable over the respiratory cycle (i.e., less negative during inspiration). In this context, avoidance of excessive negative inspiratory swings in airway (and pleural) pressure is important to mitigate the risk of negative pressure pulmonary edema, whose occurrence induces lung damage and worsens oxygenation [31].
CO2 clearance
HFOTNASAL lowers inspiratory resistance and enhances anatomical dead space clearance with CO2 washout [32, 33], finally reducing work of breathing [11, 13, 27, 34]. Our study shows that 50 L/min HFOTTRACHEAL lowers respiratory rate without changes in PaCO2, as compared to standard oxygen. A reduction in respiratory rate has been reported during HFOTNASAL [5, 35] and has been linked to anatomical dead space clearance, increased tidal volume, diminished resistive work of breathing and, in chronic obstructive pulmonary disease patients, increased positive expiratory pressure [13, 33, 36].
Work of breathing reduction by HFOTNASAL is obtained at 30 L/min and is minimally enhanced by further increases in gas flow [18]: differently, 50 L/min of HFOTTRACHEAL are needed to generate effects on respiratory rate. It is, therefore, reasonable to hypothesize that, in tracheostomized patients:
lower anatomical dead space and inspiratory resistance reduce the size effect of the intervention, that consequently requires higher flows to generate a significant effect;
inspired and expired flows are forcedly unidirectional, thus clearing anatomical dead space and improving breathing efficiency [37]: this contributes to CO2 washout independently from the device used for oxygen therapy, thereby mitigating the effect of HFOTTRACHEAL.
Our results are consistent with recent data indicating that HFOTTRACHEAL minimally affects neuro-ventilatory coupling, work of breathing and gas exchange after weaning from mechanical ventilation [19].
Differences with HFOTNASAL
Our comparison of HFOTTRACHEAL and HFOTNASAL in the same patients represented a unique opportunity to highlight the contribution of upper airway resistance to positive-pressure generation during HFOTNASAL. In fact, to our knowledge, no other data clarify the behavior of lower airway pressure during this treatment. The average expiratory pressure reported in our study is similar to what has been reported for pharyngeal pressure [11, 15, 28]. However, tracheal pressure during HFOTNASAL was not constant over the respiratory cycle and became negative during inspiration in 4 of the 5 studied patients, which is different from what has been reported on upper airway pressure [14]. Our results indicate that expiratory pressure in lower airways is higher and more inter-individually variable when high flows are delivered through nasal cannula than through tracheostomy. This suggests that the mechanism of expiratory pressure generation during high-flow oxygen is dependent not only on gas flow rate, but also on the greater resistance offered by upper airways and patient’s expiratory flow. In tracheostomized patients, resistance is limited, and the generated pressure is minimal. Patient’s expiratory flow has wide inter-individual variability according to the resistive and elastic properties of the respiratory system and to the eventual recruitment of expiratory muscles [38]: thus, the pressure produced by HFOTNASAL is variable among subjects, also if respiratory rate with HFOTTRACHEAL is similar (Fig. 3) [39].
Clinical consequences
Our study shows that the effects of HFOTTRACHEAL are milder than HFOTNASAL, likely because the dedicated interface is completely open. HFOTTRACHEAL allows to limit the negative swing in inspiratory airway pressure, but both the dead space washout and the generation of positive expiratory pressure are limited. From a clinical perspective, our findings suggest that a minimum gas flow of 50 L/min should be set during HFOTTRACHEAL to slightly improve oxygenation and reduce respiratory rate, as compared to standard oxygen. Whether these mild physiologic effects are cost-effective and may clinically benefit the management of tracheostomized patients cannot be established from our data and should be addressed in further investigations.
Limitations
First, we did not measure effectively delivered FiO2, as performed elsewhere [3]. As a result, the calculation of PaO2/FiO2 ratio may be subject to errors, especially if lower flows are used [40]. Nevertheless, our approach is clinically reproducible and we used a formula that has recently been shown to provide satisfactory correlation with actual FiO2 [24].
Second, we did not measure work of breathing by esophageal manometry [41]. However, esophageal catheter insertion in awake and spontaneously breathing patients may be challenging and eventually require some sedation. Importantly, during HFOTNASAL, changes in respiratory rate have been shown to reflect variations of the work of breathing [13, 33].
Third, there was no wash-out period between the applied interventions during HFOTTRACHEAL. However, our approach is consistent with previous investigations on the topic [18], and the randomized order of the interventions should have mitigated any carry-over effect on the observed results. Accordingly, the main outcomes of the study were not affected by the sequence of applied flow settings.
Fourth, during HFOTNASAL, absence of major leaks through the stoma was assessed by hand. Unfortunately, we had no other way to assess if minimal leaks were present. We believe, however, that even minimal leaks, if present, should not have affected tracheal pressure measurement. In fact, the tracheal pressure values we report are similar to nasopharyngeal pressure values measured in non-tracheostomized patients by others [13–15].
Finally, we showed that expiratory pressure increase due to HFOTNASAL has wide inter-individual variability. Whether and to what extent expiratory flow limitation and expiratory muscles recruitment contribute to this is unknown and remains to be established in further investigations [38, 42].
Conclusions
HFOTTRACHEAL generates small flow-dependent improvement in oxygenation and increases in tracheal expiratory pressure. When compared to standard oxygen, a minimum flow of 50 L/min is needed during HFOTTRACHEAL to improve oxygenation, increase expiratory pressure, limit inspiratory airway pressure swings and reduce respiratory rate. At same gas flow, HFOTNASAL produces higher expiratory pressure than HFOTTRACHEAL.
Acknowledgements
None.
Authors’ contributions
DN, DLG and SMM designed the study. DN, MTS, FT, GMA, DE enrolled the patients and recorded the data. LM and DLG analyzed the data. DN and PDG interpreted results and drafted the manuscript. SMM and MA critically revised the manuscript. All authors read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Support was provided solely from institutional and/or departmental sources. Outside of the present work, Dr. Grieco is supported by research Grants by SIAARTI and ESICM.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The study was approved by local Ethics Committee (ID 25533/16) and written informed consent to data analysis was obtained by all studied patients.
Competing interests
DLG has received payments for travel expenses by Maquet, Getinge and Air Liquide. MA has received payments for Board participation from Maquet, Air Liquide and Chiesi. DLG and MA disclose a research grant by General Electric Healthcare. SMM is the principal investigator of the RINO trial (clinicaltrials.gov, NCT02107183), which was supported by Fisher and Paykel healthcare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Papazian L, Corley A, Hess D, Fraser JF, Frat J-P, Guitton C, et al. Use of high-flow nasal cannula oxygenation in ICU adults: a narrative review. Intensive Care Med. 2016;42:1336–1349. doi: 10.1007/s00134-016-4277-8. [DOI] [PubMed] [Google Scholar]
- 2.Roca O, Hernández G, Díaz-Lobato S, Carratalá JM, Gutiérrez RM, Masclans JR, et al. Current evidence for the effectiveness of heated and humidified high flow nasal cannula supportive therapy in adult patients with respiratory failure. Crit Care. 2016;20:109. doi: 10.1186/s13054-016-1263-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frat J-P, Thille AW, Mercat A, Girault C, Ragot S, Perbet S, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372:2185–2196. doi: 10.1056/NEJMoa1503326. [DOI] [PubMed] [Google Scholar]
- 4.Frat J-P, Ragot S, Girault C, Perbet S, Prat G, Boulain T, et al. Effect of non-invasive oxygenation strategies in immunocompromised patients with severe acute respiratory failure: a post hoc analysis of a randomised trial. Lancet Respir Med. 2016;4:646–652. doi: 10.1016/S2213-2600(16)30093-5. [DOI] [PubMed] [Google Scholar]
- 5.Maggiore SM, Idone FA, Vaschetto R, Festa R, Cataldo A, Antonicelli F, et al. Nasal high-flow versus Venturi mask oxygen therapy after extubation. Effects on oxygenation, comfort, and clinical outcome. Am J Respir Crit Care Med. 2014;190:282–288. doi: 10.1164/rccm.201402-0364OC. [DOI] [PubMed] [Google Scholar]
- 6.Hernández G, Vaquero C, González P, Subira C, Frutos-Vivar F, Rialp G, et al. Effect of postextubation high-flow nasal cannula vs conventional oxygen therapy on reintubation in low-risk patients: a randomized clinical trial. JAMA. 2016;315:1354–1361. doi: 10.1001/jama.2016.2711. [DOI] [PubMed] [Google Scholar]
- 7.Stéphan F, Barrucand B, Petit P, Rézaiguia-Delclaux S, Médard A, Delannoy B, et al. High-flow nasal oxygen vs noninvasive positive airway pressure in hypoxemic patients after cardiothoracic surgery: a randomized clinical trial. JAMA. 2015;313:2331–2339. doi: 10.1001/jama.2015.5213. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez R, Subira C, Frutos-Vivar F, Rialp G, Laborda C, Masclans JR, et al. High-flow nasal cannula to prevent postextubation respiratory failure in high-risk non-hypercapnic patients: a randomized multicenter trial. Ann Intensive Care. 2017;7:47. doi: 10.1186/s13613-017-0270-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vourc’h M, Asfar P, Volteau C, Bachoumas K, Clavieras N, Egreteau P-Y, et al. High-flow nasal cannula oxygen during endotracheal intubation in hypoxemic patients: a randomized controlled clinical trial. Intensive Care Med. 2015;41:1538–1548. doi: 10.1007/s00134-015-3796-z. [DOI] [PubMed] [Google Scholar]
- 10.Miguel-Montanes R, Hajage D, Messika J, Bertrand F, Gaudry S, Rafat C, et al. Use of high-flow nasal cannula oxygen therapy to prevent desaturation during tracheal intubation of intensive care patients with mild-to-moderate hypoxemia. Crit Care Med. 2015;43:574–583. doi: 10.1097/CCM.0000000000000743. [DOI] [PubMed] [Google Scholar]
- 11.Parke RL, Bloch A, McGuinness SP. Effect of very-high-flow nasal therapy on airway pressure and end-expiratory lung impedance in healthy volunteers. Respir Care. 2015;60:1397–1403. doi: 10.4187/respcare.04028. [DOI] [PubMed] [Google Scholar]
- 12.Chanques G, Riboulet F, Molinari N, Carr J, Jung B, Prades A, et al. Comparison of three high flow oxygen therapy delivery devices: a clinical physiological cross-over study. Minerva Anestesiol. 2013;79:1344–1355. [PubMed] [Google Scholar]
- 13.Mauri T, Turrini C, Eronia N, Grasselli G, Volta CA, Bellani G, et al. Physiologic effects of high-flow nasal cannula in acute hypoxemic respiratory failure. Am J Respir Crit Care Med. 2017;195:1207–1215. doi: 10.1164/rccm.201605-0916OC. [DOI] [PubMed] [Google Scholar]
- 14.Parke RL, McGuinness SP. Pressures delivered by nasal high flow oxygen during all phases of the respiratory cycle. Respir Care. 2013;58:1621–1624. doi: 10.4187/respcare.02358. [DOI] [PubMed] [Google Scholar]
- 15.Parke R, McGuinness S, Eccleston M. Nasal high-flow therapy delivers low level positive airway pressure. Br J Anaesth. 2009;103:886–890. doi: 10.1093/bja/aep280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Möller W, Feng S, Domanski U, Franke K-J, Celik G, Bartenstein P, et al. Nasal high flow reduces dead space. J Appl Physiol. 2017;122:191–197. doi: 10.1152/japplphysiol.00584.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mauri T, Galazzi A, Binda F, Masciopinto L, Corcione N, Carlesso E, et al. Impact of flow and temperature on patient comfort during respiratory support by high-flow nasal cannula. Crit Care. 2018;22:120. doi: 10.1186/s13054-018-2039-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mauri T, Alban L, Turrini C, Cambiaghi B, Carlesso E, Taccone P, et al. Optimum support by high-flow nasal cannula in acute hypoxemic respiratory failure: effects of increasing flow rates. Intensive Care Med. 2017;43:1453–1463. doi: 10.1007/s00134-017-4890-1. [DOI] [PubMed] [Google Scholar]
- 19.Stripoli T, Spadaro S, Di Mussi R, Volta CA, Trerotoli P, De Carlo F, et al. High-flow oxygen therapy in tracheostomized patients at high risk of weaning failure. Ann Intensive Care. 2019;9:4. doi: 10.1186/s13613-019-0482-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corley A, Edwards M, Spooner AJ, Dunster KR, Anstey C, Fraser JF. High-flow oxygen via tracheostomy improves oxygenation in patients weaning from mechanical ventilation: a randomised crossover study. Intensive Care Med. 2017;43:465–467. doi: 10.1007/s00134-016-4634-7. [DOI] [PubMed] [Google Scholar]
- 21.Natalini D, Idone F, Grieco D, Spaziani L, Santantonio M, Toni F, et al. Impact of high-flow oxygen therapy delivered through a tracheostomy on arterial blood gases and endotracheal pressure. Crit Care. 2014;18:P321. doi: 10.1186/cc13511. [DOI] [Google Scholar]
- 22.Yurtseven N, Aydemir B, Karaca P, Aksoy T, Komurcu G, Kurt M, et al. PercuTwist: a new alternative to Griggs and Ciaglia’s techniques. Eur J Anaesthesiol. 2007;24:492–497. doi: 10.1017/S0265021506002274. [DOI] [PubMed] [Google Scholar]
- 23.Frova G, Quintel M. A new simple method for percutaneous tracheostomy: controlled rotating dilation. A preliminary report. Intensive Care Med. 2002;28:299–303. doi: 10.1007/s00134-002-1218-5. [DOI] [PubMed] [Google Scholar]
- 24.Coudroy R, Thille AW, Drouot X, Diaz V, Meurice J-C, Robert R, et al. How to assess FiO2 delivered under oxygen mask in clinical practice? Intensive Care Med Exp. 2017;5:0968. [Google Scholar]
- 25.O’Connor HH, White AC. Tracheostomy decannulation. Respir Care. 2010;55:1076–1081. [PubMed] [Google Scholar]
- 26.Reed GF, Lynn F, Meade BD. Use of coefficient of variation in assessing variability of quantitative assays. Clin Diagn Lab Immunol. 2002;9:1235–1239. doi: 10.1128/CDLI.9.6.1235-1239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Delorme M, Bouchard P-A, Simon M, Simard S, Lellouche F. Effects of high-flow nasal cannula on the work of breathing in patients recovering from acute respiratory failure. Crit Care Med. 2017;45:1981–1988. doi: 10.1097/CCM.0000000000002693. [DOI] [PubMed] [Google Scholar]
- 28.Parke R, Eccleston M, McGuinness S. The effects of flow on airway pressure during nasal high-flow oxygen therapy. Respir Care. 2011;56:1151–1155. doi: 10.4187/respcare.01106. [DOI] [PubMed] [Google Scholar]
- 29.Corley A, Caruana LR, Barnett AG, Tronstad O, Fraser JF. Oxygen delivery through high-flow nasal cannulae increase end-expiratory lung volume and reduce respiratory rate in post-cardiac surgical patients. Br J Anaesth. 2011;107:998–1004. doi: 10.1093/bja/aer265. [DOI] [PubMed] [Google Scholar]
- 30.Wong GL, Finnis ME. Use of venturi entrainment to deliver nasal high fow oxygen. Crit Care Shock. 2010;13:75–80. [Google Scholar]
- 31.Yoshida T, Fujino Y, Amato MBP, Kavanagh BP. Fifty years of research in ARDS. Spontaneous breathing during mechanical ventilation. Risks, mechanisms, and management. Am J Respir Crit Care Med. 2017;195:985–992. doi: 10.1164/rccm.201604-0748CP. [DOI] [PubMed] [Google Scholar]
- 32.Möller W, Celik G, Feng S, Bartenstein P, Meyer G, Oliver E, et al. Nasal high flow clears anatomical dead space in upper airway models. J Appl Physiol. 2015;118:1525–1532. doi: 10.1152/japplphysiol.00934.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chatila W, Nugent T, Vance G, Gaughan J, Criner GJ. The effects of high-flow vs low-flow oxygen on exercise in advanced obstructive airways disease. Chest. 2004;126:1108–1115. doi: 10.1378/chest.126.4.1108. [DOI] [PubMed] [Google Scholar]
- 34.Sklar MC, Dres M, Rittayamai N, West B, Grieco DL, Telias I, et al. High-flow nasal oxygen versus noninvasive ventilation in adult patients with cystic fibrosis: a randomized crossover physiological study. Ann Intensive Care. 2018;8:85. doi: 10.1186/s13613-018-0432-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fraser JF, Spooner AJ, Dunster KR, Anstey CM, Corley A. Nasal high flow oxygen therapy in patients with COPD reduces respiratory rate and tissue carbon dioxide while increasing tidal and end-expiratory lung volumes: a randomised crossover trial. Thorax. 2016;71:759–761. doi: 10.1136/thoraxjnl-2015-207962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mündel T, Feng S, Tatkov S, Schneider H. Mechanisms of nasal high flow on ventilation during wakefulness and sleep. J Appl Physiol. 2013;114:1058–1065. doi: 10.1152/japplphysiol.01308.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang Y, Liang Y, Kacmarek RM. The principle of upper airway unidirectional flow facilitates breathing in humans. J Appl Physiol. 2008;105:854–858. doi: 10.1152/japplphysiol.90599.2008. [DOI] [PubMed] [Google Scholar]
- 38.Junhasavasdikul D, Telias I, Grieco DL, Chen L, Gutierrez CM, Piraino T, et al. Expiratory flow limitation during mechanical ventilation. Chest. 2018;154:948–962. doi: 10.1016/j.chest.2018.01.046. [DOI] [PubMed] [Google Scholar]
- 39.Lessard MR, Lofaso F, Brochard L. Expiratory muscle activity increases intrinsic positive end-expiratory pressure independently of dynamic hyperinflation in mechanically ventilated patients. Am J Respir Crit Care Med. 1995;151:562–569. doi: 10.1164/ajrccm.151.2.7842221. [DOI] [PubMed] [Google Scholar]
- 40.Sztrymf B, Messika J, Bertrand F, Hurel D, Leon R, Dreyfuss D, et al. Beneficial effects of humidified high flow nasal oxygen in critical care patients: a prospective pilot study. Intensive Care Med. 2011;37:1780–1786. doi: 10.1007/s00134-011-2354-6. [DOI] [PubMed] [Google Scholar]
- 41.Akoumianaki E, Maggiore SM, Valenza F, Bellani G, Jubran A, Loring SH, et al. The application of esophageal pressure measurement in patients with respiratory failure. Am J Respir Crit Care Med. 2014;189:520–531. doi: 10.1164/rccm.201312-2193CI. [DOI] [PubMed] [Google Scholar]
- 42.Shi Z-H, Jonkman A, de Vries H, Jansen D, Ottenheijm C, Girbes A, et al. Expiratory muscle dysfunction in critically ill patients: towards improved understanding. Intensive Care Med. 2019;45:1061–1071. doi: 10.1007/s00134-019-05664-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.