Abstract
Breast cancer is the second in mortality rate malignancy among women. Despite the many advances in breast cancer treatment, there is still a need to improve drug efficacy and reduce non-specific effects. D-alpha-tocopheryl polyethylene glycol succinate (TPGS) is frequently used in the development of drug delivery systems to improve the pharmacokinetics of anti-cancer drugs and reduce multi-drug resistance. We have previously shown that TPGS not only acts as a carrier molecule but also exerts anti-cancer effects. As part of this study, we investigated the effect of TPGS with YM155, a small molecule suppressant of Survivin, in various breast cancer cell lines representing different subtypes of the disease. We aimed to evaluate the presumed synergistic effect of the TPGS-YM155 combination and reveal its mechanism of action. Our results show that the TPGS-YM155 combination acts synergistically to reduce specifically the viability of SKBR3 cells. The combination of these agents reduced activation of the AKT pathway, decreased Survivin and Bcl-2 levels, and induced caspase-dependent and independent apoptosis via the mitochondrial pathway. Importantly, the TPGS-YM155 combination did not significantly affect the viability of MCF-10A normal immortalized cells. In conclusion, the combination of YM155 and TPGS could be a promising approach against SKBR3-type breast cancer.
Subject terms: Breast cancer, Drug development
Introduction
Breast cancer is the most frequently diagnosed cancer among women in Europe and the United States1,2. Despite the many recent advances in breast cancer monotherapy, several issues remain including severe adverse effects caused by high concentrations of chemotherapeutic drugs and acquired drug resistance. Both of these issues can be overcome or minimized by using agents that act synergistically. Since this approach requires lower concentrations of each agent, compared to monotherapy, it produces minimal adverse effects.
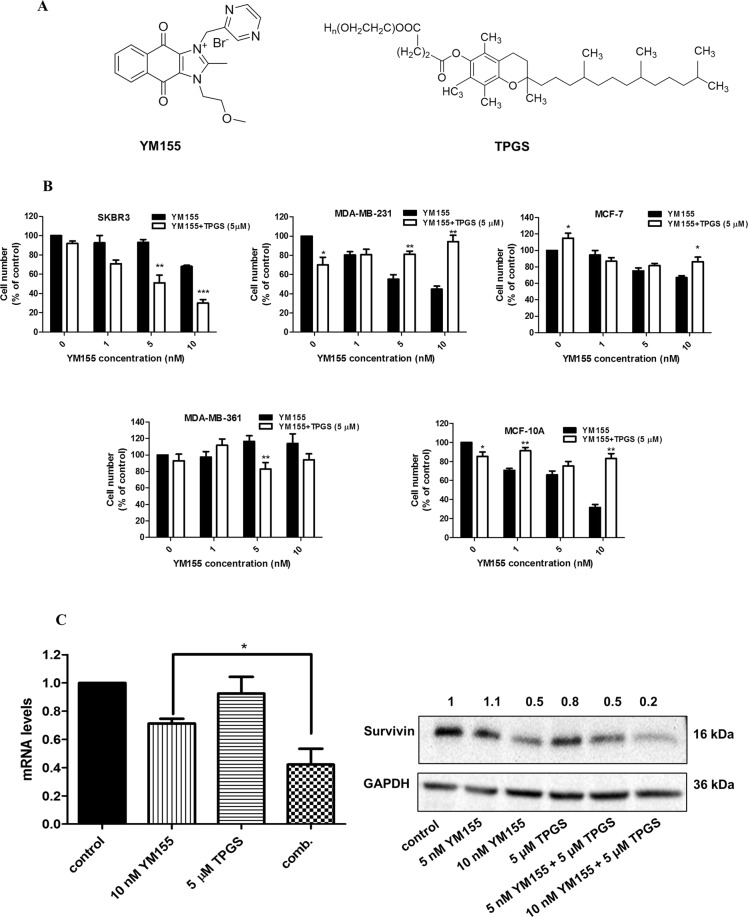
Survivin, a member of the Inhibitors of Apoptosis (IAP) family, is either undetectable or expressed at very low levels in terminally differentiated normal human tissues, but it is highly expressed in all primary tumor types3–7, making it an ideal target for cancer therapy. In cancer cells, Survivin acts as a multifunctional protein, implicated in the inhibition of apoptosis and the promotion of cell proliferation and angiogenesis8,9. YM155 (Sepantronium bromide) (Fig. 1A), is a potent small-molecule suppressant of Survivin that induces DNA damage and apoptosis in various human cancer models10.YM155 suppresses Survivin expression by binding to the C-terminal of RNA binding proteins interleukin enhancer-binding factor-3 (ILF3/NF110) and disrupts its binding to the Survivin promoter. YM155 has been evaluated in several clinical trials as a single agent or in combination therapy11,12. Even though YM155 exhibited a favorable safety/tolerability profile, it displayed modest single-agent activity in patients13.
Figure 1.
Antiproliferative effect of YM155 alone and in combination with TPGS in breast cell lines. (A) Chemical structures of YM155 and TPGS. (B) MTT assay was employed for the cytotoxicity evaluation (% cell viability) of increasing concentrations of YM155 (1, 5 and 10 nM) with or without 5 μΜ TPGS for 48 hours in SKBR3, MDA-MB-231, MCF-7, MDA-MB-361 and MCF-10A cells. IC50 of YM155 and TPGS as well the Combination Index (CI) in each cell line are shown in Table 1. (C) YM155 and TPGS effects on the mRNA and protein levels of Survivin at 48 hours of treatment (the blots were cropped; full length blots are shown in Fig. S6). The results represent the mean ± SEM of three different replicates and are representative of at least three different experiments. *P value < 0.05, **P value < 0.01, ***P value < 0.001.
D-a-tocopheryl polyethylene glycol succinate (TPGS) is a synthetic derivative of natural alpha-tocopherol, prepared from the esterification of α-TOS and polyethylene glycol (PEG) 1000 (Fig. 1A), which is frequently used in the development of drug delivery systems. The co-administration of TPGS with anti-cancer drugs improves their efficacy by enhancing their bioavailability and improving in vivo pharmacokinetics14. Combination of TPGS with other drugs leads to synergistic effects due to its ability to inhibit P-glycoprotein, an ATP-dependent drug efflux pump, also known as multidrug resistance protein 1 (MDR1) or ATP-binding cassette sub-family B member 1 (ABCB1)15,16. Also, as a single agent, TPGS has been found to inhibit the growth of human lung, prostate, and breast cancer cells by inducing apoptosis17–19.
In this study, we determined that the combination of YM155 and TPGS acted synergistically in reducing the viability of breast cancer cells. The combination of agents was effective in Her2neu-overexpressing, MDR1-wild-type SKBR3 cells but did not display synergistic effects in other breast cancer cell types or normal immortalized cells, suggesting that the mechanism of action is cell-type specific. Further mechanistic studies revealed that the compounds induce mitochondrial apoptosis via the de-activation of the AKT pathway and downregulation of Survivin. These results suggest that the markedly improved therapeutic efficacy of this combinational approach may hold significant potential for the development of future cancer treatment protocols.
Results
YM155 acts synergistically with TPGS to reduce the viability of SKBR3 cells
The effects of TPGS and YM155 on cell viability, alone and in combination, were tested on four human breast cancer cell lines (SKBR-3, MDA-MB-361, MCF-7 and MDA-MB-231) and one “normal” immortalized cell line (MCF-10A). All cell lines except MDA-MB-361 were sensitive to YM155 treatment (Fig. 1B and Table 1). The order of sensitivity to YM155 is as follows: MCF-7 < SKBR3 < MCF-10A < MDA-MB-231. TPGS at 5 μΜ did not significantly affect the viability of the cell lines tested, with the exception of MDA-MB-231 cells where the viability dropped to 70% (Figs 1B and S1). The combination of TPGS at 5 μΜ and YM155 at 5 and 10 nM displayed synergistic effect in reducing the viability of SKBR3 cells, with a CI index of 0.38 for the latter (Fig. 1B and Table 1). In the presence of 5 nM and 10 nM YM155 alone, the viability of SKBR3 dropped to 93% and 68% respectively, while its combination with TPGS (5 μΜ) reduced cell viability to 51% and 30%, respectively. Co-treatment of SKBR3 cells with the combination of TPGS-YM155 and with pan-caspase inhibitor z.vad.fmk or autophagy inhibitor Bafilomycine (Baf), were not able to restore cell viability (Supplementary Fig. S2) suggesting that caspase activation or autophagy are not solely responsible for the observed cell death.
Table 1.
Cytotoxicity of TPGS and YM155 alone and in combination in breast cell lines at 48 hours.
| Cell line | TPGS IC50 (μΜ) | ΥΜ155 IC50 (nΜ) | Combination Index (CI)* |
|---|---|---|---|
| SKBR3 | 20.77 ± 0.07 | 12 ± 0.09 | 0.38 |
| MDA-MB-361 | 15.3 ± 0.04 | N/A | 1.37 |
| MDA-MB-231 | 14.32 ± 0.08 | 7.26 ± 0.04 | 2.40 |
| MCF-7 | 10.27 ± 0.03 | 29.30 ± 0.11 | 5.74 |
| MCF-10A | 57.82 ± 0.10 | 7.73 ± 0.02 | 24.10 |
The data are expressed as the mean (±SE) of the results from at least three separate experiments. *for combination of 10 nM YM155 and 5 μΜ TPGS.
In order to examine whether the expression of HER2 is required for the synergistic effect with YM155, we evaluated the effects of the combination on MDA-MB-361, another HER2-overexpressing breast cancer cell line. Interestingly, the combination does not affect the viability of MDA-MB-361 cells (Fig. 1B) suggesting that the presence of Her2neu alone does not ensure a synergistic effect. The characteristics of each cell line used are displayed in Supplementary Table S1.
The combination of agents induces apoptosis in breast cancer cells
The selected agent concentrations were based on the calculated CI index as shown in Table 1. An additional consideration was to select YM155 concentrations that inhibit Survivin expression (Fig. 1C) without reducing the viability of SKBR3 cells below 50% of the control value. Based on these considerations we selected 5, 7.5 and 10 nM for YM155 and these were used for subsequent experiments.
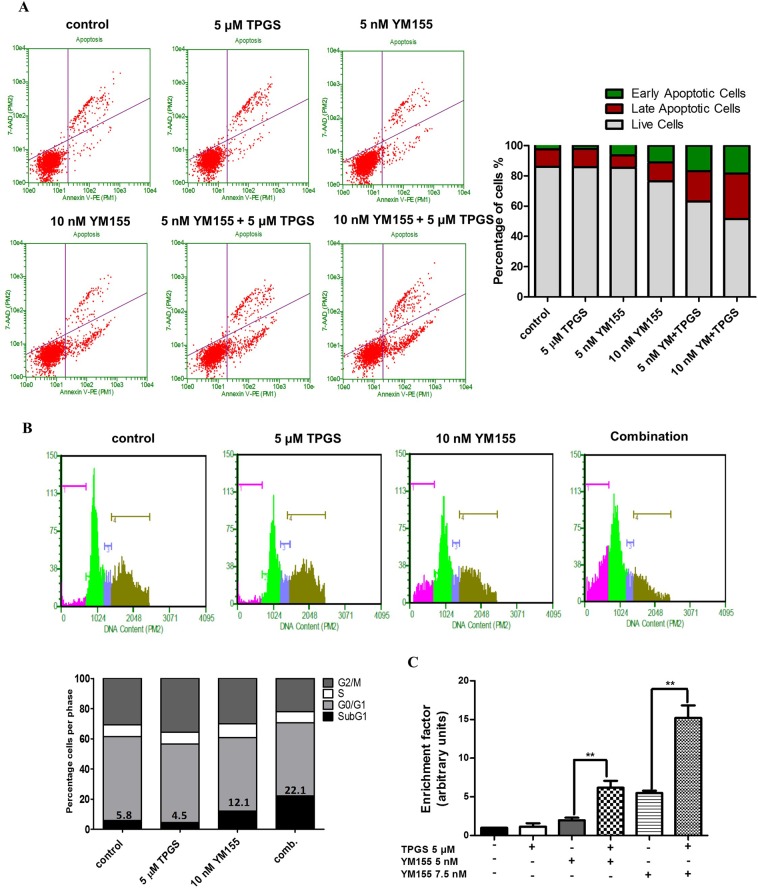
The combination of 10 nM YM155 and 5 μΜ TPGS decreased the mRNA and protein levels of Survivin in SKBR3 cells to a larger extend than each agent alone (Fig. 1C). To investigate the mechanism of action, we examined the ability of the agents to induce apoptosis. Apoptotic induction was analyzed using the Alexa FluorTM 488 Dead Cell Apoptosis Kit, where Annexin V and PI are used for determination of apoptotic cell death. Treatment with 5 μΜ TPGS and 10 nM YM155 for 48 hours resulted in about 18% of SKBR3 staining positive for Annexin V (early apoptotic) and 30% for PI (late apoptotic) (Fig. 2A). The combination of agents did not induce an increase in the early or late apoptotic population of MCF-10A cells (Supplementary Fig. S3). TPGS did not enhance the effect of apoptotic effect of YM155 in MCF-7 cells (Supplementary Fig. S4), suggesting that the synergistic effect is cell-type specific.
Figure 2.
Apoptotic effect of YM155 in combination with TPGS in SKBR3 cells. (A) Annexin V/PI staining was employed for evaluation of the apoptotic effect (% compared to control) of increasing concentrations of 5 and 10 nM YM155 with or without 5 μΜ TPGS for 48 hours. (B) Cell cycle analysis following treatment with 5 μΜ TPGS and 10 nM YM155 alone or in combination for 48 hours. (C) Elisa-based DNA fragmentation detection in SKBR3 cells treated with 5 or 7.5 nM YM155 alone or in combination with 5 μΜ TPGS at 24 hours. The results represent the mean ± SEM of three different replicates and are representative of at least three different experiments. *P value < 0.05, **P value < 0.01, ***P value < 0.001.
To determine whether the growth inhibitory effect of the agents is accompanied by cell cycle arrest, we incubated cells with 5 μΜ TPGS and 10 nM YM155 for 48 hours and performed cell cycle analysis. The test agents, when administered either alone or in combination did not induce cell cycle arrest, but rather caused an increase in the subG1 phase (12.1% in the presence of YM155 alone and 22.1% with both agents) further supporting their ability to induce apoptosis in this cell line (Fig. 2B). DNA fragmentation was also detected using an Elisa-based assay. Treatment of SKBR3 cells with 5 μΜ TPGS + 5 nM YM155 increased DNA fragmentation 3-fold compared to 5 nM YM155 alone, and 6-fold compared to control. Much higher increase was evident when TPGS was combined with 7.5 nM YM155 (Fig. 2C).
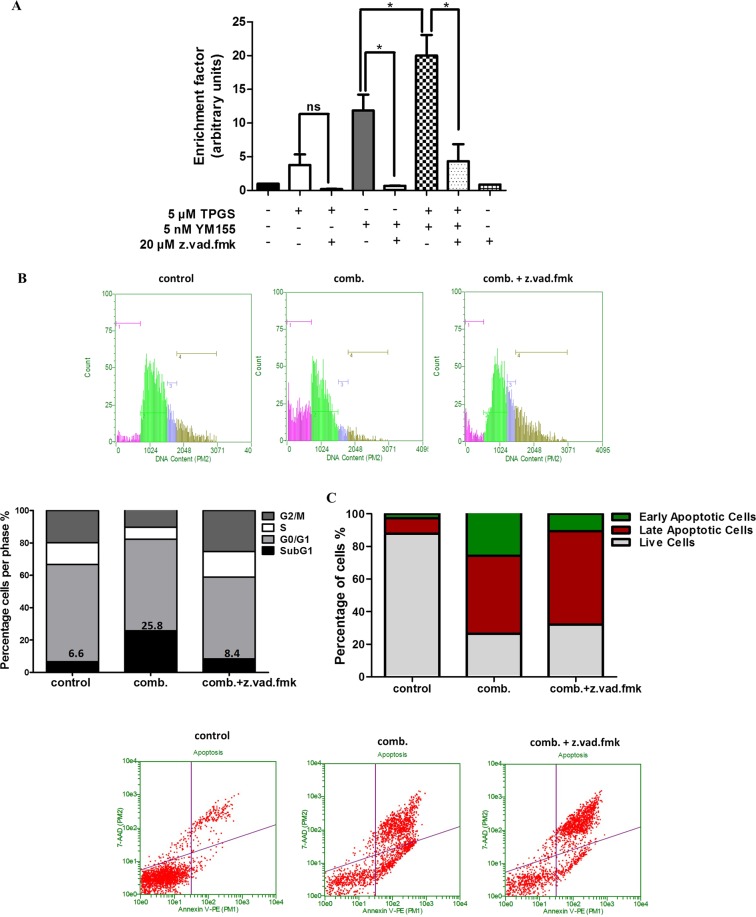
DNA fragmentation was greatly reduced in the presence of the caspase inhibitor z.vad.fmk (Fig. 3A,B). The latter indicates the activation of caspase-dependent pathways by the combination of agents. However, even though co-incubation of TPGS and YM155 with z.vad.fmk reduced DNA fragmentation it did not restore the viability of cells (Figs 3C and S2) suggesting that caspase-independent pathways of cell death may also be activated. The supernatant of treated cells was also collected and evaluated for the presence of DNA fragments to detect necrosis. The supernatant of treated cells in the presence of the agents was not significantly enriched with DNA fragments suggesting that necrosis is likely not involved in their mode of action (Supplementary Fig. S5). In addition, treatment of SKBR3 cells with 5 and 10 nM YM155 alone or in combination with 5 μΜ TPGS did not increase the intracellular Reactive Oxygen Species (ROS) levels (Supplementary Fig. S5), suggesting that the induced DNA damage is not attributed to oxidative stress.
Figure 3.
Reduction of DNA fragmentation caused by the combination of agents by z.vad.fmk. (A) Elisa-based DNA fragmentation detection in SKBR3 cells treated with 5 nM YM155, 5 μΜ TPGS with or without 20 μΜ z.vad.fmk for 48 hours. (B) Cell cycle analysis and (C) Annexin V/PI staining of SKBR3 cells treated with the combination of 5μΜ TPGS and 10 nM YM155 for 48 hours with or without z.vad.fmk. The results represent the mean ± SEM of three different replicates and are representative of at least three different experiments. *P value < 0.05, **P value < 0.01, ***P value < 0.001.
YM155 and TPGS induce the intrinsic pathway of apoptosis in SKBR3 cells
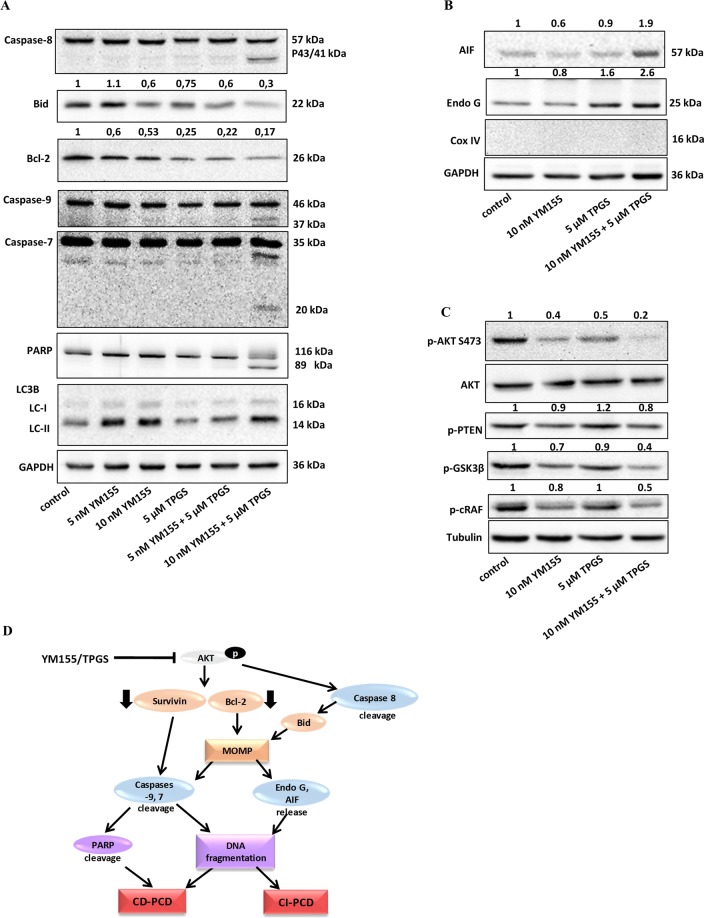
To investigate the apoptotic pathway induced by the combination of agents, we determined their ability to affect the status of proteins involved in mitochondrial apoptosis. TPGS and YM155 reduced the levels of anti-apoptotic Bcl-2, a known inhibitor of the Mitochondrial Outer Membrane Permeabilization (MOMP) process20 (Fig. 4A). Consistently, these agents induced cleavage of Caspase-9, a target of pro-apoptotic proteins released from the mitochondria, and Caspase-7, its downstream target. PARP, a known substrate of active caspase-721 was also cleaved 48 hours following the combination treatment (Fig. 4A). In addition, the combination of agents produced cleavage of initiator caspase-8 and reduced the levels of full length Bid which further enhances MOMP and mitochondrial apoptosis22. In agreement with previous reports, YM155 increased the amount of LC3B-II (Fig. 4A), a widely used autophagosomal marker, in SKBR3 cells23,24. However, co-treatment with TPGS did not further enhance the appearance of the LC-II form. This result is consistent with the inability of the autophagy inhibitor25 Bafilomycin to rescue cell viability in the combination treatment (Supplementary Fig. S2). These results indicate that autophagy is not involved in this scheme. The reduction of viability induced by the combination of YM155 and TPGS was not reversed by z.vad.fmk, indicating that capsase-independent pathways are also involved in their mode of action. To further investigate this possibility we assessed the cytosolic and nuclear levels of Apoptosis Inducing Factor (AIF) and Endo-G endonoucleases which have previously been shown to be involved in the induction of CI pathways of apoptosis26. Our results showed that the combination of agents caused an increase in the cytosolic levels of AIF and the 25 kDa form of Endo G providing strong support for the involvement of the caspace-independent apoptotic pathway (Fig. 4B).
Figure 4.
Effect of TPGS and YM155 on the levels and localization of apoptotic proteins. (A) The combination of 5 μΜ TPGS and 10 nM YM155 induces Caspase 8, 9, 7 and PARP cleavage and reduces the levels of Bcl-2 and Bid following 48 hours of treatment. YM155 alone produces the appearance of the LC-II protein (the blots were cropped; full length blots are shown in Fig. S7), (B) The combination of agents increased the levels of Endo G and AIF in the cytoplasm (the blots were cropped; full length blots are shown in Fig. S8), (C) The compounds inhibit the phosphorylation of AKT. SKBR3 cells were serum starved for 24 h, followed by incubation with 5 μM TPGS and 10 nM YM155 for 3 h and treated with 1 nM insulin, 1 hour prior to protein extraction (the blots were cropped; full length blots are shown in Fig. S9). (D) Potential mechanism of action of TPGS and YM155 in breast cancer. The agents inactivate the AKT pathway leading to the downregulation of Survivin and Bcl-2 and activation of Caspase-8. MOMP is increased causing Caspase 9 and 7 cleavage and release of Endo G and AIF to the cytosol, which lead to the activation of caspase-dependent and caspase-independent PCD. The results represent the mean ± SEM of three different replicates and are representative of at least three different experiments, *P value < 0.05, **P value < 0.01, ***P value < 0.001.
YM155 and TPGS inhibit the activation of the AKT pathway
AKT or Protein Kinase B (PKB), is often overactivated in cancer and plays a central role in the promotion of survival and inhibition of apoptosis. Akt is activated by phosphorylation within the carboxy terminus at Ser47327. Since the levels and activity of Survivin and Bcl-2 can be regulated via the PKB kinase28,29, we investigated the effects of the combination of agents on the phosphorylation status of the PI3K/AKT signaling pathway. YM155 and TPGS given as single agents reduced the levels of p-AKT at S473, while their combination almost abolished the phosphorylation of this residue (Fig. 4C). As a downstream effect, the levels of p-GSK3β, whose activity is inhibited by AKT-mediated phosphorylation at Ser930, are also reduced. Phosphorylation of c-Raf by Akt at S259, which was also blocked by the TPGS-YM155 concurrent treatment, inhibits activation of the Raf-MEK-ERK signalling pathway and shifts the cellular response from cell cycle arrest to proliferation in human breast cancer cells31. The phosphorylation status of PTEN, a major upstream regulator of the PI3K/Akt signaling pathway, was not significantly affected in the presence of the agents (Fig. 4C).
Discussion
Overexpression of Survivin has been reported in almost all human malignancies, including breast cancer, where it induces genetic instability32,33. The resulting population is resistant to apoptosis due to the overexpression of this IAP despite having extensive DNA damage34. YM155 causes DNA damage and downregulates Survivin, re-sensitizing in this manner this population of abnormal cells to apoptosis24; therefore, it is considered a promising treatment option and is being extensively investigated in a variety of cancers35–37. However, the efficacy of YM155 is hindered by its non-favorable pharmacokinetic profile38. Furthermore, short-term administration of high concentration of YM155 in the blood causes cardiotoxicity as well as nephrotoxicity39. In an attempt to lower the concentration of YM155 and improve its bioavailability, synergistic approaches as well as novel drug delivery systems have been developed. YM155 has demonstrated synergistic antitumor activity in combination with taxanes in lung cancer and melanoma models40,41 and sensitized human non-small cell lung cancer (NSCLC) cells to platinum drugs cisplatin and carboplatin42. YM155 also displayed synergistic action in neuroblastoma in in vitro and in vivo models in combination with lapatinib; the effect was attributed to lapatinib-induced inhibition of the ABCB1 efflux transporter, which allowed prolonged and elevated cytotoxicity of YM15543.
Recent studies have highlighted TPGS as an ideal molecular biomaterial in combination studies due to its multi-functional nature and its documented synergistic effectiveness with anti-cancer drugs44. Mixed micelles composed of a pH-sensitive poly(ethylene glycol)-doxorubicin conjugate prodrug and TPGS showed enhanced efficacy in multidrug resistant MCF-7/ADR cells45. The addition of TPGS in a nanocarrier loaded with Doxorubicin increased the therapeutic efficacy of the resulting nanoparticles, while a TPGS derivative was found to act synergistically with Docetaxel to reduce the viability of MCF-7 cells46,47.
In this study, we showed that the combination of YM155 and TPGS acts synergistically in SKBR3 breast cancer cells by de-activating the AKT survival pathway and inducing mitochondrial apoptosis. We also determined that the concentration of YM155 producing the highest synergy with TPGS is achievable, and well tolerated, in adult patients11. Importantly, the combination of agents did not produce significant cytotoxicity in normal immortalized breast cells. The effect of the combination of agents was specific to the SKBR3 cells that express high levels of HER2neu and have wild type PI3K/AKT and P-glycoprotein (Supplementary Table S1). HER2neu expression strongly correlates with PI3K/AKT activation48, which may support SKBR3 sensitivity to these agents. In addition, TPGS may block the activity of WT P-glycoprotein15 which is present in SKBR3 cells, thereby allowing for an enhanced effect of YM155, compared to the other cell lines (Fig. 1B). The sensitivity of SKBR3 to the combination of agents may also be attributed to the fact that they express the highest Survivin levels amongst the cell lines tested19. TPGS and YM155 act antagonistically in MCF-7, MCF-10A and MDA-MB-231 cells (Fig. 1B and Table 1); even though YM155 has been described as a Survivin suppressant, its mechanism of action was later challenged and may also act by inducing DNA damage and Mcl-1 depletion49. It is possible that the two agents compete for interaction with molecules implicated in yet unidentified mechanisms of action in cells lacking a functional HER2/PI3K/AKT/Survivin axis.
In agreement with our previous report, TPGS was found to be effective in inducing apoptosis in breast cancer cells by reducing Survivin levels via the AKT/PKB pathway, but had no significant effect in normal immortalized cells19. In addition, TPGS was shown to induce apoptosis in T cell acute lymphocytic leukemia cells (but not on human peripheral blood lymphocytes) in a dose dependent manner; TPGS increased the expression of Bax and Puma, reduced mitochondrial membrane potential, caused Caspase-3 cleavage, nuclear DNA fragmentation and cell cycle arrest in ALL cells50.
The combination of TPGS and YM155 inhibited the AKT pathway, reduced Survivin and Bcl-2 levels and induced cleavage of Caspase-8, causing translocation of AIF and Endo G to the cytosol resulting in cell death mediated through both caspase-dependent and caspase-independent programmed cell death (PCD). Inhibition of p-AKT is known to cause Caspase-8 and Bid cleavage, thereby inducing MOMP51. Previous studies showed that YM155 induces caspase-8 dependent apoptosis, in human leukemia cells, through downregulation of Survivin and Mcl-1 and, in glioma cell lines to promote loss of mitochondrial membrane potential and release of AIF to the cytosol52,53. TPGS is known to activate both caspase-dependent and caspase-independent PCD in breast cancer cell lines, inducing the downregulation of Bcl-2, the translocation of AIF and Endo G to the cytosol and the cleavage of caspase-7 and PARP19. We propose here a mechanism of synergistic action between YM155 and TPGS, by which the combination of the two agents inhibits the phosphorylation of AKT and causes downregulation of Bcl-2 and Survivin followed by Caspase-8 cleavage. The mitochondrial pathway of apoptosis is initiated, allowing the activation of Caspases −9 and −7 and the release of AIF and Endo G to the cytoplasm which cause caspase-dependent and caspase-independent PCD respectively (Fig. 4D).
Since polymeric nanoparticles for the delivery of YM155 have been developed, our data suggest that the inclusion of YM155 in a TPGS-based nano-carrier may be an effective approach against breast cancer.
Methods
Cell cultures and reagents
SKBR3, MCF-7, MDA-MB-231, MDA-MB-361 and MCF-10A cell lines were obtained from the American Type Culture Collection (ATCC) (Manassas, VA). SKBR3 cells were cultured in McCoys media supplemented with 5% FBS and 1% antibiotic/antimycotic, MCF-7 and MDA-MB-231 cells in DMEM supplemented with 10% FBS and 1% antibiotic/antimycotic, MDA-MB-361 cells in DMEM-F12 supplemented with 10% FBS, 1% antibiotic/antimycotic and 4 mM L-glutamine and MCF-10A in DMEM-F12 supplemented with 20 ng/ml EGF, 100 ng/ml Cholera Toxin, 500 ng/ml Hydrocortizone, 10 μg/ml insulin, 5% Horse Serum (HS) and 1% Antibiotic, Antimycotic. DMEM, McCoys, FBS, HS, antibiotic/antimycotic and trypsin used in cell culture were purchased from Gibco, Invitrogen (Carlsbad, California, USA). PARP, AIF, p-AKT Ser476, total-AKT, p-PTEN, p-GSK3β, p-cRAF, Caspase -7, -8, -9 and Survivin antibodies were purchased from Cell Signaling Technology (Danvers, Massachusetts, USA). α-Τubulin antibody was purchased from Sigma (St. Louis, Missouri, USA). Bcl-2, GAPDH, Cox IV and Endo G antibodies were purchased from Santa Cruz Biotechnology Inc. LC3B antibody was purchased from Novus Biologicals Inc. (Littleton, CO, USA). YM155 and z.vad.fmk was obtained from Selleck chemicals (Houston, TX, USA). TPGS was purchased from Eastman Chemical Company (Kingsport, Tennessee, USA). All other reagents were purchased from Sigma (St. Louis, Missouri, USA).
MTT assay
A total of 1 × 104 cells were seeded per well of a 96-well plate and incubated for 24 hours. At the end of the incubation period, cells were treated with different concentrations of YM155 or (and where stated) in the presence of TPGS, for the time periods and concentrations described in the figure legends. The assay was performed as described elsewhere54. Cell viability was measured across a range of dose levels for the drug pair without maintaining the ratio of dose levels constant. The combination index (CI) was used for the quantification of synergistic, antagonistic or additive effects of the drug pair as described previously55. Calculation of the CI was performed using the CompuSyn program, version 1.0.
Cell cycle analysis
Cells were seeded at a concentration of 1 × 106 cells per well of 10 mm plate. Following incubation, samples were prepared as previously described56 and analyzed for DNA content using the Guava EasyCyte™ flow cytometer and the GuavaSoft analysis software (Millipore, Watford,UK).
Cell death detection ELISA
Cells were seeded at a concentration of 1 × 104 cells per well of a 96-well plate and incubated for 24 hours. Cells were treated with TPGS or YM155 as indicated. The quantification of mono- and oligonucleosomes present in the cytoplasm of apoptotic cells was performed using the Cell Death ElisaPLUS Apoptosis Kit according to the manufacturer’s instructions (Roche).
Annexin V/Propidium iodide staining
Cells were seeded at a concentration of 1 × 105 cells per well of a 60-mm plate and treated with TPGS or YM155 as indicated. Cells were harvested and stained as described by Alexa FluorTM 488 Annexin V/Dead Cell Apoptosis kit (Life Technologies). Cell viability, death and apoptosis were evaluated using the Guava EasyCyte™ flow cytometer and the GuavaSoft analysis software (Millipore, Watford,UK). The annexin-V positive/PI negative cells were recognized as early apoptotic cells by the cytometer software whereas the annexin V positive/PI positive cells were identified as late apoptotic/dead cells. Similarly, the annexin V-negative/PI negative cells were identified as viable cells.
Western blot analysis
To determine protein levels we performed Western blot analysis as described previously18. For preparation of mitochondrial and cytosolic extracts, the Mitochondria/Cytosol Fractionation Kit (ab65320) was used according to the manufacturer’s instructions (Abcam). The intensity values from the densitometry analysis of Western blots were normalized against GAPDH or α-Tubulin using ImageJ analysis software. Intensity values were expressed as fold change compared to control.
Total RNA preparation and real-time quantitative PCR (q-PCR)
Total RNA was extracted with Trizol reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s protocol. cDNA was synthesized with random primers using the Superscript III Reverse Transcriptase (Invitrogen, Carlsbad, CA, USA). Primer sequences were designed using Primer3 and are as follows: human survivin, 5′-GACGACCCCATAGAGGAACA-3′(forward) and 5′-GACAGAAAGGAAAGCGCAAC-3′(reverse); and human GAPDH, 5´-TTGGTATCGTGGAAGGACTCA-3´(forward), 5´-TGTCATCATATTTGGCAGGTTT-3´ (reverse). Real-Time PCR was performed using the BioRad CFX96 Real-Time System and the SYBR Green PCR Master Mix (Applied Biosystems) according to the manufacturer’s instructions. The PCR products were normalized to those obtained from GAPDH mRNA amplification.
Statistical analysis
Results for continuous variables were presented as Mean ± Standard Error. Two-group differences in continuous variables were assessed by the unpaired T-test. P-values are two-tailed with confidence intervals 95%. Statistical analysis was performed by comparing treated samples with untreated control. All statistical tests were conducted using Prism software version 5.0 (Graphpad, San Diego, California, USA).
Supplementary information
Acknowledgements
This work was supported by funding from the Department of Biological Sciences of the University of Cyprus.
Author Contributions
C.N.M. wrote the main manuscript text and prepared all figures, A.M. and G.G. assisted in experimental methods, A.I.C. oversaw the project and assisted with the writing of the manuscript.
Data Availability
All materials, data and associated protocols mentioned in this manuscript will be available upon request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-50884-9.
References
- 1.Ferlay, J. et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer (2018). [DOI] [PubMed]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 3.O’Connor DS, et al. Regulation of apoptosis at cell division by p34cdc2 phosphorylation of survivin. Proc Natl Acad Sci USA. 2000;97:13103–13107. doi: 10.1073/pnas.240390697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altieri DC. Survivin and IAP proteins in cell-death mechanisms. Biochem J. 2010;430:199–205. doi: 10.1042/BJ20100814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waligorska-Stachura J, et al. Survivin–prognostic tumor biomarker in human neoplasms–review. Ginekol Pol. 2012;83:537–540. [PubMed] [Google Scholar]
- 6.Xu, C. et al. High survivin mRNA expression is a predictor of poor prognosis in breast cancer: a comparative study at the mRNA and protein level. Breast Cancer (2012). [DOI] [PubMed]
- 7.Kelly RJ, Lopez-Chavez A, Citrin D, Janik JE, Morris JC. Impacting tumor cell-fate by targeting the inhibitor of apoptosis protein survivin. Mol Cancer. 2011;10:35. doi: 10.1186/1476-4598-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gyrd-Hansen M, Meier P. IAPs: from caspase inhibitors to modulators of NF-kappaB, inflammation and cancer. Nat Rev Cancer. 2010;10:561–574. doi: 10.1038/nrc2889. [DOI] [PubMed] [Google Scholar]
- 9.Jha, K., Shukla, M. & Pandey, M. Survivin expression and targeting in breast cancer. Surg Oncol (2011). [DOI] [PubMed]
- 10.Nakahara T, et al. YM155, a novel small-molecule survivin suppressant, induces regression of established human hormone-refractory prostate tumor xenografts. Cancer Res. 2007;67:8014–8021. doi: 10.1158/0008-5472.CAN-07-1343. [DOI] [PubMed] [Google Scholar]
- 11.Kudchadkar R, et al. A phase 2, multicenter, open-label study of sepantronium bromide (YM155) plus docetaxel in patients with stage III (unresectable) or stage IV melanoma. Cancer Med. 2015;4:643–650. doi: 10.1002/cam4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papadopoulos KP, et al. A multicenter phase II study of sepantronium bromide (YM155) plus rituximab in patients with relapsed aggressive B-cell Non-Hodgkin lymphoma. Leuk Lymphoma. 2016;57:1848–1855. doi: 10.3109/10428194.2015.1113275. [DOI] [PubMed] [Google Scholar]
- 13.Giaccone G, et al. Multicenter phase II trial of YM155, a small-molecule suppressor of survivin, in patients with advanced, refractory, non-small-cell lung cancer. J Clin Oncol. 2009;27:4481–4486. doi: 10.1200/JCO.2008.21.1862. [DOI] [PubMed] [Google Scholar]
- 14.Neophytou CM, Constantinou AI. Drug Delivery Innovations for Enhancing the Anticancer Potential of Vitamin E Isoforms and Their Derivatives. Biomed Res Int. 2015;2015:584862. doi: 10.1155/2015/584862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dintaman JM, Silverman JA. Inhibition of P-glycoprotein by D-alpha-tocopheryl polyethylene glycol 1000 succinate (TPGS) Pharmaceutical research. 1999;16:1550–1556. doi: 10.1023/A:1015000503629. [DOI] [PubMed] [Google Scholar]
- 16.Collnot EM, et al. Mechanism of inhibition of P-glycoprotein mediated efflux by vitamin E TPGS: influence on ATPase activity and membrane fluidity. Mol Pharm. 2007;4:465–474. doi: 10.1021/mp060121r. [DOI] [PubMed] [Google Scholar]
- 17.Youk HJ, et al. Enhanced anticancer efficacy of alpha-tocopheryl succinate by conjugation with polyethylene glycol. J Control Release. 2005;107:43–52. doi: 10.1016/j.jconrel.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 18.Constantinou C, et al. Induction of DNA damage and caspase-independent programmed cell death by vitamin E. Nutr Cancer. 2012;64:136–152. doi: 10.1080/01635581.2012.630167. [DOI] [PubMed] [Google Scholar]
- 19.Neophytou CM, Constantinou C, Papageorgis P, Constantinou AI. D-alpha-tocopheryl polyethylene glycol succinate (TPGS) induces cell cycle arrest and apoptosis selectively in Survivin-overexpressing breast cancer cells. Biochem Pharmacol. 2014;89:31–42. doi: 10.1016/j.bcp.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Tait SW, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol. 2010;11:621–632. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- 21.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kantari C, Walczak H. Caspase-8 and bid: caught in the act between death receptors and mitochondria. Biochim Biophys Acta. 2011;1813:558–563. doi: 10.1016/j.bbamcr.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 23.Kabeya Y, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng SM, et al. YM155 down-regulates survivin and XIAP, modulates autophagy and induces autophagy-dependent DNA damage in breast cancer cells. Br J Pharmacol. 2015;172:214–234. doi: 10.1111/bph.12935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamamoto A, et al. Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells. Cell Struct Funct. 1998;23:33–42. doi: 10.1247/csf.23.33. [DOI] [PubMed] [Google Scholar]
- 26.Constantinou C, Papas KA, Constantinou AI. Caspase-independent pathways of programmed cell death: the unraveling of new targets of cancer therapy? Curr Cancer Drug Targets. 2009;9:717–728. doi: 10.2174/156800909789271512. [DOI] [PubMed] [Google Scholar]
- 27.Alessi DR, et al. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. doi: 10.1002/j.1460-2075.1996.tb01045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asanuma H, et al. Survivin expression is regulated by coexpression of human epidermal growth factor receptor 2 and epidermal growth factor receptor via phosphatidylinositol 3-kinase/AKT signaling pathway in breast cancer cells. Cancer Res. 2005;65:11018–11025. doi: 10.1158/0008-5472.CAN-05-0491. [DOI] [PubMed] [Google Scholar]
- 29.Pugazhenthi S, et al. Akt/protein kinase B up-regulates Bcl-2 expression through cAMP-response element-binding protein. J Biol Chem. 2000;275:10761–10766. doi: 10.1074/jbc.275.15.10761. [DOI] [PubMed] [Google Scholar]
- 30.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 31.Zimmermann S, Moelling K. Phosphorylation and regulation of Raf by Akt (protein kinase B) Science. 1999;286:1741–1744. doi: 10.1126/science.286.5445.1741. [DOI] [PubMed] [Google Scholar]
- 32.Jaiswal PK, Goel A, Mittal RD. Survivin: A molecular biomarker in cancer. Indian J Med Res. 2015;141:389–397. doi: 10.4103/0971-5916.159250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boidot R, et al. The expression of BIRC5 is correlated with loss of specific chromosomal regions in breast carcinomas. Genes Chromosomes Cancer. 2008;47:299–308. doi: 10.1002/gcc.20533. [DOI] [PubMed] [Google Scholar]
- 34.Rauch A, et al. Survivin antagonizes chemotherapy-induced cell death of colorectal cancer cells. Oncotarget. 2018;9:27835–27850. doi: 10.18632/oncotarget.25600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winter GE, et al. The solute carrier SLC35F2 enables YM155-mediated DNA damage toxicity. Nat Chem Biol. 2014;10:768–773. doi: 10.1038/nchembio.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang BH, et al. YM155 potently kills acute lymphoblastic leukemia cells through activation of the DNA damage pathway. J Hematol Oncol. 2015;8:39. doi: 10.1186/s13045-015-0132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pavlidou A, Kroupis C, Dimas K. Association of survivin splice variants with prognosis and treatment of breast cancer. World J Clin Oncol. 2014;5:883–894. doi: 10.5306/wjco.v5.i5.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawano H, et al. Antitumor efficacy and biodistribution of liposomal sepantronium bromide (YM155), a novel small-molecule survivin suppressant. European journal of pharmaceutics and biopharmaceutics: official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V. 2014;88:283–289. doi: 10.1016/j.ejpb.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 39.Satoh T, et al. Phase I study of YM155, a novel survivin suppressant, in patients with advanced solid tumors. Clin Cancer Res. 2009;15:3872–3880. doi: 10.1158/1078-0432.CCR-08-1946. [DOI] [PubMed] [Google Scholar]
- 40.Nakahara T, et al. YM155, a novel survivin suppressant, enhances taxane-induced apoptosis and tumor regression in a human Calu 6 lung cancer xenograft model. Anticancer Drugs. 2011;22:454–462. doi: 10.1097/CAD.0b013e328344ac68. [DOI] [PubMed] [Google Scholar]
- 41.Yamanaka K, et al. Antitumor activity of YM155, a selective small-molecule survivin suppressant, alone and in combination with docetaxel in human malignant melanoma models. Clin Cancer Res. 2011;17:5423–5431. doi: 10.1158/1078-0432.CCR-10-3410. [DOI] [PubMed] [Google Scholar]
- 42.Iwasa T, et al. Marked anti-tumour activity of the combination of YM155, a novel survivin suppressant, and platinum-based drugs. Br J Cancer. 2010;103:36–42. doi: 10.1038/sj.bjc.6605713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Radic-Sarikas B, et al. Lapatinib potentiates cytotoxicity of YM155 in neuroblastoma via inhibition of the ABCB1 efflux transporter. Sci Rep. 2017;7:3091. doi: 10.1038/s41598-017-03129-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang C, Wu T, Qi Y, Zhang Z. Recent Advances in the Application of Vitamin E TPGS for Drug Delivery. Theranostics. 2018;8:464–485. doi: 10.7150/thno.22711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun D, Lv X, Wang X, Yu A, Wang Y. Mixed micelles based on a pH-sensitive prodrug and TPGS for enhancing drug efficacy against multidrug-resistant cancer cells. Colloids Surf B Biointerfaces. 2017;159:419–426. doi: 10.1016/j.colsurfb.2017.07.082. [DOI] [PubMed] [Google Scholar]
- 46.Shieh MJ, et al. Reversal of doxorubicin-resistance by multifunctional nanoparticles in MCF-7/ADR cells. J Control Release. 2011;152:418–425. doi: 10.1016/j.jconrel.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 47.Mi Y, Liu Y, Feng SS. Formulation of Docetaxel by folic acid-conjugated d-alpha-tocopheryl polyethylene glycol succinate 2000 (Vitamin E TPGS(2k)) micelles for targeted and synergistic chemotherapy. Biomaterials. 2011;32:4058–4066. doi: 10.1016/j.biomaterials.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 48.Zhou BP, et al. HER-2/neu blocks tumor necrosis factor-induced apoptosis via the Akt/NF-kappaB pathway. The Journal of biological chemistry. 2000;275:8027–8031. doi: 10.1074/jbc.275.11.8027. [DOI] [PubMed] [Google Scholar]
- 49.Voges Y, et al. Effects of YM155 on survivin levels and viability in neuroblastoma cells with acquired drug resistance. Cell death & disease. 2016;7:e2410. doi: 10.1038/cddis.2016.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ruiz-Moreno C, Jimenez-Del-Rio M, Sierra-Garcia L, Lopez-Osorio B, Velez-Pardo C. Vitamin E synthetic derivate-TPGS-selectively induces apoptosis in jurkat t cells via oxidative stress signaling pathways: implications for acute lymphoblastic leukemia. Apoptosis. 2016;21:1019–1032. doi: 10.1007/s10495-016-1266-x. [DOI] [PubMed] [Google Scholar]
- 51.Uriarte SM, et al. Akt inhibition upregulates FasL, downregulates c-FLIPs and induces caspase-8-dependent cell death in Jurkat T lymphocytes. Cell Death Differ. 2005;12:233–242. doi: 10.1038/sj.cdd.4401549. [DOI] [PubMed] [Google Scholar]
- 52.Jane EP, Premkumar DR, Sutera PA, Cavaleri JM, Pollack IF. Survivin inhibitor YM155 induces mitochondrial dysfunction, autophagy, DNA damage and apoptosis in Bcl-xL silenced glioma cell lines. Mol Carcinog. 2017;56:1251–1265. doi: 10.1002/mc.22587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feng W, Yoshida A, Ueda T. YM155 induces caspase-8 dependent apoptosis through downregulation of survivin and Mcl-1 in human leukemia cells. Biochem Biophys Res Commun. 2013;435:52–57. doi: 10.1016/j.bbrc.2013.04.036. [DOI] [PubMed] [Google Scholar]
- 54.Charalambous C, Pitta CA, Constantinou AI. Equol enhances tamoxifen’s anti-tumor activity by induction of caspase-mediated apoptosis in MCF-7 breast cancer cells. BMC Cancer. 2013;13:238. doi: 10.1186/1471-2407-13-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 56.Savva CG, Totokotsopoulos S, Nicolaou KC, Neophytou CM, Constantinou AI. Selective activation of TNFR1 and NF-kappaB inhibition by a novel biyouyanagin analogue promotes apoptosis in acute leukemia cells. BMC Cancer. 2016;16:279. doi: 10.1186/s12885-016-2310-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All materials, data and associated protocols mentioned in this manuscript will be available upon request.