Abstract
Purpose
There is no established treatment of choice for low anterior resection syndrome (LARS). To evaluate the efficacy of biofeedback therapy for objective improvement of pelvic function in LARS, we performed the present study.
Methods
The primary endpoint was the change of Wexner score. Consenting patients between 20 and 80 years old with major LARS at least 2 months after sphincter preserving proctectomy for rectal cancer were enrolled. After recommendation of biofeedback therapy, patients who accept it were enrolled in the biofeedback group and patients who refuse were enrolled in the control group. Initial and follow-up evaluations were performed and analyzed.
Results
Fifteen and sixteen patients were evaluated in the control group and the biofeedback group, respectively. There was no statistically significant difference of LARS score between both groups. Decrease in Wexner score and increase in rectal capacity were significantly higher in the biofeedback group (odds ratio [OR], 5.386; 95% confidence interval [CI], 1.194–24.287; P = 0.028 and OR, 1.061; 95% CI, 1.002–1.123; P = 0.042).
Conclusion
Biofeedback therapy was superior for objective improvement of pelvic function to observation in LARS. It can be considered to induce more rapid improvement of major LARS.
Keywords: Fecal incontinence, Manometry, Postoperative complications, Rectal neoplasm, Rehabilitation
INTRODUCTION
Low anterior resection syndrome (LARS) is a constellation of symptoms, including frequent or fragmented bowel movements, emptying difficulties, urgency, or fecal incontinence after a sphincter-saving proctectomy and mesorectal excision. Symptoms vary in type, severity, and duration and can disturb quality of life from weakly to very severely. It is reported that 50%–71% of patients who undergo lower anterior resection for rectal cancer develop one or more symptoms of LARS, 35%–52% of them suffer from major LARS, which means LARS with more than 30 score according to LARS scoring instruction (Fig. 1) [1]. Although most improvements of the functional impairment are known to accomplish by 6 to 12 months after operation, long-term studies recently reported the duration of symptoms can last up to 15 years after operation [2,3]. Recently, as the rate of sphincter-saving surgery has increased [4], the incidence of LARS has also increased. Pelvic radiotherapy as the part of the therapeutic strategy for locally advanced rectal cancer can also negatively affect function of the remnant rectum and sphincters [5].
Fig. 1. The low anterior resection syndrome score (LARS score).
There is no established treatment of choice for LARS. Most patients are recommended for only observation with reassurance that it will improve with sufficient time. Loperamide (for diarrhea) or ramosetron (for postprandial urgency or incontinence) is generally used. Transanal irrigation has been reported to improve quality of life in patients with LARS [6]. The efficacy of pelvic floor rehabilitation using biofeedback, pelvic floor muscle training, or balloon training has been reported [7,8,9]. In the last 2 decades, sacral nerve stimulation has emerged as a treatment for LARS [10,11]. Although surgical treatments including graciloplasty and artificial sphincter have been introduced, their efficacy is unclear. Unfortunately, a few patients who suffer from LARS which is too severe to maintain daily living choose to undergo stoma formation.
Among the numerous treatments mentioned above, biofeedback is a method that can induce fundamental improvement of pelvic function by rehabilitation. It can be performed easily and safely in a defined period. Biofeedback therapy does not accompany inconvenience of continuous taking and regulating medication. Also, there is less or no concern about cost-effectiveness, feasibility, invasiveness with permanent insertion of artificial prosthesis as sacral nerve stimulation.
However, although there are several retrospective or prospective studies have reported the efficacy of biofeedback therapy in LARS [8,12,13], there is no prospective and comparative study evaluating its efficacy for objective improvement of pelvic function. To evaluate the efficacy of biofeedback therapy for objective improvement of pelvic function comparing to observation in LARS, we performed the present study.
METHODS
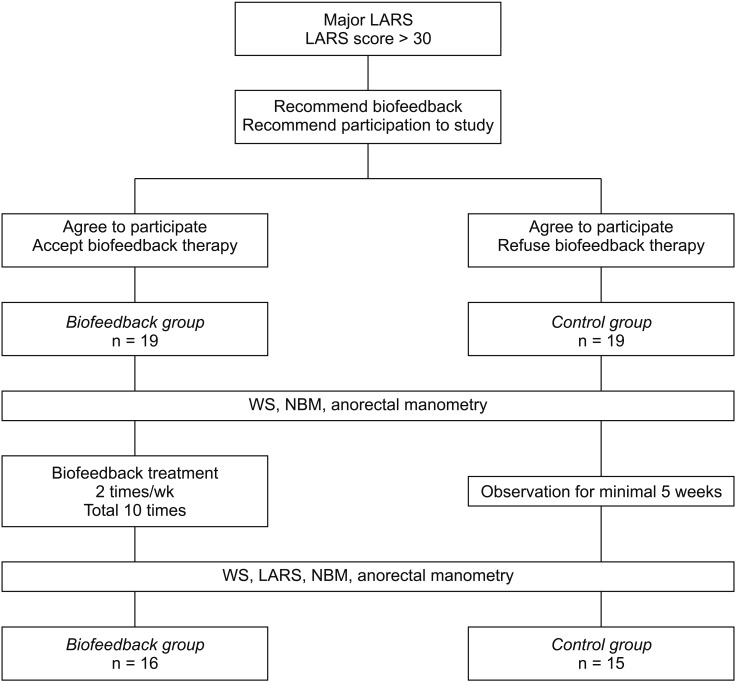
Patients were asked to participate in the study when they met the following inclusion criteria: (1) between 20 and 80 years old; (2) at least 2 months after sphincter-saving proctectomy with mesorectal excision for rectal cancer; (3) major LARS according to the LARS score (Fig. 1) [1]. After recommendation of biofeedback therapy to the consent patients, patients who accept it were enrolled in the biofeedback group and patients who refuse were enrolled in the control group (Fig. 2). We excluded patients with previous incontinence or defecatory dysfunction unrelated to rectal cancer.
Fig. 2. Flow-chart. LARS, low anterior resection syndrome; WS, Wexner score; NBM, number of bowel movement.
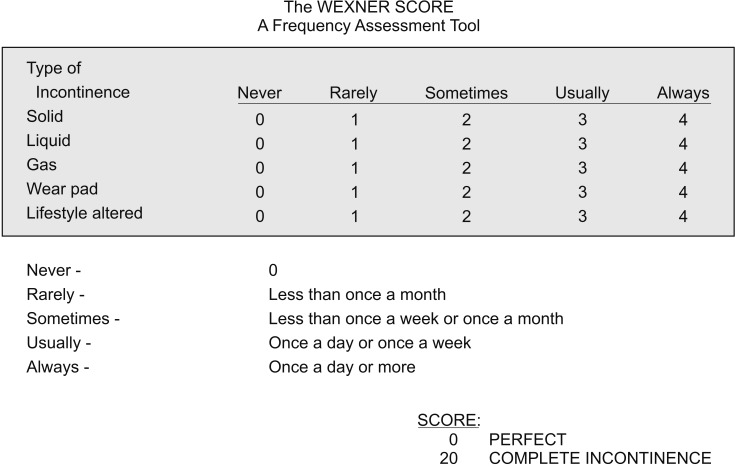
Initial Wexner score (WS) (Fig. 3), LARS score, and the number of bowel movement (NBM) per day were evaluated and anorectal manometry (ARM) was performed. The biofeedback group underwent biofeedback therapy twice per week for a total of 10 times, and the control group underwent observation. Loperamide administration was permitted in both groups, and dose and times were regulated depending on patient's individual condition and preference freely from 1 to 8 tablets per day, from 1 to 2 tablets once. After 5 weeks, follow-up WS, LARS score, and NBM were evaluated, and follow-up ARM was performed.
Fig. 3. The Wexner score (The translated version into the native language was used).
The study was approved by the Institutional Review Board (approval number: 2014-06-017) and all patients provided written informed consent.
Anorectal manometry
Patients were asked to apply a bisacodyl suppository at least 3 hours before the examination to empty the rectum. Enema shortly before ARM is avoided because it can stimulate the sphincter and consequently affect the results of examination.
With the patient in the Sims position, a radial 8-channel Micro Tip anorectal water-perfused catheter (Medtronic, Minneapolis, MN, USA) was placed into the neorectum 6 cm above the anal verge and pulled by the practitioner at the rate of 1 cm per second. The catheter was connected to an 8-channel water perfusion pump system (Medtronic) and POLYGRAM NET software (POLYGRAF ID, Alpine Biomed ApS, Skovlunde, Denmark). This continuous pull-though measurement was performed twice. Then, a spiral 8-channel anorectal water-perfused catheter with a balloon was placed in the neorectum. As the balloon was gradually inflated with air using a 50-mL syringe, existence of rectoanal inhibitory reflex was evaluated, and the volumes of the first sensation, the desire to defecate, and maximal tolerance were measured. This procedure was conducted very carefully and cautiously, especially for the patients who underwent radiotherapy, in order to prevent iatrogenic injury to the neorectum or anastomosis.
ARM data include sphincter profiles (length, mean resting pressure [MNRP], maximal resting pressure [MXRP], mean squeezing pressure [MNSP], maximal squeezing pressure [MXSP], mean asymmetry, vector volume, maximal pressure to the end of the sphincter), high pressure zone profiles (length, MNRP, MXRP, MNSP, MXSP, mean asymmetry, vector volume), rectoanal inhibitory reflex, and sensory thresholds (first sensation, desire to defecate, maximal tolerance). All ARM procedures were performed by a single experienced technician.
Biofeedback therapy
Biofeedback therapy was carried using Orion Platinum 2 biofeedback equipment (SRS Medical Systems, Inc., Redmond, WA, USA) twice per week for a total of 10 sessions by a single experienced therapist. It is considered 4–6 or more sessions are necessary for learning new patterns and sustaining the effects and usually it is performed weekly or biweekly. It can be tailored individually. Because most LARS patients have serious symptoms, we applicated more intensive program to induce more prompt and maximized effect. Electrodes for surface electromyography (EMG) were attached to the lower abdomen and an acryl sensor (Perry, Elan, SRS medical Systems, Redmond, WA, USA) was inserted in the anus. With visual feedback on the computer monitor displaying EMG activities, the patient was instructed to squeeze and relax the anal sphincters and trained to perform pelvic exercise.
Statistical analysis
The primary endpoint of the present study was the change of WS, and secondary endpoints were the changes of LARS score, NBM and ARM profiles. Using the Power and Sample Size program, the sample size of each group was calculated as 19 at the significant level 0.05, power of 0.8, mean of 4.9, and standard deviation of 6.0 [8]. Comparisons of clinical and pathological characteristics, results of initial and follow-up evaluations, and changes of those results between both groups, and multivariate analyses were performed using chisquare linear-by linear association and binary logistic regression analysis of SPSS ver. 12 (SPSS Inc., Chicago, IL, USA).
RESULTS
Nineteen patients were enrolled in each group. However, 4 and 3 patients were excluded from the control group and the biofeedback group, respectively, due to loss of follow-up, refusal of follow-up evaluation, or refusal to complete biofeedback therapy. Finally, 15 and 16 patients were evaluated in each group, respectively.
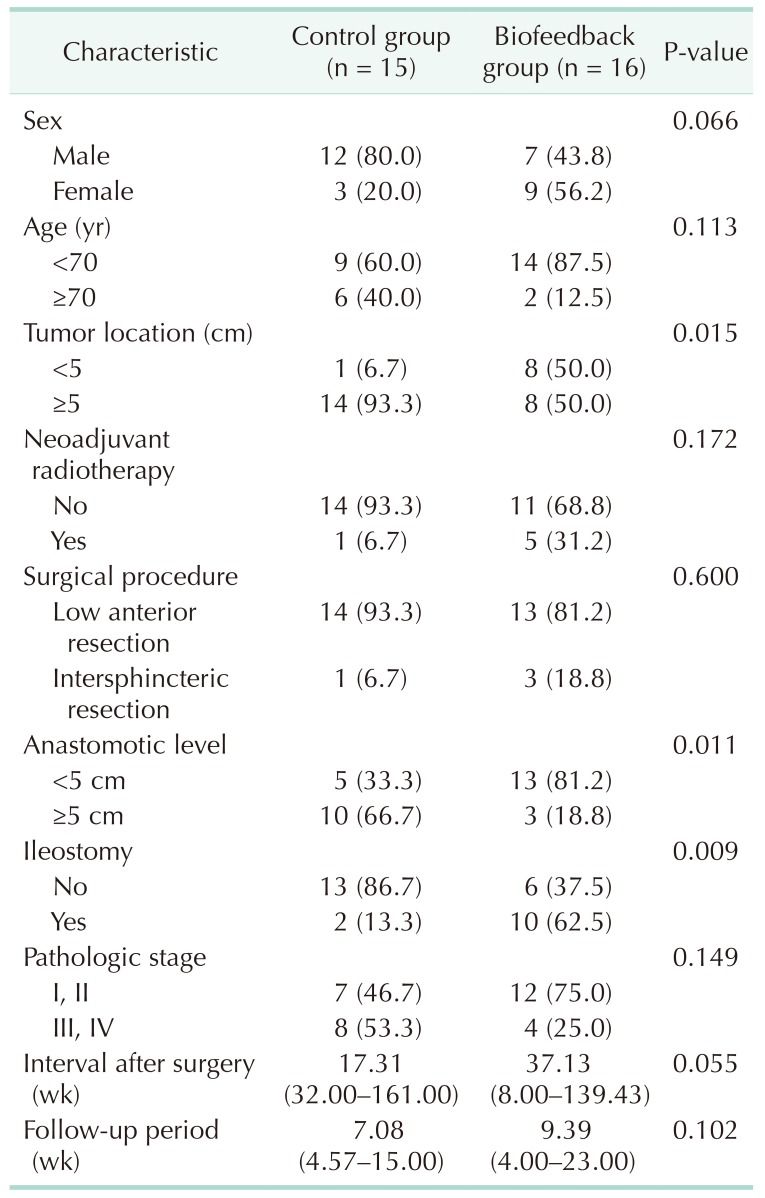
Clinical and pathological characteristic are reported in Table 1. There was no statistically significant difference in sex, age, neoadjuvant radiotherapy, type of operation, pathologic stage, interval after surgery, and follow-up period between groups. However, tumor location and anastomotic level were significantly lower in the biofeedback group (P = 0.015 and P = 0.011) and rate of ileostomy was significantly higher in the biofeedback group (P = 0.009).
Table 1. Clinical and pathologic characteristics.
Values are presented as number (%) or median (range).
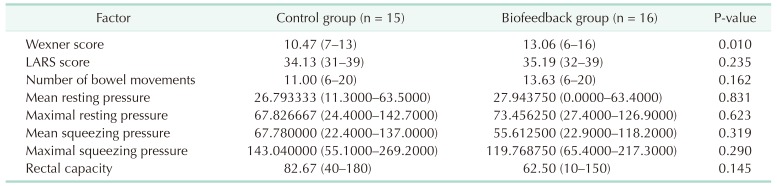
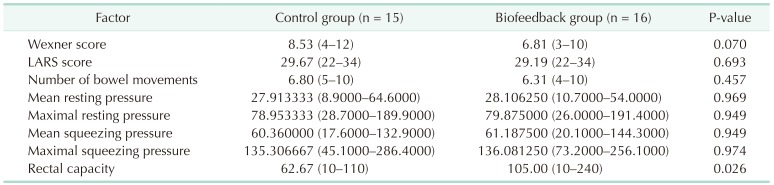
The results of initial evaluation are presented in Table 2. There was no statistically significant difference in LARS score, NBM, MNRP, MXRP, MNSP, MXSP, and rectal capacity. However, WS was significantly higher in the biofeedback group (10.47 vs. 13.06, P = 0.010).
Table 2. Initial evaluation.
Values are presented as median (range).
LARS, low anterior resection syndrome.
The results of follow-up evaluation are presented in Table 3. While initial WS was significantly higher in the biofeedback group, follow-up WS was lower in the biofeedback group with borderline significance (8.53 vs. 6.81, P = 0.070). Rectal capacity was significantly higher in the biofeedback group (62.67 vs. 105.00, P = 0.026).
Table 3. Follow-up evaluation.
Values are presented as median (range).
LARS, low anterior resection syndrome.
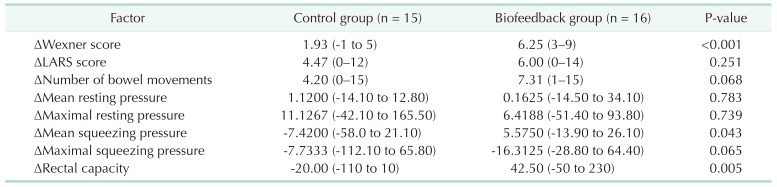
Differences between initial and follow-up evaluations are presented in Table 4. Decrease in WS, increase in MNSP, and increase in rectal capacity were significantly higher in the biofeedback group (1.93 vs. 6.25, P < 0.001; −7.4200 vs. 5.5750, P = 0.043; −20.00 vs. 42.50, P = 0.005). Decrease in number of bowel movements and increase in MXSP were higher in the biofeedback group with borderline significance (4.20 vs. 7.31, P = 0.068; −7.7333 vs. 16.3125, P = 0.065).
Table 4. Differences between initial and follow-up evaluations.
Values are presented as median (range).
LARS, low anterior resection syndrome.
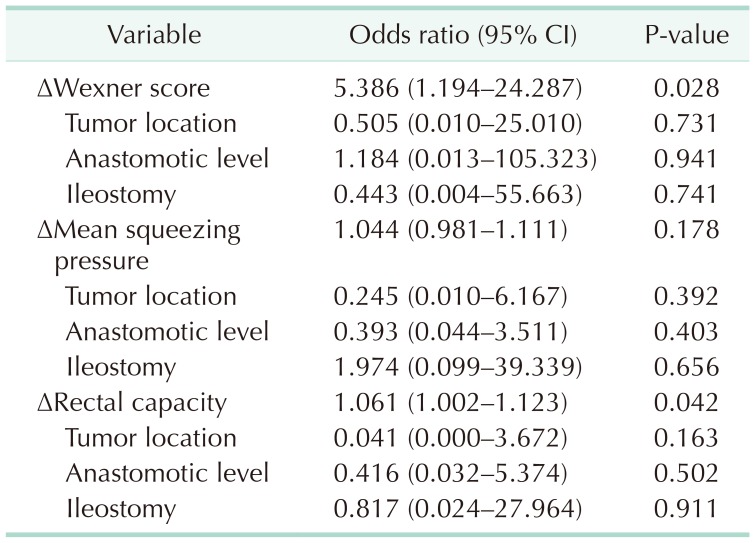
Multivariate analyses for adjustment of significant differences including tumor location, anastomotic level, and ileostomy between both groups were presented in Table 5. Decrease in WS and increase in rectal capacity were independently significant even after adjustment of above mentioned covariates (odds ratio [OR], 5.386; 95% confidence interval [CI], 1.194–24.287; P = 0.028 and OR, 1.061; 95% CI, 1.002–1.123; P = 0.042). However, increase in MNSP was not significant at the multivariate analysis.
Table 5. Multivariate analysis.
CI, confidence interval.
DISCUSSION
LARS is an inevitable result after physical loss of total or partial rectal volume. Reconstruction techniques, such as the colonic J pouch, was reported to produce only a transient effect that lasts shorter than 18 months [14], proving that LARS is not caused solely by reduction in rectal capacity and compliance. It is considered that there are several other factors contributing to the development of LARS. As pelvic dissection or radiotherapy can cause pelvic denervation, subsequent neorectal hyposensitivity can result in reservoir dysfunction of neorectum, and subsequent loss of distal negative feedback can increase proximal colonic motility. Intersphincteric resection and pelvic irradiation can cause anal sphincter dysfunction [15]. Some studies reported that the mean anal resting pressure decreased after low anterior resection and this change was irreversible [16,17]. The rate of direct injury to the internal sphincter by the transanal device during surgery has been reported to be as high as 18% [18]. Based on the above mentioned multifactorial pathology of LARS, rehabilitation can be a fundamental treatment approach that can produce physiologic improvement of pelvic function by adaptation to structural and functional changes of the pelvis after proctectomy and total mesorectal excision.
Biofeedback therapy is an established treatment for non-surgically induced fecal incontinence. In LARS, several studies have reported that biofeedback therapy could result in improvement of fecal incontinence scores, NBM, use of antidiarrheal medication, and ARM values [7,8]. Nevertheless, it is hard to distinguish whether the improvement of LARS is due to efficacy of biofeedback therapy or natural decline over time because patients with LARS show variable degrees of improvement over time without any active treatment [19]. However, there is lack of prospective study concerning the superior efficacy of biofeedback therapy compared to observation. Although there are many studies reporting efficacy of biofeedback with scoring system according to the patient's answers or questionnaires, there are few studies that have confirmed the improvement of the objective parameters. Therefore, we performed this prospective and comparative study.
In the present study, despite LARS of the biofeedback group is considered to be worse than that of the control group initially at the aspect of tumor location, anastomotic level, creation of ileostomy and initial WS, there was no statistically significant difference between initial LARS scores of both groups. Biofeedback therapy was superior for decrease of WS and increase of squeezing pressure with statistical significance, and it can also be expected to decrease of NBM and increase of rectal capacity by the borderline significances. Strengthening of pelvic function by squeezing pressure and increase of rectal capacity can increase thresholds and enhance voluntary responses to LARS symptoms as frequency, urgency, and fecal incontinence. Considering that there was no difference of resting pressure between both groups, it is considered that functional recovery of internal sphincter is hard to be induced by short-term rehabilitation. It may require more sufficient time or may be permanent as mentioned above [16].
A major limitation of the study is the small sample size with relatively marked number of drop-out and the selection bias. As described above, patients with more severe symptoms would tend to applicate biofeedback therapy and be included in the biofeedback group. However, randomization for biofeedback therapy is not feasible because patient's opinion for the necessity of multiple sessions of treatment varied depending on the patient's tolerance to LARS, social activities, or accessibility to the clinic. Despite this selection bias, LARS scores were almost equal between both groups, and some follow-up parameters and their improvement degrees were confirmed to be superior in the biofeedback group with statistical significance in the univariate and multivariate analyses. Because these parameters are objective, placebo effects are less likely to intervene to the results as in the subjective questionnaires. Therefore, it is not considered that the bias did not produce significant negative effect the reliability of the results.
The heterogeneity of the interval from surgery to treatment, ranging from 8 weeks to 161 weeks, is another limitation. Because the severity and the course of LARS are variable, there is difficulty in predicting the appropriate timing of biofeedback therapy. Martelluci insisted retrograde irrigation and pelvic floor rehabilitation as biofeedback therapy has to be considered if major LARS exists after 30 days from surgery [2]. Although many patients experience a natural improvement of symptoms after a few or several years and there may not be significant difference of long-term outcomes between both groups, biofeedback therapy can improve pelvic function sooner in the period that symptoms of LARS are most suffering and overwhelming, and decrease the prevalence length of major LARS.
In conclusion, biofeedback therapy was superior for objective improvement of pelvic function to observation in LARS. It can be considered to induce more rapid improvement of major LARS.
ACKNOWLEDGEMENTS
This study was supported by research fund of Chungnam National University.
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Emmertsen KJ, Laurberg S. Low anterior resection syndrome score: development and validation of a symptom-based scoring system for bowel dysfunction after low anterior resection for rectal cancer. Ann Surg. 2012;255:922–928. doi: 10.1097/SLA.0b013e31824f1c21. [DOI] [PubMed] [Google Scholar]
- 2.Martellucci J. Low anterior resection syndrome: a treatment algorithm. Dis Colon Rectum. 2016;59:79–82. doi: 10.1097/DCR.0000000000000495. [DOI] [PubMed] [Google Scholar]
- 3.Kupsch J, Jackisch T, Matzel KE, Zimmer J, Schreiber A, Sims A, et al. Outcome of bowel function following anterior resection for rectal cancer-an analysis using the low anterior resection syndrome (LARS) score. Int J Colorectal Dis. 2018;33:787–798. doi: 10.1007/s00384-018-3006-x. [DOI] [PubMed] [Google Scholar]
- 4.Warschkow R, Ebinger SM, Brunner W, Schmied BM, Marti L. Survival after abdominoperineal and sphincter-preserving resection in nonmetastatic rectal cancer: a population-based time-trend and propensity score-matched SEER analysis. Gastroenterol Res Pract. 2017;2017:6058907. doi: 10.1155/2017/6058907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dahlberg M, Glimelius B, Graf W, Pahlman L. Preoperative irradiation affects functional results after surgery for rectal cancer: results from a randomized study. Dis Colon Rectum. 1998;41:543–549. doi: 10.1007/BF02235256. [DOI] [PubMed] [Google Scholar]
- 6.Rosen H, Robert-Yap J, Tentschert G, Lechner M, Roche B. Transanal irrigation improves quality of life in patients with low anterior resection syndrome. Colorectal Dis. 2011;13:e335–e338. doi: 10.1111/j.1463-1318.2011.02692.x. [DOI] [PubMed] [Google Scholar]
- 7.Pucciani F, Ringressi MN, Redditi S, Masi A, Giani I. Rehabilitation of fecal incontinence after sphincter-saving surgery for rectal cancer: encouraging results. Dis Colon Rectum. 2008;51:1552–1558. doi: 10.1007/s10350-008-9312-6. [DOI] [PubMed] [Google Scholar]
- 8.Kim KH, Yu CS, Yoon YS, Yoon SN, Lim SB, Kim JC. Effectiveness of biofeedback therapy in the treatment of anterior resection syndrome after rectal cancer surgery. Dis Colon Rectum. 2011;54:1107–1113. doi: 10.1097/DCR.0b013e318221a934. [DOI] [PubMed] [Google Scholar]
- 9.Lin KY, Granger CL, Denehy L, Frawley HC. Pelvic floor muscle training for bowel dysfunction following colorectal cancer surgery: a systematic review. Neurourol Urodyn. 2015;34:703–712. doi: 10.1002/nau.22654. [DOI] [PubMed] [Google Scholar]
- 10.de Miguel M, Oteiza F, Ciga MA, Armendariz P, Marzo J, Ortiz H. Sacral nerve stimulation for the treatment of faecal incontinence following low anterior resection for rectal cancer. Colorectal Dis. 2011;13:72–77. doi: 10.1111/j.1463-1318.2009.02066.x. [DOI] [PubMed] [Google Scholar]
- 11.Schwandner O. Sacral neuromodulation for fecal incontinence and “low anterior resection syndrome” following neoadjuvant therapy for rectal cancer. Int J Colorectal Dis. 2013;28:665–669. doi: 10.1007/s00384-013-1687-8. [DOI] [PubMed] [Google Scholar]
- 12.Laforest A, Bretagnol F, Mouazan AS, Maggiori L, Ferron M, Panis Y. Functional disorders after rectal cancer resection: does a rehabilitation programme improve anal continence and quality of life? Colorectal Dis. 2012;14:1231–1237. doi: 10.1111/j.1463-1318.2012.02956.x. [DOI] [PubMed] [Google Scholar]
- 13.Visser WS, Te Riele WW, Boerma D, van Ramshorst B, van Westreenen HL. Pelvic floor rehabilitation to improve functional outcome after a low anterior resection: a systematic review. Ann Coloproctol. 2014;30:109–114. doi: 10.3393/ac.2014.30.3.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown CJ, Fenech DS, McLeod RS. Reconstructive techniques after rectal resection for rectal cancer. Cochrane Database Syst Rev. 2008;(2):CD006040. doi: 10.1002/14651858.CD006040.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen TY, Wiltink LM, Nout RA, Meershoek-Klein Kranenbarg E, Laurberg S, Marijnen CA, et al. Bowel function 14 years after preoperative short-course radiotherapy and total mesorectal excision for rectal cancer: report of a multicenter randomized trial. Clin Colorectal Cancer. 2015;14:106–114. doi: 10.1016/j.clcc.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 16.Williamson ME, Lewis WG, Finan PJ, Miller AS, Holdsworth PJ, Johnston D. Recovery of physiologic and clinical function after low anterior resection of the rectum for carcinoma: myth or reality? Dis Colon Rectum. 1995;38:411–418. doi: 10.1007/BF02054232. [DOI] [PubMed] [Google Scholar]
- 17.Pucciarelli S, Del Bianco P, Efficace F, Toppan P, Serpentini S, Friso ML, et al. Health-related quality of life, faecal continence and bowel function in rectal cancer patients after chemoradiotherapy followed by radical surgery. Support Care Cancer. 2010;18:601–608. doi: 10.1007/s00520-009-0699-y. [DOI] [PubMed] [Google Scholar]
- 18.Farouk R, Duthie GS, Lee PW, Monson JR. Endosonographic evidence of injury to the internal anal sphincter after low anterior resection: long-term follow-up. Dis Colon Rectum. 1998;41:888–891. doi: 10.1007/BF02235373. [DOI] [PubMed] [Google Scholar]
- 19.Bryant CL, Lunniss PJ, Knowles CH, Thaha MA, Chan CL. Anterior resection syndrome. Lancet Oncol. 2012;13:e403–e408. doi: 10.1016/S1470-2045(12)70236-X. [DOI] [PubMed] [Google Scholar]